Abstract
Background & Aims
The transient receptor potential ankyrin 1 (TRPA1) channel is highly expressed in the intestinal lamina propria, but its contribution to gut physiology/pathophysiology is unclear. Here, we evaluated the function of myofibroblast TRPA1 channels in intestinal remodeling.
Methods
An intestinal myofibroblast cell line (InMyoFibs) was stimulated by transforming growth factor-β1 to induce in vitro fibrosis. Trpa1 knockout mice were generated using the Clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated 9 (Cas9) system. A murine chronic colitis model was established by weekly intrarectal trinitrobenzene sulfonic acid (TNBS) administration. Samples from the intestines of Crohn’s disease (CD) patients were used for pathologic staining and quantitative analyses.
Results
In InMyoFibs, TRPA1 showed the highest expression among TRP family members. In TNBS chronic colitis model mice, the extents of inflammation and fibrotic changes were more prominent in TRPA1-/- knockout than in wild-type mice. One-week enema administration of prednisolone suppressed fibrotic lesions in wild-type mice, but not in TRPA1 knockout mice. Steroids and pirfenidone induced Ca2+ influx in InMyoFibs, which was antagonized by the selective TRPA1 channel blocker HC-030031. Steroids and pirfenidone counteracted transforming growth factor-β1–induced expression of heat shock protein 47, type 1 collagen, and α-smooth muscle actin, and reduced Smad-2 phosphorylation and myocardin expression in InMyoFibs. In stenotic intestinal regions of CD patients, TRPA1 expression was increased significantly. TRPA1/heat shock protein 47 double-positive cells accumulated in the stenotic intestinal regions of both CD patients and TNBS-treated mice.
Conclusions
TRPA1, in addition to its anti-inflammatory actions, may protect against intestinal fibrosis, thus being a novel therapeutic target for highly incurable inflammatory/fibrotic disorders.
Keywords: Crohn’s Disease, Intestinal Fibrosis, Myofibroblast, Transient Receptor Potential Ankyrin 1
Abbreviations used in this paper: α-SMA, α smooth muscle actin; AITC, allyl isothiocyanate; CD, Crohn’s disease; EGTA, ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid; HSP47, heat shock protein 47; InMyoFib, intestinal myofibroblast cell line; KO, knockout; mRNA, messenger RNA; MT, Masson trichrome; PBS, phosphate-buffered saline; PCR, polymerase chain reaction; RT-PCR, reverse-transcription polymerase chain reaction; sgRNA, single-guide RNA; siRNA, small interfering RNA; TGF, transforming growth factor; TNBS, trinitrobenzene sulfonic acid; TNF, tumor necrosis factor; TRP, transient receptor potential; TRPA1, transient receptor potential ankyrin 1; TRPC, transient receptor potential canonical; WT, wild-type
Graphical abstract
Summary.
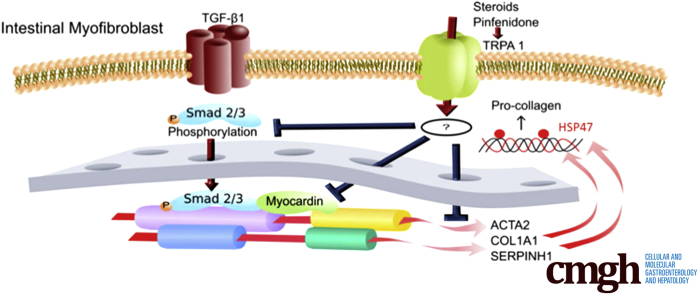
Steroids and pirfenidone activated the transient receptor potential ankyrin 1 (TRPA1) channel to inhibit fibrosis in vitro and in trinitrobenzene sulfonic acid colitis. TRPA1-positive cells accumulated within stenotic regions of Crohn’s disease intestine. The results suggest that TRPA1 channel activation could be beneficial in fibrotic inflammatory disorders.
Myofibroblasts play crucial roles in tissue repair and remodeling. The major profibrotic factor, transforming growth factor (TGF)-β, is secreted from many types of cells and is known to drive the transformation of fibroblasts to myofibroblasts. Activated myofibroblasts proliferate, migrate, and invade affected tissues to facilitate the formation of collagen-rich extracellular matrix and express α-smooth muscle actin (α-SMA) to confer contractility ability.1, 2 We previously showed that cultured intestinal myofibroblasts (InMyoFibs) stimulated by TGF-β1 actively secreted collagens to serve as an excessive fibrosis model. TGF-β1 treatment induced characteristic morphologic changes in InMyoFibs, such as enlarged size and a filamentous microstructure.3 The activated TGF-β–receptor 1 complex has been shown to phosphorylate the transcription factors Smad-2 and Smad-3, which in turn promote collagen synthesis.4 TGF-β was reported to up-regulate heat shock protein 47 (HSP47), a collagen-specific molecular chaperone that plays a critical role in intestinal fibrosis. Blocking the bioactivities of HSP47 not only alters collagen production, but also reduces fibrotic lesions, directly implicating HSP47 in fibrogenesis.5, 6 TGF-β modulates transcriptional regulation by the serum response factor and its cofactor myocardin-related transcription factor A during the induction of pathologic colonic myofibroblast differentiation.7
TGF-β levels are increased in inflamed intestines of patients with Crohn’s disease (CD) and ulcerative colitis. However, TGF-β1 also is essential for anti-inflammatory responses. Thus, the use of TGF-β1–neutralizing antibodies for antifibrotic treatment in clinical practice may exacerbate CD progression by attenuating the anti-inflammatory actions of TGF-β1. In addition, abnormal TGF-β signaling impairs intestinal immune tolerance and tissue repair.8
There is growing interest in the role of transient receptor potential (TRP) channels in the pathogenesis of tissue remodeling.9, 10, 11, 12 We recently reported that transient receptor potential canonical (TRPC)4 and TRPC6 activities in myofibroblast are functionally linked to the progression of intestinal fibrotic stenosis.3 Our preliminary experiment indicated that another TRP member, TRP ankyrin 1 (TRPA1), is expressed abundantly at the messenger RNA (mRNA) level in intestinal myofibroblasts. TRPA1 is expressed in myenteric nerves and intestinal epithelial cells,13, 14 and its activation has been shown to inhibit gut motility and protect against gut inflammation via an adrenomedullin-mediated increase in intestinal microcirculation.14, 15, 16 It also has been reported that a TRPA1 agonist allyl isothiocyanate (AITC) exerts antifibrogenic effects on hepatic stellate cells, and another TRPA1 agonist allicin prevents fibrotic changes in the oral submucosa and heart.17, 18 Moreover, TRPA1 activity was reported to have anti-inflammatory actions on dextran sulfate sodium–induced chronic colitis.19 Given the chronic nature of this colitis, T cells may contribute to its pathogenesis, with TRPA1 regulating the inflammatory potential of T cells. Indeed, genetic deletion of TRPA1 on an interleukin 10-/- background exacerbated colitis, and TRPA1-/- naive T cells were more colitogenic in a T-cell transfer model of colitis.20 In contrast, TRPA1 activation by cannabichromene was found to reduce the severity of DNBS-induced colitis.21 However, the molecular mechanisms underlying these beneficial actions of TRPA1 activation remain largely unknown. One possible approach for answering these questions is to compare the severity of gastrointestinal inflammation/fibrosis between wild-type (WT) and TRPA1-deficient mice by developing colitis models.
In our ongoing project involving screening of 103 types of food ingredients, we identified 4 plant extract components with both TRPA1-activating and antifibrotic activities. These 4 components suppressed TGF-β1–induced α-SMA and collagen production. Among them was the ethanol extract of licorice, which contains a steroid-like ingredient (glycyrrhizic acid). It is metabolized to glycyrrhetinic acid and showed the strongest TRPA1-activating/antifibrotic activities. In clinical practice, steroids and pirfenidone are widely used antifibrotic drugs that target many organs, including intestinal strictures after surgical dilatation.22, 23 These drugs also are known to mitigate inflammatory bowel disease–associated fibrosis and consequent stenosis and strictures, which are common and severe complications in CD patients.22, 23, 24 In many CD patients, surgery alone often results in only temporarily beneficial effects. Pirfenidone has been reported to suppress various types of fibrosis in animal models and was shown to be effective in clinical trials for patients with idiopathic pulmonary fibrosis. In the gastrointestinal tract, pirfenidone exerts antifibrotic effects by suppressing the expression of HSP47,25 which acts as a collagen-specific molecular chaperone and participates in the intestinal fibrosis of CD.6, 26, 27
In the present study, we examined whether myofibroblast TRPA1 is involved in the antifibrotic/antistenotic mechanisms in the intestine and whether steroids and pirfenidone exert their antifibrotic actions in the intestine by activating the myofibroblast TRPA1 channel. We prepared TRPA1-knockout mice and compared the in vivo results from mice with those of in vitro experiments using InMyoFibs. Moreover, biopsy samples from stenotic and nonstenotic regions of intestines from CD patients were used to confirm the validity for human pathogenesis. Our results suggest that TRPA1 activity in myofibroblast cells negatively regulates TGF-β1–induced α-SMA and collagen production, and thus direct stimulation of TRPA1 channels in the intestinal submucosa may be a promising intervention for relieving abdominal symptoms associated with intestinal fibrotic remodeling.
Materials and Methods
Materials
Licorice extract powder was dissolved in ethanol by sonication and purified by filtering. AITC and HC-030031 were obtained from Sigma-Aldrich (St. Louis, MO). Recombinant human TGF-β1 (Wako, Osaka, Japan) was added to cultured cells according to the manufacturer’s instructions. Human Stealth small interfering RNAs (siRNAs) for TRPA1 (TRPA1HSS113276 (5′-GGAGCAAUUGCUGUUUACUUCUAUU-3′ and 5′-AAUAGAAGUAAACAGCAAUUGCUCC-3′) were obtained from Invitrogen (Carlsbad, CA) and used for gene silencing according to the manufacturer’s instructions. Other TRPA1-siRNAs, TRPA1HSS113277 and TRPA1HSS189723, also were evaluated, but the silencing efficacy was inferior to that by TRPA1HSS113276 for InMyoFibs. Antibodies against TRPA1 (mouse, Sigma-Aldrich; rabbit, Novus, CO), HSP47 (rabbit; Abcam, Cambridge, UK), α-SMA (Abcam), β-actin (Abcam), collagen I (Abcam), Smad-2/3, and phospho-Smad-2 (Cell Signaling Technology, Danvers, MA) were used for immunoblotting and immunostaining experiments. The human TRPA1 plasmid used for single-channel recording was kindly provided by Professor Yasuo Mori (Kyoto University, Japan).
Cell Culture
Normal human InMyoFibs were purchased from Lonza (CC-2902; Basel, Switzerland) and grown in smooth muscle basal medium supplemented with 5% fetal bovine serum, antibiotics (gentamicin/amphotericin-B), and growth factors (insulin, human epidermal growth factor-β, and human fibroblastic growth factor). InMyoFibs cells were passaged 10–19 times. TGF-β1 (5 ng/mL) was used under low-serum (1% fetal bovine serum) conditions.
Expression Array Analysis
Total RNA isolation for array
The total RNA was isolated from the InMyoFibs using TRIzol Reagent (Invitrogen) and purified using the SV Total RNA Isolation System (Promega, Madison, WI) according to the manufacturer's instructions. RNA samples were quantified using an ND-1000 spectrophotometer (NanoDrop Technologies, Wilmington, DE) and the quality was confirmed using an Experion System (Bio-Rad Laboratories, Hercules, CA).
Gene expression microarrays
The complementary RNA was amplified, labeled using a GeneChip (Affymetrix, CA) WT Terminal Labeling and Control Kit, and hybridized to an Affymetrix Human Genome U133 Plus 2.0 array according to the manufacturer's instructions. All hybridized microarrays were scanned with an Affymetrix scanner. Relative hybridization intensities and background hybridization values were calculated using the Affymetrix Expression Console.
Data analysis and filter criteria
Raw signal intensities for respective probes were calculated from hybridization intensities. Next, the raw signal intensities of 2 samples were log2-transformed and normalized using the robust multi-array average and quantile algorithm with Affymetrix Expression Console 1.1 software. To identify up-regulated or down-regulated genes, we calculated Z-scores and ratios (non–log-scaled fold-change) from the normalized signal intensities of respective probes to compare control and experimental samples.
