Abstract
Background
The lack of efficacy of pharmacological treatments for cognitive and negative symptoms in schizophrenia highlights the need for new interventions. We investigated the effects of tDCS on working memory and negative symptoms in patients with schizophrenia.
Method
Double-blinded, randomized, sham-controlled clinical trial, investigating the effects of 10 sessions of tDCS in schizophrenia subjects. Stimulation used 2 mA, for 20 min, with electrodes of 25 cm2 wrapped in cotton material soaked in saline solution. Anode was positioned over the left DLPFC and the cathode in the contralateral area. Twenty-four participants were assessed at baseline, after intervention and in a three-months follow-up. The primary outcome was the working memory score from MATRICS and the secondary outcome the negative score from PANSS. Data were analyzed using generalized estimating equations.
Results
We did not find group ∗ time interaction for the working memory (p = 0.720) score or any other cognitive variable (p > 0.05). We found a significant group ∗ time interaction for PANSS negative (p < 0.001, d = 0.23, CI.95 = −0.59–1.02), general (p = 0.011) and total scores (p < 0.001). Exploratory analysis of PANSS 5 factors suggests tDCS effect on PANSS negative (p = 0.012), cognitive (p = 0.016) and depression factors (p = 0.029).
Conclusion
The results from this trial highlight the therapeutic effects of tDCS for treatment of persistent symptoms in schizophrenia, with reduction of negative symptoms. We were not able to confirm the superiority of active tDCS over sham to improve working memory performance. Larger sample size studies are needed to confirm these findings.
Keywords: Schizophrenia, Transcranial direct current stimulation, tDCS, Working memory, Negative symptoms
1. Introduction
Schizophrenia is a heterogeneous disorder with symptoms classified into four domains: positive symptoms, negative and affective symptoms and cognitive impairments. One of the mot used instrument to verify the intensity of the symptoms in this population is the Positive and Negative Scale (PANSS) and principal component analysis of PANSS suggests that the disorder is better understood through 5 factors: negative, disorganization/cognitive, excitement, positive and depressive/anxiety (Higuchi et al., 2014). Although antipsychotic medications are moderately effective for the treatment of the positive symptoms (Leucht et al., 2009), including disorganization, delusions and hallucinations, they have small-to-no effect for the cognitive and negative symptoms (Fusar-Poli et al., 2015; Green and Harvey, 2014).
The negative symptoms are associated with a reduction of the expected functioning and behavior. Symptoms include flattened affect, poverty of speech, apathy, avolition, anhedonia and asociality (American Psychiatric Association, 2013). Both cognitive and negative symptoms may persist even after stabilization of the illness (Brissos et al., 2011; Haro et al., 2015). In addition, they are strongly correlated to poor functional outcome and low recovery rates, evidencing the need for alternatives in treatment (Fusar-Poli et al., 2013; Fusar-Poli et al., 2015; Green and Harvey, 2014; Grimes et al., 2017; Haro et al., 2015).
The cognitive impairments can be observed ten years before the first psychotic episode (Goff et al., 2011; Kahn and Keefe, 2013) and have been reported in first-degree relatives (Cella et al., 2015). Among the most impaired abilities, the speed of processing, executive functioning, attention, working memory (WM) and cognitive control deficits have been associated with prefrontal cortex (PFC) dysfunction, which has been described as a consequence of illness (Lewis and Glausier, 2016; Sakurai et al., 2015).
Resting-state and task-related activation of the dorsolateral prefrontal (DLPFC) cortex have been a topic of research in schizophrenia. The DLPFC is crucial for mental representation and abstraction and its dysfunction account for WM deficits (Arnsten, 2013). In schizophrenia, DLPFC shows smaller gray matter volume (Arnsten, 2013) and reduced activation (Hill et al., 2004), which reflects a decrease in resting-state blood flow (Andreasen et al., 1997). These abnormalities account for the impairment in cognition and the pathophysiology of the disorder as well. Regardless of the specificity of the deficits in the DLPFC, PFC dysconnectivity to other brain regions is well documented and associated with both cognitive deficits and psychotic symptoms (Zhou et al., 2015). Recently, orbitofrontal cortex thickness in the left hemisphere was associated with negative symptoms severity (Walton et al., 2017).
The lack of efficacy of pharmacological treatments for the cognitive and negative symptoms, in addition to the recent findings of neurobiological studies, boosted the research on non-invasive brain stimulation techniques (NIBS), such as transcranial direct current stimulation (tDCS) (Hasan et al., 2012, Hasan et al., 2013). Following the interesting results from previous studies, we hereby present a double-blinded, randomized, sham-controlled clinical trial investigating the effects of 10 sessions of tDCS over the DLPFC in schizophrenia subjects. We hypothesize that anodal tDCS applied over the left DLPFC, with the cathode at the right contralateral area, will improve both working memory and negative symptoms. Despite their differences regarding clinical characteristic, they have been associated in the literature, and share similar brain subtracts. In this context, we believe that increasing excitability of the left DLPFC may lead to an improvement of both issues.
2. Method
2.1. Trial design
This is a parallel randomized, double-blinded sham-controlled clinical trial with two arms and 1:1 allocation ratio. The study was conducted following the principles of the Declaration of Helsinki and guidelines of Good Clinical Practice and was approved by the Ethics Committee of the Federal University of Sao Paulo (UNIFESP) and is registered in the Brazilian Clinical Trial platform under number RBR-69g952. It also follows the Consolidated Standards of Reporting Trials (CONSORT) (Turner et al., 2012).
2.2. Participants
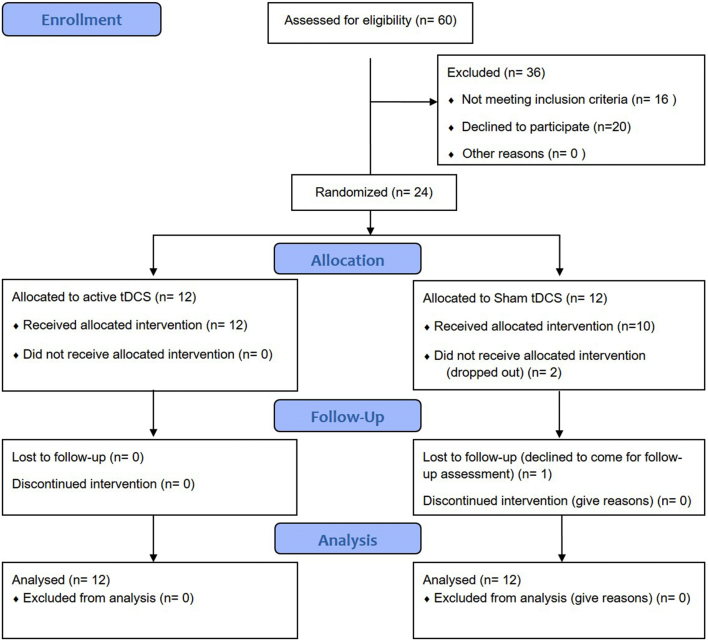
Assessments and stimulation sessions were conducted at one of the two recruitment centers enrolled in the study: Schizophrenia Program from the Federal University of Sao Paulo (PROESQ – UNIFESP) or at outpatient unity from Santa Casa School of Medical Sciences. Two trained psychiatrists performed the diagnosis of schizophrenia by using the Structured Clinical Interview of the DSM-IV (SCID-I) (American Psychiatric Association, 1994). Patients eligibility criteria included: (a) Subjects between 18 and 65 years old diagnosed with DSM-IV schizophrenia; (b) No history of substance abuse/dependence, in exception to tobacco and/or caffeine; (c) No diagnosis of any neurological conditions affecting central nervous system(e.g. Parkinson's disease); (d) No history of seizures; (e) No unexplained loss of consciousness; (f) Stability of pharmacological treatment for at least 6 weeks; (g) No contraindications to tDCS, such as metal in the head or implanted brain medical devices; (h) No pregnancy at enrollment; (i) acceptance to participate in the study and provide the written informed consent, given in the first interview. Dropout was considered after the absence in two consecutive tDCS sessions or declined consent to participate. Sixty patients were initially contacted, 16 did not meet the eligibility criteria, and 20 patients refused to participate. Twenty-four patients were included and randomized to either sham or active tDCS treatment (Fig. 1).
