Abstract
Aims
Spironolactone (SPL) improves endothelial dysfunction and survival in heart failure. Immune modulation, including poorly understood mineralocorticoid receptor (MR)-independent effects of SPL might contribute to these benefits and possibly be useful in other inflammatory cardiovascular diseases such as pulmonary arterial hypertension.
Methods and results
Using human embryonic kidney cells (HEK 293) expressing specific nuclear receptors, SPL suppressed NF-κB and AP-1 reporter activity independent of MR and other recognized nuclear receptor partners. NF-κB and AP-1 DNA binding were not affected by SPL and protein synthesis blockade did not interfere with SPL-induced suppression of inflammatory signalling. In contrast, proteasome blockade to inhibit degradation of xeroderma pigmentosum group B complementing protein (XPB), a subunit of the general transcription factor TFIIH, or XPB overexpression both prevented SPL-mediated suppression of inflammation. Similar to HEK 293 cells, a proteasome inhibitor blocked XPB loss and SPL suppression of AP-1 induced target genes in human pulmonary artery endothelial cells (PAECs). Unlike SPL, eplerenone (EPL) did not cause XPB degradation and failed to similarly suppress inflammatory signalling. SPL combined with siRNA XPB knockdown further reduced XPB protein levels and had the greatest effect on PAEC inflammatory gene transcription. Using chromatin-immunoprecipitation, PAEC target gene susceptibility to SPL was associated with low basal RNA polymerase II (RNAPII) occupancy and TNFα-induced RNAPII and XPB recruitment. XP patient-derived fibroblasts carrying an N-terminal but not C-terminal XPB mutations were insensitive to both SPL-mediated XPB degradation and TNFα-induced target gene suppression. Importantly, SPL treatment decreased whole lung XPB protein levels in a monocrotaline rat model of pulmonary hypertension and reduced inflammatory markers in an observational cohort of PAH patients.
Conclusion
SPL has important anti-inflammatory effects independent of aldosterone and MR, not shared with EPL. Drug-induced, proteasome-dependent XPB degradation may be a useful therapeutic approach in cardiovascular diseases driven by inflammation.
Keywords: Inflammation, Proteasome, Xeroderma pigmentosum, Pulmonary arterial hypertension, Endothelial dysfunction
1. Introduction
Spironolactone (SPL) increases survival in patients with left heart failure1 by improving endothelial function and reducing inflammation.2 Importantly, these extra-renal effects of SPL may also be useful in other cardiovascular diseases such as pulmonary arterial hypertension (PAH).3 Endothelial dysfunction, inflammation and pathologic vascular remodelling are prominent features of PAH. However, mineralocorticoid receptor (MR) antagonists like SPL are often only prescribed after the onset of right heart failure, a late manifestation of end-stage disease. Earlier use of SPL in PAH might slow disease progression,4 but a strong mechanistic rationale for this approach and evidence of clinical efficacy are lacking.
The anti-inflammatory effects of SPL have long been ascribed to mineralocorticoid inhibition.5 Accordingly, SPL has been widely used to impute a role for aldosterone and MR in cardiovascular inflammation.6,7 However, SPL also appears to suppress inflammation independent of MR, but the molecular basis for this activity is unclear.8–11 Unlike eplerenone (EPL), SPL binds to and exerts biological effects through several nuclear receptors other than MR including the androgen (AR), glucocorticoid (GR),12 progesterone (PR),12 pregnane X (PXR)13 and retinoid X gamma (RXRγ) receptors.14 Furthermore, SPL but not EPL induces proteasomal degradation of xeroderma pigmentosum (XP) group B-complementing protein (XPB, official gene symbol ERCC3),15 a subunit of the TFIIH core complex involved in basal transcription and nucleotide excision repair (NER).
Here, SPL and EPL were compared for effects on canonical NF-κB and AP-1 inflammatory signalling. Selective overexpression16 of MR, GR, AR, PR, PXR and RXRγ was used to examine the role of all relevant nuclear receptors. Although not previously shown to affect basal transcription,17 SPL-induced XPB loss potently suppressed NF-κB and AP-1 reporters, as well as corresponding target genes in primary human pulmonary artery endothelial cells (PAECs). Fibroblasts from a patient with XP, carrying a particular XPB mutation, were resistant to SPL, indicating structural specificity for its effect on XPB. Importantly, SPL decreased XPB protein levels in whole lung tissue from rats with monocrotaline-induced pulmonary hypertension (MCT-PH), where it has previously been shown to reduce pathologic vascular remodelling.18,19 Finally, SPL was associated with reduced serum cytokine levels in a retrospective cohort of patients with PAH. SPL is the prototype for a new class of drugs that degrade specific protein targets and XPB loss is a novel mechanism for treating inflammation.
2. Methods
More detailed methods are available as a Supplementary material online.
2.1 Cell culture
Human embryonic kidney (HEK293) cells (ATCC, Manassas, VA) were grown in high glucose DMEM supplemented with 10% heat-inactivated FBS (Life Technologies, Grand Island, NY). PAECs (Lonza, Walkersville, MD) were cultured for a maximum of 6 passages in endothelial basal medium-2 (Lonza) supplemented with growth factors containing 2% serum (Lonza) in cell culture flasks coated with type I collagen (50 μg/ml in 0.02 N acetic acid). For a list of PAEC donors refer to Supplementary material online, Table S1. Fibroblasts harbouring XPB mutations, as well as unaffected parental fibroblasts, were obtained from the NIGMS Human Genetic Cell Repository (Coriell Institute for Medical Research; Camden, NJ) and grown in high glucose DMEM supplemented with 10% FBS (Life Technologies, Grand Island, NY). For a list of fibroblast donors refer to Supplementary material online, Table S2. Cells were maintained at 37 °C in a humidified incubator with 5% CO2 and 95% air.
2.2 Cytokine, chemokine and growth factor multiplex immunoassay
Supernatant and serum cytokine, chemokine and growth factor concentrations were determined using the Bio-Plex 27-plex Suspension Array system. PAECs (1 × 105) were seeded in medium containing charcoal-stripped serum onto collagen-coated 12-well plates 24 h prior to treatments. Cell supernatants were aspirated and cleared by centrifugation (10 000 g for 5 min at 4 °C).
Human experimental guidelines of the US Department of Health and Human Services were followed and all clinical investigations were conducted according to the principles expressed in the Helsinki Declaration. The National Heart, Lung, and Blood Institute’s institutional review board approved the clinical protocol (13-CC-0012). The Office of Human Subjects Research Protections (OHSRP no. 12458) approved the use of de-identified serum samples provided by Inova Fairfax Hospital. The institutional review board of Inova Fairfax Hospital approved the clinical protocol originally associated with these samples (no. 09.147). All participants provided written informed consent.
2.3 Rat lung from the monocrotaline model of pulmonary hypertension
Animal studies were approved by the Institutional Animal Care and Use Committee at Tufts Medical School and conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA) were subcutaneously injected with 60 mg/kg of monocrotaline to induce pulmonary hypertension and starting on day 21 treated for 14 days with either placebo or SPL sustained-release pellets (40 mg/kg/d), as previously described.19 Animals were anaesthetized with ketamine (40–80 mg/kg) and xylazine (5–10 mg/kg) for subcutaneous pellet implantation. Buprenorphine (0.05–0.1 mg/kg) was given prior to incision and then continued twice daily for 3d. Following euthanasia (pentobarbital intraperitoneal injection, 120 mg/kg), lung tissue was obtained from placebo and SPL-treated, monocrotaline-exposed animals, as well as normal control rats.
2.4 Statistical analysis
Linear regression models were used in dose response analyses. ANOVA models with two-way interactions were used in all other analyses unless noted otherwise. Random effects were added to the models to account for repeated measures. Post hoc tests were chosen for the comparisons of interest without adjustment for multiple comparisons. Standard residual diagnostics were used to check model assumptions, and data were log-transformed when necessary. Analyses were performed in JMP version 12 and SAS version 9.3 (SAS Institute Inc., Cary, NC). All P-values are nominal and two-sided, and P < 0.05 was considered significant.
