Abstract
The objective of this study was to determine the effect of oven and traditional roasting on the polyphenol content, antioxidant activity, lipid quality, proximate composition and mineral content of fermented cocoa beans. Results showed that traditional roasting significantly decrease (p < 0.05) the polyphenol content and antioxidant activity of cocoa beans. The analysis of oil showed that oven and traditional roasting significantly increase (p < 0.05) the peroxide and thiobarbituric acid values of cocoa butter and that traditional roasting samples were the most deteriorated. Oven and traditional roasting for 10 min reduce the iodine value of the oil, but increase its acidity. The proximate and mineral composition of cocoa beans was also seriously affected during cooking. Drying and oven roasting for 5 and 10 min; and drying and traditional roasting for 5 min appear to be the best processing methods of cocoa beans for production of cocoa based foods like chocolate.
Keyword: Food science
1. Introduction
Cocoa is the mature fruit of the cocoa tree (Theobroma cacao L.), growing in tropical regions of Africa and South America [1]. Raw cacao beans are one of the most nutritious foods in the world and has been demonstrated to protect the body from the impact of free radicals, reduces stress and depression, protect against heart diseases and many types of cancer [2]. This health promoting effect of cocoa beans is generally attributed to the presence of polyphenols. The polyphenols in cocoa beans contribute to about 12–18% of the dry weight of the whole bean. Main classes of polyphenolic compounds found in cocoa beans are simple phenols, benzoquinones, phenolic acids, flavonoids etc. [3]. In addition, cocoa beans are a good source of nutrients (lipids, proteins, carbohydrates, minerals and vitamins) [4].
Cocoa beans are mostly consumed after several processing techniques. The cocoa pre-processing steps (harvest, breaking, fermentation and drying) are mandatory to ensure good quality of beans, but cocoa flavors develop during fermentation and roasting. The most commercial products of cocoa are made with roasted beans [5, 6]. The main goal of technological treatment is to improve the organoleptic properties of cocoa beans and eliminate its antinutrients (Hydrocyanate, Oxalate and Theobromine).
During roasting, flavor precursors that are developed during fermentation interact to produce the desired chocolate flavor, which are formed between amino acids and sugars through Maillard reaction [5, 6]. Cocoa beans that are not roasted have bitter, acidic, astringent and nutty flavor. By roasting the cocoa beans, acidity is decreased by reducing the concentration of volatile acids [7]. However, processing may also cause chemical changes that may decrease the nutritional value and the antioxidant property of the beans. This can negatively affect the potential health benefits arising from the consumption of cocoa beans. For example, high processing temperature can promote lipid oxidation and non-enzymatic browning, which have the property to decrease the nutritional value of foods by causing a loss of essential fatty acids, essential aminoacids and digestible carbohydrates. They also lead to the destruction of vitamins and the reduction of protein digestibility [8]. Besides affecting the nutritional and organoleptic properties of the beans and final products, they may generate toxic compounds which are harmful for the consumers [9].
Cameroon is the fifth worldwide and the fourth African cocoa producer. In Cameroon, the crop is cultivated in almost all regions. The seeds are used for industrial and local production of chocolate, cocoa butter, cocoa tea, other sweets etc or eaten directly after roasting.
Several studies have been reported on the effects of processing temperature and time on the nutritional value and phenolic content of edible seeds [10, 11]. In spite of the studies that have already been conducted on cocoa beans, there is almost no report on the effects of processing temperature and time on its phenolic content, antioxidant activity, lipid quality, proximate composition and mineral content. In one study, Rocha et al. [12] have demonstrated the impact of the roasting temperature and time of cocoa beans on the sensory characteristics and acceptability of chocolate. Thus, the objective of this study was to determine the effect of oven and traditional roasting on the polyphenols content, antioxidant activity, lipid quality and nutritional value of fermented cocoa beans.
2. Material and methods
2.1. Material
The freshly fermented cocoa beans (Theobroma cacao) were collected from a farmer in Mbanga, Littoral region, Cameroon, in July 2017. The variety used was Trinitario. All the chemicals and reagents used were of analytical grade.
2.2. Methods
2.2.1. Sample preparation and processing
Freshly fermented cocoa beans (4 Kg) were divided into two different groups (G1 and G2). The first group (G1) was dried in an electric air-dried oven at 45 °C for a period of 48 h. The dried beans were then divided into 05 sub-groups of 200 g each (SG0, SG1, SG2, SG3 and SG4). SG1 and SG2 samples were traditionally roasted in cooking pot by continuous stirring for 5 and 10 minutes respectively, and were affected the codes DTRCB 5 min and DTRCB 10 min respectively. The temperature between the heat source and cooking pot was ranged between 200–220 °C. SG3 and SG4 were also roasted, but in the oven at 180 °C for a period of 5 and 10 minutes respectively, and were affected the codes DORCB 5 min and DORCB 10 min respectively. SG0 served as dried control and was coded DCB. G2 was extracted fresh and served as unprocessed control and was coded FCB.