Fig. 1.
CONSORT 2010 flow diagram.
2.3. Interventions
A total of ten sessions of either sham or active tDCS was performed, with the anode placed over the left DLPFC, and the cathode in the contralateral area, following the 10/20 EEG system (Beam et al., 2009; Saletu et al., 2010). The stimulation was performed over two consecutive weeks (Monday to Friday) and was initiated immediately after the baseline assessment. For the active stimulation, the following parameters were used: 2 mA of tDCS applied for 20 min with electrodes of 25 cm2 wrapped in cotton material soaked in saline solution. For the sham stimulation, the stimulation procedures were the same, with the exception that the current remained active for the first 30 s of the session only. This is a suitable method of blinding for this technique (Brunoni and Fregni, 2011).
2.4. Outcomes
The primary outcome was the performance on working memory task. As a secondary outcome, we investigated the effects on negative symptomatology, based on PANSS negative subscale score. Other cognitive and clinical measures were analyzed as exploratory outcomes. Measures were obtained at three-time points: baseline (T0), after intervention (T1) and after a 3-month follow up (FU) (T2).
2.4.1. Clinical assessments
Patients were assessed at baseline and after the last session of tDCS using the Positive and Negative Syndrome Scale (Higuchi et al., 2014; Kay et al., 1988), the Calgary Depression Scale (CDS) (Addington et al., 1993) and the Global Assessment of Functioning Scale (GAF) (Endicott et al., 1976).
2.4.2. Cognitive assessments
The Brazilian version of the Measurement and Treatment Research to Improve Cognition in Schizophrenia Consensus Cognitive Battery (MATRICS) (Fonseca et al., 2017) was used to assess changes in cognition. The MATRICS is a standardized cognitive assessment for patients with schizophrenia composed by cognitive tasks with normative data (Green et al., 2004; Lezak et al., 2004; Strauss et al., 2006). Ten tests from the MATRICS were used, in order to evaluate the following domains (Fonseca et al., 2017; Green et al., 2004):
-
(1)
Speed of processing: Trail Making Test: Part A (TMTA), Brief Assessment of Cognition in Schizophrenia (BACS): Symbol Coding and Category Fluency Test: Animal naming (Fluency);
-
(2)
Attention: Continuous Performance Test—Identical Pairs (CPT-IP);
-
(3)
Working memory: Wechsler Memory Scale—Third Edition (WMS-III): Spatial Span (SS) and Letter-Number Span Test (LNS);
-
(4)
Verbal learning: Hopkins Verbal Learning Test—Revised (HVLT-R);
-
(5)
Visual learning: Brief Visuospatial Memory Test—Revised (BVMT-R);
-
(6)
Reasoning and problems solving: Neuropsychological Assessment Battery (NAB): Mazes;
-
(7)
Social cognition: Mayer–Salovey–Caruso Emotional Intelligence Test (MSCEIT): Managing Emotions.
In this study, we excluded the MSCEIT subtest from the MATRICS given its low-reliability coefficient in Brazilian versions (Fonseca et al., 2017). The outcome analyzed from MATRICS was the T-scores of the six remaining domains (Kern et al., 2011; McCleery et al., 2015). Intelligence coefficient (IQ) was estimated by the composite score of the subtests Vocabulary and Matrix Reasoning from the Brazilian Version of the Wechsler Adult Intelligence Scale – 3rd edition (Nascimento, 2005).
2.5. Randomization
Subjects were assigned to the active or sham stimulation in a 1:1 ratio using blocked randomization with randomly permuted blocks of four and six. A researcher not involved in the execution of the trial performed the randomization at randomization.com. Randomization was performed using www.randomizer.org.
2.6. Blinding
Both the subjects receiving the interventions and the researchers performing the assessments were blinded. The researcher administering the stimulation protocols was not blinded.
3. Statistical analysis
Data were analyzed following the intention-to-treat approach, and the missing data were inputted based on the last observation carried forward (LOCF), using IBM SPSS v.21 software. For analysis of the clinical and sociodemographic data, Fisher's exact test was used for categorical data (gender) and the t-test for independent samples for numeric data (age, duration of the illness an IQ, PANSS positive score, PANSS negative score, PANSS general score, PANSS total scale score, CDS and GAF).
The primary outcome was to investigate the effects of tDCS on working memory; as a secondary outcome, we analyzed the changes in negative symptoms. Analyses of the other cognitive measures (speed of processing, attention, verbal learning, visual learning, and problem-solving) and clinical variables (GAF, PANSS positive score, PANSS general score and PANSS total scale score, PANSS negative factor, PANSS disorganization/cognitive factor, PANSS excitement factor, PANSS positive factor and PANSS depressive/anxiety factor) were exploratory.
The main analysis consisted of a series of generalized estimating equations (GEE) (using normal distribution, a robust estimator as covariance matrix and exchangeable correlation matrix structure) comparing the effects of the intervention on tDCS and sham groups. It included three main factors: time (baseline, after intervention and FU), group (SCH and sham) and the interaction of time and group. Post-hoc pairwise comparisons were run using Bonferroni adjustment for multiple comparisons. GEE is an extension of the generalized linear model for the analysis of longitudinal data. It uses a quasi-likelihood approach, requiring fewer assumptions about the joint distribution of the repetitive outcomes and maintaining the consistency of the regression coefficients. It is known as a reliable alternative even for non-Gaussian data and small sample sizes (Guimarães and Hirakata, 2012; Zeger and Liang, 1986). The effect size was calculated with Cohen's D, based on the mean and standard deviation of the mean of T1 for tDCS and Sham.
4. Results
Participant flow is described in the Flow diagram (Fig. 1). Twenty four patients were included in the analysis. Fifteen were recruited from outpatient unity Santa Casa School of Medical Sciences and nine from the Schizophrenia Program at the Federal University of Sao Paulo (PROESQ - UNIFESP).
4.1. Baseline data
The groups were matched for age, gender, duration of the illness and IQ. Baseline scores for CDS, GAF and PANSS scores (negative, general and total), except for PANSS positive score, with the tDCS group showing higher scores (t(22) = 2.332, p < 0.029) (for demographic data, see Table 1).
Table 1.
Clinical and demographic information of the participants, and baseline statistics between groups.
| tDCS | Sham | p value | |
|---|---|---|---|
| Participant N | 12 | 12 | |
| Age (years) | 39.17, SD 9.34 | 33.75, SD 12.08 | 0.232 |
| Gender (N female) | 2 | 5 | 0.371 |
| Duration of the illness (years) | 16.00, SD 11.62 | 10.00, SD 7.32 | 0.191 |
| IQ (Mean score) | 95.15, SD 13.01 | 93.32, SD 11.30 | 0.738 |
| PANSS positive (mean score) | 16.25, SD 3.12 | 13.25, SD 3.19 | 0.029⁎ |
| PANSS negative score (mean score) | 23.75, SD 5.56 | 21.67, SD 8.40 | 0.481 |
| PANSS general scores (mean score) | 41.58, SD 9.68 | 36.08, SD 9.48 | 0.174 |
| PANSS total scale score (mean score) | 81.58, SD 16.04 | 71.00, SD 19.91 | 0.166 |
| CDS (mean score) | 3.08, SD 2.97 | 1.17, SD 1,99 | 0.077 |
| GAF (mean score) | 43.83, SD 14.14 | 50.58, SD 18.17 | 0.321 |
p < 0.05.
4.2. Outcomes
4.2.1. Cognitive assessments
There was no group ∗ time interaction for any of the cognitive variables, as shown in Table 2. A time effect was found for WM, the speed of processing, visual learning and problem solving. Further analysis with Bonferroni test was not able to identify a significant difference between the three-time points for WM and visual learning. Total mean of the speed of processing t-score significantly increased from baseline to FU, with a mean difference for tDCS group of 2.42 and of 1.6 for sham group. Problem solving also had a significant total mean increase from baseline to FU, with a mean difference for tDCS group of 2.08 and of 3.0 for sham group.
Table 2.