3. Results
3.1 SPL suppresses NF-κB and AP-1 reporter activity independent of nuclear receptor overexpression
Selective expression in HEK293 cells allowed for the separate assessment of relevant nuclear receptors. As expected and previously reported,16,20 HEK293 cells expressed only low levels of endogenous GR, but not MR or AR (Figure 1A). Likewise, PR and PXR were not detected in HEK293 cells, but low levels of RXRγ were found by Western blotting (see see Supplementary material online, Figure S1A and S1B).
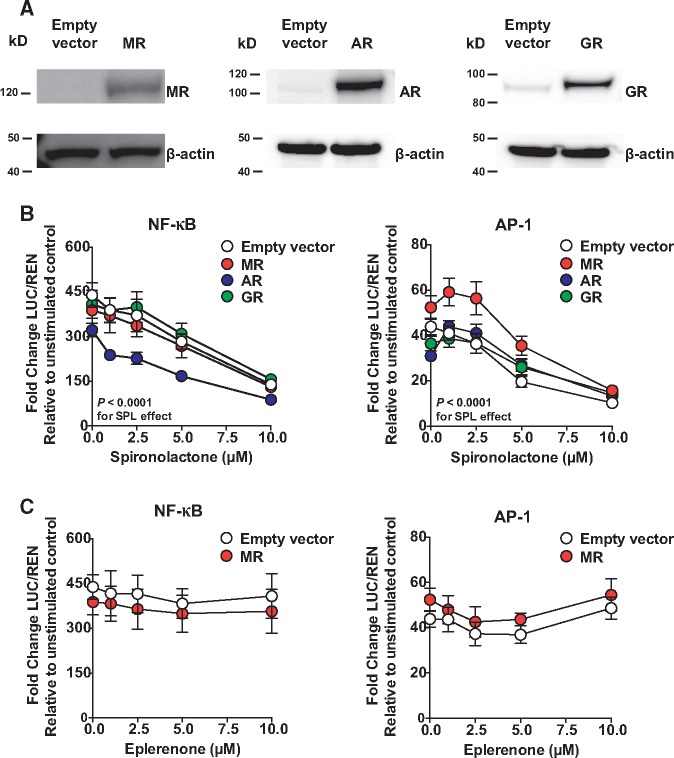
Figure 1.
SPL suppresses NF-κB and AP-1 reporters independent of MR, AR, and GR in HEK293 cells. (A) Western blots of total cell lysate demonstrate human MR, AR, and GR overexpression in HEK293 cells 24 h post-transfection. Only GR had low, but detectable baseline expression. (B) SPL dose-dependently suppressed NF-κB and AP-1 reporter activity in the absence and presence of MR, AR, and GR (P < 0.0001 for the downward trend of slopes). Expression of AR and MR suppressed overall NF-κB signalling (P < 0.0001 and P = 0.004, respectively, for main effects without significant interactions with SPL). For AP-1 signalling, MR, AR and GR expression variably shifted SPL dose-response curves and altered their shapes, particularly at low concentrations (P ≤ 0.0004 for evidence of MR-, AR-, and GR-SPL interactions), but nevertheless SPL dose-dependent suppression of AP-1 was the overall predominant effect. (C) Unlike SPL, increasing doses of EPL in the absence and presence of MR had no effect on NF-κB reporter activity (P = 0.15) and only modestly suppressed AP-1 activity at concentrations ≤5 μM (P = 0.006), but not 10 μM (P = 0.42). Independent of EPL, MR expression alone modestly shifted NF-κB activity downward (P = 0.0006 for main effect) and AP-1 upward (P = 0.0003 for main effect). Twenty-four hours following transfection, cells were treated for 1 h with vehicle control or MR antagonist followed by stimulation with either TNFα (10 ng/ml; NF-κB activation) or PMA (100 nM; AP-1 activation) for 5 h. LUC activity was normalized to the renilla (REN) control. Luciferase results from five (NF-κB) and four (AP-1) independent experiments are presented relative to unstimulated control (mean ± SE).
SPL dose-dependently suppressed NF-κB and AP-1 promoter activity independent of MR, AR, and GR (Figure 1B). Independent of SPL, AR expression alone suppressed the NF-κB reporter, while MR had a similar, but smaller effect (Figure 1B). In contrast to these effects on NF-κB, MR, AR, and GR expression variably shifted and altered the shapes of their respective SPL dose-response curves, but the overall suppressive effects of SPL were maintained. Like AR and MR, expression of PR slightly suppressed the NF-κB reporter independent of SPL (see Supplementary material online, Figure S1A). PR expression alone decreased basal AP-1 activity, but at concentrations of SPL ≤5 μM, dose-dependently increased AP-1 activity, which was then decreased at 10 μM of SPL (see Supplementary material online, Figure S1A). Although this pattern suggests PR-SPL trans-activation of AP-1 signalling,16 this possibility was not further investigated. As with MR, AR and GR, SPL dose-dependently suppressed NF-κB and AP-1 in the absence and presence of PXR, RXRγ and PXR + RXRγ (see Supplementary material online, Figure S1B). Independent of SPL, PXR overexpression shifted NF-κB reporter activity downward and AP-1 upward (see Supplementary material online, Figure S1B). Together, these results indicate that SPL directly suppresses NF-κB and AP-1 inflammatory signalling through mechanisms independent of its commonly recognized nuclear receptor targets. Unlike SPL, EPL had no effect on NF-κB promoter activity and only modestly suppressed AP-1 activity at ≤ 5 μM, but not at 10 μM (Figure 1C). Independent of EPL treatment, MR expression alone, had similar, small effects on NF-κB (suppressed) and AP-1 (enhanced) reporter activity as seen in experiments with SPL.
3.2 DNA binding of p65/p50 or cFos/cJun not altered by SPL
The effect of SPL and EPL on p65/p50 (NF-κB) and cFos/cJun (AP-1) binding activity was investigated using nuclear protein extracts from HEK293 cells. The consensus binding sequences used in these experiments were homologous to those present in the NF-κB and AP-1 reporters. As expected, p65/p50 and cFos/cJun binding activity was increased following stimulation with TNFα and phorbol 12-myristate 13-acetate (PMA), respectively (see Supplementary material online, Figure S2A and S2B). Nevertheless, DNA binding of p65/p50 and cFos/cJun was unchanged by treatment with either SPL or EPL (see Supplementary material online, Figure S2A and S2B).
3.3 Suppression of inflammatory signalling by SPL requires proteasomal degradation of XPB, but not de novo protein synthesis
Next, HEK293 cells were treated with cycloheximide (CHX) to determine whether SPL suppression of inflammatory signalling required new protein synthesis. Luciferase (LUC) activity was measured to ensure that protein synthesis had been completely blocked (data not shown) and luc2P mRNA was used to quantify the impact of SPL on NF-κB promoter activity. As previously described for NF-κB regulated gene transcription,21 TNFα stimulation in the presence of CHX led to super-induction of luc2P (>2-fold increase compared with the effect of TNFα in the absence of CHX; see Supplementary material online, Figure S2A and S2B). Nonetheless, SPL suppressed TNFα induction of NF-κB signalling as measured by luc2P mRNA by > 60% in the absence and presence of CHX (see Supplementary material online, Figure S2C). The AP-1 reporter was not investigated in the presence of CHX because blocking protein synthesis suppresses PMA-induced de novo cFos synthesis and AP-1 responses.21,22 In contrast to inhibition of protein synthesis, proteasome blockade with MG132 completely prevented SPL-meditated suppression of PMA-induced AP-1 promoter activity (see Supplementary material online, Figure S2D). Only the AP-1 reporter was used to examine the impact of MG132 on SPL-mediated suppression, because proteasome inhibitors interfere with TNFα-induced degradation of IκBα and therefore NF-κB activation.23
Suppression of two distinct inflammatory signalling cascades, as well as preserved DNA binding of p65/p50 and cFos/cJun suggested that the anti-inflammatory effects of SPL might converge downstream from NF-κB and AP-1 activation and possibly involve very basic mechanisms such as chromatin remodelling or transcriptional initiation. Notably, SPL induces proteasome-dependent degradation of XPB, a helicase subunit of the TFIIH complex.15 Importantly, while XPB and other helicases open promoters for transcriptional access,24,25 SPL-mediated degradation of XPB does not universally impair class II gene expression.17
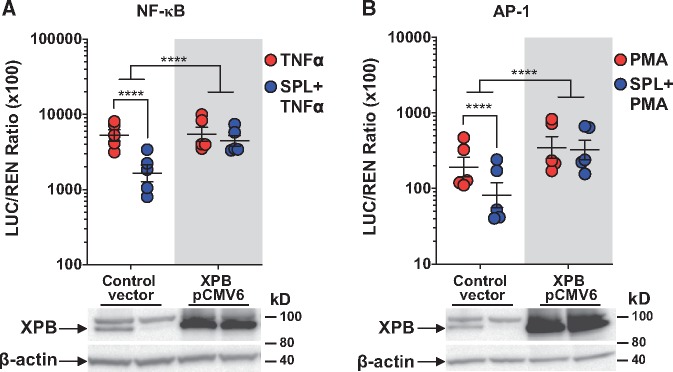
As expected and in agreement with Alekseev et al., SPL-induced XPB loss was proteasome-dependent and EPL was inactive (see Supplementary material online, Figure S3A). Furthermore, SPL had no significant effect on XPB mRNA levels (see Supplementary material online, Figure S3B). Importantly, overexpression of human XPB in HEK293 cells completely blocked SPL-mediated suppression of both TNFα-induced NF-κB (Figure 2A) and PMA-induced AP-1 (Figure 2B) reporter activation. Collectively, these findings identified XPB degradation as a shared, MR-independent mechanism by which SPL inhibits both NF-κB and AP-1 inflammatory signalling.
