Abstract
Na+/H+ exchanger regulatory factor 1 (NHERF1) is a scaffold protein, with two tandem PDZ domains and a carboxyl-terminal ezrin-binding (EB) region. This particular sticky structure is responsible for its interaction with different molecules to form multi-complexes that have a pivotal role in a lot of diseases. In particular, its involvement during carcinogenesis and cancer progression has been deeply analyzed in different tumors. The role of NHERF1 is not unique in cancer; its activity is connected to its subcellular localization. The literature data suggest that NHERF1 could be a new prognostic/predictive biomarker from breast cancer to hematological cancers. Furthermore, the high potential of this molecule as therapeutical target in different carcinomas is a new challenge for precision medicine. These evidences are part of a future view to improving patient clinical management, which should allow different tumor phenotypes to be treated with tailored therapies. This article reviews the biology of NHERF1, its engagement in different signal pathways and its involvement in different cancers, with a specific focus on breast cancer. It also considers NHERF1 potential role during inflammation related to most human cancers, designating new perspectives in the study of this “Janus-like” protein.
Introduction
NHERF1 emerged on the scientific panorama at the end of the ‘90s, and because of its sticky structure, which is able to link different molecules, the great possibilities that it retained in its nature were immediately clear. From its physiological role it was but a short step to realize its engagement in different diseases, and in particular in cancer. In fact, protein-protein interaction studies underlined the relation between NHERF1 and other molecules, many of which involved in cancer signaling [1], [2], [3], [4], [5]. Very early it became clear that NHERF1 role was not so coherent in cancer, but it fitted into the multifaceted context of tumoral disease. Further, there was already a consensus, emerging from the first few studies, that linked its activity to its subcellular localization as it was, influenced by the state of cells and different NHERF1-protein interactions. NHERF1 in the cell can play as tumor suppressor, when it is present in the apical membrane, as well as oncogene, after the switch from membrane to cytoplasm and nucleus. This opposite activity makes it a “Janus like” protein, which is a significant obstacle to study it. Here, we will review the main data regarding the biology of the NHERF1 signaling and its interplay with other prominent signaling pathways in the cell, its relevance in cancer development, and its potential role during inflammation related to most human cancers.
NHERF1 is a scaffolding protein identified independently as a co-regulator of the exchanger NHE3 in rabbit kidney epithelia [6], and as a phosphoprotein that associates with high affinity and specificity with ezrin and moesin (EBP50, ezrin-radixin-moesin binding phosphoprotein 50) [7].
Na+/H+ exchangers (NHEs) is a family of integral membrane proteins with multiple transmembrane domains and a large cytosolic carboxyl-terminal domain. This family consists of six isoforms, and every member mediates electroneutral exchange of Na+ for H+ at the plasma membrane and across the membranes of some intracellular organelles [8]. NHE3 isoform plays a central role in the (re)absorption of Na+ and HCO-3 across the epithelial layer and is mainly found at the apical membrane of polarized epithelial cells of organs such as kidney, gastrointestinal tract, and gallbladder [9].
Scaffold proteins allow the formation of protein complexes, interacting with a wide variety of cellular targets. They bring together two or more proteins, for example membrane receptors/transporters and cytoplasmic signaling molecules, in a relatively stable configuration, building macromolecular complexes. Scaffolds contribute to the coordination and the positive or negative regulation of specific signaling pathways. This can occurs by modulating, for example, the activity of specific kinase or phosphatases, by regulating the activity of membrane protein such as transporters and receptors or by concentrating and localizing transporters, receptors or enzymes in close proximity of their substrates or regulatory proteins [10], [11]. In scaffolding proteins, the association of multiple target proteins is facilitated by the presence of post-synaptic density protein/Drosophila disc large tumor suppressor/zonulaoccludens1 protein (PDZ)modular protein–protein interaction modules. These domains interact with specific carboxyl-terminal motifs on target proteins. Moreover, the scaffolding function can be enhanced by oligomerization with other PDZ domains [12].
These interactions can protect signaling molecules from inactivation and lead to a different subcellular localization of the proteins involved in the signaling pathway, giving to NHERF1 a role as coordinator of multiple signaling pathways such as those depending on tyrosine kinase (TK) receptors [13].
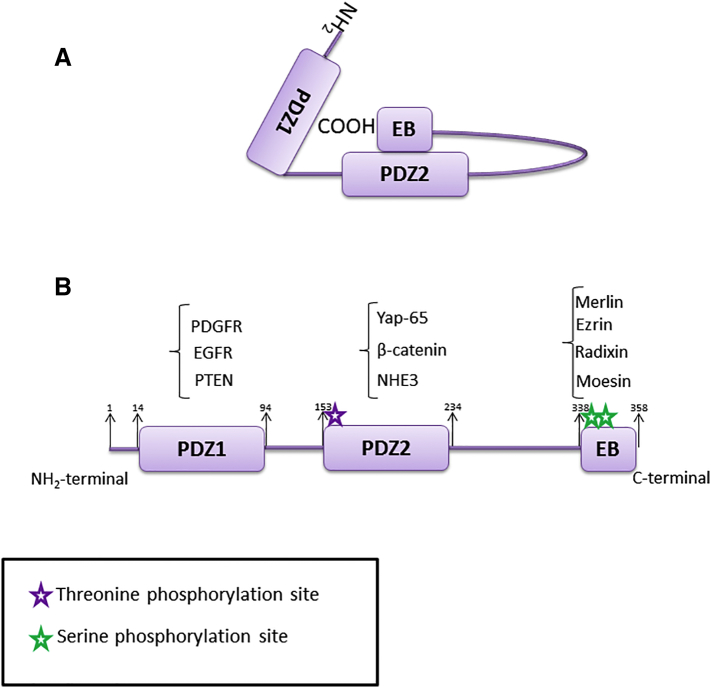
NHERF1 is encoded by SLC9A3R1 gene, localized on human chromosome 17q25.1, which contains six exons. It is a protein comprising 358 amino acids and is characterized by the presence of two NH2-terminal PDZ domains, PDZ1 (11-97 amino acids) and PDZ2 (150-237 amino acids) and a carboxyterminal ezrin–radixin–moesin (ERM) binding region [14] (Figure 1). These two PDZ domains show 74% identity to each other. Human NHERF1 presents 84% and 48% overall sequence identity to rabbit protein cofactor Na+/H+ exchanger regulatory factor (NHE-RF) and to human Tyrosine Kinase Activator Protein 1(TKA-1), respectively. NHE-RF is a protein that is involved in the regulation of the rabbit renal brush border Na+/H+ ion exchanger [15]. NHERF1 and NHE-RF align very well over their entire lengths, while the sequence of TKA-1 diverges after G261 in NHERF1, and PDZ1 together with PDZ2 are found in nearly identical versions in both protein cofactor and TKA-1.
Figure 1.
NHERF1 structure. (A) Intramolecular head-to-tail NHERF1 conformation; (B) phosphorylation sites: phosphorylations on Thr156 and Ser 339-340 disturb self-association, favoring PDZ-ligands interaction.
NHERF1 is enriched in the microvilli, which are specialized cell surface structures present in polarized epithelial cells of various tissues, including kidney, intestine, liver, and placenta. Microvilli are also enriched in members of the ERM family protein [16], [17]. ERM proteins organize protein complexes that link the membrane to the cytoskeleton. The structure of ERM proteins comprises an amino (N)-terminal FERM (band 4.1, ERM) domain and a short carboxy (C)-terminal domain. ERM proteins bind to transmembrane or membrane-associated proteins with the FERM domain and interact, with their C-terminal domains, with actin of the cytoskeleton [3].
By now is well known that NHERF1 is a physiologically relevant ezrin-binding protein, as demonstrated by Reczek et al. [7]. By immunoprecipitation experiments with extracts of purified placental microvilli they demonstrated that NHERF1 coprecipitates some of the ezrin, and vice versa. Moreover, in the placental syncytiotrophoblast, immunoelectron microscopy reveals that NHERF1, like ezrin, is specifically associated with the microvilli. Apical localization of NHERF1 and its interaction with ERM proteins plays a pivotal role in the constitution of microvilli, and consequently they are both required to maintain epithelial integrity [18]. This has been confirmed by observations of polarized epithelia of the kidney and small intestine in NHERF1−/− mice, which have shown that NHERF1 is important for stabilizing active phosphorylated ERM proteins at the apical membrane. Moreover, NHERF1−/− mice present structural defects of the intestinal brush border membrane that are similar to defects found in ezrin−/− mice [14], [19].
According to several studies, the function of NHERF1 in cancer is determined by its subcellular localization, which depends on cellular state and on proteins with which NHERF1 interacts and regulates. Physiologically, NHERF1 is localized in the sub-plasma membranous region of cells, associated with the cortical actin cytoskeleton. This interaction is possible thanks to the presence of a cholesterol-binding site in PDZ1 domain and thanks to the ezrin-binding (EB) domain [20]. The association between NHERF1 and its ligands can be prevented by an intra-molecular interaction between the N-terminal PDZ2 domain and the C-terminal EB domain. This “head-to-tail” folding conformation inhibits the association of NHERF1 PDZ domains with PDZ ligands, such as PTEN or β-catenin (Figure 1A). Thus, it is possible to find NHERF1 in a “dormant state” in the cytosol [3]. Interaction of EB domain with ERM proteins inhibits folding of NHERF1, and in this way the interaction expose the PDZ domains.
However, an aberrant nuclear localization of NHERF1 has been found in various cell lines and specimen of hepatocellular, colon, and breast cancer. PDZ and EB domains seem to be critical for NHERF1 nuclear localization. After deletion of PDZ2, NHERF1 is excluded from the nucleus, whereas loss of EB or PDZ1 domains causes NHERF1 delocalization in the nucleus [21]. Subcellular localization of NHERF1 can be regulated by phosphorylation in specific sites (Figure 1B). NHERF1 structure contains 31 Ser and 9 Thr residues, and their phosphorylation represents the main post-translational modification of NHERF1 [22].
Phosphorylation can also alter NHERF1 ability to oligomerize, that means its association with itself or with other proteins containing PDZ domains, which facilitates the formation and regulation of cellular signaling complexes [23], [24]. For example, oligomerization can potentiate the signaling of NHERF1 binding proteins, as has been well demonstrated in vitro for platelet-derived growth factor receptor (PDGFR) [25].
A large portion of NHERF1 in cells can be found in a constitutively phosphorylated state on Ser289 by G protein-coupled receptor kinase 6A (GRK6A), a kinase having a high affinity for NHERF1 [26], and this state has been demonstrated to promote NHERF1 oligomerization [27].
This latter can also be enhanced by phosphorylation on Ser339/340 by PKC, and this increases the PDZ2 accessibility to its targets [28].
Ser77 found in PDZ1 domain is phosphorylated upon stimulation with PTH and dopamine, and this disassociates NHERF1 from the sodium-dependent phosphate transporter 2a (Npt2a), which plays a pivotal role in the regulation of renal phosphate transport [29]. NHERF1 phosphorylation status is variable throughout the various phases of cell cycle and is important for its progression, in particular for correct cytokinesis. In particular, it has been found that NHERF1 is heavily phosphorylated by cdc2 on Ser280/302 during mitosis [30]. More recently, RSK1 has been identified as phosphorylating NHERF1 in HeLa cell line. RSK1 is a kinase activated downstream of the Ras-ERK pathway, and phosphorilates NHERF1 on Thr156 residue binding to its PDZ1 domain, thus leading to the nuclear localization of NHERF1. Even this phosphorylation seems to be a cell cycle-dependent event, as it is enhanced in mitotic cells [31].
Since its discovery, a broad variety of proteins interacting with NHERF1 have been identified: transporters, receptors, junction proteins and signaling molecules.
The regulation of the PI3K/AKT pathway by NHERF1 upon stimulation with PDGF, is one of the most studied pathway. NHERF1 can interact with both AKT [32] and its negative regulators PTEN [33] and PHLPP [34]. As demonstrated by Takahashi and colleagues, PTEN C-terminal tail contains a PDZ-binding motif able to interact with PDZ1 domain of NHERF1. NHERF1 binds to PDGFRβ and recruits PTEN to the membrane compartment close to PDGFR, scaffolding a complex between PDGFRβ and PTEN. This ternary complex regulates PI3K/AKT signaling in response to PDGF ligand, avoiding an overactivation of the pathway. Indeed, utilizing NHERF−/− mouse embryonic fibroblasts (MEFs) and NHERF-depleted cells, they observed that after PDGF stimulation there was a prolonged activation of the PI3K pathway. The interaction PTEN-NHERF1 enhances PTEN protein stability and depends on PTEN phosphorylation status, as PTEN phosphorylation reduces its recruitment to the plasma membrane. Normally, PTEN is degradated by means of the ubiquitin proteasome pathway, but PTEN-NHERF1 interaction prevents the binding of PTEN with NEDD4, an ubiquitin E3 ligase, thus preventing ubiquitination-dependent PTEN degradation [35]. A higher level of PTEN and a consequent reduced level of p-AKT has been observed by Cardone et al. in breast cancer cells after overexpression of NHERF1 [36].
The formation of PTEN/NHERF1/PDGFRb complex can be affected by two point mutations in the NHERF1 sequence, K172N and D301V, found in breast tumors and in the SUM149PT breast cancer cell lines.In pull-down experiments with protein extracts from COS-7 cells, NHERF1- K172N and NHERF1-D301V were both able to bind to PTEN, but their affinities with PTEN were reduced compared with that of NHERF1-wt. Moreover, NHERF1-D301V promoted up to a 2-fold increase in NHERF1-PDGFRb interaction. These results suggested that NHERF1-K172N and NHERF1-D301V mutations can compromise the formation of PTEN/NHERF1/PDGFRb complex [37].
Aberrant Wnt signaling has been described as a key player in the initiation of and/or maintenance and development of many cancers. First evidence for a possible involvement of NHERF1 in the regulation of this pathway came from a study of Songyang et al. [38], demonstrating that Frizzled (Fzd) proteins, that act as the primary receptors for Wnt signals, terminate in a canonical PDZ ligand domain.
β-Catenin is a well-established partner of NHERF1. It is member of a complex of proteins important not only for assembling and functionality of adherens junctions, but also for acting as a nuclear transcription factor in the Wnt pathway.β-catenin translocates to the nucleus, where it associates with T-cell factor/lymphoid enhancer factor (TCF/LEF) family of DNA-bound transcription factors to activate several oncogenes and other gene targets [39]. Association between NHERF1 and β-catenin has been demonstrated by Shibata et al. [40] in HepG2 cells, a human hepatocarcinoma cell line, where these proteins coimmunoprecipitate. Only PDZ2 domain of NHERF1 seems to bind β-catenin. Immunofluorescence analysis showed that in HepG2 cells β-catenin was expressed, as expected, both at the level of the plasma membrane and in the nucleus. Even NHERF1 was frequently observed in the nucleus. Although NHERF1 interacts with β-catenin through its PDZ2 domain, NHERF1 binds TCF-1B via its PDZ1 domain. Thus, NHERF1 can act as a positive regulator of Wnt signaling, binding to both β-catenin and TCF-1B, forming a ternary complex that enhances the activity of these transcription factors (Figure 2C). Moreover, in NHERF1−/− MEFs, β-catenin is delocalized from the plasma membrane to the cytoplasm and forms weaker complexes with E-cadherin. These cells present an anchorage-independent growth, indicating that NHERF1 could have a tumor suppressor role, controlling the intracellular distribution of β-catenin by stabilizing complexes with E-cadherin at the plasma membrane. Indeed, NHERF1−/− cells show a lower β-catenin membrane immunofluorescence staining compared to NHERF1+/+ cells, and a higher cytoplasmic expression in fractioned proteins [41]. These data have been confirmed in vitro in mice intestinal epithelial cells.