Cognitive measures of the participants and statistics between different time-points derived from repeated measures GEE.
| Time | Groups |
Total |
Group | Time | Group ∗ time | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| TDCS |
SHAM |
|||||||||
| Mean | SE | Mean | SE | Mean | SE | |||||
| Working memory | Baseline | 45.75 | 1.78 | 40.25 | 2.45 | 43.00# | 1.68 | 0.107 | 0.032⁎ | 0.720 |
| After | 43.08 | 1.86 | 39.08 | 2.01 | 41.03# | 1.49 | ||||
| FU | 46.08 | 2.30 | 41.25 | 3.00 | 43.67# | 2.09 | ||||
| Total | 44.97 | 1.74 | 40.19 | 2.40 | ||||||
| Speed of processing | Baseline | 43.00 | 2.70 | 39.9 | 3.18 | 41.46A | 2.09 | 0.260 | 0.020⁎ | 0.306 |
| After | 46.17 | 1.98 | 40.50 | 2.95 | 43.33AB | 1.78 | ||||
| FU | 45.42 | 2.41 | 41.50 | 2.97 | 43.46B | 1.91 | ||||
| Total | 44.86 | 2.28 | 40.64 | 2.30 | ||||||
| Attention | Baseline | 42.42 | 2.99 | 37.25 | 4.22 | 39.83 | 2.59 | 0.221 | 0.265 | 0.139 |
| After | 43.66 | 3.35 | 38.50 | 4.24 | 41.08 | 2.70 | ||||
| FU | 45.91 | 2.77 | 38.08 | 4.47 | 42.00 | 2.63 | ||||
| Total | 44.00 | 2.90 | 37.94 | 4.01 | ||||||
| Verbal learning | Baseline | 35.17 | 2.94 | 40.33 | 2.41 | 37.75 | 1.90 | 0.402 | 0.501 | 0.696 |
| After | 35.00 | 3.71 | 39.33 | 4.60 | 37.17 | 2.58 | ||||
| FU | 38.50 | 3.69 | 40.42 | 5.68 | 39.46 | 2.98 | ||||
| Total | 36.22 | 3.13 | 40.02 | 3.30 | ||||||
| Visual learning | Baseline | 29.42 | 3.87 | 28.66 | 3.27 | 29.04# | 2.54 | 0.727 | 0.047⁎ | 0.891 |
| After | 34.67 | 4.54 | 32.00 | 4.23 | 33.33# | 3.10 | ||||
| FU | 30.42 | 3.27 | 28.92 | 3.24 | 29.67# | 2.30 | ||||
| Total | 31.50 | 3.49 | 29.86 | 3.15 | ||||||
| Problem solving | Baseline | 42.17 | 2.06 | 37.67 | 2.29 | 39.91A | 1.54 | 0.233 | 0.016⁎ | 0.154 |
| After | 42.25 | 1.77 | 39.83 | 2.23 | 41.04AB | 1.42 | ||||
| FU | 44.25 | 1.96 | 40.67 | 2.53 | 42.46B | 1.60 | ||||
| Total | 42.89 | 1.83 | 39.39 | 2.56 | ||||||
Distinct lowercase letters represent statistically significant differences between groups.
Distinct capital letters represent statistically significant differences within groups. i.e. over time.
p < 0.05.
Bonferroni test did not show significant difference.
4.2.2. Clinical assessments
There was a significant group ∗ time interaction for negative symptoms. Bonferroni analysis suggests a significant reduction of 3.83 points from baseline to after intervention for tDCS group versus 0.17 points for the sham group (Fig. 2). Cohen's d suggests a small effect size for the mean differences from sham and tDCS groups (d = 0.23, CI.95 = −0.59–1.02). The differences were maintained in the FU (mean change for tDCS = 3.42 and sham = 0.25).
Fig. 2.
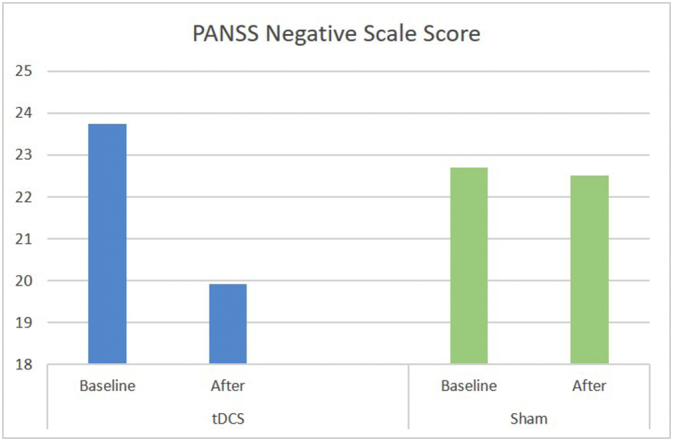
Mean score of PANSS negative scale score at baseline and after intervention for tDCS and sham groups.
The total and general PANSS scores also presented group ∗ time interaction, with greater reduction from baseline to after stimulation and to FU in the active group: 10.75 and 6 points for tDCS group compared to 0.83 and 0.59 points for the sham group, respectively. There was no interaction for PANSS positive scale. Table 3 summarizes analyses of clinical variables. Analysis of PANSS five factors showed a group ∗ time interaction for the negative, the disorganization/cognitive and for the depressive/anxiety factors. From baseline to after stimulation they reduced, respectively: 4.42, 3.58 and 3.83 points. From baseline to FU, they maintained the stastically significant reduction: 3.92, 3.42, 4.75 points. Factor analysis is presented on Table 4.
Table 3.
Clinical information of the participants and statistics between different time-points derived from repeated measures GEE.
| Time | Group |
Total |
Group | Time | Group ∗ time | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| TDCS |
SHAM |
|||||||||
| Mean | SE | Mean | SE | Mean | SE | |||||
| GAF | Baseline | 43.83 | 3.91 | 50.58 | 5.02 | 47.21 | 3.18 | 0.371 | 0.005 | 0.318 |
| After | 48.25 | 3.55 | 52.00 | 5.47 | 50.12 | 3.26 | ||||
| FU | 48.50 | 3.56 | 55.16 | 5.79 | 51.83 | 3.40 | ||||
| Total | 46.86 | 3.52 | 52.58 | 5.323 | ||||||
| Calgary | Baseline | 3.08 | 0.82 | 1.16 | 0.55 | 2.12 | 0.49 | 0.100 | 0.106 | 0.354 |
| After | 2.41 | 0.84 | 1.0 | 0.54 | 1.71 | 0.50 | ||||
| FU | 2.58 | 0.80 | 1.25 | 0.58 | 1.92 | 049 | ||||
| Total | 2.69 | 0.78 | 1.14 | 0.52 | ||||||
| PANSS Positive scale |
Baseline | 16.25 | 0.86 | 13.25 | 0.88 | 14.75 | 0.62 | 0.073 | 0.221 | 0.687 |
| After | 15.33 | 1.11 | 13.08 | 1.08 | 14.21 | 0.77 | ||||
| FU | 14.83 | 1.16 | 12.75 | 1.06 | 13.79 | 0.78 | ||||
| Total | 15.47 | 0.94 | 13.02 | 0.99 | ||||||
| PANSS Negative scale |
Baseline | 23.75aA | 1.54 | 21.67aA | 2.32 | 22.71 | 1.39 | 0.943 | <0.001⁎⁎⁎ | <0.001⁎⁎⁎ |
| After | 19.92aB | 1.45 | 21.5aA | 2.34 | 20.71 | 1.38 | ||||
| FU | 20.33aB | 1.58 | 21.42aA | 2.35 | 20.87 | 1.41 | ||||
| Total | 21.33 | 1.46 | 21.53 | 2.31 | ||||||
| PANSS General scale |
Baseline | 41.58aA | 2.68 | 36.09aA | 2.62 | 38.83 | 1.87 | 0.609 | <0.001⁎⁎⁎ | 0.011⁎ |
| After | 35.58aB | 2.45 | 35.5aA | 2.74 | 35.54 | 1.84 | ||||
| FU | 35.00aB | 2.48 | 35.08aA | 2.67 | 35.04 | 1.83 | ||||
| Total | 37.38 | 2.43 | 35.55 | 2.63 | ||||||
| PANSS Total scale |
Baseline | 81.58aA | 4.43 | 71.00aA | 5.50 | 76.29 | 3.47 | 0.569 | <0.001⁎⁎⁎ | <0.001⁎⁎⁎ |
| After | 70.83aB | 4.36 | 70.17aA | 5.85 | 70.50 | 3.66 | ||||
| FU | 70.17aB | 4.63 | 69.25aA | 5.71 | 69.71 | 3.67 | ||||
| Total | 74.19 | 4.35 | 70.14 | 5.63 | ||||||
Distinct lowercase letters represent statistically significant differences between groups.