Figure 2.
SPL suppression of NF-κB and AP-1 is reversed by XPB overexpression. XPB overexpression (five independent experiments for each pathway) blocked the ability of SPL to suppress (A) TNFα-induced NF-κB reporter activation and (B) PMA-induced AP-1 reporter activation. LUC activity was normalized to REN control and results are presented as the geometric mean LUC/REN ratio (×100) ± geometric SE plotted on a log10 scale. Total cell lysates were collected concurrent with the timing of luciferase assay experiments, resolved by SDS-PAGE and immunoblotted for XPB and β-actin to demonstrate XPB overexpression in HEK293 cells. Representative Western blots are shown below the graphs. ****P < 0.0001 (ANOVA with post hoc tests, as indicated).
3.4 SPL suppression of inflammation in primary human PAECs
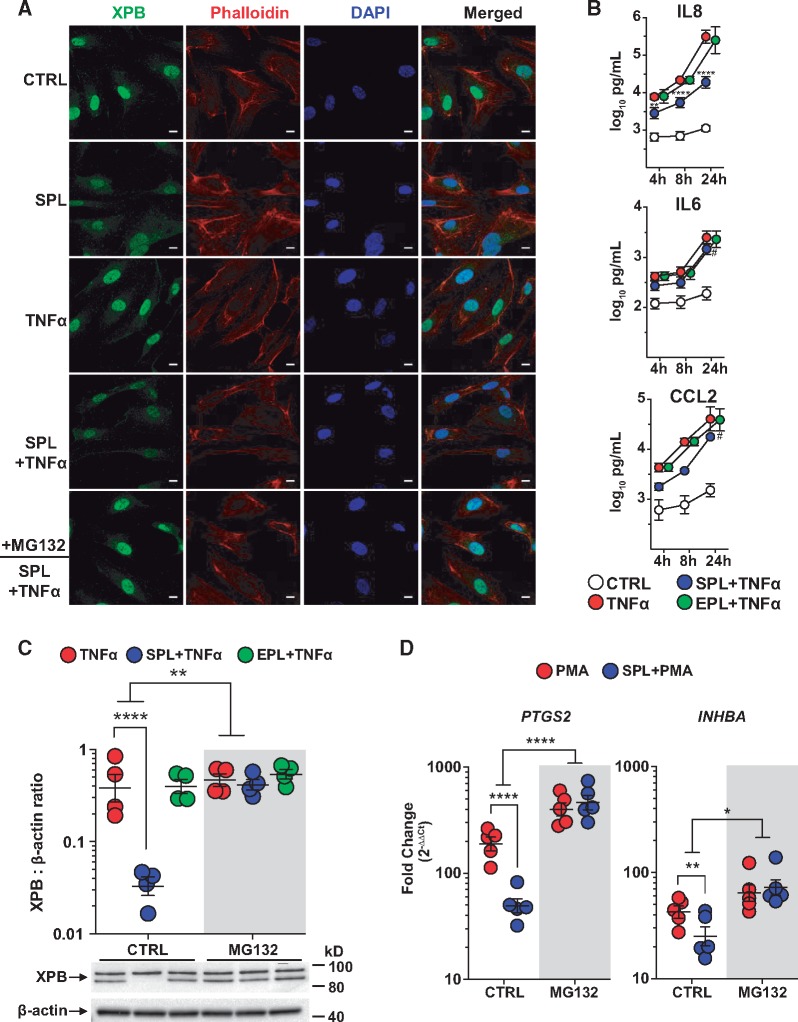
SPL reduces endothelial inflammation in cellular and animal models of left heart disease,2 while its immunomodulatory effects on pulmonary artery endothelium have not been examined previously. Therefore, primary human PAECs were used to further explore the effects of SPL-mediated XPB degradation on inflammation. As shown by immunofluorescence staining, treatment of PAECs with SPL resulted in XPB loss, an effect that was reversed by proteasome inhibition (Figure 3A), consistent with our findings above. Next, the effects of SPL compared with EPL were investigated in TNFα-challenged PAECs, focusing on inflammatory mediators associated with PAH pathogenesis. SPL was found to significantly suppress the production of many cytokines, chemokines, and growth factors at 4, 8, and 24 h, respectively, including IL8 (2.7-, 3.8-, and 20-fold decreases), IL6 (1.5-, 1.6-, and 1.8-fold decreases) and CCL2 (2.4-, 3.8-, and 3.8-fold decreases), while EPL had no significant effect on any cytokine at any time point (Figure 3B and see Supplementary material online, Table S3). Consistent with previous studies using other cell types,15,26 SPL treatment of PAECs for 24 h in the presence of TNFα did not reduce cell viability (data not shown). Western blots of whole cell lysates demonstrated that SPL, but not EPL reduced XPB protein levels in PAECs, and MG132 blocked this SPL-induced loss of XPB (Figure 3C). Similar to the findings in HEK293 cells using an AP-1 reporter assay, MG132 also reversed SPL-mediated suppression of PMA-induced targets as measured by mRNA (PTGS2 and INHBA; Figure 3D) and protein (PTGS2 and IL8; Supplementary material online, Figure S4).
Figure 3.
SPL suppression of inflammation in PAECs and the role of XPB degradation. (A) XPB protein expression, localized predominantly to the nucleus, was reduced following treatment with SPL. The effect of SPL on XPB (green) appeared similar in the absence or presence of TNFα stimulation for 2 h and was blocked by proteasome inhibition (MG132; 10 µM). Cell cytoplasm was stained with phalloidin (red) and nuclei were counterstained with DAPI (blue). Fluorescence images are representative of results from three different PAEC donors. Scale bars: 10 μm. (B) In PAECs, SPL (10 µM) significantly suppressed TNFα-induced secretion of IL8, IL6, and CCL2, while EPL (10 µM) had no effect on any of these cytokines (P ≥ 0.77 for the EPL main effect across all three time points). Data are presented as the log10-transformed mean concentration ± SE of four independent experiments using different donors. Multiplex cytokine analysis was performed on cell supernatant collected at 4, 8, and 24 h after TNFα (5 ng/ml) stimulation. **P < 0.01; ****P < 0.0001 for the effect of SPL at 4, 8, and 24 h, respectively; #P ≤ 0.0002 for the SPL main effect, when similar across all three time points. See Supplementary material online, Table S1 for the results of all cytokines analysed. Consistent with findings by immunofluorescence, (C) MG132 blocked SPL-induced XPB degradation in PAECs as determined by total cell lysate Western blots. In contrast to SPL, EPL had no effect on XPB protein levels in the absence or presence of MG132 (P ≥ 0.30 for both). Densitometric quantification of XPB protein expression relative to β-actin is presented as the geometric mean ratio ± geometric SE on log10 scale of four independent experiments using different donors. A representative Western blot is shown below the graph. (D) MG132 significantly blocked the effect of SPL on PMA-induced PTGS2 and INHBA mRNA expression. PAECs were treated with SPL for 1 h followed by stimulation with PMA (10 nM) for 4 h. Expression of mRNA measured by quantitative real-time PCR is presented as fold-change relative to unstimulated cells (geometric mean ± geometric SE) of five independent experiments, each with a different donor, plotted on log10 scale. *P < 0.05; **P < 0.01; ****P < 0.0001 (ANOVA with post hoc tests, as indicated).
