Abstract
Background
Chronic heart failure (CHF) is the most common reason for hospital admissions in Germany. For the National Disease Management Guideline (NDMG) on CHF, a multidisciplinary expert panel revised the chapters on drug therapy, invasive therapy, and care coordination, following the methods of evidence-based medicine.
Methods
Recommendations are based on international guideline adaptations or systematic literature search. They were developed by a multidisciplinary expert panel, approved in a formal consensus procedure, and tested in open consultation, as specified by the requirements for S3 guidelines.
Results
The pharmacological treatment is based on ACE inhibitors, beta-blockers and mineralocorticoid receptor antagonists as well as diuretics to treat fluid retention, if present. Sacubitril/Valsartan and ivabradine showed positive effects on mortality in large but methodologically limited RCT. They are recommended if established combination therapy is not sufficient for symptom control, or if drugs are not tolerated/contraindicated. The indications for pacemakers or defibrillators have been confined to patient subgroups in which clinical trials have shown a clear benefit. Moreover, the goals of treatment and the patient’s expectations should be aligned with each other. Structured care programs, specialized nurses, remote, or telephone monitoring showed moderate effects on patient related outcomes in RCT.
Conclusion
All patients with heart failure are suggested to be enrolled in a structured program (e.g., a disease management program) including coordinated multidisciplinary care and continuous educational interventions. In patients with a poor prognosis, more intensive care is recommended, e.g. specialized nurses, or telephone support.
At a total of 444 632 cases, heart failure (coded under ICD I50) was the most frequent single diagnosis for hospital admission in Germany in 2015; it is also one of the most frequent causes of death (1) (ICD, International Statistical Classification of Diseases and Related Health Problems). Its incidence is continuing to rise, partly owing to demographic developments and partly to improved survival after myocardial infarction and other forms of heart disease. In August 2017, the German Medical Association (BÄK, Bundesärztekammer), the Federal Association of Statutory Health Insurers (KBV, Kassenärztliche Bundesvereinigung), and the Association of Scientific Medical Societies in Germany (AWMF, Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften) published a new, revised edition of the National Disease Management Guideline (NDMG, Nationale VersorgungsLeitlinie) on chronic heart failure (2).
Method
National disease management guidelines are developed on the basis of national and international concepts and quality criteria for guidelines (3– 5). Elementary methods for the development of NDMGs are described in the general ‘Methods Report’ (6) and the detailed procedure for this guideline in the “Guideline Report’ (7). The first edition of the Chronic Heart Failure NDMG was published in 2009 (8). The second edition was prepared between October 2015 and August 2017 by a multidisciplinary guideline development group made up of representatives of patients, doctors, nurses, and pharmacists (ebox). The group’s first priority was to update the sections on medical therapy, invasive treatment, and coordination of care.
eBOX. Publishers and authors of the German National Clinical Practice Guideline for Chronic Heart Failure.
-
(Publishers
German Medical Association (BÄK, Bundesärztekammer)
Federal Association of Statutory Health Insurers (KBV, Kassenärztliche Bundesvereinigung)
Association of Scientific Medical Societies in Germany (AWMF, Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften)
-
(and
Drug Commission of the Federal Union of German Associations of Pharmacists (AMK, Arzneimittelkommission der Deutschen Apotheker)
Drug Commission of the German Medical Association (AkdÄ, Arzneimittelkommission der deutschen Ärzteschaft)
German Association of Self-Help Groups (DAG SHG, Deutsche Arbeitsgemeinschaft Selbsthilfegruppen)
German Diabetes Association (DDG, Deutsche Diabetes Gesellschaft)
German College of General Practitioners and Family Physicians (DEGAM, Deutsche Gesellschaft für Allgemeinmedizin und Familienmedizin)
German Geriatrics Society (DGG, Deutsche Gesellschaft für Geriatrie)
German Society of Internal Medicine (DGIM, Deutsche Gesellschaft für Innere Medizin)
German Cardiac Society (DGK, Deutsche Gesellschaft für Kardiologie – Herz- und Kreislaufforschung)
German Nephrology Society (DGfN, Deutsche Gesellschaft für Nephrologie)
German Society of Palliative Medicine (DGP Palliativmedizin, Deutsche Gesellschaft für Palliativmedizin)
