Abstract
A novel tetranorditerpenoid, sinubatin A (1) (having an unprecedented carbon skeleton), a new norditerpenoid, sinubatin B (2) (a 4,5-epoxycaryophyllene possessing an unusual methylfuran moiety side chain), and a known diterpenoid, gibberosin J (3) were isolated from soft coral Sinularia nanolobata. The structures of the new compounds were elucidated by extensive analysis of spectroscopic data.
Keywords: Sinularia nanolobata, tetranorditerpenoid, norditerpenoid, gibberosin J, cytotoxicity
1. Introduction
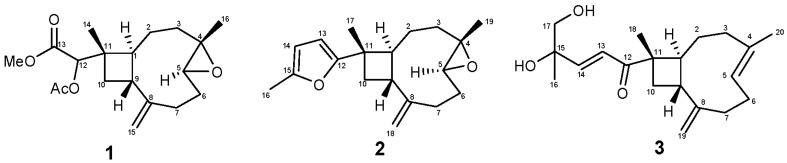
Soft corals of genus Sinularia (Alcyoniidae) have been reported to be a rich source of novel structures and bioactive terpenoids and steroids [1]. Previous studies on the sample of Sinularia nanolobata Verseveldt have resulted in the isolation of diterpenoids [2,3,4,5] and sesquiterpenoids [3,4], and steroids [5,6]. During the course of our search of bioactive compounds from marine organisms, a chemical investigation on the secondary metabolites of S. nanolobata from Taiwanese waters has afforded a novel tetranorditerpenoid, sinubatin A (1) (possessing an unprecedented carbon skeleton), a new norditerpenoid, sinubatin B (2) (a 4,5-epoxycaryophyllene possessing an unusual methylfuran moiety side chain), and gibberosin J (3) (Figure 1). The structures of 1 and 2 were determined by extensive spectroscopic analysis. The chemical structure of gibberosin J (3) was determined by comparison of its infrared (IR), high resolution electron spray ionization mass spectrum (HR-ESI-MS), and nuclear magnetic resonance (NMR) spectroscopic data with the literature data [7].
Figure 1.
Structure of Metabolites 1–3.
2. Results and Discussion
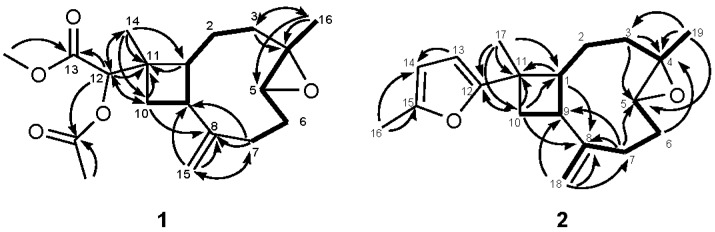
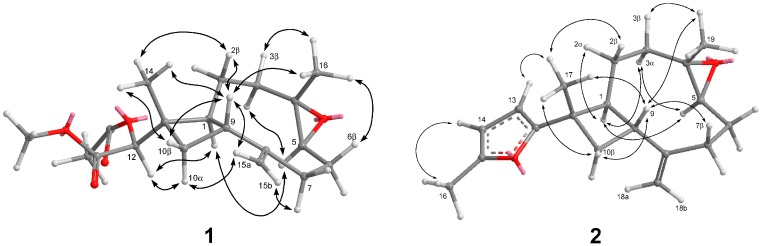
Chromatographic separation on the acetone extract resulted in the isolation of two new terpenoids, sinubatin A and B (1 and 2), as well as a known compound, gibberosin J (3). The HR-ESI-MS, 13C NMR, and DEPT spectroscopic data of sinubatin A (1) established its molecular formula as C19H28O5. 13C NMR and DEPT spectrum of 1 showed the presence of four methyl, five sp3 methylene, four sp3 methine, one sp2 methylene, two sp3 quaternary, one sp2 quaternary, and two carbonyl carbons. The presence of an exomethylene in 1 was shown by the NMR data [δH 4.90 (1H, s), 5.01 (1H, s); δC 114.0 (CH2), 150.7 (C)] (Table 1). The NMR data [δC 59.6 (C), 63.8 (CH), δH 2.92 (1H, dd, J = 10.8, 4.0 Hz)] (Table 1) indicated a trisubstituted epoxide in 1. The NMR spectrum contained signals for a secondary acetoxyl [δH 4.74 (1H, s), 2.15 (3H, s); δC 79.4 (CH), 20.6 (CH3), and 170.8 (C)] (Table 1). The presence of a methyl ester [δH 3.71 (3H, s); δC 51.9 (CH3), 169.2 (C)] was shown in the NMR spectrum. From the data of 1H–1H COSY correlations (Figure 2), we established two partial structures of consecutive proton systems extending from H-10 to H-3 through H-9 and from H-16 to H-7 through H-4. HMBC correlations of (a) CH3-16 to C-3, C-4, and C-5, (b) H2-15 to C-7, C-8, and C-9, (c) CH3-14 to C-1, C-10, C-11, and C-12, (d) CH-12 to C-10, C-11,C-13, and C-14 connected four partial structures and concluded the planar structure of 1, as shown in Figure 2. The above functionalities revealed that sinubatin A (1) possesses a novel xeniaphyllane-derived tetranorditerpene skeleton. The relative configuration of 1 was established from a NOESY experiment. NOE correlations of H3-14/H-9 and H3-16/H-9 pointed H3-14, H-9 and H3-16 to be on the β-side of the molecule. NOE correlation of H-1/H-5 suggested H-1 and H-5 were on the α-side of the molecule. (Figure 3).
