Abstract
Introduction
Given the challenges concerning the differential diagnosis of dementia, we investigated the possible added value of monoaminergic compounds to the standard cerebrospinal fluid (CSF) Alzheimer's disease (AD) biomarkers. Particularly, regarding the AD versus dementia with Lewy bodies (DLB) comparison, monoamines or their metabolites might have added discriminative value as there is a more severe neuropathological burden in the locus coeruleus of DLB patients, the principal site of noradrenaline synthesis.
Methods
We applied enzyme-linked immunosorbent assay (ELISA) to analyze CSF amyloid β peptide of 42 amino acids, total tau, and tau phosphorylated at threonine 181, in patients with AD, frontotemporal dementia, DLB/Parkinson's disease dementia, and controls. Reversed-phase high-performance liquid chromatography with electrochemical detection was implemented to study monoamine and metabolite levels in CSF and serum. Stepwise forward conditional logistic regression and receiver operating characteristic (ROC) curve analyses were performed to assess the diagnostic accuracy of these newly fitted models containing the most discriminative indicators of disease status.
Results
Most significant differences in CSF and serum were confined to the noradrenergic system. More specifically, CSF 3-methoxy-4-hydroxyphenylglycol (MHPG) levels were higher, whereas serum MHPG levels were lower, in DLB patients compared with all other groups. Addition of CSF and serum MHPG levels to the CSF AD biomarker panel significantly increased diagnostic accuracy between DLB/Parkinson's disease dementia and AD. Interestingly, a model only including CSF and serum MHPG without the classic AD biomarker panel reached similar area under the curve values.
Discussion
We hypothesize that varying degrees of neuronal loss in the locus coeruleus of DLB/Parkinson's disease dementia versus AD patients result in differentially altered MHPG levels, making this metabolite a valuable biomarker.
Keywords: MHPG, Biomarkers, Alzheimer's disease, Dementia with Lewy bodies, Monoamines, Diagnostic accuracy, RP-HPLC-ECD
1. Introduction
Alzheimer's disease (AD) and associated neurodegenerative brain disorders remain an important health-care burden [1]. Recent findings from the Alzheimer's Association indicate that in 2015, 46.8 million people suffered from dementia worldwide, while this number is expected to increase in the next couple of decades [2], giving rise to even higher health strategies. Early detection of this neurocognitive disorder, combined with treatment strategies in the initial stages of the disease could aid in its reduction [3], [4].
Although current cerebrospinal fluid (CSF) biomarkers for AD diagnosis (amyloid β [Aβ1–42], total tau (T-tau), and tau phosphorylated at threonine 181 [P-tau181P]), as comprised in the International Working Group-2 criteria [5], are widely used in clinical research, they still bring about several challenges. As such, they lack specificity to accurately discriminate between AD and dementia with Lewy bodies (DLB), which is especially complicated by the presence of AD co-pathology in patients with DLB [6]. Thus, apart from shared clinical symptoms, some dementia types can also share a common etiology. Because overlapping concentrations exist in CSF T-tau and P-tau181P between AD, frontotemporal dementia (FTD), DLB, and vascular AD [7], current diagnostics often require additional imaging investigations for differential dementia diagnosis. Another pitfall associated with CSF biomarkers is that their diagnostic performance decreases with age [8]. Given the aforementioned complications and the practical difficulties associated with CSF sampling, the search for efficient blood biomarkers is imperative [9], [10]. Nevertheless, variability of the distinct blood constituents involves supplementary challenges concerning reproducibility of biomarker analysis. In addition, questions arise about the applicability of blood biomarkers as the blood compartment is not in direct contact with the central nervous system and might therefore inaccurately reflect changes in disease progression [10]. Moreover, it was shown that plasma and CSF Aβ1–42 levels did not correlate in either patients with AD, non-AD, mild cognitive impairment, and control subjects (CONTR) [11]. Although a recently published article provided the first evidence for plasma neurofilament light as a potential blood biomarker for AD [12], such a blood biomarker for discrimination between AD and non-AD cases, to the best of our knowledge, has not been identified nor validated yet.
Recent studies, however, indicate that monoaminergic neurotransmitter profiles could represent an added value in improving etiological dementia diagnosis [13]. One of the first indications of this hypothesis was provided by Aerts et al., who proved that addition of CSF 3-methoxy-4-hydroxyphenylglycol (MHPG), a main metabolite of the monoamines adrenaline (A) and noradrenaline (NA) that aids in indication of central noradrenergic activity [14], to the classical biomarker profile of AD, could increase both sensitivity and specificity for the discrimination between AD and DLB [15], [16]. This hypothesis might be further strengthened by the notion that distinct MHPG levels between AD and DLB patients were observed in eight out of 11 brain regions, with DLB patients demonstrating significantly reduced MHPG levels [13]. Other studies investigating monoamine neurotransmitter levels in brain tissue equally gave rise to the awareness that AD and FTD differ in serotonergic and noradrenergic neurotransmitter content [17], [18], while an earlier study reported that CSF MHPG levels were considerably higher in FTD patients than those in AD patients [19]. It was also noted that CSF NA and MHPG levels were increased in patients with advanced AD as compared with subjects suffering from moderate AD or CONTR, suggesting hyperactivity of the noradrenergic system in the end stage of the disease [20]. Furthermore, extensive evidence demonstrates that the locus coeruleus (LC), the main NA-producing nucleus in the brain, is severely affected by Lewy pathology in Parkinson's disease dementia (PDD) [21] and associated with severe cell death which might affect the dopaminergic nigrostriatal pathway through loss of noradrenergic innervation [22], [23], [24], [25]. This Lewy pathology in PDD has even been shown to precede the appearance of α-synuclein inclusions and neuronal loss in the dopaminergic substantia nigra [21], [26], [27], [28], [29], [30], [31], indicating an undeniable role of noradrenergic deficits in PDD. Interestingly, MHPG easily passes the blood–brain [32] and blood-CSF [33] barrier. Taking into account all of the above, it appears that monoaminergic systems are indeed differentially implicated in distinct dementia subtypes and could potentially serve as predictive markers.
Accordingly, this study aimed at identifying predictive monoamine biomarkers in both CSF and serum derived from patients suffering from AD, FTD, DLB/PDD, age-matched CONTR, and young control (Y-CONTR) subjects. We hypothesized that these fluid monoamine markers, especially with regard to MHPG, could add significantly to the classical CSF AD biomarker panel, thus increasing diagnostic accuracy.
2. Materials & methods
2.1. Study population
Paired CSF-serum samples derived from patients with probable AD (n = 52), FTD (n = 59), DLB (n = 39), PDD (n = 14), as well as CONTR (n = 88) and Y-CONTR (n = 32), were selected from the Biobank of the Institute Born-Bunge. All patients included in the AD, FTD, DLB, PDD, and Y-CONTR groups were included in a prospective, longitudinal study on neuropsychiatric symptoms [34] between 2001 and 2011 and originally recruited at the Memory Clinic of the Hospital Network Antwerp Middelheim (ZNA) and Hoge Beuken as part of their diagnostic clinical workup. At inclusion, subjects underwent neuropsychological assessment and behavioral analysis as described earlier [34]. If consented patients died, brain autopsy was performed within 6 hour postmortem. The left hemisphere was frozen at −80°C, whereas the right hemisphere was fixated in paraformaldehyde (12%) for neuropathological examination, which was performed as described earlier [13], [17]. None of the age-matched CONTR nor Y-CONTR suffered from neurological disease. In addition, CONTR were excluded in case of psychiatric antecedents or suspicion of central nervous system pathology. Thus, the CONTR group consisted of patients requiring lumbar radiculography as they suffered from mechanical low back pain, subjects with peripheral nervous system disorders, and patients with subjective complaints which were not due to disorders of the central nor the peripheral nervous system [35]. Finally, the study was approved by the Medical Ethical Committee of the Middelheim General Hospital (Antwerp, Belgium; approval numbers 2805 and 2806) and conducted in compliance with the Helsinki Declaration.
