Abstract
Three new isocoumarins—dichlorodiaportintone (1), desmethyldichlorodiaportintone (2) and desmethyldichlorodiaportinol (3)—as well as six known analogues (4–9) were isolated from the culture of the mangrove endophytic fungus Ascomycota sp. CYSK-4 from Pluchea indica. Their structures were elucidated by analysis of spectroscopic data. The absolute configuration of compounds 1 and 2 were determined by the modified Mosher’s method. Compound 2 showed significant anti-inflammatory activity by inhibiting the production of NO in LPS-induced RAW 264.7 cells with IC50 value of 15.8 μM, while compounds 1, 5, and 6 exhibited weak activities with IC50 values of 41.5, 33.6, and 67.2 μM, respectively. In addition, compounds 1, 5, and 6 showed antibacterial effects against Staphylococcus aureus, Bacillus subtilis, Escherichia coli, Klebsiella pneumoniae, and Acinetobacter calcoaceticus with the MIC values in the range of 25–50 μg·mL−1.
Keywords: isocoumarin, endophytic fungus, Pluchea indica, Ascomycota sp., anti-inflammatory, antibacterial
1. Introduction
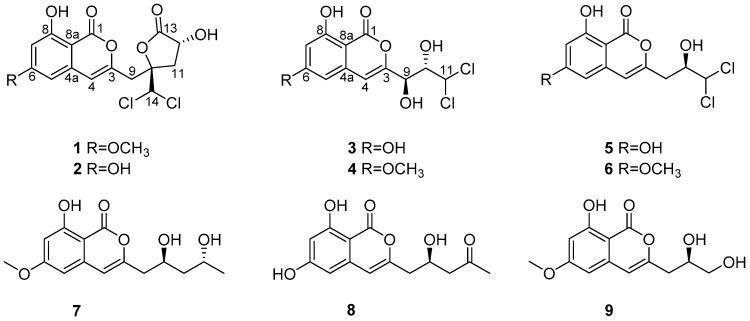
Fungi in the genus Ascomycota sp. produce various structurally novel metabolites, including ascomindones A–C and ascomfurans A–B [1], ascomycotin A [2], wortmannilactone E [3], orsellinic acid [4], isosclerone [5], and chaetocyclinone [6]. Furthermore, most of these compounds possess a wide range of biological activities. For example, polyketone ascomindones A exhibited more potent capacity in scavenging DPPH radical [1]. Diphenyl ether barceloneic acid A showed modest inhibition of FPTase enzyme [7]. As part of our ongoing investigation on bioactive natural products from mangrove-derived fungi, an endophytic fungus Ascomycota sp. CYSK-4, which was isolated from the healthy branch of the marine semimangrove Pluchea indica, attracted our attention because an EtOAc extract of the fungal culture exhibited significant anti-inflammatory activity. Bioassay-guided fractionation of the EtOAc extract led to the isolation of three new isocoumarin derivatives, compounds 1–3, together with six known isocoumarin analogues 4–9 (Figure 1). In the in vitro assays, compounds 1–2 and 5–6 showed inhibitory activities against the lipopolysaccharides (LPS) induced nitric oxide (NO) production in murine macrophage RAW 264.7. Herein, the details of the isolation, structural elucidation and anti-inflammatory evaluation of these compounds were reported.
Figure 1.
The structures of compounds 1–9.
2. Results
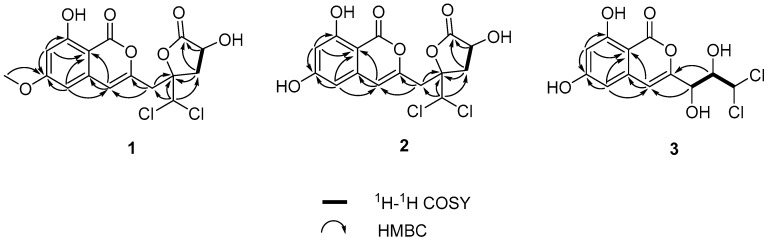
Compound 1 was obtained as an amorphous solid. Its molecular formula was established as C16H14O7Cl2 based on the HRESIMS and NMR data, containing two Cl-atoms and implying nine indices of hydrogen deficiency. The 1H NMR data of 1 (Table 1) displayed signals of two aromatic protons [δH 6.51 (1H, d, J = 2.2 Hz); 6.62 (1H, d, J = 2.2 Hz)], one olefin proton [6.68 (1H, s)], two methylenes [δH 3.33 (2H, d, J = 6.0 Hz); 3.04 (1H, dd, J = 9.5, 14.7 Hz); 2.45 (1H, dd, J = 6.9, 14.7 Hz)], two methines [δH 4.75 (1H, dd, J = 6.9, 9.5 Hz); 6.52 (1H, s)], and one methoxy group at δH 3.92 (1H, s). The 13C NMR data of 1 (Table 2) exhibited 16 carbon resonances assignable to one methyl, two methylenes, two sp3 and three sp2 methines, six quaternary carbons and two carbonyl carbons. These spectroscopic features suggested that 1 belong to the isocoumarin class [8,9,10]. Analysis of the 1H-1H COSY spectrum (Figure 2) suggested the presence of one independent spin system H2-11/H-12. Together with the HMBC cross-peaks (Figure 2) of H-12/C-13; H-11/C-10, C-13; H-9/C-3, C-10; H-14/C-9, C-10, and C-11 indicated the side chain of isocaproicacid moiety location at C-3. The chemical shift of H-14 (δH 6.52) indicated the dichloro substitution at C-14 [8]. Apart from a carbonyl group and isocoumarin group, the remaining one indices of hydrogen deficiency was proved to be α-hydroxyl-γ-lactone ring. The chemical shift of quaternary carbon δC 86.0 (C-10) confirmed the ring bridging C-10 and C-13. Moreover, the methoxy group was placed to C-6 based on the HMBC correlation of its proton to C-6. Thus, the constitution of 1 was established (Figure 1).
