Abstract
BACKGROUND & AIMS
Although treatment of T1a esophageal adenocarcinoma (EAC) is shifting from esophagectomy to endoscopic therapy, T1b EACs are considered too high risk to be treated endoscopically. We investigated the effectiveness and cost effectiveness of esophagectomy vs endoscopic therapy for T1a and T1b EACs, and the effects of age and comorbidities, using a decision analytic Markov model.
METHODS
We developed a model to simulate a hypothetical cohort of men 75 years old with Charlson comorbidity index scores of 0 and either T1aN0M0 or T1bN0M0 EAC, as a base case. We used the model to compare the effects of esophagectomy vs serial endoscopic therapy. We performed sensitivity analyses based on age at diagnosis of 60–85 years, comorbidity indices of 0–2, and utilities. Post-procedure cancer-specific mortality was derived from the Surveillance, Epidemiology, and End Results Medicare database.
RESULTS
In the T1a base case, esophagectomy yielded more unadjusted life years than endoscopic therapy (6.97 vs 6.81), but fewer quality-adjusted life years (QALYs, 4.95 for esophagectomy vs 5.22 for endoscopic therapy). In the T1b base case, esophagectomy yielded more unadjusted life years than endoscopic therapy (5.73 vs 5.01) and QALYs (4.07 vs 3.85 for endoscopic therapy), but was not cost effective (incremental cost-effectiveness ratio $156,981). Sensitivity analyses showed endoscopic therapy optimized QALYs for patients more than 80 years old with a comorbidity index of 1 or 2, or if the ratio of post-esophagectomy to post-endoscopic therapy utilities was below 0.875.
CONCLUSION
In a Markov model, we showed that endoscopic therapy of T1a EAC yields more QALYs and is more cost effective than esophagectomy for patients of all ages and comorbidity indices tested. In contrast, selection of therapy for T1b EAC depends on age and comorbidities, due to surgical mortality and the competing risk of non-cancer death.
Keywords: SEER, Esophageal Cancer, Endoscopic Resection, Esophagectomy
Although the traditional resection of esophageal adenocarcinoma (EAC) has been with esophagectomy, treatment for localized disease is moving increasingly from a surgical to endoscopic approach.1,2 National guidelines for treatment of EAC confined to the mucosa, ie, T1aN0M0 cancer, prefer endoscopic therapy (ET) over esophagectomy because of comparable survival and decreased morbidity.3–6
In contrast, esophagectomy continues to be recommended for EAC invading into the submucosa, ie, T1b cancers, because of the 15%–30% prevalence of lymph node involvement.1–3,7 However, investigations into ET for T1b are being performed. Pre-surgical staging with endoscopic ultrasound has reduced the risk of occult lymph node involvement, although its accuracy in early stage EAC varies from 65% to 93%.2,8–12 Of more use may be endoscopic mucosal resection, which allows for pre-surgical pathologic staging and identification of higher risk features, such as tumor diameter more than 3 cm, depth of invasion more than one-third into the submucosa, higher grade, and lymphovascular invasion.13,14 Despite these advances, national data suggest that to date, T1b EAC treated with ET has worse outcomes compared with esophagectomy.1,15,16 Even so, rates of ET for T1b cancers in the United States are rising; in 2010, 20.9% were treated with ET compared with 6.6% in 2004, mainly in patients aged 75 or older.1 This may be due to poor surgical candidacy in these older patients.17–20 In addition, the risk of dying from competing comorbidities should be weighed against the risk of dying of cancer.14 Finally, esophagectomy is a morbid procedure with persistently decreased quality of life.21–23 For patients with already reduced life expectancy from comorbidities, it is unclear whether the relative gain in cancer-related survival with esophagectomy is worth the cost in quality of life.
The aim of our study was to develop and analyze a decision-analytic model investigating the effectiveness of esophagectomy versus ET for T1a and T1b EAC with respect to unadjusted life years, quality-adjusted life years (QALYs), and cost-effectiveness, varying combinations of age and comorbidity burden to explore whether these factors would change the optimal therapy.
