Abstract
Introduction
Free and Cued Selective Reminding Test (FCSRT) performance identifies patients with preclinical disease at elevated risk for developing Alzheimer’s dementia, predicting diagnosis better than other memory tests.
Methods
Based on literature mapping FCSRT performance to clinical outcomes and biological markers, and on longitudinal preclinical data from the Baltimore Longitudinal Study of Aging, we developed the Stages of Objective Memory Impairment (SOMI) model. Five sequential stages of episodic memory decline are defined by Free Recall (FR) and Total Recall (TR) score ranges and years prior to dementia diagnosis. We sought to replicate the SOMI model using longitudinal assessments of 142 Einstein Aging Study participants who developed AD over 10 years.
Results
Average time to diagnosis was seven years if FR was intact, four years if TR was intact, and two years if TR was impaired, consistent with SOMI model predictions. The SOMI identified incipient dementia with excellent sensitivity and specificity.
Discussion
The SOMI model provides an efficient approach for clinical trial cognitive screening in advance of more costly biomarker studies and ultimately in clinical practice, and provides a vocabulary for understanding AD biomarker patterns and for re-analysis of existing clinical trial data.
Keywords: Free and Cued Selective Reminding Test, Preclinical AD, Dementia, Episodic memory impairment, Amnestic mild cognitive impairment, Prodromal AD, Biomarkers, Clinical trials
1. Introduction
Episodic memory is not a unitary phenomenon; encoding, storage, and retrieval are distinct processes that affect recall. Impairment of episodic memory, which is the hallmark of Alzheimer's disease (AD), begins well before the clinical diagnosis of dementia. To assess episodic memory, the International Working Group [1], [2] recommends using the Free and Cued Selective Reminding Test (FCSRT) which, unlike other memory tests, controls the learning conditions to ensure encoding and distinguish retrieval deficits from storage deficits [3], [4]. The earliest signs of memory impairment are found in free recall (FR), which reflects impaired retrieval of stored memories, progressively worsening in the prodromal stage. Storage remains unimpaired until the late prodromal stage when retrieval fails despite effective cued recall.
1.1. The Free and Cued Selective Reminding Test
The test begins with a study phase in which items (e.g., grapes) are identified in response to unique semantic cues (e.g., fruit) that are used in the test phase to prompt recall of items not retrieved by FR. In contrast to passively listening, as items are presented in conventional word list learning tests, the study phase requires active cognitive engagement and deep semantic processing. By coordinating the conditions of encoding and retrieval with category cues, the FCSRT [3] and its modification that includes immediate recall (FCSRT+IR) [4] optimizes encoding specificity and maximizes recall. The sum of free and cued recall is called total recall (TR). Test details are presented in Supplementary Material A.
Our use of FCSRT performance to define the stages of objective memory impairment (SOMI) in predementia AD is based on it outperforming typical cognitive screening measures for identifying preclinical and clinical AD including the Mini Mental State Exam [5], Selective Reminding Test [6], [7], the California Verbal Learning Test [8], and Logical Memory [9], [10]; its effectiveness at detecting amnestic mild cognitive impairment (aMCI) and dementia [11], predicting future dementia and AD [5], [9], [12], [13], [14], [15], distinguishing AD from non-AD dementias [16], as an outcome measure in ongoing clinical trials [17], [18]; and on the strong association of FR and TR with AD biomarkers summarized in Supplementary Material B.
1.2. TR impairment
Impairment of TR on the FCSRT + IR defines the core clinical phenotype of prodromal AD, which consists of a recall deficit that does not normalize with cuing [1]. The high specificity of TR for prodromal AD in clinical studies is due in part to the fact that TR (maximum score = 48) is unimpaired and remains close to ceiling (47/48) in dementia-free seniors, rendering more than one cue failure worrisome. In fact, a TR cut score of ≤46 has been adopted as an indicator of memory impairment by the Anti-Amyloid Treatment in Asymptomatic AD (“A4”) study [19]. The same cut score predicts 3-year clinical progression on the Clinical Dementia Rating (CDR) Scale to a Global CDR score of 0.5 among cognitively unimpaired subjects [20] and predicts incident dementia 3 years later among dementia-free primary care patients [21]. A TR cut score of ≤44 correctly classified 97% of a community cohort comprising seniors with mild dementia and clinically normal seniors [22].
1.3. FR decline
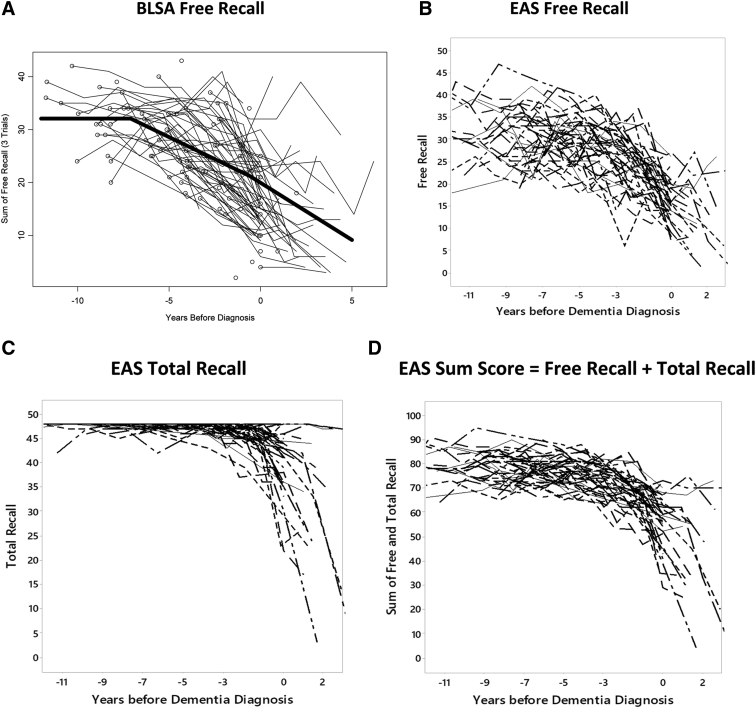
FR decline begins earlier than TR in the predementia course. The trajectory of FR decline on the picture version of the FCSRT (pFCSRT) + IR (pFCSRT + IR) was studied in 92 community residents from the Baltimore Longitudinal Study of Aging (BLSA) who went on to develop diagnosed AD over 15 years of follow-up [23]. Spline regression applied to the data from biannual assessments revealed that FR decline accelerates about 7 years before the diagnosis of dementia (Fig. 1A). Before that point, FR remained above 31. Afterward, 1.48 items/year was lost until 2–3 years before diagnosis when rate of decline of FR doubled to 2.90 items/year, coincident with the acceleration of executive dysfunction. The trajectory of continued FR decline is in stark contrast to the relatively unchanging FR of a robust sample of Einstein Aging Study (EAS) participants who remained dementia-free for 5 years. FR was 31 at baseline and decline was very slow at only 0.18 items/year [24]. At this rate, a one-point decline would take 5 years.
