Abstract
Hypertension is a major risk factor for many common chronic diseases, such as heart failure, myocardial infarction, stroke, vascular dementia, and chronic kidney disease. Pathophysiological mechanisms contributing to the development of hypertension include increased vascular resistance, determined in large part by reduced vascular diameter due to increased vascular contraction and arterial remodelling. These processes are regulated by complex-interacting systems such as the renin-angiotensin-aldosterone system, sympathetic nervous system, immune activation, and oxidative stress, which influence vascular smooth muscle function. Vascular smooth muscle cells are highly plastic and in pathological conditions undergo phenotypic changes from a contractile to a proliferative state. Vascular smooth muscle contraction is triggered by an increase in intracellular free calcium concentration ([Ca2+]i), promoting actin–myosin cross-bridge formation. Growing evidence indicates that contraction is also regulated by calcium-independent mechanisms involving RhoA-Rho kinase, protein Kinase C and mitogen-activated protein kinase signalling, reactive oxygen species, and reorganization of the actin cytoskeleton. Activation of immune/inflammatory pathways and non-coding RNAs are also emerging as important regulators of vascular function. Vascular smooth muscle cell [Ca2+]i not only determines the contractile state but also influences activity of many calcium-dependent transcription factors and proteins thereby impacting the cellular phenotype and function. Perturbations in vascular smooth muscle cell signalling and altered function influence vascular reactivity and tone, important determinants of vascular resistance and blood pressure. Here, we discuss mechanisms regulating vascular reactivity and contraction in physiological and pathophysiological conditions and highlight some new advances in the field, focusing specifically on hypertension.
Keywords: Contraction, Dilation, Calcium, Actin cytoskeleton, Rho kinase, Vascular tone
1. Introduction
Hypertension is associated with vascular changes characterized by endothelial dysfunction, increased vascular contraction, and arterial remodelling.1–3 Vascular smooth muscle cells, which constitute the bulk of the vascular wall, are critically involved in these processes through their highly plastic and dynamic features and ability to undergo phenotypic differentiation.1–3 Pro-hypertensive stimuli, such as activation of the renin-angiotensin-aldosterone system (RAAS), oxidative stress, activation of the sympathetic nervous system, haemodynamic changes, and mechanical forces stimulate vascular smooth muscle cell signalling, which promotes vasoconstriction, vascular hypertrophy, fibrosis, inflammation, and calcification, processes that underlie vascular functional, structural, and mechanical changes in hypertension.4,5
The arterial system comprises large conduit vessels, medium sized arteries, small arteries, arterioles, and capillaries. Small resistance arteries (lumen diameter < 300 μm) are responsible for regional distribution of vascular tone and blood flow and play an important role in the regulation of blood pressure, through effects on vascular resistance.6 Three fundamental parameters determine resistance to blood flow including vessel diameter (radius), arterial length, and blood viscosity. Of these, vessel diameter is the most important because it can change rapidly due to contraction and dilation of vascular smooth muscle.6 Based on Poiseuille’s Law, vessel resistance is inversely proportional to the radius (lumen diameter) to the fourth power (r4). Therefore, small changes in lumen diameter have major impact on vascular resistance.7 The lumen diameter of resistance arteries is a function of vasomotor tone (vasoconstriction/vasodilation) and the structural and mechanical properties of the vessel. Vasomotor control underlies acute rapid adaptation of vessel diameter due mainly to vasoconstriction exerted by the active contraction of vascular smooth muscle cells, while changes in vascular structure represent a dynamic process in response to chronic haemodynamic changes.8 Initially structural changes are adaptive but in chronic pathological conditions become maladaptive leading to vascular remodelling and rigid, stiff, and poorly compliant vessels, typically observed in chronic hypertension.9,10 Endothelial stiffening decreases nitric oxide generation leading to smooth muscle cell contraction and vasoconstriction and may precede hypertension. Arterial stiffness is strongly associated with high blood pressure and is an independent predictor of cardiovascular disease.11,12
At the molecular and cellular levels vascular hyperreactivity, remodelling, and stiffening involve changes in cytoskeletal organization, cell-to-cell connections, cell growth, calcification, inflammation, and rearrangement of vascular smooth muscle cells.13,14 At the extracellular level, remodelling is influenced by fibrosis, changes in matrix protein composition and reorganization of proteoglycans, collagens, and fibronectin, which provide tensile strength, and elastin, responsible for vascular elasticity. Activation of adipocytes in perivascular adipose tissue, secrete vasoactive adipokines that also influence vascular reactivity and contractility.15,16
Acute regulation of vascular diameter and consequently vascular resistance depends on the activation status of the contractile machinery involving actin: myosin interaction in vascular smooth muscle cells.17 Changes in [Ca2+]i, ion fluxes, and membrane potential lead to calcium–calmodulin-mediated phosphorylation of the regulatory myosin light chains (MLCs) and actin–myosin cross-bridge cycling with consequent rapid vasoconstriction.18 Calcium-independent mechanisms associated with altered calcium sensitization and actin filament remodelling and increased bioavailability of reactive oxygen species (ROS) (oxidative stress), also modulate vascular contraction.19
In the present review, we discuss mechanisms regulating vascular reactivity and contraction in physiological and pathophysiological conditions, with a particular focus on hypertension. The role of vascular smooth muscle function in vascular remodelling, inflammation, and calcification and the importance of other vascular cell types in vascular health and disease are discussed elsewhere in the current issue of the journal.
2. The vascular media
The arterial wall is composed of three anatomical layers, a single layer of endothelial cells, the vascular media comprising multiple layers of vascular smooth muscle cells, and the adventitia, containing fibroblasts, adipocytes, connective tissue, and extracellular matrix.20–23 Endothelial cells secrete vasoactive agents and ROS that modulate the vessel diameter by influencing vascular smooth muscle cell function.20 The muscular media of vessels is innervated by the autonomic nervous system and its contractile state is regulated by hormones, vasoactive peptides, and ROS. Vascular smooth muscle cells possess a complex cytoskeletal skeleton, structural, and functional contractile proteins and associated regulatory molecules. Individual vascular smooth muscle cells connect to neighbouring cells through gap junctions, such as connexins, which control the synchronization in ion concentration and membrane potential between neighbouring cells.22,23
Although smooth muscle contraction can be tonic (sustained) or phasic, tonic contraction is essential for maintenance of vascular tone and regulation of blood flow. In resistance arteries vessels contract in response to increasing pressure in the physiological range (70–100 mmHg). With increased blood pressure, this myogenic response is impaired and basal vascular tone and contractility are increased.24 Clinically, non-invasive approaches (vascular ultrasound, pulse wave analysis, and peripheral arterial tone) to study endothelial function and vascular tone in humans have demonstrated that patients with hypertension exhibit impaired endothelium-dependent vasodilation, enhanced vascular reactivity, and increased contractility.25
3. The plastic nature of vascular smooth muscle cells
Vascular smooth muscle cells are specialized cells that are highly plastic and multifunctional. Physiologically vascular smooth muscle cells are quiescent and exhibit low levels of growth. Normally, they express genes and proteins important for contraction/dilation, which allows them to control systemic and local pressure through the regulation of vascular tone.26 However, under stressed or pathological conditions, highly differentiated contractile cells re-enter the cell cycle and become dedifferentiated, assuming a proliferative/migratory phenotype. Although differentiated cells express specific contractile and cytoskeletal proteins [e.g. smooth muscle cell α actin (α-SMA), smooth muscle myosin heavy chain (SM-MHC), calponin, caldesmon, and sm22-α], dedifferentiated cells express low levels of contractile markers and high levels of signalling molecules associated with cell growth, migration, fibrosis, and inflammation [e.g. cell cycle regulators (cyclins), mitogen-activated protein kinases (MAPKs), pro-inflammatory transcription factors, and matrix metalloproteinases].27
3.1 Vascular smooth muscle dedifferentiation in hypertension
In hypertension and other pathological conditions associated with vascular injury, the phenotypic switch contributes to vascular dysfunction and arterial remodelling. In the early stages of hypertension and during vascular repair, the cell cycle is tightly regulated contributing to controlled vascular smooth muscle cell proliferation important in the ‘adaptive’ phase of remodelling. However when the cell cycle is unchecked and proliferation is uncontrolled, dedifferentiated smooth muscle cells accumulate in the vascular wall leading to media thickening, neointimal hyperplasia, and vascular stiffness, features found in hypertension, atherosclerosis, and pulmonary artery hypertension.28 Studies using cell-tracking approaches in in vivo models, demonstrated that >80% of vascular smooth muscle cells in sites of arterial injury or vascular remodelling exhibit features of dedifferentiation.29
3.2 Molecular mechansims of vascular smooth muscle cell dedifferentiation in hypertension
Molecular mechanisms underlying the cellular phenotypic switch in hypertension are complex and multifactorial. Vasoactive stimuli [Angiotensin II (Ang II), norepinephrine, and endothelin-1 (ET-1)], growth factors [Insulin-like growth factor 1 (IGF-1), epidermal growth factor (EGF), platelet-derived growth factor (PDGF)], mechanical forces (stretch), and physical factors (shear stress, pressure) are important.30,31 These processes induce changes in expression and function of genes that control cell membrane receptors, growth signalling pathways, extracellular matrix components, transcription factors, ion channels, and transporters, important in vascular hypertrophy in hypertension.31–33
3.3 The non-coding genome and dedifferentiation of vascular smooth muscle cells in hypertension
Recent evidence indicates that the phenotypic switch of smooth muscle cells involves the non-coding genome. Non-coding RNAs (ncRNAs), which are classified based on their size: small ncRNAs (<200 nucleotides) and long ncRNAs (lncRNA) (>200 nucleotides), regulate gene expression at multiple levels including transcription, RNA processing, and translation.26,34 Most ncRNAs do not have protein-coding abilities but they guide DNA synthesis or genome rearrangement and as such have major impact on the regulation of the genome.
