Abstract
Aims
Hypertensive cardiac hypertrophy is associated with reduced coronary flow reserve, but its impact on coronary flow regulation and vasomotor function remains incompletely understood and requires further investigation.
Methods and results
Left ventricular hypertrophy was induced in mice by transverse aortic coarctation (TAC) for 4 weeks. The left coronary artery blood velocity (LCABV) and myocardium lactate level were measured following the metabolic activation by isoproterenol. Septal coronary arterioles were isolated and pressurized for functional studies. In TAC mice, the heart-to-body weight ratio was increased by 45%, and cardiac fractional shortening and LCABV were decreased by 51 and 14%, respectively. The resting myocardial lactate level was 43% higher in TAC mice. Isoproterenol (5 µg/g, i.p.) increased heart rate by 20% in both groups of animals, but the corresponding increase in LCABV was not observed in TAC mice. The ventricular hypertrophy was associated with elevation of myocardial endothelin-1 (ET-1), increased vascular expression of rho-kinases (ROCKs), and increased superoxide production in the myocardium and vasculature. In coronary arterioles from TAC mice, the endothelial nitric oxide (NO)-mediated dilation to acetylcholine (ACh) was reversed to vasoconstriction and the vasoconstriction to ET-1 was augmented. Inhibition of ROCK by H-1152 alleviated oxidative stress and abolished enhanced vasoconstriction to ET-1. Both H-1152 and superoxide scavenger Tempol abolished coronary arteriolar constriction to ACh in a manner sensitive to NO synthase blocker NG-nitro-L-arginine methyl ester.
Conclusions
Myocardial hypertrophy induced by pressure overload leads to cardiac and coronary microvascular dysfunction and ischaemia possibly due to oxidative stress, enhanced vasoconstriction to ET-1 and compromised endothelial NO function via elevated ROCK signalling.
Keywords: Cardiac hypertrophy, Arteriole, Nitric oxide, ROCK, Superoxide
1. Introduction
Cardiac hypertrophy is generally considered as a compensatory mechanism that normalizes the increased tension of the ventricular wall resulting from hypertension/pressure overload.1 However, cardiac hypertrophy is an independent risk factor for cardiovascular morbidity and mortality,2 which can be caused by insufficient blood supply to the hypertrophied myocardium due to reduced coronary flow reserve.3–5 Coronary blood flow is regulated by coronary arterioles, the major resistance vessels in the heart, by adjusting their diameters to maintain a constant flow under resting conditions6–9 and to ensure adequate perfusion to meet the oxygen demand of the myocardium.10,11 However, the impact of hypertensive cardiac hypertrophy on coronary vasomotor function and flow regulation remains incompletely understood.
Endothelial cells play a crucial role in regulating vasomotor activity by releasing vasoactive substances such as vasodilator nitric oxide (NO)12 and vasoconstrictor endothelin-1 (ET-1).13 The impairment of NO-mediated vasodilation by elevated oxidative stress has been considered as the hallmark of endothelial dysfunction in various cardiovascular diseases including hypertension.14,15 Interestingly, the expression of prepro-ET-1, the precursor of ET-1, has been reported to be increased in the hypertrophied heart during the progression of hypertension-induced cardiac dysfunction.16 ET-1 induces vasoconstriction by increasing the intracellular Ca2+ concentration through activation of the phospholipase C pathway and by inhibiting myosin light chain phosphatase (i.e. prolonging myosin light chain phosphorylation) through activation of rho-kinase (ROCK).17 Although current evidence suggests the importance of ROCK in the pathophysiology of myocardial hypertrophy in hypertension,18 its expression in the coronary microvasculature and its role in vasomotor regulation under the disease state remains unknown. In addition, ROCK activation can be associated with elevated oxidative stress,19–21 which is known as a major risk factor for the pathogenesis of cardiovascular diseases,15,19,20,22 but its relationship with ET-1 and endothelium-dependent NO function in coronary arterioles remains unclear.
In this study, we hypothesized that the deficiency of endothelial NO, elevation of oxidative stress, and enhanced vasoconstriction to ET-1, via ROCK signalling, contribute to the coronary arteriolar dysfunction in hypertensive myocardial hypertrophy. To test this hypothesis, transverse aortic coarctation (TAC) was performed to induce cardiac hypertrophy in mice and the associated coronary blood flow changes in response to metabolic activation were assessed by ultrasound echocardiography. To directly evaluate microvascular function without the confounding influences from systemic haemodynamics and humoral factors, coronary arterioles were isolated and pressurized for vasomotor assessment using videomicroscopic tools with pharmacologic approaches. Elucidating the relationship among ET-1, ROCK, and oxidative stress in vasomotor regulation of coronary arterioles under pressure overload may help our understanding on the development of coronary deficiency in hypertensive cardiac hypertrophy.
2. Methods
2.1 TAC mouse
The animal procedures were approved by the Institutional Animal Care and Use Committee, Baylor Scott and White Health, and conform to the Guide for the Care and Use of Laboratory Animals (National Institutes of Health). Male C57BL/6 mice, 6−8 weeks old, were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) (Hospira, USA) and xylazine (8 mg/kg) (LLOYD Laboratories, USA) mixture. A 2–3 mm left-sided thoracotomy was created at the second intercostal space without cutting the ribs.23 Aorta between the innominate and left common carotid artery was located and looped with a 7.0 Prolene suture. With an overlaying 27-gauge needle, the aorta was then ligated. After removing the needle, the procedure produced a discrete region of stenosis in the aorta. The chest was then closed and the mice were allowed to recover. A group of sham control mice received the same procedure without ligating the aorta. The heart to body weight ratio reaches plateau at 3–5 weeks after the TAC procedure as previously described,23 thus all the following experiments were performed at four weeks after surgery. All chemicals were purchased from Sigma-Aldrich unless otherwise noted.
2.2 Ultrasound echocardiography
Mice were anesthetized with 2.5% (v/v) of isoflurane (Piramal Healthcare, India) in 100% oxygen in a closed chamber and the anesthesia was maintained with 2% isoflurane using a nose cone. The VisualSonics Vevo 2100 system (FujiFilm VisualSonics, Canada) was used to record electrocardiogram (ECG) and cardiac imaging. Mice were taped supine to ECG electrodes on a procedure board maintained at 37 °C. A MS-550D transducer with frequency of 32 MHz was used to measure left ventricular end-diastolic diameter (EDD), end-systolic diameter (ESD) and left coronary artery blood velocity (LCABV). Fractional shortening was calculated by (EDD − ESD)/EDD. To measure LCABV, the left main coronary artery was located by color Doppler and then the velocity waveform was recorded. The highest velocity during the diastolic phase was measured and defined as the peak LCABV. To measure the LCABV after metabolic activation, a group of mice received β-adrenergic agonist isoproterenol injection (5 µg/g body weight, i.p.) to increase myocardial activity. The peak LCABV was measured before (baseline) and after (treated) isoproterenol administration. The LCABV change was calculated as the ratio of treated and baseline peak LCABV and expressed as a percentage of change. Phosphate buffered saline (PBS) injection served as control. At the end of ultrasound protocols, the heart was removed, frozen immediately with liquid nitrogen and stored at −80 °C for the lactate assay as described below.
2.3 Lactate assay
A commercialized lactate assay kit (BioVision, USA) was used to measure lactate concentration following the manufacturer's instructions. In brief, the left ventricular tissue was homogenized with Lactate Assay Buffer and then centrifuged at 13 000 g for 10 min. The supernatant was then passed through a 10 kDa molecular weight spin filter. Lactate concentration of the sample was measured using a colorimetric assay and the optical density was read at 570 nm by the μQuant Microplate Spectrophotometer (BioTek Instruments, USA).