Fig. 1.
Spaghetti plots from the Baltimore Longitudinal Study of Aging (BLSA) and Einstein Aging Study (EAS) of cases diagnosed with Alzheimer's disease (AD): (A) BLSA free recall, (B) EAS free recall, (C) EAS total recall, (D) EAS sum score = free recall + total recall.
Both FR and TR decline with progression in mild cognitive impairment (MCI) [5], [25]. Notably, FR was sensitive to change at high levels of cognition, whereas TR was sensitive to change at low levels of cognition during the 3-year prodromal period [25]. In the GuidAge Prevention Trial, there was a yearly decrease in FR of 2.66 points in the group that progressed to AD dementia, while the group that did not develop dementia displayed a significant net increase in FR of 1.8 points between the baseline and fifth year performance [5]. Decline in TR (≤46) identified clinically normal adults (CDR = 0.0) with elevated amyloid burden on positron-emission tomography (PET) imaging who progressed (CDR = 0.5) over 3 years [20]. Each point decline from 46 was accompanied by a higher risk of progression.
1.4. Biomarker associations
FR and TR have been effective in interrogating specific brain regions that underlie episodic memory functioning and the connectivity between regions using AD antemortem biomarkers including CSF amyloid and tau burden, PET amyloid imaging, structural magnetic resonance imaging studies, and functional imaging studies. Supplementary Material B summaries the main findings of the 26 studies linking FR and TR performance with biological indices of progression, which align well with the trajectory of memory decline.
Impairment of TR on the FCSRT + IR correlated with the Cornu Ammonis 1 field (CA1) (superior region of the hippocampus) of the hippocampus and defined the amnestic syndrome of the hippocampal type [2]. Initially demonstrated in patients with mild AD, recent studies extend the pattern of neuroanatomical correlates to MCI patients with impaired TR [26], [27]. aMCI patients with impaired TR displayed greater gray matter loss in the medial temporal areas bilaterally than aMCI patients with intact TR and, over 18 months, developed gray matter atrophy within the left anterior and lateral temporal lobes. The aMCI patients who had low FR scores but intact TR developed only subcortical and frontal gray matter loss over the 18 months [27]. In another MCI cohort, there was a positive correlation between the volume of the medial temporal lobe, predominately on the left for aMCI patients with impaired TR but not for aMCI patients with intact TR. FR was correlated with prefrontal aspects [26].
Both FR and TR are associated with the CSF profile that is characteristic of AD. Impairments of either distinguished MCI patients with the profile from MCI patients with intact recall who did not display the profile [10]. Eighty-eight percent of patients with memory complaint and a CSF AD profile presented with a medial temporal amnesia profile defined by impaired TR or FR [28].
2. Methods
2.1. SOMI development
Based on the extensive literature mapping FCSRT performance to clinical outcomes and biological markers in longitudinal aging cohorts, we established levels of recall and their rates of change that we hypothesize corresponded to clinically and biologically valid disease states. In the present report, we applied the model to an additional longitudinal data set, the EAS, seeking to demonstrate the ability of our staging scheme to distinguish stages of AD progression and predict time to clinical dementia.
The SOMI system defines sequential predementia stages based on performance below a cut score, first on FR and then TR as measured on the picture version of the test (pFCSRT+IR). Although the proposal is based on extensive research, we intend for it as a starting point; we expect modifications as additional data emerge. The SOMI system is based on memory performance alone.
Until recently, the accepted view of AD biomarkers was that memory decline was driven by β-amyloidosis early in the preclinical phase, which potentiates tau deposition in the prodromal phase with subtle cognitive impairment emerging subsequently [29], [30]. The critical difference between the SOMI and the two biomarker-based preclinical staging schemes is that memory impairment can be identified by the FCSRT in the predementia stages of the SOMI (Table 1).
Table 1.
SOMI stages and expected associations with Aβ biomarkers (A), tau pathology biomarkers (T), and markers of neurodegeneration or neuronal injury (N) compared to the National Institute of Aging and Alzheimer's Association and the international working group preclinical staging systems
| SOMI | Expected biomarker associations with SOMI | Sperling et al, 2011 Preclinical stages |
Dubois et al, 2016 Stages |
||
|---|---|---|---|---|---|
| 0 No memory impairment |
Aβ? Tau− ND− |
Clinically normal | Aβ− Tau− |
||
| 1 Subtle retrieval impairment |
Aβ+ Tau− ND− |
1 Asymptomatic cerebral amyloidosis, No evidence of subtle cognitive change. |
Aβ+ Tau− ND− |
AR-AD | Aβ+ Tau− |
| 2a Moderate retrieval impairment |
Aβ+ Tau? ND− |
2 Asymptomatic amyloidosis + neurodegeneration, no cognitive change. |
Aβ+ and markers of neuronal injury (Tau, FDG, fMRI) | Preclinical Before onset of phenotype |
Aβ+ Tau+ |
| 2b Moderate retrieval and subtle storage impairment |
Aβ+ Tau+ ND? |
3 Asymptomatic amyloidosis + neurodegeneration + cognitive change |
Clinical Clinical phenotype of AD including prodromal and dementia stages |
||
| 3 Significant storage impairment compatible with dementia |
Aβ+ Tau+ ND+ |
||||
Abbreviations: Aβ, amyloid β; AD, Alzheimer's disease; SOMI, Stages of Objective Memory Impairment; FDG PET, [18F]-fluorodeoxyglucose positron-emission tomography; AR-AD, asymptomatic at risk for clinical AD; fMRI, functional magnetic resonance imaging; ND, neurodegeneration.
The revised biomarker view is agnostic with regard to the order in which these antemortem AD biomarkers emerge [31] and divides them into three binary classes based on the pathophysiology each biomarker measures: Amyloid β (Aβ) biomarkers (A), tau pathology biomarkers (T), and markers of neurodegeneration or neuronal injury (N). The second column of Table 1 represents what the expected biomarker associations would be with the SOMI stages.
2.2. Trajectory of decline: Predicting years to AD diagnosis
Based on the change point model applied to the BLSA data, we estimated the mean FR scores as a function of years before dementia diagnosis.
-
•
FR = >30, 7 years before diagnosis when FR begins its accelerated decline;
-
•
FR = ∼24 at 2–3 years before diagnosis when memory decline accelerates further and executive dysfunction accelerates heralding the prodromal period;
-
•
FR = ∼20 at the time of AD diagnosis when intellectual decline accelerates.