Materials and Methods
Model Design
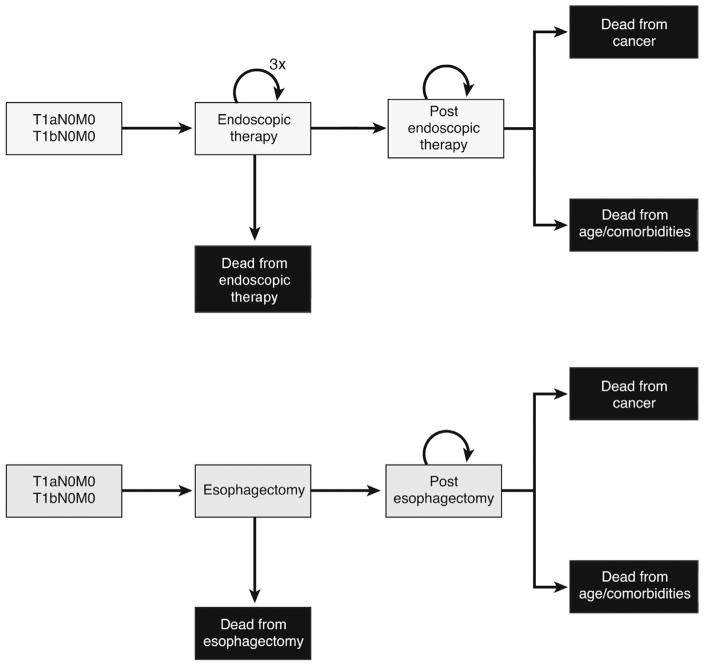
Decision-analytic Markov-state transition models were constructed in TreeAge Pro (TreeAge, Williams-town, MA) for T1aN0M0 and T1bN0M0 EAC (Figure 1). Health states included post-esophagectomy, post-ET, and dead. Possible causes of death included age- and comorbidity-related mortality, procedural mortality, and cancer-specific mortality. The Markov cycle length was 1 month. The simulation in the base case analysis began with a hypothetical cohort of 75-year-old men with T1a or T1bN0M0 EAC, not previously treated with radiation or chemotherapy, who were followed until death. In the ET arm, the simulated patient would undergo ET for the first 3 cycles.13,14 In both treatment arms, the patient could die of procedural mortality, stay in the same state, die of age- and comorbidity-related mortality, or die of cancer.
Figure 1.
Schematic of 2 treatment strategies for T1a and T1b EAC, ET and esophagectomy, and possible transition states.
We estimated the comorbidity burden and associated mortality of our patients by using the Charlson comorbidity index.24 In calculating our patients’ comorbidity index, we excluded esophageal cancer and included only 3 scores: 0, 1, and 2+. This is common practice in the surgical literature, because patients with comorbidity indices greater than 2 are usually excluded as surgical candidates.25 We did not include age in our Charlson comorbidity index in order to account for risks related to age separately.
Parameter Estimates
Model parameters were estimated from the literature. Base case values and ranges used in sensitivity analyses are summarized in Table 1.
Table 1.
Model Inputs
| Parameters | Base case estimate | Ranges used in sensitivity analysis | Sources |
|---|---|---|---|
| Age (y) | 75 | 60–85 | SEER-Medicare, see |
| Sex | Male | Female, male | Materials and Methods |
| Charlson comorbidity index | 0 | 0–2 | 24 |
| Probabilities | |||
| Death from age, comorbidities | 0.036 | 24,26 | |
| Esophagectomy surgical mortality | 0.053 | 18 | |
| ET therapy mortality | 0.000021 | 0–0.00005 | 32 |
| Annual rates of death from cancer | |||
| T1a | |||
| Esophagectomy | |||
| 0–2 y | 0.027 | SEER-Medicare, see | |
| 2+ y | 0.034 | Materials and Methods | |
| ET | |||
| 0+ y | 0.046 | ||
| T1b | |||
| Esophagectomy | |||
| 0–1 y | 0.065 | ||
| 1+ y | 0.071 | ||
| ET | |||
| 0–2 y | 0.15 | ||
| 2+ y | 0.088 | ||
| Utilities | |||
| Resectable cancer, before therapy | 0.84 | 0.68–0.92 | 27 |
| Post-esophagectomy | |||
| Postoperative recovery, 0–1 mo | 0.7 | 28 | |
| 1+ mo | 0.9 | 0.8–1 | 23,27,32 |
| Post-ET | 0.97 | 0.88–1 | 23,27,33 |
| Costs ($) | |||
| Esophagectomy | 40,163.89 | 25,000–41,000 | 27,32 |
| ET (per session) | 1037.46 | 1000–2100 | 27,32 |
| Surveillance endoscopy | 746.43 | 700–1100 | 27,32 |
| Clinic visit | 125.33 | 50–150 | 27,32,34 |
ET, endoscopic therapy; SEER, Surveillance, Epidemiology, and End Results.