2.4 Functional assessment of isolated coronary arterioles
Mice were anesthetized with ketamine (100 mg/kg) and xylazine (8 mg/kg) and the heart was excised and placed in ice-cold saline. The techniques for identification, isolation and cannulation of coronary arterioles have been described previously.24 In brief, the aorta was cannulated with PE-60 tubing (BD Biosciences, USA) and a mixture of India ink and gelatin in physiological salt solution (PSS; NaCl, 145.0 mM; KCl, 4.7 mM; CaCl2, 2.0 mM; Mg2SO4, 1.17 mM; NaH2PO4, 1.2 mM; glucose, 5.0 mM; pyruvate, 2.0 mM; EDTA, 0.02 mM; and MOPS, 3.0 mM) was back perfused into coronary arteries to visualize the coronary microvessels. Septal coronary arterioles with internal diameter about 40–60 μm in situ were dissected out from the surrounding tissue in PSS with 1% albumin at 8 °C. The isolated vessel was then transferred to a tissue chamber and cannulated onto a pair of glass micropipettes and pressurized to 60 cmH2O lumenal pressure without flow by two independent reservoir systems. After developing stable basal tone at 36–37 °C, arteriolar dilations in response to the endothelium-dependent, NO-mediated vasodilator acetylcholine (ACh, 10−7–10−3 M) and the endothelium-independent NO-donor sodium nitroprusside (SNP, 10−8–10−5 M) were examined. In another series of experiments, the vessels were incubated with NO synthase inhibitor NG-nitro-L-arginine methyl ester (L-NAME, 10−5 M) for 30 min to assess the role of NO in the coronary response of TAC animals. In addition, the contribution of superoxide to the vasomotor response was determined with superoxide scavenger 4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl (Tempol, 1 mM, 60-min incubation). In another set of experiments, the concentration-dependent arteriolar constriction to ET-1 (10−11–10−9 M) was examined. To determine the role of ROCK in arteriolar responses to ET-1 and ACh, vessels were treated with ROCK inhibitor H-1152 (10−8 M, 60-min incubation) in another series of studies. At the end of each experiment, the maximum diameter of the vessels was obtained at 10−4 M SNP in the presence of calcium-free PSS with EDTA (1 mM).
2.5 Western blotting
Total protein samples were extracted from the myocardium and coronary arterioles of the left ventricle. Proteins (10 μg) were separated by 4–15% gradient SDS-PAGE (Bio-Rad, USA), electrotransfered onto nitrocellulose membrane and blocked with skim milk. Blots were probed with goat anti-ET-1 antibody (1:250, Santa Cruz, USA), rabbit anti-ROCK1 antibody (1:250, Santa Cruz, USA), rabbit anti-ROCK2 antibody (1:250, Santa Cruz, USA), mouse anti-GAPDH antibody (1:1000, Santa Cruz, USA) or mouse anti-smooth muscle actin antibody (1:1000, Sigma-Aldrich, USA). After incubation with appropriate HRP-conjugated secondary antibodies, membranes were developed by enhanced chemiluminescence (Thermo Scientific, USA). Densitometric analyses of immunoblots were performed by Quantity One software (Bio-Rad, USA).
2.6 Detection of superoxide
Myocardial tissue and coronary arterioles were isolated from the left ventricle of sham and TAC mice and then treated with ROCK inhibitor H-1152 (10−8 M) or with PSS as control at 37 °C for 2 hours. Samples were embedded in Tissue-Tek O.C.T. Compound (Sakura Finetek, USA) and cut into 10-μm thick sections using a Leica CM1850 cryostat (Leica, Germany). Sections were stained with dihydroethidium (DHE, 4 μM) for 30 min. Fluorescence images were taken using an Axiovert 200 microscope (Zeiss, Germany). Settings for image acquisition were identical for all groups. The fluorescence intensities of DHE staining were analysed by AxioVision software (Zeiss, Germany).
2.7 Data analysis
Vessel diameters were normalized to the percentage of resting diameter. Statistical analysis was performed using Student's t test, one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test, or repeated measures two-way ANOVA followed by Bonferroni multiple-range test when appropriate. A value of P < 0.05 was considered significant. The data are expressed as means ± SEM.
3. Results
3.1 Cardiac function and LCABV
As shown in Table 1, at four weeks after surgery, there was no difference in the body weight between TAC and sham mice. However, the heart weight to body weight ratio increased by 45% in TAC mice. The heart rate (HR) in TAC mice was slightly but significantly higher than that in sham mice by 9%. The left ventricular fractional shortening and the peak LCABV were decreased by 51 and 14% in TAC mice, respectively.
Table 1.
Summary of heart weight and echocardiography data at 4 weeks after surgery
| Sham | TAC | |
|---|---|---|
| Body weight (g) | 25.5 ± 0.3 | 24.9 ± 0.3 |
| HW/BW (mg/g) | 6.88 ± 0.37 | 9.99 ± 0.56 * |
| HR (bpm) | 342.3 ± 11.9 | 372.4 ± 7.6 * |
| Fractional shortening (%) | 51.49 ± 6.64 | 25.00 ± 4.07 * |
| Peak LCA blood velocity (mm/s) | 337.48 ± 13.72 | 289.37 ± 22.95 * |
All data are expressed as Mean ± SEM, n = 20.
HW/BW, heart weight to body weight ratio; LCA, left coronary artery.
P < 0.05 vs. Sham; t-test.
3.2 Metabolic activation
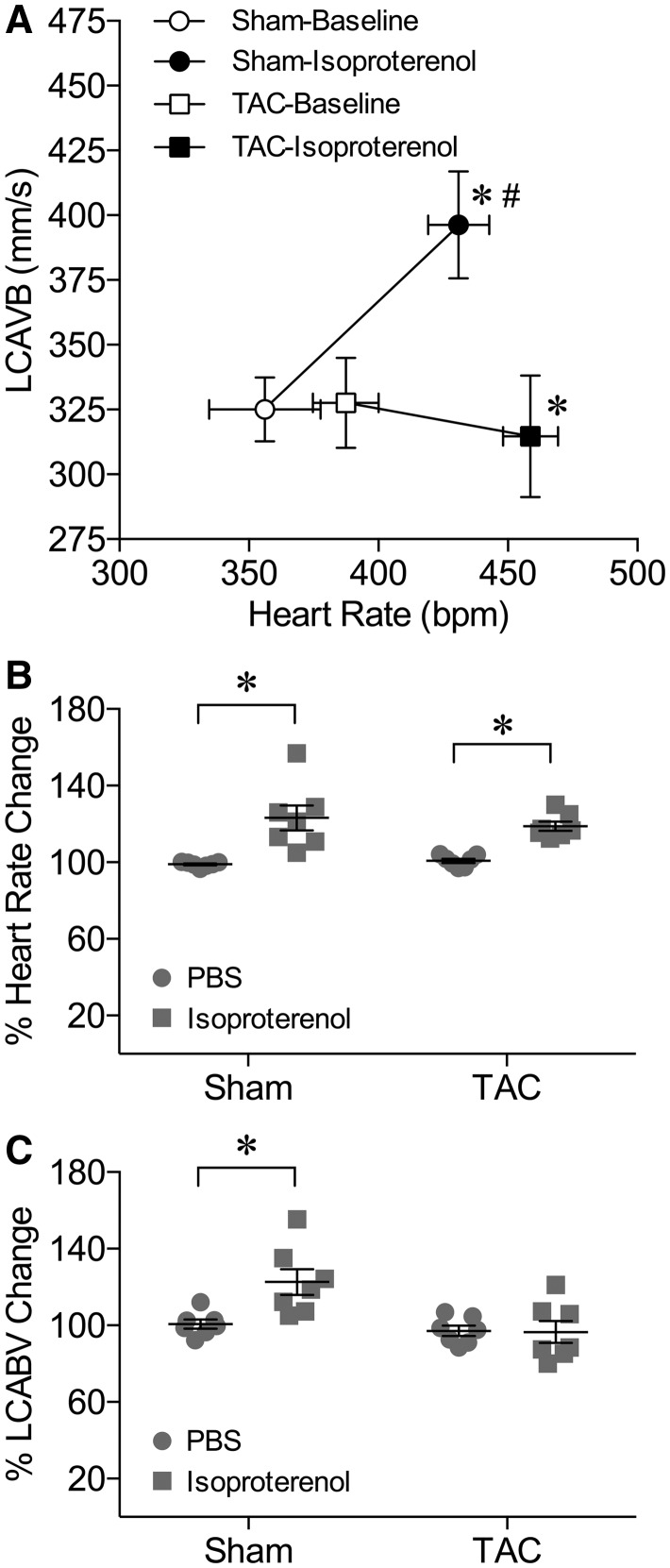
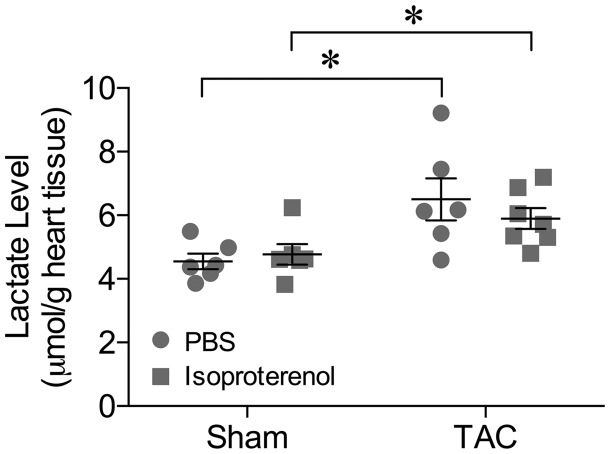
Intraperitoneal injection of β-adrenergic agonist isoproterenol (5 µg/g body weight) caused a simultaneous increase in HR and peak LCABV in sham mice (Figure 1A). Isoproterenol also induced a comparable increase in HR in TAC mice, but the peak LCABV remained unchanged (Figure 1A). When compared with PBS injection, isoproterenol comparably increased HR by 23 and 19% in sham and TAC mice, respectively (Figure 1B). However, the corresponding increase in the peak LCABV (by 23%) was only observed in sham mice (Figure 1C). To determine whether TAC leads to myocardial ischaemia under the resting state or after metabolic activation, myocardial tissue from the left ventricle was subjected to a lactate assay after treating with PBS (resting control) or isoproterenol. As shown in Figure 2, the myocardial lactate level in TAC mice was significantly higher than that in sham mice under resting conditions. Administration of isoproterenol did not alter the lactate level in either sham or TAC mice (Figure 2).
