Abstract
Aims
High salt intake markedly enhances hypertension induced by angiotensin II (Ang II). We explored central and peripheral slow-pressor mechanisms which may be activated by Ang II and salt.
Methods and results
In protocol I, Wistar rats were infused subcutaneously with low-dose Ang II (150 ng/kg/min) and fed regular (0.4%) or high salt (2%) diet for 14 days. In protocol II, Ang II-high salt was combined with intracerebroventricular infusion of mineralocorticoid receptor (MR) blockers (eplerenone, spironolactone), epithelial sodium channel (ENaC) blocker (benzamil), angiotensin II type 1 receptor (AT1R) blocker (losartan) or vehicles. Ang II alone raised mean arterial pressure (MAP) ∼10 mmHg, but Ang II-high salt increased MAP ∼50 mmHg. Ang II-high salt elevated plasma corticosterone, aldosterone and endogenous ouabain but not Ang II alone. Both Ang II alone and Ang II-high salt increased mRNA and protein expression of CYP11B2 (aldosterone synthase gene) in the adrenal cortex but not of CYP11B1 (11-β-hydroxylase gene). In the aorta, Ang II-high salt increased sodium-calcium exchanger-1 (NCX1) protein. The Ang II-high salt induced increase in MAP was largely prevented by central infusion of MR blockers, benzamil or losartan. Central blockades significantly lowered plasma aldosterone and endogenous ouabain and markedly decreased Ang II-high salt induced CYP11B2 mRNA expression in the adrenal cortex and NCX1 protein in the aorta.
Conclusion
These results suggest that in Ang II-high salt hypertension, MR-ENaC-AT1R signalling in the brain increases circulating aldosterone and endogenous ouabain, and arterial NCX1. These factors can amplify blood pressure responses to centrally-induced sympatho-excitation and thereby contribute to severe hypertension.
Keywords: Angiotensin II, Aldosterone, Brain, Endogenous ouabain, High salt diet, Central blockade
1. Introduction
Actions of circulating angiotensin II (Ang II) within the central nervous system (CNS) play a critical role in Ang II-induced hypertension.1 Elevated plasma Ang II can act on neurons in circumventricular organs in the forebrain located outside the blood–brain barrier.2–4 Angiotensinergic projections from the circumventicular organs can relay the signals of circulating Ang II to neurons in the paraventricular nucleus (PVN), supraoptic nucleus (SON) and rostral ventrolateral medulla (RVLM)5,6 and thereby increase sympathetic nerve activity (SNA),5,7,8 as well as plasma vasopressin,9 aldosterone and endogenous ouabain (EO).10,11 Chronically, circulating and brain Ang II also activate a neuromodulatory pathway involving aldosterone–mineralocorticoid receptors (MR)–epithelial sodium channels (ENaC)–EO and α2 Na+ pumps.12,13 This pathway enhances the effectiveness of Ang II-Ang type 1 receptor (AT1R) signalling in, e.g. the PVN and is essential for persistent increases in SNA,14 plasma aldosterone and EO,10,11 and the chronic hypertension.10
Ang II-induced hypertension is markedly amplified by a high salt (HS) diet.15,16 This HS-induced amplification involves central actions of Ang II.17,18 For example, CNS pathways involving the PVN are necessary to maintain SNA and elevated blood pressure (BP) in Ang II + HS hypertension.19,20 Chronic increases in plasma/CSF [Ang II] or [Na+] both activate the MR-ENaC-EO neuromodulatory pathway.12 Therefore, simultaneous increases in plasma Ang II and dietary salt might enhance the activity of the aldosterone-MR-ENaC-EO-AT1R pathway in the CNS significantly more than low-dose Ang II alone. In support of this concept, chronic intracerebroventricular (icv) infusion of the ENaC blocker, benzamil, prevents Ang II + HS induced hypertension21; suggesting that brain ENaCs may play an important role. Secondly, these central pathways enhance effective sympathetic tone16,22 by increasing peripheral key slow-pressor mechanisms involving plasma aldosterone as well as EO and its effects on arterial Ca2+-transporters.23 To test the aforementioned hypotheses, we assessed in Wistar rats; (i) the effects of infusion of sc Ang II at the minimally effective dose of 150 ng/kg/min, alone or combined with HS on BP, plasma and tissue aldosterone and EO and the arterial calcium transport protein, Na+/Ca2+ exchanger-1 (NCX1), and (ii) the effects of chronic icv infusion of an MR antagonist, ENaC blocker or AT1R blocker on these parameters in rats with Ang II + HS hypertension.
The results reveal that the severe hypertension caused by the combination of a minor increase in plasma Ang II and a HS intake depends largely upon a pathway that involves MR-ENaC-AT1R in the CNS. Stimulation of this CNS pathway activates a humoral axis that increases circulating aldosterone and EO, and arterial myocyte NCX1 protein expression.
2. Methods
2.1 Animals
Male Wistar rats weighing 200–250 g were obtained from Charles River Breeding Laboratories (Montreal, PQ, Canada), housed on a 12 h light/dark cycle at constant room temperature, and provided with a standard laboratory chow (0.4% NaCl, RS) and tap water ad libitum. All surgeries and experiments for the present study were approved by the University of Ottawa Animal Care Committee and conform with the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (11th Edition, 2011). For all surgeries, rats were anesthetized with 2% isoflurane in oxygen. Effective levels of anesthesia were maintained by observing reactions to physical stimulation such as toe-pinch, as well as monitoring the pattern of respiration. For pain relief, sustained release buprenorphine (1 mg/kg) was injected subcutaneously (sc) 1 h before surgery, which provides adequate analgesia for 3 days.
2.2 Ang II-salt model of hypertension
Wistar rats were placed on high salt diet (2% NaCl, HS), or continued regular salt (0.4% NaCl, RS) and the same day infusion of Ang II was started at a dose of 150 ng/kg/min sc by osmotic minipump (Model 2002, ALZET, DURECT Corp, Cupertino, CA, USA). This dose of Ang II was adopted from other studies16,21 and causes only a minimal increase in plasma Ang II levels.24 All experiments were performed in male rats, because female rats exhibit marked attenuation of the hypertension induced by Ang II.25 Different sets of animals were used according to the following protocols.
2.3 Protocol I: effects of AngII-HS on BP, plasma and tissue aldosterone and EO
An initial experiment was performed to obtain primary data for the Ang II-HS model. Three groups of rats were used (n = 4–6 per group) for measurements of BP, plasma Ang II, and plasma and tissue aldosterone and corticosterone. One group stayed on RS and two groups were fed HS; one of the latter also received a sc infusion of Ang II for 2 weeks.
For the main experiment, two subsets of animals were studied (first and second study of first experiment). In the first study, four groups of rats were used for BP and heart rate (HR), plasma Ang II, plasma and tissue aldosterone, corticosterone and EO. Groups 1 and 2 (n = 6) stayed on RS, whereas groups 3 and 4 (n = 8) were placed on HS. Groups 2 and 4 also were infused sc with Ang II for 2 weeks. Sham surgery was done for groups 1 and 3, without implantation of a pump. Body weight (BW) was measured at the start and at the end of the treatment and 24 h water intake at the end of week 1 and 2 of treatment. In a follow-up study, the same groups were studied to assess effects on BP and aldosterone as well as plasma electrolytes and adrenal mRNA and proteins. An additional fifth group was included for HS + sc vehicle. Results for this group were the same as for HS alone (data not shown).
In the morning of days 12–13 of the Ang II infusion, the right femoral artery was cannulated with a PE-50/10 tubing filled with heparinized saline (1000 U/ml in 0.9% NaCl). After recovery for 2–3 h, arterial lines were connected to a pressure transducer linked to a data acquisition system. Digital signals of BP and HR were processed by a personal computer equipped with Acknowledge 3.8. After a 20–30 min rest, resting BP and HR were recorded for 20 min. In rats of the follow-up experiment, 1 ml arterial blood was collected for measurement of plasma electrolytes by ion-selective electrode (Roche/Hitachi “Cobas C” system, Roche Diagnostics, Laval, QC, Canada). Rats then were returned to original cages and kept in a quiet room until the next morning. The conscious rats were rapidly decapitated in order to obtain sufficient trunk blood for plasma Ang II, aldosterone, corticosterone and EO without causing ACTH release. The heart was rapidly removed, rinsed in ice-cold saline, and the right ventricle (RV) was separated from the left ventricle (LV), blotted and weighed. In the first study of the main experiment, the aortas were then removed and placed in ice-cold physiological salt solution. The aortas were cleaned of fat and connective tissue, de-endothelialized and frozen in liquid nitrogen. Adrenals were collected for CYP11B1 (11-β-hydroxylase gene) and CYP11B2 (aldosterone synthase gene) mRNA expression levels and kidneys for EO levels. Adrenal protein expression levels were measured in the follow-up experiment. All tissues were stored at −80 °C until used.
2.4 Protocol II: effects of central blockades on Ang II-salt induced hypertension
Under anesthesia, a telemetry transmitter (model TA11PA-C40; DSI-St. Paul, MN, USA) was placed into the abdominal cavity and secured to the ventral abdominal wall with the catheter inserted into the abdominal aorta. The telemetry signal was transmitted, wirelessly, to a receiver placed under the cage, connected to a data acquisition system to record BP and HR. Data collection was initiated 5 days after surgery using Scheduled Sampling Mode with a 1 min recording duration at the beginning of each hour. After recording baseline BP and HR for 2 days, icv cannulae and osmotic minipumps (Model 2004, ALZET) were implanted for chronic icv infusion in eight groups of rats with n = 5–6/group for the four active treatment groups, and n = 3/group for four control groups. All responses were similar in the four vehicle groups that were therefore combined. Four rats across the groups exhibited transmitter or icv cannula dysfunction, and data from these rats were excluded. Rats were allocated to the following treatments: (1) MR blocker, eplerenone (10 μg/kg/day, in aCSF with 4% acetonitrile); (2) MR blocker, spironolactone (10 μg/kg/day in aCSF with 2% ethanol); (3) ENaC blocker, benzamil (4 µg/kg/h, in aCSF with 15% propylene glycol); (4) AT1R blocker, losartan (1 mg/kg/day, in aCSF); and groups 5–8) controls (the vehicles for groups 1–3 respectively, and aCSF for group (4) all at an infusion rate of 0.25 μl/h. The doses of the various antagonists were based on previous studies. Eplerenone is specific for MR but has low affinity, whereas spironolactone has high affinity but is not specific for MR. However, responses to the 2 MR blockers were similar as shown for the BP in Figure 1B, and other results for the 2 MR blockers were combined. Two days after the start of icv infusions, all rats were fed HS and infused sc with Ang II at 150 ng/kg/min. BW and water intake were recorded at the start and at the end of the 2 week infusion period. At the end, trunk blood was collected for plasma Ang II, aldosterone, corticosterone and EO, and heart, adrenals and kidneys were collected.
