Abstract
Human epidermal growth factor receptor 2 (HER2) is overexpressed in approximately 20% to 30% of breast cancers and various other types of cancers, which plays a vital role in the cancer progression. Monoclonal antibodies targeting Her2 are now used in the clinic to treat Her2 overexpression cancer patients. However, relapse or resistance is frequent with the current therapies. To generate a new treatment avenue against Her2, we immunized and selected a specific anti-Her2 single domain antibody C3 for further studies. The C3-Fc antibody drove antibody-dependent cell-mediated cytotoxicity against Her2-positive tumor cells in vitro and resulted in potent antitumor growth in vivo. These data suggest that the C3-Fc antibody may provide an alternative avenue for Her2-positive cancer therapy.
Introduction
HER2 is a member of the human epidermal growth factor receptor (HER/EGFR/ERBB) family and is also known as CD340 (cluster of differentiation 340), proto-oncogene Neu, or ERBB2 (human) [1]. The overexpression of Her2 leads to cell proliferation and tumorigenesis and occurs in approximately 20% to 30% of breast cancers [2]. Her2 is also overexpressed in approximately 7% to 34% of patients with gastric cancer [3], [4] and in 30% of salivary duct carcinomas [5] and various other human cancers, such as ovarian, adenocarcinoma of the lung, and aggressive forms of uterine cancer [6]. Her2-positive tumors are generally correlated with a faster growth rate and a poorer prognosis. Several therapeutic methods were developed to block Her2 activity to suppress tumor growth, including monoclonal antibodies (mAbs) [7] such as trastuzumab. However, although these therapeutics show significant clinical benefits, their efficacy remains variable and modest, for example, with no benefit against Her2-positive head and neck cancer [8]. Thus, it is necessary to develop new therapeutic avenues to improve the current Her2-targeting therapy.
One avenue to increase the therapeutic effect of the Her2 antibody is to employ a smaller antibody format to increase tumor penetration. A variety of mAb fragments, such as Fab, scFv, and nanobody, can be employed. However, Fabs and scFvs have a low production yield, poor stability and solubility, and a short half-life in vivo [9]. Nanobodies are also known as a single-domain antibody (sdAb) or VHH fragments. VHHs are the smallest fully functional antigen-binding fragments at approximately 15 kDa [10]. Their smaller size may enhance tissue penetration and epitope access sterically. VHHs also exhibit high thermal and conformational stability [11] and solubility and are resistant to acids and alkalines [12]. These excellent properties make VHHs valuable and attractive tools for various applications.
In this study, we generated anti-Her2 VHHs after immunizing a llama and employed a phage display screen. One of the anti-Her2 VHHs, the C3 clone, exhibited a high affinity to Her2. C3 was then fused with a human IgG1 Fc to generate the C3-Fc antibody. In vitro and in vivo analyses were then performed to characterize the C3-Fc antibody. The in vitro and in vivo analyses suggested that the C3-Fc antibody might provide a better therapeutic efficacy than trastuzumab.
Material and Methods
Immunized VHH Phage Display Library Construction and Screening
To generate an anti-Her2 single-domain antibody, a llama was immunized with a Her2-His protein (Genscript), as described previously [13]. The immunization was monitored by checking the serum titer against Her2-His using enzyme-linked immunosorbent assay (ELISA). A high titer was achieved after four immunizations. Then, peripheral blood was extracted, and the lymphocytes were isolated by gradient centrifugation. Total RNA was extracted from the lymphocytes using Trizol reagent (Invitrogen). After reverse-transcription to generate the first-strand cDNA, the VHH fragments were amplified with specific primer sets and ligated to the pMECS phagemid vector. A VHH phage library was created by transforming the ligation products into XL1-Blue E. coli cells [14], [15], [16].
To amplify the library, 200 μl of the Her2-VHH phage library was inoculated into 40 ml of super broth medium (10 g of MOPS Sigma, 30 g of tryptone BD-Bioscience, and 20 g of yeast extract BD-Bioscience; 1 l total volume with ddH2O) containing 100 μg/ml ampicillin and 10 μg/ml tetracycline at 37°C and 220 rpm/min until OD600 = 0.6-0.8. Then, approximately 4×1011 colony-forming units (cfu) of helper phage VCSM13 was added and incubated at 37°C without agitation for 15 minutes. Then, at 37°C, the culture was incubated at 220 rpm/min for 1.5 to 2 hours. After the incubation, the bacteriophages were collected by centrifuging at 4000 rpm/ min for 10 minutes and then resuspended in 40 ml of the fresh super broth medium with 100 μg/ml ampicillin, 10 μg/ml tetracycline, and 50 μg/ml kanamycin and incubated at 30°C overnight. The bacterial cells were discarded by centrifugation at 4000 rpm/min for 10 minutes at 4°C or room temperature. The phages were precipitated from the supernatant with 5× PEG/NaCl (20% PEG/2.5 M NaCl) and resuspended in 1 ml of PBS and then precipitated again to remove the bacterial cells. The phages were then resuspended in 100 to 200 μl of PBS+1% BSA [16], [17], [18].