2.2.2. Oil extraction
2.2.2.1. Maceration method
This method was used for oil extraction from dried samples (DCB, DTRCB 5 min, DTRCB 10 min, DORCB 5 min and DORCB 10 min) as described by Womeni et al. [13]. The beans were separately grinded to pass 1 mm sieve. Eighty grams of each power was separately macerated in 400 mL of hexane at room temperature for 24 h with constant shaking. After that, the mixture was filtered using the Whatman paper N°1, and the filtrate was concentrated on a rotatory evaporator at 40 °C. The extracted oils were stored in the refrigerator at 4 °C for further analysis. The remaining solid fractions were dried in the oven at 50 °C for the determination of their proximate composition.
2.2.2.2. Bligh and Dyer method
Oils were extracted from fresh cocoa beans (FCB) by the method described by Bligh and Dyer [14]. About 80 g of beans were introduced in a grinding machine (Moulinex) to which 100 mL of chloroform and 200 mL of methanol were subsequently added. The mixture was grinded for 3 min; followed by the addition of 100 mL of chloroform and 100 mL of distilled water. The mixture was again grinded for 1 min, and filtered. The final extraction was ensured by the addition of chloroform, in order to respect the following proportion: 2:2:1.8 for chloroform, methanol and water respectively. After separating the different phases in a funnel, the organic phase was collected and dried using sodium anhydrous. The organic solvent was then eliminated by evaporation on a rotatory evaporator at 45 °C under reduced pressure. The extracted oil was stored in the refrigerator at 4 °C for further analysis.
2.2.3. Extraction of cocoa beans polyphenols
The extraction of cocoa beans was done as described by Womeni et al. [13]. Twenty grams of each processed cocoa powder (DCB, DTRCB 5min, DTRCB 10 min, DORCB 5 min and DORCB 10 min) was extracted with 400 mL of methanol for 48 h at room temperature. The mixture was regularly subjected to shaking during the extraction. The extract was filtered with a Whatman No. 1 filter paper, and residue was again extracted with 200 mL of methanol to ensure maximum extraction of phenolic compounds. The combined filtrates were subjected to rotary evaporation at 40 °C under reduced pressure for the removal of the solvent. The dried extract was used for further analysis.
2.2.4. Effect of processing on the total polyphenol of cocoa beans
The effect of processing on the total phenolic content of cocoa beans was determined using the Folin-Ciocalteu colorimetric method, as described by Gao et al. [15]. In a test tube of 5 mL volume, 20 μl of a 2 mg/mL extract solution of cocoa beans was added, followed by the Folin–Ciocalteu reagent (0.2 mL) and distilled water (2 mL). After 3 min incubation of the solution mixture at room temperature, 1 mL of 20% sodium carbonate solution was added and the mixture re-incubated for 20 min under the same conditions. The absorbance of the resulting blue-coloured solution was measured at 765 nm using a spectrophotometer. The total phenolic content of the extract was calculated from the gallic acid standard curve, and expressed as milligrams equivalents gallic acid per gram of extract.
2.2.5. Effect of processing on the antioxidant activity of cocoa beans
The ability of each extract to scavenge the DPPH radical was determined according to the method of Braca et al. [16]. A total of 4.5 mL of 0.002% alcoholic solution of DPPH was added to 0.5 mL of different concentrations (250, 500, 1000, and 2000 μg/mL) of samples and standard solutions separately, in order to have final concentrations of products of 25–200 μg/mL. The samples were kept at room temperature in the dark and after 30 min and the absorbance of the resulting solution was measured at 517 nm. The absorbance of the samples, control, and blank was measured in comparison with methanol. The antioxidant activity (AA) was calculated according to the formula:
2.2.6. Effect of processing on the quality of cocoa beans oil
The determination of the peroxide value of cocoa butter samples was made following the spectrophotometrical IDF standard method, 74A: 1991 [17]. Its iodine and acid values were determined according to the procedure of AOCS Official Method CD 1-25 and CD 3d-63 respectively [18]. Finally, its thiobarbituric acid value was evaluated as described by Draper and Hadley [19].
2.2.7. Changes in the proximate composition of cocoa beans during processing
The proximate composition of Theobroma cacao beans was determined by evaluating the following parameters: Moisture, fat, ash and protein contents, using the standard methods described by AOAC [20]. The moisture content was evaluated by drying cocoa beans in the oven at 103 °C until constant weight, according to the AOAC method 925.40 [20]. The ash content was obtained by incineration of the beans at 550 °C, following the AOAC procedure 924.05 [20]. The Nitrogen (N) content was determined using the micro-Kjeldahl, as described in the AOAC procedure 984.13. The protein content was then calculated as N x 6.25. The lipid contain was determined on a Soxhlet apparatus using hexane, following the AOAC 963.15 method [20]. The total carbohydrate content was determined by difference as reported by Onyeike et al. [21]. This technique involves adding the total values of crude fat, proteins, moisture and ash constituents of the sample and subtracting it from 100. All samples were analyzed in triplicate.
2.2.8. Effect of processing on the mineral composition of cocoa beans
Theobroma cacao beans were ashed at 550 °C and the ash boiled with 10 mL HCl (10%). After filtration, the following mineral elements: calcium (Ca), magnesium (Mg), sodium (Na), potassium (K) and iron (Fe) were, determined using an atomic absorption spectrophotometer (Varian 220FS Spectra AA, Les Ulis, France). Phosphorus (P) was determined by colorimetry, using the vanado molybdate, following the AOAC method 965.17 [22]. The mineral content of all samples were determined using the calibration curve of standards minerals. All samples were analyzed in triplicate.