Distinct capital letters represent statistically significant differences within groups. i.e. over time.
p < 0.05.
p < 0.001.
Table 4.
Sensitivity analysis of 5 PANSS factors and statistics between different time-points derived from repeated measures GEE.
| Time | Group |
Total |
Group | Time | Group ∗ time | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| TDCS |
SHAM |
|||||||||
| Mean | SE | Mean | SE | Mean | SE | |||||
| PANSS Negative factor |
Baseline | 29.92A | 1.99 | 27.58A | 3.08 | 28.75 | 1.84 | |||
| After | 25.50B | 1.95 | 26.92A | 3.03 | 26.21 | 1.80 | 0.994 | 0.001⁎⁎⁎ | 0.012⁎ | |
| FU | 26.00B | 2.02 | 26.83A | 3.07 | 26.42 | 1.84 | ||||
| Total | 27.14 | 1.94 | 27.11 | 3.01 | ||||||
| PANSS Cognition factor |
Baseline | 24.92A | 1.49 | 22.50A | 2.20 | 23.71 | 1.33 | 0.840 | 0.001⁎⁎⁎ | 0.016⁎ |
| After | 21.33B | 1.54 | 21.92A | 2.18 | 21.62 | 1.34 | ||||
| FU | 21.50B | 1.67 | 21.75A | 2.15 | 21.62 | 1.36 | ||||
| Total | 22.58 | 1.48 | 22.05 | 2.16 | ||||||
| PANSS Excitement factor |
Baseline | 17.08 | 1.37 | 16.41 | 1.37 | 16.75 | 1.19 | 0.851 | 0.389 | 0.574 |
| After | 16.33 | 1.29 | 15.67 | 1.29 | 16.00 | 0.95 | ||||
| FU | 15.42 | 1.29 | 15.67 | 1.29 | 15.54 | 0.98 | ||||
| Total | 16.28 | 1.43 | 15.92 | 1.28 | ||||||
| PANSS Positive factor |
Baseline | 23.33 | 1.24 | 17.83 | 1.28 | 20.58 | 0.89 | 0.023⁎ | 0.258 | 0.662 |
| After | 21.58 | 1.69 | 17.75 | 1.58 | 19.67 | 1.16 | ||||
| FU | 21.00 | 1.77 | 17.00 | 1.69 | 19.00 | 1.23 | ||||
| Total | 21.97a | 1.34 | 17.53b | 1.42 | ||||||
| PANSS Depression factor |
Baseline | 22.92A | 1.81 | 19.67A | 1.26 | 21.04 | 1.10 | 0.778 | 0.092 | 0.029⁎ |
| After | 19.08B | 1.79 | 19.67A | 1.43 | 19.37 | 1.15 | ||||
| FU | 18.16B | 1.75 | 19.58A | 1.52 | 18.87 | 1.16 | ||||
| Total | 20.05 | 1.58 | 19.47 | 1.33 | ||||||
Distinct lowercase letters represent statistically significant differences between groups.
Distinct capital letters represent statistically significant differences within groups. i.e. over time.
p < 0.05.
p < 0.001.
We did not find interaction effects for CDS. GAF had a time effect, with a total mean increase of 3.06 points from baseline to the end of the intervention. A group effect was observed for positive symptoms, with the tDCS group showing higher scores. There was no group difference for any other variables.
4.2.3. Safety
Two participants from the sham group dropped out after the first stimulation session. Both declared to have felt uncomfortable with the sensations from the stimulation. No subject in the intervention group dropped out.
5. Discussion
The main aim of this randomized controlled trial was to evaluate the efficacy of tDCS, a non-pharmacological intervention, for the treatment of cognitive deficits in chronic patients with schizophrenia. From our data, we are not able to confirm the superiority of applying real stimulation over sham stimulation to achieve this goal. As a secondary outcome, we also investigated the efficacy for the treatment of negative symptoms. The notable improvement in the psychopathology scores, including negative symptoms, highlights the effects of tDCS as a potential intervention to treat persistent symptoms that affect functioning and quality of life of schizophrenia subjects.
The hypothesis about passing direct current through the scalp is that it may induce spontaneous electric activity of the neurons, increasing or inhibiting excitability on the target brain area and, consequently, producing neuroplasticity effects (Brunoni et al., 2012; Zhao et al., 2017). PFC is a critical region involved in coordinating cognition (Lewis and Glausier, 2016), planned and motivated behaviors (Horan et al., 2014) and negative symptoms (Haro et al., 2015), and inducing excitability through different NIBS techniques seems to be an intuitive rationale to improve PFC related manifestations in schizophrenia.
The most common finding associated to improvement of symptomatology in schizophrenia is the normalization of the frontal cortex functional activation, including those from pharmacology and NIBS (Kani et al., 2017). Our choice for stimulating the left DLPFC and inhibiting the right contralateral area took into account the described under activation of the prefrontal area, called hypofrontality (Hill et al., 2004), the frequently reported deficits in hemisphere lateralization in schizophrenia (Núñez et al., 2017; Oertel-Knöchel and Linden, 2011), and the association of those brain abnormalities to cognitive deficits (Oertel-Knöchel and Linden, 2011) and to negative symptomatology in schizophrenia (Núñez et al., 2017; Shaffer et al., 2015; Walton et al., 2017; Wible et al., 2001).
Emergent research on non-pharmacological approaches have highlighted the effects of noninvasive brain stimulation (NIBS) on brain functioning (Farzan et al., 2012; Gomes et al., 2016; Hoffmann et al., 2000; Zhao et al., 2017) and in the improvement of refractory symptoms (Brunelin et al., 2012; Cordes et al., 2010; Hoffmann et al., 2000; Holi et al., 2004; Lee et al., 2005; Mondino et al., 2015). Transcranial magnetic stimulation (TMS) in the temporoparietal cortex has been associated with clinical improvement of positive symptoms, but it seems to have no effects on negative symptoms (Cordes et al., 2010; Lee et al., 2005; Rosenberg et al., 2012). However, modulating the prefrontal cortex (PFC) appears to be effective, at least when the left dorsolateral prefrontal cortex (DLPFC) was stimulated using 10 Hz TMS (Cordes et al., 2010; Prikryl et al., 2007; Schneider et al., 2008; Shi et al., 2014), with a moderate to large effect size (Shi et al., 2014).
Although some authors reported inconsistent results with transcranial direct current stimulation (tDCS) for negative symptoms (Fitzgerald et al., 2014; Fröhlich et al., 2016; Smith et al., 2015), when anodal tDCS is applied to left DLPFC, it appears to have similar effects to 10 Hz TMS in the same region. Ten sessions (twice a day for five days) of left DLPFC anodal stimulation, with the cathode at the temporoparietal region, was effective to reduce auditory hallucination and the authors also verified a reduction on negative symptoms (Brunelin et al., 2012). Our team (Gomes et al., 2015) and others (Palm et al., 2016) also reported improvement of negative symptoms after ten sessions of 2 mA anodal stimulation on the left DLPFC, with the cathode in the contralateral area. This protocol has been associated with changes in DLPFC connectivity to other brain networks, such as thalamic and temporoparietal regions (Palm et al., 2016) and to structural brain changes. However these effects were observed only for the responders (i.e., patients showing improvement equal or higher than 20% in PANSS negative scale) (Hasan et al., 2017).
