Abstract
Adoptive cellular therapy using T cells with tumor specificity derived from either natural T cell receptors (TCRs) or an artificial chimeric antigen receptor (CAR) has reached late phase clinical testing, with two CAR T cell therapies achieving regulatory approval within the United States in 2017. The effective use of these therapies depends upon an understanding of their pharmacology, which is quite divergent from traditional small molecule or biologic drugs. We review the different types of T cell therapy under clinical development, the factors affecting cellular kinetics following infusion, and the relationship between these cellular kinetics and anti-cancer activity. We also discuss the toxicity associated with T cell therapies, with an emphasis on cytokine release syndrome and neurotoxicity, and the gaps in knowledge regarding these frequent and unique adverse effects.
Keywords: T cell, chimeric antigen receptor, cellular therapy, gene therapy, immunotherapy
Main Text
For the past century, the concept of “a drug” has largely been confined to low molecular weight organic compounds, so-called “small molecules” and larger biomolecules. However, over the past decade, these traditional concepts have been challenged with the advent of new drugs based upon cells, which we refer to here as cellular therapies. Despite their complexity, cellular therapies have incredible potential for the treatment of human disease. This potential is illustrated by the recent FDA approvals of tisagenlecleucel (CTL019, Kymriah) and axicabtagene ciloleucel (Yescarta), genetically engineered T cell therapies that are able to induce durable, complete remission of acute lymphoblastic leukemia (ALL) and diffuse large B cell lymphoma (DLBCL) in individuals with otherwise highly chemotherapy-refractory disease.1, 2 Like all drugs, understanding the pharmacology of T cell therapies is critical to their effective application in the clinical setting.
Overview of Adoptive Cellular Therapy Using T Cells
T cell therapy is hardly a new concept. In fact, it has been practiced for over 50 years in the form of allogeneic bone marrow transplantation, in which T cells passively transferred in the cellular product used to reconstitute the ablated hematopoietic system mediate important graft-versus-leukemia effects that are critical to long-term success of this therapy.3 Over the past 2 decades, this approach has been refined greatly by the use of cell culture and gene therapy tools to create T cells with defined antigenic specificity for cancer therapy. At least two distinct approaches are currently being developed for controlling antigen specificity.
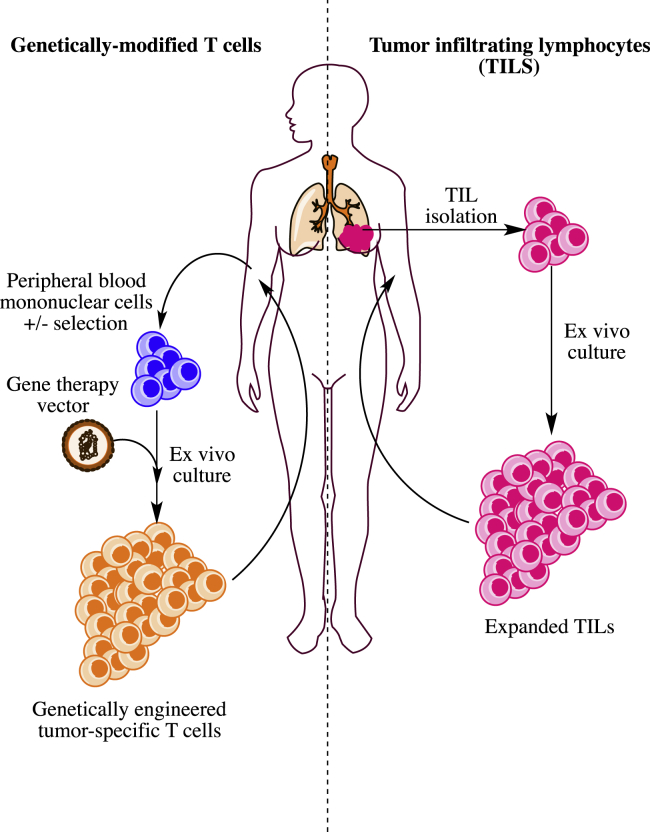
The first approach relies upon isolation of naturally formed, tumor-specific T cells such as tumor infiltrating lymphocytes (TILs). Pioneered by Steven Rosenberg and his research team at the National Cancer Institute, this therapy relies upon the isolation of T cells from surgically resected tumor fragments that are propagated ex vivo by repeated rounds of stimulation with an agonist monoclonal antibody to CD3ε and recombinant human interleukin 2 (rhIL-2).4 After extensive expansion of the tumor-derived lymphocytes, these cells are then re-infused back into the patient from which they were originally derived. The overall process is depicted schematically in Figure 1. TIL therapy has shown some remarkable anti-tumor activity, especially in melanoma, with 20%–30% of patients with metastatic melanoma showing deep and durable clinical remissions lasting years and even decades.5, 6, 7
Figure 1.
Schematic Depiction of the Adoptive T Cell Immunotherapy Process for Tumor-Infiltrating Lymphocyte or Engineered T Cell Therapy
The second approach for generating tumor-specific T cell therapies capitalizes on our ability to transfer genetic material encoding either a cloned T cell receptor (TCR) or a synthetic receptor formed by combining the antigen-binding portions of an antibody molecule with signaling components from immunoreceptors and costimulatory molecules, which has been referred to as chimeric antigen receptors (CARs) or T-bodies.8 Although there are many different approaches used for generating genetically modified T cells, most of these approaches share the same basic schema, as shown in Figure 1. In general, T cells are obtained from peripheral blood, most commonly by leukopheresis. After activation by mitogenic signals similar to those used for TILs, the cells are genetically modified and then expanded prior to their reinfusion back into the patient. Genetic modification can be accomplished by use of retroviral vectors,9, 10 lentiviral vectors,11 transposons,12 or, most recently, homologous recombination following gene editing.13, 14
Defining the “Drug” in T Cell Therapies
Drugs used in the clinical setting require production that adheres to current Good Manufacturing Practices (cGMP) to ensure the safety, purity, and potency from batch to batch. Although the approach to assessing purity and potency are fairly straightforward for small molecule drugs and most biomolecules, defining these characteristics for a cellular product, such as a genetically modified T cell or TIL, is challenging at best and perhaps impossible using present technology.
Unlike traditional molecular drugs that can be chemically defined, cellular therapies are composed of highly complex mixtures of thousands of proteins, lipids, nucleic acids, and other organic compounds. A cellular therapy product generated for therapeutic purposes also typically contains hundreds of millions or billions of cells, which vary greatly in their chemical composition from one cell to another. How then does one define purity in the context of this complexity? Embracing practicality, the purity of most cellular products is currently defined by the analysis of a few highly selected proteins using flow cytometric approaches that define the T cell and T cell subset composition of the cells within a product. However, the advent of new single cell transcriptomic approaches (e.g., single cell RNA-seq) show that even highly selected “homogenous” populations of T cells or bone marrow progenitor cells exhibit a great deal more variation than is obvious from the limited set of surface markers typically used to define these populations.15, 16 Diversity is also created during the genetic engineering process. Most gene delivery approaches in current use for cell therapy employ viral vectors that produce insertions of the genetic material into random locations within the genome. The effect of insertion location on the expression and function of T cells is largely unknown, but a recent study by Eyquem et al.13 using site-directed insertion of a CAR into the TCR gene locus suggests that gene location may have large effects on CAR-T cell function in vivo.
Similar to purity, potency is also quite difficult to delineate for a cellular therapy product. Potency is generally described as the quantity of a drug required to achieve a defined effect. It seems logical to believe that the number of T cells required to kill a defined number of tumor cells in vitro might be a good test of potency for a cancer-targeted T cell therapy. Unfortunately, cytotoxic activity in vitro as well as several other assays of T cell function (e.g., interferon-γ [IFN-γ] production) appear to have little correlation with the in vivo potency of CD19-specific CAR-T cells.17 There are many factors that might limit the in vivo activity and overall efficacy of a T cell therapy. However, it is important to recognize that cellular therapies are uniquely “living drugs,” with the capacity to replicate themselves. The cells that carry out the majority of the cytotoxicity in vivo are unlikely to be the cells in the original infused cell product, but rather the descendants of these cells. In the extreme situation, even a single T cell clone was reported to have mediated the majority of the antileukemic effects in a patient with ALL treated with CTL019 who experienced a delayed response to the therapy, suggesting that T cell therapies may rely upon a large amount of T cell proliferation to achieve their desired anticancer effects.18
The “Pharmacokinetics” and “Pharmacodynamics” of Engineered T Cells
Genetically Engineered T Cell Kinetics following Adoptive Transfer
Most of our knowledge regarding T cell engraftment kinetics is derived from correlative studies performed in clinical trials of genetically modified T cells, in particular CD19-specific CAR-T cells. Two methods, qPCR and flow cytometry, are commonly employed to track the genetically modified T cells following adoptive transfer. Although it is possible for gene silencing or receptor internalization to limit concordance of these methods by reducing expression of the immunoreceptor transgene,19 these methods appear to show good correlation, especially during peak levels of T cell engraftment.20 Overall, the data obtained across more than 10 clinical trials of CAR-modified T cells show that engineered T cell concentrations rapidly decline within hours following infusion (see Table 1). Following this initial decline, which likely represents cellular redistribution into tissues, the concentrations of engineered T cells then rise to reach a maximum concentration (Cmax) in blood that typically occurs within the 2nd week following infusion. This peak is subsequently followed by a second, slower decline in concentration that occurs over a variable period of days to several months. However, the concentration of functional engineered T cells can be maintained at detectable concentrations in many individuals for years.21, 22 Although more limited, data, primarily from multiple myeloma, indicates that engineered T cell kinetics within the tumor compartment appear to parallel concentrations in blood.23
Table 1.