To select the Her2-specific VHH binder, conventional plate panning was used [19], [20]. Briefly, the desired human Her2 antigen was prebound onto a 96-well microplate. Then, the phage library, approximately 1011 cfu phages, was incubated in the coated plates for 15 minutes at 37°C on a shaker. The weakly bound phages or excess of nonbinding phages was rinsed away with 0.1% PBST. The specific binder phages were eluted with a Glycine-BSA buffer (pH 2.2) and immediately neutralized with 2 M Tris buffer (pH 9.0). The resulting phage collection was named the output. The eluted phage binders were used to infect competent E. coli XL1-blue (OD600 = 0.6) and amplified, which was named the input and used for further panning rounds. The panning was performed for three cycles, with more stringent conditions in each cycle to enrich the positive binders. After phage panning, 48 clones were picked, expanded in a 96-well deep block, and rescued by the addition of the VCSM13 helper phage. A phage ELISA was performed with the phage-containing medium supernatant to further determine the positive clones. The positive phage clones were then precipitated by a PEG/NaCl solution and resuspended in PBS. The phage concentration was determined by measuring the OD at A280 [21].
ELISA
The ELISA was performed by immobilizing 2 μg/ml of the immunogen onto the Maxi-sorp microplate (Nunc, Thermo Scientific) in 0.1 M NaHCO3 (pH 8.9) buffer and then blocking with a PBS/0.2% BSA solution. After washing four times with PBS containing 0.05% Tween-20 (pH 7.2), the plate was then probed with the corresponding phages or antibodies and developed with the tetramethylbenzidine substrate (Cell Signaling Technology). The absorbance of the wells was measured on a plate reader (Tecan). For the phage ELISA, an anti–M13-HRP mAb (GE Healthcare Life Science, 1:5000 v/v) was used. For other ELISAs, a goat anti-human IgG HRP (BioLegend, 1:5000 v/v) was used [22]. The data were processed using Graphpad Prism 5.
Flow Cytometry Analysis
For the flow cytometry analysis, SKOV3 cells were grown to 80% to 90% confluence at harvesting. The cells were digested with 0.25% trypsin and collected. A total of 5×105 cells per sample were collected by centrifugation at 1000 rpm for 5 minutes and then washed with 1 ml of ice cold PBS+0.2% BSA twice. The pellet was resuspended in 200 μl of ice-cold PBS+0.2% BSA. In each tube, 1 μg of phage, as the primary antibody, was added. An anti-M13 Ms mAb (SinoBio, 11973-MM05T) was used as the secondary antibody. Additionally, goat anti-Ms IgG-AF488 (Invitrogen, A11001) was the tertiary antibody. For the other FACS analyses, a goat anti-human IgG (H+L) AF488 (Invitrogen, A11013) was used as the secondary antibody. Flow cytometry analysis was performed on an FC500 (Beckman Coulter) after washing the cells twice and filtering with a 70-μm filter.
Immunofluorescence Assay
For further analyzing the binding of the antibodies to the Her2-positive cell surface, an immunofluorescence assay was performed as described previously [23]. The CHO and SKBR3 cells were plated on the glass bottom dish (Cellvis) prior to the day of the experiments. Before fixing the cells with 4% paraformaldehyde, they were washed with PBS three times. After blocking with PBS and 1% BSA for 1 hour at room temperature, the cells were incubated with the Her2 antibodies and then with the goat anti-Hu IgG(H + L)-AF488 (Invitrogen, A11013) for 1 hour at 4°C. After washing with PBST, samples were examined using a Zeiss EC Plan-Neofluar 40×/1.30 Oil DIC M27 objective and finally analyzed by ZEN software.
Production of the VHH Protein
The selected phage clones were sequenced, fused with Fc at the c-terminal, and subcloned into the pCDNA3.1+ vector (Novagen). The constructed Her2-VHH plasmids were transfected into Human Embryonic Kidney (HEK293f) cells [24]. Proteins were then purified with Protein-A affinity chromatography. Proteins were analyzed by SDS-PAGE and Western blot for purity.
Cell Lines and Animals
The HER2-positive cancer cell lines LS174T (human colon cancer cell lines), SKBR3 (human breast cancer cells), MCF7 (human breast cancer cells), and SKOV3 (human ovary cancer cells) and the HER2-negative cancer cell line Chinese hamster ovary (CHO) were purchased from the Type Culture Collection of the Chinese Academy of Sciences, Shanghai, China. The SKBR3, MCF7, and SKOV3 lines were cultured in Dulbecco’s modified Eagle medium (Gibco, Life Technologies, China) with 10% HI fetal bovine serum (Gibco, Life Technologies, USA) and 1% penicillin/streptomycin (HyClone). The LS174T and CHO lines were cultured in RPMI-1640 medium (Gibco, Life Technologies, China) also with 10% HI fetal bovine serum and 1% penicillin/ streptomycin.
Nonobese diabetic–severe combined immunodeficiency disease (NOD/SCID) female, 4- to 5-week-old, 18- to 22-g mice were purchased from the Vital River Laboratory Animal Technology Co., Ltd. (Beijing). The animals were housed in the animal experiment center at Sun Yat-Sen University (20°C-26°C room temperature, 40%-70% relative humidity, and 12-hour light/dark rhythm). All of the animals were provided with sterile food and water and maintained under pathogen-free conditions.
