Abstract
Patients with locally advanced rectal cancer (LARC) are at tremendous risk of metastatic diseases. To improve the prognoses of LARC patients, the efficacy of adding targeted agents to neoadjuvant therapy has been investigated by many researchers but remains controversial. A literature search of relevant databases was conducted through December 2016, 804 studies were identified and 32 investigations were ultimately included. A total of 1196 patients from 31 cohorts of 29 studies were eligible for quantitative synthesis in this single‐arm setting meta‐analysis. As pathologic complete response (pCR) shows promise as a prognosis indicator, we focused on pCR rates to evaluate whether adding targeted agents to neoadjuvant therapies improves the outcome of LARC patients. In our study, we revealed pooled estimates of pCR of 27% (95%CI, 21–34%) and 14% (95%CI, 9–21%) for bevacizumab‐relevant cohorts and cetuximab‐relevant cohorts, respectively. The safety of adding targeted agents to neoadjuvant therapy was also evaluated by pooling the data of Grade 3/4 toxicity. In conclusion, our study revealed that adding bevacizumab to the neoadjuvant therapy regimens provides appreciable pCR for LARC patients. Meanwhile, the efficacy of cetuximab remains inconclusive, RCTs with larger scale and better study design that stress more on mutational status are needed.
Keywords: Efficacy, neoadjuvant therapy, pathologic complete response, rectal cancer, targeted agents
Introduction
Rectal cancer is one of the most commonly diagnosed and deadliest cancers around the world 1. Patients with locally advanced rectal cancer (LARC) are at tremendous risk of metastatic diseases due to high rates of local and distant recurrence 2. In recent years, neoadjuvant chemoradiotherapy (nCRT) has proven its efficacy in tumor downstaging and local control 3, 4. Tumor downstaging, usually indicated by the endpoint of pathologic complete response (pCR) which is defined as the complete remission of tumor cells in the resected specimen, can increase the success of radical surgery, provide better opportunity for sphincter preservation, and may be associated with increased benefit from adjuvant therapy for LARC patients 4, 5, 6. Thus, nCRT followed by total mesorectal excision (TME) and adjuvant chemotherapy has been highly recommended in the National Comprehensive Cancer Network (NCCN) guidelines as a standard treatment for LARC patients 7. However, the pCR rates reported in many studies investigating the efficacy of nCRT are far from satisfying. The FFCD trial 8 showed a pCR rate of merely 11.4% for 375 patients in the nCRT arm, while only 13.7% of enrolled patients receiving nCRT reached pCR in the EORTC 22921 trial 9. pCR rates in other studies were also reported to be around 15% after the conduction of nCRT, indicating that improved nCRT regimens are necessary 10, 11, 12.
In the past decade, numerous emerging strategies for adding various targeted agents to nCRT regimens gained attention from oncologists. Targeted vascular endothelial growth factor (VEGF) inhibitors or epidermal growth factor receptor (EGFR) monoclonal antibodies such as bevacizumab, aflibercept, cetuximab, and panitumumab have been demonstrated to increase pCR rates and improve prognoses for metastatic colorectal cancer (mCRC) patients 13, 14, 15, 16. However, the NCCN recommends against the addition of bevacizumab, cetuximab, or panitumumab to nCRT regimens for resectable mCRC patients due to the higher incidences of wound‐healing complications, treatment‐related mortality, and reduced progression‐free survival (PFS) 7, 17, 18, 19, 20. On the contrary, targeted agents are recommended to be added to nCRT for unresectable mCRC patients despite the blurred standards for regimens 7.
In recent years, the efficacy of adding targeted agents to neoadjuvant therapies for LARC patients has been studied by abundant phase II trials, with pCR being the primary endpoint 21, 22, 23, 24. Yet, with few randomized controlled trials (RCTs) or clinical controlled trials (CCTs) available, we lack head‐to‐head data of time‐to‐event endpoints such as overall survival (OS) and PFS to evaluate the survival status of LARC patients receiving targeted agents in their nCRT regimens compared with those receiving nCRT alone. Thus, we focused on the pCR rates of LARC patients to study the efficacy of adding targeted agents to their neoadjuvant therapies. pCR has become a widely accepted prognostic indicator in LARC patients 25. Maas et al. 26. conducted a meta‐analysis of a large amount of individual patient data provided by 14 investigators and concluded that rectal cancer patients with pCR have better local control, a lower rate of distant recurrence, and improved survival compared to those without pCR. Several other investigations have also recommended pCR as an indicator of better outcome concerning local or distant recurrence, disease‐free survival (DFS), and OS 27, 28, 29, 30, 31. However, the reported pCR rates in the current studies vary, ranging from approximately 39.1% 32 to merely 4.3% 33. The sample sizes of these studies are also relatively small, the largest being 8334 and the smallest consisting of only eight patients 35. Therefore, the efficacy of adding targeted agents to the nCRT for LARC patients is still controversial.
Since pCR shows promise as a prognosis indicator, in this meta‐analysis we pooled the data of pCR rates extracted from the included studies to evaluate whether adding targeted agents to neoadjuvant therapies improves the outcome of LARC patients.
Methods
Study selection
This meta‐analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta‐analysis (PRISMA) statements checklist 36.
The predefined criteria for eligible studies were as follow: (1) Patients with locally advanced rectal cancer (cT3‐4 primary rectal cancer and/or lymph node metastasis, without evidence of distant metastatic diseases). (2) Application of approved targeted agents in neoadjuvant therapy. (3) Endpoint of interest was pCR. (4) Original studies only (case reports, reviews, pooled‐analyses, and letters to the editor were excluded). Phase I clinical trials, which aim to evaluate the safety of novel agents, were also ruled out. (5) If investigations presented overlapping cohorts, studies which were more recently published and of higher quality were chosen.
Search strategy
PubMed, Embase, and Web of Science were searched using a combination of the following terms: “rectal,” “rectum,” “colorectal,” “tumor,” “cancer,” “neoplasm,” “neoadjuvant,” “preoperative,” “perioperative,” “targeted,” “VEGF,” “EGFR,” “bevacizumab,” “cetuximab,” “C225,” “panitumumab,” “ramucirumab,” and “aflibercept” for relevant publications up to December 17, 2016. The references of the relevant studies were also screened for potential pertinent articles. There were no language restrictions used during the search.
Data extraction
The primary endpoint was pCR and the second endpoint was the proportion of patients who encountered any Grade 3/4 toxic effects during preoperative chemoradiotherapy (preoperative Grade 3/4 toxicity). Data were manually extracted by two independent reviewers (X Zhong and Z.H. Wu) using standardized sheets. Any discrepancies between them were resolved by a third senior author.
The baseline details of the included studies were extracted by the same two reviewers and listed in the sheets mentioned above, and all the data entries were reviewed by the third senior author. The following data were extracted from VEGF‐inhibitor‐relevant studies: author and year of publication, study design, enrollment, regimen of neoadjuvant therapy, median age, tumor staging of included patients at enrollment, and the distance of primary tumor from anal verge. The following data were extracted from EGFR‐inhibitor‐relevant studies: author and year of publication, study design, enrollment, regimen of neoadjuvant therapy, median age, tumor staging of included patients at enrollment, the distance of primary tumor from anal verge, and KRAS status. The Newcastle‐Ottawa quality assessment scale (NOS) was applied to assess the quality of eligible studies for meta‐analysis 37. Studies which scored five or more were considered as moderate‐quality trials, whereas those with seven or more were regarded as high‐quality trials.
Statistical analysis
All statistical analyses were performed using STATA version 12.0 (STATA, College Station, TX). Meta‐analyses were conducted by calculating the pooled estimates of pCR and preoperative Grade 3/4 toxicity, and a random‐effect model was used which provides more conservative estimates for the inevitable heterogeneity of included multicenter studies 38. To evaluate heterogeneity, the Cochrane's Q test and inconsistent index (I2) were performed, with I2 < 40% considered acceptable 19, 39, 40. Potential origins of heterogeneity were detected by performing sensitivity analysis. Publication biases were evaluated via funnel plots, Begg's funnel plot, and Egger linear regression test for further confirmation 19.
Results
Study selection and the characteristics of included studies
We identified 804 publications through the initial database search and screening the references of relevant studies, and 788 remained after removing duplicates. We excluded 740 records after reading their titles and abstracts, leaving 48 potentially eligible studies for full‐text review. A total of 32 studies were ultimately included, after ruling out 16 ineligible investigations which failed to meet the inclusion and exclusion criteria for this meta‐analysis. The included studies consisted of 21 for the VEGF inhibitor, bevacizumab 21, 22, 23, 32, 33, 35, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, and 11 for EGFR inhibitors (eight for cetuximab 34, 56, 57, 58, 59, 60, 61, 62, one for nimotuzumab 63 and two for panitumumab 64, 65). These included one randomized clinical trial (RCT) 54 and three clinical controlled trials (CCT) 34, 53, 55, but we only analyzed cohorts which tested the addition of targeted agents to their neoadjuvant therapy regimens for this meta‐analysis. There were also two bevacizumab‐relevant studies 22, 52 consisting of two arms with bevacizumab in their neoadjuvant regimens, and we included all four cohorts for the meta‐analysis. Additionally, there was one study 49 consisting of two cohorts testing addition of bevacizumab, one in the neoadjuvant setting and the other in the postoperative setting, and we included only the former. The rest of the remaining studies were all single‐arm investigations. After the search, we determined that there were inadequate nimotuzumab‐relevant and panitumumab‐relevant studies to conduct a meta‐analysis. Thus, a total of 1196 subjects from 31 cohorts of 29 studies were eligible for quantitative synthesis. The whole selection process is presented in a flow diagram (Fig. 1). The baseline characteristics and data regarding the primary and secondary endpoints of the included studies for meta‐analysis are shown in Table 1 (bevacizumab‐relevant studies) and Table 2 (cetuximab‐relevant studies). The NOS quality assessment of the included investigations for meta‐analysis is shown in Table 3. Among the 29 studies, three scored seven points and were regarded as high‐quality studies and the remaining 26 all scored six points and were considered as studies of moderate quality.