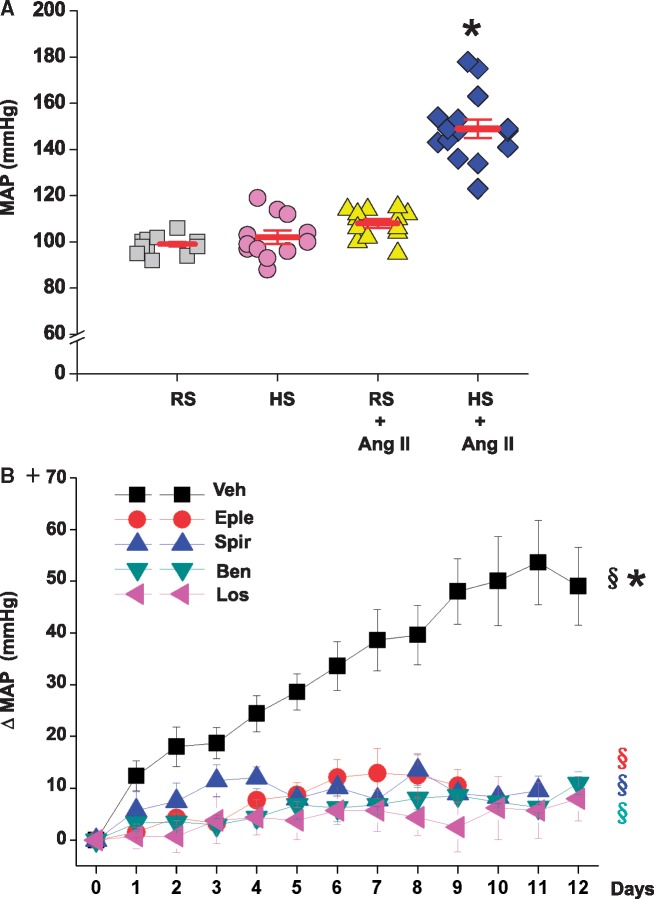
Figure 1.
Sc infusion of Ang II at 150 ng/kg/min does not significantly increase MAP in rats on regular (RS) diet but causes severe hypertension in rats on 2% high salt (HS) diet (panel A). Central infusion of an MR blocker (eplerenone or spironolactone), ENaC blocker (benzamil) or AT1R blocker (losartan) prevents most of this increase in MAP (panel B). Panel A: values are means ± SE (n = 12–15/group, two studies combined). BP was measured by intra-arterial line. Two-way ANOVA: *P<0.05 vs. others. Panel B: values are means ± SE for changes in MAP from baseline (days -2-0). n = 11 for vehicles (Veh), n = 5 for eplerenone (Eple), n = 4 for spironolactone (Spir), n = 6 for benzamil (Ben), and n = 4 for losartan (Los). BP was measured by telemetry. One-way repeated measures ANOVA: §P<0.05 vs. baseline MAP. Factorial repeated measures ANOVA: *P<0.05 vs. others.
2.5 Real-time RT-PCR for mRNA levels in the adrenal cortex
Adrenal cortex and medulla were separated on ice under a microscope, and the cortex was homogenized in QIAzol Lysis Reagent (Qiagen, Toronto, ON, Canada) on ice using a pestle (Bel-Art-Product, Pequannock, NJ, USA) driven by a pellet pestle motor for total RNA extraction. 20 µg of total RNA was treated by DNase I (Thermo Scientific Inc., Rockford, IL, USA). 5 μg of DNase I treated RNA was reverse transcribed into cDNAs by RevertAid H Minus First Strand cDNA Synthesis Kit using oligo dT primers (Thermo Scientific Inc. Waltham, MA, USA).
The primers, PCR conditions and external standards for CYP11B1 and CYP11B2 and the house keeping gene phosphoglycerate kinase 1 (PGK 1) were the same as previously described.26 Real-time PCR was performed with a Roche Light Cycler LC480 using LC480 SYBR Green I master mix (Roche Diagnostics, Quebec City, QC, Canada). The PCR conditions were set as follows: initial at 95 °C for 10 min followed by 45 cycles of denaturation at 95 °C for 10 s; annealing at 62 °C for 15 s (CYP11B2), at 55 °C for 18 s (CYP11B1); extension at 72 °C. The specificity of the real-time PCR products was determined by both melting curve analysis and verified by agarose gel electrophoresis. mRNA abundance of target genes was normalized against PGK1 levels as the endogenous reference.
2.6 Biochemical assays for Ang II, aldosterone, corticosterone and EO
The whole hypothalamus and hippocampus were dissected according to Glowinski and Iversen.27 Aldosterone and corticosterone were measured by radioimmunoassay (RIA) as described previously.10 For the standard curve of the aldosterone assay, the lowest detectable value was 0.5 pg per tube. Since the average weight of hypothalamic tissue was 80–-90 mg and the amount in each RIA tube was ∼14 mg, the sensitivity for hypothalamic aldosterone was 0.5/14 = 35 pg/g. The average weight for the hippocampus was 180–-190 mg, the amount in each RIA tube was ∼36 mg, and the sensitivity was 0.5/36 = 14 pg/g. Observed levels of hypothalamic and hippocampus aldosterone (>50 pg/g) were higher than the detection limit. The aldosterone antibody (MP Biomedicals, NY, USA; product #07-108216) exhibits <0.1% cross-reactivity with other steroids. Corticosterone was determined using a corticosterone 125I RIA kit (MP Biomedicals, NY, USA; product #07-120103). Plasma Ang II concentrations were measured by RIA after extraction on C18 Sep-Pak cartridges and separation by HPLC as previously described.9
For EO, all plasma samples were first subjected to solid phase extraction (SPE). Tissue samples were weighed, homogenized in methanol-water mixtures, vacuum dried and extracted using C18 columns (200 mg, Bond Elut, Agilent Technologies, Santa Clara, CA, USA). Routine SPE-RIA was used; reconstituted samples were employed in duplicate for ouabain RIA using the R7 antiserum described previously.28 All measurements were made blind with coded samples.
2.7 Immunoblotting for arterial and adrenal proteins
2.7.1 Arterial proteins
Membrane proteins were solubilized in sodium dodecyl sulfate buffer containing 5% 2-mercaptoethanol and separated by polyacrylamide gel electrophoresis. The blot was incubated overnight at 4 °C with mouse monoclonal anti-NCX1 (R3F1, diluted 1: 500; a generous gift from Dr. K.D. Philipson, UCLA, USA). Gel loading was controlled with monoclonal anti-β actin antibodies (dilution 1:10 000; Sigma-Aldrich, St. Louis, MO, USA). After washing, membranes were incubated with anti-rabbit horseradish peroxidase (HRP)-conjugated IgG for 1 h at room temperature. The immune complexes on the membranes were detected by Enhanced Chemiluminescence Plus’ (Amersham Biosciences, Piscataway, NJ, USA) and exposure to X-ray film (Eastman Kodak, Rochester, NY, USA). Quantitative analysis of immunoblots was performed by using a Kodak DC120 digital camera and 1 D Image Analysis Software (Eastman Kodak).
NCX1 immunoblots usually show prominent bands at 120 and 70 kDa, often with a less prominent band at 160 kDa that is normally ignored. In non-reducing gels (β-mercaptoethanol omitted), the 160 kDa band is usually more prominent than the 120 kDa band.29 Nevertheless, the various treatments generally affect the relative densities of the 160 and 120 kDa bands in similar fashion. Chymotrypsin decreases the density of both large bands and increases that of the 70 kDa band, suggesting that the 70 kDa band is a proteolytic fragment.29 In the experiment of protocol I, for reasons that are not clear, the immunoblot behaved like a non-reducing gel (i.e. as if β-mercaptoethanol had been omitted). The 160 kDa band was analyzed for protocol I, and the 120 kDa band for protocol II to minimize the variance in each of the analyses.
2.7.2 Adrenal proteins
Adrenal cortex was homogenized in lysis buffer (50 mM Tris-HCl, pH 7.4; 1% Nonidet P-40; 0.25% sodium deoxycholate; 150 mM NaCl; 1 mM EDTA; 1 mM phenylmethylsulfonyl fluoride; 1 mM Na3VO4, 1 mM NaF plus 1% of protease inhibitor cocktail (Sigma) for protein extraction. 40 μg proteins were separated by a 10% SDS-PAGE gel. The blot was incubated with monoclonal anti-CYP11B1 antibody (1:1000; clone 80-7, Millipore, Temecula, CA, USA) or anti-CYP11B2 antibody (1:1000; a generous gift from Dr. Mitani. Tokyo, Japan)30 overnight at 4 °C. After washing, the membrane was incubated with the secondary antibody goat anti-rat IgG-HRP (1:5000, Millipore) for CYP11B1 or goat anti-rabbit IgG-HRP (1:2000, R&D systems. Minneapolis, MN, USA) for CYP11B2. For protein loading control, the membrane was re-probed with anti-β-actin (1:10 000; Sigma) and sheep anti-mouse IgG-HRP (1:10 000; GE Healthcare. Mississauga, ON, Canada) as secondary antibody. The signal was developed with the Luminata Forte Western HRP Substrate (EMD Millipore. Etobicoke, ON Canada) and visualized by an Alpha Innotech imager (Alpha Innotech, San Leandro, CA, USA). Band densities were quantified by Alpha Ease software.