Figure 1.
Myocardial activation fails to increase coronary blood flow velocity in TAC mice. (A) Administration of isoproterenol (5 µg/g, i.p.) induced a simultaneous increase in HR and LCABV in sham mice. Administration of isoproterenol also increased HR in TAC mice but LCABV remained unchanged. * P < 0.05 vs. Baseline in HR, n = 7, t-test; #P < 0.05 vs. Baseline in LCABV, n = 7, t-test. (B) As compared to PBC injection, isoproterenol induced a significant increase in HR in both sham and TAC mice. (C) Administration of isoproterenol increased LCABV in sham mice but not in TAC mice. *P < 0.05, n = 7, t-test.
Figure 2.
Myocardial ischaemia in TAC mice. The myocardium lactate level was elevated in TAC mice as compared to sham control under resting conditions (PBS injection). The myocardial lactate level in either sham or TAC mice was not altered by isoproterenol. *P < 0.05, n = 7, one-way ANOVA followed by Tukey’s multiple comparisons test.
3.3 Endothelium-dependent, NO-mediated arteriolar dilation
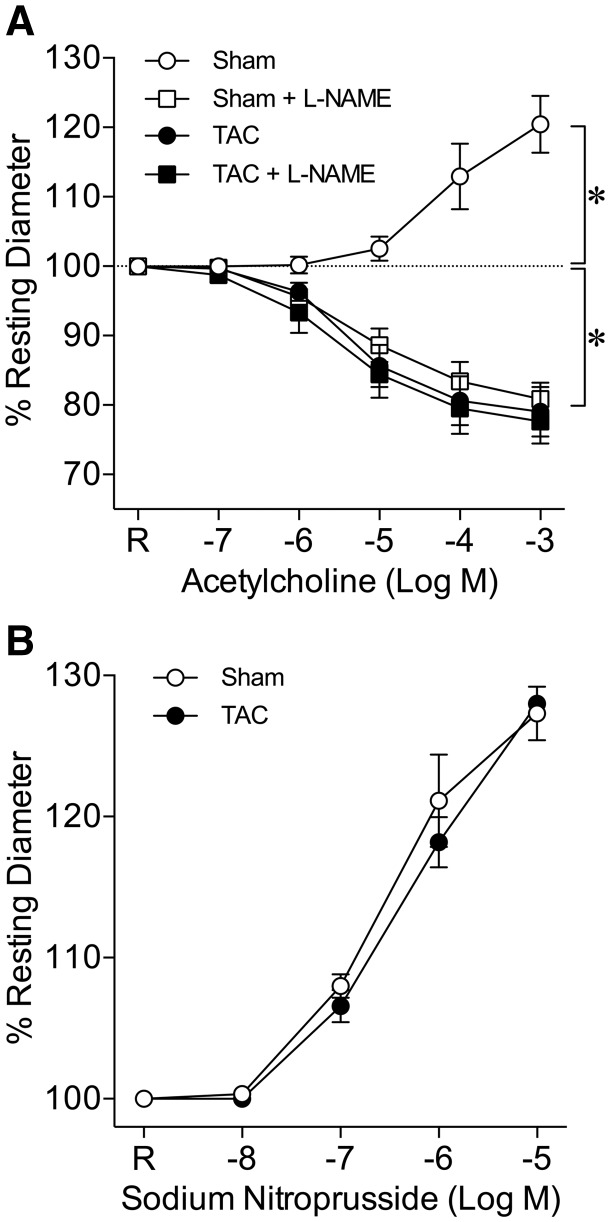
Coronary arterioles isolated from sham and TAC mice developed stable and comparable basal tone (Sham: 76.4 ± 0.8% vs. TAC: 75.8 ± 0.9% of maximum diameter) at 60 cmH2O lumenal pressure with no difference in either resting or maximum diameter (Table 2). ACh induced a concentration-dependent dilation of coronary arterioles from sham mice, and this response was reversed to vasoconstriction in the presence of NO synthase inhibitor L-NAME (Figure 3A). Coronary arterioles from TAC mice constricted in response to ACh, and L-NAME had no effect on this vasoconstriction (Figure 3A). In contrast to ACh-induced responses, the vasodilation to endothelium-independent vasodilator SNP was not different in vessels from sham and TAC mice (Figure 3B).
Table 2.
Coronary arteriolar diameter and basal tone
| Sham (n = 31) | TAC (n = 34) | |
|---|---|---|
| Resting diameter (μm) | 65.6 ± 2.1 (42–100) | 62.1 ± 2.4 (37–89) |
| Maximum diameter (μm) | 85.9 ± 2.6 (55–128) | 81.8 ± 2.9 (49–106) |
| Basal tone (%) | 76.4 ± 0.8 (67–83) | 75.8 ± 0.9 (68–88) |
All data are expressed as mean ± SEM (range).
Figure 3.
Deficiency of endothelium-dependent NO-mediated coronary arteriolar dilation in TAC mice. (A) ACh induced concentration-dependent dilation of coronary arterioles from sham mice (Sham, n = 6). In the presence of NO synthase inhibitor L-NAME (10−5 M), the vasodilation was reversed to vasoconstriction (Sham + L-NAME, n = 6). Coronary arterioles from TAC mice constricted to ACh (TAC, n = 9) and L-NAME had no effect on this vasoconstriction (TAC + L-NAME, n = 6). *P < 0.05 vs. resting diameter (dashed line), repeated measures two-way ANOVA with Bonferroni multiple-range test. (B) The endothelium-independent vasodilation induced by SNP was not different in coronary arterioles from sham and TAC mice (n = 5, repeated measures two-way ANOVA with Bonferroni multiple-range test). R, Resting Diameter.