Table 1.
1H NMR (nuclear magnetic resonance) (ppm, mult, (J in Hz)) data of compounds 1–3 in acetone-d6 (500 MHz).
| Position | 1 | 2 | 3 |
|---|---|---|---|
| 4 | 6.68, s | 6.63, s | 6.69, s |
| 5 | 6.62, d (2.2) | 6.43, d (2.0) | 6.51, d (2.1) |
| 7 | 6.51, d (2.2) | 6.50, d (2.0) | 6.44, d (2.1) |
| 9 | 3.33, d (6.0) | 3.31, m | 4.38, d (8.8) |
| 10 | 4.28, dd (1.6, 8.8) | ||
| 11α | 3.04, dd (9.5, 14.7) | 3.04, dd (9.5, 14.7) | 6.43, d (1.6) |
| 11β | 2.45, dd (6.9, 14.7) | 2.45, dd (6.8, 14.4) | |
| 12 | 4.75, dd (6.9, 9.5) | 4.75, dd (6.9, 9.3) | |
| 14 | 6.52, s | 6.52, s | |
| 6-OCH3 | 3.92, s | ||
| 8-OH | 11.0, br s | 11.0, br s | 10.9, br s |
Table 2.
13C NMR (ppm, mult) data of compounds 1–3 in acetone-d6 (125 MHz).
| Position | 1 | 2 | 3 |
|---|---|---|---|
| 1 | 166.5, C | 166.5, C | 166.6, C |
| 3 | 151.7, C | 151.5, C | 156.1, C |
| 4 | 109.8, CH | 109.7, CH | 107.5, CH |
| 4a | 139.9, C | 140.1, C | 140.1, C |
| 5 | 102.6, CH | 102.9, CH | 104.3, CH |
| 6 | 167.9, C | 166.4, C | 164.6, C |
| 7 | 101.6, CH | 104.1, CH | 103.0, CH |
| 8 | 164.2, C | 164.4, C | 166.6, C |
| 8a | 100.9, C | 100.1, C | 100.3, C |
| 9 | 41.2, CH2 | 41.2, CH2 | 73.1, CH |
| 10 | 86.0, C | 86.0, C | 76.7, CH |
| 11 | 37.4, CH2 | 37.4, CH2 | 76.1, CH |
| 12 | 68.4, CH | 68.4, CH | |
| 13 | 175.6, C | 175.5, C | |
| 14 | 77.2, CH | 77.2, CH | |
| 6-OCH3 | 56.3, CH3 |
Figure 2.
Key COSY and HMBC correlations of compounds 1–3.
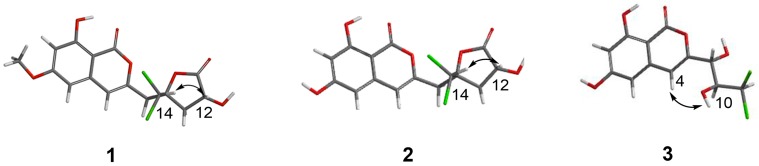
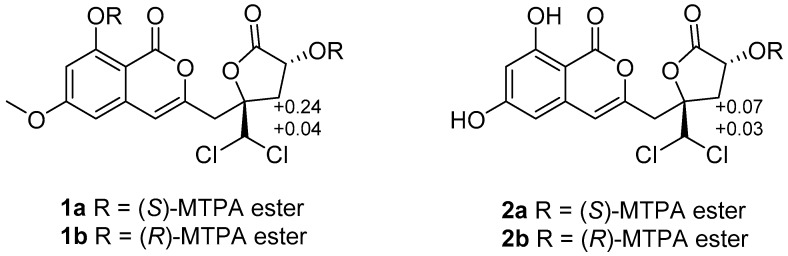
The relative configuration of 1 was determined by NOESY data (Figure 3). The cross-peak of H-12 and H-14 indicated the syn relationship between H-12 and H-14. The absolute configuration of C-12 was further confirmed by the modified Mosher ester method [11]. The (S)- and (R)-MTPA esters of 1 (1a and 1b) were prepared using (R)- and (S)-MTPA chloride, respectively. The differences in the 1H NMR chemical shifts of 1a and 1b were summarized to determine the absolute configuration of this position, which was clearly established as 12R. Taking the data discussed above into account, the absolute configuration of 1 was assigned as 10R,12R (Figure 4). Thus, compound 1 was determined as dichlorodiaportintone (Figure 1).
Figure 3.
Key NOESY correlations of compounds 1–3.
Figure 4.
