Abstract
Matricellular proteins are secreted molecules that have affinities for both extracellular matrix and cell surface receptors. Through interaction with structural proteins and the cells that maintain the matrix these proteins can alter matrix strength. Matricellular proteins exert control on cell activity primarily through engagement of membrane receptors that mediate outside-in signaling. An example of this group is thrombospondin-1 (TSP1), first identified as a component of the secreted product of activated platelets. As a result, TSP1 was initially studied in relation to coagulation, growth factor signaling and angiogenesis. More recently, TSP1 has been found to alter the effects of the gaseous transmitter nitric oxide (NO). This latter capacity has provided motivation to study TSP1 in diseases associated with loss of NO signaling as observed in cardiovascular disease and pulmonary hypertension (PH). PH is characterized by progressive changes in the pulmonary vasculature leading to increased resistance to blood flow and subsequent right heart failure. Studies have linked TSP1 to pre-clinical animal models of PH and more recently to clinical PH. This review will provide analysis of the vascular and non-vascular effects of TSP1 that contribute to PH, the experimental and translational studies that support a role for TSP1 in disease promotion and frame the relevance of these findings to therapeutic strategies.
Keywords: Thrombospondin-1, CD47, Pulmonary hypertension, Nitric oxide, eNOS, Endothelin-1, ROS, Nox1, cMyc, Vasorelaxation
1. Introduction
Matricellular proteins are a diverse group of molecular entities that are secreted into the extracellular milieu from where they modify cell responses. The term matricellular protein has been employed for over 20 years and was coined to embrace the properties of secreted proteins that were noted to alter cell attachment to matrix and cell-cell attachments.1 Contained within the term is the idea that these proteins interact with matrix, other secreted proteins including growth factors, enzymes such as proteases, calcium, and with the cell membrane and membrane-located receptors, but do not serve as structural elements of the extracellular matrix.2 A founding member of this group is thrombospondin-1 (TSP1), a trimeric glycoprotein composed of 150-KDa monomers linked through disulfide bonds.3 These later play a role in TSP1-mediated adhesion via integrins,4 in growth factor signaling5 and in Ca2+ binding.6 Each TSP1 monomer consists of an N-terminal globular domain that binds heparin, type I, II, and III repeats, and a C-terminal globular domain.3 TSP1 was first described as a protein released from thrombin-activated platelets a characteristic incorporated in its name.7 TSP1 regulates multiple cellular activities, in part, due to its multi-domain structure permitting interaction with several cell receptors and matrix proteins8 (Table 1). Initially assigned a role in modifying the tumor micro-environment, further work suggests a role for TSP1 in vascular9 and metabolic diseases,10 although it also has a role in health and homeostasis. This review will consider the systemic and the pulmonary vascular effects of TSP1 signaling and the implications of these for pulmonary hypertension (PH).
Table 1.
TSP1 domains with receptor and interacting partners
| N-terminal domain | TSRs domains | C-terminal domain | |
|---|---|---|---|
| Molecular interaction | |||
| Functions |
|
|
|
2. Thrombospondin-1 (TSP1)–in health and homeostasis
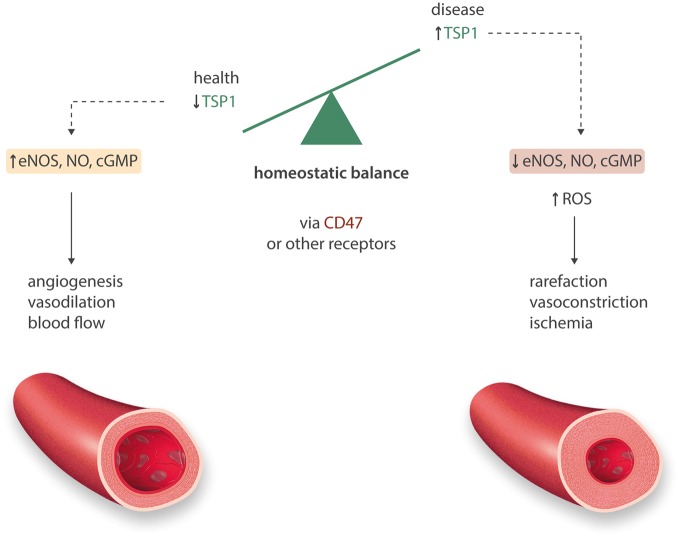
TSP1 has under-appreciated functions in health including the maintenance of vascular structure and homeostasis through the regulation of cell proliferation, apoptosis, and adhesion.3,11 In development TSP1 supports synapse formation in the central nervous system,12 while in adult mice absence of TSP1 is associated with increased seizure susceptibility.13 TSP1 is necessary for maintaining normal lacrimal gland homeostasis14 and the immune privileged status of the ocular region.15 TSP1 influences von Willebrand Factor (VWF) multimer size and may control vWF release.16 TSP1 has a beneficial role in modulating inflammation through tempering macrophage17 and T cell18,19 responses. In left ventricle pressure overload induced by aortic constriction, mice lacking TSP1 showed more injury compared to mice expressing TSP1,20 whereas after cutaneous thermal injury the rate of wound closure was decreased in the absence of TSP1.21 Together these findings speak to a homeostatic role for TSP1 in wound healing.
3. TSP1–a natural anti-angiogenic
TSP1 limited endothelial cell chemotaxis to fibroblast growth factor β (FGF-β) and FGF-β-stimulated angiogenic outgrowth in rat corneas.22 Concurrently, it was reported that TSP1 limited endothelial cells proliferation23 identifying it as a natural inhibitor of angiogenesis. In endothelial cells TSP1 inhibited the effects of vascular endothelial growth factor (VEGF) by suppressing VEGF’s24 ability to increase cyclic guanosine monophosphate (cGMP), a downstream effector of the biogas nitric oxide (NO).25 This latter activity was thought to be through engagement of cell receptor CD36 on endothelial cells.25 However the specificity of this interaction was in doubt as other CD36-interacting partners including collagen and oxidized LDL mimicked the inhibitory actions of TSP1 on endothelial cells.26 TSP1 can also bind soluble VEGF and displace cell membrane-bound VEGF27 to limit endothelial cell angiogenic activity. Insight was provided through the observation that minimal concentrations of TSP1 (0.2–2 nM TSP1) interacting with the cell receptor CD47 inhibited VEGF-mediated activation of receptor VEGF-R2 on endothelial cells,28 and this was absent in endothelial cells lacking CD47. Over-expression of TSP1 in mice confirmed the anti-angiogenic activity of TSP1.29,30 Not surprisingly, enhancing TSP1 signaling with domain-derived peptides and molecules that enhance specific activities of the protein are in development as therapies for excessive ocular angiogenesis,31 and to inhibit tumor-driven angiogenesis.32
4. TSP1 and nitric oxide (NO)
Nitric oxide (NO) is a gaseous transmitter produced under physiologic and pathologic conditions by several synthetic enzymes (reviewed in Ref. 33). In response to blood flow, increased NO production at low concentrations by endothelial nitric oxide synthase (eNOS)34 is rapid, intermittent and limits endothelial inflammation,35 thrombosis formation36,37 and vasoconstriction38 to increase blood flow. Low concentrations of NO regulate cell signaling by binding to soluble guanylate cyclase to increase soluble guanylate cyclase (sGC).39 In inflammation, NO produced by inducible nitric oxide synthase40 is sustained, substantial and invokes transcriptional changes in cells that are distinct from those mediated by eNOS-derived NO.41
The effect of TSP1 to inhibit NO was first appreciated in experiments that assessed the anti-angiogenic activity of TSP1. Skeletal muscle from young male Thbs1-/- mice explanted in collagen matrix had increased vascular cell outgrowth compared to wild type (TSP1+/+) samples following treatment with an NO donor.25 Further, TSP1 inhibited NO-induced cell outgrowth in explants from wild type mice.25In vitro, NO-mediated effects on endothelial cell proliferation, cGMP levels and cGMP-sensitive kinase activation were inhibited by 1 μg/mL (2.2 nM) TSP1.25 Thus, in endothelial cells TSP1 exerts upstream and downstream control of NO (Figure 1). Subsequent studies found that TSP1 inhibited NO signaling in other vascular cells including arterial vascular smooth muscle cells (VSMC)42 and platelets.43 As noted, TSP1 binds to several cell receptors including calreticulin/low-density lipoprotein (LDL) receptor-related protein, CD148, syndecan-3, neuroligin-1, very-low-density-lipoprotein receptor, six different integrins, CD36 and CD47 (reviewed in Ref. 44). It was not clear which of these receptors was responsible for transducing TSP1 inhibition of NO. Initial attention again focused on CD36. NO-stimulated increases in endothelial cell cGMP were inhibited by treating with TSP1, a CD36 agonist antibody and a domain of TSP1 that binds to CD36.25 Proximate to NO activation of sGC, TSP1 limits eNOS activation and production of NO through alteration in calcium signaling45 and in some circumstances via CD36 restricting uptake of the fatty acid myristate.46 However, TSP1 also inhibits NO signaling in CD36-/- VSMC47 that express the TSP1 receptor CD47.48 This finding was important, as vascular cells from Cd47-/- mice, that express CD36, were insensitive to TSP1-mediated inhibition of NO signaling.47 Studies employing the recombinant CD47 binding domain of TSP1, agonist and antagonist CD47 antibodies, and vascular cells and tissues from CD36-/- and Cd47-/- mice confirmed that while CD36 is sufficient for TSP1 inhibition of NO only CD47 is necessary47 (Figure 1). It was noted that TSP1 did not bind to a bacterial-derived recombinant CD47 extracellular domain raising concerns that TSP1 was not a ligand of CD47.49,50 Subsequent studies demonstrated that TSP1 binds with high affinity to CD47 to limit NO signaling48 and that in some cells this binding requires a glycosylation that is absent in bacterial-expressed CD47.51 At baseline Thsp1-/- and Cd47-/- mice have elevated levels of phosphorylated (active) eNOS,45 cGMP, and cAMP in tissues compared to controls.52 Conversely, cultured human umbilical vein endothelial cells (HUVEC) treated with the NO donor (DETA/NO, 0.1 μM, 24 h) showed a 50% decrease in TSP1 protein vs. untreated.53 The finding of NO-mediated suppression of TSP1 has yet to be confirmed in vivo. Still these findings are important in light of the broad anti-angiogenic activity of TSP1 and have implications in regard to pulmonary hypertension (PH) as this is a disease of low NO signaling and elevated TSP1.