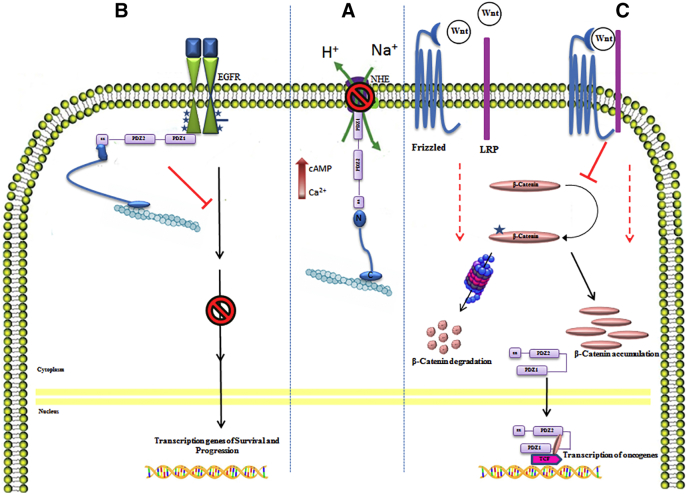
Figure 2.
NHERF1 pathways. In the middle part (A), in physiological conditions NHERF1 phosphorylates Na+/H+ exchangers (NHEs) affecting their activity and microenvironment acidification. On the left (B), NHERF1 acts as an oncosuppressor protein negative regulating the proliferative activity of EGFR pathway, when it is localized at the plasma membrane. On the right (C), NHERF1 appears as oncogene, in fact it imports β-catenin into the nucleus to form a bridge complex with the trancription factor TCF. This complex activates oncogenes transcription such as cMyc, CD1 etc.
Further evidence of NHERF1 regulating the canonical Wnt signaling and acting as a tumor suppressor was found by Wheeler and his research group [42]. They first demonstrated, in CHO-N10 cell line, that NHERF1 can bind directly to Fzd receptors, such as Fzd4, via its PDZ2 domain, allowing an anchorage of the receptor to the cortical actin cytoskeleton. Afterwards, MCF7 and MDA MB-231 human breast cancer cell lines (expressing respectively high and very low levels of NHERF1) were used to show that upon stimulation with Wnt proteins, β-catenin activation, cyclin D1 levels and proliferation rate were higher in cells lacking NHERF1.This could explain the increased duct density and nuclear β-catenin levels that the authors observed in the mammary glands of NHERF1 knockout mice. Moreover, in breast cancer biopsies of varying stages and ER/PR status, stained for NHERF1 and β-catenin, a negative correlation between these two proteins, where an increased expression of β-catenin was accompanied by an increase in the percent of nuclear β-catenin, has been observed. The expression level of NHERF1 has been demonstrated to regulate expression and function of the epidermal growth factor receptor(EGFR) in normal cells [43].
EGFR is a member of the tyrosine kinase receptor family, and its overexpression represents one of the primary mechanisms involved in the pathogenesis and progression of different carcinoma types. Upon binding with its ligand, epidermal growth factor (EGF), signaling pathways involved in cell growth, survival and migration are activated [44]. In basal conditions there is not a co-localization at the plasma membrane of breast cancer cells between NHERF1 and EGFR, while stimulation with EGF brings back NHERF1 at membrane, where if forms a complex with EGFR [45].
Lazar et al. [43] have demonstrated that PDZ1 domain of NHERF1 specifically binds to an internal peptide motif within the COOH-terminal regulatory domain of EGFR, retaining the receptor at the cell surface and retarding its down-regulation, as demonstrated by inserting a point mutation in the binding site. This interaction, that seems to be independent of ligand binding and autophosphorylation of EGFR, enhances EGFR signaling through inhibiting ligand-induced endocytosis. This prolongs the activated state of EGFR and the subsequent activation of the downstream signaling protein ERK.
Yao and colleagues [46] studied the effect of NHERF1 expression on EGFR signaling pathway in the breast cancer cell lines MDA-MB-231 and MCF-7. They found that NHERF1 expression inhibits the autophosphorylation of the receptor and the activation of downstream effectors ERK1/2 and AKT, suppressing EGF-induced proliferation of BC cells. The NHERF1-EGFR complex formation is altered by E43G mutation, localized in PDZ1 domain and identified in BCs [47]. In cholangiocarcinomas a delocalization of NHERF1 to the cytoplasm can be often observed, and this associates with EGFR expression. In vitro experiments carried out on biliary carcinoma cell lines showed that NHERF1 silencing enhances the expression of EGFR at plasma membrane and its downstream signaling, furthermore leading to acquisition of epithelial mesenchymal transition (EMT) [48] (Figure 2B).
Given the regulatory role of NHERF1 in pathways which disregulation plays an evident role in tumorigenesis, various research groups in the last years have studied the behavior of this adaptor protein in different cancer types (Figure 3). In particular, our group have focused its attention on analyzing the biological role of NHERF1 in different cancers: colorectal, gastric, lung and breast, finding evidence of its involvement in these diseases.
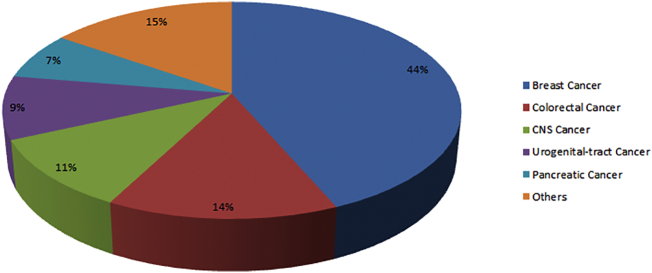
Figure 3.
Frequency of researches about NHERF1 divided for cancer. The majority of the studies explore the role of NHERF1 in breast and colorectal cancer, followed by tumors of the central nervous system, urogenital tract and pancreas. 15% includes studies about: hepatocarcinoma, melanoma, esophageal, biliary, gastric and lung cancer.
The following paragraphs will illustrate the "state of the art" of NHERF1 knowledge and its future study perspectives.
NHERF1 in Gastrointestinal Tract Cancers
Hepatocellular Carcinoma (HCC)
The initial investigations have shown the involvement of NHERF1 during development and progression of solid tumors. Shibata et al., showed a close association between NHERF1 and β-catenin in HCC model. Binding assay has demonstrated that β-catenin carboxyl terminal region is associated to the carboxyl PDZ2 NHERF1 domain. The co-localization of NHERF1 and β-catenin in the nucleus, their protein overexpression in clinical cases and the high mRNA levels of NHERF1 in different HCC cell lines, highlighted it as a possible cause of HCC development [4]. Peng et al., supposed that NHERF1 overexpression could inhibit the growth of two HCC cell lines and stimulate apoptosis by β-catenin/E-cadherin pathway [49]. Moreover, high NHERF1 mRNA levels in tumor compared to non-tumor tissues have been detected in HCC [50]. Thus, NHERF1 may be considered a potential therapeutic target in HCC.
Colorectal Cancer (CRC)
In CRC it has been demonstrated a significant positive correlation between cytoplasmic β-catenin and nuclear NHERF1 (nNHERF1) to support the close relationship of these two protein and their association in carcinogenesis. Further, new evidences in favor of involvement of NHERF1 in Wnt signaling pathway and consequently in CRC development have been collected. A positive correlation among RAS-association domain family 1 methylation, isoform A (RASSF1A) and nuclear β-catenin has been found too, noting that Wnt pathway activation is mediated by RASSF1A, a putative tumor suppressor RAS effector, whose epigenetic silencing by promoter methylation has been reported during cancer progression [51], [52]. These observations support the NHERF1 limelight in the cancer development and its trigger role, given by its intrinsic nature, that makes it a highly versatile molecule.
Evidence of NHERF1 dynamism has been provided by Hayashi and colleagues. They reported a heterogeneous pattern of NHERF1 in primary colorectal cancer (CRC) during the colorectal adenoma-to-carcinoma transition. The study showed the loss of the normal apical membrane arrangement of this protein and an ectopic cytoplasmic overexpression. In normal-like cellular model of CaCo2 cells they mimed the same membrane NHERF1 depletion, and observed morphological and biochemical changes characteristic of EMT, and an increase of cellular migration and invasion. Only the ectopic NHERF1 re-expression at the apical membrane restored the initial morphology and reduced cellular motility, clarifying for the first time the importance of NHERF1 location in CRC development, and indicating NHERF1 as a probable diagnostic marker in this tumor type [53]. Then, these results have been confirmed in a further study on a 3D model of human intestinal gland formation. High similarities have been found between the above colorectal adenoma-to-carcinoma transition and normal gland morphology–to-tumor-like 3D gland formation for NHERF1 membrane loss. These tumor-like 3D glands were characterized by aberrant growth, enlarged nuclei and increased migration, consistent with high grade dysplasia and observed in CRC progression [54].
Independently, our group have demonstrated that nuclear NHERF1 expression, which is present in the early stages of carcinogenesis, may contribute to the onset of malignant phenotype in CRC patients. Colorectal cancer samples, including non-neoplastic tissue, primary tumors, synchronous lymph node and liver metastases, were used in this study. A shift of NHERF1 expression from membrane to nucleus has been observed, and it was associated at the adenoma-carcinoma sequence. Interestingly, the loss of the apical membrane expression and occurrence of a cytoplasmic and nuclear staining, had already been observed in normal adjacent mucosa, indicating it as an early marker of pre-morphological triggering of carcinogenesis [55] (Figure 4). Further analysis has confirmed the importance of nNHERF1 localization, dynamism and its ability to correlate with different molecules, which emphasizes the oncogenic role of NHERF1 in CRC. We demonstrated an overexpression of nuclear NHERF1 in no longer polarized epithelial cells, converted to a mesenchymal phenotype in hypoxic colonic areas. Furthermore, we reported that nNHERF1 nuclear expression was related to poor differentiation grade and to high HIF-1α (Hypoxia-inducible factor) and TWIST1 expression, which supports a more invasive phenotype. NHERF1 close relation with these two oncogenic transcription factor linked it to a wide range of genes involved in cellular responses to different signals, linked to tumoral microenvironment [56], tumor invasion and metastasis [57]. However, in this report only the nuclear presence of NHERF1 seemed to be a more potent marker of aggressiveness in advanced CRC [58]. A positive linear correlation between cytoplasmic NHERF1 (cNHERF1) and protease-activated receptor-2 (PAR-2), and a significant co-expression of the two proteins mostly in the margin of the tumor mass, has been observed too. Cytoplasmic PAR-2/NHERF1 expression immunophenotype predicted poor prognosis for CRC patients, being associated with the presence of nodal and distant metastasis, poor differentiation grade and lymphovascular invasion [59]. NHERF1 identification as potential targeted biomarker is reflected in another study, in which its expression was positively correlated with VEGFR2 expression. Rapid NHERF1 up-regulation in hypoxia suggested NHERF1 as a rapid response element to the tumor microenvironment and as regulator of VEGF/VEGFR signaling pathway in metastatic progression [60]. This result fits in a prospective view that hopes to increase patient-oriented clinical management, permitting different immunophenotypes to be treated with tailor-made therapies, to improve therapeutical response and prognosis.
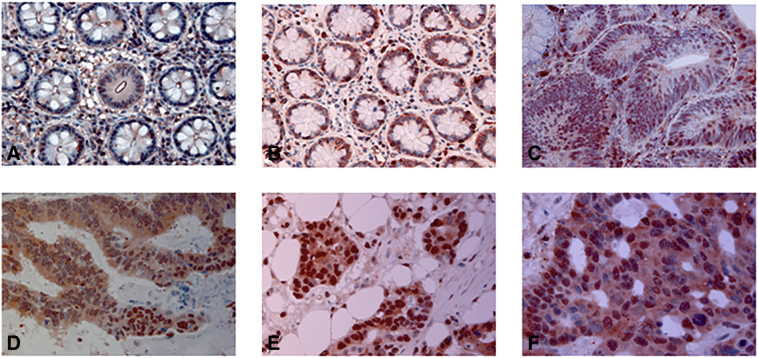
Figure 4.
Representative images of NHERF1 immunoreactivity and localization in metastatic CRC. (A) NHERF1 staining is present mostly in membrane of DNT mucosa; (B) in the cytoplasmic and nuclear of SNT and (C) in ADN; (D) over-expression of cytoplasmic and nuclear NHERF1 is present in T, (E) LnM and (F) LM. DNT: distant non-neoplastic tissue, SNT: surrounding non-neoplastic tissue, ADN: adenoma, T: primary tumor, LnM: synchronous lymph node metastasis, LM: liver metastasis.
Controversial NHERF1 behavior as a tumor suppressor or promoter has been found by Lin Y.Y and colleagues too, relating to cellular confluence in tumor area. In fact, they reported NHERF1 in the nucleus at the invasive front of CRC samples, while it was retained at the membranous and cytosolic portions in the central corpus of tumors, a comparable distribution pattern with β-catenin [61]. They linked also NHERF1 to EMT, suggesting that it could play a regulatory role in acquiring mesenchymal characteristics through a possible ternary bridge-formation among NHERF1- β-catenin-TCF-1, that facilitates its entry into the nucleus. A detailed analysis to clarify the interacting pathway of NHERF1 during intestinal neoplasia onset has highlighted its inhibitory activity on Wnt-β-catenin pathway. The complete absence of membrane expression (NHERF1−/−) was necessary to trigger a tumorigenic process in a mouse in vivo model, while heterozygosis condition did not abolished its onco-suppressor activity. Tumor incidence and size was increased in this model and the tumors showed an high nuclear expression of cyclin D1, a protein connected to cell cycle progression and growth. A screening of possible upstream cyclin D1 pathways revealed an increase of β-catenin and Yap expression, showing the link between NHERF1−/− phenotype and intestinal tumorigenesis. The Hippo-Yap pathway implication in tumorigenesis is a recent founding, and un-phosphorylated Yap increase has been observed in CRC too [62], [63], [64].
Pancreatic Cancer (PC)
The role of NHERF1 has been studied in other cancer sites with different results depending on tumors, sites, pharmacological treatment etc.