Figure 1.
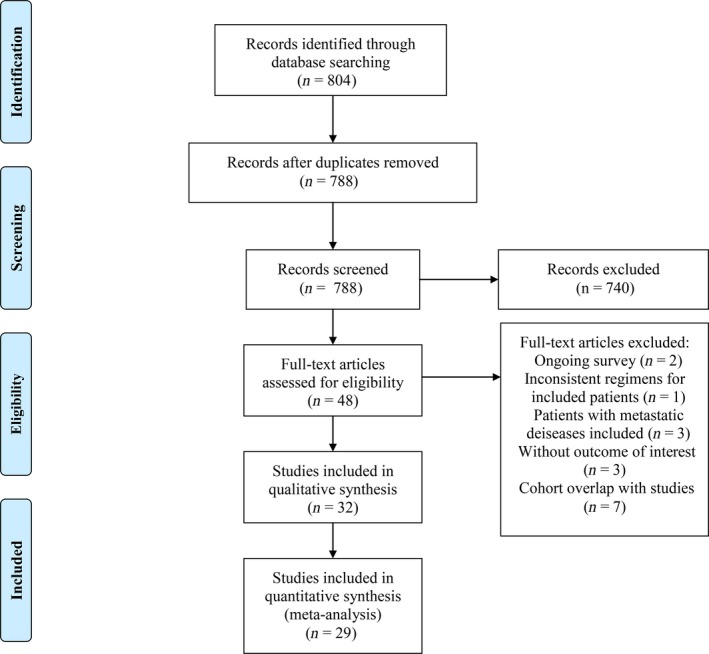
Selection of studies. Flow diagram showing the selection process for the included studies.
Table 1.
Baseline characteristics of cohort groups of bevacizumab for meta‐analysis
| Study | Study design | Enrollment, n | Neoadjuvant therapy | Median age, year | Stage at enrollment, n | Distance from anal verge, cm | PreoperativeGrade3/4 toxicity | pCR |
|---|---|---|---|---|---|---|---|---|
| Blaszkowsky 2014 | Prospective Phase I/II | 32 | 5‐FU + Erlotinib + bevacizumab + RT | NR | cT3N0: 6; cT3N1: 15; cT3N2: 4; cT3Nx: 4; cT4N0: 2; cT4N1: 1 | NR | 46.9% (15/32) | 33.3% (9/27) |
| Borg 2014 | Prospective Phase II | 46 | Folfox‐4 + bevacizumab | 60.6 | cT3N0: 10; cT3N1: 31; Tc3N2: 5 | NR | 50% (23/46) | 23.8% (10/42) |
| 45 | 5‐FU + bevacizumab + RT | 60.1 | cT3N0: 8; cT3N1: 28; cT3N2: 9 | NR | 20% (9/45) | 11.4% (5/44) | ||
| Crane 2010 | Prospective Phase II | 25 | Capecitabine + bevacizumab + RT | 54.0 | cT3N0: 5; cT3N0+: 20 | ≤5 cm: 15; >5 cm: 10 | NR | 32% (8/25) |
| Dellas 2013 | Prospective Phase II | 69 | Capox + bevacizumab + RT | 61.0 | cT2Nx: 2; cT3N0: 12; cT3N0 + : 44; cT4N0: 3; cT4N+: 4: | 5.92 ± 3.68 (Mean ± SD) | 11.6% (8/69) | 17.4% (12/69) |
| Dipetrillo2012 | Prospective Phase II | 25 | mFOLFOX6 + bevacizumab + RT | 50.0 | T2: 2; T3: 20; T4: 3; N‐: 7; N+: 16; Nx: 2 | NR | 76% (19/25) | 20% (5/25) |
| Fernandez‐Martos 2014 | Prospective Phase II | 46 | Capox + bevacizumab | NR | cT3: 46 | NR | NR | 19.6% (9/46) |
| Garcia 2015 | Prospective Phase II | 41 | Capecitabine + bevacizumab + RT | 63.0 | cT3a: 32; cT3a: 3; cT3b: 1; cT3c: 2; cT4: 2 | NR | 7.3% (3/41) | 7.5% (3/40) |
| Gasparini 2012 | Prospective Phase II | 43 | Capecitabine + bevacizumab + RT | 64.0 | cT2N1M0: 4; cT3N0M0: 14; cT3N1M0: 20; cT3NxM0: 1; cT4N1M0: 1; cT4N1M0: 1; cT4N2M0: 1; cTxN1M0: 1; cT4N2M1: 1 | NR | NR | 14.0%(6/43) |
| Hasegawa 2014 | Prospective Pilot study | 25 | Capox + bevacizumab | 63.0 | cT4aN0M0: 1; cT4bN0M0: 3; cT2,cT3N2M0: 3; cT3,cT4aN1M0: 10; cT4aN2M0: 1; cT4bN1/N2M0: 7 | 5.0 (Median) | 28% (7/25) | 4.3% (1/23) |
| Landry 2015 | Prospective Phase II | 54 | Capox + bevacizumab + RT | 54.0 | cT3: 50; cT4: 4; cNx: 2; cN0: 17; cN1: 30; cN2: 5 | NR | NR | 17.0% (9/53) |
| Nogue 2011 | Prospective Phase II | 47 | Capox + bevacizumab + RT | 58.5 | cT3N0: 5; cT3N1: 22; cT3N2: 14; cT4N0: 2; cT4N1: 2; cT4N2: 2 | NR | NR | 35.6% (16/45) |
| Resch 2012 | Prospective Phase II | 8 | Capecitabine + bevacizumab + RT | 70.0 | cT3: 8; cN0: 1; cN1: 4; cN2: 1; cNx: 2 | NR | 37.5% (3/8) | 25% (2/8) |
| Sadahiro 2015 | Prospective Phase II | 52 | S‐1 + bevacizumab + RT | 59.0 | cT2: 2; cT3: 49; cT4: 1; cN0: 16; cN1: 36 | 5.5 (Median) | 1.9% (1/52) | 19.2% (10/52) |
| Spigel 2012 | Prospective Phase II | 35 | 5‐FU + bevacizumab + RT | 57.0 | II: 11; III: 24 | NR | NR | 28.6% (10/35) |
| Uehara 2013 | Prospective Phase II | 32 | Capox + bevacizumab | 62.0 | cT3: 13; cT4a: 9; cT4b: 10; cN0: 6; cN1: 14; cN2: 12 | 4.7 (Median) | 25% (8/32) | 13.3% (4/30) |
| Velenik 2011 | Prospective Phase II | 61 | Capecitabine + bevacizumab + RT | 60.0 | cT3N0: 12; cT2N1: 1; cT3N1: 19; cT2N2: 2; cT3N2: 22; cT4N2: 5 | 6.0 (Median) | NR | 13.3% (8/60) |
| Wang 2014 | Prospective Phase II | 12 | FOLFOX + bevacizumab + RT/5‐FU + bevacizumab + RT | 52.5 | cT2: 1; cT3: 8; cT4: 3; cN0: 2; cN1: 2; cN2: 8 | 5 cm: 5; 5‐10 cm: 7; ≥10: 0 | 16.7% (2/12) | 33.3% (4/12) |
| 6 | FOLFOX + bevacizumab + RT | 57.5 | cT2: 0; cT3: 5; cT4: 1; cN0: 2; cN1: 4; cN2: 0 | 5 cm: 1; 5‐10 cm: 4; ≥10: 1 | 16.7% (1/6) | 25% (1/4) | ||
| Xiao 2015 | Prospective Phase II | 25 | 5‐FU + oxaliplatin + bevacizumab + RT | 45.0 | cT2: 2; cT3: 9; cT4a: 8; cT4b: 6; cN‐: 4; cN+: 21 | ≤5 cm: 7; >5 cm: 18 | NR | 39.1% (9/23) |
| Koukourakis 2011 | Prospective Phase II | 19 | Capecitabine + bevacizumab + RT | 68.0 | pT3: 19; pT4: 0; pN1: 12 | NR | NR | 36.8% (7/19) |
| Salazar 2015 | Prospective Phase II | 44 | Capecitabine + bevacizumab + RT | 64.0 | II A: 6; II B: 1; III B: 18; III C: 19 | 6.5 (Median) | 15.9% (7/44) | 15.9% (7/44) |
| Willett 2010 | Prospective Phase II | 32 | 5‐FU + bevacizumab + RT | 51.0 | cT3: 28; cT4: 4; cN0: 9; cN1‐2: 23 | NR | 21.9% (7/32) | 15.6% (5/32) |
pCR, pathologic complete response; RT: radiotherapy; 5‐FU, fluorouracil; FOLFOX, leucovorin plus fluorouracil plus oxaliplatin; Capox, capecitabine plus oxaliplatin; S‐1, tegafur plus gimeracil plus potassium oxonate; NR, not reported.
It was not specified if the cT3 status was cT3a, cT3b or cT3c.
Table 2.
