Abstract
Background
Oral glucose tolerance test (OGTT) is a traditional diagnostic tool for diabetes. Hemoglobin A1c (HbA1c) is an alternative method used in adults; however, its application in youths has been controversial. We evaluated the diagnostic performance of HbA1c and determined optimal cutoff points for detecting prediabetes and diabetes in youth.
Methods
This retrospective study included 389 obese children (217 boys, 55.8%) who had undergone simultaneous OGTT and HbA1c testing at six hospitals, Korea, between 2010 and 2016. Subjects were diagnosed with diabetes (fasting glucose ≥ 7.0 mmol/L; 2-hour glucose ≥ 11.1 mmol/L) or prediabetes (fasting glucose 5.6–6.9 mmol/L; 2-hour glucose 7.8–11.0 mmol/L). The diagnostic performance of HbA1c for prediabetes and diabetes was determined using the area under the receiver operating characteristic curve (AUC).
Results
At diagnosis, 197 (50.6%) subjects had normoglycemia, 121 (31.1%) had prediabetes, and 71 (18.3%) had diabetes. The kappa coefficient for agreement between OGTT and HbA1c was 0.464. The optimal HbA1c cutoff points were 5.8% (AUC, 0.795; a sensitivity of 64.1% and a specificity of 83.8%) for prediabetes and 6.2% (AUC, 0.972; a sensitivity of 91.5% and a specificity of 93.7%) for diabetes. When HbA1c (≥ 6.2%) and 2-hour glucose level were used to diagnose diabetes, 100% were detected.
Conclusion
Pediatric criteria for HbA1c remain unclear, therefore, we recommend the combination of fasting and 2-hour glucose levels, in addition to HbA1c, in the diagnosis of childhood prediabetes and diabetes.
Keywords: Diabetes Mellitus, Diagnosis, Glucose Tolerance Test, HbA1c, Prediabetic State
Graphical Abstract
INTRODUCTION
Early detection of prediabetes and early diabetes is crucial to enable preventive management of cardiovascular disease. Diabetes-related microvascular complications are 2–20 times higher and mortality is three times higher than in adults without diabetes, leading to a major financial burden in individuals with diabetes.1 Several studies have shown a rising trend in the worldwide prevalence of type 2 diabetes mellitus (DM) in the pediatric population.2,3,4 In Korean children and adolescents, the proportion of type 2 DM has also shown a rapid increase over the past 20 years.5
Traditionally, plasma glucose level obtained from the oral glucose tolerance test (OGTT) has been used for the diagnosis of prediabetes and diabetes.6 Hemoglobin A1c (HbA1c) has recently been recommended as an alternative diagnostic method for diabetes in adults.7 HbA1c is easy to use without the requirement for fasting; however, it is insensitive in the detection of impaired glucose tolerance, and is affected by age and ethnicity.8 Postprandial glucose usually increases in advance of fasting plasma glucose (FPG) below an HbA1c level of 7.3%, whereas beyond a level of 8.4%, the contribution of basal hyperglycemia to overall hyperglycemia becomes predominant.9 This means that most children with impaired glucose tolerance may have near-normal HbA1c levels and may be neglected if the OGTT including 2-hour plasma glucose (2-hr PG) is not performed.
The use of HbA1c for the diagnosis of prediabetes and diabetes in the pediatric population is controversial, because of its low sensitivity and specificity, and poor diagnostic performance in children compared to adults.10 Only a few studies have investigated the capability of HbA1c for diabetes screening in children. However, the number of participants in these studies was small, or they did not undergo an OGTT to screen for impaired glucose tolerance. Therefore, we aimed to evaluate the diagnostic performance of HbA1c and to compare the results with those of the OGTT. We also determined the optimal cutoff points for detection of prediabetes and diabetes in a large number of children and adolescents.
METHODS
Subjects
We retrospectively reviewed the medical records of 390 children and adolescents less than 20 years of age, who had undergone simultaneous OGTT and HbA1c testing for evaluation of obesity and related complications between January 2010 and June 2016, at the Pediatric Endocrinology Clinic of six university hospitals in Korea. The inclusion criteria were the following: 1) age 10 years and above or at the onset of puberty, 2) overweight or obese (body mass index [BMI] ≥ 85th percentile for age and gender), and 3) two or more additional risk factors for diabetes, consistent with American Diabetes Association (ADA) recommendations for type 2 DM screening, such as family history of type 2 DM, race or ethnicity, signs of insulin resistance or its associated conditions, maternal history of DM or gestational DM.11 Children and adolescents with known diabetes or newly diagnosed type 1 DM (low C-peptide levels and the presence of beta-cell autoantibodies) or anemia (hemoglobin [Hb] < 11.5 g/dL in subjects under the age of 12 years; Hb < 13.0 g/dL and Hb < 12.0 g/dL in boys and girls aged 12 years and over, respectively) were excluded (n = 1). After applying of the exclusion criteria, 389 children and adolescents (217 boys, 55.8%) were included in the present study. Participants underwent an OGTT (1.75 g/kg of anhydrous glucose solution, maximum 75 g) after an 8-hour overnight fast. They were categorized as follows: normoglycemia (FPG < 5.6 mmol/L and 2-hr PG < 7.8 mmol/L), prediabetes (FPG 5.6–6.9 mmol/L or 2-hr PG 7.8–11.0 mmol/L) or type 2 DM (FPG ≥ 7.0 mmol/L or 2-hr PG ≥ 11.1 mmol/L).