Many classes of small ncRNAs have been identified, of which miRNAs are particularly important in the phenotypic regulation of smooth muscle cells. Mature miRNA derives from precursor miRNA through cleavage by the enzyme Dicer. Evidence supporting a pathophysiological role for miRNA in smooth muscle cell differentiation and proliferation derives from in vivo studies in conditional knockout mice, where smooth muscle cell-specific knockout of Dicer was associated with dilated, thin-walled arteries, reduced vascular smooth muscle cell proliferation, decreased expression of contractile genes, and decreased blood pressure.34,35 Similar features were observed in mice deficient in smooth muscle cell miR-143/145 cluster.36 Other miRNAs associated with vascular smooth muscle cell differentiation include miR-21, miR-22, miR-26a, miR-34a, miR-146a, and miR-221/222.26,34–36
Although the list of miRNAs involved in vascular smooth muscle cell differentiation is growing, there is a paucity of information on vascular cell lncRNAs. LncRNA regulate gene expression by stimulating or repressing gene transcription, translation, and signalling. They also regulate the structure and function of chromosomes. Multiple lncRNAs, including H19, ANRIL, lncRNA-p21, lncRNA-362, and GAS5, have been associated with vascular smooth muscle cell differentiation/proliferation and various vascular pathologies.37 Most lncRNA are widely expressed, however, smooth muscle and endothelial cell enriched migration/differentiation-associated lncRNA (SENCR) seems to be specifically expressed in smooth muscle cells and endothelial cells.35 Numerous Ang II-regulated lncRNAs have been identified in vascular remodelling in experimental hypertension.38 In particular, lncRNA-GAS5 (growth arrest-specific 5) has been identified as a regulator of hypertension-induced vascular remodelling, which when knocked down causes hypertension.39
4. Vascular contraction and hypertension
Dynamic changes in vascular diameter depend in large part on the contractile activation and inactivation (phosphorylation/dephosphorylation) of contractile proteins in vascular smooth muscle cells. The contractile machinery of vascular smooth muscle includes actin and myosin and a highly organized cytoskeleton.
4.1 A primer in vascular smooth muscle contraction: Ca2+-dependent mechanisms
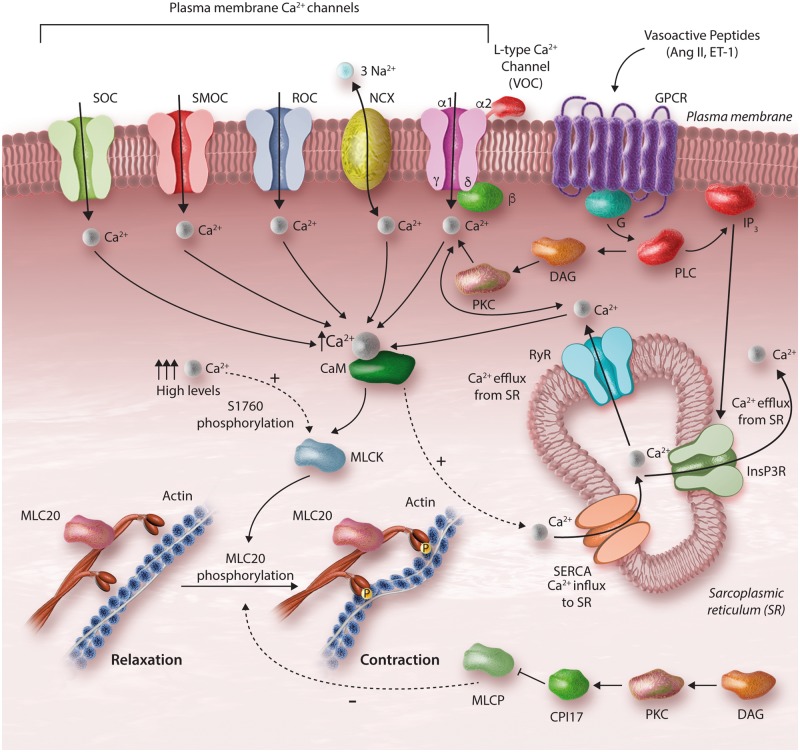
The key event in vascular smooth muscle excitation-contraction coupling is an increase in [Ca2+]i in response to mechanical, humoral, or neural stimuli40,41 (Figure 1). [Ca2+]i and calcium signalling control the key functions of vascular smooth muscle cells and are finely tuned by plasma membrane calcium-permeable channels, exchangers, and transporters and by intracellular sources, including the sarcoplasmic reticulum, mitochondria, and calcium-binding proteins. The extracellular concentration of calcium is 2–4 mM with a basal [Ca2+]i of 90–110 nM.40–42 In hypertension, these processes are altered leading to increased [Ca2+]i and a hypercontractile state and vascular remodelling.
Figure 1.
Calcium-dependent regulation of vascular smooth muscle cell (VSMC) contraction. Vasoconstrictors induce VSMC contraction by increasing the intracellular levels of Ca2+. Vasoactive peptides, such as Ang II, bind to G protein-coupled receptors (GPCRs) activating PLC leading to (i) production of IP3 and (ii) formation of DAG. IP3 binds to the IP receptor Ca2+ channel (InsP3R) and induces Ca2+ release from the sarcoplasmic reticulum (SR). DAG causes activation of PKC, which influences Ca2+ channels, such as store-operated Ca2+ channel (SOC), second messenger-operated Ca2+ channel (SMOC), receptor-operated Ca2+ channel (ROC), voltage-gated Ca2+ channel (VOC), and the Na+–Ca2+ exchanger (NCX). PKC also stimulates activity of the ryanodine Ca2+ channel (RyR) inducing release of Ca2+ from the SR. MLCP activity is reduced by CPI-17 phosphorylation. Ca2+ binds to calmodulin and activates the MLCK, leading to MLC20 phosphorylation at Ser19, actin polymerization, and vascular contraction.
Stimulation of vascular smooth muscle cells by pro-hypertensive factors such as neurohumoral stimuli (acetylcholine, norepinephrine) and vasoactive peptides (Ang II, ET-1), induces activation of receptors coupled to Phospholipase C (PLC), leading to generation of the second messengers inositol trisphosphate (IP3) and diacylglycerol (DAG).31,40–42 Circulating non-cellular factors such as cytokines, diffusible ROS (nitric oxide and hydrogen peroxide), and miRNAs and cellular-derived factors such as microparticles and endothelial progenitor cells also stimulate membrane receptors or cross the plasma membrane to regulate pathways that control [Ca2+]i.43–45 Moreover, endothelium-secreted vasoconstrictors regulate vascular contraction. Endothelial-derived ET-1 and prostanoids activate vascular smooth muscle cell receptors leading to activation of pro-contractile signalling.46
PLC-induced IP3 production stimulates intracellular calcium release from the sarcoplasmic reticulum and DAG causes activation of protein kinase C (PKC). In addition, numerous calcium channels, such as voltage-operated (VOC), receptor-operated channels (ROC), store-operated channels (SOC), transient receptor potential cation (TRP) channels and Ca2+-permeable non-selective cation channels are activated, promoting calcium influx and increased [Ca2+]i.47–51 Calcium diffuses to the contractile machinery and binds to calmodulin. The calcium–calmodulin complex induces a conformational change in MLC kinase (MLCK) converting it from an inactive to an active state.40–43 Activated MLCK induces phosphorylation of MLC20, stimulates myosin–actin interaction, which generates force and shortening and consequent vascular contraction.
4.2 Calcium-dependent mechanisms of vascular smooth muscle cell contraction in hypertension
In hypertension, many of the mechanisms regulating intracellular calcium homeostasis are perturbed, with experimental models and hypertensive patients demonstrating abnormal vascular calcium handling and high [Ca2+]i. Increased calcium influx, augmented sarcoplasmic reticular calcium release and decreased sarcoplasmic reticular calcium reuptake, activation of the PLC-DAG-IP3 pathway, increased calcium signalling, vascular hyperreactivity, and exaggerated contractile responses to vasoactive agonists have been demonstrated in genetic [spontaneously hypertensive rats (SHRs), stroke-prone SHR], experimental (deoxycorticosterone acetate (DOCA)-salt, Ang II-infused, L-NAME-induced), and human hypertension.47–54 Processes triggering these events in hypertension likely involve activation of the sympathetic nervous system and up-regulation of the RAAS.
