Abstract
We present a case of aggressive angiomyxoma of the vulva. The patient presented with a persistent, enlarging vulvar mass, initially misdiagnosed as a Bartholin gland cyst. The patient underwent wide local excision, which resulted in total resection of the mass. Final pathology was consistent with aggressive angiomyxoma, a rare soft tissue tumor with a predilection for the female pelvis. Though rare, it is important to consider in the differential diagnosis of a pelvic mass, given the locally aggressive nature of this tumor and propensity for recurrence.
Keywords: Angiomyxoma, Pelvic mass
Highlights
-
•
Aggressive angiomyxoma is a rare soft tissue tumor occurring in the pelvic region of females.
-
•
Differential diagnosis for a persistent vulvar mass should include consideration of this tumor.
-
•
Gynecologic oncologists may manage these tumors due to propensity for deep invasion.
1. Introduction
Aggressive angiomyxoma (AAM) is a rare soft tissue tumor, first named and described by Steeper and Rosai in their quintessential case series in 1983 (Steeper and Rosai, 1983). It typically occurs in the pelvic and perineal region in females, with six times the incidence in females compared to males (Chen et al., 2017). The World Health Organization defines AAM as a “tumor of uncertain differentiation” (Fletcher et al., 2002). Prevalence in the population is unknown due to its rarity, making management and counseling difficult (Wang et al., 2016). Most reports of this disease come from case reports, with approximately 350 known cases (Sutton and Laudadio, 2012). However, it seems that AAM has a tendency for recurrence and can be a locally aggressive and invasive tumor.
As it has a predilection for the pelvic and perineal regions, AAM is often misdiagnosed as a Bartholin gland cyst, vulvar abscess, lipoma, or hernia, as much of the tumor itself is frequently hidden deep within the soft tissues of the pelvis (Sutton and Laudadio, 2012). It does not typically invade local structures such as the rectum, vagina, bladder, and urethra; thus, patients rarely experience obstructive symptoms from even quite large growths, but rather have nonspecific symptoms. Interestingly, there have been 7 cases reporting AAM originating outside the pelvic region (Sato et al., 2017). It is managed primarily with wide surgical resection (Steeper and Rosai, 1983).
2. Case
A 47-year-old female presented as a referral to Gynecologic Oncology with a new diagnosis of angiomyxoma of the vulva. She was initially seen by her gynecologist for a small <2 cm lesion thought to be a Bartholin gland cyst. Over the course of a year, it grew significantly in size. A biopsy was eventually done that revealed angiomyxoma.
The initial pathology from the biopsy was described as a moderately cellular, spindle-cell, myxoid lesion with variably sized blood vessels, and without nuclear pleomorphism, mitotic activity, or necrosis. Stromal tissue demonstrated bland cells intermixed with myxoid background and thin walled capillaries. Immunohistochemistry was positive for desmin and estrogen receptors, and negative for S-100. Based on these morphological and histological features, in combination with the clinical presentation, the diagnosis of aggressive angiomyxoma was made. This was confirmed by pathology review at a second institution.
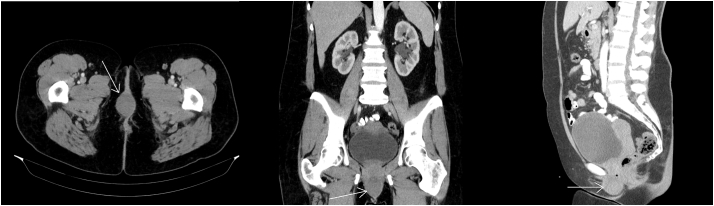
Due to persistence and progressive enlargement of the mass, and concern for deep invasion, the patient was referred to Gynecologic Oncology. She had no symptoms of dysuria, pain, bleeding, or change in bowel and bladder habits. Physical exam demonstrated a 5 cm spherical, mobile mass protruding from the right labia (Fig. 1). It did not appear to involve the rectum or urethra. No nodules were palpated internally on vaginal or rectal exam. Computed tomography (CT) with IV and oral contrast demonstrated soft tissue enlargement at the midline of the perineum, measuring approximately 4.3 × 3.6 cm (Fig. 2). The remainder of her imaging was unremarkable. The patient was consented for surgical excision of the mass. At the time of excision, 2 cm margins on the palpable mass were obtained.