Model Transition Probabilities and Calibration
Cancer-specific survival for T1b EAC treated with ET has only been reported in small studies with a wide range of estimates. Therefore, we calculated our own estimates of cancer-specific death for esophagectomy and ET on the basis of data from the Surveillance, Epidemiology, and End Results (SEER) database (Supplementary Methods). Because of small numbers of T1b tumors in the database, tumors categorized as “T1NOS” were combined into the T1b group (Supplementary Figure 1). Kaplan-Meier curves were generated to compare overall survival between treatment groups (Figure 2). Hazard rates were calculated on the basis of the Kaplan-Meier curves of cancer-specific survival. Thirty-day surgical mortality was estimated from the Steyerberg score, a validated risk score for mortality from esophagectomy by using SEER-Medicare data (Supplementary Methods).18 Non–cancer-related death was predicted from the probability of death on the basis of age multiplied by the relative risk of death based on Charlson comorbidity index.24,26
Figure 2.
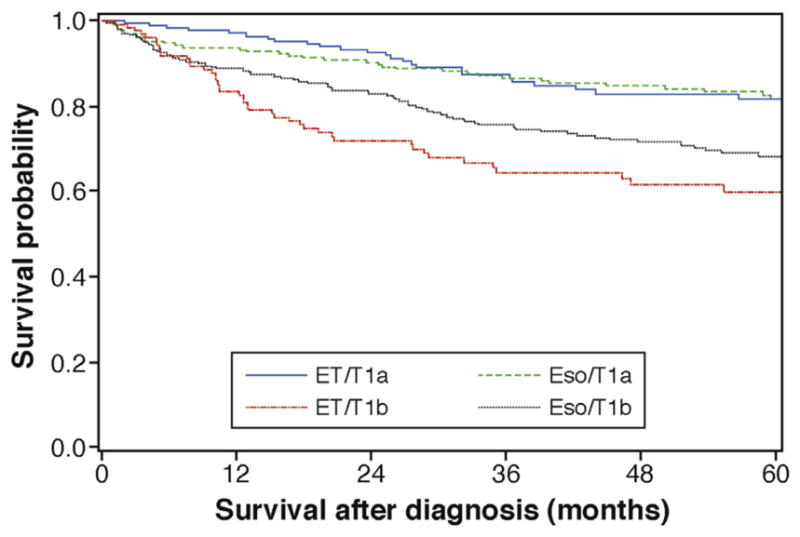
Kaplan-Meier curves of cancer-specific survival for T1a and T1b cancers treated with ET or esophagectomy. Data from SEER-Medicare. Eso, esophagectomy.
Costs and Utilities
Costs were based on published literature, converted to 2017 U.S. dollars by using the Consumer Price Index (U.S. Bureau of Labor Statistics). The cost estimate for ET of $1037.46 is the cost for one session. Quality-of-life measures were adjusted to utility scores for the specific health states: resectable cancer before therapy, 0.84; post-esophagectomy, 0.7 for the first postoperative month and 0.9 thereafter; and post-ET, 0.97.23,27,28 These utilities were age-adjusted by multiplying by quality of life by age.29 Costs and utilities were discounted at an annual rate of 3%.30
Outcomes
The primary outcomes of the analysis were unadjusted life years (life expectancy), QALYs, and incremental cost-effectiveness ratio (ICER) between competing treatment strategies. The willingness-to-pay threshold was set at $100,000/QALY.31
Analyses Performed
A base case analysis using best estimates for all model parameters was performed separately for T1a and T1b EAC. One-way sensitivity analyses were performed to investigate the effects of each model parameter on estimated outcomes, including for age at diagnosis, Charlson comorbidity index, sex, health state utilities, and costs of procedure. To explore the interaction between age and comorbidity, two-way sensitivity analyses were performed for age at diagnosis and Charlson comorbidity index. Finally, because the relative utility estimates for post-esophagectomy and post-ET states were also key inputs for the model, two-way sensitivity analyses were also performed for these utilities.
Results
Base Case Results
The base case analyses of the T1a and T1b EAC cohorts are presented in Table 2. For the T1a analysis, treatment with esophagectomy yielded 0.16 more unadjusted life years compared with ET but returned 0.27 fewer QALYs and cost $34,834 more. Therefore, the ET treatment strategy dominated esophagectomy for T1a EAC (resulted in more QALYs and cost less). For the T1b analysis, treatment with esophagectomy yielded 0.72 more unadjusted life years and 0.22 more QALYs, making it the more effective treatment strategy. However, esophagectomy was not cost-effective, with an ICER of $156,981/QALY.
Table 2.
Base Case Results for T1a and T1b Patientsa
| Therapy | Cost ($) | Unadjusted life years | QALYs | ICER, $ |
|---|---|---|---|---|
| T1a | ||||
| Esophagectomy | 47,812.09 | 6.97 | 4.95 | Dominated |
| ET | 12,977.74 | 6.81 | 5.22 | |
| T1b | ||||
| Esophagectomy | 46,345.74 | 5.73 | 4.07 | 156,980.91 |
| ET | 11,366.51 | 5.01 | 3.85 | |
ET, endoscopic therapy; ICER, incremental cost-effective ratio; QALY, quality-adjusted life year.