2.2. CSF and serum sampling
Sampling of CSF was performed according to Vermeiren et al. [36]. In short, a lumbar puncture was performed at the L3/L4 or L4/L5 intervertebral space between 8.00 and 10.00 am. Patients and CONTR fasted overnight and abstained from smoking for at least 12 hours. In total, 16.5 mL CSF was collected in five fractions using polypropylene vials as described by Engelborghs et al. [35].
Total blood was sampled into two serum gel tubes coated with clotting activator (S-Monovette 7.5 mL Z-gel [Sarstedt, Nümbrecht, Germany]) and centrifuged at 3000 rpm for 10 minutes. Serum aliquots were subsequently distributed to marked polypropylene vials and frozen in liquid nitrogen. Of note, part of the CONTR group (n = 43) followed the protocol specified previously (i.e., matched CSF-serum samples after overnight fasting) [34], whereas serum-only samples of the remaining 45 CONTR were obtained at different time points and under a distinct clinical setting.
All samples were stored in the Biobank of the Institute Born-Bunge at −80°C.
2.3. CSF Aβ1–42, T-tau, and P-tau181P analysis using ELISA
CSF analyses of Aβ1–42, T-tau, and P-tau181P were performed by means of ELISA, as part of a clinical diagnostic workup. Cutoff values were derived from the lower and upper detection limits inherent to the ELISA kits (INNOTEST β amyloid(1–42), INNOTEST hTAU-Ag, and INNOTEST PHOSPHO-TAU(181P) for CSF Aβ1–42, T-tau, and P-tau181P, respectively [Fujirebio, Ghent, Belgium]), that is, 125 pg/mL and 2000 pg/mL for Aβ1–42, 75 pg/mL and 1200 pg/mL for T-tau, and 15.6 pg/mL and 500 pg/mL for P-tau181P. The detailed CSF analysis protocol has already been published by Le Bastard et al. [37].
2.4. Reversed-phase high-performance liquid chromatography with electrochemical detection
Tryptophan and the monoamines, dopamine (DA), serotonin (5-HT), A and NA, as well as their respective metabolites (homovanillic acid and 3,4-dihydroxyphenylacetic acid, 5-hydroxyindoleacetic acid [5-HIAA], and MHPG) were analyzed in paired CSF-serum samples by means of an optimized and validated reversed-phase high-performance liquid chromatography system with electrochemical detection (ALEXYS Monoamine Analyzer; Antec Leyden B.V., Zoeterwoude, Netherlands) [38]. The sample preparation protocol was standardized and consisted of a precolumn purification using Amicon Ultra 0.5 Centrifugal Filters (cutoff 3000 Da; Millipore, Ireland), washed twice with 450 μL sample buffer during centrifugation (14,000 × g, 25 minutes, 4°C). Subsequently, CSF and serum samples were loaded onto the prewetted columns and centrifuged at 4°C for 40 minutes at 14,000 × g. The filtrate was diluted 1:2 and 1:7 for CSF, while 1:4 and 1:15 dilutions were implemented for serum. Finally, diluted samples were injected automatically onto an ALF-125 column (C18; 250 mm × 1.0 mm, 3 μm particle size). Further specifications of the reversed-phase high-performance liquid chromatography with electrochemical detection procedure have been described by Van Dam et al. [38].
2.5. Statistics
As our data set was characterized by non-normally distributed variables, nonparametric statistical tests were performed. All continuous variables were tested for differences between diagnostic classes using the Kruskal–Wallis test, followed by a post hoc analysis using the Mann–Whitney U tests with a Bonferroni correction for multiple comparisons (P < .005). In addition, the association between the diagnostic classes and categorical variables such as gender was tested using the chi-square test. If the expected cell count was less than five, Fisher's exact test was used.
To identify the most discriminative indicators of disease status, stepwise forward conditional logistic regression analysis was applied, with disease status as dependent variable and a combination of standard AD biomarkers and monoamines or metabolites as explanatory variables. Age was included in every regression model. Subsequently, area under the curve (AUC) values belonging to the respective ROC curves of models fitted with and without the addition of monoamines and/or metabolites were compared by performing DeLong tests. Finally, optimal cutoff values were determined by maximization of the Youden's index [39].
All statistical analyses, except for DeLong tests, were performed using SPSS 24.0 for Windows (IBM SPSS Software; IBM Corp, Armonk, NY). The DeLong test was carried out as implemented in a ROC package for R, version 3.4.0 for Windows, specifically designed to investigate partial AUC values (pROC). [40].
3. Results
3.1. Demographical and clinical data
Table 1 contains corresponding demographical and clinical information.
Table 1.