Methods
We recorded the chronological age, gender, birth weight, pubertal status, height, weight, BMI (kg/m2), waist circumference, systolic and diastolic blood pressure, and family history of DM in first- and second-degree relatives. The height, body weight, and BMI were expressed as standard deviation scores (SDSs) using the Korean Growth Standard for the same age and gender.12 We also reviewed Hb, aspartate transaminase, alanine transaminase, total cholesterol, triglycerides, high-density lipoprotein-cholesterol, FPG, 2-hr PG during OGTT, and HbA1c.
PG levels were measured using the hexokinase method, and HbA1c levels were measured with high-performance liquid chromatography, which are methods certified by the National Glycohemoglobin Standardization Program (Supplementary Table 1).13
Statistical analysis
Statistical analyses were performed using Stata 14.2 software (StataCorp LP, College Station, TX, USA). One-way analysis of variance was performed to compare the clinical and biochemical parameters of the three groups. Post hoc analysis was performed using the Bonferroni method. The χ2 test was used for the comparison of categorical variables. Data were expressed as mean ± standard deviation or number (percent). Geometric mean ± standard error was calculated for parameters that were log transformed. Kappa coefficients were calculated to assess agreement between glucose test results obtained from the OGTT and HbA1c levels. The kappa coefficient is scaled as 0 (poor) to 1 (perfect agreement), and intermediate values are interpreted as fair (0.21–0.40), moderate (0.41–0.60), or substantial agreement (0.61–0.80).14 The diagnostic performance of HbA1c was investigated using sensitivity, specificity, positive predictive value, and negative predictive value at thresholds of 5.7% for prediabetes and 6.5% for diabetes, as recommended by the ADA. The area under the receiver operating characteristic curve (AUC) was generated to assess the predictive capability of HbA1c for prediabetes and diabetes. The optimal cutoff points were determined as the points at which the distance between the AUC curve and the point with a sensitivity of 1 and a specificity of 0 was minimized. A P < 0.05 was considered statistically significant.
Ethics statement
This retrospective study was performed in accordance with the Declaration of Helsinki and the Institutional Review Boards of each hospital. The Institutional Review Boards waived informed consent.
RESULTS
Clinical and biochemical characteristics of study participants
The study participants consisted of 389 children (48 overweight and 341 obese) and there were more boys (217, 55.8%) than girls. The mean age was 13.0 ± 2.5 years. The mean height SDS, body weight SDS, and BMI SDS were 0.9 ± 1.2, 2.2 ± 0.8, and 2.2 ± 0.6, respectively. About half of the children (203, 52.2%) had a family history of DM in first- and second-degree relatives. Their mean FPG, 2-hr PG and HbA1c levels were 6.1 ± 2.6 mmol/L, 9.0 ± 5.2 mmol/L, and 6.3% ± 2.1%, respectively.
Based on the results of the OGTT, 197 (50.6%) subjects had normoglycemia, 121 (31.1%) had prediabetes, and 71 (18.3%) had type 2 DM (Table 1). Children with type 2 DM were the oldest among the three groups (14.5 ± 2.3 years, P < 0.001), had lower BMI SDS than those with normoglycemia (2.0 ± 0.5, P = 0.002), and were more likely to have a family history of DM (71.8%). The proportions of boys and of obesity were similar between the three groups. FPG, 2-hr PG, HbA1c, and total cholesterol levels were higher, and high-density lipoprotein-cholesterol was lower in children with type 2 DM than in those with normoglycemia and prediabetes. Hb, aspartate transaminase, and alanine transaminase were similar between the three groups.
Table 1. Clinical and biochemical characteristics of study participants.