The importance of calcium channels in abnormal calcium handling in hypertension is evidenced by the effective antihypertensive actions of L-type calcium channel blockers, which target L-type CaV1.2 calcium channels.55,56 In experimental and clinical hypertension, activation of L-type VOCs is increased and sensitivity to calcium channel blockers is greater in hypertensive compared with normotensive individuals.55,56 Processes underlying this include increased expression and phosphorylation of subunits of L-type calcium channels.55 Other mechanisms involving regulatory factors have also been identified. In particular, recent studies demonstrated that the splicing factor Rbfox2 plays a regulatory role in CaV1.2 calcium channels and calcium influx in vascular smooth muscle cell contraction. In hypertension, aberrant splicing by dysregulated Rbfox2 induces enhanced activity of CaV1.2 calcium channels leading to increased vascular myogenic tone.57
ROCs, TRP channels (TRPC3, TRPC6, and TRPC7), and the Na+/Ca2+ exchanger (NCX) have also been shown to play a role in altered calcium handling and vascular dysfunction in hypertension.50 Moreover, processes that stimulate store-operated calcium entry (SOCE) may be important. SOCE, a mechanism whereby reduced sarcoplasmic reticular calcium stores stimulate calcium influx, is influenced by stromal interaction molecule 1 (STIM1) and Orai1 (pore subunit of calcium release-activated calcium channels). In hypertension, increased expression of vascular STIM1/Orai1 is associated with augmented aortic contraction, especially in males.50,58
In hypertension, vascular smooth muscle calcium homeostasis is also modulated by activation of signalling pathway not typically associated with contration, such as MAPKs, tyrosine kinases, transcription factors, and nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (Noxs).59–63 We showed that c-Src is a point of cross-talk between calcium- and oxidation/reduction (redox)-signalling in vascular cells and that in human hypertension, up-regulation of this system contributes to increased vascular [Ca2+]i and contraction.64 Redox-regulated calcium-sensitive transcription factors, including serum response factor (SRF), nuclear factor of activated T-cells (NFAT), and cyclic adenosine monophosphate (cAMP) responsive element-binding protein, promote expression of genes encoding contractile proteins, further influencing vascular contractility in hypertension.50
Involvement of immune and inflammatory systems has also been shown to influence vascular calcium homeostasis in hypertension and target organ damage in hypertension. Activated immune cells migrate into target organs, including the vasculature, and factors released by these cells, including ROS, metalloproteinases, cytokines, and chemokines promote dysfunction and cause damage.65 In vessels, these factors enhance constriction, remodelling, and rarefaction. Vascular toll-like receptors (TLRs) and damage-associated molecular patterns (DAMPs) have also been shown to influence vascular reactivity in hypertension. TLR4 is up-regulated in resistance arteries of SHR and contributes to hypercontractile responses through DAMP and cyclooxygenase-dependent pathways.66 In hypertension, increased DAMP-TLR activation may influence vascular reactivity as well as elicit a vascular inflammatory response, also important in the pathophysiology of hypertension. Involvement of immune cells in the perivascular adventitial and adipose tissue has also been implicated in hypertension-associated vascular dysfunction.67
4.3 Calcium-independent mechanisms of vascular smooth muscle cell contraction in hypertension: calcium sensitization
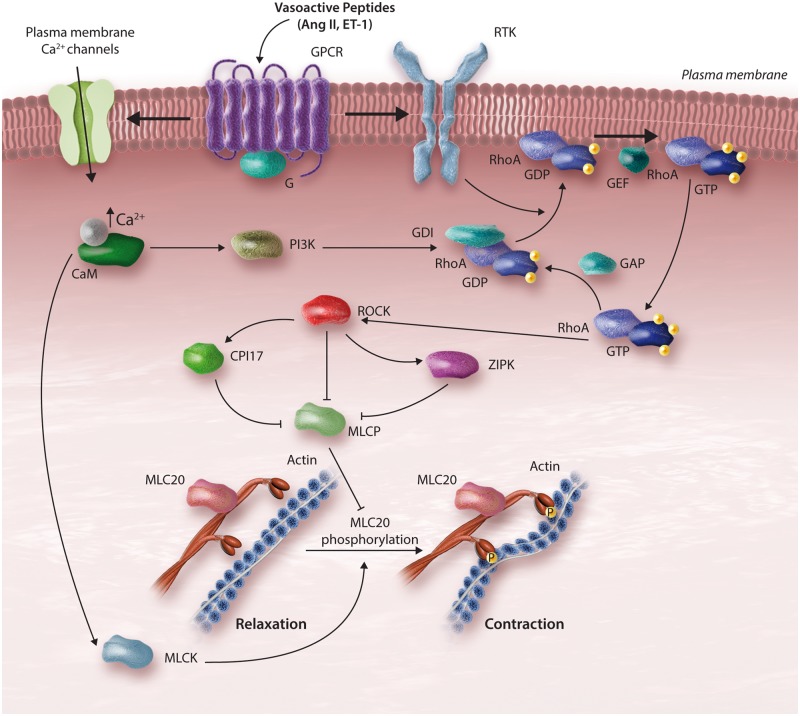
In addition to calcium-dependent mechanisms, calcium-independent processes regulate vascular smooth muscle contraction, by influencing the sensitivity of MLC to calcium. The calcium sensitization process maintains force generation following dissipation of the initial calcium signal. Two major signalling pathways have been implicated in calcium sensitization, including the DAG-PLC-PKC pathway and the RhoA-Rho kinase (ROCK) pathway67,68 (Figure 2). Other kinases also play a role including integrin-linked kinase (ILK), p21-activated protein kinase and zipper-interacting protein kinase (ZIPK).69,70 The calcium sensitization mechanism regulates the phosphorylation state of MLC20 independently of calcium–calmodulin–MLCK signalling.
Figure 2.
ROCK-induced contraction of VSMCs. Vasoactive agents bind to their respective GPCRs leading to release of RhoA from a guanine nucleotide dissociation inhibitor (GDI). RhoA translocates to the membrane. Mechanisms involving RhoA activation also involve transactivation of receptor tyrosine kinases (RTKs). GEFs promote exchange of Guanosine diphosphate (GDP) to Guanosine triphosphate (GTP), activating the RhoA-ROCK pathway. Activated ROCK renders MLCP inactive by phosphorylation of CPI-17 and/or zipper-interacting protein (ZIPK), facilitating MLC20 phosphorylation and vascular contraction. Increased Ca2+ may directly activate ROCK through phosphatidylinositol-4, 5-bisphosphate 3-kinase (PI3K)-dependent pathways.
Hydrolysis of phosphatidylinositol 4, 5-bisphosphate (PIP2) by PLC produces IP3 and DAG. IP3 stimulates release of calcium from the sarcoplasmic reticulum, while DAG acts as a second messenger inducing activation of PKC and C-kinase potentiated protein Phosphatase 1 inhibitor, molecular mass 17 kDa (CPI-17). PKCs are classified in three major groups; conventional or classic PKCs, novel PKCs, and atypical PKCs. Vascular smooth muscle cells variably express multiple isoforms, although the classic isoforms, α, β, and γ appear to be most abundant. Activated PKC induces phosphorylation of downstream targets including ion channels, transporters, and nuclear protein.71 It also phosphorylates CPI-17, a smooth muscle-specific inhibitor of MLC phosphatase (MLCP), which binds to its catalytic domain, inhibiting phosphatase activity, facilitating persistent contraction. α-PKC also induces phosphorylation of actin-binding proteins, such as calponin and calmodulin, which promote actin–myosin interaction and vascular smooth muscle cell contraction.72
Increased PKC activity, associated with vascular hypercontractility, has been demonstrated in experimental and clinical hypertension. Agonist-stimulated vasoconstriction is more potently inhibited by PKC inhibitors in aorta from SHR vs. control Wistar Kyoto rats.73 Moreover, hindlimb perfusion of a phorbol ester that activates PKC, caused prolonged vasoconstriction, and increased perfusion pressure in SHR compared with normotensive WKY, effects that were blocked by PKC inhibitors.74 Human vascular smooth muscle cells from hypertensive patients exhibit increased Ang II-induced proliferation and contraction through processes that involve Phospholipase D and PKC.75 Several PKC inhibitors have been developed, including non-specific staurosporine and chelerythrine, which reduce contraction in hypertension. Isoform-specific PKC inhibitors, such as ruboxistaurin have been tested in clinical trials for diabetic retinopathy76 and may have potential in the treatment of other vascular diseases.