Based on the success of using a TR cutoff of ≤46 to indicate impairment in the “A4” study as described above, we propose that
-
•
TR is intact (>46) until the late prodromal period (∼2 years) when it begins to decline (TR<=46).
-
•
TR = ∼44 at the time of AD diagnosis.
2.3. SOMI description
The FR scores at the BLSA change points and TR scores from the “A4” study divided the preclinical AD period into the stages shown in Table 2:
SOMI 0 (no memory impairment): This group is operationally defined by FR of >30 and TR of 47 or 48 on the pFCSRT + IR. This is a stage where pFCSRT + IR performance alone does not differ between cognitively normal persons who will remain clinically normal and those who will go on to develop AD.
SOMI 1 (subtle retrieval impairment): Operationally, FR scores between 30 and 25 define this stage combined with intact TR (>46). This is the first predementia stage when amyloid is likely accumulating and affecting retrieval. Patients are experiencing increasing difficulty carrying out internally driven cognitive process needed for effective FR. FR of the BLSA cases was 27.7 five years before diagnosis [23]. A cut score of 28 predicted AD cases 4 years later in a community-based cohort with memory complaints [9].
SOMI 2a, 2b (moderate retrieval impairment): Operationally, FR scores between 20 and 24 define this stage that typically begins 2 or 3 years before diagnosis when the rate of FR decline doubles and executive dysfunction accelerates, consistent with the scores of community and clinic participants who developed dementia 2–3 years later [32], [33] and with FR of ≤24 that predicted incident dementia among clinic participants over 3 years [21]. FR was 20 when the AD cases from the BLSA met criteria for dementia.
SOMI 2b (subtle storage impairment): In contrast to SOMI 0 and SOMI 1, executive dysfunction is accelerating and may contribute to the decline in FR observed in the prodromal stages. We were uncertain about the effect of executive dysfunction on TR. Was TR still normal (>46) or was it impaired? Would this difference affect prediction of time to diagnosis? To allow for this possibility, the prodromal stage was divided into SOMI 2a operationally defined by FR between 20 and 24 with TR > 46 and SOMI 2b with the same FR but TR between 45 and 46.
SOMI 3 (significant storage impairment): Operationally, this stage is defined by TR ≤ 44, the cut score that had high sensitivity and specificity in distinguishing patients with and without prevalent dementia [22]. When TR falls below 44, FR should no longer matter for classification purposes. SOMI 3 is the clinical dementia stage that reflects significant storage impairment compatible with dementia. Intellectual decline accelerated at this stage for the BLSA cases, heralding instrumental activities of daily living (IADLs) impairment and clinical diagnosis [23]. TR ≤ 32 indicates progression to moderately severe impairment, which is outside the scope of the SOMI system.
Table 2.
SOMI stages and memory impairment defined by pFCSRT + IR performance with respect to time to diagnosis
| SOMI | Free recall scores Maximum score 48 |
Total recall scores Maximum score 48 |
Years to diagnosis Mean (SD) |
|---|---|---|---|
| 0 No memory impairment None detected by pFCSRT + IR |
>30 | >46 | 6.90 (2.62) |
| 1 Subtle retrieval impairment Free recall declines as patients experience increasing difficulty carrying out internally driven cognitive processes needed to effectively search memory. Storage is preserved as reflected by normal performance on cued recall. |
25–30 | >46 | 4.89 (2.48) |
| 2a Moderate retrieval impairment Rate of free recall decline doubles, and the rate of executive dysfunction accelerates. Storage is preserved. |
20–24 | >46 | 4.03 (2.62) |
| 2b Moderate retrieval impairment and subtle storage impairment Cuing fails to normalize total recall. |
20–24 | 45–46 | 2.35 (2.04) |
| 3 Significant storage impairment compatible with dementia For persons with dementia, intellectual decline accelerates heralding IADL impairment. |
Any | 33–44 | 0.98 (1.35) |
Abbreviations: AD, Alzheimer's disease; FR, free recall; pFCSRT + IR, picture version of the Free and Cued Selective Reminding Test with immediate recall; SOMI, stages of objective memory impairment severity.
2.4. The EAS cohort
The sample used to evaluate the proposed SOMI model includes EAS participants, a systematically recruited sample of Bronx community elders aged over 70 years and free of dementia at baseline who underwent testing with the pFCSRT + IR at yearly evaluations since 1992 [34]. Some subjects have been followed since 1985 and their data were included in the analyses. Subjects with no follow-up visit were excluded.
One hundred forty-two incident AD cases including 50 with probable AD and 92 with possible AD developed during follow-up. One thousand three hundred seventy-seven of the participants who remained dementia free during follow-up comprised the robust control group (Table 3). The AD group was older with fewer years of education and made more errors on the Blessed test (Ps < .005), administered at every assessment, providing an independent index of cognitive status and dementia severity [35].
Table 3.
Demographic characteristics of the Einstein Aging Study (EAS) cohort by group at baseline
| Robust normal controls∗ |
AD cases |
||
|---|---|---|---|
| N = 1377 | Mean (SD) | N = 142 | Mean (SD) |
| Age at wave | 77.2 (5.6) | Age at wave | 79.5 (5.9) |
| Gender (%F) | 61 | Gender (%F) | 66 |
| Education (years) | 13.5 (3.5) | Education (years) | 12.5 (3.7) |
| Ethnicity (%) | Ethnicity (%) | ||
| White | 71 | White | 67 |
| Black | 23 | Black | 28 |
| Other | 6 | Other | 5 |
| Years to last follow-up | 4.9 (3.7) | Years to diagnosis | 5.1 (3.7) |
| Blessed errors | 2.05 (2.0) | Blessed errors | 4.1 (3.3) |
Abbreviation: AD, Alzheimer's disease.
EAS subjects who remained dementia-free for up to 5 years of follow-up.
2.5. Does SOMI predict time to diagnosis?
The primary issue was whether the temporal trajectory of FR and TR decline in the participants who developed AD was consistent with the proposed SOMI stages. To answer this, each participant's assessments were assigned to a SOMI stage using the FR and TR cut scores presented in Table 2. For example, at a particular assessment, if the FR score was 34 and TR was 47, that assessment was assigned to SOMI 0 (no memory impairment). If FR was 22 and TR was 45, that assessment was assigned to SOMI 2b. The dependent measure was predicted time to clinical dementia defined by the difference in years between the assessment date and the date of dementia diagnosis.
Generalized estimating equations evaluated the association between SOMI stage and predicted time to diagnosis controlling for within person correlation. Due to the longitudinal nature of the data, subjects had different numbers of measurements. These models use the “all available pairs” method, in which all nonmissing pairs of data are used in estimating the working correlation parameters. With this method, a subject is not required to have every time point to be included in the analysis. Models were conducted with and without the covariates of age, gender, years of education, and AD diagnosis (probable AD vs. possible AD).