NHERF1 delocalization and its role in cancer progression is confirmed by a study in PC. By quantum dot-immunohistochemistry assay, NHERF1 expression was observed at the apical membranes of normal pancreatic tissue, with a decrease of membrane staining and cytoplasmic overexpression moving on to tumor area. In in vitro tests, NHERF1 down-regulation stimulated the cell proliferation, supporting its role as important tumor suppressor in PC. Further, the authors have explored the possible molecular mechanism involved in this behavior considering the expression of phosphorylated Rb (Retinoblastoma protein), cyclin E, p27 and β-catenin, which play an important role in the growth and proliferation of human cancer cells. They found increase of phosphorylation of Rb and cyclin E and reduction of p27 protein expression following NHERF1 silencing. This indicates a potential effect of NHERF1 alterations on regulation of cell growth, repression, proliferation and tumor formation, in which these proteins are involved [65]. A subsequent in vitro and in vivo study provided that NHERF1 overexpression inhibited the growth of the PC tumors inducing cell apoptosis, by decreasing Bcl-2 expression [66]. The same research group have improved this results, demonstrating as NHERF1 overexpression inhibited PC cell growth and invasion targeting the β-catenin/E-cadherin pathway, and indicating NHERF1 as a potential tumor suppressor and a possible therapeutic target [67]. In a 2013 study it has been demonstrated that NHERF1 is a bridge between CXCR2 (CXC chemokine receptor 2), a receptor for the CXC chemokines, involved in cancer progression, and PLC-β3 (phosphatidylinositide-specific phospholipase C), an activator of protein kinase C and Ca2+ release, both possessing a consensus PDZ at their carboxyl terminals [68], [69]. This complex has been associated to proliferation, invasion and tumor growth. In fact, its disruption reverted malignant cellular functions [70].
Gastric and Esophageal Carcinomas
Non canonical NHERF1 expression has been observed in a series of advanced gastric cancers (GC), treated with the epirubicin, oxaliplatin, and capecitabine chemotherapy regimen. In these samples was found an increase of cytoplasmic and nuclear pattern of NHERF1, in association with multidrug resistance proteins, such as P-gp and sorcin. These patients showed lower nNHERF1 and tended to have a high expression of P-gp and sorcin, although not statistically significant. This relates to chemotherapy-resistance and worse outcome and indirectly to mechanisms of drug resistance. A multivariate analysis revealed a significant correlation between nNHERF1 and clinical response, indicating it as an independent predictive factor of therapeutic response in these patients [71]. Previously, in a cohort of Chinese nationality patients, NHERF1 had been associated with several malignant clinicopathological features of GC, but it had not been evaluated as a predictive outcome factor for GC patient prognosis [72]. A chemogene therapy regarding NHERF1 was hypothesized in 2012, following the study of Lv XG and colleagues. They found an apoptotic increase induced by 5-Fluorouracil (5-FU) in GC cells overexpressing NHERF1 compared to wild type cell line, and they proposed a combination of adenovirus-NHERF1 and 5-FU as a better possible treatment for GC [73]. In esophageal squamous cell carcinoma has been reported a decrease of mRNA and protein NHERF1 expression in tumor compared to non-tumor tissues. In these patients the loss of membranous (mNHERF1) was associated with malignant progression and poor prognosis. Furthermore, the NHERF1 knockdown in in vitro experiments promoted cell growth and cycle progression [74].
Biliary Carcinoma
A comparative proteomic analysis of protein expression profiles in four histologically different cholangiocarcinoma cell lines, to represent cholangiocarcinoma development (from moderately differentiated adenocarcinoma to adenosquamous cell carcinoma) has been carried out. The analysis has permitted to identify protein differently expressed in the four cell lines, including NHERF1. Verification using IHC analysis on tissues has been performed and NHERF1 overexpression has been detected in the cytoplasm and in the membrane of cholangiocarcinoma tissues. Its expression was also associated with tumor invasion of lymphatic and blood vessels and with reduced survival after surgery [75].
Loss of NHERF1 at the plasma membrane of biliary cancer cells contributed to biliary carcinogenesis through EGFR activation. In normal biliary epithelium a mNHERF1 expression has been reported, whereas a cytoplasm delocalization has been observed in about 60% of the tumors, associated to EGFR expression, suggesting a defective interaction NHERF1/EGFR when NHERF1 ectopic expression was present. In fact, in cells expressing mNHERF1 there is a close interaction, just reported, with EGFR and β-catenin [4], [43].
To define the NHERF1/EGFR relation has been used a biliary carcinoma cell line, expressing endogenous EGFR and mNHERF1, in which NHERF1 was silenced by small interfering RNA (siRNA) [48]. In normal conditions, mNHERF1 links EGFR and β-catenin [33], [40], [43], thus stabilizing the β-catenin/E-cadherin complex [41]. During cancerogenesis, the loss of NHERF1 at the plasma membrane destroys NHERF1-EGFR connection and increases EGFR expression/activation, triggering its downstream effectors transcription and nuclear translocation of β-catenin, which results in EMT program activation [76], [77]. A recent work reports NHERF1 down-regulated expression levels in extra-hepatic bile duct carcinoma tissue, in relation with an increase of pathological stage and malignant phenotype. To confirm and elucidate tissue observations, the authors knocked down NHERF1 by siRNA in an extra-hepatic bile duct carcinoma cell line, observing cell proliferation and migration. In conclusion it has been hypothesized a link between NHERF1 and incidence and development of extra-hepatic bile duct carcinoma [78].
The overview of the gastrointestinal tract allows us to indicate mNHERF1 as a realistic expected prognostic/predictive biomarker for these tumors from colorectal cancer to extra-hepatic bile duct carcinoma, although extensive studies and clear guidelines need to standardize real application.
NHERF1 in Lung Cancer
For the first time, we have reported a loss of mNHERF1 staining in non-small cell lung (NSCLC) cancer too [79]. By analyzing different types of sample, fine needle aspiration cytology (FNAC) and surgical specimens, it has been demonstrated that in lung parenchyma tissue, NHERF1 immunoreactivity was mainly at apical membrane in the bronchial epithelial cells and in contiguous non-tumor lung tissue of the surgical samples, whereas it was negative in the alveoli. Considering the three different compartments, NHERF1expression resulted higher in cytoplasm and nucleus compared to plasma membrane, both in FNACs and NSCLC samples. A statistically significant correlation was found between cNHERF1 expression and tumor stage, and between nNHERF1 expression and histotypes. This latter finding might indicate NHERF1 expression as a diagnostic tool to distinguish the different phenotypes and a probable marker of aggressiveness in lung cancer. A non-congruent data showed a negative cytoplasmic NHERF1 expression in almost all IV stage tumors, indicating that cytoplasmic NHERF1 expression might be associated to a less aggressive phenotype, which is in contrast with previously results in breast cancer studies [80], [81]. The different microenvironment and cancer biology of this tumor might explain the different behavior and the different function for the multitasking NHERF1 [79]. Recent in vitro studies examined the role of NHERF1 during EMT in non-small cell lung cancer cells. NHERF1 down-regulation was involved in EMT regulated by TGF-β1, reverted by its re-expression in cellular system. In conclusion, the authors proposed an inhibitory function of NHERF1 on migration and invasion malignant phenotype of lung cancer cells, modulating EMT-associated markers, including E-cadherin and N- cadherin, snail family transcriptional repressor 1 and snail family transcriptional repressor 2 [82].
NHERF1 in Brain Cancers
In the last decade, the design of stable, safe and effective molecules has been significantly improved preclinical in clinical trials for cancer therapy. Despite that, there are still some tumors associated with significant morbidity and mortality, such as central nervous system (CNS) tumors. Understanding the molecular mechanism of these cancers is important to improve diagnosis and to successfully use novel targeted therapies and improve patient outcomes. Increased NHERF-1 gene expression has been observed in invasive glioma cells in vivo, isolated from tumor core or invasive rim, of 19 samples of glioblastoma multiforme (GBM). GBM is the most frequent form of primary brain cancer characterized by an aggressive phenotype and a lethal nature [83]. By gene expression profiling, genes that were differentially regulated in the core and the rim of the tumors were analyzed, and NHERF1 was overexpressed in the rim of more than 50% of the tumors. Its overexpression in invasive cancer rim links NHERF1 to migration activity of tumor cells in GBM. Higher levels of NHERF-1 expression in the invasive rim compared with the core has been detected by immunohistochemistry too. In vitro experiments in glioma cells showed inhibition of migration, increased cellular adhesion and morphological change after NHERF1 knockdown. Further, NHERF1 inhibition amplified apoptotic cell death caused by pharmacological treatments [84], [85]. These results permit us to indicate NHERF1 as a possible therapeutic target for treatment of GBM. Subsequently, the involvement of NHERF1 and PTEN, its ligand by PDZ motif, has been studied in normal adjacent brain, grade III anaplastic astrocytoma (AA) and in grade IV GBM samples. In normal region NHERF1 was observed at plasma membrane level, while in tumor areas a double pattern both cytoplasmic and membranous has been seen. Further, cNHERF1 has been associated with AKT activation and PTEN cytoplasmic translocation. AKT is a signal transduction pathway that promotes survival and growth in response to extracellular signals and is a part of PI3K/AKT pathway. The re-expression of NHERF1 in membrane restored normal membranous PTEN and suppressed cell proliferation triggered by Akt activity [86]. Consequently, the mechanism underpinning NHERF1-PI3K interaction in this type of cancer has been investigated in depth. A cellular model of glioblastoma doubly silenced for NHERF1 and PTEN showed a synergic effect on Akt activation, already observed for NHERF1 only. Further, to validate the PI3K/Akt inhibitory activities of NHERF1, has been studied a PI3K-Akt suppressor molecule with PDZ-binding motifs, that means PH domain leucine-rich repeat protein phosphatase 1 (PHLPP1). It is a new NHERF1 ligand, that regulates its plasma membrane enrollment and growth suppressive effect. The PTEN-NHERF1-PHLPP1 network was distorted in glioblastoma, when comparing high grade to low-grade gliomas or normal samples a significant reduction of all three suppressors and an increase of activated Akt was detected [87]. These data demonstrated that NHERF1 was involved in PTEN redistribution in cancer cells and this supposed a crucial role in controlling of PI3K/Akt pathway, thus opening a new area of molecular targeted therapy for GBM. Recently, it has been observed that NHERF1 has high sensitivity and specificity for microlumen in ependymal tumors. Ependymomas are well-delineated tumors resulting from uncontrolled growth of ependymal cells or precursors and characterized by perivascular and ependymal rosettes [88]. They are categorized as subependymomas (grade I), ependymomas (grade II) and anaplastic ependymomas (grade III), on the basis of their mitotic proliferative activity. In ependymomas, neoplastic cells arrange in characteristic polarized perinuclear dot-like structures, corresponding to microlumens, and in rosettes delimiting a lumen. In both structures, NHERF1 stained the apical membrane of cancer cells, where it builds protein complexes with moesin and PTEN. To establish the diagnostic role of NHERF1, more than 100 primary brain tumors (ependymomas, anaplastic ependymomas, and lower grade ependymal tumors) have been screened. NHERF1 was diffused in all grade I subependymomas, while its loss was detected in anaplastic ependymomas, associated to differentiation loss. So these results indicated that NHERF1 can be a reliable diagnostic marker for these tumors [89]. NHERF1 has been also associated to epithelial membrane antigen (EMA) expression in a cohort of ependymoma and non-ependymoma tumors, showing a major sensitivity for grade I sub and myxopapillary ependymomas. While, EMA and NHERF1 positivity in non-ependymomas (glioblastoma multiforme and meningioma) contributed to the lowered specificity. Only EMA positivity was more sensitive in grade III ependymomas. So, NHERF1 was considered a good diagnostic marker for grade I/II ependymomas, while a combined panel of EMA and NHERF1 was suggested for grade III ependymomas [90]. In choroid plexus (CP) neoplasms an immunohistochemistry panel formed by NHERF1 and its associated ezrin-radixin-moesin-merlin/ neurofibromin-2 (ERM-NF2) protein has been analyzed. NHERF1 showed a high apical plasma membrane mark in grade I CP papilloma and cytoplasmic expression in grade III CP carcinoma. Similarly to CRC, NHERF1 could be involved in a papilloma-to-ependymoma morphology sequence. NF2 showed polarized membranous staining in all CP tumors. Taken together, NHERF1 and NF2 expression showed highest sensitivity and specificity for CP tumors compared to commonly used biomarkers [91]. All these results support the real possibility of a diagnostic application of NHERF1, alone or in combination, in tumors of the CNS.
NHERF1 in Melanoma
Melanoma aggressiveness has been associated to increase of Phosphatase of regenerating liver-3 (PRL-3) expression, together with an increase of Akt phosphorylation and a decrease of PTEN. NHERF1 has been found into the nucleus of stage I, II, and III melanoma, but not in the nucleus of stage IV or lymph node metastatic melanoma. The loss of nNHERF1 during cancer progression in melanoma reflects the same behavior observed in the advanced breast cancer, where the loss of nNHERF1 has been linked to more aggressive phenotype [81]. Furthermore, a NHERF1/PTEN co-localization in stage I melanoma nucleus and a cytoplasmic shift in lymph node metastatic melanoma has been determined. As a result of tissue observation, the hypothesis of a central role of NHERF1/PTEN during malignant melanoma progression has been explored in cell lines. It has been demonstrated that PRL-3 regulates the phosphorylation of NHERF1 at Ser residue, promoting the translocation of NHERF1 and PTEN from the nucleus to the cytoplasm during cancer progression. Knockdown of cNHERF1 repressed tumor growth, demonstrating a NHERF1 therapeutic significance in melanoma too [92].
NHERF1 in Urogenital Tract Cancers
Ovarian Cancer (OC)
In primary ovarian mucinous carcinomas of the intestinal type a preliminary study demonstrated NHERF1 expression in 73% of the cases in association with poor prognosis [93]. Mutational analysis in the NHERF1 gene of epithelial OCs has showed the presence of new somatic mutations in 26% of analyzed samples, and in silico related analysis indicated a possible effect during the splicing process, affecting tumorigenesis [94]. NHERF1 and Ezrin expression was analyzed in cyst adenofibromas, serous borderline tumors, and serous OC. A high immunoreactivity was observed at the membrane of borderline tumors and papillary structures of cancers. In no-papillary tumors, apical NHERF1 expression at the limits of the luminal spaces was observed, while membrane Ezrin, but no NHERF1 expression, was detected in solid tumor area. In OCs, the different behavior of these proteins, usually close linked each other, is due to high molecular complexity of these cancers [95]. To underline the key role of NHERF1 pathway during ovarian cancer progression, a recent study has associated NHERF1 to cortical protrusion structures in OC cells. In fact, in an OC cell line, cytoplasmic rapidly moved to the plasma membrane after lysophosphatidic acid (LPA) stimulation, and interacted with C-terminally phosphorylated ERM proteins (cpERM). NHERF1 depletion by siRNA down-regulated cpERM and inhibited chemotactic cell migration in the direction of a LPA gradient. The authors highlighted the high dynamism of cytosolic NHERF1 and its role as a regulator of chemotactic migration, which is crucial for OC progression [96].