Blood Cell Fractionation and Cytotoxic Assays
Peripheral blood mononuclear cells (PBMCs) were freshly prepared using a gradient centrifugation method with Ficoll-Plaque Plus (GE health) after drawing from health donors. NK cells were then isolated from the PBMCs using an EasySep Human NK cell enrichment Kit (STEMCELL Technologies, Inc., Vancouver, Canada) according to the manufacturer's instructions. Cytotoxicity assays were performed as described previously [25], [26]. Briefly, the SKOV3, MCF7, LS174T, and CHO cancer cells were plated into 96-well plates at approximately 2500 cells per well and incubated for 6 hours or overnight at 37°C in 5% CO2. A total of 25,000 NK cells per well without prior stimulation were added as effector cells. Different concentrations of anti-Her2 proteins were mixed in growth medium and added to each well. After 72 hours of incubation, the Cell Counting Kit-8 reagent (Dojindo, CK04) was applied to quantify cell viability. After 1 to 4 hours of incubation, the OD at 450 nm was measured using a TECAN microplate reader. The survival rate (%) of the target cells was calculated using the following formula: [(live target cells (sample) medium)/ (live target cells (control) medium)].
In Vivo Tumor Growth Inhibition Assay
For the in vivo studies, LS174T human colon carcinoma cells were harvested from the cell culture and washed twice and resuspended in PBS. The cell suspensions were injected subcutaneously into the right flank of NOD/SCID mice in a total volume of 200 μl per mouse containing 1×106 LS174T cells. After the cell engraftment, 1×107 human PBMCs, freshly isolated from healthy donors, and different antibodies or vehicle control (PBS) were administered intraperitoneally when the tumor size grew to 50 to 100 mm3. The animals were treated over the following 10 days every 2 days. The weight of the mice and the tumor volume were measured with calipers. The condition of the mice was observed every day. The tumor volume was measured in two perpendicular dimensions and calculated using the formula (width2×length)/2. The experiments were terminated, and the mice were euthanized when the tumor volumes reached 1000 mm3. All the results are presented as the arithmetic mean for each group.
Results
Phage Display Library Construction and Single-Domain Antibody Screening
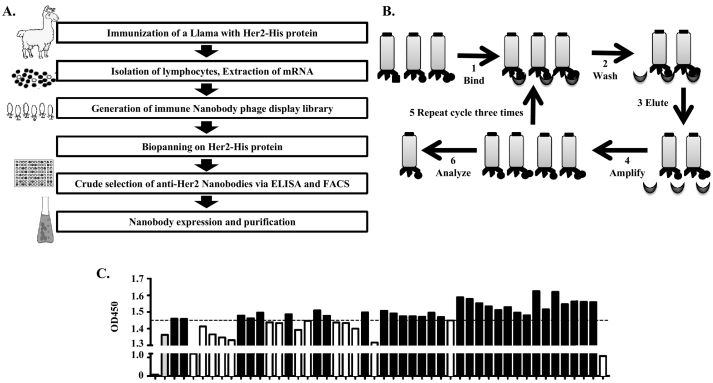
To obtain anti-Her2 single-domain antibodies, a llama was immunized with 300 μg of the His-tagged Her2 extracellular part of the protein seven times. A phage library was then constructed from the lymphocytes isolated from the immunized llama (Figure 1A). After three rounds of panning (Figure 1B), about a ten-thousand-fold enrichment was achieved (Table 1). A total of 48 clones were randomly picked after the panning, and an ELISA was performed using the phage-containing medium. Forty-seven clones showed high affinity to the Her2-His antigen. The positive phage clones were then precipitated by a PEG/NaCl solution and resuspended in PBS. The phage concentration was determined by measuring the OD at A280. These purified and quantified 47 clones were then used in another quantitative ELISA. The ELISA experiments demonstrated that 31 of these phages specifically recognized Her2 (Figure 1C).
Figure 1.
Phage display library construction and single-domain antibody screening. (A) Schematic overview of the library construction, panning of the captured specific antibodies, and validation of the positive clones. (B) For the panning procedure, the desired antigen was immobilized on the micro plate wells, and then the phage displayed library, with a pool of different antibodies, was incubated with it. The phage particles that were bound weakly and the excess nonbinding phages were rinsed away. The specific binding phages were then eluted by a glycine elution buffer. The eluted phages were then used for the infection of E. coli. The amplified phages were used for further panning rounds until a significant enrichment was achieved. Three rounds were performed to achieve the enrichment. (C) ELISA with 0.5 μg of purified and quantified phage. OD450=1.45 was used as the cutoff to select 31 clones (black bars).
Table 1.
Enrichment Data for the Her2-VHH Library
| 1st Round |
2nd Round |
3rd Round |
|
|---|---|---|---|
| 1 μg rhHER2 | 200 ng rhHER2 | 40 ng rhHER2 | |
| Input phage (cfu) | 5.00*1012 | 1.00*1011 | 1.00*1011 |
| Output phage (cfu) | 3.74*107 | 2.20*108 | 3.26*109 |
| Output/Input | 7.48*10−6 | 2.20*10−3 | 3.26*10−2 |
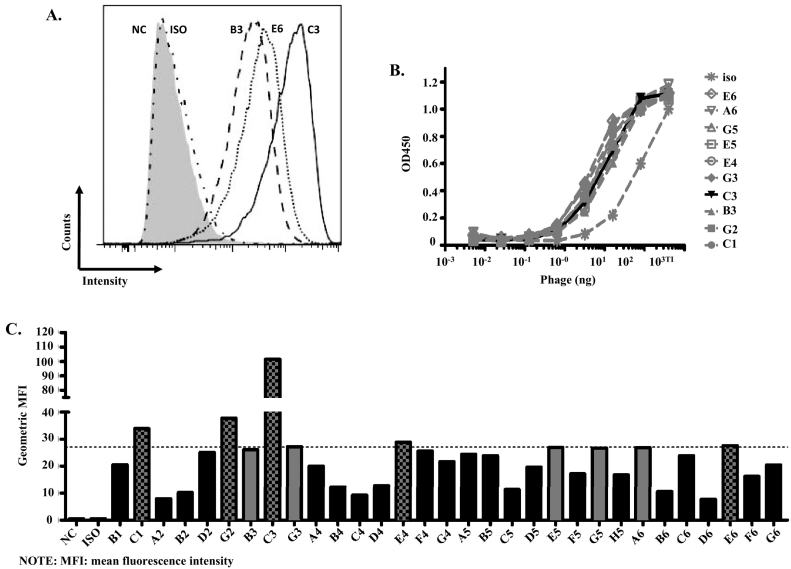
To further analyze the positive clones, a flow cytometry analysis was performed to analyze the binding of the VHH fragments to the SKOV3 cells that had Her2 overexpression [27]. A total of 10 of the 31 clones had an obvious shift (Figure 2, A and C). To rank the 31 clones, an ELISA with a serial dilution of the antigen was also performed (Figure 2B). However, all the clones showed a similar binding curve and no obvious difference between the different clones (Figure 2B). Thus, flow cytometry was performed to rank the 31 clones based on the binding intensity on the SKOV3 cells (Figure 2C). Based on the results, clones C1, G2, E4, E6, and C3 were further analyzed (Figure 2C).