A secondary analysis assessed the sensitivity and specificity of the SOMI system for distinguishing persons with incipient AD from dementia-free persons.
3. Results
Figs. 1B and 1C illustrate the decline in FR and TR for the 48 of the 142 persons with a clinical diagnosis of AD who had at least 7 years of follow-up before diagnosis. FR appears to decline steadily, whereas TR remains above 46 until 2–3 years before diagnosis. Thereafter, TR begins to decline.
In the sample of 142 AD cases, we performed 620 annual assessments (4.4/AD case) conducted from 10 years before clinical diagnosis up to and including the diagnostic assessment. For 527 of these 620 (85%) assessments, we were able to classify participants into a discrete SOMI stage. For 220 assessments, an individual contributed more than one assessment to a single stage. To avoid multiple observations per person for any given stage, we eliminated these replicate assessments leading to a final sample of 307 assessments from a total of 142 distinct cases. When multiple assessments for one case were classified into the same stage, the earliest assessment was selected for inclusion in the analysis.
3.1. Time to diagnosis
The 307 assessments were assigned to the appropriate stage. Table 4 shows the group means for time to diagnosis, FR, TR, and Blessed Test errors by SOMI stage. The number of Blessed errors increased with progression to more advanced stages (Ps < .000).
Table 4.
Characteristics of the assessments of the 142 EAS AD cases by SOMI stage
| SOMI stage | N | Mean | Standard deviation | Standard error | 95% CI |
|
|---|---|---|---|---|---|---|
| Lower bound | Upper bound | |||||
| Free recall | ||||||
| 0 | 43 | 34.28 | 3.15 | 0.48 | 33.31 | 35.25 |
| 1 | 68 | 27.52 | 1.55 | 0.19 | 27.14 | 27.89 |
| 2a | 56 | 22.45 | 1.33 | 0.18 | 22.09 | 22.80 |
| 2b | 29 | 21.97 | 1.45 | 0.27 | 21.41 | 22.52 |
| 3 | 111 | 16.17 | 5.85 | 0.56 | 15.06 | 17.28 |
| Total | 307 | |||||
| Total recall | ||||||
| 0 | 43 | 47.81 | 0.39 | 0.06 | 47.69 | 47.94 |
| 1 | 68 | 47.69 | 0.47 | 0.06 | 47.58 | 47.80 |
| 2a | 56 | 47.57 | 0.50 | 0.07 | 47.44 | 47.71 |
| 2b | 29 | 45.52 | 0.51 | 0.09 | 45.32 | 45.71 |
| 3 | 111 | 39.42 | 5.15 | 0.49 | 38.44 | 40.40 |
| Total | 307 | |||||
| SUM score = free recall + total recall | ||||||
| 0 | 43 | 82.09 | 3.24 | 0.49 | 81.10 | 83.09 |
| 1 | 68 | 75.21 | 1.59 | 0.19 | 74.82 | 75.59 |
| 2a | 56 | 70.02 | 1.43 | 0.19 | 69.63 | 70.40 |
| 2b | 29 | 67.48 | 1.55 | 0.29 | 66.89 | 68.07 |
| 3 | 111 | 55.59 | 9.90 | 0.95 | 53.71 | 57.47 |
| Total | 307 | |||||
| Blessed errors | ||||||
| 0 | 43 | 2.84 | 2.71 | 0.41 | 2.00 | 3.67 |
| 1 | 68 | 2.94 | 2.28 | 0.28 | 2.39 | 3.49 |
| 2a | 55 | 4.60 | 3.28 | 0.44 | 3.72 | 5.49 |
| 2b | 29 | 6.31 | 3.42 | 0.64 | 5.01 | 7.61 |
| 3 | 111 | 7.19 | 4.00 | 0.39 | 6.42 | 7.95 |
| Total | 307 | |||||
| Time to diagnosis | ||||||
| 0 | 43 | 6.90 | 2.62 | 0.40 | 6.12 | 7.67 |
| 1 | 68 | 4.89 | 2.48 | 0.30 | 4.30 | 5.47 |
| 2a | 56 | 4.03 | 2.62 | 0.34 | 3.35 | 4.70 |
| 2b | 29 | 2.35 | 2.04 | 0.37 | 1.62 | 3.08 |
| 3 | 111 | 0.96 | 1.35 | 0.13 | 0.71 | 1.21 |
| Total | 307 | |||||
Abbreviations: AD, Alzheimer's disease; CI, confidence interval; EAS, Einstein Aging Study; SOMI, stages of objective memory impairment.
The time to diagnosis at each stage was estimated using generalized estimating equation models with and without covariates. In the simple model, the temporal trajectory of FR and TR decline in the entire cohort of AD cases was largely consistent with the proposed SOMI stages.
-
•
Persons whose assessments were classified as SOMI 0 developed dementia 6.9 years later (95% confidence interval [CI]: 6.1, 7.7);
-
•
Persons whose assessments were classified as SOMI 1 developed dementia significantly in 4.9 years (95% CI: 4.3, 5.5) (P < .000).
-
•
Persons with assessments classified into SOMI 2 who had intact TR (SOMI 2a) developed dementia 4.0 years later (95% CI: 3.3, 4.7) compared to persons who had impaired TR (SOMI 2b) who developed dementia 2.4 years (95% CI: 1.6, 3.1) later (P < .000).
-
•
Persons with assessments classified into SOMI 3 were diagnosed with clinical dementia 1 year later.
-
•
Predicted time to diagnosis did not differ significantly between the SOMI 1 and 2a stages (4.9 vs. 4.0, P < .32), despite the considerable difference in FR.
The addition of covariates did not materially affect the time to diagnosis at each stage. Older persons developed dementia sooner than younger persons (P < .001), and probable AD cases developed dementia 7 months sooner than possible AD cases (P < .04), despite equivalent ages. Neither gender (P = .23) nor years of education (P = .12) influenced time to diagnosis.
Of the original 620 assessments, 93 (15%) were not otherwise classified. All had TR scores from 45 to 48; 71% had impaired TR (45, 46); 62% had low FR (<20). They developed dementia 2.6 years later, the same as those assessments classified into the 2b stage (2.4 years, P < .35).
3.2. SOMI sensitivity and specificity
The sensitivity and specificity of the SOMI system for distinguishing persons with incipient AD from dementia-free persons were estimated by sorting into their respective SOMI stages the diagnostic assessment of the AD cases and the last assessment of the robust controls. Nine percent of these assessments were unclassified and thus not included in the calculations. At the last assessment, conducted about 5 years from their baseline visit, 20% of the robust controls were diagnosed with an MCI subtype. Based on the notion that impairment of TR defines the core clinical phenotype of AD, the assessments with impaired TR (SOMI stages 2b and 3) were considered positive for dementia (Table 5). Sensitivity and specificity were both excellent (93%).