Cervical Cancer (CC)
In cervical cancer it has been demonstrated that human papillomavirus type16 (HPV16) E6 binds NHERF1 with its PDZ-binding motif, inducing NHERF1 degradation via the proteasome pathway. Further, it seems like E7 interacts with E6 to reinforce this activity, triggering NHERF1 phosphorylation by CDK1/2. As a consequence of NHERF1 degradation PI3K/AKT pathway activation has been reaffirmed too. NHERF-1 was down-regulated in different CC-derived cell lines with high levels of E6 and E7 genes/proteins. In accordance with in vitro evidences, NHERF1 down-regulation was observed in HPV16-positive cervical intra-epithelial neoplasia grade III only, compared to premalignant lesions [97]. An independent study has demonstrated that NHERF1 expression was down-regulated in CC tissues, and this low levels were related with cell proliferation, cell cycle and with ERK signaling activation by EGFR, suggesting an involvement in EGFR signaling in this tumor type. To verify this, it was produced a mutated NHERF1, which destroyed the interaction with EGFR, and this demonstrated that its overexpression reduced NHERF1 inhibition on EGFR signaling. Furthermore, NHERF1 expression was associated with poor prognosis of a sub-set of CC patients, with continuous EGFR activation [98]. A recent work focused on the anti-metastatic effect of NHERF1 action in breast and CC cells. Overexpression of NHERF1 in cervical cell line inhibited adhesion, wound-healing and invasion, while NHERF1 down-regulation supported them. Moreover, its over-expression inhibited the MMP-2 activity, whereas its down-regulation endorsed it, proposing NHERF1 participation to MMP-2-mediated cell metastasis suppression. In conclusion NHERF1 might be a potential precise therapeutic target or prognostic marker for CC patients [99]. In human cisplatin-resistant cervical cancer cells, NHERF1 overexpression repressed proliferation and increased apoptosis, while NHERF1 down-regulation had inverse effects. In wild type cells its down-regulation increased cisplatin resistance. In cisplatin-resistant cells these studies also revealed an AKT and ERK signaling pathways inhibition. This work demonstrates for the first time that NHERF1 can be involved in cisplatin-resistance of cervical cancer cells [100]. These results are consistent with our previously data published regarding gastric cancer [71].
Prostatic Cancer
In prostatic cancer an immunohistochemical study has revealed a different NHERF1 expression in relation with normal-to metastatic adenocarcinoma sequence, showing a combination of membranous/apical and cytoplasmic staining. A main membranous/apical staining of NHERF1 was present in the benign and pre-neoplastic specimens, while a cytoplasmic staining was observed in primary and metastatic tumors. So, NHERF1 is proving as a potential prognostic marker for patients with prostate cancer too [101].
NHERF1 in Hematological Cancers
Leukemia, lymphomas and myeloma are hematological malignancies originated in the bone marrow and lymph nodes. The incidence of these tumors is high among elder people, and the prognosis and responsiveness show a great variability. NHERF1 engagement in these diseases has been little investigated, but considering recent publications it is possible to hypothesize its involvement in hematological cancer too.
In fact, NHERF1 has been found up-regulated in acute myeloid leukemia (AML) cells treated with Histone Deacetylase Inhibitors (HDACi), altering osteoblast-mediated protection of AML cells. The survival of AML stem cells seems guaranteed by bone marrow microenvironment that plays a protective role towards standard chemotherapeutics. So eradicating this protective environment is crucial for more efficient drug therapies. In this study osteoblasts preserved AML cells from apoptosis in a co-culture model of bone marrow microenvironment, while HDACi treatment reverted this protection, inducing up-regulation of NHERF1 and its interaction with Protein phosphatase-1 (PP1). The interaction between NHERF1-PP1facilitates PP-mediated TAZ dephosphorylation, which is involved in protection of leukemic cells [102].
NHERF1 has been found up-regulated in chronic myeloid leukemia (CML) too. In fact, by proteomic assay, its up-regulation has been observed in imatinib-resistant CML-T1/IR cellular subclones, mimicking acquired drug resistance. Further, in these resistant cells have been detected an altered cytosolic pH and reduced calcium levels, suggesting that NHERF1 may be linked to these alterations and to fundamental signaling pathways such as Wnt pathway. These findings could indicate a new potential therapeutic approach and an improvement of prognosis for imatinib- resistant CML cases [103]. A recent study has reported a putative interaction between NHERF1 and multidrug resistance protein 4 (MRP4). In addition, a redistribution of MRP4 from intracellular structures to the plasma membrane in leukemia cells after NHERF1 silencing has been reported [104].
These first evidences draw a new prospective road for NHERF1, in order to better understand hematological cancer mechanisms and so to tailor the therapeutical management in this field.
NHERF1 in Breast Cancer (BC)
A possible NHERF1 involvement in breast cancerogenesis has been reported for the first time by Stemmer-Rachamimov and colleagues. They showed its overexpression in tumor compared with the non-tumor counterparts of BC samples.
They analyzed 18 infiltrating breast adenocarcinomas by immunohistochemistry and showed a positive NHERF1 immunoreactivity in tumor cells compared to adjacent stroma. Both a membranous and a cytoplasmic staining was shown, with a specific membranous reactivity at persisted ductal-like structures. This was one of the first evidences of aberrant NHERF1 detection in BC, although in a small cohort [105].
To clarify NHERF1 role in BC progression, its expression has been measured by western blot in human breast tumors compared to contiguous healthy tissue, highlighting NHERF1 overexpression in tumor tissues. In this case as well NHERF1 immunolocalization was limited to the apical membrane region of cells in normal lobules, while in tumor lobules a diffuse cytoplasmic distribution could be observed. Furthermore, NHERF1 protein expression was significantly related to increasing tumor cyto-histological dedifferentiation and to poor prognosis [106].
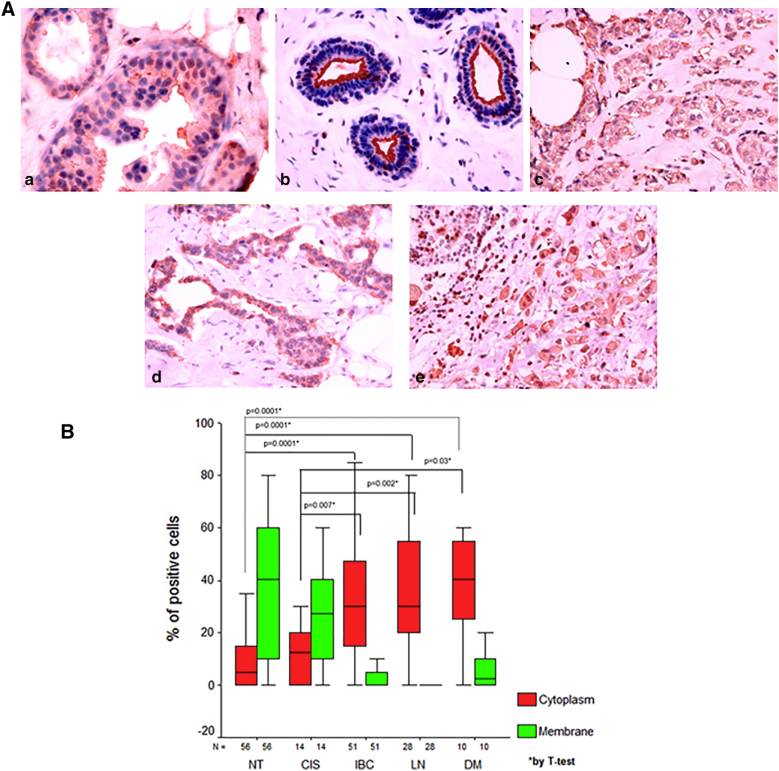
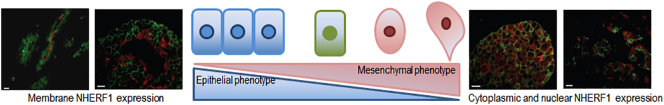
Subsequently, our group [107] has performed an immunohistochemical analysis of NHERF1 expression in a wider cohort of 215 samples including normal breast, ductal carcinoma in situ (DCIS), invasive breast carcinoma (IBC), synchronous metastatic lymph node and metachronous distant metastases. We found that cNHERF1 expression in DCIS, invasive and metastatic tissues was significantly increased compared with the corresponding cytoplasmic expression observed in normal tissues, while the percentage of positive cells with mNHERF1 immunoreactivity in DCIS, IBC and metachronous distant metastasis tissues was significantly decreased compared with normal tissues. An even greater increase in the amount of cNHERF1 in cancerous epithelial cells was observed moving from DCIS to more aggressive IBC and metastases. Furthermore, levels of mNHERF1 were higher in DCIS compared to both IBC and metachronous distant metastases tumor tissues (Figure 5). So, we have demonstrated a significant change in the pattern of cellular NHERF1 distribution from normal to in situ and invasive BC tissue, showing that cNHERF1 staining accumulation could suggest an important role in BC development and tumor progression (Figure 6). This behavior has been confirmed in other tumor types such as colorectal cancer [53], [55] and cholangiocarcinoma [75].
Figure 5.
A. Representative images of NHERF1 immunoreactivity and localization in breast cancer. (a) In ductal carcinoma in situ comedo type NHERF1 presents a cytoplasmic and membrane immunoreactivity; (b) In morphologically normal tissue NHERF1 immunoreactivity is present mostly as apical membranous. In both invasive breast carcinomas (c) and metachronous distant metastases (d), cytoplasmic accumulation is present. (e) In synchronous metastatic lymph node tissues NHERF1 immunoreactivity is present only as cytoplasmic accumulation. B. Cellular distribution of NHERF1 in breast tissues; NT: non tumor, CSI: carcinoma in situ, IBC: invasive breast carcinoma, LN: synchronous metastatic lymph node; DM: metachronous distant metastases.
Figure 6.
NHERF1 representative switch during breast carcinogenesis from normal epithelia to cancer: red signals is NHERF1, Green signal is HEr2/neu. Bar = 16 μm. (Immunofluorescence images were already included in Mangia A. et al., Histopathology 2009)
Given the central role of estrogens in breast physiology in promoting the proliferation of both normal and neoplastic breast epithelium [108], a possible interaction between these hormones and NHERF1 has been investigated. Estrogen receptor-positive (ER+) BC is the most common subtype to be diagnosed, and in vitro and in vivo data show that it is associated to a more favorable prognosis, due to availability of anti-estrogenic endocrine therapy. However, ER+ BCs frequently acquire resistance to endocrine therapy, although ER continues to be expressed. First findings regarding a positive regulation of NHERF1 by estrogens were illustrated in vitro by Ediger [109]. They observed that stimulating MCF-7 cells (an ER+ human BC cell line) with estradiol, there is a 4-5 fold up-regulation of NHERF1 mRNA. To confirm these data were used MDA-MB-231 cells, an ER-negative (ER-) BC cell line, which normally shows low basal levels of NHERF1. Stimulation of these cells with estradiol didn’t show an increase in NHERF1 mRNA. When the same cells were treated with estradiol containing a stably integrated ER expression construct, a 5- to 6-fold increase of NHERF mRNA, compared to untreated cells was observed.
Afterwards, Stemmer-Rachamimov and colleagues [105] confirmed these findings performing quantitative Western blot analysis. They found that levels of NHERF1 were higher in three ER+ BC cell lines, MCF-7, ZR-75-B, and T-47D, compared to normal mammary lines HBL-100 and MCF-12-F. Low levels of NHERF1 were expressed only in estrogen receptor-negative breast cancer lines MDA-MB-231. Moreover, by immunohistochemistry performed on infiltrating BCs, a strong correlation was found between positive immunostaining for ER and high expression of NHERF1. In MCF-7 cells an increased expression of NHERF1 upon stimulation with 17-β-estradiol (E2) has been observed. Interacting with PTEN, NHERF1 enhances its stability and retards its degradation via the ubiquitin-proteasome system, causing an up-regulation of the expression of this signaling molecule [35].
Then, NHERF1 immunostaining has been evaluated in two cohorts of BC patients, the first comprising the whole tissue sections of 49 cases and the second including 120 tissue microarrayed cases [110]. NHERF1 immunopositivity was present in 73,5% and 80% of the cases, respectively. In both cohorts, immunoreactivity was significantly associated with tumor stage, lymph node invasion and ER positivity, and the staining could be observed in normal and cancerous epithelial cells, but not on adjacent stromal cells. In cancer cells could be observed an increase of cytoplasmic accumulation of the protein and an increase of NHERF1 mRNA, whereas in normal cells the staining was mainly apical and membranous. Also a correlation analysis reported in Cardone’s study linked NHERF1 protein expression levels with increasing ER levels in ER+ tumors [106].
An association between NHERF1 and ER was found also by Karn and collaborators [111]. In their study, NHERF1 expression was first examined in a set of 171 tumor samples by immunohistochemistry, finding that NHERF1 expression was higher in the luminal B subtype of BCs compared to any other subtypes. These tumors are characterized by high proliferation and ER positivity, associated with a poor prognosis. The clinical relevance of NHERF1 was subsequently assessed in a database of 3030 microarrays from primitive BCs. In line with previous studies, indicating that NHERF1 is a gene regulated by estrogens, higher levels of NHERF1 transcripts were found in ER+BCs, compared to ER−. Kaplan–Meier analyses of event free survival according to the expression of NHERF1 were performed separately in the ER+ and ER- subgroups of BCs, and a prognostic value of NHERF1 overexpression was only observed among ER+ tumors, which showed a poorer survival. Furthermore, NHERF1 overexpression in ER+ tumors was correlated with a more aggressive phenotype. A recent work showed that NHERF1 is positively related to G protein-coupled receptor (GPER) downstream signaling in ER+ invasive BC specimens, since NHERF1 increased GPER protein stability. GPER signaling was higher in ER+ tumor compared to normal breast tissues, according to high levels of GPER protein in clinical samples, in association with poor prognosis [112].
What makes the study of this protein interesting is that evidences from our laboratory and from other studies indicate that subcellular localization of this protein seems to have different biological significance and role in BC, acting as a tumor suppressor when it is localized at the apical level of the membrane, and as an oncogenic protein when it silocalized in the cytoplasm or nucleus. An analysis in a cohort of 222 IBCs with long-term clinical follow-up showed a prognostic significance between the expression of NHERF1 in the different compartments and clinicopathological characteristics [81]. Cytoplasmic NHERF1 was significantly associated with negative PgR tumors and with HER2 overexpression, while nNHERF1 was associated with small tumor size and positive ER tumors. In this work, the relationship between NHERF1 expression and BC survival has been investigated for the first time. Patients with positive nNHERF1 expression tended toward a higher DFS compared to patients with negative nuclear expression, whereas there was no difference in OS between the two groups.
Differences in DFS were also found when NHERF1 expression was examined in association with ER expression. Indeed, Kaplan–Meier curves showed that patients with nNHERF1-/ER- immunophenotype, having a large tumor size, high histological grade, PgR-negativity and high Ki67 index, had worse DFS compared with patients with the nNHERF1+/ER+ immunophenotype. And interestingly, these patients presented more frequently distant metastases. The loss of nNHERF1 expression is associated with reduced survival, and a link exists between nNHERF1 and ER status as a prognostic marker for the routine clinical management of breast cancer.