Figure 1.
Thrombospondin-1 negatively intersects vascular NO-ROS. Under healthy conditions TSP1 is almost undetectable. In acute and chronic injury and disease, TSP1 is induced in the systemic vasculature to engage CD47 and limit NO signaling through decreasing eNOS activity, and separately by rendering sGC and PKG resistant to activation. Separately, TSP1 limits cAMP signaling. TSP1 also activates NADPH oxidase 1 (Nox1) increasing superoxide production. Acting via these several pathways TSP1 promotes vascular rarefaction, vasoconstriction and ischaemia.
5. TSP1 and reactive oxygen species (ROS)
NO has a half-life of seconds39 and is more transient in conditions of inflammation where the reactive oxygen species (ROS) superoxide is abundant. Treating U937 cells, a human monocyte cell line, with TSP1 (20 μg/mL) enhanced phorbol myristate-mediated superoxide production, and this was abrogated by superoxide dismutase or a α6β1 integrin blocker.54 Aortic VSMC from young Cd47-/- mice had lower ROS production compared to wild type cells, when quantified by dihydroethidium (DHE), dichlorofluorescein and Mitosox fluorescence.55 Human pulmonary arterial endothelial cells challenged with hypoxia (1% O2, 12 h) displayed increased TSP1 protein expression and increased superoxide production quantified by DHE fluorescence.56 Treatment with a human CD47 antibody (clone B6H12, 1 μg/mL) that blocks TSP1 binding,48 suppressed the hypoxia-mediated increase in superoxide.56 In hearts from 30 month old mice TSP1 was increased compared to hearts from 2 month old animals,57 while in the skin of aged wild type mice TSP1 and CD47 protein expression was increased and associated with decreased blood flow vs. young animals.58 Together, these findings suggest that TSP1 promotes ROS production and that aging may upregulate TSP1-CD47 signaling. Expanding upon this, studies in arterial VSMC59 and renal tubule epithelial cells (rTEC)60 demonstrated that treatment with TSP1 at relevant concentrations (2.2 nM) increased nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 1-derived superoxide production (Nox1). Further, TSP1 via both CD4759 as well as cell membrane receptor signal regulatory protein-α (SIRP-α)60 phosphorylated the key Nox1 organizer subunit p47ph°x and increased superoxide production. It remains to be determined if these ligand-receptor interactions stimulate Nox1 in a mutually dependent or independent manner. Nonetheless, involvement of SIRP-α61 in vascular and epithelial cell signaling anticipates a role beyond phagocytosis.
6. TSP1 inhibits transplant healing and blood flow
Skin grafting is a standard model of angiogenic tissue healing.62 In patients with skin grafts to burn wounds, soluble TSP1 was found in the wound fluid,63 however it was not clear if this was associated in any way with graft healing. Skin grafts transplanted from wild type C57Bl/6 mice to wounds in Thsp1-/- and Cd47-/- mice (both strains on a C57BL/6 background) resulted in improved healing vs. transplants of wild type skin grafts to wild type recipients.64 Treatment with a TSP1-CD47 antagonist antibody or a CD47 targeting morpholino oligonucleotide that decreased CD47 protein levels increased healing rates of wild type grafts.64 Curiously, skin grafts from Cd47-/- mice displayed better healing when transplanted onto wild type recipients vs. wild type grafts. In light of the ability of wild type (SIRP-α +) macrophages to phagocytize circulating cells lacking CD47,65 these data suggest that SIRP-α-CD47-mediated phagocytosis is less relevant for parenchymal cells.
A role for TSP1 in regulating blood flow was hinted at in relation to Sickle Cell Disease (SCD). Flow chambers coated with TSP1 had increased adhesion of SCD red blood cells (RBCs).66 In a translational study, plasma TSP1 levels positively correlate with some vascular complications in SCD patients.67 Under flow conditions TSP1 via CD47 promoted platelet adhesion to endothelial cells68 and contributed to endothelial cell apoptosis.69 Also, TSP1 promoted platelet adhesion under pathologic shear stress.70 In other experiments, TSP1 expression increased in endothelial cells maintained in static (no-flow) conditions but was suppressed under laminar flow.71 Finally, HUVEC exposed to shear stress (13 dynes/cm2, 2 h) demonstrated increased TSP1 expression in the extracellular matrix compared to matrix from cells under static conditions.72 These results suggest the intriguing idea that lowering of blood flow (and hence eNOS-derived NO production), through upregulation of TSP1 promotes vascular occlusion.
Evidence that TSP1 has a role in acutely controlling blood flow was first shown in young male wild type and Thsp1-/- mice. Challenging anesthetized animals with the NO donor (DEA/NO) resulted in increased hind limb skeletal muscle blood flow (quantified by BOLD-MRI) that was faster and greater in Thsp1-/- as compared to wild type mice.73 In 14–18 month old male wild type, Thsp1-/- and Cd47-/- mice subjected to surgically-induced soft tissue ischaemia, laser Doppler demonstrated enhanced tissue blood flow immediately after injury in mice lacking TSP1 and CD47.74 Interestingly, BOLD-MRI found that aged Thsp1-/- mice had improved NO-mediated blood flow changes 72 h after femoral artery ligation compared to aged wild type.74 Potential differences in capillary density among mouse strains might have accounted for some of these effects. Nonetheless, young75 as well as aged male wild type mice74 and young pigs76 treated with a CD47 suppressing morpholino oligonucleotide had less tissue injury and improved blood flow after ischaemia compared to controls. Acute ischaemia followed by reperfusion (IR) increases TSP1 expression in organs,77 while animals lacking TSP177 and CD4778,79 and wild types treated with CD47 blocking agents78,80,81 are resistant to IR injury. Similarly, intravenous TSP1 impedes reperfusion in rats and this is corrected by pre-treatment with a CD47 blocking antibody (clone OX101).59 In keeping with a role for TSP1 to acutely inhibit blood flow in vivo, studies in isolated systemic arteries found that TSP1 (0.22 nM), as well as a recombinant portion of the protein that contains the CD47 binding domain, inhibited vasorelaxation by the eNOS activator acetylcholine (Ach)45 in arteries from wild type mice, whereas TSP1 inhibited Ach-stimulated vasorelaxation in arteries from Thsp1-/- but not Cd47-/- mice.45 In other studies, TSP1 inhibition of Ach-mediated vasorelaxation in wild type vessels was partially ameliorated by treatment with the ROS scavenger Tempol.60 Conversely, TSP1-/- and CD47-/- arteries were less sensitive to phenylephrine-mediated vasoconstriction compared to wild type arteries.45 TSP1 also inhibited NO-mediated vasorelaxation of coronary arterioles from 24 month old, but not 4 month old, female rats.82 Treatment with a CD47 blocking antibody (clone OX101) and ROS scavengers limited inhibition by TSP1, although the CD47 blocking antibody was more effective at increasing NO sensitivity.82 Finally, TSP1 mRNA levels were increased in amputated limbs from individuals with peripheral vascular disease (PVD),83 while plasma TSP1 levels positively correlated with PVD.84
7. Pulmonary hypertension–a disease of lung ischaemia
Pulmonary hypertension (PH) is a progressive fatal process characterized by among other things deterioration in NO signaling, hyperactive ROS production, progressive loss of micro-vascularity, ischaemia, absence of angiogenesis, proliferative remodeling of pulmonary macro-vessels and increased resistance to pulmonary blood flow (reviewed in Refs. 85 and 86). As the circulatory system is an integrated unit, PH is predicted to have systemic manifestations. Yet it remains to be understood why in PH ischaemia primarily presents in the lung and why angiogenesis is suppressed.
8. TSP1 and PH–cell and animal studies
Over the last decade an interest in TSP1 in relation to PH has been pursued. In a pig model of pulmonary arterial (PA) IR lung injury, PA sensitivity to Ach-mediated vasorelaxation was decreased post-IR and this was associated with increased TSP1 mRNA levels vs. sham PAs.87 This study is important in linking TSP1 and vascular dysfunction in PAs. Proliferation of human PA VSMC was inhibited by treatment with TSP1 (25 ng/mL).88 Chronic hypoxia increases pulmonary vascular resistance in mammals. Young male mice exposed to hypoxia (10% FiO2) for 1 and 7 days showed increased TSP1 and decreased CD36 transcript in micro-dissected pulmonary vessels compared to normoxic mice.89 Unfortunately, cardiopulmonary pressure and heart morphometric data was not provided. Thsp1-/- mice exposed to 10% FiO2 for 6 weeks displayed less increase in right ventricular systolic pressure (RVSP) and Fulton index (weight of right ventricle/left ventricle and septum) vs. wild type.90 Pulmonary vascular remodeling was also less pronounced in lungs from Thsp1-/- mice.90 Normoxic Thsp1-/- mice had less elevation in RVSP after treatment with the thromboxane A2 receptor agonist U-46619 and no elevation in RVSP following 15 min of hypoxia (10% FiO2) compared to wild type. In these studies, Thsp1-/- mice received sulfamethoxazole and trimethoprim putatively to preempt possible lung infection.11 It remains unknown what effect treatment with these drugs had on the outcomes. Others confirmed that pulmonary TSP1 is increased in hypoxia-56 as well as bleomycin-mediated91 PH, and that Thsp1-/- mice are resistant to cardiopulmonary changes following chronic hypoxia.56 However, in bleomycin-mediated PH the data require careful interpretation as rat neonatal pups were employed. Interestingly, in normoxic pulmonary arterial endothelial cells TSP1 limited VEGF-stimulated angiogenic activity,91 similar to results in systemic arterial vascular endothelial cells,25,28 while treatment with a Rho-kinase inhibitor partially limited thrombin (10 U/mL)-stimulated TSP1 production in pulmonary cells.91 Together, these data suggest that absence of TSP1 provides protection from acute and chronic pulmonary vascular changes and that TSP1 has a presser effect in the pulmonary circulation. Ex vivo myography studies of PAs confirmed the presser activity of TSP1. Normoxic PAs from mice and rats treated with TSP1 (2.2 nM) were less sensitive to endothelial (Ach)- and VSMC (sodium nitroprusside, SNP)-mediated vasorelaxation, and in the case of rat PAs more sensitive to a vasoconstrictor.92 When PAs were studied under hypoxic conditions, endothelial-mediated vasorelaxation was reduced in vessels from wild type but not Thps1-/- mice93 (Figure 2).