Figure 2.
Single-domain antibody characterization. (A) Flow cytometry analysis of the different phage clones using SKOV3 cells. A total of 5×105 SKOV3 cells were used for per sample, and 1 μg of purified phage binders and 1 μg M13 Ms mAb were used as the primary antibodies. Goat anti-mouse IgG-AF488 used as the detection antibody. For simplicity, only clones B3, E6, and C3 are shown. NC refers to negative control, and ISO refers to isotype. (B) The relatively strong binders were selected for the ELISA with a serial dilution of the Her2 antigen. (C). Ranking of the VHHs based on the flow cytometry analysis, as described in Figure 2A; 10 of the clones (gray bars) showed an obvious shift, and 5 of them were further analyzed (gray bars with black dots).
C3 VHH Had High Affinity and Specificity to Her2
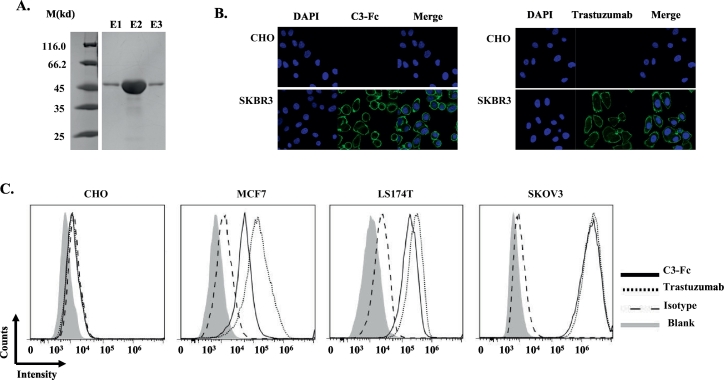
To study the function of the Her2-VHHs, the different VHH clones were fused with the human IgG1 Fc to make the VHH-Fc antibody. The VHH-Fc fusions were then cloned into the pCDNA3.1+ vector and expressed in the HEK293f cells. After purification with Protein A affinity chromatography, the VHH-Fc antibodies with molecular weights of ~45 kDa were obtained (Figure 3A).
Figure 3.
C3 VHH has a high affinity and specificity to Her2. (A) Her2-Fc was expressed and purified from HEK293f cells efficiently. Coomassie blue staining of the SDS-PAGE showed that C3-Fc was approximately 45 kDa. M indicates the molecular weight; E1, E2, and E3 refer to elution 1, 2, and 3 from the protein A affinity chromatograph. (B) Immunofluorescence analysis of Her2 binding. CHO and SKBR3 cells were fixed and stained with DAPI (nucleus staining), C3-Fc (left) or trastuzumab (right) were the primary antibodies, and then, Alex488-conjugated goat anti-human IgG1 was the secondary antibody. (C) Flow cytometry analysis of Her2-Fc proteins. The tumor cells CHO, MCF7, LS174T, and SKOV3 were studied. The gray area (blank) indicates cells with no staining; the dashed line indicates cells with human IgG1 and Alex488-conjugated goat anti-human IgG1 staining; the solid line indicates C3-Fc staining, then with Alex488-conjugated goat anti-human IgG1 staining; the dotted line indicates trastuzumab staining, then with Alex488-conjugated goat anti-human IgG1 staining.
To confirm that the cloned VHH-Fc antibodies bound Her2, an immunofluorescence analysis was performed. Similar to trastuzumab, C3-Fc showed strong positive staining on the surface of SKBR3 cells and negative staining on CHO cells, confirming that Her2-Fc specifically bound to Her2-positive cells (Figure 3B). A flow cytometry analysis was also performed with the different Her2-positive cell lines, including the SKOV3, MCF7, and LS174T cells, and the Her2-negative cell line, CHO, as a control. For the positive control, trastuzumab, strong binding to Her2-positive cells was observed (Figure 3C) but not in the Her2-negative CHO cell line (Figure 3C). Similar to trastuzumab, the different VHH-Fc antibodies exhibited strong binding to the Her2-positive cells but not the Her2-negative CHO cell line, indicating the specific binding of the Her2 VHHs. Similar to the VHH-only results (Figure 2), C3-Fc showed the highest binding among the different VHHs (data not shown). Compared with trastuzumab, C3-Fc showed only slightly reduced binding, especially on low–Her2 expression MCF7 cells (Figure 3C). Using a biolayer interferometry analysis, C3-Fc also showed high affinity to Her2 with a Kd ~3 nM. Thus, C3-Fc was selected for further analysis.