In a recent study, the different role of the sublocalization of NHERF1 has been reported in a retrospective series of 308 invasive BCs too. It has been found that nNHERF1 expression was associated with nBRCA1 expression, which is in line with our previous study in which low nBRCA1 and nNHERF1 expression was related to familial story. Furthermore, in the whole cohort there was a direct correlation between cNHERF1 and nPARP1, and the nNHERF1+/ nPARP1+ phenotype showed a shorter 5-year OS. Interestingly, in the subgroup of triple negative breast cancers the association of cNHERF1 with nPARP1 was linked to a shorter survival. These data indicate a new potential biomarker role of NHERF1 in combination with PARP1 and BRCA1 expression to stratify BC patients [113].
NHERF1 and HER2/neu
Among the different proteins that interact with NHERF1, a relevant position is surely that of human epidermal growth factor receptor 2 (HER2/neu).
HER2 is a tyrosine kinase receptor, a member of the epidermal growth factor (EGF) receptor family involved in cell proliferation and survival. Its overexpression is present in approximately 20–30% of BCs, mainly because of an overamplification of its gene. HER2+ BCs are associated with a more aggressive phenotype, with higher recurrence rate and a lower survival, respect to HER2- cancers [114], [115]. The interaction between NHERF1 and HER2/neu has been analyzed by immunofluorescence [107]. In normal epithelial cells, intense NHERF1 immunoreactivity was mainly found at the apical membrane, whereas HER2/neu was found basolaterally and at the intraepithelial junctions. This localization was maintained in DCIS not overexpressing the receptor, while a membranous co-localization with NHERF1 was evident in DCIS overexpressing HER2/neu. Only in IBC and metachronous distant metastases a low membrane staining of NHERF1 could be observed, colocalized with HER2/neu only when the receptor was overexpressed.
In invasive tumor, the cell architecture is completely overturned and cells are able to receive different extracellular signals from the tumor microenvironment. In this pathological state, NHERF1 loses its exclusively apical domain function, is overexpressed in the cytosol and starts to coordinate intracellular pathways [107]. Further, a positive correlation between NHERF1 expression and HER2 status was observed in ER negative and positive tumors.70% of the ER+/ HER2+ and 80 % of the ER -/ HER2+ samples were found in high NHERF1expressing tumors [111]. A similar finding has been observed by Jeong et al. Examining NHERF1 expression by immunofluorescence in 16 HER2+and 4 HER2- DCIS. Furthermore, in a wider cohort of 652 microarrayed invasive BCs, they reported a correlation between NHERF1 expression and HER2+ status [116].
In a study performed on 187 microarrayed BCs from 94 familial and 93 sporadic BCs patients we have found that in multivariate analysis a “new-biomarker” signature, comprising HER2- status, mNHERF1- and nBRCA1+, significantly correlated with family history of BC [117].
NHERF1 and Microenvironment
Over the last decade, the progress in tumor biology knowledge has been able to evaluate the tumors as a complex multicellular group, in close contact with its microenvironment. Many studies have reported the crucial role of tumor microenvironment in cancer progression, including different cellular and molecular players, such as extracellular matrix, tumor-associated fibroblast and macrophages, mast and immune cells [80], [118], [119].
Tumor microenvironment changes, such as hypoxia, lymphocytes infiltration etc., play a key role in tumor development and progression [120]. Affecting gene expression and leading to EMT. The association between NHERF1 and Hypoxia inducible-factor α (HIFα) has been observed by correlation and confocal analysis in invasive BCs, indicating a possible interaction between the metabolic microenvironment and NHERF1. To further clarify this link, BC cell lines MCF-7 and MDA-MB-435 were subjected to hypoxia and serum deprivation experiments. These two conditions not only induced a marked up-regulation of NHERF1 expression in both cell lines, but particularly in MDA-MB-435 induced a change in the shape of cells, that formed long leading-edge pseudopodia in which NHERF1 expression was strong at the tip [106]. At the tip of pseudopodia, NHERF1 colocalizes with NHE1, a protein whose disregulation is a hallmark of cells, undergoing tumorigenesis and metastasis [121].
The role of NHERF1, and in particular the specific role of its PDZ domains in the regulation of the metastatic process, have been better defined in a subsequent study [37]. MDA-MB-231 cells were transfected with wild-type NHERF1 or with NHERF1 mutated in PDZ1 or PDZ2 domain, finding that PDZ domains differentially regulates the expression of two phenotypic programs, thus conferring a specific metastatic organotropism to the cell.
In vitro, PDZ1 mutated cells exhibit a mesenchymal/invasive phenotype with restricted vasculogenic capacity. In vivo this favors the formation of visceral metastases, whereas the PDZ2 mutated cells in vivo exhibit a marked osteotropism due to increased formation of podosomes and stimulation of neoangiogenesis and of osteoclastogenesis.
During the study of possible implication of NHERF1 in cancer evolution, our group has also analyzed its role in relation to microenvironment modifications related to progression, aggressiveness, hypoxic response and invasion in a primary invasive cohort of BCs, including a sub-group of grade 2 cancers. Traditional prognostic factors and a panel of biomarkers not used in routine diagnosis has been analyzed [NHERF1, VEGFR1, HIF-1a, TWIST1 and perivascular tumor invasion (PVI)] [122], [58]. Our results demonstrated that PVI and mNHERF1 categorized grade 2 tumors into two distinct subgroups, exhibiting significantly different prognosis. The PVI+/mNHERF1-expression phenotype was associated to an adverse prognosis. This phenotype was also associated with poor prognosis tumors in the whole cohort of BCs, that also showed cNHERF1 expression co-localization and positively correlation to VEGFR1 [58]. In metastatic colorectal cancer we also reported a closed link between NHERF1 and microenvironment biomarkers. In fact, a co-localization and a positive correlation between nNHERF1 and nuclear HIF-1α and between NHERF1-TWIST1 has been detected too [55].
NHERF1 and Inflammation
Pathological inflammation is a hallmark of numerous diseases and can maintain a cycle of damage/ healing that has been linked to many forms of cancer [123]. Many factors can trigger inflammatory response in tumors, counting infection, tissue damage, activation of oncogenes, and loss of tumor suppressors [124], [125]. Tumor-associated inflammation is often low in grade and chronic and allows nascent tumors to escape immune-surveillance [126] thus taking a pivotal role in cancer growth: from initiation to metastasis development, up to therapy response [127]. The recruitment of inflammatory cells (neutrophils and macrophages) and the production of cytokines and chemokines are at the basis of this response [128]. The inflammatory cellular component makes up a significant cellular percentage of tumor mass, involving resident and infiltrating immune cells [129]. The relationship between tumor cells and infiltrating immune cells is dynamic and can have different effects, positive or negative, on tumor development, feeding a continuous proliferation and cell renewal described as “wounds that do not heal” by Dvorak [130]. Given these opposed activities on cancer progression, it is important to understand the interaction between inflammatory constituents and tumors in order to improve the therapeutic strategies, as well as to incentivize and improve tailored medicine.
Recent studies have underlined the role of tumor-infiltrating lymphocytes (TILs) during carcinogenesis, progression and response to therapy in many solid tumors, including breast cancer [131], [132], [133]. TILs are a composite group of mononuclear immune cells that infiltrate tumor tissue, consisting of cytotoxic T cells and helper T cells, B cells, macrophages, dendritic and natural killer cells, known since the ’70s. During the years TILs have been identified as lymphocytes moving from bloodstream to tumor site [134]. Their involvement is not new in cancer, in fact since 1922 it has been hypothesized a certain contribution of infiltrating lymphocytes related to favorable outcome [135]. This was just a first observation and analogous reports in other cancer types came in following years, up to the recent concept of onco-immunology and immunotherapy in addition to standard treatments in cancer management.
More than one hundred papers about the role of TILs in different cancers have been published during the last years [136], [137], [138], [139], [140], [141], [142], [143], [144], [145], [146], [147], [148], [149], [150], [151], [152], [153], [154], [155]. The theory of the mediation of the immune system on antitumoral effects of traditional anticancer agents (cytotoxic, radiation and antibody-based therapy) has found answers in the new findings of recent translational research. Thus, TILs have acquired a clinical relevance and a key role as they are prognostic or predictive of response to standard cytotoxic or immuno-modulatory treatments. The presence of TILs has been associated to positive patient outcome in many tumors, although the prognostic significance will still remain interconnected with the various TILs subpopulations, density and location and according to tumor type and stage [133], [137], [138], [147].
In a recent immunohistochemical study, a cohort of advanced stage of non-small cell lung cancer patients showed high stromal CD8+ TILs, associated to a partial response to therapy and had a better progression-free-survival and overall survival [137].
Little is known about NHERF1 involvement during inflammation. Some studies reported its down regulation in intestinal bowel disease (IBD) patients and in mouse model of colitis [156], [157], [158]. In inflammatory diseases has been observed the formation of a macromolecular signaling complex NHERF1-CXCR2-PLCβ2, with a PDZ-based interaction, which controls neutrophil infiltration [159], [160]. Furthermore, Leslie and colleagues demonstrated that NHERF1 triggered macrophages activation and increased the reaction of vessels to inflammation [161].
Our pioneeristic study [162] in a group of 55 BC patients confirmed a marked overexpression of NHERF1 protein by western blotting in both primary tumors and metastatic lymph nodes, compared to the non-tumor compartment. Immunostaining in paraffin-embedded tissue showed that NHERF1 expression was limited to the apical membrane regions in the normal lobules, whereas NHERF1 was also expressed in the cytoplasm of the tumor cells and of the majority of the lymphocytes which were present in tumoral and lymph-nodal stroma. Furthermore, we analyzed the expression of NHERF1 in peripheral blood lymphocytes between patients and healthy control group, and found a significantly higher expression in patients. NHERF1 was significantly more expressed in tumor tissues and lymphocytes from grade 3 patients compared to grade 1 and in the poor-prognosis group compared to the good-prognosis one. These evidences underlined an hypothetical involvement of NHERF1 in immunological events associated with neoplastic disease. This is the only study that measures NHERF1 levels in circulating blood lymphocytes and lays the basis of potential use of NHERF1 as an early tumor biomarker in breast cancer.
At the present time, our group is conducting a study about a possible correlation between NHERF1 and inflammatory microenvironment in BC, and preliminary data are extremely heartening and let us insert a new piece in the puzzle of the NHERF1 story.
Conflicts of Interests
The authors declare no conflict of interest.
Acknowledgements
The authors are grateful to Caterina Farina for manuscript language revision.
Footnotes
Funding: This work was supported by founding from Italian Ministry of Health “Ricerca Corrente 2017”.
References
- 1.Hernando N, Déliot N, Gisler SM, Lederer E, Weinman EJ, Biber J, Murer H. PDZ-domain interactions and apical expression of type IIa Na/P(i) cotransporters. Proc Natl Acad Sci U S A. 2002;99(18):11957–11962. doi: 10.1073/pnas.182412699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sneddon WB, Syme CA, Bisello A, Magyar CE, Rochdi MD, Parent JL, Weinman EJ, Abou-Samra AB, Friedman PA. Activation-independent parathyroid hormone receptor internalization is regulated by NHERF1 (EBP50) J Biol Chem. 2003;278(44):43787–43796. doi: 10.1074/jbc.M306019200. [DOI] [PubMed] [Google Scholar]
- 3.Morales FC, Takahashi Y, Momin S, Adams H, Chen X, Georgescu MM. NHERF1/EBP50 head-to-tail intramolecular interaction masks association with PDZ domain ligands. Mol Cell Biol. 2007;27(7):2527–2537. doi: 10.1128/MCB.01372-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shibata T, Chuma M, Kokubu A, Sakamoto M, Hirohashi S. EBP50, a beta-catenin-associating protein, enhances Wnt signaling and is over-expressed in hepatocellular carcinoma. Hepatology. 2003;38(1):178–186. doi: 10.1053/jhep.2003.50270. [DOI] [PubMed] [Google Scholar]
- 5.Fraenzer JT, Pan H, Minimo L, Jr., Smith GM, Knauer D, Hung G. Overexpression of the NF2 gene inhibits schwannoma cell proliferation through promoting PDGFR degradation. Int J Oncol. 2003;23(6):1493–1500. [PubMed] [Google Scholar]
- 6.Weinman EJ, Steplock D, Shenolikar S. cAMP-mediated inhibition of the renal brush border membrane Na+-H+ exchanger requires a dissociable phospho- protein cofactor. J Clin Invest. 1993;92:1781–1786. doi: 10.1172/JCI116767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reczek D, Berryman M, Bretscher A. Identification of NHERF1: A PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family. J Cell Biol. 1997;139(1):169–179. doi: 10.1083/jcb.139.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orlowski J, Grinstein S. Na+/H+ exchangers of mammalian cells. J Biol Chem. 1997;272(36):22373–22376. doi: 10.1074/jbc.272.36.22373. [DOI] [PubMed] [Google Scholar]
