Abstract
Meningitis caused by the zoonotic pathogen Campylobacter fetus in immunocompetent adults is rare. We report a 48-year-old Japanese woman with no underlying disease who was found to have meningitis caused by C. fetus. Both C. fetus subsp. fetus and C. fetus subsp. venerealis were isolated from the cerebrospinal fluid culture. The mode of infection in our patient was considered to be associated with the consumption of raw beef and raw cattle liver on a regular basis. Public awareness and education to avoid the consumption of raw or undercooked meat might help prevent C. fetus meningitis.
Keywords: Campylobacter fetus, Meningitis, Raw meat, Immunocompetent
Introduction
Campylobacter fetus (formerly called as Spirillum serpens or Vibrio fetus) is a zoonotic pathogen with major reservoirs of cattle and sheep. C. fetus is a rare cause of bacterial meningitis. Thus far, C. fetus meningitis has been reported to occur in those who frequently chew khat (an alkaloid containing plant) in an animal sanctuary, come in contact with domestic animals, or consume the raw meat or raw liver of cattle and sheep [1].
C. fetus infections frequently occur among patients with impaired immunity including conditions such as chronic alcoholism, liver disease, old age, diabetes mellitus, and malignancies [2]. There are only a few case reports of C. fetus bacteremia and meningitis in healthy adults [[3], [4], [5], [6]]. However, immunosuppression may not be the sole risk factor [7]. In this study, we report a case of C. fetus meningitis in a healthy adult and conducted a literature review.
Case
While in the emergency department of our hospital, she was alert and oriented, and not in acute distress. Her blood pressure was 132/60 mmHg, her heart rate was 64/min, her respiratory rate was 30/min, and her body temperature was 38.4 °C. The physical examination revealed nuchal rigidity without focal neurological abnormalities. Her laboratory tests revealed a white blood cell count of 14,200/μL; she tested negative for human immunodeficiency virus (HIV) antigens and antibodies and her electrolyte and aminotransferase levels were within normal limits. Cerebrospinal fluid (CSF) testing revealed leukocytosis with high protein and low glucose levels (Table 1). Her CSF showed increased white blood cells with neutrophil dominance with no organisms seen on Gram stain. Dexamethasone, ceftriaxone, ampicillin, vancomycin, and acyclovir were administered to treat both bacterial and viral meningitis. In addition, minocycline was administered to treat rickettsiosis. The serum cryptococcal antigen and serum nontreponemal and treponemal tests were negative. The acid-fast bacilli smear test and tuberculosis polymerase chain reaction (PCR) of the CSF were both negative.
Table 1.
Cerebrospinal fluid test.
| Leukocytes | 1219/μL |
|---|---|
| Polynuclear cells | 799/μL (65%) |
| Mononuclear cells | 418/μL (34%) |
| Protein | 80 mg/dL |
| Glucose | 51 mg/dL |
| (Blood glucose) | 134 mg/dL |
On day three of admission, the patient’s headaches began to recede. Vancomycin and dexamethasone were discontinued as meningitis due to Streptococcus pneumoniae was thought to be less likely as the CSF cultures were negative. On day five of admission, Gram-negative spiral bacilli were isolated from the CSF culture. Acyclovir, ampicillin, and minocycline were discontinued, and only ceftriaxone was continued. On day 12 of admission, matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF-MS) identified the organisms isolated from the CSF specimen as C. fetus subsp. venerealis (score 2.378) and C. fetus subsp. fetus (score 2.334). At this point, repeated history taking revealed that she had been consuming raw beef and raw cattle liver every weekend. Thus, the diagnosis of C. fetus as a cause of meningitis was made.
Ceftriaxone was changed to meropenem as she developed generalized skin rash most likely as a side-effect of ceftriaxone. She was discharged home after she received four weeks of intravenous antimicrobial treatment, and she did not show any signs of recurrence. 16S rRNA gene sequencing was performed to confirm the identification of the organisms. Gene sequencing revealed 100% coincidence with C. fetus subsp. venerealis and 99% coincidence with C. fetus subsp. fetus.