German Society of Nursing Science (DGP Pflegewissenschaft, Deutsche Gesellschaft für Pflegewissenschaft)
German Respiratory Society (DGP Pneumologie Deutsche Gesellschaft für Pneumologie und Beatmungsmedizin)
German Society of Cardiovascular Prevention and Rehabilitation (DGPR, Deutsche Gesellschaft für Prävention und Rehabilitation von Herz-Kreislauferkrankungen)
German Society of Psychosomatic Medicine and Medical Psychotherapy (DGPM, Deutsche Gesellschaft für Psychosomatische Medizin und Ärztliche Psychotherapie)
German Association of Rehabilitation Sciences (DGRW, Deutsche Gesellschaft für Rehabilitationswissenschaften)
German Society for Thoracic and Cardiovascular Surgery (DGTHG, Deutsche Gesellschaft für Thorax-, Herz- und Gefäßchirurgie)
German College for Psychosomatic Medicine (DKPM, Deutsches Kollegium für Psychosomatische Medizin)
-
(Authors of the second edition
Prof. Dr. rer. nat. Martin Schulz, Prof. Dr. med. Ulrich Laufs (AMK)
Prof. Dr. med. Klaus Mörike, Dr. med. Gisela Schott, MPH (AkdÄ)
Roland Keuchen (DAG SHG)
Prof. Dr. med. Diethelm Tschöpe (DDG)
Dr. med. Christiane Muth, MPH, Prof. Dr. med. Martin Scherer (DEGAM)
Prof. Dr. med. Roland Hardt, PD Dr. med. Philipp Bahrmann (DGG)
Prof. Dr. med. Rolf Wachter, Prof. Dr. med. Frank Edelmann (DGIM)
Prof. Dr. med. Georg Ertl, Prof. Dr. med. Stefan Störk (DGK)
Prof. Dr. med. Gunnar Heine, PD Dr. med. Sarah Seiler-Mußler (DGfN)
Prof. Dr. med. Bernd Alt-Epping (DGP Palliativmedizin)
Nina Kolbe, MScN (DGP Pflegewissenschaften)
PD Dr. med. Mathias M. Borst (DGP)
Prof. Dr. med. Axel Schlitt, MHA, Prof. Dr. med. Martin Halle (DGPR, DGRW)
Prof. Dr. med. Jan Gummert, Prof. Dr. med. Christoph Knosalla (DGTHG)
Prof. Dr. med. Christiane Waller (DGPM, DKPM)
-
(Methodological support, coordination, and editorial support
Dr. med. Monika Nothacker, MPH (AWMF)
Peggy Prien, Corinna Schaefer, Dr. rer. nat. Susanne Schorr, Dr. rer. medic. Sabine Schwarz, Svenja Siegert, Isabell Vader, MPH (German Agency for Quality in Medicine – ÄZQ, Ärztliches Zentrum für Qualität in der Medizin)
Conflicts of interest
Conflicts of interest were declared in writing by all participants at the beginning of the guideline development process, discussed and assessed within the development group, and published in the guideline report (7). It was not found necessary to exclude any participant. The guideline development group decided that participants should abstain from voting in cases of conflict of interest in the category “Fees received as expert witness, consultant, or lecturer” concerning a specific topic of the NDMG. Overall, there were 14 abstentions relating to 8 of the 55 new recommendations owing to conflicts of interest (7).
Evidence base
The NDMG update is based on a guideline synopsis by the Institute for Quality and Efficiency in Health Care (IQWiG, Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen) (9). The 2016 European Society of Cardiology (ESC) guideline was taken as the source guideline (10). Additionally, 14 systematic searches were carried out by the German Agency for Quality in Medicine (ÄZQ, Ärztliches Zentrum für Qualität in der Medizin) on topics that included structured care concepts, sacubitril/valsartan, ivabradine, implantable cardioverter-defibrillators (ICDs), and cardiac resynchronization treatment (CRT) in special patient groups. A total of 1965 hits were screened in a two-stage procedure, and of these 192 full texts were compiled into evidence tables and evaluated for methodological quality (for search strategies, evaluations, and evidence tables, see the guideline report [7]).
Recommendation levels and consensus
In assigning recommendation class (“recommended”, “not recommended”, “is suggested”, “should not be used” or “may”), the strength of the underlying evidence, ethical considerations, the clinical relevance of the research results and their applicability to the target patient group, patient preferences, and practicability in everyday clinical routine and within German health care structures were all taken into account. Against the background of shared decision-making, the recommendations place an emphasis on collaborative consultation. They were agreed in a consensus conference (nominal group process). Comments received during a 1-month consultation open to the public were investigated by the guideline development group and any possible implications discussed (7).
Results
In addition to measures targeting the causes of heart failure, treatment of prognostically unfavorable factors, and non-drug interventions, therapy for chronic heart failure is based on symptom-orientated medical therapy and, if required, invasive treatment.