Discounted at 3%/y.
Sensitivity Analysis
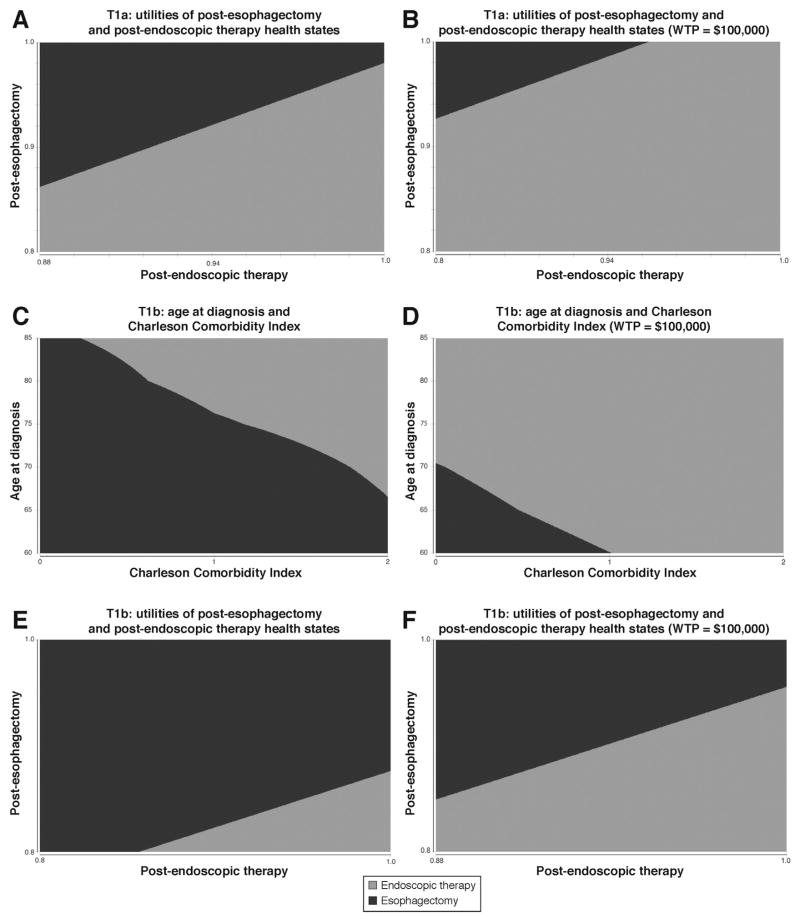
For the T1a EAC cohort, the results were only sensitive (preferred strategy changed) to relative utilities of post-esophagectomy and post-ET health states (Figure 3A and B). Esophagectomy yielded the same or more QALYs if the ratio of post-esophagectomy utility to post-ET utility was 0.97 or greater. Esophagectomy was cost-effective if the ratio of post-esophagectomy utility to post-ET utility was 1.05 or greater. We also generated a model incorporating misdiagnosis, where a patient’s T1a EAC was actually T1b. Sensitivity analysis was performed for probability of misdiagnosis between 0% and 100% because of the uncertainty in the true prevalence of this misclassification. At a probability of 55%, QALYs achieved with esophagectomy and ET become equal at 4.47; at a probability higher than 55%, esophagectomy provided more QALYs. There was no threshold at which esophagectomy was cost-effective compared with ET, even at a misdiagnosis probability of 100%. Other variables, including age and Charlson comorbidity index, did not change the optimal treatment strategy for the T1a cohort (Supplementary Table 2). Probabilistic sensitivity analysis did not find any willingness-to-pay threshold up to $300,000 for which esophagectomy was cost-effective compared to ET (Supplementary Figure 2, Table 4).
Figure 3.
Two-way sensitivity analyses of T1a EAC post-esophagectomy and post-ER state utilities with respect to (A) QALYs and (B) cost-effectiveness; T1b EAC age at diagnosis and Charlson comorbidity index with respect to (C) QALYs and (D) cost-effectiveness; T1b EAC post-esophagectomy and post-ER state utilities with respect to (E) QALYs and (F) cost-effectiveness. WTP, willingness-to-pay.
For the T1b EAC cohort, results of key sensitivity analyses are summarized in Table 3. The optimal treatment strategy was not affected by age at diagnosis alone, except with regard to cost-effectiveness. For age 70 at diagnosis, esophagectomy became cost-effective with an ICER of $96,630.
