Abstract
Older individuals typically display stronger regional brain activity than younger subjects during motor performance. However, knowledge regarding age-related changes of motor network interactions between brain regions remains scarce. We here investigated the impact of ageing on the interaction of cortical areas during movement selection and initiation using dynamic causal modelling (DCM). We found that age-related psychomotor slowing was accompanied by increases in both regional activity and effective connectivity, especially for ‘core’ motor coupling targeting primary motor cortex (M1). Interestingly, younger participants within the older group showed strongest connectivity targeting M1, which steadily decreased with advancing age. Conversely, prefrontal influences on the motor system increased with advancing age, and were inversely correlated with reduced parietal influences and core motor coupling. Interestingly, higher net coupling within the prefrontal-premotor-M1 axis predicted faster psychomotor speed in ageing. Hence, as opposed to a uniform age-related decline, our findings are compatible with the idea of different age-related compensatory mechanisms, with an important role of the prefrontal cortex compensating for reduced coupling within the core motor network.
Keywords: fMRI, Ageing, Motor control, Effective connectivity
Highlights
-
•
Enhanced motor network activity and connectivity in ageing
-
•
Parietal-premotor and premotor-M1 coupling decreases with advancing age.
-
•
Prefrontal influences on the motor system increase with advancing age.
-
•
Prefrontal cortex compensates for age-related decline in other motor connections.
-
•
Prefrontal-premotor-M1 coupling predicts psychomotor speed in ageing.
1. Introduction
Ageing is associated with decline of various cognitive functions (Grady, 2012). Moreover, older people often display deterioration of motor performance such as psychomotor slowing or reduced fine motor skills (Salthouse, 2000; Seidler et al., 2010). One important factor contributing to age-related performance decline is neurodegeneration as represented by, e.g., grey matter atrophy (Draganski et al., 2013). However, functional neuroimaging studies revealed substantial evidence for adaptive plasticity paralleling structural decline.
Typically, older subjects display both enhanced and more widespread brain activation than their younger counterparts during motor performance (Mattay et al., 2002; Rowe et al., 2006). Notwithstanding, the exact functional role of increased regional brain activity for motor control in older individuals remains poorly understood. On the one hand, non-selective recruitment of brain activity could reflect a loss of neural specificity or efficiency in the ageing brain, i.e., dedifferentiation (Li and Lindenberger, 1999; Logan et al., 2002; Riecker et al., 2006). On the other hand, numerous studies point to a compensatory role in that stronger recruitment of brain activity is beneficial for motor performance in ageing (Mattay et al., 2002; Naccarato et al., 2006; Wu and Hallett, 2005). However, from a systems-level perspective, enhanced regional activity could as well depict a compensatory mechanism to account for age-related reduction in network connectivity, similar to what has been observed in neurodegenerative diseases such as Parkinson's disease or in stroke (Grefkes et al., 2008; Rowe et al., 2002). Here, studies of resting-state functional connectivity revealed that reduced motor performance in older individuals is associated with both increased and diminished interregional coupling within the motor network (Langan et al., 2010; Seidler et al., 2015; Solesio-Jofre et al., 2014). However, resting-state analyses do not allow direct conclusions about how brain areas interact during a given task, thereby limiting insights into the relationship between network changes underlying a specific behaviour and age-related performance decline (Rehme et al., 2013; Sala-Llonch et al., 2015). Nevertheless, the wealth of studies demonstrating age-related motor deficits is contrasted by the dearth of studies that addressed the question of how brain areas interact in the ageing brain during motor performance. The evidence thus far available from task-based studies suggests that interregional connectivity is enhanced in older as compared to young subjects, especially coupling among ‘core’ motor regions like premotor cortex and primary motor cortex (M1) (Boudrias et al., 2012; Heitger et al., 2013; Rowe et al., 2006). Furthermore, interindividual variability in premotor-M1 coupling has been shown to predict motor performance in older individuals (Stewart et al., 2014).
Nevertheless, motor actions do not only depend on such core motor regions, but also on activity in anterior/prefrontal and posterior/parietal brain regions, i.e., areas which typically show increased activity in older subjects even in simple motor tasks (Heuninckx et al., 2005, Heuninckx et al., 2008; Mattay et al., 2002). Particularly enhanced prefrontal activity has consistently been shown in older subjects during motor performance (Heuninckx et al., 2005, Heuninckx et al., 2008; Wu and Hallett, 2005). This is at first sight at odds with the frontal lobe hypothesis stating that age-related behavioural deficits are primarily due to the structural and functional deterioration of frontal parts of the ageing brain (Moscovitch and Winocur, 1992; West, 1996). Yet paradoxically, multiple neuroimaging studies have linked increased activity in anterior brain regions associated with higher-order cognitive demands to better behavioural performance in ageing individuals across multiple cognitive domains (Cabeza et al., 2002; Grady et al., 2005; Reuter-Lorenz et al., 2000). Intriguingly, this enhancement of top-down modulation seems to compensate for dysfunctional sensory-driven bottom-up processing in posterior brain regions of ageing individuals, a phenomenon termed the ‘Posterior to Anterior Shift in Ageing’ (PASA; Davis et al., 2008).
To date, it remains, however, to be elucidated how the PASA theory relates to motor network connectivity, i.e., how anterior and posterior brain regions change their influence on the core motor system. It is currently poorly understood how the balance between top-down influences from regions anterior to and bottom-up influences from regions posterior to core motor regions affects motor performance in ageing individuals. To address this question, we assessed effective connectivity in an extended cortical motor network underlying psychomotor processes in young and older subjects using functional magnetic resonance imaging (fMRI) and dynamic causal modelling (DCM; Friston et al., 2003). We used a reaction paradigm that enabled us to study the neural mechanisms of both basic motor aspects such as movement initiation as well as higher-order movement preparation, selection and visuomotor integration within the same experimental setting (Hoffstaedter et al., 2013; Michely et al., 2015). Moreover, such psychomotor processes, that are typically slowed in ageing individuals, strongly rely on the integrity of neural coupling between both top-down modulation from anterior/prefrontal and bottom-up modulation from posterior/parietal brain regions onto the core motor system (Berchicci et al., 2012; Stewart et al., 2014; Vallesi et al., 2011). We expected that ageing is associated not only with changes in interregional coupling within the core motor network, but also with differences in the influence that prefrontal and parietal areas exert onto (pre)motor regions. In line with the PASA theory, we hypothesized that age-related reduction in bottom-up modulation from posterior/parietal regions might be compensated by increasing top-down modulation from anterior/prefrontal regions onto the core motor system. Finally, in order to address this compensation theory, we tested whether age-related coupling changes related to the PASA theory are linked to behavioural parameters of psychomotor speed in ageing individuals.
2. Materials and methods
2.1. Subjects
Twenty-four healthy male subjects participated in the study after providing informed written consent (12 younger subjects, mean age 27.4 ± 4.2, range 21–35; 12 older subjects, mean age 62.1 ± 6.3, range 52–74). The underlying rationale for the inclusion of subjects with this particular age range was two-fold: First, we wanted to assess general ageing effects by comparing two distinct age groups, i.e., young and older subjects. Second, we aimed to characterize how changes in neural coupling relate to progressive structural atrophy and behavioural performance in advancing age, i.e., within our older subgroup between 52 and 74 years of age.
All participants underwent a comprehensive clinical interview to exclude a history of any neurological or psychiatric disease or other chronic disabling medical problem. According to the Edinburg handedness inventory (Oldfield, 1971), all subjects were right-handed (mean 81.0 ± 20.2). In order to exclude cognitive deficits in older participants, subjects were additionally tested by the means of a comprehensive cognitive test battery, assessing executive functions, working memory, attention, and visuospatial functions, i.e., the Parkinson Neuropsychometric Dementia Assessment (Kalbe et al., 2008). Importantly, all subjects scored well above the cut-off score for cognitive impairment, hence, there was no indication of cognitive impairment in our older participants (mean score 25.6 ± 3.7, range 20–30, cut-off score < 18). FMRI data of the older subjects was previously used as healthy control data in a study on Parkinson's disease (Michely et al., 2015). However, all analyses, models and results in the present study are new, hence, there is no overlap with previously presented results. The study was in accordance with the Declaration of Helsinki and approved by the local ethics committee.
2.2. FMRI paradigm
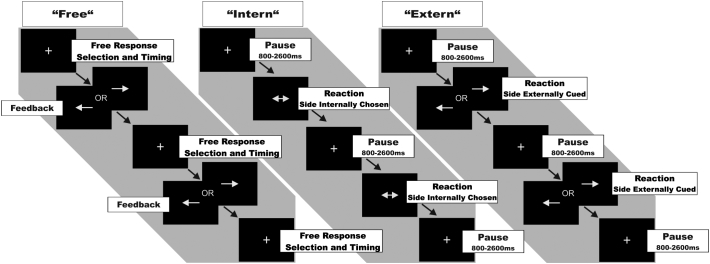
The experimental paradigm (Fig. 1) was equivalent to our previous studies on motor control in healthy subjects (Hoffstaedter et al., 2013), patients suffering from Parkinson's disease (Michely et al., 2012, Michely et al., 2015) and major depression (Hoffstaedter et al., 2012). The task comprised three conditions and an imbedded functional localizer. Subjects responded via button presses on a MRI compatible response device using the right or left index finger. Visual stimuli were generated using the ‘Presentation’ software package (Version 10.3, Neurobehavioral Systems Inc., Albany, CA). Each condition was presented in blocks of 20 s duration separated by resting baselines of 16 s during which subjects watched a blank screen. Each block was introduced by a one-word instruction presented for 2.5 s, informing the subject about which of the four conditions followed next.
Fig. 1.
FMRI paradigm.