The other system that sensitizes myosin to calcium involves ROCKs (ROCK1, ROCK2), which are serine/threonine kinases and downstream effectors of the small GTPase RhoA.77,78 RhoA, a member of the Rho family of small GTPase-binding proteins, is abundantly expressed in vascular smooth muscle cells and is rapidly activated by vasoconstrictors, such as Ang II. ROCK influences calcium sensitization through two main mechanisms. First, it stimulates phosphorylation of myosin phosphatase target subunit 1 (MYPT1) at T695 or T853. Secondly, it phosphorylates ZIPK (also known as DAPK3), which stimulates phosphorylation of MYPT1 at T696 and T18/S19.79,80 MYPT1 phosphorylation in turn interferes with binding of MLCP to MLC, and accordingly reduces phosphatase activity leading to sustained contraction. In addition to MYPT1, ROCK phosphorylates CPI-17.81 Hyperactivation of the RhoA-ROCK leads to decreased MLCP activation and consequent sustained vasoconstriction and blood pressure elevation. ROCK-dependent calcium sensitization is particularly important in the acute phase of vasoconstriction, as evidenced by studies demonstrating that blockade of this pathway reduces the initial vasoconstrictor action of mechanical, humoral, and neural stimuli.82
Experimental models of hypertension exhibit increased vascular RhoA/ROCK activation,76,83–86 processes that are augmented with high salt diet.83 A number of mechanisms have been implicated in ROCK hyperactivation in hypertension, including dysregulation of Rho guanine nucleotide exchange factors (Rho-GEFs).84 In experimental hypertension, activation of ROCK signalling generates a negative feedback loop on the expression of vascular RhoA-GEFs, which influences ROCK-dependent contraction or remodelling in vessels under pathophysiological conditions, including hypertension.85–88 In clinical studies, mononuclear cell p63RhoGEF gene and protein expression and MYPT1 phosphorylation were increased in patients with essential hypertension.88 ROCK2 also plays an important role in Ang II-induced hypertension and cardiac hypertrophy, through processes that involve formin Homology 2 domain containing 3 (FHOD3), crucial in regulating myofibrillogenesis in cardiomyocytes.87 In human vascular smooth muscle cells, Ang II activates ROCK-mediated contractile signalling through the RhoA exchange factor Arhgef1.88 In addition to modulating vascular contraction in hypertension, ROCK activation is associated with increased vascular stiffness through processes that increase SRF/myocardin signalling.89
Pharmacological inhibition of ROCK with fasudil or Y27632 suppresses acute pressor responses of Ang II but does not reduce blood pressure chronically, supporting the role of RhoA/ROCK in acute vasoconstriction, rather than in mechanisms associated with adaptive vascular remodelling in hypertension that occur chronically with Ang II infusion.90–93 Clinical studies have demonstrated beneficial effects of ROCK inhibitors. Fasudil has been used clinically and prevents vasospasm associated with subarachnoid haemorrhage, acute ischaemic stroke, angina, coronary artery spasm, atherosclerosis, and in the regulation of vascular tone in hypertensive renal transplant recipients.91–96 Increasing evidence indicates a pathophysiological role for ROCK in pulmonary arterial hypertension, with clinical studies suggesting ROCK inhibitors as potential therapeutic agents.96 Use of ROCK inhibitors in human essential hypertension is not yet approved but may be an interesting strategy.
5. Reversal of vascular smooth muscle contraction—importance of vascular relaxation
Vascular smooth muscle relaxation occurs as a result of decreased [Ca2+]i due to inactivation of L-type calcium channels with reduced calcium influx, increased plasmalemmal Ca2+ATPase and activation of the sodium–calcium exchanger with increased calcium efflux and activation of sarcoplasmic/endoplasmic reticulum Ca2+ATPase (SERCA), which stimulates reuptake of calcium into the sarcoplasmic reticulum. As calcium dissociates from calmodulin, MLCK becomes inactivated and MLC20 is dephosphorylated by the serine/threonine phosphatase MLCP.97,98 The overall magnitude of MLC20 phosphorylation and resultant vascular smooth muscle contraction are determined by the relative activities of MLCK and MLCP. Another pathway promoting vascular relaxation involves endothelial-derived nitric oxide that regulates cyclic guanosine monophosphate and cAMP, which signal through protein kinase G (PKG) and protein kinase A (PKA) to increase MLCP activity and decrease calcium sensitivity of the contractile machinery.99,100
Impaired vasorelaxation, with sustained contraction and altered MLCP activity have been demonstrated in human and experimental hypertension.101 In smooth muscle from mice with specific knockout of the MLCP regulatory subunit MYPT1, phosphorylation of MLC and contractile force in isolated mesenteric arteries were enhanced, indicating an important role for MLCP and its regulatory subunit MYPT1 in vascular contraction and development of hypertension.102
6. The actin cytoskeleton and contraction
In addition to the calcium-dependent and -independent modulation of MLC20 phosphorylation and actin–myosin cross-bridge formation, reorganization of actin filaments, intermediate filaments, and microtubules play an important role in vascular contraction. Increased polymerization of actin, tyrosine phosphorylation of paxillin, activation of small GTP-binding proteins, and conformational changes in focal adhesion sites result in stiffening and reorganization of the cytoskeleton in vascular smooth muscle cells.103 This dynamic rearrangement of the actin cytoskeleton is key in maintaining vascular tone and plasticity, especially important in the regulation of vascular diameter related to pressure-dependent myogenic tone in hypertension. Cytoskeletal remodelling occurs over time, with actin polymerization increasing as vasoconstriction persists.104 This involves cellular reorganization and formation of new intercellular adhesions through integrins and focal adhesion molecules.105,106 Prolonged vasoconstriction and associated actin polymerization participate in the initial phases of vascular remodelling in hypertension, processes that involve ROCK and ROS.107 Vascular smooth muscle cell length adaptation, with reorganization of the actin cytoskeleton also contributes to small artery eutrophic remodelling, typically observed in essential hypertension.108
7. Reactive oxygen species, calcium, and contraction in hypertension
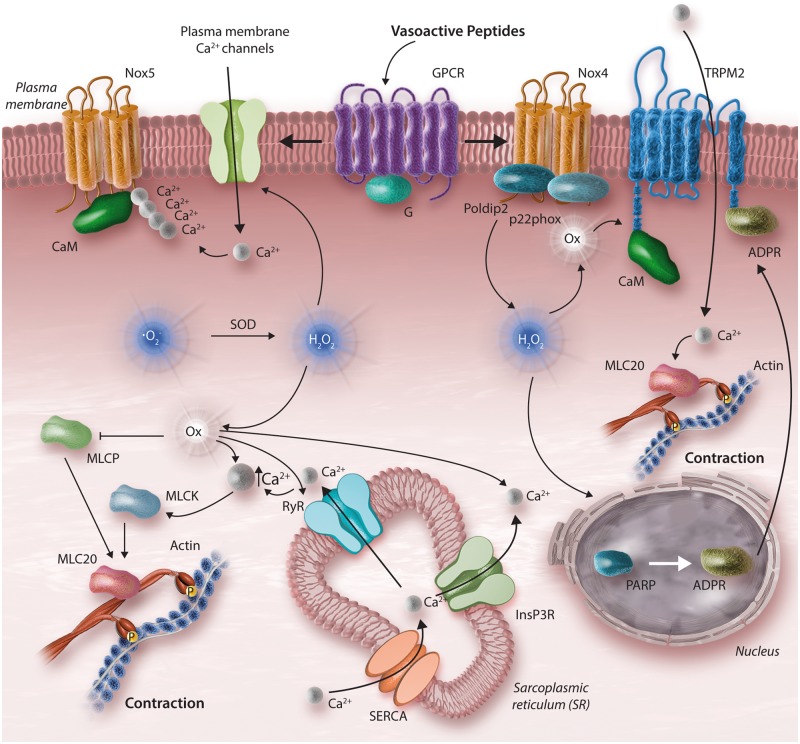
Similar to intracellular calcium, ROS are now considered important second messenger molecules in vascular smooth muscle cells in that (i) they are generated within cells in a regulated manner, (ii) they act on downstream signalling molecules, and (iii) their effects on protein targets reversibly influence cellular function.109 ROS (superoxide anion and hydrogen peroxide) and reactive nitrogen species (nitric oxide and peroxynitrite) are biologically important O2 derivatives that play a role in vascular (patho)biology through their redox potential. The major enzymatic source of ROS in endothelial and vascular smooth muscle cells is Nox. Seven Nox isoforms have been identified, of which Nox1, 2, 4, and 5 are expressed and functionally active in human vascular smooth muscle cells.110–112 Noxs are activated by vasoactive agents and activation is increased in hypertension.113,114 Superoxide is converted to the more stable hydrogen peroxide by superoxide dismutase (SOD). Hydrogen peroxide is further reduced to water and oxygen by catalase and peroxidases. In endothelial cells, superoxide reacts with nitric oxide producing peroxynitrite. An imbalance in redox state where pro-oxidants overwhelm antioxidant capacity leads to oxidative stress, which in turn causes vascular inflammation, fibrosis and arterial remodelling in hypertension.115
Vascular ROS effects are mediated through redox-sensitive signalling pathways. ROS regulate protein kinases, phosphatases, MAPKs, and transcription factors and are important modulators of [Ca2+]i, ROCK, and the contractile machinery (Figure 3). ROS increase vascular [Ca2+]i by stimulating IP3-mediated calcium mobilization, by increasing cytosolic calcium accumulation through SERCA inhibition, and by stimulating calcium channels.116,117 Increased Nox-derived ROS generation enhances calcium signalling, up-regulates ROCK and modulates the actin cytoskeleton, thereby promoting vascular contraction and increasing vascular tone.
Figure 3.