Table 5.
Distribution of assessments classified into SOMI stages at the diagnostic wave of 118 AD cases and last follow-up for 1263 robust controls from the EAS∗
| SOMI stage | AD cases (n = 118) at diagnosis | Robust normals (n = 1263) at last follow-up |
|---|---|---|
| Intact total recall SOMI 0–2a |
9 | 1179 |
| Impaired total recall SOMI 2b, 3 |
109 | 84 |
| Total assessments | 118 | 1263 |
Abbreviations: AD, Alzheimer's disease; EAS, Einstein Aging Study; SOMI, stages of objective memory impairment.
NOTE. Sensitivity: 109/118 = 93%; specificity: 1179/1263 = 93%.
Nine percent of the assessments were unclassified. Including them as errors reduces sensitivity to 81% and specificity to 86%.
4. Discussion
The SOMI system describes the temporal unfolding of declining episodic verbal memory in predementia and clinical AD using FR and TR cut scores on pFCSRT + IR to divide the memory impairment continuum into five sequential stages. As expected, the predementia phase transitioned in a nonlinear fashion from early-stage impairment at 6–7 years to the prodromal stage at 2–3 years before memory impairment consistent with dementia was diagnosed. As expected, impaired FR was a marker for early-stage disease, whereas impairment of TR emerged later in the prodromal stage.
The assessments of 142 AD cases conducted in the 10 years before clinical diagnosis were sorted into SOMI stages according to the proposed cutoffs. Predicted time to diagnosis at each SOMI stage was largely consistent with the model's expected temporal trajectory. Cases with intact FR and TR (SOMI 0) developed clinical dementia 6.9 years later, whereas cases with subtle retrieval impairment (SOMI 1) developed clinical dementia 4.9 years later. Dividing the later prodromal phase into two stages demonstrated the value of TR: when TR was intact (47, 48), time to diagnosis was at least 4 years, whereas when TR was impaired, time to diagnosis was significantly sooner, 2.4 years. Older cases developed dementia sooner than younger cases, and probable AD cases developed dementia 7 months earlier than possible AD cases. Sensitivity and specificity of the SOMI system for distinguishing persons with impaired TR from dementia-free persons were excellent.
pFCSRT+IR differs from most episodic memory tests not only because it controls the conditions of learning but also because pathological levels of performance are determined by cut scores that have high predictive and discriminative validity for dementia and AD rather than by normative data adjusted for age and education as is typically done to diagnose MCI. Although MCI was intended to capture predementia states, its success has been limited by variation in operational diagnostic criteria, leading to heterogeneity in clinical course [36], [37]. In some MCI studies, delayed story recall served as the sole objective evidence of memory impairment, which helps to explain why 40% of MCI patients in the Alzheimer's Disease Neuroimaging Initiative had normal cognition when assessed with a neuropsychological algorithm [38].
4.1. Utility of TR and FR for prediction
The study findings are consistent with other clinical studies indicating that impaired TR is a harbinger of dementia 2 or 3 years later [12], [39]. Impaired TR constitutes the core clinical feature of the typical clinical phenotype of AD [1], [2]. While impaired TR alone greatly increases AD risk, FR alone may not distinguish at-risk persons from cognitively normal persons because of the considerable overlap in their distributions 7 years before dementia is diagnosed. Even in the present study, time to diagnosis did not differ between those with subtle or moderate retrieval impairment (4.9 vs. 4.0 years) as long as TR was intact.
We expect that identifying high-risk persons early will improve when FR is combined with a biomarker of amyloid or tau.
This combination approach has promise. In the Longitudinal Cognitive Impairment and Dementia study, subjective memory complaints (SMC) cases with biomarker evidence of abnormal Aβ displayed lower FR scores than cases without biomarker evidence [40]. FR decline preceded TR decline in cognitively normal participants with positive Aβ imaging by 2 years [20]. FR detected declining cognition in the high CSF tau/Aβ group earlier than eight other neuropsychological measures [41]. Nonetheless, if someone has a FR deficit but does not have biomarkers for amyloid or tau, they could have lifelong poor retrieval, a non-AD cause of acquired retrieval impairment including frontal executive deficits that are remediated by cuing but exposed during FR.
4.2. Clinical vocabulary for staging biomarker associations
The SOMI system provides a clinical vocabulary for describing the type and severity of episodic memory impairment in preclinical AD that can be used alongside the latest iteration of a classification scheme for AD biomarkers that unlike earlier versions makes no assumptions about the order in which biomarkers emerge [31]. The 7 major antemortem AD biomarkers are divided into three binary classes based on the pathophysiology that each biomarker measures: Aβ biomarker (A: amyloid PET or CSF Aβ 42), tau pathology biomarker (T: CSF p-tau or tau PET), or neurodegeneration or neuronal injury (N: CSF t-tau, [18F]-fluorodeoxyglucose positron-emission tomography [FDG PET], structural magnetic resonance imaging).
The second column of Table 1 shows the hypothesized association of each SOMI stage with the A, T, and N biomarkers largely based biomarker associations with FCSRT performance summarized in Supplementary Material B. The absence of FR or TR impairment in SOMI 0 may indicate the absence of any biomarker abnormality. A positive Aβ marker at this stage would not be surprising, given the presence of amyloid deposition up to 20 years before clinical dementia [29], [41]. The combination of impaired FR and intact TR in SOMI 1 is presumed to reflect amyloid deposition and the absence of tau pathology or neurodegeneration. The status of tau pathology is uncertain in the first prodromal stage (SOMI 2a) when TR is still intact but FR has declined further and executive dysfunction accelerates. Tau pathology is abnormal in the second prodromal stage (SOMI 2b) when TR is impaired. Evidence of neurodegeneration may be present at this stage as well. At SOMI 3, all three biomarkers are predicted to be present. Whether or not the expected associations between SOMI stages and the three classes of biomarkers are observed in future studies, the SOMI system provides the needed clinical vocabulary for understanding the pattern of AD biomarkers.
4.3. Implications for clinical trials
In clinical trials, to screen for preclinical AD in asymptomatic older adults using biomarkers is expensive (amyloid or tau PET) or invasive (CSF assessment of amyloid and tau biomarkers) and costly for large-scale population-based interventions. In addition, the rate of clinical decline in the presence of cerebral amyloid is highly variable, affected by known (e.g., apolipoprotein E [apoE] carriage) and unknown factors. Thus, a staging system like the SOMI that reliably identifies points of transition in the emergence of cognitive impairment is a desirable alternative or addition to the use of invasive and expensive biomarker studies as part of enrichment strategies to identify patients more likely to experience disease progression during the trial.
