Abstract
PURPOSE: Tumor-infiltrating lymphocytes (TILs) have an established impact on the prognosis of high-grade serous ovarian carcinoma (HGSOC), however, their role in recurrent ovarian cancer is largely unknown. We therefore systematically investigated TIL densities and MHC class I and II (MHC1, 2) expression in the progression of HGSOC. EXPERIMENTAL DESIGN: CD3+, CD4+, CD8+ TILs and MHC1, 2 expression were evaluated by immunohistochemistry on tissue microarrays in 113 paired primary and recurrent HGSOC. TILs were quantified by image analysis. All patients had been included to the EU-funded OCTIPS FP7 project. RESULTS: CD3+, CD4+, CD8+ TILs and MHC1 and MHC2 expression showed significant correlations between primary and recurrent tumor levels (Spearman rho 0.427, 0.533, 0.361, 0.456, 0.526 respectively; P<.0001 each). Paired testing revealed higher CD4+ densities and MHC1 expression in recurrent tumors (Wilcoxon P=.034 and P=.018). There was also a shift towards higher CD3+ TILs levels in recurrent carcinomas when analyzing platinum-sensitive tumors only (Wilcoxon P=.026) and in pairs with recurrent tumor tissue from first relapse only (Wilcoxon P=.031). High MHC2 expression was the only parameter to be significantly linked to prolonged progression-free survival after first relapse (PFS2, log-rank P=.012). CONCLUSIONS: This is the first study that analyzed the development of TILs density and MHC expression in paired primary and recurrent HGSOC. The level of the antitumoral immune response in recurrent tumors was clearly dependent on the one in the primary tumor. Our data contribute to the understanding of temporal heterogeneity of HGSOC immune microenvironment and have implications for selection of samples for biomarker testing in the setting of immune-targeting therapeutics.
Introduction
Epithelial ovarian cancer (EOC) is one of the most common causes of gynecological cancer deaths and ranks fifth in the causes of overall cancer deaths in women. The low 5-year survival rate of 38% can be attributed to a majority (70%) of the aggressive high-grade serous ovarian carcinoma (HGSOC) subtype. The poor prognosis of HGSOC is mainly due to patients being diagnosed in advanced stage (75% in FIGO III/IV) [1] and to primary or secondary chemotherapy resistance that develops in almost all patients [2], [3]. Cure by radical tumor resection and platinum-based therapy is only seen in early-stage tumors and rarely in advanced-stage tumors.
Apart from the two most important established prognostic parameters of tumor stage and residual disease after surgery, the level of tumor-infiltrating lymphocytes (TILs) has repeatedly been shown to be a valid prognostic factor for prolonged survival (for meta-analysis, see [4]). Notably, high numbers of CD3+ and CD8+ TILs are linked to prolonged survival [5], [6], [7], [8], [9]. This applies particularly to intratumoral lymphocytes, which are in direct contact with tumor cells and less strongly to stromal lymphocytes [5], [6], [7]. Apart from progression-free and overall survival, the number of TILs may also affect therapeutic success since tumors with low CD3+ and CD8+ TILs numbers are more likely to be chemoresistant and patients with low CD8+ TILs benefit from aggressive cytoreduction [10], [11], [12]. Cytotoxic T cells (CD8+) are activated by major histocompatibility complex class I (MHC1) molecules that perform antigen presentation of aberrant peptides, e.g., viral but also tumoral antigens, while T helper cells (CD4+) interact with MHC2 molecules that are most often expressed by antigen-presenting cells. A high expression of MHC1 and MHC2 in ovarian cancer environment has been found to correlate with prolonged survival and to be associated with an increased chemoresponse [10], [11], [13], [14].
These previous findings suggest that the immune system is able to identify and attack ovarian cancer cells. The inhibition of immune checkpoints, such as programmed cell death protein 1 (PD1), PD-1 ligand (PD-L1), and cytotoxic T-lymphocyte associated antigen (CTLA4), was found to mediate cancer regression and prolong survival in metastatic melanoma and for PD1 blockade also in non–small cell lung cancer and renal cancer [15], [16], [17]. Several clinical trials on checkpoint inhibitors in EOC are ongoing (for review see, [18]). Plus, adoptive cell transfer (ACT) has been successfully executed on metastatic melanoma and showed 50% objective response up to total tumor regression [19], [20], [21] and was also associated with prolonged survival in EOC [22], [23]. Former trials showed that the tumoral environment might influence the success of such therapeutics, as for example a brisk CD8+ TIL expression correlated with a higher response to PD-1 blockade in melanoma [17].
To fully understand the role of the immune system in HGSOC and thereby the potential of immunotherapy, a further elucidation of immunological mechanisms is necessary. In particular, recurrent HGSOC has not been examined in previous studies; therefore, the composition of the tumoral microenvironment during cancer progression remains unanswered. We therefore systematically investigated the dynamics of tumoral TILs density and MHC class I and II expression during the progression of HGSOC by analyzing paired primary and recurrent tumors.
Material and Methods
Patient Cohort and Characteristics
All patients had been included in the OCTIPS project (Ovarian Cancer Therapy–Innovative Models Prolong Survival, www.octips.eu) supported by European Community’s Seventh Framework Programme under grant agreement No. 279113-2. Ethical approval has been given by the ethics committees of all project partners (EK207/2003, ML2524, 05/Q0406/178, EK366/2003, EK260/2003, 06/S1101/16). A total of 158 patients with paraffin-embedded, formalin-fixed tissue blocks of resection and biopsy specimens were evaluable. However, in 21 cases, no tumor pair could be established; 14 turned out not to be HGSOC after histopathological review, 12 had been treated with neoadjuvant chemotherapy (not chemonaive) and were excluded from this study. The final study group included 113 patients with paired samples. Most of the patients (n=67) were recruited at Charité University Hospital Berlin, Germany. The other specimens were provided by the OCTIPS partners University Hospital Leuven, Belgium (n=20); The University of Edinburgh, United Kingdom (n=16); and London Imperial College of Science, United Kingdom (n=10). Every included sample for this study was paired, namely, tissue of primary and recurrent ovarian cancer. Tissue from the first recurrence was used for most of the cases (74.3 %). Specimens had been obtained from 1985 until 2015. Data on 53 patients’ germline and/or tumoral BRCA status were retrieved from the OCTIPS Consortium database [24], [25] Platinum sensitivity and platinum resistance were defined, according to the Gynecologic Cancer Intergroup, as a relapse occurring after or before 6 months following the last platinum-based chemotherapy, respectively [26]. Recurrence was defined based on Response Evaluation Criteria In Solid Tumors [27]. Clinicopathological parameters of the study group are outlined in Table 1.