Summary of Reported Pharmacokinetic Data from Clinical Trials of CD19-Specific CAR-T Cells
| Reference | Trial | Indication (Number of Subjects) | Vector, Transgene, and Cells | Conditioning Regimen | Dose | Response | Cmax (Blood) | Time to Cmax | Persistence | t1/2 | AUC | Comment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lee et al., Lancet 201585 | NCT01593696, CD19 CAR T | B-ALL (20), DLBCL (1) | γ retrovirus (FMC63)-28z leukopheresis bulk T cells | Cytoxan + fludarabine | median: 1 × 106 CAR+/kg (mean, range: 1.3, 0.03–3.6) | 70% CR (ALL) | mean, ∼20 cells/μL (range, 0 to ∼45) by FCMa | 14 days | absent by D+68 (in evaluable non-HSCT subjects) | not reported | not reported | |
| Locke et al., 201763 | ZUMA-1, CD19 CAR T | DLBCL(7) | γ retrovirus (FMC63)-28z leukopheresis bulk T cells | Cytoxan + fludarabine | median: 2 × 106 CAR+/kg (mean, range: 1.7, 1.1–2) | 57% CR; 71% OR | not reported | 7–14 days | detectable at 12 months in 3, with ongoing response by qPCR | not reported | not reported | patient with lowest Cmax experienced CR, ongoing at 12 months |
| Ali et al., 201624 | NCT02215967, BCMA CAR T | MM (12) | γ retrovirus (11D-5-3)-28z leukopheresis bulk T cells | Cytoxan + fludarabine | median: 2 × 106 CAR+/kg (mean, range: 2.8, 0.3–9) | 8.3% CR; 25% PR | <10 cells/μL (dose 0.3 × 106 to 1 × 106 CAR+/kg); <10/μL to >250/μL (dose 3 × 106 to 9 × 106 CAR+/kg)a | not reported | CAR+ ≤0.1% of PBMCs by 3 months | not reported | not reported | patients with highest Cmax experienced best response |
| Wang et al., Blood 201686 | NCT01318317, CD19 CAR T day +2 post auto-HSCT | DLBCL (7), MCL (1) | lentivirus (FMC63)-z leukopheresis CD4-CD45RA-CD14-depleted, CD62L-enriched T cells | HSCT conditioning: bis-chloroethylnitrosourea; etoposide; Ara-C; melphalan | median: 50 × 106 total CAR+ (mean, range: 65.6, 25–100) | 63% CR, 25% PR | median, 280 (range, 0–925) CAR copies/mL (qPCR); mean, 1.6 CAR copies/μg gDNA | ∼2 weeks | mean persistence: 18.25 days (range 0–28) by qPCR | not reported | AUC25: mean 25.4 log10 CAR copies/μg gDNA | |
| Wang et al., Blood 201686 | NCT01815749, CD19 CAR T day +2 post auto-HSCT | DLBCL (4), MCL (4) | lentivirus (FMC63)-28z leukopheresis CD25-CD45RA-CD14-depleted, CD62L-enriched T cells | HSCT conditioning: bis-chloroethylnitrosourea; etoposide; Ara-C; melphalan | median: 200 × 106 total CAR+ (mean, range: 143.8, 50–200) | 100% CR | median, 692 (range, 267–27,790) CAR copies/mL (qPCR); mean, 2.79 CAR copies/μg gDNA | ∼2 weeks | mean persistence: 20.5 days (range 7–27) by qPCR | not reported | AUC25: mean 40.2 log10 CAR copies/μg DNA | |
| Gardner et al., 201725 | NCT02028455, CD19 CAR T | B-ALL, pediatric (43) | lentivirus (FMC63)-41BBz + EGFRt leukopheresis, CD4s and CD8s expanded separately, enriched for transgene, mixed 1:1 for infusion | Cytoxan (27); Cytoxan/etoposide (1); fludarabine (1); Cytoxan + fludarabine (13) | median: 1 × 106 CAR+/kg (mean, range: 2.55, 0.5–10) | 93% CR | mean 100–400 CAR+/μL (FCM)a | median 10 days (range, 7–18 days) | median 3 months (95% CI 2.07–6.44), by B cell aplasia | not reported | not reported | Cmax and AUC correlate with antigen burden but not with dose |
| Brentjens et al., 201159 | NCT00466531, NCT01044069, CD19 CAR T | CLL (8), B-ALL (1) | γ retrovirus (SJ25C1)-28z leukopheresis bulk T cells | no conditioning (3); Cytoxan (6) | median: 1.1 × 109 total CAR+ (mean, range: 1.24 × 109, 1.8 × 108 to 3.2 × 109) | reduction in lymphadenopathy (1 subject, CLL); persistent B cell aplasia (1 subject, ALL) | not reported | not reported | up to 8 weeks in 2 patients by IHC | not reported | not reported | presence of CAR+ cells determined by qPCR/flow cytometry after ex vivo restimulation |
| Mueller et al., 201720 | NCT01626495, NCT01029366, NCT01747486, CD19 CAR T | B-ALL, pediatric (55), adult (6), CLL (42) | lentivirus (FMC63)-41BBz leukopheresis bulk T cells | Cytoxan; Cytoxan/etoposide; clofarabine; fludarabine/Cytoxan; CVAD; bendamustine; no conditioning | range: 0.76 × 106 to 20.6 × 106 CAR+/kg, ALL; median: 1.6 × 108 total CAR+ (range: 0.14 × 108 to 11 × 108), CLL | ALL: 82%–93% CR; CLL: 35% CR, 18% PR | geometric mean (CV%), peds-ALL: 48,000 copies/μg gDNA (132) in CR/CRi patients, 17,200 copies/μg gDNA (779) in NR | CR: 11 days (range, 1–32); NR: 15 days (range, 1–32) | CR: median 192 days (range, 18–780 days); NR: median 28.5 (range, 1–32) by qPCR | CR: median 18.8 days (range, 0.7–400); NR 8.8 days (range, 1.2–11.8) | AUC0–28: CR- 328,212 copies/μg × days (CV% 208.5); NR: 8,688 copies/μg × days (CV% 1,910) | |
| Hu et al., 201787 | ChiCTR-OCC-15007008, CD19 CAR T | B-ALL (15) | lentivirus (FMC63)-41BBz leukopheresis bulk T cells | Cytoxan + fludarabine | median: 3.7 × 106 CAR+/kg (mean, range: 4.4, 1.1–9.8) | 40% CR | median, 342 CAR+ cells/mL (95% CI, 140–532) in grade 3 CRS group; median, 96 CAR+ cells/mL (95% CI, 61.5–132.8) in grade 1 to 2 CRS/non-CRS group | not reported | up to 7 months in one patient | not reported | not reported | |
| Turtle et al., 201631 | NCT01865617, CD19 CAR T | B-ALL (30) | lentivirus (FMC63)-41BBz + EGFRt leukopheresis, CD4+ and CD8+/CD8+Tcm-enriched expanded separately, enriched for transgene, mixed 1:1 for infusion | Cytoxan (11); Cytoxan + etoposide (2); Cytoxan + fludarabine (17) | median: 2 × 106 CAR+/kg (mean, range: 2.42, 0.2–20) | 93% remission by flow cytometry, 86% MRD-negative CR | not reported | 7–14 daysa | not reported | not reported | not reported | |
| Turtle et al., 201788 | NCT01865617, CD19 CAR T | CLL (24) | lentivirus (FMC63)-41BBz + EGFRt leukopheresis, CD4+ and CD8+/CD8+Tcm-enriched expanded separately, enriched for transgene, mixed 1:1 for infusion | Cytoxan (1); fludarabine (2); Cytoxan + fludarabine (21) | median: 2 × 106 CAR+/kg (mean, range: 2.42, 0.2– 20) | 21% CR, 53% PR by IWCLL criteria | not reported | not reported | not reported | not reported | not reported | |
| Pan et al., 201789 | ChiCTR-IIh-16008711, CD19 CAR T | B-ALL (51) | lentivirus (FMC63)-41BBz leukopheresis bulk T cells | Cytoxan + fludarabine; no conditioning (1) | mean, range: 1 × 105 CAR+/kg, 0.05–14 | 90% CR | not reported | 8–11 days | undetectable in blood on day +30 (42 subjects), present at day 40, 45, and 60 (3 subjects) by FCM | not reported | not reported | |