Each block of trials started with the presentation of a fixation cross. ‘Free’- condition: Upon appearance of the fixation cross, subjects were instructed to press the left or right button with the respective index finger at any self-chosen time. Every response was followed by a visual feedback pointing to the side of the button-press. Thereafter, the fixation cross re-appeared until the next response was given by the subject. Thus, subjects were free in terms of both movement lateralization and timing. ‘Intern’- condition: Subjects were instructed to react as fast as possible and press the left or right button upon appearance of a double-headed arrow pointing to both sides. Hence, subjects were restricted with re. to the timing of movement initiation but free in terms of movement lateralization. The fixation cross re-appeared for the time between stimuli. ‘Extern’- condition: Subjects were instructed to react as fast as possible and press the left button upon appearance of an arrow pointing to the left or the right button upon appearance of an arrow pointing to the right. Thus, subjects were restricted with re. to both timing and movement lateralization.
2.2.1. Condition ‘Free’: self-timed movement selection
In the ‘Free’- condition, subjects were instructed to press either the left or right button at any self-chosen time. Hence, subjects were free in terms of both movement lateralization and timing. Every response was followed by an immediate visual feedback consisting of an arrow pointing to the side of the button-press (duration: 400 ms; Fig. 1). By providing a feedback arrow, we kept this condition comparable to the reactive ones in terms of visual input and display delays. Moreover, during feedback, no further response was allowed to prevent repetitive finger tapping. Since subjects were not allowed to press any button whilst the feedback arrow was presented, response times in the ‘Free’-condition reflect the interval between the end of the presentation of the feedback arrow and the next self-initiated button press. Subjects were instructed to roughly balance between left and right button presses, and to avoid extensive periods of rest between button presses.
2.2.2. Condition ‘Intern’: reaction to a non-informative cue
Subjects were asked to respond to a double-headed arrow, i.e., non-informative cue (displayed for 400 ms; Fig. 1) with a button press of either their left or right index finger. Since subjects were prompted to press the right or left button as fast as possible, they were restricted with regard to the timing of movement execution, but free in terms of movement lateralization. Twelve to 14 stimuli were presented per block with varying stimulus onset asynchrony (ranging from 800 to 2600 ms), thereby minimizing anticipation of the cue. As in the ‘Free’- condition, subjects were instructed to roughly balance between left- and right-sided responses.
2.2.3. Condition ‘Extern’: reaction to an informative cue
In the ‘Extern’-condition, subjects were instructed to respond as fast as possible to a single-headed arrow (displayed for 400 ms; Fig. 1), pointing either to the left or right side. Hence, movements were purely reactive, and thus restricted with regard to both timing and lateralization. As in the ‘Intern’- condition, 12–14 cues with varying stimulus onset asynchrony were presented per block.
2.2.4. Condition ‘Tapping’: repetitive finger tapping (functional localizer)
In the ‘Tapping’- condition, subjects were asked to perform vertical tapping movements at maximum speed using the right or left index finger. A white arrow presented in the centre of a black screen pointed to the left or right and thereby indicated which finger to use. This cue was presented throughout the entire tapping period. As in earlier studies (Michely et al., 2015; Wang et al., 2011), we used short finger tapping periods of 3 s followed by a 2.5 s break instead of continuous tapping throughout the entire 20 s block in order to prevent fatigue. In each block, four tapping periods had to be performed with fingers balanced, i.e., two right, two left.
The ‘Tapping’- condition served as functional localizer to identify ‘core’ (pre)motor areas for the connectivity analyses at the single subject level. The other three conditions probed different aspects of higher motor control such as movement preparation, selection and initiation. In contrast to the ‘Free’-condition where subjects were not reacting to any external cue, conditions ‘Extern’ and ‘Intern’ constituted externally and internally triggered choice reaction time (RT) tasks (Jahanshahi et al., 1992). Prior to scanning, subjects were trained outside and inside the scanner to warrant stable task performance. A single fMRI run lasted 21 min including 8 repetitions of each condition. The four conditions were presented consecutively in blocks, within these blocks the order was pseudorandomized yet equal for all subjects to account for ordering effects and to maintain comparability.
2.3. Statistical analysis of behavioural data
In the RT conditions, i.e., ‘Intern’ and ‘Extern’, we first eliminated outliers which were unlikely to represent physiologically interpretable reactions to the visual stimuli: RTs longer than 1000 ms and shorter than 150 ms were regarded as random or anticipatory responses. Furthermore, for each subject, all RTs exceeding the individual mean RT by more than three standard deviations were excluded from further analysis. Together, these steps removed 1.5% ± 0.7 in young and 1.4% ± 1.0 of the data in older participants with no between-group differences (p = 0.728). Moreover, we defined error responses when subjects pressed more than one, the wrong, or no button. The percentage error rate, i.e., the ratio between error responses and presented stimuli was calculated as a measure of task accuracy. Subsequently, we computed the mean individual RT for all subjects for the ‘Extern’ and ‘Intern’ condition as a measure of psychomotor speed. Independent two-sample t-tests were used to compare performance differences between young and older subjects regarding error rates and psychomotor speed.
2.4. FMRI image acquisition and preprocessing
Functional MR images were acquired using a Siemens Trio 3 T scanner (Siemens Medical Solutions) We employed a gradient echo planar imaging (EPI) sequence with the following blood oxygenation level-dependent (BOLD) imaging parameters: repetition time (TR) = 2200 ms, echo time (TE) = 30 ms, field of view (FOV) = 200 mm, 33 axial slices, slice thickness = 3.1 mm, voxel size = 3.1 mm isotropic, flip angle = 90°, distance factor = 20%. The slices covered the whole brain from the vertex to lower parts of the cerebellum. Each fMRI time series consisted of 574 images preceded by four dummy images allowing tissue magnetization to reach a steady state. In addition, high-resolution T1-weighted structural images were acquired (MPRAGE-sequence, TR = 2250 ms, TE = 3.93 ms, FOV = 256 mm, 176 sagittal slices, voxel size = 1.0 mm3, flip angle = 9°). FMRI data were analyzed using Statistical Parametric Mapping (SPM8; Wellcome Trust Centre for Neuroimaging, London, UK, http://www.fil.ion.ucl.ac.uk). After discarding the dummy images, the EPI volumes were realigned to the mean image of each time series. The structural T1-weighted image was co-registered to the mean EPI image. Spatial normalization of all images into the space of the Montreal Neurological Institute (MNI) was achieved via the unified segmentation approach using the individual mean EPI image (Ashburner and Friston, 2005). After spatial normalization, the voxel size was resampled to 1.5 mm3. Finally, data were smoothed using an isotropic Gaussian kernel of 8-mm full-width at half-maximum to suppress noise and effects due to residual interindividual differences in functional and gyral anatomy.
2.5. FMRI statistical analysis
Statistical analysis was performed within the framework of the general linear model. The four experimental conditions and the instructions were separately modelled using boxcar stimulus functions convolved with a canonical hemodynamic response function. The time series in each voxel were high-pass filtered at 1/128 Hz. The six head motion parameters, as assessed by the realignment algorithm, were treated as covariates to remove movement-related variance from the image time series. We computed a full factorial ANOVA second level analysis. Main effects for each condition were computed by contrasting task-related activity (‘Free’/‘Intern’/‘Extern’; ‘Tapping’ as functional localizer) with the resting baselines for each subject. Moreover, we compared contrasts for all three higher motor control conditions between young and older subjects.
2.6. Dynamic causal modelling
Deterministic, bilinear DCM as implemented in SPM8 models changes in neuronal states over time as
where x is the state vector, A represents the endogenous (intrinsic) connectivity, B(j) represents the modulatory influence of the experimental manipulation u(j) onto the endogenous connections among the network nodes, and C denotes the influence of direct inputs to the system. Deterministic DCM requires the definition of an external driving input that modulates activity of a given area (DCM-C matrix) and propagates within the entire system. In the DCM formula, the driving input is represented by ‘u’ (which is either 0 at baseline or 1 for the presence of a given condition). Note that due to the block design of the present study, the input function u is not locked to single events but covers visual cues, motor responses and also the cognitive state induced by the instruction of a current condition during the blocks. It is important to note that the definition of the DCM-A coupling values have changed across different DCM versions. As used here in DCM within SPM8, endogenous connectivity (DCM-A) is always present during the experiment and reflects the context-independent (i.e., constant) component of interregional coupling across the entire experimental setting. Hence, it is not equivalent to the resting-conditions only but also considers coupling values that were consistent during the movement conditions (see also Rehme et al., 2013). The context-dependent modulations are represented in DCM-B and reflect changes in interregional coupling evoked by a particular higher motor control condition assessing psychomotor speed (‘Free’/‘Intern’/‘Extern’). The tapping condition was not included into the DCM analysis as this condition served as independent functional localizer for (pre)motor regions. The DCM-C matrix represents the direct experimental input to the system that drives regional activity. Note that DCM models also accounted for temporal differences in image acquisition, i.e., slice-timing.
