Abstract
Vascular dysfunction and impaired endothelial mediated relaxation are powerful underlying abnormalities in the pathogenesis of hypertension, coronary heart disease, and stroke. Obesity, type 2 diabetes mellitus, and other metabolic abnormalities are associated with activation of mineralocorticoid receptor (MRs) in the vasculature and adipose tissue. While MR signaling is involved in the normal physiological differentiation and maturation of adipocyte, enhanced activation of MRs also contributes to increase oxidative stress, release of pro-inflammatory adipokines, and dysregulation of adipocyte autophagy. This, in turn, increases the maladaptive expansion of subcutaneous, visceral and perivascular adipose tissue, resulting in systemic and cardiovascular (CV) insulin resistance and increased CV stiffness and impaired vascular and cardiac relaxation. This review summarizes the normal role of MR activation in adipose tissues and explores the mechanisms by which excessive MR activation mediates adipose tissue inflammation and vascular dysfunction. Potential preventative and therapeutic strategies directed in the prevention of MR activation and CV disease are also discussed.
Keywords: Obesity, Adipocyte tissue, Mineralocorticoid receptors, Adipokines, Metabolic disorders, Cardiovascular disease
1. Introduction
Increased activation of the systemic and tissue renin–angiotensin-aldosterone system (RAAS) exists in states of obesity and insulin resistance, and this activation plays an important role in the development of hypertension, coronary heart disease, heart failure, and overall cardiovascular disease (CVD). Data from epidemiological studies and randomized controlled clinical trials support the notion that elevated aldosterone levels and associated mineralocorticoid receptor (MR) activation increase CVD risk, and that MR antagonists (MRAs) reduce CVD events.1 For example, the RALES, EPHESUS, and EMPHASIS clinical trials have demonstrated that MRA administration decreases morbidity and mortality in patients with heart failure.2 Further, the Joint National Committee-8 and the European Society of Hypertension recommend that MRAs may be considered as the ‘fourth-line’ drug therapy in the treatment of the resistant hypertension following standard multi-drug regimen including diuretic, angiotensin-converting enzyme (ACE) inhibitor, or angiotensin receptor blocker and calcium (Ca2+) channel blocker.3 Classically, MRAs may exert their antihypertensive effects through diuresis and reduction of sympathetic activity, vascular tone, and vascular stiffness. In this regard, aldosterone is traditionally regarded as the primary ligand for MRs and has been considered a ‘renal’ hormone that modulates plasma volume, electrolyte homeostasis, and blood pressure.4,5 However, aldosterone also has extra-renal actions since MRs also exists in other tissues including heart, vessels, brain, immune cells, and adipose tissue.4,5 Studies conducted by our group6 and others2 have demonstrated that cell-specific MR activation in adipocytes, endothelial cells (ECs), vascular smooth muscle cells (VSMCs), cardiomyocytes, and macrophages may impair insulin metabolic signaling and prompt oxidative stress and inflammation, resulting in adipose and cardiovascular (CV) tissue stiffness in conjunction with insulin resistance and associated CVD. However, the precise mechanisms by which enhanced activation of MRs promotes dysfunctional cross-talk between adipocytes, ECs, VSMCs, cardiomyocytes, and macrophages in obese persons with insulin resistance and T2DM remains to be elucidated. In this review, we focus on recent studies exploring the complex interplay of cell specific MR activation in obesity and vascular dysfunction and the contemporary understanding of potential therapeutic strategies that address this dysfunctional MR activation.
2. Aldosterone and MR in adipose tissue
Adipose tissue not only serves as a site for fat storage of triglycerides but is also a metabolically active endocrine organ that synthesizes and secretes hormones, such as aldosterone, which has extra-renal actions mediated by MR activation. Vascular tissues, including both endothelial and smooth muscle cells, express the enzyme 11-beta hydroxysteroid dehydrogenase 2 (11βHSD2) that allows aldosterone to selectively activate MR by inactivating cortisol.6 This contrasts with cardiomyocytes, immune cells and adipose tissue where the main ligand for MRs is cortisol.6
2.1. MR signaling in adipose tissue
Although aldosterone is primarily synthesized in adrenocortical cells of the zona glomerulosa of the adrenal cortex, it is also produced by adipocytes, including perivascular adipose tissue (PVAT), where it exerts autocrine and paracrine effects resulting in vascular remodeling and dysfunction. Furthermore, human adipocytes secrete mineralocorticoid-releasing factors which increase aldosterone secretion.7 Angiotensin II, including that produced by adipocytes, induces the expression of aldosterone synthase (CYP11B2) and prompts the adipocyte-secretion of aldosterone in a calcineurin/nuclear factor of activated T (NFAT)-dependent manner.8 In addition, various factors such as cholesteryl ester-transfer protein (CETP) inhibitors increase aldosterone biosynthesis in adipocytes in a Nox-dependent manner.9 Upon being activated, the MR is translocated to the nucleus and further regulates gene transcription and translation of proteins such as adipokines and serum-and-glucocorticoid-induced protein kinase 1 (SGK1) by binding to DNA hormone/steroid stimulatory or negative response elements.10 Aldosterone also exerts rapid non-genomic effects that mediate CV tissue remodeling by the activation of extracellular receptor kinase, Rho kinase, and protein kinase C, thereby resulting in increased cytosolic Ca2+ and reactive oxygen species (ROS) production, and endothelial sodium channel (EnNAC) activation, which, in turn, promotes tissue remodeling and vascular stiffening.6,10
2.2. MR activation regulates adipose differentiation
Adipocyte tissue consists mainly of adipocytes interspersed with fibroblasts, immune cells, multipotent stem cells, connective tissue, vessels, and nerves.4,5 Pre-adipocytes can be induced to develop into mature adipocytes through the adipocyte proliferation and differentiation that dictate their different physiological and pathological properties. Adipocyte tissue is generally classified into three main types: white adipocyte tissue (WAT), brown adipocyte tissue (BAT), and beige or brite adipocyte tissue which contains interspersed brown and white adipocytes while maintaining many of the characteristics of BAT.11 Peripheral subcutaneous adipose tissue encompasses ∼80% of total body fat in healthy individuals.12 Another 10% of fat tissue is present as either abdominal visceral adipose tissue or that in and around organs including the vasculature, heart, liver, and kidneys.13 Typically, visceral and abdominal subcutaneous adipose tissue is expanded in obesity, and this is associated with the development of insulin resistance in rodents and humans.11 The abdominal aorta, mesenteric and femoral arteries are traditionally surrounded by WAT.11 While WAT is the primary site for its energy storage and expansion, this fat is associated with inflammation, insulin resistance, and development of the MS,11 BAT has beneficial functions, including production of heat through non-shivering thermogenesis that is mediated by uncoupling protein 1 (UCP1) activity.14 In this regard, The functional BAT in humans is typically presented in the portions of cervical, paravertebral, supraclavicular, interscapular, and mediastinal areas.15 The beige adipose tissue is usually locate in the inguinal region interspersed with WAT.15
Both aldosterone and cortisol can activate MRs and induce direct and non-direct transcriptional effects in various cells in adipose tissue. The action of glucocorticoid receptors on adipose tissue was first reported in 1977,16 and adipose tissue MRs were described in 1998.17 The relevance of MRs in adipose tissue was ascertained using a transgenic mice model expressing the SV40 large T antigen that overexpresses MR promoter activity and induces liposarcoma development, thus demonstrating that MR was transcriptionally active in adipocytes and providing a new model to explore molecular mechanisms of mineralocorticoid action.17 Enhanced MR activation has been found to induce the expression of adipocyte markers and promote adipogenesis. To this point, aldosterone induces increases in WAT, characterized by the accumulation of intracytoplasmic lipid droplets and intracellular triglyceride induction.17 Aldosterone also inhibits expression and activity of UCP1 in adipocytes.18 Furthermore, MRAs reduce adipocyte differentiation in both 3T3L1 cells and primary human adipocytes, and MR knockout mice show defective adipogenesis.19 MRA also induces browning of WAT through impairment of autophagy and conversion of WAT into thermogenic fat. Indeed, MRA administration prevents adipocyte dysfunction in diet-induced obesity.20 Collectively, these studies demonstrate a major role of MR in the regulation of adipose tissue differentiation and relative expansion of WAT relative to BAT.