| Davila et al., 201458 | NCT01044069, CD19 CAR T | B-ALL (16) | γ retrovirus (SJ25C1)-28z leukopheresis bulk T cells | Cytoxan | 3 × 106 CAR T cells/kg (15 subjects); 4.8 × 105 CAR+ cells/kg (1 subject) | 88% overall CR | not reported | 7–14 days | low undetectable by 2 to 3 months, complicated by progression to allo-HSCT in 7 subjects | not reported | not reported | |
| Rossig et al., 201790 | CD19TPALL, CD19 CAR T, in vivo vaccination to boost CAR T cells in cohort 2 | B-ALL, pediatric (11) | γ retrovirus (FMC63)-28z EBV-specific CTL | fludarabine; fludarabine + vincristine + dexamethasone | median: 4.55 × 107 CAR+/m2 (mean, range: 4.62, 1.08– 7.2) | 36.4% continued, 9.1% CR, 9.1% PR | not reported | not reported | cohort 1 (no vaccination): median, 0 days (range 0–28); cohort 2 (vaccination): median, 56 days (range, 0–221) | not reported | not reported | |
| Kochenderfer et al., 201748b | NCT00924326, CD19 CAR T | DLBCL (19), FL (2), MCL (1) | γ retrovirus (FMC63)-28z leukopheresis bulk T cells | Cytoxan + fludarabine | median: 2 × 106 CAR+/kg (mean, range: 1.86, 1–6) | 55% CR, 18% PR | median, 98 CAR+/μL in subjects with remission; median, 15 CAR+/μL in subjects without remission | median, 8.5 days (range, 6–35 days) | 0 to 1 CAR+/μL by 3 months | not reported | not reported | |
| Kochenderfer et al., 201591b | NCT00924326, CD19 CAR T | DLBCL (9), CLL (4), other indolent lymphoma (2) | γ retrovirus (FMC63)-28z leukopheresis bulk T cells | Cytoxan + fludarabine | median: 2.5 × 106 CAR+/kg (mean, range: 2.3, 1–5) | 53% CR, 26.7% PR | range, 9–777 CAR+ cells/μL | 7–17 days | not reported | not reported | not reported |
B-ALL, B cell acute lymphoblastic leukemia; FCM, flow cytometry; HSCT, hematopoietic stem cell transplant; OR, overall response; MM, multiple myeloma; MCL, mantle cell lymphoma; PBMC, peripheral blood mononuclear cell; gDNA, genomic DNA; CI, confidence interval; NR, non-responder; MRD, minimal residue disease; IWCLL, international workshop on chronic lymphocytic leukemia; FL, follicular lymphoma.
Estimate from graphical data presented in report, without numerical values explicitly reported.
There is likely some patient overlap between these last two reports by Kochenderfer et al. (neither is a complete subset of the other).
Factors Affecting T Cell Engraftment Kinetics
Perhaps not unexpected, T cell engraftment kinetics varies considerably across patients and across clinical trials. Recently reported data from 103 individuals with ALL and chronic lymphocytic leukemia (CLL) treated with tisagenlecleucel (CTL019) show a coefficient of variation (%CV) for Cmax and area under the concentration time curve from day 0 to day 28 (AUC0–28d) of 167% and 209%, respectively, in responding patients. In non-responding patients, which were primarily individuals with CLL, the variation was even wider, with the %CV for both Cmax and AUC0–28d exceeding 1,000%.20 Although these statistics are not reported in other published trials, the variability in both kinetic parameters shown within these studies appear to be considerable.
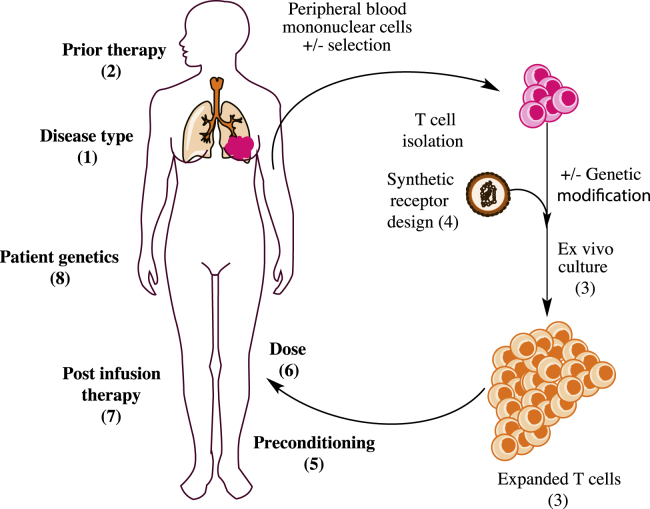
There are multiple factors that likely contribute to the observed variability in engineered T cell engraftment kinetics (Figure 2). T cell dose is a logical variable to consider. Unfortunately, few studies have directly compared engraftment in relation to the engineered T cell dose, and those studies have shown conflicting results.24, 25, 26 In many ways, T cell therapy is akin to hematopoietic stem cell transplantation, in which the number of CD34+ cells transferred is correlated with both the engraftment success as well as the time to recovery of mature hematopoietic cells.27 However, it is still challenging to identify the T cells within an infused product with the greatest “stemness.” Studies in both murine and primate models of adoptive cellular therapy (ACT) over the past decade have shown that T cells vary in their ability to support long-term engraftment following adoptive transfer. Memory T cells with a naive-like and central memory immunophenotype appear to have “stem-cell-like” properties with the greatest replicative and engraftment capacity.28, 29 However, it is largely unknown whether the immunophenotypes described for quiescent T cells freshly isolated from blood are informative when applied to T cells that have been ex vivo activated and cultured. The surface markers used for identifying the naive-like, stem cell memory (Tscm) or central memory (Tcm) T cells change significantly during the culture process such that T cell immunophenotype is much more homogeneous at the end of the culture compared with the cells used at the culture’s start. Recent retrospective analysis of CTL019 data suggest that immunophenotypic characterization of T cell composition of both the starting leukopheresis material as well as the infused T cell product are predictive of engraftment and response.30 In addition, efforts to generate T cell products with a more defined composition have shown good engraftment results.31 However, because they have also not been directly compared to products with less defined composition, it is difficult to know how much benefit is derived from these approaches. These approaches are also more complicated and costly to implement, and a substantial proportion of patients in the study by Turtle et al.31 failed to achieve a defined composition using Tcm-selected T cells due to low lymphocyte counts.
Figure 2.
Variables Impacting Efficacy of T Cell Therapy
Many recipient and product factors are thought to impact the efficacy of adoptive T cell therapy. Although effects have largely not been tested in human trials, in vitro, preclinical in vivo, and human trial evidence implicate parameters at all points of the T cell therapy “life cycle”: (1) disease type, (2) prior therapy, (3) expansion culture conditions (e.g., cytokine and length of culture), including T cell phenotype of the product, (4) synthetic antigen receptor design, (5) pre-conditioning regimen and tumor burden, (6) T cell dose, and (7) post-infusion therapy. (8) Recipient genetics, especially those related to immunity, likely also impact efficacy.