Electrophysiological Study
Whole-cell voltage clamp recording was conducted using an EPC-10 patch-clamp amplifier (HEKA Electronics, Lambrecht, Germany). Recording electrodes with resistances of 2–4 MΩ were pulled from glass capillaries. An Ag–AgCl wire was used as a reference electrode. Capacitive currents were compensated, and linear leak and residual capacitance were subtracted by the P/4 protocol. More than 70% series resistance was compensated to minimize voltage errors. Data analysis was performed offline using a versatile analysis software (Clampfit v.9.2; Axon Instruments, Union City, CA; and Origin 9.1; OriginLab Corporation, Northampton, MA). Long-term recordings were performed using an A/D, D/A converter PowerLab/400 (ADInstruments, Sydney, Australia; sampling rate, 100 Hz) and analyzed with the accessory software Chart v.5.0 (ADInstruments, Sydney, Australia). A solenoid valve-driven fast solution change device similar to the Y-tube system was used to rapidly apply drugs to the cells as described previously.28 Membrane capacitance was measured to normalize current amplitudes and minimize the variance caused by differences in cell size. To obtain current density–voltage relationships, cells were held at a holding potential of -60 mV and then subjected to ramp pulses of 100 ms from -100 to 100 mV at a rate of 4 mV/ms. To prevent rapid Ca2+-dependent inactivation of TRPA1 currents, a Ca2+ chelator was added to both the bath solution and pipette solution.29 The extracellular solution contained 140 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L MgCl2, 5 mmol/L ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 10 mmol/L HEPES, and 10 mmol/L glucose. K+ currents were eliminated by adding Cs+ and tetraethylammonium (TEA) to the patch pipette solution. Patch pipettes were filled with 130 mmol/L Cs-aspartate, 10 mmol/L TEA-Cl, 5 mmol/L BAPTA, 1.374 mmol/L Ca-gluconate, 1 mmol/L MgCl2, 2 mmol/L MgSO4, 2 mmol/L Na2 adenosine triphosphate, and 10 mmol/L HEPES. For cell-attached recording of expressed HEK293 cells, the pipette solution contained 140 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L MgCl2, 5 mmol/L EGTA, 10 mmol/L HEPES, and 10 mmol/L glucose. The extracellular solution contained 145 KCl, 1.2 MgCl2, 5 mmol/L EGTA, 10 mmol/L HEPES, and 10 mmol/L glucose.
Measurement of [Ca2+]i
[Ca2+]i in myofibroblasts was monitored using a digital fluorescence imaging technique with Fura-2. Briefly, InMyoFibs were dispersed onto a poly-L-lysine–coated (Sigma-Aldrich) glass chamber placed on the stage of an inverted fluorescent microscope (DMI600B; Leica, Wetzlar, Germany). InMyoFibs cells then were loaded with 5 μmol/L Fura-2/AM in the dark at 37°C for 30 minutes. The intensity of Fura-2 emissions at 510 nm (±10 nm) resulting from excitation at 340 or 380 nm was measured using a fluorescent microscope (DMI600B) and a Cascade EMCCD camera (Nippon Roper, Tokyo, Japan). Data acquisition and analysis were performed using SlideBook 4.2 software (Intelligent Imaging Innovation, Inc, Denver, CO). Fluorescence intensities were corrected for background fluorescence and changes in [Ca2+]i were defined as the ratio of corrected fluorescence intensities resulting from excitation at 340 and 380 nm (F340/F380). The normal external solution for Ca2+-imaging experiments contained the following: 140 mmol/L NaCl, 5 mmol/L KCl, 1 mmol/L CaCl2, 1.2 mmol/L MgCl2, 10 mmol/L HEPES, and 10 mmol/L glucose (pH 7.4, adjusted with Tris base).
Immunoblot Analysis
Immunoblotting experiments were performed to examine the protein levels of type I collagen, α-SMA, β-actin, Smad-2/3, and phospho–Smad-2 in InMyoFibs as described previously. Total cell lysates were prepared in sample buffer and diluted in 5% (vol/vol) 2-mercaptoethanol and 1% (wt/vol) bromophenol blue before electrophoresis. Proteins were resolved on 10% (wt/vol) sodium dodecyl sulfate–polyacrylamide gels and transferred to polyvinyldifluoridine membranes. The membranes were blocked with Blocking One (Nacalai Tesque, Kyoto, Japan) and incubated overnight (4°C) with appropriate primary antibodies. Protein levels were detected by incubating the polyvinyldifluoridine membranes with appropriate species-specific, horseradish peroxidase–conjugated secondary antibodies (20°C, 45 min), and visualized using the ECL Western Blotting Detection System (GE Healthcare, Little Chalfont, UK).
Real-time Reverse-Transcription Polymerase Chain Reaction
Total RNA for quantitation was extracted from InMyoFibs, using the RNeasy RNA Extraction Kit (Qiagen, Hilden, Germany). To quantify mRNA expression levels, real-time polymerase chain reaction (PCR) was performed using a BioMark HD System (Fluidigm, South San Francisco, CA). Thermocycling was performed using an initial denaturation at a hot start phase of 60 seconds at 95°C. This was followed by 35 cycles of 5 seconds at 96°C and 20 seconds at 60°C. The following TaqMan Gene Expression Assay kits from Life Technologies (Carlsbad, CA) were used for real-time PCR reactions: TRPA1 (Hs00175798_m1, Mm01227437_m1), MYOCD (myocardin; Hs00538071_m1), SERPINH1 (HSP47; Hs01060397_g1), ACTA2 (α-SMA; Hs00426835_g1), and COL1A1 (Hs00164004_m1).
Immunostaining
InMyoFibs cells and tissues and were fixed in 4% formaldehyde for 15 minutes and permeabilized with 0.3% Triton X-100 in the presence of 5% normal goat serum (Wako). Cells then were incubated overnight with primary antibodies (1:200 dilution) at 4°C, washed with phosphate-buffered saline (PBS), and incubated with an Alexa Fluor–conjugated secondary antibody (1:200 dilution; Life Technologies) for an additional 1 hour. Immunostained cells were analyzed using a Zeiss LSM 710 Confocal Microscope (Oberkochen, Germany). Images were acquired through a 40× objective lens, using default settings for pinhole width, laser intensity, and detector gain. 3,3′-Diaminobenzidine tetra hydrochloride staining was performed with the R.T.U.Vectastain Universal Elite ABCkit (Vector Lab, Inc, Burlingame, CA).
Generation of Trpa1 Knockout Mice Using the CRISPR/Cas9 System
The validation of in vitro cleavage activity of single-guide RNA (sgRNA) and generation of knockout mice by pronuclear injection of the circular pX330 plasmid were performed as described previously.30 Super-ovulated B6D2F1 female mice were briefly mated with B6D2F1 males and then fertilized eggs were collected. Circular pX330-sgRNA-S02 and pX330-sgRNA-AS02 plasmids targeting Trpa1 were co-injected into the pronuclei of fertilized eggs. Off-target analysis was performed using Bowtie software (http://bowtie-bio.sourceforge.net/index.shtml).31 sgRNA sequences used for pX330 plasmid construction and injection were as follows: 5′-CAATGAAGCGCGGCTTGAGG-3′ for sgRNA-S02 and 5′-CGCCGCGATAGACAACGCCC-3′ for sgRNA-AS02. Primer sequences used for EGxxFP plasmid construction and genotyping were as follows: 5′-GCAGAGCTTAGTTGTAGGTCCCAAGTCCGG-3′ and 5′-TGATACTGTCTCCTGCCATTTCCATGTTCC-3′. Genotyping was performed by direct sequencing after PCR. Littermate mice generated from heterozygous crosses were used in the present study.
Trinitrobenzene Sulfonic Acid Chronic Colitis Model
Chronic trinitrobenzene sulfonic acid (TNBS)-associated colitis was induced as previously reported. Mice (ages, 8–9 wk; n = 8) were injected weekly with TNBS solution in 30% ethanol/PBS (10 mg/mL; 50 μL) intracolonically for 6 weeks. The vehicle group received 30% ethanol/PBS (50 μL) for 6 weeks. Intracolonic administration was achieved after 24-hour fasting by inserting a polyethylene (PE10 Polyethylene; Braintree Scientific, Inc, Massachusetts) catheter 4 cm into the rectum under pentobarbital (40 mg/kg injected intraperitoneally with saline) anesthesia. Prednisolone was administered by enema for 1 week (5 mg/kg/day; anesthetized by isoflurane) after the last TNBS treatment. Changes in body weight were monitored. Colonic tissues were excised from the anus to the cecum at week 6 after cervical dislocation. All animal experiments were conducted in accordance with the guidelines of the Animal Center of Fukuoka University.
Histologic Evaluation
The mouse distal colon samples and patient biopsy specimens were fixed in 10% buffered formalin, embedded in paraffin, and cut into 4-μm–thick sections for routine H&E and Masson trichrome (MT) staining. Stained colon tissues were microphotographed at 200× magnification at 6 randomly chosen locations on each tissue and analyzed by a pathologist (K.K.) in a blinded manner. For all mice, the fibrosis score was measured at the tissue located 1 cm from the anus, where the most obvious fibrosis was observed. The optical density of blue-stained collagen fibers was measured and expressed as a fibrosis score on a scale of 0–3, as follows: 0, no fibrosis WT vehicle mouse; 1, mild fibrosis (focal mucosal/submucosal collagen deposition without architectural distortion); 2, moderate fibrosis (significant mucosal/submucosal collagen deposition with modest distortion of mucosal/submucosal architecture but without obscuring the mucosal/submucosal border); and 3, severe fibrosis (extensive mucosal/submucosal collagen deposition with marked architectural distortion obscuring the mucosal/submucosal border).32
Quantification of Fecal Lipocalin-2 by Enzyme-Linked Immunosorbent Assay
Fecal Lipocalin-2 (Lcn-2) levels were measured in WT and TRPA1-/- mice before TNBS treatment, TNBS 5 weeks (just before the last TNBS challenge), and TNBS 6 weeks (1 week after the last TNBS challenge), (n = 6–8). Freshly collected or frozen fecal samples were reconstituted in PBS containing 0.1% Tween 20 at a concentration of 100 mg feces/mL and vortexed for 20 minutes to yield a homogenous suspension. The samples were centrifuged at 12,000 rpm at 4°C for 10 minutes. Clear supernatant was collected and stored at -20°C until analysis. Lipocalin-2 levels were quantified using the Mouse Lipocalin-2/Neutrophil Gelatinase-associated Lipocalin (NGAL) enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN).
Patient Biopsy and Surgical Resected Tissues
As described previously,3 we studied biopsy samples from 8 CD patients with colonic lesions. The profiles of CD patients are summarized in Table 1. In all cases, the patients had, in addition to co-existing stenosis, intra-abdominal or perianal fistula, and/or an abscess, during the disease course. Four patients had mucosal ulcerations, as determined by endoscopic evaluation. The Fukuoka University Hospital Ethics Committee approved the protocol, and written informed consent was obtained from all patients. After undergoing colonoscopies, 2 paired biopsy samples were obtained from each patient, 1 from stenotic areas and the other from nonstenotic areas 3 cm away from each stenotic area. Biopsy samples and surgically resected tissues for histologic evaluation were fixed in 10% buffered formalin. Samples for RNA quantitation were stabilized in RNAlater solution (Life Technologies) and homogenized for total RNA isolation. TaqMan real-time reverse-transcription PCR (RT-PCR) was performed on these samples (n = 8).3
Table 1.
Summary of the Profiles of CD Patients Whose Clinical Tissues Were Used to Compare Stenosis and Nonstenosis
| Patient number | Age, y | Sex | Diagnosis | Stenosis area | Severity of stenosis | Clinical history | Past treatment | Sampling |
|---|---|---|---|---|---|---|---|---|
| 01 | 22 | M | CD, small and large intestine type | Sigmoid colon | Moderate | 10 | Anti-TNFα+AZA | Endoscope |
| 02 | 29 | M | CD, small and large intestine type | Anastomosis | Moderate–severe | 8 | Anti-TNFα | Endoscope |
| 03 | 43 | F | CD, small and large intestine type | Transverse colon | Moderate–severe | 3 | Anti-TNFα+AZA | Endoscope |
| 04 | 33 | M | CD, large intestine type | Ascending colon | Moderate | 8 | Anti-TNFα | Endoscope |
| 05 | 51 | M | CD, large intestine type | Descending colon | Moderate | 5 | Anti-TNFα | Endoscope |
| 06 | 40 | M | CD, small and large intestine type | Sigmoid colon | Moderate–severe | 15 | Anti-TNFα | Endoscope |
| 07 | 49 | M | CD, small and large intestine type | Sigmoid colon, anastomosis | Severe | 26 | Anti-TNFα | Surgery |
| 08 | 51 | M | CD, small and large intestine type | Ascending colon | Severe | 24 | TPN, Mesalazine | Surgery |
NOTE. Age, sex, diagnosis, stenosis area, severity of stenosis, clinical history, past main treatment, and sampling methods are listed.