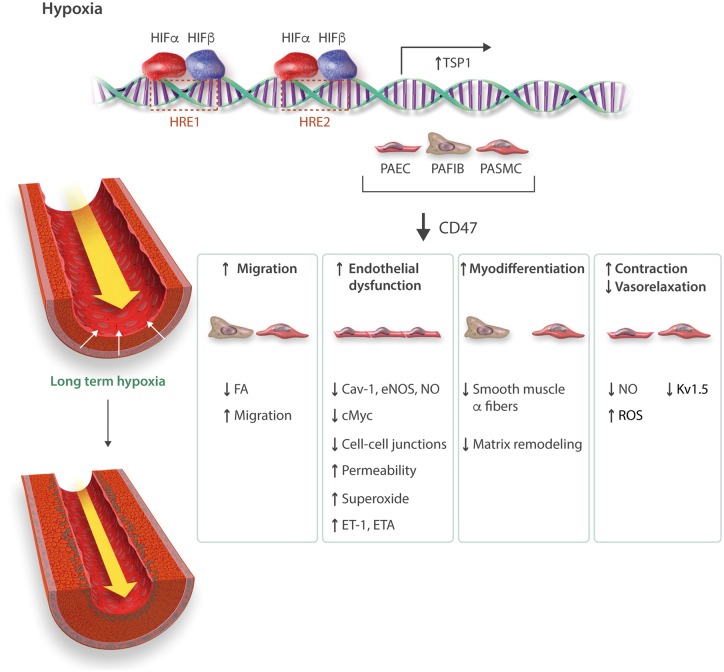
Figure 2.
Thrombospondin-1 in PH. Hypoxia inducible factor-2α (HIF-2α) targets hypoxia response elements (HRE) to increase THSP1 transcription in pulmonary vascular cells. In pulmonary arterial endothelial cells (PAEC) TSP1 via CD47 promotes PH by (1) targeting Cav-1 to dysregulate eNOS while increasing ROS, (2) suppressing cMyc to de-repress ET-1/ETA, and (3) decreasing cell-cell adhesion. TSP1 also increases pulmonary smooth muscle cell (PASMC) migration and fibroblast (PAFIB) activity. In pulmonary arteries, TSP1 inhibits vasorelaxation, increases vasoconstriction and in hypoxia decreases Kv1.5 ion channel effects.
Under low oxygen conditions, cells respond through hypoxia inducible transcription factors (HIFs).94 TSP1 mRNA and protein are increased in lungs from mice within 7 h of hypoxia93 concurrent with increased pulmonary expression of hypoxia inducible factor 2 alpha (HIF-2α). In vitro, TSP1 expression was also increased in murine fibroblasts and murine and human PA endothelial and VSMC exposed to 1% FiO2 for 24 h.93 Mutant mice that lack the Von Hippel-Lindau (VHL) protein, which mediates proteasomal degradation of HIF-α protein under normoxia95 and mimic wild type mice under hypoxia, showed upregulation of pulmonary TSP1.93 In contrast, vhl-/-/hif-2α-/- double mutant mice had decreased pulmonary TSP1 protein and mRNA levels that were restored in vhl-/-/hif-1α-/- mice, while suppression of HIF-2α in pulmonary vascular cells abrogated hypoxia-stimulated upregulation of TSP1.93 Chinese hamster ovarian cells transfected with a luciferase-TSP1 hypoxia response element and a constitutively active HIF-2α construct had significant luciferase induction.93 These data give genetic evidence for HIF-2α in the pulmonary induction of TSP1 (Figure 2).
Loss of NO signaling in PH includes decreased eNOS expression in human PAs, and this correlates inversely with the extent of vasculopathy and the degree of elevation in pulmonary vascular resistance,96 although eNOS expression may be increased in pulmonary plexi-form lesions.97 Further, NO and NO byproducts are decreased in lungs of PH subjects.98 In keeping with data from systemic vascular cells, eNOS activation, as characterized by phosphorylation at serine 1176 (the murine equivalent of the human residue serine 117999), was constitutively increased in lungs from normoxic young male Thps1-/- vs. wild type mice.56 However, challenging Thsp1-/- mice with hypoxia (10% FiO2, 3 weeks) resulted in decreased levels of phosphorylated eNOS, suggesting TSP1 does not directly control eNOS activity in hypoxic PH lungs.56 In pulmonary endothelial cells Cav-1 interacts with CD47 and this interaction is suppressed by TSP1 and hypoxia,56 while in lungs from hypoxic Thsp1-/- and Cd47-/- mice Cav-1 protein levels are increased and ROS levels decreased compared to lungs from hypoxic wild type mice. This is important as loss of Cav-1 results in PH.100,101 Conversely, blocking TSP1-CD47 signaling resulted in increased Cav-1 expression and decreased eNOS-derived superoxide production by hypoxic pulmonary arterial endothelial cells.45 These findings indicate that TSP1 inhibits the ability of Cav-1 to mitigate eNOS dysfunction under chronic hypoxia. However, it remains to be determined if TSP1 is an activator of Nox1 in the pulmonary vasculature. This is a valid question as Nox-derived superoxide contributes to pre-clinical PH102 while Nox1, a target of TSP1 in systemic vascular cells,59,60 is increased in PAs from hypoxic piglets.103 Interestingly, hypoxia-stimulated proliferation of human PA VSMC was inhibited by treatment with a TSP1 antibody and by rosiglitazone (a peroxisome proliferator-activated receptor gamma blocker), while treatment with exogenous TSP1 (2.2 nM) for 72 h increased Nox4 protein expression.104 Conversely, overexpression of Nox1 in A549 cells resulted in accumulation of HIF-1α,105 suggesting the hypothesis that Nox1 via HIFs may act in a feed-forward fashion to increase TSP1.
While experiments in isolated pulmonary vascular cells and rodents point to a role for TSP1 to promote PH, the possible receptors transducing these effects have only started to be confirmed. Initial information was derived from studies in rats given the pyrrolizidine alkaloid monocrotaline (mct, 50 mg/kg). This induces a delayed but significant injury to the pulmonary circulation with increased pulmonary vascular resistance and RV hypertrophy.106 Four weeks after treatment male rats treated with mct displayed increased expression of pulmonary TSP1 and CD47 protein and elevated RVSP and Fulton index compared to controls.56 Treating mct injured animals with a CD47 blocking antibody (clone OX101, 1 μg/gram body weight on day 1 and day 14 via i.p. injection) partially normalized RVSP and Fulton index and was associated with increased pulmonary phosphorylated eNOS and Cav-1 protein expression.56 Also, Cd47-/- mice are resistant to hypoxia-mediated cardiopulmonary aberrations compared to wild type.92 These results indicate that absence of ligand TSP1 and receptor CD47 or blockade of TSP1-CD47 signaling provides protection from hypoxic- and mct-derived PH. Studies in pulmonary arterial endothelial cells and whole lungs indicate that the protection gained in the absence of CD47 is, in part, secondary to suppression of ET-1 signaling. It was noted that TSP1, via CD47, acted to constitutively suppress pulmonary cMyc which, being a key transcription factor regulator, significantly inhibited ET-1 and its pulmonary VSMC receptor endothelin-A (ETA).92 In the setting of high TSP1-CD47 signaling cMyc is suppressed allowing for upregulation of pulmonary ET-1/ETA and VSMC hypertrophy. In the setting of low or absent TSP1-CD47 signaling cMyc is upregulated leading to the suppression of ET-1/ETA (Figure 2). A repercussion of this is that treating pulmonary VSMC with a CD47 blocking antibody limits the hypertrophic effects of exogenous ET-1.92 ET-1 modifies NO signaling in vascular cells to both increase and decrease NO effects.107 It remains to be seen if TSP1-CD47, via cMyc and ET-1, alters pulmonary NO signaling. It also remains to be determined why in the face of chronically elevated pulmonary cMyc, Cd47-/- mice have minimal vascular remodeling and overgrowth.