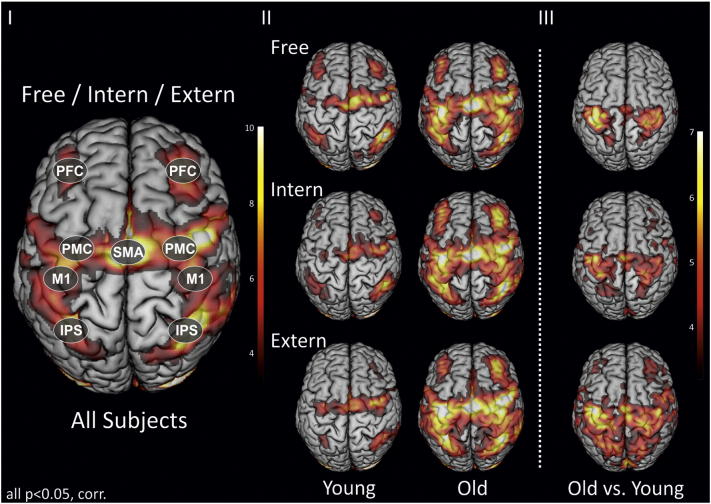
For our connectivity models, regions were selected based on different criteria. Besides generating a model that is biologically plausible, we selected brain regions that were significantly activated by all tasks whilst also considering between-group differences in in brain activation (cf. Fig. 2). Moreover, the selected regions are known to be crucially involved in movement selection and initiation as well as visuomotor transformation processes. Note that the number of ROIs for DCM is limited to prevent dramatic increase of the number of free parameters requiring more stringent shrinkage priors to ensure system stability, and hence result in a reduction of the conditional precision for any of the estimated parameters. We tried to overcome this issue by focusing our analysis on an extended cortical motor system in accordance with the network suggested by the GLM group analysis yielding strongest activity at the cortical level. Dorsal premotor cortex (PMC), supplementary motor area (SMA) and primary motor cortex (M1) feature core regions of the motor system and were hence included in the connectivity models (Boudrias et al., 2012; Grefkes et al., 2008). Furthermore, the intraparietal sulcus (IPS) as part of the dorsal visual stream is an important region for the integration of visuospatial information into motor plans, i.e., ‘bottom-up’ processes (Cieslik et al., 2011; Grefkes et al., 2004; Rushworth et al., 2003) and was therefore included into the models. Moreover, a prefrontal region was included in the connectivity matrix. Specifically the prefrontal ROI represents the dorsolateral prefrontal cortex, given its crucial role in executive control over motor output and movement preparation, i.e., in ‘top-down’ processes (Nishitani and Hari, 2000; Rowe et al., 2010) and the strong activation of the dorsolateral prefrontal cortex in our task (see Fig. 2). For simplification, we use the abbreviation PFC for this region throughout the manuscript. We extracted the first eigenvariate of the effects-of-interest adjusted time series for all nodes using 4-mm radius spheres centred on the subject-specific individual activation maxima (p < 0.05) in the respective region based on functional and anatomical criteria. The group maximum MNI coordinate was set as origin to search for the closest local maximum in the individual SPM maps. The mean number of voxels per ROI was 78.9 ± 6.8 across all subjects. The anatomical landmarks used for region identification and coordinates of all ROIs are provided in the Supplementary data.
Fig. 2.
BOLD activation pattern and between-group activity differences.
(I) Conjunction analysis of the neural networks activated by all three higher motor control conditions (‘Free’/‘Intern’/‘Extern’) across all subjects, i.e., n = 24. ROIs used for DCM analysis are highlighted. PFC = prefrontal cortex, PMC = premotor cortex, SMA = supplementary motor area, M1 = primary motor cortex, IPS = intraparietal sulcus. (II) Activity for young (n = 12) and old (n = 12) subjects for each condition separately. (III) Between-group activity differences. Significantly enhanced BOLD activity in old as compared to young individuals for each condition separately. All p < 0.05, family-wise error (FWE) corrected at the cluster level.
The model space used for DCM constitutes a set of network hypotheses that are considered plausible explanations for the observed regional responses. For each model, we assumed a network based on known anatomical connectivity among the ROIs as derived from invasive tract-tracing studies in primates. Firstly, we constructed two different sets (families) of models. For our first set of models (Family 1), we constructed an endogenous connectivity matrix (DCM-A) between IPS/PFC and premotor regions (Bates and Goldman-Rakic, 1993; Cavada and Goldman-Rakic, 1989; Lu et al., 1994; Miyachi et al., 2005; Tanji and Hoshi, 2008) as well as premotor regions (PMC/SMA) and M1 (Rouiller et al., 1994). Moreover, we assumed interhemispheric transcallosal connections between homologous regions (Leichnetz, 1986; Marconi et al., 2003; McGuire et al., 1991; Padberg et al., 2005; Rouiller et al., 1994). The second set (Family 2) was similarly constructed, yet with the difference that we omitted endogenous connectivity between PFC and premotor regions, but instead assumed connectivity between PFC and IPS. Hence, in this family, only IPS was assumed to directly modulate activity in premotor regions, whilst activity in PFC merely modulated premotor regions indirectly via IPS. Note, however, that although we constructed our network on anatomical plausible connections as informed by primate studies, connectivity parameters in DCM do to necessarily reflect monosynaptic anatomical connections but rather the net effect a region exerts on activity of other regions. This can theoretically be transmitted via direct anatomical connections, a single relay region or more complex network loops. Moreover, condition-specific modulations of interregional coupling may not necessarily affect all intrinsic anatomical connections. We, therefore, constructed several alternative models (similar for both families) with varying complexity representing plausible hypotheses on interregional coupling (Supplementary data). First, we omitted interhemispheric coupling between homologous areas for both families (A–E). Moreover, for family 1, we removed modulatory effects between PFC and premotor areas (F), and modulatory effects between PFC and IPS (F), for family 2 respectively. Moreover, for both families, we removed modulatory effects between IPS and premotor areas (G). As alternative, we excluded modulatory effects of premotor areas onto M1 (H). Finally, modulatory effects of task conditions were allowed to modulate all intrinsic connections (I) for both families. Next, we used random-effects Bayesian model selection first to compare model evidence between the two families, and then to determine the model providing the best trade-off between accuracy and generalizability/complexity (Penny et al., 2004; Stephan et al., 2009). Following earlier DCM studies, we assumed that activity across conditions was driven and propagated to other regions by the PFC and IPS due to their roles in ‘top-down’ and ‘bottom-up’ control over motor output and core motor network activity (DCM-C; Cieslik et al., 2011; Grefkes et al., 2010; Rowe et al., 2010; Wang et al., 2010).
2.7. Statistical analysis of connectivity data
To test for general effects of ageing on neural coupling, i.e., differences between young and older subjects, coupling estimates of all connections were compared using independent two-sample t-tests, separately for endogenous connections (DCM-A) and condition-specific coupling for the three task conditions (DCM-B for ‘Free’/‘Intern’/‘Extern’). Due to the significant age gape between groups and the missing “middle-aged” subjects in our sample, we did not compute linear correlation with age across the entire sample. However, to investigate the effects of advancing age on neural coupling and to further differentiate whether putative group differences were further increasing or diminishing with older age, we additionally computed Pearson's correlations between age and coupling strength for all connections in the older group only. The false discovery rate (FDR, Benjamini and Hochberg, 1995) approach was used to correct for multiple comparisons, both for group comparisons and correlation analyses.
2.8. Confound removal: structural atrophy
As also healthy ageing is associated with regional grey matter atrophy, which in turn may contribute to changes in effective connectivity, we performed additional analyses of age-related structural changes using voxel-based morphometry (VBM). The structural analyses were conducted using the VBM8 toolbox (dbm.neuro.uni-jena.de/vbm.html) within SPM8 with standard settings for bias-field correction, segmentation of grey matter, white matter and cortico-spinal fluid, partial volume effect adjustment and spatial normalization into MNI-space within a unified segmentation model (Ashburner and Friston, 2005). The segmented images were non-linearly modulated for normalization to the group mean template. The resulting voxel-wise amount of expansion or contraction was used to estimate grey matter volume for all ROIs as identified from the functional analysis in all subjects. Thereby, regionally specified grey matter volume was corrected for individual brain size as it represents the non-linear modulation of the grey matter of each individual brain in relation to the group template. The structural parameters obtained with VBM were subsequently used to control for the influence of atrophy on age-related functional changes as observed in our connectivity analysis. Hence, for connections displaying significant differences between young and older subjects, and for coupling parameters showing significant correlations with advancing age, connectivity analyses were repeated including individual grey matter parameters of the particular two ROIs for a connection as covariates of no interest.
2.9. Regional BOLD activity
The main focus of this study was to assess age-related network effects. However, in addition to our whole-brain BOLD analysis, we also assessed regional differences in brain activity for the 9 regions used for the DCM analysis using the MarsBar toolbox (Brett et al., 2002). Similar to our connectivity analysis, we used grey matter parameters to control for the effect of structural atrophy.
3. Results
3.1. Behavioural data
There were strong between-group differences with respect to RT in both internally and externally cued responses, with a significant slowing of psychomotor speed in older compared to young subjects (‘Extern’: young 309.1 ms ± 21.2, old 353.9 ms ± 36.3, p = 0.001; ‘Intern’: young 243.7 ms ± 17.5, old 281.2 ± 37.7, p = 0.005). In contrast, performance accuracy as assessed by error rates was only marginally different between groups in the reaction conditions (‘Extern’: young 6.4% ± 3.2, old 9.8% ± 6.2, p = 0.109; ‘Intern’: young 1.8% ± 1.4, old 3.8% ± 3.2, p = 0.059). Notably, there was no correlation between psychomotor speed (RTs) and accuracy (error rates) in older participants (‘Extern’: r = −0.381, p = 0.222; ‘Intern’: r = −0.279, p = 0.381). Moreover, there was no correlation with scores from the cognitive test battery and RTs in the older group (Extern: r = 0.134, p = 0.678; Extern: r = 0.044, p = 0.892). In the ‘Free’ condition, subjects on average pressed a button 15.4 times per block, i.e., participants performed a slightly higher number of executed movements as in the reaction conditions in which they were forced by the cues to perform on average 13 button presses per block. Importantly, the two groups showed a comparable timing with re. to initiating a button press in the ‘Free’ condition (young 858.3 ms ± 262.0, old 956.3 ms ± 269.2, p = 0.376). Hence, there was no significant between-group difference for the number of self-initiated motor responses. Moreover, there was no significant between-group difference for the distribution of right- and left-handed responses in the conditions with self-chosen response lateralization, i.e., ‘Free’ and ‘Intern’ (proportion of right-handed responses out of all responses: ‘Free’: young 0.514 ± 0.025, old 0.523 ± 0.046, p = 0.545; ‘Intern’: young 0.520 ± 0.045, old 0.510 ± 0.065, p = 0.644).
3.2. Structural atrophy
Older subjects displayed a significant reduction of total grey matter volume, adjusted for individual intracranial volume, compared to young subjects (young 50.8% ± 1.3, old 46.5% ± 1.8, p < 0.001). Additionally, within the group of older subjects, i.e., between 52 and 74 years in our sample, grey matter volume displayed a significant negative correlation with advancing age (r = −0.79, p = 0.002). Hence, as expected, grey matter volume was significantly reduced in older as compared to young subjects.