Table 1.
Characteristics of the Study Group
| n (%) | |
|---|---|
| Total pairs | 113 (100%) |
| Age | |
| <60 years | 76 (67.3) |
| >60 years | 37 (32.7) |
| Median | 55 years |
| FIGO stage primary | |
| FIGO I | 2 (1.8) |
| FIGO II | 6 (5.3) |
| FIGO III | 93 (82.3) |
| FIGO IV | 12 (10.6) |
| Recurrence used for IHC | |
| 1st | 98 (86.7) |
| 2nd | 6 (5.3) |
| 3rd | 7 (6.2) |
| Other | 2 (1.8) |
| Postoperative residual tumor | |
| None | 79 (69.9) |
| Any | 34 (30.1) |
| First-line chemotherapy | |
| Taxol/carboplatin | 88 (77.9) |
| Other platinum-based | 19 (16.8) |
| Other | 6 (5.3) |
| Platinum sensitivity status after 1st-line treatment | |
| Sensitive | 89 (78.8) |
| Resistant | 16 (14.2) |
| Missing | 8 |
| Platinum sensitivity status after 2nd-line treatmenta | |
| Sensitive | 58 (84.1) |
| Resistant | 11 (15.9) |
| Missing | 29 |
| BRCA germline status | |
| wt | 12 (57.1) |
| BRCA1 mt | 7 (33.3) |
| BRCA2 mt | 2 (9.5) |
| Missing | 94 |
| BRCA tumor status (includes germline and somatic mt)b | |
| wt | 31 (58.5) |
| BRCA1 mt | 16 (30.2) |
| BRCA2 mt | 6 (11.3) |
| Missing | 60 |
First recurrence only
BRCA status in tumor tissue was identical in all pairs of primary and recurrent tumors.
Immunohistochemistry
Immunohistochemistry was performed on tissue microarrays with two 1-mm tumor cores per case with a Ventana Discovery XT autostainer (Ventana Medical Systems, Inc. Tucson, AZ). The following antibodies were used: CD3 (1:200, Dako Denmark A/S, Ref. No. A0452), CD4 (1:50, Zytomed, Ref. No. 503-3354), CD8 (1:25, Dako/Denmark, Ref. No. M7103), MHC class 1 (HLA-A, B, C) (1:6.000, Dako/Denmark A/S, Ref. No. D-226-3), and MHC class 2 (1:200, MBL, Ref. No. M0746). Diaminobenzidine was used as a chromogen. Antibody detection and counterstaining were performed according to the manufacturer's protocols.
Evaluation of TILs
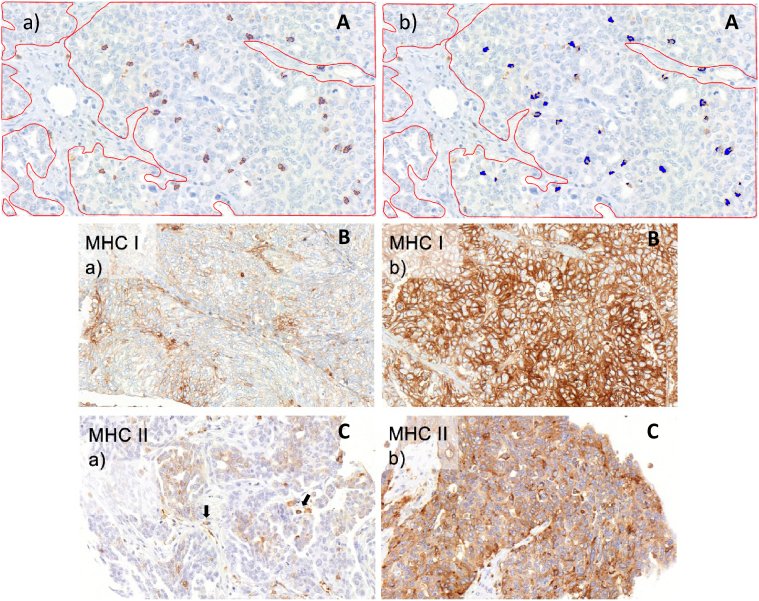
For the evaluation of CD3+, CD4+, and CD8+ TILs density, five fields for each tumor sample were selected and photographed in 400× magnification (=high-power field) on scanned slides using the VM Slide explorer 2.2 (VM Scope, Berlin, Germany). Areas with high density of the marker of interest were favored. With the use of ROI Manager (CognitionMaster, VM Scope), tumoral areas in the high-power fields were visually discriminated against nontumoral areas (such as stroma, necrosis) and labeled, enabling the ROI Manager to calculate the pure-tumor area for each case. The count of stained TILs was then performed automatically with CD3 Quantifier (VM Scope). Only intratumoral TILs which were in direct contact with tumor cells were evaluated. Absolute CD3+, CD4+, and CD8+ TILs numbers and tumor areas were then used to calculate TILs density per 1 mm2 tumor area. As we had previously seen that cutoff values for a prognostic and thereby biologically relevant effect of TILs density were in the lower range [28], we also used a low cutoff for the present study (25% percentile of TILs count in primary tumor) for categorization of cases in low- and high-TILs density groups. In a previous study from our group TILs were assessed in a similar way, except that lymphocytes were identified and labeled by a trained pathologist instead of the CD3 Quantifier software [28]. To guarantee that automatic TILs detection was as reliable as the visual method previously performed, comparative studies using n=209 HGSOC showed a very strong correlation between the data obtained by both methods (Spearman’s rho 0.850, P<.0001). Figure 1A shows a representative CD8 stain with annotation by the CD3 Quantifier software.
Figure 1.
Immunohistochemistry. (A) Representative CD8 stain after annotation by CD3 Quantifier image analysis. Stromal areas have been manually encircled (red) and were not evaluated. (a) Stained lymphocytes are labeled by a thin blue line; (b) same picture as in a, with annotated lymphocytes shown as blue areas. (B) MHC1 expression in HGSOC. (a) Example of a tumor with low, focal expression; (b) example of a tumor with strong diffuse expression. A membranous and cytoplasmic expression pattern is evident. (C) MHC2 expression in HGSOC. (a) Weak and focal expression; single cells with strong expression are intratumoral immune cells (which were not evaluated; arrows); (b) tumor with diffuse expression with varying intensity revealing a mosaic-like pattern; expression is mainly cytoplasmic in these examples.