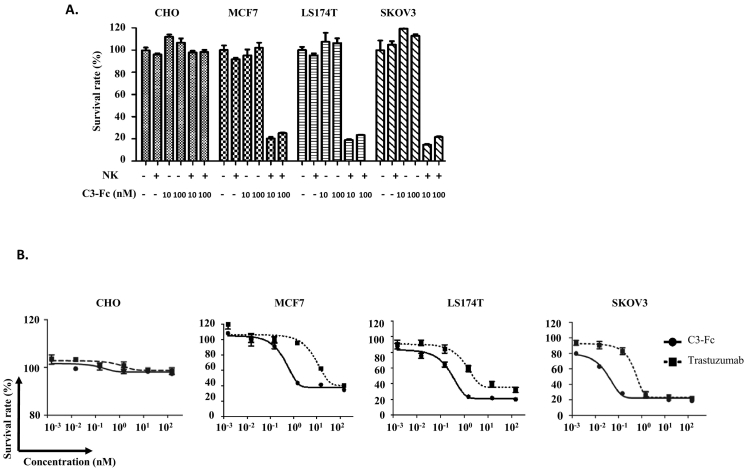
C3-Fc Induces NK Cell-Mediated Cytotoxicity
Antibody-mediated tumor cell killing is the major mode of trastuzumab’s ability to inhibit Her2-positive tumor growth. To determine whether C3-Fc mediates the killing of tumor cells, cytotoxic assays were performed for cells with or without Her2 expression. Similar to trastuzumab, when C3-Fc alone was incubated with Her2-positive or -negative cells, no tumor cell growth inhibition was observed (Figure 4A). In the presence of NK cells, C3-Fc induced a strong antibody-dependent cell cytotoxicity (ADCC) to kill Her2-positive tumor cells (Figure 4A). To further analyze the cytotoxicity of C3-Fc on tumor cells, its dose-dependent cell death capacity was studied in the presence of effector cells. Increased cytotoxicity was observed for the Her2-positive SKOV3 cells but not for the Her2-negative CHO cells for both C3-Fc and trastuzumab (Figure 4B). Similar to previous studies [28], C3-Fc and Trastuzumab showed higher cytotoxic activities against the higher–Her2 expression tumor SKOV3 cells than the medium–Her2 expression cell line LS174T and the low–Her2 expression MCF7 cells (Figure 4B). Unexpectedly, in all three different Her2 expression tumor cells, C3-Fc exhibited a higher cytotoxicity than trastuzumab (Figure 4B) even though they have similar or lower Her2 binding activity (Figure 3), suggesting that C3-Fc might have a stronger therapeutic potential in Her2-overexpression tumors.
Figure 4.
C3-Fc induced NK cell–mediated cytotoxicity. (A) The cytotoxic activities of C3-Fc were dependent on the presence of NK cells. Different cell lines were treated with C3-Fc with or without fresh isolated NK cells. (B) Dose-dependent cytotoxicity assays were performed with human NK cells as effector cells and CHO, MCF7, LS174T, and SKOV3 as target cells (E/T=10:1) in the presence of Her2-Fc proteins. The concentration of Her2-Fc was from 150 nM to 0.0015 nM. The mixtures were incubated for 72 hours before the cytotoxicity measurement. All the data are the mean of triplicates, with the error bars representing the standard deviation.
C3-Fc Has a More Potent Antitumor Effect In Vivo
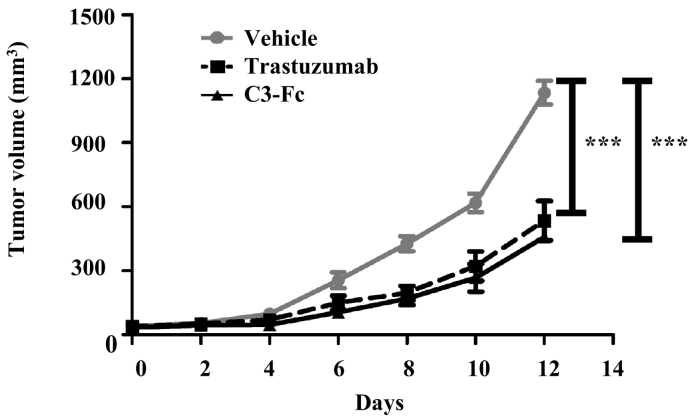
The higher in vitro cytotoxicity of C3-Fc led us to investigate whether C3-Fc also has a more potent antitumor activity in vivo. To study the in vivo antitumor effect of C3-Fc, a xenograft mouse model using LS174T cells, which have medium Her2 expression, was employed [28]. The NOD/SCID mice were engrafted subcutaneously with LS174T cells. When the tumor volume reached 50 to 100 mm3, the mice were grafted with freshly isolated human PBMCs and treated with PBS, trastuzumab, or C3-Fc. Both trastuzumab and C3-Fc exhibited a significant tumor inhibition (Figure 5). C3-Fc also exhibited consistently more tumor inhibition in this xenograft model. No obvious toxicity and weight loss were observed for all the animals, including the C3-Fc–treated animals. These data demonstrated that C3-Fc inhibited tumor growth in a xenograft mouse model and exhibited a slightly higher potent antitumor effect than trastuzumab.
Figure 5.