2.8 Statistical analysis
Values are expressed as mean ± SE. The effects of sc infusion of Ang II and HS were analyzed by two-way ANOVA. F values are provided for the main effect of the two factors and their effect modification. Student–Newman–Keuls test was performed as post hoc test for multiple comparisons. For protocol II, factorial repeated measures ANOVA was performed to analyze BP and HR changes over time. Student t-test was performed to analyze data for vehicles combined compared to all blockers combined. Correlations analyses were performed by Pearson’s correlation analysis. Statistical significance was defined as P≤0.05. F values and specific P values are provided in the Supplementary material online.
3. Results
3.1 Low-dose Ang II with high salt diet markedly increases systolic and diastolic BP and LV weight
Two weeks of HS alone caused a modest (not significant, NS) decrease in plasma Ang II (Table 1). Consistent with our previous study,24 sc infusion of Ang II at the low rate of 150 ng/kg/min did not cause a detectable increase in plasma Ang II levels (Table 1). As expected, 2 weeks of HS alone had little effect on MAP, while sc infusion of Ang II alone increased BP modestly, for MAP from 99 ± 1 to 108 ± 2 mm Hg, as measured by intra-arterial line (Figure 1A). Systolic, but not diastolic BP increased significantly (Table 1). In contrast, the combination of Ang II with HS increased MAP markedly to 160 ±6 mmHg in the preliminary experiment and to 152 ±4 mmHg in the main experiment (Figure 1A). Both systolic and diastolic BP increased by ∼50% and LV weight increased significantly (Table 1). None of the treatments affected HR, RV weight or BW gain (Table 2). HS alone modestly increased water intake but more combined with Ang II (Table 2). Plasma Na+, K+ and Cl− did not change (Table 2).
Table 1.
Effect of low-dose Ang II combined with regular or 2% high salt diet on plasma Ang II, systolic and diastolic BP and LV weight (top panel) and effects of central MR-ENaC-AT1R blockade on responses to Ang II + salt (bottom panel)
| Regular salt | High salt | Regular salt + Ang II | High salt + Ang II | |
|---|---|---|---|---|
| Plasma Ang II (pg/ml) | 14 ± 2 | 7 ± 2 | 12 ± 3 | 14 ± 2 |
| Systolic BP (mmHg) | 127 ± 1 | 131 ± 3 | 142 ± 2** | 185 ± 4* |
| Diastolic BP (mmHg) | 85 ± 2 | 87 ± 3 | 91 ± 2 | 131 ± 4* |
| LV weight (mg/100g BW) | 187 ± 9 | 204 ± 5 | 211 ± 7 | 238 ± 6* |
| High salt + Ang II | ||||
| + | + | + | + | |
| Veh (n = 11) | MR inh (n = 9) | Ben (n = 6) | Los (n = 4) | |
| Plasma Ang II (pg/ml) | 24 ± 6 | 18 ± 4 | 24 ± 2 | 14 ± 5 |
| Systolic BP (mmHg) | ||||
| Baseline | 120 ± 3 | 117 ± 1 | 116 ± 2 | 119 ± 2 |
| Average of last 3 days | 177 ± 7*,*** | 129 ± 3*** | 124 ± 2*** | 128 ± 5 |
| Diastolic BP (mmHg) | ||||
| Baseline | 88 ± 2 | 81 ± 1 | 80 ± 1 | 85 ± 3 |
| Average of last 3 days | 138 ± 8*,*** | 91 ± 2*** | 88 ± 2*** | 90 ± 5 |
| LV weight (mg/100g BW) | 262 ± 11* | 210 ± 5 | 203 ± 4 | 215 ± 6 |
Values are means ± SE.
Top panel: Values for systolic and diastolic BP, n = 12–15/group (2 studies combined); for LV weight and plasma Ang II, n = 8–13/group (preliminary experiment + first study of first experiment).
Lower panel: Values for plasma Ang II and for LV weight at the end of experiment, and for systolic and diastolic BP at baseline (days -2-0) and last 3 days of treatments for vehicles (Veh), combined eplerenone and spironolactone (MR inh), benzamil (Ben) and losartan (Los).
BP was measured in the first experiment (top panel) by intra-arterial catheter after 2 weeks of treatment, and in the second experiment (bottom panel) by continuous telemetry recording. BP responses to the different icv vehicles and to the 2 MR blockers are comparable, and the data for these are shown as the combined data.
P < 0.05 vs. others.
P < 0.05 vs. regular salt.
P < 0.05 vs. baseline.
Table 2.
Effect of low-dose Ang II combined with regular or 2% high salt diet on heart rate, RV weight, BW gain, water intake and plasma electrolytes
| Regular salt | High salt | Regular salt + Ang II | High salt + Ang II | |
|---|---|---|---|---|
| HR (bpm) | 444 ± 11 | 421 ± 10 | 441 ± 14 | 433 ± 15 |
| RV weight (mg/100g BW) | 47 ± 3 | 45 ± 2 | 49 ± 2 | 52 ± 2 |
| BW gain (g) | 79 ± 5 | 70 ± 4 | 78 ± 4 | 82 ± 4 |
| Water intake (ml/100g BW) | ||||
| End of first week | 14 ± 1 | 17 ± 1 | 14 ± 1 | 21 ± 1* |
| End of second week | 12 ± 1 | 15 ± 1** | 12 ± 1 | 19 ± 1* |
| Plasma Na+ (mmol/L) | 144 ± 1 | 146 ± 1 | 145 ± 1 | 142 ± 1 |
| Plasma K+ (mmol/L) | 5.1 ± 0.2 | 5.6 ± 0.1 | 5.2 ± 0.1 | 5.1 ± 0.2 |
| Plasma Cl− (mmol/L) | 100 ± 1 | 103 ± 1 | 101 ± 1 | 101 ± 1 |
Values are means ± SE. For HR, BW gain and water intake, n = 12–16/group (2 studies combined). For RV weight, n = 8–13/group (preliminary experiment + first study of first experiment). For Na+, K+ and Cl−, n = 6–10/group.
HR, heart rate;RV, right ventricle; BW, body weight.
P < 0.05 vs. others.
P < 0.05 vs. regular salt.
3.2 Low-dose Ang II with high salt diet increases plasma and tissue aldosterone, corticosterone and EO
3.2.1 Plasma hormone levels and adrenal gene expression
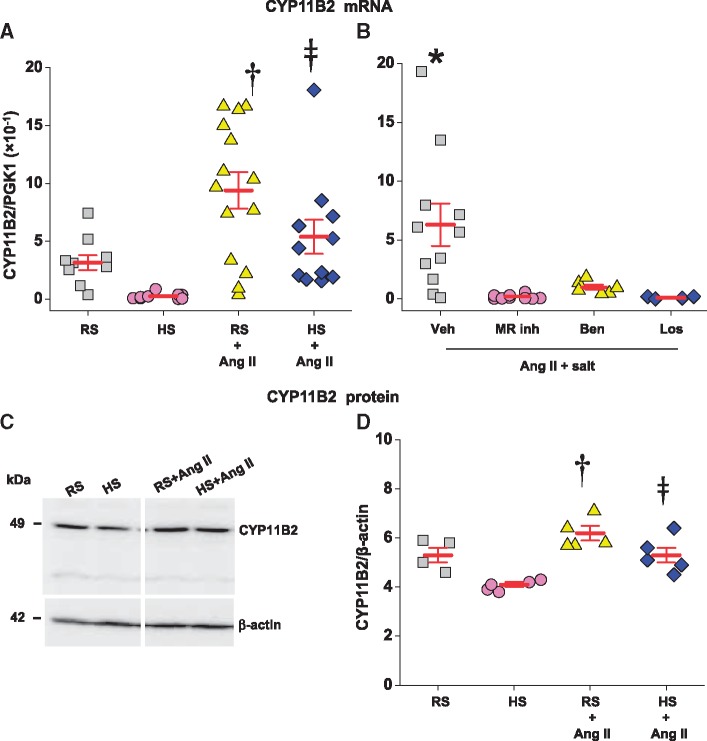
Ang II activates and HS decreases steroid synthesis in the adrenal cortex. Therefore, we measured the mRNA and protein levels of two key steroid biosynthetic enzymes and the plasma levels of three adrenal steroids. Two weeks of HS alone had a negligible effect on CYP11B1 mRNA and protein (Table 3) but caused a marked decrease in CYP11B2 mRNA (Figure 2A). The decline in CYP11B2 mRNA was associated with only minor (NS) decreases in CYP11B2 protein (Figure 2C and D) and plasma aldosterone (Figure 3A).
Table 3.
Effect of low-dose Ang II combined with regular or 2% high salt diet on brain and plasma aldosterone and corticosterone and adrenal CYP11B1 expression (top panel); and effect of central MR-ENaC-AT1R blockade on plasma aldosterone and corticosterone (bottom panel)
| Regular salt | High salt | Regular salt + Ang II | High salt + Ang II | |
|---|---|---|---|---|
| Brain aldosterone (pg/g) | ||||
| Hypothalamus | 69 ± 20 | 101 ± 34 | 135 ± 19 | 295 ± 79* |
| Hippocampus | 53 ± 9 | 63 ± 16 | 113 ± 21 | 211 ± 39* |
| Brain corticosterone | ||||
| (ng/g) | ||||
| Hypothalamus | 3.1 ± 0.7 | 9.4 ± 4.7 | 8.0 ± 2.1 | 36.8 ± 11.4* |
| Hippocampus | 3.7 ± 0.9 | 8.5 ± 3.6 | 8.6 ± 2.3 | 32.9 ± 7.6* |
| Plasma aldosterone | 91 ± 15 | 90 ± 21 | 206 ± 42 | 383 ± 100* |
| (pg/ml) | ||||
| Plasma corticosterone | 98 ± 25 | 115 ± 32 | 75 ± 27 | 198 ± 43* |
| (ng/ml) | ||||
| Adrenal cortex CYP11B1 | ||||
| mRNA (CYP11B1/PGK1) | 5.9 ± 0.6 | 6.7 ± 0.3 | 5.7 ± 0.3 | 6.3 ± 0.5 |
| Protein (CYP11B1/β-actin) | 1.9 ± 0.3 | 2.1 ± 0.4 | 1.8 ± 0.5 | 1.2 ± 0.1 |
| High salt + Ang II | ||||
| + | + | + | + | |
| Veh (n = 11) | MR inh (n = 9) | Ben (n = 6) | Los (n = 4) | |
| Plasma aldosterone (pg/ml) | 179 ± 84* | 41 ± 9 | 69 ± 31 | 44 ± 13 |
| Plasma corticosterone (ng/ml) | 108 ± 35 | 72 ± 24 | 113 ± 57 | 53 ± 17 |
Values are means ± SE.