AZA, azathioprine; TPN, total parenteral nutrition.
Statistical Analysis
The results of electrophysiological study, quantitative PCR, Western blot, and [Ca2+]i measurement are expressed as the means ± SEM. Experimental protocols were repeated with at least 4 different batches of cells under each condition, and pooled data were averaged and subjected to statistical analyses. Statistically significant differences among groups were evaluated by analysis of variance (1-way and 2-way analysis of variance), and the Dunnett test was used for multiple comparisons where appropriate. P values <.05 were considered statistically significant.
Results
Functional Expression of TRPA1 Channel in InMyoFibs
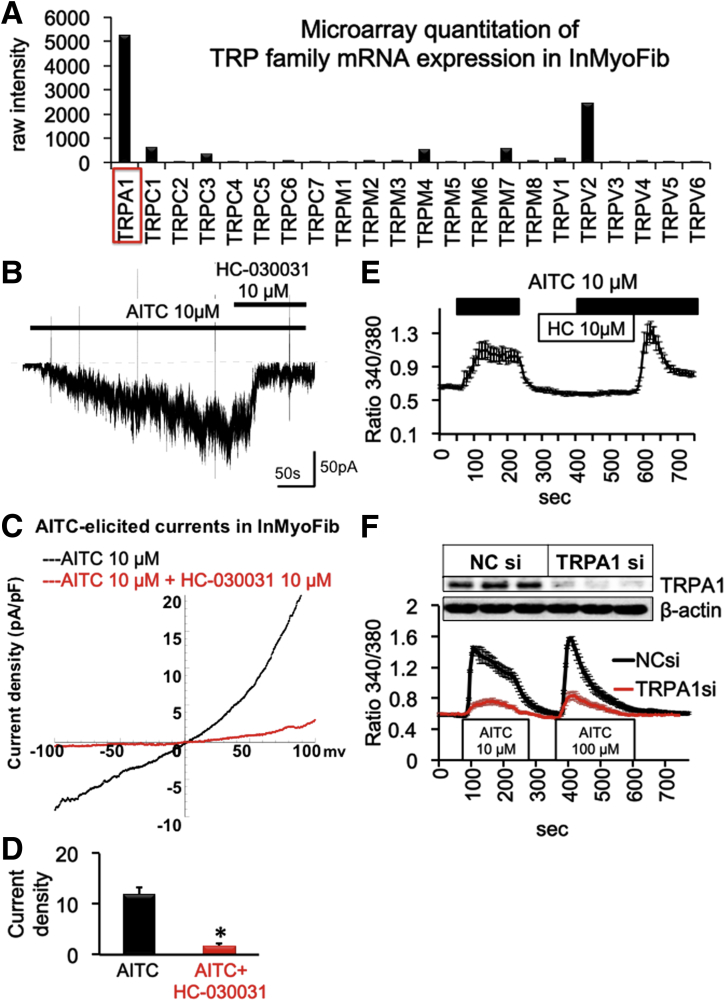
Microarray quantitation of InMyoFibs indicated that the mRNA level of TRPA1 was highest among TRP family members (Figures 1A and 2). Consistent with this result, whole-cell patch clamp experiments showed robust induction of a nonselective cation current in response to the potent TRPA1 agonist AITC (10 μmol/L) in InMyoFibs (Figure 1B). The AITC-induced current showed typical features of TRPA1 channel, that is, prominent outward rectification (Figure 1C) and potent blockade by the TRPA1-selective antagonist HC-030031 (10 μmol/L) (Figure 1D). The same concentration of this compound nearly completely suppressed the intracellular Ca2+ increase ([Ca2+]i) elicited by AITC (Figure 1E). To further confirm that the responses induced by AITC represented TRPA1 channel activities, we specifically knocked down TRPA1 in InMyoFibs, using an siRNA strategy. As shown in Figure 1F, 24-hour treatment with TRPA1-targeting siRNA in InMyoFibs resulted in substantial elimination of TRPA1 protein expression as well as AITC-induced [Ca2+]i increase. These results indicate that InMyoFibs express functional TRPA1 channels that permeate Ca2+.
Figure 1.
(A) Microarray quantitation of TRP family mRNA expression and (B–D) electrophysiological characterization of TRPA1 currents in InMyoFibs cells. (B) Representative traces of whole-cell currents recorded from InMyoFibs cells at a holding potential of -60 mV. TRPA1 nonselective cation currents are activated by AITC (10 μmol/L) and inhibited by HC-030031 (10 μmol/L). (C) Current–voltage relationships of TRPA1 currents after application of AITC and HC-030031. (D) Peak current density of inward currents (-60 mV) after application of AITC and HC-030031 (n = 4) *P < .01. (E) Representative [Ca2+]i responses in InMyoFibs cells. Cells were exposed to AITC (10 μmol/L), and TRPA1 antagonist HC-030031 (10 μmol/L) was added to examine whether Ca2+ influx is involved in TRPA1 channels. (F) Representative [Ca2+]i responses in Negative control siRNA (NCsi) and TRPA1 siRNA (TRPA1si)-pretreated InMyoFibs cells. Knockdown of TRPA1 was confirmed by Western blot (upper panel). The vertical axis indicates the magnitude of Ca2+ influx expressed as the 340/380 fluorescence ratio. Data points represent the means ± SEM from > 30 cells.
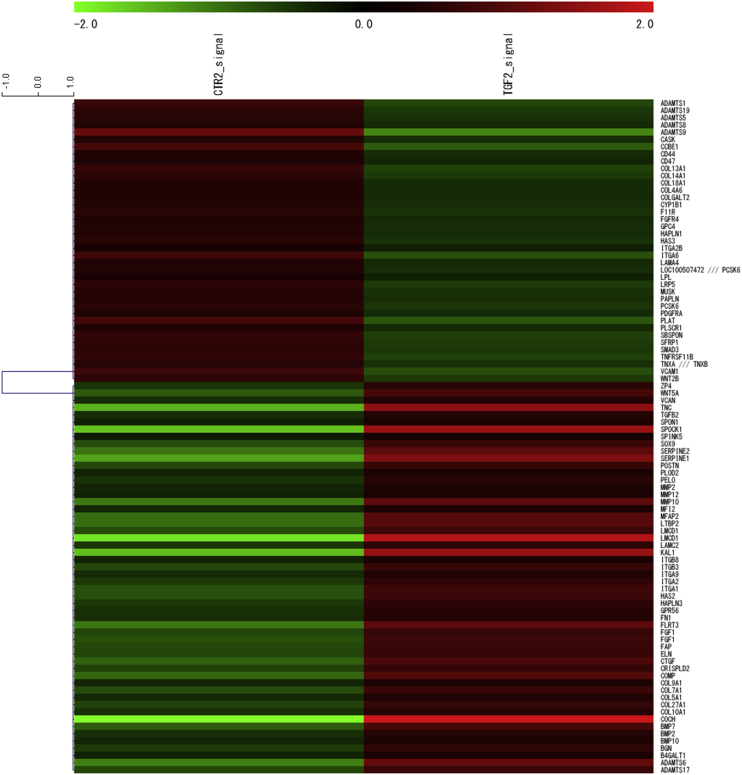
Figure 2.
Fold-change heat map of fibrosis-associated molecule mRNAs in InMyoFibs by expression array analysis. Control vs TGF-β1-treated (5 ng/mL, 24 h) InMyoFibs. The criteria for regulated genes were as follows: up-regulated genes, Z-score ≥ 2.0 and ratio ≥ 1.5-fold; down-regulated genes, Z-score ≤ -2.0 and ratio ≤ 0.66.
TRPA1 Channel Activation Attenuates TGF-β1–Induced Stress-Fiber Formation and Collagen Synthesis
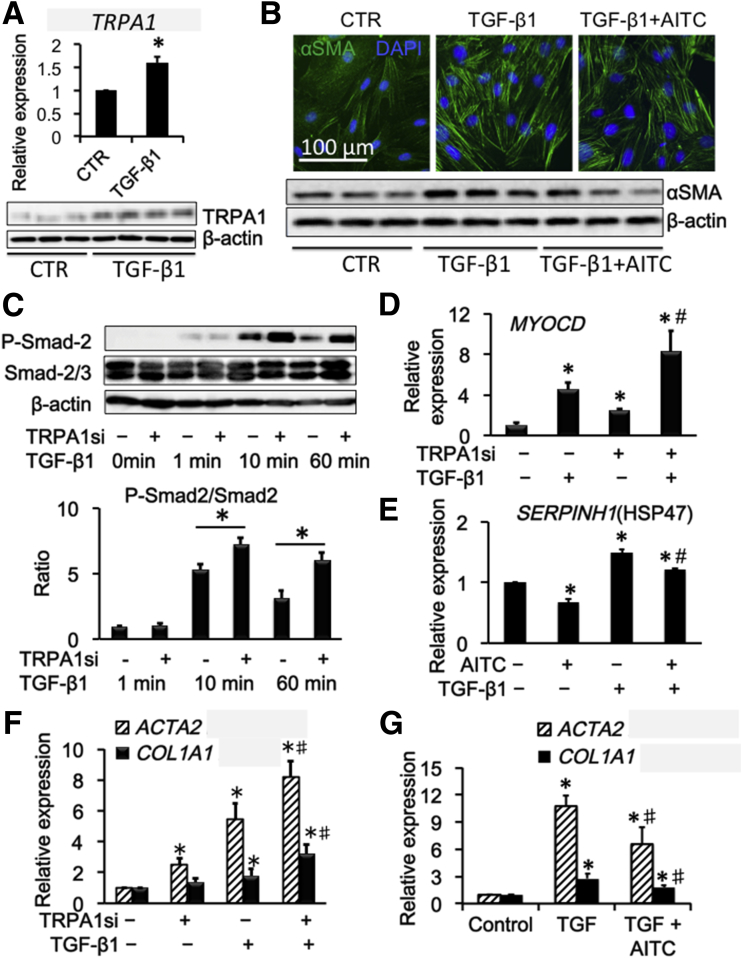
We then explored the possible contribution of TRPA1 to fibrotic changes induced by TGF-β1 treatment. Twenty-four–hour treatment of InMyoFibs with 5 ng/mL TGF-β1 enhanced the expression of TRPA1 mRNA and protein (Figure 3A). Concomitantly, TGF-β1 facilitated stress fiber formation and protein expression of α-SMA, which were suppressed effectively by co-treatment with 10 μmol/L AITC (Figure 3B). Analyses of the downstream cascade of TGF-β1–meditated signaling showed that increased mRNA expression of the master transcription regulator MYOCD by TGF-β1 was enhanced further by TRPA1 knockdown, with increased phosphorylation of Smad-2 (Figure 3C and D). In addition, TRPA1 agonist AITC effectively suppressed the up-regulation of HSP47 by TGF-β1 (Figure 3E). siRNA knockdown of TRPA1 enhanced the expression of α-SMA (ACTA2) and collagen type 1A1 (COL1A1) both in the presence and absence of TGF-β1 (Figure 3F). TGF-β1 treatment also increased the mRNA expression of ACTA2 and COL1A1, which were antagonized by AITC (Figure 3G). These results collectively suggest that TRPA1 activity negatively regulates TGF-β1–meditated, fibrosis-associated signaling in InMyoFibs.
Figure 3.
Antifibrotic drugs induced Ca2+influx via TRPA1 in InMyoFibs. (A) Relative quantification of TRPA1/18S ribosomal RNA mRNA expression (upper panel) and Western blot (lower panel) of control (CTR) and TGF-β1–stimulated (5 ng/mL, 24 h) InMyoFib cells. (B) Immunostaining images of InMyoFibs cells stained with anti–α-SMA (green) antibody and 4′,6-diamidino-2-phenylindole (DAPI) (blue) in untreated controls, after 24-hour TGF-β1 (5 ng/mL) or TGF-β1 (5 ng/mL)+AITC (10 μmol/L) treatment (upper panel), and Western blot of α-SMA and β-actin were performed (lower panel). (C) TGF-β1–induced phosphorylation of Smad-2 was measured by Western blot, with or without TRPA1-siRNA pretreatment. The ratios of phosphorylated/unphosphorylated Smad-2 proteins in TGF-β1–treated cells are shown. (D and E) Myocardin (MYOCD) and HSP47 (SERPINH1) mRNA expression by real-time PCR in Negative control siRNA (NCsi) and TRPA1 siRNA (TRPA1si)-pretreated InMyoFibs cells. (F) Relative quantitation of COL1A1 and ACTA2 mRNA expression by real-time PCR in NCsi and TRPA1si-pretreated InMyoFibs cells. (G) Relative quantitation of COL1A1 and ACTA2 mRNA expression, AITC (10 μmol/L) was co-administered with TGF-β1. *P < .05 vs control cells; #P < .05 vs TGF-β1–treated cells (n = 4).