Table 1.
NMR spectral data of 1.
| Position | δH a | (J in Hz) | δC b | Type | COSY | HMBC | NOESY |
|---|---|---|---|---|---|---|---|
| 1 | 2.34 | m | 45.3, | CH | 2, 9 | 11, 12 | 5 |
| 2α | 1.45 | m | 27.7, | CH2 | 1 | - | - |
| 2β | 1.57 | m | 1, 3β | - | 9 | ||
| 3α | 1.00 | td (12.8, 4.8) | 38.4, | CH2 | 2β | 1, 4, 16 | 3β, 5 |
| 3β | 2.09 | m | 2β | - | 3α | ||
| 4 | - | - | 59.6, | C | - | - | |
| 5 | 2.92 | dd (10.8, 4.0) | 63.8, | CH | 6β, 16 | - | 1, 3α |
| 6α | 2.30 | m | 30.2, | CH2 | - | - | - |
| 6β | 1.31 | m | 5 | - | 6α | ||
| 7α | 2.16 | m | 29.2, | CH2 | 6α | 6, 9 | 6β |
| 7β | 2.32 | m | 6α | 8, 15 | - | ||
| 8 | - | - | 150.7, | C | - | - | |
| 9 | 2.71 | td (9.6, 9.2) | 48.7, | CH | 1, 10α, 10β | - | 2β, 10β, 14, 15a |
| 10α | 1.85 | dd (10.4, 9.6) | 36.2, | CH2 | 9 | 9, 11, 12, 14 | - |
| 10β | 1.74 | dd (10.4, 9.2) | 9 | 8 | 9, 14 | ||
| 11 | - | - | 38.3, | C | - | - | - |
| 12 | 4.74 | s | 79.4, | CH | - | 10, 11, 13, 14, carbonyl (OAc-12) | 1, 10α |
| 13 | - | - | 169.2, | qC | - | - | - |
| 14 | 1.14 | s | 15.2, | CH3 | - | 1, 10, 11, 12 | 2α, 2β, 9, 10β |
| 15a | 5.01 | s | 114.0, | CH2 | - | 7, 8, 9 | 9, 10α, 15b |
| 15b | 4.90 | s | - | 7, 9 | 7α | ||
| 16 | 1.19 | s | 17.1, | CH3 | 5 | 3, 4, 5 | 3β, 6β, 9 |
| OAc-12 | 2.15 | s | 20.6, | CH3 | OMe-13 | carbonyl (OAc-12) | - |
| - | - | 170.8, | C | - | - | - | |
| OMe-13 | 3.71 | s | 51.9, | CH3 | OAc-12 | 13 | - |
a Spectrum recorded at 400 MHz in CDCl3. b Spectrum recorded at 100 MHz in CDCl3.
Figure 2.
Selected 1H–1H COSY (bold lines) and HMBC (arrows) correlations of 1 and 2.
Figure 3.
Key NOESY Correlations of 1 and 2.