| Characteristics | Normoglycemia (n = 197, 50.6%) | Prediabetes (n = 121, 31.1%) | Type 2 DM (n = 71, 18.3%) | P valuea |
|---|---|---|---|---|
| Age, yr | 12.3 ± 2.4b,c | 13.1 ± 2.3b,d | 14.5 ± 2.3c,d | < 0.001 |
| Sex (boys) | 115 (58.4) | 65 (53.7) | 37 (52.1) | 0.567 |
| Height SDS | 1.1 ± 1.2b | 0.6 ± 1.2b | 0.9 ± 1.3 | 0.001 |
| Weight SDS | 2.3 ± 0.8b,c | 2.1 ± 0.8b | 2.1 ± 0.7c | 0.008 |
| BMI SDS | 2.3 ± 0.6b | 2.2 ± 0.7 | 2.0 ± 0.5b | 0.002 |
| Obesity | 177 (90.0) | 101 (83.5) | 63 (88.7) | 0.233 |
| Waist circumference, cm | 94.2 ± 12.7 | 94.3 ± 12.1 | 92.3 ± 11.5 | 0.586 |
| Family history of DM | 88 (44.7) | 64 (52.9) | 51 (71.8) | < 0.001 |
| Fasting glucose, mmol/L | 5.0 ± 0.4b | 5.5 ± 0.5c | 10.1 ± 3.8b,c | < 0.001 |
| 2-hour glucose, mmol/L | 6.3 ± 0.8b,c | 7.9 ± 1.0b,d | 18.5 ± 5.8c,d | < 0.001 |
| HbA1c, % | 5.5 ± 0.3b | 5.8 ± 0.8c | 9.7 ± 2.8b,c | < 0.001 |
| Hb, g/dL | 13.9 ± 1.0 | 13.9 ± 1.1 | 14.2 ± 1.0 | 0.073 |
| Total cholesterol, mg/dLe | 171.7 ± 2.1b | 175.3 ± 3.1 | 186.6 ± 5.1b | 0.008 |
| Triglyceride, mg/dLe | 112.4 ± 4.1 | 124.3 ± 5.6 | 131.9 ± 11.2 | 0.069 |
| HDL-cholesterol, mg/dLe | 44.5 ± 0.7b | 43.6 ± 1.0 | 40.9 ± 1.1b | 0.035 |
Data are expressed as mean ± standard deviation or number (%).
DM = diabetes mellitus, SDS = standard deviation score, BMI = body mass index, HbA1c = hemoglobin A1c, Hb = hemoglobin, HDL = high-density lipoprotein.
aP < 0.05 among 3 groups; b,c,dSame superscript means significant difference between groups; eGeometric mean ± standard error was calculated for parameters that were log transformed.
Agreement between fasting glucose, 2-hour glucose level, and HbA1c
Table 2 shows the agreement between glucose results from the OGTT and HbA1c levels based on ADA criteria. The kappa coefficients for agreement between the OGTT, FPG, 2-hr PG, and HbA1c results were 0.464 (95% confidence interval [CI], 0.417–0.527), 0.396 (95% CI, 0.356–0.459), and 0.476 (95% CI, 0.463–0.500), respectively. These were interpreted as fair to moderate agreement.
Table 2. Fasting glucose and 2-hour glucose level according to HbA1c category.
| Glucose category | HbA1c category | Total | Kappa coefficient (95% CI) | |||
|---|---|---|---|---|---|---|
| < 5.7% | 5.7%–6.4% | ≥ 6.5% | ||||
| Fasting glucose or 2-hour glucose | 0.464 (0.417–0.527) | |||||
| Normal | 145 (73.6) | 52 (26.4) | 0 (0.0) | 197 (100.0) | ||
| Prediabetes | 58 (47.9) | 55 (45.5) | 8 (6.6) | 121 (100.0) | ||
| Type 2 diabetes | 2 (2.8) | 7 (9.9) | 62 (87.3) | 71 (100.0) | ||
| Fasting glucose, mmol/L | 0.396 (0.356–0.459) | |||||
| < 5.6 | 170 (66.9) | 78 (30.7) | 6 (2.4) | 254 (100.0) | ||
| ≥ 5.6 and < 7.0 | 34 (42.0) | 34 (42.0) | 13 (16.0) | 81 (100.0) | ||
| ≥ 7.0 | 1 (1.9) | 2 (3.7) | 51 (94.4) | 54 (100.0) | ||
| 2-hour glucose, mmol/L | 0.476 (0.463–0.500) | |||||
| < 7.8 | 168 (70.9) | 66 (27.8) | 3 (1.3) | 237 (100.0) | ||
| ≥ 7.8 and < 11.1 | 35 (41.7) | 42 (50.0) | 7 (8.3) | 84 (100.0) | ||
| ≥ 11.1 | 2 (2.9) | 6 (8.8) | 60 (88.2) | 68 (100.0) | ||
| Total | 205 (52.7) | 114 (29.3) | 70 (18.0) | 389 (100.0) | ||
Data are expressed as number (%).
HbA1c = hemoglobin A1c, CI = confidence interval.