Evaluation of MHC1 and MHC2 Expression
The evaluation of MHC1 and MHC2 expression in cancer cells was performed with VM Slide explorer 2.2 and VM TMA Evaluator (VM Scope). Two cores per specimen were visually assessed regarding the percentage of stained tumor cells [0% (0 point), 1%-10% (1 point), 11%-50% (2 points), 51%-80% (3 points), 81%-100% (4 points)] and the intensity of staining [scored negative (0 point), weak (1 point), moderate (2 points), strong (3 points)]. Both were then summarized to a semiquantitative immunoreactivity score (IRS). For statistical analysis, the cases were grouped in low- and high-IRS score classes using a lower-level cutoff value (IRS3) similarly to the TILs cutoff. Figure 1, B and C shows representative pictures for MHC1 and MHC2 stainings with each low and high expression, respectively.
Statistical Evaluation
The IBM SPSS Statistics Version 23.0.0.2 (Armonk, NY) and GraphPad Prism v.5 (La Jolla, CA) were used for statistical analyses. Spearman rank test was used for correlations between variables. Due to the wide distribution of TIL counts especially within high ranges (positively skewed distribution), we also used lg10 values of TILs density for some calculations. Associations of paired samples were examined using Wilcoxon signed ranks test; comparison of groups was performed with Pearson's χ2 (using Fisher's Exact Test) or Mann-Whitney test. For survival analysis, the Kaplan-Meyer method with log-rank test was used. Tests were considered statistically significant with a P value <.05, regarding 2-sided tests.
Results
TILs Densities and MHC Expression Patterns
All TILs subsets (CD3+, CD4+, and CD8+ TILs) could be found in both primary and recurrent tumors. Informative data on CD3+ TILs were available on 97 tumor pairs. The median number of CD3+ TILs in primary tumors was 158/mm2 (range 0-2.454) and in recurrences 247/mm2 (range 0-3.550). Data on CD4+ TILs were available for n=100 pairs with a median number of TILs of 82/mm2 (range 0-2.252) in primary and 153/mm2 (range 0-2.098) in recurrent tumors. In n=98 pairs with data on CD8+ TILs, the median number of TILs in primaries was 122/mm2 (range 6-2.221) and in recurrences 144/mm2 (range 0-2.123; Table 2).
Table 2.
CD3+, CD4+, and CD8+ TILs MHC Class I and Class II Categories in Primary and Recurrent Tumors
| Primary |
Recurrent |
Total |
Fisher’s Exact P (Kappa) | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | |
| CD3 low | CD3 high | |||
| CD3 low | 10 (41.7) | 14 (58.3) | 24 (100) | .059 |
| CD3 high | 15 (20.5) | 58 (79.5) | 73 (100) | (0.196) |
| CD4 low | CD4 high | |||
| CD4 low | 10 (40.0) | 15 (60.0) | 25 (100) | .006 |
| CD4 high | 9 (12.0) | 66 (88.0) | 75 (100) | (0.192) |
| CD8 low | CD8 high | |||
| CD8 low | 10 (41.7) | 14 (58.3) | 24 (100) | .122 |
| CD8 high | 18 (24.3) | 56 (75.7) | 74 (100) | (0.092) |
| MHC1 low | MHC1 high | |||
| MHC1 low | 1 (20.0) | 4 (80.0) | 5 (100) | .262 |
| MHC1 high | 5 (5.1) | 94 (94.9) | 99 (100) | (0.137) |
| MHC2 low | MHC2 high | |||
| MHC2 low | 27 (65.9) | 14 (34.1) | 41 (100) | <.0001 |
| MHC2 high | 18 (29.5) | 43 (70.5) | 61 (100) | (0.358) |
We observed that both MHC1 and MHC2 were expressed on the membrane and cytoplasm of tumor cells and that expression in both cellular compartments was not easily distinguishable. We therefore evaluated total cellular MHC expression. For MHC1, n=104 paired samples were evaluable; for MHC2, n=102. MHC1 expression was strong and diffuse in most cases; the most frequent IRS was 12 (in both primary and recurrent tumors), and no sample was completely negative. MHC2 was also expressed in tumor cells, however, in a significantly lower rate than MHC1; the most frequent IRS being 2 (in both primary and recurrent tumors), 16 (15.7%) were completely negative (IRS0) in primary and 17 (16.7%) in recurrent tumors (see Table 2 for detailed data).
All immunological factors were positively correlated with each other both within and across primary and recurrent tumors (Supplementary Table 1).