Baseline characteristics of cohort groups of cetuximab for meta‐analysis
| Study | Study design | Enrollment | Neoadjuvant therapy | Median age, year | Stage at enrollment | Distance from anal verge, cm | KRAS status | Preoperative Grade3/4 toxicity | pCR |
|---|---|---|---|---|---|---|---|---|---|
| Bengala 2009 | Prospective Phase II | 40 | 5‐FU + cetuximab + RT | 61 | uT3N0: 12; uT3N1: 25; uT4N1: 3 | NR | Wild‐type: 30; Mutated: 9 | NR | 7.7% (3/39) |
| Horisberger 2009 | Prospective Phase II | 50 | Capecitabine+Irinotecan+cetuximab+RT | 57 | cT2: 5; T3: 42; cT4: 2; Local relapse: 1; cN0: 13; cN+: 37 | 7.5 (Median) (1–13, Range) | NR | NR | 8% (4/50) |
| Kim 2011 | Prospective Phase II | 40 | CapIri + cetuximab + RT | 56.5 | cT3N0: 6; cT3N+: 30; cT4N0: 2; cT4N+: 2 | ≤5: 19 > 5: 21 5.5 (Median) (0–8.0, Range) | Wild‐type: 33; Mutated: 5 | 17.9% (7/39) | 23.1% (9/39) |
| Machiels 2007 | Prospective Phase I/II | 40 | Capecitabine + cetuximab + RT | 61 | cT2N+: 2; cT3N0: 18; cT3N+: 13; cT4N0: 5; cT4N+: 2 | <6 cm: 25 6–10 cm: 10 > 10 cm: 5 | NR | NR | 5% (2/40) |
| Rodel 2008 | Prospective Phase I/II | 60 | Capox + cetuximab + RT | 61.5 | cT2N1‐2: 1; cT3N0: 7; cT3N1‐2: 43; cT4N0: 2; cT4N1‐2: 7 | 7 ± 3.5 (Mean ± SD) 0–14 (Range) Lower third (≤6 cm): 27 Middle third (6–12 cm): 27 Upper third (≥12 cm): 6 | NR | NR | 8.9% (4/45) |
| Sun 2012 | Prospective Phase II | 63 | Capecitabine + cetuximab + RT | 64 | cT3N0: 8; cT3N1: 21; cT3N2: 26; cT4N0: 2; cT4N1: 2; cT4N2: 4 | 5 (Median) (1–9, Range) | Wild‐type: 44; Mutated: 19 | NR | 12.7% (8/63) |
| Velenik 2012 | Prospective Phase II | 47 | Capecitabine + cetuximab + RT | 55 | cT3N0: 3; cT2N1: 1; cT3N1: 13; cT2N2: 1; cT3N2: 15; cT4N2: 4 | 6 (Median) (1–11, Range) | Wild‐type: 30; Mutated: 7 | NR | 8.1% (3/37) |
| Dewdney 2012 | Prospective Phase II | 83 | Capecitabine + cetuximab + RT | 61 | cT3c‐ T3d: 47; T4: 21 | NR | aWild‐type: 46; Mutated: 37 | NR | 18% (15/83) |
pCR, pathologic complete response; RT, radiotherapy; 5‐FU, fluorouracil; CapIri, capecitabine plus irinotecan; Capox, capecitabine plus oxaliplatin; NR, not reported.
KRAS/BRAF status.
Table 3.
The NOS quality of included studies
| Study | Selection | Comparability | Outcome | Total | Quality | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| REC | SNEC | AE | DO | SC | AF | AO | FU | AFU | |||
| Blaszkowsky 2014 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Borg 2014 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 7 | High |
| Crane 2010 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Dellas 2013 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Dipetrillo 2012 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Fernandez‐Martos 2014 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Garcia 2015 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Gasparini 2012 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Hasegawa 2014 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Landry 2015 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Nogue 2011 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Resch 2012 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Sadahiro 2015 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Spigel 2012 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Uehara 2013 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Velenik 2011 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Wang 2014 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Xiao 2015 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Koukourakis 2011 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Salazar 2015 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 7 | High |
| Willett 2010 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Bengala 2009 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Horisberger 2009 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Kim 2011 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Machiels 2007 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Rodel 2008 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Sun 2012 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Velenik 2012 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 6 | Moderate |
| Dewdney 2012 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 7 | High |
REC, representativeness of the exposed cohort; SNEC, selection of the nonexposed cohort; AE, ascertainment of exposure; DO, demonstration that outcome of interest was not present at start of study; SC, study controls for age, sex; AF, study controls for any additional factors; AO, assessment of outcome; FU: follow‐up long enough (36M) for outcomes to occur; AFU, adequacy of follow‐up of cohorts (≥90%). “1″ means that the study satisfies the item and “0” means the opposite situation.
The efficacy and safety of VEGF inhibitor
The pooled estimate of pCR for bevacizumab‐relevant cohorts was 27% (95%CI, 21–34%) (Fig. 2A). Meanwhile, the pooled estimate of preoperative Grade 3/4 toxicity for bevacizumab‐relevant cohorts was 36% (95% CI, 20–63%) (Fig. 3A). To better learn about the increased risk of clinically relevant toxicities, we listed the incidences of anti‐VEGF‐relevant toxicity focusing on bleeding, gastrointestinal perforation, and wound‐healing complication (shown in Table 4). The pooled estimates of Grade 3/4 bleeding, Grade 3/4 gastrointestinal perforation, and Grade 3/4 wound‐healing complication were also calculated and the results were 2.1% (95% CI, 1.0–4.7%) for Grade 3/4 bleeding, 1.9% (95% CI, 0.7–5.4%) for Grade 3/4 gastrointestinal perforation and 2.4% (95% CI, 1.0–6.2%) for Grade 3/4 wound‐healing complication.
Figure 2.
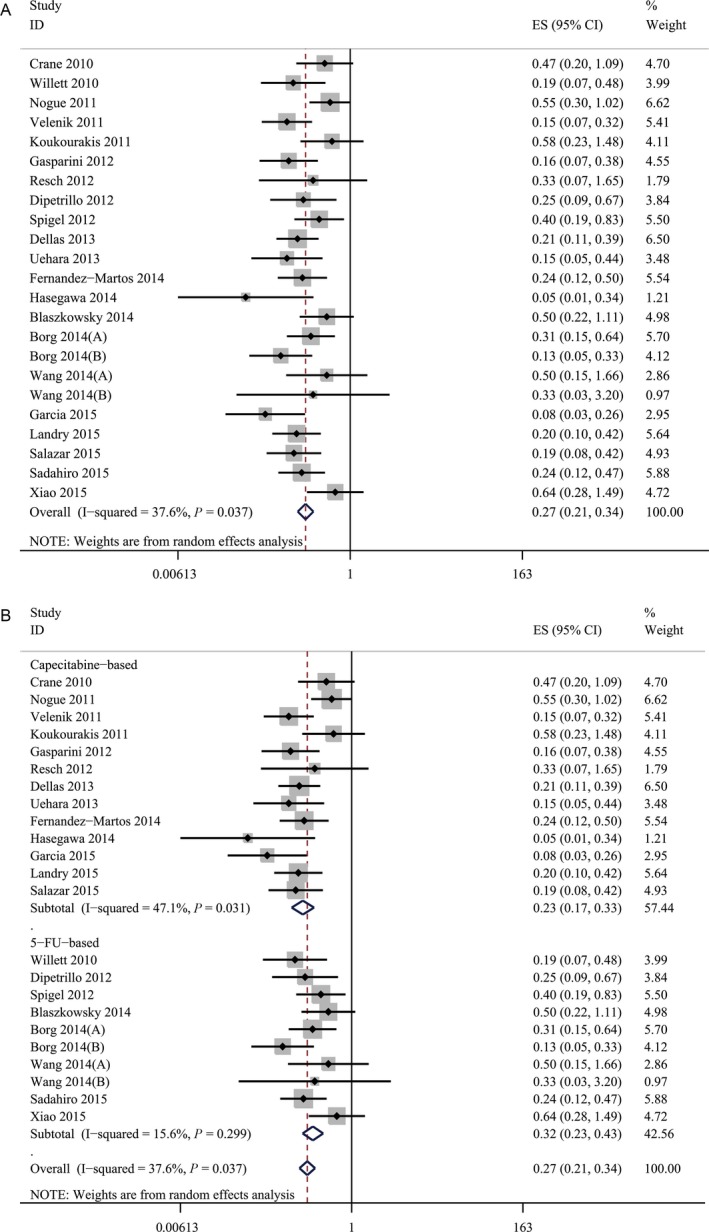
(A) The pooled estimate of pCR for bevacizumab‐relevant cohorts. (B) The results of subgroup analysis of bevacizumab‐relevant cohorts. The pooled estimates of pCR. pCR, pathologic complete response.
Figure 3.
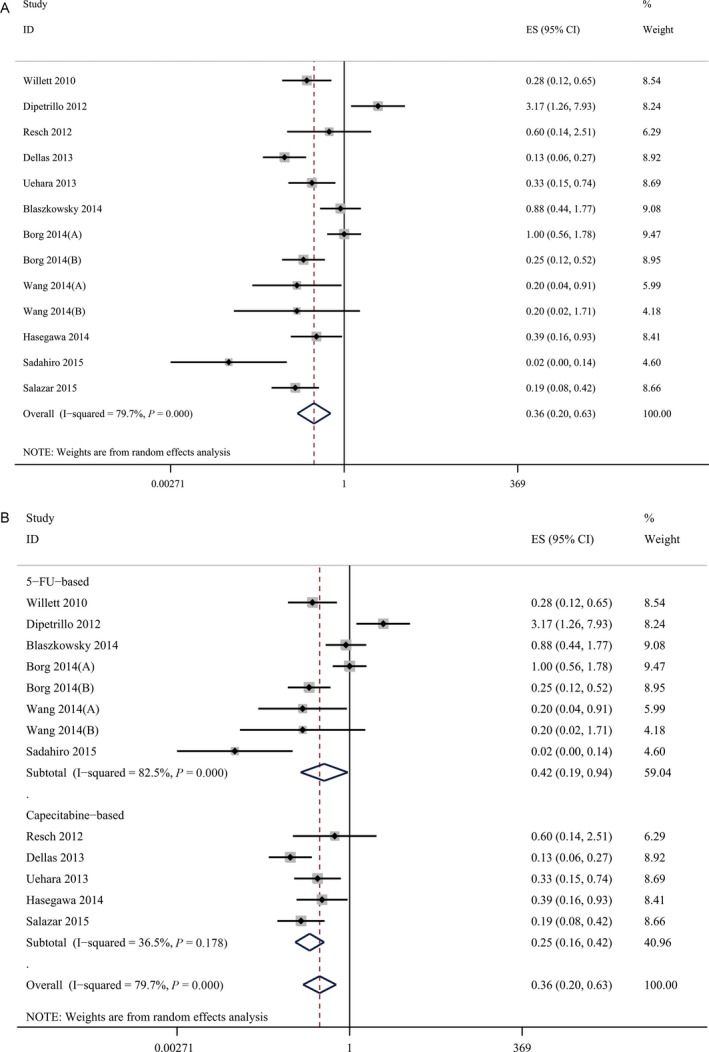
(A) The pooled estimate of preoperative Grade 3/4 toxicity for bevacizumab‐relevant cohorts. (B) The results of subgroup analysis of bevacizumab‐relevant cohorts. The pooled estimates of preoperative Grade 3/4 toxicity.