3.5 Combining XPB knockdown with SPL in PAECs increases the suppression of TNFα-induced target genes
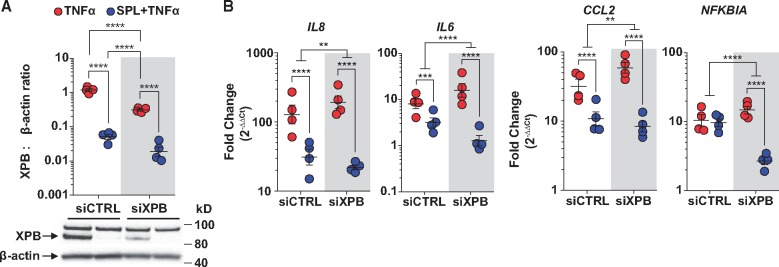
Gene specific siRNA knockdown was performed in PAECs in order to further characterize the impact of XPB on inflammatory gene transcription. Both SPL treatment and XPB knockdown significantly reduced XPB protein levels, however, the effect of SPL was significantly greater (24-fold decrease for SPL vs. 4-fold decrease for siXPB; Figure 4A). Consistent with effects of SPL on TNFα-induced inflammatory cytokine secretion in PAECs (Figure 3B), SPL significantly suppressed TNFα-induced IL8, IL6 and CCL2 mRNA expression (Figure 4B) in cells transfected with control siRNA. In contrast, SPL did not alter TNFα-induction of NFKBIA (Figure 4B). Although modest XPB knockdown alone slightly increased rather than decreased TNFα-induced IL8, IL6, CCL2, and NFKBIA expression levels, SPL and XPB knockdown combined further suppressed all four transcripts compared with SPL alone (Figure 4B). The failure of XPB gene knockdown alone to suppress inflammatory responses suggests that TNFα-induced gene transcription is not disrupted until XPB protein levels fall below a threshold level. XPB knockdown combined with SPL produced a larger drop in XPB protein levels than either intervention alone and had the strongest suppressive effect on inflammatory gene transcription (Figure 4B). Importantly, these findings were confirmed using a second siRNA (see Supplementary material online, Figure S5).
Figure 4.
SPL in combination with XPB knockdown further suppresses TNFα target genes in PAECs. (A) XPB knockdown and SPL treatment both significantly reduced XPB protein levels, but the impact of SPL was significantly greater and similar in control siRNA (siCTRL) and XPB siRNA transfected PAECs (P = 0.27 for the interaction between SPL and XPB knockdown). Densitometric quantification of XPB protein expression relative to β-actin is presented as the geometric mean ratio ± geometric SE on log10 scale of four independent experiments, each with a different donor. A representative Western blot is shown below the graph. (B) SPL suppressed TNFα-induced IL8, IL6, and CCL2, but not NFKBIA (P = 0.39) mRNA expression in PAECs transfected with siCTRL. XPB knockdown alone in PAECs did not suppress, but rather modestly increased TNFα-induced IL8 (P = 0.03), IL6 (P = 0.003), CCL2 (P = 0.005), and NFKBIA (P = 0.008) expression levels compared with siCTRL. However, compared with SPL alone, the combination of XPB knockdown and SPL had the strongest suppressive effect on TNFα-induced IL8, IL6, CCL2, and NFKBIA. Expression of mRNA measured by quantitative real-time PCR is presented as the fold-change relative to unstimulated cells transfected with siCTRL (geometric mean ± geometric SE) of four independent experiments, each with a different donor, plotted on log10 scale. **P < 0.01; ***P < 0.001; ****P ≤ 0.0001 (ANOVA with post hoc tests, as indicated).
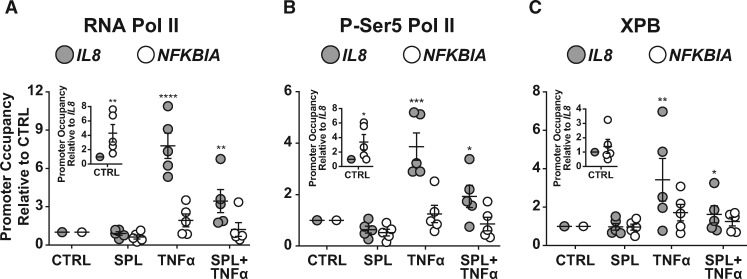
3.6 RNA polymerase II, pSer5-RNA polymerase II, and XPB differences in occupancy and recruitment distinguish genes with variable susceptibility to SPL-mediated suppression
Using chromatin-immunoprecipitation in PAECs, basal occupancy and TNFα-induced recruitment of RNA polymerase II (RNAPII) and XPB were examined for possible differences comparing the IL8 and NFKBIA promoter regions. TNFα stimulation had a much smaller effect on RNAPII recruitment to NFKBIA compared with IL8 (1.9 ± 0.5 vs. 7.8 ± 1.0 fold-change relative to control; Figure 5A). Notably, basal RNAPII occupancy at NFKBIA, a gene that is both constitutively expressed and inducible, was already more than 4-fold higher relative to the IL8 promoter (Figure 5A, inset). Consistent with effects on IL8 transcription, SPL decreased TNFα-induced RNAPII recruitment to the IL8 promoter region by > 50% (Figure 5A). In comparison, the impact of SPL at the NFKBIA promoter was more modest (Figure 5A).
Figure 5.
Target gene susceptibility to SPL-mediated suppression is associated with low basal levels of RNAPII occupancy and greater TNFα-induced recruitment. (A) TNFα stimulation significantly increased total RNAPII recruitment to the IL8 promoter region in PAECs, an effect that was suppressed by SPL treatment. In contrast, TNFα stimulation only modestly and non-significantly (P = 0.14) increased total RNAPII occupancy at the NFKBIA promoter, where basal RNAPII occupancy was already significantly higher compared with basal RNAPII occupancy at the IL8 promoter (inset). (B) Similar to total RNAPII, active pSer5-RNAPII occupancy at the IL8 promoter region was significantly increased following TNFα stimulation and suppressed by SPL treatment. However again, TNFα stimulation did not significantly increase pSer5-RNAPII occupancy at the NFKBIA promoter (P = 0.78) where basal pSer5-RNAPII occupancy was already significantly higher compared with the IL8 promoter (inset). (C) Although basal XPB occupancy was similar at the IL8 and NFKBIA promoter regions (P = 0.80; inset), TNFα stimulation significantly increased XPB recruitment to the IL8 but not the NFKBIA promoter region (P = 0.17). Similar to RNAPII and pSer5-RNAPII, XPB recruitment to the IL8, but not the NFKBIA promoter region was significantly suppressed by SPL. PAECs were treated with SPL (10 μM) or vehicle control (CTRL) for 2 h and then stimulated with TNFα (5 ng/ml) or vehicle control for 1 h. Precipitated protein/DNA complexes were eluted and crosslinks reversed for DNA purification, followed by quantitative real-time PCR analysis. RNAPII, pSer5-RNAPII, and XPB promoter enrichment from five independent experiments, each with a different donor were calculated as a percentage of input DNA normalized to unstimulated cells (mean ± SE). RNAPII, pSer5-RNAPII, and XPB basal NFKBIA promoter occupancy (inset) from five independent experiments, each with a different donor was normalized to CTRL IL8 occupancy (mean ± SE). *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Similar to total RNAPII, active pSer5-RNAPII occupancy was significantly increased following TNFα stimulation at the IL8, but not the NFKBIA promoter region (Figure 5B). Again, basal occupancy of pSer5-RNAPII at the NFKBIA promoter was >3-fold higher compared with IL8 (Figure 5Binset). Likewise, SPL suppressed TNFα-induced recruitment of pSer5-RNAPII to the IL8 promoter by > 50% (Figure 5B) while its effect at NFKBIA was less pronounced (Figure 5B). These results suggest that the reduced impact of SPL on some inflammatory response genes correlates with high total and active RNAPII basal occupancy rates and less TNFα-induced recruitment.