Pairwise Comparison of TIL Densities and MHC Expression in Primary and Recurrent Tumors
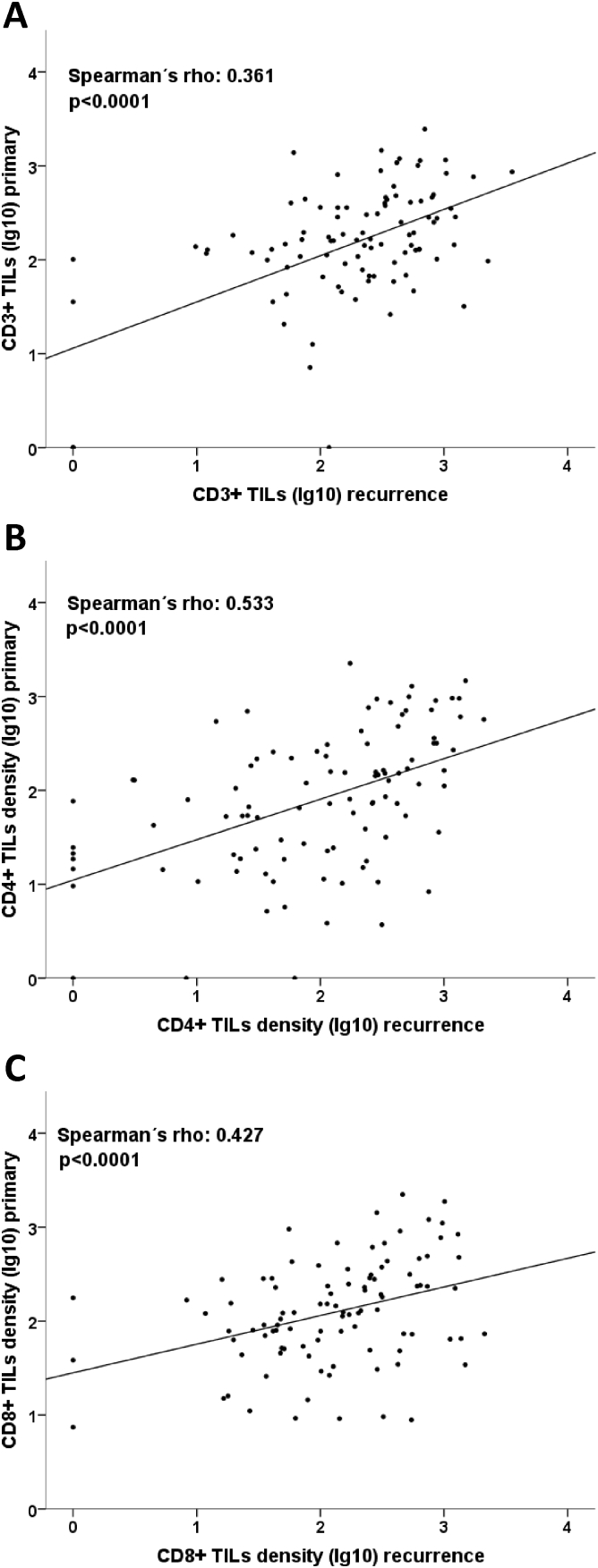
All TIL subsets were moderately but significantly correlated between primary and recurrent tumors (CD3: Spearman rho 0.427, P<.0001, CD4: Spearman rho 0.533, P<.0001, CD8: Spearman rho 0.361, P<.0001; Supplementary Table 1, Figure 2). Paired testing (Wilcoxon) showed that CD4+ TIL densities in recurrent tumors were frequently higher than in their respective primaries (P=.034). A similar trend was seen for CD3 (P=.077) but not for CD8 (P=.624). Comparing categorized TIL data in primary and recurrent tumors, it became evident that the vast majority of primary tumors with a high TIL density also had high TILs in the recurrent tumor (CD3: 79.5%, CD4: 88.0%, CD8: 75.7%; Table 2). In contrast, primary tumors with low TIL densities often exhibited high rather than low TILs in recurrent tumors (CD3: 58.3%, CD4: 60.0%, CD8: 58.3%). This correlation was significant for CD4+ TILs (P=.006), borderline significant for CD3+ (P=.059), and only seen as a trend for CD8 (P=.122). When using the medians of primary tumor TILs densities as a cutoff (instead of the 25th percentiles, which, due to their prognostic effects, we considered as biologically more relevant [27]), we found similar results; however, the shifts toward high categories in recurrence were not so pronounced (Supplementary Table 1): while primaries of low CD3 TILs category had an approximately 50% chance of either low or high category in the recurrent tumor, primaries of high CD3 category stayed in the high category in the recurrent tumor in 73.3%. For CD4 TILs, 60% of primary low category stayed low in the recurrence, while 80% of primaries with high TILs stayed high. For CD8, there was a weaker trend towards a switch to the high category in recurrence for primarily high tumors. Analyzing pairs with tissue from first recurrence only, which might be considered to constitute a more homogeneous group, the shift to higher TILs levels in relapse samples became significant for CD3 (n=74, Wilcoxon P=.031) and even more significant for CD4 (n=76, Wilcoxon P=.014) but not for CD8 (n=76, Wilcoxon P=.286).
Figure 2.
Correlation of TILs levels between primary and recurrent tumors. (A-C) A moderate, significant correlation between CD3+, CD4+, and CD8+ TILs density in primary and recurrent tumors is seen. TILs data were logarithmized to deskew the diagram.
MHC1 and MHC2 IRS values in primary tumors significantly correlated with those in recurrent tumors (Spearman rho 0.456, P<.0001, and Spearman rho 0.526, P<.0001, respectively, Supplementary Table 1). As for the TIL rates, we compared the MHC1 and MHC2 expression in the tumor pairs. Wilcoxon testing showed a directed change of MHC1 expression to higher IRS values from primary to recurrent tissue (P = .018), while no significant change was seen for MHC2 (P=.803). For further investigation, the data for MHC intensity were split at the cutoff of 3 to obtain two groups: low expression (IRS0-2) and high expression (IRS3-12). Similarly to TILs, high MHC1 expression in primary tumors was linked to high expression in recurrent tumors (94.9%), and primary tumors with low MHC1 expression were linked to high expression in recurrent tumors also (80.0%); however, this was not significant probably due to a low sample size (n=5) in MHC1 low-expressing tumors (P=.262; Figure 3D). Increasing the number of MHC1 low-expressing cases by the use of a higher cutoff point (IRS0-4 vs IRS6-12) resulted in a significant association (P=.016, not shown). Still, 89.7% of cases with high MHC1 expression in the primary were also MHC1 high in the recurrent tumor, while 64.7% of cases with low MHC1 expression in the primary were MHC1 high in the relapse sample. Unlike MHC1, MHC2 status in the primary tumor was strongly linked to the same expression status in recurrences: 1) Primary with low MHC2 expression was more likely to have low scores in the paired recurrent tumor as well (73.8% remained low), and 2) high MHC2 expression in primary was correlated with high scores in recurrent tumor (63.4% remained high), indicating that the groups (low and high expression) remained stable during tumor progression (P<.001, Table 2). Analyzing pairs with tissue from first recurrence only, the trend towards higher MHC1 levels in recurrences was only of borderline significance (n=98, Wilcoxon P=.072), and the analysis for MHC2 remained nonsignificant (n=88, Wilcoxon P=.770).
Figure 3.
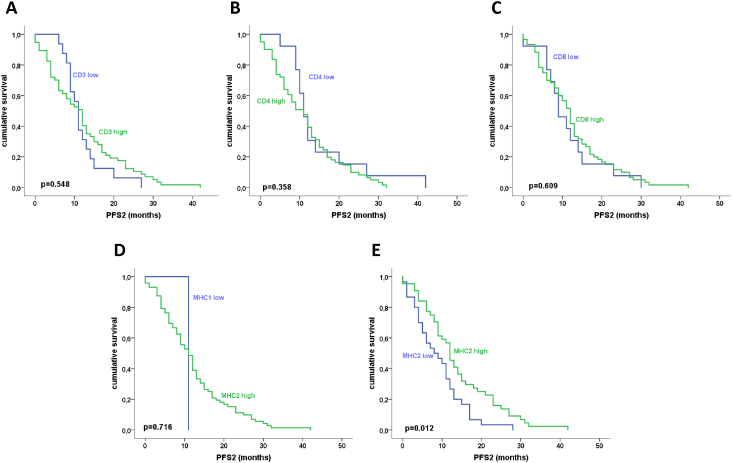
Progression-free survival from first to second recurrence (PFS2) in dependence of TILs levels (A-C) and MHC expression (D, E). There was a trend towards longer PFS2 in recurrent tumors with high CD4+ TILs densities (B). MHC2 expression was significantly linked to longer PFS2 in recurrent HGSOC (E).