Δδ = δS − δR values in ppm for the MTPA (Methoxy–trifluoromethyl phenylacetic acid) esters of 1 and 2.
Compound 2 was isolated as a white amorphous powder, having the molecular formula C15H12O7Cl2 based on the HREIMS at m/z 372.9885 [M − H]−. The NMR data (Table 1 and Table 2) resembled those of 1, except for the disappearence of a methoxy group (δC 56.3, δH 3.92). The NOESY correlation (Figure 3) from H-12 to H-14 proved that compounds 2 and 1 shared the same relative configuration. The absolute configuration at C-12 was also determined as R by the modified Mosher’s method. Thus, the absolute configuration of 2 was determined as 10R,12R (Figure 4). Therefore, the compound 2 was assigned as desmethyldichlorodiaportintone (Figure 1).
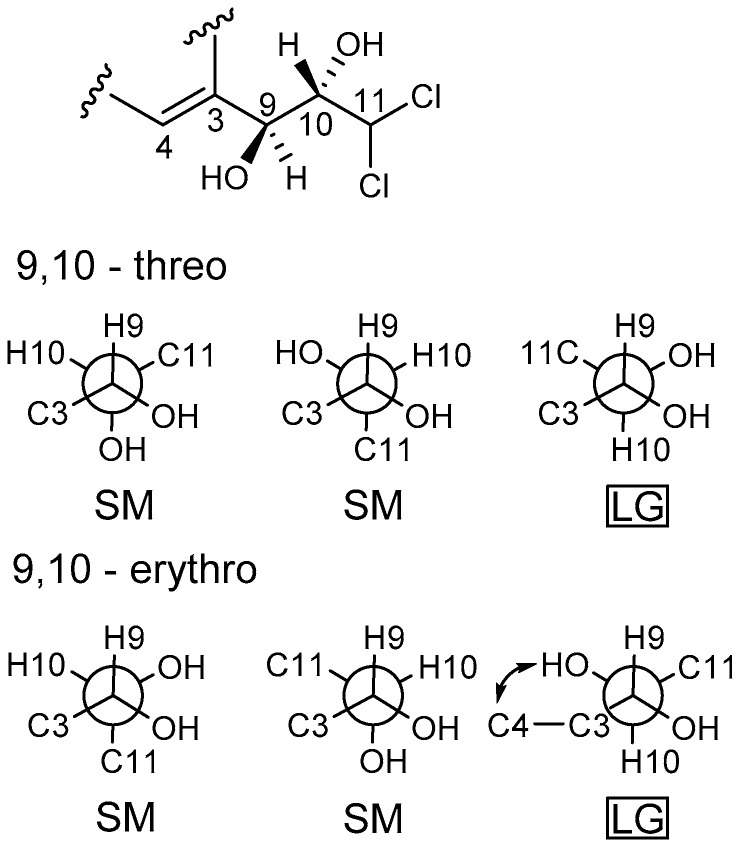
Compound 3 was obtained as a white powder. The molecular formula was determined as C12H10O6Cl2 by HRESIMS. The 1H NMR spectrum (Figure S18) of 3 showed signals for a 1,2,3,5-tetrasubstituted aromatic unit [δH 6.44 (1H, d, J = 2.1 Hz) and δH 6.51 (1H, d, J = 2.1 Hz)], one methine signal at δH 6.43 (1H, d, J = 1.6 Hz), two oxygenated methine signals at δH 4.38 (1H, dd, J = 1.6, 8.8 Hz) and δH 4.28 (1H, d, J = 8.8 Hz). The 13C NMR spectrum (Figure S19) exhibited 13 carbon signals, indicating a carbonyl carbon, three methines, eight aromatic carbons. The 1H and 13C NMR spectra (Table 1 and Table 2) of 3 were similar to those of dichlorodiaportinol A (4), except for the absence of the methoxy group (δC 56.3, δH 3.90) at C-6 in 3. The structure of 3 was also confirmed using 1H-1H COSY and HMBC spectra (Figure 2). The configuration of two stereocenters (C-9 and C-10) were determined by coupling constants and NOESY experiments (Figure 3). Protons H-9 and H-10 displayed a large coupling constant (3JH-9,H-10 = 8.8 Hz), indicating them to be in an anti configuration. This allowed for only two of the six possible relative configuration for C-9 and C-10 could be satisfied. As well as the NOE correlation of H-4/OH-10 in DMSO, the relative configuration of C-9 and C-10 was unambiguously determined as 9R* and 10S* (Figure 5) and named desmethyldichlorodiaportinol (Figure 1).
Figure 5.
Newman projections for C-9/C-10. Box indicates conformation that consistent with coupling constant. LG: large; SM: small.
The other known compounds were identified as dichlorodiaportinol (4) [9], desmethyldichlorodiaportin (5) [8], dichlorodiaportin (6) [10], mucorisocoumarin B (7) [12], citroisocoumarin (8) [13], and diaportinol (9) [11] by comparison with NMR data in the literature.