3.4 ROCK-dependent arteriolar constriction to ET-1
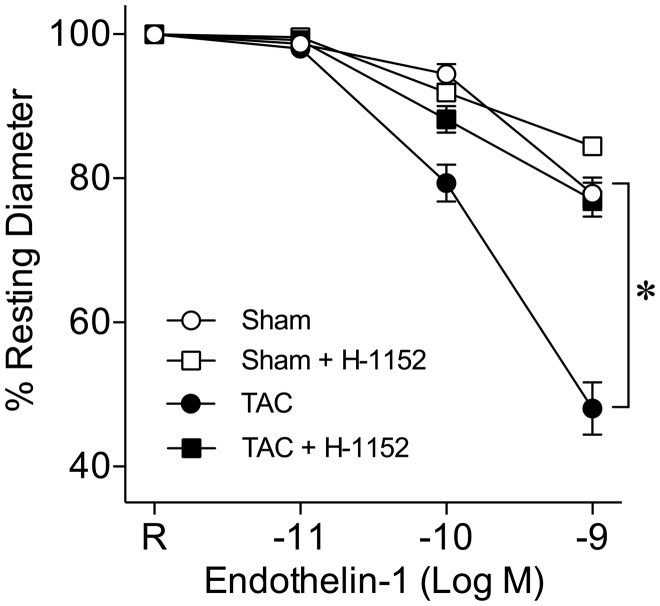
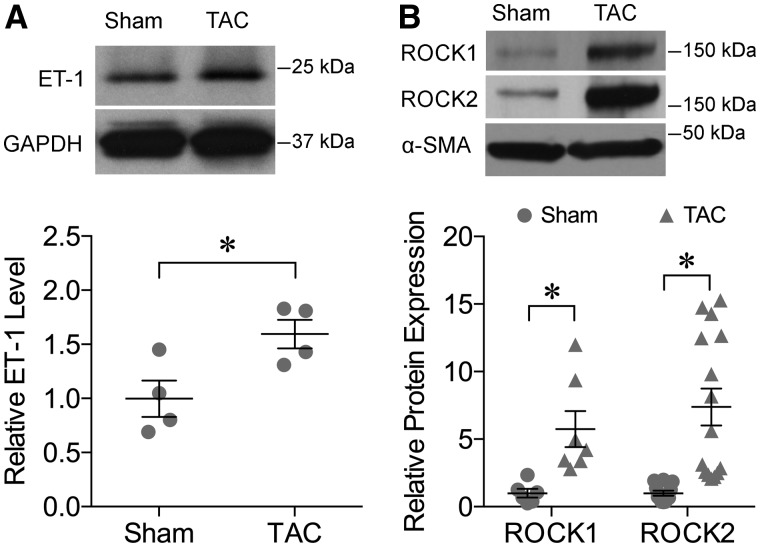
Coronary arterioles constricted in a concentration-dependent manner to ET-1, and this response was enhanced in vessels isolated from TAC mice (Figure 4). In the presence of ROCK inhibitor H-1152 (10−8 M), the vasoconstriction to ET-1 was not altered in the vessels from sham mice but the enhanced vasoconstriction to ET-1 in TAC mice was abolished (Figure 4). Expression of the ET-1 peptide in the myocardium was elevated in TAC mice (Figure 5A). The ROCK1 and ROCK2 protein expressions in coronary arterioles from TAC mice were also increased significantly (Figure 5B).
Figure 4.
ROCK activation potentiates coronary arteriolar constriction to ET-1 in TAC mice. ET-1 induced concentration-dependent constriction of coronary arterioles from sham mice (Sham, n= 9). Arteriolar constriction to ET-1 was enhanced in TAC mice (TAC, n = 11). Pre-treatment with H-1152 (10−8 M) had no effect on ET-1-induced vasoconstriction in sham mice (Sham + H-1152, n = 6) but abolished the enhanced vasoconstriction in TAC mice (TAC + H-1152, n = 6). R, Resting Diameter; *P < 0.05, repeated measures two-way ANOVA with Bonferroni multiple-range test.
Figure 5.
Upregulation of ET-1 and ROCK expression in TAC mice. (A) Upper Panel: Protein expression of ET-1 in the myocardium tissue was increased in TAC mice. The expression of GAPDH was used as an internal control. Lower Panel: The quantitative results of four independent experiments. *P < 0.05, t-test. (B) Upper Panel: The protein expression of ROCK1 and ROCK2 was increased in coronary arterioles from TAC mice. Lower Panel: The quantitative results of ROCK1 (n = 7) and ROCK2 (n = 15). *P < 0.05, t-test.
3.5 Myocardial and coronary superoxide production
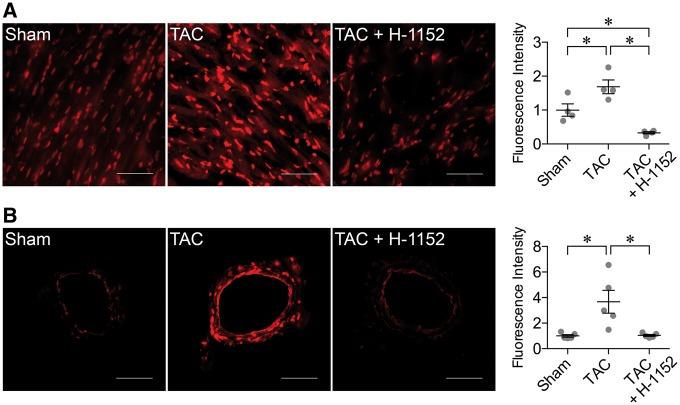
The fluorescence signals for superoxide were significantly elevated in the myocardial tissue (Figure 6A) and coronary arterioles (Figure 6B) from TAC mice. In the presence of H-1152, the elevated superoxide in these tissues was reversed to the level similar to that found in sham mice (Figure 6).
Figure 6.
Elevation of ROCK-mediated oxidative stress in TAC mice. (A) Left Panel: The superoxide level in the myocardium was increased in TAC mice. ROCK inhibitor H-1152 significantly decreased superoxide production in the myocardium. Scale bar: 50 μm. Right Panel: The quantitative results from four sets of experiments. *P < 0.05, one-way ANOVA followed by Tukey’s multiple comparisons test. (B) Left Panel: The superoxide level was increased in the isolated coronary arterioles from TAC mice. H-1152 abolished the increased superoxide (TAC + H-1152). Scale bar: 50 μm. Right Panel: The quantitative results from five sets of experiments. *P < 0.05, one-way ANOVA followed by Tukey’s multiple comparisons test.
3.6 ROCK and NO-related arteriolar function
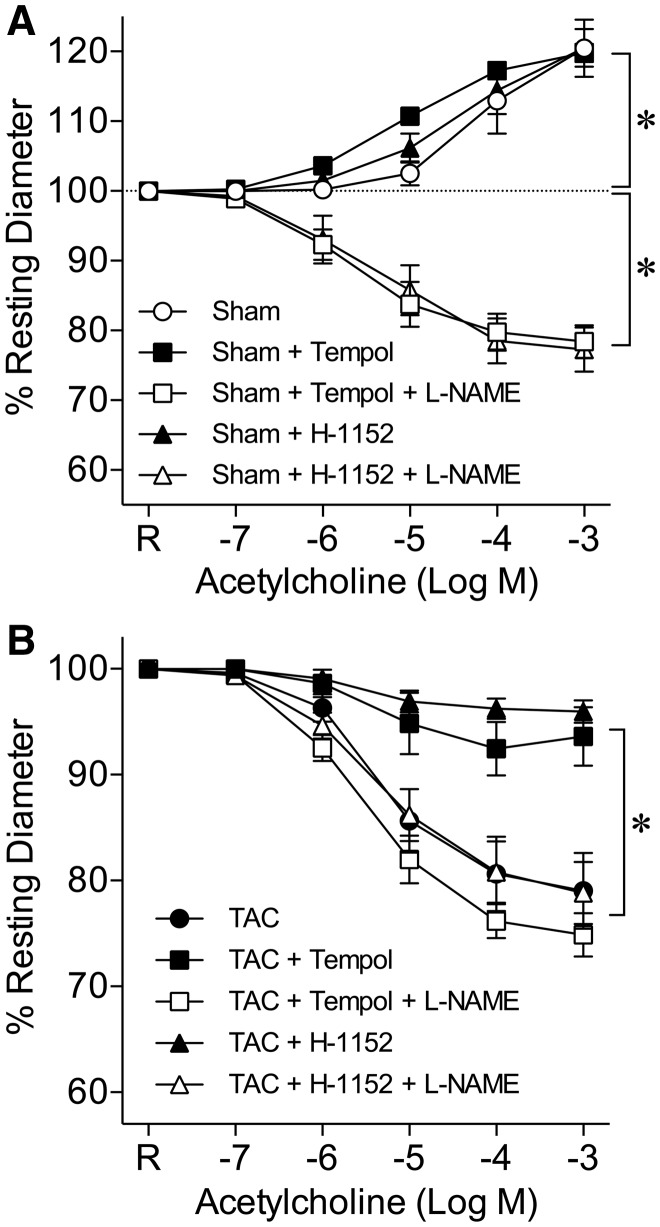
Incubation of coronary arterioles from sham mice with superoxide scavenger Tempol or ROCK inhibitor H-1152 did not affect ACh-induced vasodilation, which was sensitive to L-NAME (Figure 7A). However, Tempol significantly attenuated vasoconstriction to ACh in TAC mice (Figure 7B). The effect of Tempol was abolished in the presence of L-NAME. Treating coronary arterioles from TAC mice with H-1152 almost abolished arteriolar constriction to ACh, and the effect of H-1152 was reversed by L-NAME (Figure 7B).