Table 3.
Sensitivity Analyses of Selected Parameters for T1b Patients
| Parameter for T1b | Unadjusted life years change | QALYs change | ICERs change |
|---|---|---|---|
| Age (y) | — | ||
| 60 | — | — | Esophagectomy cost-effective |
| 65 | — | — | Esophagectomy cost-effective |
| 70 | — | — | Esophagectomy cost-effective |
| 80 | — | — | — |
| 85 | — | — | — |
| CCI | |||
| 1 | — | — | — |
| 2 | — | ET preferred | — |
| Sex | |||
| Female | — | — | — |
| Utility of post-esophagectomy state | |||
| 0.8 | — | ET preferred | — |
| 1 | — | — | Esophagectomy cost-effective |
| Utility of post-ET state | |||
| 0.88 | — | — | Esophagectomy cost-effective |
| 1 | — | — | — |
| Cost of esophagectomy, $ | |||
| 27,000 | — | — | Esophagectomy cost-effective |
| 28,000 | — | — | — |
| Age (y) and CCI | |||
| 60, 0 | — | — | Esophagectomy cost-effective |
| 60, 1 | — | — | Esophagectomy cost-effective |
| 60, 2 | — | — | — |
| 65, 0 | — | — | Esophagectomy cost-effective |
| 65, 1 | — | — | — |
| 65, 2 | — | — | — |
| 70, 0 | — | — | Esophagectomy cost-effective |
| 70, 1 | — | — | — |
| 70, 2 | — | ET preferred | — |
| 80, 0 | — | — | — |
| 80, 1 | — | ET preferred | — |
| 80, 2 | ET preferred | ET preferred | — |
| 85, 0 | — | — | — |
| 85, 1 | — | ET preferred | — |
| 85, 2 | ET preferred | ET preferred | — |
| Utilities of post-esophagectomy and post-ET states | |||
| 0.8, 0.88 | — | — | — |
| 1, 0.88 | — | — | Esophagectomy cost-effective |
| 0.8, 1.0 | — | ET preferred | — |
| 1.0, 1.0 | — | — | Esophagectomy cost-effective |
CCI, Charlson comorbidity index; ET, endoscopic therapy; ICER, incremental cost-effective ratio; QALY, quality-adjusted life year.
QALYs for the T1b cohort were affected by increasing Charlson comorbidity index. For Charlson comorbidity index of 1, esophagectomy continued to yield slightly more QALYs compared with ET (2.90 versus 2.88). For index of 2, ET became the optimal strategy, resulting in 2.03 QALYs compared with 1.93 for esophagectomy.
Varying combinations of age at diagnosis and Charlson comorbidity index had significant impact on the optimal treatment strategy (Figure 3C and D). For age 70 and comorbidity index of 2 and ages 80–85 with comorbidity index of 1–2, ET yielded more QALYs than esophagectomy. For ages 80 and 85 with comorbidity index of 2, ET also resulted in more unadjusted life years compared with esophagectomy (Supplementary Table 3). Esophagectomy was cost-effective for age 60 with comorbidity index 0–1, but not at higher comorbidity indices. For ages 65 and 70, esophagectomy was cost-effective only for comorbidity index of 0.
As in the T1a analysis, the T1b model was also sensitive to relative utilities of the post-esophagectomy and post-ET health states (Figure 3E and F). ET became the optimal treatment strategy if the ratio of post-esophagectomy to post-ET utilities was 0.875 or less. Esophagectomy became cost-effective if the ratio of post-esophagectomy to post-ET utilities was 0.95 or greater. Probabilistic sensitivity analysis found esophagectomy cost-effective compared to ET in greater than 50% of iterations at a willingness-to-pay threshold of $220,000 (Supplementary Figure 3, Table 4).
Discussion
Our modeling analysis confirmed that for T1a EAC, ET is the preferred treatment strategy across a range of ages and comorbidity indices, providing more QALYs and at a lower cost than esophagectomy. Although esophagectomy yielded slightly more unadjusted life years than ET for the T1a base case, we believe QALYs are a more important end point because of the significant morbidity associated with esophagectomy. With QALYs as the end point, the results were only sensitive to relative utilities of the post-esophagectomy and post-ET health states at a ratio of 0.97. Thus, if a patient considered his/her quality of life post-esophagectomy nearly equal to, or preferable to, his/her quality of life post-ET, esophagectomy would be the optimal treatment strategy. An example would be the patient who would rather have an esophagectomy than worry about recurrence with ET.