Consistent with concepts borrowed from the hematopoietic stem cell (HSC) transplant field, prior lymphodepleting chemotherapy (so-called “conditioning”) appears to also have important effects on both the Cmax and maintenance of adoptively transferred T cells over time.32, 33, 34, 35 The mechanisms that underlie the effect of conditioning on T cell kinetics are not fully understood. Reduced competition for homeostatic cytokines required for T cell maintenance has been postulated as one important part of the mechanism;36, 37, 38, 39, 40 however, there is evidence to suggest that cytokines induced by gut microflora translocation may also be an important contributing factor to the enhanced engraftment observed with conditioning.41 Modulation of the tumor microenvironment, including depletion of regulatory T cells and other suppressive cells, have also been attributed to the enhancing effects of conditioning on adoptive T cell therapy.42, 43
It is clear that additional factors beyond conditioning contribute to the variable kinetics of proliferation and trafficking within the host. Although no clinical studies directly comparing the kinetics of CAR-T cell engraftment and expansion for CARs with different costimulatory domains have been reported, pre-clinical studies indicate that CD137 (4-1BB) and CD28 domains may have important differences in kinetics. We reported that CD137-domain-containing CARs exhibit greater persistence of T cell concentration over time compared with CD28-costimulated CARs using a xenograft model of ALL.44 Improved persistence of T cells has also been reported with CARs bearing domains from ICOS, OX-40, as well as 3rd generation designs incorporating CD28 and CD137 or OX-40.45, 46, 47
Evidence for Pharmacodynamic Relationships in CAR-T Cell Therapy
Efficacy
Not surprisingly, there is increasing evidence for a relationship between exposure to CAR-T cells and clinical efficacy. Evaluation of data from the phase I and II clinical trials of tisagenlecleucel in ALL and CLL show that individuals achieving a complete response (CR) exhibit a higher overall exposure to CAR-T cells as measured by the AUC and Cmax of vector copies/mg of genomic DNA over the first 48 days following infusion compared to non-responding patients.20 Similar relationships between CAR-T cell exposure have been described for CD19-specific CAR-T cells in non-Hodgkin lymphoma (NHL) as well as B cell maturation antigen (BCMA)-specific CAR-T cells in multiple myeloma.24, 48
Numerous strategies are being investigated to improve the efficacy of T cell therapies; details of each of these are beyond the scope of this review and readers are referred to recent reviews.49, 50, 51 Chief among these are methods to neutralize effects of checkpoint signaling. For example, preclinical and early clinical evidence suggests that antibody therapy targeting the PD-1/PD-L1 axis may enhance CAR-T cell effects.52, 53 Additionally, CAR-T cell intrinsic systems have been designed to disrupt checkpoint signaling; as examples, a “switch receptor” consisting of the PD-1 extracellular domains fused to CD28 transmembrane and cytoplasmic domains can convert inhibitory interactions into T cell stimulatory signals.54 Other cell-intrinsic approaches include CAR-T cell-secreted checkpoint inhibitors, PD-1 dominant-negative receptors, and genetic disruption of inhibitory receptor expression.53, 55, 56 It is likely that the success of therapeutic T cells, particularly against solid tumors, will depend upon combination approaches that address checkpoint inhibition in addition to immune-receptor activation.
Cytokine Release Syndrome
Cytokine release syndrome (CRS) represents the most prominent adverse event associated with CD19-specific CAR-T cell therapy. CRS, which occurs in the majority of patients with ALL, is characterized in its mild form by a limited, influenza-like syndrome that includes fever, chills, and myalgia. Symptoms of CRS typically occur during the first 2 weeks following CAR-T cell infusion, with reported onset as early as 2 days following CAR-T cell administration to as late as several weeks after infusion. The onset and severity of CRS coincides with the kinetics of T cell engraftment and a rise in the concentration of cytokines within the serum.57, 58 More severe CRS (sCRS) is associated with hemodynamic instability, which, in the most severe manifestation, requires vasopressor and ventilator support and can lead to death. Some differences in the grading system exist between studies, which influences the frequencies of sCRS; however, sCRS is reported to occur in 8%–43% of patients treated with CD19 CAR-T cells.31, 58, 59, 60, 61, 62, 63 Although CRS is the most frequent adverse event reported with CD19 CAR-T cell therapies, the frequency of CRS in other CAR-T cell therapies is less clear. CRS, including sCRS, has been reported with BCMA-specific24, 64 and CD123-specific65 CAR-T cell therapies. However, little data on CRS have been reported from trials of CAR-T cells targeting other antigens in solid tumors. In addition, the nature and severity of CRS in these settings may be different.66
Many factors influence the risk for CRS. The most comprehensive analysis of CRS is reported by Turtle and colleagues.67, 68 In an effort to better understand and predict CRS and neurotoxicity following CD19 CAR-T cell treatment, they analyzed 133 patients treated for ALL, CLL, or NHL. Higher tumor burden and higher CAR-T cell dose were significantly associated with sCRS, which is consistent with data reported from other CD19 CAR-T cell studies.61 The relationship of CRS severity to CAR-T cell exposure was supported by logistic regression that demonstrated a correlation between peak CAR-T cell engraftment and occurrence and severity of CRS. Additional factors affecting sCRS risk included conditioning chemotherapy, the use of CAR T cells produced using bulk CD8+ T cells rather than central memory selected CD8+ cells, and thrombocytopenia prior to conditioning. The latter finding is intriguing in light of the additional evidence demonstrating disseminated intravascular coagulation in patients suffering from sCRS, and it suggests that endothelial injury may be an important underlying mechanism contributing to CRS severity that remains to be explored.
Limiting the dose of infused cells may serve to mitigate CRS toxicity. However, both efficacy and toxicity are linked to the engraftment (i.e., Cmax) of CAR-T cells. The therapeutic index of CD19 CAR-T cell therapy may therefore be narrow. Reduction of T cell dose in the setting of high tumor burden has been advocated, and this strategy is used in some studies. Because patients with sCRS often exhibit fever within the first 2 days of infusion, split dosing administered over multiple days and held with evidence of CRS is also employed to mitigate the risk of sCRS. However, none of these strategies has been studied prospectively. Tocilizumab (anti-interleukin-6 [IL-6]) therapy is quite effective at reversing the clinical signs of severe CRS, forming the basis for its Food and Drug Administration (FDA) approval in conjunction with tisagenlecleucel. Clinical studies are underway to determine whether early intervention with tocilizumab can mitigate the risk of sCRS (ClinicalTrials.gov: NCT02906371). Biomarkers that predict CRS following CD19-directed CAR T cell therapy have been identified by multiple groups.57, 67 Prospective studies of treatment algorithms utilizing biomarkers to guide preventative intervention are needed. Ultimately, multiple strategies, including modifications in product manufacturing, patient pre-treatment, and synthetic receptor engineering, may need to be combined to prevent occurrence of CRS. Additional mechanistic insights into the pathophysiology of CRS in conjunction with a better understanding of the critical elements of anti-tumor T cell functions are also needed to overcome the challenge of controlling toxicity, without interfering with efficacy. For additional information on CRS, including current management strategies, readers are referred to additional recent publications.60, 67, 69, 70, 71
Neurotoxicity
Encephalopathy characterized by confusion, delirium, and aphasia that can also be associated with seizures and cerebral edema is a less frequent, but still relatively common, toxicity associated with CAR-T cell therapy in approximately 40% of treated patients. Most cases of encephalopathy are mild to moderate in severity, without evidence of neuropathology on imaging, and appear largely reversible. However, the data on neurotoxicity are relatively sparse and lack an established grading system. Neurologic toxicity was a prominent feature in 6 of 15 deaths reported in relation to CAR-T cell therapy, and severe neurotoxicity is clearly an important toxicity of this therapy. Severe neurotoxicity has also been reported in trials of TCR-engineered T cell therapies targeting MAGE-A3.72
The mechanism(s) by which T cell therapy leads to neurologic toxicity is poorly understood. In the case of the MAGE-A3 TCR studies, the severe neurotoxicity was attributed to direct recognition of MAGE protein within the CNS.72 Neurotoxicity occurs even in those with no evidence of tumor cells within the CSF prior to CAR-T cell infusion, and there is no clear evidence for a role of target antigen in the CNS for CD19 or BCMA-specific CAR-T cell therapies.68, 73 In these therapies, the severity of neurotoxicity appears related to the severity of CRS and is associated with both early and high cytokine concentrations as well as endothelial activation.67, 68 The similarity of sCRS to macrophage activation syndrome/hemophagocytic lymphohistiocytosis (MAS/HLH), which also frequently has associated neurologic symptoms that portend a poorer prognosis, suggests a common mechanism that might relate to inflammatory cytokines that are highly elevated in both conditions and predictive. However, the temporal relationships between CRS and neurotoxicity are not entirely synchronous with the onset of CRS, which generally precedes the signs of neurotoxicity by several days. IL-6 blocking therapy also fails to reverse neurotoxicity in some individuals who are treated for CRS. Whether this is due to limited antibody penetration into the CNS due to the blood-brain barrier, important roles for other cytokines or cytokine-independent mechanisms is unknown. At present, an optimal strategy for managing patients with severe neurotoxicity associated with CAR-T cell therapy has not been defined and has largely relied upon management of CRS with supportive care.