- 9.Kurashima K, D'Souza S, Szászi K, Ramjeesingh R, Orlowski J, Grinstein S. The apical Na(+)/H(+) exchanger isoform NHE3 is regulated by the actin cytoskeleton. J Biol Chem. 1999;274(42):29843–29849. doi: 10.1074/jbc.274.42.29843. [DOI] [PubMed] [Google Scholar]
- 10.Good MC, Zalatan JG, Lim WA. Scaffold proteins: hubs for controlling the flow of cellular information. Science. 2011;332(6030):680–686. doi: 10.1126/science.1198701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langeberg LK, Scott JD. Signalling scaffolds and local organization of cellular behaviour. Nat Rev Mol Cell Biol. 2015;16(4):232–244. doi: 10.1038/nrm3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cortese MS, Uversky VN, Dunker AK. Intrinsic disorder in scaffold proteins: getting more from less. Prog Biophys Mol Biol. 2008;98(1):85–106. doi: 10.1016/j.pbiomolbio.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinman EJ, Hall RA, Friedman PA, Liu-Chen LY, Shenolikar S. The association of NHERF adaptor proteins with g protein-coupled receptors and receptor tyrosine kinases. Annu Rev Physiol. 2006;68:491–505. doi: 10.1146/annurev.physiol.68.040104.131050. [DOI] [PubMed] [Google Scholar]
- 14.Morales FC, Takahashi Y, Kreimann EL, Georgescu MM. Ezrin-radixin-moesin (ERM)-binding phosphoprotein 50 organizes ERM proteins at the apical membrane of polarized epithelia. Proc Natl Acad Sci U S A. 2004;101:17705–17710. doi: 10.1073/pnas.0407974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinman EJ, Steplock D, Wang Y, Shenolikar S. Characterization of a protein cofactor that mediates protein kinase A regulation of the renal brush border membrane Na(+)-H+ exchanger. J Clin Invest. 1995;95:2143–2149. doi: 10.1172/JCI117903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berryman M, Franck Z, Bretscher A. Ezrin is concentrated in the apical microvilli of a wide variety of epithelial cells whereas moesin is found primarily in endothelial cells. J Cell Sci. 1993;105(Pt 4):1025–1043. doi: 10.1242/jcs.105.4.1025. [DOI] [PubMed] [Google Scholar]
- 17.Amieva MR, Wilgenbus KK, Furthmayr H. Radixin is a component of hepatocyte microvilli in situ. Exp Cell Res. 1994;210(1):140–144. doi: 10.1006/excr.1994.1021. [DOI] [PubMed] [Google Scholar]
- 18.Garbett D, LaLonde DP, Bretscher A. The scaffolding protein NHERF1 regulates microvillar assembly in a phosphorylation-dependent manner. J Cell Biol. 2010;191:397–413. doi: 10.1083/jcb.201004115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saotome I, Curto M, McClatchey AI. Ezrin is essential for epithelial organization and villus morphogenesis in the developing intestine. Dev Cell. 2004;6:855–864. doi: 10.1016/j.devcel.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 20.Sheng R, Chen Y, Yung Gee H, Stec E, Melowic HR, Blatner NR, Tun MP, Kim Y, Källberg M, Fujiwara TK. Cholesterol modulates cell signaling and protein networking by specifically interacting with PDZ domain-containing scaffold proteins. Nat Commun. 2012;3:1249. doi: 10.1038/ncomms2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voltz JW, Brush M, Sikes S, Steplock D, Weinman EJ, Shenolikar S. Phosphorylation of PDZI domain attenuates NHERF-1 binding to cellular targets. J Biol Chem. 2007;282:33879–33887. doi: 10.1074/jbc.M703481200. [DOI] [PubMed] [Google Scholar]
- 22.Ardura JA, Friedman PA. Regulation of G protein-coupled receptor function by Na+/H+ exchange regulatory factors. Pharmacol Rev. 2011;63(4):882–900. doi: 10.1124/pr.110.004176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fouassier L, Yun CC, Fitz JG, Doctor RB. Evidence for ezrin-radixin-moesinbinding phosphoprotein 50 (EBP50) self-association through PDZ-PDZ interactions. J Biol Chem. 2000;275:25039–25045. doi: 10.1074/jbc.C000092200. [DOI] [PubMed] [Google Scholar]
- 24.Lau AG, Hall RA. Oligomerization of NHERF-1 and NHERF-2 PDZ domains: differential regulation by association with receptor carboxyl-termini and by phosphorylation. Biochemistry. 2001;40:8572–8580. doi: 10.1021/bi0103516. [DOI] [PubMed] [Google Scholar]
- 25.Maudsley S, Zamah AM, Rahman N, Blitzer JT, Luttrell LM, Lefkowitz RJ, Hall RA. Platelet-derived growth factor receptor association with Na(+)/H(+) exchanger regulatory factor potentiates receptor activity. Mol Cell Biol. 2000;20:8352–8363. doi: 10.1128/mcb.20.22.8352-8363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hall RA, Spurney RF, Premont RT, Rahman N, Blitzer JT, Pitcher JA, Lefkowitz RJ. G protein-coupled receptor kinase 6A phosphorylates the Na(+)/H(+) exchanger regulatory factor via a PDZ domain-mediated interaction. J Biol Chem. 1999;274(34):24328–24334. doi: 10.1074/jbc.274.34.24328. [DOI] [PubMed] [Google Scholar]
- 27.Fouassier L, Nichols MT, Gidey E, McWilliams RR, Robin H, Finnigan C, Howell KE, Housset C, Doctor RB. Proteinkinase C regulates the phosphorylation and oligomerization of ERM binding phosphoprotein 50. Exp Cell Res. 2005;306(1):264–273. doi: 10.1016/j.yexcr.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Poulikakos PI, Dai Z, Testa JR, Callaway DJ, Bu Z. Protein kinase C phosphorylation disrupts Na+/H+ exchanger regulatory factor 1 autoinhibition and promotes cystic fibrosis transmembrane conductance regulator macromolecular assembly. J Biol Chem. 2007;282:27086–27099. doi: 10.1074/jbc.M702019200. [DOI] [PubMed] [Google Scholar]
- 29.Weinman EJ, Biswas RS, Peng G, Shen L, Turner CL, Xiaofei E, Steplock D, Shenolikar S, Cunningham R. Parathyroid hormone inhibits renal phosphate transport by phosphorylation of serine 77 of sodium-hydrogen exchanger regulatory factor-1. J Clin Invest. 2007;117(11):3412–3420. doi: 10.1172/JCI32738. [Erratum in: J Clin Invest. 2008 Jan;118(1):387. Peng, Quihong [corrected to Peng, Guihong]. J Clin Invest. 2013 Jun;123(6):2752] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 30.Sun C, Zheng J, Cheng S, Feng D, He J. EBP50 phosphorylation by Cdc2/Cyclin B kinase affects actin cytoskeleton reorganization and regulates functions of human breast cancer cell line MDA-MB-231. Mol Cells. 2013;36:47–54. doi: 10.1007/s10059-013-0014-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim H, Jou TS. Ras-activated RSK1 phosphorylates NHERF1 to regulate its nuclear localization and promote cell proliferation. Oncotarget. 2016;7:10283–10296. doi: 10.18632/oncotarget.7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang B, Yang Y, Friedman PA. Na/H Exchange regulatory factor 1, a novel akt associating protein, regulates extracellular signal-regulated kinase signaling through a B-raf-mediated pathway. Mol Biol Cell. 2008;19:1637–1645. doi: 10.1091/mbc.E07-11-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takahashi Y, Morales FC, Kreimann EL, Georgescu MM. PTEN tumor suppressor associates with NHERF proteins to attenuate PDGF receptor signaling. EMBO J. 2006;25:910–920. doi: 10.1038/sj.emboj.7600979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molina JR, Agarwal NK, Morales FC, Hayashi Y, Aldape KD, Cote G, Georgescu MM. PTEN, NHERF1 and PHLPP form a tumor suppressor network that is disabled in glioblastoma. Oncogene. 2012;31:1264–1274. doi: 10.1038/onc.2011.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang L, Wang Y, Chen P, Hu J, Xiong Y, Feng D, Liu H, Zhang H, Yang H, He J. Na(+)/H(+) exchanger regulatory factor 1 (NHERF1) is required for the estradiol-dependent increase of phosphatase and tensin homolog (PTEN) protein expression. Endocrinology. 2011;152:4537–4549. doi: 10.1210/en.2011-1207. [DOI] [PubMed] [Google Scholar]
- 36.Cardone RA, Greco MR, Capulli M, Weinman EJ, Busco G, Bellizzi A, Casavola V, Antelmi E, Ambruosi B, Dell'Aquila ME. NHERF1 acts as a molecular switch to program metastatic behavior and organotropism via its PDZ domains. Mol Biol Cell. 2012;23:2028–2040. doi: 10.1091/mbc.E11-11-0911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng S, Li Y, Yang Y, Feng D, Yang L, Ma Q, Zheng S, Meng R, Wang S, Wang S. Breast cancer-derived K172N, D301V mutations abolish Na+/H+ exchanger regulatory factor 1 inhibition of platelet-derived growth factor receptor signaling. FEBS Lett. 2013;587:3289–3295. doi: 10.1016/j.febslet.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 38.Songyang Z, Fanning AS, Fu C, Xu J, Marfatia SM, Chishti AH, Crompton A, Chan AC, Anderson JM, Cantley LC. Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science. 1997;275(5296):73–77. doi: 10.1126/science.275.5296.73. [DOI] [PubMed] [Google Scholar]
- 39.MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shibata T, Chuma M, Kokubu A, Sakamoto M, Hirohashi S. NHERF1, a beta-catenin associating protein, enhances Wnt signaling and is over-expressed in hepatocellular carcinoma. Hepatology. 2003;38:178–186. doi: 10.1053/jhep.2003.50270. [DOI] [PubMed] [Google Scholar]
- 41.Kreimann EL, Morales FC, de Orbeta-Cruz J, Takahashi Y, Adams H, Liu TJ, McCrea PD, Georgescu MM. Cortical stabilization of beta-catenin contributes to NHERF1/NHERF1 tumor suppressor function. Oncogene. 2007;26(36):5290–5299. doi: 10.1038/sj.onc.1210336. [DOI] [PubMed] [Google Scholar]
- 42.Wheeler DS, Barrick SR, Grubisha MJ, Brufsky AM, Friedman PA, Romero G. Direct interaction between NHERF1 and Frizzled regulates β-catenin signaling. Oncogene. 2011;30(1):32–42. doi: 10.1038/onc.2010.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lazar CS, Cresson CM, Lauffenburger DA, Gill GN. The Na+/H+ exchanger regulatory factor stabilizes epidermal growth factor receptors at the cell surface. Mol Biol Cell. 2004;15:5470–5480. doi: 10.1091/mbc.E04-03-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F, Salomon DS. Epidermal growth factor receptor (EGFR) signaling in cancer.Gene. 2006;366(1):2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 45.Bellizzi A, Greco MR, Rubino R, Paradiso A, Forciniti S, Zeeberg K, Cardone RA, Reshkin SJ. The scaffolding protein NHERF1 sensitizes EGFR-dependent tumor growth, motility and invadopodia function to gefitinib treatment in breast cancer cells. Int J Oncol. 2015;46:1214–1224. doi: 10.3892/ijo.2014.2805. [DOI] [PubMed] [Google Scholar]
- 46.Yao W, Feng D, Bian W, Yang L, Li Y, Yang Z, Xiong Y, Zheng J, Zhai R, He J. NHERF1 inhibits EGF-induced breast cancer cell proliferation by blocking EGFR phosphorylation. Amino Acids. 2012;43:2027–2035. doi: 10.1007/s00726-012-1277-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DU G, Hao C, Gu Y, Wang Z, Jiang WG, He J, Cheng S. A novel NHERF1 mutation in human breast cancer inactivates inhibition by NHERF1 protein in EGFR signaling. Anticancer Res. 2016;36:1165–1173. [PubMed] [Google Scholar]
- 48.Clapéron A, Guedj N, Mergey M, Vignjevic D, Desbois-Mouthon C, Boissan M, Saubaméa B, Paradis V, Housset C, Fouassier L. Loss of NHERF1 stimulates EGFR activity to induce EMT phenotypic features in biliary cancer cells. Oncogene. 2012;31:1376–1388. doi: 10.1038/onc.2011.334. [DOI] [PubMed] [Google Scholar]
- 49.Peng XL, Ji MY, Yang ZR, Song J, Dong WG. Tumor suppressor function of ezrin-radixin-moesin-binding phosphoprotein-50 through β-catenin/E-cadherin pathway in human hepatocellular cancer. World J Gastroenterol. 2013;19(8):1306–1313. doi: 10.3748/wjg.v19.i8.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nguyen Ho-Bouldoires T, Clapéron A, Mergey M, Wendum D, Desbois-Mouthon C, Tahraoui S, Fartoux L, Chettouh H. Mitogen-activated protein kinase-activated protein kinase 2 mediates resistance to hydrogen peroxide-induced oxidative stress in human hepatobiliary cancer cells. Free Radic Biol Med. 2015;89:34–46. doi: 10.1016/j.freeradbiomed.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 51.Schirosi L, Mazzotta A, Opinto G, Pinto R, GrazianoG Tommasi S, Fucci L, Simone G, Mangia A. β-catenin interaction with NHERF1 and RASSF1A methylation in metastatic colorectal cancer patients. Oncotarget. 2016;7(42):67841–67850. doi: 10.18632/oncotarget.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richter AM, Pfeifer GP, Dammann RH. The RASSF proteins in cancer; from epigenetic silencing to functional characterization. Biochim Biophys Acta. 2009;1796(2):114–128. doi: 10.1016/j.bbcan.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 53.Hayashi Y, Molina JR, Hamilton SR, Georgescu MM. NHERF1/EBP50 is a new marker in colorectal cancer. Neoplasia. 2010;12(12):1013–1022. doi: 10.1593/neo.10780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Georgescu MM, Cote G, Agarwal NK, White CL., III NHERF1/EBP50 controls morphogenesis of 3D colonic glands by stabilizing PTEN and ezrin-radixin-moesin proteins at the apical membrane. Neoplasia. 2014;16(4):365–374. doi: 10.1016/j.neo.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mangia A, Saponaro C, Malfettone A, Bisceglie D, Bellizzi A, Asselti M, Popescu O, Reshkin SJ, Paradiso A, Simone G. Involvement of nuclear NHERF1 in colorectal cancer progression. Oncol Rep. 2012;28(3):889–894. doi: 10.3892/or.2012.1895. [DOI] [PubMed] [Google Scholar]