Medical therapy
Patients with heart failure are often elderly and have multiple morbidities (11). For this reason, some basic recommendations are devoted to the geriatric aspects of medical therapy and the issue of polypharmacy: For all patients with heart failure a medication plan is recommended that is regularly reviewed and updated by doctors and pharmacists, and includes non-prescription medications, in order to avoid problems related to polypharmacy such as drug interactions. Moreover, it is recommended to regularly review current or planned (co-)medication for drug substances that can cause or exacerbate heart failure. Such review includes asking the patient about his or her use of nonprescription drugs such as nonsteroidal anti-inflammatory drugs (NSAIDs). It should also cover a review of whether all the medications are still appropriate, especially when new comorbidities arise, such as atrial fibrillation or renal disease. Over the longer term, it is important to regularly monitor the patient’s response to heart failure medication, in order to avoid potentially fatal unwanted effects such as hyperkalemia (table 1).
Table 1. Recommended monitoring in patients receiving medical treatment for chronic heart failure.
| Physical exams*1 | Laboratory values | ||||
| Intervals | Adjusted to the individual patient’s current status, but at least as frequent as mandatory lab tests | Before treatment; 1–2 weeks after each dose increase; after 3 months; thereafter at 6-monthly intervals*2 or on any alteration of treatment, during every hospital stay | |||
| Parameter | Body weight*3 |
Heart rate | Blood pressure |
Serum electrolytes (esp. potassium and sodium) |
Serum values of renal function markers; esp. estimated GFR (or creatinine) |
| Drug or drug class | |||||
| Diuretics | +++ | ++ | +++ | +++ | |
| ACE inhibitors | +++ | +++ | +++ | ||
| ARB | +++ | +++ | +++ | ||
| Sacubitril/valsartan | +++ | +++ | +++ | ||
| Beta receptor blockers | +++ | +++ | |||
| Ivabradine | +++ | ++ | |||
| MRA | ++ | ++ | +++*4 | +++*4 | |
| Cardiac glycosides*5 | +++ | +++ | +++ (for digoxin or its derivatives) |
||
+++, very important; ++, moderately important
*1 May also be carried out by nursing staff as instructed by a physician
*2 Maximum acceptable intervals in clinically stable patients; intervals should be shorter in patients with known preexisting renal dysfunction or electrolyte disturbances or receiving concomitant treatment with nephrotoxic drugs
*3 Intra-individual time course
*4 4-monthly intervals; especially in patients with impaired renal function
*5 Also: determine drug concentration in order to monitor target plasma levels
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; GFR, glomerular filtration rate; MRA, mineralocorticoid receptor antagonists
Medical therapy for heart failure varies depending on the size of the left ventricular ejection fraction (LVEF). Although around 50% of heart failure patients have preserved ejection fraction (LVEF = 50%), there is still no evidence-based medical treatment for this patient group. In this group, we recommend treating comorbidities that influence prognosis, especially hypertension, and also symptomatic treatment with diuretics. In our estimation, however, patients with mildly reduced ejection fraction (LVEF 40% to 49%), especially if symptomatic, should receive the same treatment as is indicated for reduced LVEF.
All patients with a reduced ejection fraction (LVEF <40%) should receive treatment appropriate to their disease stage (New York Heart Association [NYHA] classification). This basic treatment includes ACE inhibitors or angiotensin receptor blockers (sartans) and beta blockers; for NYHA class II disease and higher, mineralocorticoid receptor antagonists (MRAs: spironolactone, eplerenone) are added and, in patients with fluid retention, diuretics as well (table 2). If symptoms do not improve adequately on this basic treatment, or if the patient does not tolerate it or contraindications are present, other drugs can be used.
Table 2. Staged medical therapy for patients with heart failure with reduced left-ventricular (LV) ejection fraction.