Discussion
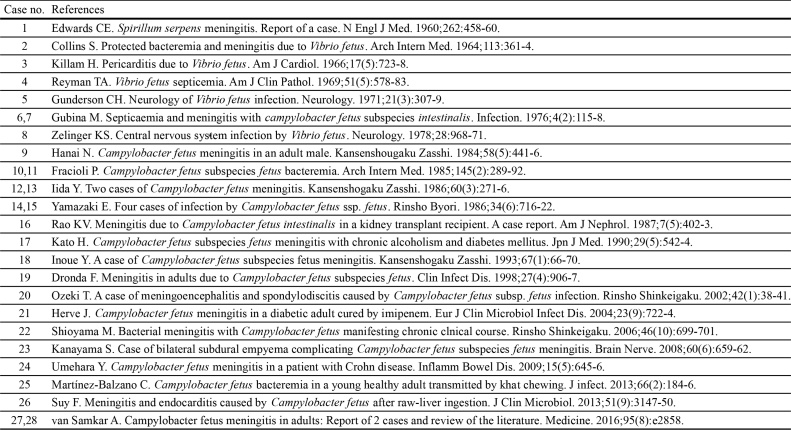
A literature search in Pubmed was performed, and all clinical cases of C. fetus meningitis in adults published in English and Japanese were reviewed. The following keywords were used: “meningitis AND Campylobacter fetus,” “meningitis AND Vibrio fetus,” and “Spirillum serpens AND meningitis.” The major findings are summarized in Table 2.
Table 2.
Summary of previously reported Campylobacter fetus meningitis.
| Case no. | Year | Author | Country | Age | Sex | Underlying conditions | Cause | Bacteriology | Method to identify the organism | Blood culture | CSF culture | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1960 | Edwards CE | United States | 50 | F | Hypertention | Handling fecal discharges of rats | Spirillum serpens | Biochemical | + | + | Cured |
| 2 | 1964 | Collins S | United States | 55 | M | Chronic lymphatic leukemia | Unknown | Vibrio fetus | Biochemical | + | + | Relapsed → Cured |
| 3 | 1966 | Killam H | United States | 48 | F | Healthy | Frequent contact with domestic animals | Vibrio fetus | Biochemical | + | – | Hemiparesis |
| 4 | 1969 | Reyman TA | United States | 69 | F | Diabetes mellitus, Thrombocytopenia | Unkown | Vibrio fetus | Biochemical | + | + | Died |
| 5 | 1971 | Gunderson CH | United States | 53 | M | Drug abuse | Unknown | Vibrio fetus | Biochemical | + | + | Deeply comatose |
| 6 | 1976 | Gubina M | Yugoslavia | 46 | M | Healthy | Frequent contact with domestic animals | C. fetus subsp. intestinalisa | Biochemical | + | – | Cured |
| 7 | 1976 | Gubina M | Yugoslavia | 40 | M | Healthy | Frequent contact with domestic animals | C. fetus subsp. intestinalisa | Biochemical | – | + | Cured |
| 8 | 1978 | Zelinger KS | United States | 50 | M | Healthy | Handling raw meat | Vibrio fetus | Biochemical | + | – | Cured |
| 9 | 1984 | Hanai N | Japan | 53 | M | Liver dysfunction | Unknown | C. fetus subsp. fetus | Biochemical | + | + | Cured |
| 10 | 1985 | Fracioli P | Switzerland | 68 | M | Adenocarcinoma of rectum with hepatic metastasis | Unknown | C. fetus subsp. fetus | Biochemical | + | + | Died |
| 11 | 1985 | Fracioli P | Switzerland | 65 | M | Alcoholic cirrhosis | Unknown | C. fetus subsp. fetus | Biochemical | + | – | Relapsed → Cured |
| 12 | 1986 | Iida Y | Japan | 30 | M | Healthy (Appendectomy history, Herniated disc) | Ingesting raw cattle liver | C. fetus subsp. fetus | Biochemical | – | + | Cured |
| 13 | 1986 | Iida Y | Japan | 42 | M | Healthy | Unknown | C. fetus subsp. fetus | Biochemical | – | + | Cured |
| 14 | 1986 | Yamazaki E | Japan | 53 | M | Healthy | Unknown | C. fetus subsp. fetus | Biochemical | + | + | No data |
| 15 | 1986 | Yamazaki E | Japan | 53 | M | Healthy | Unknown | C. fetus subsp. fetus | Biochemical | + | + | No data |