Figure 7.
ROCK-dependent superoxide production contributes to NO deficiency and coronary vasomotor dysfunction. (A) Incubation of coronary arterioles with Tempol (Sham + Tempol, n = 6) or H-1152 (Sham + H-1152, n = 6) did not affect acetylcholine-induced vasodilation in sham mice. The vasodilation was reversed to vasoconstriction in the presence of L-NAME (Sham + Tempol + L-NAME, n = 6; Sham + H-1152 + L-NAME, n = 6). R, Resting Diameter; *P < 0.05, repeated measures two-way ANOVA with Bonferroni multiple-range test. (B) Acetylcholine induced concentration-dependent constriction of isolated coronary arterioles from TAC mice. Superoxide scavenger Tempol significantly attenuated the vasoconstriction (TAC + Tempol, n = 6). The effect of Tempol was abolished by L-NAME (TAC + Tempol + L-NAME, n = 6). ROCK inhibitor H-1152 also significantly attenuated the vasoconstriction to acetylcholine (TAC + H-1152, n = 6) in a manner sensitive to L-NAME (TAC + H-1152 + L-NAME, n = 6). R, Resting Diameter; *P < 0.05, repeated measures two-way ANOVA with Bonferroni multiple-range test.
4. Discussion
This study characterized coronary microvascular dysfunction in the mouse model of hypertensive myocardial hypertrophy. Ultrasound echocardiography demonstrated decreased resting LCABV and compromised coronary blood flow in response to metabolic activation, which was associated with elevated myocardial lactate level. Coronary arterioles exhibited impaired endothelium-dependent NO-mediated vasodilation, enhanced ET-1-induced vasoconstriction, and elevated superoxide production. The overexpression of ROCK and its signalling appears to be responsible for the altered microvascular function via elevated oxidative stress in the vascular wall.
The TAC mouse model has been adopted for studying the mechanism of pressure overload-induced cardiac hypertrophy and myocardial dysfunction.25–28 Although the structural and functional changes and the mechanism of myocardial hypertrophy in response to pressure overload have been well studied,27,29 its impact on the coronary microcirculation remains unclear despite that myocardial ischaemia is commonly observed.30,31 In this study, TAC significantly increased heart to body weight ratio by 45% at 4 weeks post-operation, a result comparable to that reported by others.23,32,33 Decreased left ventricular fractional shortening was also observed in the TAC mice, indicating the decrease in cardiac function toward the decompensated phase of heart failure. This is consistent with the results reported in a similar mouse model at 4 weeks after TAC.22 Although coronary perfusion pressure was not measured in this study, the observed reduction in baseline LCABV (by ∼14%), in association with elevated aortic pressure by coarctation, suggested that coronary perfusion might be compromised due to increased coronary resistance in TAC mice. This context is supported by a recent finding that the reduction in left ventricular blood flow under resting condition correlates positively with the development of cardiac hypertrophy and left ventricular dysfunction in TAC mice.34 The myocardial hypertrophy derived by hypertension is expected to increase cardiac workload and oxygen demand, which should be met by increased myocardial perfusion. However, the deficiency of resting coronary blood flow might have led to ischaemia in the hypertrophied heart as shown by elevation of the left ventricular lactate level in this study (Figure 2). It is speculated that the reduced oxygen supply might have direct functional impact on myocardial contractility (Table 1) and may promote myocardial apoptosis and fibrosis toward heart failure.30
It is well documented that coronary blood flow is closely regulated to meet the metabolic demand of the cardiac tissue. Myocardial activation by isoproterenol caused an increase in HR and a corresponding LCABV increase in sham mice. However, isoproterenol failed to increase LCABV in TAC mice despite the HR was elevated comparably with sham mice (Figure 1), indicating the impairment of coronary flow regulation of hypertrophied heart in response to increased metabolic demand. Because the diminished coronary flow reserve is generally observed in the pathological cardiac hypertrophy,30,33,35 it is likely that the already exhausted coronary reserve under the resting condition might have limited the capacity of flow increase during metabolic activation in our TAC mice. The observed general increase in minimal coronary vascular resistance and ischaemia in most forms of cardiac hypertrophy36 appears to support this view. Moreover, the changes in vascular density31,36 and/or remodelling of coronary vascular structure37 during the development of ventricular hypertrophy might also physically hinder coronary blood flow. Another possible contribution is the dysfunction of coronary resistance arterioles that fail to regulate coronary blood flow during metabolic activation as discussed below.
The recent study from van Nierip et al.34 demonstrated the correlation of left ventricular hypertrophy and decline in left ventricular function with a general diminish in regional myocardial perfusion in the mouse TAC model. Similarly, this study demonstrated the deficiency of metabolic regulation of coronary blood flow in TAC mice. However, the potential mechanism contributing to the observed perfusion deficiency remains unclear. In this study, we characterized the vasomotor function of isolated coronary arterioles without confounding influences from systemic or local neural, humoral, haemodynamic, and metabolic changes. We first examined the endothelium-dependent NO-mediated vasodilation of coronary arterioles, since this vasodilator mechanism plays a critical role in coronary flow regulation.10,15,24 We found that ACh caused concentration-dependent dilation of coronary arterioles isolated from sham mice and this vasodilation was converted to vasoconstriction by the NO synthase inhibitor L-NAME (Figure 3A). This is consistent with the reports in mice that ACh triggers NO-mediated vasodilation in the coronary circulation.38,39 In contrast, coronary arterioles isolated from TAC mice constricted exclusively to ACh and this vasoconstrictor response was not altered by L-NAME (Figure 3). Moreover, both sham and TAC vessels exhibited similar dilation to the smooth muscle relaxing agent SNP, suggesting the selective loss of endothelium-dependent NO-mediated vasodilation while preserving normal smooth muscle contraction and relaxation in these TAC vessels. Since endothelial NO is released upon shear stress (flow) stimulation7,40,41 and plays an important role in not only counteracting myogenic vasoconstriction24 but also improving functional hyperemia in the heart,10 the absence of this NO-mediated vasodilator mechanism in the hypertensive heart in vivo is conceivable to enhance myogenic tone (i.e. vascular resistance) and compromise flow regulation during metabolic activation.42,43 This may, in part, explain the absence of isoproterenol-induced coronary flow recruitment in TAC mice in our study.
In the myocardium, the ET-1 expression is generally elevated in the ventricles subjected to pressure overload44,45 and consequently contributes to myocardial hypertrophy via autocrine and paracrine regulation.29,46,47 Disruption of the ET-1 gene or blockage of endothelin receptors mitigates the development of cardiac hypertrophy elicited by either hormonal stimulation48 or haemodynamic stress.49 Moreover, the endogenous cardiac ET-1 exerts a positive inotropic effect on cardiac contraction.29,50 We found that the expression of ET-1 in the left ventricular tissue was elevated in TAC mice (Figure 5A), which might be helpful to sustain cardiac function during adaptation to pressure overload. In the vasculature, the ET-1 peptide is produced mainly by the endothelium as one of the most potent coronary vasoconstrictors13 with half maximal effective concentration at the nanomolar range from various mammalian species, including mice (∼1.0 nM)51 and humans (∼3.5 nM).52,53 The normal circulatory level of ET-1 in the peripheral blood is about 1–3 pM (2–8 pg/ml) and rises two- to four-fold in patients with hypertension or ischaemic heart disease.54–58 Therefore, the coronary response to ET-1 at concentrations from picomolar to nanomolar was examined in this study. Interestingly, the ET-1-induced vasoconstriction was augmented in coronary arterioles isolated from TAC mice (Figure 4). The increased vascular sensitivity and responsiveness to ET-1, in addition to the elevation of myocardial ET-1, is expected to accentuate the vasomotor tone and vascular resistance, especially in the absence of opposed endothelial vasodilator NO (Figure 3). It appears that the positive inotropic and mitogenic properties of ET-1 might benefit myocardial function and survival at the expense of jeopardizing coronary vascular function and regulation by evoking arteriolar constriction, and thus reducing resting coronary blood flow and limiting metabolic hyperemia as observed in this study.