Diagnostic performance of HbA1c for glucose category by OGTT
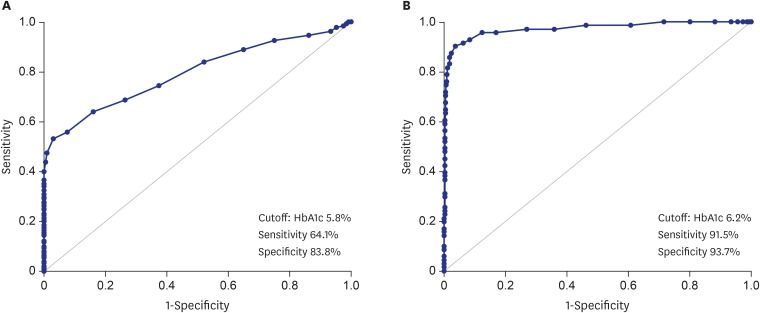
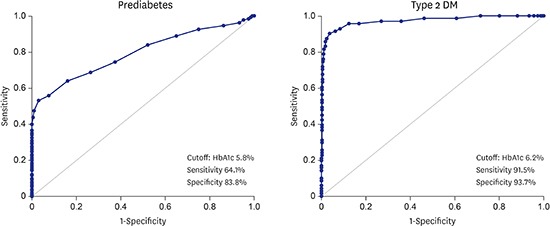
AUC was used to determine the diagnostic performance of HbA1c for prediabetes and diabetes (Fig. 1). The statistically optimal HbA1c cutoff point for prediabetes was 5.8% (AUC, 0.795; 95% CI, 0.750–0.840), with a sensitivity of 64.1% and a specificity of 83.8%. The statistically optimal HbA1c cutoff point for diabetes was 6.2% (AUC, 0.972; 95% CI, 0.949–0.995), with a sensitivity of 91.5% and a specificity of 93.7%.
Fig. 1.
The receiver operating characteristic curve for HbA1c. (A) In the diagnosis of prediabetes and (B) type 2 DM, which corresponds to the AUC (95% CI) of 0.795 (0.750–0.840) for prediabetes and 0.972 (0.949–0.995) for type 2 diabetes.
HbA1c = hemoglobin A1c, DM = diabetes mellitus, AUC = area under the receiver operating characteristic curve, CI = confidence interval.
Table 3 shows a comparison of sensitivity, specificity, positive predictive value, and negative predictive value between ADA criteria and the thresholds in this study. The sensitivity of this study was lower and the specificity was higher than that of ADA criteria at the prediabetic cutoff (64.1% vs. 68.8% and 83.8% vs. 73.6%, respectively). The sensitivity of this study was higher and the specificity was lower than that of ADA criteria at the diabetic cutoff (91.5% vs. 87.3% and 93.7% vs. 97.5%, respectively).
Table 3. Diagnostic performance of HbA1c for glucose category by OGTT.
| Criteria | HbA1c cutoff, % | No. (%) | Sensitivity, % | Specificity, % | PPV, % | NPV, % | |
|---|---|---|---|---|---|---|---|
| ADA | |||||||
| Prediabetes | ≥ 5.7 | 184 (47.3) | 68.8 | 73.6 | 71.7 | 70.7 | |
| Type 2 DM | ≥ 6.5 | 70 (18.0) | 87.3 | 97.5 | 88.6 | 97.2 | |
| The present study | |||||||
| Prediabetes | ≥ 5.8 | 155 (39.9) | 64.1 | 83.8 | 79.4 | 70.5 | |
| Type 2 DM | ≥ 6.2 | 85 (21.9) | 91.5 | 93.7 | 76.5 | 98.0 | |
HbA1c = hemoglobin A1c, OGTT = oral glucose tolerance test, PPV = positive predictive value, NPV = negative predictive value, ADA = American Diabetes Association, DM = diabetes mellitus.
Consistency rates between fasting glucose and 2-hour glucose levels during OGTT, and HbA1c
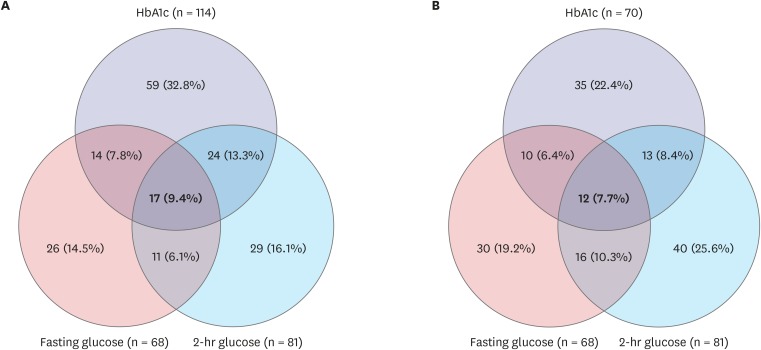
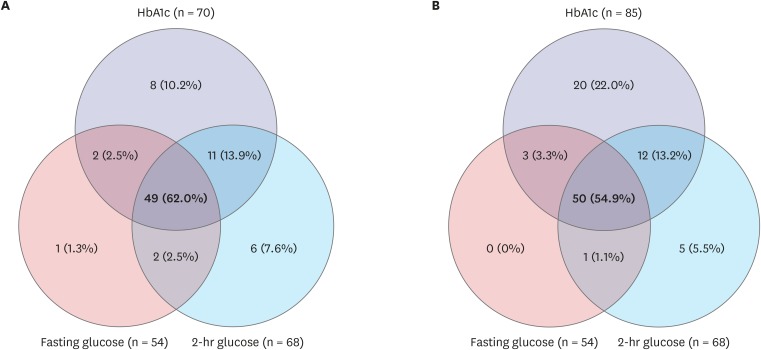
Figs. 2 and 3 show the consistency rates between FPG, 2-hr PG, and HbA1c level in prediabetes and type 2 DM, respectively. Based on the ADA cutoff point for HbA1c of 5.7%–6.4%, 17 (9.4%) of 180 children with prediabetes satisfied all three diagnostic criteria. Twenty-nine (16.1%) were omitted without 2-hr PG. Based on the cutoff point for HbA1c of 5.8%–6.1% in the present study, 12 (7.7%) of 156 children with prediabetes satisfied all three diagnostic criteria; 40 (25.6%) were omitted without 2-hr PG (Fig. 2). For type 2 DM, 49 (62.0%) of 79 children satisfied all three diagnostic criteria based on the ADA cutoff point of HbA1c ≥ 6.5%. Six (7.6%) were omitted without 2-hr PG. Based on the cutoff point of HbA1c ≥ 6.2% in the present study, 50 (54.9%) of 91 children with diabetes satisfied all three diagnostic criteria; five (5.5%) were omitted without 2-hr PG. When two of these criteria were used to diagnose diabetes, 78.0% were detected by OGTT results and 94.5% were detected by HbA1c and FPG level, while 100% were detected by HbA1c and 2-hr PG level (Fig. 3).