There is a growing body of evidence demonstrating the potential role of the FCSRT + IR in enriching clinical trial subject pools. TR was found to be one of the best univariate predictors of progression in the CSF Aβ-positive prodromal AD group in a phase-3 study [42], [43], and FR showed significant utility predicting Aβ status in the entire cohort [44]. Both FR and TR declined in clinically normal older adults with elevated amyloid burden on PET imaging [20]. FR in Aβ+ subjects declined at 2 years postbaseline, whereas TR declined 4 years postbaseline.
There is increased interest in the use of the sum of FR plus TR rather than TR alone in clinical trials. This index, the sum of FR and TR, is a component of the cognitive composite, which was highly predictive of the 5-year risk of AD in the GuidAge Prevention Trial [17]. Because of the long duration of early AD clinical trials, sensitivity to both the earliest signs of episodic memory impairment and to longitudinal change across a wide range of impairment severity as participants decline is needed for a measure to be effective. This index, the sum of FR and TR, doubles the weight of FR, which is sensitive to early-stage impairment and may increase sensitivity to change over the long clinical trial compared to TR alone.
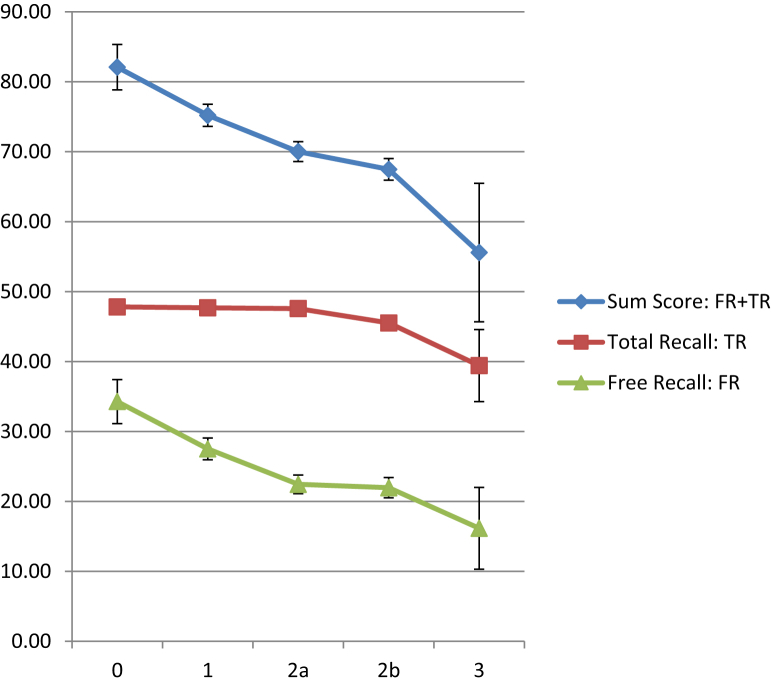
The means and standard deviations for this sum score (FR + TR) were calculated for the EAS AD cases, tabulated along with the means and standard deviations for FR and TR in Table 4. The means and standard deviations for FR, TR, and the sum score are plotted in Fig. 2 against each SOMI stage. Fig. 1D is the spaghetti plot of decline in the sum score across years before diagnosis.
Fig. 2.
FR, TR, and sum score means and SDs at each SOMI stage (0, 1, 2a, 2b, 3). Abbreviations: FR, free recall; SOMI, Stages of Objective Memory Impairment; SDs, standard deviations; TR, total recall.
4.4. Reanalysis of existing data
For completed or ongoing MCI/prodromal/early AD clinical trials using the FCSRT with brain amyloidosis as the biomarker inclusion criterion, given the variability in rates of progression with amyloidosis, applying the SOMI stages to the data retrospectively may help in the interpretation of existing clinical trial data and for potential modification of subject selection criteria for future studies.
In these analyses, one must be mindful that there are four versions of the FCSRT and that the SOMI was based on the pFCSRT + IR. While they have similar operating characteristics, the scores on the versions are not equivalent [45]. Thus, cut scores that define the stages of the SOMI using performance on other versions of the test will likely differ. Of the four versions, pFCSRT + IR produces the highest scores, possibly reflecting the use of both pictures and immediate recall in the study phase enabling additional semantic and nonverbal processing. The predictive and discriminative validity of the word and picture versions that include immediate recall are similar for distinguishing mild AD patients from cognitively healthy controls.
4.5. Use of SOMI in primary care
Another role of the SOMI is to provide an empirical bridge to primary care settings where most of the older adults receive their medical care. pFCSRT + IR's overall profile of freedom from influence by demographic variables and major influence by clinical status makes it a useful clinical tool in primary care screening [11], [21], [33], [46], [47], [48]. Furthermore, the International Working Group recommends the FCSRT + IR as a reliable tool for diagnosing typical AD among various neurodegenerative diseases [16]. Cut scores indicating impairment on FR or TR distinguished typical AD from all other conditions with a sensitivity of 100% and a specificity of 75%. In a world where secondary prevention becomes available, we will need to identify efficiently cases among elders with memory complaints who have a higher likelihood of testing positive for amyloid or tau. Simple cost-effective strategies like the pFCSRT + IR may prove useful for such clinical decision making, assuring maximal availability of preventive treatments for those who will benefit from them.
4.6. Limitations
We acknowledge that the SOMI stages are apparent only in retrospect, after we have followed persons to AD, introducing a degree of circularity into our replication of FR and TR trajectories. To have utility for clinical trial screening, future studies will need to determine how well a mixed sample of individuals can be prospectively classified without knowledge of their outcomes to predict time to diagnosis. Time-dependent receiver operating characteristic curve analysis [9] and multistate transition modeling [49] are planned to assess the predictive and discriminative validities of the SOMI system and to expose errors in its current formulation.
There are other limitations. pFCSRT + IR performance was disclosed to EAS raters when assigning clinical diagnoses, thereby compromising the test's independence as a predictor, although a variety of other indicators were available on which raters based their diagnoses [34]. The finding that mental status declined with progression from one SOMI stage to the next provides a mitigating factor for the compromised independence. Another limitation is that the SOMI system will miss predementia cases who do not have memory impairment but have other clinical phenotypes for AD. This limitation is significant because criteria for dementia due to AD no longer require memory impairment [50]. Finally, 15% of the assessments were not otherwise classified into any SOMI stage. Forty-five percent of them occurred at the assessment wave that proceeded the wave at which clinical dementia was diagnosed. Seventy-one percent had impaired TR and developed dementia approximately 2 years later. Another 10% occurred the first time the participant encountered the test (i.e., baseline). We hope that more of these cases will be classified in future SOMI formulations. One possible factor contributing to the not insignificant fraction of unclassified assessments is that those not classified may have a pattern of memory impairment that is not due to AD alone. For subject selection in AD prevention clinical trials, if this were true, these exclusions could be a benefit of the SOMI model.