2.3. Statistical analysis
Results obtained in the present study were subjected to one-way analysis of variance (ANOVA) with Student-Newman-Keuls tests using the software Graphpad-Instat version 3.05, to evaluate the statistical significance of the data. A probability value at p < 0.05 was considered statistically significant.
3. Results and discussion
3.1. Effect of processing on the phenolic content and antioxidant activity of cocoa beans
3.1.1. Total phenolic content
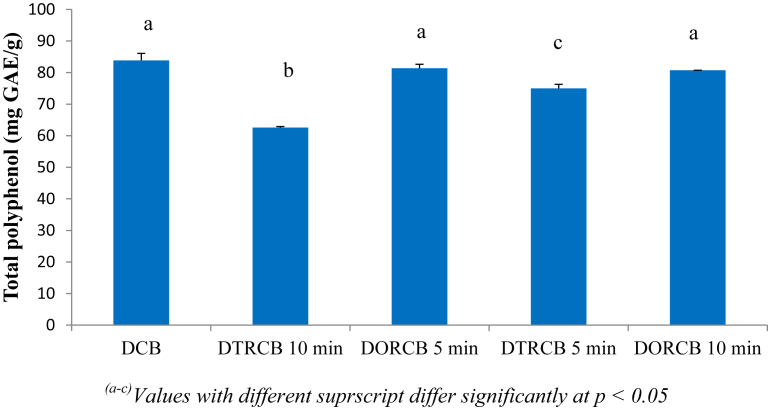
The changes in total polyphenol of processed cocoa beans samples compared to the control (DCB) are presented in Fig. 1. A significant reduction (p < 0.05) in polyphenol content was registered in DTRCB 5 min and DTRCB 10 min, meaning that the traditional roasting has significantly affected the polyphenol of this sample. It is also observed that, the rate of reduction of the total polyphenol was increasing with roasting time. However, no significant difference was observed between the samples roasted in the oven (DORCB 5 min and DORCB 10 min) compared to the control (DCB). From these observations, it clearly appears that, oven roasting preserve better the total polyphenol of cocoa beans compared to the traditional roasting. A significant reduction (p < 0.05) in total polyphenol registered in DTRCB 5 min and DTRCB 10 min compared to DORCB 5 min and DORCB 10 min might be attributed to the high processing temperature, because the traditional roasting temperature (200–220 °C) was higher than the oven roasting temperature (180 °C). It has been proven that at high temperature, low molecular weight phenolic compounds easily volatilize. These results are in agreement with those of Endraiyani [23] who demonstrated that the total polyphenol of cocoa pulps significantly decrease with the number of pasteurization. They are also in accordance with those of Rizki et al. [10] who showed that, the total phenolic content of sesame seeds significantly decrease with roasting time.
Fig. 1.
Changes in total phenolic content of cocoa beans during processing [DCB = Oven dried cocoa beans; DTRCB 5 min = Dried and traditionally roasted cocoa beans (5 min); DORCB 5 min = Dried and oven roasted cocoa beans (5 min); DTRCB 10 min = Dried and traditionally roasted cocoa beans (10 min); DORCB 10 min = Dried and oven roasted cocoa beans (10 min)].
3.1.2. Antioxidant activity
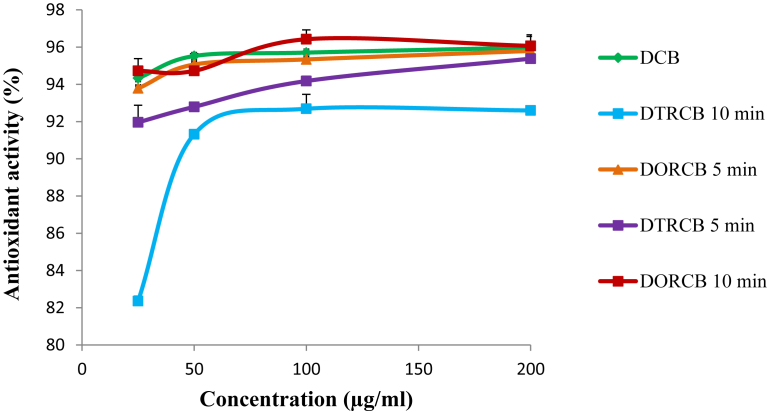
The effect of different roasting conditions on the antioxidant property of cocoa beans is illustrated in Fig. 2. As previously observed with the total polyphenol, DTRCB 5 min and DTRCB 10 min have exhibited the lowest (p < 0.05) antioxidant activities compared to DCB, DORCB 5 min and DORCB 10 min. No significant difference (p > 0.05) was registered between the antioxidant activity of DCB, DORCB 5 min and DORCB 10 min. These results are in accordance with those previously obtained with the total polyphenol, because samples which exhibited low polyphenol content also exhibited lowest antiradical activity. In previous studies, the relation between polyphenol concentration and antioxidant activity has been demonstrated [13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24]. From these observations, is appears clear that traditional roasting significantly reduce both polyphenol concentration and antioxidant activity of cocoa beans These observations are in agreement with that reported by Rizki et al., [10] who showed that the decrease in antioxidant activity of cocoa beans during roasting was related to the destruction of their polyphenols.
Fig. 2.