Table 4.
The treatment‐related toxicity status of patients who received additional anti‐VEGF or anti‐EGFR agents in neoadjuvant treatment
| Study | Enrollment, n | Neoadjuvant therapy | Grade 3/4 treatment‐related toxicitya |
|---|---|---|---|
| Blaszkowsky 2014 | 32 | 5‐FU + Erlotinib + bevacizumab + RT | NR |
| Borg 2014 | 46 | Folfox‐4 + bevacizumab | Grade 3/4 gastrointestinal perforation: 1/46 (2.17%)Grade 3/4 bleeding/hemorrhage: 2/46 (4.35%)Grade 3/4 wound‐healing complication: 0 |
| 45 | 5‐FU + bevacizumab + RT | Grade 3/4 gastrointestinal perforation: 0Grade 3/4 bleeding/hemorrhage: 0Grade 3/4 wound‐healing complication: 2/45 (4.44%) | |
| Crane 2010 | 25 | Capecitabine + bevacizumab + RT | NR |
| Dellas 2013 | 69 | Capox + bevacizumab + RT | Grade 3/4 delayed wound‐healing: 1/69 (1.45%) |
| Dipetrillo2012 | 25 | mFOLFOX6 + bevacizumab + RT | Grade 3/4 bleeding: 1/25 (4%) |
| Fernandez‐Martos 2014 | 46 | Capox + bevacizumab | NR |
| Garcia 2015 | 41 | Capecitabine + bevacizumab + RT | NR |
| Gasparini 2012 | 43 | Capecitabine + bevacizumab + RT | Grade 3/4 rectal hemorrhage: 0 |
| Hasegawa 2014 | 25 | Capox + bevacizumab | NR |
| Landry 2015 | 54 | Capox + bevacizumab + RT | Grade 3/4 CNS hemorrhage: 1/54 (1.85%) |
| Nogue 2011 | 47 | Capox + bevacizumab + RT | Grade 3/4 hemorrhage: 0 |
| Resch 2012 | 8 | Capecitabine + bevacizumab + RT | NR |
| Sadahiro 2015 | 52 | S‐1 + bevacizumab + RT | NR |
| Spigel 2012 | 35 | 5‐FU + bevacizumab + RT | Grade 3/4 wound complication: 0 |
| Uehara 2013 | 32 | Capox + bevacizumab | Grade 3/4 perforation: 1/32 (3.13%) |
| Velenik 2011 | 61 | Capecitabine + bevacizumab + RT | Grade 3/4 bleeding: 10/61 (16.39%) |
| Wang 2014 | 12 | FOLFOX + bevacizumab + RT/5‐FU + bevacizumab + RT | NR |
| 6 | FOLFOX + bevacizumab + RT | NR | |
| Xiao 2015 | 25 | 5‐FU + oxaliplatin + bevacizumab + RT | NR |
| Koukourakis 2011 | 19 | Capecitabine + bevacizumab + RT | NR |
| Salazar 2015 | 44 | Capecitabine + bevacizumab + RT | NR |
| Willett 2010 | 32 | 5‐FU + bevacizumab + RT | NR |
| Bengala 2009 | 40 | 5‐FU + cetuximab + RT | NR |
| Horisberger 2009 | 50 | Capecitabine + Irinotecan + cetuximab + RT | NR |
| Kim, S. Y 2011 | 40 | CapIri + cetuximab + RT | Grade 3/4 diarrhea: 2/40 (12.5%)Grade 3/4 hand‐foot syndrome: 0Grade 3/4 skin rash: 2/40 (5%) |
| Machiels 2007 | 40 | Capecitabine + cetuximab + RT | Grade 3/4 diarrhea: 6/40(15%);Grade 3/4 hand‐foot syndrome: 1/40 (2.5%);Grade 3/4 acneiform rash: 0 |
| Rodel 2008 | 60 | Capox + cetuximab + RT | Grade 3/4 diarrhea: 9/60 (15%)Grade 3/4 hand‐foot syndrome: 0Grade 3/4 radiation dermatitis: Grade 3: 4/60 (6.67%);Grade 3/4 acneiform rash: 2/60 (3.33%) |
| Sun 2012 | 63 | Capecitabine + cetuximab + RT | Grade 3/4 diarrhea: 0Grade 3/4 hand and foot syndrome: 0Grade 3/4 radiodermatitis: 10/63 (15.87%)Grade 3/4 acneiform rash: 4/63 (6.35%) |
| Velenik 2012 | 47 | Capecitabine + cetuximab + RT | Grade 3/4 diarrhea: 4/47 (8.51%)Grade 3/4 hand‐foot syndrome: 0Grade 3/4 acneiform rash: 0 |
| Dewdney 2012 | 83 | Capecitabine + cetuximab + RT | NR |
| Jin 2015 | 21 | Capecitabine + nimotuzumab + RT | Grade 3/4 diarrhea: 2/21 (9.52%)Grade 3/4 hand‐foot skin reaction: 0Grade 3/4 radiation dermatitis: 0Grade 3/4 acneiform rash: 0 |
| Helbling 2013 | 40 | Capecitabine + panitumumab + RT | Grade 3/4 diarrhea: 4/40 (10%)Grade 3/4 hand‐foot syndrome: 1/40 (2.5%)Grade 3/4 acneiform rash: 1/40 (2.5%) |
| Pinto 2011 | 60 | 5‐FU + oxaliplatin + panitumumab + RT | Grade 3/4 diarrhea: 23/60 (38.33%)Grade 3/4 hand‐foot syndrome: 0Grade 3/4 acneiform rash: 11/60 (18.33%) |
RT, radiotherapy; 5‐FU, fluorouracil; FOLFOX, leucovorin plus fluorouracil plus oxaliplatin; Capox, capecitabine plus oxaliplatin; S‐1, tegafur plus gimeracil plus potassium oxonate; NR, not reported.
We focused on bleeding and bowel perforation and impaired wound‐healing for anti‐VEGF‐relevant cohorts and diarrhea and skin changes in the affected area of the skin involved in radiotherapy for anti‐EGFR‐relevant cohorts.
To further evaluate the efficacy and safety of bevacizumab, we performed a subgroup analysis by separating the bevacizumab‐relevant cohorts into two subgroups: the 5‐fluorouracil‐based (5‐FU‐based) bevacizumab group and the capecitabine‐based bevacizumab group. The results of the subgroup analysis showed that the 5‐FU‐based bevacizumab group had a pooled estimate for pCR of 32% (95% CI, 23–43%) (Fig. 2B) and the pooled estimate of preoperative Grade 3/4 toxicity reached 42% (95% CI, 19–94%) (Fig. 3B). For capecitabine‐based bevacizumab group, a pooled pCR of 23% (95% CI, 17–33%) was achieved (Fig. 2B) along with a pooled estimate of preoperative Grade 3/4 toxicity of 25% (95% CI, 16–42%) (Fig. 3B).
The efficacy and safety of EGFR inhibitors
The pooled estimate of pCR for cetuximab‐relevant studies was 14% (95% CI, 9–21%) (Fig. 4). One study 58 reported a preoperative Grade 3/4 toxicity of approximately 17.9%, while the others did not report toxicity in this manner.
Figure 4.
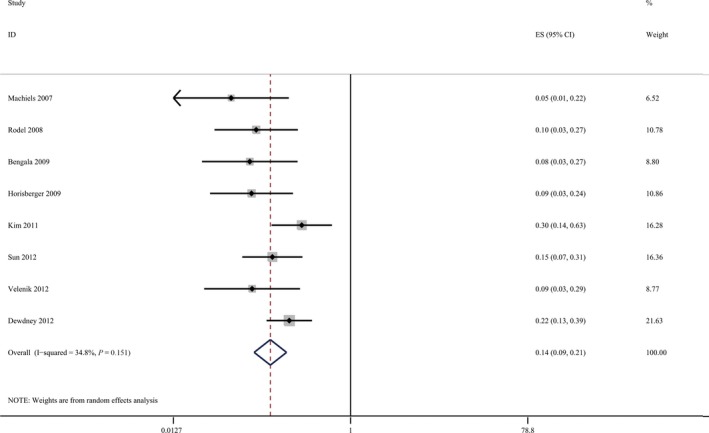
The pooled estimate of pathologic complete response for cetuximab‐relevant cohorts.
We reviewed the few studies involving the other EGFR inhibitors, although their low numbers made additional analysis unavailable. In the only study 63 focusing on nimotuzumab, four (19%) of 21 enrolled patients achieved pCR. For the two studies studying panitumumab, one 65 reported a pCR rate of 21.1% in 57 eligible patients and the other, a RCT 64 showed a 10% pCR rate for patients receiving panitumumab in addition with nCRT versus 18% for patients treated with nCRT alone.
To comprehensively evaluate the increased risk of clinically relevant toxicities, we listed the incidences of anti‐EGFR‐relevant toxicity focusing on diarrhea and skin changes in the affected area of the skin involved in radiotherapy (shown in Table 4). The pooled estimates of Grade 3/4 diarrhea, Grade 3/4 hand‐foot syndrome, Grade 3/4 rash, and Grade 3/4 radiodermatitis were also calculated and the results were 13.3% (95% CI, 6.4–27.9%) for Grade 3/4 diarrhea, 1.5% (95% CI, 0.6–3.7%) for Grade 3/4 hand‐foot syndrome, 5.2% (95% CI, 2.2–11.9%) for Grade 3/4 rash and 10.7% (95% CI, 4.2–27.1%) for Grade 3/4 radiodermatitis.