Top panel: Values for hypothalamus and hippocampus aldosterone and corticosterone, n = 6–8/group (second study of first experiment); for plasma aldosterone, n = 12–16/group for two studies combined; for plasma corticosterone, n = 8–12/group (preliminary experiment + first study of first experiment); for CYP11B1/PGK1, n = 11–14/group (two studies combined), and for CYP11B1/β-actin, n = 5–6/group (second study of first experiment).
Lower panel: Values for plasma aldosterone and corticosterone for vehicles (Veh), combined eplerenone and spironolactone (MR inh), benzamil (Ben) and losartan (Los).
P < 0.05 vs. others.
Figure 2.
Sc infusion of Ang II at 150 ng/kg/min similarly increases CYP11B2 mRNA (panel A) and protein (panels C and D) in the adrenal cortex of rats on regular (RS) or 2% high salt (HS) diet. Central infusion of an MR blocker (eplerenone or spironolactone), ENaC blocker (benzamil) or AT1R blocker (losartan) prevents the increase in CYP11B2 mRNA caused by Ang II in rats on HS diet (panel B). A representative immunoblot shows ∼49 kDa CYP11B2 protein in the adrenal cortex (panel C). Panels A and D: values are means ± SE (for CYP11B2 mRNA, n = 11–14/group for 2 studies combined; for protein levels, n = 5–6/group for second study only). Two-way ANOVA: †P<0.05 vs. regular salt. ‡P<0.05 vs. high salt. Panel B: values are means ± SE. n = 11 for vehicles (Veh), n = 9 for combined eplerenone and spironolactone (MR inh), n = 6 for benzamil (Ben), and n = 4 for losartan (Los). One-way ANOVA or Student t-test for Veh vs. blockers combined: *P<0.05 vs. others. In panel A, reference gene PGK1 mRNA in the first study was in the range of 6.7–7.2, and in the second study in the range of 6.2–6.7. In panel B, PGK1 mRNA was in the range of 6.5–6.7 (not different between groups).
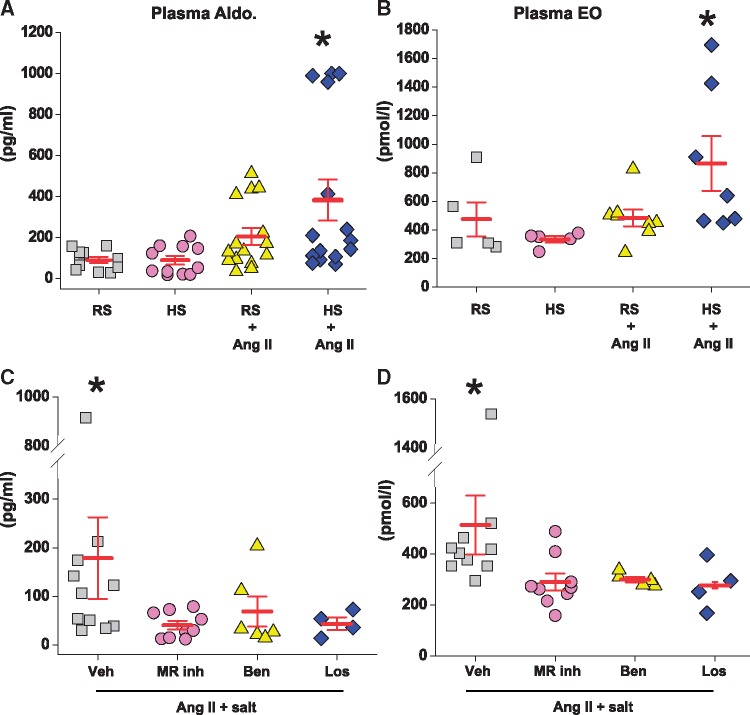
Figure 3.
Sc infusion of Ang II at 150 ng/kg/min significantly increases plasma aldosterone and EO in rats on high salt (HS) diet but not in rats on regular salt (RS) diet (panels A and B). Central infusion of an MR blocker (eplerenone or spironolactone), ENaC blocker (benzamil) or AT1R blocker (losartan) significantly lowers plasma aldosterone and EO in rats with Ang II on HS diet (panels C and D). Panels A and B: values are means ± SE (for plasma aldosterone, n = 12–16/group for 2 studies combined; for plasma EO, n = 6–8/group). Two-way ANOVA: *P<0.05 vs. others. Panels C and D: values are means ± SE. Veh, n = 11 for plasma aldosterone and n = 6 for plasma EO, MR inh, n = 9 for plasma aldosterone and n = 6 for plasma EO, benzamil (Ben), n = 6 and losartan (Los), n = 4. Student t-test for Veh vs. blockers combined. *P≤0.05 vs. others. In the first experiment (panels A and B), blood samples were obtained after recent surgery, likely explaining the higher values for plasma aldosterone, EO and corticosterone (Table 3) compared to the second experiment with more distant surgery (panels C and D).
Ang II with or without HS had no effect on CYP11B1 mRNA and protein (Table 3) but significantly increased CYP11B2 mRNA and protein (Figure 2A and D). In rats on RS, sc infusion of Ang II did not change plasma corticosterone (Table 3), or EO (Figure3B) and caused a modest (NS) increase in plasma aldosterone (Figure 3A). Despite similar changes in adrenal gene expression, the combination of Ang II + HS caused a distinctly different pattern of changes in the levels of the 3 plasma steroids than Ang II or HS alone. Ang II + HS increased plasma aldosterone by ∼four-fold (Figure 3A, Table 3), corticosterone by ∼two-fold (Table 3) and EO by ∼two-fold (Figure 3B). Increases in plasma corticosterone and aldosterone showed highly significant correlations (Figure 4A).
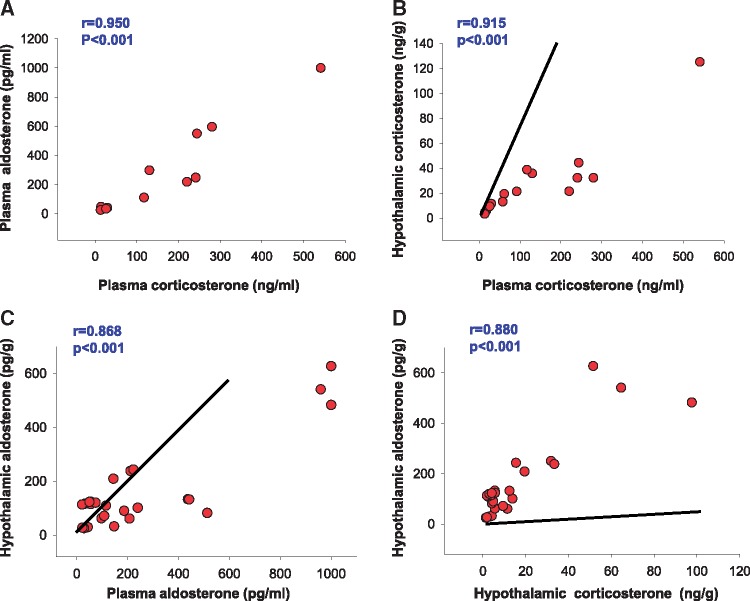
Figure 4.
Correlations between plasma and hypothalamic corticosterone and aldosterone levels in protocol I. Lines in B and C represent the expected slope of relationship if plasma corticosterone or aldosterone can freely enter the hypothalamus. Line in D represents the expected slope for 100 ng corticosterone measuring as 40 pg aldosterone in aldosterone assay. Conclusion: measured aldosterone immunoreactivity is ∼90% aldosterone not corticosterone. Pearson’s correlation analysis.
3.2.2 Tissue hormone levels
Because Ang II also activates a steroid pathway in the brain,10 we also measured brain aldosterone and corticosterone. Indeed, whereas Ang II + RS caused only modest (NS) increases, low-dose sc Ang II + HS significantly increased aldosterone levels in the hypothalamus and the hippocampus four-fold and corticosterone levels nearly 10-fold (Table 3). Plasma and hypothalamus levels for each of the two steroids correlated significantly (Figure 4B, C). In addition, hypothalamic corticosterone and aldosterone levels are correlated significantly (Figure 4D).
Since circulating ouabain/EO can accumulate in the kidney,31,32 we assessed the effects of Ang II and HS on renal EO levels. Whereas HS alone had no effect on renal EO levels, Ang II alone or together with HS, increased the renal EO content up to ∼three-fold (Table 4).
Table 4.
Effect of low-dose Ang II combined with regular or 2% high salt diet on renal EO (µg/kg) (top panel); and effect of central MR-ENaC-AT1R blockade on renal cortex, medulla and papilla EO (µg/kg) (bottom panel)
| Regular salt | High salt | Regular salt + Ang II | High salt + Ang II | |
|---|---|---|---|---|
| (n = 6) | (n = 6) | (n = 8) | (n = 8) | |
| Kidney | 1.3 ± 0.1 | 1.3 ± 0.1 | 3.3 ± 0.5* | 4.0 ± 0.5** |
| High salt + Ang II | ||||
| + | + | + | + | |
| Veh (n = 11) | MR inh (n = 9) | Ben (n = 6) | Los (n = 4) | |
| Renal cortex | 2.2 ± 0.4 | 3.5 ± 1.1 | 4.0 ± 1.2 | Δ |
| Renal medulla | 39.5 ± 9.2*** | 16.5 ± 4.6 | 6.0 ± 0.9 | 16.6 ± 8.9 |
| Renal papilla | 7.6 ± 1.3 | 6.2 ± 1.4 | 4.2 ± 1.9 | 6.6 ± 1.6 |
Values are means ± SE.
Lower panel: Values are for vehicles (Veh), combined eplerenone and spironolactone (MR inh), benzamil (Ben) and losartan (Los). Δ: sample size is too small and data are not included.
P < 0.05 vs. regular salt.
P < 0.05 vs. high salt.
P < 0.05 vs. others.