Changes in antioxidant activity of cocoa beans extracts during processing [DCB = Oven dried cocoa beans; DTRCB 5 min = Dried and traditionally roasted cocoa beans (5 min); DORCB 5 min = Dried and oven roasted cocoa beans (5 min); DTRCB 10 min = Dried and traditionally roasted cocoa beans (10 min); DORCB 10 min = Dried and oven roasted cocoa beans (10 min)].
3.2. Changes in quality of cocoa bean oil during processing
3.2.1. Peroxide value
The determination of the peroxide value of oils generally inform on their primary oxidation state, characterized by the formation of hydroperoxides which are the main oxidation products [9, 13]. The changes in peroxide value of cocoa bean oil samples are presented in Table 1. A significant increase (p < 0.05) in PV was registered in almost all the processed samples compared to the controls (FCB and DCB). No significant difference (p > 0.05) was observed between the PV of controls (FCB and DCB) and that of DORCB 5 min. Among the processed samples, DTRCB 5 min and DTRCB 10 min were significantly (p < 0.05) primarily oxidized compared to DORCB 5 min and DORCB 10 min respectively. Apart of DTRCB 10 min who exhibited a PV higher than the recommended value which is 10 meq O2/Kg [25]; all samples have presented a PV in the accepted range 1–10 meq O2/Kg. This indicates that the formation rate of hydroperoxides was elevated with traditional roasting than oven roasting. It has been demonstrated that, heat facilitates the initiation of lipid oxidation which leads to the formation of free radicals, which can easily react with molecular oxygen and form hydroperoxides at the propagation stage [24]. So, the high PV of traditionally roasted cocoa beans oil indicates its poor resistance towards peroxidation during storage. These results are in line with those reported by Tenyang et al. [11], who showed that the peroxide values of oils extracted from two varieties of sesame seeds was increasing with the roasting temperature and time.
Table 1.
Changes in peroxide, TBA, Iodine and Acid values of cocoa bean oil samples during processing.
| Samples | Peroxide value (meq O2/Kg) | TBA value (ppm) | Iodine value (g I2/100 g) | Acid value (mg KOH/g) |
|---|---|---|---|---|
| FCB | 7.6 ± 0.0a | 0.8 ± 0.0a | 31.9 ± 0.0a | 0.2 ± 0.0a |
| DCB | 7.8 ± 1.1a | 1.4 ± 0.1b | 31.8 ± 0.1a | 0.2 ± 0.0a |
| DTRCB 5 min | 9.9 ± 0.6b | 3.8 ± 0.2c | 31.8 ± 0.0a | 0.3 ± 0.1a |
| DORCB 5 min | 7.7 ± 0.1a | 1.2 ± 0.9be | 31.9 ± 0.2a | 0.3 ± 0.0a |
| DTRCB 10 min | 12.4 ± 0.1c | 4.1 ± 0.5d | 31.1 ± 0.0c | 0.4 ± 0.0b |
| DORCB 10 min | 9.8 ± 0.9b | 2.0 ± 0.4e | 31.0 ± 0.0b | 0.4 ± 0.0b |
Data are presented as mean ± SD (n = 3) (a–e) Means within each column with different superscripts are significantly (p < 0.05) different. FBC = Fresh cocoa beans; DCB = Oven dried cocoa beans; DTRCB 5 min = Dried and traditionally roasted cocoa beans (5 min); DORCB 5 min = Dried and oven roasted cocoa beans (5 min); DTRCB 10 min = Dried and traditionally roasted cocoa beans (10 min); DORCB 10 min = Dried and oven roasted cocoa beans (10 min).
3.2.2. Thiobarbituric acid value (TBA value)
The TBA test is an easy and quick technique widely used in the assessment of the secondary oxidation state of oils and fats. It especially gives an idea on the concentration of malonaldehyde present, which is one of the secondary oxidation products of oils and fats [26]. The changes in TBA value of cocoa bean oil samples are presented in Table 1. DTRCB 10 min and DTRCB 5 min which previously exhibited the highest peroxide values have also showed highest TBA values (4.11 and 3.81 ppm respectively) compared to the controls (FCB and DCB), DORCB 5 min and DORCB 10 min (0.84, 1.35, 1.22 and 2.02 respectively). This is the proof that the amount of malonaldehyde significantly increases (p < 0.05) during traditional roasting. Malonaldehyde is generally obtained from the decomposition of hydroperoxides under the effect of heat [13]. The traditional roasting temperature might catalyze the formation of these products by enhancing the decomposition of primary oxidation products. These results are in accordance with those of Tenyang et al. [11] who showed that the amount of secondary oxidation products significantly increases with roasting time and temperature.
3.2.3. Iodine value
The degree of unsaturation of oils and fats is generally measured through the determination of the iodine value. Generally, during lipid oxidation, the double bonds of unsaturated fatty acids are attacked by free radicals, resulting in the reduction of the number of unsaturation [27]. The changes in IV of cocoa bean oil samples are presented in Table 1. A significant decrease (p < 0.05) in Iodine value was registered in DTRCB 10 min and DORCB 10 min compared to DTRCB 5 min, DORCB 5 min and controls (FCB and DCB). This indicates that traditional and oven roasting for 10 min significantly alter (p < 0.05) the double bonds of unsaturated fatty acids of cocoa bean oil. However, the iodine values of DORCB 5 min and DTRCB 5 min, were similar (p > 0.01) to those of controls (FCB and DCB), meaning that these oil samples were less affected by the treatment. It is however important to note that the iodine values obtained in this work were ranged between 30–35 gI2/100 g as recommended by the regulation [25]. These results are in agreement with those reported by Djikeng et al. [9] who demonstrated that heating of palm olein at 180 °C significantly reduce its iodine value.