Fig. 1.
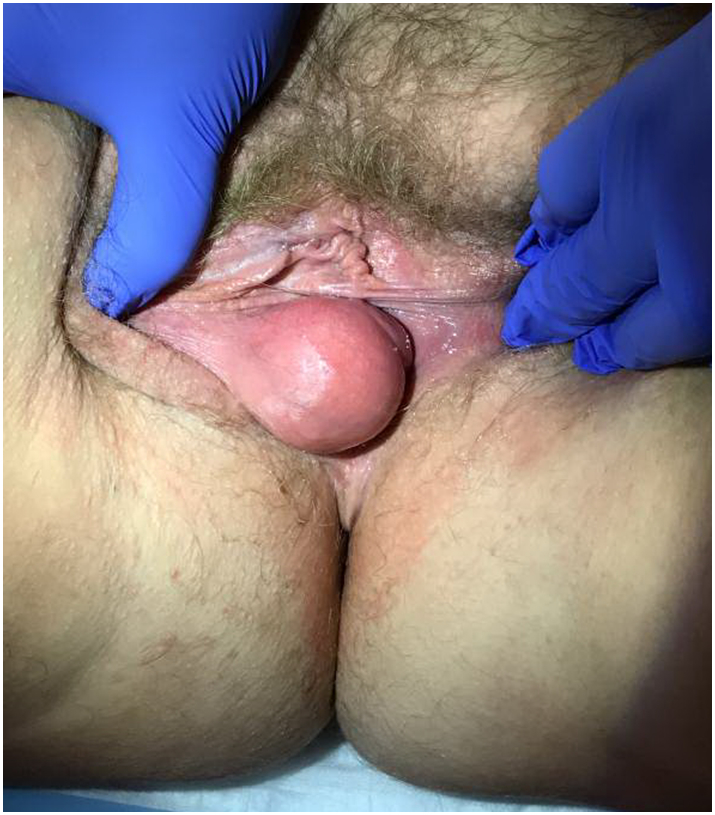
Approximately 5 cm spherical, mobile vulvar mass protruding from right labia.
Fig. 2.
CT scan demonstrated soft tissue enlargement at the midline of the perineum without other abnormalities. Tumor indicated by arrows.
Intraoperative consultation to pathology was obtained at the time of excision. The gross appearance of the lesion was described as a tan, whorled, rubbery nodule deep to the skin; it was excised in its entirety with negative margins. They reported difficulty interpreting the specimen as the lesion had low cellularity and no significant atypia. Some spindle cells were seen, favored to represent reactive stromal cells. Final pathology again confirmed AAM (Fig. 3). The patient did well postoperatively and has not had recurrence for 6 months. She will continue to follow with Gynecologic Oncology to ensure no local or distant recurrence.
Fig. 3.
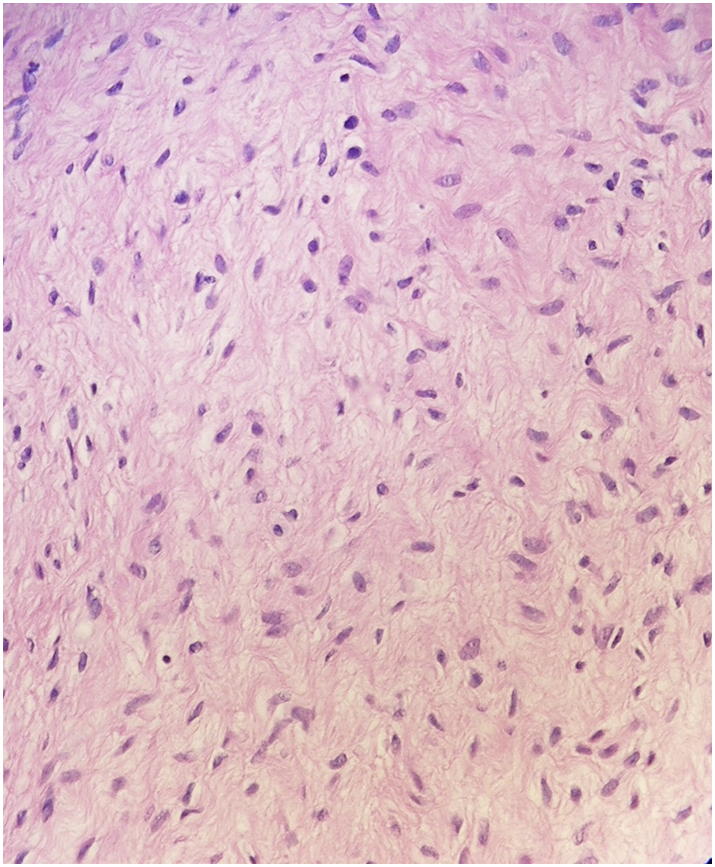
Bland appearing tissue with spindle to stellate shaped cells, myxoid background. Low cellularity, no significant atypia, and no mitotic activity noted.