Demographic and clinical data of the study population
| Parameter | AD (n = 52) | FTD (n = 59) | DLB/PDD (n = 53) | CONTR (n = 88) | Y-CONTR (n = 32) | Test statistics |
|---|---|---|---|---|---|---|
| Age at sampling (y) | 75.5 ± 8.2aa,ddd (56.0–89.1) (n = 52) |
68.8 ± 9.5aa,eee,f,ggg (40.8–83.3) (n = 59) |
76.6 ± 6.0eee,iii (61.5–88.5) (n = 53) |
73.8 ± 10.7f,jjj (50.4–92.8) (n = 88) |
38.2 ± 8.8ddd,ggg,iii,jjj (17.1–50.0) (n = 32) |
X2 = 100.2 P < .00001 |
| MMSE (/30) | 13.7 ± 5.8aa,ccc (3.0–25.0) (n = 39) |
19.1 ± 7.3aa,fff (1.0–30.0) (n = 43) |
17.2 ± 6.6hhh (3.0–28.0) (n = 45) |
28.2 ± 1.6ccc,fff,hhh (24.0–30.0) (n = 39) |
N/A | X2 = 84.2 P < .00001 |
| HDS (/10) | 6.9 ± 1.3ccc (4.2–9.1) (n = 28) |
7.3 ± 1.7fff (2.9–9.8) (n = 31) |
8.0 ± 5.0hhh (2.4–34.0) (n = 32) |
9.7 ± 0.3ccc,fff,hhh (9.1–10.0) (n = 17) |
N/A | X2 = 40.1 P < .00001 |
| BNT (/60) | 24.2 ± 12.5a,bbb,ccc (4.0–48.0) (n = 28) |
34.9 ± 13.4a,fff (1.0–57.0) (n = 35) |
38.3 ± 10.3bbb,hhh (13.0–54.0) (n = 38) |
51.2 ± 4.7ccc,fff,hhh (36.0–57.0) (n = 23) |
N/A | X2 = 53.9 P < .00001 |
| VFT | 20.2 ± 10.6ccc (2.0–45.0) (n = 25) |
25.2 ± 15.2fff (0.0–56.0) (n = 31) |
24.1 ± 9.6hhh (6.0–49.0) (n = 34) |
51.0 ± 13.3ccc,fff,hhh (34.0–90.0) (n = 24) |
N/A | X2 = 48.2 P < .00001 |
| GDetS (/7) | 5.7 ± 0.7aaa,ccc (4.0–7.0) (n = 38) |
4.9 ± 1.0aaa,fff (3.0–7.0) (n = 33) |
5.2 ± 1.0hhh (3.0–7.0) (n = 38) |
1.5 ± 0.6ccc,fff,hhh (1.0–3.0) (n = 39) |
N/A | X2 = 97.5 P < .00001 |
| Gender (Male/Female) | 30/22 | 30/29 | 37/16 | 46/42 | 15/17 | Pearson X2 = 6.4 P = .47 |
| Psychotropic medication (N/Y) | 13/39 | 16/43 | 10/43 | 52/36 | 25/7 | Pearson X2 = 51.1 P < .00001 |
| Anti-Alzheimer's medication (N/Y) | 33/19 | 46/13 | 38/15 | 88/0 | 32/0 | Pearson X2 = 45.4 P < .00001 |
| Anti-Parkinson's medication (N/Y) | 50/2 | 55/4 | 28/25 | 88/0 | 32/0 | Pearson X2 = 90.1 P < .00001 |
| Hypnotic, sedative or anxiolytic medication (N/Y) | 42/10 | 46/13 | 41/12 | 69/19 | 29/3 | Pearson X2 = 2.8 P = .59 |
| Antidepressant medication (N/Y) | 34/18 | 41/18 | 34/19 | 67/21 | 27/5 | Pearson X2 = 6.0 P = .20 |
| Antipsychotic medication (N/Y) | 29/23 | 34/25 | 40/13 | 86/2 | 32/0 | Pearson X2 = 58.1 P < .00001 |
| Antiepileptic medication (N/Y) | 52/0 | 57/2 | 53/0 | 82/6 | 32/0 | Fisher's exact test = 6.6 P = .078 |
NOTE. Data are represented as mean ± SD with minimum-maximum ranges between brackets. Test statistics of the Kruskal–Wallis and chi-square (or Fisher's exact) analysis can be found in the rightmost column, while statistically significant differences with P ≤ .005, P ≤ .001, and P ≤ .0001 after M-W U analysis with post hoc Bonferroni corrections are depicted by one, two, or three superscript letters, respectively. Superscript letters denote differences between following groups, a: AD and FTD, b: AD and DLB/PDD, c: AD and CONTR, d: AD and Y-CONTR, e: FTD and DLB/PDD, f: FTD and CONTR, g: FTD and Y-CONTR, h: DLB/PDD and CONTR, i: DLB/PDD and Y-CONTR, and j: CONTR and Y-CONTR, respectively. Only cognitive test scores of no more than 4 months before date of sampling were included in the analyses.
Abbreviations: AD, Alzheimer's disease; BNT, Boston Naming Test; CONTR, controls; DLB/PDD, dementia with Lewy bodies/Parkinson's disease dementia; FTD, frontotemporal dementia; GDetS, Global Deterioration Scale; HDS, Hierarchic Dementia Scale; MMSE, Mini–Mental State Examination; N/A, not applicable; VFT, Verbal Fluency Test; Y-CONTR, young controls.
Whereas all patient groups were gender matched (P = .174), age of CSF and serum sampling was only comparable between the AD and DLB/PDD groups (P = .519), as well as between the AD and CONTR groups (P = .660) and DLB/PDD and CONTR groups (P = .469). Furthermore, significant differences were detected for cognitive test scores (P < .001) between all study groups. For instance, Mini–Mental State Examination scores were much lower in the AD group than those in DLB/PDD, FTD, and CONTR groups. Indication of disease stage by global deterioration scale scores indicated that AD patients were somewhat more advanced than DLB/PDD and FTD counterparts. Scores of the neuropsychological assessment were only included if there were no more than 4 months between the moment of testing and sampling. In total, 42 out of 52 AD, 15 out of 59 FTD, and, nine out of 53 DLB/PDD patients, respectively, had neuropathological confirmation of their clinical diagnoses. In addition, out of the nine neuropathologically defined DLB patients, seven had concomitant AD pathology.
Administered classes of psychotropic medication can be found in Table 1 for each diagnostic category.
3.2. Neurochemical comparisons of biomarkers and monoamines
We found statistically significant differences in CSF levels of Aβ1–42 (P < .0001), T-tau (P < .0001), and P-Tau181P (P = .001) for the AD versus FTD comparison, as well as between AD and DLB/PDD (P = .001, P < .0001, and P < .0001 for Aβ1–42, T-tau, and P-Tau181P, respectively), as well as between the AD and Y-CONTR groups (P < .0001 for all three biomarkers). Differences in concentrations of Aβ1–42 (P < .0001) and T-tau (P < .0001), but not P-Tau181P (P = .021), in AD versus CONTR subjects were also deemed significant. A complete description of biomarker levels between diagnostic categories can be found in Table 2.
Table 2.
Concentrations of classic CSF AD biomarkers
| Parameter | AD (n = 52) | FTD (n = 59) | DLB/PDD (n = 53) | CONTR (n = 88) | Y-CONTR (n = 32) | Test statistics |
|---|---|---|---|---|---|---|
| Aβ1–42 (pg/mL) | 432.3 ± 172.2aaa,bb,ccc,ddd (125.0–1159.0) (n = 47) |
680.1 ± 245.2aaa (282.0–1200.0) (n = 58) |
584.9 ± 235.4bb (274.0–1327.0) (n = 51) |
803.7 ± 275.9ccc (302.0–1212.0) (n = 19) |
1014.6 ± 162.2ddd (725.0–1265.0) (n = 16) |
X2 = 62.8 P < .00001 |
| T-tau (pg/mL) | 581.5 ± 285.2aaa,bbb,ccc,ddd (108.0–1200.0) (n = 47) |
362.8 ± 190.9aaa (97.0–956.0) (n = 58) |
304.2 ± 189.7bbb (17.0–1200.0) (n = 50) |
295.7 ± 144.7ccc (126.0–680.0) (n = 19) |
185.6 ± 57.0ddd (96.0–320.0) (n = 16) |
X2 = 49.3 P < .00001 |
| P-tau181P (pg/mL) | 71.0 ± 31.7aa,bbb,ddd (16.0–152.0) (n = 47) |
52.7 ± 24.6aa (19.0–116.0) (n = 58) |
50.1 ± 24.9bbb (19.0–151.0) (n = 50) |
53.4 ± 21.7 (23.0–110.0) (n = 19) |
37.1 ± 9.7ddd (21.0–52.0) (n = 16) |
X2 = 24.1 P < .0001 |
NOTE. Data represented as mean ± SD with minimum-maximum ranges between brackets. Test statistics of the Kruskal–Wallis analysis can be found in the rightmost column, while statistically significant differences with P ≤ .005, P ≤ .001, and P ≤ .0001 after M–W U analysis with Bonferroni post hoc corrections (P ≤ .0125) are depicted by one, two, and three superscript letters, respectively. Superscript letters denote differences between following groups, a: AD and FTD, b: AD and DLB/PDD, c: AD and CONTR, and, d: AD and Y-CONTR, respectively.