2.3. MR activation regulates adipogenesis
Adipogenesis is modulated by various transcription factors including the peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT-enhancer-binding protein (C/EBP)β, C/EBP δ, and C/EBPα (Figure 2).21 Adiponectin, fatty acid synthase, and fatty acid binding protein 4 induce and regulate the late stages of adipocyte maturation.21 Enhanced activation of the RAAS, including that of MRs in adipose tissue, induces adipogenesis through stimulation of the mammalian target of rapamycin (mTOR)/ribosomal S6 kinase (S6K1) signaling pathway which, in turn, activates PPARγ and C/EBPα signaling, thereby promoting adipogenesis under condition of over-nutrition and diet induced obesity (Figure 2).22 To this point, mTOR1 and its complexes positively activate the sterol regulatory element-binding proteins to promote adipogenesis in both an mTOR/S6K1-dependent and mTOR/S6K1-independent manner.23 Meanwhile, mTORC2 also modulates the activity of protein kinase B (Akt) and plays an important role in the early stages of adipogenesis.21,23 Furthermore, increased accumulation of triacylglycerol, translocation of glucose transporter type 4 (GLUT4) to the plasma membrane, and activation of the enzyme glycerol 3 phosphate dehydrogenase promote adipogenesis.24 The activation of MR in adipogenesis of BAT or beige is still uncertain. One study found that MRA induced increases in brown adipocyte-specific transcripts and UCP1 level in visceral and inguinal fat depots, suggesting that inhibition of MR signaling induces the ‘browning’ of WAT. Therefore, activation of MR might have a different role in the adipogenesis of WAT and BAT.20
Figure 2.
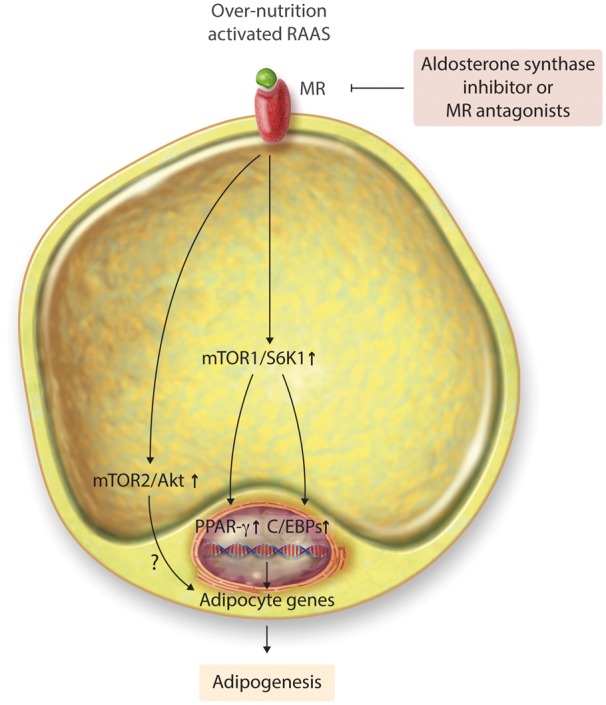
MR mediates chronic over-nutrition and activated renin–angiotensin-aldosterone system-induced adipogenesis through mTOR1/S6K1 signaling pathways. MR, mineralocorticoid receptor; mTOR, rapamycin; S6K, ribosomal S6 kinase; Akt, protein kinase B; PPAR-γ, peroxisome proliferator-activated receptor γ; C/EBPs, CCAAT-enhancer-binding proteins.
2.4. Adipose-derived factors prompts aldosterone secretion and MR activation
Elevated aldosterone levels and enhanced MR activity occur in obese patients with insulin resistance and hypertension.5 Interestingly, recent studies suggest that an adipocyte-derived factor may stimulate adipocyte aldosterone synthesis and MR activation in an angiotensin independent manner.25 For example, obese patients usually present with hypertension, glucose intolerance, and a metabolic dyslipidemia. Most importantly, high leptin levels associated with adipose tissue expansion are positively associated with increases in blood pressure and aldosterone levels in patients with resistant hypertension.5 One study found that aldosterone production by adipose tissue is increased by adipocyte-derived factors such as leptin, and this increase is not inhibited by ACE inhibitors or angiotensin II receptor antagonists.25 Indeed, leptin directly promotes CYP11B2 expression and enhances aldosterone production via a Ca2+-dependent mechanism.26 Further, this increased adipocyte production of aldosterone is accompanied by vascular dysfunction and CVD. Therefore, a direct link exists between altered adipose tissue metabolism and adipose mineralocorticoid secretion that may be responsible for obesity-related vascular and cardiac fibrosis and impaired relaxation and associated CVD.
3. Role of enhanced activation of MR in adipose tissue pathophysiology
Maladaptive adipose tissue expansion contributes to inappropriate activation of MRs and subsequently oxidative stress, release of pro-inflammatory adipokines, and dysregulation of adipocyte autophagy, resulting impaired insulin metabolic signaling and associated CVD.
3.1. Oxidative stress
Oxidative stress is increased in maladaptive adipose tissue expansion, and this is mediated by an increase in ROS production and reduction of antioxidant capacity. The Framingham study found that urinary levels of 8-epi-prostaglandin F2α (8-epi-PGF2α), a systemic oxidative stress marker, were significantly associated with body mass index in 2828 obese patients, suggesting an important role of oxidative stress in the deleterious impact of obesity related CVD.27 Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase is one of the important enzymes that is responsible for much of the generation of ROS in adipose tissue. NADPH oxidase is comprised of several cytosolic and membrane subunits that can be mobilized and activated by over-nutrition and RAAS activation. To this point, aldosterone stimulates expression of Nox2 and p22phox through an MR-dependent mechanism and expression of p47phox through both angiotensin II type 1 receptor-dependent and MR-dependent mechanisms in rat vascular tissues.28 Furthermore, both Nox1 and Nox4 are also involved in ROS-sensitive aldosterone production in cultured adipocytes.9 We previously found that oxidative stress plays a key role in impaired insulin metabolic signaling and associated cardiac dysfunction in obese rodent models.10 Indeed, MRA decreases oxidative stress, which provides protection against cardiac tissue fibrosis, remodeling, cardiac diastolic dysfunction,28 supporting a role for enhanced MR activation of NADPH oxidase in adipose tissue in promoting excess oxidative stress and associated CVD.