Fig. 2.
The consistency rates among three diagnostic criteria for prediabetes. Based on (A) ADA criteria (HbA1c 5.7%–6.4%) and (B) the results of the present study (HbA1c 5.8%–6.1%). Prediabetes defined as fasting glucose 5.6–6.9 mmol/L or 2-hour glucose 7.8–11.0 mmol/L.
ADA = American Diabetes Association, HbA1c = hemoglobin A1c.
Fig. 3.
The consistency rates among three diagnostic criteria for type 2 DM. Based on (A) ADA criteria (HbA1c ≥ 6.5%) and (B) the results of the present study (HbA1c ≥ 6.2%). Type 2 DM defined as fasting glucose ≥ 7.0 mmol/L or 2-hour glucose ≥ 11.1 mmol/L.
DM = diabetes mellitus, ADA = American Diabetes Association, HbA1c = hemoglobin A1c.
DISCUSSION
This retrospective study showed that about half of overweight and obese children at risk had prediabetes or diabetes based on OGTT results. The present study revealed moderate agreement between OGTT and HbA1c results when applying ADA criteria and identified optimal HbA1c cutoff points for prediabetes (5.8%) and diabetes (6.2%) in children. The adult HbA1c cutoff point of 6.5% underestimated the prevalence of diabetes, compared with that determined using the threshold of this study. All subjects with diabetes were detected with the combined use of 2-hr PG and HbA1c level.
In 2009, an International Expert Committee composed of members from the International Diabetes Federation, the European Association for the Study of Diabetes, and the ADA recommended using HbA1c at the level of ≥ 6.5% for the diagnosis of diabetes.15 Owing to several advantages such as the lack of requirement for fasting, accurate measurement of chronic glycemic levels, and low between- and within-subject variation, HbA1c has been incorporated in the ADA guidelines as a diagnostic tool for diabetes since 2010.7
The adult HbA1c threshold of 6.5% is based on the cutoff point for detection of retinopathy identified by Colaguiuri.15 Considering the slow, asymptomatic progression of type 2 DM, screening for diabetic complications is recommended at the time of initial diagnosis, and prediabetes is regarded as a high-risk state for diabetes.16 Thus, early detection and preemptive intervention of type 2 DM would decrease future cardiovascular disease in the pediatric population.1 The pediatric research, which investigated HbA1c level for the diagnosis of diabetes, reported lower HbA1c cutoff point than that in adults.10 In this study, children with type 2 DM were older, more likely to have a family history of DM, and had lower BMI SDS than those with normoglycemia. Lower BMI SDS in children with type 2 DM might reflect the inclusion of some symptomatic children. The statistical HbA1c cutoff point for diagnosis of diabetes was 6.2%, which showed discrepancy from the value suggested by ADA. When applying the cutoff point of 6.2%, sensitivity for detecting type 2 DM increased (87.3% vs. 91.5%).