Regarding cognition, the reported findings are still controversial, with a limited number of studies exploring this issue. A systematic review on NIBS from 2016 retrieved only three clinical trials evaluating the efficacy of tDCS as a primary or secondary outcome. Even including repetitive TMS trials, with a total of 33 studies, a particular effect of NIBS on cognition could not be driven, in particular for the heterogeneity of the studies protocol and the variability of cognitive measures evaluated (Hasan et al., 2016). A more recent and specific review on effects of tDCS on cognition included six studies and suggested a small to positive effects of this NIBS modality on working memory and attention (Mervis et al., 2017). However, the data for attention was extracted from one study alone (Smith et al., 2015), which may represent a bias to confirm the specificity of tDCS on this cognitive measure.
The effect of working memory, on the other hand, is more consistent across different studies (Hoy et al., 2014; Nienow et al., 2016; Orlov et al., 2016; Smith et al., 2015). However, a metanalysis comparing tDCS effects on WM changes for health and neuropsychiatric population suggest a trend for improvement for the former but no differences for the latter, when the WM was applied ‘offline’ (not concomitant with tDCS) (Hill et al., 2016). Different reasons may account for our negative results. Three out the four studies (Hoy et al., 2014; Nienow et al., 2016; Orlov et al., 2017) assessed WM using a different instrument, a computerized 2-back (Hoy et al., 2014; Nienow et al., 2016) or n-back (2 and 3) (Orlov et al., 2017) task. However, even using the same standardized cognitive battery applied by others (Smith et al., 2015), our study was not able to replicate this finding.
The effects of tDCS on cognition in clinical population seems to be challenged by inter-individual variability, which may include from baseline cognitive characteristics and background, being affected by practice effect affecting both active and sham groups (Hasan et al., 2016), to genetic variables (Wiegand et al., 2016). The neuroplasticity effect of NIBS has been reinforced by investigation about their effects on neurotransmitters levels in the brain. Anodal tDCS is associated with reduction in gamma-aminobutyric acid (GABA) neurotransmitters in young (Bachtiar et al., 2015) and older healthy adults (Antonenko et al., 2017) although its mechanism of action is still unclear. Moreover, the role of GABA in negative and cognitive symptoms of schizophrenia has been discussed (Wassef et al., 2003) and reducing glutamatergic neurotransmission is suggested as a promising intervention (Merritt et al., 2016) for persistent symptoms. From our data, we may speculate that the sustained reduction of the negative symptoms may be associated to neuroplasticity effects of the neuromodulation.
Although the cognitive tests did not improve with the intervention, the disorganized/cognitive dimension of the PANSS has shown a significant improvement. Two explanations can be offered. First, the disorganized dimension of the PANSS and cognitive function are not the same construct. In a meta-analysis of 104 studies (n = 80,150) conducted by others (Ventura et al., 2010), disorganized symptoms were more strongly linked to neurocognitive deficits than positive symptoms. However, the relationship between disorganization and neurocognition were at most moderate (r = −0.23; p < 0.01). Despite the considerable overlap between disorganization and cognitive impairment, they might represent different symptom dimensions (Klingberg et al., 2006). Moreover, the roles of specific disorganized symptoms in the neurocognition, such as social cognition relationship, were less clear (Minor et al., 2015). Interestingly, their findings suggested that disorganized symptoms seemed to respond to treatment interventions differently than cognitive dysfunction. However, improvement in disorganization could affect cognitive impairments, hence being an important treatment consideration when aiming to improve cognitive impairments. Another explanation is the possible relationship between disorganization symptoms and functioning of the dorsolateral prefrontal cortex. A review of literature suggests that disorganization is more associated with dorsolateral prefrontal cortex than negative symptoms (Goghari et al., 2010).
In this trial, we verified that negative symptoms have improved after left DLPFC anodal stimulation, but we could not confirm the effect on cognition. These results should be interpreted with caution, since different limitations were present. The small sample size have prevented the detection of cognitive changes, leading to a type II error. Moreover, although Brazilian version of the MATRICS battery has small and non-significant practice effects for repeated measures assessment (Fonseca et al., 2017), the effect of being observed induces changes in behavior (McCambridge et al., 2014). Larger sample sizes warranted to confirm or refute the hypothesis that tDCS facilitate the cognitive process in patients with schizophrenia.
A selection bias may exist considering that we recruited chronic patients from two ambulatories, by convenience. It may represent only part of the population suffering from schizophrenia. Sample characteristics, as the duration of the disease, level of functioning and baseline clinical and cognitive profile, should be explored in further investigations. Moreover, we did not control for the levels of caffeine neither to nicotine intake, which may be considered as intervenient variables for cognitive performance. Another limitation accounts to the fact that the investigator performing the stimulation protocol was not blinded. Moreover, we did not assess the effectiveness of the blinding directly.
In conclusion, although major advances have been registered in treatment of schizophrenia in last decades, treatment of negative symptoms and cognitive deficits still represents a major unmet need in the care of this population. The present results suggest the efficacy of tDCS for treatment of negative symptoms in schizophrenia. Further investigations of tDCS as an adjunct treatment should be done, including the association with other remediation approaches, as cognitive training.
Funding
JSG received scholarship from CAPES-PROEX during her PhD.
Author contributions
Gomes, J.S.: contributed with definitions of the study design and protocol procedures; she also contributed collecting data and analyzing the results; she contributed writing the first draft of the manuscript and in the final version.
Trevizol, A.P.: contributed to the clinical assessment and interpretation of results; he also contributed to the first draft and the final version of the manuscript.
Ducos, D.V.: contributed with definitions of the neuropsychological evaluation procedures; she also contributed collecting data. She reviewed and contributed to the final version of the manuscript.
Gadelha, A.: contributed with definitions of the study design, with psychiatric evaluation procedures and selecting patients; he also contributed collecting data. He reviewed and contributed to the final version of the manuscript.
Ortiz, B.B.: contributed with definitions regarding the psychiatric evaluation procedures and collecting data; He contributed to the interpretation of results and also reviewed the final version of the manuscript.
Fonseca, A.O.: contributed selecting the patients and collecting data. She reviewed and contributed to the final version of the manuscript.
Akiba, H.T.: contributed with definitions of the protocol procedures; He reviewed and contributed to the final version of the manuscript.
Azevedo, C.C.: contributed collecting and organizing the data. She reviewed and contributed to the final version of the manuscript.
Guimaraes, L.S.P.: contributed to the statistical analysis and description of the results. He also reviewed and contributed to the final version of the manuscript.
Shiozawa, P: contributed with definitions regarding the psychiatric evaluation procedures and collecting data; he reviewed the final version of the manuscript.
Cordeiro, Q: contributed with definitions of the study. He reviewed and contributed to the final version of the manuscript.
Lacerda, A: contributed to study design. He contributed to interpretation of results and in the final version of the manuscript.
Dias, AM: contributed with definitions of the study design and protocol procedures. He contributed to interpretation of results and the final version of the manuscript.
Acknowledgements
We would like to thank all the patients enrolled in this study. Special acknowledgments to the neuropsychologists who supported the assessment: Valquiria Oliveira Barbara e Ana Luiza Franco.