On-Target Off-Tumor Toxicity
All patients who experience complete tumor response to CD19-directed CAR T cell therapy experience concomitant elimination of non-malignant B cells. This loss of B cells is associated with a decrease in total immunoglobulin concentrations in serum but preservation of many vaccine-related antibody responses, suggesting that the defects in humoral immunity following CD19-specific CAR-T cell therapy are incomplete.74 B cell aplasia has been used as a pharmacologic biomarker of drug persistence and efficacy. Indeed, early return of B cells has prompted investigators to re-infuse patients with additional doses of CD19-directed CAR T cells.61 The minimum duration of B cell aplasia, reflective of CAR-T cell persistence, required for durable (i.e., decades-long) tumor remission is not unknown and may be different across tumor types. Long-term follow up of patients following CD19 CAR-T cell therapy will eventually shed light on this and guide approaches to limit CAR-T cell persistence to reverse B cell aplasia without impairing efficacy.
Similarly, it is reasonable to expect that elimination of normal plasma cells (PCs) occurs in the setting of multiple myeloma treated with CAR-T cells that target antigens such as BCMA, which are shared between non-malignant PCs and myeloma cells. Consistent with this notion, Ali et al.24 demonstrate absence of any CD138+ cells in a bone marrow biopsy from a patient who experienced a stringent complete remission after BCMA-directed CAR-T cell infusion. Whether the absence of PCs within the bone marrow serves as a reliable marker of ongoing anti-myeloma efficacy may not be as clinically useful as B cells following CD19-directed CAR therapy because other measures of CAR-T cell presence and disease status are easily performed on peripheral blood. Unlike CD19-CAR therapy, elimination of normal PCs with BCMA-directed therapies is likely to more severely impact humoral immunity to pathogens, and this off-tumor effect of therapy requires further investigation.
Off-tumor targeting of immunotherapy antigen has also been associated with T cell immunotherapies for melanoma, in which some responding patients develop vitiligo, immune-mediated destruction of skin melanocytes. This toxicity appears due to targeting that includes MART-1, TRP-1/2, and gp100, which are shared between normal melanocytes and melanoma cells. In some cases, melanocytic cells within the eye can also be targeted, leading to ophthalmic complications.75 Patients who develop vitiligo after melanoma therapy, including adoptive T cell therapy, demonstrate increased survival compared to those who do not develop vitiligo.76 Severe toxicity due to T cell targeting of tissues beyond the desired tumor has also been observed with TCRs, recognizing the MAGE family of cancer-testis proteins.72, 77 In a case of fatal cardiotoxicity associated with an affinity-enhanced, MAGE-A6-specific TCR, the TCR recognized a peptide derived from the muscle protein, titin, which exhibited similarities to the targeted peptide from MAGE-A6.78
Toxicity could also occur when genetically introduced TCRa and TCRb chains form heterologous pairs with endogenous TCR chains. In this manner, new TCRs may be generated. These new TCRs have not been subject to the usual process of thymic selection, which prunes autoreactive receptors from the functional T cell repertoire. In addition to generating potentially pathogenic new receptors, chain mispairing may limit therapeutic efficacy by decreasing the expression level of the intended TCR. Although in vivo toxicity due to chain mispairing has not yet been reported in patients, several strategies have been developed to mitigate this theoretical complication. Approaches can be divided into two broad categories. One aims to reduce or eliminate endogenous TCR chains using small hairpin RNA (shRNA) or CRISPR.79 A phase I trial at the University of Pennsylvania (ClinicalTrials.gov: NCT03399448) will apply CRISPR to delete endogenous TCRa and TCRb in a TCR-based cell therapy targeting MART-1. The second category encompasses several protein engineering strategies that biochemically favor intended pairing over mispairing. Examples that have shown merit in preclinical studies include use of murine constant domains in the TCRa and TCRb chains, introduction of inter-chain disulfide bonds, mutations that create inter-chain “knob-in-hole” interactions, and domain swapping between TCRa and TCRb and substitution with TCRg and TCRd transmembrane and cytoplasmic domains.79, 80
Conclusions
We have entered an exciting time in the field of immunotherapy. A plethora of new tools that enable the insertion, deletion, or modification of genes within T cells has become available over the last decade. Combined with novel synthetic control systems that can more precisely regulate T cell survival and function,81 it is now possible to create T cell-enhanced function and improved safety. In parallel with improved technologies to create T cells, new technologies are enabling high-resolution characterization of T cell products and highly sensitive techniques are available to detect therapeutic T cells in vivo and measure their pharmacologic effects (i.e., target elimination). The first generation of T cell therapies, such as the recently approved tisagenlecleucel and axicabtagene ciloleucel, are leading the way for more advanced cellular therapies.
Universal donor T cells that utilize gene editing technologies to eliminate the TCR and MHC required for graft-versus-host disease and donor cell rejection, respectively, represents one of the most notable new technologies to enter clinical phase testing.82 Not only do these approaches have the potential to markedly reduce cost and improve the efficiency of manufacturing, but a universal donor T cell might also reduce the significant variation in engraftment kinetics and efficacy that arise from the variable nature of T cells across individuals. The use of gene editing technology also affords the potential to eliminate important negative regulatory pathways, such as the immune checkpoint molecule PD-1, which limits T cell immunity, especially within solid tumors.83 Synthetic molecular switches such as an inducible caspase 9 that regulate T cell survival through administration of a second drug have also entered the clinic.84 These latter approaches, while adding complexity, are often included in T cell therapies as a fail-safe mechanism to mitigate the risk of off-tumor toxicity.
Finally, there is still much to learn, especially about the long-term safety of these novel therapies. No case of insertional oncogenesis has been reported to our knowledge, with either retroviral or lentiviral vector engineering of mature T cells compared with the observed leukemogenesis associated with retroviral engineering of hematopoietic stem cells. Nevertheless, the possibility that this complication may occur with low frequency remains given the limited number of patients treated overall. Current FDA guidance recommends at least a 15-year follow up for individuals treated with gene therapy, and both tisagenlecleucel and axicabtagene ciloleucel were approved with the requirement for long-term monitoring of treated subjects during the post-marketing phase.
Author Contributions
M.C.M. wrote the original draft and V.G.B. contributed to the writing, review, and editing of the final manuscript, including compilation of data for Table 1.
References
- 1.Novartis AG. (2017). Kymriah package insert. https://www.fda.gov/downloads/UCM573941.pdf.
- 2.Kite Pharma, Inc. (2017). Yescarta package insert. https://www.fda.gov/downloads/UCM581226.pdf.