The anti-inflammatory activities of all compounds were evaluated against nitric oxide (NO) production in the lipopolysaccharide (LPS)-stimulated mouse macrophage RAW 264.7. The results suggest that the compound 2 showed potent inhibitory activities with IC50 value of 15.8 μM, and compounds 1, 5, and 6 exhibited weak inhibitory activity in comparison with the indomethacin (the positive control, IC50 = 37.5 μM). Other compounds showed no inhibitory effect (IC50 > 100 μM) (Table 3). All compounds showed no cytotoxic effect at the tested concentration. Compounds 2 and 5 which have a hydroxyl group at C-6 showed batter than compounds 1 and 6 with a methoxy group at C-6. The structure-activity relationships of these dichloroisocoumarins indicated that a hydroxyl group was more significance than a methoxy group on anti-inflammatory activity. Isocoumarins were previously reported to have radical scavenging and antioxidant [14], anti-HIV [15], antimicrobial [16], anti-γ-secretase [17], antitumor [18], immunomodulatory [19], antifungal [20], toxicity to zebrafish embryos [13], and α-glucosidase inhibitory activities [21]. This is the first report of anti-inflammatory activity of dichloroisocoumarins.
Table 3.
Inhibitory effects of isolated compounds on lipopolysaccharide (LPS)-stimulated nitric oxide (NO) production in RAW (mice macrophage) 264.7 cells.
| Compound | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Indometacin a |
|---|---|---|---|---|---|---|---|---|---|---|
| IC50 (μM) | 41.5 | 15.8 | >100 | >100 | 33.6 | 67.2 | >100 | >100 | >100 | 37.5 |
a Positive control.
The antibacterial activities of the isolated compounds 1–9 against two Gram-positive bacteria (Staphylococcus aureus and Bacillus subtilis) and three Gram-negative bacteria (Escherichia coli, Klebsiella pneumoniae, and Acinetobacter calcoaceticus) were tested (Table 4). Compounds 5 and 6 showed antibacterial activities with the MIC values between 25 and 50 μg·mL–1 against S. aureus, B. subtilis, E. coli, K. pneumoniae, and A. calcoaceticus. Compound 1 exhibited antibacterial activities with the MIC values at 50 μg·mL−1 against S. aureus, E. coli and K. pneumoniae. Other compounds did not exhibit obvious activity at 50 μg·mL−1.
Table 4.
Antibacterial activities of compounds 1–9.
| Compound a | MIC (μg·mL−1) | ||||
|---|---|---|---|---|---|
| Staphylococcus aureus | Bacillus subtilis | Escherichia coli | Klebsiella pneumoniae | Acinetobacter calcoaceticus | |
| 1 | 50 | >50 | 50 | 50 | >50 |
| 5 | 25 | 25 | 25 | 25 | 50 |
| 6 | 25 | 25 | 50 | 50 | 50 |
| Ciprofloxacin b | 0.25 | 0.50 | 0.50 | 0.25 | 0.25 |
| Gentamicin b | 0.10 | 0.25 | 0.25 | 0.25 | 0.25 |
a Compounds 2, 3, 4, 7, 8, and 9 showed no activities (MIC > 50 μg·mL–1); b Positive control.
3. Materials and Methods
3.1. General Experimental Procedures
Optical rotations were measured on an MCP 300 (Anton Paar, Shanghai, China) polarimeter at 25 °C. UV spectra were recorded in MeOH using a PERSEE TU-1900 spectrophotometer (Persee, Beijing, China). IR spectra were recorded on a Nicolet Nexus 670 spectrophotometer (Nicolet, Madison, WI, USA) in KBr discs. NMR spectra were carried out on Bruker Avance 400 spectrometer (1H 400 MHz, 13C 100 MHz) (Bruker Bio Spin Corporation, Bellerica, MA, USA) and Bruker Avance 500 spectrometer (1H 500 MHz, 13C 125 MHz) (Bruker Bio Spin Corporation, Bellerica, MA, USA). ESIMS spectra were measured on a Finnigan LCQ-DECA mass spectrometer (Finnigan, Beijing, China), and HRESIMS spectra were obtained on a Thermo Fisher Scientic Q-TOF mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Column chromatography (CC) was conducted using silica gel (200–300 mesh, Qingdao Marine Chemical Factory, Qingdao, China) and Sephadex LH-20 (Amersham Pharmacia, Piscataway, NJ, USA). Thin-layer chromatography (TLC) was performed on silica gel plates (Qingdao Huang Hai Chemical Group Co., G60, F-254, Qingdao, China).
3.2. Fungal Material and Fermentation
The fungus CYSK-4 used in this study was isolated from healthy branch of the marine semimangrove Pluchea indica, which was collected in July 2015 from Shankou Mangrove Nature Reserve in Guangxi Province, China. It was obtained using the standard protocol for isolation [21]. Initially, the plant tissue was washed with sterile water and surface-sterilized in a 200 mL beaker with 75% ethanol for 1 min. This was followed by dipping the sample into 5% sodium hypochlorite for 1 min, then the plant parts were rinsed with sterile water, cut into 3 mm sections, and plated on PDA with penicillin (100 units per mL) and streptomycin (0.08 mg·mL−1). The plates were incubated at 25 ± 1 °C. The endophytic fungal strains were isolated by routine microbiological methods. The fungal isolates were numbered and stored at 4 °C in triplicate on PDA slants. Fungal identification was carried out using a molecular biological protocol by DNA amplification and sequencing of the ITS region [22]. The sequence data obtained from the fungal strain have been deposited at Gen Bank with accession no. MG571637. A BLAST search result showed that the sequence was the most similar (99%) to the sequence of Ascomycota sp. (compared to KT240142.1 EF060747.1). A voucher strain was deposited in the Guangdong Microbial Culture Center under patent depository number GDMCC 60100. The fungus Ascomycota sp. CYSK-4 was cultured on autoclaved rice solid-substrate medium (60 × 500 mL Erlenmeyer flasks, each containing 50 g rice and 50 mL 3‰ of saline water) for 30 days at room temperature.