3.2.4. Acid value
This test generally inform on the amount of free fatty acids present in the oil. It measures the extend of decomposition of triglyceride by lipase or high temperature [11]. The variations in acid value of oil samples during processing of the cocoa beans are presented in Table 1. No significant difference (p > 0.05) was observed between the acid value of FCB, DCB, DTRCB 5 min and DORCB 5 min; meaning that the hydrolytic activities were less in these samples. However, the acid value of DTRCB 10 min and DORCB 10 min were significantly higher (p < 0.05). This might be attributed to the thermal hydrolysis of triglycerides of these samples. The acid value in all the samples was lower than 4 mg KOH/g which is the recommended acid value in crude oils [25]. The fact that the acidity of oils increases with the temperature has previously been demonstrated [11, 26].
3.3. Effect of roasting on the nutritional composition of cocoa beans
3.3.1. Proximate composition
The changes in proximate composition of cocoa beans during processing are presented in Table 2. The moisture content obtained in this study varies from 3.98 to 6.40% and is similar to that reported by Ajala and Ojewande [28]. These authors reported that the amount of moisture in cocoa beans falls between 4.96 and 7.00% during drying at different temperatures. A significant decrease (p < 0.05) in moisture content of cocoa beans was registered with DTRCB 5 min, DTRCB 10 min and DORCB 10 min compared to the control (DCB) and DORCB 5 min. However, no difference (p > 0.05) was observed between the amount of moisture of DCB and DORCB 5 min. High temperatures generally contribute to the reduction of the moisture content of foods to a minimum value. Abiola and Tewe [29] have reported that, the key factors for long shelf life of cocoa powder are the reduction of its moisture content which will reduce the water activity and inhibit several chemical reactions and biological activities which can reduce the quality of food. From the same table (Table 2), it appears that the amount of ash varied from 7.25 to 9.15%. Similar range was reported in cocoa beans by Ndjife et al. [30]. No significant difference was observed between the ash content of the control (DCB), DTRCB 5 min, and DORCB 5 min. However, the total ash content of DTRCB 10 min and DORCB 10 min were significantly higher (p < 0.05) compared to those above mentioned. This suggests that oven and traditional roasting increase the amount of ash in cocoa beans. These results are in agreement with those of Amaiz et al. [31] who demonstrated that, the ash content of cocoa beans was increasing with roasting. However, these results were not in accordance with those of Ajala and Ojewande [28] who showed that the ash content of cocoa beans decreases with the elevation of the temperature.
Table 2.
Proximate composition (dry basis) of raw and processed cocoa beans.
| Samples | Moisture (%) | Ash (%) | Lipid (%) | Protein (%) | Carbohydrate (%) |
|---|---|---|---|---|---|
| DCB | 5.9 ± 0.6a | 7.3 ± 0.4a | 41.2 ± 0.8a | 17.9 ± 0.3a | 27.7 ± 0.1a |
| DTRCB 5 min | 4.0 ± 0.0b | 7.7 ± 0.2a | 39.0 ± 0.1bc | 17.6 ± 0.2a | 31.7 ± 0.1b |
| DORCB 5 min | 6.4 ± 0.0a | 7.6 ± 0.4a | 38.2 ± 0.6b | 17.9 ± 0.1a | 30.0 ± 0.2c |
| DTRCB 10 min | 4.1 ± 0.3b | 9.2 ± 0.5b | 41.0 ± 0.6a | 15.5 ± 0.1b | 30.2 ± 0.3c |
| DORCB 10 min | 4.5 ± 0.3b | 9.1 ± 0.3b | 39.0 ± 0.1c | 16.6 ± 0.1c | 30.8 ± 0.1d |
Data are presented as mean ± SD (n = 3) (a–d) Means within each column with different superscripts are significantly (p < 0.05) different. DCB = Oven dried cocoa beans; DTRCB 5 min = Dried and traditionally roasted cocoa beans (5 min); DORCB 5 min = Dried and oven roasted cocoa beans (5 min); DTRCB 10 min = Dried and traditionally roasted cocoa beans (10 min); DORCB 10 min = Dried and oven roasted cocoa beans (10 min).