The diagnostic performances of FPG, 2-hr PG, and HbA1c levels vary depending on the desired clinical outcomes.17 Yang et al.18 reported that the HbA1c cutoff points for prediabetes and diabetes in Chinese adults were 5.9% and 6.2%, respectively. Addition of an OGTT was suggested for adults with HbA1c ≥ 6.1%.19
The use of adult HbA1c criteria in pediatric populations has been controversial. Based on increasing HbA1c levels according to age in nondiabetic populations,20 several studies have reported that a lower HbA1c cutoff point should be applied in children.10,21 Hosking et al.22 described a weak correlation between FPG and HbA1c level, and suggested an optimal cutoff point for prediabetes at HbA1c of 5.4%. However, this cutoff point did not reflect impaired glucose tolerance, because OGTT had not been performed. Even though it is known that HbA1c level differs according to ethnic background,23 four previous studies suggested that the HbA1c cutoff points for impaired glucose tolerance were 5.5%, with a sensitivity of 85.7% and a specificity of 56.9% in 209 children; 5.5%, with a sensitivity of 63% and a specificity of 70% in 106 children; 5.8%, with a sensitivity of 64.7% and a specificity of 61.6% in 126 children; and 5.9%, with a sensitivity of 80% and a specificity of 64% in 98 children.24,25,26,27 While the previous four studies in the pediatric populations only suggested an optimal cutoff point for prediabetes, we also evaluated the cutoff point for type 2 DM. In a recent study, Ehehalt et al.28 also reported an optimal HbA1c cutoff point for diabetes of 6.0%, with a sensitivity of 94.0% and a specificity of 92.9% in 4,848 German children. The differences in HbA1c cutoff points may reflect differences in study populations. Ethnicity and the definition of obesity were different from those in our study. They included new-onset type 1 DM and maturity-onset diabetes of the young, and screened for diabetes in overweight youth. However, we performed simultaneous OGTT and HbA1c testing in children with two or more additional risk factors for diabetes, consistent with ADA recommendations for type 2 DM screening. Regardless of these differences, all agreed that both HbA1c and OGTT are needed in overweight children and adolescents.
Several studies have reported limited overlap between FPG, 2-hr PG from OGTT results, and HbA1c levels. There was only a weak correlation between FPG, 2-hr PG, and HbA1c in German children and adolescents.29 Only 7% of 4,004 European adults satisfied all three tests.30 The discrepancy between HbA1c level and OGTT results was more remarkable in prediabetes than in diabetes.27 According to population-based estimates from Canada, the prevalence of undiagnosed type 2 DM is 1.13% and 3.09% with the use of FPG or HbA1c alone, respectively.31 The OGTT has some disadvantages: it is time-consuming, requires prior fasting, and lacks reproducibility32,33; however, it can identify insulin resistance related to other metabolic disorders. HbA1c alone without OGTT-based glucose results may miss the diagnosis of patients with a high-risk of metabolic syndrome.34 In the present study, the diagnosis of prediabetes would have been missed in nearly half of children without OGTT results. Our results showing greater postprandial hyperglycemia to basal hyperglycemia ratios in both prediabetes and diabetes also supported these findings. The undiagnosed rate was higher in children without 2-hr PG results than in those without FPG results, especially in the prediabetic state. Interestingly, simultaneous application of 2-hr PG and HbA1c cutoff point of 6.2% detected all children with diabetes, which notes that the importance of OGTT in the pediatric population.
There are some limitations to the present study, including possible selection bias or use of data from symptomatic children. Our study was conducted in a Korean population; hence, HbA1c cutoff points might not be generalizable to other populations. While the ADA requires a second test to confirm diabetes owing to the lack of reproducibility of the OGTT, the test was not repeated in the majority of subjects in this study. There are no available data concerning the reliability of OGTT in Korean children, and further studies are necessary. Due to the discrepancy between ADA criteria and those used in the present study for diagnosing prediabetes, subjects with prediabetes might be overlooked with use of a cutoff value of 5.8%. Whether early detection using a lower cutoff point will reduce morbidity and mortality remains unclear; therefore, further longitudinal studies including children with HbA1c levels of 5.8%–6.1% and 6.2%–6.4% will be required. Nevertheless, this multicenter study included a large number of Asian children and adolescents who underwent simultaneous OGTT and HbA1c, thereby strengthening the validity of the results.
In conclusion, use of adult HbA1c criteria for diagnosis of prediabetes and diabetes in children and adolescents remains controversial, owing to disparities between the results of OGTT- and HbA1c-based tests. Screening for prediabetes and diabetes in high-risk children is still important. A follow-up screening is required at short intervals for children with HbA1c levels of 5.8%–6.1%. Especially for children with HbA1c levels of 6.2%–6.4%, an OGTT would be a useful method for diabetes screening. Combination of OGTT and HbA1c level might reduce the possibility to miss the diagnosis of diabetes in children and adolescents.
Footnotes
Disclosure: The authors have no potential conflicts of interest to disclose.
Author Contributions: Conceptualization: Nam HK, Kim JH. Data curation: Nam HK, Cho WK, Kim JH, Rhie YJ, Chung S, Lee KH, Suh BK. Investigation: Kim JH. Writing - original draft: Nam HK. Writing - review & editing: Nam HK, Cho WK, Kim JH, Rhie YJ, Chung S, Lee KH, Suh BK.