Chronic TNBS Fibrosis Model of TRPA1-Knockout Mice
The Trpa1 knockout (KO) mice were generated by the CRISPR/Cas9 system (Figure 4). Chronic colitis was induced by repeated administration of TNBS over 6 weeks (Figure 5A). TNBS is known to be more suitable than dextran sulfate sodium for investigating the pathogenesis of fibrosis in inflammatory bowel disease because the former has been found to cause much slower and more moderate progression of gut inflammation and fibrosis in mouse colitis models.33, 34
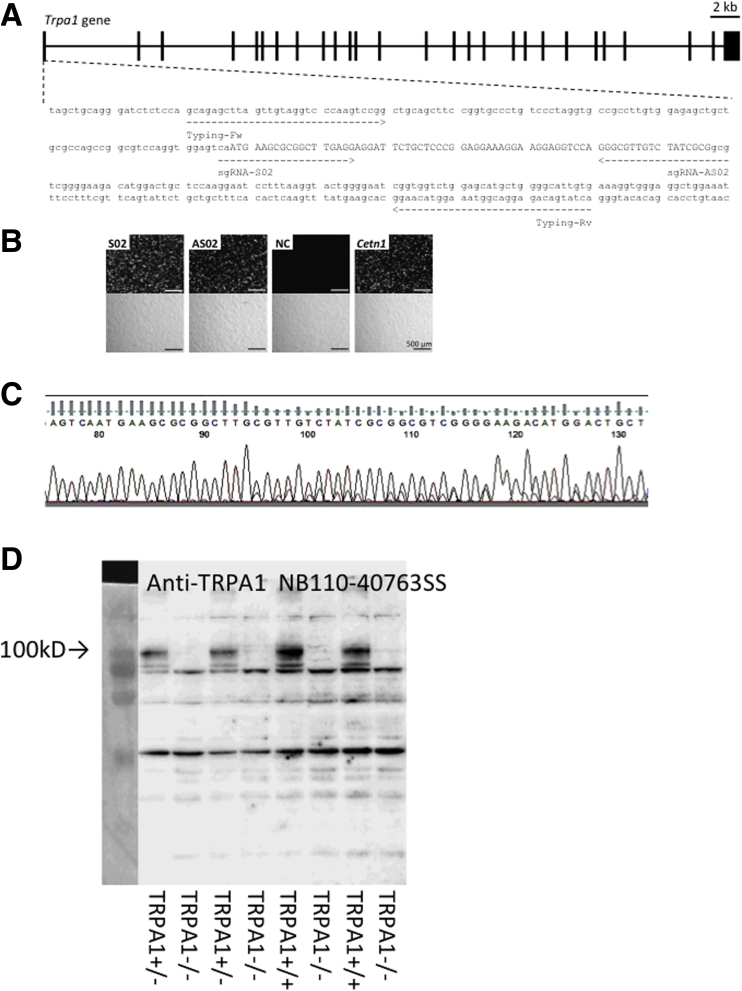
Figure 4.
CRISPR/Cas9-mediated Trpa1 gene mutation. (A) Complex of CAS9 and sgRNAs, S02 and AS02, recognized exon 1 of Trpa1 and induced genomic mutations. Indels introduced by the sgRNA/CAS9-expressing plasmid (pX330) were identified by PCR using the primer set, typing forward and reverse. The region of capital letters indicates the coding region of Trpa1. (B) The pCAG-EGxxFP-Trpa1 plasmid was co-transfected with pX330 plasmids containing sgRNA sequences, S02 and AS02, into HEK293T cells. Reconstituted enhanced green fluorescent protein (EGFP) fluorescence was observed at 48 hours after transfection. Fluorescence intensity was compared with the pCAG-EGxxFP-Cetn1 and pX330-Cetn1/sgRNA1 as the positive control. Transfection of pCAG-EGxxFP-Trpa1 and pX330 without sgRNA was used as the negative control (NC). Generation of Trpa1 mutant mice was injected with the circular pX330 plasmid with sgRNA-S02 and sgRNA-AS02. (C) Genotype of Trpa1 mutant mice by sequence analysis. Raw data of DNA sequence of TRPA1+/- mouse is shown. (D) TRPA1 knockout was analyzed by Western blot using anti-TRPA1 antibody NB110-40763SS.
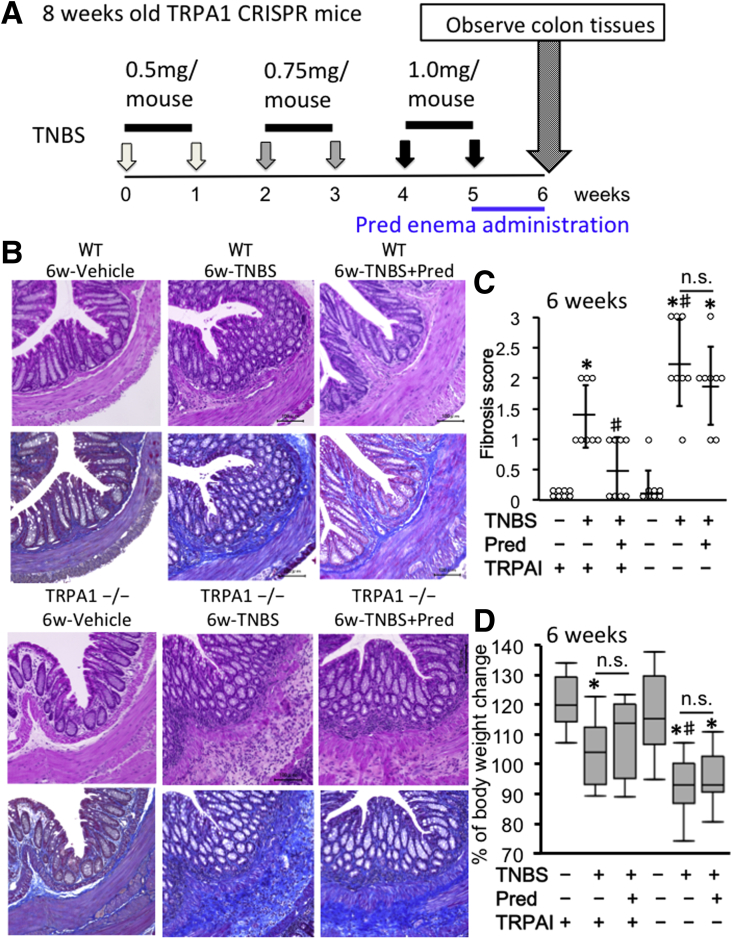
Figure 5.
Validation of chronic TNBS-induced colitis in WT and TRPA1-/-CRISPR mouse. Chronic colitis was induced by weekly intrarectal injection of TNBS for 6 weeks. (A) TNBS injection scheme. (B) H&E- and MT-stained tissues in vehicle, TNBS, and TNBS+Prednisolone (Pred) groups of WT and TRPA1-/- mice. In the TNBS sections, chronic active inflammation and fibrosis. (C) Histologic fibrosis score from 0 (no fibrosis) to 3 (severe fibrosis) was shown in dot plot graph. The leftmost circles (n = 8) show absolutely zero values. Values are the means ± SD. (D) Body weight change in each experimental group was shown in box plot graph. *P < .01 vs WT vehicle; #P < 0.01 vs WT TNBS (n = 8).
At 6 weeks of TNBS treatment, as compared with WT mice, TRPA1-KO mice treated with TNBS showed severer signs of inflammation/fibrosis characterized by prominent cell infiltration in some mucosal and submucosal layers and lower body weight increase (Figure 5B–D). To assess the total inflammation level, we quantified fecal lipocalin-2 by enzyme-linked immunosorbent assay (Figure 6A). The level of fecal lipocalin-2 significantly increased by repeated TNBS administration. At 5 and 6 weeks of TNBS administration when fibrosis occurred mildly and moderately/severely, respectively, increases in fecal lipocalin-2 level by TNBS treatment were not statistically different between WT and TRPA1-KO mice by 2-way analysis of variance. In TRPA1-KO mice treated with TNBS for 6 weeks, mucosal destruction was occasionally observed in a few parts of severely inflamed areas of intestine (Figure 6B).
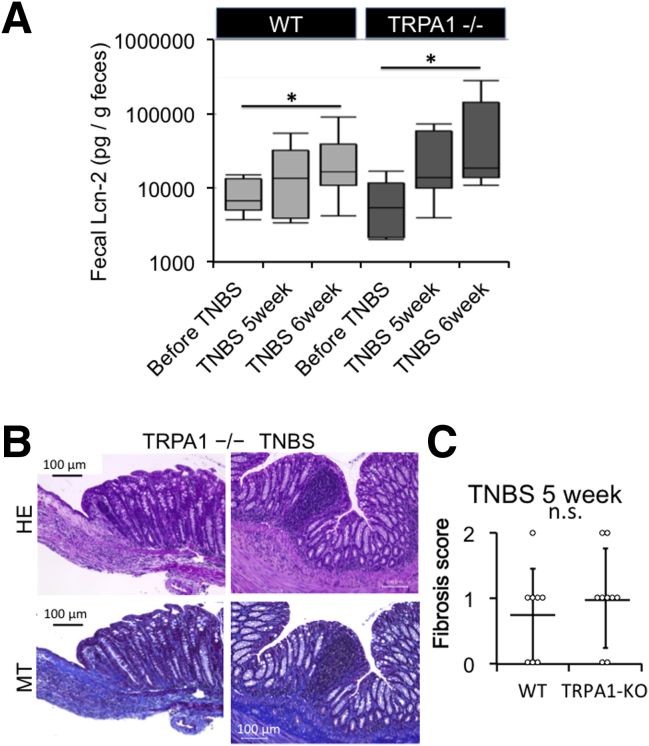
Figure 6.
Support data of inflammation and fibrosis. (A) Box plots of fecal lipocalin-2 level quantified using an enzyme-linked immunosorbent assay kit. Fecal lipocalin (Lcn-2) levels were measured before and 5 or 6 weeks after TNBS administration, in WT mouse and TRPA1-/- mouse before TNBS treatment. TNBS 5 weeks (just before last treatment), TNBS 6 weeks, respectively. By using 2-way analysis of variance with TNBS treatment and TRPA1 knockout as 2 independent variables, only the former turned out to be a statistically significant factor (∗P < .05, n = 6–8). (B) Severe inflammation and fibrosis in TNBS-treated TRPA1–CRISPR knockout mouse. H&E-stained and MT-stained TRPA1-/- CRISPR mouse intestine. (C) Histologic fibrosis score from 0 (no fibrosis) to 3 (severe fibrosis) of WT and TRPA1-/- mouse before last TNBS challenge (TNBS 5 weeks) was shown in dot plot graph (n = 8). Values are means ± SD.
At 5 weeks of TNBS treatment (just before prednisolone administration), no statistically significant differences could be detected in the extent of fibrosis among WT and TRPA1-KO mice (Figures 6C and 7), probably because of mild and slow progression of fibrotic changes. However, after 6 weeks, fibrosis scores assessed by histopathologic examination were significantly higher in TRPA1-KO than in WT mice (P < .01 for both) (Figure 5B and C), with pronounced extension of collagen fibers into the submucosal layer (Figures 5B and 6B). Just after the final TNBS treatment (ie, at 5 weeks TNBS treatment), we administered a prednisolone enema for 1 week. With this procedure, the fibrosis score was remarkably reduced in WT mice, whereas no change was observed in TRPA1 KO mice (Figure 5B and C). Overall views of MT staining at 1–4 cm from the anus of vehicle- or TNBS-treated mice (vehicle 6 weeks, TNBS 5 weeks and 6 weeks) are shown in Figure 7. These observations strongly suggest that TRPA1 channel activity is crucial for counteracting the inflammatory/fibrogenic responses in chronic colitis.
Figure 7.
An overall view of MT staining of 1 cm, 2 cm, 3 cm, and 4 cm from the anus of the vehicle mouse or TNBS mouse is shown. Evaluation of fibrosis was performed with a stained image 1 cm from the anus.