HR-ESI-MS of sinubatin B (2) showed a pseudomolecular ion peak at m/z 309.1842 [M + Na]+, consistent with the molecular formula C19H26O2, and seven degrees of unsaturation. The 13C NMR spectrum (Table 2) of 2 displayed 19 carbon signals, and a DEPT experiments indicated the presence of three methyl, five sp3 methylene, three sp3 methine, two sp2 methine, one sp2 methylene, two sp3 quaternary, and three sp2 quaternary carbons. The 13C and 1H NMR spectra (Table 2) revealed the presence of a trisubstituted epoxides [δH 2.92 (dd, J = 10.4, 4.0 Hz); δC 63.7 (CH) and 59.7 (C)], a 2,5-disubstituted furan [δH 5.83 (d, J = 4.0 Hz), 5.84 (dd, J = 4.0, 0.8 Hz), and 2.28 (d, J = 0.8 Hz); δC 103.7 (CH), 105.8 (CH), 150.6 (C), 160.2 (C), 13.6 (CH3)] [8] and an exomethylene [δH 5.09 (s) and 4.93 (s); δC 113.5 (CH2) and 151.4 (C)]. Thus, the tetracyclic structure of 2 was revealed. From the 1H–1H COSY spectrum of 2, it was also possible to identify two different structural units (Figure 2), which were assembled with the assistance of an HMBC experiments. Key HMBC correlations (Figure 2) of H3-19 to C-3, C-4, and C-5; H3-18 to C-7, C-8, and C-9; H3-17 to C-1, C-10, C-11, and C-12 indicated that compound 2 was a 4,5-epoxycaryophyllene having a methylfuran on C-11. The relative configuration of 2 was determined from a NOESY experiment. NOE correlations of H3-19/H-9 and H3-17/H-9 suggested H3-19, H-9 and H3-17 to be on the β-side of the molecule. NOE correlation of H-1/H-5 indicated H-1 and H-5 were on the α-side of the molecule. (Figure 3). Compound 2 was the first caryophyllene possessing a methylfuran on C-11.
Table 2.
NMR spectral data of 2.
| Position | δH a | (J in Hz) | δC b | Type | COSY | HMBC | NOESY |
|---|---|---|---|---|---|---|---|
| 1 | 2.47 | td (10.0,8.4) | 49.3, | CH | 2β, 9 | 3, 8, 9, 11, 17 | 2α, 3α, 5 |
| 2α | 1.72 | m | 27.2, | CH2 | 3α, 3β | 1, 3, 11 | 1, 3α, 3β |
| 2β | 1.54 | m | 1, 3α, 3β | 1 | 3β, 9 | ||
| 3α | 0.98 | td (13.2, 5.2) | 38.8, | CH2 | 2α, 2β, 19 | 2, 4, 5, 19 | 1, 2α, 5 |
| 3β | 2.07 | dt (13.2,3.6) | 2α, 2β | - | 2α, 2β, 19 | ||
| 4 | - | - | 59.7, | C | - | - | - |
| 5 | 2.92 | dd (10.4, 4.0) | 63.7, | CH | 6α, 6β | 3, 6 | 1, 3α, 6α |
| 6α | 2.28 | m | 30.1, | CH2 | 5 | 4, 5, 7 | 5 |
| 6β | 1.37 | m | 5, 7α, 7β | - | 7β | ||
| 7α | 2.44 | ddd (12.8, 8.0, 4.0) | 29.8, | CH2 | 6β | 5, 6, 8, 9 | 18b |
| 7β | 2.17 | ddd (12.8, 8.0, 4.4) | 6α, 6β | 5, 6, 8, 9 | 9 | ||
| 8 | - | - | 151.4, | C | - | - | - |
| 9 | 2.74 | td (9.6, 8.4) | 47.9, | CH | 1, 10α, 10β | 1, 7, 8, 10 | 2β, 7β, 10β, 18a, 19 |
| 10α | 2.33 | dd (10.8, 9.6) | 37.8, | CH2 | 9, 10β | 9, 11, 12, 17 | 18a |
| 10β | 1.87 | dd (10.8, 8.4) | 9, 10α | 1, 16 | 9, 10α, 17 | ||
| 11 | - | - | 36.9, | C | - | - | - |
| 12 | - | - | 160.2, | C | - | - | - |
| 13 | 5.83 | d (4.0) | 103.7, | CH | - | - | 17 |
| 14 | 5.84 | dd (4.0, 0.8) | 105.8, | CH | - | - | 16 |
| 15 | - | - | 150.6, | C | - | - | - |
| 16 | 2.28 | d (0.8) | 13.6, | CH3 | - | 14, 15 | 14 |
| 17 | 1.35 | s | 17.9, | CH3 | - | 1, 10, 11, 12 | 2α, 2β, 9, 13 |
| 18a | 5.09 | s | 113.5, | CH2 | 7β | 7, 8, 9 | 9, 10α |
| 18b | 4.93 | s | 7β | 7, 8, 9 | 7β | ||
| 19 | 1.23 | s | 17.0, | CH3 | 3 | 3, 4, 5 | 3β, 9 |
a Spectrum recorded at 400 MHz in CDCl3. b Spectrum recorded at 100 MHz in CDCl3.