Abbreviations: Aβ1–42, amyloid β peptide of 42 amino acids; AD, Alzheimer's disease; CONTR, controls; DLB/PDD, dementia with Lewy bodies/Parkinson's disease dementia; FTD, frontotemporal dementia; P-tau181P, tau phosphorylated at threonine 181; T-tau, total tau; Y-CONTR, young controls.
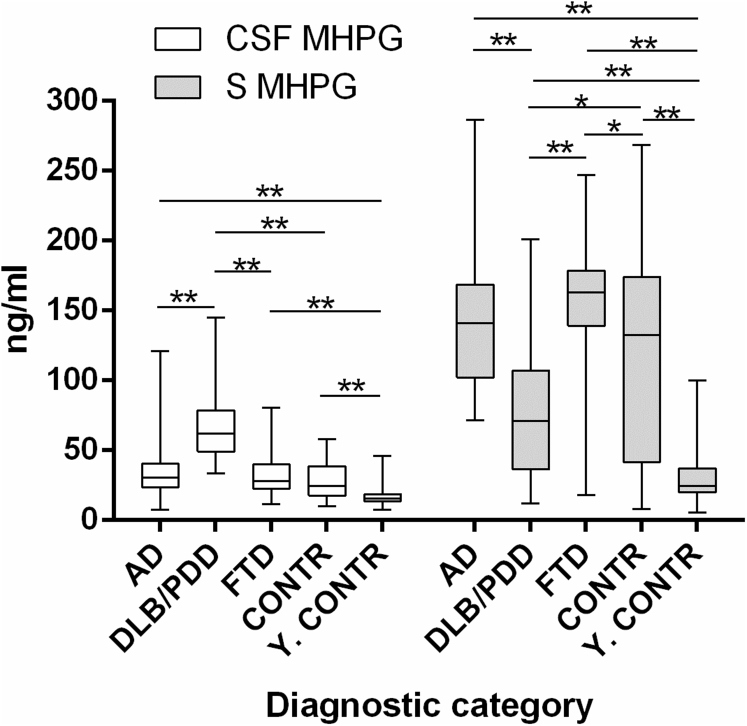
Similar analyses were performed in paired CSF and serum samples, taking into account all monoamines, their metabolites, and calculated ratios. In CSF, we found 48 significant differences between diagnostic categories, whereas 59 statistically significant distinctions could be identified in serum. Most of these differences were identified in the noradrenergic system and, therefore, we will only focus on MHPG in CSF and serum (Fig. 1). Significant differences in CSF MHPG were found when we compared all diagnostic categories (for all: P < .0001), except for the comparisons between AD and FTD, AD and CONTR, and FTD and CONTR. Likewise, distinct serum MHPG levels could be observed for all group comparisons (P ≤ .001), other than those between AD and FTD and AD and CONTR.
Fig. 1.
CSF and serum MHPG levels in all diagnostic categories. Data are represented as median ± IQR. Significant differences between groups after M-W U tests with post hoc Bonferroni correction (P < .005) are indicated by one (P = .001) or two asterisks (P < .001). Abbreviations: AD, Alzheimer's disease; CONTR, controls; CSF, cerebrospinal fluid; DLB/PDD, dementia with Lewy bodies/Parkinson's disease dementia; FTD, frontotemporal dementia; IQR, interquartile range; MHPG, 3-methoxy-4-hydroxyphenylglycol; M-W U, Mann–Whitney U; S, serum; Y-CONTR, young controls.
We refer to the Supplementary Table for the complete data set of CSF and serum monoamines for each diagnostic category.
3.3. Added value of monoamines on standard biomarkers
Table 3 contains diagnostic accuracy values corresponding to each fitted model.
Table 3.
Results of DeLong tests indicating discriminative power between distinct diagnostic categories
| BM-based discrimination between | Selected CSF BM | Selected CSF and/or serum MA or MT | AUC | 95% CI | S (%) | Sp (%) |
|---|---|---|---|---|---|---|
| Without MA-MT | ||||||
| AD and FTD | Aβ1–42/P-tau181P ratio* | N/A | 0.81 | 0.73–0.90 | 66 | 85 |
| AD and DLB/PDD | Aβ1–42, T-tau & P-tau181P | N/A | 0.82a(a) | 0.73–0.91 | 88 | 73 |
| AD and CONTR | Aβ1–42/T-tau ratio* | N/A | 0.88 | 0.75–1.00 | 89 | 82 |
| AD versus non-AD | Aβ1–42, T-tau & P-tau181P | N/A | 0.87a | 0.80–0.94 | 90 | 76 |
| With inclusion of MA-MT | ||||||
| AD and FTD | Aβ1–42/P-tau181P ratio* | CSF NA | 0.85 | 0.78–0.93 | 69 | 90 |
| AD and DLB/PDD | Aβ1–42, T-tau & P-tau181P N/A (without BM) |
CSF & serum MHPG CSF & serum MHPG |
0.99aa 0.98a |
0.97–1.0 0.95–1.0 |
98 98 |
95 95 |
| AD and CONTR | Aβ1–42/T-tau ratio* | CSF NA | 0.94 | 0.88–1.0 | 89 | 92 |
| AD versus non-AD | Aβ1–42, T-tau & P-tau181P | CSF NA & TRP and serum 5-HIAA | 0.95a | 0.91–0.99 | 97 | 82 |
NOTE. Optimal cutoff values were determined by maximizing the Youden's index. Superscript letters “a” added to the AUC values indicate statistical significance: aP < .005; aaP < .001. An asterisk indicates the inclusion of biomarker ratios rather than separate biomarker levels for comparisons between AD and both FTD and CONTR, as described earlier in Struyfs et al. [41].
Abbreviations: 5-HIAA, 5-hydroxyindoleacetic acid; Aβ1–42, amyloid β peptide of 42 amino acids; AD, Alzheimer's disease; AUC, area under the curve; BM, biomarkers; CI, confidence interval; CONTR, controls; CSF, cerebrospinal fluid; DLB/PDD, dementia with Lewy bodies/Parkinson's disease dementia; FTD, frontotemporal dementia; MHPG, 3-methoxy-4-hydroxyphenylglycol; MA & MT, monoamines and metabolites; N/A, not applicable; NA, noradrenaline; P-tau181P, tau phosphorylated at threonine 181; S, sensitivity; Sp, specificity; TRP, tryptophan; T-tau, total tau.
Besides the classic CSF biomarker set for AD, that is, Aβ1–42, T-tau, and P-Tau181P, as well as age of sampling, CSF and serum NA and its metabolite MHPG were among the most predictive markers. In case of distinctions between AD and DLB/PDD, we found that the AUC values differed significantly (P < .001) between the fitted models with and without addition of CSF (P < .001) and serum (P = .001) MHPG. When concentrations of only CSF and serum MHPG were included in a model without the classic AD biomarker panel, this difference could even be maintained (P = .002). Subanalysis in the group of neuropathologically characterized AD (n = 42) and DLB (n = 9) subjects confirmed previous finding, with the AUC value increasing from 0.70 to 0.99 (P < .001) if solely CSF and serum MHPG were included in the model instead of the core CSF AD biomarkers. Models fitted for comparisons between the AD group and both the FTD and CONTR groups could not be significantly improved by addition of CSF or serum monoamines. When an overall distinction between AD and non-AD was made, the model with addition of CSF NA (P = .002), serum 5-HIAA (P = .015), and CSF tryptophan (P = .020) was characterized by a significantly raised AUC (P = .004). Finally, none of the distinctions including Y-CONTR could be improved by addition of monoamines and/or metabolites, as sensitivity and specificity values already reached 100%.