Stratification According to Platinum Sensitivity and BRCA Status
TILs levels (CD3, CD4, CD8) and MHC1 or MHC2 expression (IRS) were not significantly associated with platinum sensitivity in primary tumors (Mann-Whitney P>.1). In recurrent tumors, there was a borderline significance for higher MHC2 IRS values in platinum-sensitive tumors (after second-line treatment, n=63, Mann-Whitney P=.067). Analyzing patients with platinum-sensitive status after first-line treatment only (n=89), paired Wilcoxon testing showed significantly higher CD4+ TILs and MHC1 levels in recurrent tumors compared to primaries (P=.010, and P=.015), similarly to the total study group; however, there was also a significant shift towards higher CD3+TILs numbers in relapses (P=.026). Interestingly, in patients with platinum sensitivity after both first- and second-line therapy (n=45), the effect was even more significant for CD3+ TILs and CD4+ TILs (both Wilcoxon P=.003) but not for MHC1 or MHC2 (P>.05). Small sample size (n=16) precluded subgroup analysis in platinum-resistant tumors.
Combining germline and tumoral BRCA status to two categories, we obtained n=31 BRCAwt tumors and n=22 BRCAmt tumors. BRCAmt primaries had higher MHC1 and MHC2 expression levels as compared to BRCAwt primaries (borderline significance P=.055 and P=.056, respectively; for TILs: P>.1). BRCAmt relapses (first recurrences only) had a significantly higher expression of MHC1 as compared to BRCAwt relapses (P=.024; MHC2 and TILs: P>.1). Explorative paired analysis stratified according to BRCA status showed higher levels of MHC1 expression in recurrent tumors as compared to primaries in with wild-type BRCA status as the only significant result (Wilcoxon P=.026; MHC1 in BRCAmt as well as MHC2 and TILs in BRCAwt and BRCAmt: P>.05).
Prognostic Effect of TIL Density and MHC Expression
To determine the prognostic impact of TIL density, data were split as described before. Kaplan-Meier analysis confirmed the previously reported association between CD3+ and CD8+ TIL rates and longer progression-free survival after primary diagnosis (PFS1). Patients with CD3+ TILs low primaries had a median survival time of 13.4 months (standard error 1.1) as opposed to 21.3 months in CD3+ TILs high tumors (standard error 2.2; P<.001). For CD8+ TILs, median survival for patients with primaries of the high category was 20.4 months (standard error 1.1) and was 13.6 months for tumors with low TILs (standard error 2.8, P=.026, not shown). For CD4+ TILs, MHC1 and MHC2 expression was not significantly associated with survival (P>.05, not shown).
Data on progression-free survival after the first recurrence (PFS2) were available for n=74 tumor pairs (only cases with first recurrence samples were included). Interestingly, high MHC2 expression in the recurrent tumor was associated with a longer PFS2 [median survival 12.0 months (standard error 2.4) vs 9.0 months (standard error 0.9), P=.019, Figure 3E]. No significance was obtained for CD3, CD4, CD8, and MHC1 expression (Figure 3, A-D).
Discussion
In this study, we compared tumor-infiltrating lymphocytes and MHC expression in primary and recurrent high-grade serous EOC. We found that TIL infiltrations and MHC expression levels correlated between primary and relapse samples, and there was a suggestion that immune engagement might be elevated in many recurrent tumors.
To our knowledge, this is the first study that analyzed immunological parameters during ovarian cancer progression. There are however reports on spatial heterogeneity of TILs in breast cancer that compared different areas of the primary tumor [29] or primary tumors with corresponding distant metastases [30]. The authors described that TILs scores were similar in different primary tumors regions [29] and that, although TILs rates in primary tumors were higher than in metastases, the composition of the immunological infiltrate, as to stromal and intraepithelial TILs and different TILs subpopulations, was comparable in tumor sites [30]. Taking together these and our findings, it seems that spatial and temporal intratumor heterogeneity of the immune microenvironment might not be a major characteristic.
Our findings of a correlation between major subsets of T cells in primary and recurrent samples are not necessarily predictable. The manifestations of primary and recurrent HGSC were separated by months or years, and in addition to the temporal aspect, chemotherapy may cause tumor evolution, potentially resulting in a significant change of tumor biology [31]. Temporal heterogeneity has been shown to occur in several biological levels of cancer, also ovarian cancer, such as on the genomic level. Transcriptomic or epigenetic landscapes seem to be more affected by temporal heterogeneity, which might be explained by the greater fluctuation and instability of these systems [32]. The immunological microenvironment for sure also belongs to these fluctuant and flexible systems; however, our data suggest that the molecular constitution regulating TIL levels in a tumor site seems rather to be inherent to an individual tumor. MHC1 expression is very likely one of the important factors attracting TILs. One interesting finding in our study was that—after dichotomization into low– and high–TIL level categories—cases with a high TIL level in the primary were more likely to retain high levels in the recurrence than cases with low TIL levels in the primary. Thus, tumors with high-level TILs have an immunological constitution that seems to be more stable during tumor progression. Tumors with low-level TILs in contrast have a relatively high chance to switch to a higher-level immunological constitution in the recurrence.