Compounds 1–3 were tested for cytotoxicity against mouse lymphocytic leukemia (P-388), human colon adenocarcinoma (HT-29), and human lung epithelial carcinoma (A-549) tumor cell lines. Compound 3 exhibited cytotoxicity against P-388, A549, and HT-29 with ED50 values of of 1.0, 1.2, and 0.5 μg/mL, respectively. However, compounds 1 and 2 were not cytotoxic to P-388, A549 and HT-29 cell lines. Compounds 1–3 were also examined for antiviral activity against human cytomegalovirus (HCMV) and did not show anti-HCMV activity.
3. Experimental Section
3.1. General Experimental Procedures
Optical rotations were obtained on a JASCO P1020 digital polarimeter (Tokyo, Japan). UV and IR spectra were determined on JASCO V-650 (JASCO, Tokyo, Japan) and JASCO FT/IR-4100 spectrophotometers (JASCO, Tokyo, Japan), respectively. NMR spectra were recorded on a Varian MR 400 NMR spectrometer (Santa Clara, CA, USA) at 400 MHz for 1H and 100 MHz for 13C. 1H NMR chemical shifts are expressed in δ (ppm) referring to the solvent peak δH 7.27 for CHCl3 and coupling constants are expressed in Hertz (Hz). 13C NMR chemical shifts are expressed in δ (ppm) referring to the solvent peak δC 77.0 for CDCl3. MS were obtained by a Bruker APEX II mass spectrometer (Bruker, Bremen, Germany). Precoated silica gel plates (Merck, Kieselgel 60 F254, 0.25 mm) and precoated RP-18 F254s plates (Merck) were used for thin-layer chromatography (TLC) analysis. Silica gel 60 (Merck, Darmstadt, Germany, 230–400 mesh) and LiChroprep RP-18 (Merck, 40–63 µm) were used for column chromatography. High-performance liquid chromatography (HPLC) (Hitachi, Tokyo, Japan) was carried out using a Hitachi L-7100 pump (Hitachi, Tokyo, Japan) equipped with a Hitachi, L-7400 UV detector (Hitachi, Tokyo, Japan) at 220 nm together with a semi-preparative reversed-phased column (Merck, Hibar LiChrospher RP-18e, 5 µm, 250 mm × 25 mm).
3.2. Animal Material
The soft coral S. nanolobata was collected by hand using scuba at San-Shin-Tai, Taitong County, Taiwan, in July 2008, at a depth of 7 m. A voucher specimen (SST-009) was deposited in the Department of Marine Biotechnology and Resources, National Sun Yat-sen University.
3.3. Extraction and Separation
The frozen soft coral (3.0 kg) was chopped into small pieces (about 1 cm) and extracted with acetone in a percolator at room temperature. The acetone extract (30.0 g) of S. nanolobata was concentrated under reduced pressure to a brown gum, which was partitioned between EtOAc and H2O. The EtOAc-soluble fraction (30 g) was applied to Si 60 CC using n-hexane–EtOAc mixtures of increasing polarity for elution. Fraction 12, eluted with n-hexane–EtOAc (6:1), was further purified by reverse-phase HPLC (MeOH–H2O, 60:40) to obtain 1 (1.5 mg). Fraction 3, eluted with n-hexane–EtOAc (80:1), was further purified by reverse-phase HPLC (MeOH–H2O, 85:15) to afford 2 (2.6 mg). Fraction 18, eluted with n-hexane–EtOAc (1:4), was further purified by reverse-phase HPLC (MeOH–H2O, 65:35) to obtain 3 (5.0 mg).
Sinubatin A (1): Colorless oil; −19.2 (c 0.38, CHCl3); IR (neat) νmax 2934, 1742, 1442, 1373 and 1420 cm−1; 1H and 13C NMR data, see Table 1; ESI-MS m/z 359 [M + Na]+; HR-ESI-MS m/z 359.1837 (calcd. for C19H28O5Na, 359.1834).