3.4. Psychotropic medication comparisons: Effect on monoamines
Concerning the influence of medication on monoaminergic compounds, we found an expected effect of antidepressants on serotonergic compounds and ratios in all diagnostic categories except Y-CONTR. Significant differences could also be detected in DA levels in FTD (serum DA; P = .035) and DLB/PDD (CSF DA; P = .004) between patients taking and not taking anti-Alzheimer's medication. Overall, concentrations of DA were altered between patients taking and not taking psychotropic medication in CSF samples of the FTD group (P = .012) and serum samples of the DLB/PDD group (P = .025). In addition, noradrenergic alterations could be observed between patients on anti-Parkinson's medication and patients free of such medication, with CSF MHPG/NA ratios being significantly higher in patients not taking anti-Parkinson's drugs in AD (P = .048) and DLB/PDD groups (P = .005). Likewise, serum MHPG/NA ratios in DLB/PDD subjects were higher in patients not taking anti-Parkinson's drugs (P < .0001). In the same study group, CSF and serum NA levels were higher (P = .002 and P < .0001, respectively), in patients free of anti-Parkinson's medication. Finally, use of antipsychotic medication significantly influenced CSF (P = .015) and serum (P = .034) NA levels in the DLB/PDD group, as well as CSF (P = .027) and serum (P = .022) MHPG/NA ratios.
4. Discussion
Similar to our results, CSF MHPG was reported to increase discriminative power between AD and DLB, although this effect was not apparent in comparisons with other dementia types [15], [16]. Besides monoamines in CSF, we investigated whether such compounds in serum could equally serve as valuable blood biomarkers. Indeed, addition of serum MHPG and 5-HIAA proved to be useful in discriminating AD versus non-AD, increasing sensitivity and specificity values. Several studies have already indicated that noradrenergic cell loss in the LC is apparent in both AD [42], [43], [44] and DLB/PDD [45], [46]. Although both dementias are characterized by LC degeneration, cell loss in the brainstem has been reported to be more prominent in DLB/PDD [47]. The authors found a significantly lower number of tyrosine-hydroxylase labeled neurons at the 70% level of the LC in DLB patients compared with their AD counterparts, with the most rostral (0%) section defined as the beginning of the trochlear nucleus, and the most caudal end defined as the rostral edge of the trigeminal motor nucleus [48]. Moreover, two other studies also reported more severe, albeit not significant, cell loss in DLB/PDD compared with AD [45], [46]. Further evidence supporting these findings can be found in the fact that the LC is initially involved in DLB/PDD neuropathology, while AD patients at first show Aβ plaques in neo- and allocortical brain regions, with amyloid pathology only reaching the brainstem nuclei in more advanced stages, that is, Thal stages 4 and 5 [49], [50]. Tau pathology affects brainstem nuclei only in mid-to-late AD stages, coinciding with Braak stages IV–VI [51], [52]. Most patients suffering from DLB, as well as about half of PDD subjects, also show AD pathology [53], possibly exacerbated by the notion that the A53T mutant of α-synuclein, although rare [54], is able to promote the association of tau fibrils [55]. Taken together, these factors might result in a more severe noradrenergic cell loss in the LC due to a heavier neuropathological load. It was shown that in patients suffering from AD, this cell loss corresponded with an increased noradrenergic turnover (as a compensatory mechanism) in brain areas receiving efferent projections from the LC [20], [56], such as the hippocampus and amygdala [57]. Our results support this finding in CSF of AD and DLB patients, with increased MHPG/NA ratios, indicating increased noradrenergic turnover, in both conditions. Furthermore, we observed that these ratios were higher in DLB compared with AD patients (Supplementary Table). Latter event might be explained by a more severe neuropathological load in DLB patients, resulting in a more extensive compensatory mechanism.
Although a preceding study questioned the use of CSF MHPG as a reliable indicator of central noradrenergic activity given the diffusion of MHPG into spinal cord tissue [32], it was suggested that both CSF NA and MHPG levels reflected NA metabolism in the brain [20], [33], [58], [59], while plasma NA was hypothesized to mirror noradrenergic turnover of peripheral sympathetic neurons [20], [60]. A more recent study confirmed that MHPG, unlike NA [20], passes the blood–brain, as well as the blood-CSF barrier [61], thus strengthening latter theory. Still others suggested that degeneration of peripheral noradrenergic neurons may occur as a prodromal state of DLB and Parkinson's disease [62], [63], preceding neuropathological lesions in the LC. Another study reported that patients originally diagnosed with pure autonomic failure may later on convert to multiple system atrophy or DLB/PDD [63], [64]. Our results indeed indicated lowered NA and increased MHPG levels in the CSF of the DLB/PDD group, possibly reflecting the hypothesis that extensive damage to the LC results in lowered and increased brain NA and MHPG levels, respectively, which are mirrored in the CSF of these patients. In AD, we found a trend toward increased CSF MHPG levels, in addition to significantly decreased NA levels compared with CONTR.
The direction of change of NA and MHPG levels has been the subject of previous debate. For instance, levels of CSF NA and MHPG were found to be unchanged or enhanced in patients suffering from (severe) AD [16], [20], [65]. It has also been reported that CSF MHPG levels were unchanged or decreased in PDD [66] and decreased in DLB patients [16]. However, CSF NA levels between AD and DLB/PDD patients did not differ, which was in concordance with previous findings [36]. We hypothesize that we could not corroborate previous findings regarding CSF NA because this compound was influenced by several types of psychotropic medication in the DLB/PDD group, while little information pertaining to medication effects is provided in the study conducted by Herbert et al. (2014). Lastly, next to the proposed hypothesis, differences in study population characteristics and a considerable variation in CSF MHPG levels in the DLB group reported by Herbert et al. (2014), might also have caused this discrepancy compared with preceding studies. In serum, both diagnostic categories showed increased MHPG and NA concentrations, which may be explained by additional NA release by peripheral noradrenergic neurons, as well as peripheral NA metabolism. Conversely, patients suffering from pure autonomic failure are characterized by lowered plasma NA levels [63], as a result of autonomic dysfunction. This finding could not be corroborated in our study, possibly because serum is a large compartment in which several confounding biological processes occur, possibly masking previously mentioned effects.
One of the limitations of this study is the variation of sampling procedures in the control group, which could give rise to a considerable amount of variation in serum MHPG levels in this population (Fig. 1). In addition, the biochemical analysis of MHPG and NA, which are sensitive to preanalytical variability effects of temperature and oxidation, still remains challenging. We also found that medication use was not comparable between diagnostic categories, and, moreover, that psychotropic drug use might have influenced various monoaminergic compounds, such as the serotonergic and noradrenergic ones (see section 3.4). However, it should be taken into account that none of the serotonergic parameters influenced by antidepressant medication were included in the logistic regression models, with the sole exception of serum 5-HIAA for the distinction between AD and non-AD. Finally, none of the drug classes influenced concentrations of serum nor CSF MHPG, a crucial compound included in the newly fitted models. This is a strength of the study in addition to the considerable amount of available clinical and neuropsychological data, as well as the additional neuropathological confirmation.