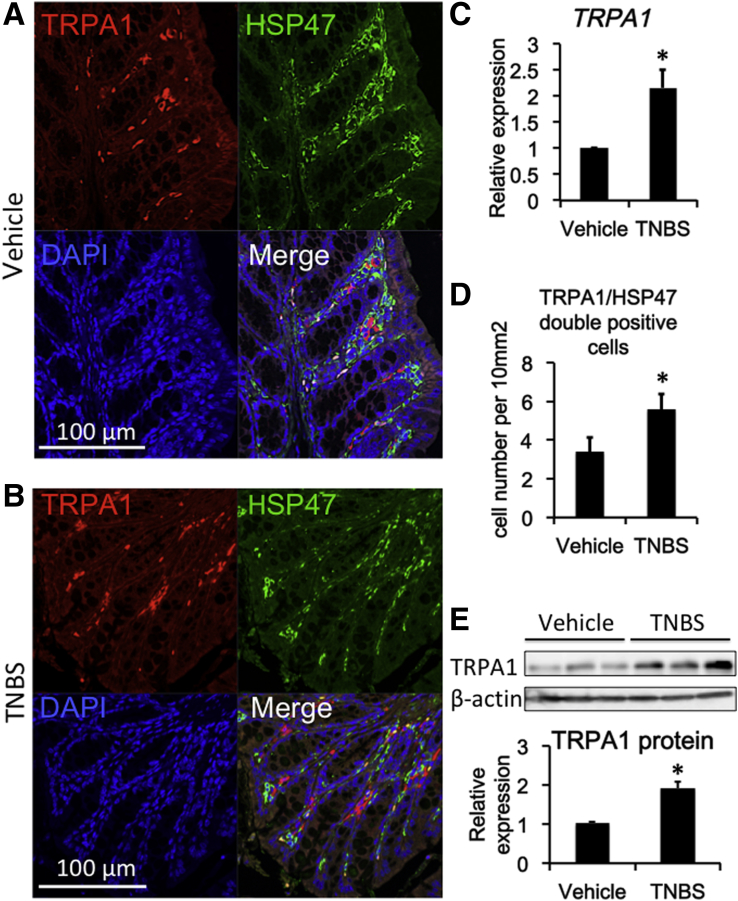
Double-immunostaining experiments indicated a high co-incidence of immunoreactivities against TRPA1 and HSP47, an endoplasmic reticulum-resident molecule essential for correct procollagen folding (Figure 8A and B). TNBS treatment significantly increased the expression level of TRPA1 mRNA in wild-type mouse colons (P < .05 for TNBS vs vehicle group) (Figure 8C). The number of TRPA1/HSP47 double-positive cells per unit area greatly increased in the TNBS-treated group compared with in the vehicle groups (Figure 8D). The protein level of TRPA1 was significantly up-regulated by TNBS treatment (Figure 8E).
Figure 8.
Co-localization of TRPA1 channel and HSP47 in mouse intestine. (A) Vehicle, (B) TNBS: Immunostaining images of mouse intestine stained with anti-TRPA1 (red) antibody, anti-HSP47 (green), and 4′,6-diamidino-2-phenylindole (DAPI) (blue). (C) TRPA1 mRNA expression in vehicle and TNBS-treated colitis mouse (n = 5). (D) Number of TRPA1/HSP47 double-positive cells per unit area: 10 mm3 counted from immune-staining data (n = 5). (E) Western blot analysis of TRPA1 and β-actin in WT vehicle and WT TNBS mouse distal colon (n = 5). *P < .05 vs vehicle.
Steroids and Pirfenidone Induce Ca2+ Influx via TRPA1 and Suppress Collagen Synthesis in InMyoFibs
In our ongoing project involving screening 103 types of food ingredients, we identified that the steroid-like active principle of licorice (glycyrrhetinic acid) has antifibrotic activity in InMyoFibs (see Introduction). As described earlier, steroids and pirfenidone are widely used antifibrotic drugs for various fibrotic diseases. We therefore hypothesized that the antifibrotic effects of these compounds may in part involve activation of the TRPA1 channel. To test this hypothesis, we first examined the Ca2+-mobilizing effects of these molecules on InMyoFibs.
As shown in Figures 9B, 10A, 11A, 11B, and 12A, licorice, and its active principle glycyrrhetinic acid, and steroids methylprednisolone, prednisolone, and pirfenidone, all evoked robust [Ca2+]i increases in InMyoFibs. These [Ca2+]i increases were comparable in magnitude with that evoked by 10 μmol/L AITC (Figure 1E), and were completely inhibited by prior application of the TRPA1-selective antagonist HC-03001, suggesting that the Ca2+-mobilizing effects of these compounds are mediated by TRPA1 channel activation. Application of methylprednisolone (100 μmol/L) and AITC (5 μmol/L) to HEK293 cells expressing TRPA1 also induced TRPA1 channel activities in the cell-attached patch clamp recording (membrane potential [Vm = –40 mV]) (Figure 11C).
Figure 9.
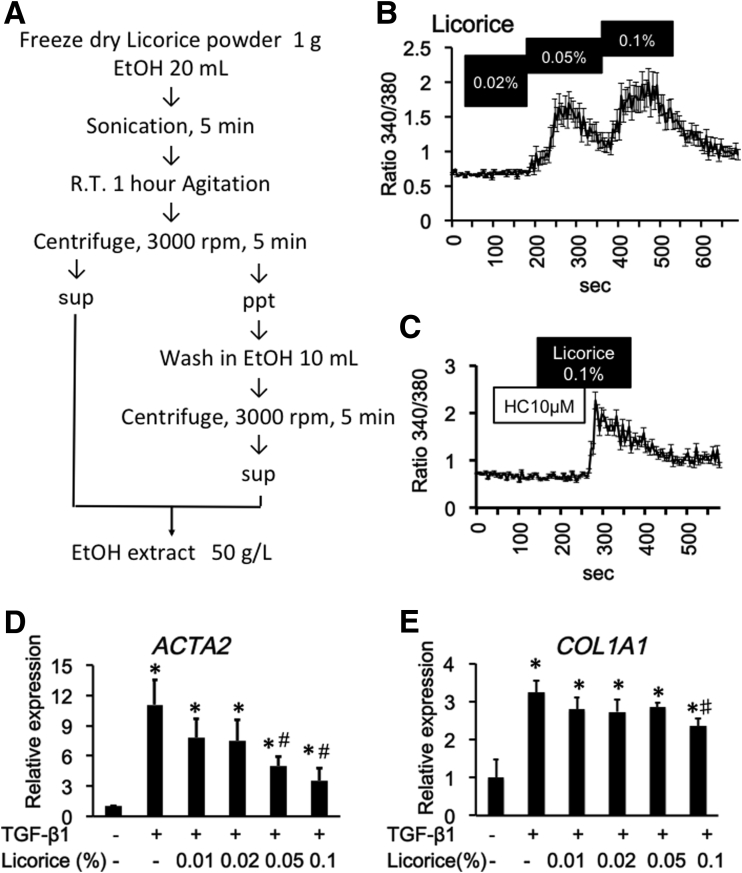
Licorice extracts activate the TRPA1 channel and suppresses TGF-β1–induced fibrosis mediators in InMyoFibs. (A) Method for extracting active ingredient from licorice by ethanol. (B and C) Licorice-induced Ca2+ influx in InMyoFibs. The vertical axis indicates the magnitude of Ca2+ influx expressed as the 340/380 fluorescence ratio. TRPA1 antagonist HC-030031 (10 μmol/L) was added to examine whether Ca2+ influx is involved in TRPA1 channels. Data points represent the means ± SEM from > 30 cells. (D and E) Real-time PCR analysis of mRNA expression levels of α-SMA and type Ⅰ collagen after treatment with TGF-β1 (5 ng/mL, 24 h). Licorice extracts 0.01%, 0.02%, 0.05%, and 0.1% were co-administered with TGF-β1. *P < .05 vs control cells; #P < .05 vs TGF-β1–treated cells (n = 4).
Figure 10.
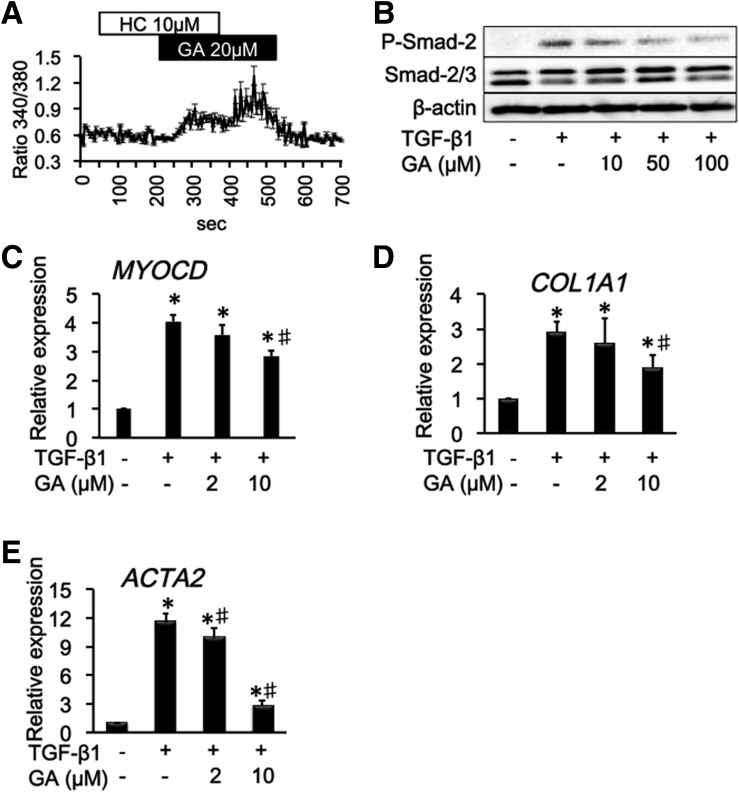
Glycyrrhetinic acid (GA) activates the TRPA1 channel and suppresses TGF-β1–induced fibrosis mediators in InMyoFibs. (A) GA induced Ca2+ influx in InMyoFibs. The vertical axis indicates the magnitude of Ca2+ influx expressed as the 340/380 fluorescence ratio. TRPA1 antagonist HC-030031 (10 μmol/L) was added to examine whether Ca2+ influx is involved in TRPA1 channels. Data points represent the means ± SEM from > 30 cells. (B) TGF-β1–induced phosphorylation of Smad-2 was measured by Western blot, with or without GA pretreatment. (C–E) Real-time PCR analysis of mRNA expression levels of MYOCD, α-SMA, and type Ⅰ collagen after treatment with TGF-β1 (5 ng/mL, 24 h). GA was co-administered with TGF-β1. *P < .05 vs control cells; #P < .05 vs TGF-β1–treated cells (n = 4).
Figure 11.
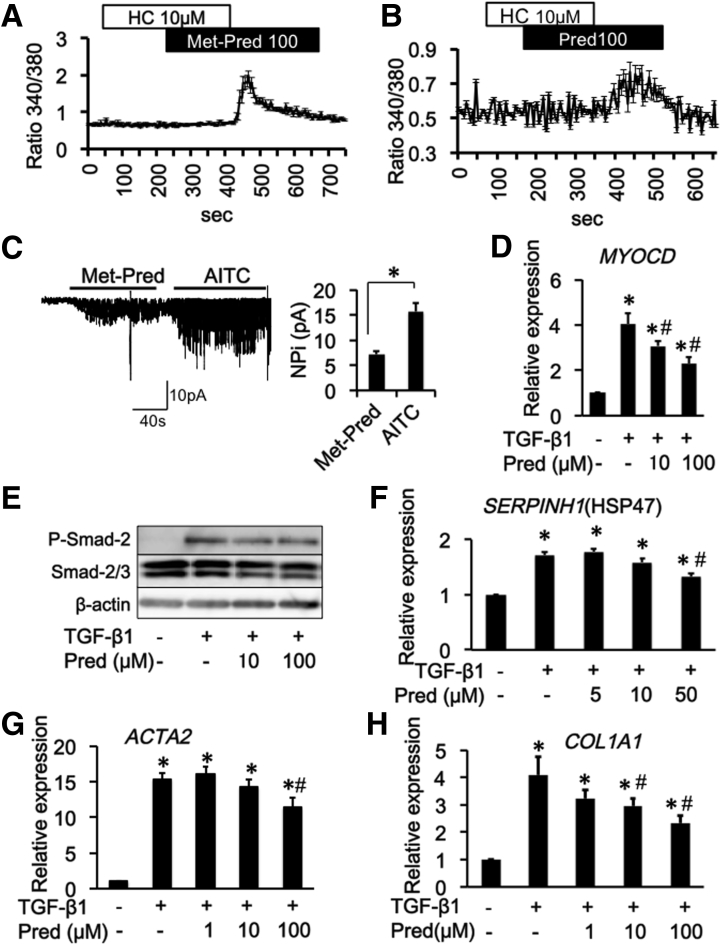
Steroids activate the TRPA1 channel and suppress TGF-β1–induced fibrosis mediators in InMyoFibs. (A and B) Representative [Ca2+]i responses in InMyoFibs cells. Cells were exposed to steroids, TRPA1 antagonist HC-030031 (10 μmol/L) was added. Data points represent the means ± SEM from > 30 cells. (C) Activated single-channel currents in TRPA1 expressed HEK293 cells. Representative traces of cell-attached recorded at a holding potential of –40 mV. The averaged density of single ITRPA1 at –40 mV (expressed as NPi) by application of Met-pred (100 μmol/L) and AITC (10 μmol/L) (n = 6). P < .01. (D–H) Predonisolone (Pred) was co-administered with TGF-β1 in InMyoFib cells. (D) Myocardin (MYOCD) mRNA expression, (E) TGF-β1–induced phosphorylation of Smad-2 was measured by Western blot, (F–H) HSP47 (SERPINH) and type 1 collagen (COL1A1), and α-SMA (ACTA2) mRNA expression measured by real-time PCR in InMyoFibs cells. *P < .05 vs control cells; #P < .05 vs TGF-β1–treated cells (n = 4). Met-Pred, methylprednisolone.