9. TSP1 and PH–human data
Studies in isolated mammalian pulmonary vascular cells, and rodent and pig models, indicate a role for TSP1 in PH. Beyond this, several recent studies have linked TSP1 to clinical PH. Analysis of lungs and distal PA (5th order) segments from patients undergoing transplant for end-stage PH confirmed upregulation of TSP1 and CD47 protein and TSP1 mRNA compared to non-PH lungs and PAs.56,92 Immunofluorescent TSP1 and CD47 were increased in the medial and adventitial layers of distal PAs from end-stage PH lungs along with increased matrix protein mRNA.92 Of functional relevance, TSP1 (2.2 nM) inhibited SNP-mediated vasorelaxation and potentiated ET-1-stimulated vasoconstriction of healthy 5th order PAs. Diseased distal PAs from PH lungs were diminished in vasorelaxation to Ach and SNP, whereas treating diseased PAs with a CD47 antibody (clone B6H12, 1 μg/mL) improved endothelial- and VSMC- mediated vasorelaxation.92 In 93 individuals with isolated PH and 19 healthy controls, plasma TSP1 levels were assessed.108 The disease cohort included idiopathic pulmonary arterial hypertension, lung disease-associated and chronic thromboembolic PH. Plasma TSP1 levels were elevated in all PH groups compared to controls and increased with worsening World Health Organization functional classification.108 Although 61% of PH subjects were women, no difference in TSP1 in relation to gender was found. Plasma TSP1 levels positively correlated with mean PA pressure and negatively correlated with cardiac output. Non-linear regression analysis found a bi-phasic pattern to plasma TSP1 levels with an initial increase as a function of increasing pulmonary vascular resistance that plateaued and then declined as resistance peaked, although the reason for this unclear. Noteworthy, chronically hypoxic wild type mice displayed a significant increase in plasma TSP1, whereas plasma TSP1 was not elevated in hypoxic Cd47-/- mice.92 Finally, 5 year mortality in the PH cohort positively correlated with the degree in elevation in plasma TSP1.108 Interestingly, TSP1 is increased in other pulmonary diseases including asthma,109 idiopathic interstitial pneumonia,110 and chronic obstructive pulmonary disease.111 These and other studies (Table 2) suggest that TSP1 is a possible biomarker of pulmonary and cardiovascular disease.
Table 2.
Select studies implicating TSP1 as a possible biomarker
| Target | Cohort | Readout | Correlation | Control for platelet activation | Reference |
|---|---|---|---|---|---|
| [CD142+/TSP1+] platelet microparticles from blood | clinical and genetic diagnosis of heterozygous Familial Hypercholesterolemia (FH) (n = 37) and non-FH secondary hypercholesterolemic patients (n = 37) | burden of atherosclerosis by MRI | TSP1+/CD142+ platelet-derived microparticles characterize young patients with high cardiovascular risk | unknown | 136 |
| TSP1 mRNA analysis - cardiac needle biopsies | 11 cardiac allografts, 15 normal hearts | vasculopathy on angiography | + correlation increased TSP1 mRNA and transplant vasculopathy | N/A | 137 |
| TSP1 in plasma | non-diabetics with fasting glucose, 2 h glucose and fasting insulin data, or who had type 2 diabetes | nano-liquid chromatography MS in SRM mode | + correlation with TSP1 and pre-diabetes | unknown | 138 |
| TSP1 in cerebrospinal fluid | 31 acute subarachnoid haemorrhage patients | transcranial Doppler of cerebral vasospasm | elevated TSP1 post subarachnoid haemorrhage correlated with cerebral vasospasm and poor outcome | N/A | 139 |
| TSP1 in plasma | 61 chronic haemodialysis patients with diagnosis of cardiovascular disease including cerebrovascular, coronary and peripheral vascular disease | outcomes analysis of all-cause mortality and cardiovascular mortality | elevated TSP1 associated with CVD highest TSP1 level patients had a 5.32- and 6.75-fold more risk for all-cause and cardiovascular mortality | unknown | 140 |
Genetic links between THBS1 and PH have also been reported. In familial pulmonary arterial hypertension several mutations of the type 1 repeat domain of TSP1 (which engages CD36 and mediates activation of latent transforming growth factor beta, TGF-β) were found in about 5% of individuals.112 Recombinant mutant TSP1 protein or peptide mimics of the mutated region were less effective in activating latent TGF-β and at inhibiting cell proliferation.112 Another report analyzed patients with homozygous haemoglobin SS (HbSS, SCD) from the multicentre walk-PHaSST (Treatment of Pulmonary Hypertension and Sickle Cell Disease with Sildenafil Therapy) trial and identified two single nucleotide polymorphisms (SNPs) of TSP1 that negatively associated with tricuspid value regurgitant velocity (TRV) ≥ 2.5 m/s, an echocardiographic marker of PH associated with increased mortality.113 Both SNPs localized to the 5′ untranslated region. Limited but applicable data from explanted lungs from a SCD patient with end-stage PH found upregulation of TSP1 and CD47 in parenchymal tissues, 5th order PAs and cultured VSMC from PAs.114 Also, in 5th order PAs from the SCD lungs (that over-express TSP1-CD47) vasorelaxation to Ach was decreased compared to PAs from non-PH lungs.114
10. Therapeutic opportunities for targeting TSP1 signaling in PH
PH treatments focus on elevating NO signaling, limiting ET-1 signaling, increasing cyclic adenosine monophosphate (cAMP), and increasing cardiac function. TSP1 intersects NO-cGMP and ET-1 to promote adverse cellular responses in the pulmonary vasculature. TSP1 also dysregulates cAMP in arterial VSMC.115 Thus, TSP1 exerts negative regulatory activity on current PH therapies, including heme-independent sGC activators.116 However, TSP1 exists in large quantities pre-formed in platelets supporting targeting TSP1 receptors, and in particular CD47, in PH. CD47 antibodies are in clinical trials albeit for cancer (see: https://clinicaltrials.gov/ct2/results?term=CD47&Search=Search). These CD47 antibodies will likely have effects upon the vascular compartment in PH. Indeed, published ex vivo studies in diseased PAs from human PH lungs found that treatment with a commercially available human CD47 antibody enhanced vasorelaxation to endothelial and VSMC activators.92 However, it is not known what effects targeting CD47 will have upon vascular remodeling and ischaemia in PH. Also, antibodies have low tissue penetration, although this may be circumvented by small molecule inhibitors or morpholino oligonucleotides. This strategy would have the additional benefit of avoiding the ‘antibody sink’ of CD47 expressed on circulating RBCs.117 While small molecules targeting CD47 await development, morpholino oligonucleotides that effectively suppress CD47 have been demonstrated to provide injury protection in pre-clinical studies. As a class, these agents are in the clinic with the third-generation morpholino oligonucleotide Eteplirsen approved by the FDA for treating patients with Duchene muscular dystrophy.
11. Future directions and concluding remarks
TSP1 is emerging as a possible promoter of PH. Yet many important questions deserve attention. First, is TSP1 a primary or secondary contributor to PH? In animals, pulmonary TSP1 is upregulated rapidly following hypoxic challenge, well before gross cardiovascular and pulmonary injury, but what does this mean in relation to clinical disease. Second, the cellular source(s) of pulmonary TSP1 remain to be characterized. While in vitro results confirm that multiple pulmonary vascular cell types including endothelial, VSMC and fibroblasts can produce TSP1, it is also possible that inflammatory cells and platelets contribute to pulmonary TSP1 expression. Cell-specific TSP1 and CD47 null mutant animals will be instrumental in addressing such questions. The role of other TSP1 receptors and signaling pathways warrants additional consideration. For example, Systemic Sclerosis is associated with PH and increased expression of CD36.118 The finding of a link between TSP1 and pulmonary cMyc is possibly far reaching, but pre-clinical data should be complemented with clinical results. TSP1 is also an activator of latent TGF-β, and in rats mct-driven PH is partially ameliorated by treatment with the TGF-β blocker SD-208.119 Interestingly, treatment of mice with a CD47 morpholino oligonucleotide decreased TGF-β mRNA levels associated with thermal injury,21 suggesting a further benefit in PH from targeting TSP1-CD47. As a regulator of both NO and superoxide, TSP1 would be likely to have significant effects upon cardiac function. This notion is supported by the finding that Cd47-/- mice are protected from LV pressure overload secondary to transverse aortic constriction via regulation of Ca2+-calmodulin‐dependent protein kinase II.120 The role of TSP1 to promote RV disease independent of pulmonary effects needs to be determined in a model of RV injury such as pulmonary arterial banding. Also, TSP1 has been involved in dysregulating Ca2+ signaling and this may have implication to cardiomyocyte function. The role of TSP1 to increase tissue remodeling and to alter matrix quality and quantity should be considered in relation to PH. Additional clinical studies of plasma TSP1 and mutations of TSP1 in PH are justified. In these future studies factors such as circadian rhythms, medications especially those that elevate NO and suppress ROS, sex, age and others would be useful to investigate. Lastly, it remains to be tested if TSP1 or one of the TSP1 receptors can be successfully suppressed, for how long and in which patients to obtain optimum therapeutic advantage in PH.
Published studies reviewed herein indicating a role for TSP1 in PH are relatively recent and are far fewer compared to the scientific literature implicating other factors promoting PH. Nonetheless, building upon a foundation of bench-top, animal and human studies from multiple groups it seems that TSP1 exerts negative effects in PH that are at the cross roads of angiogenesis, oxidative stress and hypertrophic growth signaling, supporting the need for continued basic, translation and clinical study.
Acknowledgement
The authors wish to thank Dr. David D. Roberts (National Cancer Institute, NIH) for critical reading of the manuscript.
Conflict of interest: J.S.I. serves as Chair of the Scientific Advisory Board of Radiation Control Technologies, Inc. (RCTI, Garden City, NJ) and has equity interest in RCTI and Tioma Therapeutics (St. Louis, MO) that have licensed CD47 technology for development. The other authors have no COI to report.
Funding
This work was funded by 2P01HL103455, R01 HL-108954, and 1R01HL112914. This work was also supported by the Institute for Transfusion Medicine, the Hemophilia Center of Western Pennsylvania and the Heart, Lung, Blood and Vascular Medicine Institute of the University of Pittsburgh School of Medicine.