| 16 | 1987 | Rao KV | United States | 47 | M | Cadaver kidney transplant recipient | Ingesting raw cattle liver | C. fetus subsp. intestinalisa | Biochemical | + | + | Cured |
| 17 | 1990 | Kato H | Japan | 55 | M | Chronic alcoholism, Diabetes mellitus, Lung tuberculosis | Unknown | C. fetus | Biochemical | – | + | Cured |
| 18 | 1993 | Inoue Y | Japan | 40 | M | Healthy (Appendectomy history) | Ingesting raw beef | C. fetus subsp. fetus | Biochemical | – | + | Cured |
| 19 | 1996 | Dronda F | United States | 47 | M | Chronic alcoholism, Previous infection with HBV | Unknown | C. fetus subp. fetus | Biochemical, PCR | + | + | Relapsed → Cured |
| 20 | 1998 | Ozeki T | Japan | 49 | M | Alcoholic liver disease | Unknown | C. fetus subsp. fetus | Biochemical | + | + | Hemiparesis |
| 21 | 2002 | Herve J | France | 71 | M | Diabetes mellitus | Unknown | C. fetus subsp. fetus | Biochemical, 16S rRNA gene sequencing | + | + | Cured |
| 22 | 2004 | Shioyama M | Japan | 43 | M | Healthy | Unkown | C. fetus | Biochemical | – | + | Cured |
| 23 | 2006 | Kanayama S | Japan | 51 | M | Healthy | Unkown | C. fetus subsp. fetus | Biochemical | + | + | Cured |
| 24 | 2008 | Umehara Y | Japan | 40 | M | Crohn's disease | Unkown | C. fetus | Biochemical | + | + | Cured |
| 25 | 2010 | Martínez-Balzano C | United States | 28 | M | Healthy | Khat chewing | C. fetus subsp. fetus | 16S rRNA gene sequencing | + | – | Cured |
| 26 | 2013 | Suy F | France | 75 | M | Diabetes mellitus, Adenomatous sigmoid polyps | Ingesting raw sheep liver | C. fetus subsp. fetus | MALDI-TOF-MS, 16S rRNA gene sequencing | + | + | Cured |
| 27 | 2016 | van Samkar A | The Netherlands | 23 | F | Healthy | Frequent contact with domestic animals | C. fetus subsp. fetus | No data | – | + | Concentration problems |
| 28 | 2016 | van Samkar A | The Netherlands | 52 | M | Healthy | Frequent contact with domestic animals | C. fetus subsp. fetus | No data | + | + | Relapsed → Cured |
| 29 | 2017 | Present case | Japan | 48 | F | Healthy | Ingesting raw beef and cattle liver | C. fetus subsp. fetus/venrealis | No data | + | + | Relapsed → Cured |
C. fetus subsp. fetus is formerly described as C. fetus subsp. intesinalis [10].
Two subspecies of C. fetus were identified: C. fetus subsp. fetus and C. fetus subsp. venerealis. C. fetus subsp. fetus is associated with abortion in cattle and sheep and also causes infections in humans [2]. Conversely, C. fetus subsp. veneralis is associated with abortion in cattle [8], but its role in humans is uncertain. C. fetus subsp. veneralis has only been isolated from the stools of two homosexual men in Australia and from two women with bacterial vaginosis [2].