3. Discussion
Although AAM is a rare entity, it is important to be aware of its clinical features. Patients diagnosed with AAM often present with a single mass that can range in size and can be otherwise asymptomatic. Overlying skin may appear normal, and practitioners may mistake AAM for a benign entity. The mass may be discovered upon routine gynecologic examination, or by the patient herself. Due to its propensity to invade deep into the soft tissue of the pelvis, a true appreciation for the size of the tumor may be difficult to evaluate on physical exam (Sutton and Laudadio, 2012). Symptoms range from pelvic fullness, to vulvar or perineal pain, to dyspareunia. It may have mass effect, but rarely invades rectal, vaginal, or urothelial tissue.
On excision, the tumor is noted to be un-encapsulated or partially capsulated, lobular, with borders that are not well circumscribed (Chen et al., 2017). The tumor nodule appears rubbery and bland in color. Histologically, the tumor demonstrates stellate-to-spindle-shaped cells distributed sparsely throughout the myxoid stroma and aggregated around blood vessels. Mitotic figures are infrequent and not atypical, and there is no necrosis within the tumor (Wang et al., 2016; Bai et al., 2013). Immunohistochemistry commonly is positive for CD34, SMA, desmin, vimentin, estrogen receptors (ER), and progesterone receptors (PR), and is typically negative for S-100; ER and/or PR positivity is one of the most characteristic features (Sutton and Laudadio, 2012; Bai et al., 2013). AAM can also present as multiple masses but this is rarely described. A 13-patient case series noted only one such case; an additional report of a patient with distant thoracic and lung metastases characterized a complex of several pelvic masses (Bai et al., 2013; Siassi et al., 1999).
For timely diagnosis, it is important to have a high index of suspicion for AAM. Misdiagnosis rates have been reported as high as 82% (Bai et al., 2013). Though rare, there can be high morbidity for patients when large growths require wide resection. Clinical diagnosis prior to biopsy, or excision and histological examination, can be difficult precisely because it is rare, and presentation is similar to many other genitourinary pathologies. Multiple imaging modalities have been used to evaluate AAM preoperatively, including CT (as in the described case), magnetic resonance imaging (MRI), and ultrasonography. Appearance on imaging may be variable, but has been noted to have a distinct “swirling” pattern on MRI, related to its myxoid composition. On CT, it may be hypodense to muscle, or have both cystic and solid components. Ultrasound may show a homogeneous hypoechoic mass with internal blood flow. It has been suggested that MRI may be preferred, due to characteristic swirling pattern observed, better depiction of tissue, and avoidance of radiation exposure. Pre-operative imaging may thus provide clues to increase suspicion for AAM (Chen et al., 2017; Sutton and Laudadio, 2012; Bai et al., 2013; Li and Ye, 2011).