Even more surprising than the detection of a correlation between primary and recurrent tumor TILs and MHC expression is that there seems to be a shift towards higher immunogenicity in recurrent tumors as compared to primaries. This shift was seen for CD4+ TILs as well as MHC1 expression and, in trend, for CD3+ TILs. Analyzing more homogeneous groups, such as platinum-sensitive tumors only or pairs with first recurrences only, this effect for CD3+ TILs even became significant. However, earlier trials reported that MHC class I was prone to downregulation to evade immunological elimination in ovarian cancer and other tumor types; especially advanced disease stages showed this immunoescape mechanism (for review see, [33], [34]). Therefore, we rather expected the MHC1expression in recurrent tumors to be lower than in the primaries. However, we did not find such downregulation during tumor progression in our study group. On the contrary, the recurrent tumors tended to show higher expression values than primary lesions, independent of their expression level in the primary tumor. Thus, the vast majority of cases with a high expression in the primary retained a high expression in the relapse sample; however, cases with a low expression in the primary most often changed to high levels in the recurrence. Similarly as for TILs, our MHC1 data indicate that the higher immunogenicity in the primary, the more likely that it will also be high at recurrence. Interestingly, a recent study on paired pre– and post–neoadjuvant chemotherapy (NACT) EOC specimens detected an upward shift of TILs and PD-L1 expression after NACT [35], and a comparable study reported enhanced IFNγ production by CD4+ TILs and increased antitumor Th1 gene signatures in omental tumor biopsies after NACT [36]. Of note, CD8+ TILs densities were not affected by NACT in the latter study similarly to our data. Lo et al. also found higher levels of TILs subsets after NACT of HGSOC; however, interestingly, there were no changes in MHC1 expression in tumor cells [37]. These data parallel our findings; however, it is unclear if the same mechanisms account for our data and results from the 2 other groups since recurrent tumor samples in our study were retrieved months or years after chemotherapy, while in the latter studies, they were retrieved immediately after chemotherapy.
The potential reasons for an upward shift of tumor immunogenicity are unclear to date. Hypothetically, during primary tumor development, the immune system might adapt to the tumor by generating memory effectors that recognize a tumor recurrence, which leads to an even more intense, however not necessarily more effective, reaction to the recurrent tumor tissue in a significant number of cases. It is also conceivable that the CD4+ TILs we found to be increased in recurrent tumors might be constituted in the major part of regulatory cells that inhibit or attenuate the immune reaction. This is supported by the fact that cytotoxic CD8+ TILs were not significantly affected by an upregulation during tumor recurrence. Of note, the shifts towards higher immune effector levels in recurrences we found were rather subtle, and validations in independent and preferably larger cohorts are therefore necessary.
A parallel study on OCTIPS samples investigated gene expression profiling in paired fresh-frozen samples [38]. The authors found differences in the expression of immune-related genes to be the predominant distinguishing feature in HGSC and accordingly grouped the study group as immune-active and immune-silent. Interestingly, 51% of cases with a silent phenotype in the primary switched to an active immunological phenotype in the recurrence as compared to 36% of cases with an active phenotype that switched to silent. This parallels our findings of a tendency of TIL-high tumors to remain high in the recurrence. Interestingly, there were no relevant differences in gene expression between primary and recurrent tumor samples within the active-active and within the silent-silent groups, indicating that, in immunologically concordant cases, the phenotypic constitution remains similar. This on the morphological level is paralleled by our study.
The relative stability of immunological features during ovarian cancer progression we detected in this study has implications for the assessment of immunological biomarkers in histopathological diagnosis. As immune checkpoint inhibitors such as anti-PD-1 and anti-PD-L1 antibodies are clinically investigated in many malignancies as well as in EOC [17], the question on which tumor sample to use for companion diagnostics or translational analyses became quite urgent. PD-L1 expression and TILs are important candidate markers in this regard, but of note, they are not validated markers for response to checkpoint inhibition in EOC yet. Some trials require novel biopsies for inclusion of patients, which frequently constitute a problem because of the invasive procedure in often significantly sick patients. Our data suggest that primary tumor samples that are available for almost all patients might be used for biomarker analysis; at least primary tumors with high TIL densities might be considered sufficient as a decrease in TILs levels is rare in these cases, while in case of primaries with low TILs levels, a retesting of recurrent samples might be considered.
In contrast to the well-established prognostic impact of immunological features in primary HGSOC, the relevance for the recurrent situation remains unclear. Our data give a hint that certain markers (MHC2) might have a certain relevance in the relapse situation, too. TILs, which have an established strong impact on prognosis in the primary setting, were not prognostic in the recurrence setting. Interestingly, the lack of a prognostic information of TILs was reported in ovarian cancer samples post-NACT, too [37]. However, the low sample size of n=68 was too small to draw strong conclusions of our findings on PFS2 in our study and was particularly prone to false-negative results.
Our study has several strengths and weaknesses. One limitation is the sample size that hampers especially subgroup analyses, which might be of interest (e.g., comparison of tumors that change the immunological class during progression to those that do not or comparisons of BRCA mutant and wild-type tumors). Unfortunately, our paraffin-embedded, formalin-fixed study cohort only partially overlapped with the OCTIPS fresh-frozen cohort, for which several molecular data are available. Furthermore, our study has no independent validation cohort. It is however the largest study to investigate immunological features in paired ovarian cancer samples. Another limitation is the fact that these patients due to the fact that surgery was possible in relapse situation are a highly preselected cohort that might not be representative for all HGSOC, e.g., median patient age (55 years) was relatively low. Furthermore, due to the relatively long ascertainment period, changes in treatment (introduction of taxanes, development of surgical methods) might have impacted the homogeneity of the study cohort.
As a conclusion, our observations are in line with previous reports. TILs subgroups and MHC classes correlated with each other, and a higher immunogenicity was associated with prolonged survival. However, we made a further step into the investigation of tumor progression in EOC. Our study showed a connection of the immunologic pattern between primary and recurrent lesions; especially tumors with a high immunogenicity may have a similar molecular composition during relapse. Exploring and understanding the immunological profile and its development will provide a basis for the establishment of new therapeutics in EOC, such as checkpoint inhibitors or adoptive cell transfer. Further analyses are needed to validate these findings, preferentially as translational protocols in clinical trials cohorts, where the data could directly be investigated as to therapy response. Further molecular characterization of paired tumor samples, e.g., as to clonal evolution, and, e.g., neoantigen expression with regard to the immunological phenotype, should give valuable insights into the temporal heterogeneity of mechanisms regulating the immunological microenvironment.
The following are the supplementary data related to this article.
Correlations of Immune Parameters.
CD3+, CD4+ and CD8+ TILs (Using Medians as CutOff) and MHC Class I and Class II Categories (Using IRS6 as Cutoff) in Primary and Recurrent Tumors.
Conflict of Interest
All authors state that they have no conflicts of interest.