3.2. Adipokines
Adipose tissue is an endocrine/autocrine/paracrine organ and releases more than 600 peptide hormones that present various biological activities including regulation of appetite, energy expenditure, inflammation, insulin sensitivity, glucose and lipid metabolism, and fat distribution.29 Adipose tissue produces both pro-inflammatory and anti-inflammatory adipokines. For example, adipocytes release some protective substances such as adiponectin and omentin that help maintain normal insulin metabolic signaling and normal physiological CV structure and function (Table 1).29 However, in obese individuals, there is increased expression of pro-inflammatory cytokines, including tumour necrosis factor alpha (TNF-α), interleukin-6 (IL-6), IL-8, monocyte chemoattractant protein-1 (MCP-1), toll-like receptor-4 (TLF-4), and associated CVD (Table 1).29 Indeed, adipose tissue is composed of a variety of immune cells, such as macrophages, dendritic cells, B cells, T lymphocytes, mast cells, and neutrophils.29 To this point, macrophages are an important driver of adipose tissue inflammation and associated metabolic abnormalities and CVD. Macrophage polarization is traditionally dichotomized into M1 macrophages (F4/80+ CD11c+) and M2 macrophage phenotypes (F4/80+ CD11c- CD301+ Arg1+ CD206+).10,30 The macrophage M1 phenotype is generally associated with an increase of pro-inflammatory responses, and the macrophage M2 phenotype plays an important role in anti-inflammatory responses and tissue repair.6 Further, M2 macrophages have been showed to positively support BAT function in obese individules.10,31 Therefore, the pro-inflammatory response of the macrophage M1 phenotype in adipose tissue is important in mediating systemic inflammation and related CVD. MR activation in adipose tissue promotes adipose tissue inflammation, in part, via increasing pro-inflammatory adipokines including TNF-α, MCP-1, and IL-6 with concomitant reduction of the UCP-1 transcription and thermogenic activity in BAT.8,32 Indeed, MRA has been found to decrease the expression of TNF-α, MCP-1, IL-6, macrophage M1 marker markers CD68 and CD11c, underscoring the role of MRA in reducing adipose tissue inflammation.33 For example, eplerenone significantly reduced immune cell infiltration, including macrophage and ROSs production in 3T3-L1 adipocytes.34 Our recent data also suggest that low dose spironolactone inhibited macrophage infiltration and M1 polarization in aortic and cardiac tissue.35,36. Interestingly, cell specific MR deletion in endothelium (ECMR KO) and macrophage displayed protection against cardiac fibrosis with a reduction in macrophage M1 macrophage markers and a parallel increase in M2 markers in cardiac tissue.6,37 To this point, consumption of a western diet high in saturated fat and refined carbohydrates induced an increase in CD11b, a total macrophage marker in cardiac tissue. ECMR KO prevented this abnormality by Inhibition of pro-inflammatory cytokines (IL17 and CD11b) and an increase in macrophage M2 markers (CD206 and IL10) and thus the ratio of M2/M1 marker gene expression.6 These data suggest that cell specific MR activation affects the expression of pro-inflammatory adipokines and macrophage recruitment and thus plays an important role in the pathogenesis of the chronic inflammatory responses observed in obese individuals. However, one study found that globally overexpressing MR in the transgenic mice with high fat diet displays the beneficial metabolic effects with a trend toward higher M2 differentiation compared with wild type mice.38 Therefore, further studies are warranted to clarify the cell specific role of MR activation in the promotion of systemic inflammation and associated CVD in conditions of obesity and insulin resistance.
Table 1.
Adipokines involved in vascular function
| Proinflammatory | Anti-inflammatory | Vasorelaxation | Vasocontraction | Vasorelaxation and vasocontraction |
|---|---|---|---|---|
| Leptin | Adiponectin | Adiponectin | Ang II | Aldosterone |
| TNF-α, IL-6, IL-8, MCP-1, TLR-4 | Omentin | Omentin | Resistin | H2O2 |
| Osteopontin | M2 macrophage | Adipocyte-derived relaxing factor | Leptin | |
| M1 macrophage | Apelin |
TNF-α, tumour necrosis factor α; IL, interleukin; MCP-1, monocyte chemoattractant protein-1; TLF-4, toll-like receptor-4; Ang II, angiotensin II; H2O2, hydrogen peroxide.
Adipokines also regulate vascular function (Table 1). For example, factors such as adiponectin, omentin, and adipocyte-derived relaxing factor (ADRF) promote vasorelaxation. Adiponectin is a specific adipocyte-secreted protein from both BAT and WAT, and it promotes insulin metabolic signaling and is anti-oxidative, and anti-inflammatory.4 Adiponectin has been shown to increase endothelial nitric oxide (NO) production by binding to either the adiponectin type 2 receptor or T-cadherin on the endothelial surface,39 resulting in endothelium dependent relaxation as well as potassium (K+) channel-mediated vasorelaxation in rodent models. 39 ADRF is released from PVAT to reduce vascular contractions by promoting the opening of different K+ channels in VSMCs.40 Therefore, reduction of ADRF and other adipokines is involved in vascular dysfunction in obese individuals. Angiotensin II and resistin are adipokines that promote vasocontraction and are proatherogenic effects. Some adipokines are associated with both vasorelaxation and vasocontraction under different conditions (Table 1).41 For example, short term aldosterone treatment enhances vascular relaxation mediated by endothelium-derived NO, but longer exposure induces vasoconstriction through non-genomic signaling.42 Chronic exposure to aldosterone also induces impairment of endothelial function by reducing bioavailable NO and increasing endothelin-1 (ET-1) expression, resulting in vascular constriction via both non-genomic and genomic effects.42 H2O2 production in PVAT induces vascular relaxation by the release of vasodilating cyclooxygenase metabolites and NO from ECs and via endothelium-independent mechanisms involving activation of K+ channels on VSMCs.43 However, enhanced adipocyte MR activation induces overexpression of H2O2 and thus results impairment of vascular contraction.44 Our previous study found regional variation in obesity-induced arterial stiffening. To this point, western diet feeding increased aorta and femoral stiffness and endothelium dependent relaxation while western diet feeding increased coronary arterial vasoconstrictor responsiveness.35,45 The contractile activity of small arterial VSMCs is induced by the influx of extracellular Ca2+ that activates myosin light-chain kinase and initiates contraction.43 Very little is known about Ca2+ signaling in large artery stiffness.
3.3. Autophagy
Autophagy, an event of ‘self-eating’ cellular components, regulates cellular homeostasis through the elimination of damaged organelles, abnormal aggregated proteins, bulk cytoplasmic contents, and intracellular pathogens under physiological conditions.46 However, over-nutrition and associated obesity disrupts autophagosome maturation and inhibits autophagy through alterations of AMP-activated protein kinase (AMPK), sirtuin, and mTOR1 signaling pathways in CV tissues.47 Impaired macrophage autophagy increases macrophage M1 polarization and associated inflammatory responses in obese mice.47 Furthermore, consumption of a high fat diet increases serum levels of triglycerides and small dense low-density lipoprotein cholesterol particles that can lead to defects in autophagosome and lysosome fusion and impair macrophage autophagy.48 To this point, inhibition of autophagy leads to metabolic abnormalities by promoting hepatocyte lipid accumulation since autophagy is critical for the lipolysis of triglycerides stored in the liver.49 However, the precise effects of autophagy stimulation in adipose tissue from patients with obesity and T2DM remain unclear. Enhanced autophagy activity is usually regarded as necessary to maintain normal cell homeostasis and physiological function. In this regard, one study found that adipocyte-specific autophagy gene 7 (atg7)-knockout mice reduced WAT size with an increase in the amount of interscapular BAT.46 Moreover, WAT presented increased protein levels of UCP1 and PPARγ co-activator 1alpha (PGC1α), which are the master regulators of mitochondrial biogenesis,46 thereby suggesting that defective autophagy in obese mice decreases WAT and enhances insulin sensitivity. A recent study has shown that adipocyte MR activation by aldosterone prompts adipocyte autophagy.20 MRA reduces adipose tissue autophagic flux and induces browning of WAT in mice fed a high fat diet.20 Thus, activated MR is involved in the dysregulation of autophagy in adipose tissue that lead to metabolic abnormalities and associated CVD.