Figure 12.
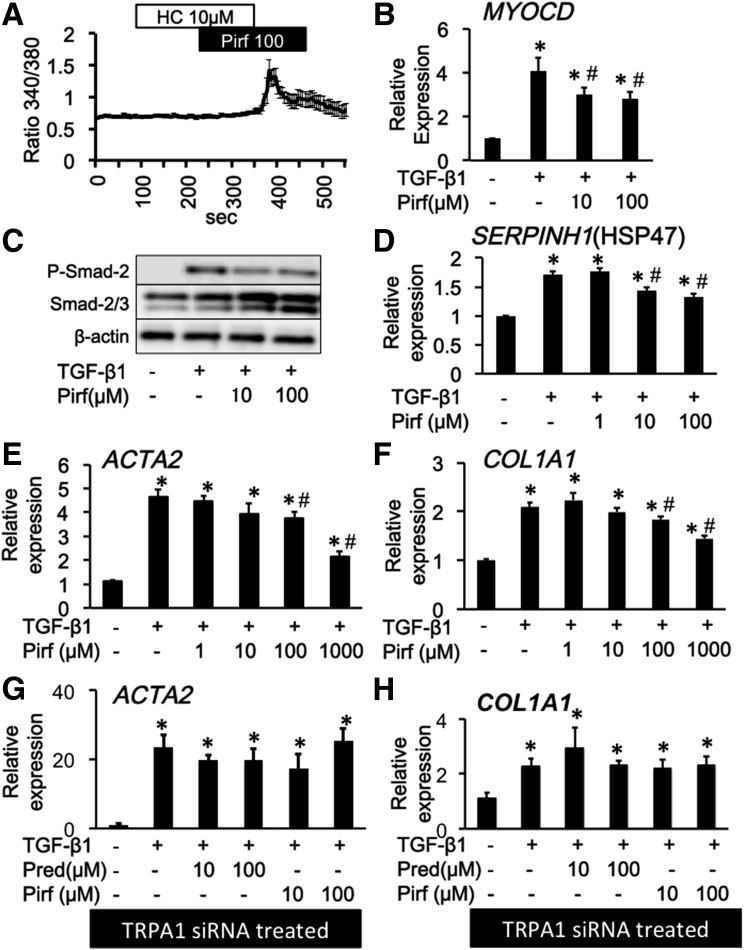
Pirfenidone activates the TRPA1 channel and suppresses TGF-β1–induced fibrosis mediators in InMyoFibs. (A) Representative [Ca2+]i responses in InMyoFibs cells. Cells were exposed to pirfenidone (Pirf), TRPA1 antagonist HC-030031 (10 μmol/L) was added. Data points represent the means ± SEM from > 30 cells. (B–F) Pirfenidone was co-administered with TGF-β1 in InMyoFib cells. (B) Myocardin (MYOCD) mRNA expression, (C) TGF-β1–induced phosphorylation of Smad-2 was measured by Western blot, (D–F) HSP47 (SERPINH) and type 1 collagen (COL1A1) and α-SMA (ACTA2) mRNA expression measured by real-time PCR in InMyoFibs cells. *P < .05 vs control cells; #P < .05 vs TGF-β1–treated cells (n = 4). (G–H) Type 1 collagen (COL1A1) and α-SMA (ACTA2) mRNA expression measured by real-time PCR in TRPA1 siRNA-pretreated InMyoFibs cells. *P < .05 vs TRPA1 siRNA-treated control cells.
We found that the traditional herbal medicine licorice can activate the TRPA1 channel and inhibit TGF-β1–induced α-SMA and type 1 collagen production (Figure 9D and E). In real-time RT-PCR analyses of InMyoFibs, glycyrrhetinic acid, prednisolone, and pirfenidone dose-dependently inhibited the enhanced mRNA expression of ACTA2 and COL1A1 by TGF-β1 (Figures 10D, 10E, 11G, 11H, 12E, and 12F). The maximum extent of this inhibition was similar to that produced by AITC (Figure 3G). Importantly, these inhibitory effects of prednisolone, pirfenidone, glycyrrhetinic acid, and AITC were well-correlated with suppression of Smad-2 phosphorylation and HSP47 and MYOCD expression induced by TGF-β1 treatment (Figures 3C–E, 10B and C, 11D–F, and 12B–D). However, in TRPA1-siRNA–treated InMyoFibs, the enhanced mRNA expression of COL1A1 and ACTA2 by TGF-β1 were no longer suppressed by prednisolone and pirfenidone (Figure 12G and H).
Enhanced TRPA1 Expression in Stenotic Tissues of CD Patients
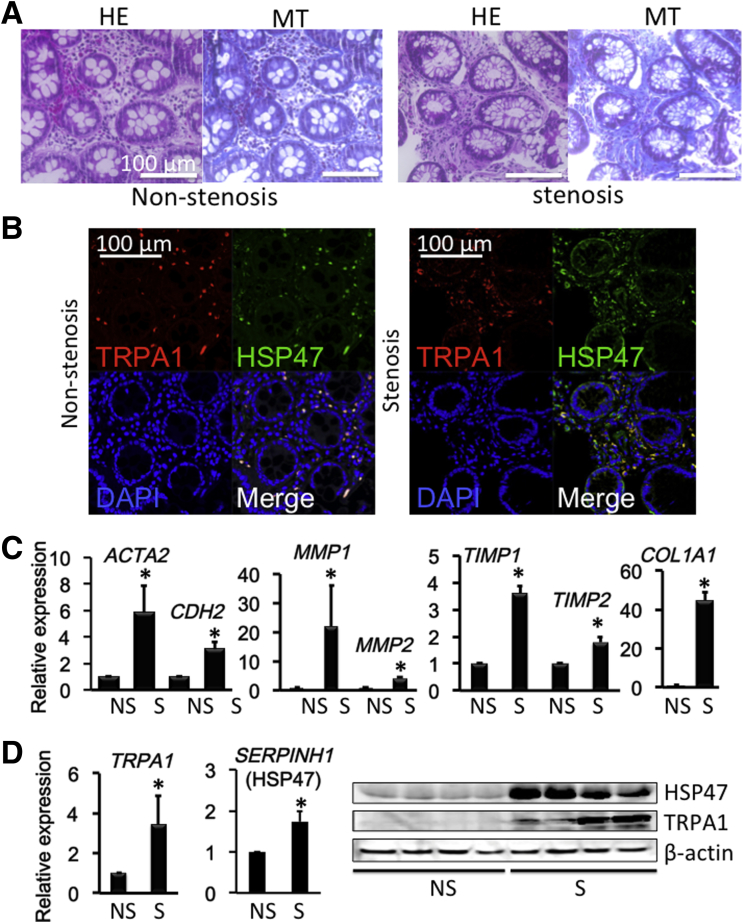
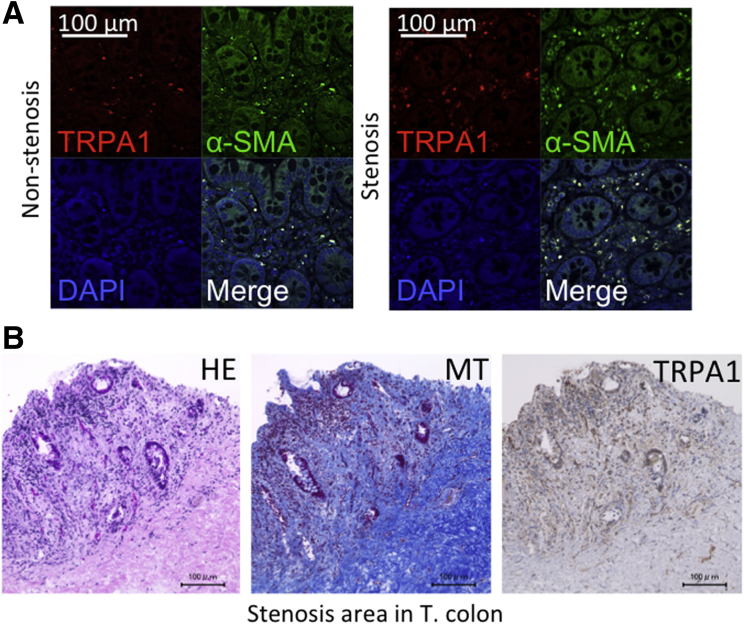
Because intestinal stricture formation in CD is driven by local excessive accumulation of myofibroblasts,2, 35 we examined whether TRPA1 is up-regulated in highly fibrotic areas of human CD patients. Biopsy samples obtained from the patients’ intestines were subjected to histologic examination, immunostaining, and real-time RT-PCR to detect fibrosis-associated molecules. Histologic examination with H&E and MT staining showed much denser deposition of collagen fibers in the mucosal layer of the fibrotic stenotic area compared with in the nonstenotic area (Figure 13A). Moreover, double-immunostaining of biopsy tissues showed that the number of TRPA1/HSP47 double-positive cells in the mucosal layer of the stenotic area appeared larger than that in the nonstenotic area (Figure 13B). As summarized in Figure 13C, the mRNA expression of TRPA1 and HSP47 were significantly enhanced in stenotic compared with nonstenotic regions. In agreement with this, the mRNA levels of fibrosis factors, that is, ACTA2, CDH2, COL1A1, COL3A1, matrix metalloproteinase 1 (MMP1), MMP2, tissue inhibitor of metalloproteinase 1, and tissue inhibitor of metalloproteinase 2 were much higher in stenotic than nonstenotic areas of CD patients (Figure 13C).3 Increased mRNA and protein expression of TRPA1 and SERPINH1 (HSP47) in the stenotic area was observed (Figure 13D). TRPA1/α-SMA double-positive cells suggested TRPA1 expressed intestinal myofibroblast (Figure 14A); TRPA1 signals detected by 3,3′-diaminobenzidine tetra hydrochloride staining were distributed more diffusely in the mucosal and submucosal layers of stenotic regions in the intestines of CD patients (Figure 14B).
Figure 13.
Colonoscopy and biopsy sampling was performed and showed a fibrotic lesion responsible for colon stenosis. (A) H&E-stained and MT-stained CD patient biopsy specimens of nonstenotic or stenotic intestinal areas. (B) Immunostaining of TRPA1/HSP47 in nonstenosis and stenosis biopsy samples. (C) mRNA levels (n = 8) of ACTA2 (α-SMA), CDH2 (N-cadherin), MMP1, MMP2, tissue inhibitor of metalloproteinase 1 (TIMP1), TIMP2, and COL1A1 mRNA in biopsy specimens were examined by real-time RT-PCR in nonstenotic or stenotic inflamed mucosal tissues of CD patients. (D) TRPA1 and HSP47 mRNA (n = 8) and protein (n = 4) levels were examined in nonstenotic or stenotic inflamed mucosal tissues of CD patients. *P < .05 vs nonstenotic sample (16 paired samples obtained from 8 patients). DAPI, 4′,6-diamidino-2-phenylindole.
Figure 14.
TRPA1 localization in CD patient intestine. (A) Immunostaining of TRPA1/α-SMA in nonstenosis and stenosis biopsy samples. (B) H&E-, MT-, and TRPA1–3,3′-diaminobenzidine tetra hydrochloride (DAB)–stained stenosis surgical tissue from a CD patient transverse colon. DAPI, 4′,6-diamidino-2-phenylindole. T. colon, transverse colon.