Our ultimate goal is to identify at-risk individuals in clinical settings for early intervention and to ensure a consistently and accurately characterized population for secondary AD clinical trials.
Acknowledgments
The FCSRT + IR is copyrighted by the Albert Einstein College of Medicine and is made freely available for noncommercial purposes. EG receives a small percentage of any royalties on the FCSRT + IR when it is used for commercial purposes.
The authors thank Drs. Mark Bondi and Judith Jaeger for their comments on an earlier draft of the manuscript.
Footnotes
Dr. Grober receives a small percentage of any royalties on the FCSRT 1 IR when it is used for commercial purposes. No conflict of interest exists for Drs. Veroff and Lipton.
Supplementary data related to this article can be found online at https://doi.org/10.1016/j.dadm.2017.12.004.
Supplementary data
References
- 1.Dubois B., Feldman H.H., Jacova C., DeKosky S.T., Barberger-Gateau P., Cummings J. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 2.Dubois B., Feldman H.H., Jacova C., Hampel H., Molinuevo J.L., Blennow K. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. 2014;13:614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 3.Buschke H. Cued recall in Amnesia. J Clin Exp Neuropsychol. 1984;6:433–440. doi: 10.1080/01688638408401233. [DOI] [PubMed] [Google Scholar]
- 4.Grober E., Buschke H. Genuine memory deficits in dementia. Dev Neuropsychol. 1987;3:13–36. [Google Scholar]
- 5.Di Stefano F.F. Prediction of Alzheimer's disease dementia: data from the GuidAge Prevention Trial. J Alzheimer's Dis. 2015;48:793–804. doi: 10.3233/JAD-150013. [DOI] [PubMed] [Google Scholar]
- 6.Lemos R., Afonso A., Martins C., Waters J.H., Blanco F.S., Simoes M.R. Selective reminding and free and cued selective reminding in mild cognitive impairment and alzheimer disease. Appl Neuropsychol Adult. 2015;16:1–9. doi: 10.1080/23279095.2015.1012761. [DOI] [PubMed] [Google Scholar]
- 7.Grober E., Merling A., Heimlich T., Lipton R.B. Comparison of selective reminding and free and cued selective reminding in the elderly. J Clin Exp Neuropsychol. 1997;19:643–654. doi: 10.1080/01688639708403750. [DOI] [PubMed] [Google Scholar]
- 8.Tounsi H., Deweer B., Ergis A., Van der Linden M., Pillon B., Michon A. Sensitivity to semantic cuing: an index of episodic memory dysfunction in early Alzheimer disease. Alzheimer Dis Assoc Disord. 1999;13:38–46. doi: 10.1097/00002093-199903000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Derby C.A., Burns L.C., Wang C., Katz M.J., Zimmerman M.E., L'Italien G. Screening for predementia AD: time-dependent operating characteristics of episodic memory tests. Neurology. 2013;80:1307–1314. doi: 10.1212/WNL.0b013e31828ab2c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wagner M., Wolf S., Reischies F.M., Daerr M., Wolfsgruber S., Jessen F. Biomarker validation of a cued recall memory deficit in prodromal Alzheimer disease. Neurology. 2012;78:379–386. doi: 10.1212/WNL.0b013e318245f447. [DOI] [PubMed] [Google Scholar]
- 11.Grober E., Wakefield D., Ehrlich A.R., Mabie P., Lipton R. Identifying memory impairment and early dementia in primary care. Alzheimers Dement. 2017;6:188–195. doi: 10.1016/j.dadm.2017.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarazin M., Berr C., De Rotrou J., Fabrigoule C., Pasquier F., Legrain S. Amnestic syndrome of the medial temporal type identifies prodromal AD: a longitudinal study. Neurology. 2007;69:1859–1867. doi: 10.1212/01.wnl.0000279336.36610.f7. [DOI] [PubMed] [Google Scholar]
- 13.Grober E., Lipton R.B., Hall C., Crystal H. Memory impairment on free and cued selective reminding predicts dementia. Neurology. 2000;54:827–832. doi: 10.1212/wnl.54.4.827. [DOI] [PubMed] [Google Scholar]
- 14.Vos S.J., Xiong C., Visser P.J., Jasielec M.S., Hassenstab J., Grant E.A. Preclinical Alzheimer's disease and its outcome: a longitudinal cohort study. Lancet Neurol. 2013;12:957–965. doi: 10.1016/S1474-4422(13)70194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Auriacombe S., Helmer C., Amieva H., Berr C., Dubois B., Dartigues J.F. Validity of the free and cued Selective Reminding Test in predicting dementia: The 3C Study. Neurology. 2010;74:1760–1767. doi: 10.1212/WNL.0b013e3181df0959. [DOI] [PubMed] [Google Scholar]
- 16.Teichmann M., Epelbaum S., Samri D., Levy Nogueira M., Michon A., Hampel H. Free and cued Selective Reminding Test - accuracy for the differential diagnosis of Alzheimer's and neurodegenerative diseases: a large-scale biomarker-characterized monocenter cohort study (ClinAD) Alzheimers Dement. 2017;13:913–923. doi: 10.1016/j.jalz.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 17.Coley N., Gallini A., Ousset P.J., Vellas B., Andrieu S. Evaluating the clinical relevance of a cognitive composite outcome measure: an analysis of 1414 participants from the 5-year GuidAge Alzheimer's prevention trial. Alzheimers Dement. 2016;12:1216–1225. doi: 10.1016/j.jalz.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 18.Sevigny J., Chiao P., Bussiere T., Weinreb P.H., Williams L., Maier M. The antibody aducanumab reduces Aβ plaques in Alzheimer's disease. Nature. 2016;537:50–56. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- 19.Donohue M.C., Sperling R.A., Salmon D.P., Rentz D.M., Raman R., Thomas R.G. The preclinical alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol. 2014;71:961–970. doi: 10.1001/jamaneurol.2014.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papp K., Rentz D., Mormino E.C., Schultz A.P., Amariglio R.E., Quiroz Y. Cued memory decline in biomarker defined preclinical Alzheimer's Disease. 2017;88:1431–1438. doi: 10.1212/WNL.0000000000003812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grober E., Sanders A.E., Hall C., Lipton R.B. Free and cued selective reminding identifies very mild dementia in primary care. Alzheimer Dis Assoc Disord. 2010;24:284–290. doi: 10.1097/WAD.0b013e3181cfc78b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grober E., Buschke H., Crystal H., Bang S., Dresner R. Screening for dementia by memory testing. Neurology. 1988;38:900–903. doi: 10.1212/wnl.38.6.900. [DOI] [PubMed] [Google Scholar]