4. Activation of MRs leads to adipose tissue dysregulation and associated vascular dysfunction
4.1 Adipose tissue expansion in vascular function
There is a positive relationship between obesity, especially visceral obesity and CVD. For example, obese patients exhibit increased vascular stiffness compared with non-obese individuals, and weight loss increases vascular compliance in these obese individuals.50 A recent population study found that skin-fold thickness is a predictor of vascular stiffness in patients with hypertension.51 Furthermore, greater VAT was associated with increased dyslipidemia, increased risk of atherosclerosis and CVD in a study of 382 patients with T2DM.52 While abdominal adipose tissue expansion is associated with obesity-related metabolic abnormalities and associated CVD, lower-body (gluteal and femoral) subcutaneous adipose tissue may be protective.53 Therefore, adipose tissue expansion regulates vascular function.
4.2. Role of peri-vascular adipose tissue in vascular stiffness
PVAT, a special local deposit of adipose tissue surrounding blood vessels, provides mechanical protection, and regulation of blood vessel tone.54 PVAT is also a source of adipokines with varied paracrine effects in the different vascular beds.55,56 For example, the thoracic aorta is surrounded by both WAT and BAT, but the abdominal aorta is surrounded only by WAT.55 In rodents, femoral and mesenteric arteries are surrounded by WAT, which is traditionally expanded in obese individuals with insulin resistance and T2DM.55 In normal physiological conditions, PVAT releases vasodilator substances, including adiponectin and ADRF that promote normal vascular relaxation.57 However, in the setting of obesity, insulin resistance, and T2DM, NADPH oxidase-derived ROS is up-regulated in WAT and thereby results in an increase in pro-inflammatory adipokines including resistin, TNF-α, IL-6, and IL-8, leading to the vascular insulin resistance and impaired relaxation.55,56 Data from the Framingham Offspring and Third Generation cohorts support the notion that altered PVAT volume is linked with higher thoracic and abdominal aortic dimensions and increased stiffness even after adjusting for sex, age, and CVD risk factors including BMI and VAT volume.58 Thus, PVAT is an independent risk factor for vascular dysfunction and other CVD.
4.3. Role of MR activation in the cross-talk between adipose tissue and altered vascular function
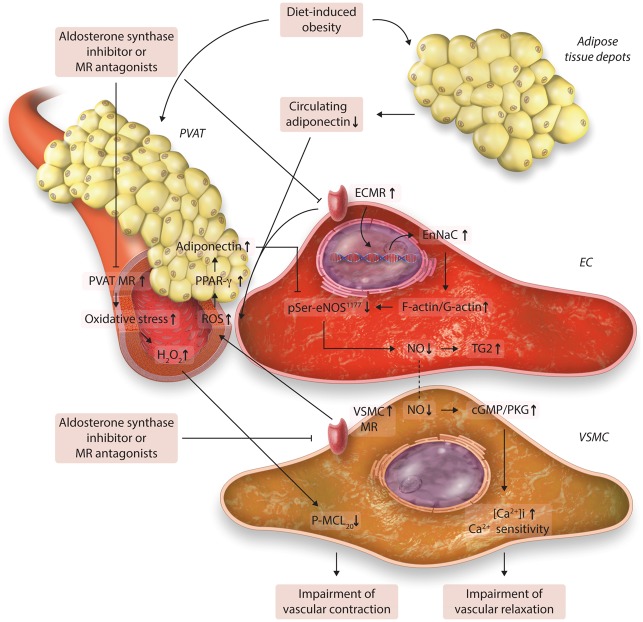
Typically, endothelial dysfunction and vascular stiffness are thought to be the earliest manifestation of vascular dysfunction in obesity and precede the development of hypertension.5 In this regard, enhanced activation of MRs in adipose tissue prompts activation of the ECMR, leading to increases in EnNaC surface abundance, intra-endothelial Na+, F actin polymerization and endothelial cortical stiffness, which in turn lead to subsequent decreases in endothelial NO synthase (eNOS) activation and NO bioavailability (Figure 1).5,10 A decrease in bioavailable NO results in activation and cellular release of tissue transglutaminase (TG2), which plays an important role in vascular and cardiac fibrosis and remodeling. To this point, TG2 is abundantly expressed in cardiac and vascular tissues.4,5 Normally, TG2 undergoes intracellular s-nitrosylation by NO, which retains TG2 within the cytosolic compartment. Reduced bioavailable NO leads to TG2 translocation to the extracellular compartment to induce crosslinking of extracellular matrix proteins, resulting in enhanced connective tissue cross linking activity and tissue fibrosis.10 Furthermore, reduced NO decreases cyclic guanosine 3′,5′-monophosphate (cGMP) and activates kinases responsible for increases in VSMC intracellular Ca2+ and Ca2+ sensitization that induces vascular constriction.5 Indeed, VSMC MR activation is also involved in the vascular dysfunction and stiffness by increasing VSMC proliferation, migration, calcification, was well as L-type Ca2+ channel activation (Figure 1).59 Resident vascular macrophage MR activation mediates macrophage M1 polarization, chemotaxis, and pro-inflammatory responses.60 It has been reported that enhanced adipocyte MR activation, not only induces significant metabolic abnormalities such as increased dyslipidemia, insulin resistance, and visceral adiposity, but also promotes altered arteriolar VSMC contractility involving up-regulation of vascular redox-sensitive protein kinase G (PKG)-1 activity and down-regulation of redox-sensitive Rho kinase (ROCK) activity in a conditionally adipocyte overexpression MR mouse model.44 The resultant changes in PKG-1 and ROCK decrease myosin light chain (MLC) kinase phosphorylation/activation and VSMC Ca2+ sensitivity contractile machinery (Figure 1).44 Moreover, enhanced vascular MR activation signaling further activates both growth signaling and the pro-fibrotic transforming growth factor beta 1 signaling pathways, leading to fibrosis and tissue remodeling.61 Thus, enhanced adipocyte MR signaling induces expression of adipocyte-derived H2O2 and an altered vascular phenotype providing insight into the complicated interaction of adipose-vascular tissue in conditions of obesity-induced MR signaling.
Figure 1.
Proposed the role of mineralocorticoid receptor mediated the signaling in the cross-talk between adipose tissue and the vascular dysfunction. ECMR increases EnNaC surface abundance, intra-endothelial sodium, F actin polymerization and cell stiffness, leading to subsequent decreases in eNOS activation and NO bioavailability. Reduced NO decreases cGMP/PKG and activates kinases responsible for promotion of VSMC levels of intracellular Ca2+ and Ca2+ sensitization that induces impairment of vascular relaxation. Meanwhile, enhanced adipocyte MR activation promotes an increase in H2O2 and impaired VSMC contractility involving down-regulation of MLC kinase phosphorylation/activation and VSMC Ca2+ sensitivity contractile machinery. Furthermore, Over-nutrition and obesity decrease circulating adiponectin and increase the activation of MRs in PVAT, ECs, and VSMCs that prompts vascular oxidative stress. The increased ROS up-regulate adiponectin expression in PVAT via a PPAR-γ with a feedback manner, resulting in increases in eNOS activity and bioavailable NO. Abbreviations: MR, mineralocorticoid receptor; PVAT, perivascular adipose tissue; ECs, endothelial cells; VSMCs, vascular smooth muscle cells; ROS, reactive oxygen species; PPAR-γ, peroxisome proliferator-activated receptor γ; NO, nitric oxide; eNOS, endothelial NO synthase; ECMR, endothelial cell mineralocorticoid receptor; EnNaC, endothelial epithelial sodium channel; cGMP/PKG, cyclic guanosine 3′,5′-monophosphate/protein kinase G; Ca2+, calcium; H2O2, hydrogen peroxide; TG2, transglutaminase 2; P-MLC20, phosphorylated myosin regulatory light chain.