The analysis of the macronutrients composition showed that cocoa beans are rich in lipids (38.16–41.23%), proteins (15.54–17.91%) and carbohydrates (27.67–30.78 %). The amount of lipids was similar to that reported by Amaiz et al. [31] who showed that, the lipids content of cocoa beans varied between 45.42 and 46.27%. Those of proteins and carbohydrates were closed to the data reported by Rucker [4] who demonstrated that the protein and carbohydrate content of cocoa beans fall in the ranges 18–19% and 45–55% respectively. Generally, the amount of lipids was significantly reducing (p < 0.05) with the treatments. These results are in agreement with those reported by Amaiz et al. [31]. These results are in line with those reported by Amaiz et al. [31] and Ajala and Ojewande [28] who have proven that the amount of lipids of cocoa beans reduces with processing. Similar tendencies were observed with the protein content of these samples. This can be attributed to the involvement of proteins in non-enzymatic browning reactions. Similar results were obtained by Ajala and Ojewande [28] during drying of fermented cocoa beans. Concerning the total carbohydrates content, its amount was significantly increasing (p < 0.05) in the oven and traditionally roasted cocoa beans samples compared to the control. These results are not in agreement with those of Ihemeje et al. [32] who demonstrated that the amount of carbohydrate of walnut seeds significantly decreases (p < 0.05) with the treatments. However, they are in accordance with those of Tenyang et al. [11] who showed that roasting of white sesame seeds at 120 °C for 30 min considerably increases its carbohydrates content.
3.3.2. Mineral content
The changes in mineral composition of cocoa beans during processing are presented in Table 3. Results show that cocoa beans contain various minerals element, that play important functions in the human body such as many enzymatic reactions, energy production, transmission of nerve impulses and multiple biological reactions [33]. It is clearly observed that the amount of iron has significantly increased (p < 0.05) with traditional roasting (59.12 and 53.78 mg/100g for DTRCB 5 min and DTRCB 10 min respectively) compared to the control (DCB) (51.11 mg/100 g). However, oven drying significantly reduced the concentration in this mineral. The increase in iron registered with traditional roasting can be attributed to the fact that traditional roasting temperature increases the digestibility of walnut seeds, and then initiates the release and increase of iron [34]. The amount of iron was reducing with processing time. Globally, the amount of iron of all the samples was ranged between 42.26–59.12 mg/100g, which is higher than 10–15 mg/100 g as reported by Rucker [4] in dried cocoa beans. This mineral is an essential part of the respiratory pigments, myoglobin, haemoglobin and many enzymes. Its deficiency leads to anaemia, which is one of the most severe nutritional disorders in the world [35].
Table 3.
Changes in mineral content of cocoa beans during processing.
| Samples | Fe (mg/100g) | P (mg/100g) | K (mg/100g) | Na (mg/100g) | Ca (mg/100g) | Mg (mg/100g) |
|---|---|---|---|---|---|---|
| DCB | 51.1 ± 0.8a | 24.8 ± 0.4a | 947.2 ± 1.8a | 76.5 ± 2.1a | 229.3 ± 10.1a | 219.6 ± 1.3a |
| DTRCB 5 min | 59.1 ± 1.0b | 21.5 ± 0.6b | 949.0 ± 4.3a | 75.8 ± 1.1a | 125.0 ± 7.1b | 681.7 ± 1.8b |
| DORCB 5 min | 48.4 ± 0.6ad | 16.7 ± 0.1c | 947.0 ± 1.4a | 75.9 ± 1.3a | 203.4 ± 5.1c | 344.6 ± 6.2c |
| DTRCB 10 min | 53. 8 ± 0.8c | 27.0 ± 0.4d | 745.9 ± 3.7b | 81.9 ± 6.9a | 125.5 ± 7.7b | 268.7 ± 1.9d |
| DORCB 10 min | 47.3 ± 0.9d | 11. 6 ± 0.6e | 745.5 ± 3.2b | 51.0 ± 1.4b | 126.0 ± 8.5b | 536.1 ± 2.1e |
Data are presented as mean ± SD (n = 3) (a–e) Means within each column with different superscripts are significantly (p < 0.05) different. DCB = Oven dried cocoa beans; DTRCB 5 min = Dried and traditionally roasted cocoa beans (5 min); DORCB 5 min = Dried and oven roasted cocoa beans (5 min); DTRCB 10 min = Dried and traditionally roasted cocoa beans (10 min); DORCB 10 min = Dried and oven roasted cocoa beans (10 min).
Concerning the phosphorus, a significant decrease (p < 0.05) in its concentration compared to the control (DCB) was registered in DORCB 5 min and DORCB 10 min, and were respectively 16.72 and 11.56 mg/100 g, while that of DTRCB 10 min considerably increased to 26.97 mg/100 g. This shows that traditional roasting for 10 min is the best treatment to increase the amount of phosphorus in cocoa beans. Together with calcium, they plays crucial roles in bones mineralization [36]. The changes in potassium content revealed that traditional and oven roasting for 10 min significantly reduce the concentration in this mineral in cocoa beans compared to DCB, DTRCB 5 min and DORCB 5 min. The amount of potassium in dried cocoa beans (947.22 mg/100 g) was lower than 1248.62 and 1199.66 mg/100 g as reported by Torres-Moreno et al. [37] in cocoa beans from Ecuador and Ghana respectively. Talking about the sodium, no significant difference was observed between its concentrations in DCB, DTRCB 5 min, DORCB 5min and DTRCB 10 min, while it was significantly low in DORCB 10 min (51.00 mg/100 g). This indicates that oven roasting of cocoa beans for 10 min or more is not suitable for the preservation of sodium. The amount of calcium was significantly decreasing in all the roasted samples compared to the control (DCB). The calcium content of DCB was 229.25 mg/100 g and was higher than to that reported by Torres-Moreno et al. [37] in the same beans (122.22 mg/100 g). However, the magnesium content in all the roasted samples was significantly increasing compared to that of the control (DCB), and falls in the range 500–600 mg/100 g, as reported by Rucker [4] in cocoa beans.