SUPPLEMENTARY MATERIAL
List of machines for glucose and HbA1c level
References
- 1.Task Force Team for Basic Statistical Study of Korean Diabetes Mellitus of Korean Diabetes Association Diabetes epidemics in Korea: reappraise nationwide survey of diabetes “diabetes in Korea 2007”. Diabetes Metab J. 2013;37(4):233–239. doi: 10.4093/dmj.2013.37.4.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dabelea D, Mayer-Davis EJ, Saydah S, Imperatore G, Linder B, Divers J, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311(17):1778–1786. doi: 10.1001/jama.2014.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitagawa T, Owada M, Urakami T, Yamauchi K. Increased incidence of non-insulin dependent diabetes mellitus among Japanese schoolchildren correlates with an increased intake of animal protein and fat. Clin Pediatr (Phila) 1998;37(2):111–115. doi: 10.1177/000992289803700208. [DOI] [PubMed] [Google Scholar]
- 4.Alberti G, Zimmet P, Shaw J, Bloomgarden Z, Kaufman F, Silink M, et al. Type 2 diabetes in the young: the evolving epidemic: the international diabetes federation consensus workshop. Diabetes Care. 2004;27(7):1798–1811. doi: 10.2337/diacare.27.7.1798. [DOI] [PubMed] [Google Scholar]
- 5.Kwon EB, Lee HS, Shim YS, Jeong HR, Hwang JS. The changes of subtypes in pediatric diabetes and their clinical and laboratory characteristics over the last 20 years. Ann Pediatr Endocrinol Metab. 2016;21(2):81–85. doi: 10.6065/apem.2016.21.2.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care. 2007;30(Suppl 1):S42–S47. doi: 10.2337/dc07-S042. [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association Standards of medical care in diabetes--2011. Diabetes Care. 2011;34(Suppl 1):S11–S61. doi: 10.2337/dc11-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubowitz N, Xue W, Long Q, Ownby JG, Olson DE, Barb D, et al. Aging is associated with increased HbA1c levels, independently of glucose levels and insulin resistance, and also with decreased HbA1c diagnostic specificity. Diabet Med. 2014;31(8):927–935. doi: 10.1111/dme.12459. [DOI] [PubMed] [Google Scholar]
- 9.Monnier L, Colette C. Postprandial and basal hyperglycaemia in type 2 diabetes: contributions to overall glucose exposure and diabetic complications. Diabetes Metab. 2015;41(6 Suppl 1):6S9–6S15. doi: 10.1016/S1262-3636(16)30003-9. [DOI] [PubMed] [Google Scholar]
- 10.Lee JM, Wu EL, Tarini B, Herman WH, Yoon E. Diagnosis of diabetes using hemoglobin A1c: should recommendations in adults be extrapolated to adolescents? J Pediatr. 2011;158(6):947–952.e1-3. doi: 10.1016/j.jpeds.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Standards of medical care in diabetes-2016: summary of revisions. Diabetes Care. 2016;39(Suppl 1):S4–S5. doi: 10.2337/dc16-S003. [DOI] [PubMed] [Google Scholar]
- 12.Korea Centers for Disease Control and Prevention; The Korean Pediatric Society, Committee for Korean Children and Adolescents Growth Standard. 2007 Korean Children and Adolescents Growth Standard. Seoul, Korea: Korea Centers for Disease Control and Prevention; 2007. [Google Scholar]
- 13.National glycohemoglobin standardization program. [Updated 2016]. [Accessed November 4, 2016]. http://www.ngsp.org/certified.asp.
- 14.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 15.Gillett MJ. International Expert Committee report on the role of the A1c assay in the diagnosis of diabetes: Diabetes Care 2009; 32: 1327-1334. Clin Biochem Rev. 2009;30:197–200. [PMC free article] [PubMed] [Google Scholar]
- 16.Perreault L, Pan Q, Mather KJ, Watson KE, Hamman RF, Kahn SE, et al. Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: results from the Diabetes Prevention Program Outcomes Study. Lancet. 2012;379(9833):2243–2251. doi: 10.1016/S0140-6736(12)60525-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warren B, Pankow JS, Matsushita K, Punjabi NM, Daya NR, Grams M, et al. Comparative prognostic performance of definitions of prediabetes: a prospective cohort analysis of the Atherosclerosis Risk in Communities (ARIC) study. Lancet Diabetes Endocrinol. 2017;5(1):34–42. doi: 10.1016/S2213-8587(16)30321-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang C, Liu Y, Li X, Liang H, Jiang X. Utility of hemoglobin A1c for the identification of individuals with diabetes and prediabetes in a Chinese high risk population. Scand J Clin Lab Invest. 2012;72(5):403–409. doi: 10.3109/00365513.2012.689324. [DOI] [PubMed] [Google Scholar]