Unlike RNAPII, basal XPB occupancy was not significantly different at the IL8 and NFKBIA promoter regions (Figure 5C, inset). However, consistent with differences in basal promoter openness, TNFα stimulation led to significantly more XPB recruitment to the IL8 compared with NFKBIA promoter region (3.4 ± 1.1 vs. 1.7 ± 0.4 fold-change; Figure 5C). Likewise, SPL suppressed TNFα-induced recruitment of XPB to the IL8 promoter by more than 50% (Figure 5C), whereas its effect at the NFKBIA promoter was again much less (Figure 5C).
3.7 Effects of SPL on patient-derived fibroblasts with XPB mutations
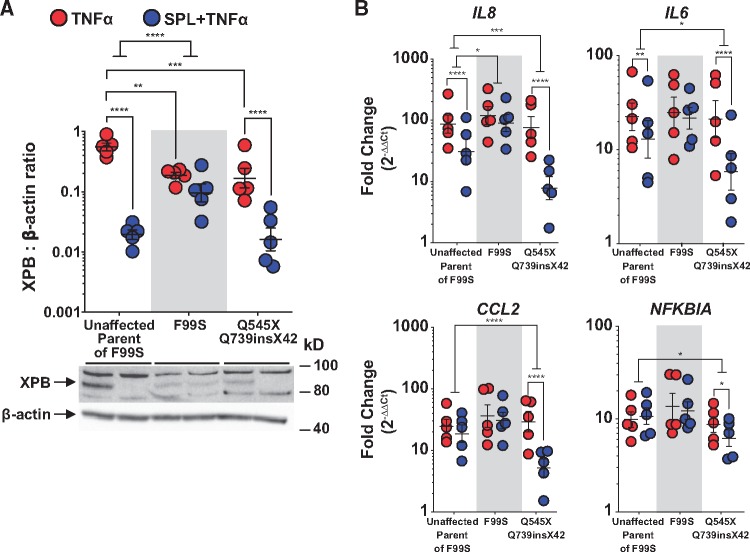
Patient-derived fibroblasts harbouring different XPB mutations were used to further investigate the interaction between SPL and XPB. The unaffected mother of a patient with mild XP/Cockayne syndrome (CS) complex, associated with a phenylalanine to serine amino acid substitution (F99S), served as a control as she was not a carrier of the F99S allele.27 Another patient with severe XP/CS complex was heterozygous for a nonsense mutation that converts the glutamine at position 545 to a premature stop codon (Q545X) and a splice acceptor mutation in intron 14 resulting in a 42-amino acid insertion at codon 739 followed by a stop codon (Q739insX42).28
Fibroblasts harbouring XPB mutations expressed lower amounts of XPB relative to control fibroblasts from the unaffected mother of the F99S patient (Figure 6A). In contrast to normal fibroblasts and those harbouring the compound C-terminal mutations, SPL treatment did not reduce XPB protein levels in F99S mutant fibroblasts (Figure 6A). Thus, the N-terminal region of wild type XPB appears to be important for SPL-induced degradation. Unlike fibroblasts from the unaffected parent, but consistent with the lack of effect on XPB, SPL did not suppress TNFα-induced inflammatory gene transcription in F99S fibroblasts (Figure 6B). Supporting a possible threshold effect, TNFα-induced IL8, IL6, CCL2 and NFKBIA mRNA transcription was not blunted in either F99S or Q545X + Q739insX742 fibroblasts, despite moderately lower levels of XPB protein in these cells (Figure 6B). In addition to NFKBIA, CCL2 was also not significantly suppressed by SPL in normal fibroblasts possibly reflecting a cell-specific difference with PAECs. Unlike its lack of effect on the F99S mutant, SPL had a significantly greater suppressive effect on TNFα-induced IL8, IL6, CCL2, and NFKBIA mRNA levels in Q545X + Q739insX742 fibroblasts compared with the unaffected control (Figure 6B), mirroring the combined effects of both SPL and XPB knockdown in PAECs.
Figure 6.
XP patient-derived fibroblasts harbouring an N-terminal, but not C-terminal XPB mutations were insensitive to SPL. (A) F99S and Q545X + Q739insX42 mutant fibroblasts expressed lower levels of XPB compared with control fibroblasts from an unaffected parent of the F99S patient. SPL induced XPB degradation in both normal fibroblasts and those expressing C-terminal mutations in XPB. In contrast, F99S mutant fibroblasts were resistant to SPL-mediated XPB degradation. Densitometric quantification of XPB protein expression relative to β-actin is presented as the geometric mean ratio ± geometric SE on log10 scale of five independent experiments. A representative Western blot is shown below the graph. (B) In contrast to the unaffected parent, F99S mutant fibroblasts were also resistant to SPL-mediated suppression of IL8 and IL6. TNFα-induced CCL2 and NFKBIA gene transcription was not significantly suppressed by SPL in either normal or F99S mutant fibroblasts. However, in fibroblasts heterozygous for the compound C-terminal mutations (Q545X + Q739insX42), SPL synergistically suppressed TNFα-induced IL8, IL6, CCL2, and NFKBIA. Fibroblasts were treated with SPL for 1 h followed by stimulation with TNFα (10 ng/ml) for 4 h. Expression of mRNA measured by quantitative real-time PCR is presented as the fold-change relative to unstimulated normal fibroblasts (geometric mean ± geometric SE) of five independent experiments, plotted on log10 scale. *P < 0.05; **P < 0.01; ***P < 0.001; ****P ≤ 0.0001 (ANOVA with post hoc tests, as indicated).
3.8 In vivo effects of SPL on XPB in rat lung and serum inflammatory markers in patients with PAH
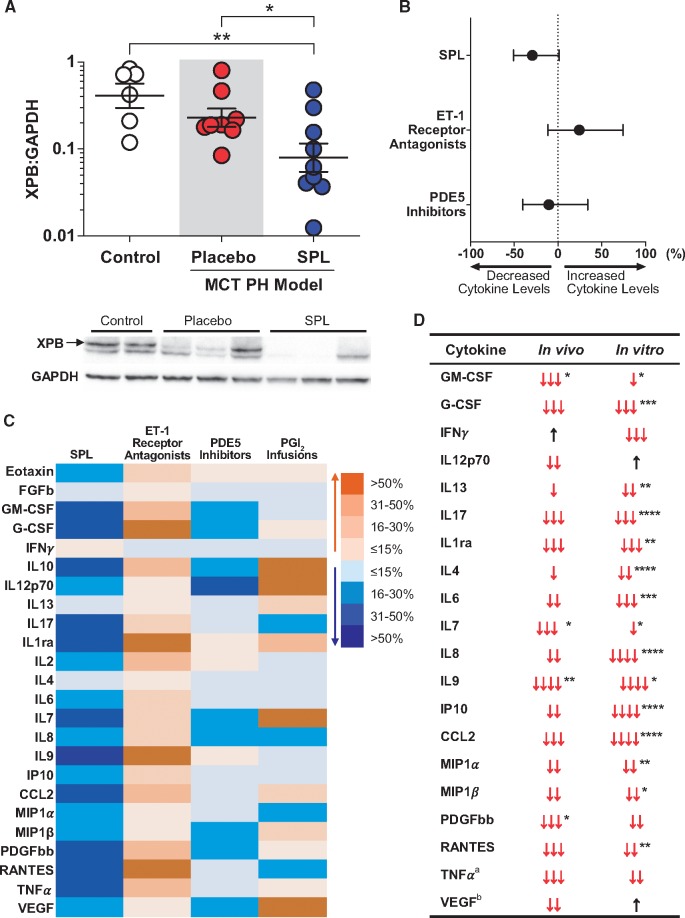
SPL was previously shown to have beneficial effects on pulmonary vascular remodelling in the MCT-PH rat model of pulmonary hypertension.19 Western blots of whole lung tissue from animals in this study demonstrated that MCT-PH rats treated with SPL (40 mg/kg/d) for 14 days had lower XPB protein levels compared with both control and MCT-PH rats treated with placebo (Figure 7A).