C3-Fc inhibits tumor growth in vivo. NOD/SCID mice (n=6/group) were engrafted subcutaneously with LS174T cells (1×106 per animal). When the tumor grew to 50 to 100 mm3, the mice were then grafted with freshly isolated human PBMCs (1×106 per animal) and treated intraperitoneally with vehicle PBS (gray line), trastuzumab (dashed line), or C3-Fc (solid line) every 2 days. The tumor volume was then measured. The data represent the average tumor volume of six mice. The error bars represent the standard deviation (*P<.05, t test, vehicle vs C3-Fc, vehicle vs trastuzumab).
Discussion
In this study, a single-domain anti-Her2 antibody, C3, was obtained and analyzed for its function in tumor inhibition. The VHH-Fc antibody C3-Fc showed specific and strong binding to Her2-positive tumor cells. Although the binding of C3-Fc to Her2 was slightly lower than Trastuzumab, C3-Fc showed a higher tumor cell killing ability both in vitro and in vivo, suggesting that C3-Fc may present an alternative therapeutic approach to Her2-positive tumors.
Therapeutic antibodies are a major force in the fight against tumors. Most current antibodies in the clinic are derived from regular IgGs, with a full-size antibody of approximately 150 kDa. Recently, single-domain antibodies or variable-domain (VHH) antibodies have attracted increased attention due to their small size and superior biochemical properties. The single-domain antibodies VHHs are devoid of a light chain. The antigen-binding module is the VHH only, with a size of approximately 12.5 to 15 kDa. Compared with the full-size IgG antibodies, which have poor penetration into tissues, especially solid tumors, the VHH antibodies are much smaller and penetrate into specific tissues more easily [29], [30], with better tumor penetration [31], [32]. However, because of its relatively small molecular size, VHH has a very short serum half-life of approximately 2 to 4 hours, limiting its application as a therapeutic. One solution to improve the protein’s short half-life is to fuse it to the IgG Fc domain [33], [34]. With the Fc fusion, VHH-Fc is approximately 75 to 80 kDa, which is close to half the size of regular IgG, and has reasonable half-life in vivo [35].
In addition to their smaller sizes, VHHs also exhibit a high thermal and conformational stability [11], a high solubility, and acid and alkaline resistance [12]. These properties make VHH-Fc likely to be easier to manufacture and store, thus reducing the cost of production. Indeed, C3-Fc reached a much higher expression level than trastuzumab in the transient 293T expression system (data not shown).
In this study, we immunized a llama and applied phage display screening [36] to obtained C3 VHH. C3 exhibited specific and high Her2 binding (Figure 2, Figure 3). The higher ADCC activity of C3-Fc was unexpected, as C3-Fc had a slightly lower binding by a flow cytometry analysis (Figure 4). The higher ADCC activity of C3-Fc was probably not due to the single-domain VHH per se, as another anti-Her2 VHH-Fc, which showed a similar affinity to C3-Fc and cell surface binding by flow cytometry analysis to C3-Fc, did not have higher ADCC activity (data not shown). Based on the internalization assay, C3-Fc had a similar rate to trastuzumab, suggesting that the longer cell membrane retention time was not the cause of the higher ADCC either. More detailed analyses are underway to understand the higher cytotoxicity of C3-Fc.
The epitope mapping analysis suggested that C3 did not have the same binding epitope as trastuzumab on the Her2 protein (data not shown). This provides an opportunity for the use of C3-Fc alone or the combination of C3-Fc with trastuzumab in the treatment of Her2-positive tumors. The combination therapy of trastuzumab with another anti-Her2 antibody, pertuzumab, which binds to a different epitope than trastuzumab, is a more effective therapy in the clinic [37], [38]. Studies suggest that the combination of anti-Her2 antibodies with different mechanisms may be more effective in the treatment of Her2-positive tumors [39]. Currently, we are assessing whether C3-Fc has additive or synergistic effects in the treatment of Her2-positive tumors with trastuzumab, or pertuzumab, or trastuzumab and pertuzumab in combination. These studies will likely provide more effective combinations than the current treatments of Her2-positive tumors.
In conclusion, a single domain–based anti-Her2 antibody was developed in this study. Due to its unique biochemical and stronger antitumor effects, the C3-Fc may present an alternative approach to combat Her2-overexpression tumors. It might also be used to treat Her2-overexpression tumors together with current anti-Her2 therapeutics.
Conflict of Interest
The authors declare no competing financial interests.
Acknowledgements
This work was financially supported by the Department of Science and Technology of Guangdong Province (PR China) (2016A050503028).
Contributor Information
Xiaoqiong Wu, Email: wuxq23@mail2.sysu.edu.cn.
Siqi Chen, Email: chen.sqi@outlook.com.
Limin Lin, Email: linlm3@mail2.sysu.edu.cn.
Jiayu Liu, Email: liujy53@mail2.sysu.edu.cn.
Yanlan Wang, Email: wangylan3@mail2.sysu.edu.cn.
Yumei Li, Email: liym45@mail2.sysu.edu.cn.
Qing Li, Email: liqing66@mail.sysu.edu.cn.
Zhong Wang, Email: wangzh357@mail.sysu.edu.cn.