Recently, studies have provided evidence that there is bidirectional cross talk from both outside-in and inside-out between the PVAT and vessels, and that altered adiponectin production may play an important role in the dysfunctional cross-talk between adipose and vascular tissue, leading to vascular dysfunction coronary artery disease patients.62,63 To this point, reduced circulating adiponectin levels have been associated with increased insulin resistance and development of vascular stiffness and atherosclerosis.64 The protective role of adiponectin is reinforced by the observation that loss of function single nucleotide polymorphism variants of the adiponectin gene may be responsible for lowered adiponectin in individuals with insulin resistance and coronary heart disease.65 Meanwhile, reduced adiponectin was independently associated with reduced bioavailable NO and increased O2(-) production/eNOS uncoupling in both internal mammary arteries and saphenous veins.62,63 Altered local adiponectin gene expression/release in PVAT was positively correlated with O2(-) and eNOS uncoupling in the vascular tissue.62 Furthermore, increased vascular oxidative stress leads to a compensatory release of peroxidation products that up-regulate adiponectin gene expression in PVAT via a PPAR-γ-dependent mechanism (Figure 1).62 The increased adiponectin further induced eNOS phosphorylation and increased tetrahydrobiopterin bioavailability and improved eNOS coupling and NO production in vascular tissues with a feedback manner (Figure 1).62,63 Therefore, reduced adiponectin in patients with obesity and T2DM induces an increase in vascular oxidative stress that increases NADPH oxidase activity and up-regulates adiponectin gene expression in PVAT and vascular tissues, suggesting that increased vascular NADPH oxidase-derived ROS from the artery wall causes ‘a rescue signal’ to increase adiponectin in PVAT. Therefore, adiponectin provides an exquisite self-regulatory mechanism under the setting of obesity and T2DM (Figure 1).
5. Role of enhanced adipocyte and vascular MR signaling in development of vascular stiffness and hypertension
There is a link between obesity, vascular stiffness, and increased blood pressure. For example, overweight or obesity was estimated to independently contribute to vascular stiffness51 and 11–17% of the total hypertension population in the multinational studies.66 Indeed, vascular stiffening, a normal aging phenomenon, is significantly linked with damage to target organs including the vasculature, heart, kidney, and brain.67 Recent studies indicate that vascular stiffness precedes hypertension and is characterized by progressive structural and functional abnormalities, which exist prior to the development of increased blood pressure.68 To this point, vascular stiffening and decreased compliance are associated with increases in systolic blood pressure and decreases in diastolic blood pressure, resulting in increased pulse pressure and pulse wave velocity. There are numerous factors that promote obesity-induced vascular stiffness and hypertension, including pro-inflammatory adipokines, oxidative stress, MR activation, as well as dysregulation of adipocyte apoptosis, autophagy, and immune modulation.4 For example, expanded adipose tissue increases leptin, which is related to increased adrenal and fat production of aldosterone that induces vascular injury, inflammation, fibrosis, remodeling, and dysfunction.26 Leptin also directly induces vascular oxidative stress, pro-inflammatory responses, as well as vascular stiffness and subsequent hypertension that are associated with obesity.69 We recently reported that MRA with very low dose spironolactone administered to female mice prevents development of vascular insulin resistance and stiffness in a very translational model of obesity-induced CV dysfunction.35 This suggests a role for cell specific MR activation in the pathogenesis of aortic stiffness. ECMR activation was associated with pathological changes in immune responses, oxidative stress, vascular cell dysfunction and structural remodeling.35 Other factors such as VSMC calcification, increased vascular extracellular matrix, elastin dysfunction also contribute to vascular dysfunction.10
6. Therapeutic strategies in prevention of MR activation in obese patients with vascular dysfunction
MRA includes spironolactone, eplerenone, and canrenoate. While spironolactone and eplerenone are oral agents, canrenoate is available in both intravenous and oral formulations. Spironolactone and eplerenone have been widely studied in large randomized controlled trials, and MRA decreases morbidity and mortality in patients with heart failure.2 Treatment with MRA reduces blood pressure in patients with hypertension, especially in resistant hypertension.3 Spironolactone treatment prevented vascular fibrosis and remodeling independently of lowing blood pressure in spontaneously hypertensive rats.70 Our recent data also suggest that low dose spironolactone is a potential strategy in the prevention of obesity-induced vascular inflammation, oxidative stress, and vascular stiffening.35 Meanwhile, MRA regulates the differentiation of 3T3-L1 adipocytes via of DNA synthesis and PPARγ expression, leading to inhibition of clonal expansion and interference with the transcriptional control in adipose tissue.19 MRA also inhibits the pro-inflammatory adipokine expression and increases adipose tissue insulin sensitivity in diabetic mice71 and mice fed high-fat diets.35 Furthermore, MRA increases the expression of brown fat-specific transcripts with up-regulation of UCP-1 through a direct control of autophagic rate in WAT.20 These data demonstrated that the inhibition of MR activation plays a key role in maintaining normal adipose and vascular tissue physiology and function (Figure 2).
Lifestyle modifications including caloric restriction, weight loss, and aerobic physical exercise can positively improve metabolic function and prevent insulin resistance, T2DM and associated CVD.72 In a randomized controlled clinical trial, long term aerobic exercise has positive effects on systolic and diastolic blood pressure and heart rate in patients with T2DM. Exercise can improve cardiac function and increase left ventricular ejection fraction by 2–5% in patients with obesity and T2DM.72 FAD286, an aldosterone synthase inhibitor, has been reported to reduce vascular inflammation and atherosclerosis and apolipoprotein E-deficient mice (Figures 1 and 2).73 In db/db and ob/ob mice, pharmacological treatment with MRA such as eplerenone improved insulin sensitivity and reversed the obesity-related changes in adipose tissue gene expression (Figure 2).33 Other therapies such as lipid lowering drugs, β-adrenergic blockers, and RAAS inhibitors are regarded as effective strategies in the prevention of vascular insulin resistance and dysfunction.4 Recently, the therapy in modulating adipokines is also proposed as a novel pharmacologic strategy in obesity-related CVD.74 Therefore, further studies are needed to show the potential of these therapeutically approaches in the prevention of obesity-related disorders.
7. Conclusion
The expression of MR in tissues such as the adipose tissue and blood vessels and their consequent effects upon activation have extended the traditional notion of aldosterone mediated MR activation beyond that of epithelial tissue. Thus, activated MRs in adipose tissue as well as CV tissue are not only linked to metabolic disorders, inflammation, and oxidative stress but also to adipocyte and endothelial dysfunction, increased vascular stiffness, and hypertension. Therefore, diet-induced obesity and associated MR activation is an important and independent risk factor in the pathogenesis of vascular stiffness and impaired vascular relaxation that are predictive for development of CVD. A deeper understanding of the molecular mechanisms in MR activation and responses in the bidirectional cross-talk between adipose and vascular tissue may lead to the discovery of clinically effective targets for preventing obesity related CVD.