4. Conclusion
Results of the present study indicate that, traditional roasting significantly reduce the polyphenol content and the antioxidant activity of cocoa beans. The analysis of oil showed that both oven and traditional roasting significantly affect the peroxide and TBA values of cocoa beans oil. However, traditional roasting is the most severe treatment. Oven and traditional roasting for 10 min decrease the iodine value of cocoa beans oil, but increase their acidity. The proximate composition of Theobroma cacao beans was also significantly affected by the roasting techniques. Oven and traditional roasting significantly increase the ash content of cocoa beans, but reduce its protein concentration. All the cooking techniques increase the carbohydrate content of these beans, while slightly decreasing their lipid content. Traditional roasting increases the iron, phosphorus and sodium contents of cocoa beans. Oven roasting preserve the potassium content of the beans while traditional roasting leads to its significant reduction. All these treatments significantly reduce the concentration of calcium, but increase that of magnesium.
Declarations
Author contribution statement
Fabrice T. Djikeng: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
William T. Teyomnou: Performed the experiments; Analyzed and interpreted the data.
Noël Tenyang, Bernard Tiencheu, Mallampalli S. L. Karuna, François Z. Ngoufack, Hilaire Macaire Womeni: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Azia Theresia Morfor, Blaise A. H. Touko: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Serges N. Houketchang, Gires Teboukeu Boungo: Performed the experiments.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Ibric, Cavar . vol. 42. 2014. Phenolic compounds and antioxidant activity of cocoa and chocolate products; pp. 37–40. (Bulletin of the Chemists and Technologists of Bosnia and Herzegovina). [Google Scholar]
- 2.Latif R. Health benefits of cocoa. Curr. Opin. Clin. Nutr. Metab. Care. 2013;16:669–674. doi: 10.1097/MCO.0b013e328365a235. [DOI] [PubMed] [Google Scholar]
- 3.Hii C.L., Law C.L., Suzannah S., Cloke M. Polyphenols in cocoa (Theobroma cacao L.) AJOFAI. 2009;2:702–722. [Google Scholar]
- 4.Rucker R. Nutritional properties of cocoa. Appendix 10. In: Grivetti, Shapiro, editors. Chocolate: History, Culture, and Heritage. John Wiley & Sons, Inc; 2009. Copyright © 2009. [Google Scholar]
- 5.Ramli N., Hassan O., Said M., Idris N.A. Influence of roasting conditions on volatile flavour of roasted Malaysia cocoa bean (abstract) J. Food Process. Preserv. 2008;30:280–298. [Google Scholar]
- 6.Sacchetti G., Ioannone F., Gregorio M., Mattia C., Serafini M., Mastrocola D. Non enzymatic browning during cocoa roasting as affected by processing time and temperature. J. Food Eng. 2015;169:44–52. [Google Scholar]
- 7.Beckett S.T. 4th ed. Blackwell Publishing; Hoboken: 2009. Industrial Chocolate Manufacture and Use. 732 p. [Google Scholar]
- 8.Cuvelier M.E., Maillard M.N. Stabilité des huiles alimentaires au cours de leur stockage. OCL. 2012;19:125–132. [Google Scholar]
- 9.Djikeng T.F., Womeni H.M., Anjaneyulu E., Boungo T.G., Karuna M.S.L., Prasad R.B.N., Linder M. Performance of Green Tea leaves methanolic extract in stabilizing refined, bleached and deodorized palm olein during storage at frying temperature. European J. Nutr. Food Saf. 2017;7:144–154. [Google Scholar]
- 10.Rizki H., Kzaiber F., Elharfi M., Ennahli S., Hanine H. Effects of roasting temperature and time on the physicochemical properties of sesame (Sesamum indicum L.) seeds. IJIAS. 2015;11:148–155. [Google Scholar]
- 11.Tenyang N., Ponka R., Tiencheu B., Djikeng F.T., Azmeera T., Karuna M.S.L., Prasad R.B.N., Womeni H.M. Effects of boiling and roasting on proximate composition, lipid oxidation, fatty acid profile and mineral content of two sesame varieties commercialized and consumed in Far-North Region of Cameroon. Food Chem. 2017;221:1308–1316. doi: 10.1016/j.foodchem.2016.11.025. [DOI] [PubMed] [Google Scholar]
- 12.Rocha I.S., Santana L.R.R., Soares S.E., Bispo E.S. Food Sci. Technol; Campinas: 2017. Effect of the Roasting Temperature and Time of cocoa Beans on the Sensory Characteristics and Acceptability of Chocolate. 10 p. [Google Scholar]
- 13.Womeni H.M., Tonfack D.F., Anjaneyulu B., Karuna M.S.L., Prasad R.B.N., Linder M. Oxidative stabilization of RBD palm olein under forced storage conditions by old Cameroonian green tea leaves methanolic extract. NFS J. 2016;3:33–40. [Google Scholar]