3.3. Extraction and Isolation
Following incubation. The mycelia and solid rice medium were extracted three times with EtOAc. The extract was evaporated under reduced pressure to yield 60 g of residue. The residue was subjected to a silica gel column chromatography, eluting with a gradient of petroleum ether/EtOAc from 1:0 to 0:1, to obtain 36 fractions. Fraction 8 (120 mg) was subjected to silica gel CC (CH2Cl2/MeOH v/v, 98:2) to yield compounds 1 (8.2 mg), 3 (3.8 mg), and 7 (2.5 mg). Fraction 12 (90 mg) was applied to Sephadex LH-20 CC (CH2Cl2/MeOH v/v, 1:1) to give compounds 2 (5.1 mg) and 5 (3.4 mg). Fraction 16 (68 mg) was purified by Sephadex LH-20 CC (CH2Cl2/MeOH v/v, 1:1) and silica gel CC (CH2Cl2/MeOH v/v, 88:12) afford 4 (4.6 mg) and 8 (2.2 mg). Fraction 18 (62 mg) was chromatographed on Sephadex LH-20 CC (100% MeOH) to obtain compound 6 (1.8 mg). Fraction 20 was separated by silica gel CC (CH2Cl2/MeOH v/v, 86:14) to give subfraction fraction 20.2, which was purified by Sephadex LH-20 CC (CH2Cl2/MeOH v/v, 1:1) to yield 9 (2 mg).
3.3.1. Dichlorodiaportintone (1)
Amorphous solid; [α = +11.9 (c 0.12, acetone); UV (MeOH) λmax (log ε): 332 (3.78), 280 (3.87), 245 (4.61) nm; IR (KBr) νmax: 3319, 1800, 1681, 1621, 1386, 1238, 1209, 1167, 856, 795, 712 cm−1; HRESIMS m/z 387.0041 [M − H]– (calcd. for C16H13O7Cl2, 387.0048); 1H NMR (500 MHz, acetone-d6) data, see Table 1; 13C NMR (125 MHz, acetone-d6) data, see Table 2.
3.3.2. Desmethyldichlorodiaportintone (2)
White amorphous powder; [α = +6.9 (с 0.06, acetone); UV (MeOH) λmax (log ε): 331 (3.85), 262 (4.20), 246 (4.44) nm; IR (KBr) νmax: 3232, 1781, 1679, 1630, 1455, 1372, 1248, 1166, 1071, 781 cm−1; HRESIMS m/z 372.9885 [M − H]− (calcd. for C15H11O7Cl2, 372.9887); 1H NMR (500 MHz, acetone-d6) data, see Table 1; 13C NMR (125 MHz, acetone-d6) data, see Table 2.
3.3.3. Desmethyldichlorodiaportinol (3)
White powder; [α = +18.3 (с 0.1, acetone); UV (MeOH) λmax (log ε): 329 (3.62), 268 (3.65), 245 (3.26) nm; IR (KBr) νmax: 3543, 3461, 3232, 1683, 1631, 1497, 1399, 1293, 1242, 1188, 1071, 1038, 837, 703 cm−1; HRESIMS m/z 318.9782 [M − H]− (calcd. for C12H9O6Cl2, 318.9782); 1H NMR (500 MHz, acetone-d6) data, see Table 1; 13C NMR (125 MHz, acetone-d6) data, see Table 2.
3.3.4. Preparation of the (S)- and (R)-MTPA Esters 1a and 1b
Compound 1 (1 mg) was treated with (R)-MTPACl (10 μL) and pyridine (0.5 mL). The mixture was reacted at room temperature for 24 h. The solution was extracted with 5 mL of CH2Cl2, and the organic phase was concentrated under reduced pressure. The residue was purified by preparative TLC (CH2Cl2) to yield the (S)-MTPA ester 1a (0.8 mg). In a similar way, (R)-MTPA ester 1b (0.5 mg) was obtained from compound 1 (1 mg) reacted with (S)-MTPACl (10 μL).
(S)-MTPA ester 1a: 1H NMR (acetone-d6, 400 MHz) δH: 6.74 (1H, s, H-4), 6.65 (1H, s, H-14), 7.12 (1H, d, J = 2.2 Hz, H-5), 6.72 (1H, d, J = 2.2 Hz, H-7), 5.95 (1H, dd, J = 8.0, 10.3 Hz, H-12), 3.98 (3H, s, 6-OCH3), 3.76 (3H, s, OCH3-MTPA), 3.52 (3H, s, OCH3-MTPA), 3.43 (2H, d, J = 4.6 Hz, H-9), 3.33 (1H, dd, J = 7.8, 15.0 Hz, H-11a), 2.85 (1H, dd, J = 10.4, 15.0 Hz, H-11b). ESIMS m/z 821.0 [M + 1]+.