5. Conclusion
We observed that CSF and serum monoamines have an added value in the differential diagnosis of dementia, next to the classical CSF AD biomarker panel. In particular, we found that CSF and serum MHPG were the most valuable markers to discriminate DLB/PDD from AD. To verify these findings, future studies should focus on age-matched, and, preferably, medication-free patient populations. Beforehand, various methodological and confounding aspects that might interfere with the analysis of CSF/serum MHPG, such as sample handling, lumbar puncture, and dietary effects, should be vigorously investigated.
Research in context.
-
1.
Systematic review: The establishment of dementia biomarkers in body fluids still faces difficulties. Previous work led to the discovery that cerebrospinal fluid (CSF) and serum 3-methoxy-4-hydroxyphenylglycol (MHPG) levels are altered in Alzheimer's disease (AD) and dementia with Lewy bodies/Parkinson's disease dementia patients and that addition of CSF MHPG to the AD biomarker panel improved diagnostic accuracy between AD and dementia with Lewy bodies/Parkinson's disease dementia.
-
2.
Interpretation: Our findings confirmed the added value of CSF MHPG in distinguishing AD and dementia with Lewy bodies/Parkinson's disease dementia, and, moreover, extended this conclusion to serum MHPG. These results correspond to the distinct degrees of neuropathological damage to the locus coeruleus.
-
3.
Future directions: To corroborate our conclusion, it is advisable that forthcoming studies investigate larger, fully neuropathologically characterized, medication-free patient populations. The quantification of the degree of locus coeruleus neuronal loss combined with CSF and serum MHPG analyses in various dementia subgroups might strengthen this hypothesis.
Acknowledgments
This research was supported by the Alzheimer Research Foundation Belgium (SAO-FRA; grant P#16003), Research Foundation Flanders (FWO), Interuniversity Poles of Attraction (IAP Network P7/16) of the Belgian Federal Science Policy Office, Methusalem excellence grant of the Flemish Government, agreement between Institute Born-Bunge and University of Antwerp, the Medical Research Foundation Antwerp, the Thomas Riellaerts research fund, Neurosearch Antwerp, and the Alzheimer Research Center of the University Medical Center Groningen (ARCG-UMCG).
The authors gratefully acknowledge the contribution and support of all patients, control subjects, relatives, caregivers, nursing and administrative personnel, and clinical staff involved.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.dadm.2018.01.002.
Supplementary data
References
- 1.Dharmarajan T.S., Gunturu S.G. Alzheimer's disease: a healthcare burden of epidemic proportion. Am Health Drug Benefits. 2009;2:39–47. [PMC free article] [PubMed] [Google Scholar]
- 2.Prince M., Wimo A., Guerchet M., Ali G.C., Wu Y., Prina A.M. Alzheimer's Disease International; London: 2015. World Alzheimer Report 2015. The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends. [Google Scholar]
- 3.Brookmeyer R., Johnson E., Ziegler-Graham K., Arrighi H.M. Forecasting the global burden of Alzheimer's disease. Alzheimers Dement. 2007;3:186–191. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 4.Robinson M., Lee B.Y., Hane F.T. Recent progress in Alzheimer's disease research, part 2: genetics and epidemiology. J Alzheimers Dis. 2017;57:317–330. doi: 10.3233/JAD-161149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubois B., Feldman H.H., Jacova C., Hampel H., Molinuevo J.L., Blennow K. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 6.Toledo J.B., Brettschneider J., Grossman M., Arnold S.E., Hu W.T., Xie S.X. CSF biomarkers cutoffs: the importance of coincident neuropathological diseases. Acta Neuropathol. 2012;124:23–35. doi: 10.1007/s00401-012-0983-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Deyn P.P. Dementia: Cerebrospinal fluid biomarkers in dementias. Nat Rev Neurol. 2015;11:549–550. doi: 10.1038/nrneurol.2015.175. [DOI] [PubMed] [Google Scholar]
- 8.Mattsson N., Rosen E., Hansson O., Andreasen N., Parnetti L., Jonsson M. Age and diagnostic performance of Alzheimer disease CSF biomarkers. Neurology. 2012;78:468–476. doi: 10.1212/WNL.0b013e3182477eed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Humpel C. Identifying and validating biomarkers for Alzheimer's disease. Trends Biotechnol. 2011;29:26–32. doi: 10.1016/j.tibtech.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Snyder H.M., Carrillo M.C., Grodstein F., Henriksen K., Jeromin A., Lovestone S. Developing novel blood-based biomarkers for Alzheimer's disease. Alzheimers Dement. 2014;10:109–114. doi: 10.1016/j.jalz.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Le Bastard N., Aerts L., Leurs J., Blomme W., De Deyn P.P., Engelborghs S. No correlation between time-linked plasma and CSF Abeta levels. Neurochem Int. 2009;55:820–825. doi: 10.1016/j.neuint.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Mattsson N., Andreasson U., Zetterberg H., Blennow K. Alzheimer's Disease Neuroimaging I. Association of plasma neurofilament light with neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 2017;74:557–566. doi: 10.1001/jamaneurol.2016.6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vermeiren Y., Van Dam D., Aerts T., Engelborghs S., Martin J.J., De Deyn P.P. The monoaminergic footprint of depression and psychosis in dementia with Lewy bodies compared to Alzheimer's disease. Alzheimers Res Ther. 2015;7:7. doi: 10.1186/s13195-014-0090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chase T.N., Gordon E.K., Ng L.K. Norepinephrine metabolism in the central nervous system of man: studies using 3-methoxy-4-hydroxyphenylethylene glycol levels in cerebrospinal fluid. J Neurochem. 1973;21:581–587. doi: 10.1111/j.1471-4159.1973.tb06003.x. [DOI] [PubMed] [Google Scholar]
- 15.Aerts M.B., Esselink R.A., Claassen J.A., Abdo W.F., Bloem B.R., Verbeek M.M. CSF tau, Abeta42, and MHPG differentiate dementia with Lewy bodies from Alzheimer's disease. J Alzheimers Dis. 2011;27:377–384. doi: 10.3233/JAD-2011-110482. [DOI] [PubMed] [Google Scholar]
- 16.Herbert M.K., Aerts M.B., Kuiperij H.B., Claassen J.A., Spies P.E., Esselink R.A. Addition of MHPG to Alzheimer's disease biomarkers improves differentiation of dementia with Lewy bodies from Alzheimer's disease but not other dementias. Alzheimers Dement. 2014;10:448–455.e2. doi: 10.1016/j.jalz.2013.05.1775. [DOI] [PubMed] [Google Scholar]