For T1b EAC, the optimal treatment strategy hinged on the combination of age at diagnosis and Charlson comorbidity index and again on relative utilities of post-treatment health states. For younger patients aged 60–70, esophagectomy was the preferred strategy at all comorbidity indices. However, for ages older than 70 with rising comorbidity index, the optimal strategy changed to ET. For the sickest patients, those aged 80 and older with comorbidity index of 2, ET not only provided more QALYs but more unadjusted life years as well. For the T1b cohort as well as the T1a cohort, relative utilities of post-esophagectomy and post-ET health states were a key driver of the optimal treatment strategy, reflecting the importance of the patient’s perspective on quality of life after esophagectomy. With regard to cost-effectiveness, esophagectomy was only cost-effective for younger, healthier patients (ie, age 60–70 with comorbidity index of 0–1). In summary, for patients with local T1b cancers, the decision to treat with esophagectomy versus ET should be an individualized decision factoring in the patient’s age, comorbidities, and quality of life preferences.
Our model has several limitations. For the T1a model, T1a tumors with more high-risk features, such as high-grade or lymphovascular invasion, may not be identified on endoscopic mucosal resection because of lack of standardization in pathology reporting of T1a cancers. Reporting these tumor features would allow for identification of higher-risk T1a patients, and a separate model should be performed to explore whether the preferred treatment strategy may differ for high-risk versus low-risk T1a tumors.
Our estimates of cancer-specific death were derived from the SEER-Medicare database. Because of limited numbers of T1b cancers treated with ET in the database, we chose to pool T1NOS cancers into our T1b cohort (Supplementary Methods). The T1NOS cohort performed the worst compared with T1a and T1b, likely representing tumors misclassified as T1b that may have been higher stage (Supplementary Figure 1). This would have biased our analysis against ET because T1NOS patients made up 31% of the ET group compared with 11% of the esophagectomy group and overestimated the probability of cancer-related death for T1b patients treated with ET. Similarly, the staging of the SEER-Medicare database patients was heterogeneous because of practice variation in use of staging endoscopic mucosal resection, endoscopic ultrasound, and computed tomography or positron emission tomography imaging. This likely also biased the analysis against ET, because those patients were more likely to be under-staged because of lack of interrogation into regional lymph nodes that would be standard with esophagectomy. Also biasing against ET in our model was the inability to distinguish between “high risk” and “low risk” T1b tumors.9,13,14 Because of successful outcomes of ET for low risk T1b cancers, generation of a separate model for low risk T1b adenocarcinomas might favor ET for a wider spectrum of patients. However, with its heterogeneous patient population, our analysis more closely reflects actual clinical practice.
Limitations of the model biasing against esophagectomy are largely related to cost. Our model did not incorporate costs of adjunctive therapies such as chemotherapy and radiation or palliative care, which are more likely in patients who do not undergo esophagectomy.15,16 In addition, we were unable to distinguish patients who failed serial ET and proceeded to esophagectomy from patients treated only with esophagectomy. In an intention-to-treat analysis, these patients would have been classified as ET (rather than as esophagectomy, as in our analysis), and the cost of esophagectomy would have been included in the total cost of their treatment.
Lastly, our model did not capture more granular decrements in utilities such as stricture, metastatic disease, and complications of either treatment or progressive disease. These omissions likely overestimate the utility of the post-ET health state because stricture is a known risk of ET and progressive disease is more likely with ET.
Our model is overall biased against ET with regard to effectiveness, but despite this bias, it identifies a large cohort of patients for whom ET would provide more QALYs and even, for the oldest comorbid patients, more unadjusted life years. Our analysis supports the increasingly common practice of recommending ET for older comorbid patients, although it clarifies that age alone should not determine treatment, because non-comorbid older patients continue to have better outcomes with esophagectomy compared with ET. As improved estimates of survival for T1b EAC treated with ET emerge, an updated analysis would be warranted that might identify an even greater population of patients who would benefit from ET compared with esophagectomy.
In conclusion, our model analysis of T1a and T1b EAC supports the use of ET for T1a cancers and recognizes that optimal treatment for T1b cancer may be an individualized decision that should consider a patient’s combination of age and comorbidity and his/her preferences regarding quality of life.
Supplementary Material
Acknowledgments
Funding
Supported by National Institutes of Health grants NCI U01 CA 199336 and R01 CA 140574.
Abbreviations used in this paper
- EAC
esophageal adenocarcinoma
- ET
endoscopic therapy
- ICER
incremental cost-effectiveness ratio
- QALY
quality-adjusted life year
- SEER
Surveillance, Epidemiology, and End Results
Footnotes
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2017.10.024.