Due to its aggressive and infiltrative nature, surgical management is the gold standard for treatment. As it grows, AAM tends to first displace rather than invade adjacent organs, though invasion does occur (Li and Ye, 2011). Deeper pelvic tumors may endanger pelvic organs at the time of removal to achieve desired wide margins (Chen et al., 2017). Incomplete removal is acceptable when significant morbidity is anticipated with broader excision (e.g. preservation of bladder or fertility), especially considering the generally indolent course of AAM (Sutton and Laudadio, 2012). Recurrence may occur either due to intentional or unintentional subtotal removal, as its borders are difficult to identify grossly, or despite adequate resection. Surgeons may consider medical management of residual disease with hormone modulating therapy (described below), or alternatively simply with close observation, which has been shown to be a viable option with long follow up and no subsequent recurrence of tumor. Recurrence rates range from 25% to 47%, with 85% recurring within 5 years of initial surgery (Chen et al., 2017; Sutton and Laudadio, 2012). For this report, the literature reviewed involved 55 individual cases out of approximately 350 known cases. All were primarily treated with surgical resection, 16 experienced recurrence, 6 were treated with adjuvant therapy, and 3 had distant metastatic disease. Time to recurrence ranged from 2 months to 15 years. Local recurrence is common, but distant metastases are rare.
In reviewing current literature, we found only one instance where lymphadenectomy was performed as part of surgical management of this rare tumor; the mass was initially misdiagnosed as recurrent urethral rhabdomyosarcoma, and only final pathology revealed AAM (Gonzaga et al., 2005). Due to the paucity of cases, there is not a well-established protocol for management beyond wide excision, and no established role for lymphadenectomy. Thus there is no evidence at this time for performing lymphadenectomy for this disease.
Additionally, there is not a well-established role for chemotherapy or radiation therapy in the treatment of AAM, though there are a few cases described where radiation was utilized pre-or-post-operatively to decrease tumor size or minimize risk of recurrence (Sutton and Laudadio, 2012). AAM has been shown to possess estrogen receptors and progesterone receptors, suggesting growth is hormonally responsive. This is supported by evidence of rapid tumor growth or recurrence during pregnancy (Orfanelli et al., 2016). Recently, medical treatment using hormonal agents, in particular gonadotropin releasing hormone (GNRH) agonists, sometimes in combination with selective estrogen receptor modulators (SERM), has been described in treatment of recurrent tumors (Palomba et al., 2011). Use of hormone-modulating therapies has shown success in both neoadjuvant and adjuvant modality; it has been used to preoperatively shrink tumors to minimize surgical morbidity, and post-operatively to treat recurrent disease. Both post-operative radiation and GNRH-agonists have demonstrated complete resolution of residual or recurrent tumor growth, and will continue to be valuable treatment tools to be explored in recurrent and possibly metastatic disease (Bai et al., 2013; Shinohara et al., 2004).
Metastatic disease has been reported in only three cases to date. One case described a patient with pulmonary metastatic disease, concomitant with initial discovery of pelvic masses (Siassi et al., 1999). Another described a patient that experienced several recurrences over a 10-year period, culminating with pulmonary metastatic AAM confirmed at autopsy (Blandamura et al., 2003). Lastly, there is case of pulmonary metastases with invasion along the vena cava and right atrium of the heart (Geng et al., 2012). Only the second report noted patient outcome – death from disease. Only the last case described treatment, which involved resection of tumor burden. There is no consensus on treatment of metastases, though as with recurrent disease, surgical resection where able appears to be a viable course of action. In addition, the success of hormonal therapies in treating recurrences heralds another potential modality to explore in the treatment of metastatic illness.
4. Summary
AAM is a rare but clinically relevant tumor with high potential morbidity without timely treatment. It has a predilection for the genitourinary, perineal, and pelvic areas in females, though a few cases have been reported in males, and rare instances originating outside the pelvis. Biopsy and imaging are conducive for preoperative diagnosis of this neoplasm. Wide surgical resection is the gold standard for treatment, though exceptions can be made for preservation of pelvic organ function and fertility, or to limit morbidity. Recent reports have also demonstrated potential benefit of medical therapies. Long-term follow up is necessary due to the high frequency of recurrence of AAM, the need to avoid increased morbidity from larger excision, and monitor for rare but deadly metastatic disease.
Conflicts of interest
The authors have no conflicts of interest to disclose.