Our literature review revealed that all cases of meningitis were caused by C. fetus subsp. fetus. Our patient was unique as her CSF culture showed two subspecies: C. fetus subsp. fetus and C. fetus subsp. venerealis. MALDI-TOF-MS and 16S rRNA gene sequencing identified both subspecies. We considered two hypotheses. One was that our patient was infected by both the subspecies C. fetus subsp. fetus and venerealis. The other was that MALDI-TOF-MS and 16S rRNA gene sequencing failed to distinguish the two subspecies. Differentiation between the two subspecies has traditionally been determined by the 1% glycin tolerance test, and PCR assays have also been reported as a valuable adjunctive technique [9]. We did not perform these tests; however, C. fetus subsp. venerealis reported a higher score on performing MALDI-TOF-MS and a higher coincidence on performing 16S rRNA gene sequencing. C. fetus subsp. venerealis, an extremely rare organism to cause infections in humans, could be the pathogen that caused meningitis in our patient.
Another remarkable point in our literature review is that five patients were infected by consuming raw meat or raw liver and that three of them were Japanese with no past medical history, including our patient. It is not a rare occasion for people in Japan and other Asian countries to consume raw beef and raw cattle liver. Therefore, eating habits can be a major risk factor for these people even if they are immunocompetent.
In 2012, the Japanese Ministry of Health, Labour and Welfare prohibited serving raw cattle liver at restaurants. However, self-barbecue restaurants still provide raw meat and raw liver, and there are no legal restrictions regarding how restaurant customers cook raw meat and raw liver that was provided. Public awareness and education to prevent C. fetus meningitis should be warranted not only in Japan but also in other Asian countries where these eating habits exist.
Conflict of interest
All authors do not have any conflict of interest.
Acknowledgments
We thank Prof. Longzhu Cui, Professor of Microbiology, Jichi Medical University, Tochigi, Japan, for his insightful advice.
Appendix A.
References cited in Table 2.
References
- 1.van Samkar Anusha. Campylobacter fetus meningitis in adults: report of 2 cases and review of the literature. Medicine (Baltimore) 2016;95(8):e2858. doi: 10.1097/MD.0000000000002858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.8th ed. Elsevier; Saunders: 2015. Mandell, Douglas, and Bennett’s principles and practice of infections diseases. [Google Scholar]
- 3.Chavez A.C. Campylobacter fetus bacteremia in a healthy patient returning from a trip to the Ecuadorian Amazonia. Zoonoses Public Health. 2017;64(5):391–393. doi: 10.1111/zph.12338. [DOI] [PubMed] [Google Scholar]
- 4.Mikals K. Campylobacter fetus bacteremia in an immunocompetent traveler. Am J Trop Med Hyg. 2014;91(4):766. doi: 10.4269/ajtmh.14-0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nagy M.T. Campylobacter fetus sepsis in an immunocompetent patient with haematological complication. BMJ Case Rep. 2013 doi: 10.1136/bcr-2013-008610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zonios D.I. Campylobacter fetus bacteremia in a healthy individual: clinical and therapeutical implications. J Infect. 2005;51(4):329–332. doi: 10.1016/j.jinf.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Balzano C. Campylobacter fetus bacteremia in a young healthy adult transmitted by khat chewing. J Infect. 2013;66(2):184–186. doi: 10.1016/j.jinf.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 8.Salama S.M. Differentiation of the subspecies of Campylobacter fetus by genomic sizing. Int J Syst Bacteriol. 1992;42(3):446–450. doi: 10.1099/00207713-42-3-446. [DOI] [PubMed] [Google Scholar]
- 9.Schulze F. Identification of Campylobacter fetus subspecies by phenotypic differentiation and PCR. J Clin Microbiol. 2006;44(6):2019–2024. doi: 10.1128/JCM.02566-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veron M., Chatelain R. Taxonomic study of genus Campylobacter Sebald and Veron and designation of the neotype strain for the type species, Campylobacter fetus(Smith and Taylor) Sebald and Veron. Int J Syst Bacteriol. 1973;23(2):122–134. [Google Scholar]