Discussion
Activation of TGF-β–mediated signaling pathways underlies the stenotic complications of CD patients. However, simple neutralizing therapy targeting this signaling is not beneficial and can even exacerbate the inflammatory response.8 In this respect, the present study provides 2 novel and clinically important insights. First, in both in vitro and in vivo experiments as well as in human biopsy sample examination, the obtained evidence strongly supports that increased activity of TRPA1 channels in myofibroblasts protects against intestinal fibrogenesis. Second, we found that TRPA1 channel activation underlies the antifibrotic effects of steroids, pirfenidone, and licorice (and its active principle glycyrrhetinic acid). These conclusions are supported by several different observations. First, TRPA1 mRNA was highly expressed in myofibroblasts and remarkably up-regulated in stenotic regions of human CD patient intestines. Second, TNBS-treated mice showed stromal fibrosis in the mucosal and submucosal layers of colon with active inflammatory cell infiltration. Fibrosis scores assessed by using this colitis model were significantly higher in TRPA1-KO mice compared with wild-type mice, and 1-week prednisolone treatment after the last TNBS administration suppressed fibrosis changes only in wild-type mice. Third, pirfenidone and steroids were able to elicit Ca2+ influx via TRPA1 channel activation with comparable efficacies to the TRPA1-selective agonist AITC. These compounds also counteracted TGF-β–induced fibrotic changes (ie, reduced the expression of fibrosis markers) and suppressed HSP47 expression in InMyoFibs. Fourth, TRPA1/HSP47 double-positive cells accumulated in the submucosal layer of intestines of both CD patients and TNBS mice. In the stenotic area of CD patients, many cells were doubly immunopositive for TRPA1 and HSP47 in the submucosal layer, where numerous collagen fibers were stained and fibrosis markers were significantly up-regulated. Notably, both TRPA1 and HSP47 are known to be activated not only by temperature increases, but also by many other physicochemical stimuli. Overall, these findings support the antifibrotic actions of steroids, pirfenidone, and possibly licorice or its active principle glycyrrhetinic acid in intestinal myofibroblasts and indicate their therapeutic potential for CD-associated intestinal stenosis.
However, TRPA1 channel activation in the digestive tract clearly causes anti-inflammatory actions by modifying immune responses. Bertin et al20 reported that TRPV1-mediated CD4+–T-cell activation and the resultant colitogenic response were restrained by TRPA1 activity in the same cell, ultimately retarding fibrotic processes. Although this mechanism may have affected our experiments to some extent, the results of the present study clearly showed an additional mechanism operating through myofibroblast TRPA1 activation. This mechanism prevents fibrogenesis during the sustained phase of inflammatory colitis, as indicated by the fact that steroids introduced in the fifth week of the 6-week TNBS administration protocol (which should have already developed fibrosis) was sufficient for abrogating fibrotic changes, likely via TRPA1 activation.
Previous studies have shown that TRPA1 participates in tissue inflammation and remodeling in various manners.15 TRPA1 is functional in both neuronal and non-neuronal cells to modulate local tissue inflammation in animal models of acute pulmonary inflammation induced by acrolein and cigarette smoke, experimental colitis, or carrageenan-induced paw edema.13, 16, 36 In mouse corneal stroma, TRPA1 is required for TGF-β signaling, and its genetic deletion abrogates inflammatory fibrosis.37 Wound healing is a physiological process triggered by inflammation that may lead to tissue repair with reconstitution of normal morphology and function or fibrotic replacement depending on the balance between the production and degradation of extracellular matrix proteins.38, 39 Thus, the negative regulation of intestinal TGF-receptor–mediated profibrotic signaling by myofibroblast TRPA1 identified in the present study also may be involved in such multifaceted actions of TRPA1 in tissue inflammation/remodeling.
Steroids are widely used to treat fibrotic disorders. However, direct molecular targets of their antifibrotic effects remain unclear. The therapeutic concentrations of steroids for fibrotic diseases are thought to be much higher than those required to saturate the glucocorticoid receptors (genomic effects).40 This raises the question of whether there is an additional target(s) for the nongenomic effects of glucocorticoids. The results of the present study clearly show that steroids (and pirfenidone) target TRPA1, the activation of which leads to the negative regulation of TGF-β1–Smad signaling and eventually the down-regulation of antifibrotic factors such as collagen type I and α-SMA (Figures 9, 10, 11, and 12). However, our previous study showed that activation of the myofibroblast TRPC6 channel also can suppress fibrotic changes by inhibiting TGF-β1–Smad signaling via the calcineurin-mediated pathway.3 This raises the question of whether TRPA1 and TRPC6 mediate myofibroblast fibrotic signaling in a redundant manner. However, the process mediated by TRPC6 requires a longer time than TRPA1 because the former is critically dependent on up-regulation of TRPC6 expression by TGF-β1.3 In contrast, the TRPA1 channel is constitutively active in the gut and further up-regulated by TGF-βl stimulation. This indicates that targeting of the TRPA1 channel may be prophylactic against and/or beneficial in an earlier stage of inflammatory fibrosis as compared with TRPC6. According to the recent study of TRPA1 structure, the pre-S1 region of N-terminal domain would be suited to detect reactive chemical agonists and interact with the TRP-like domain causing allosteric modulation. Thus, through a similar mechanism, steroid may induce a conformational change of TRPA1 channel leading to channel opening.
The higher prevalence of fibrosis in CD is likely the consequence of transmural bowel inflammation that exposes all mesenchymal cells capable of producing the extracellular matrix to fibrotic mediators.41 The findings from stenotic and nonstenotic biopsy samples suggest that the same mechanism as that involved in InMyoFib may operate in the stenotic lesion of intestines from CD patients. Of the 8 CD patients with stenotic lesions investigated in the present study, 7 patients already received anti–tumor necrosis factor (TNF) therapy. Although steroids and pirfenidone should be the first-line therapy in patients with mild-to-moderate CD without poor prognostic factors or complicated disease, anti-TNF treatment is regarded as a better choice for CD patients with complications or bowel damage with poor prognostic factors and/or severe disease.42 However, anti-TNF treatment may worsen stenotic fibrosis because rapid mucosal healing induced by TNFα antibody may facilitate excessive fibrosis.43 Therefore, therapeutic strategies that distinguish between these states may yield more beneficial outcomes than currently available therapeutic regimens. For instance, topical administration of steroid is used as an initial treatment for CD.44, 45 Our finding that myofibroblast TRPA1 contributes to intestinal fibrosis is of great therapeutic significance, and may be generalized to other fibrotic lesions such as those in the skin, lung, and liver, where several TRP channels, including TRPA1, are highly expressed. In addition, the collagen-specific molecular chaperone HSP47, which was co-expressed with TRPA1 in the present study, is a stress-inducible protein up-regulated by heat, shear stress, oxidation, and other stimuli, and thus is another candidate molecule related to intestinal fibrosis in CD. This raises another intriguing question that should be addressed in future studies: whether and how these molecules functionally interact to contribute to the pathogenesis of fibrotic complications of inflammatory bowel disease.
In summary, the present study showed a new role for the myofibroblast TRPA1 channel in intestinal fibrosis and that steroids and pirfenidone activate this channel to exert its antifibrotic effects. Identification of this new antifibrotic molecular target TRPA1 will lead to additional studies of the pharmacologic actions of steroids and pirfenidone, as well as improve our understanding of the additive/synergistic effects and side effects of distinct antifibrotic drugs. The present findings not only provide a novel molecular target for antifibrotic therapy of inflammatory bowel diseases, but also a framework for re-interpreting the theory of inflammation and fibrosis in the gut.
Acknowledgments
The authors thank NPO Biotechnology Research and Development for technical assistance. The authors thank the Biotechnology and Food Research Institute, Fukuoka Industrial Technology Centre, for providing the 103 herb library for screening; Professor Keiichro Haga coordinated this collaboration. The authors thank Professor Yasuo Mori (Kyoto University, Japan), who kindly provided TRPA1 plasmids. The authors also would like to thank Editage (www.editage.jp) for English language editing. The authors thank cell-innovator supported Microarray analysis.
Footnotes
Author contributions Lin Hai Kurahara designed the experiments and wrote the paper; Lin Hai Kurahara and Keizo Hiraishi performed most experiments and analyzed the data; Yaopeng Hu conducted patch clamp experiments and analyzed the obtained data; Kaori Koga and Miki Onitsuka performed pathologic analysis; Mayumi Doi and Yuwen Jian performed immunostaning experiments and collected data; Kunihiko Aoyagi, Hidetoshi Takedatsu, and Daibo Kojima gave clinical advice, recruited study participants, performed biopsies, and surgically resected tissues; Yoshitaka Fujihara generated Trpa1 knockout mice with the Clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated 9 (Cas9) system; Yuwen Jian created a graphical abstract; and Ryuji Inoue supervised the study and provided comments during the preparation of this manuscript.
Conflicts of interest The authors disclose no conflicts.
Funding This study was supported by The Ministry of Education, Culture, Sports, Science and Technology (MEXT) KAKENHI (15K08978, 22790677, and 25860571); the Fukuoka Foundation for Sound Health Cancer Research Fund; the MEXT-Supported Program supporting the research activities of female researchers; the Clinical Research Foundation; and the Central Research Institute of Fukuoka University (151045 and 147104).