Acknowledgement
We would like to thank Mrs. Ines Koch and Mrs. Barbara-Meyer-Bartell for their excellent technical assistance. The documentation of clinical and patient's data was managed with "AlcedisTRIAL the web based documentation system" of Alcedis GmbH, Winchesterstr. 3, 35394 Giessen, Germany.
Footnotes
Funding: This work was funded by European Community’s Seventh Framework Programme under grant agreement no. 279113-2.
Contributor Information
Mandy Stanske, Email: mandy.stanske@charite.de.
Stephan Wienert, Email: stephanwienert@gmx.de.
Dan Cacsire Castillo-Tong, Email: dan.cacsire-castillo@meduniwien.ac.at.
Caroline Kreuzinger, Email: caroline.kreuzinger@meduniwien.ac.at.
Ignace Vergote, Email: ignace.vergote@uzleuven.be.
Sandrijne Lambrechts, Email: sandrijne.lambrechts@gmail.com.
Hani Gabra, Email: h.gabra@imperial.ac.uk.
Charlie Gourley, Email: charlie.gourley@ed.ac.uk.
Ram N. Ganapathi, Email: ram.ganapathi@carolinashealthcare.org.
Ivonne Kolaschinski, Email: ivonne.kolaschinski@charite.de.
Jan Budczies, Email: jan.budczies@charite.de.
Jalid Sehouli, Email: jalid.sehouli@charite.de.
Ilary Ruscito, Email: ilary.ruscito@uniroma1.it.
Carsten Denkert, Email: carsten.denkert@charite.de.
Hagen Kulbe, Email: hagen.kulbe@charite.de.
Wolfgang Schmitt, Email: wolfgang.schmitt@charite.de.
Korinna Jöhrens, Email: korinna.joehrens@charite.de.
Ioana Braicu, Email: ioana@braicu.de.
Silvia Darb-Esfahani, Email: silvia.darb-esfahani@charite.de.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20(5):1248–1259. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 3.Heintz AP, Odicino F, Maisonneuve P, Quinn MA, Benedet JL, Creasman WT, Ngan H.Y., Pecorelli S, Beller U. Carcinoma of the ovary. FIGO 26th annual report on the results of treatment in gynecological cancer. Int J Gynaecol Obstet. 2006;95(Suppl. 1):S161–S192. doi: 10.1016/S0020-7292(06)60033-7. [DOI] [PubMed] [Google Scholar]
- 4.Hwang WT, Adams SF, Tahirovic E, Hagemann IS, Coukos G. Prognostic significance of tumor-infiltrating T cells in ovarian cancer: a meta-analysis. Gynecol Oncol. 2012;124(2):192–198. doi: 10.1016/j.ygyno.2011.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, Makrigiannakis A., Gray H., Schlienger K., Liebman M.N. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 6.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, Jungbluth A.A., Frosina D., Gnjatic S., Ambrosone C. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102(51):18538–18543. doi: 10.1073/pnas.0509182102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke B, Tinker AV, Lee CH, Subramanian S, van de Rijn M, Turbin D, Kalloger S, Han G, Ceballos K, Cadungog MG. Intraepithelial T cells and prognosis in ovarian carcinoma: novel associations with stage, tumor type, and BRCA1 loss. Mod Pathol. 2009;22(3):393–402. doi: 10.1038/modpathol.2008.191. [DOI] [PubMed] [Google Scholar]
- 8.Milne K, Köbel M, Kalloger SE, Barnes RO, Gao D, Gilks CB, Watson PH, Nelson BH. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS One. 2009;4(7) doi: 10.1371/journal.pone.0006412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ovarian Tumor Tissue Analysis (OTTA) Consortium Dose-response association of CD8+ tumor-infiltrating lymphocytes and survival time in high-grade serous ovarian cancer. JAMA Oncol. 2017;3(12) doi: 10.1001/jamaoncol.2017.3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santoiemma PP, Reyes C, Wang LP, McLane MW, Feldman MD, Tanyi JL, Powell D.J., Jr Systematic evaluation of multiple immune markers reveals prognostic factors in ovarian cancer. Gynecol Oncol. 2016;143(1):120–127. doi: 10.1016/j.ygyno.2016.07.105. [DOI] [PubMed] [Google Scholar]
- 11.Mariya T, Hirohashi Y, Torigoe T, Asano T, Kuroda T, Yasuda K, Mizuuchi M., Sonoda T., Saito T., Sato N. Prognostic impact of human leukocyte antigen class I expression and association of platinum resistance with immunologic profiles in epithelial ovarian cancer. Cancer Immunol Res. 2014;2(12):1220–1229. doi: 10.1158/2326-6066.CIR-14-0101. [DOI] [PubMed] [Google Scholar]
- 12.Adams SF, Levine DA, Cadungog MG, Hammond R, Facciabene A, Olvera N. Intraepithelial T cells and tumor proliferation: impact on the benefit from surgical cytoreduction in advanced serous ovarian cancer. Cancer. 2009;115(13):2891–2902. doi: 10.1002/cncr.24317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Callahan MJ, Nagymanyoki Z, Bonome T, Johnson ME, Litkouhi B, Sullivan EH, Hirsch M.S., Matulonis U.A., Liu J., Birrer M.J. Increased HLA-DMB expression in the tumor epithelium is associated with increased CTL infiltration and improved prognosis in advanced-stage serous ovarian cancer. Clin Cancer Res. 2008;14(23):7667–7673. doi: 10.1158/1078-0432.CCR-08-0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shehata M, Mukherjee A, Deen S, Al-Attar A, Durrant LG, Chan S. Human leukocyte antigen class I expression is an independent prognostic factor in advanced ovarian cancer resistant to first-line platinum chemotherapy. Br J Cancer. 2009;101(8):1321–1328. doi: 10.1038/sj.bjc.6605315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R., Robert C., Schadendorf D., Hassel J.C. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16(5):275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turner TB, Buchsbaum DJ, Straughn JM, Jr., Randall TD, Arend RC. Ovarian cancer and the immune system—the role of targeted therapies. Gynecol Oncol. 2016;142(2):349–356. doi: 10.1016/j.ygyno.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson D.R., Seipp C.A., Einhorn J.H., White D.E. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst. 1994;86(15):1159–1166. doi: 10.1093/jnci/86.15.1159. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348(6230):62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Besser MJ, Shapira-Frommer R, Treves AJ, Zippel D, Itzhaki O, Hershkovitz L, Levy D., Kubi A., Hovav E., Chermoshniuk N. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2010;16(9):2646–2655. doi: 10.1158/1078-0432.CCR-10-0041. [DOI] [PubMed] [Google Scholar]