VSMC contraction and oxidative stress. Nox-derived ROS influence cellular Ca2+ homeostasis and pro-contractile signalling. Calcium also influences Nox-derived ROS generation, through Nox5, a Ca2+-sensitive Nox isoform that produces . Nox4 activation leads to production of H2O2. High levels of ROS, cause oxidative modifications of Ca2+ channels, ryanodine receptors (RyR), actin, actin-binding proteins as well as TRP cation channel, subfamily M, Member 2 (TRPM2). TRPM2, a redox-sensitive Ca2+ channel indirectly activated by H2O2 through poly (ADP-ribose) polymerase (PARP) activation in the nucleus and consequent Adenosine diphosphate-ribose (ADPR) production. Once released to the cytosol, ADPR binds and activates TRPM2, stimulating Ca2+ influx. Dashed line indicates that reduced MLCP activity results in icreased MLC20 activation.
Superoxide causes vasoconstriction while hydrogen peroxide induces both vasodilation and vasoconstriction, depending on the vascular bed and the Nox isoform involved. In cerebral arteries, coronary arteries, and pulmonary vessels, hydrogen peroxide is a potent vasodilator,118,119 whereas in peripheral arteries and aorta it causes vasoconstriction.120,121 Hydrogen peroxide acts as an endothelium-derived hyperpolarizing factor and induces vasodilation through PKG1α-mediated pathways122,123 and inhibition of intracellular calcium mobilization, while ROS mediates vasoconstriction by increasing [Ca2+]i.52
8. Oxidative stress, protein oxidation, and vascular contraction
ROS influence pro-contractile signalling through post-translational oxidative modification of proteins. Cysteine and methionine residues on proteins are highly redox-sensitive and undergo oxidative modification when ROS production is increased, such as in hypertension.124 Under physiological conditions, protein oxidation is usually reversible, while in pathological conditions associated with oxidative stress, protein oxidation may be irreversible resulting in oxidative damage of proteins and cell death. Many proteins that regulate vascular smooth muscle cell calcium homeostasis, including calcium channels, SERCA, TRP channels, and ROCK, undergo post-translational oxidative modifications.125–127 In addition, actin and actin-binding proteins, myosin and cofilin, are directly oxidized by ROS.128,129 Accordingly, increased vascular Nox-derived ROS generation in hypertension causes an increase in [Ca2+]i and cytoskeletal rearrangement leading to altered vascular reactivity and enhanced contraction.
9. Conclusions
Vascular smooth muscle cells are highly differentiated and normally maintain a contractile phenotype. Vascular contraction/relaxation is regulated by many processes that are both calcium-dependent and -independent and involve calcium channels and signalling pathways such as IP3-PKC-DAG and ROCK. In hypertension, these processes are dysregulated, and signalling pathways not typically associated with contraction, such as MAPKs, tyrosine kinases, and transcription factors are activated. These phenomena lead to a hypercontractile state and dedifferentiation of vascular smooth muscle cells to a proliferative/migratory phenotype with consequent vascular remodelling. Emerging evidence also implicates a role for the immune/inflammatory system and the non-coding genome in vascular dysfunction in hypertension. Unravelling the complex interactions between traditional pro-contractile calcium-regulated signalling pathways and non-traditional contractile mechanisms will provide better insights into processes underlying the vascular phenotype in hypertension.
Conflict of interest: none declared.
Funding
R.M.T. was supported through a British Heart Foundation Chair award (CH/4/29762). A.C.M. was supported through a University of Glasgow Walton fellowship. R.A.-L. was supported through a BHF Award of Excellence (RE/13/5/30177).
References
- 1. Savoia C, Burger D, Nishigaki N, Montezano A, Touyz RM.. Angiotensin II and the vascular phenotype in hypertension. Expert Rev Mol Med 2011; 13:11–20. [DOI] [PubMed] [Google Scholar]
- 2. Intengan HD, Schiffrin EL.. Structure and mechanical properties of resistance arteries in hypertension role of adhesion molecules and extracellular matrix determinants. Hypertension 2000; 36:312–318. [DOI] [PubMed] [Google Scholar]
- 3. Montezano AC, Tsiropoulou S, Dulak-Lis M, Harvey A, Camargo LDL, Touyz RM.. Redox signaling, Nox5 and vascular remodeling in hypertension. Curr Opin Nephrol Hypertens 2015; 24:425–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Touyz RM, Schiffrin EL.. Signal transduction mechanisms mediating the physiological and pathophysiological actions of angiotensin II in vascular smooth muscle cells. Pharmacol Rev 2000; 52:639–672. [PubMed] [Google Scholar]
- 5. Endemann DH, Schiffrin EL.. Endothelial dysfunction. J Am Soc Nephrol 2004; 15:1983–1992. [DOI] [PubMed] [Google Scholar]
- 6. Christensen KL, Mulvany MJ.. Location of resistance arteries. J Vasc Res 2001; 38:1–12. [DOI] [PubMed] [Google Scholar]
- 7. Welsh DG, Tran CHT, Hald BO, Sancho M.. The conducted vasomotor response: function, biophysical basis, and pharmacological control. Annu Rev Pharmacol Toxicol 2017; 58:391–410. [DOI] [PubMed] [Google Scholar]
- 8. Brozovich FV, Nicholson CJ, Degen CV, Gao YZ, Aggarwal M, Morgan KG.. Mechanisms of vascular smooth muscle contraction and the basis for pharmacologic treatment of smooth muscle disorders. Pharmacol Rev 2016; 68:476–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Quintavalle M, Condorelli G, Elia L.. Arterial remodeling and atherosclerosis: miRNAs involvement. Vascul Pharmacol 2011; 55:106–110. [DOI] [PubMed] [Google Scholar]
- 10. Intengan HD, Deng LY, Li JS, Schiffrin EL.. Mechanics and composition of human subcutaneous resistance arteries in essential hypertension. Hypertension 1999; 33:569–574. [DOI] [PubMed] [Google Scholar]
- 11. Sehgel NL, Vatner SF, Meininger GA.. Smooth muscle cell stiffness syndrome -revisiting the structural basis of arterial stiffness. Front Physiol 2015; 6:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Torjesen A, Cooper LL, Rong J, Larson MG, Hamburg NM, Levy D, Benjamin EJ, Vasan RS, Mitchell GF.. Relations of arterial stiffness with postural change in mean arterial pressure in middle-aged adults: the Framingham heart study. Hypertension 2017; 69:685–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tuna BG, Bakker EN, VanBavel E.. Smooth muscle biomechanics and plasticity: relevance for vascular calibre and remodelling. Basic Clin Pharmacol Toxicol 2012; 110:35–41. [DOI] [PubMed] [Google Scholar]
- 14. Ma KT, Li XZ, Li L, Jiang XW, Chen XY, Liu WD, Zhao L, Zhang ZS, Si JQ.. Role of gap junctions in the contractile response to agonists in the mesenteric artery of spontaneously hypertensive rats. Hypertens Res 2014; 37:110–115. [DOI] [PubMed] [Google Scholar]
- 15. Nguyen Dinh Cat A, Briones AM, Callera GE, Yogi A, He Y, Montezano AC, Touyz RM.. Adipocyte-derived factors regulate vascular smooth muscle cells through mineralocorticoid and glucocorticoid receptors. Hypertension 2011; 58:479–488. [DOI] [PubMed] [Google Scholar]
- 16. Neves KB, Nguyen Dinh Cat A, Lopes RA, Rios FJ, Anagnostopoulou A, Lobato NS, de Oliveira AM, Tostes RC, Montezano AC, Touyz RM.. Chemerin regulates crosstalk between adipocytes and vascular cells through nox. Hypertension 2015; 66:657–666. [DOI] [PubMed] [Google Scholar]
- 17. Sacharidou A, Stratman AN, Davis GE.. Molecular mechanisms controlling vascular lumen formation in three-dimensional extracellular matrices. Cells Tissues Organs 2012; 195:122–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim HR, Appel S, Vetterkind S, Gangopadhyay SS, Morgan KG.. Smooth muscle signalling pathways in health and disease. J Cell Mol Med 2008; 12:2165–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matchkov VV, Kudryavtseva O, Aalkjaer C.. Intracellular Ca2+ signalling and phenotype of vascular smooth muscle cells. Basic Clin Pharmacol Toxicol 2012;110:42–48. [DOI] [PubMed] [Google Scholar]
- 20. Kietadisorn R, Juni RP, Moens AL.. Tackling endothelial dysfunction by modulating NOS uncoupling: new insights into its pathogenesis and therapeutic possibilities. Am J Physiol Endocrinol Metab 2012; 302:E481–E495. [DOI] [PubMed] [Google Scholar]