References
- 1. Sage EH, Bornstein P.. Extracellular proteins that modulate cell-matrix interactions. SPARC, tenascin, and thrombospondin. J Biol Chem 1991;266:14831–14834. [PubMed] [Google Scholar]
- 2. Bornstein P. Matricellular proteins: an overview. Matrix Biol 2000;19:555–556. [DOI] [PubMed] [Google Scholar]
- 3. Adams JC, Lawler J.. The thrombospondins. Int J Biochem Cell Biol 2004;36:961–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sun X, Skorstengaard K, Mosher DF.. Disulfides modulate RGD-inhibitable cell adhesive activity of thrombospondin. J Cell Biol 1992;118:693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hogg PJ, Hotchkiss KA, Jimenez BM, Stathakis P, Chesterman CN.. Interaction of platelet-derived growth factor with thrombospondin 1. Biochem J 1997; 326 : 709–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Misenheimer TM, Mosher DF.. Calcium ion binding to thrombospondin 1. J Biol Chem 1995;270:1729–1733. [DOI] [PubMed] [Google Scholar]
- 7. Baenziger NL, Brodie GN, Majerus PW.. A thrombin-sensitive protein of human platelet membranes. Proc Natl Acad Sci USA 1971;68:240–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Calzada MJ, Roberts DD.. Novel integrin antagonists derived from thrombospondins. Curr Pharm Des 2005;11:849–866. [DOI] [PubMed] [Google Scholar]
- 9. Isenberg JS, Roberts DD, Frazier WA.. CD47: a new target in cardiovascular therapy. Arterioscler Thromb Vasc Biol 2008;28:615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miller TW, Soto-Pantoja DR, Schwartz AL, Sipes JM, DeGraff WG, Ridnour LA, Wink DA, Roberts DD.. CD47 receptor globally regulates metabolic pathways that control resistance to ionizing radiation. J Biol Chem 2015;290:24858–24874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lawler J, Sunday M, Thibert V, Duquette M, George EL, Rayburn H, Hynes RO.. Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. J Clin Invest 1998;101:982–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA.. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 2005;120:421–433. [DOI] [PubMed] [Google Scholar]
- 13. Mendus D, Rankin-Gee EK, Mustapha M, Porter BE.. Increased sensitivity to kindling in mice lacking TSP1. Neuroscience 2015;305:302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shatos MA, Hodges RR, Morinaga M, McNay DE, Islam R, Bhattacharya S, Li D, Turpie B, Makarenkova HP, Masli S, Utheim TP, Dartt DA.. Alteration in cellular turnover and progenitor cell population in lacrimal glands from thrombospondin 1-/- mice, a model of dry eye. Exp Eye Res 2016;153:27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Masli S, Vega JL.. Ocular immune privilege sites. Methods Mol Biol 2011;677:449–458. [DOI] [PubMed] [Google Scholar]
- 16. Pimanda JE, Ganderton T, Maekawa A, Yap CL, Lawler J, Kershaw G, Chesterman CN, Hogg PJ.. Role of thrombospondin-1 in control of von Willebrand factor multimer size in mice. J Biol Chem 2004;279:21439–21448. [DOI] [PubMed] [Google Scholar]
- 17. Zhao Y, Xiong Z, Lechner EJ, Klenotic PA, Hamburg BJ, Hulver M, Khare A, Oriss T, Mangalmurti N, Chan Y, Zhang Y, Ross MA, Stolz DB, Rosengart MR, Pilewski J, Ray P, Ray A, Silverstein RL, Lee JS.. Thrombospondin-1 triggers macrophage IL-10 production and promotes resolution of experimental lung injury. Mucosal Immunol 2014;7:440–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grimbert P, Bouguermouh S, Baba N, Nakajima T, Allakhverdi Z, Braun D, Saito H, Rubio M, Delespesse G, Sarfati M.. Thrombospondin/CD47 interaction: a pathway to generate regulatory T cells from human CD4+ CD25- T cells in response to inflammation. J Immunol 2006;177:3534–3541. [DOI] [PubMed] [Google Scholar]
- 19. Lamy L, Foussat A, Brown EJ, Bornstein P, Ticchioni M, Bernard A.. Interactions between CD47 and thrombospondin reduce inflammation. J Immunol 2007;178:5930–5939. [DOI] [PubMed] [Google Scholar]
- 20. Xia Y, Dobaczewski M, Gonzalez-Quesada C, Chen W, Biernacka A, Li N, Lee DW, Frangogiannis NG.. Endogenous thrombospondin 1 protects the pressure-overloaded myocardium by modulating fibroblast phenotype and matrix metabolism. Hypertension 2011;58:902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Soto-Pantoja DR, Shih HB, Maxhimer JB, Cook KL, Ghosh A, Isenberg JS, Roberts DD.. Thrombospondin-1 and CD47 signaling regulate healing of thermal injury in mice. Matrix Biol 2014;37:25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Good DJ, Polverini PJ, Rastinejad F, Le Beau MM, Lemons RS, Frazier WA, Bouck NP.. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci USA 1990;87:6624–6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bagavandoss P, Wilks JW.. Specific inhibition of endothelial cell proliferation by thrombospondin. Biochem Biophys Res Commun 1990;170:867–872. [DOI] [PubMed] [Google Scholar]
- 24. Papapetropoulos A, Garcia-Cardena G, Madri JA, Sessa WC.. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest 1997;100:3131–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Isenberg JS, Ridnour LA, Perruccio EM, Espey MG, Wink DA, Roberts DD.. Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent manner. Proc Natl Acad Sci USA 2005;102:13141–13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dawson DW, Pearce SF, Zhong R, Silverstein RL, Frazier WA, Bouck NP.. CD36 mediates the In vitro inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol 1997;138:707–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gupta K, Gupta P, Wild R, Ramakrishnan S, Hebbel RP.. Binding and displacement of vascular endothelial growth factor (VEGF) by thrombospondin: effect on human microvascular endothelial cell proliferation and angiogenesis. Angiogenesis 1999;3:147–158. [DOI] [PubMed] [Google Scholar]
- 28. Kaur S, Martin-Manso G, Pendrak ML, Garfield SH, Isenberg JS, Roberts DD.. Thrombospondin-1 inhibits VEGF receptor-2 signaling by disrupting its association with CD47. J Biol Chem 2010;285:38923–38932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kragh M, Quistorff B, Tenan M, Van Meir EG, Kristjansen PE.. Overexpression of thrombospondin-1 reduces growth and vascular index but not perfusion in glioblastoma. Cancer Res 2002;62:1191–1195. [PubMed] [Google Scholar]
- 30. Streit M, Velasco P, Brown LF, Skobe M, Richard L, Riccardi L, Lawler J, Detmar M.. Overexpression of thrombospondin-1 decreases angiogenesis and inhibits the growth of human cutaneous squamous cell carcinomas. Am J Pathol 1999;155:441–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang S, Neekhra A, Albert DM, Sorenson CM, Sheibani N.. Suppression of thrombospondin-1 expression during uveal melanoma progression and its potential therapeutic utility. Arch Ophthalmol 2012;130:336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miao WM, Seng WL, Duquette M, Lawler P, Laus C, Lawler J.. Thrombospondin-1 type 1 repeat recombinant proteins inhibit tumor growth through transforming growth factor-beta-dependent and -independent mechanisms. Cancer Res 2001;61:7830–7839. [PubMed] [Google Scholar]
- 33. Feletou M, Kohler R, Vanhoutte PM.. Nitric oxide: orchestrator of endothelium-dependent responses. Ann Med 2012;44:694–716. [DOI] [PubMed] [Google Scholar]
- 34. Pollock JS, Forstermann U, Mitchell JA, Warner TD, Schmidt HH, Nakane M, Murad F.. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc Natl Acad Sci USA 1991;88:10480–10484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. De Caterina R, Libby P, Peng HB, Thannickal VJ, Rajavashisth TB, Gimbrone MA Jr, Shin WS, Liao JK.. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Invest 1995;96:60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Just M, Martorana PA.. Effect of molsidomine on thrombus formation in stenosed coronary arteries of dogs and pigs. J Cardiovasc Pharmacol 1989;14(Suppl. 11):S129–S136. [PubMed] [Google Scholar]
- 37. Loscalzo J. Antiplatelet and antithrombotic effects of organic nitrates. Am J Cardiol 1992;70:18B–22B. [DOI] [PubMed] [Google Scholar]
- 38. Hilgers RH, De Mey JG.. Myoendothelial coupling in the mesenteric arterial bed; segmental differences and interplay between nitric oxide and endothelin-1. Br J Pharmacol 2009;156:1239–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ignarro LJ. Endothelium-derived nitric oxide: actions and properties. Faseb J 1989;3:31–36. [DOI] [PubMed] [Google Scholar]
- 40. Yui Y, Hattori R, Kosuga K, Eizawa H, Hiki K, Kawai C.. Purification of nitric oxide synthase from rat macrophages. J Biol Chem 1991;266:12544–12547. [PubMed] [Google Scholar]
- 41. Thomas DD, Espey MG, Ridnour LA, Hofseth LJ, Mancardi D, Harris CC, Wink DA.. Hypoxic inducible factor 1alpha, extracellular signal-regulated kinase, and p53 are regulated by distinct threshold concentrations of nitric oxide. Proc Natl Acad Sci USA 2004;101:8894–8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Isenberg JS, Wink DA, Roberts DD.. Thrombospondin-1 antagonizes nitric oxide-stimulated vascular smooth muscle cell responses. Cardiovasc Res 2006;71:785–793. [DOI] [PubMed] [Google Scholar]