- 22.Fujita K, Ikarashi H, Takakuwa K, Kodama S, Tokunaga A, Takahashi T, Tanaka K. Prolonged disease-free period in patients with advanced epithelial ovarian cancer after adoptive transfer of tumor-infiltrating lymphocytes. Clin Cancer Res. 1995;1(5):501–507. [PubMed] [Google Scholar]
- 23.Liu J, Li H, Cao S, Zhang X, Yu J, Qi J, An X., Yu W., Ren X., Hao X. Maintenance therapy with autologous cytokine-induced killer cells in patients with advanced epithelial ovarian cancer after first-line treatment. J Immunother. 2014;37(2):115–122. doi: 10.1097/CJI.0000000000000021. [DOI] [PubMed] [Google Scholar]
- 24.Lambrechts S, Smeets D, Moisse M, Braicu EI, Vanderstichele A, Zhao H, Van Nieuwenhuysen E, Berns E, Sehouli J, Zeillinger R. Genetic heterogeneity after first-line chemotherapy in high-grade serous ovarian cancer. Eur J Cancer. 2016;53:51–64. doi: 10.1016/j.ejca.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Patel J.N., Sehouli J., Timms K., Solimeno C., Reid J.R., Lanchbury J.S., Braicu I., Darb-Esfahani S., Ganapathi M., Ganapathi R.N. Characteristics of homologous recombination deficiency (HRD) in paired primary and recurrent high-grade serous ovarian cancer (HGSOC) J Clin Oncol. 2015;33:5534. doi: 10.1038/s41416-018-0268-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedlander M, Trimble E, Tinker A, Alberts D, Avall-Lundqvist E, Brady M, Harter P, Pignata S, Pujade-Lauraine E, Sehouli J. Stuart GC; Gynecologic Cancer InterGroup. Clinical trials in recurrent ovarian cancer. Int J Gynecol Cancer. 2011;21(4):771–775. doi: 10.1097/IGC.0b013e31821bb8aa. [DOI] [PubMed] [Google Scholar]
- 27.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 28.Darb-Esfahani S, Kunze CA, Kulbe H, Sehouli J, Wienert S, Lindner J, Budczies J, Bockmayr M, Dietel M, Denkert C. Prognostic impact of programmed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor-infiltrating lymphocytes in ovarian high grade serous carcinoma. Oncotarget. 2016;7(2):1486–1499. doi: 10.18632/oncotarget.6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mani NL, Schalper KA, Hatzis C, Saglam O, Tavassoli F, Butler M, Chagpar AB, Pusztai L, Rimm DL. Quantitative assessment of the spatial heterogeneity of tumor-infiltrating lymphocytes in breast cancer. Breast Cancer Res. 2016;18(1):78. doi: 10.1186/s13058-016-0737-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sobottka B, Pestalozzi B, Fink D, Moch H, Varga Z. Similar lymphocytic infiltration pattern in primary breast cancer and their corresponding distant metastases. Oncoimmunology. 2016;5(6) doi: 10.1080/2162402X.2016.1153208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jamal-Hanjani M, Quezada SA, Larkin J, Swanton C. Translational implications of tumor heterogeneity. Clin Cancer Res. 2015;21(6):1258–1266. doi: 10.1158/1078-0432.CCR-14-1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marusyk A, Almendro V, Polyak K. Intra-tumour heterogeneity: a looking glass for cancer? Nat Rev Cancer. 2012;12(5):323–334. doi: 10.1038/nrc3261. [DOI] [PubMed] [Google Scholar]
- 33.Aptsiauri N, Cabrera T, Mendez R, Garcia-Lora A, Ruiz-Cabello F, Garrido F. Role of altered expression of HLA class I molecules in cancer progression. Adv Exp Med Biol. 2007;601:123–131. doi: 10.1007/978-0-387-72005-0_13. [DOI] [PubMed] [Google Scholar]
- 34.Chang CC, Ferrone S. Immune selective pressure and HLA class I antigen defects in malignant lesions. Cancer Immunol Immunother. 2007;56(2):227–236. doi: 10.1007/s00262-006-0183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mesnage SJL, Auguste A, Genestie C, Dunant A, Pain E, Drusch F, Gouy S, Morice P, Bentivegna E, Lhomme C. Neoadjuvant chemotherapy (NACT) increases immune infiltration and programmed death-ligand 1 (PD-L1) expression in epithelial ovarian cancer (EOC) Ann Oncol. 2017;28(3):651–657. doi: 10.1093/annonc/mdw625. [DOI] [PubMed] [Google Scholar]
- 36.Böhm S, Montfort A, Pearce OM, Topping J, Chakravarty P, Everitt GL, Clear A, McDermott JR, Ennis D, Dowe T. Neoadjuvant chemotherapy modulates the immune microenvironment in metastases of tubo-ovarian high-grade serous carcinoma. Clin Cancer Res. 2016;22(12):3025–3036. doi: 10.1158/1078-0432.CCR-15-2657. [DOI] [PubMed] [Google Scholar]
- 37.Lo CS, Sanii S, Kroeger DR, Milne K, Talhouk A, Chiu DS, Rahimi K, Shaw PA, Clarke BA, Nelson BH. Neoadjuvant chemotherapy of ovarian cancer results in three patterns of tumor-infiltrating lymphocyte response with distinct implications for immunotherapy. Clin Cancer Res. 2017;23(4):925–934. doi: 10.1158/1078-0432.CCR-16-1433. [DOI] [PubMed] [Google Scholar]
- 38.Kreuzinger C., Geroldinger A., Smeets D., Braicu E.I., Sehouli J., Koller J., Wolf A., Darb-Esfahani S., Jöhrens K., Vergote I., Vanderstichele A., Boeckx B., Lambrechts D., Gabra H., Wisman G.B.A., Trillsch F., Heinze G., Horvat R., Polterauer S., Berns E., Theillet C., Cacsire Castillo-Tong D. A complex network of tumor microenvironment in human high grade serous ovarian cancer. Clin Cancer Res. 2017;23(24):7621–7632. doi: 10.1158/1078-0432.CCR-17-1159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Correlations of Immune Parameters.
CD3+, CD4+ and CD8+ TILs (Using Medians as CutOff) and MHC Class I and Class II Categories (Using IRS6 as Cutoff) in Primary and Recurrent Tumors.