|
NYHA I (asymptomatic LV dysfunction) |
NYHA II | NYHA III |
NYHA IV (only in close collaboration with a cardiologist) |
|
| To improve prognosis | ||||
| ACE inhibitors | Indicated | Indicated | Indicated | Indicated |
| Angiotensin receptor blockers (ARB) |
In patients who cannot tolerate ACE inhibitors | In patients who cannot tolerate ACE inhibitors | In patients who cannot tolerate ACE inhibitors | In patients who cannot tolerate ACE inhibitors |
| Beta receptor blockers (BRB) |
After myocardial infarction or patients with hypertension | Indicated | Indicated | Indicated |
| Mineralocorticoid receptor antagonists |
Indicated | Indicated | Indicated | |
| Ivabradin | In patients who cannot tolerate BRBs, or additionally in patients with a heart rate ≥ 75/min | In patients who cannot tolerate BRBs, or additionally in patients with a heart rate ≥ 75/min | In patients who cannot tolerate BRBs, or additionally in patients with a heart rate ≥ 75/min | |
| Sacubitril/valsartan | To replace ACE inhibitors/ARBs in patients with persistent symptoms* | To replace ACE inhibitors/ARBs in patients with persistent symptoms* | To replace ACE inhibitors/ARBs in patients with persistent symptoms* | |
| To improve symptoms | ||||
| Diuretics | In patients with fluid retention | Indicated | Indicated | |
| Cardiac glycosides | As a reserve drug (with low target serum concentration) in patients with sinus rhythm | As a reserve drug (with low target serum concentration) in patients with sinus rhythm | ||
| In patients with uncontrolled tachyarrhythmic atrial fibrillation | ||||
* Despite combination therapy with ACE(angiotensin-converting enzyme) inhibitors /angiotensin receptor blockers, beta receptor blockers, and mineralocorticoid receptor antagonists according to guideline
LV dysfunction, left ventricular dysfunction; NYHA, New York Heart Association classification; BRB, beta receptor blocker
In patients with a resting heart rate of = 75/min despite taking the maximum tolerated or target dose of beta blockers, and in those who cannot tolerate beta blockers or in whom these drugs are contraindicated, ivabradine is suggested. In the pivotal trial, the If channel blocker reduced the absolute risk for the composite primary endpoint—cardiovascular mortality or hospital admission for worsening heart failure—by 5% in comparison to standard treatment + placebo (24% versus 29%; hazard ratio [HR]: 0.82; 95% confidence interval: [0.75; 0.90]; number needed to treat [NNT]: 20) (12). However, in patients taking at least 50% of the target dose of beta blockers, no significant effect was seen (12, 13). Ivabradine is not recommended in patients with cardiac arrhythmias. Because of the increased risk of atrial fibrillation (number needed to harm [NNH]: 208 per treatment year) (14), we recommend regular monitoring of heart rhythm in patients taking ivabradine (table 1), and to cease ivabradine treatment if atrial fibrillation does occur.
In regard to sacubitril-valsartan, licensed for the treatment of heart failure in 2015, our recommendation is cautious compared to international guidelines (10, 15): we suggest to change from ACE inhibitors to this angiotensin receptor–neprilysin inhibitor only for patients still symptomatic under basic medical treatment carried out in accordance with the guidelines. In the pivotal trial, the sacubitril-valsartan group showed a 4.7% absolute improvement in the composite primary endpoint—death from cardiovascular causes or hospitalization for heart failure—after a median follow-up of 27 months in comparison to the enalapril group (21.8% versus 26.5%; HR: 0.80 [0.73; 0.87]; NNT: 22) (16). We regard it as a weakness in the data that results have so far been reported from only one large study in which considerable preselection of patients had taken place on the basis of exclusion criteria and a run-in phase. Decision making about this treatment should also take into account the uncertainty that exists about the long-term tolerability and safety profile of sacubitril/valsartan.
Invasive treatment
Like international guidelines (10), we recommend cardiac resynchronization treatment (CRT) for patients with reduced ejection fraction grouped according to bundle branch block morphology and QRS duration (table 3), as these criteria are predictors of therapeutic success. Since most patients in the CRT studies have not been preselected in this respect, the recommendations largely rely on meta-analyses of post hoc defined subgroups: for example, Cleland et al. in 2013 calculated a relative mortality risk reduction of 34% for patients with left bundle branch block receiving CRT (n = 3036; HR: 0.66 [0.55; 0.78]), whereas in patients with right bundle branch block there was no significant improvement (n = 346; HR: 0.74 [0.44; 1.23]) (17).
Table 3. Recommendations for cardiac resynchronization therapy in patients with sinus rhythm and left ventricular ejection fraction =35%.
| QRS (ms) | Left bundle branch block | Non-left bundle branch block |
| <130 | ↓↓ | ↓↓ |
| 130–149 | ↑↑ | ↔ |
| ≥ 150 | ↑↑ | ↑ |
↓↓, Is not recommended; ↑↑, Is recommended; ↔, may be considered; ↑, is suggested
The evidence for CRT in patients with atrial fibrillation is poorer than the evidence for the same treatment in patients with sinus rhythm, as patients with atrial fibrillation are excluded from most randomized controlled studies (RCTs) and the findings of retrospective observation studies are inconsistent. For this reason, we regard CRT as indicated only in exceptional cases and provided a nearly complete bi-ventricular capture (usually achieved by atrioventricular node ablation). In regard to patients with pre-existing atrioventricular block, we have more reservations about the evidence for CRT than does the ESC guideline (10) and therefore do not state a recommendation. The main reason for this is that one study showing a positive effect (18) is counterbalanced by another study, so far unpublished (19, 20), that shows no effect.