- 3.Horowitz M.M., Gale R.P., Sondel P.M., Goldman J.M., Kersey J., Kolb H.J., Rimm A.A., Ringdén O., Rozman C., Speck B. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–562. [PubMed] [Google Scholar]
- 4.Topalian S.L., Muul L.M., Solomon D., Rosenberg S.A. Expansion of human tumor infiltrating lymphocytes for use in immunotherapy trials. J. Immunol. Methods. 1987;102:127–141. doi: 10.1016/s0022-1759(87)80018-2. [DOI] [PubMed] [Google Scholar]
- 5.Dréno B., Nguyen J.M., Khammari A., Pandolfino M.C., Tessier M.H., Bercegeay S., Cassidanius A., Lemarre P., Billaudel S., Labarrière N. Randomized trial of adoptive transfer of melanoma tumor-infiltrating lymphocytes as adjuvant therapy for stage III melanoma. Cancer Immunol. Immunother. 2002;51:539–546. doi: 10.1007/s00262-002-0315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Figlin R.A., Thompson J.A., Bukowski R.M., Vogelzang N.J., Novick A.C., Lange P., Steinberg G.D., Belldegrun A.S. Multicenter, randomized, phase III trial of CD8(+) tumor-infiltrating lymphocytes in combination with recombinant interleukin-2 in metastatic renal cell carcinoma. J. Clin. Oncol. 1999;17:2521–2529. doi: 10.1200/JCO.1999.17.8.2521. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg S.A., Yannelli J.R., Yang J.C., Topalian S.L., Schwartzentruber D.J., Weber J.S., Parkinson D.R., Seipp C.A., Einhorn J.H., White D.E. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J. Natl. Cancer Inst. 1994;86:1159–1166. doi: 10.1093/jnci/86.15.1159. [DOI] [PubMed] [Google Scholar]
- 8.Hinrichs C.S., Rosenberg S.A. Exploiting the curative potential of adoptive T-cell therapy for cancer. Immunol. Rev. 2014;257:56–71. doi: 10.1111/imr.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotterman M.A., Chalberg T.W., Schaffer D.V. Viral vectors for gene therapy: translational and clinical outlook. Annu. Rev. Biomed. Eng. 2015;17:63–89. doi: 10.1146/annurev-bioeng-071813-104938. [DOI] [PubMed] [Google Scholar]
- 10.Schott J.W., Hoffmann D., Schambach A. Retrovirus-based vectors for transient and permanent cell modification. Curr. Opin. Pharmacol. 2015;24:135–146. doi: 10.1016/j.coph.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Naldini L., Trono D., Verma I.M. Lentiviral vectors, two decades later. Science. 2016;353:1101–1102. doi: 10.1126/science.aah6192. [DOI] [PubMed] [Google Scholar]
- 12.Tipanee J., VandenDriessche T., Chuah M.K. Transposons: moving forward from preclinical studies to clinical trials. Hum. Gene Ther. 2017;28:1087–1104. doi: 10.1089/hum.2017.128. [DOI] [PubMed] [Google Scholar]
- 13.Eyquem J., Mansilla-Soto J., Giavridis T., van der Stegen S.J., Hamieh M., Cunanan K.M., Odak A., Gönen M., Sadelain M. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature. 2017;543:113–117. doi: 10.1038/nature21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacLeod D.T., Antony J., Martin A.J., Moser R.J., Hekele A., Wetzel K.J., Brown A.E., Triggiano M.A., Hux J.A., Pham C.D. Integration of a CD19 CAR into the TCR alpha chain locus streamlines production of allogeneic gene-edited CAR T cells. Mol. Ther. 2017;25:949–961. doi: 10.1016/j.ymthe.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gaublomme J.T., Yosef N., Lee Y., Gertner R.S., Yang L.V., Wu C., Pandolfi P.P., Mak T., Satija R., Shalek A.K. Single-cell genomics unveils critical regulators of Th17 cell pathogenicity. Cell. 2015;163:1400–1412. doi: 10.1016/j.cell.2015.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nestorowa S., Hamey F.K., Pijuan Sala B., Diamanti E., Shepherd M., Laurenti E., Wilson N.K., Kent D.G., Göttgens B. A single-cell resolution map of mouse hematopoietic stem and progenitor cell differentiation. Blood. 2016;128:e20–e31. doi: 10.1182/blood-2016-05-716480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Novartis. (2017). Tisagenlecleucel. Report of the Oncologic Drugs Advisory Committee Meeting, U.S. Food & Drug Administration. November 9, 2017, BLA 125646. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/OncologicDrugsAdvisoryCommittee/UCM566166.pdf.
- 18.Motz, G., Bushman, F.D., Fraietta, J.A., June, C.H., Melenhorst, J.J., Nobles, C.L., and Young, R.M. March 23, 2017. Car t cell therapies with enhanced efficacy. U.S. patent WO2017049166 A1.
- 19.Ellis J. Silencing and variegation of gammaretrovirus and lentivirus vectors. Hum. Gene Ther. 2005;16:1241–1246. doi: 10.1089/hum.2005.16.1241. [DOI] [PubMed] [Google Scholar]
- 20.Mueller K.T., Maude S.L., Porter D.L., Frey N., Wood P., Han X., Waldron E., Chakraborty A., Awasthi R., Levine B.L. Cellular kinetics of CTL019 in relapsed/refractory B-cell acute lymphoblastic leukemia and chronic lymphocytic leukemia. Blood. 2017;130:2317–2325. doi: 10.1182/blood-2017-06-786129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Porter D.L., Hwang W.T., Frey N.V., Lacey S.F., Shaw P.A., Loren A.W., Bagg A., Marcucci K.T., Shen A., Gonzalez V. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci. Transl. Med. 2015;7:303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scholler J., Brady T.L., Binder-Scholl G., Hwang W.T., Plesa G., Hege K.M., Vogel A.N., Kalos M., Riley J.L., Deeks S.G. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci. Transl. Med. 2012;4:132ra53. doi: 10.1126/scitranslmed.3003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rapoport A.P., Stadtmauer E.A., Binder-Scholl G.K., Goloubeva O., Vogl D.T., Lacey S.F., Badros A.Z., Garfall A., Weiss B., Finklestein J. NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma. Nat. Med. 2015;21:914–921. doi: 10.1038/nm.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ali S.A., Shi V., Maric I., Wang M., Stroncek D.F., Rose J.J., Brudno J.N., Stetler-Stevenson M., Feldman S.A., Hansen B.G. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016;128:1688–1700. doi: 10.1182/blood-2016-04-711903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gardner R.A., Finney O., Annesley C., Brakke H., Summers C., Leger K., Bleakley M., Brown C., Mgebroff S., Kelly-Spratt K.S. Intent-to-treat leukemia remission by CD19 CAR T cells of defined formulation and dose in children and young adults. Blood. 2017;129:3322–3331. doi: 10.1182/blood-2017-02-769208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frey N.V., Shaw P.A., Hezner E.O., Gill S., Marcucci K., Luger S.M., Mangan J., Grupp S., Maude S., Ericson S. Optimizing chimeric antigen receptor (CAR) T cell therapy for adult patients with relapsed or refractory (r/r) acute lymphoblastic leukemia (ALL) J Clin Oncol. 2016;34(Suppl) 7002–7002. [Google Scholar]
- 27.Mavroudis D., Read E., Cottler-Fox M., Couriel D., Molldrem J., Carter C., Yu M., Dunbar C., Barrett J. CD34+ cell dose predicts survival, posttransplant morbidity, and rate of hematologic recovery after allogeneic marrow transplants for hematologic malignancies. Blood. 1996;88:3223–3229. [PubMed] [Google Scholar]
- 28.Berger C., Jensen M.C., Lansdorp P.M., Gough M., Elliott C., Riddell S.R. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J. Clin. Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gattinoni L., Lugli E., Ji Y., Pos Z., Paulos C.M., Quigley M.F., Almeida J.R., Gostick E., Yu Z., Carpenito C. A human memory T cell subset with stem cell-like properties. Nat. Med. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fraietta J.A., Lacey S.F., Wilcox N.S., Bedoya F., Chen F., Orlando E., Brogdon J.L., Hwang W.-T., Frey N., Young R.M. Biomarkers of response to anti-CD19 chimeric antigen receptor (CAR) T-cell therapy in patients with chronic lymphocytic leukemia. Blood. 2016;128:57. [Google Scholar]