References
- Addington D., Addington J., Maticka-Tyndale E. The British journal of psychiatry; 1993. Assessing depression in schizophrenia: the Calgary Depression Scale. [PubMed] [Google Scholar]
- American Psychiatric Association . American Psychiatric Pub; 2013. Diagnostic and statistical manual of mental disorders (DSM-5®) [Google Scholar]
- Andreasen N.C., O'Leary D.S., Flaum M., Nopoulos P., Watkins G.L., Boles Ponto L.L., Hichwa R.D. Hypofrontality in schizophrenia: distributed dysfunctional circuits in neuroleptic-naive patients. Lancet. 1997;349:1730–1734. doi: 10.1016/s0140-6736(96)08258-x. [DOI] [PubMed] [Google Scholar]
- Antonenko D., Schubert F., Bohm F., Ittermann B., Aydin S., Hayek D., Grittner U., Flöel A. tDCS-induced modulation of GABA levels and resting-state functional connectivity in older adults. J. Neurosci. 2017;79–17 doi: 10.1523/JNEUROSCI.0079-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten A.F.T. The neurobiology of thought: the groundbreaking discoveries of patricia Goldman-Rakic 1937–2003. Cereb. Cortex. 2013;23:2269–2281. doi: 10.1093/cercor/bht195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtiar V., Near J., Johansen-Berg H., Stagg C.J. Modulation of GABA and resting state functional connectivity by transcranial direct current stimulation. elife. 2015;4:1–9. doi: 10.7554/eLife.08789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beam W., Borckardt J.J., Reeves S.T., George M.S. An efficient and accurate new method for locating the F3 position for prefrontal TMS applications. Brain Stimul. 2009;2:50–54. doi: 10.1016/j.brs.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissos S., Dias V.V., Balanzá-Martinez V., Carita A.I., Figueira M.L. Symptomatic remission in schizophrenia patients: relationship with social functioning, quality of life, and neurocognitive performance. Schizophr. Res. 2011;129(2):133–136. doi: 10.1016/j.schres.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Brunelin J., Mondino M., Gassab L., Haesebaert F., Gaha L., Suaud-Chagny M.F., Saoud M., Mechri A., Poulet E. Examining transcranial direct-current stimulation (tDCS) as a treatment for hallucinations in schizophrenia. Am. J. Psychiatry. 2012;169:719–724. doi: 10.1176/appi.ajp.2012.11071091. [DOI] [PubMed] [Google Scholar]
- Brunoni A.R., Fregni F. Clinical trial design in non-invasive brain stimulation psychiatric research. Int. J. Methods Psychiatr. Res. 2011;20:e19–30. doi: 10.1002/mpr.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni A.R., Nitsche M.A., Bolognini N., Bikson M., Wagner T., Merabet L., Edwards D.J., Valero-Cabre A., Rotenberg A., Pascual-Leone A., Ferrucci R., Priori A., Boggio P.S., Fregni F. Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul. 2012;5:175–195. doi: 10.1016/j.brs.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M., Hamid S., Butt K., Wykes T. Cognition and social cognition in non-psychotic siblings of patients with schizophrenia. Cogn. Neuropsychiatry. 2015;20(3):232–242. doi: 10.1080/13546805.2015.1014032. [DOI] [PubMed] [Google Scholar]
- Cordes J., Thunker J., Agelink M.W., Arends M., Mobascher A., Wobrock T., Schneider-Axmann T., Brinkmeyer J., Mittrach M., Regenbrecht G., Wolwer W., Winterer G., Gaebel W. Effects of 10 Hz repetitive transcranial magnetic stimulation (rTMS) on clinical global impression in chronic schizophrenia. Psychiatry Res. 2010;177:32–36. doi: 10.1016/j.psychres.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Endicott J., Spitzer R.L., Fleiss J.L., Cohen J. The Global Assessment Scale: a procedure for measuring overall severity of psychiatric disturbance. Arch. Gen. Psychiatry. 1976;33:766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- Farzan F., Barr M.S., Sun Y., Fitzgerald P.B., Daskalakis Z.J. Transcranial magnetic stimulation on the modulation of gamma oscillations in schizophrenia. Ann. N. Y. Acad. Sci. 2012;1265:25–35. doi: 10.1111/j.1749-6632.2012.06543.x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P.B., McQueen S., Daskalakis Z.J., Hoy K.E. A negative pilot study of daily bimodal transcranial direct current stimulation in schizophrenia. Brain Stimul. 2014;7:813–816. doi: 10.1016/j.brs.2014.08.002. [DOI] [PubMed] [Google Scholar]
- Fonseca A.O., Berberian A.A., de Meneses-Gaya C., Gadelha A., Vicente M. de O., Nuechterlein K.H., Bressan R.A., Lacerda A.L.T. The Brazilian standardization of the MATRICS consensus cognitive battery (MCCB): psychometric study. Schizophr. Res. 2017;185:148–153. doi: 10.1016/j.schres.2017.01.006. [DOI] [PubMed] [Google Scholar]
- Fröhlich F., Burrello T.N., Mellin J.M., Cordle A.L., Lustenberger C.M., Gilmore J.H., Jarskog L.F. Exploratory study of once-daily transcranial direct current stimulation (tDCS) as a treatment for auditory hallucinations in schizophrenia. Eur. Psychiatry. 2016;33:54–60. doi: 10.1016/j.eurpsy.2015.11.005. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Kempton M.J., Rosenheck R.A. Efficacy and safety of second-generation long-acting injections in schizophrenia: a meta-analysis of randomized-controlled trials. Int. Clin. Psychopharmacol. 2013;28(2):57–66. doi: 10.1097/YIC.0b013e32835b091f. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P., Papanastasiou E., Stahl D., Rocchetti M., Carpenter W., Shergill S., McGuire P. Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr. Bull. 2015;41(4):892–899. doi: 10.1093/schbul/sbu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff D.C., Hill M., Barch D. The treatment of cognitive impairment in schizophrenia. Pharmacol. Biochem. Behav. 2011;99(2):245–253. doi: 10.1016/j.pbb.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goghari V.M., Sponheim S.R., MacDonald A.W. The functional neuroanatomy of symptom dimensions in schizophrenia: a qualitative and quantitative review of a persistent question. Neurosci. Biobehav. Rev. 2010;34:468–486. doi: 10.1016/j.neubiorev.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes J.S., Shiozawa P., Dias Á.M., Valverde Ducos D., Akiba H., Trevizol A.P., Bikson M., Aboseria M., Gadelha A., De Lacerda A.L.T., Cordeiro Q. Left dorsolateral prefrontal cortex anodal tDCS effects on negative symptoms in schizophrenia. Brain Stimul. 2015 doi: 10.1016/j.brs.2015.07.033. [DOI] [PubMed] [Google Scholar]
- Gomes J.S., Akiba H., Dias A.M., Soares A., Taiar I., Gadelha A.A., Cordeiro Q., Shiozawa P. Trigeminal nerve stimulation for olfactory hallucinations in schizophrenia: case study. Schizophr. Res. 2016;176:203–205. doi: 10.1016/j.schres.2016.08.002. [DOI] [PubMed] [Google Scholar]
- Green M.F., Nuechterlein K.H., Gold J.M., Barch D.M., Cohen J., Essock S., Fenton W.S., Frese F., Goldberg T.E., Heaton R.K., Keefe R.S.E., Kern R.S., Kraemer H., Stover E., Weinberger D.R., Zalcman S., Marder S.R. Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol. Psychiatry. 2004;56:301–307. doi: 10.1016/j.biopsych.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Green M.F., Harvey P.D. Cognition in schizophrenia: Past, present, and future. Schizophr. Res. Cogn. 2014;1(1):e1–e9. doi: 10.1016/j.scog.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes K.M., Zanjani A., Zakzanis K.K., Psych C. Memory impairment and the mediating role of task difficulty in patients with schizophrenia. Psychiatry Clin. Neurosci. 2017 doi: 10.1111/pcn.12520. [DOI] [PubMed] [Google Scholar]
- Guimarães L.S.P., Hirakata V.N. Uso do Modelo de Equações de Estimações Generalizadas na análise de dados longitudinais. 2012;32(4):503–511. http://seer.ufrgs.br/index.php/hcpa/article/view/36971/23993 Rev HCPA[Internet] [cited 2016 May 15] [Google Scholar]