Acknowledgement
The authors would like to thank Brenda Hunter for her editorial assistance.
Conflict of interest: none declared.
Funding
J.R.S. received funding from NIH (R01 HL73101-01A and R01 HL107910-01) and the Veterans Affairs Merit System (0018). G.J. received funding from American Diabetes Association (Innovative Basic Science Award #1-17-IBS-201).
References
- 1. Ivanes F, Susen S, Mouquet F, Pigny P, Cuilleret F, Sautiere K, Collet JP, Beygui F, Hennache B, Ennezat PV, Juthier F, Richard F, Dallongeville J, Hillaert MA, Doevendans PA, Jude B, Bertrand M, Montalescot G, Van Belle E.. Aldosterone, mortality, and acute ischaemic events in coronary artery disease patients outside the setting of acute myocardial infarction or heart failure. Eur Heart J 2012;33:191–202. [DOI] [PubMed] [Google Scholar]
- 2. Shen JZ, Young MJ.. Corticosteroids, heart failure, and hypertension: a role for immune cells? Endocrinology 2012;153:5692–5700. [DOI] [PubMed] [Google Scholar]
- 3. Glicklich D, Frishman WH.. Drug therapy of apparent treatment-resistant hypertension: focus on mineralocorticoid receptor antagonists. Drugs 2015;75:473–485. [DOI] [PubMed] [Google Scholar]
- 4. Jia G, Aroor AR, Martinez-Lemus LA, Sowers JR.. Overnutrition, mTOR signaling, and cardiovascular diseases. Am J Physiol Regul Integr Comp Physiol 2014;307:R1198–R1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jia G, DeMarco VG, Sowers JR.. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat Rev Endocrinol 2016;12:144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jia G, Habibi J, DeMarco VG, Martinez-Lemus LA, Ma L, Whaley-Connell AT, Aroor AR, Domeier TL, Zhu Y, Meininger GA, Barrett Mueller K, Jaffe IZ, Sowers JR.. Endothelial mineralocorticoid receptor deletion prevents diet-induced cardiac diastolic dysfunction in females. Hypertension 2015;66:1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ehrhart-Bornstein M, Lamounier-Zepter V, Schraven A, Langenbach J, Willenberg HS, Barthel A, Hauner H, McCann SM, Scherbaum WA, Bornstein SR.. Human adipocytes secrete mineralocorticoid-releasing factors. Proc Natl Acad Sci U S A 2003;100:14211–14216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dinh Cat AN, Friederich-Persson M, White A, Touyz RM.. Adipocytes, aldosterone and obesity-related hypertension. J Mol Endocrinol 2016;57:F7–F21. [DOI] [PubMed] [Google Scholar]
- 9. Rios FJ, Neves KB, Nguyen Dinh Cat A, Even S, Palacios R, Montezano AC, Touyz RM.. Cholesteryl ester-transfer protein inhibitors stimulate aldosterone biosynthesis in adipocytes through Nox-dependent processes. J Pharmacol Exp Ther 2015;353:27–34. [DOI] [PubMed] [Google Scholar]
- 10. Jia G, Habibi J, Aroor AR, Martinez-Lemus LA, DeMarco VG, Ramirez-Perez FI, Sun Z, Hayden MR, Meininger GA, Mueller KB, Jaffe IZ, Sowers JR.. Endothelial mineralocorticoid receptor mediates diet-induced aortic stiffness in females. Circ Res 2016;118:935–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pellegrinelli V, Carobbio S, Vidal-Puig A.. Adipose tissue plasticity: how fat depots respond differently to pathophysiological cues. Diabetologia 2016;59:1075–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee MJ, Wu Y, Fried SK.. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med 2013;34:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shen W, Wang Z, Punyanita M, Lei J, Sinav A, Kral JG, Imielinska C, Ross R, Heymsfield SB.. Adipose tissue quantification by imaging methods: a proposed classification. Obes Res 2003;11:5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chouchani ET, Kazak L, Jedrychowski MP, Lu GZ, Erickson BK, Szpyt J, Pierce KA, Laznik-Bogoslavski D, Vetrivelan R, Clish CB, Robinson AJ, Gygi SP, Spiegelman BM.. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature 2016;532:112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gil A, Olza J, Gil-Campos M, Gomez-Llorente C, Aguilera CM.. Is adipose tissue metabolically different at different sites? Int J Pediatr Obes 2011;6:13–20. [DOI] [PubMed] [Google Scholar]
- 16. Feldman D, Loose D.. Glucocorticoid receptors in adipose tissue. Endocrinology 1977;100:398–405. [DOI] [PubMed] [Google Scholar]
- 17. Zennaro MC, Le Menuet D, Viengchareun S, Walker F, Ricquier D, Lombès M.. Hibernoma development in transgenic mice identifies brown adipose tissue as a novel target of aldosterone action. J Clin Invest 1998;101:1254–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marzolla V, Armani A, Zennaro MC, Cinti F, Mammi C, Fabbri A, Rosano GM, Caprio M.. The role of the mineralocorticoid receptor in adipocyte biology and fat metabolism. Mol Cell Endocrinol 2012;350:281–288. [DOI] [PubMed] [Google Scholar]
- 19. Caprio M, Antelmi A, Chetrite G, Muscat A, Mammi C, Marzolla V, Fabbri A, Zennaro MC, Feve B.. Antiadipogenic effects of the mineralocorticoid receptor antagonist drospirenone: potential implications for the treatment of metabolic syndrome. Endocrinology 2011;152:113–125. [DOI] [PubMed] [Google Scholar]
- 20. Armani A, Cinti F, Marzolla V, Morgan J, Cranston GA, Antelmi A, Carpinelli G, Canese R, Pagotto U, Quarta C, Malorni W, Matarrese P, Marconi M, Fabbri A, Rosano G, Cinti S, Young MJ, Caprio M.. Mineralocorticoid receptor antagonism induces browning of white adipose tissue through impairment of autophagy and prevents adipocyte dysfunction in high-fat-diet-fed mice. FASEB J 2014;28:3745–3757. [DOI] [PubMed] [Google Scholar]
- 21. Yoon MS, Zhang C, Sun Y, Schoenherr CJ, Chen J.. Mechanistic target of rapamycin controls homeostasis of adipogenesis. J Lipid Res 2013;54:2166–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kim JA, Jang HJ, Martinez-Lemus LA, Sowers JR.. Activation of mTOR/p70S6 kinase by ANG II inhibits insulin-stimulated endothelial nitric oxide synthase and vasodilation. Am J Physiol Endocrinol Metab 2012;302:E201–E208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Haissaguerre M, Saucisse N, Cota D.. Influence of mTOR in energy and metabolic homeostasis. Mol Cell Endocrinol 2014;397:67–77. [DOI] [PubMed] [Google Scholar]
- 24. Rodríguez A, Ezquerro S, Méndez-Giménez L, Becerril S, Frühbeck G.. Revisiting the adipocyte: a model for integration of cytokine signaling in the regulation of energy metabolism. Am J Physiol Endocrinol Metab 2015;309:E691–E714. [DOI] [PubMed] [Google Scholar]
- 25. Nagase M, Yoshida S, Shibata S, Nagase T, Gotoda T, Ando K, Fujita T.. Enhanced aldosterone signaling in the early nephropathy of rats with metabolic syndrome: possible contribution of fat-derived factors. J Am Soc Nephrol 2006;17:3438–3446. [DOI] [PubMed] [Google Scholar]