Figure 7.
SPL treatment decreases XPB in the MCT-PH rat lung and is associated with lower serum concentrations of inflammatory markers in PAH patients. (A) Western blots of homogenized whole lung tissue demonstrated reduced XPB protein levels in MCT-PH rats treated with SPL (40 mg/kg/d) for 14 days (n = 9) compared with either control rats (n = 6) or MCT-PH rats treated with placebo (n = 8). Densitometric quantification of XPB protein expression relative to GAPDH is presented on log10 scale for each animal as well as the geometric mean ± geometric SE. A representative Western blot is shown below. (B) In a multivariate analysis, treatment with SPL was associated with an overall trend toward reduced serum cytokine concentrations in an observational cohort of PAH patients [mean (95% CI) across all cytokines: −29.3% (−50.8, 1.5); P = 0.06]. In contrast, treatment with ET-1 receptor antagonists [24.3% (−11.4, 74.4); P = 0.21], and PDE5 inhibitors [−10.5 (−40.2, 34.0); P = 0.59] did not alter cytokine levels. Discordant effects on individual cytokines precluded a meaningful aggregate estimate of effect size for PGI2 infusions. (C) Effects of SPL, ET-1 receptor antagonists, PGI2 infusions, and PDE5 inhibitors on individual cytokines are depicted as a heatmap. Orange indicates percent increase and blue percent decrease in serum cytokine concentrations. (D) Directional effects of SPL treatment on measured cytokines in PAH patients compared with TNFα-stimulated PAECs in vitro revealed a substantial degree of concordance. ↓:≤15% decrease; ↓↓: 16–30% decrease; ↓↓↓: 31–50% decrease; ↓↓↓↓:>50% decrease; ↑:≤15% increase; *P < 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001. For significant interactions between SPL treatment and time in vitro, the largest effect is reported, otherwise main effects are used. Analytes not depicted were either not induced by TNFα in vitro (FGFb and IL10) or were otherwise not detected in vitro (Eotaxin, IL2, IL5) or in vivo (IL1β, IL5, and IL15). See Supplementary material online for exclusion criteria. aUsed to stimulate cells. bComponent of cell culture media.
Patients with PAH have intense local inflammatory cell infiltrates adjacent to areas of pulmonary vascular remodelling.29,30 Likewise, PAH patients have elevated levels of circulating inflammatory markers and several, including IL6, IL8, IL10, and IL12p70, correlated with decreased survival.31 In order to investigate the effects of SPL therapy on in vivo inflammatory mediator production, we obtained stored serum samples from all PAH patients (n = 53; see Supplementary material online, Table S4) participating in National Heart, Lung and Blood Institute-sponsored biorepository studies at either the NIH Clinical Center (n = 13; NCT01730092) or a regional lung transplant referral center (n = 40). A multivariate analysis of patient demographics and clinical data recorded from the date of blood collection was used to adjust for potential confounding factors (see see Supplementary material online, Table S5 for complete analysis). When compared with PAH-specific therapy with endothelin-1 (ET-1) receptor antagonists (P = 0.21) or phosphodiesterase 5 (PDE5) inhibitors (P = 0.59), SPL was associated with a more consistent tendency to suppress serum markers of inflammation (P = 0.06; Figure 7B). Prostacyclin (PGI2) infusions had significantly different effects on individual serum mediators (Figure 7C), precluding the calculation of an overall effect. SPL therapy was consistently associated with decreased concentrations of serum mediators of inflammation, whereas the three classes of PAH-specific therapy had largely non-significant, mixed effects (Figure 7C). Interestingly, a high degree of concordance was seen between the in vitro effects of SPL on TNFα-induced cytokine production in PAECs and the directional effects of SPL therapy in vivo (Figure 7D). Importantly, patients treated with SPL also more frequently received hydroxychloroquine, an immunomodulatory agent used in patients with connective tissue disease, and PGI2 infusions than patients not treated with SPL (see Supplementary material online, Table S4). In addition to adjusting for these imbalances in the multivariate model, several sensitivity analyses were performed including restriction of patient-level covariates to only those that reached statistical significance (i.e. PGI2 infusion, hydroxychloroquine use, and obesity; see Supplementary material online, Table S4), propensity score adjustment and removing the five patients treated with hydroxychloroquine, which all similarly indicated that SPL was uniquely anti-inflammatory. Nevertheless, these results are largely hypothesis generating and need to be confirmed in a well-controlled clinical trial.
4. Discussion
SPL-induced degradation of the XPB subunit of TFIIH suppresses inflammatory responses independent of MR and may contribute to the benefits of SPL in cardiovascular diseases. SPL was found to suppress NF-κB and AP-1 inflammatory signalling independent of all recognized nuclear receptor binding partners. Importantly, blocking XPB degradation or overexpressing XPB reversed SPL suppression of NF-κB and AP-1 reporters. Likewise, a proteasome inhibitor blocked both XPB loss and SPL suppression of PMA-induced genes in human PAECs. In addition, SPL in combination with XPB knockdown had the strongest suppressive effect on TNFα target genes in PAECs. Unlike SPL, EPL did not cause XPB degradation and failed to suppress these inflammatory signalling pathways. Gene susceptibility to SPL was associated with low basal RNAPII occupancy and robust TNFα-induced recruitment of XPB and RNAPII. In patient fibroblasts carrying an N-terminal mutation in XPB, SPL neither induced XPB degradation nor suppressed TNFα-induced target genes. Finally, SPL treatment decreased whole lung XPB protein levels in rats and reduced serum inflammatory markers in an observational cohort of PAH patients.
Putative evidence that SPL might suppress inflammation independent of MR had been previously based largely on the failure of other MR ligands (e.g. aldosterone, canrenone, 7α-thiomethyl-SPL and EPL) to either block or mimic some of the immune modulating effects of SPL.8–11 Here, the activation of NF-κB and AP-1 reporters were used to individually test the role of not only MR, but also AR, GR, PR, PXR, and RXRγ. SPL, but not EPL, displayed unambiguous anti-inflammatory effects on NF-κB- and AP-1 signalling independent of nuclear receptor expression. SPL had been reported to reduce p65 and inhibitor of kappa B (IκB) kinase activation,32 as well as modestly blunt IκBα phosphorylation.11 However, a mechanistic explanation has not emerged for these previous findings, which were only evident at SPL concentrations 10-fold higher than those used here. In contrast, we found that SPL had no effect on NF-κB binding to DNA and SPL-induced XPB degradation was shown to more broadly impact inflammatory signalling by similarly affecting both NF-κB- and AP-1-driven transcription.
The selectivity of XPB loss for inflammatory response genes was unexpected. Recently, basal and all-trans retinoic acid-induced transcription was found to be relatively spared in a human lung fibroblast line following SPL treatment.17 This indicates that SPL-induced XPB depletion primarily impacts NER and largely spares RNAPII-dependent transcription. However, an earlier study in human peripheral blood mononuclear cells found that SPL, through an unclear MR-independent mechanism, suppressed a substantial subset of LPS-induced target genes.10 This was perhaps the first indication that SPL has specificity for inhibiting inflammatory gene transcription. Consistent with this emerging paradigm, we found that SPL-induced XPB loss broadly, but not universally affected the transcription of inflammatory response genes in human PAECs. Thus, cell- and gene-specific effects, as well as context (basal vs. inducible) influence the transcriptional consequences of drug-induced XPB depletion. Importantly, small molecule-induced protein degradation is an emerging pharmaceutical strategy for targeting parts of the proteome that have been otherwise inaccessible to traditional drug development.33 As such, SPL can serve as the prototype for new drug classes with clinically important in vivo effects on inflammation due to the induced loss of specific proteins.