- Haro J.M., Altamura C., Corral R., Elkis H., Evans J., Malla A., Krebs M., Zink M., Bernasconi C., Lalonde J., Nordstroem A. Understanding the impact of persistent symptoms in schizophrenia: cross-sectional fi ndings from the pattern study. Schizophr. Res. 2015 doi: 10.1016/j.schres.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Hasan A., Aborowa R., Nitsche M.A., Marshall L., Schmitt A., Gruber O., Falkai P., Wobrock T. Abnormal bihemispheric responses in schizophrenia patients following cathodal transcranial direct stimulation. Eur. Arch. Psychiatry Clin. Neurosci. 2012;262:415–423. doi: 10.1007/s00406-012-0298-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan A., Falkai P., Wobrock T. Transcranial brain stimulation in schizophrenia: targeting cortical excitability, connectivity and plasticity. Curr. Med. Chem. 2013;20:405–413. [PubMed] [Google Scholar]
- Hasan A., Strube W., Palm U., Wobrock T. Repetitive noninvasive brain stimulation to modulate cognitive functions in schizophrenia: a systematic review of primary and secondary outcomes. Schizophr. Bull. 2016;42:S95–S109. doi: 10.1093/schbul/sbv158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan A., Wobrock T., Guse B., Langguth B., Landgrebe M., Eichhammer P., Frank E., Cordes J., Wölwer W., Musso F., Winterer G., Gaebel W., Hajak G., Ohmann C., Verde P.E., Rietschel M., Ahmed R., Honer W.G., Dechent P., Malchow B., Castro M.F.U., Dwyer D., Cabral C., Kreuzer P.M., Poeppl T.B., Schneider-Axmann T., Falkai P., Koutsouleris N. Structural brain changes are associated with response of negative symptoms to prefrontal repetitive transcranial magnetic stimulation in patients with schizophrenia. Mol. Psychiatry. 2017;22:857–864. doi: 10.1038/mp.2016.161. [DOI] [PubMed] [Google Scholar]
- Higuchi C.H., Ortiz B., Berberian A.A., Noto C., Cordeiro Q., Belangero S.I., Pitta J.C., Gadelha A., Bressan R.A. Factor structure of the positive and negative syndrome scale (PANSS) in Brazil: convergent validation of the Brazilian version. Rev. Bras. Psiquiatr. 2014;36:336–339. doi: 10.1590/1516-4446-2013-1330. [DOI] [PubMed] [Google Scholar]
- Hill K., Mann L., Laws K.R., Stephenson C.M.E., Nimmo-Smith, McKenna P.J. Hypofrontality in schizophrenia: a meta-analysis of functional imaging studies. Acta Psychiatr. Scand. 2004;110:243–256. doi: 10.1111/j.1600-0447.2004.00376.x. [DOI] [PubMed] [Google Scholar]
- Hill A.T., Fitzgerald P.B., Hoy K.E. Effects of anodal transcranial direct current stimulation on working memory: a systematic review and meta-analysis of findings from healthy and neuropsychiatric populations. Brain Stimul. 2016;9:197–208. doi: 10.1016/j.brs.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Hoffmann R.E., Boutros N.N., Hu S., Berman R.M., Krystal J.H., Charney D.S. Transcranial magnetic stimulation for auditory hallucinations in schizophrenia. Psychiatry Res. 2000;335:1073–1075. doi: 10.1016/S0140-6736(00)02043-2. [DOI] [PubMed] [Google Scholar]
- Holi M., Eronen M., Toivonen K., Toivonen P., Marttunen M., Naukkarinen H. Left prefrontal repetitive transcranial magnetic stimulation in schizophrenia. Schizophr. Bull. 2004;30:429–434. doi: 10.1093/oxfordjournals.schbul.a007089. [DOI] [PubMed] [Google Scholar]
- Horan W.P., Wynn J.K., Mathis I., Miller G.A., Green M.F. Approach and withdrawal motivation in schizophrenia: an examination of frontal brain asymmetric activity. PLoS One. 2014;9:1–7. doi: 10.1371/journal.pone.0110007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy K.E., Arnold S.L., Emonson M.R., Daskalakis Z.J., Fitzgerald P.B. An investigation into the effects of tDCS dose on cognitive performance over time in patients with schizophrenia. Schizophr. Res. 2014;155:96–100. doi: 10.1016/j.schres.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Kani A.S., Shinn A.K., Lewandowski K.E., Öngür D. Converging effects of diverse treatment modalities on frontal cortex in schizophrenia: a review of longitudinal functional magnetic resonance imaging studies. J. Psychiatr. Res. 2017;84:256–276. doi: 10.1016/j.jpsychires.2016.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn R.S., Keefe R.S. Schizophrenia is a cognitive illness: time for a change in focus. JAMA psychiatry. 2013;70(10):1107–1112. doi: 10.1001/jamapsychiatry.2013.155. [DOI] [PubMed] [Google Scholar]
- Kay S.R., Opler L.A., Lindenmayer J.P. Reliability and validity of the positive and negative syndrome scale for schizophrenics. Psychiatry Res. 1988;23(1):99–110. doi: 10.1016/0165-1781(88)90038-8. [DOI] [PubMed] [Google Scholar]
- Kern R.S., Gold J.M., Dickinson D., Green M.F., Nuechterlein K.H., Baade L.E.…Sugar C.A. The MCCB impairment profile for schizophrenia outpatients: results from the MATRICS psychometric and standardization study. Schizophr. Res. 2011;126(1):124–131. doi: 10.1016/j.schres.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg S., Wittorf A., Wiedemann G. Disorganization and cognitive impairment in schizophrenia: independent symptom dimensions? Eur. Arch. Psychiatry Clin. Neurosci. 2006;256:532–540. doi: 10.1007/s00406-006-0704-0. [DOI] [PubMed] [Google Scholar]
- Lee S.H., Kim W., Chung Y.C., Jung K.H., Bahk W.M., Jun T.Y., Kim K.S., George M.S., Chae J.H. A double blind study showing that two weeks of daily repetitive TMS over the left or right temporoparietal cortex reduces symptoms in patients with schizophrenia who are having treatment-refractory auditory hallucinations. Neurosci. Lett. 2005;376:177–181. doi: 10.1016/j.neulet.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Lewis D.A., Glausier J.R. The Neuropsychopathology of Schizophrenia. Vol. 63. Springer International Publishing; 2016. Alterations in prefrontal cortical circuitry and cognitive dysfunction in schizophrenia; pp. 31–75. [DOI] [PubMed] [Google Scholar]
- Leucht S., Corves C., Arbter D., Engel R.R., Li C., Davis J.M. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. The Lancet. 2009;373(9657):31–41. doi: 10.1016/S0140-6736(08)61764-X. [DOI] [PubMed] [Google Scholar]
- Lezak M.D., Howieson D.B., Loring D.W. Oxford University Press; New York: 2004. Neuropsychological Assessment. [Google Scholar]
- McCambridge J., Witton J., Elbourne D.R. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. J. Clin. Epidemiol. 2014;67:267–277. doi: 10.1016/j.jclinepi.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleery A., Green M.F., Hellemann G.S., Baade L.E., Gold J.M., Keefe R.S.…Ventura J. Latent structure of cognition in schizophrenia: a confirmatory factor analysis of the MATRICS Consensus Cognitive Battery (MCCB) Psychol. Med. 2015;45(12):2657–2666. doi: 10.1017/S0033291715000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merritt K., Egerton A., Kempton M.J., Taylor M.J., Mcguire P.K. 2016. Nature of Glutamate Alterations in Schizophrenia A Meta-analysis of Proton Magnetic Resonance Spectroscopy Studies. [DOI] [PubMed] [Google Scholar]
- Mervis J.E., Capizzi R.J., Boroda E., MacDonald A.W. Transcranial direct current stimulation over the dorsolateral prefrontal cortex in schizophrenia: a quantitative review of cognitive outcomes. Front. Hum. Neurosci. 2017;11 doi: 10.3389/fnhum.2017.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor K.S., Marggraf M.P., Davis B.J., Luther L., Vohs J.L., Buck K.D., Lysaker P.H. Conceptual disorganization weakens links in cognitive pathways: disentangling neurocognition, social cognition, and metacognition in schizophrenia. Schizophr. Res. 2015;169:153–158. doi: 10.1016/j.schres.2015.09.026. [DOI] [PubMed] [Google Scholar]
- Mondino M., Brunelin J., Palm U., Brunoni A.R., Poulet E., Fecteau S. Transcranial direct current stimulation for the treatment of refractory symptoms of schizophrenia. Current evidence and future directions. Curr. Pharm. Des. 2015;21:3373–3383. doi: 10.2174/1381612821666150619093648. [DOI] [PubMed] [Google Scholar]