- 56.Nagaraju GP, Bramhachari PV, Raghu G, El-Rayes BF. Hypoxia inducible factor-1α: Its role in colorectal carcinogenesis and metastasis. Cancer Lett. 2015;366(1):11–18. doi: 10.1016/j.canlet.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 57.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117(7):927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 58.Malfettone A, Silvestris N, Paradiso A, Mattioli E, Simone G, Mangia A. Overexpression of nuclear NHERF1 in advanced colorectal cancer: association with hypoxic microenvironment and tumor invasive phenotype. Exp Mol Pathol. 2012;92(3):296–303. doi: 10.1016/j.yexmp.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 59.Malfettone A, Silvestris N, Saponaro C, Ranieri G, Russo A, Caruso S, Popescu O, Simone G, Paradiso A, Mangia A. High density of tryptase-positive mast cells in human colorectal cancer: a poor prognostic factor related to protease-activated receptor 2 expression. J Cell Mol Med. 2013;17(8):1025–1037. doi: 10.1111/jcmm.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gu Y, Yu H, Hao C, Martin TA, Hargest R, He J, Cheng S, Jiang WG. NHERF1 regulates the progression of colorectal cancer through the interplay with VEGFR2 pathway. Oncotarget. 2017;8(5):7753–7765. doi: 10.18632/oncotarget.13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin YY, Hsu YH, Huang HY, Shann YJ, Huang CY, Wei SC, Chen CL, Jou TS. Aberrant nuclear localization of EBP50 promotes colorectal carcinogenesis in xenotransplanted mice by modulating TCF-1 and β-catenin interactions. J Clin Invest. 2012;122(5):1881–1894. doi: 10.1172/JCI45661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, Knuechel R, Kirchner T. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci U S A. 2001;98(18):10356–10361. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Georgescu MM, Gagea M, Cote G. NHERF1/EBP50 suppresses Wnt-β-Catenin Pathway-Driven Intestinal Neoplasia. Neoplasia. 2016;18(8):512–523. doi: 10.1016/j.neo.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ehmer U, Sage J. Control of Proliferation and Cancer Growth by the Hippo Signaling Pathway. Mol Cancer Res. 2016;14(2):127–140. doi: 10.1158/1541-7786.MCR-15-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ji MY, Fan DK, Lv XG, Peng XL, Lei XF, Dong WG. The detection of EBP50 expression using quantum dot immunohistochemistry in pancreatic cancer tissue and down-regulated EBP50 effect on PC-2 cells. J Mol Histol. 2012;43(5):517–526. doi: 10.1007/s10735-012-9424-0. [DOI] [PubMed] [Google Scholar]
- 66.Ji M, Yuan L, Lv X, Dong W, Peng X. EBP50 regulates the apoptosis of pancreatic cancer cells by decreasing the expression levels of Bcl-2. Exp Ther Med. 2014;8(3):919–924. doi: 10.3892/etm.2014.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ji M, Fan D, Yuan L, Zhang Y, Dong W, Peng X. EBP50 inhibits pancreatic cancer cell growth and invasion by targeting the β-catenin/E-cadherin pathway. Exp Ther Med. 2015;10(4):1311–1316. doi: 10.3892/etm.2015.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baugher PJ, Richmond A. The carboxyl-terminal PDZ ligand motif of chemokine receptor CXCR2 modulates post-endocytic sorting and cellular chemotaxis. J Biol Chem. 2008;283(45):30868–30878. doi: 10.1074/jbc.M804054200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hwang JI, Kim HS, Lee JR, Kim E, Ryu SH, Suh PG. The interaction of phospholipase C-beta3 with Shank2 regulates mGluR-mediated calcium signal. J Biol Chem. 2005;280(13):12467–12473. doi: 10.1074/jbc.M410740200. [DOI] [PubMed] [Google Scholar]
- 70.Wang S, Wu Y, Hou Y, Guan X, Castelvetere MP, Oblak JJ, Banerjee S, Filtz TM, Sarkar FH, Chen X. CXCR2 macromolecular complex in pancreatic cancer: a potential therapeutic target in tumor growth. Transl Oncol. 2013;6(2):216–225. doi: 10.1593/tlo.13133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mangia A, Caldarola L, Dell'Endice S, Scarpi E, Saragoni L, Monti M, Santini D, Brunetti O, Simone G, Silvestris N. The potential predictive role of nuclear NHERF1 expression in advanced gastric cancer patients treated with epirubicin/oxaliplatin/capecitabine first line chemotherapy. Cancer Biol Ther. 2015;16(8):1140–1147. doi: 10.1080/15384047.2015.1056414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lv XG, Lei XF, Ji MY, Guo XF, Wang J, Dong WG. Clinical significance of EBP50 overexpression assessed by quantum dot analysis in gastric cancer. Oncol Lett. 2013;5(6):1844–1848. doi: 10.3892/ol.2013.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lv XG, Ji MY, Dong WG, Lei XF, Liu M, Guo XF, Wang J, Fang C. EBP50 gene transfection promotes 5-fluorouracil-induced apoptosis in gastric cancer cells through Bax- and Bcl-2-triggered mitochondrial pathways. Mol Med Rep. 2012;5(5):1220–1226. doi: 10.3892/mmr.2012.805. [DOI] [PubMed] [Google Scholar]
- 74.Wang L, Du YR, Ji MY, Wang W, Zhan N, Zhou QS, Dong WG. Reduced EBP50 expression or mis-localization of the EBP50 protein is associated with the malignant progression of esophageal squamous cell carcinoma. Eur Rev Med Pharmacol Sci. 2014;18(24):3854–3863. [PubMed] [Google Scholar]
- 75.Yonglitthipagon P, Pairojkul C, Chamgramol Y, Loukas A, Mulvenna J, Bethony J, Bhudhisawasdi V, Sripa B. Prognostic significance of peroxiredoxin 1 and ezrin-radixin-moesin-binding phosphoprotein 50 in cholangiocarcinoma. Hum Pathol. 2012;43(10):1719–1730. doi: 10.1016/j.humpath.2011.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clapéron A, Guedj N, Mergey M, Vignjevic D, Desbois-Mouthon C, Boissan M, Saubaméa B, Paradis V, Housset C, Fouassier L. Loss of EBP50 stimulates EGFR activity to induce EMT phenotypic features in biliary cancer cells. Oncogene. 2012;31(11):1376–1388. doi: 10.1038/onc.2011.334. [DOI] [PubMed] [Google Scholar]
- 77.Lo HW, Hsu SC, Xia W, Cao X, Shih JY, Wei Y, Abbruzzese JL, Hortobagyi GN, Hung MC. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial–mesenchimal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Res. 2007;67:9066–9076. doi: 10.1158/0008-5472.CAN-07-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Feng D, Xiong Y, Peng Z, Ma Q, Tao T, Liu H, Liang J, Wei Z, Zheng J, Wang L. Reduced EBP50 expression levels are correlated with unfavorable clinicopathological features of extrahepatic bile duct carcinoma and promote the proliferation and migration of QBC939 cells. Oncol Lett. 2017;13(4):2758–2764. doi: 10.3892/ol.2017.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mangia A, Partipilo G, Schirosi L, Saponaro C, Galetta D, Catino A, Scattone A, Simone G. Fine Needle Aspiration Cytology: A Tool to Study NHERF1 Expression as a Potential Marker of Aggressiveness in Lung Cancer. Mol Biotechnol. 2015;57(6):549–557. doi: 10.1007/s12033-015-9848-3. [DOI] [PubMed] [Google Scholar]
- 80.Mangia A, Malfettone A, Rossi R, Paradiso A, Ranieri G, Simone G, Resta L. Tissue remodelling in breast cancer: human mast cell tryptase as an initiator of myofibroblast differentiation. Histopathology. 2011;58(7):1096–1106. doi: 10.1111/j.1365-2559.2011.03842.x. [DOI] [PubMed] [Google Scholar]
- 81.Paradiso A, Scarpi E, Malfettone A, Addati T, Giotta F, Simone G, Amadori D, Mangia A. Nuclear NHERF1 expression as a prognostic marker in breast cancer. Cell Death Dis. 2013;4(11):e904. doi: 10.1038/cddis.2013.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang F, Gu Y, Zhao Z, Huang J, Jiang WG, Cheng S. NHERF1 Suppresses Lung Cancer Cell Migration by Regulation of Epithelial-Mesenchymal Transition. Anticancer Res. 2017;37(8):4405–4414. doi: 10.21873/anticanres.11835. [DOI] [PubMed] [Google Scholar]
- 83.Giese A, Bjerkvig R, Berens ME, Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol. 2003;21(8):1624–1636. doi: 10.1200/JCO.2003.05.063. [DOI] [PubMed] [Google Scholar]
- 84.Gilbert MR. New treatments for malignant gliomas: careful evaluation and cautious optimism required. Ann Intern Med. 2006;144(5):371–373. doi: 10.7326/0003-4819-144-5-200603070-00015. [DOI] [PubMed] [Google Scholar]
- 85.Kislin KL, McDonough WS, Eschbacher JM, Armstrong BA, Berens ME. NHERF-1: modulator of glioblastoma cell migration and invasion. Neoplasia. 2009;11(4):377–387. doi: 10.1593/neo.81572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Molina JR, Morales FC, Hayashi Y, Aldape KD, Georgescu MM. Loss of PTEN binding adapter protein NHERF1 from plasma membrane in glioblastoma contributes to PTEN inactivation. Cancer Res. 2010;70(17):6697–6703. doi: 10.1158/0008-5472.CAN-10-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Molina JR, Agarwal NK, Morales FC, Hayashi Y, Aldape KD, Cote G, Georgescu MM. PTEN, NHERF1 and PHLPP form a tumor suppressor network that is disabled in glioblastoma. Oncogene. 2012;31(10):1264–1274. doi: 10.1038/onc.2011.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reni M, Mazza E, Zanon S, Gatta G, Vecht CJ. Central nervous system gliomas. Crit Rev Oncol Hematol. 2017;113:213–234. doi: 10.1016/j.critrevonc.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 89.Georgescu MM, Yell P, Mobley BC, Shang P, Georgescu T, Wang SH, Canoll P, Hatanpaa KJ, White CL, 3rd, Raisanen JM. NHERF1/EBP50 is an organizer of polarity structures and a diagnostic marker in ependymoma. Acta Neuropathol Commun. 2015;3:11. doi: 10.1186/s40478-015-0197-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nambirajan A, Sharma MC, Rajeshwari M, Kakkar A, Suri V, Sarkar C. A Comparative Immunohistochemical Study of Epithelial Membrane Antigen and NHERF1/EBP50 in the Diagnosis of Ependymomas. Appl Immunohistochem Mol Morphol. 2018;26(1):71–78. doi: 10.1097/PAI.0000000000000384. [DOI] [PubMed] [Google Scholar]
- 91.Georgescu MM, Mobley BC, Orr BA, Shang P, Lehman NL, Zhu X, O'Neill TJ, Rajaram V, Hatanpaa KJ, Timmons CF. NHERF1/EBP50 and NF2 as diagnostic markers for choroid plexus tumors. Acta Neuropathol Commun. 2016;4(1):55. doi: 10.1186/s40478-016-0329-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fang XY, Song R, Chen W, Yang YY, Gu YH, Shu YQ, Wu XD, Wu XF, Sun Y, Shen Y. PRL-3 Promotes the Malignant Progression of Melanoma via Triggering Dephosphorylation and Cytoplasmic Localization of NHERF1. J Invest Dermatol. 2015;135(9):2273–2282. doi: 10.1038/jid.2015.154. [DOI] [PubMed] [Google Scholar]
- 93.Tabrizi AD, Kalloger SE, Köbel M, Cipollone J, Roskelley CD, Mehl E, Gilks CB. Primary ovarian mucinous carcinoma of intestinal type: significance of pattern of invasion and immunohistochemical expression profile in a series of 31 cases. Int J Gynecol Pathol. 2010;29(2):99–107. doi: 10.1097/PGP.0b013e3181bbbcc1. [DOI] [PubMed] [Google Scholar]
- 94.Kreimann EL, Ratajska M, Kuzniacka A, Demacopulo B, Stukan M, Limon J. A novel splicing mutation in the SLC9A3R1 gene in tumors from ovarian cancer patients. Oncol Lett. 2015;10(6):3722–3726. doi: 10.3892/ol.2015.3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Demacopulo B, Lema BE, Cabrini RL, Kreimann EL. Similar expression pattern of NHERF1 and EZRIN in papillary but not in solid areas of human serous ovarian carcinomas. Acta Histochem. 2016;118(8):797–805. doi: 10.1016/j.acthis.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 96.Oh YS, Heo K, Kim EK, Jang JH, Bae SS, Park JB, Kim YH, Song M, Kim SR, Ryu SH. Dynamic relocalization of NHERF1 mediates chemotactic migration of ovarian cancer cells toward lysophosphatidic acid stimulation. Exp Mol Med. 2017;49(7):e351. doi: 10.1038/emm.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Accardi R, Rubino R, Scalise M, Gheit T, Shahzad N, Thomas M, Banks L, Indiveri C, Sylla BS, Cardone RA. E6 and E7 from human papillomavirus type 16 cooperate to target the PDZ protein Na/H exchange regulatory factor 1. J Virol. 2011;85(16):8208–8216. doi: 10.1128/JVI.00114-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peng Z, Wang Q, Zhang Y, He J, Zheng J. EBP50 interacts with EGFR and regulates EGFR signaling to affect the prognosis of cervical cancer patients. Int J Oncol. 2016;49(4):1737–1745. doi: 10.3892/ijo.2016.3655. [DOI] [PubMed] [Google Scholar]
- 99.Wang L, Qi Y, Xiong Y, Peng Z, Ma Q, Zhang Y, Song J, Zheng J. Ezrin-Radixin-Moesin Binding Phosphoprotein 50 (EBP50) Suppresses the Metastasis of Breast Cancer and HeLa Cells by Inhibiting Matrix Metalloproteinase-2 Activity. Anticancer Res. 2017;37(8):4353–4360. doi: 10.21873/anticanres.11829. [DOI] [PubMed] [Google Scholar]
- 100.Tao T, Yang X, Qin Q, Shi W, Wang Q, Yang Y, He J. NHERF1 Enhances Cisplatin Sensitivity in Human Cervical Cancer Cells. Int J Mol Sci. 2017;18(1):5. doi: 10.3390/ijms18010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bartholow TL, Chandran UR, Becich MJ, Parwani AV. Immunohistochemical staining of radixin and moesin in prostatic adenocarcinoma. BMC Clin Pathol. 2011;11:1. doi: 10.1186/1472-6890-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kremer KN, Dudakovic A, Hess AD, Smith BD, Karp JE, Kaufmann SH, Westendorf JJ, van Wijnen AJ, Hedin KE. Histone Deacetylase Inhibitors Target the Leukemic Microenvironment by Enhancing a Nherf1-Protein Phosphatase 1α-TAZ Signaling Pathway in Osteoblasts. J Biol Chem. 2015;290(49):29478–29492. doi: 10.1074/jbc.M115.668160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Toman O, Kabickova T, Vit O, Fiser R, Polakova KM, Zach J, Linhartova J, Vyoral D, Petrak J. Proteomic analysis of imatinib-resistant CML-T1 cells reveals calcium homeostasis as a potential therapeutic target. Oncol Rep. 2016;36(3):1258–1268. doi: 10.3892/or.2016.4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Schaletzki Y, Kromrey ML, Bröderdorf S, Hammer E, Grube M, Hagen P, Sucic S, Freissmuth M, Völker U, Greinacher A. Several adaptor proteins promote intracellular localisation of the transporter MRP4/ABCC4 in platelets and haematopoietic cells. Thromb Haemost. 2016;117(1):105–115. doi: 10.1160/TH16-01-0045. [DOI] [PubMed] [Google Scholar]
- 105.Stemmer-Rachamimov AO, Wiederhold T, Nielsen GP, James M, Pinney-Michalowski D, Roy JE, Cohen WA, Ramesh V, Louis DN. NHE-RF, a merlin-interacting protein, is primarily expressed in luminal epithelia, proliferative endometrium, and estrogen receptor-positive breast carcinomas. Am J Pathol. 2001;158:57–62. doi: 10.1016/S0002-9440(10)63944-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cardone RA, Bellizzi A, Busco G, Weinman EJ, Dell’Aquila ME, Casavola V, Azzariti A, Mangia A, Paradiso A, Reshkin SJ. The NHERF1 PDZ2 domain regulates PKA-RhoA-p38-mediated NHE1 activation and invasion in breast tumor cells. Mol Biol Cell. 2007;18:1768–1780. doi: 10.1091/mbc.E06-07-0617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mangia A, Chiriatti A, Bellizzi A, Malfettone A, Stea B, Zito FA, Reshkin SJ, Simone G, Paradiso A. Biological role of NHERF1 protein expression in breast cancer. Histopathology. 2009;55(5):600–608. doi: 10.1111/j.1365-2559.2009.03424.x. [DOI] [PubMed] [Google Scholar]
- 108.Russo J, Russo IH. The role of estrogen in the initiation of breast cancer. J Steroid Biochem Mol Biol. 2006;102(1-5):89–96. doi: 10.1016/j.jsbmb.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ediger TR, Kraus WL, Weinman EJ, Katzenellenbogen BS. Estrogen receptor regulation of the Na+/H+ exchange regulatory factor. Endocrinology. 1999;140:2976–2982. doi: 10.1210/endo.140.7.6885. [DOI] [PubMed] [Google Scholar]