- 26. Huby A-C, Antonova G, Groenendyk J, Gomez-Sanchez CE, Bollag WB, Filosa JA, Belin de Chantemèle EJ.. Adipocyte-derived hormone leptin is a direct regulator of aldosterone secretion, which promotes endothelial dysfunction and cardiac fibrosis. Circulation 2015;132:2134–2145. [DOI] [PubMed] [Google Scholar]
- 27. Keaney JF Jr, Larson MG, Vasan RS, Wilson PW, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ, Framingham S.. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol 2003;23:434–439. [DOI] [PubMed] [Google Scholar]
- 28. Hirono Y, Yoshimoto T, Suzuki N, Sugiyama T, Sakurada M, Takai S, Kobayashi N, Shichiri M, Hirata Y.. Angiotensin II receptor type 1-mediated vascular oxidative stress and proinflammatory gene expression in aldosterone-induced hypertension: the possible role of local renin–angiotensin system. Endocrinology 2007;148:1688–1696. [DOI] [PubMed] [Google Scholar]
- 29. Fasshauer M, Bluher M.. Adipokines in health and disease. Trends Pharmacol Sci 2015;36:461–470. [DOI] [PubMed] [Google Scholar]
- 30. Apostolopoulos V, de Courten MP, Stojanovska L, Blatch GL, Tangalakis K, de Courten B.. The complex immunological and inflammatory network of adipose tissue in obesity. Mol Nutr Food Res 2016;60:43–57. [DOI] [PubMed] [Google Scholar]
- 31. Fujisaka S, Usui I, Bukhari A, Ikutani M, Oya T, Kanatani Y, Tsuneyama K, Nagai Y, Takatsu K, Urakaze M, Kobayashi M, Tobe K.. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes 2009;58:2574–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kargi AY, Iacobellis G.. Adipose tissue and adrenal glands: novel pathophysiological mechanisms and clinical applications. Int J Endocrinol 2014;2014:614074.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guo C, Ricchiuti V, Lian BQ, Yao TM, Coutinho P, Romero JR, Li J, Williams GH, Adler GK.. Mineralocorticoid receptor blockade reverses obesity-related changes in expression of adiponectin, peroxisome proliferator-activated receptor-gamma, and proinflammatory adipokines. Circulation 2008;117:2253–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hirata A, Maeda N, Nakatsuji H, Hiuge-Shimizu A, Okada T, Funahashi T, Shimomura I.. Contribution of glucocorticoid-mineralocorticoid receptor pathway on the obesity-related adipocyte dysfunction. Biochem Biophys Res Commun 2012;419:182–187. [DOI] [PubMed] [Google Scholar]
- 35. DeMarco VG, Habibi J, Jia G, Aroor AR, Ramirez-Perez FI, Martinez-Lemus LA, Bender SB, Garro M, Hayden MR, Sun Z, Meininger GA, Manrique C, Whaley-Connell A, Sowers JR.. Low-dose mineralocorticoid receptor blockade prevents western diet-induced arterial stiffening in female mice. Hypertension 2015;66:99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bostick B, Habibi J, DeMarco VG, Jia G, Domeier TL, Lambert MD, Aroor AR, Nistala R, Bender SB, Garro M, Hayden MR, Ma L, Manrique C, Sowers JR.. Mineralocorticoid receptor blockade prevents Western diet-induced diastolic dysfunction in female mice. Am J Physiol Heart Circ Physiol 2015;308:H1126–H1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rickard AJ, Morgan J, Tesch G, Funder JW, Fuller PJ, Young MJ.. Deletion of mineralocorticoid receptors from macrophages protects against deoxycorticosterone/salt-induced cardiac fibrosis and increased blood pressure. Hypertension 2009;54:537–543. [DOI] [PubMed] [Google Scholar]
- 38. Kuhn E, Bourgeois C, Keo V, Viengchareun S, Muscat A, Meduri G, Le Menuet D, Fève B, Lombès M.. Paradoxical resistance to high-fat diet-induced obesity and altered macrophage polarization in mineralocorticoid receptor-overexpressing mice. Am J Physiol Endocrinol Metab 2014;306:E75–E90. [DOI] [PubMed] [Google Scholar]
- 39. Greenstein AS, Khavandi K, Withers SB, Sonoyama K, Clancy O, Jeziorska M, Laing I, Yates AP, Pemberton PW, Malik RA, Heagerty AM.. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation 2009;119:1661–1670. [DOI] [PubMed] [Google Scholar]
- 40. Lohn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM.. Periadventitial fat releases a vascular relaxing factor. FASEB J 2002;16:1057–1063. [DOI] [PubMed] [Google Scholar]
- 41. Maenhaut N, Van de Voorde J.. Regulation of vascular tone by adipocytes. BMC Med 2011;9:25.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schmidt BM, Oehmer S, Delles C, Bratke R, Schneider MP, Klingbeil A, Fleischmann EH, Schmieder RE.. Rapid nongenomic effects of aldosterone on human forearm vasculature. Hypertension 2003;42:156–160. [DOI] [PubMed] [Google Scholar]
- 43. Gao YJ, Hirota S, Zhang DW, Janssen LJ, Lee RM.. Mechanisms of hydrogen-peroxide-induced biphasic response in rat mesenteric artery. Br J Pharmacol 2003;138:1085–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Nguyen Dinh Cat A, Antunes TT, Callera GE, Sanchez A, Tsiropoulou S, Dulak-Lis MG, Anagnostopoulou A, He Y, Montezano AC, Jaisser F, Touyz RM.. Adipocyte-specific mineralocorticoid receptor overexpression in mice is associated with metabolic syndrome and vascular dysfunction: role of redox-sensitive PKG-1 and Rho kinase. Diabetes 2016;65:2392–2403. [DOI] [PubMed] [Google Scholar]
- 45. Bender SB, Castorena-Gonzalez JA, Garro M, Reyes-Aldasoro CC, Sowers JR, DeMarco VG, Martinez-Lemus LA.. Regional variation in arterial stiffening and dysfunction in Western diet-induced obesity. Am J Physiol Heart Circ Physiol 2015;309:H574–H582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Singh R, Xiang Y, Wang Y, Baikati K, Cuervo AM, Luu YK, Tang Y, Pessin JE, Schwartz GJ, Czaja MJ.. Autophagy regulates adipose mass and differentiation in mice. J Clin Invest 2009;119:3329–3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kandadi MR, Panzhinskiy E, Roe ND, Nair S, Hu D, Sun A.. Deletion of protein tyrosine phosphatase 1B rescues against myocardial anomalies in high fat diet-induced obesity: Role of AMPK-dependent autophagy. Biochim Biophys Acta 2015;1852:299–309. [DOI] [PubMed] [Google Scholar]
- 48. Liu K, Zhao E, Ilyas G, Lalazar G, Lin Y, Haseeb M, Tanaka KE, Czaja MJ.. Impaired macrophage autophagy increases the immune response in obese mice by promoting proinflammatory macrophage polarization. Autophagy 2015;11:271–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ.. Autophagy regulates lipid metabolism. Nature 2009;458:1131–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Villacorta L, Chang L.. The role of perivascular adipose tissue in vasoconstriction, arterial stiffness, and aneurysm. Hormone Mol Biol Clin Investig 2015;21:137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Selcuk A, Bulucu F, Kalafat F, Cakar M, Demirbas S, Karaman M, Ay SA, Saglam K, Balta S, Demirkol S, Arslan E.. Skinfold thickness as a predictor of arterial stiffness: obesity and fatness linked to higher stiffness measurements in hypertensive patients. Clin Exp Hypertens 2012;35:459–464. [DOI] [PubMed] [Google Scholar]