- Nascimento E. Casa do Psicólogo; São Paulo: 2005. WAIS-III: Escala de Inteligência Wechsler para Adultos-manual técnico. [Google Scholar]
- Nienow T.M., MacDonald A.W., Lim K.O. TDCS produces incremental gain when combined with working memory training in patients with schizophrenia: a proof of concept pilot study. Schizophr. Res. 2016;172:218–219. doi: 10.1016/j.schres.2016.01.053. [DOI] [PubMed] [Google Scholar]
- Núñez C., Paipa N., Senior C., Coromina M., Siddi S., Ochoa S., Brébion G., Stephan-Otto C. Global brain asymmetry is increased in schizophrenia and related to avolition. Acta Psychiatr. Scand. 2017;135:448–459. doi: 10.1111/acps.12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel-Knöchel V., Linden D.E.J. Cerebral asymmetry in schizophrenia. Neuroscientist. 2011;17:456–467. doi: 10.1177/1073858410386493. [DOI] [PubMed] [Google Scholar]
- Orlov N.D., Tracy D.K., Joyce D., Patel S., Rodzinka-pasko J., Dolan H., Hodsoll J., Collier T., Rothwell J., Shergill S.S. Brain stimulation stimulating cognition in schizophrenia: a controlled pilot study of the effects of prefrontal transcranial direct current stimulation upon memory and learning. Brain Stimul. 2016:6–12. doi: 10.1016/j.brs.2016.12.013. [DOI] [PubMed] [Google Scholar]
- Orlov N.D., Tracy D.K., Joyce D., Patel S., Rodzinka-Pasko J., Dolan H., Hodsoll J., Collier T., Rothwell J., Shergill S.S. Stimulating cognition in schizophrenia: a controlled pilot study of the effects of prefrontal transcranial direct current stimulation upon memory and learning. Brain Stimul. 2017;10:560–566. doi: 10.1016/j.brs.2016.12.013. [DOI] [PubMed] [Google Scholar]
- Palm U., Keeser D., Hasan A., Kupka M.J., Blautzik J., Sarubin N., Kaymakanova F., Unger I., Falkai P., Meindl T., Ertl-Wagner B., Padberg F. Prefrontal transcranial direct current stimulation for treatment of schizophrenia with predominant negative symptoms: a double-blind, sham-controlled proof-of-concept study. Schizophr. Bull. 2016;42:1253–1261. doi: 10.1093/schbul/sbw041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prikryl R., Kasparek T., Skotakova S., Ustohal L., Kucerova H., Ceskova E. Treatment of negative symptoms of schizophrenia using repetitive transcranial magnetic stimulation in a double-blind, randomized controlled study. Schizophr. Res. 2007;95:151–157. doi: 10.1016/j.schres.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Rosenberg O., Gersner R., Klein L.D., Kotler M., Zangen A., Dannon P. Deep transcranial magnetic stimulation add-on for the treatment of auditory hallucinations: a double-blind study. Ann. General Psychiatry. 2012;11:13. doi: 10.1186/1744-859X-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T., Gamo N.J., Hikida T., Kim S.H., Murai T., Tomoda T., Sawa A. Converging models of schizophrenia–Network alterations of prefrontal cortex underlying cognitive impairments. Prog. Neurobiol. 2015;134:178–201. doi: 10.1016/j.pneurobio.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saletu B., Anderer P., Saletu-Zyhlarz G.M. EEG topography and tomography (LORETA) in diagnosis and pharmacotherapy of depression. Clin. EEG Neurosci. Off. J. EEG Clin. Neurosci. Soc. 2010;41:203–210. doi: 10.1177/155005941004100407. [DOI] [PubMed] [Google Scholar]
- Schneider A.L., Schneider T.L., Stark H. Repetitive transcranial magnetic stimulation (rTMS) as an augmentation treatment for the negative symptoms of schizophrenia: a 4-week randomized placebo controlled study. Brain Stimul. 2008;1:106–111. doi: 10.1016/j.brs.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Shaffer J.J., Peterson M.J., McMahon M.A., Bizzell J., Calhoun V., van Erp T.G.M., Ford J.M., Lauriello J., Lim K.O., Manoach D.S., McEwen S.C., Mathalon D.H., O'Leary D., Potkin S.G., Preda A., Turner J., Voyvodic J., Wible C.G., Belger A. Neural correlates of schizophrenia negative symptoms: distinct subtypes impact dissociable brain circuits. Mol. Neuropsychiatry. 2015;1:191–200. doi: 10.1159/000440979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C., Yu X., Cheung E.F., Shum D.H., Chan R.C. Revisiting the therapeutic effect of rTMS on negative symptoms in schizophrenia: a meta-analysis. Psychiatry Res. 2014;215:505–513. doi: 10.1016/j.psychres.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R.C., Boules S., Mattiuz S., Youssef M., Tobe R.H., Sershen H., Lajtha A., Nolan K., Amiaz R., Davis J.M. Effects of transcranial direct current stimulation (tDCS) on cognition, symptoms, and smoking in schizophrenia: a randomized controlled study. Schizophr. Res. 2015;168:260–266. doi: 10.1016/j.schres.2015.06.011. [DOI] [PubMed] [Google Scholar]
- Strauss E., Sherman E.M.S., Spreen O. 3rd ed. Oxford University Press; New Yourk: 2006. A Compendium of Neuropsychological Tests: Administration Norms and Commentary. [Google Scholar]
- Turner L., Shamseer L., Altman D.G., Weeks L., Peters J., Kober T.…Moher D. The Cochrane Library; 2012. Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura J., Thames A.D., Wood R.C., Guzik L.H., Hellemann G.S. Disorganization and reality distortion in schizophrenia: a meta-analysis of the relationship between positive symptoms and neurocognitive deficits. Schizophr. Res. 2010;121:1–14. doi: 10.1016/j.schres.2010.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton E., Hibar D.P., van Erp T.G.M., Potkin S.G., Roiz-Santiañez R., Crespo-Facorro B., Suarez-Pinilla P., van Haren N.E.M., de Zwarte S.M.C., Kahn R.S., Cahn W., Doan N.T., Jørgensen K.N., Gurholt T.P., Agartz I., Andreassen O.A., Westlye L.T., Melle I., Berg A.O., Morch-Johnsen L., Færden A., Flyckt L., Fatouros-Bergman H., Jönsson E.G., Hashimoto R., Yamamori H., Fukunaga M., Jahanshad N., De Rossi P., Piras F., Banaj N., Spalletta G., Gur R.E., Gur R.C., Wolf D.H., Satterthwaite T.D., Beard L.M., Sommer I.E., Koops S., Gruber O., Richter A., Krämer B., Kelly S., Donohoe G., McDonald C., Cannon D.M., Corvin A., Gill M., Di Giorgio A., Bertolino A., Lawrie S., Nickson T., Whalley H.C., Neilson E., Calhoun V.D., Thompson P.M., Turner J.A., Ehrlich S., Ehrlich S., Karolinska Schizophrenia Project consortium (KaSP) Prefrontal cortical thinning links to negative symptoms in schizophrenia via the ENIGMA consortium. Psychol. Med. 2017;1–13 doi: 10.1017/S0033291717001283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassef A., Baker J., Kochan L.D. Vol. 23. 2003. GABA and Schizophrenia: A Review of Basic Science and Clinical Studies. [DOI] [PubMed] [Google Scholar]
- Wible C.G., Anderson J., Shenton M.E., Kricun A., Hirayasu Y., Tanaka S., Levitt J.J., O'Donnell B.F., Kikinis R., Jolesz F.A., McCarley R.W. NIH public access. Psychiatry Res. 2001;108:65–78. doi: 10.1016/s0925-4927(01)00109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand A., Nieratschker V., Plewnia C. Genetic modulation of transcranial direct current stimulation effects on cognition. Front. Hum. Neurosci. 2016;10 doi: 10.3389/fnhum.2016.00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger S.L., Liang K.Y. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- Zhao H., Qiao L., Fan D., Zhang S., Turel O., Li Y., Li J., Xue G., Chen A., He Q. Modulation of brain activity with noninvasive transcranial direct current stimulation (tDCS): clinical applications and safety concerns. Front. Psychol. 2017;8 doi: 10.3389/fpsyg.2017.00685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Fan L., Qiu C., Jiang T. Prefrontal cortex and the dysconnectivity hypothesis of schizophrenia. Neurosci. Bull. 2015;31(2):207–219. doi: 10.1007/s12264-014-1502-8. [DOI] [PMC free article] [PubMed] [Google Scholar]