3.3. BOLD activation pattern
Fig. 2 depicts the neural activation pattern evoked by the three motor control conditions. All regions included in the connectivity model were significantly activated by all three conditions of interest across the entire sample of subjects. Note that differences in brain activity at the subcortical level between young and older individuals were most pronounced in the thalamus, especially for the ‘Extern’ condition, which strongly relies on sensory input. In contrast, between-group differences were considerably weaker -if not absent- in the basal ganglia (Supplementary data). At the cortical level, older participants displayed widespread enhancement of activity (Fig. 2). Here, the BOLD analysis confirmed findings from earlier studies showing that older subjects feature enhanced activity not only in core motor regions but also in parietal and prefrontal cortex. As expected from the results of the whole-brain analyses, there were strong increases in BOLD activity for our regions of interest in older subjects for all three conditions (p < 0.05, corrected, Supplementary data). Notably, differences in regional BOLD activity for our regions of interest persisted when correcting for grey matter atrophy in the respective regions. Moreover, there was a positive correlation with advancing age for right prefrontal cortex activation in the ‘Extern’ condition (p < 0.05, corrected; r = 0.777). However this prefrontal overactivation did not correlate with behavioural performance in older participants. Therefore, the key question of the present study was to investigate whether and to what degree changes in activity found within this extended cortical motor network can be explained by changes in network connectivity using DCM.
3.4. Bayesian model selection
Firstly, the random-effects Bayesian model selection revealed that the set of models involving connections from both PFC and IPS onto premotor regions (Family 1) clearly outperformed the set of models involving connections between PFC and IPS but only assuming IPS to modulate premotor regions (Family 2). Moreover, the model selection showed that out of all models tested model ‘I’ was the most likely one given the data. This was true when testing across all 24 subjects as well as when testing for each group separately, i.e., young and older subjects (Supplementary data). The winning model assumed modulatory effects from both PFC and IPS onto premotor regions as well as interhemispheric connectivity between homologous areas.
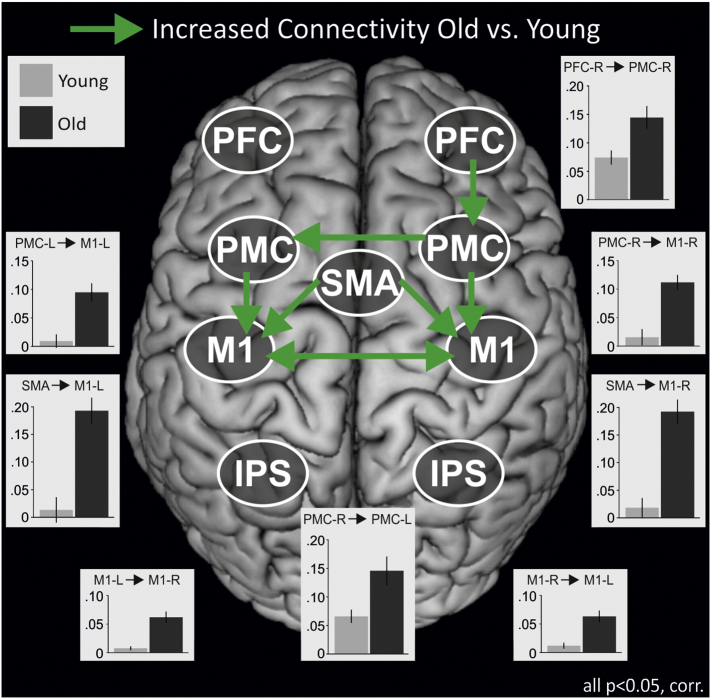
3.5. Endogenous connectivity (DCM-A)
We first analyzed interregional coupling that was constant across all three tasks of interest, i.e., ‘Intern’, ‘Extern’ and ‘Free’. Here, older subjects showed significantly stronger coupling for several connections compared to young subjects (all p < 0.05, corrected, Fig. 3). Excitatory influences from PMC and SMA targeting M1 were significantly enhanced in the older group in both hemispheres. Moreover, we found increases in interhemispheric coupling between both homologous PMC and M1. Thus, especially coupling between core motor regions was significantly enhanced in the older group. In addition, there was a stronger excitatory influence exerted by right-hemispheric PFC upon PMC in older subjects. When using the individual GM parameters of the particular ROIs as covariates to control for putative effects of regional atrophy on effective connectivity, all previously reported differences between groups remained significant.
Fig. 3.
Between-group connectivity differences.
Green arrows indicate significantly enhanced endogenous connectivity (DCM-A) between two regions in old as compared to young individuals. Note that all differences between groups remained significant when controlling for structural atrophy as informed by the VBM analysis. PFC = prefrontal cortex, PMC = premotor cortex, SMA = supplementary motor area, M1 = primary motor cortex, IPS = intraparietal sulcus. R = right-hemispheric, L = left-hemispheric. p < 0.05, FDR-corrected for multiple comparisons. Bars represent coupling strength in 1/s. Error bars: SEM.
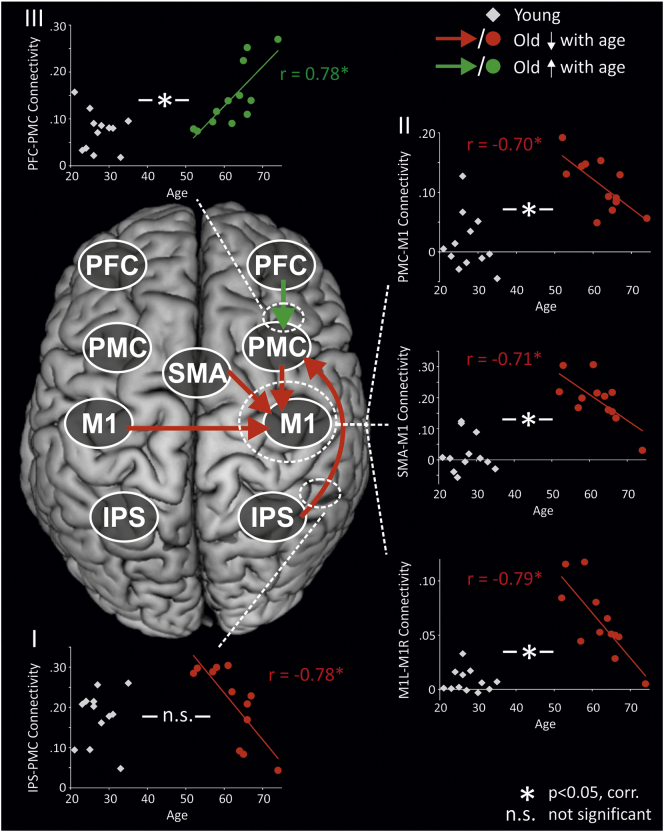
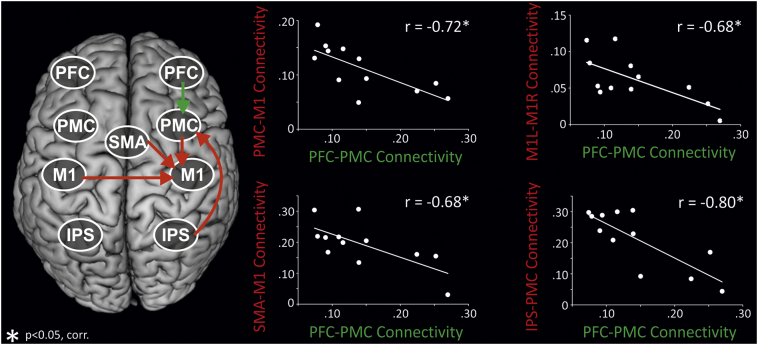
We next tested whether coupling strengths correlated with higher age in the group of older subjects. That is, whether connectivity in the extended motor network of interest further changes with advancing age, i.e., between 52 and 74 in our subjects sample. Interestingly, only right-hemispheric connections (PFC-PMC, IPS-PMC, SMA-M1, PMC-M1) and interhemispheric connectivity between homologous regions from the left targeting the right hemisphere (M1, PFC) showed a relationship with advancing age (all p < 0.05, corrected). Except for interhemispheric PFC coupling, correlations with age persisted when correcting for grey matter atrophy in the respective regions (p < 0.05, range of r = 0.66–0.89). Hence, age-related changes in connectivity occurred independent of the degree of structural atrophy. Accordingly, we identified three patterns of differential connectivity changes (Fig. 4): First, parietal-premotor connectivity showed no group difference, yet a negative correlation with age in older subjects. Second, connectivity targeting M1 was increased in older subjects at the group level, but featured a negative correlation with advancing age in the group of older subjects. Hence, group difference seemed to be driven by younger subjects within the older group. As this connectivity pattern was compatible with an inverted U-shaped association with age, we specifically tested this relationship. Indeed, for all three tested connections, there was a significant, negative quadratic association between coupling parameters and age across the entire subject sample (PMC-M1: r = −0.68, p = 0.002; SMA-M1: r = −0.79, p ≤0.001; interhemispheric M1: r = −0.77, p < 0.001). Third, prefrontal-premotor coupling showed an increase in older as compared to young subjects at the group level, and in addition featured a positive correlation with advancing age in the group of older subjects. Hence, in contrast to coupling targeting M1 the group difference was driven by the older subjects within the older group.
Fig. 4.
Network changes with advancing age.
Correlations between advancing age and coupling parameters (DCM-A) in old individuals. Coupling parameters of young subjects are indicated by grey diamonds and shown for illustrative purposes to underline between-group differences for the respective connections. Coupling parameters for older subjects are indicated by red circles for connections displaying a negative correlation with age, and by green circles for connections showing a positive correlation with age. Three different patterns of differential connectivity changes emerged: (I) IPS-PMC: no group difference between young and old subjects, negative correlation with age in old subjects. (II) Coupling targeting M1: enhanced connectivity in older individuals at the group level, negative correlation with age in old subjects. (III) PFC-PMC: enhanced connectivity in older individuals at the group level, positive correlation with age in older subjects. Note that all correlations shown remained significant when controlling for structural atrophy as informed by the VBM analysis. PFC = prefrontal cortex, PMC = premotor cortex, SMA = supplementary motor area, M1 = primary motor cortex, IPS = intraparietal sulcus. *p < 0.05, FDR-corrected for multiple comparisons; n.s. = not significant. Coupling strength in 1/s.