- 21. Cahill PA, Redmond EM.. Vascular endothelium - gatekeeper of vessel health. Atherosclerosis 2016; 248:97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wede OK, Löfgren M, Li Z, Paulin D, Arner A.. Mechanical function of intermediate filaments in arteries of different size examined using desmin deficient mice. J Physiol 2002; 540:941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fisher SA. Vascular smooth muscle phenotypic diversity and function. Physiol Genomics 2010; 42A:169–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Davis MJ. Perspective: physiological role(s) of the vascular myogenic response. Microcirculation 2012; 19:99–114. [DOI] [PubMed] [Google Scholar]
- 25. Flammer A, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Lüscher TM, Shechter M, Taddei S, Vita JA, Lerman A.. The assessment of endothelial function from research into clinical practice. Circulation 2012; 126:753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coll-Bonfill N, de la Cruz-Thea B, Pisano MV, Musri MM.. Noncoding RNAs in smooth muscle cell homeostasis: implications in phenotypic switch and vascular disorders. Pflugers Arch 2016; 468:1071–1087. [DOI] [PubMed] [Google Scholar]
- 27. Owens GK, Kumar MS, Wamhoff BR.. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 2004; 84:767–801. [DOI] [PubMed] [Google Scholar]
- 28. Nemenoff RA, Horita H, Ostriker AC, Furgeson SB, Simpson PA, VanPutten V, Crossno J, Offermanns S, Weiser-Evans MC.. SDF-1alpha induction in mature smooth muscle cells by inactivation of PTEN is a critical mediator of exacerbated injury-induced neointima formation. Arterioscler Thromb Vasc Biol 2011; 31:1300–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC, Isakson B, Randolph GJ, Owens GK.. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med 2015; 21:628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kennedy AJ, Yang P, Read C, Kuc RE, Yang L, Taylor EJ, Taylor CW, Maguire JJ, Davenport AP.. Chemerin elicits potent constrictor actions via chemokine-like receptor 1 (CMKLR1), not G-protein-coupled receptor 1 (GPR1), in human and rat vasculature. J Am Heart Assoc 2016; 5:pii: e004421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wynne BM, Chiao CW, Webb RC.. Vascular smooth muscle cell signaling mechanisms for contraction to angiotensin II and endothelin-1. J Am Soc Hyperten 2009; 3:84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Touyz RM, El Mabrouk M, He G, Wu XH, Schiffrin EL.. Mitogen-activated protein/extracellular signal-regulated kinase inhibition attenuates angiotensin II-mediated signaling and contraction in spontaneously hypertensive rat vascular smooth muscle cells. Circ Res 1999; 84:505–515. [DOI] [PubMed] [Google Scholar]
- 33. Montezano AC, Zimmerman D, Yusuf H, Burger D, Chignalia AZ, Wadhera V, van Leeuwen FN, Touyz RM.. Vascular smooth muscle cell differentiation to an osteogenic phenotype involves TRPM7 modulation by magnesium. Hypertension 2010; 56:453–462. [DOI] [PubMed] [Google Scholar]
- 34. Albinsson S, Suarez Y, Skoura A, Offermanns S, Miano JM, Sessa WC.. MicroRNAs are necessary for vascular smooth muscle growth, differentiation, and function. Arterioscler Thromb Vasc Biol 2010; 30:1118–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bell RD, Long X, Lin M, Bergmann JH, Nanda V, Cowan SL, Zhou Q, Han Y, Spector DL, Zheng D, Miano JM.. Identification and initial functional characterization of a human vascular cell-enriched long noncoding RNA. Arterioscler Thromb Vasc Biol 2014; 34:1249–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Boettger T, Beetz N, Kostin S, Schneider J, Kruger M, Hein L, Braun T.. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Invest 2009; 119:2634–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gomes CPC, Spencer H, Ford KL, Michel LYM, Baker AH, Emanueli C, Balligand JL, Devaux Y.. Cardiolinc Network. The function and therapeutic potential of long non-coding RNAs in cardiovascular development and disease. Mol Ther Nucleic Acids 2017; 8:494–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gangwar RS, Rajagopalan S, Natarajan R, Deiuliis JA.. Non-coding RNAs in cardiovascular disease: pathological relevance and emerging role as biomarkers and therapeutics. Am J Hypertens 2018; 31:150–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang Y-N-Z, Shan K, Yao M-D, Yao J, Wang J-J, Li X, Liu B, Zhang Y-Y, Ji Y, Jiang Q, Yan B.. Long noncoding RNA-GAS5: a novel regulator of hypertension-induced vascular remodeling. Hypertension 2016; 68:736–748. [DOI] [PubMed] [Google Scholar]
- 40. Allen BG, Walsh M.. The biochemical basis of the regulation of smooth-muscle contraction. Trends Biochem Sci 1994; 19:362–368. [DOI] [PubMed] [Google Scholar]
- 41. Ikebe M. Regulation of the function of mammalian myosin and its conformational change. Biochem Biophys Res Commun 2008; 369:157–164. [DOI] [PubMed] [Google Scholar]
- 42. Walsh M. Vascular smooth muscle myosin light chain diphosphorylation: mechanism, function, and pathological implications. IUBMB Life 2011; 63:987–1000. [DOI] [PubMed] [Google Scholar]
- 43. Hill MA, Meininger GA.. Small artery mechanobiology: roles of cellular and non-cellular elements. Microcirculation 2016; 23:611–613. [DOI] [PubMed] [Google Scholar]
- 44. Chironi GN, Boulanger CM, Simon A, Dignat-George F, Freyssinet J-M, Tedgui A.. Endothelial microparticles in diseases. Cell Tissue Res 2009; 335:143–151. [DOI] [PubMed] [Google Scholar]
- 45. Burger D, Turner M, Munkonda MN, Touyz RM.. Endothelial microparticle-derived reactive oxygen species: role in endothelial signaling and vascular function. Oxid Med Cell Longev 2016;2016:1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Félétou M, Verbeuren TJ, Vanhoutte PM.. Endothelium-dependent contractions in SHR: a tale of prostanoid TP and IP receptors. Br J Pharmacol 2009; 156:563–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Matsuda K, Lozinskaya I, Cox R. H. Augmented contributions of voltage-gated Ca2+ channels to contractile responses in spontaneously hypertensive rat mesenteric arteries. Am J Hypertens 1997;10:1231–1239. [DOI] [PubMed] [Google Scholar]
- 48. Misárková E, Behuliak M, Bencze M, Zicha J.. Excitation-contraction coupling and excitation-transcription coupling in blood vessels: their possible interactions in hypertensive vascular remodeling. Physiol Res 2016; 65:173–191. [DOI] [PubMed] [Google Scholar]
- 49. Bazan E, Campbell AK, Rapoport RM.. Protein kinase C activity in blood vessels from normotensive and spontaneously hypertensive rats. Eur J Pharmacol 1992; 227:343–348. [DOI] [PubMed] [Google Scholar]
- 50. Goulopoulou S, Webb RC.. Symphony of vascular contraction: how smooth muscle cells lose harmony to signal increased vascular resistance in hypertension. Hypertension 2014; 63:e33–e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Touyz RM, Deng LY, Schiffrin EL.. Ca2+ and contractile responses of resistance vessels of WKY rats and SHR to endothelin-1. J Cardiovasc Pharmacol 1995; 26:S193–S196. [PubMed] [Google Scholar]
- 52. Tabet F, Savoia C, Schiffrin EL, Touyz RM.. Differential calcium regulation by hydrogen peroxide and superoxide in vascular smooth muscle cells from spontaneously hypertensive rats. J Cardiovasc Pharmacol 2004; 44:200–208. [DOI] [PubMed] [Google Scholar]
- 53. Matchkov VV, Boedtkjer DM, Aalkjaer C.. The role of Ca(2+) activated Cl(-) channels in blood pressure control. Curr Opin Pharmacol 2015; 21:127–137. [DOI] [PubMed] [Google Scholar]
- 54. Kosch M, Hausberg M, Barenbrock M, Posadzy-Malaczynska A, Rahn KH, Kisters K.. Increased membraneous calcium concentrations in primary hypertension: a causal link to pathogenesis? J Hum Hypertens 2001; 15:37–40. [DOI] [PubMed] [Google Scholar]
- 55. Godfraind T. Discovery and development of calcium channel blockers. Front Pharmacol 2017; 8:286.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zamponi GW, Striessnig J, Koschak A, Dolphin AC.. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol Rev 2015; 67:821–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhou Y, Fan J, Zhu H, Ji L, Fan W, Kapoor I, Wang Y, Wang Y, Zhu G, Wang J.. Aberrant splicing induced by dysregulated Rbfox2 produces enhanced function of CaV12 calcium channel and vascular myogenic tone in hypertension. Hypertension 2017; 70:1183–1193. [DOI] [PubMed] [Google Scholar]