ROCK has been implicated in mediating diverse biological functions of various cells and the abnormal activation of ROCK can contribute to the development of cardiovascular diseases, including hypertension and cardiac hypertrophy.59 The myocardial expression of ROCK1 and ROCK2 isoforms was found to increase in the ventricular tissue subjected to pressure overload.22,60 In the mice, deletion of ROCK1 appears to reduce the development of fibrosis60 and improves contractile function61 without altering pressure-induced ventricular hypertrophy. On the other hand, ROCK2 deletion reduces ventricular fibrosis, cardiac apoptosis and the development of cardiac hypertrophy in response to neurohumoral or haemodynamic stress.62 Although these studies have demonstrated the importance of ROCK signalling in the pathogenesis of ventricular hypertrophy, the role of ROCKs in pathophysiological regulation of coronary microvasculature remains unknown. We found that both ROCK isoforms were significantly upregulated in the coronary arterioles isolated from TAC mice (Figure 5B) and administration of ROCK inhibitor H-1152 abolished the enhanced vasoconstriction in response to ET-1 (Figure 4). These results demonstrated for the first time on ROCK expression in the coronary microvasculature and its contribution to the alteration of vasomotor function in the hypertrophied heart.
Although the signalling mechanism underlying the vasoconstriction of coronary microvessels to ET-1 remains unclear, ROCK has been shown to modulate calcium sensitivity63 and phosphorylation17 of contractile myofilaments, and thus regulates the force of smooth muscle contraction. In view that increased mechanical stress (e.g. hypertension) is known to activate ROCK signalling in the vascular wall,64 the enhancement of contractile dynamics of myofilaments by ROCK in the vascular smooth muscle adapted to high blood pressure may likely contribute to the enhanced vasoconstriction to ET-1. Interestingly, the oxidative stress that generally associates with ventricular hypertrophy in pressure overload22,62,65 is significantly attenuated, along with the reduction of ventricular hypertrophy, in the mice overexpressing dominant-negative ROCK in the heart.22 This in vivo study suggested the critical link of ROCK signalling to the oxidative insult in the hypertrophied myocardium.22 However, it is unclear whether the reduced oxidative stress in the ventricular tissue is a direct result of ROCK inhibition or a secondary effect associated with the reduction of cardiac hypertrophy by ROCK. In this study, the signals for superoxide production in TAC mice were significantly elevated in both myocardium and coronary arterioles in a manner sensitive to ROCK inhibition (Figure 6). These results suggest that ROCK activation might have contributed to the oxidative stress observed in the myocardium and vasculature in the heart subjected to pressure overload.
Overexpression of cytoplasmic superoxide dismutase in the heart has been reported to ameliorate pressure overload-induced cardiac hypertrophy,65 indicating the pivotal role of redox activity in mediating the development of this adaptation. Interestingly, administration of superoxide scavenger Tempol with a concentration that is known to reduce superoxide production in the myocardium or isolated vessels in our previous studies66,67 significantly attenuated ACh-induced constriction of coronary arterioles from TAC mice (Figure 7B). It appears that the beneficial action of Tempol on vasomotor activity is to improve the bioavailability of endothelial NO because L-NAME effectively abolished the response (Figure 7B). Our data agree with the well-documented findings on the induction of endothelium-dependent vasomotor dysfunction by oxidative stress through modulating NO bioavailability.15,68,69 Consistent with the observed anti-oxidant action of H-1152 in this study (Figure 6), inhibition of ROCK by H-1152 attenuated ACh-induced arteriolar constriction in TAC mice in a manner sensitive to L-NAME, an effect similar to that exerted by Tempol (Figure 7B). It is worth noting that both Tempol and H-1152 did not alter coronary vascular response to ACh in sham mice (Figure 7A). Collectively, our findings support the role of ROCK activation in mediating superoxide production and consequently compromising vasomotor activity by reducing endothelial NO for vasodilation in the heart subjected to pressure overload. Although we did not address the source of superoxide in this study, the investigation on cardiovascular hypertrophy in the rat suggested that the activation of NAD(P)H oxidase is responsible for the angiotensin II-elicited oxidative stress in the vascular cells via ROCK signalling.19 However, it remains unclear whether the same pathway is utilized in the myocardium. It is worth noting that exposure of coronary arterioles to a pathophysiological, sub-vasomotor concentration of ET-1 can lead to vascular dysfunction by impairing endothelium-dependent NO-mediated dilation via superoxide production from redox oxidase.67 The elevated level of ET-1 in the hypertrophied heart might evoke oxidative stress in both myocardium and vasculature in our study and thus contribute to the observed coronary dysfunction. Although it has been suggested that ROCK can be the downstream effector of ET-1,17,18 it remains unclear in this study whether ET-1 triggers ROCK activation for superoxide production in the model of ventricular hypertrophy.
In conclusion, we have demonstrated the impairment of cardiac and coronary microvascular function in the mouse model of hypertensive myocardial hypertrophy. Activation of ROCK signalling may play a central role in the mechanism leading to oxidative stress and coronary arteriolar dysfunction by enhancing vasoconstriction to ET-1 and impairing endothelial NO-mediated vasodilation. These microvascular changes may explain the observed reduction in resting coronary blood flow and impairment of flow recruitment during metabolic activation. This vascular maladaptation is expected to promote ventricular decompensation and consequently lead to heart failure in pressure overload. ROCK inhibition might benefit both cardiac and coronary function by improving vasodilation to endothelial NO, normalizing vasoconstriction to ET-1, and alleviating oxidative stress posed on the hypertrophied heart.
Conflict of interest : none declared.
Funding
This work was supported by a National Institutes of Health Grant (R01EY18420 to T.W.H.), the Baylor Scott and White Research Foundation (to L.K.) and the Kruse Chair Endowment Fund (to L.K.).