Sinubatin B (2): Colorless oil; +17.2 (c 0.65, CHCl3); IR (neat) νmax 2961, 2925, 1261, 1094, 1020, 799 cm−1; 1H and 13C NMR data, see Table 2; ESIMS m/z 309 [M + Na]+; HR-ESI-MS m/z 309.1832 (calcd. for C19H26O2Na, 309.1830).
3.4. Biological Assay
Cytotoxicity assay and anti-HCMV assay were conducted as previously described [9].
4. Conclusions
The chemical study of soft coral S. nanolobata led to the isolation of a novel tetranorditerpenoid, sinubatin A (1) (having an unprecedented carbon skeleton), a new norditerpenoid, sinubatin B (2) (a 4,5-epoxycaryophyllene possessing an unusual methylfuran moiety side chain), and gibberosin J (3). Compound 3 exhibited cytotoxicity toward P-388, A549, and HT-29 with ED50 values of 1.0, 1.2. and 0.5 μg/mL, respectively. However, compounds 1 and 2 were not cytotoxic to P-388, A549 and HT-29 cell lines. Compounds 1–3 did not show anti-HCMV activity.
Acknowledgments
This research was supported by grants from Ministry of Science and Technology (MOST105-2320-B-110-003-MY3), NSYSUNKMU Joint Project (106-P010), and NSYSUKMU Joint Project (106-P016). We thank Chang-Feng Dai, Institute of Oceanography, National Taiwan University, for the identification the soft coral specimen.
Author Contributions
Conceived of and designed the experiments: Chang-Yih Duh, Shang-Kwei Wang. Performed the experiments: Fu-Yun Hsu.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Blunt J.W., Copp B.R., Keyzers R.A., Munro M.H.G., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2017;34:235–294. doi: 10.1039/C6NP00124F. [DOI] [PubMed] [Google Scholar]
- 2.Yamada K., Ujiie T., Yoshida K., Miyamoto T., Higuchi R. Sinulobatins A–D, new amphilectane-type diterpenoids from the Japanese soft coral Sinularia nanolobata. Tetrahedron. 1997;53:4569–4578. doi: 10.1016/S0040-4020(97)00169-5. [DOI] [Google Scholar]
- 3.Ahmed A.F., Su J.H., Shiue R.T., Pan X.J., Dai C.F., Kuo Y.H., Sheu J.H. New β-caryophyllene-derived terpenoids from the Soft Coral Sinularia nanolobata. J. Nat. Prod. 2004;67:592–597. doi: 10.1021/np030286w. [DOI] [PubMed] [Google Scholar]
- 4.Tseng Y.J., Wen Z.H., Dai C.F., Chiang M.Y., Sheu J.H. Nanolobatolide, a New C18 metabolite from the Formosan soft coral Sinularia nanolobata. Org. Lett. 2009;11:5030–5032. doi: 10.1021/ol901990c. [DOI] [PubMed] [Google Scholar]
- 5.Tseng Y.J., Wang S.K., Duh C.-Y. Secosteroids and norcembranoids from the soft coral Sinularia nanolobata. Mar. Drugs. 2013;11:3288–3296. doi: 10.3390/md11093288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ngoc N.T., Huong P.T.M., Thanh N.V., Cuong N.X., Nam N.H., Thung D.C., Kiem P.V., Minh C.V. Steroid constituents from the soft coral Sinularia nanolobata. Chem. Pharm. Bull. 2016;64:1417–1419. doi: 10.1248/cpb.c16-00385. [DOI] [PubMed] [Google Scholar]
- 7.Chen S.P., Su J.H., Ahmed A.F., Dai C.F., Wu Y.C., Sheu J.H. Xeniaphyllane-derived terpenoids from the Formosan soft coral Sinularia gibberosa. Chem. Pharm. Bull. 2007;55:1471–1475. doi: 10.1248/cpb.55.1471. [DOI] [PubMed] [Google Scholar]
- 8.Kel’in A.V., Gevorgyan V. Efficient synthesis of 2-mono- and 2,5-disubstituted furans via the CuI-catalyzed cycloisomerization of alkynyl ketones. J. Org. Chem. 2002;67:95–98. doi: 10.1002/chin.200227141. [DOI] [PubMed] [Google Scholar]
- 9.Lee Y.-S., Duh T.-H., Siao S.-S., Chang R.-C., Wang S.-K., Duh C.-Y. New cytotoxic terpenoids from soft corals Nephthea chabroli and Paralemnalia thyrsoides. Mar. Drugs. 2017;15:392. doi: 10.3390/md15120392. [DOI] [PMC free article] [PubMed] [Google Scholar]