Conflicts of interest
These authors disclose the following: N. S. Nishioka has received research support from CSA Medical and has been on the clinical advisory board of Ninepoint. J. A. Abrams has done consulting for C2 Therapeutics and Medtronic and received research support from C2 Therapeutics, Medtronic, and Trio Medicines. J. M. Inadomi has received research support from Ninepoint. The remaining authors disclose no conflicts.
References
- 1.Merkow RP, Bilimoria KY, Keswani RN, et al. Treatment trends, risk of lymph node metastasis, and outcomes for localized esophageal cancer. J Natl Cancer Inst. 2014;106:dju133. doi: 10.1093/jnci/dju133. [DOI] [PubMed] [Google Scholar]
- 2.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349:2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 3.Esophageal and esophagogastric junction cancers. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): National Comprehensive Cancer Network. 2017. [Google Scholar]
- 4.Pech O, May A, Manner H, et al. Long-term efficacy and safety of endoscopic resection for patients with mucosal adenocarcinoma of the esophagus. Gastroenterology. 2014;146:652–660. e1. doi: 10.1053/j.gastro.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 5.Ell C, May A, Pech O, et al. Curative endoscopic resection of early esophageal adenocarcinomas (Barrett’s cancer) Gastrointest Endosc. 2007;65:3–10. doi: 10.1016/j.gie.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 6.Moss A, Bourke MJ, Hourigan LF, et al. Endoscopic resection for Barrett’s high-grade dysplasia and early esophageal adenocarcinoma: an essential staging procedure with long-term therapeutic benefit. Am J Gastroenterol. 2010;105:1276–1283. doi: 10.1038/ajg.2010.1. [DOI] [PubMed] [Google Scholar]
- 7.Molena D, Schlottmann F, Boys JA, et al. Esophagectomy following endoscopic resection of submucosal esophageal cancer: a highly curative procedure even with nodal metastases. J Gastrointest Surg. 2017;21:62–67. doi: 10.1007/s11605-016-3210-3. [DOI] [PubMed] [Google Scholar]
- 8.DaVee T, Ajani JA, Lee JH. Is endoscopic ultrasound examination necessary in the management of esophageal cancer? World J Gastroenterol. 2017;23:751–762. doi: 10.3748/wjg.v23.i5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buskens CJ, Westerterp M, Lagarde SM, et al. Prediction of appropriateness of local endoscopic treatment for high-grade dysplasia and early adenocarcinoma by EUS and histopathologic features. Gastrointest Endosc. 2004;60:703–710. doi: 10.1016/s0016-5107(04)02017-6. [DOI] [PubMed] [Google Scholar]
- 10.Gotink AW, Spaander MCW, Doukas M, et al. Exploring diagnostic and therapeutic implications of endoscopic mucosal resection in EUS-staged T2 esophageal adenocarcinoma. Endoscopy. 2017;49:941–948. doi: 10.1055/s-0043-112492. [DOI] [PubMed] [Google Scholar]
- 11.Young PE, Gentry AB, Acosta RD, et al. Endoscopic ultrasound does not accurately stage early adenocarcinoma or high-grade dysplasia of the esophagus. Clin Gastroenterol Hepatol. 2010;8:1037–1041. doi: 10.1016/j.cgh.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 12.Thosani N, Singh H, Kapadia A, et al. Diagnostic accuracy of EUS in differentiating mucosal versus submucosal invasion of superficial esophageal cancers: a systematic review and meta-analysis. Gastrointest Endosc. 2012;75:242–253. doi: 10.1016/j.gie.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 13.Manner H, May A, Pech O, et al. Early Barrett’s carcinoma with “low-risk” submucosal invasion: long-term results of endoscopic resection with a curative intent. Am J Gastroenterol. 2008;103:2589–2597. doi: 10.1111/j.1572-0241.2008.02083.x. [DOI] [PubMed] [Google Scholar]
- 14.Manner H, Pech O, Heldmann Y, et al. Efficacy, safety, and long-term results of endoscopic treatment for early stage adenocarcinoma of the esophagus with low-risk sm1 invasion. Clin Gastroenterol Hepatol. 2013;11:630–635. doi: 10.1016/j.cgh.2012.12.040. quiz e45. [DOI] [PubMed] [Google Scholar]