- 17.Vermeiren Y., Janssens J., Aerts T., Martin J.J., Sieben A., Van Dam D. Brain serotonergic and noradrenergic deficiencies in behavioral variant frontotemporal dementia compared to early-onset Alzheimer's disease. J Alzheimers Dis. 2016;53:1079–1096. doi: 10.3233/JAD-160320. [DOI] [PubMed] [Google Scholar]
- 18.Bowen D.M., Procter A.W., Mann D.M., Snowden J.S., Esiri M.M., Neary D. Imbalance of a serotonergic system in frontotemporal dementia: implication for pharmacotherapy. Psychopharmacology (Berl) 2008;196:603–610. doi: 10.1007/s00213-007-0992-8. [DOI] [PubMed] [Google Scholar]
- 19.Sjogren M., Minthon L., Passant U., Blennow K., Wallin A. Decreased monoamine metabolites in frontotemporal dementia and Alzheimer's disease. Neurobiol Aging. 1998;19:379–384. doi: 10.1016/s0197-4580(98)00086-4. [DOI] [PubMed] [Google Scholar]
- 20.Raskind M.A., Peskind E.R., Halter J.B., Jimerson D.C. Norepinephrine and MHPG levels in CSF and plasma in Alzheimer's disease. Arch Gen Psychiatry. 1984;41:343–346. doi: 10.1001/archpsyc.1984.01790150033006. [DOI] [PubMed] [Google Scholar]
- 21.Del Tredici K., Braak H. Dysfunction of the locus coeruleus-norepinephrine system and related circuitry in Parkinson's disease-related dementia. J Neurol Neurosurg Psychiatry. 2013;84:774–783. doi: 10.1136/jnnp-2011-301817. [DOI] [PubMed] [Google Scholar]
- 22.Gesi M., Soldani P., Giorgi F.S., Santinami A., Bonaccorsi I., Fornai F. The role of the locus coeruleus in the development of Parkinson's disease. Neurosci Biobehav Rev. 2000;24:655–668. doi: 10.1016/s0149-7634(00)00028-2. [DOI] [PubMed] [Google Scholar]
- 23.Mavridis M., Degryse A.D., Lategan A.J., Marien M.R., Colpaert F.C. Effects of locus coeruleus lesions on parkinsonian signs, striatal dopamine and substantia nigra cell loss after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine in monkeys: a possible role for the locus coeruleus in the progression of Parkinson's disease. Neuroscience. 1991;41:507–523. doi: 10.1016/0306-4522(91)90345-o. [DOI] [PubMed] [Google Scholar]
- 24.Fornai F., Bassi L., Torracca M.T., Scalori V., Corsini G.U. Norepinephrine loss exacerbates methamphetamine-induced striatal dopamine depletion in mice. Eur J Pharmacol. 1995;283:99–102. doi: 10.1016/0014-2999(95)00313-a. [DOI] [PubMed] [Google Scholar]
- 25.Marien M., Briley M., Colpaert F. Noradrenaline depletion exacerbates MPTP-induced striatal dopamine loss in mice. Eur J Pharmacol. 1993;236:487–489. doi: 10.1016/0014-2999(93)90489-5. [DOI] [PubMed] [Google Scholar]
- 26.Del Tredici K., Rub U., De Vos R.A., Bohl J.R., Braak H. Where does parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol. 2002;61:413–426. doi: 10.1093/jnen/61.5.413. [DOI] [PubMed] [Google Scholar]
- 27.van de Berg W.D., Hepp D.H., Dijkstra A.A., Rozemuller J.A., Berendse H.W., Foncke E. Patterns of alpha-synuclein pathology in incidental cases and clinical subtypes of Parkinson's disease. Parkinsonism Relat Disord. 2012;18 Suppl 1:S28–30. doi: 10.1016/S1353-8020(11)70011-6. [DOI] [PubMed] [Google Scholar]
- 28.Miki Y., Mori F., Wakabayashi K., Kuroda N., Orimo S. Incidental Lewy body disease restricted to the heart and stellate ganglia. Mov Disord. 2009;24:2299–2301. doi: 10.1002/mds.22775. [DOI] [PubMed] [Google Scholar]
- 29.Markesbery W.R., Jicha G.A., Liu H., Schmitt F.A. Lewy body pathology in normal elderly subjects. J Neuropathol Exp Neurol. 2009;68:816–822. doi: 10.1097/NEN.0b013e3181ac10a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dickson D.W., Uchikado H., Fujishiro H., Tsuboi Y. Evidence in favor of Braak staging of Parkinson's disease. Mov Disord. 2010;25 Suppl 1:S78–82. doi: 10.1002/mds.22637. [DOI] [PubMed] [Google Scholar]
- 31.Bloch A., Probst A., Bissig H., Adams H., Tolnay M. Alpha-synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol. 2006;32:284–295. doi: 10.1111/j.1365-2990.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- 32.Kessler J.A., Fenstermacher J.D., Patlak C.S. 3-Methoxy-4-hydroxyphenylethyleneglycol (mhpg) transport from the spinal cord during spinal subarachnoid perfusion. Brain Res. 1976;102:131–141. doi: 10.1016/0006-8993(76)90579-5. [DOI] [PubMed] [Google Scholar]
- 33.Kopin I.J., Gordon E.K., Jimerson D.C., Polinsky R.J. Relation between plasma and cerebrospinal fluid levels of 3-methoxy-4-hydroxyphenylglycol. Science. 1983;219:73–75. doi: 10.1126/science.6849119. [DOI] [PubMed] [Google Scholar]
- 34.Engelborghs S., Maertens K., Nagels G., Vloeberghs E., Marien P., Symons A. Neuropsychiatric symptoms of dementia: cross-sectional analysis from a prospective, longitudinal Belgian study. Int J Geriatr Psychiatry. 2005;20:1028–1037. doi: 10.1002/gps.1395. [DOI] [PubMed] [Google Scholar]
- 35.Engelborghs S., De Vreese K., Van de Casteele T., Vanderstichele H., Van Everbroeck B., Cras P. Diagnostic performance of a CSF-biomarker panel in autopsy-confirmed dementia. Neurobiol Aging. 2008;29:1143–1159. doi: 10.1016/j.neurobiolaging.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 36.Vermeiren Y., Le Bastard N., Van Hemelrijck A., Drinkenburg W.H., Engelborghs S., De Deyn P.P. Behavioral correlates of cerebrospinal fluid amino acid and biogenic amine neurotransmitter alterations in dementia. Alzheimers Dement. 2013;9:488–498. doi: 10.1016/j.jalz.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 37.Le Bastard N., Aerts L., Sleegers K., Martin J.J., Van Broeckhoven C., De Deyn P.P. Longitudinal stability of cerebrospinal fluid biomarker levels: fulfilled requirement for pharmacodynamic markers in Alzheimer's disease. J Alzheimers Dis. 2013;33:807–822. doi: 10.3233/JAD-2012-110029. [DOI] [PubMed] [Google Scholar]
- 38.Van Dam D., Vermeiren Y., Aerts T., De Deyn P.P. Novel and sensitive reversed-phase high-pressure liquid chromatography method with electrochemical detection for the simultaneous and fast determination of eight biogenic amines and metabolites in human brain tissue. J Chromatogr A. 2014;1353:28–39. doi: 10.1016/j.chroma.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 39.Youden W.J. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 40.Robin X., Turck N., Hainard A., Tiberti N., Lisacek F., Sanchez J.C. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;77 doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Struyfs H., Niemantsverdriet E., Goossens J., Fransen E., Martin J.J., De Deyn P.P. Cerebrospinal fluid P-Tau181P: biomarker for improved differential dementia diagnosis. Front Neurol. 2015;6:138. doi: 10.3389/fneur.2015.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kelly S.C., He B., Perez S.E., Ginsberg S.D., Mufson E.J., Counts S.E. Locus coeruleus cellular and molecular pathology during the progression of Alzheimer's disease. Acta Neuropathol Commun. 2017;5:8. doi: 10.1186/s40478-017-0411-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iversen L.L., Rossor M.N., Reynolds G.P., Hills R., Roth M., Mountjoy C.Q. Loss of pigmented dopamine-beta-hydroxylase positive cells from locus coeruleus in senile dementia of Alzheimer's type. Neurosci Lett. 1983;39:95–100. doi: 10.1016/0304-3940(83)90171-4. [DOI] [PubMed] [Google Scholar]