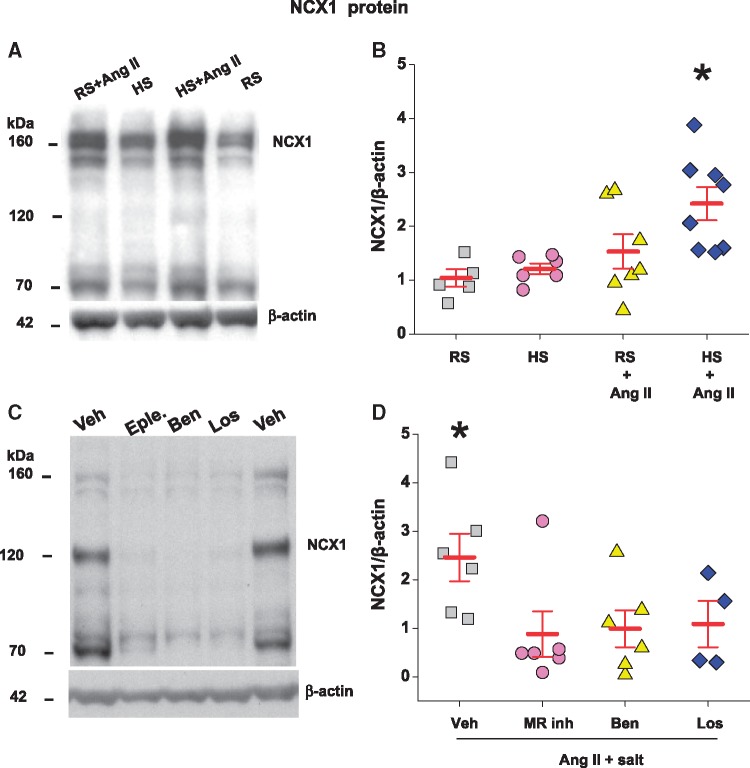
3.2.3 NCX1 expression in the aorta
In many models of hypertension, including icv Ang II-induced hypertension, NCX1 and several other arterial myocyte Ca2+ transporter proteins are markedly up-regulated.11,33 HS or low-dose sc Ang II alone caused a minor increase in NCX1 protein. In contrast, Ang II + HS increased myocyte NCX1 by >two-fold compared to controls (Figure 5B).
Figure 5.
Sc Ang II at 150 ng/kg/min significantly increases NCX1 protein in the aorta of rats on 2% high salt (HS), but not in rats on regular salt (RS) diet (panels A and B). Central infusion of an MR blocker (eplerenone or spironolactone), ENaC blocker (benzamil) or AT1R blocker (losartan) prevents the increase in NCX1 protein in the aorta caused by Ang II in rats on HS diet (panels C and D). Panels A and B: the summary data are for changes in NCX1 relative to the control group. A representative immunoblot shows ∼160 kDa NCX1 protein under non-reducing conditions. Values are means ± SE (n = 5–8/group). Two-way ANOVA: *P<0.05 vs. others. Panels C and D: the summary data are for changes in NCX1 protein levels relative to the vehicle; n = 6 for vehicle (Veh), n = 6 for MR blocker, n = 6 for benzamil (Ben), and n = 4 for losartan (Los). A representative immunoblot shows effect of central MR-ENaC-AT1R blockade on NCX1 protein. Student t-test: *P<0.05 vs. others
3.3. Central MR-ENaC-AT1R blockades prevent hormonal and cardiovascular responses to sc Ang II + HS
A critical test of our hypothesis that the central neurohumoral pathway mediates the actions of circulating Ang II and HS on BP regulation is the effect of specific blockade of that pathway. Therefore, we tested the effects of icv infusion of very small amounts of the AT1R blocker, losartan, the two MR inhibitors, spironolactone and eplerenone, and the ENaC blocker, benzamil, in Ang II + HS-treated rats; some of the Ang II + HS rats were infused icv with vehicles.
3.3.1 Hormonal responses to CNS pathway blockade
All the icv blockers reduced adrenal CYP11B2 mRNA expression (Figure 2B) but had no effect on CYP11B1 mRNA (Table 3). The reductions in adrenal CYP11B2 were all reflected by reduced plasma aldosterone levels in sc Ang II + HS-treated rats (Figure 3C). The lack of effect of the icv blockers on plasma corticosterone (Table 3, bottom panel) fits with the unaltered adrenal CYP11B1. Furthermore, plasma EO in the Ang II + HS rats was significantly reduced by all the icv blockers (Figure 3D). This decline in plasma EO likely accounts for the reduction of renal medullary EO in the presence of the icv blockers (Table 4, bottom panel), because renal medullary EO is often a reflection of the plasma level.32
3.3.2 Cardiovascular responses to CNS pathway blockade
The combination of sc Ang II (150 ng/kg/min) with 2% HS and icv vehicles increased MAP by ∼50 mm Hg after 10 days (Figure 1B) and similarly increased systolic and diastolic BP (Table 1). Concurrent icv infusion of eplerenone, spironolactone, benzamil or losartan prevented most of the increase in MAP (Figure 1B) as well as the increase in LV weight (Table 1). The blockers also greatly reduced aortic NCX1 protein expression relative to that in Ang II + HS with vehicles (Figure 5C). The icv blockers caused a minor (NS) decrease in water intake (end of first week: 24 ± 4 vs. 18 ± 2 ml/100 g BW, t = 1.7, p = 0.1; end of second week: 21 ± 2 vs. 17 ± 1 ml/100 g BW, t = 1.6, p = 0.1, vehicles vs. combined blockers).
4. Discussion
The present study provides two major new findings regarding the pathophysiology of Ang II + HS hypertension in rats, a model for salt-sensitive essential hypertension.15,16,34 First, the gradual development of severe hypertension by the combination of a low-dose infusion of Ang II given in the periphery, and a modest increase in salt intake depends critically on a CNS pathway involving MR-ENaC-AT1R signalling. Second, in addition to the well-established CNS-mediated increase in sympathetic activity,16,34 activation of this CNS pathway also increases plasma aldosterone and EO, and arterial NCX1 protein expression. Together, the elevated aldosterone and EO likely amplify sympathetic actions on the kidneys and arteries.
4.1 A novel CNS neurohumoral pathway mediates Ang II + salt hypertension
The synergy between circulating Ang II and high dietary salt in the elevation of BP is well documented in humans,35 dogs15,36 and rodents.37,38 We employed the low-dose Ang II + HS model to elucidate central and peripheral mechanisms that mediate the elevation of BP.
As reported previously,24 sc infusion of Ang II at the low dose of 150 ng/kg/min does not cause a detectable increase in plasma Ang II and causes only a minor increase in BP. When combined with a 2% salt diet, however, this same Ang II infusion rate induces severe hypertension and significantly increases LV weight. The extent of these effects is similar to the effect of infusing sc Ang II at the high rate of 500 ng/kg/min in rats on a regular (0.4%) salt diet.24 Moreover, in both the low-dose Ang II + HS and the high-dose Ang II + RS models, central AT1R blockade prevents or reverses most of the hypertension (present study and Refs. 14 and 39). This indicates that the activation of central angiotensinergic pathways by circulating Ang II, either with or without HS, plays a critical role in the pathogenesis of the hypertension.
Central MR or ENaC blockade also prevents/reverses most of the hypertension in the high-dose Ang II + RS and low-dose Ang II + HS models (present study, Refs. 10, 14, 21, 39, 40). This implies that the same CNS MR-ENaC-AT1R pathway contributes to the hypertension in both models. Both Ang II and [Na+] can activate this pathway,12 and their effects are synergistic.36,37 Thus, it is not surprising that a high dietary salt intake, that, by itself, has little effect on BP, can augment the pressor response to low-dose Ang II via the MR-ENaC-AT1R-mediated pathway. Circulating aldosterone may initiate activation of this pathway through MR in the SFO.41 Studies in other rat models of Ang II and/or salt-induced hypertension using central infusion of an aldosterone synthase inhibitor have demonstrated in addition an important role for aldosterone, produced locally in the hypothalamus, and MR in eg the PVN.24,39,42,43 Whether MR activation by plasma aldosterone as well as locally in the CNS produced aldosterone also plays a role in the low-dose Ang II + HS model, has not yet been studied. Both plasma and hypothalamic corticosterone and aldosterone levels increased with highly significant correlations. Uptake from the circulation may therefore also play a role in this model.
The CNS blockade data indicate that the central actions of Ang II, and not its peripheral actions, are primarily responsible for the BP elevation. This is consistent with the evidence that: (i) The plasma Ang II level is not detectably elevated by the low dose sc Ang II infusion (Table 1); and (ii) Blockade of arterial Ang II receptors has negligible effect on vascular tone or BP in the Ang II + HS model; virtually all of the tone depends on SNA and α-adrenoceptors.44 Peripheral effects of Ang II at this low infusion rate and the 2% HS on eg kidneys and arteries may be amplifying the central actions, but on their own, i.e. in the presence of central blockade, may cause only minimal increases in BP. Na+ appears first to amplify CNS responses to Ang II (and vice versa) and subsequently to enhance the secondary activation of peripheral pressor mechanisms.
4.2 CNS neurohumoral pathway-dependent regulation of plasma aldosterone and EO
Sc infusion of Ang II in rats on regular dietary salt doubled the adrenal CYP11B2 mRNA and protein levels but caused only a modest (NS) increase in plasma aldosterone, suggesting that net enzymatic activity of the aldosterone synthase increased only minimally. In contrast, the high (2%) salt diet alone decreased plasma Ang II, adrenal CYP11B2 mRNA and protein and plasma aldosterone, as expected.45 Nevertheless, the combination of Ang II+HS not only increased CYP11B2 mRNA and protein to the same levels as did Ang II alone but also significantly increased plasma aldosterone four-fold. An increase in ACTH release seems unlikely to explain this increase in plasma aldosterone because the adrenal CYP11B1 mRNA and protein levels did not increase, and ACTH tends to elevate plasma aldosterone only transiently.46,47 On the other hand, enhanced sympathetic drive to the adrenals evoked by Ang II + HS may, via β-receptor stimulation, increase aldosterone48,49 secretion. In addition, increases in plasma EO or vasopressin induced by Ang II + HS may stimulate aldosterone secretion.50–52 These explanations seem especially plausible because all blockers of the CNS neurohumoral pathway suppressed elevation of CYP11B2 mRNA (Figure 2B), plasma aldosterone (Figure 3C) and plasma EO (Figure 3D).