(R)-MTPA ester 1b: 1H NMR (acetone-d6, 400 MHz) δH: 6.65 (1H, s, H-4), 6.63 (1H, s, H-14), 6.57 (1H, d, J = 2.2 Hz, H-5), 6.52 (1H, d, J = 2.2 Hz, H-7), 6.03 (1H, dd, J = 8.0, 10.3 Hz, H-12), 3.92 (3H, s, 6-OCH3), 3.58 (3H, s, OCH3-MTPA), 3.52 (3H, s, OCH3-MTPA), 3.38 (2H, d, J = 4.6 Hz, H-9), 3.29 (1H, dd, J = 7.8, 15.0 Hz, H-11a), 2.61 (1H, dd, J = 10.4, 15.0 Hz, H-11b). ESIMS m/z 821.0 [M + 1]+.
3.3.5. Preparation of the (S)- and (R)-MTPA Esters 2a and 2b
Following the same way as described for compound 1, R- and S- MTPA ester derivatives 2a and 2b were prepared from 2.
(S)-MTPA ester 2a: 1H NMR (CHCl3, 400 MHz) δH: 6.96 (1H, s, H-4), 6.95 (1H, s, H-14), 7.01 (1H, s, H-5), 6.69 (1H, s, H-7), 6.35 (1H, d, J = 2.31 Hz, H-12), 3.53 (3H, s, OCH3-MTPA), 3.46 (2H, m, H-9), 2.98 (1H, dd, J = 10.6, 14.9 Hz, H-11a), 2.55 (1H, dd, J = 7.7, 14.9 Hz, H-11b). ESIMS m/z 591.0 [M + 1]+.
(R)-MTPA ester 2b: 1H NMR (CHCl3, 400 MHz) δH: 6.91 (1H, s, H-4), 6.93 (1H, s, H-14), 7.0 (1H, s, H-5), 6.62 (1H, s, H-7), 6.33 (1H, d, J = 2.31 Hz, H-12), 3.53 (3H, s, OCH3-MTPA), 3.40 (2H, m, H-9), 2.91 (1H, dd, J = 10.6, 14.9 Hz, H-11a), 2.52 (1H, dd, J = 7.7, 14.9 Hz, H-11b). ESIMS m/z 591.0 [M + 1]+.
3.4. Nitric Oxide Production Assay
Murine macrophage RAW 264.7 cells purchased from the Shanghai Institutes for Biological Sciences in DMEM (high glucose) medium supplemented with 10% (v/v) fetal bovine serum, 100 μg·mL‒1 penicillin and streptomycin, and 10 mM HEPES at 37 °C in a 5% CO2 atmosphere [23]. Cells were pretreated with different samples dissolved in serum-free culture medium containing 0.5% DMSO (10, 5, 2.5, 1.25, and 0.625 μM) for 4 h, followed by stimulation with 1 μg·mL–1 LPS for 24 h. Fifty μL of cell culture medium was mixed with 100 μL of Griess reagent I and II and incubated at room temperature for 10 min with horizontal shaking, after which the absorbance at 540 nm was measured in a microplate reader. Indomethacin was used as a positive control and was purchased from Sigma-Aldrich Co. (CAS number: 53-86-1, EINECS number: 200-186-5; Buchs, Switzerland). Wells with DMSO were used as a negative control (final DMSO concentration was 0.1%). The NO production inhibition rate was calculated by the flowing formula:
| (1) |
IC50 was defined as the concentration of compound that inhibited 50% NO production relative to the LPS group and was calculated using SPSS 16.0 software. All assays were performed in triplicate.
3.5. Antimicrobial Activity
Antimicrobial activities were evaluated by the conventional broth dilution assay [24]. Two Gram-positive—S. aureus (ATCC 12228) and B. subtilis (ATCC 6633)—and three Gram-negative—E. coli (ATCC 25922), K. pneumoniae (ATCC 13883), and A. calcoaceticus (ATCC 23055)—were used. Overnight cultures of five bacterial strains were made up in 0.9% saline to an inoculum density of 5 × 105 cfu by comparison with a MacFarland standard. All compounds were dissolved in DMSO and diluted by Mueller Hinton broth to a starting concentration of 2 mg·mL–1. Ninety-five μL of MHB and 5 μL of test compounds or the antibiotic were dispensed into wells as well as the 100 μL bacterial suspension. After incubation at 37 °C for 24 h, the inhibitory effect was evaluated by optical density measurement. The MIC was determined as the concentration which the growth was inhibited 80% of bacterial. One hundred μL bacterial suspension were added to the solutions in 96-well to achieve a final volume of 200 μL and final sample concentrations from 50 to 0.125 μg·mL–1. The blank well was also incubated with only medium under the same conditions. OD measurement was record at 595 nm. All experiments were performed in triplicate and with ciprofloxacin and gentamicin as the positive control.