We recommend the use of implantable cardioverter-defibrillators (ICDs) for secondary prevention in all patients who have survived near-fatal ventricular fibrillation or experience sustained ventricular tachycardia causing severe symptoms without an avoidable cause and with an estimated life expectancy of >1 year (absolute risk reduction [ARR] for mortality after 3 years: between 3.7% [21] and 11.3% [22]). For prevention of sudden cardiac death (“primary prevention”), implantation of an ICD is also recommended for patients with NYHA II/III disease, LVEF =35%, an estimated life expectancy of >1 year, and good functional status (e.g., MADIT II study: ARR for mortality: 5.6% [23]). However, among these patients we limit our recommendation to those with ischemic cardiomyopathy. The rationale for this comes from the DANISH study (24), which investigated the efficacy of ICDs in patients with nonischemic cardiomyopathy and found no significant effect on mortality (21.6% versus 23.4%; HR: 0.87 [0.68; 1.12], p = 0.28). We do not categorically rule out implantation in these patients, but because the evidence is unclear, neither do we specifically recommend it.
Patients often overestimate the benefit to be gained from an ICD, so it is important to explain to them the goal of treatment with an ICD, and the fact that when the ICD generator needs replacing, there will be a review as to whether continuing this treatment is appropriate. Inappropriate shocks can be a burden both for a patient who is dying and for the patient’s relatives, and for this reason the subject of “turning off the ICD” should be raised early on and should be repeated during follow-up visits for monitoring after implantation of the device. Conversations should include legal aspects and the particular form of words needed in the patient’s advance directive (“living will”).
Regarding the choice of implant, we recommend caution, as more complicated types of implant are associated with a higher complication rate: implantation of a combined CRT-ICD system may in our opinion only be considered in a few individual patients who fulfill the criteria for both biventricular stimulation and implantation of a defibrillator, as current evidence does not allow the additional value of a combined device (CRT-ICD) over CRT alone to be assessed: no direct comparisons from RCTs are available, and the findings of retrospective cohort studies, indirect comparisons, and meta-analyses are inconsistent (25– 27). Dual-chamber ICDs should, in our opinion, not be used except in the presence of an additional indication (e.g., anti-bradycardia pacing); here, too, a meta-analysis of RCTs failed to find any additional benefit in comparison to single-chamber devices (28).
Ventricular assist devices (including “total artificial hearts”) are currently being implanted in about 1000 patients a year in Germany, with an upward trend. If medical and CRT/ICD therapy according to guidelines fails to control symptoms, we suggest to discuss VAD implantation with the patient and referral to a specialist center for confirming indication at an early date, before irreversible damage has occurred to the kidneys, liver, or lungs.
Some surgical or catheter-based procedures offer the possibility of causal treatment of the heart failure. The benefit for patients of myocardial revascularization by means of a bypass procedure—especially for patients with heart failure (LVEF =35%)—has been regarded as proven since publication of the 10-year data from the STICH study: after 10 years, the absolute risk for mortality of patients in the bypass group was reduced by 7.2% compared to the control group (p = 0.02; NNT = 14) (29). Ventricular reconstruction or LV aneurysmectomy may be appropriate in selected patients, as may surgical treatment of valve disease.
Coordination of care
The NDMG emphasizes aspects of care within the German health care system. We therefore make recommendations for the transition between outpatient and inpatient care, and about managing the intersection between primary and specialist care. First and foremost is the importance of active communication between all health professionals involved, and we recommend to coordinate diagnostic assessments, treatment measures, the length of monitoring intervals, and other information, and to exchange these in written form. For all patients—including those with cardiac dysfunction with few or no symptoms—regular check-ups by a cardiologist are recommended; the intervals between check-ups should be suggested by the cardiologist and appropriate to the severity of disease. Because patients with heart failure often have comorbidities, we have compiled a list of typical or prognostically relevant patterns of symptoms and signs, in the presence of which the family doctor or cardiologist is suggested to collaborate with specialists in other disciplines (e.g., nephrology, diabetology, pneumology, psychiatry) or refer the patient for co-treatment (table 4).
Table 4. Patterns of symptoms and signs suggesting that consultation with or referral to a medical specialist is required.