Evaluation of publication bias
To evaluate publication bias, we performed Begg's test and Egger's test. The P values of Begg's test and Egger's test for the pooled pCR of bevacizumab‐relevant cohorts were 0.303 and 0.277 (Fig. S2). The P values of Begg's test and Egger's test for the pooled preoperative Grade 3/4 toxicity of bevacizumab‐relevant cohorts were 0.714 and 0.257 (Fig. S3). The P values of Begg's test and Egger's test for the pooled pCR of cetuximab‐relevant cohorts were 0.048 and 0.005 (Fig. S4). To further evaluate the potential publication bias detected from the pooled pCR of cetuximab‐relevant cohorts, we performed sensitivity analysis, the results are shown in Figure S5.
Discussion
Since the use of neoadjuvant therapies began, a tremendous amount of work has been done to improve the regimens. Abundant clinical trials and two meta‐analyses have revealed the efficacy of preoperative radiotherapy granting better local control and a lower rate of local recurrence for LARC patients compared with surgery alone 66, 67. Subsequently, the addition of 5‐FU or capecitabine to neoadjuvant radiotherapy was demonstrated to significantly increase the incidence of pCR, and they have been widely accepted as first‐line anticancer regimens in the clinic 8, 68. More recently, researchers have studied the roles of various targeted agents added to the nCRT setting in pursuit of higher pCR rates for LARC patients. However, whether or not the addition of targeted agents to the nCRT regimens provides increased efficacy remains controversial and requires further investigation.
Until now, there have been limited RCTs and CCTs investigating the roles of targeted agents in nCRT regimens for LARC patients, and most of the studies in this field were single‐arm phase II studies. These single‐arm phase II studies basically focus on the pCR rates to demonstrate the efficacy of a certain targeted agent, and often lack data regarding patient survival status 35, 46, 47. Under these circumstances, a benchmark pCR rate would be necessary to be able to evaluate the efficacy of the additional targeted agents to the nCRT regimens. However, single‐arm phase II clinical trials lack a putative benchmark and usually evaluate the efficacy by comparing their pCR results with their predefined goal for pCR rate or the results of pCR in other studies 21, 22, 23. To help evaluate the efficacy of bevacizumab when added to the neoadjuvant therapy for LARC patients, we established a benchmark by quantitatively synthesizing the pCR rates of neoadjuvant therapy regimens without added targeted agents for LARC patients. We extracted pCR rates from ten cohorts that met our patient enrollment criteria and without any targeted agents in their nCRT regimens from the pooled analysis of Maas et al. 26. The baseline characteristics of these cohorts are shown in Table S1 and the pooled estimate of pCR of these cohorts was 17% (95% CI, 15–20%) (Fig. S1). This benchmark is also in the range of the pCR rates reported in several other previous studies 3, 4, 8, 10, 11, 12. Therefore, we believe that 17% is an adequate benchmark that can help reasonably evaluate the efficacy of adding targeted agents to the nCRT for LARC patients.
Willett et al. 69 were the pioneers in investigating the role of bevacizumab in 5‐FU‐based nCRT, and they achieved a feasible pCR rate of 16%. Other researchers also devoted themselves to evaluating the efficacy of bevacizumab in nCRT for LARC patients 21, 22, 23. In our study, we achieved a pooled pCR rate (27%) over the benchmark (17%) and thus, demonstrated an appreciable pCR for the addition of bevacizumab to neoadjuvant therapy for LARC patients. Moreover, the results of the subgroup analysis showed that the 5‐FU‐based group achieved a higher pooled estimate of pCR (32%) than capecitabine‐based group (23%), yet the pCR rates for both groups were higher than the benchmark (17%). One previous study demonstrated 70 that capecitabine‐based nCRT was superior to 5‐FU‐based nCRT in 5‐year overall survival, 3‐year DFS, reduction in distant metastasis, and pCR rate. Alternatively, a more recent meta‐analysis comparing the efficacies of oral capecitabine and infusional 5‐FU 71 demonstrated no significant difference between the pCR rates of the two groups in a neoadjuvant setting. The NCCN guidelines also comment that the efficacy of these two drugs is “equivalent” 7. In pursuit of a plausible explanation, we extracted and evaluated the RT status of bevacizumab‐relevant cohorts considering the tumor‐downsizing nature of RT (shown in Table S2). Three of the 13 capecitabine‐based cohorts do not include RT in their neoadjuvant therapy while only one of the 10 5‐FU‐based cohorts does not include RT. And the pCR of two of these three capecitabine‐based cohorts are distinctly low, merely 4.3% (1/23) and 13.3% (4/30). Besides, a total of 53 individuals who did not receive RT hold over a tenth of the whole capecitabine‐based group population. These may help explain the controversial result of this subgroup analysis to some extent. In summary, bevacizumab shows appreciable efficacy in nCRT for LARC patients, and this efficacy is consistent in 5‐FU‐based nCRT and capecitabine‐based nCRT. As our enrolled studies are mostly phase II clinical trials, this efficacy can encourage more incoming phase III clinical trials and serve as evidence of a promising outlook for future clinical applications of bevacizumab in nCRT regimens for LARC patients.
It is well‐known that chemotherapy can cause toxicity in patients. Thus, it is inevitable that adding targeted agents in nCRT regimens could result in extra toxicity. Sauer et al. 4 reported a Grade 3/4 toxicity rate of 27% in 399 rectal cancer patients receiving preoperative chemoradiotherapy. Two other important RCTs 3, 8 showed that Grade 3/4 toxicity occurred in 13.9% and 14.6% of their enrolled patients, respectively, in the duration of nCRT. A previous study 72 also demonstrated that LARC patients in two cohorts with different nCRT regimens without any targeted agents reached pCR rates of 17% and 13% at the cost of Grade 3/4 toxicities of 23% and 20%, respectively. In our study, the pooled estimates of preoperative Grade 3/4 toxicity (36% for total bevacizumab‐relevant cohorts, 42% for 5‐FU‐based bevacizumab group cohorts, and 25% for capecitabine‐based bevacizumab group cohorts) are reasonable considering the high rates of pooled pCR (27%, 32%, and 23%, respectively) in bevacizumab‐relevant cohorts. Additionally, the incidences of anti‐VEGF‐relevant toxicity listed in Table 4 and the pooled estimates indicate that anti‐VEGF treatment‐relevant toxicities are relatively mild. Thus, we presume that the safety of bevacizumab is acceptable.
The role of cetuximab, an anti‐EGFR monoclonal antibody, in nCRT for LARC patients has been investigated by many researchers in recent years 56, 59. In our study, we found that the pooled estimate for pCR in cetuximab‐relevant cohorts is less than the benchmark, which may indicate an inadequate efficacy of adding cetuximab to the nCRT for LARC patients. Increasing evidences have demonstrated that KRAS‐mutated patients cannot benefit from anti‐EGFR treatments 34, 73, 74, 75. It is also a well‐known fact that anti‐EGFR activity might be also strictly dependent on the presence/lack of mutations in NRAS or BRAF genes 7, 76, 77. In our study, most of the included cetuximab‐relevant studies only focus on KRAS status and did not report their pCR rates according to the KRAS status of the enrolled patients. Thus, the inadequate pooled pCR rate of cetuximab‐relevant cohorts may be due to the lack of published mutation status. As such, additional investigations are needed to explore the efficacy of adding cetuximab to the neoadjuvant therapy specifically for RAS and BRAF wild‐type LARC patients.
The few studies 63, 64, 65 investigating the roles of nimotuzumab and panitumumab in the nCRT for LARC patients did not show convincing evidence for efficacy or safety, so more investigations regarding nimotuzumab and panitumumab are urgently needed. Two ongoing surveys 78, 79 focusing on bevacizumab and lapatinib are expected to provide more evidence on the outcome of LARC patients in a couple of years.
Despite the inadequate pCR, the addition of anti‐EGFR agents presents acceptable safety and this safety may facilitate more anti‐EGFR‐oriented clinical trials.
No publication bias was detected in the meta‐analysis for bevacizumab‐relevant cohorts. However, the results of Begg's and Egger's tests concerning the pCR for cetuximab‐relevant cohorts suggested the existence of potential publication bias. The results of sensitivity analysis, as shown in Figure S5, seem to indicate that the pooled pCR of cetuximab‐relevant cohorts deviates from the current value most when Dewdney et al's study is omitted. Thus, we comprehensively reviewed this well‐designed RCT of Dewdney et al's and found that the pCR rate of their cetuximab‐relevant arm (18%) was higher than most of the other included cetuximab‐relevant cohorts. Meanwhile, this cohort held the largest weight in the quantitative analysis due to the largest sample size (83) among all of the inclusions. Besides, over half of the population (46) in this cohort are KRAS/BRAF wild type which is previously reported to present good response to anti‐EGFR treatment. All of the above accounts for the higher pCR presented in this cohort and explains why this pCR influences the pooled estimate most.
Our study is the first meta‐analysis to evaluate the efficacy of targeted agents in the nCRT for LARC patients. However, several limitations exist in our study. First, due to the lack of relevant RCTs and CCTs, we conducted this meta‐analysis in a single‐arm setting. Second, we only focused on pCR and its indicative role in our meta‐analysis and we lack data regarding perioperative and postoperative outcomes including operation time, perioperative complication rate, and postoperative recovery time so that we cannot directly evaluate the potential influences that adding targeted agents may have on the following curative surgical resection and postoperative recovery of LARC patients which highly concern clinical practitioners in this field. Third, cohort numbers from single‐arm studies included in this study are mostly small‐scale, which can lead to over‐reporting of the efficacy of these neoadjuvant regimens. Meanwhile, heterogeneity is, to an extent, inevitable among these multi‐center studies. Fourth, most of the anti‐EGFR cohorts are small‐scale and stress KRAS status only. To the best of our knowledge, however, anti‐EGFR activity might be also strictly determined by the mutational statuses of NRAS and BRAF. Fifth, when conducting this study, we only focused on published studies and extracted data available in the text, thus, we did not have access to relevant individual patient data, which could help us improve the analysis of the treatment effects of the targeted agents. Despite these limitations, we found that there is increased efficacy when adding bevacizumab to nCRT for LARC patients.