There is extensive evidence for the synthesis of ouabain and other cardiotonic steroids in both the CNS and adrenals.11,53 CNS EO via secretion of EO, possibly by the pituitary can be a source of circulating EO.11 Adrenal production and secretion of EO is generally considered the major source of plasma EO50,54 and can be stimulated by ACTH, Ang II and catecholamines.55 Further, activation of the hypothalamic aldosterone-MR-ENaC pathway by, e.g. Ang II or salt,11,55,56 increases hypothalamic EO, and may, thereby, also contribute to the elevated plasma EO.57 The parallel elevation of plasma aldosterone and EO in Ang II + HS hypertension (Figure 3A and B) and in a large portion of patients with essential hypertension that are also salt-sensitive58 suggests a common link in their regulation and biosynthesis.
4.3 CNS-mediated actions of Ang II + HS on peripheral pressor mechanisms
If SNA, rather than Ang II, directly activates arterial myocytes and is primarily responsible for the elevated vascular tone in Ang II + HS hypertension,44 we must ask whether the SNA alone, mediates the BP elevation. Experimental evidence suggests that this is not the case. Before addressing this issue, however, we need to consider the effects of acute and chronic EO elevation on arterial myocytes.
As recently reviewed,23 nanomolar ouabain/EO directly inhibits arterial myocyte high ouabain affinity α2 Na+ pumps in rats and thereby presumably elevates the sub-plasma membrane (PM) cytosolic Na+ concentration ([Na+]SPM) in the vicinity of “junctional sarcoplasmic reticulum(SR).”59 PM Na+/Ca2+ exchangers (NCX) are also located in these microdomains. Thus, the net effect is a rise in cytosolic Ca2+, an increase in the Ca2+ stored in the sarcoplasmic reticulum (SR), and an amplification of Ca2+ signalling (the acute ‘vasotonic effect’). When the EO/ouabain elevation is sustained, it also activates a protein kinase signalling cascade that includes C-Src. This induces an increase in the expression of several arterial Ca2+ transporter proteins, including NCX type 1 (NCX1, Figure 5) and the SR Ca2+ pump, SERCA2.11,32,59 The net effect of this chronic action of EO is a further amplification of Ca2+ signalling in response to vasoconstrictors such as SNA.
These considerations imply that, in addition to SNA, the CNS neuromodulatory pathway signals to the arteries via circulating EO, and that arterial α2 Na+ pumps and NCX1 also may play a crucial role in chronic BP elevation. Experimental support for this view comes from studies on mice with genetically engineered arterial α 2 Na+ pumps and NCX1 and mice in which EO is immuno-neutralized. Mice with reduced smooth muscle (SM)-specific α2 Na+ pump expression (i.e. fewer than normal EO receptors) have elevated basal BP and enhanced sensitivity to Ang II- and HS-induced BP elevation.60 Also, mice with SM-specific overexpression of NCX1 are hypertensive and exhibit enhanced sensitivity to HS (Ang II was not tested).61 Finally, in Ang II + HS hypertensive mice, BP can be reduced by “DigiFab” (fab fragments that selectively immuno-neutralize EO).62–64 These reports, plus the present results and data from icv Ang II-infused rats,11 suggest that chronic activation of CNS mechanisms by Ang II + HS causes hypertension in part by amplifying the effective sympathetic tone via EO-dependent reprogramming of arterial function. This peripheral effect of EO would enable minimal increases in sympathetic drive to sustain high BP.
5. Limitations
The present study provides novel evidence that central mechanisms mediate the strong synergistic actions of dietary salt and circulating Ang II. However, the mechanisms and specific locations within the CNS by which high dietary salt intake amplifies the central actions of Ang II require further studies.
Central infusions of different blockers were performed to assess the functional role of the brain MR-ENaC-AT1R pathway. The 2 MR blockers combined and the AT1R blocker provide specific evidence for the role of brain MR and AT1R. On the other hand, benzamil can affect several Na+-channels or transporters,65 but as discussed previously26 at the current infusion rate likely causes primarily ENaC blockade.
The aldosterone antibody exhibits low cross-reactivity for other steroids (0.04% for corticosterone). However, the concentration of corticosterone is in the ng range vs. pg for aldosterone. Thus, 100 ng/ml or ng/g of corticosterone would be measured as 40 pg/ml or pg/g of “aldosterone” in the aldosterone RIA. However, comparison of the absolute levels and their changes by Ang II + HS (Table 3) indicates that most of the RIA values indeed reflect aldosterone per se.
6. Clinical perspectives
6.1 Pathophysiology
Under normal physiological conditions, the imposition of low or high salt diets either enhances or diminishes, respectively, the circulating levels of Ang II, aldosterone and EO with little net effect on BP. The animal model of moderate high salt intake combined with a minimal increase in plasma Ang II bears a striking resemblance to those patients with salt-sensitive essential hypertension in whom plasma renin activity is marginally increased and the circulating levels of EO and aldosterone are co-elevated.58 The inability to suppress Ang II on high salt intake results in an increase in plasma aldosterone and EO and rather severe hypertension. All of these effects depend critically on activation of a CNS pathway that involves MR-ENaC-AT1R signalling (Figure 6). This central pathway increases SNA, per se, but, in addition, apparently requires the participation of circulating EO and peripheral pressor mechanisms that enhance the effect of sympathetic tone on the arteries in order to effect chronic BP elevation (Figure 6).
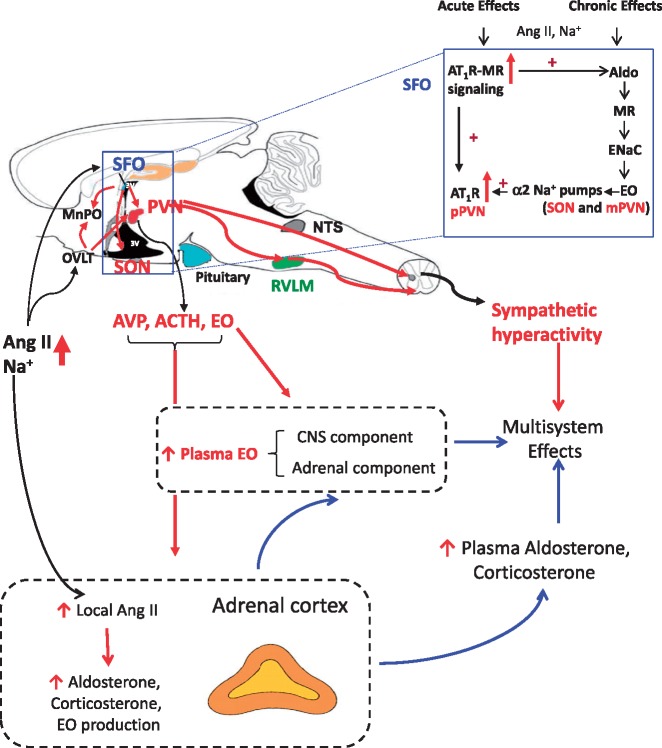
Figure 6.
Proposed central and peripheral pathways/mechanisms being activated by circulating Ang II and high dietary salt intake. Central components: Both circulating Ang II and dietary salt, presumably by (transiently) increasing plasma [Na+], can activate Ang II-AT1R-ROS signaling in circumventricular organs such as the SFO and OVLT which are located outside of the BBB, followed by activation of angiotensinergic projections to the PVN, SON and RVLM (red lines). Prolonged stimulation of AT1R - MR in the SFO also activates a slow neuromodulatory pathway (box at upper right) that involves locally in the hypothalamus produced aldosterone that binds MR in, e.g. SON and PVN and via ENaC increases hypothalamic EO. Ouabain or EO acutely inhibit α2 Na+ pumps and chronically also activate an α2 Na+ pump associated protein kinase cascade that increases expression of ACE, AT1R and NADPH oxidase subunits. As a consequence, chronic Ang II-AT1R-ROS signaling is maintained/enhanced. Peripheral components: Chronic activation of brain angiotensinergic pathways can cause a persistent increase in sympathetic tone and in addition may stimulate the production and secretion of CNS EO as well as of AVP and ACTH into the circulation. These factors can all contribute to enhanced adrenal production and secretion of aldosterone, corticosterone and EO. A chronic increase in plasma EO will activate an α2 Na+ pump-associated protein kinase cascade (e.g. C-Src-mediated) that increases arterial smooth muscle cell NCX expression, and arterial sarcoplasmic reticulum (SR) Ca2+ pump (SERCA2) expression. The EO-induced NCX and SERCA2 up-regulation enhances Ca2+ signaling and thereby effects of SNA to increase vascular tone and elevate BP.
The CNS can stimulate adrenocortical steroid production via a mediator that is likely to be independent of ACTH or Ang II. Although the identity of this mediator is not known, it could underlie the subtle adrenocortical dysfunction in patients with essential hypertension.66,67
6.2 Therapeutic implications
If similar CNS pathways also play a role in patients with, e.g. salt-sensitive hypertension, obviously central infusions are not a relevant treatment strategy, and the key question is whether oral treatment is able to target this CNS signalling pathway. A number of animal studies have demonstrated that oral treatment with MR or AT1R blockers can cause substantial central blockade and can prevent salt-induced hypertension.68 In humans, oral treatment can lower sympathetic hyperactivity, consistent with central actions.69,70 Moreover, aminopeptidase A (APA) inhibitors such as RB150, can cross the BBB and inhibit conversion of Ang II to Ang III in the CNS. They are presently being tested in clinical studies as a new class of antihypertensive drugs to specifically target the brain RAS.71,72
Supplementary Material
Acknowledgements
Frans Leenen holds the Pfizer Chair in Hypertension Research, an endowed chair supported by Pfizer Canada, University of Ottawa Heart Institute Foundation, and Canadian Institutes of Health Research.
Funding
This study was supported by operating Grant no. FRN: MOP-74432 from the Canadian Institutes of Health Research.