References
- 1. Cooper G. Cardiocyte adaptation to chronically altered load. Annu Rev Physiol 1987;49:501–518. [DOI] [PubMed] [Google Scholar]
- 2. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP.. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med 1990;322:1561–1566. [DOI] [PubMed] [Google Scholar]
- 3. Houghton JL, Frank MJ, Carr AA, von Dohlen TW, Prisant LM.. Relations among impaired coronary flow reserve, left ventricular hypertrophy and thallium perfusion defects in hypertensive patients without obstructive coronary artery disease. J Am Coll Cardiol 1990;15:43–51. [DOI] [PubMed] [Google Scholar]
- 4. Misawa K, Nitta Y, Matsubara T, Oe K, Kiyama M, Shimizu M, Mabuchi H.. Difference in coronary blood flow dynamics between patients with hypertension and those with hypertrophic cardiomyopathy. Hypertens Res 2002;25:711–716. [DOI] [PubMed] [Google Scholar]
- 5. Hamasaki S, Al Suwaidi J, Higano ST, Miyauchi K, Holmes DR Jr, Lerman A.. Attenuated coronary flow reserve and vascular remodeling in patients with hypertension and left ventricular hypertrophy. J Am Coll Cardiol 2000;35:1654–1660. [DOI] [PubMed] [Google Scholar]
- 6. Kuo L, Davis MJ, Chilian WM.. Myogenic activity in isolated subepicardial and subendocardial coronary arterioles. Am J Physiol 1988;255:H1558–H1562. [DOI] [PubMed] [Google Scholar]
- 7. Kuo L, Davis MJ, Chilian WM.. Endothelium-dependent, flow-induced dilation of isolated coronary arterioles. Am J Physiol 1990;259:H1063–H1070. [DOI] [PubMed] [Google Scholar]
- 8. Marcus ML, Chilian WM, Kanatsuka H, Dellsperger KC, Eastham CL, Lamping KG.. Understanding the coronary circulation through studies at the microvascular level. Circulation 1990;82:1–7. [DOI] [PubMed] [Google Scholar]
- 9. Kuo L, Davis MJ, Chilian WM.. Longitudinal gradients for endothelium-dependent and -independent vascular responses in the coronary microcirculation. Circulation 1995;92:518–525. [DOI] [PubMed] [Google Scholar]
- 10. Jones CJ, Kuo L, Davis MJ, DeFily DV, Chilian WM.. Role of nitric oxide in the coronary microvascular responses to adenosine and increased metabolic demand. Circulation 1995;91:1807–1813. [DOI] [PubMed] [Google Scholar]
- 11. Hein TW, Kuo L.. cAMP-independent dilation of coronary arterioles to adenosine: role of nitric oxide, G proteins, and KATP channels. Circ Res 1999;85:634–642. [DOI] [PubMed] [Google Scholar]
- 12. Kuo L, Davis MJ, Cannon MS, Chilian WM.. Pathophysiological consequences of atherosclerosis extend into the coronary microcirculation. Restoration of endothelium-dependent responses by L-arginine. Circ Res 1992;70:465–476. [DOI] [PubMed] [Google Scholar]
- 13. Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T.. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988;332:411–415. [DOI] [PubMed] [Google Scholar]
- 14. Giles TD. Aspects of nitric oxide in health and disease: a focus on hypertension and cardiovascular disease. J Clin Hypertens (Greenwich) 2006;8:2–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kuo L, Hein TW.. Vasomotor regulation of coronary microcirculation by oxidative stress: role of arginase. Front Immunol 2013;4:237.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koyanagi T, Wong LY, Inagaki K, Petrauskene OV, Mochly-Rosen D.. Alteration of gene expression during progression of hypertension-induced cardiac dysfunction in rats. Am J Physiol Heart Circ Physiol 2008;295:H220–H226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chitaley K, Weber D, Webb RC.. RhoA/Rho-kinase, vascular changes, and hypertension. Curr Hypertens Rep 2001;3:139–144. [DOI] [PubMed] [Google Scholar]
- 18. Wirth A. Rho kinase and hypertension. Biochim Biophys Acta 2010;1802:1276–1284. [DOI] [PubMed] [Google Scholar]
- 19. Higashi M, Shimokawa H, Hattori T, Hiroki J, Mukai Y, Morikawa K, Ichiki T, Takahashi S, Takeshita A.. Long-term inhibition of Rho-kinase suppresses angiotensin II-induced cardiovascular hypertrophy in rats in vivo: effect on endothelial NAD(P)H oxidase system. Circ Res 2003;93:767–775. [DOI] [PubMed] [Google Scholar]
- 20. Soliman H, Gador A, Lu YH, Lin G, Bankar G, MacLeod KM.. Diabetes-induced increased oxidative stress in cardiomyocytes is sustained by a positive feedback loop involving Rho kinase and PKCβ2. Am J Physiol Heart Circ Physiol 2012;303:H989–H1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ma Z, Zhang J, Ji E, Cao G, Li G, Chu L.. Rho kinase inhibition by fasudil exerts antioxidant effects in hypercholesterolemic rats. Clin Exp Pharmacol Physiol 2011;38:688–694. [DOI] [PubMed] [Google Scholar]
- 22. Ikeda S, Satoh K, Kikuchi N, Miyata S, Suzuki K, Omura J, Shimizu T, Kobayashi K, Kobayashi K, Fukumoto Y, Sakata Y, Shimokawa H.. Crucial role of rho-kinase in pressure overload-induced right ventricular hypertrophy and dysfunction in mice. Arterioscler Thromb Vasc Biol 2014;34:1260–1271. [DOI] [PubMed] [Google Scholar]
- 23. Hill JA, Karimi M, Kutschke W, Davisson RL, Zimmerman K, Wang Z, Kerber RE, Weiss RM.. Cardiac hypertrophy is not a required compensatory response to short-term pressure overload. Circulation 2000;101:2863–2869. [DOI] [PubMed] [Google Scholar]
- 24. Kuo L, Chilian WM, Davis MJ.. Interaction of pressure- and flow-induced responses in porcine coronary resistance vessels. Am J Physiol 1991;261:H1706–H1715. [DOI] [PubMed] [Google Scholar]
- 25. Rockman HA, Ross RS, Harris AN, Knowlton KU, Steinhelper ME, Field LJ, Ross J Jr, Chien KR.. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc Natl Acad Sci U S A 1991;88:8277–8281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Patten RD, Hall-Porter MR.. Small animal models of heart failure: development of novel therapies, past and present. Circ Heart Fail 2009;2:138–144. [DOI] [PubMed] [Google Scholar]
- 27. Wakatsuki T, Schlessinger J, Elson EL.. The biochemical response of the heart to hypertension and exercise. Trends Biochem Sci 2004;29:609–617. [DOI] [PubMed] [Google Scholar]
- 28. deAlmeida AC, van Oort RJ, Wehrens XH.. Transverse aortic constriction in mice. J Vis Exp 2010;38:e1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Drawnel FM, Archer CR, Roderick HL.. The role of the paracrine/autocrine mediator endothelin-1 in regulation of cardiac contractility and growth. Br J Pharmacol 2013;168:296–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vatner SF, Hittinger L.. Coronary vascular mechanisms involved in decompensation from hypertrophy to heart failure. J Am Coll Cardiol 1993;22:34a–40a. [DOI] [PubMed] [Google Scholar]
- 31. Friehs I, del Nido PJ.. Increased susceptibility of hypertrophied hearts to ischemic injury. Ann Thorac Surg 2003;75:S678–S684. [DOI] [PubMed] [Google Scholar]
- 32. Barrick CJ, Rojas M, Schoonhoven R, Smyth SS, Threadgill DW.. Cardiac response to pressure overload in 129S1/SvImJ and C57BL/6J mice: temporal- and background-dependent development of concentric left ventricular hypertrophy. Am J Physiol Heart Circ Physiol 2007;292:H2119–H2130. [DOI] [PubMed] [Google Scholar]
- 33. Hartley CJ, Reddy AK, Madala S, Michael LH, Entman ML, Taffet GE.. Doppler estimation of reduced coronary flow reserve in mice with pressure overload cardiac hypertrophy. Ultrasound Med Biol 2008;34:892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van Nierop BJ, Coolen BF, Bax NA, Dijk WJ, van Deel ED, Duncker DJ, Nicolay K, Strijkers GJ.. Myocardial perfusion MRI shows impaired perfusion of the mouse hypertrophic left ventricle. Int J Cardiovasc Imaging 2014;30:619–628. [DOI] [PubMed] [Google Scholar]
- 35. Wu J, Zhou YQ, Zou Y, Henkelman M.. Evaluation of bi-ventricular coronary flow patterns using high-frequency ultrasound in mice with transverse aortic constriction. Ultrasound Med Biol 2013;39:2053–2065. [DOI] [PubMed] [Google Scholar]
- 36. Marcus ML, Harrison DG, Chilian WM, Koyanagi S, Inou T, Tomanek RJ, Martins JB, Eastham CL, Hiratzka LF.. Alterations in the coronary circulation in hypertrophied ventricles. Circulation 1987;75:I19–I25. [PubMed] [Google Scholar]