- 52. Sam S, Haffner S, Davidson MH, D'agostino RB Sr., Feinstein S, Kondos G, Perez A, Mazzone T.. Relationship of abdominal visceral and subcutaneous adipose tissue with lipoprotein particle number and size in type 2 diabetes. Diabetes 2008;57:2022–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. White UA, Fitch MD, Beyl RA, Hellerstein MK, Ravussin E.. Differences in in vivo cellular kinetics in abdominal and femoral subcutaneous adipose tissue in women. Diabetes 2016;65:1642–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Xia N, Horke S, Habermeier A, Closs EI, Reifenberg G, Gericke A, Mikhed Y, Munzel T, Daiber A, Forstermann U, Li H.. Uncoupling of endothelial nitric oxide synthase in perivascular adipose tissue of diet-induced obese mice. Arterioscler Thromb Vasc Biol 2016;36:78–85. [DOI] [PubMed] [Google Scholar]
- 55. Omar A, Chatterjee TK, Tang Y, Hui DY, Weintraub NL.. Proinflammatory phenotype of perivascular adipocytes. Arterioscler Thromb Vasc Biol 2014;34:1631–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gil-Ortega M, Somoza B, Huang Y, Gollasch M, Fernández-Alfonso MS.. Regional differences in perivascular adipose tissue impacting vascular homeostasis. Trends Endocrinol Metab 2015;26:367–375. [DOI] [PubMed] [Google Scholar]
- 57. Fitzgibbons TP, Czech MP.. Epicardial and perivascular adipose tissues and their influence on cardiovascular disease: basic mechanisms and clinical associations. J Am Heart Assoc 2014;3:e000582.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Thanassoulis G, Massaro JM, Corsini E, Rogers I, Schlett CL, Meigs JB, Hoffmann U, O'donnell CJ, Fox CS.. Periaortic adipose tissue and aortic dimensions in the Framingham Heart Study. Journal of the American Heart Association 2012;1:e000885.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. McCurley A, Pires PW, Bender SB, Aronovitz M, Zhao MJ, Metzger D, Chambon P, Hill MA, Dorrance AM, Mendelsohn ME, Jaffe IZ.. Direct regulation of blood pressure by smooth muscle cell mineralocorticoid receptors. Nat Med 2012;18:1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shen JZ, Morgan J, Tesch GH, Rickard AJ, Chrissobolis S, Drummond GR, Fuller PJ, Young MJ.. Cardiac tissue injury and remodeling is dependent upon MR regulation of activation pathways in cardiac tissue macrophages. Endocrinology 2016;157:3213–3223. [DOI] [PubMed] [Google Scholar]
- 61. Jia G, Habibi J, Bostick BP, Ma L, DeMarco VG, Aroor AR, Hayden MR, Whaley-Connell AT, Sowers JR.. Uric acid promotes left ventricular diastolic dysfunction in mice fed a Western diet. Hypertension 2015;65:531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Margaritis M, Antonopoulos AS, Digby J, Lee R, Reilly S, Coutinho P, Shirodaria C, Sayeed R, Petrou M, De Silva R, Jalilzadeh S, Demosthenous M, Bakogiannis C, Tousoulis D, Stefanadis C, Choudhury RP, Casadei B, Channon KM, Antoniades C.. Interactions between vascular wall and perivascular adipose tissue reveal novel roles for adiponectin in the regulation of endothelial nitric oxide synthase function in human vessels. Circulation 2013;127:2209–2221. [DOI] [PubMed] [Google Scholar]
- 63. Antonopoulos AS, Margaritis M, Coutinho P, Shirodaria C, Psarros C, Herdman L, Sanna F, De Silva R, Petrou M, Sayeed R, Krasopoulos G, Lee R, Digby J, Reilly S, Bakogiannis C, Tousoulis D, Kessler B, Casadei B, Channon KM, Antoniades C.. Adiponectin as a link between type 2 diabetes and vascular NADPH oxidase activity in the human arterial wall: the regulatory role of perivascular adipose tissue. Diabetes 2015;64:2207–2219. [DOI] [PubMed] [Google Scholar]
- 64. Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB.. Plasma adiponectin levels and risk of myocardial infarction in men. JAMA 2004;291:1730–1737. [DOI] [PubMed] [Google Scholar]
- 65. Chung CM, Lin TH, Chen JW, Leu HB, Yang HC, Ho HY, Ting CT, Sheu SH, Tsai WC, Chen JH, Lin SJ, Chen YT, Pan WH.. A genome-wide association study reveals a quantitative trait locus of adiponectin on CDH13 that predicts cardiometabolic outcomes. Diabetes 2011;60:2417–2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Geleijnse JM, Grobbee DE, Kok FJ.. Impact of dietary and lifestyle factors on the prevalence of hypertension in Western populations. J Hum Hypertens 2005;19(Suppl. 3):S1–S4. [DOI] [PubMed] [Google Scholar]
- 67. Schiffrin EL. Vascular stiffening and arterial compliance. Implications for systolic blood pressure. Am J Hypertens 2004;17:39S–48S. [DOI] [PubMed] [Google Scholar]
- 68. Weisbrod RM, Shiang T, Al Sayah L, Fry JL, Bajpai S, Reinhart-King CA, Lob HE, Santhanam L, Mitchell G, Cohen RA, Seta F.. Arterial stiffening precedes systolic hypertension in diet-induced obesity. Hypertension 2013;62:1105–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Werner N, Nickenig G.. From fat fighter to risk factor: the zigzag trek of leptin. Arterioscler Thromb Vasc Biol 2004;24:7–9. [DOI] [PubMed] [Google Scholar]
- 70. Benetos A, Lacolley P, Safar ME.. Prevention of aortic fibrosis by spironolactone in spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol 1997;17:1152–1156. [DOI] [PubMed] [Google Scholar]
- 71. Hirata A, Maeda N, Hiuge A, Hibuse T, Fujita K, Okada T, Kihara S, Funahashi T, Shimomura I.. Blockade of mineralocorticoid receptor reverses adipocyte dysfunction and insulin resistance in obese mice. Cardiovasc Res 2009;84:164–172. [DOI] [PubMed] [Google Scholar]
- 72. Schrauwen-Hinderling VB, Hesselink MK, Meex R, van der Made S, Schar M, Lamb H, Wildberger JE, Glatz J, Snoep G, Kooi ME, Schrauwen P.. Improved ejection fraction after exercise training in obesity is accompanied by reduced cardiac lipid content. J Clin Endocrinol Metab 2010;95:1932–1938. [DOI] [PubMed] [Google Scholar]
- 73. Gamliel-Lazarovich A, Gantman A, Coleman R, Jeng AY, Kaplan M, Keidar S.. FAD286, an aldosterone synthase inhibitor, reduced atherosclerosis and inflammation in apolipoprotein E-deficient mice. J Hypertens 2010;28:1900–1907. [DOI] [PubMed] [Google Scholar]
- 74. Vecchiola A, Lagos CF, Carvajal CA, Baudrand R, Fardella CE.. Aldosterone production and signaling dysregulation in obesity. Curr Hypertens Rep 2016;18:20. [DOI] [PubMed] [Google Scholar]