Eight different XPB mutations have been associated with three distinct but related diseases, XP, XP/CS, and trichothiodystrophy.28 Some XPB mutants have been associated with transcriptional deficiencies, but responses to inflammatory stimuli were not tested.25,34–36 We found that TNFα-induced IL6, IL8, CCL2, and NFKBIA mRNA expression was preserved or even increased in fibroblasts harbouring XPB mutations, results similar to moderate XPB knockdown in PAECs (Figure 4). Consistent with our findings, fibroblasts from patients with trichothiodystrophy have been reported to express higher basal and PMA-induced levels of MMP-1 mRNA compared with normal fibroblasts.37 Additionally, peripheral blood lymphocytes from XP patients exhibit normal basal and IL2-induced TNFα production.38
Here, SPL exposure combined with susceptible XPB mutations or siRNA knockdown resulted in very low levels of XPB and the strongest suppressive effect on target genes, even those that had been resistant to SPL-induced XPB depletion alone. The strongest interactions between SPL and XPB knockdown or C-terminal mutants of XPB were observed for NFKBIA, which is both constitutively expressed and inducible.39 In normal PAECs, the inability of SPL to suppress NFKBIA correlated with high basal total and activated RNAPII promoter occupancy and only modest TNFα-induced RNAPII and XPB recruitment. In contrast, IL8 had much less pre-positioned RNAPII and required significantly more recruitment of RNAPII and XPB. Persistently low levels of XPB in cells harbouring mutations may favor a more ‘closed’ chromatin conformation.25 Likewise, XPB knockdown has relatively long-term effects that may reduce basal promoter occupancy, thereby augmenting the impact of factor recruitment. However, we also cannot exclude the possibility that SPL induces the degradation of unknown proteins besides XPB that interact with XPB and/or the transcriptional machinery to produce the findings reported here. For example, XPB could simply interfere with SPL-induced inflammatory suppression. Nonetheless, XPB degradation was shown to have a central mechanistic role in the MR-independent anti-inflammatory effects of SPL. Notably, the N-terminal F99S mutant form of XPB was resistant to SPL-induced degradation suggesting that this effect has substantial structural specificity. Similar to our results, a recent paper found that a one amino acid substitution blocked sulfonamide-mediated selective degradation of the splicing factor CAPERα.40
In two separate rodent models of pulmonary hypertension, SPL reduced right ventricular systolic pressure, pulmonary vascular resistance, and pulmonary vascular remodelling.18,19 Although pulmonary vascular fibrosis was also decreased in both models, neither study comprehensively investigated the effects of SPL on inflammation. Nevertheless, Preston et al. found less perivascular inflammation in treated animals. Here we demonstrate that SPL treatment decreased whole lung XPB protein levels in MCT-PH rats. Although not examined in vivo, SPL-induced degradation of XPB is not dependent on the presence of an inflammatory stimulus in vitro (Figure 3A) and therefore is not expected to be specific to MCT-challenged rats. In models of aldosterone excess, MR blockade alone is also likely beneficial as EPL, which does not induce XPB degradation, has been shown to reduce vascular inflammation and fibrosis in some models of PAH.2
In heart failure patients as well as patients with rheumatoid arthritis, SPL improves endothelial function and reduces inflammatory markers.8,41,42 Likewise, here in a small, retrospective cohort of PAH patients, SPL therapy was associated with an overall suppressive effect on circulating cytokines (Figure 7B and C). Although this analysis is largely hypothesis generating, directional concordance with effects of SPL and XPB degradation on PAECs in vitro is striking. Preliminary safety results were recently reported for the first of three studies investigating the effects of SPL in patients with PAH.43 Two additional SPL studies, a randomized, double-blind, placebo-controlled trial4 and a cross-over design in combination with the ET-1 antagonist ambrisentan (NCT02253394) are currently ongoing. These studies may lend support for the early use of SPL in PAH. SPL is a prototype for drugs that induce the degradation of protein targets and XPB depletion is a previously unrecognized anti-inflammatory mechanism that may prove therapeutically useful in diseases characterized by vascular inflammation.
Supplementary Material
Acknowledgements
We thank Drs. Gomez-Sanchez at the University of Mississippi Medical Center for sharing their monoclonal antibody against the mineralocorticoid receptor and Merte Lemma at the Inova Heart and Vascular Institute for collecting and organizing clinical data. Our dedicated research nurses, Bonnie Harper, RN and Grace Graninger, RN assisted with patient care as well as sample and data collection. We also greatly appreciate Kelly Byrne for editing and formatting the manuscript and figures. This research was supported by intramural NIH funds.
Conflict of interest: none declared.
Funding
This research was supported by the NIH Intramural Research Program, the NIH Clinical Center and the National Heart, Lung and Blood Institute.
Time for primary review: 38 days
References
- 1. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J.. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med 1999;341:709–717. [DOI] [PubMed] [Google Scholar]
- 2. Moss ME, Jaffe IZ.. Mineralocorticoid Receptors in the Pathophysiology of Vascular Inflammation and Atherosclerosis. Front Endocrinol (Lausanne) 2015;6:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maron BA, Leopold JA.. The role of the renin-angiotensin-aldosterone system in the pathobiology of pulmonary arterial hypertension (2013 Grover Conference series). Pulm Circ 2014;4:200–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Elinoff JM, Rame JE, Forfia PR, Hall MK, Sun J, Gharib AM, Abd-Elmoniem K, Graninger G, Harper B, Danner RL, Solomon MA.. A pilot study of the effect of spironolactone therapy on exercise capacity and endothelial dysfunction in pulmonary arterial hypertension: study protocol for a randomized controlled trial. Trials 2013;14:91.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bajusz E, Jasmin G.. Effect of aldactone, an antiminera-locorticoid steroid spironolactone, on inflammation. Rev Can Biol 1961;20:829–832. [PubMed] [Google Scholar]
- 6. Fiebeler A, Schmidt F, Muller DN, Park JK, Dechend R, Bieringer M, Shagdarsuren E, Breu V, Haller H, Luft FC.. Mineralocorticoid receptor affects AP-1 and nuclear factor-kappab activation in angiotensin II-induced cardiac injury. Hypertension 2001;37:787–793. [DOI] [PubMed] [Google Scholar]
- 7. Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT.. Aldosterone-induced inflammation in the rat heart: role of oxidative stress. Am J Pathol 2002;161:1773–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bendtzen K, Hansen PR, Rieneck K.. Spironolactone/Arthritis Study G. Spironolactone inhibits production of proinflammatory cytokines, including tumour necrosis factor-alpha and interferon-gamma, and has potential in the treatment of arthritis. Clin Exp Immunol 2003;134:151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hansen PR, Rieneck K, Bendtzen K.. Spironolactone inhibits production of proinflammatory cytokines by human mononuclear cells. Immunol Lett 2004;91:87–91. [DOI] [PubMed] [Google Scholar]
- 10. Sonder SU, Mikkelsen M, Rieneck K, Hedegaard CJ, Bendtzen K.. Effects of spironolactone on human blood mononuclear cells: mineralocorticoid receptor independent effects on gene expression and late apoptosis induction. Br J Pharmacol 2006;148:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sonder SU, Woetmann A, Odum N, Bendtzen K.. Spironolactone induces apoptosis and inhibits NF-kappaB independent of the mineralocorticoid receptor. Apoptosis 2006;11:2159–2165. [DOI] [PubMed] [Google Scholar]
- 12. Fagart J, Hillisch A, Huyet J, Barfacker L, Fay M, Pleiss U, Pook E, Schafer S, Rafestin-Oblin ME, Kolkhof P.. A new mode of mineralocorticoid receptor antagonism by a potent and selective nonsteroidal molecule. J Biol Chem 2010;285:29932–29940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rigalli JP, Ruiz ML, Perdomo VG, Villanueva SS, Mottino AD, Catania VA.. Pregnane X receptor mediates the induction of P-glycoprotein by spironolactone in HepG2 cells. Toxicology 2011;285:18–24. [DOI] [PubMed] [Google Scholar]