- 44.Bondareff W., Mountjoy C.Q., Roth M., Rossor M.N., Iversen L.L., Reynolds G.P. Neuronal degeneration in locus ceruleus and cortical correlates of Alzheimer disease. Alzheimer Dis Assoc Disord. 1987;1:256–262. doi: 10.1097/00002093-198701040-00005. [DOI] [PubMed] [Google Scholar]
- 45.Brunnstrom H., Friberg N., Lindberg E., Englund E. Differential degeneration of the locus coeruleus in dementia subtypes. Clin Neuropathol. 2011;30:104–110. doi: 10.5414/npp30104. [DOI] [PubMed] [Google Scholar]
- 46.Haglund M., Friberg N., Danielsson E.J., Norrman J., Englund E. A methodological study of locus coeruleus degeneration in dementing disorders. Clin Neuropathol. 2016;35:287–294. doi: 10.5414/NP300930. [DOI] [PubMed] [Google Scholar]
- 47.Szot P., White S.S., Greenup J.L., Leverenz J.B., Peskind E.R., Raskind M.A. Compensatory changes in the noradrenergic nervous system in the locus ceruleus and hippocampus of postmortem subjects with Alzheimer's disease and dementia with Lewy bodies. J Neurosci. 2006;26:467–478. doi: 10.1523/JNEUROSCI.4265-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hoogendijk W.J., Feenstra M.G., Botterblom M.H., Gilhuis J., Sommer I.E., Kamphorst W. Increased activity of surviving locus ceruleus neurons in Alzheimer's disease. Ann Neurol. 1999;45:82–91. doi: 10.1002/1531-8249(199901)45:1<82::aid-art14>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 49.Montine T.J., Phelps C.H., Beach T.G., Bigio E.H., Cairns N.J., Dickson D.W. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta Neuropathol. 2012;123:1–11. doi: 10.1007/s00401-011-0910-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thal D.R., Rub U., Orantes M., Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 51.Dugger B.N., Tu M., Murray M.E., Dickson D.W. Disease specificity and pathologic progression of tau pathology in brainstem nuclei of Alzheimer's disease and progressive supranuclear palsy. Neurosci Lett. 2011;491:122–126. doi: 10.1016/j.neulet.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 53.Marsh S.E., Blurton-Jones M. Examining the mechanisms that link beta-amyloid and alpha-synuclein pathologies. Alzheimers Res Ther. 2012;4:11. doi: 10.1186/alzrt109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klein C., Westenberger A. Genetics of Parkinson's disease. Cold Spring Harb Perspect Med. 2012;2:a008888. doi: 10.1101/cshperspect.a008888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kotzbauer P.T., Giasson B.I., Kravitz A.V., Golbe L.I., Mark M.H., Trojanowski J.Q. Fibrillization of alpha-synuclein and tau in familial Parkinson's disease caused by the A53T alpha-synuclein mutation. Exp Neurol. 2004;187:279–288. doi: 10.1016/j.expneurol.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 56.Winblad B., Adolfsson R., Carlsson A., Gottfries C.G. Biogenic amines in brains of patients with Alzheimer's disease. In: Corkin S., Davis K.L., Growdon J.H., Usolin E., Wurtman R.J., editors. Alzheimer's Disease: A Report of Progress in Research. Raven Press; New York: 1982. pp. 25–33. [Google Scholar]
- 57.Foote S.L., Bloom F.E., Aston-Jones G. Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev. 1983;63:844–914. doi: 10.1152/physrev.1983.63.3.844. [DOI] [PubMed] [Google Scholar]
- 58.Ziegler M.G., Lake C.R., Wood J.H., Ebert M.H. Norepinephrine in cerebrospinal fluid: basic studies, effects of drugs and disease. In: Wood J.H., editor. Neurobiology of Cerebrospinal Fluid. Plenum Press; New York: 1980. pp. 141–152. [Google Scholar]
- 59.Elsworth J.D., Redmond D.E., Jr., Roth R.H. Plasma and cerebrospinal fluid 3-methoxy-4-hydroxyphenylethylene glycol (MHPG) as indices of brain norepinephrine metabolism in primates. Brain Res. 1982;235:115–124. doi: 10.1016/0006-8993(82)90200-1. [DOI] [PubMed] [Google Scholar]
- 60.Ziegler M.G., Wood J.H., Lake R., Kopin I.J. Norepinephrine and 3-methoxy-4-hydroxyphenyl glycol gradients in human cerebrospinal fluid. Am J Psychiatry. 1977;134:565–568. doi: 10.1176/ajp.134.5.565. [DOI] [PubMed] [Google Scholar]
- 61.Sharma R.P., Javaid J.I., Faull K., Davis J.M., Janicak P.G. CSF and plasma MHPG, and CSF MHPG index: pretreatment levels in diagnostic groups and response to somatic treatments. Psychiatry Res. 1994;51:51–60. doi: 10.1016/0165-1781(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 62.Kaufmann H., Nahm K., Purohit D., Wolfe D. Autonomic failure as the initial presentation of Parkinson disease and dementia with Lewy bodies. Neurology. 2004;63:1093–1095. doi: 10.1212/01.wnl.0000138500.73671.dc. [DOI] [PubMed] [Google Scholar]
- 63.Kaufmann H., Norcliffe-Kaufmann L., Palma J.A., Biaggioni I., Low P.A., Singer W. The natural history of pure autonomic failure: a United States prospective cohort. Ann Neurol. 2017;81:287–297. doi: 10.1002/ana.24877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Singer W., Berini S.E., Sandroni P., Fealey R.D., Coon E.A., Suarez M.D. Pure autonomic failure: predictors of conversion to clinical CNS involvement. Neurology. 2017;88:1129–1136. doi: 10.1212/WNL.0000000000003737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martignoni E., Bono G., Blandini F., Sinforiani E., Merlo P., Nappi G. Monoamines and related metabolite levels in the cerebrospinal fluid of patients with dementia of Alzheimer type. Influence of treatment with L-deprenyl. J Neural Transm Park Dis Dement Sect. 1991;3:15–25. doi: 10.1007/BF02251133. [DOI] [PubMed] [Google Scholar]
- 66.Jimenez-Jimenez F.J., Alonso-Navarro H., Garcia-Martin E., Agundez J.A. Cerebrospinal fluid biochemical studies in patients with Parkinson's disease: toward a potential search for biomarkers for this disease. Front Cell Neurosci. 2014;8:369. doi: 10.3389/fncel.2014.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.