References
- 1.Owen SC, Patel N, Logie J, Pan G, Persson H, Moffat J, Sidhu SS, Shoichet MS. Targeting HER2+breast cancer cells: lysosomal accumulation of anti-HER2 antibodies is influenced by antibody binding site and conjugation to polymeric nanoparticles. J Control Release. 2013;172:395–404. doi: 10.1016/j.jconrel.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 2.Burstein HJ. The distinctive nature of HER2-positive breast cancers. N Engl J Med. 2005;353:1652–1654. doi: 10.1056/NEJMp058197. [DOI] [PubMed] [Google Scholar]
- 3.Ruschoff J, Hanna W, Bilous M, Hofmann M, Osamura RY, Penault-Llorca F, van de Vijver M, Viale G. HER2 testing in gastric cancer: a practical approach. Mod Pathol. 2012;25:637–650. doi: 10.1038/modpathol.2011.198. [DOI] [PubMed] [Google Scholar]
- 4.Meza-Junco J, Au HJ, Sawyer MB. Critical appraisal of trastuzumab in treatment of advanced stomach cancer. Cancer Manag Res. 2011;3:57–64. doi: 10.2147/CMR.S12698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiosea SI, Williams L, Griffith CC, Thompson LDR, Weinreb I, Bauman JE, Luvison A, Roy S, Seethala RR, Nikiforova MN. Molecular characterization of apocrine salivary duct carcinoma. Am J Surg Pathol. 2015;39:744–752. doi: 10.1097/PAS.0000000000000410. [DOI] [PubMed] [Google Scholar]
- 6.Santin AD, Bellone S, Roman JJ, McKenney JK, Pecorelli S. Trastuzumab treatment in patients with advanced or recurrent endometrial carcinoma overexpressing HER2/neu. Int J Gynecol Obstet. 2008;102:128–131. doi: 10.1016/j.ijgo.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 7.Vasconcellos FA, Vasconcellos FA, Aleixo PB, Stone SC, Conceicao FR, Dellagostin OA, Aleixo JA. Generation and characterization of new HER2 monoclonal antibodies. Acta Histochem. 2013;115:240–244. doi: 10.1016/j.acthis.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Pollock NI, Grandis JR. HER2 as a therapeutic target in head and neck squamous cell carcinoma. Clin Cancer Res. 2015;21:526–533. doi: 10.1158/1078-0432.CCR-14-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doshi R, Chen BR, Vibat CRT, Huang N, Lee CW, Chang G. In vitro nanobody discovery for integral membrane protein targets. Sci Rep. 2014;4 doi: 10.1038/srep06760. [Artn 6760] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Van de B, Devoogdt N, D'Hollander A, Gijs HL, Jans K, Lagae L, Muyldermans S, Maes G, Borghs G. Specific cell targeting with nanobody conjugated branched gold nanoparticles for photothermal therapy. ACS Nano. 2011;5:4319–4328. doi: 10.1021/nn1023363. [DOI] [PubMed] [Google Scholar]
- 11.van der Linden RH, Frenken LG, de Geus B, Harmsen MM, Ruuls RC, Stok W, de Ron L, Wilson S, Davis P, Verrips CT. Comparison of physical chemical properties of llama VHH antibody fragments and mouse monoclonal antibodies. Biochim Biophys Acta. 1999;1431:37–46. doi: 10.1016/s0167-4838(99)00030-8. [DOI] [PubMed] [Google Scholar]
- 12.Stewart CS, MacKenzie CR, Hall JC. Isolation, characterization and pentamerization of alpha-cobrotoxin specific single-domain antibodies from a naive phage display library: preliminary findings for antivenom development. Toxicon. 2007;49:699–709. doi: 10.1016/j.toxicon.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 13.Vaneycken I, Devoogdt N, Van Gassen N, Vincke C, Xavier C, Wernery U, Muyldermans S, Lahoutte T, Caveliers V. Preclinical screening of anti-HER2 nanobodies for molecular imaging of breast cancer. FASEB J. 2011;25:2433–2446. doi: 10.1096/fj.10-180331. [DOI] [PubMed] [Google Scholar]
- 14.Shahsavarian MA, Le Minoux D, Matti KM, Kaveri S, Lacroix-Desmazes S, Boquet D, Friboulet A, Avalle B, Padiolleau-Lefevre S. Exploitation of rolling circle amplification for the construction of large phage-display antibody libraries. J Immunol Methods. 2014;407:26–34. doi: 10.1016/j.jim.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Arbabi Ghahroudi M, Desmyter A, Wyns L, Hamers R, Muyldermans S. Selection and identification of single domain antibody fragments from camel heavy-chain antibodies. FEBS Lett. 1997;414:521–526. doi: 10.1016/s0014-5793(97)01062-4. [DOI] [PubMed] [Google Scholar]
- 16.Vincke C, Gutierrez C, Wernery U, Devoogdt N, Hassanzadeh-Ghassabeh G, Muyldermans S. Generation of single domain antibody fragments derived from camelids and generation of manifold constructs. Methods Mol Biol. 2012;907:145–176. doi: 10.1007/978-1-61779-974-7_8. [DOI] [PubMed] [Google Scholar]
- 17.Pavoni E, Vaccaro P, Anastasi AM, Minenkova O. Optimized selection of anti-tumor recombinant antibodies from phage libraries on intact cells. Mol Immunol. 2014;57:317–322. doi: 10.1016/j.molimm.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Yuan Q, Jordan R, Brlansky RH, Istomina O, Hartung J. Development of single chain variable fragment (scFv) antibodies against Xylella fastidiosa subsp. pauca by phage display. J Microbiol Methods. 2015;117:148–154. doi: 10.1016/j.mimet.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 19.Aghebati-Maleki L, Bakhshinejad B, Baradaran B, Motallebnezhad M, Aghebati-Maleki A, Nickho H, Yousefi M, Majidi J. Phage display as a promising approach for vaccine development. J Biomed Sci. 2016;23:66. doi: 10.1186/s12929-016-0285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hust M, Maiss E, Jacobsen HJ, Reinard T. The production of a genus-specific recombinant antibody (scFv) using a recombinant potyvirus protease. J Virol Methods. 2002;106:225–233. doi: 10.1016/s0166-0934(02)00166-0. [DOI] [PubMed] [Google Scholar]