- 14. Leung WH, Vong QP, Lin W, Janke L, Chen T, Leung W.. Modulation of NKG2D ligand expression and metastasis in tumors by spironolactone via RXRgamma activation. J Exp Med 2013;210:2675–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alekseev S, Ayadi M, Brino L, Egly JM, Larsen AK, Coin F.. A small molecule screen identifies an inhibitor of DNA repair inducing the degradation of TFIIH and the chemosensitization of tumor cells to platinum. Chem Biol 2014;21:398–407. [DOI] [PubMed] [Google Scholar]
- 16. Dougherty EJ, Elinoff JM, Ferreyra GA, Hou A, Cai R, Sun J, Blaine KP, Wang S, Danner RL.. Mineralocorticoid Receptor (MR) trans-Activation of Inflammatory AP-1 Signaling: dependence on DNA sequence, MR conformation, and AP-1 family member expression. J Biol Chem 2016;291:23628–23644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alekseev S, Nagy Z, Sandoz J, Weiss A, Egly JM, Le May N, Coin F.. Transcription without XPB establishes a unified helicase-independent mechanism of promoter opening in eukaryotic gene expression. Mol Cell 2017;65:504–514 e504. [DOI] [PubMed] [Google Scholar]
- 18. Maron BA, Zhang YY, White K, Chan SY, Handy DE, Mahoney CE, Loscalzo J, Leopold JA.. Aldosterone inactivates the endothelin-B receptor via a cysteinyl thiol redox switch to decrease pulmonary endothelial nitric oxide levels and modulate pulmonary arterial hypertension. Circulation 2012;126:963–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Preston IR, Sagliani KD, Warburton RR, Hill NS, Fanburg BL, Jaffe IZ.. Mineralocorticoid receptor antagonism attenuates experimental pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2013;304:L678–L688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fu M, Liu M, Sauve AA, Jiao X, Zhang X, Wu X, Powell MJ, Yang T, Gu W, Avantaggiati ML, Pattabiraman N, Pestell TG, Wang F, Quong AA, Wang C, Pestell RG.. Hormonal control of androgen receptor function through SIRT1. Mol Cell Biol 2006;26:8122–8135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Newton R, Adcock IM, Barnes PJ.. Superinduction of NF-kappa B by actinomycin D and cycloheximide in epithelial cells. Biochem Biophys Res Commun 1996;218:518–523. [DOI] [PubMed] [Google Scholar]
- 22. Roux P, Blanchard JM, Fernandez A, Lamb N, Jeanteur P, Piechaczyk M.. Nuclear localization of c-Fos, but not v-Fos proteins, is controlled by extracellular signals. Cell 1990;63:341–351. [DOI] [PubMed] [Google Scholar]
- 23. Palombella VJ, Rando OJ, Goldberg AL, Maniatis T.. The ubiquitin-proteasome pathway is required for processing the NF-kappa B1 precursor protein and the activation of NF-kappa B. Cell 1994;78:773–785. [DOI] [PubMed] [Google Scholar]
- 24. Fishburn J, Tomko E, Galburt E, Hahn S.. Double-stranded DNA translocase activity of transcription factor TFIIH and the mechanism of RNA polymerase II open complex formation. Proc Natl Acad Sci U S A 2015;112:3961–3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Singh A, Compe E, Le May N, Egly JM.. TFIIH subunit alterations causing xeroderma pigmentosum and trichothiodystrophy specifically disturb several steps during transcription. Am J Hum Genet 2015;96:194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lacombe B, Morel M, Margottin-Goguet F, Ramirez BC.. Specific Inhibition of HIV Infection by the Action of Spironolactone in T Cells. J Virol 2016;90:10972–10980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vermeulen W, Scott RJ, Rodgers S, Muller HJ, Cole J, Arlett CF, Kleijer WJ, Bootsma D, Hoeijmakers JH, Weeda G.. Clinical heterogeneity within xeroderma pigmentosum associated with mutations in the DNA repair and transcription gene ERCC3. Am J Hum Genet 1994;54:191–200. [PMC free article] [PubMed] [Google Scholar]
- 28. Oh KS, Khan SG, Jaspers NG, Raams A, Ueda T, Lehmann A, Friedmann PS, Emmert S, Gratchev A, Lachlan K, Lucassan A, Baker CC, Kraemer KH.. Phenotypic heterogeneity in the XPB DNA helicase gene (ERCC3): xeroderma pigmentosum without and with Cockayne syndrome. Hum Mutat 2006;27:1092–1103. [DOI] [PubMed] [Google Scholar]
- 29. Stacher E, Graham BB, Hunt JM, Gandjeva A, Groshong SD, McLaughlin VV, Jessup M, Grizzle WE, Aldred MA, Cool CD, Tuder RM.. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med 2012;186:261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Savai R, Pullamsetti SS, Kolbe J, Bieniek E, Voswinckel R, Fink L, Scheed A, Ritter C, Dahal BK, Vater A, Klussmann S, Ghofrani HA, Weissmann N, Klepetko W, Banat GA, Seeger W, Grimminger F, Schermuly RT.. Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2012;186:897–908. [DOI] [PubMed] [Google Scholar]
- 31. Soon E, Holmes AM, Treacy CM, Doughty NJ, Southgate L, Machado RD, Trembath RC, Jennings S, Barker L, Nicklin P, Walker C, Budd DC, Pepke-Zaba J, Morrell NW.. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation 2010;122:920–927. [DOI] [PubMed] [Google Scholar]
- 32. Kato Y, Kamiya H, Koide N, Odkhuu E, Komatsu T, Dagvadorj J, Watarai A, Kondo M, Kato K, Nakamura J, Yokochi T.. Spironolactone inhibits production of proinflammatory mediators in response to lipopolysaccharide via inactivation of nuclear factor-kappaB. Immunopharmacol Immunotoxicol 2014;36:237–241. [DOI] [PubMed] [Google Scholar]
- 33. Lai AC, Crews CM.. Induced protein degradation: an emerging drug discovery paradigm. Nat Rev Drug Discov 2017;16:101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Coin F, Bergmann E, Tremeau-Bravard A, Egly JM.. Mutations in XPB and XPD helicases found in xeroderma pigmentosum patients impair the transcription function of TFIIH. Embo J 1999;18:1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dianov GL, Houle JF, Iyer N, Bohr VA, Friedberg EC.. Reduced RNA polymerase II transcription in extracts of cockayne syndrome and xeroderma pigmentosum/Cockayne syndrome cells. Nucleic Acids Res 1997;25:3636–3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hwang JR, Moncollin V, Vermeulen W, Seroz T, van Vuuren H, Hoeijmakers JH, Egly JM.. A 3’ –> 5’ XPB helicase defect in repair/transcription factor TFIIH of xeroderma pigmentosum group B affects both DNA repair and transcription. J Biol Chem 1996;271:15898–15904. [DOI] [PubMed] [Google Scholar]
- 37. Arseni L, Lanzafame M, Compe E, Fortugno P, Afonso-Barroso A, Peverali FA, Lehmann AR, Zambruno G, Egly JM, Stefanini M, Orioli D.. TFIIH-dependent MMP-1 overexpression in trichothiodystrophy leads to extracellular matrix alterations in patient skin. Proc Natl Acad Sci U S A 2015;112:1499–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gaspari AA, Fleisher TA, Kraemer KH.. Impaired interferon production and natural killer cell activation in patients with the skin cancer-prone disorder, xeroderma pigmentosum. J Clin Invest 1993;92:1135–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Scott ML, Fujita T, Liou HC, Nolan GP, Baltimore D.. The p65 subunit of NF-kappa B regulates I kappa B by two distinct mechanisms. Genes Dev 1993;7:1266–1276. [DOI] [PubMed] [Google Scholar]
- 40. Uehara T, Minoshima Y, Sagane K, Sugi NH, Mitsuhashi KO, Yamamoto N, Kamiyama H, Takahashi K, Kotake Y, Uesugi M, Yokoi A, Inoue A, Yoshida T, Mabuchi M, Tanaka A, Owa T.. Selective degradation of splicing factor CAPERalpha by anticancer sulfonamides. Nat Chem Biol 2017;13:675–680. [DOI] [PubMed] [Google Scholar]
- 41. Farquharson CA, Struthers AD.. Spironolactone increases nitric oxide bioactivity, improves endothelial vasodilator dysfunction, and suppresses vascular angiotensin I/angiotensin II conversion in patients with chronic heart failure. Circulation 2000;101:594–597. [DOI] [PubMed] [Google Scholar]
- 42. Macdonald JE, Kennedy N, Struthers AD.. Effects of spironolactone on endothelial function, vascular angiotensin converting enzyme activity, and other prognostic markers in patients with mild heart failure already taking optimal treatment. Heart 2004;90:765–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Safdar Z, Tamez E, Thakur A, Entman M, Basant A, Frost A.. Effects of Spironolactone in Pulmonary Arterial Hypertension. Am J Respir Crit Care Med 2016;193:A7380. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.