- 58. Giachini FR, Lima VV, Filgueira FP, Dorrance AM, Carvalho MH, Fortes ZB, Webb RC, Tostes RC.. STIM1/Orai1 contributes to sex differences in vascular responses to calcium in spontaneously hypertensive rats. Clin Sci 2012; 122:215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Callera GE, Antunes TT, He Y, Montezano AC, Yogi A, Savoia C, Touyz RM.. c-Src inhibition improves cardiovascular function but not remodeling or fibrosis in angiotensin II-induced hypertension. Hypertension 2016; 68:1179–1190. [DOI] [PubMed] [Google Scholar]
- 60. Watanabe S, Matsumoto T, Ando M, Adachi T, Kobayashi S, Iguchi M, Takeuchi M, Taguchi K, Kobayashi T.. Multiple activation mechanisms of serotonin-mediated contraction in the carotid arteries obtained from spontaneously hypertensive rats. Pflugers Arch 2016; 468:1271–1282. [DOI] [PubMed] [Google Scholar]
- 61. Touyz RM, Montezano AC, Rios F, Widlansky ME, Liang M.. Redox stress defines the small artery vasculopathy of hypertension: how do we bridge the bench-to-bedside gap? Circ Res 2017; 120:1721–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. García-Redondo AB, Briones AM, Martínez-Revelles S, Palao T, Vila L, Alonso MJ, Salaices M.. c-Src, ERK1/2 and Rho kinase mediate hydrogen peroxide-induced vascular contraction in hypertension: role of TXA2, NAD(P)H oxidase and mitochondria. J Hypertens 2015; 33:77–87. [DOI] [PubMed] [Google Scholar]
- 63. Harvey AP, Montezano AC, Hood KY, Lopes RA, Rios F, Ceravolo G, Graham D, Touyz RM.. Vascular dysfunction and fibrosis in stroke-prone spontaneously hypertensive rats: the aldosterone-mineralocorticoid receptor-Nox1 axis. Life Sci 2017; 179:110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Touyz RM, Wu X-H, He G, Park JB, Chen X, Vacher J, Rajapurohitam V, Schiffrin EL.. Role of c-Src in the regulation of vascular contraction and Ca2+ signaling by angiotensin II in human vascular smooth muscle cells. J Hypertens 2001;19:441–449. [DOI] [PubMed] [Google Scholar]
- 65. Norlander AE, Madhur MS, Harrison DG.. The immunology of hypertension. J Exp Med 2018; 215:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bomfim GF, Dos Santos RA, Oliveira MA, Giachini FR, Akamine EH, Tostes RC, Fortes ZB, Webb RC, Carvalho MH.. Toll-like receptor 4 contributes to blood pressure regulation and vascular contraction in spontaneously hypertensive rats. Clin Sci 2012; 122:535–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Guzik TJ, Skiba DS, Touyz RM, Harrison DG.. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc Res 2017; 113:1009–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Loirand G, Pacaud P.. The role of Rho protein signaling in hypertension. Nat Rev Cardiol 2010; 7:637–647. [DOI] [PubMed] [Google Scholar]
- 69. Endo A, Surks HK, Mochizuki S, Mochizuki N, Mendelsohn ME.. Identification and characterization of zipper-interacting protein kinase as the unique vascular smooth muscle myosin phosphatase-associated kinase. J Biol Chem 2004; 279:42055–42061. [DOI] [PubMed] [Google Scholar]
- 70. Deng JT, Van Lierop JE, Sutherland C, Walsh MP.. Ca2+-independent smooth muscle contraction. A novel function for integrin-linked kinase. J Biol Chem 2001;276:16365–16373. [DOI] [PubMed] [Google Scholar]
- 71. Ringvold HC, Khalil RA.. Protein kinase C as regulator of vascular smooth muscle function and potential target in vascular disorders. Adv Pharmacol 2017; 78:203–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Khalil RA. Protein kinase C inhibitors as modulators of vascularfunction and their application in vascular disease. Pharmaceuticals 2013; 6:407–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Chan CK, Mak JC, Man RY, Vanhoutte PM.. Rho kinase inhibitors prevent endothelium-dependent contractions in the rat aorta. J Pharmacol Exp Ther 2009; 329:820–826. [DOI] [PubMed] [Google Scholar]
- 74. Bilder GE, Kasiewski CJ, Perrone MH.. Phorbol-12, 13-dibutyrate-induced vasoconstriction in vivo: characterization of response in genetic hypertension. J Pharmacol Exp Ther 1990; 252:526–530. [PubMed] [Google Scholar]
- 75. Touyz RM, Schiffrin EL.. Ang II-stimulated generation of reactive oxygen species in human vascular smooth muscle cells is mediated via PLD-dependent pathways. Hypertension 1999; 34:976–982. [DOI] [PubMed] [Google Scholar]
- 76. Calò LA, Davis PA, Pagnin E, Dal Maso L, Maiolino G, Seccia TM, Pessina AC, Rossi GP.. Increased level of p63RhoGEF and RhoA/Rho kinase activity in hypertensive patients. J Hypertens 2014; 32:331–338. [DOI] [PubMed] [Google Scholar]
- 77. Deissler HL, Lang GE.. The protein kinase C inhibitor: ruboxistaurin. Dev Ophthalmol 2016; 55:295–301. [DOI] [PubMed] [Google Scholar]
- 78. Seko T, Ito M, Kureishi Y, Okamoto R, Moriki N, Onishi K, Isaka N, Hartshorne DJ, Nakano T.. Activation of RhoA and inhibition of myosin phosphatase as important components in hypertension in vascular smooth muscle. Circ Res 2003; 92:411–418. [DOI] [PubMed] [Google Scholar]
- 79. Rahman A, Davis B, Lövdahl C, Hanumaiah VT, Feil R, Brakebusch C, Arner A.. The small GTPase Rac1 is required for smooth muscle contraction. J Physiol 2014; 592:915–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Behuliak M, Bencze M, Vaněčková I, Kuneš J, Zicha J.. Basal and activated calcium sensitization mediated by RhoA/Rho kinase pathway in rats with genetic and salt hypertension. Biomed Res Int 2017; 2017:8029728.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M, Narumiya S.. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature 1997; 389:990–994. [DOI] [PubMed] [Google Scholar]
- 82. Huveneers S, Daemen MJL, Hordijk PL.. Between Rho(k) and a hard place the relation between vessel wall stiffness, endothelial contractility, and cardiovascular disease. Circ Res 2015; 116:895–908. [DOI] [PubMed] [Google Scholar]
- 83. Crestani S, Webb RC, da Silva-Santos JE.. High-salt intake augments the activity of the RhoA/ROCK pathway and reduces intracellular calcium in arteries from rats. Am J Hypertens 2017; 30:389–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Komers R, Oyama TT, Beard DR, Anderson S.. Effects of systemic inhibition of Rho kinase on blood pressure and renal haemodynamics in diabetic rats. Br J Pharmacol 2011; 162:163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cario-Toumaniantz C, Ferland-McCollough D, Chadeuf G, Toumaniantz G, Rodriguez M, Galizzi JP, Lockhart B, Bril A, Scalbert E, Loirand G, Pacaud P.. RhoA guanine exchange factor expression profile in arteries: evidence for a Rho kinase-dependent negative feedback in angiotensin II-dependent hypertension. Am J Physiol Cell Physiol 2012; 302:C1394–C1404. [DOI] [PubMed] [Google Scholar]
- 86. Jernigan NL, Resta TC.. Calcium homeostasis and sensitization in pulmonary arterial smooth muscle. Microcirculation 2014; 21:259–271. [DOI] [PubMed] [Google Scholar]
- 87. Zhou Q, Wei SS, Wang H, Wang Q, Li W, Li G, Hou JW, Chen XM, Chen J, Xu WP, Li YG, Wang YP.. Crucial role of ROCK2-mediated phosphorylation and upregulation of FHOD3 in the pathogenesis of angiotensin II-induced cardiac hypertrophy. Hypertension 2017; 69:1070–1083. [DOI] [PubMed] [Google Scholar]
- 88. Carbone ML, Brégeon J, Devos N, Chadeuf G, Blanchard A, Azizi M, Pacaud P, Jeunemaître X, Loirand G.. Angiotensin II activates the RhoA exchange factor Arhgef1 in humans. Hypertension 2015; 65:1273–1278. [DOI] [PubMed] [Google Scholar]
- 89. Zhou N, Lee JJ, Stoll S, Ma B, Costa KD, Qiu H.. Rho kinase regulates aortic vascular smooth muscle cell stiffness via actin/SRF/myocardin in hypertension. Cell Physiol Biochem 2017; 44:701–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Mukai Y, Shimokawa H, Matoba T, Kandabashi T, Satoh S, Hiroki J, Kaibuchi K, Takeshita A.. Involvement of Rho-kinase in hypertensive vascular disease: a novel therapeutic target in hypertension. FASEB J 2001; 15:1062–1064. [DOI] [PubMed] [Google Scholar]
- 91. Zhao J, Zhou D, Guo J, Ren Z, Zhou L, Wang S, Xu B, Wang R.. Effect of fasudil hydrochloride, a protein kinase inhibitor, on cerebral vasospasm and delayed cerebral ischemic symptoms after aneurysmal subarachnoid hemorrhage. Neurol Med Chir 2006; 46:421–428. [DOI] [PubMed] [Google Scholar]
- 92. Fukumoto Y, Mohri M, Inokuchi K, Ito A, Hirakawa Y, Masumoto A, Hirooka Y, Takeshita A, Shimokawa H.. Anti-ischemic effects of fasudil, a specific Rho-kinase inhibitor, in patients with stable effort angina. J Cardiovasc Pharmacol 2007; 49:117–121. [DOI] [PubMed] [Google Scholar]
- 93. Masumoto A, Mohri M, Shimokawa H, Urakami L, Usui M, Takeshita A.. Suppression of coronary artery spasm by the Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation 2002; 105:1545–1547. [DOI] [PubMed] [Google Scholar]