- 43. Isenberg JS, Romeo MJ, Yu C, Yu CK, Nghiem K, Monsale J, Rick ME, Wink DA, Frazier WA, Roberts DD.. Thrombospondin-1 stimulates platelet aggregation by blocking the antithrombotic activity of nitric oxide/cGMP signaling. Blood 2008;111:613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Resovi A, Pinessi D, Chiorino G, Taraboletti G.. Current understanding of the thrombospondin-1 interactome. Matrix Biol 2014;37:83–91. [DOI] [PubMed] [Google Scholar]
- 45. Bauer EM, Qin Y, Miller TW, Bandle RW, Csanyi G, Pagano PJ, Bauer PM, Schnermann J, Roberts DD, Isenberg JS.. Thrombospondin-1 supports blood pressure by limiting eNOS activation and endothelial-dependent vasorelaxation. Cardiovasc Res 2010;88:471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Isenberg JS, Jia Y, Fukuyama J, Switzer CH, Wink DA, Roberts DD.. Thrombospondin-1 inhibits nitric oxide signaling via CD36 by inhibiting myristic acid uptake. J Biol Chem 2007;282:15404–15415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Isenberg JS, Ridnour LA, Dimitry J, Frazier WA, Wink DA, Roberts DD.. CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J Biol Chem 2006;281:26069–26080. [DOI] [PubMed] [Google Scholar]
- 48. Isenberg JS, Annis DS, Pendrak ML, Ptaszynska M, Frazier WA, Mosher DF, Roberts DD.. Differential interactions of thrombospondin-1, -2, and -4 with CD47 and effects on cGMP signaling and ischemic injury responses. J Biol Chem 2009;284:1116–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Adams JC, Bentley AA, Kvansakul M, Hatherley D, Hohenester E.. Extracellular matrix retention of thrombospondin 1 is controlled by its conserved C-terminal region. J Cell Sci 2008;121:784–795. [DOI] [PubMed] [Google Scholar]
- 50. Kvansakul M, Adams JC, Hohenester E.. Structure of a thrombospondin C-terminal fragment reveals a novel calcium core in the type 3 repeats. Embo J 2004;23:1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kaur S, Kuznetsova SA, Pendrak ML, Sipes JM, Romeo MJ, Li Z, Zhang L, Roberts DD.. Heparan sulfate modification of the transmembrane receptor CD47 is necessary for inhibition of T cell receptor signaling by thrombospondin-1. J Biol Chem 2011;286:14991–15002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Isenberg JS, Qin Y, Maxhimer JB, Sipes JM, Despres D, Schnermann J, Frazier WA, Roberts DD.. Thrombospondin-1 and CD47 regulate blood pressure and cardiac responses to vasoactive stress. Matrix Biol 2009;28:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ridnour LA, Isenberg JS, Espey MG, Thomas DD, Roberts DD, Wink DA.. Nitric oxide regulates angiogenesis through a functional switch involving thrombospondin-1. Proc Natl Acad Sci USA 2005;102:13147–13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Martin-Manso G, Galli S, Ridnour LA, Tsokos M, Wink DA, Roberts DD.. Thrombospondin 1 promotes tumor macrophage recruitment and enhances tumor cell cytotoxicity of differentiated U937 cells. Cancer Res 2008;68:7090–7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Frazier EP, Isenberg JS, Shiva S, Zhao L, Schlesinger P, Dimitry J, Abu-Asab MS, Tsokos M, Roberts DD, Frazier WA.. Age-dependent regulation of skeletal muscle mitochondria by the thrombospondin-1 receptor CD47. Matrix Biol 2011;30:154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bauer PM, Bauer EM, Rogers NM, Yao M, Feijoo-Cuaresma M, Pilewski JM, Champion HC, Zuckerbraun BS, Calzada MJ, Isenberg JS.. Activated CD47 promotes pulmonary arterial hypertension through targeting caveolin-1. Cardiovasc Res 2012;93:682–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cai H, Yuan Z, Fei Q, Zhao J.. Investigation of thrombospondin-1 and transforming growth factor-beta expression in the heart of aging mice. Exp Ther Med 2012;3:433–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rogers NM, Roberts DD, Isenberg JS.. Age-associated induction of cell membrane CD47 limits basal and temperature-induced changes in cutaneous blood flow. Ann Surg 2013;258:184–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Csanyi G, Yao M, Rodriguez AI, Al Ghouleh I, Sharifi-Sanjani M, Frazziano G, Huang X, Kelley EE, Isenberg JS, Pagano PJ.. Thrombospondin-1 regulates blood flow via CD47 receptor-mediated activation of NADPH oxidase 1. Arterioscler Thromb Vasc Biol 2012;32:2966–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yao M, Rogers NM, Csanyi G, Rodriguez AI, Ross MA, St Croix C, Knupp H, Novelli EM, Thomson AW, Pagano PJ, Isenberg JS.. Thrombospondin-1 activation of signal-regulatory protein-alpha stimulates reactive oxygen species production and promotes renal ischemia reperfusion injury. J Am Soc Nephrol 2014;25:1171–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Barclay AN. Signal regulatory protein alpha (SIRPalpha)/CD47 interaction and function. Curr Opin Immunol 2009;21:47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Capla JM, Ceradini DJ, Tepper OM, Callaghan MJ, Bhatt KA, Galiano RD, Levine JP, Gurtner GC.. Skin graft vascularization involves precisely regulated regression and replacement of endothelial cells through both angiogenesis and vasculogenesis. Plast Reconstr Surg 2006;117:836–844. [DOI] [PubMed] [Google Scholar]
- 63. Krishnaswami S, Ly QP, Rothman VL, Tuszynski GP.. Thrombospondin-1 promotes proliferative healing through stabilization of PDGF. J Surg Res 2002;107:124–130. [DOI] [PubMed] [Google Scholar]
- 64. Isenberg JS, Pappan LK, Romeo MJ, Abu-Asab M, Tsokos M, Wink DA, Frazier WA, Roberts DD.. Blockade of thrombospondin-1-CD47 interactions prevents necrosis of full thickness skin grafts. Ann Surg 2008;247:180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Matozaki T, Murata Y, Okazawa H, Ohnishi H.. Functions and molecular mechanisms of the CD47-SIRPalpha signalling pathway. Trends Cell Biol 2009;19:72–80. [DOI] [PubMed] [Google Scholar]
- 66. Barabino GA, Wise RJ, Woodbury VA, Zhang B, Bridges KA, Hebbel RP, Lawler J, Ewenstein BM.. Inhibition of sickle erythrocyte adhesion to immobilized thrombospondin by von Willebrand factor under dynamic flow conditions. Blood 1997;89:2560–2567. [PubMed] [Google Scholar]
- 67. Novelli EM, Kato GJ, Ragni MV, Zhang Y, Hildesheim ME, Nouraie M, Barge S, Meyer MP, Hassett AC, Gordeuk VR, Gladwin MT, Isenberg JS.. Plasma thrombospondin-1 is increased during acute sickle cell vaso-occlusive events and associated with acute chest syndrome, hydroxyurea therapy, and lower hemolytic rates. Am J Hematol 2012;87:326–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lagadec P, Dejoux O, Ticchioni M, Cottrez F, Johansen M, Brown EJ, Bernard A.. Involvement of a CD47-dependent pathway in platelet adhesion on inflamed vascular endothelium under flow. Blood 2003;101:4836–4843. [DOI] [PubMed] [Google Scholar]
- 69. Freyberg MA, Kaiser D, Graf R, Vischer P, Friedl P.. Integrin-associated protein and thrombospondin-1 as endothelial mechanosensitive death mediators. Biochem Biophys Res Commun 2000;271:584–588. [DOI] [PubMed] [Google Scholar]
- 70. Jurk K, Clemetson KJ, de Groot PG, Brodde MF, Steiner M, Savion N, Varon D, Sixma JJ, Van Aken H, Kehrel BE.. Thrombospondin-1 mediates platelet adhesion at high shear via glycoprotein Ib (GPIb): an alternative/backup mechanism to von Willebrand factor. faseb J 2003;17:1490–1492. [DOI] [PubMed] [Google Scholar]
- 71. Bongrazio M, Da Silva-Azevedo L, Bergmann EC, Baum O, Hinz B, Pries AR, Zakrzewicz A.. Shear stress modulates the expression of thrombospondin-1 and CD36 in endothelial cells in vitro and during shear stress-induced angiogenesis in vivo. Int J Immunopathol Pharmacol 2006;19:35–48. [PubMed] [Google Scholar]
- 72. Gomes N, Legrand C, Fauvel-Lafeve F.. Shear stress induced release of von Willebrand factor and thrombospondin-1 in HUVEC extracellular matrix enhances breast tumour cell adhesion. Clin Exp Metastasis 2005;22:215–223. [DOI] [PubMed] [Google Scholar]
- 73. Isenberg JS, Hyodo F, Matsumoto K, Romeo MJ, Abu-Asab M, Tsokos M, Kuppusamy P, Wink DA, Krishna MC, Roberts DD.. Thrombospondin-1 limits ischemic tissue survival by inhibiting nitric oxide-mediated vascular smooth muscle relaxation. Blood 2007;109:1945–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Isenberg JS, Hyodo F, Pappan LK, Abu-Asab M, Tsokos M, Krishna MC, Frazier WA, Roberts DD.. Blocking thrombospondin-1/CD47 signaling alleviates deleterious effects of aging on tissue responses to ischemia. Arterioscler Thromb Vasc Biol 2007;27:2582–2588. [DOI] [PubMed] [Google Scholar]