- 21.Sharifzadeh Z, Rahbarizadeh F, Shokrgozar MA, Ahmadvand D, Mahboudi F, Rahimi Jamnani F, Aghaee Bakhtiari SH. Development of oligoclonal nanobodies for targeting the tumor-associated glycoprotein 72 antigen. Mol Biotechnol. 2013;54:590–601. doi: 10.1007/s12033-012-9601-0. [DOI] [PubMed] [Google Scholar]
- 22.Suo S, Wang X, Zarlenga D, Bu RE, Ren YD, Ren XF. y Phage display for identifying peptides that bind the spike protein of transmissible gastroenteritis virus and possess diagnostic potential. Virus Genes. 2015;51:51–56. doi: 10.1007/s11262-015-1208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xing JY, Lin LM, Li L, Liu JY, Zhou CH, Pan HT, Shu R, Dong B, Cao DL, Li Q. BiHC, a T-cell–engaging bispecific recombinant antibody, has potent cytotoxic activity against Her2 tumor cells. Transl Oncol. 2017;10:780–785. doi: 10.1016/j.tranon.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subedi GP, Johnson RW, Moniz HA, Moremen KW, Barb A. High yield expression of recombinant human proteins with the transient transfection of HEK293 Cells in suspension. J Vis Exp. 2015:e53568. doi: 10.3791/53568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li L, He P, Zhou C, Jing L, Dong B, Chen S, Zhang N, Liu Y, Miao J, Wang Z. A novel bispecific antibody, S-Fab, induces potent cancer cell killing. J Immunother. 2015;38:350–356. doi: 10.1097/CJI.0000000000000099. [DOI] [PubMed] [Google Scholar]
- 26.Dong B, Zhou C, He P, Li J, Chen S, Miao J, Li Q, Wang Z. A novel bispecific antibody, BiSS, with potent anti-cancer activities. Cancer Biol Ther. 2016 doi: 10.1080/15384047.2016.1139266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bagheri S, Yousefi M, Safaie Qamsari E, Riazi-Rad F, Abolhassani M, Younesi V, Dorostkar R, Movassaghpour AA, Sharifzadeh Z. Selection of single chain antibody fragments binding to the extracellular domain of 4-1BB receptor by phage display technology. Tumour Biol. 2017;39 doi: 10.1177/1010428317695924. [1010428317695924] [DOI] [PubMed] [Google Scholar]
- 28.Li AF, Xing JY, Li L, Zhou CH, Dong B, He P, Li Q, Wang Z. A single-domain antibody-linked Fab bispecific antibody Her2-S-Fab has potent cytotoxicity against Her2-expressing tumor cells. AMB Express. 2016;6 doi: 10.1186/s13568-016-0201-4. [ARTN 32] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xenaki KT, Oliveira S, van Bergen En Henegouwen PMP. Antibody or antibody fragments: implications for molecular imaging and targeted therapy of solid tumors. Front Immunol. 2017;8:1287. doi: 10.3389/fimmu.2017.01287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kijanka M, Dorresteijn B. S Oliveira and PM van Bergen En Henegouwen, Nanobody-based cancer therapy of solid tumors. Nanomedicine. 2015;10:161–174. doi: 10.2217/nnm.14.178. [DOI] [PubMed] [Google Scholar]
- 31.Cortez-Retamozo V, Backmann N, Senter PD, Wernery U, De Baetselier P, Muyldermans S. Efficient cancer therapy with a nanobody-based conjugate. Cancer Res. 2004;64:2853–2857. doi: 10.1158/0008-5472.can-03-3935. [DOI] [PubMed] [Google Scholar]
- 32.Dumoulin M, Conrath K, Van Meirhaeghe A, Meersman F, Heremans K, Frenken LGJ, Muyldermans S, Wyns L, Matagne A. Single-domain antibody fragments with high conformational stability. Protein Sci. 2002;11:500–515. doi: 10.1110/ps.34602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen S, Huang Q, Liu J, Xing J, Zhang N, Liu Y, Wang Z, Li Q. A targeted IL-15 fusion protein with potent anti-tumor activity. Cancer Biol Ther. 2015;16:1415–1421. doi: 10.1080/15384047.2015.1071739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 35.Mary C, Coulon F, Poirier N, Dilek N, Martinet B, Blancho G, Vanhove B. Antagonist properties of monoclonal antibodies targeting human CD28: role of valency and the heavy-chain constant domain. MAbs. 2013;5:47–55. doi: 10.4161/mabs.22697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith GP. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985;228:1315–1317. doi: 10.1126/science.4001944. [DOI] [PubMed] [Google Scholar]
- 37.Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A, Heeson S. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372:724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swain SM, Kim SB, Cortes J, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A, Knott A. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14:461–471. doi: 10.1016/S1470-2045(13)70130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rockberg J, Schwenk JM, Uhlen M. Discovery of epitopes for targeting the human epidermal growth factor receptor 2 (HER2) with antibodies. Mol Oncol. 2009;3:238–247. doi: 10.1016/j.molonc.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]