Conclusion
In conclusion, our study revealed that adding bevacizumab to the neoadjuvant therapy regimens provides an appreciable pCR for LARC patients. However, more RCTs are needed for further validation. Meanwhile, the efficacy of cetuximab remains inconclusive, RCTs with larger scale and better study design that stress more on mutational status are needed.
Conflict of Interest
The authors declare that they have no conflict of interest.
Supporting information
Figure S1. The establishment of benchmark for pCR.
Figure S2. (a) The Begg's funnel plots concerning the pCR for bevacizumab‐relevant studies. (b) The Egger's publication bias plot concerning the pCR for bevacizumab‐relevant studies.
Figure S3. (a) The Begg's funnel plots concerning the preoperative Grade 3/4 toxicity for bevacizumab‐relevant studies. (b) The Egger's publication bias plot concerning the preoperative Grade 3/4 toxicity for bevacizumab‐relevant studies.
Figure S4. (a) The Begg's funnel plots concerning the pCR for cetuximab‐relevant studies. (b) The Egger's publication bias plot concerning the pCR for cetuximab‐relevant studies.
Figure S5. The results of the sensitivity analysis concerning the pCR for cetuximab‐relevant studies.
Table S1. Data from Maas, et al's pooled analysis.
Table S2. The radiotherapy status of Bevacizumab‐relevant cohorts.
Acknowledgments
This work was supported by Clinical Capability Construction Project for Liaoning Provincial Hospitals (LNCCC‐A01‐2014), the Key Laboratory Programme of Education Department of Liaoning Province (LZ2015076) and Scientific Programme of Science & Technology Department of Liaoning Province(2015225002).
Contributor Information
Zhenning Wang, Email: josieon826@sina.cn.
Yongxi Song, Email: songyongxi840309@126.com.
References
- 1. Torre, L. A. , Bray F., Siegel R. L., Ferlay J., Lortet‐Tieulent J., and Jemal A.. 2015. Global cancer statistics, 2012. CA Cancer J. Clin. 65:87–108. [DOI] [PubMed] [Google Scholar]
- 2. Schrag, D. , Weiser M. R., Goodman K. A., Gonen M., Hollywood E., Cercek A., et al. 2014. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J. Clin. Oncol. 32:513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bosset, J. F. , Collette L., Calais G., Mineur L., Maingon P., Radosevic‐Jelic L., et al. 2006. Chemotherapy with preoperative radiotherapy in rectal cancer. N. Engl. J. Med. 355:1114–1123. [DOI] [PubMed] [Google Scholar]
- 4. Sauer, R. , Becker H., Hohenberger W., Rodel C., Wittekind C., Fietkau R., et al. 2004. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N. Engl. J. Med. 351:1731–1740. [DOI] [PubMed] [Google Scholar]
- 5. Janjan, N. A. , Crane C., Feig B. W., Cleary K., Dubrow R., Curley S., et al. 2001. Improved overall survival among responders to preoperative chemoradiation for locally advanced rectal cancer. Am. J. Clin. Oncol. 24:107–112. [DOI] [PubMed] [Google Scholar]
- 6. Collette, L. , Bosset J. F., den Dulk M., Nguyen F., Mineur L., Maingon P., et al. 2007. Patients with curative resection of cT3‐4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil‐based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group. J. Clin. Oncol. 25:4379–4386. [DOI] [PubMed] [Google Scholar]
- 7. Rectal cancer V.3 . 2017. NCCN Clinical Practical Guidelines in Oncology. Available at: http://www.nccn.org/professionals/physician_gls/
- 8. Gerard, J. P. , Conroy T., Bonnetain F., Bouche O., Chapet O., Closon‐Dejardin M. T., et al. 2006. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3‐4 rectal cancers: results of FFCD 9203. J. Clin. Oncol. 24:4620–4625. [DOI] [PubMed] [Google Scholar]
- 9. Bosset, J. F. , Calais G., Mineur L., Maingon P., Radosevic‐Jelic L., Daban A., et al. 2005. Enhanced tumorocidal effect of chemotherapy with preoperative radiotherapy for rectal cancer: preliminary results–EORTC 22921. J. Clin. Oncol. 23:5620–5627. [DOI] [PubMed] [Google Scholar]
- 10. Bazarbashi, S. , El‐Bassiouni M., Abdelsalam M., Soudy H., Sanea N. A., Jabbar A. A., et al. 2008. A modern regimen of pre‐operative concurrent chemo‐radiation therapy in locally advanced rectal cancer. J. Surg. Oncol. 98:167–174. [DOI] [PubMed] [Google Scholar]
- 11. Chan, A. K. , Wong A. O., and Jenken D. A.. 2010. Preoperative capecitabine and pelvic radiation in locally advanced rectal cancer–is it equivalent to 5‐FU infusion plus leucovorin and radiotherapy? Int. J. Radiat. Oncol. Biol. Phys. 76:1413–1419. [DOI] [PubMed] [Google Scholar]
- 12. Craven, I. , Crellin A., Cooper R., Melcher A., Byrne P., and Sebag‐Montefiore D.. 2007. Preoperative radiotherapy combined with 5 days per week capecitabine chemotherapy in locally advanced rectal cancer. Br. J. Cancer 97:1333–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hurwitz, H. , Fehrenbacher L., Novotny W., Cartwright T., Hainsworth J., Heim W., et al. 2004. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 350:2335–2342. [DOI] [PubMed] [Google Scholar]
- 14. Jonker, D. J. , O'Callaghan C. J., Karapetis C. S., Zalcberg J. R., Tu D., Au H. J., et al. 2007. Cetuximab for the treatment of colorectal cancer. N. Engl. J. Med. 357:2040–2048. [DOI] [PubMed] [Google Scholar]
- 15. Douillard, J. Y. , Siena S., Cassidy J., Tabernero J., Burkes R., Barugel M., et al. 2010. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first‐line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J. Clin. Oncol. 28:4697–4705. [DOI] [PubMed] [Google Scholar]
- 16. Van Cutsem, E. , Tabernero J., Lakomy R., Prenen H., Prausova J., Macarulla T., et al. 2012. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin‐based regimen. J. Clin. Oncol. 30:3499–3506. [DOI] [PubMed] [Google Scholar]
- 17. Ranpura, V. , Hapani S., and Wu S.. 2011. Treatment‐related mortality with bevacizumab in cancer patients: a meta‐analysis. JAMA 305:487–494. [DOI] [PubMed] [Google Scholar]
- 18. Scappaticci, F. A. , Fehrenbacher L., Cartwright T., Hainsworth J. D., Heim W., Berlin J., Kabbinavar F., et al. 2005. Surgical wound healing complications in metastatic colorectal cancer patients treated with bevacizumab. J. Surg. Oncol. 91:173–180. [DOI] [PubMed] [Google Scholar]
- 19. Higgins, T J. P., Green S. eds. 2011. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, Available from http://handbookcochraneorg (last accessed March 2011)
- 20. Primrose, J. , Falk S., Finch‐Jones M., Valle J., O'Reilly D., Siriwardena A., et al. 2014. Systemic chemotherapy with or without cetuximab in patients with resectable colorectal liver metastasis: the New EPOC randomised controlled trial. Lancet Oncol. 15:601–611. [DOI] [PubMed] [Google Scholar]
- 21. Garcia, M. , Martinez‐Villacampa M., Santos C., Navarro V., Teule A., Losa F., et al. 2015. Phase II study of preoperative bevacizumab, capecitabine and radiotherapy for resectable locally‐advanced rectal cancer. BMC Cancer 15:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Borg, C. , Andre T., Mantion G., Boudghene F., Mornex F., Maingon P., et al. 2014. Pathological response and safety of two neoadjuvant strategies with bevacizumab in MRI‐defined locally advanced T3 resectable rectal cancer: a randomized, noncomparative phase II study. Ann. Oncol. 25:2205–2210. [DOI] [PubMed] [Google Scholar]
- 23. Blaszkowsky, L. S. , Ryan D. P., Szymonifka J., Borger D. R., Zhu A. X., Clark J. W., et al. 2014. Phase I/II study of neoadjuvant bevacizumab, erlotinib and 5‐fluorouracil with concurrent external beam radiation therapy in locally advanced rectal cancer. Ann. Oncol. 25:121–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee, J. J. , and Feng L.. 2005. Randomized phase II designs in cancer clinical trials: current status and future directions. J. Clin. Oncol. 23:4450–4457. [DOI] [PubMed] [Google Scholar]
- 25. Capirci, C. , Valentini V., Cionini L., De Paoli A., Rodel C., Glynne‐Jones R., et al. 2008. Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: long‐term analysis of 566 ypCR patients. Int. J. Radiat. Oncol. Biol. Phys. 72:99–107. [DOI] [PubMed] [Google Scholar]
- 26. Maas, M. , Nelemans P. J., Valentini V., Das P., Rodel C., Kuo L. J., et al. 2010. Long‐term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 11:835–844. [DOI] [PubMed] [Google Scholar]
- 27. Chari, R. S. , Tyler D. S., Anscher M. S., Russell L., Clary B. M., Hathorn J., et al. 1995. Preoperative radiation and chemotherapy in the treatment of adenocarcinoma of the rectum. Ann. Surg. 221:778–786;discussion 86‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Diaz‐Gonzalez, J. A. , Calvo F. A., Cortes J., Garcia‐Sabrido J. L., Gomez‐Espi M., Del Valle E., et al. 2006. Prognostic factors for disease‐free survival in patients with T3‐4 or N+ rectal cancer treated with preoperative chemoradiation therapy, surgery, and intraoperative irradiation. Int. J. Radiat. Oncol. Biol. Phys. 64:1122–1128. [DOI] [PubMed] [Google Scholar]