References
- 1.Rieder F., Brenmoehl J., Leeb S., Scholmerich J., Rogler G. Wound healing and fibrosis in intestinal disease. Gut. 2007;56:130–139. doi: 10.1136/gut.2006.090456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- 3.Kurahara L.H., Sumiyoshi M., Aoyagi K., Hiraishi K., Nakajima K., Nakagawa M., Hu Y., Inoue R. Intestinal myofibroblast TRPC6 channel may contribute to stenotic fibrosis in Crohn's disease. Inflamm Bowel Dis. 2015;21:496–506. doi: 10.1097/MIB.0000000000000295. [DOI] [PubMed] [Google Scholar]
- 4.Medina C., Santos-Martinez M.J., Santana A., Paz-Cabrera M.C., Johnston M.J., Mourelle M., Salas A., Guarner F. Transforming growth factor-beta type 1 receptor (ALK5) and Smad proteins mediate TIMP-1 and collagen synthesis in experimental intestinal fibrosis. J Pathol. 2011;224:461–472. doi: 10.1002/path.2870. [DOI] [PubMed] [Google Scholar]
- 5.Inoue K., Naito Y., Takagi T., Hayashi N., Hirai Y., Mizushima K., Horie R., Fukumoto K., Yamada S., Harusato A., Hirata I., Omatsu T., Yoshida N., Uchiyama K., Ishikawa T., Handa O., Konishi H., Wakabayashi N., Yagi N., Ichikawa H., Kokura S., Yoshikawa T. Daikenchuto, a Kampo medicine, regulates intestinal fibrosis associated with decreasing expression of heat shock protein 47 and collagen content in a rat colitis model. Biol Pharm Bull. 2011;34:1659–1665. doi: 10.1248/bpb.34.1659. [DOI] [PubMed] [Google Scholar]
- 6.Kitamura H., Yamamoto S., Nakase H., Matsuura M., Honzawa Y., Matsumura K., Takeda Y., Uza N., Nagata K., Chiba T. Role of heat shock protein 47 in intestinal fibrosis of experimental colitis. Biochem Biophys Res Commun. 2011;404:599–604. doi: 10.1016/j.bbrc.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 7.Johnson L.A., Rodansky E.S., Haak A.J., Larsen S.D., Neubig R.R., Higgins P.D. Novel Rho/MRTF/SRF inhibitors block matrix-stiffness and TGF-beta-induced fibrogenesis in human colonic myofibroblasts. Inflamm Bowel Dis. 2014;20:154–165. doi: 10.1097/01.MIB.0000437615.98881.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Babyatsky M.W., Rossiter G., Podolsky D.K. Expression of transforming growth factors alpha and beta in colonic mucosa in inflammatory bowel disease. Gastroenterology. 1996;110:975–984. doi: 10.1053/gast.1996.v110.pm8613031. [DOI] [PubMed] [Google Scholar]
- 9.Inoue R., Jensen L.J., Shi J., Morita H., Nishida M., Honda A., Ito Y. Transient receptor potential channels in cardiovascular function and disease. Circ Res. 2006;99:119–131. doi: 10.1161/01.RES.0000233356.10630.8a. [DOI] [PubMed] [Google Scholar]
- 10.Nishida M., Onohara N., Sato Y., Suda R., Ogushi M., Tanabe S., Inoue R., Mori Y., Kurose H. Galpha12/13-mediated up-regulation of TRPC6 negatively regulates endothelin-1-induced cardiac myofibroblast formation and collagen synthesis through nuclear factor of activated T cells activation. J Biol Chem. 2007;282:23117–23128. doi: 10.1074/jbc.M611780200. [DOI] [PubMed] [Google Scholar]
- 11.Onohara N., Nishida M., Inoue R., Kobayashi H., Sumimoto H., Sato Y., Mori Y., Nagao T., Kurose H. TRPC3 and TRPC6 are essential for angiotensin II-induced cardiac hypertrophy. EMBO J. 2006;25:5305–5316. doi: 10.1038/sj.emboj.7601417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du J., Xie J., Zhang Z., Tsujikawa H., Fusco D., Silverman D., Liang B., Yue L. TRPM7-mediated Ca2+ signals confer fibrogenesis in human atrial fibrillation. Circ Res. 2010;106:992–1003. doi: 10.1161/CIRCRESAHA.109.206771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poole D.P., Pelayo J.C., Cattaruzza F., Kuo Y.M., Gai G., Chiu J.V., Bron R., Furness J.B., Grady E.F., Bunnett N.W. Transient receptor potential ankyrin 1 is expressed by inhibitory motoneurons of the mouse intestine. Gastroenterology. 2011;141:565–575. doi: 10.1053/j.gastro.2011.04.049. e561–e564. [DOI] [PubMed] [Google Scholar]
- 14.Kono T., Kaneko A., Omiya Y., Ohbuchi K., Ohno N., Yamamoto M. Epithelial transient receptor potential ankyrin 1 (TRPA1)-dependent adrenomedullin upregulates blood flow in rat small intestine. Am J Physiol Gastrointest Liver Physiol. 2013;304:G428–G436. doi: 10.1152/ajpgi.00356.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bautista D.M., Pellegrino M., Tsunozaki M. TRPA1: a gatekeeper for inflammation. Annu Rev Physiol. 2013;75:181–200. doi: 10.1146/annurev-physiol-030212-183811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nassini R., Pedretti P., Moretto N., Fusi C., Carnini C., Facchinetti F., Viscomi A.R., Pisano A.R., Stokesberry S., Brunmark C., Svitacheva N., McGarvey L., Patacchini R., Damholt A.B., Geppetti P., Materazzi S. Transient receptor potential ankyrin 1 channel localized to non-neuronal airway cells promotes non-neurogenic inflammation. PLoS One. 2012;7:e42454. doi: 10.1371/journal.pone.0042454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee B.H., Hsu W.H., Hsu Y.W., Pan T.M. Suppression of dimerumic acid on hepatic fibrosis caused from carboxymethyl-lysine (CML) by attenuating oxidative stress depends on Nrf2 activation in hepatic stellate cells (HSCs) Food Chem Toxicol. 2013;62:413–419. doi: 10.1016/j.fct.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Jiang X., Zhang Y., Li F., Zhu Y., Chen Y., Yang S., Sun G. Allicin as a possible adjunctive therapeutic drug for stage II oral submucous fibrosis: a preliminary clinical trial in a Chinese cohort. Int J Oral Maxillofac Surg. 2015;44:1540–1546. doi: 10.1016/j.ijom.2015.06.015. [DOI] [PubMed] [Google Scholar]
- 19.Kun J., Szitter I., Kemeny A., Perkecz A., Kereskai L., Pohoczky K., Vincze A., Godi S., Szabo I., Szolcsanyi J., Pinter E., Helyes Z. Upregulation of the transient receptor potential ankyrin 1 ion channel in the inflamed human and mouse colon and its protective roles. PLoS One. 2014;9:e108164. doi: 10.1371/journal.pone.0108164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bertin S., Aoki-Nonaka Y., Lee J., de Jong P.R., Kim P., Han T., Yu T., To K., Takahashi N., Boland B.S., Chang J.T., Ho S.B., Herdman S., Corr M., Franco A., Sharma S., Dong H., Akopian A.N., Raz E. The TRPA1 ion channel is expressed in CD4+ T cells and restrains T-cell-mediated colitis through inhibition of TRPV1. Gut. 2017;66:1584–1596. doi: 10.1136/gutjnl-2015-310710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romano B., Borrelli F., Fasolino I., Capasso R., Piscitelli F., Cascio M., Pertwee R., Coppola D., Vassallo L., Orlando P., Di Marzo V., Izzo A. The cannabinoid TRPA1 agonist cannabichromene inhibits nitric oxide production in macrophages and ameliorates murine colitis. Br J Pharmacol. 2013;169:213–229. doi: 10.1111/bph.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brooker J.C., Beckett C.G., Saunders B.P., Benson M.J. Long-acting steroid injection after endoscopic dilation of anastomotic Crohn's strictures may improve the outcome: a retrospective case series. Endoscopy. 2003;35:333–337. doi: 10.1055/s-2003-38145. [DOI] [PubMed] [Google Scholar]
- 23.Van Assche G. Intramural steroid injection and endoscopic dilation for Crohn's disease. Clin Gastroenterol Hepatol. 2007;5:1027–1028. doi: 10.1016/j.cgh.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Meier R., Lutz C., Cosin-Roger J., Fagagnini S., Bollmann G., Hunerwadel A., Mamie C., Lang S., Tchouboukov A., Weber F.E., Weber A., Rogler G., Hausmann M. Decreased fibrogenesis after treatment with pirfenidone in a newly developed mouse model of intestinal fibrosis. Inflamm Bowel Dis. 2016;22:569–582. doi: 10.1097/MIB.0000000000000716. [DOI] [PubMed] [Google Scholar]
- 25.Inoue Y.N., Takagi T., Hayashi N., Hirai Y., Mizushima K., Tsuji T., Yoriki H., Kugai M., Horie R., Fukumoto K., Yamada S., Harusato A., Yoshida N., Uchiyama K., Ishikawa T., Handa O., Konishi H., Wakabayashi N., Yagi N., Ichikawa H., Kokura S., Yoshikawa T. Pirfenidone regulates intestinal fibrosis through the inhibition of HSP47 expression in a rat colitis model. Gastroenterology. 2011;140(Suppl 1):S-770. [Google Scholar]
- 26.Honzawa Y., Nakase H., Takeda Y., Nagata K., Chiba T. Heat shock protein 47 can be a new target molecule for intestinal fibrosis related to inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:2004–2006. doi: 10.1002/ibd.21243. [DOI] [PubMed] [Google Scholar]
- 27.Nakase H., Honzawa Y., Chiba T. Heat shock protein 47 is a new candidate molecule as anti-fibrotic treatment of Crohn's disease. Aliment Pharmacol Ther. 2010;31:926–928. doi: 10.1111/j.1365-2036.2010.04240.x. [DOI] [PubMed] [Google Scholar]
- 28.Shi J., Mori E., Mori Y., Mori M., Li J., Ito Y., Inoue R. Multiple regulation by calcium of murine homologues of transient receptor potential proteins TRPC6 and TRPC7 expressed in HEK293 cells. J Physiol. 2004;561:415–432. doi: 10.1113/jphysiol.2004.075051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi N., Kuwaki T., Kiyonaka S., Numata T., Kozai D., Mizuno Y., Yamamoto S., Naito S., Knevels E., Carmeliet P., Oga T., Kaneko S., Suga S., Nokami T., Yoshida J., Mori Y. TRPA1 underlies a sensing mechanism for O2. Nat Chem Biol. 2011;7:701–711. doi: 10.1038/nchembio.640. [DOI] [PubMed] [Google Scholar]
- 30.Fujihara Y., Ikawa M. CRISPR/Cas9-based genome editing in mice by single plasmid injection. Methods Enzymol. 2014;546:319–336. doi: 10.1016/B978-0-12-801185-0.00015-5. [DOI] [PubMed] [Google Scholar]
- 31.Mashiko D., Fujihara Y., Satouh Y., Miyata H., Isotani A., Ikawa M. Generation of mutant mice by pronuclear injection of circular plasmid expressing Cas9 and single guided RNA. Sci Rep. 2013;3:3355. doi: 10.1038/srep03355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson L.A., Luke A., Sauder K., Moons D.S., Horowitz J.C., Higgins P.D. Intestinal fibrosis is reduced by early elimination of inflammation in a mouse model of IBD: impact of a “Top-Down” approach to intestinal fibrosis in mice. Inflamm Bowel Dis. 2012;18:460–471. doi: 10.1002/ibd.21812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fichtner-Feigl S., Fuss I.J., Young C.A., Watanabe T., Geissler E.K., Schlitt H.J., Kitani A., Strober W. Induction of IL-13 triggers TGF-beta1-dependent tissue fibrosis in chronic 2,4,6-trinitrobenzene sulfonic acid colitis. J Immunol. 2007;178:5859–5870. doi: 10.4049/jimmunol.178.9.5859. [DOI] [PubMed] [Google Scholar]
- 34.Rieder F., Fiocchi C., Rogler G. Mechanisms, management, and treatment of fibrosis in patients with inflammatory bowel diseases. Gastroenterology. 2017;152:340–350 e346. doi: 10.1053/j.gastro.2016.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hinz B., Gabbiani G. Fibrosis: recent advances in myofibroblast biology and new therapeutic perspectives. F1000 Biol Rep. 2010;2:78. doi: 10.3410/B2-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moilanen L.J., Laavola M., Kukkonen M., Korhonen R., Leppanen T., Hogestatt E.D., Zygmunt P.M., Nieminen R.M., Moilanen E. TRPA1 contributes to the acute inflammatory response and mediates carrageenan-induced paw edema in the mouse. Sci Rep. 2012;2:380. doi: 10.1038/srep00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okada Y., Shirai K., Reinach P.S., Kitano-Izutani A., Miyajima M., Flanders K.C., Jester J.V., Tominaga M., Saika S. TRPA1 is required for TGF-beta signaling and its loss blocks inflammatory fibrosis in mouse corneal stroma. Lab Invest. 2014;94:1030–1041. doi: 10.1038/labinvest.2014.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Speca S., Giusti I., Rieder F., Latella G. Cellular and molecular mechanisms of intestinal fibrosis. World J Gastroenterol. 2012;18:3635–3661. doi: 10.3748/wjg.v18.i28.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Latella G., Di Gregorio J., Flati V., Rieder F., Lawrance I.C. Mechanisms of initiation and progression of intestinal fibrosis in IBD. Scand J Gastroenterol. 2015;50:53–65. doi: 10.3109/00365521.2014.968863. [DOI] [PubMed] [Google Scholar]
- 40.Adcock I.M., Nasuhara Y., Stevens D.A., Barnes P.J. Ligand-induced differentiation of glucocorticoid receptor (GR) trans-repression and transactivation: preferential targeting of NF-kappaB and lack of I-kappaB involvement. Br J Pharmacol. 1999;127:1003–1011. doi: 10.1038/sj.bjp.0702613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Latella G., Sferra R., Speca S., Vetuschi A., Gaudio E. Can we prevent, reduce or reverse intestinal fibrosis in IBD? Eur Rev Med Pharmacol Sci. 2013;17:1283–1304. [PubMed] [Google Scholar]
- 42.Peyrin-Biroulet L., Fiorino G., Buisson A., Danese S. First-line therapy in adult Crohn's disease: who should receive anti-TNF agents? Nat Rev Gastroenterol Hepatol. 2013;10:345–351. doi: 10.1038/nrgastro.2013.31. [DOI] [PubMed] [Google Scholar]
- 43.Condino G., Calabrese E., Zorzi F., Onali S., Lolli E., De Biasio F., Ascolani M., Pallone F., Biancone L. Anti-TNF-alpha treatments and obstructive symptoms in Crohn's disease: a prospective study. Dig Liver Dis. 2013;45:258–262. doi: 10.1016/j.dld.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 44.Kuenzig M.E., Rezaie A., Seow C.H., Otley A.R., Steinhart A.H., Griffiths A.M., Kaplan G.G., Benchimol E.I. Budesonide for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev. 2014;8:CD002913. doi: 10.1002/14651858.CD002913.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coward S., Kuenzig M.E., Hazlewood G., Clement F., McBrien K., Holmes R., Panaccione R., Ghosh S., Seow C.H., Rezaie A., Kaplan G.G. Comparative effectiveness of mesalamine, sulfasalazine, corticosteroids, and budesonide for the induction of remission in Crohn's disease: a Bayesian network meta-analysis. Inflamm Bowel Dis. 2017;23:461–472. doi: 10.1097/MIB.0000000000001023. [DOI] [PubMed] [Google Scholar]