- 15.Ballard DD, Choksi N, Lin J, et al. Outcomes of submucosal (T1b) esophageal adenocarcinomas removed by endoscopic mucosal resection. World J Gastrointest Endosc. 2016;8:763–769. doi: 10.4253/wjge.v8.i20.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian J, Prasad GA, Lutzke LS, et al. Outcomes of T1b esophageal adenocarcinoma patients. Gastrointest Endosc. 2011;74:1201–1206. doi: 10.1016/j.gie.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Stahl CC, Hanseman DJ, Wima K, et al. Increasing age is a predictor of short-term outcomes in esophagectomy: a propensity score adjusted analysis. J Gastrointest Surg. 2014;18:1423–1428. doi: 10.1007/s11605-014-2544-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steyerberg EW, Neville BA, Koppert LB, et al. Surgical mortality in patients with esophageal cancer: development and validation of a simple risk score. J Clin Oncol. 2006;24:4277–4284. doi: 10.1200/JCO.2005.05.0658. [DOI] [PubMed] [Google Scholar]
- 19.Ra J, Paulson EC, Kucharczuk J, et al. Postoperative mortality after esophagectomy for cancer: development of a preoperative risk prediction model. Ann Surg Oncol. 2008;15:1577–1584. doi: 10.1245/s10434-008-9867-4. [DOI] [PubMed] [Google Scholar]
- 20.O’Grady G, Hameed AM, Pang TC, et al. Patient selection for oesophagectomy: impact of age and comorbidities on outcome. World J Surg. 2015;39:1994–1999. doi: 10.1007/s00268-015-3072-y. [DOI] [PubMed] [Google Scholar]
- 21.Daster S, Soysal SD, Stoll L, et al. Long-term quality of life after Ivor Lewis esophagectomy for esophageal cancer. World J Surg. 2014;38:2345–2351. doi: 10.1007/s00268-014-2576-1. [DOI] [PubMed] [Google Scholar]
- 22.Djarv T, Lagergren J, Blazeby JM, et al. Long-term health-related quality of life following surgery for oesophageal cancer. Br J Surg. 2008;95:1121–1126. doi: 10.1002/bjs.6293. [DOI] [PubMed] [Google Scholar]
- 23.Hur C, Wittenberg E, Nishioka NS, et al. Quality of life in patients with various Barrett’s esophagus associated health states. Health Qual Life Outcomes. 2006;4:45. doi: 10.1186/1477-7525-4-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 25.Backemar L, Lagergren P, Johar A, et al. Impact of co-morbidity on mortality after oesophageal cancer surgery. Br J Surg. 2015;102:1097–1105. doi: 10.1002/bjs.9854. [DOI] [PubMed] [Google Scholar]
- 26.Arias E, Heron M, Xu JQ. United States life tables, 2012. National Vital Statistics Reports. 2012:65. [PubMed] [Google Scholar]
- 27.Hu Y, Puri V, Shami VM, et al. Comparative effectiveness of esophagectomy versus endoscopic treatment for esophageal high-grade dysplasia. Ann Surg. 2016;263:719–726. doi: 10.1097/SLA.0000000000001387. [DOI] [PubMed] [Google Scholar]
- 28.Hur C, Nishioka NS, Gazelle GS. Cost-effectiveness of photodynamic therapy for treatment of Barrett’s esophagus with high grade dysplasia. Dig Dis Sci. 2003;48:1273–1283. doi: 10.1023/a:1024146823549. [DOI] [PubMed] [Google Scholar]
- 29.Hanmer J, Lawrence WF, Anderson JP, et al. Report of nationally representative values for the noninstitutionalized US adult population for 7 health-related quality-of-life scores. Med Decis Making. 2006;26:391–400. doi: 10.1177/0272989X06290497. [DOI] [PubMed] [Google Scholar]
- 30.Weinstein MC, Siegel JE, Gold MR, et al. Recommendations of the panel on cost-effectiveness in health and medicine. JAMA. 1996;276:1253–1258. [PubMed] [Google Scholar]
- 31.Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness: the curious resilience of the $50,000-per-QALY threshold. N Engl J Med. 2014;371:796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- 32.Inadomi JM, Sampliner R, Lagergren J, et al. Screening and surveillance for Barrett esophagus in high-risk groups: a cost-utility analysis. Ann Intern Med. 2003;138:176–186. doi: 10.7326/0003-4819-138-3-200302040-00009. [DOI] [PubMed] [Google Scholar]
- 33.Boger PC, Turner D, Roderick P, et al. A UK-based cost-utility analysis of radiofrequency ablation or oesophagectomy for the management of high-grade dysplasia in Barrett’s oesophagus. Aliment Pharmacol Ther. 2010;32:1332–1342. doi: 10.1111/j.1365-2036.2010.04450.x. [DOI] [PubMed] [Google Scholar]
- 34.Provenzale D, Schmitt C, Wong JB. Barrett’s esophagus: a new look at surveillance based on emerging estimates of cancer risk. Am J Gastroenterol. 1999;94:2043–2053. doi: 10.1111/j.1572-0241.1999.01276.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.