- 110.Song J, Bai J, Yang W, Gabrielson EW, Chan DW, Zhang Z. Histopathology. Expression and clinicopathological significance of oestrogen-responsive ezrin-radixin-moesin- binding phosphoprotein 50 in breast cancer. 2007;51(1):40–53. doi: 10.1111/j.1365-2559.2007.02730.x. [DOI] [PubMed] [Google Scholar]
- 111.Karn T, Ruckhäberle E, Hanker L, Müller V, Schmidt M, Solbach C, Gätje R, Gehrmann M, Holtrich U, Kaufmann M. Gene expression profiling of luminal B breast cancers reveals NHERF1 as a new marker of endocrine resistance. Breast Cancer Res Treat. 2011;130(2):409–420. doi: 10.1007/s10549-010-1333-x. [DOI] [PubMed] [Google Scholar]
- 112.Meng R, Qin Q, Xiong Y, Wang Y, Zheng J, Zhao Y, Tao T, Wang Q, Liu H, Wang S. NHERF1, a novel GPER associated protein, increases stability and activation of GPER in ER-positive breast cancer. Oncotarget. 2016;7(34):54983–54997. doi: 10.18632/oncotarget.10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mangia A, Scarpi E, Partipilo G, Schirosi L, Opinto G, Giotta F, Simone G. NHERF1 together with PARP1 and BRCA1 expression as a new potential biomarker to stratify breast cancer patients. Oncotarget. 2017;8(39):65730–65742. doi: 10.18632/oncotarget.19444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yarden Y. The EGFR family and its ligands in human cancer. Signalling mechanisms and therapeutic opportunities. Eur J Cancer. 2001;37(Suppl. 4):S3–S8. doi: 10.1016/s0959-8049(01)00230-1. [DOI] [PubMed] [Google Scholar]
- 115.Salamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 116.Jeong J, VanHouten JN, Kim W, Dann P, Sullivan C, Choi J, Sneddon WB, Friedman PA, Wysolmerski JJ. The scaffolding protein NHERF1 regulates the stability and activity of the tyrosine kinase HER2. J Biol Chem. 2017;292(16):6555–6568. doi: 10.1074/jbc.M116.770883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mangia A, Malfettone A, Saponaro C, Tommasi S, Simone G, Paradiso A. Human epidermal growth factor receptor 2, Na+/H+ exchanger regulatory factor 1, and breast cancer susceptibility gene-1 as new biomarkers for familial breast cancers. Hum Pathol. 2011;42(11):1589–1595. doi: 10.1016/j.humpath.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 118.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49-6. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Novikova MV, Khromova NV, Kopnin PB. Components of the Hepatocellular Carcinoma Microenvironment and Their Role in Tumor Progression. Biochemistry (Mosc) 2017;82(8):861–873. doi: 10.1134/S0006297917080016. [DOI] [PubMed] [Google Scholar]
- 120.Spill F, Reynolds DS, Kamm RD, Zaman MH. Impact of the physical microenvironment on tumor progression and metastasis. Curr Opin Biotechnol. 2016;40:41–48. doi: 10.1016/j.copbio.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Amith SR, Fong S, Baksh S, Fliegel L. Na (+)/H (+)exchange in the tumour microenvironment: does NHE1 drive breast cancer carcinogenesis? Int J Dev Biol. 2015;59(7-9):367–377. doi: 10.1387/ijdb.140336lf. [DOI] [PubMed] [Google Scholar]
- 122.Sabatier R, Jacquemier J, Bertucci F, Esterni B, Finetti P, Azario F, Birnbaum D, Viens P, Gonçalves A, Extra JM. Peritumoural vascular invasion: a major determinant of triple-negative breast cancer outcome. Eur J Cancer. 2011;47(10):1537–1545. doi: 10.1016/j.ejca.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 123.Medzhitov R. Inflammation: new adventures of an old flame. Cell. 2010;140:771–776. doi: 10.1016/j.cell.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 124.Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 125.Kuraishy A, Karin M, Grivennikov SI. Tumor promotion via injury- and death-induced inflammation. Immunity. 2011;35(4):467–477. doi: 10.1016/j.immuni.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Elinav E, Nowarski R, Thaiss CA, Hu B, Jin C, Flavell RA. Inflammation-induced cancer: crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 127.Yang L, Lin PC. Mechanisms that drive inflammatory tumor microenvironment, tumor heterogeneity, and metastatic progression. Semin Cancer Biol. 2017;47:185–195. doi: 10.1016/j.semcancer.2017.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315(26):1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 131.Su X, Ye J, Hsueh EC, Zhang Y, Hoft DF, Peng G. Tumor microenvironments direct the recruitment and expansion of human Th17 cells. J Immunol. 2010;184(3):1630–1641. doi: 10.4049/jimmunol.0902813. [DOI] [PubMed] [Google Scholar]
- 132.Stanton SE, Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J Immunother Cancer. 2016;4:59. doi: 10.1186/s40425-016-0165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dieci MV, Criscitiello C, Goubar A, Viale G, Conte P, Guarner V, Ficarra G, Mathieu MC, Delaloge S, Curigliano G. Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Ann Oncol. 2015;26:1518. doi: 10.1093/annonc/mdv241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sackstein R, Schatton T, Barthel SR. T-lymphocyte homing: an underappreciated yet critical hurdle for successful cancer immunotherapy. Lab Investig. 2017;97(6):669–697. doi: 10.1038/labinvest.2017.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F. (2014). The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group. Ann Oncol. 2015;26:259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chang YL, Yang CY, Huang YL, Wu CT, Yang PC. High PD-L1 expression is associated with stage IV disease and poorer overall survival in 186 cases of small cell lung cancers. Oncotarget. 2017;8(11):18021–18030. doi: 10.18632/oncotarget.14935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.He Y, Yu H, Rozeboom L, Rivard CJ, Ellison K, Dziadziuszko R, Suda K, Ren S, Wu C, Hou L. LAG-3 Protein Expression in Non-Small Cell Lung Cancer and Its Relationship with PD-1/PD-L1 and Tumor-Infiltrating Lymphocytes. J Thorac Oncol. 2017;12(5):814–823. doi: 10.1016/j.jtho.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 138.Koh J, Kim S, Kim MY, Go H, Jeon YK, Chung DH. Prognostic implications of intratumoral CD103+ tumor-infiltrating lymphocytes in pulmonary squamous cell carcinoma. Oncotarget. 2017;8(8):13762–13769. doi: 10.18632/oncotarget.14632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rashed HE, Abdelrahman AE, Abdelgawad M, Balata S, Shabrawy ME. Prognostic Significance of Programmed Cell Death Ligand 1 (PD-L1), CD8+ Tumor-Infiltrating Lymphocytes and p53 in Non-Small Cell Lung Cancer: An Immunohistochemical Study. Turk Patoloji Derg. 2017;1(1):1–12. doi: 10.5146/tjpath.2017.01398. [DOI] [PubMed] [Google Scholar]
- 140.Altan M, Pelekanou V, Schalper KA, Toki M, Gaule P, Syrigos K, Herbst RS, Rimm DL. B7-H3 Expression in NSCLC and Its Association with B7-H4, PD-L1 and Tumor-Infiltrating Lymphocytes. Clin Cancer Res. 2017;23(17):5202–5209. doi: 10.1158/1078-0432.CCR-16-3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tas F, Erturk K. Tumor Infiltrating Lymphocytes (TILs) May be Only an Independent Predictor of Nodal Involvement but not for Recurrence and Survival in Cutaneous Melanoma Patients. Cancer Investig. 2017;11:1–5. doi: 10.1080/07357907.2017.1351984. [DOI] [PubMed] [Google Scholar]
- 142.Rajabi P, Bagheri A, Hani M. Intratumoral and Peritumoral Mast Cells in Malignant Melanoma: An Immunohistochemical Study. Adv Biomed Res. 2017;6:39. doi: 10.4103/2277-9175.204592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li J, Wang J, Chen R, Bai Y, Lu X. The prognostic value of tumor-infiltrating T lymphocytes in ovarian cancer. Oncotarget. 2017;8(9):15621–15631. doi: 10.18632/oncotarget.14919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mesnage SJL, Auguste A, Genestie C, Dunant A, Pain E, Drusch F, Gouy S, Morice P, Bentivegna E, Lhomme C. Neoadjuvant chemotherapy (NACT) increases immune infiltration and programmed death-ligand 1 (PD-L1) expression in epithelial ovarian cancer (EOC) Ann Oncol. 2017;28(3):651–657. doi: 10.1093/annonc/mdw625. [DOI] [PubMed] [Google Scholar]
- 145.Fang W, Chen Y, Sheng J, Zhou T, Zhang Y, Zhan J, Liu L, Huang J, Peng P, Zhang L. Association between PD-L1 Expression on Tumour-Infiltrating Lymphocytes and Overall Survival in Patients with Gastric Cancer. J Cancer. 2017;8(9):1579–1585. doi: 10.7150/jca.18729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kawazoe A, Kuwata T, Kuboki Y, Shitara K, Nagatsuma AK, Aizawa M, Yoshino T, Doi T, Ohtsu A, Ochiai A. Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein-Barr virus status in a large cohort of gastric cancer patients. Gastric Cancer. 2017;20(3):407–415. doi: 10.1007/s10120-016-0631-3. [DOI] [PubMed] [Google Scholar]
- 147.Shibutani M, Maeda K, Nagahara H, Fukuoka T, Nakao S, Matsutani S, Hirakawa K, Ohira M. The Prognostic Significance of the Tumor-infiltrating Programmed Cell Death-1+to CD8+Lymphocyte Ratio in Patients with Colorectal Cancer. Anticancer Res. 2017;37(8):4165–4417. doi: 10.21873/anticanres.11804. [DOI] [PubMed] [Google Scholar]
- 148.Inoue Y, Hazama S, Suzuki N, Tokumitsu Y, Kanekiyo S, Tomochika S, Tsunedomi R, Tokuhisa Y, Iida M, Sakamoto K. Cetuximab strongly enhances immune cell infiltration into liver metastatic sites in colorectal cancer. Cancer Sci. 2017;108(3):455–460. doi: 10.1111/cas.13162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Li Y, Liang L, Dai W, Cai G, Xu Y, Li X, Li Q, Cai S. Prognostic impact of programed cell death-1 (PD-1) and PD-ligand 1 (PD-L1) expression in cancer cells and tumor infiltrating lymphocytes in colorectal cancer. Mol Cancer. 2016;15(1):55. doi: 10.1186/s12943-016-0539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shen T, Zhou L, Shen H, Shi C, Jia S, Ding GP, Cao L. Prognostic value of programmed cell death protein 1 expression on CD8+ T lymphocytes in pancreatic cancer. Sci Rep. 2017;7(1):7848. doi: 10.1038/s41598-017-08479-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Taylor NA, Vick SC, Iglesia MD, Brickey WJ, Midkiff BR, McKinnon KP, Reisdorf S, Anders CK, Carey LA, Parker JS. Treg depletion potentiates checkpoint inhibition in claudin-low breast cancer. J Clin Invest. 2017;127(9):3472–3483. doi: 10.1172/JCI90499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Pelekanou V, Carvajal-Hausdorf DE, Altan M, Wasserman B, Carvajal-Hausdorf C, Wimberly H, Brown J, Lannin D, Pusztai L, Rimm DL. Effect of neoadjuvant chemotherapy on tumor-infiltrating lymphocytes and PD-L1 expression in breast cancer and its clinical significance. Breast Cancer Res. 2017;19(1):91. doi: 10.1186/s13058-017-0884-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kitano A, Ono M, Yoshida M, Noguchi E, Shimomura A, Shimoi T, Kodaira M, Yunokawa M, Yonemori K, Shimizu C. Tumour-infiltrating lymphocytes are correlated with higher expression levels of PD-1 and PD-L1 in early breast cancer. ESMO Open. 2017;2(2):e000150. doi: 10.1136/esmoopen-2016-000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Park IA, Hwang SH, Song IH, Heo SH, Kim YA, Bang WS, Park HS, Lee M, Gong G, Lee HJ. Expression of the MHC class II in triple-negative breast cancer is associated with tumor-infiltrating lymphocytes and interferon signaling. PLoS One. 2017;12(8):e0182786. doi: 10.1371/journal.pone.0182786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Liu S, Chen B, Burugu S, Leung S, Gao D, Virk S, Kos Z, Parulekar WR, Shepherd L, Gelmon KA. Role of Cytotoxic Tumor-Infiltrating Lymphocytes in Predicting Outcomes in Metastatic HER2-Positive Breast Cancer: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2017;27:e172085. doi: 10.1001/jamaoncol.2017.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sullivan S, Alex P, Dassopoulos T, Zachos NC, Iacobuzio-Donahue C, Donowitz M, Brant SR, Cuffari C, Harris ML, Datta LW. Downregulation of sodium transporters and NHERF proteins in IBD patients and mouse colitis models: potential contributors to IBD-associated diarrhea. Inflamm Bowel Dis. 2009;15(2):261–274. doi: 10.1002/ibd.20743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lenzen H, Lünnemann M, Bleich A, Manns MP, Seidler U, Jörns A. Downregulation of the NHE3-binding PDZ-adaptor protein PDZK1 expression during cytokine-induced inflammation in interleukin-10-deficient mice. PLoS One. 2012;7(7):e40657. doi: 10.1371/journal.pone.0040657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yeruva S, Chodisetti G, Luo M, Chen M, Cinar A, Ludolph L, Lünnemann M, Goldstein J, Sing AK, Riederer B. Evidence for a causal link between adaptor protein PDZK1 downregulation and Na+/H+ exchanger NHE3 dysfunction in human and murine colitis. Pflugers Arch. 2015;467(8):1795–1807. doi: 10.1007/s00424-014-1608-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Wu Y, Wang S, Farooq SM, Castelvetere MP, Hou Y, Gao JL, Navarro JV, Oupicky D, Sun F, Li C. A chemokine receptor CXCR2 macromolecular complex regulates neutrophil functions in inflammatory diseases. J Biol Chem. 2012;287(8):5744–5755. doi: 10.1074/jbc.M111.315762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Lu G, Wu Y, Jiang Y, Wang S, Hou Y, Guan X, Brunzelle J, Sirinupong N, Sheng S, Li C. Structural insights into neutrophilic migration revealed by the crystal structure of the chemokine receptor CXCR2 in complex with the first PDZ domain of NHERF1. PLoS One. 2013;8(10):e76219. doi: 10.1371/journal.pone.0076219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Leslie KL, Song GJ, Barrick S, Wehbi VL, Vilardaga JP, Bauer PM, Bisello A. Ezrin-radixin-moesin-binding phosphoprotein 50 (EBP50) and nuclear factor-κB (NF-κB): a feed-forward loop for systemic and vascular inflammation. J Biol Chem. 2013;288(51):36426–36436. doi: 10.1074/jbc.M113.483339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bellizzi A, Mangia A, Malfettone A, Cardone RA, Simone G, Reshkin SJ, Paradiso A. Na+/H+ exchanger regulatory factor 1 expression levels in blood and tissue predict breast tumour clinical behaviour. Histopathology. 2011;7:1086–1095. doi: 10.1111/j.1365-2559.2011.03844.x. [DOI] [PubMed] [Google Scholar]