- 19.Kim DL, Kim SD, Kim SK, Park S, Song KH. Is an oral glucose tolerance test still valid for diagnosing diabetes mellitus? Diabetes Metab J. 2016;40(2):118–128. doi: 10.4093/dmj.2016.40.2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pani LN, Korenda L, Meigs JB, Driver C, Chamany S, Fox CS, et al. Effect of aging on A1C levels in individuals without diabetes: evidence from the Framingham Offspring Study and the National Health and Nutrition Examination Survey 2001–2004. Diabetes Care. 2008;31(10):1991–1996. doi: 10.2337/dc08-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nowicka P, Santoro N, Liu H, Lartaud D, Shaw MM, Goldberg R, et al. Utility of hemoglobin A(1c) for diagnosing prediabetes and diabetes in obese children and adolescents. Diabetes Care. 2011;34(6):1306–1311. doi: 10.2337/dc10-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosking J, Metcalf BS, Jeffery AN, Streeter AJ, Voss LD, Wilkin TJ. Divergence between HbA1c and fasting glucose through childhood: implications for diagnosis of impaired fasting glucose (Early Bird 52) Pediatr Diabetes. 2014;15(3):214–219. doi: 10.1111/pedi.12082. [DOI] [PubMed] [Google Scholar]
- 23.Ziemer DC, Kolm P, Weintraub WS, Vaccarino V, Rhee MK, Twombly JG, et al. Glucose-independent, black-white differences in hemoglobin A1c levels: a cross-sectional analysis of 2 studies. Ann Intern Med. 2010;152(12):770–777. doi: 10.7326/0003-4819-152-12-201006150-00004. [DOI] [PubMed] [Google Scholar]
- 24.Tsay J, Pomeranz C, Hassoun A, Zandieh SO, Rutledge J, Vogiatzi MG, et al. Screening markers of impaired glucose tolerance in the obese pediatric population. Horm Res Paediatr. 2010;73(2):102–107. doi: 10.1159/000277625. [DOI] [PubMed] [Google Scholar]
- 25.Yeşiltepe Mutlu G, Özsu E, Çizmecioğlu FM, Hatun Ş. Can HbA1c and one-hour glucose concentration in standard OGTT be used for evaluation of glucose homeostasis in childhood? J Clin Res Pediatr Endocrinol. 2013;5(2):80–84. doi: 10.4274/Jcrpe.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee HS, Park HK, Hwang JS. HbA1c and glucose intolerance in obese children and adolescents. Diabet Med. 2012;29(7):e102–e105. doi: 10.1111/j.1464-5491.2012.03596.x. [DOI] [PubMed] [Google Scholar]
- 27.Al Amiri E, Abdullatif M, Abdulle A, Al Bitar N, Afandi EZ, Parish M, et al. The prevalence, risk factors, and screening measure for prediabetes and diabetes among Emirati overweight/obese children and adolescents. BMC Public Health. 2015;15(1):1298. doi: 10.1186/s12889-015-2649-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ehehalt S, Wiegand S, Korner A, Schweizer R, Liesenkotter KP, Partsch CJ, et al. Diabetes screening in overweight and obese children and adolescents: choosing the right test. Eur J Pediatr. 2017;176(1):89–97. doi: 10.1007/s00431-016-2807-6. [DOI] [PubMed] [Google Scholar]
- 29.Ehehalt S, Wiegand S, Körner A, Schweizer R, Liesenkötter KP, Partsch CJ, et al. Low association between fasting and OGTT stimulated glucose levels with HbA1c in overweight children and adolescents. Pediatr Diabetes. 2017;18(8):734–741. doi: 10.1111/pedi.12461. [DOI] [PubMed] [Google Scholar]
- 30.Gyberg V, De Bacquer D, Kotseva K, De Backer G, Schnell O, Sundvall J, et al. Screening for dysglycaemia in patients with coronary artery disease as reflected by fasting glucose, oral glucose tolerance test, and HbA1c: a report from EUROASPIRE IV--a survey from the European Society of Cardiology. Eur Heart J. 2015;36(19):1171–1177. doi: 10.1093/eurheartj/ehv008. [DOI] [PubMed] [Google Scholar]
- 31.Rosella LC, Lebenbaum M, Fitzpatrick T, Zuk A, Booth GL. Prevalence of prediabetes and undiagnosed diabetes in Canada (2007–2011) according to fasting plasma glucose and HbA1c screening criteria. Diabetes Care. 2015;38(7):1299–1305. doi: 10.2337/dc14-2474. [DOI] [PubMed] [Google Scholar]
- 32.Libman IM, Barinas-Mitchell E, Bartucci A, Robertson R, Arslanian S. Reproducibility of the oral glucose tolerance test in overweight children. J Clin Endocrinol Metab. 2008;93(11):4231–4237. doi: 10.1210/jc.2008-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chan CL, Pyle L, Newnes L, Nadeau KJ, Zeitler PS, Kelsey MM. Continuous glucose monitoring and its relationship to hemoglobin A1c and oral glucose tolerance testing in obese and prediabetic youth. J Clin Endocrinol Metab. 2015;100(3):902–910. doi: 10.1210/jc.2014-3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang L, Shen X, Yan S, Xu F, Wu P. The effectiveness of age on HbA1c as a criterion for the diagnosis of diabetes in Chinese different age subjects. Clin Endocrinol (Oxf) 2015;82(2):205–212. doi: 10.1111/cen.12494. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of machines for glucose and HbA1c level