3.6. Association between prefrontal coupling and other connections
In line with the PASA theory, we hypothesized that anterior/prefrontal connectivity increases alongside age-related reduction of posterior/parietal connectivity and core motor connectivity. To specifically address this hypothesis, we tested whether the aforementioned age-related increase in PFC-PMC coupling correlated with a decrease in the other connections displaying a relationship with advancing age in older subjects (as informed by the results shown in Fig. 4). Indeed, consistent with our hypothesis, we found a significant negative correlation between prefrontal-premotor connectivity and parietal-premotor and premotor-M1 coupling (all p < 0.05, corrected, Fig. 5). Hence, older subjects featuring the lowest parietal-premotor-M1 coupling showed the strongest increase in prefrontal-premotor connectivity. Thus, prefrontal influences on the motor system increase as parietal influences and coupling within the core motor system decreases with advancing age.
Fig. 5.
Association between increased prefrontal coupling and decreased coupling in other parts of the network.
Negative correlations between individual PFC-PMC coupling and coupling parameters of other connections displaying a relationship with advancing age in older individuals (cf. Fig. 5). Subjects featuring weaker parietal-premotor-M1 coupling with advancing age show the strongest increase in prefrontal-premotor connectivity. PFC = prefrontal cortex, PMC = premotor cortex, SMA = supplementary motor area, M1 = primary motor cortex, IPS = intraparietal sulcus. *p < 0.05, FDR-corrected for multiple comparisons. Coupling strength in 1/s.
3.7. Association between prefrontal coupling and performance
In the next step, we tested whether age-related connectivity changes as informed by the previous analyses (cf. Fig. 4) were related to behavioural performance, i.e., psychomotor speed. However, there was no correlation with individual RT for the five connections tested, also when correcting for grey matter atrophy. Hence, there was no one-to-one mapping between RTs and single coupling parameters.
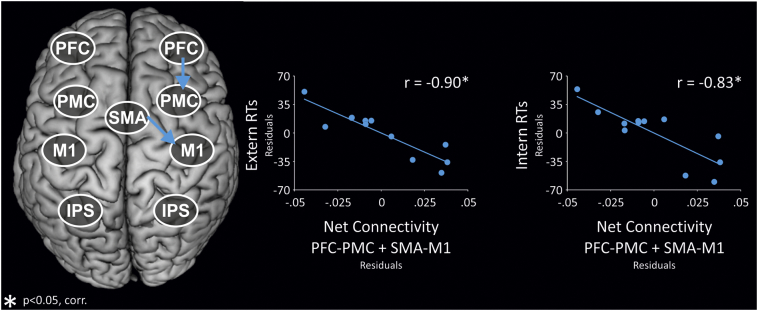
However, in line with the PASA theory, we expected prefrontal coupling to be positively correlated with good performance. Moreover, as there were differential changes with advancing age, i.e., both increases and decreases of connectivity, we tested for a net effect of different connections that showed the aforementioned changes with advancing age. Interestingly, we found a significant negative correlation between RTs in both the ‘Intern’ and ‘Extern’ condition and the sum of PFC-PMC and SMA-M1 coupling (p < 0.05, corrected for multiple comparisons and grey matter atrophy, Fig. 6). Hence, older subjects with stronger positive coupling within the prefrontal-premotor-M1 axis, i.e., strong increase of PFC-PMC coupling accompanied by less pronounced decrease of SMA-M1 coupling, displayed faster RT, i.e., better behavioural performance. Other combinations of connectivity did not correlate with behavioural measures. Importantly, there was no such relationship between connectivity and performance accuracy as indexed by error rates.
Fig. 6.
Association between increased prefrontal-premotor-M1 coupling and behavioural performance.
When controlling for grey matter atrophy as informed by the VBM analysis, increased net coupling within the prefrontal-premotor-M1 axis (PFC-PMC + SMA-M1 connectivity) negatively correlates with RTs in both the ‘Intern’ and ‘Extern’ condition. Hence, stronger positive coupling is associated with faster RT, i.e., better behavioural performance. Note that in both panels, data points of two subjects are very close, giving rise to the impression that the plots only contain 11 data points. However, in conformity with previous figures, all 12 data points are displayed in both panels. PFC = prefrontal cortex, PMC = premotor cortex, SMA = supplementary motor area, M1 = primary motor cortex, IPS = intraparietal sulcus. *p < 0.05, FDR-corrected for multiple comparisons.
3.8. Condition-specific connectivity (DCM-B)
We next tested whether condition-specific connectivity (‘Intern’, ‘Extern’, ‘Free’) showed differential effects between young and older subjects. In the ‘Free’ condition, older subjects displayed enhanced coupling between PMC and M1 in both hemispheres, as well as stronger interhemispheric connectivity from left M1 targeting right M1 (all p < 0.05, corrected). No such effects were found for the ‘Extern’ and ‘Intern’ condition. In contrast to changes in endogenous connectivity, there were no significant correlations with advancing age in the older group. Moreover, there were no significant correlations between coupling parameters and behavioural measures.
4. Discussion
We assessed age-related effects on grey matter volume as well as local brain activity and motor network connectivity underlying psychomotor processes. Behaviourally, older subjects showed significant psychomotor slowing. However, despite pronounced structural atrophy, indicated by both between-group differences as well as correlations with advancing age, older participants displayed increases in both regional activity and effective connectivity within an extended cortical motor network. Notably, ageing most prominently affected endogenous connectivity, yet not condition-specific connectivity. Importantly, endogenous connectivity is not equivalent to the experimental baseline activity as it is estimated from the whole time-series. Indeed, we have recently shown that resting-state functional connectivity fMRI parameters correlated only weakly with activity-dependent connectivity (both functional connectivity and effective connectivity as computed in DCM-A; Rehme et al., 2013). The endogenous connectivity is, however, specific for the setting of an fMRI experiment and is likely to reflect task-specific components (Friston et al., 2003). Hence, these results are indicative of global changes in the functional architecture of the ageing motor network. The global nature of network changes was also reflected on the behavioural level: we observed behavioural slowing to the same extent in both the ‘Extern’ and ‘Intern’ condition in older participants. This conformity between behavioural and neural findings supports the idea that ageing might result in a global change in the functional network architecture underlying psychomotor performance, irrespective of older subjects being internally or externally cued to select and initiate movement.
Interestingly, we found hints for differential connectivity changes at different stages of the ageing process. Specifically, younger participants within the older group showed highest coupling values for core motor connectivity targeting M1, which steadily decreased with advancing age. In contrast, prefrontal-premotor coupling increased with advancing age. Notably, age-related increases of prefrontal influences on the motor system occurred irrespective of age-related grey matter atrophy and were inversely correlated with parietal influences and core motor coupling. Although these findings rely on a relatively small sample only, they are perfectly in line with the PASA theory, supporting the validity of our findings. Interestingly, higher connectivity within the prefrontal-premotor-M1 axis correlated with faster psychomotor speed, implying that older participants with stronger neural coupling were faster to select and initiate movements.
4.1. Core motor connectivity in older participants
In line with previous studies, we found regional BOLD activity to be enhanced in older subjects, especially for core (pre)motor areas (Heuninckx et al., 2008; Mattay et al., 2002; Rowe et al., 2006; Ward et al., 2008). Notably, older subjects also displayed stronger interhemispheric connectivity between homologous PMC and M1 compared to young subjects. Moreover, connectivity from premotor regions such as SMA and PMC targeting M1 was elevated in the older group. Similar DCM effects were reported by Boudrias et al., 2012 who found that older subjects display stronger facilitatory coupling onto M1 from premotor areas and homologous contralateral M1. In a PET study, Rowe et al., 2006 also showed that older individuals feature enhanced coupling between PMC and M1 as compared to younger adults during motor performance. Although subjects in the present study were confronted with more complex motor demands such as visuomotor transformation, movement selection and speeded movement initiation, the similarity of findings between studies suggests enhanced coupling between core motor regions to represent a general property of the ageing motor system. One interesting finding of the present study is that despite a general effect of ageing as depicted as an increase of connectivity targeting M1 at the group level, connectivity again decreased in the oldest participants of our study. This pattern resembles an inverted U-shaped relationship with age. Interestingly, comparable effects have already been shown for brain activity underlying memory performance in older subjects, increasing from healthy ageing to mild cognitive impairment, yet decreasing in the transition to manifest Alzheimer's disease (Dickerson et al., 2005; Wierenga and Bondi, 2007). Therefore, it seems reasonable to assume that similar effects might occur in the motor system, i.e., connectivity increases with incipient age-related degeneration, but eventually decreases again when compensatory resources are exhausted within specific parts of the network (Cabeza and Dennis, 2012; Scheller et al., 2014). However, this issue needs to be addressed specifically in future studies including a wider age range.
4.2. PASA in motor network connectivity
In contrast to the decrease in connectivity targeting M1 with advancing age, prefrontal-premotor connectivity was not only enhanced in older individuals at the group level, but also steadily increased with higher age. Enhanced recruitment of prefrontal cortex in older adults has frequently been shown across a variety of fMRI studies assessing attentional processes, working memory or executive functions (Cabeza et al., 2004; Gunning-Dixon and Raz, 2003; Madden et al., 1997). Likewise, Heuninckx et al., 2008 found PFC overactivation during a complex interlimb coordination task to positively correlate with better motor performance in older subjects. Moreover, Berchicci et al., 2012 found that older individuals engage more PFC activity during response preparation in a visuomotor discrimination task, enabling them to reach comparable accuracy as young subjects, yet with slower response speed. These results might reflect increased cognitive control and performance monitoring during movement execution (Seidler-Dobrin and Stelmach, 1998).