| Specialty | Pattern suggesting consultation or referral |
| Nephrology | – Markedly impaired or markedly deteriorating renal function – Novel occurrence of protenuria |
| Pneumology | – Inadequate response to treatment for asthma/COPD despite increased intensity of treatment – Dyspnea of suspected pulmonary origin – When long-term treatment with oral corticosteroids is to be begun or ended |
| Diabetology | – Metabolic control/differential antidiabetic therapy – When individually agreed therapeutic goals (e.g., target HbA 1c levels) are not achieved |
| Psychosomatic medicine/ psychiatry/ psychotherapy |
– Suspected or persistent psychological or psychosomatic disorders (especially depression, adjustment disorder, anxiety disorder, somatic symptom disorder, posttraumatic stress syndrome) – Problems with drug interactions between antidepressants and heart failure medication – Etiologically relevant addiction – Increasing cognitive impairment |
| Geriatrics | – When extensive diagnostic tests and treatment in the inpatient setting are required to maintain the patient’s active participation and autonomy – When multimorbidity and polypharmacy result in complex problems |
| Specialized palliative care |
– When more intensive care is needed, e.g.: – Disease course characterized by crises (e.g., frequent decompensation and hospital admissions) – Uncontrolled physical symptoms (e.g., dyspnea, progressive weakness) – Increased need for care support in activities of daily living (ADL) – High degree of psychosocial problems (e.g., in the home environment) |
COPD, chronic obstructive pulmonary disease
We regard the care of patients with heart failure as a multidisciplinary task in which not just medical specialists, but also nursing staff and pharmacists are involved with the aim of improving patient prognosis and preventing repeated hospital admissions. Pharmacists can make an important contribution to the safety of drug treatment, for example by checking the prescriptions from the various medical specialists and advising patients on potential problems with any self-medication. Nursing staff can take on important tasks such as monitoring clinical parameters or medication management.
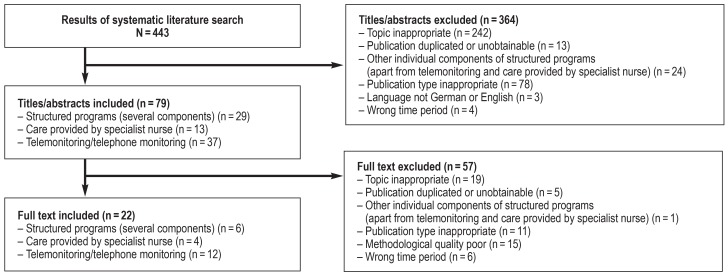
Based on the results of two systematic literature searches on structured concepts in the international (figure) and national context, we suggest to enroll all patients with heart failure in a structured treatment program that not only ensures diagnosis and treatment in accordance with guidelines, but encompasses coordinated multidisciplinary care with regular appointments and direct doctor–patient contact and continuous patient education. We recommend a more intensive treatment program for patients at increased risk of death or hospital admission, e.g., after cardiac decompensation; or patients with comorbidities that tend to produce complications, e.g., hypotension or diabetes mellitus; or if the heart failure has progressed to NYHA class III or higher. Apart from increased home visiting by the primary care physician, an intensified program of this kind could include various additional components. Reviews of RCTs show a positive influence on hospital admission rates and mortality for:
Figure.
PRISMA flow chart of literature search for structured care programs for patients with chronic heart failure
Specialist nurses (e.g., [30] rehospitalizations: ARR: 10.11%, NNT: 10, or mortality: ARR: 3.12%, NNT: 33)
Structured telephone support (e.g., [31] overall mortality: ARR 1.37%, NNT: 73, or hospital admissions for heart failure: ARR 3.17%, NNT: 32)
Noninvasive telemonitoring by means of telemetric scales, sphygmomanometers, and ECG recorders (e.g., [31] mortality: ARR: 2.49%, NNT: 41, or hospital admissions for heart failure: ARR: 7.44%, NNT: 14).
Which of these components are deployed when will generally depend on what is available regionally and what is appropriate to the patient’s individual case.
In Germany at present various structured programs exist for patients with chronic heart failure, but they are available only in certain regions or to patients insured with particular health insurers; they are set up in very different ways and are rarely evaluated. In the new independent disease management program (DMP) for heart failure expected in 2018, therefore, quality standards for this mode of care should be defined, e.g., regarding its integration with primary care and the appropriate level of qualification of non-physician treating personnel.
Information for patients
The Guideline for Patients, a required element of all NDMGs, is currently being updated on the basis of the second edition. Patient information material is also being developed for particular decision-making or patient information scenarios. The aim of these materials is to support health professionals in implementing the recommendations of the NDMG and contribute to shared decision making (free download at www.leitlinien.de/nvl/ herzinsuffizienz).
Key messages.
A medication plan is recommended for all patients with heart failure.
We suggest ivabradine for symptomatic patients with sinus rhythm who have already reached the maximum tolerated or target dose of beta blockers or who cannot tolerate beta blockers, or in whom beta blockers are contraindicated. We recommend treatment to be stopped if atrial fibrillation occurs.
In patients who do not achieve adequate symptom control on combination therapy with ACE inhibitors, beta blockers, and mineralocorticoid receptor antagonists we suggest changing from ACE inhibitor or ARB to sacubitril-valsartan.