- 37. Higashiyama H, Sugai M, Inoue H, Mizuyachi K, Kushida H, Asano S, Kinoshita M.. Histopathological study of time course changes in inter-renal aortic banding-induced left ventricular hypertrophy of mice. Int J Exp Pathol 2007;88:31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Muller-Delp JM, Lubahn DB, Nichol KE, Philips BJ, Price EM, Curran EM, Laughlin MH.. Regulation of nitric oxide-dependent vasodilation in coronary arteries of estrogen receptor-α-deficient mice. Am J Physiol Heart Circ Physiol 2003;285:H2150–H2157. [DOI] [PubMed] [Google Scholar]
- 39. Lamping KG, Nuno DW, Shesely EG, Maeda N, Faraci FM.. Vasodilator mechanisms in the coronary circulation of endothelial nitric oxide synthase-deficient mice. Am J Physiol Heart Circ Physiol 2000;279:H1906–H1912. [DOI] [PubMed] [Google Scholar]
- 40. Bagi Z, Koller A, Kaley G.. Superoxide-NO interaction decreases flow- and agonist-induced dilations of coronary arterioles in Type 2 diabetes mellitus. Am J Physiol Heart Circ Physiol 2003;285:H1404–H1410. [DOI] [PubMed] [Google Scholar]
- 41. Muller JM, Davis MJ, Kuo L, Chilian WM.. Changes in coronary endothelial cell Ca2+ concentration during shear stress- and agonist-induced vasodilation. Am J Physiol 1999;276:H1706–H1714. [DOI] [PubMed] [Google Scholar]
- 42. Liao JC, Kuo L.. Interaction between adenosine and flow-induced dilation in coronary microvascular network. Am J Physiol 1997;272:H1571–H1581. [DOI] [PubMed] [Google Scholar]
- 43. Jones CJ, Kuo L, Davis MJ, Chilian WM.. Myogenic and flow-dependent control mechanisms in the coronary microcirculation. Basic Res Cardiol 1993;88:2–10. [DOI] [PubMed] [Google Scholar]
- 44. Yorikane R, Sakai S, Miyauchi T, Sakurai T, Sugishita Y, Goto K.. Increased production of endothelin-1 in the hypertrophied rat heart due to pressure overload. FEBS Lett 1993;332:31–34. [DOI] [PubMed] [Google Scholar]
- 45. Schunkert H, Orzechowski HD, Bocker W, Meier R, Riegger GA, Paul M.. The cardiac endothelin system in established pressure overload left ventricular hypertrophy. J Mol Med 1999;77:623–630. [DOI] [PubMed] [Google Scholar]
- 46. Shubeita HE, McDonough PM, Harris AN, Knowlton KU, Glembotski CC, Brown JH, Chien KR.. Endothelin induction of inositol phospholipid hydrolysis, sarcomere assembly, and cardiac gene expression in ventricular myocytes. A paracrine mechanism for myocardial cell hypertrophy. J Biol Chem 1990;265:20555–20562. [PubMed] [Google Scholar]
- 47. Ito H, Hirata Y, Hiroe M, Tsujino M, Adachi S, Takamoto T, Nitta M, Taniguchi K, Marumo F.. Endothelin-1 induces hypertrophy with enhanced expression of muscle-specific genes in cultured neonatal rat cardiomyocytes. Circ Res 1991;69:209–215. [DOI] [PubMed] [Google Scholar]
- 48. Shohet RV, Kisanuki YY, Zhao XS, Siddiquee Z, Franco F, Yanagisawa M.. Mice with cardiomyocyte-specific disruption of the endothelin-1 gene are resistant to hyperthyroid cardiac hypertrophy. Proc Natl Acad Sci U S A 2004;101:2088–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ito H, Hiroe M, Hirata Y, Fujisaki H, Adachi S, Akimoto H, Ohta Y, Marumo F.. Endothelin ETA receptor antagonist blocks cardiac hypertrophy provoked by hemodynamic overload. Circulation 1994;89:2198–2203. [DOI] [PubMed] [Google Scholar]
- 50. Piuhola J, Makinen M, Szokodi I, Ruskoaho H.. Dual role of endothelin-1 via ETA and ETB receptors in regulation of cardiac contractile function in mice. Am J Physiol Heart Circ Physiol 2003;285:H112–H118. [DOI] [PubMed] [Google Scholar]
- 51. Bender SB, Klabunde RE.. Altered role of smooth muscle endothelin receptors in coronary endothelin-1 and α1-adrenoceptor-mediated vasoconstriction in Type 2 diabetes. Am J Physiol Heart Circ Physiol 2007;293:H2281–H2288. [DOI] [PubMed] [Google Scholar]
- 52. Wiley KE, Davenport AP.. Comparison of the effects of atherosclerosis and nitrate therapy on responses to nitric oxide and endothelin-1 in human arteries in vitro. Clin Sci 2002;103(Suppl 48):124s–127s. [DOI] [PubMed] [Google Scholar]
- 53. Maguire JJ, Davenport AP.. ETA receptor-mediated constrictor responses to endothelin peptides in human blood vessels in vitro. Br J Pharmacol 1995;115:191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hoffmann E, Assennato P, Donatelli M, Colletti I, Valenti TM.. Plasma endothelin-1 levels in patients with angina pectoris and normal coronary angiograms. Am Heart J 1998;135:684–688. [DOI] [PubMed] [Google Scholar]
- 55. Kaski JC, Elliott PM, Salomone O, Dickinson K, Gordon D, Hann C, Holt DW.. Concentration of circulating plasma endothelin in patients with angina and normal coronary angiograms. Br Heart J 1995;74:620–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yasuda M, Kohno M, Tahara A, Itagane H, Toda I, Akioka K, Teragaki M, Oku H, Takeuchi K, Takeda T.. Circulating immunoreactive endothelin in ischemic heart disease. Am Heart J 1990;119:801–806. [DOI] [PubMed] [Google Scholar]
- 57. Fujii H, Takiuchi S, Kamide K, Horio T, Niizuma S, Tanaka N, Hashimoto S, Nakatani S, Fukagawa M, Kawano Y.. Clinical implications of assessing coronary flow velocity reserve and plasma endothelin-1 in hypertensive patients. Hypertens Res 2005;28:911–916. [DOI] [PubMed] [Google Scholar]
- 58. Shichiri M, Hirata Y, Ando K, Emori T, Ohta K, Kimoto S, Ogura M, Inoue A, Marumo F.. Plasma endothelin levels in hypertension and chronic renal failure. Hypertension 1990;15:493–496. [DOI] [PubMed] [Google Scholar]
- 59. Loirand G, Guerin P, Pacaud P.. Rho kinases in cardiovascular physiology and pathophysiology. Circ Res 2006;98:322–334. [DOI] [PubMed] [Google Scholar]
- 60. Zhang YM, Bo J, Taffet GE, Chang J, Shi J, Reddy AK, Michael LH, Schneider MD, Entman ML, Schwartz RJ, Wei L.. Targeted deletion of ROCK1 protects the heart against pressure overload by inhibiting reactive fibrosis. FASEB J 2006;20:916–925. [DOI] [PubMed] [Google Scholar]
- 61. Shi J, Zhang YW, Summers LJ, Dorn GW 2nd, Wei L.. Disruption of ROCK1 gene attenuates cardiac dilation and improves contractile function in pathological cardiac hypertrophy. J Mol Cell Cardiol 2008;44:551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Okamoto R, Li Y, Noma K, Hiroi Y, Liu PY, Taniguchi M, Ito M, Liao JK.. FHL2 prevents cardiac hypertrophy in mice with cardiac-specific deletion of ROCK2. FASEB J 2013;27:1439–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Somlyo AP, Somlyo AV.. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol 2000;522:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hassona MD, Abouelnaga ZA, Elnakish MT, Awad MM, Alhaj M, Goldschmidt-Clermont PJ, Hassanain H.. Vascular hypertrophy-associated hypertension of profilin1 transgenic mouse model leads to functional remodeling of peripheral arteries. Am J Physiol Heart Circ Physiol 2010;298:H2112–H2120. [DOI] [PubMed] [Google Scholar]
- 65. Hingtgen SD, Li Z, Kutschke W, Tian X, Sharma RV, Davisson RL.. Superoxide scavenging and Akt inhibition in myocardium ameliorate pressure overload-induced NF-κB activation and cardiac hypertrophy. Physiol Genomics 2010;41:127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hein TW, Qamirani E, Ren Y, Xu X, Thengchaisri N, Kuo L.. Selective activation of lectin-like oxidized low-density lipoprotein receptor-1 mediates C-reactive protein-evoked endothelial vasodilator dysfunction in coronary arterioles. Circ Res 2014;114:92–100. [DOI] [PubMed] [Google Scholar]
- 67. Thengchaisri N, Hein TW, Ren Y, Kuo L.. Endothelin-1 impairs coronary arteriolar dilation: Role of p38 kinase-mediated superoxide production from NADPH oxidase. J Mol Cell Cardiol 2015;86:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hein TW, Kuo L.. LDLs impair vasomotor function of the coronary microcirculation: role of superoxide anions. Circ Res 1998;83:404–414. [DOI] [PubMed] [Google Scholar]
- 69. Qamirani E, Ren Y, Kuo L, Hein TW.. C-reactive protein inhibits endothelium-dependent NO-mediated dilation in coronary arterioles by activating p38 kinase and NAD(P)H oxidase. Arterioscler Thromb Vasc Biol 2005;25:995–1001. [DOI] [PubMed] [Google Scholar]