References
- Bai H.M., Yang J.X., Huang H.F., Cao D.Y., Chen J., Yang N., Lang J.H., Shen K. Individualized managing strategies of aggressive angiomyxoma of female genital tract and pelvis. Eur. J. Surg. Oncol. 2013;39(10):1101–1108. doi: 10.1016/j.ejso.2013.06.013. Oct. [DOI] [PubMed] [Google Scholar]
- Blandamura S., Cruz J., Faure Vergara L., Machado Puerto I., Ninfo V. Aggressive angiomyxoma: a second case of metastasis with patient's death. Hum. Pathol. 2003;34(10):1072–1074. doi: 10.1053/s0046-8177(03)00419-2. Oct. [DOI] [PubMed] [Google Scholar]
- Chen H., Zhao H., Xie Y., Jin M. Clinicopathological features and differential diagnosis of aggressive angiomyxoma of the female pelvis: 5 case reports and literature review. Medicine (Baltimore) 2017 May;96(20) doi: 10.1097/MD.0000000000006820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher C.D.M., Unni K.K., Mertens F., editors. World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and BoneIARC Press: Lyon. 2002:189–190. [Google Scholar]
- Geng J., Cao B., Wang L. Aggressive angiomyxoma: an unusual presentation. Korean J. Radiol. 2012;13(1):90–93. doi: 10.3348/kjr.2012.13.1.90. Jan–Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzaga L.F., Freitas F.C., Tavares J.M. Aggressive vaginal angiomyxoma mimicking urethral tumor. Int. Braz. J. Urol. 2005;31(5):475–476. doi: 10.1590/s1677-55382005000500011. Sep–Oct. [DOI] [PubMed] [Google Scholar]
- Li X., Ye Z. Aggressive angiomyxoma of the pelvis and perineum: a case report and review of the literature. Abdom. Imaging. 2011;36(6):739–741. doi: 10.1007/s00261-010-9677-6. Dec. [DOI] [PubMed] [Google Scholar]
- Orfanelli T., Kim C.S., Vitez S.F., Van Gurp J., Misra N. A case report of aggressive angiomyxoma in pregnancy: do hormones play a role? Case Rep. Obstet. Gynecol. 2016;2016 doi: 10.1155/2016/6810368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomba S., Oppedisano R., Annunziata G., Zullo F., Amorosi A. Leuprolide acetate depot plus high-dose raloxifene hydrochloride before and after surgery for recurrent vaginal aggressive angiomyxoma: a case report. Gynecol. Oncol. 2011;123(1):172–173. doi: 10.1016/j.ygyno.2011.06.013. Oct. [DOI] [PubMed] [Google Scholar]
- Sato K., Ohira M., Shimizu S., Kuroda S., Ide K., Ishiyama K., Kobayashi T., Tahara H., Shiroma N., Arihiro K., Imamura M., Chayama K., Ohdan H. Aggressive angiomyxoma of the liver: a case report and literature review. Surg. Case Rep. 2017;3(1):92. doi: 10.1186/s40792-017-0365-4. Aug 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara N., Nonomura K., Ishikawa S., Seki H., Koyanagi T. Medical management of recurrent aggressive angiomyxoma with gonadotropin-releasing hormone agonist. Int. J. Urol. 2004;11(6):432–435. doi: 10.1111/j.1442-2042.2004.00816.x. Jun. [DOI] [PubMed] [Google Scholar]
- Siassi R.M., Papadopoulos T., Matzel K.E. Metastasizing aggressive angiomyxoma. N. Engl. J. Med. 1999;341(23):1772. doi: 10.1056/nejm199912023412315. Dec 2. [DOI] [PubMed] [Google Scholar]
- Steeper T.A., Rosai J. Aggressive angiomyxoma of the female pelvis and perineum. Report of nine cases of a distinctive type of gynecologic soft-tissue neoplasm. Am. J. Surg. Pathol. 1983;7(5):463–475. doi: 10.1097/00000478-198307000-00009. Jul. [DOI] [PubMed] [Google Scholar]
- Sutton B.J., Laudadio J. Aggressive angiomyxoma. Arch. Pathol. Lab. Med. 2012;136(2):217–221. doi: 10.5858/arpa.2011-0056-RS. Feb. [DOI] [PubMed] [Google Scholar]
- Wang Y.F., Qian H.L., Jin H.M. Local recurrent vaginal aggressive angiomyxoma misdiagnosed as cellular angiomyofibroblastoma: a case report. Exp. Ther. Med. 2016;11(5):1893–1895. doi: 10.3892/etm.2016.3097. May. [DOI] [PMC free article] [PubMed] [Google Scholar]