- 23.Grober E., Hall C., Lipton R.B., Zonderman A., Resnick S., Kawas C. Memory impairment, executive dysfunction, and intellectual decline. J Int Neuropsychol Soc. 2008;14:266–278. doi: 10.1017/S1355617708080302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mowrey W., Grober E., Zimmerman M., Katz M., Hall C., Sliwinski M. Estimating episodic memory within-subject decline among nondemented older adults: Results from the Einstein Aging Study (EAS) Alzheimers Dement. 2015;11:714. [Google Scholar]
- 25.Mura T., Proust-Lima C., Jacqmin-Gadda H., Akbaraly T.N., Touchon J., Dubois B. Measuring cognitive change in subjects with prodromal Alzheimer's disease. J Neurol Neurosurg Psychiatr. 2014;85:363–370. doi: 10.1136/jnnp-2013-305078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Philippi N., Noblet V., Duron E., Cretin B., Boully C., Wisniewski I. Exploring anterograde memory: a volumetric MRI study in patients with mild cognitive impairment. Alzheimers Res Ther. 2016;8:26. doi: 10.1186/s13195-016-0190-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koric L., Ranjeva J.P., Felician O., Guye M., de Anna F., Soulier E. Cued recall measure predicts the progression of gray matter atrophy in patients with amnesic mild cognitive impairment. Dement Geriatr Cogn Disord. 2013;36:197–210. doi: 10.1159/000351667. [DOI] [PubMed] [Google Scholar]
- 28.Xie J., Gabelle A., Dorey A., Garnier-Crussard A., Perret-Liaudet A., Delphin-Combe F. Initial memory deficit profiles in patients with a cerebrospinal fluid Alzheimer's disease signature. J Alzheimers Dis. 2014;41:1109–1116. doi: 10.3233/JAD-131916. [DOI] [PubMed] [Google Scholar]
- 29.Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubois B., Hampel H., Feldman H.H., Scheltens P., Aisen P., Andrieu S. Preclinical Alzheimer's disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 2016;12:292–323. doi: 10.1016/j.jalz.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jack C.R., Jr., Bennett D.A., Blennow K., Carrillo M.C., Feldman H.H., Frisoni G.B. A/T/N: An unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology. 2016;87:539–547. doi: 10.1212/WNL.0000000000002923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holtzer R., Goldin Y., Zimmerman M., Katz M., Buschke H., Lipton R.B. Robust norms for selected neuropsychological tests in older adults. Arch Clin Neuropsychol. 2008;23:531–541. doi: 10.1016/j.acn.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grober E., Ehrlich A., Troche Y., Hahn S., Lipton R.B. Screening older Latinos for dementia in the primary care setting. J Int Neuropsychol Soc. 2014;20:1–8. doi: 10.1017/S1355617714000708. [DOI] [PubMed] [Google Scholar]
- 34.Katz M.J., Lipton R.B., Hall C.B., Zimmerman M.E., Sanders A.E., Verghese J. Age-specific and sex-specific prevalence and incidence of mild cognitive impairment, dementia, and Alzheimer dementia in blacks and whites: a report from the Einstein Aging Study. Alzheimer Dis Assoc Disord. 2012;26:335–343. doi: 10.1097/WAD.0b013e31823dbcfc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blessed G., Tomlinson B.E., Roth M. The association between quantitative measures and senile changes in the cerebral gray matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 36.Visser P.J., Brodaty H. MCI is not a clinically useful concept. Int Psychogeriatr. 2006;18:402–409. 409–414. [PubMed] [Google Scholar]
- 37.Ritchie L.J., Tuokko H. Patterns of cognitive decline, conversion rates, and predictive validity for 3 models of MCI. Am J Alzheimers Dis Other Demen. 2010;25:592–603. doi: 10.1177/1533317510382286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bondi M.W., Edmonds E.C., Jak A.J., Clark L.R., Delano-Wood L., McDonald C.R. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. J Alzheimer's Dis. 2014;42:275–289. doi: 10.3233/JAD-140276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Auriacombe S., Helmer C., Amieva H., Berr C., Dubois B., Dartigues J.F. Validity of the free and cued selective reminding test in predicting dementia: the 3C study. Neurology. 2010;74:1706–1707. doi: 10.1212/WNL.0b013e3181df0959. [DOI] [PubMed] [Google Scholar]
- 40.Polcher A, Peters O, Dichgans M, Priller J, Laske C, Teipel S, et al. Alzheimer's Disease International Conference (Torono, Canada) 2016.
- 41.Schindler S.E., Jasielec M.S., Weng H., Hassenstab J.J., Grober E., McCue L.M. Neuropsychological measures that detect early impairment and decline in preclinical Alzheimer disease. Neurobiol Aging. 2017;56:25–32. doi: 10.1016/j.neurobiolaging.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith JS, Edgar CJ, Lasser RA, Shuguang S, Model F, Blaettler T. Alzheimer's Association International Conference 2016.
- 43.Sun S, Model F, Nikolcheva T, Lasser RA. Alzheimer's Association International Conference (Toronto, Canada) 2016.
- 44.Doherty TA, Abbott RA, Zavitz K, Edgar CJ, Cormack FK, Ashford E, et al. Alzheimer's Association International Conference (Toronto, Canada) 2016.
- 45.Zimmerman M.E., Katz M.J., Wang C., Burns L.C., Berman R.M., Derby C.A. Comparison of “Word” vs. "Picture" version of the Free and Cued Selective Reminding Test (FCSRT) in older adults. Alzheimers Dement. 2015;1:94–100. doi: 10.1016/j.dadm.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grober E., Hall C., Lipton R.B., Teresi J. Primary care screen for early dementia. J Am Geriatr Soc. 2008;56:206–213. doi: 10.1111/j.1532-5415.2007.01553.x. [DOI] [PubMed] [Google Scholar]
- 47.Mura T., Baramova M., Gabelle A., Artero S., Dartigues J.F., Amieva H. Predicting dementia using socio-demographic characteristics and the Free and Cued Selective Reminding Test in the general population. Alzheimers Res Ther. 2017;9:21. doi: 10.1186/s13195-016-0230-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grober E., Mowrey W.B., Ehrlich A.R., Mabie P., Hahn S., Lipton R.B. Two-stage screening for early dementia in primary care. J Clin Exp Neuropsychol. 2016;38:1038–1049. doi: 10.1080/13803395.2016.1187117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brookmeyer R., Abdalla N., Kawas C.H., Corrada M.M. 2017. Forecasting the prevalence of preclinical and clinical Alzheimer’s disease in the United States. Alzheimers Dement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr., Kawas C.H. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.