Interestingly, in the present study, increases in prefrontal connectivity occurred irrespective of the degree of grey matter atrophy. Indeed, PFC has frequently been shown to display the greatest evidence for age-related atrophy (Driscoll et al., 2009; Raz et al., 2005), yet paradoxically, PFC constitutes the part of the brain where evidence for functional compensation is most consistently and most prominently observed across neuroimaging studies (Reuter-Lorenz and Cappell, 2008; Cabeza and Dennis, 2012). Strikingly, we here found that prefrontal-premotor coupling was inversely correlated with parietal-premotor and premotor-M1 coupling in older individuals. Hence, in line with the PASA theory, top-down control from PFC seemingly increases in response to a functional impairment of posterior brain regions involved in sensory bottom-up processing (Davis et al., 2008; Madden et al., 2014).
Moreover, in contrast to prefrontal-premotor-M1 coupling, posterior/parietal influences on premotor cortex were not enhanced in older participants at the group level, rather these influences even decreased with higher age. Thus, there seems to be no increase in parietal-premotor connectivity at any point of the ageing process, at least for the motor tasks tested in the present study. Since parietal-premotor information processing is crucial for visuomotor transformation and movement planning (Grefkes et al., 2010; Rushworth et al., 2003), the age-related decrease of parietal-premotor coupling might provide a neural mechanism for the reduction of perceptual motor speed in older individuals. Interestingly, we found strongest correlations with advancing age for right-hemispheric connections. In line with that, other studies have described that behavioural tasks, which more strongly rely on right-hemispheric processing are more susceptible to age-related deterioration (Gerhardstein et al., 1998; Lamb and Robertson, 1988), supporting the notion of a right-hemispheric ageing model (Dolcos et al., 2002; Hellige, 1993). Notably, solely right-handed subjects participated in our study. Therefore, another possible explanation for the pronounced right-hemispheric effects might be that advancing age potentially impacts more strongly on the functional network architecture of the non-dominant motor hemisphere. Previous neuroimaging studies assessing age-related motor control have shown a loss of lateralized brain activity in ageing (Mattay et al., 2002; Ward and Frackowiak, 2003), in line with the HAROLD model of reduced hemispheric asymmetry in ageing during non-motor tasks (Cabeza, 2002). Note that, however, due to the block design of our experiment, we were not able to distinguish between left- and right-handed responses on a trial by trial basis. Thus, the hemispheric specificity of our findings could be investigated in future studies that aim at thoroughly dissecting effects for both hands separately, and optimally include both right- and left-handedness subjects (Pool et al., 2014).
4.3. Different stages of age-related compensation?
As outlined above, increased prefrontal coupling in older subjects was inversely correlated with decreased coupling between other network nodes. These correlations might indicate that influences exerted by the PFC upon the motor system steadily increase in order to compensate for reduced functioning in other parts of the motor network (Cabeza and Dennis, 2012; Davis et al., 2008).
However, whether or not brain activity and connectivity can be considered compensatory, implying a causal effect is difficult to establish on the basis of functional and structural imaging data alone. One attractive way of interpreting the data is that the observed coupling changes may be interpreted in terms of functional compensation occurring at different stages of the ageing process. In our study, group comparisons indicated that both prefrontal-premotor and premotor-M1 coupling was significantly enhanced in older subjects. However, additional analyses were indicative of differential effects at different stages of the ageing process, i.e., enhanced coupling within parts of the network increasing during a ‘first stage’ (premotor-M1) and a ‘second stage’ (prefrontal-premotor) of the ageing process. Hence, data suggest that core motor coupling initially ramps up, then with further ageing this functional mechanism breaks down and seems to be replaced by increasing prefrontal influences that make up for reduced functioning of the core motor network.
This is particularly interesting as enhanced activity of brain regions, and also increased connectivity between brain regions is often interpreted as functional compensation in older individuals (Reuter-Lorenz and Cappell, 2008; Grady, 2012). For example, age-related ‘hyperactivity’ and ‘hyperconnectivity’ of the PFC has often been associated with better performance in older subjects for both motor and higher cognitive functions (Davis et al., 2008; Heuninckx et al., 2008; Rossi et al., 2004). Moreover, also enhanced premotor-M1 coupling has recently been associated with preserved motor performance in older subjects (Stewart et al., 2014). Our data complement these findings by revealing that increasing prefrontal influences on the motor system are primarily associated with advancing age. However, only older individuals with stronger coupling within the prefrontal-premotor-M1 axis featured faster psychomotor speed. This was indicated by the correlation between higher net coupling within the prefrontal-premotor-M1 axis (PFC-PMC and SMA-M1) and faster movement initiation in older subjects. Thus, our findings support the view of a compensatory role for the PFC in motor behaviour (Berchicci et al., 2012; Heuninckx et al., 2008). However, only older subjects in which this compensatory mechanism is flanked by preserved integrity of core motor coupling display faster psychomotor speed at the behavioural level.
4.4. Limitations
The most important limitation of the study is the small sample size. Notably, the sample size is particularly small for the correlations with advancing age in the older group only, which certainly limits the aforementioned interpretation with regard to compensatory mechanisms. However, the use of appropriate correction methods and the strong effects for endogenous connectivity for both group differences and correlations with advancing age underline the robustness of our findings and the fact that the sample size is large enough to detect meaningful age-related connectivity changes. Moreover, the between-group differences are very well in line with previous studies on motor network activity and connectivity in ageing (Heitger et al., 2013; Heuninckx et al., 2005, Heuninckx et al., 2008; Mattay et al., 2002; Rowe et al., 2006). However, although the connectivity pattern for coupling targeting M1 was reminiscent of an inverted U-shaped relationship with age in our study, we cannot exactly determine the peak of this trend due to the restricted age range. Although it is tempting to speculate about such multiple age-related stages of compensation, it is important to note that we used a cross-sectional design in this study. Due to the cross-sectional study of our design, we were unfortunately also not able to assess how increases in connectivity in older subjects evolve over time, for which more age-related longitudinal brain imaging studies are needed in the future (Nyberg et al., 2010). Thus, such hypotheses derived from our data need to be confirmed in future ageing studies by applying longitudinal designs, assessing a wider age range, and most importantly larger samples. Moreover, although we found a relationship between neural coupling and psychomotor performance in ageing individuals, we were unfortunately not in a position to systematically assess effects of task difficulty on neural coupling within our experimental paradigm. This limitation makes it difficult to establish a causal link between brain connectivity and compensation in ageing individuals, and underlines the importance for future studies to disentangle the complex interactions between ageing and task demand/performance on pattern of brain activity and connectivity (Ankudowich et al., 2017; Cabeza and Dennis, 2012). Note that although we used grey matter parameters to control for age-related atrophy, there might have been other microstructural changes contributing to the neural findings. Here, especially assessing the relationship between white matter degeneration and age-related changes in task-based connectivity constitutes an interesting avenue for future research (Salami et al., 2014; Stewart et al., 2014). Notably, we only assessed male participants which diminishes the generalisability of our findings to the entire population.
5. Conclusion
The results of our study are compatible with the idea that, as opposed to a uniform functional impairment, age-related changes within the motor network occur with anatomical specificity and at different stages of the ageing process. One novel finding is that prefrontal influences on the motor system seem to emerge to compensate for reduced connectivity in other parts of the network, yet only the combination of this phenomenon with preserved core motor coupling is associated with better motor performance in ageing individuals. Finally, our results provide plausible candidate regions within the prefrontal-premotor-M1 axis to be targeted by means of non-invasive brain stimulation in order to further elucidate their compensatory role for motor behaviour as described for PFC activity in memory function (Manenti et al., 2011; Rossi et al., 2004).
Acknowledgments
J.M., G.R.F. and C.G. are supported by the German Research Foundation (Clinical Research Group KFO219 ‘Basal-Ganglia-Cortex-Loops: Mechanisms of Pathological Interactions and Therapeutic Modulation’; GR 3285/5–1). J.M. is additionally supported by a fellowship from the German Research Foundation (MI 2158/1-1). G.R.F. and C.G. receive additional funding from the University of Cologne Emerging Groups Initiative (CONNECT group) implemented into the Institutional Strategy of the University of Cologne and the German Excellence Initiative. G.R.F. gratefully acknowledges additional support from the Marga and Walter Boll Stiftung. S.B.E. acknowledges funding by the Helmholtz Initiative on Systems-Biology ‘The Human Brain Model’ and the NIH (R01-MH074457).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2018.02.001.