- 14.Bligh E.G., Dyer W.J. A rapid method for total lipid extraction and purification. Can. J. Physiol. Pharmacol. 1959;37:11–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 15.Gao X., Ohlander M., Jeppsson N., Björk L., Trajkovski V. Changes in antioxidant effects and their relationship to phytonutrients in fruits of sea buckthorn (Hippophae rhamnoides L) during maturation. Can. J. Physiol. Pharmacol. 2000;48:1485–1490. doi: 10.1021/jf991072g. [DOI] [PubMed] [Google Scholar]
- 16.Braca A., Sortino C., Politi M., Morelli I., Mendez J. Antioxidant activity of flavonoids from Licania licaniaeflora. J. Ethnopharmacol. 2002;79:379–381. doi: 10.1016/s0378-8741(01)00413-5. [DOI] [PubMed] [Google Scholar]
- 17.IDF . Belgium; Brussels: 1991. International IDF Standard 74A. [Google Scholar]
- 18.AOCS . 5th ed. American Oil Chemist's Society; Champaign, IL, USA: 2003. Official Methods and Recommended Practices of the American Oil Chemist's Society. Methods Cd 8–53, Cd 18–90, Cd 19–90. [Google Scholar]
- 19.Draper H.H., Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–431. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- 20.AOAC . 16th ed. Association of Official Analytical Chemists; Washington, DC: 1990. Official Methods of Analysis. [Google Scholar]
- 21.Onyeike E.N., Anyalogbu E.A., Monanu M.O. Effect of heat processing on the proximate composition and energy values of African walnut (Plukenetia conophora) and African Elemi (Canarium schweinfurthii) consumed as masticatories in Nigeria. IJSTR. 2015;4:295–301. [Google Scholar]
- 22.AOAC . 21st ed. Association of Official Analytical Chemists; Washington, DC, USA: 1999. Official Methods of Analysis. [Google Scholar]
- 23.Endraiyani V. University of New Jersey; USA: 2011. Total Phenolic and Antioxidant Capacity of cocoa Pulp: Processing and Storage Study. (MSc thesis) 105p. [Google Scholar]
- 24.Anwar F., Qayyum H.M.A., Hussian A.I., Iqbal S. Antioxidant activity of 100% and 80% methanol extracts from barley seeds (Hordeumvulgare L.): stabilization of sunflower oil. Grassa Aceites. 2010;61:237–243. [Google Scholar]
- 25.FAO/WHO . 2009. Report of the 21st Session of the Codex Alimentarius Committee on Fats and Oils. Kola kinabala, Malaysia. [Google Scholar]
- 26.Iqbal S., Bhanger M.I. Stabilization of sunflower oil by garlic extract during accelerated storage. Food Chem. 2007;100:246–254. [Google Scholar]
- 27.Tynek M., Hazuka Z., Pawlowicz R., Dudek M. Changes in the frying medium during deep-frying of food rich in proteins and carbohydrates. J. Food Lipids. 2001;8:251–261. [Google Scholar]
- 28.Ajala A.S., Ojewande K.O. Study on drying of fermented cocoa beans (Theobroma cacao) IJIAS. 2014;6:931–936. [Google Scholar]
- 29.Abiola S.S., Tewe O.O. Chemical evaluation of cocoa by-products. Trop. Agric. 1991;68:4–6. [Google Scholar]
- 30.Ndjife J., Bolaji P., Atoyebi D., Umezuruike C. Production and quality evaluation of cocoa products (plain cocoa powder and chocolate) AJFN. 2013;3:31–38. [Google Scholar]
- 31.Amaiz L., Carmen M.D., Gutierrez R., Perez E., Alvarez C. Effect of roasting process on physical and physicochemical properties, proximate composition and fatty acid profile of the cocoa bean from Miranda state, Venezuela. Rev. Científica UDO Agrícola. 2012;2:439–446. [Google Scholar]
- 32.Ihemeje A., Ukauwa O., Ekwe C.C. Effects of cooking and germination on physiochemical properties and sensory attributes of African walnut (Tetracarpidium Conophorum) Int. J. Pharmacol. Phytochem. Ethnomed. 2015;1:93–102. [Google Scholar]
- 33.Steinberg F.M., Bearden M.M., Keen C.L. Cocoa and chocolate flavonoids: implications for cardiovascular health. J. Am. Diet Assoc. 2003;103:215–223. doi: 10.1053/jada.2003.50028. [DOI] [PubMed] [Google Scholar]
- 34.Umoren U.E., Effrong O.O., Akpan I.A. Nutritional evaluation of horse eye bean (Mucumaurens): effect of processing in the chemical composition. J. Food Agric. Environ. 2005;3:128–131. [Google Scholar]
- 35.Loumouamou B., Silou T.H., Desobry S. Characterization of seeds and oil of sesame (Sesamum indicu L.) and the kinetics of degradation of the oil during heating. RJASET. 2010;2:227–232. [Google Scholar]
- 36.Yokota T., Matsuzak Y., Koyama M., Hitomi T., Kawanaka M., Enoki Kochini M., Okuyama Y. Sesamin, a lignan of sesame, down regulates cyclin DL protein expression in human tumor cells. Cancer Sci. 2007;98:1447–1453. doi: 10.1111/j.1349-7006.2007.00560.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Torres-Moreno M., Torres-Casana E., Salas-Salvados J., Blanch C. Nutritional composition and fatty acids profile in cocoa beans and chocolates with different geographical origin and processing conditions. Food Chem. 2015;166:125–132. doi: 10.1016/j.foodchem.2014.05.141. [DOI] [PubMed] [Google Scholar]