- 31.Turtle C.J., Hanafi L.A., Berger C., Gooley T.A., Cherian S., Hudecek M., Sommermeyer D., Melville K., Pender B., Budiarto T.M. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J. Clin. Invest. 2016;126:2123–2138. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dudley M.E., Wunderlich J.R., Yang J.C., Sherry R.M., Topalian S.L., Restifo N.P., Royal R.E., Kammula U., White D.E., Mavroukakis S.A. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J. Clin. Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dudley M.E., Yang J.C., Sherry R., Hughes M.S., Royal R., Kammula U., Robbins P.F., Huang J., Citrin D.E., Leitman S.F. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J. Clin. Oncol. 2008;26:5233–5239. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wallen H., Thompson J.A., Reilly J.Z., Rodmyre R.M., Cao J., Yee C. Fludarabine modulates immune response and extends in vivo survival of adoptively transferred CD8 T cells in patients with metastatic melanoma. PLoS One. 2009;4:e4749. doi: 10.1371/journal.pone.0004749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goff S.L., Dudley M.E., Citrin D.E., Somerville R.P., Wunderlich J.R., Danforth D.N., Zlott D.A., Yang J.C., Sherry R.M., Kammula U.S. Randomized, prospective evaluation comparing intensity of lymphodepletion before adoptive transfer of tumor-infiltrating lymphocytes for patients with metastatic melanoma. J. Clin. Oncol. 2016;34:2389–2397. doi: 10.1200/JCO.2016.66.7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gattinoni L., Finkelstein S.E., Klebanoff C.A., Antony P.A., Palmer D.C., Spiess P.J., Hwang L.N., Yu Z., Wrzesinski C., Heimann D.M. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J. Exp. Med. 2005;202:907–912. doi: 10.1084/jem.20050732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown I.E., Blank C., Kline J., Kacha A.K., Gajewski T.F. Homeostatic proliferation as an isolated variable reverses CD8+ T cell anergy and promotes tumor rejection. J. Immunol. 2006;177:4521–4529. doi: 10.4049/jimmunol.177.7.4521. [DOI] [PubMed] [Google Scholar]
- 38.Kline J., Brown I.E., Zha Y.Y., Blank C., Strickler J., Wouters H., Zhang L., Gajewski T.F. Homeostatic proliferation plus regulatory T-cell depletion promotes potent rejection of B16 melanoma. Clin. Cancer Res. 2008;14:3156–3167. doi: 10.1158/1078-0432.CCR-07-4696. [DOI] [PubMed] [Google Scholar]
- 39.Proietti E., Greco G., Garrone B., Baccarini S., Mauri C., Venditti M., Carlei D., Belardelli F. Importance of cyclophosphamide-induced bystander effect on T cells for a successful tumor eradication in response to adoptive immunotherapy in mice. J. Clin. Invest. 1998;101:429–441. doi: 10.1172/JCI1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bracci L., Moschella F., Sestili P., La Sorsa V., Valentini M., Canini I., Baccarini S., Maccari S., Ramoni C., Belardelli F. Cyclophosphamide enhances the antitumor efficacy of adoptively transferred immune cells through the induction of cytokine expression, B-cell and T-cell homeostatic proliferation, and specific tumor infiltration. Clin. Cancer Res. 2007;13:644–653. doi: 10.1158/1078-0432.CCR-06-1209. [DOI] [PubMed] [Google Scholar]
- 41.Paulos C.M., Wrzesinski C., Kaiser A., Hinrichs C.S., Chieppa M., Cassard L., Palmer D.C., Boni A., Muranski P., Yu Z. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J. Clin. Invest. 2007;117:2197–2204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baba J., Watanabe S., Saida Y., Tanaka T., Miyabayashi T., Koshio J., Ichikawa K., Nozaki K., Koya T., Deguchi K. Depletion of radio-resistant regulatory T cells enhances antitumor immunity during recovery from lymphopenia. Blood. 2012;120:2417–2427. doi: 10.1182/blood-2012-02-411124. [DOI] [PubMed] [Google Scholar]
- 43.Kodumudi K.N., Weber A., Sarnaik A.A., Pilon-Thomas S. Blockade of myeloid-derived suppressor cells after induction of lymphopenia improves adoptive T cell therapy in a murine model of melanoma. J. Immunol. 2012;189:5147–5154. doi: 10.4049/jimmunol.1200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Milone M.C., Fish J.D., Carpenito C., Carroll R.G., Binder G.K., Teachey D., Samanta M., Lakhal M., Gloss B., Danet-Desnoyers G. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol. Ther. 2009;17:1453–1464. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guedan S., Chen X., Madar A., Carpenito C., McGettigan S.E., Frigault M.J., Lee J., Posey A.D., Jr., Scholler J., Scholler N. ICOS-based chimeric antigen receptors program bipolar TH17/TH1 cells. Blood. 2014;124:1070–1080. doi: 10.1182/blood-2013-10-535245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carpenito C., Milone M.C., Hassan R., Simonet J.C., Lakhal M., Suhoski M.M., Varela-Rohena A., Haines K.M., Heitjan D.F., Albelda S.M. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc. Natl. Acad. Sci. USA. 2009;106:3360–3365. doi: 10.1073/pnas.0813101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hombach A.A., Abken H. Costimulation by chimeric antigen receptors revisited the T cell antitumor response benefits from combined CD28-OX40 signalling. Int. J. Cancer. 2011;129:2935–2944. doi: 10.1002/ijc.25960. [DOI] [PubMed] [Google Scholar]
- 48.Kochenderfer J.N., Somerville R.P.T., Lu T., Yang J.C., Sherry R.M., Feldman S.A., McIntyre L., Bot A., Rossi J., Lam N., Rosenberg S.A. Long-duration complete remissions of diffuse large B cell lymphoma after anti-CD19 chimeric antigen receptor t cell therapy. Mol. Ther. 2017;25:2245–2253. doi: 10.1016/j.ymthe.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fesnak A.D., June C.H., Levine B.L. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat. Rev. Cancer. 2016;16:566–581. doi: 10.1038/nrc.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li H., Zhao Y. Increasing the safety and efficacy of chimeric antigen receptor T cell therapy. Protein Cell. 2017;8:573–589. doi: 10.1007/s13238-017-0411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Srivastava S., Riddell S.R. Chimeric antigen receptor T cell therapy: challenges to bench-to-bedside efficacy. J. Immunol. 2018;200:459–468. doi: 10.4049/jimmunol.1701155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chong E.A., Melenhorst J.J., Lacey S.F., Ambrose D.E., Gonzalez V., Levine B.L., June C.H., Schuster S.J. PD-1 blockade modulates chimeric antigen receptor (CAR)–modified T cells: refueling the CAR. Blood. 2017;129:1039–1041. doi: 10.1182/blood-2016-09-738245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cherkassky L., Morello A., Villena-Vargas J., Feng Y., Dimitrov D.S., Jones D.R., Sadelain M., Adusumilli P.S. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J. Clin. Invest. 2016;126:3130–3144. doi: 10.1172/JCI83092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu X., Ranganathan R., Jiang S., Fang C., Sun J., Kim S., Newick K., Lo A., June C.H., Zhao Y. A chimeric switch-receptor targeting PD1 augments the efficacy of second-generation CAR T cells in advanced solid tumors. Cancer Res. 2016;76:1578–1590. doi: 10.1158/0008-5472.CAN-15-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li S., Siriwon N., Zhang X., Yang S., Jin T., He F., Kim Y.J., Mac J., Lu Z., Wang S. Enhanced cancer immunotherapy by chimeric antigen receptor-modified T cells engineered to secrete checkpoint inhibitors. Clin. Cancer Res. 2017;23:6982–6992. doi: 10.1158/1078-0432.CCR-17-0867. [DOI] [PubMed] [Google Scholar]
- 56.Suarez E.R., Chang K., Sun J., Sui J., Freeman G.J., Signoretti S., Zhu Q., Marasco W.A. Chimeric antigen receptor T cells secreting anti-PD-L1 antibodies more effectively regress renal cell carcinoma in a humanized mouse model. Oncotarget. 2016;7:34341–34355. doi: 10.18632/oncotarget.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Teachey D.T., Lacey S.F., Shaw P.A., Melenhorst J.J., Maude S.L., Frey N., Pequignot E., Gonzalez V.E., Chen F., Finklestein J. Identification of predictive biomarkers for cytokine release syndrome after chimeric antigen receptor T-cell therapy for acute lymphoblastic leukemia. Cancer Discov. 2016;6:664–679. doi: 10.1158/2159-8290.CD-16-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Davila M.L., Riviere I., Wang X., Bartido S., Park J., Curran K., Chung S.S., Stefanski J., Borquez-Ojeda O., Olszewska M. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci. Transl. Med. 2014;6:224ra25. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brentjens R.J., Rivière I., Park J.H., Davila M.L., Wang X., Stefanski J., Taylor C., Yeh R., Bartido S., Borquez-Ojeda O. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood. 2011;118:4817–4828. doi: 10.1182/blood-2011-04-348540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fitzgerald J.C., Weiss S.L., Maude S.L., Barrett D.M., Lacey S.F., Melenhorst J.J., Shaw P., Berg R.A., June C.H., Porter D.L. Cytokine release syndrome after chimeric antigen receptor T cell therapy for acute lymphoblastic leukemia. Crit. Care Med. 2017;45:e124–e131. doi: 10.1097/CCM.0000000000002053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Maude S.L., Frey N., Shaw P.A., Aplenc R., Barrett D.M., Bunin N.J., Chew A., Gonzalez V.E., Zheng Z., Lacey S.F. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Turtle C.J., Hanafi L.A., Berger C., Hudecek M., Pender B., Robinson E., Hawkins R., Chaney C., Cherian S., Chen X. Immunotherapy of non-Hodgkin’s lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci. Transl. Med. 2016;8:355ra116. doi: 10.1126/scitranslmed.aaf8621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Locke F.L., Neelapu S.S., Bartlett N.L., Siddiqi T., Chavez J.C., Hosing C.M., Ghobadi A., Budde L.E., Bot A., Rossi J.M. Phase 1 results of ZUMA-1: a multicenter study of KTE-C19 anti-CD19 CAR T cell therapy in refractory aggressive lymphoma. Mol. Ther. 2017;25:285–295. doi: 10.1016/j.ymthe.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cohen A.D., Garfall A.L., Sadtmauer E.A., Lacey S.F., Lancaster E., Vogl D.T., Dengel K., Ambrose D.E., Chen F., Plesa G. B-cell maturation antigen (BCMA)-specific chimeric antigen receptor T cells (CART-BCMA) for multiple myeloma (mm): initial safety and efficacy from a phase I study. Blood. 2016;128:1147. [Google Scholar]