- 29. Rodel, C. , Martus P., Papadoupolos T., Fuzesi L., Klimpfinger M., Fietkau R., et al. 2005. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J. Clin. Oncol. 23:8688–8696. [DOI] [PubMed] [Google Scholar]
- 30. Valentini, V. , Coco C., Picciocchi A., Morganti A. G., Trodella L., Ciabattoni A., et al. 2002. Does downstaging predict improved outcome after preoperative chemoradiation for extraperitoneal locally advanced rectal cancer? A long‐term analysis of 165 patients. Int. J. Radiat. Oncol. Biol. Phys. 53:664–674. [DOI] [PubMed] [Google Scholar]
- 31. Vecchio, F. M. , Valentini V., Minsky B. D., Padula G. D., Venkatraman E. S., Balducci M., et al. 2005. The relationship of pathologic tumor regression grade (TRG) and outcomes after preoperative therapy in rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 62:752–760. [DOI] [PubMed] [Google Scholar]
- 32. Xiao, J. , Chen Z., Li W., Yang Z., Huang Y., Zheng J., et al. 2015. Sandwich‐like neoadjuvant therapy with bevacizumab for locally advanced rectal cancer: a phase II trial. Cancer Chemother. Pharmacol. 76:21–27. [DOI] [PubMed] [Google Scholar]
- 33. Hasegawa, J. , Nishimura J., Mizushima T., Miyake Y., Kim H. M., Takemoto H., Tamagawa H., Noura S., Fujii M., Fujie Y., Kato T., Miwa H., et al. 2014. Neoadjuvant capecitabine and oxaliplatin (XELOX) combined with bevacizumab for high‐risk localized rectal cancer. Cancer Chemother. Pharmacol. 73:1079–1087. [DOI] [PubMed] [Google Scholar]
- 34. Dewdney, A. , Cunningham D., Tabernero J., Capdevila J., Glimelius B., Cervantes A., Tait D., Brown G., Wotherspoon A., Gonzalez de Castro D., Chua Y. J., Wong R., et al. 2012. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high‐risk rectal cancer (EXPERT‐C). J. Clin. Oncol. 30:1620–1627. [DOI] [PubMed] [Google Scholar]
- 35. Resch, G. , De Vries A., Ofner D., Eisterer W., Rabl H., Jagoditsch M., Gnant M., and Thaler J.. 2012. Preoperative treatment with capecitabine, bevacizumab and radiotherapy for primary locally advanced rectal cancer–a two stage phase II clinical trial. Radiother. Oncol. 102:10–13. [DOI] [PubMed] [Google Scholar]
- 36. Moher, D. , Liberati A., Tetzlaff J., and Altman D. G.. 2010. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Int. J. Surg. 8: 336–341. [DOI] [PubMed] [Google Scholar]
- 37. Stang, A. 2010. Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. Eur. J. Epidemiol. 25:603–605. [DOI] [PubMed] [Google Scholar]
- 38. Liebig, C. , Ayala G., Wilks J., Verstovsek G., Liu H., Agarwal N., Berger D. H., and Albo D.. 2009. Perineural invasion is an independent predictor of outcome in colorectal cancer. J. Clin. Oncol. 27:5131–5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. DerSimonian, R. , and Laird N.. 1986. Meta‐analysis in clinical trials. Control. Clin. Trials 7:177–188. [DOI] [PubMed] [Google Scholar]
- 40. Higgins, J. P. , Thompson S. G., Deeks J. J., and Altman D. G.. 2003. Measuring inconsistency in meta‐analyses. BMJ 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Crane, C. H. , Eng C., Feig B. W., Das P., Skibber J. M., Chang G. J., Wolff R. A., Krishnan S., Hamilton S., Janjan N. A., Maru D. M., Ellis L. M., et al. 2010. Phase II trial of neoadjuvant bevacizumab, capecitabine, and radiotherapy for locally advanced rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 76:824–830. [DOI] [PubMed] [Google Scholar]
- 42. Dellas, K. , Hohler T., Reese T., Wurschmidt F., Engel E., Rodel C., Wagner W., Richter M., Arnold D., and Dunst J.. 2013. Phase II trial of preoperative radiochemotherapy with concurrent bevacizumab, capecitabine and oxaliplatin in patients with locally advanced rectal cancer. Radiat. Oncol. 8: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dipetrillo, T. , Pricolo V., Lagares‐Garcia J., Vrees M., Klipfel A., Cataldo T., Sikov W., McNulty B., Shipley J., Anderson E., Khurshid H., Oconnor B., et al. 2012. Neoadjuvant bevacizumab, oxaliplatin, 5‐fluorouracil, and radiation for rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 82:124–129. [DOI] [PubMed] [Google Scholar]
- 44. Fernandez‐Martos, C. , Brown G., Estevan R., Salud A., Montagut C., Maurel J., Safont M. J., Aparicio J., Feliu J., Vera R., Alonso V., Gallego J., et al. 2014. Preoperative chemotherapy in patients with intermediate‐risk rectal adenocarcinoma selected by high‐resolution magnetic resonance imaging: the GEMCAD 0801 Phase II Multicenter Trial. Oncologist 19:1042–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gasparini, G. , Torino F., Ueno T., Cascinu S., Troiani T., Ballestrero A., Berardi R., Shishido J., Yoshizawa A., Mori Y., Nagayama S., Morosini P., et al. 2012. A phase II study of neoadjuvant bevacizumab plus capecitabine and concomitant radiotherapy in patients with locally advanced rectal cancer. Angiogenesis 15:141–150. [DOI] [PubMed] [Google Scholar]
- 46. Landry, J. C. , Feng Y., Prabhu R. S., Cohen S. J., Staley C. A., Whittington R., Sigurdson E. R., Nimeiri H., Verma U., and Benson A. B.. 2015. Phase II trial of preoperative radiation with concurrent capecitabine, oxaliplatin, and bevacizumab followed by surgery and postoperative 5‐fluorouracil, leucovorin, oxaliplatin (FOLFOX), and bevacizumab in patients with locally advanced rectal cancer: 5‐year clinical outcomes ECOG‐ACRIN cancer research group E3204. Oncologist 20:615–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nogue, M. , Salud A., Vicente P., Arrivi A., Roca J. M., Losa F., Ponce J., Safont M. J., Guasch I., Moreno I., Ruiz A., and Pericay C.. 2011. Addition of bevacizumab to XELOX induction therapy plus concomitant capecitabine‐based chemoradiotherapy in magnetic resonance imaging‐defined poor‐prognosis locally advanced rectal cancer: the AVACROSS study. Oncologist 16:614–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sadahiro, S. , Suzuki T., Tanaka A., Okada K., Saito G., Kamijo A., Akiba T., and Kawada S.. 2015. Phase II study of preoperative concurrent chemoradiotherapy with S‐1 plus bevacizumab for locally advanced resectable rectal adenocarcinoma. Oncology 88:49–56. [DOI] [PubMed] [Google Scholar]
- 49. Spigel, D. R. , Bendell J. C., McCleod M., Shipley D. L., Arrowsmith E., Barnes E. K., Infante J. R., Burris H. A. 3rd, Greco F. A., and Hainsworth J. D.. 2012. Phase II study of bevacizumab and chemoradiation in the preoperative or adjuvant treatment of patients with stage II/III rectal cancer. Clin. Colorectal Cancer 11:45–52. [DOI] [PubMed] [Google Scholar]
- 50. Uehara, K. , Hiramatsu K., Maeda A., Sakamoto E., Inoue M., Kobayashi S., Tojima Y., Yoshioka Y., Nakayama G., Yatsuya H., Ohmiya N., Goto H., et al. 2013. Neoadjuvant oxaliplatin and capecitabine and bevacizumab without radiotherapy for poor‐risk rectal cancer: N‐SOG 03 Phase II trial. Jpn. J. Clin. Oncol. 43:964–971. [DOI] [PubMed] [Google Scholar]
- 51. Velenik, V. , Ocvirk J., Music M., Bracko M., Anderluh F., Oblak I., Edhemovic I., Brecelj E., Kropivnik M., and Omejc M.. 2011. Neoadjuvant capecitabine, radiotherapy, and bevacizumab (CRAB) in locally advanced rectal cancer: results of an open‐label phase II study. Radiat. Oncol. 6: 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang, C. C. , Liang J. T., Tsai C. L., Chen Y. H., Lin Y. L., Shun C. T., and Cheng J. C.. 2014. Neoadjuvant bevacizumab and chemoradiotherapy in locally advanced rectal cancer: early outcome and technical impact on toxicity. World J. Surg. Oncol. 12:329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Koukourakis, M. I. , Giatromanolaki A., Tsoutsou P., Lyratzopoulos N., Pitiakoudis M., Kouklakis G., Chloropoulou P. A., Manolas K., and Sivridis E.. 2011. Bevacizumab, capecitabine, amifostine, and preoperative hypofractionated accelerated radiotherapy (HypoArc) for rectal cancer: a Phase II study. Int. J. Radiat. Oncol. Biol. Phys. 80:492–498. [DOI] [PubMed] [Google Scholar]
- 54. Salazar, R. , Capdevila J., Laquente B., Manzano J. L., Pericay C., Villacampa M. M., Lopez C., Losa F., Safont M. J., Gomez A., Alonso V., Escudero P., et al. 2015. A randomized phase II study of capecitabine‐based chemoradiation with or without bevacizumab in resectable locally advanced rectal cancer: clinical and biological features. BMC Cancer 15:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Willett, C. G. , Duda D. G., Ancukiewicz M., Shah M., Czito B. G., Bentley R., Poleski M., Fujita H., Lauwers G. Y., Carroll M., Tyler D., Mantyh C., et al. 2010. A safety and survival analysis of neoadjuvant bevacizumab with standard chemoradiation in a phase I/II study compared with standard chemoradiation in locally advanced rectal cancer. Oncologist 15:845–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bengala, C. , Bettelli S., Bertolini F., Salvi S., Chiara S., Sonaglio C., Losi L., Bigiani N., Sartori G., Dealis C., Malavasi N., D'Amico R., et al. 2009. Epidermal growth factor receptor gene copy number, K‐ras mutation and pathological response to preoperative cetuximab, 5‐FU and radiation therapy in locally advanced rectal cancer. Ann. Oncol. 20:469–474. [DOI] [PubMed] [Google Scholar]