Appendix A. Supplementary data
Supplementary material
References
- Ankudowich E., Pasvanis S., Rajah M.N. Changes in the correlation between spatial and temporal source memory performance and BOLD activity across the adult lifespan. Cortex. 2017;91:234–249. doi: 10.1016/j.cortex.2017.01.006. [DOI] [PubMed] [Google Scholar]
- Ashburner J., Friston K.J. Unified segmentation. NeuroImage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Bates J.F., Goldman-Rakic P.S. Prefrontal connections of medial motor areas in the rhesus monkey. J. Comp. Neurol. 1993;336:211–228. doi: 10.1002/cne.903360205. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995;57:289–300. [Google Scholar]
- Berchicci M., Lucci G., Pesce C., Spinelli D., Di Russo F. Prefrontal hyperactivity in older people during motor planning. NeuroImage. 2012;62:1750–1760. doi: 10.1016/j.neuroimage.2012.06.031. [DOI] [PubMed] [Google Scholar]
- Boudrias M.H., Goncalves C.S., Penny W.D., Park C.H., Rossiter H.E., Talelli P., Ward N.S. Age-related changes in causal interactions between cortical motor regions during hand grip. NeuroImage. 2012;59:3398–3405. doi: 10.1016/j.neuroimage.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M., Anton J.L., Valabregue R., Poline J.B. Presented at the 8th International Conference on Functional Mapping of the Human Brain. 2002. Region of interest analysis using an SPM toolbox [abstract] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol. Aging. 2002;17(1):85–100. doi: 10.1037//0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- Cabeza R., Dennis N.A. Principles of Frontal Lobe Function. Oxford University Press; New York: 2012. Frontal lobes and aging deterioriation and compensation. [Google Scholar]
- Cabeza R., Anderson N.D., Locantore J.K., McIntosh A.R. Aging gracefully: compensatory brain activity in high-performing older adults. NeuroImage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cabeza R., Daselaar S.M., Dolcos F., Prince S.E., Budde M., Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb. Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Cavada C., Goldman-Rakic P.S. Posterior parietal cortex in rhesus monkey: I. Parcellation of areas based on distinctive limbic and sensory corticocortical connections. J. Comp. Neurol. 1989;287:393–421. doi: 10.1002/cne.902870402. [DOI] [PubMed] [Google Scholar]
- Cieslik E.C., Zilles K., Grefkes C., Eickhoff S.B. Dynamic interactions in the fronto-parietal network during a manual stimulus-response compatibility task. NeuroImage. 2011;58:860–869. doi: 10.1016/j.neuroimage.2011.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S.W., Dennis N.A., Daselaar S.M., Fleck M.S., Cabeza R. Que PASA? The posterior-anterior shift in aging. Cereb. Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson B.C., Salat D.H., Greve D.N., Chua E.F., Rand-Giovannetti E., Rentz D.M., Bertram L., Mullin K., Tanzi R.E., Blacker D., Albert M.S., Sperling R.A. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F., Rice H.J., Cabeza R. Hemispheric asymmetry and aging: right hemisphere decline or asymmetry reduction. Neurosci. Biobehav. Rev. 2002;26:819–825. doi: 10.1016/s0149-7634(02)00068-4. [DOI] [PubMed] [Google Scholar]
- Draganski B., Lutti A., Kherif F. Impact of brain aging and neurodegeneration on cognition: evidence from MRI. Curr. Opin. Neurol. 2013;26:640–645. doi: 10.1097/WCO.0000000000000029. [DOI] [PubMed] [Google Scholar]
- Driscoll I., Davatzikos C., An Y., Wu X., Shen D., Kraut M., Resnick S.M. Longitudinal pattern of regional brain volume change differentiates normal aging from MCI. Neurology. 2009;72:1906–1913. doi: 10.1212/WNL.0b013e3181a82634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Harrison L., Penny W. Dynamic causal modelling. NeuroImage. 2003;19:1273–1302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Gerhardstein P., Peterson M.A., Rapcsak S.Z. Age-related hemispheric asymmetry in object discrimination. J. Clin. Exp. Neuropsychol. 1998;20:174–185. doi: 10.1076/jcen.20.2.174.1162. [DOI] [PubMed] [Google Scholar]
- Grady C. The cognitive neuroscience of ageing. Nat. Rev. Neurosci. 2012;13:491–505. doi: 10.1038/nrn3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady C.L., McIntosh A.R., Craik F.I. Task-related activity in prefrontal cortex and its relation to recognition memory performance in young and old adults. Neuropsychologia. 2005;43:1466–1481. doi: 10.1016/j.neuropsychologia.2004.12.016. [DOI] [PubMed] [Google Scholar]
- Grefkes C., Ritzl A., Zilles K., Fink G.R. Human medial intraparietal cortex subserves visuomotor coordinate transformation. NeuroImage. 2004;23:1494–1506. doi: 10.1016/j.neuroimage.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Grefkes C., Nowak D.A., Eickhoff S.B., Dafotakis M., Kust J., Karbe H., Fink G.R. Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann. Neurol. 2008;63:236–246. doi: 10.1002/ana.21228. [DOI] [PubMed] [Google Scholar]
- Grefkes C., Wang L.E., Eickhoff S.B., Fink G.R. Noradrenergic modulation of cortical networks engaged in visuomotor processing. Cereb. Cortex. 2010;20:783–797. doi: 10.1093/cercor/bhp144. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon F.M., Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults: a prospective MRI study. Neuropsychologia. 2003;41:1929–1941. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Heitger M.H., Goble D.J., Dhollander T., Dupont P., Caeyenberghs K., Leemans A., Sunaert S., Swinnen S.P. Bimanual motor coordination in older adults is associated with increased functional brain connectivity—a graph-theoretical analysis. PLoS One. 2013;8 doi: 10.1371/journal.pone.0062133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellige J.B. Harvard University Press; Cambridge, MA: 1993. Hemispheric Asymmetry: What's Right and What's Left. [Google Scholar]
- Heuninckx S., Wenderoth N., Debaere F., Peeters R., Swinnen S.P. Neural basis of aging: the penetration of cognition into action control. J. Neurosci. 2005;25:6787–6796. doi: 10.1523/JNEUROSCI.1263-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuninckx S., Wenderoth N., Swinnen S.P. Systems neuroplasticity in the aging brain: recruiting additional neural resources for successful motor performance in elderly persons. J. Neurosci. 2008;28:91–99. doi: 10.1523/JNEUROSCI.3300-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstaedter F., Sarlon J., Grefkes C., Eickhoff S.B. Internally vs. externally triggered movements in patients with major depression. Behav. Brain Res. 2012;228:125–132. doi: 10.1016/j.bbr.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffstaedter F., Grefkes C., Zilles K., Eickhoff S.B. The “what” and “when” of self-initiated movements. Cereb. Cortex. 2013;23:520–530. doi: 10.1093/cercor/bhr391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi M., Brown R.G., Marsden C.D. Simple and choice reaction time and the use of advance information for motor preparation in Parkinson's disease. Brain. 1992;115(Pt 2):539–564. doi: 10.1093/brain/115.2.539. [DOI] [PubMed] [Google Scholar]
- Kalbe E., Calabrese P., Kohn N., Hilker R., Riedel O., Wittchen H.U., Dodel R., Otto J., Ebersbach G., Kessler J. Screening for cognitive deficits in Parkinson's disease with the Parkinson neuropsychometric dementia assessment (PANDA) instrument. Parkinsonism Relat. Disord. 2008;14:93–101. doi: 10.1016/j.parkreldis.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Lamb M.R., Robertson L.C. The processing of hierarchical stimuli: effects of retinal locus, locational uncertainty, and stimulus identity. Percept. Psychophys. 1988;44:172–181. doi: 10.3758/bf03208710. [DOI] [PubMed] [Google Scholar]
- Langan J., Peltier S.J., Bo J., Fling B.W., Welsh R.C., Seidler R.D. Functional implications of age differences in motor system connectivity. Front. Syst. Neurosci. 2010;4:17. doi: 10.3389/fnsys.2010.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leichnetz G.R. Afferent and efferent connections of the dorsolateral precentral gyrus (area 4, hand/arm region) in the macaque monkey, with comparisons to area 8. J. Comp. Neurol. 1986;254:460–492. doi: 10.1002/cne.902540403. [DOI] [PubMed] [Google Scholar]
- Li S.C., Lindenberger U. Cross-level unification: a computational exploration of the link between deterioration of neurotransmitter systems and the dedifferentiation of cognitive abilities in old age. In: Nilsson L.G., Markowitsch H.J., editors. Cognitive Neuroscience of Memory. Hogrefe & Huber; 1999. [Google Scholar]
- Logan J.M., Sanders A.L., Snyder A.Z., Morris J.C., Buckner R.L. Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron. 2002;33:827–840. doi: 10.1016/s0896-6273(02)00612-8. [DOI] [PubMed] [Google Scholar]
- Lu M.T., Preston J.B., Strick P.L. Interconnections between the prefrontal cortex and the premotor areas in the frontal lobe. J. Comp. Neurol. 1994;341:375–392. doi: 10.1002/cne.903410308. [DOI] [PubMed] [Google Scholar]
- Madden D.J., Turkington T.G., Provenzale J.M., Hawk T.C., Hoffman J.M., Coleman R.E. Selective and divided visual attention: age-related changes in regional cerebral blood flow measured by H2(15)O PET. Hum. Brain Mapp. 1997;5:389–409. doi: 10.1002/(SICI)1097-0193(1997)5:6<389::AID-HBM1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Madden D.J., Parks E.L., Davis S.W., Diaz M.T., Potter G.G., Chou Y.H., Chen N.K., Cabeza R. Age mediation of frontoparietal activation during visual feature search. NeuroImage. 2014;102(Pt 2):262–274. doi: 10.1016/j.neuroimage.2014.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manenti R., Cotelli M., Miniussi C. Successful physiological aging and episodic memory: a brain stimulation study. Behav. Brain Res. 2011;216:153–158. doi: 10.1016/j.bbr.2010.07.027. [DOI] [PubMed] [Google Scholar]
- Marconi B., Genovesio A., Giannetti S., Molinari M., Caminiti R. Callosal connections of dorso-lateral premotor cortex. Eur. J. Neurosci. 2003;18:775–788. doi: 10.1046/j.1460-9568.2003.02807.x. [DOI] [PubMed] [Google Scholar]
- Mattay V.S., Fera F., Tessitore A., Hariri A.R., Das S., Callicott J.H., Weinberger D.R. Neurophysiological correlates of age-related changes in human motor function. Neurology. 2002;58:630–635. doi: 10.1212/wnl.58.4.630. [DOI] [PubMed] [Google Scholar]