- 75. Isenberg JS, Romeo MJ, Abu-Asab M, Tsokos M, Oldenborg A, Pappan L, Wink DA, Frazier WA, Roberts DD.. Increasing survival of ischemic tissue by targeting CD47. Circ Res 2007;100:712–720. [DOI] [PubMed] [Google Scholar]
- 76. Isenberg JS, Romeo MJ, Maxhimer JB, Smedley J, Frazier WA, Roberts DD.. Gene silencing of CD47 and antibody ligation of thrombospondin-1 enhance ischemic tissue survival in a porcine model: implications for human disease. Ann Surg 2008;247:860–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Thakar CV, Zahedi K, Revelo MP, Wang Z, Burnham CE, Barone S, Bevans S, Lentsch AB, Rabb H, Soleimani M.. Identification of thrombospondin 1 (TSP-1) as a novel mediator of cell injury in kidney ischemia. J Clin Invest 2005;115:3451–3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Isenberg JS, Maxhimer JB, Powers P, Tsokos M, Frazier WA, Roberts DD.. Treatment of liver ischemia-reperfusion injury by limiting thrombospondin-1/CD47 signaling. Surgery 2008;144:752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Rogers NM, Yao M, Novelli EM, Thomson AW, Roberts DD, Isenberg JS.. Activated CD47 regulates multiple vascular and stress responses: implications for acute kidney injury and its management. Am J Physiol Renal Physiol 2012;303:F1117–F1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Rogers NM, Zhang ZJ, Wang JJ, Thomson AW, Isenberg JS.. CD47 regulates renal tubular epithelial cell self-renewal and proliferation following renal ischemia reperfusion. Kidney Internat 2016;90:334–347. [DOI] [PubMed] [Google Scholar]
- 81. Xiao Z, Banan B, Xu M, Jia J, Manning PT, Hiebsch RR, Gunasekaran M, Upadhya GA, Frazier WA, Mohanakumar T, Lin Y, Chapman WC.. Attenuation of Ischemia-Reperfusion Injury and Improvement of Survival in Recipients of Steatotic Rat Livers Using CD47 Monoclonal Antibody. Transplantation 2016;100:1480–1489. [DOI] [PubMed] [Google Scholar]
- 82. Nevitt C, McKenzie G, Christian K, Austin J, Hencke S, Hoying J, LeBlanc A.. Physiological levels of thrombospondin-1 decrease NO-dependent vasodilation in coronary microvessels from aged rats. Am J Physiol Heart Circ Physiol 2016;310:H1842–H1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Favier J, Germain S, Emmerich J, Corvol P, Gasc JM.. Critical overexpression of thrombospondin 1 in chronic leg ischaemia. J Pathol 2005;207:358–366. [DOI] [PubMed] [Google Scholar]
- 84. Smadja DM, D'audigier C, Bieche I, Evrard S, Mauge L, Dias JV, Labreuche J, Laurendeau I, Marsac B, Dizier B, Wagner-Ballon O, Boisson-Vidal C, Morandi V, Duong-Van-Huyen JP, Bruneval P, Dignat-George F, Emmerich J, Gaussem P.. Thrombospondin-1 is a plasmatic marker of peripheral arterial disease that modulates endothelial progenitor cell angiogenic properties. Arterioscler Thromb Vasc Biol 2011;31:551–559. [DOI] [PubMed] [Google Scholar]
- 85. Tuder RM, Archer SL, Dorfmuller P, Erzurum SC, Guignabert C, Michelakis E, Rabinovitch M, Schermuly R, Stenmark KR, Morrell NW.. Relevant issues in the pathology and pathobiology of pulmonary hypertension. J Am Coll Cardiol 2013;62:D4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. MacIver DH, Adeniran I, MacIver IR, Revell A, Zhang H.. Physiological mechanisms of pulmonary hypertension. Am Heart J 2016;180:1–11. [DOI] [PubMed] [Google Scholar]
- 87. Sage E, Mercier O, Van den Eyden F, de Perrot M, Barlier-Mur AM, Dartevelle P, Eddahibi S, Herve P, Fadel E.. Endothelial cell apoptosis in chronically obstructed and reperfused pulmonary artery. Respir Res 2008;9:19.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ochoa CD, Baker H, Hasak S, Matyal R, Salam A, Hales CA, Hancock W, Quinn DA.. Cyclic stretch affects pulmonary endothelial cell control of pulmonary smooth muscle cell growth. Am J Respir Cell Mol Biol 2008;39:105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kwapiszewska G, Wilhelm J, Wolff S, Laumanns I, Koenig IR, Ziegler A, Seeger W, Bohle RM, Weissmann N, Fink L.. Expression profiling of laser-microdissected intrapulmonary arteries in hypoxia-induced pulmonary hypertension. Respir Res 2005;6:109.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ochoa CD, Yu L, Al-Ansari E, Hales CA, Quinn DA.. Thrombospondin-1 null mice are resistant to hypoxia-induced pulmonary hypertension. J Cardiothorac Surg 2010;5:32.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Lee AH, Dhaliwal R, Kantores C, Ivanovska J, Gosal K, McNamara PJ, Letarte M, Jankov RP.. Rho-kinase inhibitor prevents bleomycin-induced injury in neonatal rats independent of effects on lung inflammation. Am J Respir Cell Mol Biol 2014;50:61–73. [DOI] [PubMed] [Google Scholar]
- 92. Rogers NM, Sharifi-Sanjani M, Yao M, Ghimire K, Bienes-Martinez R, Mutchler SM, Knupp HE, Baust J, Novelli EM, Ross M, St Croix C, Kutten JC, Czajka CA, Sembrat JC, Rojas M, Labrousse-Arias D, Bachman TN, Vanderpool RR, Zuckerbraun BS, Champion HC, Mora AL, Straub AC, Bilonick RA, Calzada MJ, Isenberg JS.. TSP1-CD47 signaling is upregulated in clinical pulmonary hypertension and contributes to pulmonary arterial vasculopathy and dysfunction. Cardiovasc Res 2017;113:15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Labrousse-Arias D, Castillo-Gonzalez R, Rogers NM, Torres-Capelli M, Barreira B, Aragones J, Cogolludo A, Isenberg JS, Calzada MJ.. HIF-2alpha-mediated induction of pulmonary thrombospondin-1 contributes to hypoxia-driven vascular remodelling and vasoconstriction. Cardiovasc Res 2016;109:115–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Semenza GL. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu Rev Pathol 2014;9:47–71. [DOI] [PubMed] [Google Scholar]
- 95. Elorza A, Soro-Arnaiz I, Melendez-Rodriguez F, Rodriguez-Vaello V, Marsboom G, de Carcer G, Acosta-Iborra B, Albacete-Albacete L, Ordonez A, Serrano-Oviedo L, Gimenez-Bachs JM, Vara-Vega A, Salinas A, Sanchez-Prieto R, Martin del Rio R, Sanchez-Madrid F, Malumbres M, Landazuri MO, Aragones J.. HIF2alpha acts as an mTORC1 activator through the amino acid carrier SLC7A5. Mol Cell 2012;48:681–691. [DOI] [PubMed] [Google Scholar]
- 96. Giaid A, Saleh D.. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med 1995;333:214–221. [DOI] [PubMed] [Google Scholar]
- 97. Mason NA, Springall DR, Burke M, Pollock J, Mikhail G, Yacoub MH, Polak JM.. High expression of endothelial nitric oxide synthase in plexiform lesions of pulmonary hypertension. J Pathol 1998;185:313–318. [DOI] [PubMed] [Google Scholar]
- 98. Kaneko FT, Arroliga AC, Dweik RA, Comhair SA, Laskowski D, Oppedisano R, Thomassen MJ, Erzurum SC.. Biochemical reaction products of nitric oxide as quantitative markers of primary pulmonary hypertension. Am J Respir Crit Care Med 1998;158:917–923. [DOI] [PubMed] [Google Scholar]
- 99. Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC.. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature 1999;399:597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Drab M, Verkade P, Elger M, Kasper M, Lohn M, Lauterbach B, Menne J, Lindschau C, Mende F, Luft FC, Schedl A, Haller H, Kurzchalia TV.. Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 2001;293:2449–2452. [DOI] [PubMed] [Google Scholar]
- 101. Zhao YY, Liu Y, Stan RV, Fan L, Gu Y, Dalton N, Chu PH, Peterson K, Ross J Jr, Chien KR.. Defects in caveolin-1 cause dilated cardiomyopathy and pulmonary hypertension in knockout mice. Proc Natl Acad Sci USA 2002;99:11375–11380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Liu JQ, Zelko IN, Erbynn EM, Sham JS, Folz RJ.. Hypoxic pulmonary hypertension: role of superoxide and NADPH oxidase (gp91phox). Am J Physiol Lung Cell Mol Physiol 2006;290:L2–10. [DOI] [PubMed] [Google Scholar]
- 103. Dennis KE, Aschner JL, Milatovic D, Schmidt JW, Aschner M, Kaplowitz MR, Zhang Y, Fike CD.. NADPH oxidases and reactive oxygen species at different stages of chronic hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Lung Cell Mol Physiol 2009;297:L596–L607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Green DE, Kang BY, Murphy TC, Hart CM.. Peroxisome proliferator-activated receptor gamma (PPARgamma) regulates thrombospondin-1 and Nox4 expression in hypoxia-induced human pulmonary artery smooth muscle cell proliferation. Pulm Circ 2012;2:483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Goyal P, Weissmann N, Grimminger F, Hegel C, Bader L, Rose F, Fink L, Ghofrani HA, Schermuly RT, Schmidt HH, Seeger W, Hanze J.. Upregulation of NAD(P)H oxidase 1 in hypoxia activates hypoxia-inducible factor 1 via increase in reactive oxygen species. Free Radic Biol Med 2004;36:1279–1288. [DOI] [PubMed] [Google Scholar]
- 106. Kay JM, Smith P, Heath D, Will JA.. Effects of phenobarbitone, cinnarizine, and zoxazolamine on the development of right ventricular hypertrophy and hypertensive pulmonary vascular disease in rats treated with monocrotaline. Cardiovasc Res 1976;10:200–205. [DOI] [PubMed] [Google Scholar]