4. Conclusions
In conclusion, nine secondary metabolites including three new dichloroisocumarins—dichlorodiaportintone (1), desmethyldichlorodiaportintone (2), and desmethyldichloro-diaportinol (3)—and six known compounds—dichlorodiaportinol (4), desmethyldichlorodiaportin (5), dichlorodiaportin (6), mucorisocoumarin B (7), citroisocoumarin (8), and diaportinol (9)—were isolated from the marine mangrove-derived endophytic fungus Ascomycota sp. CYSK-4. Their structures were clarified by analysis of NMR data. Compounds 1–2 and 5–6 showed anti-inflammatory activities by inhibiting the production of NO in LPS-induced RAW 264.7 cells with IC50 values of 41.5, 15.8, 33.6, and 67.2 μM, respectively. Compounds 1, 5, and 6 showed inhibitory activity against S. aureus, B. subtilis, E. coli, K. pneumoniae and A. calcoaceticus with MIC values of 25–50 μg·mL–1. The results above proved that the dichloroisocumarins have the potential to be used as natural anti-inflammatory agents and antibiotics through appropriate structural modification.
Acknowledgments
We thank the National Natural Science Foundation of China (21472251, 41276146, 81741162), the Science & Technology Plan Project of Guangdong Province of China (2013B021100011), the key project of Natural Science Foundation of Guangdong Province (2016A040403091), and the Special Promotion Program for Guangdong Provincial Ocean and Fishery Technology (A201701C06) for generous support.
Supplementary Materials
The following are available online at http://www.mdpi.com/1660-3397/16/2/54/s1, Figure S1. 1H NMR spectrum of compound 1 (500 MHz, acetone-d6), Figure S2. 1H NMR spectrum of compound 1 (500 MHz, DMSO-d6), Figure S3. 13C NMR spectrum of compound 1 (125 MHz, acetone-d6), Figure S4. HSQC spectrum of compound 1 (acetone-d6), Figure S5. HMBC spectrum of compound 1 (acetone-d6), Figure S6. 1H-1H COSY spectrum of compound 1 (acetone-d6), Figure S7. NOESY spectrum of compound 1 (acetone-d6), Figure S8. NOESY spectrum of compound 1 (DMSO-d6), Figure S9. HRESIMS spectrum of compound 1, Figure S10. 1H NMR spectrum of compound 2 (500 MHz, acetone-d6), Figure S11. 13C NMR spectrum of compound 2 (125 MHz, acetone-d6), Figure S12. HSQC spectrum of compound 2 (acetone-d6), Figure S13. HMBC spectrum of compound 2 (acetone-d6), Figure S14. 1H-1H COSY spectrum of compound 2 (acetone-d6), Figure S15. NOESY spectrum of compound 2 (acetone-d6), Figure S16. HRESIMS spectrum of compound 2, Figure S17. 1H NMR spectrum of compound 3 (500 MHz, acetone-d6), Figure S18. 1H NMR spectrum of compound 3 (500 MHz, DMSO-d6), Figure S19. 13C NMR spectrum of compound 3 (125 MHz, acetone-d6), Figure S20. HSQC spectrum of compound 3 (acetone-d6), Figure S21. HMBC spectrum of compound 3 (acetone-d6), Figure S22. 1H-1H COSY spectrum of compound 3 (acetone-d6), Figure S23. NOESY spectrum of compound 3 (acetone-d6), Figure S24. NOESY spectrum of compound 3 (DMSO-d6), Figure S25. HRESIMS spectrum of compound 3, Figure S26. Experiment ECD spectrum of 1 and 2.
Author Contributions
Yan Chen contributes to isolation, and wrote the paper; Yan Chen and Zhaoming Liu contributes to extraction, characterization of all the compounds and supplementary NMR date preparament; Hongju Liu and Jing Li contributes to the biological tests; Zhigang She, Lan Liu, and Yahong Pan guided the whole experiment and revised the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Tan C., Liu Z., Chen S. Antioxidative Polyketones from the Mangrove-Derived Fungus Ascomycota sp. SK2YWS-L. Sci. Rep. 2016;6 doi: 10.1038/srep36609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tian Y., Lin X., Liu J. Ascomycotin A, a new citromycetin analogue produced by Ascomycota sp. Ind19F07 isolated from deep sea sediment. Nat. Prod. Res. 2015;29:820–826. doi: 10.1080/14786419.2014.988620. [DOI] [PubMed] [Google Scholar]
- 3.Dong Y., Lin J., Lu X. Cathepsin B inhibitory tetraene lactones from the fungus Talaromyces wortmannii. Helv. Chim. Acta. 2009;92:567–574. doi: 10.1002/hlca.200800333. [DOI] [Google Scholar]
- 4.Thadhani V.M., Choudhary M.I., Ali S. Antioxidant activity of some lichen metabolites. Nat. Prod. Res. 2011;25:1827–1837. doi: 10.1080/14786419.2010.529546. [DOI] [PubMed] [Google Scholar]
- 5.Lin T., Lin X., Lu C. Secondary metabolites of Pyrenochaeta sp. B36, an endophytic fungus from Annona squamosa L. Nat. Prod. Res. 2011;25:1008–1013. doi: 10.1080/14786419.2010.534997. [DOI] [PubMed] [Google Scholar]