- 65.Luo Y., Chang L.-J., Hu Y., Dong L., Wei G., Huang H. First-in-man CD123-specific chimeric antigen receptor-modified T cells for the treatment of refractory acute myeloid leukemia. Blood. 2015;126:3778. [Google Scholar]
- 66.Tanyi J.L., Stashwick C., Plesa G., Morgan M.A., Porter D., Maus M.V., June C.H. Possible compartmental cytokine release syndrome in a patient with recurrent ovarian cancer after treatment with mesothelin-targeted CAR-T cells. J. Immunother. 2017;40:104–107. doi: 10.1097/CJI.0000000000000160. [DOI] [PubMed] [Google Scholar]
- 67.Hay K.A., Hanafi L.A., Li D., Gust J., Liles W.C., Wurfel M.M., López J.A., Chen J., Chung D., Harju-Baker S. Kinetics and biomarkers of severe cytokine release syndrome after CD19 chimeric antigen receptor-modified T-cell therapy. Blood. 2017;130:2295–2306. doi: 10.1182/blood-2017-06-793141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gust J., Hay K.A., Hanafi L.A., Li D., Myerson D., Gonzalez-Cuyar L.F., Yeung C., Liles W.C., Wurfel M., Lopez J.A. Endothelial activation and blood-brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 2017;7:1404–1419. doi: 10.1158/2159-8290.CD-17-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Obstfeld A.E., Frey N.V., Mansfield K., Lacey S.F., June C.H., Porter D.L., Melenhorst J.J., Wasik M.A. Cytokine release syndrome associated with chimeric-antigen receptor T-cell therapy: clinicopathological insights. Blood. 2017;130:2569–2572. doi: 10.1182/blood-2017-08-802413. [DOI] [PubMed] [Google Scholar]
- 70.Frey N.V., Porter D.L. The promise of chimeric antigen receptor T-cell therapy. Oncology (Williston Park) 2016;30:880–888. 890. [PubMed] [Google Scholar]
- 71.Neelapu S.S., Tummala S., Kebriaei P., Wierda W., Gutierrez C., Locke F.L., Komanduri K.V., Lin Y., Jain N., Daver N. Chimeric antigen receptor T-cell therapy - assessment and management of toxicities. Nat. Rev. Clin. Oncol. 2018;15:47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Morgan R.A., Chinnasamy N., Abate-Daga D., Gros A., Robbins P.F., Zheng Z., Dudley M.E., Feldman S.A., Yang J.C., Sherry R.M. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J. Immunother. 2013;36:133–151. doi: 10.1097/CJI.0b013e3182829903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garfall A.L., Lancaster E., Stadtmauer E.A., Lacey S.F., Dengel K., Ambrose D.E., Chen F., Gupta M., Kulikovskaya I., Vogl D.T. Posterior reversible encephalopathy syndrome (PRES) after infusion of anti-Bcma CAR T cells (CART-BCMA) for multiple myeloma: successful treatment with cyclophosphamide. Blood. 2016;128:5702. [Google Scholar]
- 74.Bhoj V.G., Arhontoulis D., Wertheim G., Capobianchi J., Callahan C.A., Ellebrecht C.T., Obstfeld A.E., Lacey S.F., Melenhorst J.J., Nazimuddin F. Persistence of long-lived plasma cells and humoral immunity in individuals responding to CD19-directed CAR T-cell therapy. Blood. 2016;128:360–370. doi: 10.1182/blood-2016-01-694356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Johnson L.A., Morgan R.A., Dudley M.E., Cassard L., Yang J.C., Hughes M.S., Kammula U.S., Royal R.E., Sherry R.M., Wunderlich J.R. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Byrne K.T., Turk M.J. New perspectives on the role of vitiligo in immune responses to melanoma. Oncotarget. 2011;2:684–694. doi: 10.18632/oncotarget.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Linette G.P., Stadtmauer E.A., Maus M.V., Rapoport A.P., Levine B.L., Emery L., Litzky L., Bagg A., Carreno B.M., Cimino P.J. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 2013;122:863–871. doi: 10.1182/blood-2013-03-490565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cameron B.J., Gerry A.B., Dukes J., Harper J.V., Kannan V., Bianchi F.C., Grand F., Brewer J.E., Gupta M., Plesa G. Identification of a Titin-derived HLA-A1-presented peptide as a cross-reactive target for engineered MAGE A3-directed T cells. Sci. Transl. Med. 2013;5:197ra103. doi: 10.1126/scitranslmed.3006034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schmitt T.M., Ragnarsson G.B., Greenberg P.D. T cell receptor gene therapy for cancer. Hum. Gene Ther. 2009;20:1240–1248. doi: 10.1089/hum.2009.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bethune M.T., Gee M.H., Bunse M., Lee M.S., Gschweng E.H., Pagadala M.S., Zhou J., Cheng D., Heath J.R., Kohn D.B. Domain-swapped T cell receptors improve the safety of TCR gene therapy. eLife. 2016;5 doi: 10.7554/eLife.19095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lim W.A., June C.H. The principles of engineering immune cells to treat cancer. Cell. 2017;168:724–740. doi: 10.1016/j.cell.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qasim W., Zhan H., Samarasinghe S., Adams S., Amrolia P., Stafford S., Butler K., Rivat C., Wright G., Somana K. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aaj2013. [DOI] [PubMed] [Google Scholar]
- 83.Ren J., Zhang X., Liu X., Fang C., Jiang S., June C.H., Zhao Y. A versatile system for rapid multiplex genome-edited CAR T cell generation. Oncotarget. 2017;8:17002–17011. doi: 10.18632/oncotarget.15218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Di Stasi A., Tey S.K., Dotti G., Fujita Y., Kennedy-Nasser A., Martinez C., Straathof K., Liu E., Durett A.G., Grilley B. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 2011;365:1673–1683. doi: 10.1056/NEJMoa1106152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee D.W., Kochenderfer J.N., Stetler-Stevenson M., Cui Y.K., Delbrook C., Feldman S.A., Fry T.J., Orentas R., Sabatino M., Shah N.N. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang X., Popplewell L.L., Wagner J.R., Naranjo A., Blanchard M.S., Mott M.R., Norris A.P., Wong C.W., Urak R.Z., Chang W.C. Phase 1 studies of central memory-derived CD19 CAR T-cell therapy following autologous HSCT in patients with B-cell NHL. Blood. 2016;127:2980–2990. doi: 10.1182/blood-2015-12-686725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hu Y., Wu Z., Luo Y., Shi J., Yu J., Pu C., Liang Z., Wei G., Cui Q., Sun J. Potent anti-leukemia activities of chimeric antigen receptor-modified t cells against CD19 in Chinese patients with relapsed/refractory acute lymphocytic leukemia. Clin Cancer Res. 2017;23:3297–3306. doi: 10.1158/1078-0432.CCR-16-1799. [DOI] [PubMed] [Google Scholar]
- 88.Turtle C.J., Hay K.A., Hanafi L.A., Li D., Cherian S., Chen X., Wood B., Lozanski A., Byrd J.C., Heimfeld S. Durable molecular remissions in chronic lymphocytic leukemia treated with CD19-specific chimeric antigen receptor-modified t cells after failure of Ibrutinib. J Clin Oncol. 2017;35:3010–3020. doi: 10.1200/JCO.2017.72.8519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pan J., Yang J.F., Deng B.P., Zhao X.J., Zhang X., Lin Y.H., Wu Y.N., Deng Z.L., Zhang Y.L., Liu S.H. High efficacy and safety of low-dose CD19-directed CAR-T cell therapy in 51 refractory or relapsed B acute lymphoblastic leukemia patients. Leukemia. 2017;31:2587–2593. doi: 10.1038/leu.2017.145. [DOI] [PubMed] [Google Scholar]
- 90.Rossig C., Pule M., Altvater B., Saiagh S., Wright G., Ghorashian S., Clifton-Hadley L., Champion K., Sattar Z., Popova B. Vaccination to improve the persistence of CD19CAR gene-modified T cells in relapsed pediatric acute lymphoblastic leukemia. Leukemia. 2017;31:1087–1095. doi: 10.1038/leu.2017.39. [DOI] [PubMed] [Google Scholar]
- 91.Kochenderfer J.N., Dudley M.E., Kassim S.H., Somerville R.P., Carpenter R.O., Stetler-Stevenson M., Yang J.C., Phan G.Q., Hughes M.S., Sherry R.M. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]