- McGuire P.K., Bates J.F., Goldman-Rakic P.S. Interhemispheric integration: I. Symmetry and convergence of the corticocortical connections of the left and the right principal sulcus (PS) and the left and the right supplementary motor area (SMA) in the rhesus monkey. Cereb. Cortex. 1991;1:390–407. doi: 10.1093/cercor/1.5.390. [DOI] [PubMed] [Google Scholar]
- Michely J., Barbe M.T., Hoffstaedter F., Timmermann L., Eickhoff S.B., Fink G.R., Grefkes C. Differential effects of dopaminergic medication on basic motor performance and executive functions in Parkinson's disease. Neuropsychologia. 2012;50:2506–2514. doi: 10.1016/j.neuropsychologia.2012.06.023. [DOI] [PubMed] [Google Scholar]
- Michely J., Volz L.J., Barbe M.T., Hoffstaedter F., Viswanathan S., Timmermann L., Eickhoff S.B., Fink G.R., Grefkes C. Dopaminergic modulation of motor network dynamics in Parkinson's disease. Brain. 2015;138:664–678. doi: 10.1093/brain/awu381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyachi S., Lu X., Inoue S., Iwasaki T., Koike S., Nambu A., Takada M. Organization of multisynaptic inputs from prefrontal cortex to primary motor cortex as revealed by retrograde transneuronal transport of rabies virus. J. Neurosci. 2005;25:2547–2556. doi: 10.1523/JNEUROSCI.4186-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M., Winocur G. The neuropsychology of memory and aging. In: Craik F.I.M., Salthouse T.A., editors. The Handbook of Aging and Cognition. Erlbaum; Hillsdale, NJ: 1992. [Google Scholar]
- Naccarato M., Calautti C., Jones P.S., Day D.J., Carpenter T.A., Baron J.C. Does healthy aging affect the hemispheric activation balance during paced index-to-thumb opposition task? An fMRI study. NeuroImage. 2006;32:1250–1256. doi: 10.1016/j.neuroimage.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Nishitani N., Hari R. Temporal dynamics of cortical representation for action. Proc. Natl. Acad. Sci. U. S. A. 2000;97:913–918. doi: 10.1073/pnas.97.2.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L., Salami A., Andersson M., Eriksson J., Kalpouzos G., Kauppi K., Lind J., Pudas S., Persson J., Nilsson L.G. Longitudinal evidence for diminished frontal cortex function in aging. Proc. Natl. Acad. Sci. U. S. A. 2010;107(52):22682–22686. doi: 10.1073/pnas.1012651108. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Padberg J., Disbrow E., Krubitzer L. The organization and connections of anterior and posterior parietal cortex in titi monkeys: do new world monkeys have an area 2? Cereb. Cortex. 2005;15:1938–1963. doi: 10.1093/cercor/bhi071. [DOI] [PubMed] [Google Scholar]
- Penny W.D., Stephan K.E., Mechelli A., Friston K.J. Comparing dynamic causal models. Neuroimage. 2004;22(3):1157–1172. doi: 10.1016/j.neuroimage.2004.03.026. Jul. [DOI] [PubMed] [Google Scholar]
- Pool E.M., Rehme A.K., Fink G.R., Eickhoff S.B., Grefkes C. Handedness and effective connectivity of the motor system. NeuroImage. 2014;99:451–460. doi: 10.1016/j.neuroimage.2014.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N., Lindenberger U., Rodrigue K.M., Kennedy K.M., Head D., Williamson A., Dahle C., Gerstorf D., Acker J.D. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb. Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Rehme A.K., Eickhoff S.B., Grefkes C. State-dependent differences between functional and effective connectivity of the human cortical motor system. NeuroImage. 2013;67:237–246. doi: 10.1016/j.neuroimage.2012.11.027. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz P.A., Cappell K.A. Neucognitive aging and the compensation hypothesis. Curr. Dir. Psychol. Sci. 2008;17:177–182. [Google Scholar]
- Reuter-Lorenz P.A., Jonides J., Smith E.E., Hartley A., Miller A., Marshuetz C., Koeppe R.A. Age differences in the frontal lateralization of verbal and spatial working memory revealed by PET. J. Cogn. Neurosci. 2000;12:174–187. doi: 10.1162/089892900561814. [DOI] [PubMed] [Google Scholar]
- Riecker A., Groschel K., Ackermann H., Steinbrink C., Witte O., Kastrup A. Functional significance of age-related differences in motor activation patterns. NeuroImage. 2006;32:1345–1354. doi: 10.1016/j.neuroimage.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Rossi S., Miniussi C., Pasqualetti P., Babiloni C., Rossini P.M., Cappa S.F. Age-related functional changes of prefrontal cortex in long-term memory: a repetitive transcranial magnetic stimulation study. J. Neurosci. 2004;24:7939–7944. doi: 10.1523/JNEUROSCI.0703-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouiller E.M., Babalian A., Kazennikov O., Moret V., Yu X.H., Wiesendanger M. Transcallosal connections of the distal forelimb representations of the primary and supplementary motor cortical areas in macaque monkeys. Exp. Brain Res. 1994;102:227–243. doi: 10.1007/BF00227511. [DOI] [PubMed] [Google Scholar]
- Rowe J., Stephan K.E., Friston K., Frackowiak R., Lees A., Passingham R. Attention to action in Parkinson's disease: impaired effective connectivity among frontal cortical regions. Brain. 2002;125:276–289. doi: 10.1093/brain/awf036. [DOI] [PubMed] [Google Scholar]
- Rowe J.B., Siebner H., Filipovic S.R., Cordivari C., Gerschlager W., Rothwell J., Frackowiak R. Aging is associated with contrasting changes in local and distant cortical connectivity in the human motor system. NeuroImage. 2006;32:747–760. doi: 10.1016/j.neuroimage.2006.03.061. [DOI] [PubMed] [Google Scholar]
- Rowe J.B., Hughes L.E., Barker R.A., Owen A.M. Dynamic causal modelling of effective connectivity from fMRI: are results reproducible and sensitive to Parkinson's disease and its treatment? NeuroImage. 2010;52:1015–1026. doi: 10.1016/j.neuroimage.2009.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth M.F., Johansen-Berg H., Gobel S.M., Devlin J.T. The left parietal and premotor cortices: motor attention and selection. NeuroImage. 2003;20(Suppl. 1):S89–100. doi: 10.1016/j.neuroimage.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Sala-Llonch R., Bartres-Faz D., Junque C. Reorganization of brain networks in aging: a review of functional connectivity studies. Front. Psychol. 2015;6:663. doi: 10.3389/fpsyg.2015.00663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salami A., Pudas S., Nyberg L. Elevated hippocampal resting-state connectivity underlies deficient neurocognitive function in aging. Proc. Natl. Acad. Sci. U. S. A. 2014;111(49):17654–17659. doi: 10.1073/pnas.1410233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse T.A. Aging and measures of processing speed. Biol. Psychol. 2000;54:35–54. doi: 10.1016/s0301-0511(00)00052-1. [DOI] [PubMed] [Google Scholar]
- Scheller E., Minkova L., Leitner M., Kloppel S. Attempted and successful compensation in preclinical and early manifest neurodegeneration - a review of task FMRI studies. Front. Psychiatry. 2014;5:132. doi: 10.3389/fpsyt.2014.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler R.D., Bernard J.A., Burutolu T.B., Fling B.W., Gordon M.T., Gwin J.T., Kwak Y., Lipps D.B. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci. Biobehav. Rev. 2010;34:721–733. doi: 10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler R., Erdeniz B., Koppelmans V., Hirsiger S., Merillat S., Jancke L. Associations between age, motor function, and resting state sensorimotor network connectivity in healthy older adults. NeuroImage. 2015;108:47–59. doi: 10.1016/j.neuroimage.2014.12.023. [DOI] [PubMed] [Google Scholar]
- Seidler-Dobrin R.D., Stelmach G.E. Persistence in visual feedback control by the elderly. Exp. Brain Res. 1998;119:467–474. doi: 10.1007/s002210050362. [DOI] [PubMed] [Google Scholar]
- Solesio-Jofre E., Serbruyns L., Woolley D.G., Mantini D., Beets I.A., Swinnen S.P. Aging effects on the resting state motor network and interlimb coordination. Hum. Brain Mapp. 2014;35:3945–3961. doi: 10.1002/hbm.22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan K.E., Penny W.D., Daunizeau J., Moran R.J., Friston K.J. Bayesian model selection for group studies. Neuroimage. 2009;46(4):1004–1017. doi: 10.1016/j.neuroimage.2009.03.025. Jul 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart J.C., Tran X., Cramer S.C. Age-related variability in performance of a motor action selection task is related to differences in brain function and structure among older adults. NeuroImage. 2014;86:326–334. doi: 10.1016/j.neuroimage.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji J., Hoshi E. Role of the lateral prefrontal cortex in executive behavioral control. Physiol. Rev. 2008;88:37–57. doi: 10.1152/physrev.00014.2007. [DOI] [PubMed] [Google Scholar]
- Vallesi A., McIntosh A.R., Stuss D.T. Overrecruitment in the aging brain as a function of task demands: evidence for a compensatory view. J. Cogn. Neurosci. 2011;23:801–815. doi: 10.1162/jocn.2010.21490. [DOI] [PubMed] [Google Scholar]
- Wang L., Liu X., Guise K.G., Knight R.T., Ghajar J., Fan J. Effective connectivity of the fronto-parietal network during attentional control. J. Cogn. Neurosci. 2010;22:543–553. doi: 10.1162/jocn.2009.21210. [DOI] [PubMed] [Google Scholar]
- Wang L.E., Fink G.R., Diekhoff S., Rehme A.K., Eickhoff S.B., Grefkes C. Noradrenergic enhancement improves motor network connectivity in stroke patients. Ann. Neurol. 2011;69:375–388. doi: 10.1002/ana.22237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward N.S., Frackowiak R.S. Age-related changes in the neural correlates of motor performance. Brain. 2003;126(Pt 4):873–888. doi: 10.1093/brain/awg071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward N.S., Swayne O.B., Newton J.M. Age-dependent changes in the neural correlates of force modulation: an fMRI study. Neurobiol. Aging. 2008;29:1434–1446. doi: 10.1016/j.neurobiolaging.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West R.L. An application of prefrontal cortex function theory to cognitive aging. Psychol. Bull. 1996;120:272–292. doi: 10.1037/0033-2909.120.2.272. [DOI] [PubMed] [Google Scholar]
- Wierenga C.E., Bondi M.W. Use of functional magnetic resonance imaging in the early identification of Alzheimer's disease. Neuropsychol. Rev. 2007;17:127–143. doi: 10.1007/s11065-007-9025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T., Hallett M. A functional MRI study of automatic movements in patients with Parkinson's disease. Brain. 2005;128:2250–2259. doi: 10.1093/brain/awh569. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material