Conflict of interest: none declared.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
References
- 1. Leenen FHH. Actions of circulating angiotensin II and aldosterone in the brain contributing to hypertension. Am J Hypertens 2014; 27:1024–1032. 10.1093/ajh/hpu066 [DOI] [PubMed] [Google Scholar]
- 2. McKinley MJ, Allen AM, Burns P, Colvill LM, Oldfield BJ.. Interaction of circulating hormones with the brain: the roles of the subfornical organ and the organum vasculosum of the lamina terminalis. Clin Exp Pharmacol Physiol 1998; 25:S61–S67. [DOI] [PubMed] [Google Scholar]
- 3. Smith PM, Ferguson AV.. Circulating signals as critical regulators of autonomic state: central roles for the subfornical organ. Am Physiol Regul Integr Comp Physiol 2010; 299:R405–R415. [DOI] [PubMed] [Google Scholar]
- 4. Biancardi VC, Stern JE.. Compromised blood brain barrier permeability: novel mechanism by which circulating angiotensin II signals to sympathoexcitatory centres during hypertension. J Physiol 2016; 15:1591–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cato MJ, Toney GM.. Angiotensin II excites paraventricular nucleus neurons that innervate the rostral ventrolateral medulla: an in vitro patch-clamp study in brain slices. J Neurophysiol 2005; 93:403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li DP, Chen SR, Pan HL.. Angiotensin II stimulates spinally projecting paraventricular neurons through presynaptic disinhibition. J Neurosci 2003; 23:5041–5049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carmichael CY, Wainford RD.. Hypothalamic signaling mechanisms in hypertension. Curr Hypertens Rep 2015; 17:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qi J, Zhang D-M, Suo Y-P, Song X-A, Yu X-J, Elks C, Lin Y-X, Xu Y-Y, Zang W-J, Zhu Z, Kang Y-M.. Renin-angiotensin system modulates neurotransmitters in the paraventricular nucleus and contributes to angiotensin II-induced hypertensive response. Cardiovasc Toxicol 2013; 13:48–54. [DOI] [PubMed] [Google Scholar]
- 9. Culman J, Höhle S, Qadri F, Edling O, Blume A, Lebrun C, Unger T.. Angiotensin as neuromodulator/neurotransmitter in central control of body fluid and electrolyte homeostasis. Clin Exp Hypertens 1995; 17:281–293. [DOI] [PubMed] [Google Scholar]
- 10. Huang BS, White RA, Ahmad M, Leenen FHH.. Role of brain corticosterone and aldosterone in central angiotensin II-induced hypertension. Hypertension 2013; 62:564–571. 10.1161/HYPERTENSIONAHA.113.01557 [DOI] [PubMed] [Google Scholar]
- 11. Hamlyn JM, Linde CI, Gao J, Huang BS, Golovina VA, Blaustein MP, Leenen FHH, Karamyan V.. Neuroendocrine humoral and vascular components in the pressor pathway for brain angiotensin II: A new axis in long term blood pressure control. PLoS One 2014; 9:e108916.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gabor A, Leenen FHH.. Central neuromodulatory pathways regulating sympathetic activity in hypertension. J Appl Physiol 2012; 113:1294–1303. 10.1152/japplphysiol.00553.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Van Huysse JW, Dostanic I, Lingrel JB, Hou X, Wu H.. Hypertension from chronic central sodium chloride in mice is mediated by the ouabain-binding site on the Na, K-ATPase α2-isoform. Am J Physiol Heart Circ Physiol 2011;301:H2147–H2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gabor A, Leenen FHH.. Central mineralocorticoid receptors and the role of angiotensin II and glutamate in the paraventricular nucleus of rats with angiotensin II-induced hypertension. Hypertension 2013; 61:1083–1090. 10.1161/HYPERTENSIONAHA.111.00797 [DOI] [PubMed] [Google Scholar]
- 15. Cowley AW, McCaa RE.. Acute and chronic dose-response relationships for angiotensin, aldosterone, and arterial pressure at varying levels of sodium intake. Circ Res 1976; 39:788–797. 10.1161/01.RES.39.6.788 [DOI] [PubMed] [Google Scholar]
- 16. King AJ, Fink GD.. Chronic low-dose angiotensin II infusion increases venomotor tone by neurogenic mechanisms. Hypertension 2006; 48:927–933. 10.1161/01.HYP.0000243799.84573.f8 [DOI] [PubMed] [Google Scholar]
- 17. Osborn JW, Fink GD, Kuroki MT.. Neural mechanisms of angiotensin II-salt hypertension: implications for therapies targeting neural control of the splanchnic circulation. Curr Hypertens Rep 2011; 13:221–228. 10.1007/s11906-011-0188-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Guild S-J, McBryde FD, Malpas SC, Barrett CJ.. High dietary salt and angiotensin II chronically increase renal sympathetic nerve activity a direct telemetric study. Hypertension 2012; 59:614–620. 10.1161/HYPERTENSIONAHA.111.180885 [DOI] [PubMed] [Google Scholar]
- 19. Bardgett ME, Holbein WW, Herrera-Rosales M, Toney GM.. Ang II-salt hypertension depends on neuronal activity in the hypothalamic paraventricular nucleus but not on local actions of tumor necrosis factor-α. Hypertension 2014;63:527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Larson RA, Gui L, Huber MJ, Chapp AD, Zhu J, LaGrange LP, Shan Z, Chen Q-H.. Sympathoexcitation in AngII-salt hypertension involves reduced SK channel function in the hypothalamic paraventricular nucleus. Am J Physiol Heart Circ Physiol 2015; 308:H1547–H1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Osborn JW, Olson DM, Guzman P, Toney GM, Fink GD.. The neurogenic phase of angiotensin II-salt hypertension is prevented by chronic intracerebroventricular administration of benzamil. Physiol Rep 2014; 2:e00245.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. King AJ, Osborn JW, Fink GD.. Splanchnic circulation is a critical neural target in angiotensin II salt hypertension in rats. Hypertension 2007; 50:547–556. 10.1161/HYPERTENSIONAHA.107.090696 [DOI] [PubMed] [Google Scholar]
- 23. Blaustein MP, Chen L, Hamlyn JM, Leenen FHH, Lingrel JB, Wier WG, Zhang J.. Pivotal role of α2 Na+ pumps and their high affinity ouabain binding site in cardiovascular health and disease. J Physiol 2016;594:6079–6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang BS, Ahmadi S, Ahmad M, White RA, Leenen FHH.. Central neuronal activation and pressor responses induced by circulating ang II: role of the brain aldosterone-ouabain-pathway. Am J Physiol Heart Circ Physiol 2010; 299:H422–H430. [DOI] [PubMed] [Google Scholar]
- 25. Xue B, Pamidimukkala J, Hay M.. Sex differences in the development of angiotensin II-induced hypertension in conscious mice. Am J Physiol Heart and Circ Physiol 2005; 288:H2177–H2184. [DOI] [PubMed] [Google Scholar]
- 26. Wang H-W, Amin MS, El-Shahat E, Huang BS, Tuana BS, Leenen FHH.. Effects of central sodium on epithelial sodium channels in rat brain. Am. Physiol Regul Integr Comp Physiol 2010;299:R222–R233. [DOI] [PubMed] [Google Scholar]
- 27. Glowinski J, Iversen LL.. Regional studies of catecholamines in the rat brain. J Neurochem 1966; 13:655–669. 10.1111/j.1471-4159.1966.tb09873.x [DOI] [PubMed] [Google Scholar]
- 28. Harris DW, Clark MA, Fisher JF, Hamlyn JM, Kolbasa KP, Ludens JH, DuCharme DW.. Development of an immunoassay for endogenous digitalis like factor. Hypertension 1991; 17:936–943. [DOI] [PubMed] [Google Scholar]
- 29. Philipson KD, Longoni S, Ward R.. Purification of the cardiac Na+-Ca2+ exchange protein. Biochim Biophys Acta 1988;945:298–306. [DOI] [PubMed] [Google Scholar]
- 30. Ogishima T, Suzuki H, Hata J, Mitani F, Ishimura Y.. Zone-specific expression of aldosterone synthase cytochrome P-450 and cytochrome P-45011 beta in rat adrenal cortex: histochemical basis for the functional zonation. Endocrinology 1992; 130:2971–2977. [DOI] [PubMed] [Google Scholar]
- 31. Yuan CM, Manunta P, Hamlyn JM, Chen S, Bohen E, Yeun J, Haddy FJ, Pamnani MB.. Long-term ouabain administration produces hypertension in rats. Hypertension 1993; 22:178–187. [DOI] [PubMed] [Google Scholar]
- 32. Manunta P, Rogowski AC, Hamilton BP, Hamlyn JM.. Ouabain-induced hypertension in the rat: relationships among plasma and tissue ouabain and blood pressure. J Hypertens 1994; 12:549–560. [PubMed] [Google Scholar]
- 33. Pulina MV, Zulian A, Berra-Romani R, Beskina O, Mazzocco-Spezzia A, Baryshnikov SG, Papparella I, Hamlyn JM, Blaustein MP, Golovina VA.. Upregulation of Na+ and Ca2+ transporters in arterial smooth muscle from ouabain-induced hypertensive rats. Am J Physiol Heart Circ Physiol 2010; 298:H263–H274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. King AJ, Novotny M, Swain GM, Fink GD.. Whole body norepinephrine kinetics in ang II-salt hypertension in the rat. Am Physiol Regul Integr Comp Physiol 2008; 294:R1262–R1267. [DOI] [PubMed] [Google Scholar]
- 35. Hollenberg NK, Chenitz WR, Adams DF, Williams GH.. Reciprocal influence of salt intake on adrenal glomerulosa and renal vascular responses to angiotensin II in normal man. J Clin Invest 1974; 54:34–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. DeClue JW, Guyton AC, Cowley AW, Coleman TG, Norman RA, McCaa RE.. Subpressor angiotensin infusion, renal sodium handling, and salt-induced hypertension in the dog. Circ Res 1978; 43:503–512. [DOI] [PubMed] [Google Scholar]
- 37. Osborn JW, Fink GD, Sved AF, Toney GM, Raizada MK.. Circulating angiotensin II and dietary salt: converging signals for neurogenic hypertension. Curr Sci Inc 2007; 9:228–235. [DOI] [PubMed] [Google Scholar]