- 57. Horisberger, K. , Treschl A., Mai S., Barreto‐Miranda M., Kienle P., Strobel P., Erben P., Woernle C., Dinter D., Kahler G., Hochhaus A., Post S., et al. 2009. Cetuximab in combination with capecitabine, irinotecan, and radiotherapy for patients with locally advanced rectal cancer: results of a Phase II MARGIT trial. Int. J. Radiat. Oncol. Biol. Phys. 74:1487–1493. [DOI] [PubMed] [Google Scholar]
- 58. Kim, S. Y. , Hong Y. S., Kim D. Y., Kim T. W., Kim J. H., Im S. A., Lee K. S., Yun T., Jeong S. Y., Choi H. S., Lim S. B., Chang H. J., et al. 2011. Preoperative chemoradiation with cetuximab, irinotecan, and capecitabine in patients with locally advanced resectable rectal cancer: a multicenter Phase II study. Int. J. Radiat. Oncol. Biol. Phys. 81:677–683. [DOI] [PubMed] [Google Scholar]
- 59. Machiels, J. P. , Sempoux C., Scalliet P., Coche J. C., Humblet Y., Van Cutsem E., Kerger J., Canon J. L., Peeters M., Aydin S., Laurent S., Kartheuser A., et al. 2007. Phase I/II study of preoperative cetuximab, capecitabine, and external beam radiotherapy in patients with rectal cancer. Ann. Oncol. 18:738–744. [DOI] [PubMed] [Google Scholar]
- 60. Rodel, C. , Arnold D., Hipp M., Liersch T., Dellas K., Iesalnieks I., Hermann R. M., Lordick F., Hinke A., Hohenberger W., and Sauer R.. 2008. Phase I‐II trial of cetuximab, capecitabine, oxaliplatin, and radiotherapy as preoperative treatment in rectal cancer. Int. J. Radiat. Oncol. Biol. Phys. 70:1081–1086. [DOI] [PubMed] [Google Scholar]
- 61. Sun, P. L. , Li B., and Ye Q. F.. 2012. Effect of neoadjuvant cetuximab, capecitabine, and radiotherapy for locally advanced rectal cancer: results of a phase II study. Int. J. Colorectal Dis. 27:1325–1332. [DOI] [PubMed] [Google Scholar]
- 62. Velenik, V. , Ocvirk J., Oblak I., and Anderluh F.. 2012. Cetuximab in preoperative treatment of rectal cancer ‐ term outcome of the XERT trial. Radiol. Oncol. 46:252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jin, T. , Zhu Y., Luo J. L., Zhou N., Li D. C., Ju H. X., Fan Y. T., Liu Y., Zhu Y. P., Feng H. Y., and Liu L. Y.. 2015. Prospective phase II trial of nimotuzumab in combination with radiotherapy and concurrent capecitabine in locally advanced rectal cancer. Int. J. Colorectal Dis. 30:337–345. [DOI] [PubMed] [Google Scholar]
- 64. Helbling, D. , Bodoky G., Gautschi O., Sun H., Bosman F., Gloor B., Burkhard R., Winterhalder R., Madlung A., Rauch D., Saletti P., Widmer L., et al. 2013. Neoadjuvant chemoradiotherapy with or without panitumumab in patients with wild‐type KRAS, locally advanced rectal cancer (LARC): a randomized, multicenter, phase II trial SAKK 41/07. Ann. Oncol. 24:718–725. [DOI] [PubMed] [Google Scholar]
- 65. Pinto, C. , Di Fabio F., Maiello E., Pini S., Latiano T., Aschele C., Garufi C., Bochicchio A., Rosati G., Aprile G., Giaquinta S., Torri V., et al. 2011. Phase II study of panitumumab, oxaliplatin, 5‐fluorouracil, and concurrent radiotherapy as preoperative treatment in high‐risk locally advanced rectal cancer patients (StarPan/STAR‐02 Study). Ann. Oncol. 22:2424–2430. [DOI] [PubMed] [Google Scholar]
- 66. Colorectal Cancer Collaborative Group . 2001. Adjuvant radiotherapy for rectal cancer: a systematic overview of 8,507 patients from 22 randomised trials. Lancet (London, England) 358: 1291–1304. [DOI] [PubMed] [Google Scholar]
- 67. Camma, C. , Giunta M., Fiorica F., Pagliaro L., Craxi A., and Cottone M.. 2000. Preoperative radiotherapy for resectable rectal cancer: a meta‐analysis. JAMA 284:1008–1015. [DOI] [PubMed] [Google Scholar]
- 68. Ben‐Josef, E. 2007. Capecitabine and radiotherapy as neoadjuvant treatment for rectal cancer. Am. J. Clin. Oncol. 30:649–655. [DOI] [PubMed] [Google Scholar]
- 69. Willett, C. G. , Duda D. G., di Tomaso E., Boucher Y., Ancukiewicz M., Sahani D. V., Lahdenranta J., Chung D. C., Fischman A. J., Lauwers G. Y., Shellito P., Czito B. G., et al. 2009. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: a multidisciplinary phase II study. J. Clin. Oncol. 27:3020–3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hofheinz, R. D. , Wenz F., Post S., Matzdorff A., Laechelt S., Hartmann J. T., Muller L., Link H., Moehler M., Kettner E., Fritz E., Hieber U., et al. 2012. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non‐inferiority, phase 3 trial. Lancet Oncol. 13:579–588. [DOI] [PubMed] [Google Scholar]
- 71. Zou, X. C. , Wang Q., and Zhang J.. 2017. Comparison of 5‐FU‐based and Capecitabine‐based Neoadjuvant Chemoradiotherapy in Patients With Rectal Cancer: a Meta‐analysis. Clin. Colorectal Cancer 000:000. [DOI] [PubMed] [Google Scholar]
- 72. Rodel, C. , Liersch T., Becker H., Fietkau R., Hohenberger W., Hothorn T., Graeven U., Arnold D., Lang‐Welzenbach M., Raab H. R., Sulberg H., Wittekind C., et al. 2012. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO‐04 randomised phase 3 trial. Lancet Oncol. 13:679–687. [DOI] [PubMed] [Google Scholar]
- 73. Maughan, T. S. , Adams R. A., Smith C. G., Meade A. M., Seymour M. T., Wilson R. H., Idziaszczyk S., Harris R., Fisher D., Kenny S. L., Kay E., and Mitchell J. K., et al. 2011. Addition of cetuximab to oxaliplatin‐based first‐line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet (London, England) 377: 2103–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Van Emburgh, B. O. , Sartore‐Bianchi A., Di Nicolantonio F., Siena S., and Bardelli A.. 2014. Acquired resistance to EGFR‐targeted therapies in colorectal cancer. Mol. Oncol. 8:1084–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Grimminger, P. P. , Danenberg P., Dellas K., Arnold D., Rodel C., Machiels J. P., Haustermans K., Debucquoy A., Velenik V., Sempoux C., Bracko M., Holscher A. H., et al. 2011. Biomarkers for cetuximab‐based neoadjuvant radiochemotherapy in locally advanced rectal cancer. Clin. Cancer Res. 17:3469–3477. [DOI] [PubMed] [Google Scholar]
- 76. Sorich, M. J. , Wiese M. D., Rowland A., Kichenadasse G., McKinnon R. A., and Karapetis C. S.. 2015. Extended RAS mutations and anti‐EGFR monoclonal antibody survival benefit in metastatic colorectal cancer: a meta‐analysis of randomized, controlled trials. Ann. Oncol. 26:13–21. [DOI] [PubMed] [Google Scholar]
- 77. Sanz‐Garcia, E. , Argiles G., Elez E., and Tabernero J.. 2017. BRAF mutant colorectal cancer: prognosis, treatment, and new perspectives. Ann. Oncol. 28:2648–2657. [DOI] [PubMed] [Google Scholar]
- 78. Glynne‐Jones, R. , Hava N., Goh V., Bosompem S., Bridgewater J., Chau I., Gaya A., Wasan H., Moran B., Melcher L., MacDonald A., Osborne M., et al. 2015. Bevacizumab and Combination Chemotherapy in rectal cancer Until Surgery (BACCHUS): a phase II, multicentre, open‐label, randomised study of neoadjuvant chemotherapy alone in patients with high‐risk cancer of the rectum. BMC Cancer 15:764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Sousa, N. , Sousa O., Santos L. L., Henrique R., Teixeira M. R., Dinis‐Ribeiro M., and Teixeira‐Pinto A.. 2016. Lapatinib‐capecitabine versus capecitabine alone as radiosensitizers in RAS wild‐type resectable rectal cancer, an adaptive randomized phase II trial (LaRRC trial): study protocol for a randomized controlled trial. Trials 17:459. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The establishment of benchmark for pCR.
Figure S2. (a) The Begg's funnel plots concerning the pCR for bevacizumab‐relevant studies. (b) The Egger's publication bias plot concerning the pCR for bevacizumab‐relevant studies.
Figure S3. (a) The Begg's funnel plots concerning the preoperative Grade 3/4 toxicity for bevacizumab‐relevant studies. (b) The Egger's publication bias plot concerning the preoperative Grade 3/4 toxicity for bevacizumab‐relevant studies.
Figure S4. (a) The Begg's funnel plots concerning the pCR for cetuximab‐relevant studies. (b) The Egger's publication bias plot concerning the pCR for cetuximab‐relevant studies.
Figure S5. The results of the sensitivity analysis concerning the pCR for cetuximab‐relevant studies.
Table S1. Data from Maas, et al's pooled analysis.
Table S2. The radiotherapy status of Bevacizumab‐relevant cohorts.