The decision as to whether implantation of a device (CRT, ICD, ventricular assistance devices) is indicated should be made on the basis of clinical features (e.g., for CRT, bundle branch block morphology and QRS duration) and keeping the complication risks in mind. The treatment goals should be balanced with the patient’s expectations of the treatment, which often are too high.
Patients with heart failure should be enrolled in a structured program; those with poorer prognoses should receive a greater intensity of care, e.g., from specialist nurses or via structured monitoring by telephone.
Acknowledgments
Translated from the original German by Kersti Wagstaff, M.A.
Footnotes
Conflict of interest statement
Professor Edelmann has received consultancy fees from Bayer, Vifor, and Novartis. He has received lecture fees and reimbursement of travel costs and conference registration fees from Berlin Chemie, Novartis Servier, Astra Zeneca, BG Medicine,
Bayer, Abbott, Merck, MSD, Vifor, and Boehringer Ingelheim. He has received financial support for carrying out clinical studies from Servier, Vifor, Berlin Chemie, Novartis, Astra Zeneca, BG Medicine, Bayer, MSD, Merck, Abbott, and Boehringer Ingelheim.
Professor Störk has received consultancy fees from Boston Scientific and Thermo Fisher Scientific BRAHMS. He has received lecture fees and reimbursement of travel costs and conference registration fees from ResMed, Bayer, Novartis, Astra Zeneca, Boehringer Ingelheim, and Zoll CMS. He has received financial support for carrying out clinical studies from Novartis, Bayer, MSD, and Boehringer Ingelheim.
The other authors declare that no conflict of interest exists.
References
- 1.Deutsche Herzstiftung. Deutsche Herzstiftung. Frankfurt/Main: 2016. Deutscher Herzbericht 2016. 28. Sektorenübergreifende Versorgungsanalyse zur Kardiologie und Herzchirurgie in Deutschland. [Google Scholar]
- 2.Bundesärztekammer (BÄK), Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF), Kassenärztliche Bundesvereinigung (KBV) Nationale VersorgungsLeitlinie Chronische Herzinsuffizienz - Langfassung, Version 2. doi.org/10.6101/AZQ/000390 (last accessed on 20 September 2017) (2) [Google Scholar]
- 3.Bundesärztekammer (BÄK), Kassenärztliche Bundesvereinigung (KBV) Beurteilungskriterien für Leitlinien in der medizinischen Versorgung - Beschlüsse der Vorstände der Bundesärztekammer und Kassenärztlicher Bundesvereinigung, Juni 1997. Dtsch Arztebl. 1997;94:A2154–A2155. [Google Scholar]
- 4.Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF) München Zuckschwerdt: 2012. Das AWMF-Regelwerk Leitlinien. [Google Scholar]
- 5.Qaseem A, Forland F, Macbeth F, Ollenschläger G, Phillips S, van der Wees P. Guidelines international network: toward international standards for clinical practice guidelines. Ann Intern Med. 2012;156:525–531. doi: 10.7326/0003-4819-156-7-201204030-00009. [DOI] [PubMed] [Google Scholar]
- 6.Bundesärztekammer (BÄK), Kassenärztliche Bundesvereinigung (KBV), Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF) Nationales Programm für VersorgungsLeitlinien. Methoden-Report, edition. leitlinien.de/mdb/downloads/nvl/methodik/mr-aufl-4-version-1.pdf (last accessed on 26 June 2017) (4) [Google Scholar]
- 7.Bundesärztekammer (BÄK), Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF), Kassenärztliche Bundesvereinigung (KBV) Nationale VersorgungsLeitlinie Chronische Herzinsuffizienz - Leitlinienreport, Version 2. doi.org/10.6101/AZQ/000391 (last accessed on 20 September 2017) (2) [Google Scholar]
- 8.Bundesärztekammer (BÄK), Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften (AWMF), Kassenärztliche Bundesvereinigung (KBV) Nationale VersorgungsLeitlinie Chronische Herzinsuffizienz - Langfassung, Version 7. doi.org/10.6101/AZQ/000166 (last accessed on 20 September 2017) (1) [Google Scholar]
- 9.Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen (IQWiG) Systematische Leitlinienrecherche und -bewertung sowie Extraktion relevanter Empfehlungen für ein DMP Chronische Herzinsuffizienz. Abschlussbericht. Auftrag V14-01. Version 1.0. IQWiG-Berichte, Nr. 342. www.iqwig.de/download/V14-01_Abschlussbericht_Leitlinienrecherche-und-bewertung-fuer-ein-DMP-Chronische-Herzinsuffizienz.pdf (last accessed on 16 March 2016) [Google Scholar]
- 10.Ponikowski P, Anker SD, Voors AA, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs J, Busch M, Lange C, Scheidt-Nave C. Prevalence and patterns of morbidity among adults in Germany Results of the German telephone health interview survey German Health Update (GEDA) 2009. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2012;55:576–586. doi: 10.1007/s00103-012-1464-9. [DOI] [PubMed] [Google Scholar]