- 6.Lösgen S., Schlörke O., Meindl K. Structure and biosynthesis of chaetocyclinones, new polyketides produced by an endosymbiotic fungus. Eur. J. Org. Chem. 2007;2007:2191–2196. doi: 10.1002/ejoc.200601020. [DOI] [Google Scholar]
- 7.Jayasuriya H., Ball R.G., Zink D.L. Barceloneic acid A, a new farnesyl-protein transferase inhibitor from a Phoma species. J. Nat. Prod. 1995;58:986–991. doi: 10.1021/np50121a002. [DOI] [PubMed] [Google Scholar]
- 8.Aly A.H., Edrada-Ebel R.A., Wray V. Bioactive metabolites from the endophytic fungus Ampelomyces sp. isolated from the medicinal plant Urospermum picroides. Phytochemistry. 2008;69:1716–1725. doi: 10.1016/j.phytochem.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Li C., Gong B., Cox D.G. Dichlorodiaportinol A-A new chlorine-containing isocoumarin from an endophytic fungus Trichoderma sp. 09 from Myoporum bontioides A. Gray and its cytotoxic activity. Pharmacogn. Mag. 2014;10:S153–S158. doi: 10.4103/0973-1296.127367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Larsen T.O., Breinholt J. Dichlorodiaportin, diaportinol, and diaportinic acid: Three novel isocoumarins from Penicillium nalgiovense. J. Nat. Prod. 1999;62:1182–1184. doi: 10.1021/np990066b. [DOI] [PubMed] [Google Scholar]
- 11.Ohtani I., Kusumi T., Kashman Y. High-field FT NMR application of Mosher's method. The absolute configurations of marine terpenoids. J. Am. Chem. Soc. 1991;113:4092–4096. doi: 10.1021/ja00011a006. [DOI] [Google Scholar]
- 12.Feng C.C., Chen G.D., Zhao Y.Q. New Isocoumarins from a Cold–Adapted Fungal Strain Mucor sp. and Their Developmental Toxicity to Zebrafish Embryos. Chem. Biodivers. 2014;11:1099–1108. doi: 10.1002/cbdv.201400005. [DOI] [PubMed] [Google Scholar]
- 13.LAI S., Shizuri Y., Yamamura S. Three new phenolic metalolites from Penicillium species. Heterocycles. 1991;32:297–305. [Google Scholar]
- 14.Tianpanich K., Prachya S., Wiyakrutta S. Radical scavenging and antioxidant activities of isocoumarins and a phthalide from the endophytic fungus Colletotrichum sp. J. Nat. Prod. 2010;74:79–81. doi: 10.1021/np1003752. [DOI] [PubMed] [Google Scholar]
- 15.Napolitano E. The synthesis of isocoumarins over the last decade. A review. Org. Prep. Proced. Int. 1997;29:631–664. doi: 10.1080/00304949709355245. [DOI] [Google Scholar]
- 16.Liu S.W., Jin J., Chen C. PJS, a novel isocoumarin with hexahydropyrimidine ring from Bacillus subtilis PJS. J. Antibiot. 2013;66:281–284. doi: 10.1038/ja.2012.118. [DOI] [PubMed] [Google Scholar]
- 17.Petit A., Pasini A., Alves Da Costa C. JLK isocoumarin inhibitors: Selective γ -secretase inhibitors that do not interfere with notch pathway in vitro or in vivo. J. Neurosci. Res. 2003;74:370–377. doi: 10.1002/jnr.10747. [DOI] [PubMed] [Google Scholar]
- 18.Kawano T., Agata N., Kharbanda S. A novel isocoumarin derivative induces mitotic phase arrest and apoptosis of human multiple myeloma cells. Cancer. Chemother. Pharmacol. 2007;59:329–335. doi: 10.1007/s00280-006-0274-x. [DOI] [PubMed] [Google Scholar]
- 19.Roy S., Roy S., Neuenswander B., Hill D., Larock R.C. Solution-phase synthesis of a diverse isocoumarin library. J. Comb. Chem. 2009;11:1128–1135. doi: 10.1021/cc9001197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li W., Xu J., Li F. A new antifungal isocoumarin from the endophytic fungus Trichoderma sp. 09 of Myoporum bontioides A. gray. Pharmacogn. Mag. 2016;12:259–261. doi: 10.4103/0973-1296.192204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen S., Liu Y., Liu Z. Isocoumarins and benzofurans from the mangrove endophytic fungus Talaromyces amestolkiae possess α-glucosidase inhibitory and antibacterial activities. RSC. Adv. 2016;6:26412–26420. doi: 10.1039/C6RA02566H. [DOI] [Google Scholar]
- 22.Liu Y., Yang Q., Xia G. Polyketides with α-glucosidase inhibitory activity from a mangrove endophytic fungus, Penicillium sp. HN29-3B1. J. Nat. Prod. 2015;78:1816–1822. doi: 10.1021/np500885f. [DOI] [PubMed] [Google Scholar]
- 23.Morikawa T., Matsuda H., Toguchida I. Absolute Stereostructures of Three New Sesquiterpenes from the Fruit of Alpinia oxyphylla with Inhibitory Effects on Nitric Oxide Production and Degranulation in RBL-2H3 Cells. J. Nat. Prod. 2002;65:1468–1474. doi: 10.1021/np020078o. [DOI] [PubMed] [Google Scholar]
- 24.Appendino G., Gibbons S., Giana A. Antibacterial cannabinoids from Cannabis sativa: A structure-activity study. J. Nat. Prod. 2008;71:1427–1430. doi: 10.1021/np8002673. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.