- 38. Kopkan L, Huskov Á. Z, Jíchová Š, Červenková L, Červenka L, Saifudeen Z, El-Dahr SS.. Conditional knockout of collecting duct bradykinin b2 receptors exacerbates angiotensin II-induced hypertension during high salt intake. Clin Exp Hypertens 2016; 38:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen A, Huang BS, Wang H-W, Ahmad M, Leenen FHH.. Knockdown of mineralocorticoid or angiotensin II type 1 receptor gene expression in the paraventricular nucleus prevents angiotensin II hypertension in rats. J Physiol (London) 2014; 592:3523–3536. 10.1113/jphysiol.2014.275560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen J, Gomez-Sanchez CE, Penman A, May PJ, Gomez-Sanchez E.. Expression of mineralocorticoid and glucocorticoid receptors in preautonomic neurons of the rat paraventricular nucleus. Am Physiol Regul Integr Comp Physiol 2014; 306:R328–R340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang HW, Huang BS, White RA, Chen A, Ahmad M, Leenen FH.. Mineralocorticoid and angiotensin II type 1 receptors in the subfornical organ mediate angiotensin II-induced hypothalamic reactive oxygen species and hypertension. Neuroscience 2016; 329:112–121. [DOI] [PubMed] [Google Scholar]
- 42. Huang BS, White RA, Jeng AY, Leenen FHH.. Role of central nervous system aldosterone synthase and mineralocorticoid receptors in salt-induced hypertension in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 2009; 296:R994–R1000. [DOI] [PubMed] [Google Scholar]
- 43. Gomez-Sanchez EP, Gomez-Sanchez CM, Plonczynski M, Gomez-Sanchez CE.. Aldosterone synthesis in the brain contributes to Dahl salt-sensitive rat hypertension. Exp Physiol 2010; 95:120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang Y, Chen L, Wier WG, Zhang J.. Intravital Förster resonance energy transfer imaging reveals elevated [Ca2+]i and enhanced sympathetic tone in femoral arteries of angiotensin II-infused hypertensive biosensor mice. J Physiol 2013;591:5321–5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ye P, Kenyon CJ, MacKenzie SM, Seckl JR, Fraser R, Connell JMC, Davies E.. Regulation of aldosterone synthase gene expression in the rat adrenal gland and central nervous system by sodium and angiotensin II. Endocrinology 2003; 144:3321–3328. [DOI] [PubMed] [Google Scholar]
- 46. Fuchs-Hammoser R, Schweiger M, Oelkers W.. The effect of chronic low-dose infusion of ACTH (1-24) on renin, renin-substrate, aldosterone and other corticosteroids in sodium replete and deplete man. Acta Endocrinol (Copenh) 1980; 95:198–206. [DOI] [PubMed] [Google Scholar]
- 47. Holland OB, Carr B.. Modulation of aldosterone synthase messenger ribonucleic acid levels by dietary sodium and potassium and by adrenocorticotropin. Endocrinology 1993; 132:2666–2673. 10.1210/endo.132.6.8389287 [DOI] [PubMed] [Google Scholar]
- 48. Pojoga L, Kolatkar NS, Williams JS, Perlstein TS, Jeunemaitre X, Brown NJ, Hopkins PN, Raby BA, Williams GH.. Beta-2 adrenergic receptor diplotype defines a subset of salt-sensitive hypertension. Hypertension 2006; 48:892–900. [DOI] [PubMed] [Google Scholar]
- 49. Ehrhart-Bornstem M, Bornstein SR, González-Hernández J, Holst JJ, Waterman MR, Scherbaum WA.. Sympathoadrenal regulation of adrenocortical steroidogenesis. Endocr Res 1995; 21:13–24. [DOI] [PubMed] [Google Scholar]
- 50. Manunta P, Ferrandi M, Bianchi G, Hamlyn JM.. Endogenous ouabain in cardiovascular function and disease. J Hypertens 2009; 27:9–18. 10.1097/HJH.0b013e32831cf2c6 [DOI] [PubMed] [Google Scholar]
- 51. Tamura M, Piston DW, Tani M, Naruse M, Landon EJ, Inagami T.. Ouabain increases aldosterone release from bovine adrenal glomerulosa cells: role of renin-angiotensin system. Am J Physiol-Endo Met 1996; 270:E27–E35. [DOI] [PubMed] [Google Scholar]
- 52. Payet N, Lehoux JG.. A comparative study of the role of vasopressin and ACTH in the regulation of growth and function of rat adrenal glands. J Steroid Biochem 1980; 12:461–467. 10.1016/0022-4731(80)90307-6 [DOI] [PubMed] [Google Scholar]
- 53. Hamlyn JM, Blaustein MP.. Endogenous ouabain recent advances and controversies. Hypertension 2016; 68:526–532. 10.1161/HYPERTENSIONAHA.116.06599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sophocleous A, Elmatzoglou I, Souvatzoglou A.. Circulating endogenous digitalis-like factor (s)(EDLF) in man is derived from the adrenals and its secretion is ACTH-dependent. J Endocrinol Invest 2003; 26:668–674. [DOI] [PubMed] [Google Scholar]
- 55. Blaustein MP, Leenen FHH, Chen L, Golovina VA, Hamlyn JM, Pallone TL, Van Huysse JW, Zhang J, Wier WG.. How NaCl raises blood pressure: a new paradigm for the pathogenesis of salt-dependent hypertension. Am J Physiol Heart Circ Physiol 2012; 302:H1031–H1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Leenen FHH. The central role of the brain aldosterone-ouabain pathway in salt-sensitive hypertension. Biochim Biophys Acta 2010; 1802:1132–1139. 10.1016/j.bbadis.2010.03.004 [DOI] [PubMed] [Google Scholar]
- 57. Leenen FHH, Huang BS, Harmsen E.. Role of brain ouabain-like activity in the central effects of sodium in rats. J Cardiovasc Pharmacol 1993; 22:S72–S74. [DOI] [PubMed] [Google Scholar]
- 58. Tentori S, Messaggio E, Brioni E, Casamassima N, Simonini M, Zagato L, Hamlyn JM, Manunta P, Lanzani C.. Endogenous ouabain and aldosterone are coelevated in the circulation of patients with essential hypertension. J Hypertens 2016; 34:2074–2080. [DOI] [PubMed] [Google Scholar]
- 59. Zulian A, Linde CI, Pulina MV, Baryshnikov SG, Papparella I, Hamlyn JM, Golovina VA.. Activation of c-SRC underlies the differential effects of ouabain and digoxin on Ca2+ signaling in arterial smooth muscle cells. Am J Physiol Cell Physiol 2013;304:C324–C333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Chen L, Song H, Wang Y, Lee JC, Kotlikoff MI, Pritchard TJ, Paul RJ, Zhang J, Blaustein MP.. Arterial α2-Na+ pump expression influences blood pressure: lessons from novel, genetically engineered smooth muscle-specific α2mice. Am J Physiol Heart Circ Physiol 2015;309:H958–H968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Iwamoto T, Kita S, Zhang J, Blaustein MP, Arai Y, Yoshida S, Wakimoto K, Komuro I, Katsuragi T.. Salt-sensitive hypertension is triggered by Ca2+ entry via Na+/Ca2+ exchanger type-1 in vascular smooth muscle. Nat Med 2004;10:1193–1199. [DOI] [PubMed] [Google Scholar]
- 62. Chen L, Hamlyn JM, Blaustein MP.. Abstract P145: immuno-neutralization of endogenous ouabain lowers blood pressure in Angiotensin II-dependent models. Hypertension 2015; 66:AP145–AP145. [Google Scholar]
- 63. Pullen MA, Brooks DP, Edwards RM.. Characterization of the neutralizing activity of digoxin-specific Fab toward ouabain-like steroids. J Pharmacol Exp Ther 2004; 310:319–325. 10.1124/jpet.104.065250 [DOI] [PubMed] [Google Scholar]
- 64. Pullen MA, Harpel MR, Danoff TM, Brooks DP.. Comparison of non-digitalis binding properties of digoxin-specific Fabs using direct binding methods. J Immunol Methods 2008; 336:235–241. 10.1016/j.jim.2008.05.005 [DOI] [PubMed] [Google Scholar]
- 65. Kleyman TR, Cragoe EJ.. Amiloride and its analogs as tools in the study of ion transport. J Membrain Biol 1988; 105:1–21. 10.1007/BF01871102 [DOI] [PubMed] [Google Scholar]
- 66. Connell JMC, MacKenzie SM, Freel EM, Fraser R, Davies E.. A lifetime of aldosterone excess: long-term consequences of altered regulation of aldosterone production for cardiovascular function. Endocri Rev 2008; 29:133–154. [DOI] [PubMed] [Google Scholar]
- 67. Lanzani C, Gatti G, Citterio L, Messaggio E, Carpini SD, Simonini M, Casamassima N, Zagato L, Brioni E, Hamlyn JM.. Lanosterol synthase gene polymorphisms and changes in endogenous ouabain in the response to low sodium intake. Hypertension 2016; 67:342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Leenen FHH, Yuan B.. Prevention of hypertension by irbesartan in Dahl S rats relates to central AT1 receptor blockade. Hypertension 2001; 37:981–984. 10.1161/01.HYP.37.3.981 [DOI] [PubMed] [Google Scholar]
- 69. Raheja P, Price A, Wang Z, Arbique D, Adams-Huet B, Auchus RJ, Vongpatanasin W.. Spironolactone prevents chlorthalidone induced sympathetic activation and insulin resistance in hypertensive patients. Hypertension 2012; 60:319–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ruzicka M, Floras J, McReynolds AG, Coletta E, Haddad H, Davies R, Leenen FHH.. Do high doses of AT1 receptor blockers attenuate central sympathetic outflow in humans with chronic heart failure? Clin Sci 2013;124:589–695. [DOI] [PubMed] [Google Scholar]
- 71. Bodineau L, Frugiere A, Marc Y, Inguimbert N, Fassot C, Balavoine F, Roques B, Llorens-Cortes C.. Orally active aminopeptidase A inhibitors reduce blood pressure: a new strategy for treating hypertension. Hypertension 2008; 51:1318–1325. [DOI] [PubMed] [Google Scholar]
- 72. Balavoine F, Azizi M, Bergerot D, De Mota N, Patouret R, Roques BP, Llorens-Cortes C.. Randomized, double-blind, placebo-controlled, dose escalating Phase I study of QGC001, a centrally acting aminopeptidase A inhibitor pro-drug. Clin Pharmacokinet 2014; 53:385–395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.