- 107. Rossi GP, Seccia TM, Nussdorfer GG.. Reciprocal regulation of endothelin-1 and nitric oxide: relevance in the physiology and pathology of the cardiovascular system. Int Rev Cytol 2001;209:241–272. [DOI] [PubMed] [Google Scholar]
- 108. Kaiser R, Frantz C, Bals R, Wilkens H.. The role of circulating thrombospondin-1 in patients with precapillary pulmonary hypertension. Respir Res 2016;17:96.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Agarwal AR, Mih J, George SC.. Expression of matrix proteins in an in vitro model of airway remodeling in asthma. Allergy Asthma Proc 2003;24:35–42. [PubMed] [Google Scholar]
- 110. Ide M, Ishii H, Mukae H, Iwata A, Sakamoto N, Kadota J, Kohno S.. High serum levels of thrombospondin-1 in patients with idiopathic interstitial pneumonia. Respir Med 2008;102:1625–1630. [DOI] [PubMed] [Google Scholar]
- 111. Smadja DM, Nunes H, Juvin K, Bertil S, Valeyre D, Gaussem P, Israel-Biet D.. Increase in both angiogenic and angiostatic mediators in patients with idiopathic pulmonary fibrosis. Pathol Biol 2014;62:391–394. [DOI] [PubMed] [Google Scholar]
- 112. Maloney JP, Stearman RS, Bull TM, Calabrese DW, Tripp-Addison ML, Wick MJ, Broeckel U, Robbins IM, Wheeler LA, Cogan JD, Loyd JE.. Loss-of-function thrombospondin-1 mutations in familial pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2012;302:L541–L554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Jacob SA, Novelli EM, Isenberg JS, Garrett ME, Chu Y, Soldano K, Ataga KI, Telen MJ, Ashley-Koch A, Gladwin MT, Zhang Y, Kato GJ.. Thrombospondin-1 Gene Polymorphism is Associated with Estimated Pulmonary Artery Pressure in Patients with Sickle Cell Anemia. Am J Hematol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Rogers NM, Yao M, Sembrat J, George MP, Knupp H, Ross M, Sharifi-Sanjani M, Milosevic J, St Croix C, Rajkumar R, Frid MG, Hunter KS, Mazzaro L, Novelli EM, Stenmark KR, Gladwin MT, Ahmad F, Champion HC, Isenberg JS.. Cellular, pharmacological, and biophysical evaluation of explanted lungs from a patient with sickle cell disease and severe pulmonary arterial hypertension. Pulm Circ 2013;3:936–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Yao M, Roberts DD, Isenberg JS.. Thrombospondin-1 inhibition of vascular smooth muscle cell responses occurs via modulation of both cAMP and cGMP. Pharmacol Res 2011;63:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Miller TW, Isenberg JS, Roberts DD.. Thrombospondin-1 is an inhibitor of pharmacological activation of soluble guanylate cyclase. Br J Pharmacol 2010;159:1542–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Liu J, Wang L, Zhao F, Tseng S, Narayanan C, Shura L, Willingham S, Howard M, Prohaska S, Volkmer J, Chao M, Weissman IL, Majeti R.. Pre-Clinical Development of a Humanized Anti-CD47 Antibody with Anti-Cancer Therapeutic Potential. PloS One 2015;10:e0137345.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Bassyouni IH, Gheita TA, Talaat RM.. Clinical significance of serum levels of sCD36 in patients with systemic sclerosis: preliminary data. Rheumatology (Oxford) 2011;50:2108–2112. [DOI] [PubMed] [Google Scholar]
- 119. Zaiman AL, Podowski M, Medicherla S, Gordy K, Xu F, Zhen L, Shimoda LA, Neptune E, Higgins L, Murphy A, Chakravarty S, Protter A, Sehgal PB, Champion HC, Tuder RM.. Role of the TGF-beta/Alk5 signaling pathway in monocrotaline-induced pulmonary hypertension. Am J Respir Crit Care Med 2008;177:896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Sharifi-Sanjani M, Shoushtari AH, Quiroz M, Baust J, Sestito SF, Mosher M, Ross M, McTiernan CF, St Croix CM, Bilonick RA, Champion HC, Isenberg JS.. Cardiac CD47 drives left ventricular heart failure through Ca2+-CaMKII-regulated induction of HDAC3. J Am Heart Assoc 2014;3:e000670.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Calzada MJ, Annis DS, Zeng B, Marcinkiewicz C, Banas B, Lawler J, Mosher DF, Roberts DD.. Identification of novel beta1 integrin binding sites in the type 1 and type 2 repeats of thrombospondin-1. J Biol Chem 2004;279:41734–41743. [DOI] [PubMed] [Google Scholar]
- 122. Calzada MJ, Zhou L, Sipes JM, Zhang J, Krutzsch HC, Iruela-Arispe ML, Annis DS, Mosher DF, Roberts DD.. Alpha4beta1 integrin mediates selective endothelial cell responses to thrombospondins 1 and 2 in vitro and modulates angiogenesis in vivo. Circ Res 2004;94:462–470. [DOI] [PubMed] [Google Scholar]
- 123. Calzada MJ, Sipes JM, Krutzsch HC, Yurchenco PD, Annis DS, Mosher DF, Roberts DD.. Recognition of the N-terminal modules of thrombospondin-1 and thrombospondin-2 by alpha6beta1 integrin. J Biol Chem 2003;278:40679–40687. [DOI] [PubMed] [Google Scholar]
- 124. Chandrasekaran L, He CZ, Al-Barazi H, Krutzsch HC, Iruela-Arispe ML, Roberts DD.. Cell contact-dependent activation of alpha3beta1 integrin modulates endothelial cell responses to thrombospondin-1. Mol Biol Cell 2000;11:2885–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Tan K, Duquette M, Liu JH, Zhang R, Joachimiak A, Wang JH, Lawler J.. The structures of the thrombospondin-1 N-terminal domain and its complex with a synthetic pentameric heparin. Structure 2006;14:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Yu H, Tyrrell D, Cashel J, Guo NH, Vogel T, Sipes JM, Lam L, Fillit HM, Hartman J, Mendelovitz S, Panel A, Roberts DD.. Specificities of heparin-binding sites from the amino-terminus and type 1 repeats of thrombospondin-1. Arch Biochem Biophys 2000;374:13–23. [DOI] [PubMed] [Google Scholar]
- 127. Elzie CA, Murphy-Ullrich JE.. The N-terminus of thrombospondin: the domain stands apart. Int J Biochem Cell Biol 2004;36:1090–1101. [DOI] [PubMed] [Google Scholar]
- 128. Merle B, Malaval L, Lawler J, Delmas P, Clezardin P.. Decorin inhibits cell attachment to thrombospondin-1 by binding to a KKTR-dependent cell adhesive site present within the N-terminal domain of thrombospondin-1. J Cell Biochem 1997;67:75–83. [PubMed] [Google Scholar]
- 129. Carlson CB, Lawler J, Mosher DF.. Structures of thrombospondins. Cell Mol Life Sci 2008;65:672–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Asch AS, Silbiger S, Heimer E, Nachman RL.. Thrombospondin sequence motif (CSVTCG) is responsible for CD36 binding. Biochem Biophys Res Commun 1992;182:1208–1217. [DOI] [PubMed] [Google Scholar]
- 131. Sipes JM, Guo N, Negre E, Vogel T, Krutzsch HC, Roberts DD.. Inhibition of fibronectin binding and fibronectin-mediated cell adhesion to collagen by a peptide from the second type I repeat of thrombospondin. J Cell Biol 1993;121:469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Bein K, Simons M.. Thrombospondin type 1 repeats interact with matrix metalloproteinase 2. Regulation of metalloproteinase activity. J Biol Chem 2000;275:32167–32173. [DOI] [PubMed] [Google Scholar]
- 133. Schultz-Cherry S, Chen H, Mosher DF, Misenheimer TM, Krutzsch HC, Roberts DD, Murphy-Ullrich JE.. Regulation of transforming growth factor-beta activation by discrete sequences of thrombospondin 1. J Biol Chem 1995;270:7304–7310. [DOI] [PubMed] [Google Scholar]
- 134. Lawler J, Hynes RO.. An integrin receptor on normal and thrombasthenic platelets that binds thrombospondin. Blood 1989;74:2022–2027. [PubMed] [Google Scholar]
- 135. Hogg PJ, Jimenez BM, Chesterman CN.. Identification of possible inhibitory reactive centers in thrombospondin 1 that may bind cathepsin G and neutrophil elastase. Biochem 1994;33:6531–6537. [DOI] [PubMed] [Google Scholar]
- 136. Suades R, Padro T, Alonso R, Mata P, Badimon L.. High levels of TSP1+/CD142+ platelet-derived microparticles characterise young patients with high cardiovascular risk and subclinical atherosclerosis. Thromb Haemost 2015;114:1310–1321. [DOI] [PubMed] [Google Scholar]
- 137. Zhao XM, Hu Y, Miller GG, Mitchell RN, Libby P.. Association of thrombospondin-1 and cardiac allograft vasculopathy in human cardiac allografts. Circulation 2001;103:525–531. [DOI] [PubMed] [Google Scholar]
- 138. Qin Q, Qian J, Ma J, Ge L, Ge J.. Relationship between thrombospondin-1, endostatin, angiopoietin-2, and coronary collateral development in patients with chronic total occlusion. Medicine 2016;95:e4524.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Chen Q, Ye ZN, Liu JP, Zhang ZH, Zhou CH, Wang Y, Hang CH.. Elevated cerebrospinal fluid levels of thrombospondin-1 correlate with adverse clinical outcome in patients with aneurysmal subarachnoid hemorrhage. J Neurol Sci 2016;369:126–130. [DOI] [PubMed] [Google Scholar]
- 140. Huang CL, Jong YS, Wu YW, Wang WJ, Hsieh AR, Chao CL, Chen WJ, Yang WS.. Association of Plasma Thrombospondin-1 Level with Cardiovascular Disease and Mortality in Hemodialysis Patients. Acta Cardiol Sin 2015;31:113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]