- 94. Nohria A, Grunert ME, Rikitake Y, Noma K, Prsic A, Ganz P, Liao JK, Creager MA.. Rho kinase inhibition improves endothelial function in human subjects with coronary artery disease. Circ Res 2006; 99:1426–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Büssemaker E, Herbrig K, Pistrosch F, Palm C, Passauer J.. Role of Rho-kinase in the regulation of vascular tone in hypertensive renal transplant recipients. Atherosclerosis 2009; 207:567–572. [DOI] [PubMed] [Google Scholar]
- 96. Zhang Y, Wu S.. Effects of fasudil on pulmonary hypertension in clinical practice. Pulm Pharmacol Ther 2017; 46:54–63. [DOI] [PubMed] [Google Scholar]
- 97. Qiao YN, He WQ, Chen CP, Zhang CH, Zhao W, Wang P, Zhang L, Wu YZ, Yang X, Peng YJ, Gao JM, Kamm KE, Stull JT, Zhu MS.. Myosin phosphatase target subunit 1 (MYPT1) regulates the contraction and relaxation of vascular smooth muscle and maintains blood pressure. J Biol Chem 2014; 289:22512–22523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Butler T, Paul J, Europe-Finner N, Smith R, Chan EC.. Role of serine-threonine phosphoprotein phosphatases in smooth muscle contractility. Am J Physiol Cell Physiol 2013; 304:C485–C504. [DOI] [PubMed] [Google Scholar]
- 99. Vanhoutte PM, Shimokawa H, Feletou M, Tang EH.. Endothelial dysfunction and vascular disease - a 30th anniversary update. Acta Physiol 2017; 219:22–29. [DOI] [PubMed] [Google Scholar]
- 100. Bonnevier J, Arner A.. Actions downstream of cyclic GMP/protein kinase G can reverse protein kinase C-mediated phosphorylation of CPI-17 and Ca2+ sensitization in smooth muscle. J Biol Chem 2004;279:28998–29003. [DOI] [PubMed] [Google Scholar]
- 101. Takeya K, Wang X, Sutherland C, Kathol I, Loutzenhiser K, Loutzenhiser RD, Walsh MP.. Involvement of myosin regulatory light chain diphosphorylation in sustained vasoconstriction under pathophysiological conditions. J Smooth Muscle Res 2014; 50:18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Khromov A, Choudhury N, Stevenson AS, Somlyo AV, Eto M.. Phosphorylation-dependent autoinhibition of myosin light chain phosphatase accounts for Ca2+ sensitization force of smooth muscle contraction. J Biol Chem 2009; 284:21569–21579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Martinez-Lemus LA, Hill MA, Meininger GA.. The plastic nature of the vascular wall: a continuum of remodeling events contributing to control of arteriolar diameter and structure. Physiology 2009; 24:45–55. [DOI] [PubMed] [Google Scholar]
- 104. Rembold CM, Tejani AD, Ripley ML, Han S.. Paxillin phosphorylation, actin polymerization, noise temperature, and the sustained phase of swine carotid artery contraction. Am J Physiol Cell Physiol 2007; 293:C993–C100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Hong Z, Sun Z, Li Z, Mesquitta WT, Trzeciakowski JP, Meininger GA.. Coordination of fibronectin adhesion with contraction and relaxation in microvascular smooth muscle. Cardiovasc Res 2012; 96:73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Sun Z, Li Z, Meininger GA.. Mechanotransduction through fibronectin-integrin focal adhesion in microvascular smooth muscle cells: is calcium essential? Am J Physiol Heart Circ Physiol 2012; 302:H1965–H1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Stålhand J, Holzapfel GA.. Length adaptation of smooth muscle contractile filaments in response to sustained activation. J Theor Biol 2016; 397:13–21. [DOI] [PubMed] [Google Scholar]
- 108. Staiculescu MC, Galiñanes EL, Zhao G, Ulloa U, Jin M, Beig MI, Meininger GA, Martinez-Lemus LA.. Prolonged vasoconstriction of resistance arteries involves vascular smooth muscle actin polymerization leading to inward remodelling. Cardiovasc Res 2013; 98:428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Al Ghouleh I, Khoo NK, Knaus UG, Griendling KK, Touyz RM, Thannickal VJ, Barchowsky A, Nauseef WM, Kelley EE, Bauer PM, Darley-Usmar V, Shiva S, Cifuentes-Pagano E, Freeman BA, Gladwin MT, Pagano PJ.. Oxidases and peroxidases in cardiovascular and lung disease: new concepts in reactive oxygen species signaling. Free Radic Biol Med 2011; 51:1271–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Touyz RM, Briones AM.. Reactive oxygen species and vascular biology: implications in human hypertension. Hypertens Res 2011; 34:5–14. [DOI] [PubMed] [Google Scholar]
- 111. Brown DI, Griendling KK.. Nox proteins in signal transduction. Free Radic Biol Med 2009; 47:1239–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Montezano AC, Burger D, Ceravolo GS, Yusuf H, Montero M, Touyz RM.. Novel noxes homologues in the vasculature: focusing on Nox4 and Nox5. Clin Sci 2011; 120:131–141. [DOI] [PubMed] [Google Scholar]
- 113. Montezano AC, Dulak-Lis M, Tsiropoulou S, Harvey A, Briones AM, Touyz RM.. Oxidative stress and human hypertension: vascular mechanisms, biomarkers, and novel therapies. Can J Cardiol 2015; 31:631–641. [DOI] [PubMed] [Google Scholar]
- 114. Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW.. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res 1994; 74:1141–1148. [DOI] [PubMed] [Google Scholar]
- 115. Lopes RA, Neves KB, Tostes RC, Montezano AC, Touyz RM.. Downregulation of nuclear factor erythroid 2-related factor and associated antioxidant genes contributes to redox-sensitive vascular dysfunction in hypertension. Hypertension 2015; 66:1240–1250. [DOI] [PubMed] [Google Scholar]
- 116. Touyz RM. Reactive oxygen species as mediators of calcium signaling by angiotensin II: implications in vascular physiology and pathophysiology. Antioxid Redox Signal 2005; 7:1302–1308. [DOI] [PubMed] [Google Scholar]
- 117. Zima AV, Blatter LA.. Redox regulation of cardiac calcium channels and transporters. Cardiovasc Res 2006; 71:310–321. [DOI] [PubMed] [Google Scholar]
- 118. Kadlec AO, Chabowski DS, Ait-Aissa K, Hockenberry JC, Otterson MF, Durand MJ, Freed JK, Beyer AM, Gutterman DD.. PGC-1α (peroxisome proliferator-activated receptor γ coactivator 1-α) overexpression in coronary artery disease recruits NO and hydrogen peroxide during flow-mediated dilation and protects against increased intraluminal pressure. Hypertension 2017; 70:166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Jackson WF. Arteriolar oxygen reactivity: where is the sensor and what is the mechanism of action? J Physiol 2016; 594:5055–5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Tanaka LY, Laurindo FRM.. Vascular remodelling: a redox-modulated mechanism of vessel calibre regulation. Free Rad Biol Med 2017; 109:11–21. [DOI] [PubMed] [Google Scholar]
- 121. Rasmussen HH, Hamilton EJ, Liu CC, Figtree GA.. Reversible oxidative modification: implications for cardiovascular physiology and pathophysiology. Trends Cardiovasc Med 2010; 20:85–90. [DOI] [PubMed] [Google Scholar]
- 122. Ellinsworth DC, Sandow SL, Shukla N, Liu Y, Jeremy JY, Gutterman DD.. Endothelium-derived hyperpolarization and coronary vasodilation: diverse and integrated roles of epoxyeicosatrienoic acids, hydrogen peroxide, and gap junctions. Microcirculation 2016; 23:15–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Burgoyne JR, Madhani M, Cuello F, Charles RL, Brennan JP, Schröder E, Browning DD, Eaton P.. Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science 2007; 317:1393–1397. [DOI] [PubMed] [Google Scholar]
- 124.Gu SX, Stevens JW, Lentz SR. Regulation of thrombosis and vascular function by protein methionine oxidation. Blood 2015; 125:3851–3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pasha S, Tveen Jensen K, Pitt AR, Spickett CM. Detection and quantification of protein oxidation in sarcopenic models: a mass spectrometry study. Free Radic Biol Med 2014; 75:Suppl 1:S44. [DOI] [PubMed] [Google Scholar]
- 126.Voolstra O, Huber A. Post-Translational Modifications of TRP Channels. Cells 2014; 3:258–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hobbs GA, Zhou B, Cox AD, Campbell SL. Rho GTPases, oxidation, and cell redox control. Small GTPases 2014; 5:e28579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fedorova M, Kuleva N, Hoffmann R. Identification of cysteine, methionine and tryptophan residues of actin oxidized in vivo during oxidative stress. J Proteome Res 2010; 9:1598–1609. [DOI] [PubMed] [Google Scholar]
- 129.Klamt F, Zdanov S, Levine RL, Pariser A, Zhang Y, Zhang B, Yu LR, Veenstra TD, Shacter E. Oxidant-induced apoptosis is mediated by oxidation of the actin-regulatory protein cofilin. Nat Cell Biol 2009; 11:1241–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]