- 12.Swedberg K, Komajda M, Bohm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875–885. doi: 10.1016/S0140-6736(10)61198-1. [DOI] [PubMed] [Google Scholar]
- 13.Swedberg K, Komajda M, Bohm M, et al. Effects on outcomes of heart rate reduction by ivabradine in patients with congestive heart failure: is there an influence of beta-blocker dose? Findings from the SHIFT (Systolic Heart failure treatment with the I(f) inhibitor ivabradine Trial) study. J Am Coll Cardiol. 2012;59:1938–1945. doi: 10.1016/j.jacc.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 14.Martin RI, Pogoryelova O, Koref MS, Bourke JP, Teare MD, Keavney BD. Atrial fibrillation associated with ivabradine treatment: meta-analysis of randomised controlled trials. Heart. 2014;100:1506–1510. doi: 10.1136/heartjnl-2014-305482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2016;68:1476–1488. doi: 10.1016/j.jacc.2016.05.011. [DOI] [PubMed] [Google Scholar]
- 16.McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 17.Cleland JG, Abraham WT, Linde C, et al. An individual patient meta-analysis of five randomized trials assessing the effects of cardiac resynchronization therapy on morbidity and mortality in patients with symptomatic heart failure. Eur Heart J. 2013;34:3547–3556. doi: 10.1093/eurheartj/eht290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curtis AB, Worley SJ, Adamson PB, et al. Biventricular pacing for atrioventricular block and systolic dysfunction. N Engl J Med. 2013;368:1585–1593. doi: 10.1056/NEJMoa1210356. [DOI] [PubMed] [Google Scholar]
- 19.Funck RC, Blanc JJ, Mueller HH, Schade-Brittinger C, Bailleul C, Maisch B. Biventricular stimulation to prevent cardiac desynchronization: rationale, design, and endpoints of the ‚Biventricular Pacing for Atrioventricular Block to Prevent Cardiac Desynchronization (Biopace)‘ study. Europace. 2006;8:629–635. doi: 10.1093/europace/eul075. [DOI] [PubMed] [Google Scholar]
- 20.Blanc JJ. Biopace trial preliminary results ESC congress 2014: presentation. www.clinicaltrialresults.org/Slides/TCT%202014/Blanc_Biopace.pdf (last accessed on 30 January 2017) [Google Scholar]
- 21.Connolly SJ, Gent M, Roberts RS, et al. Canadian Implantable Defibrillator Study (CIDS): a randomized trial of the implantable cardioverter defibrillator against amiodarone. Circulation. 2000;101:1297–1302. doi: 10.1161/01.cir.101.11.1297. [DOI] [PubMed] [Google Scholar]
- 22.Zipes DP, Wyse DG, Friedman PL. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. N Engl J Med. 1997;337:1576–1583. doi: 10.1056/NEJM199711273372202. [DOI] [PubMed] [Google Scholar]
- 23.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 24.Kober L, Thune JJ, Nielsen JC, et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;375:1221–1230. doi: 10.1056/NEJMoa1608029. [DOI] [PubMed] [Google Scholar]
- 25.Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 26.Woods B, Hawkins N, Mealing S, et al. Individual patient data network meta-analysis of mortality effects of implantable cardiac devices. Heart. 2015;101:1800–1806. doi: 10.1136/heartjnl-2015-307634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barra S, Providencia R, Tang A, Heck P, Virdee M, Agarwal S. Importance of implantable cardioverter-defibrillator back-up in cardiac resynchronization therapy recipients: a systematic review and meta-analysis. J Am Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.002539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu ZY, Zhang J, Xu ZT, et al. Efficiencies and complications of dual chamber versus single chamber implantable cardioverter defibrillators in secondary sudden cardiac death prevention: a meta-analysis. Heart Lung Circ. 2016;25:148–154. doi: 10.1016/j.hlc.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Velazquez EJ, Lee KL, Jones RH, et al. Coronary-artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med. 2016;374:1511–1520. doi: 10.1056/NEJMoa1602001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feltner C, Jones CD, Cene CW, et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta-analysis. Ann Intern Med. 2014;160:774–784. doi: 10.7326/M14-0083. [DOI] [PubMed] [Google Scholar]
- 31.Inglis SC, Clark RA, Dierckx R, Prieto-Merino D, Cleland JGF. Structured telephone support or non-invasive telemonitoring for patients with heart failure. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD007228.pub3. CD007228. [DOI] [PMC free article] [PubMed] [Google Scholar]