Abstract
Aims
The heart contraction is controlled by the Ca2+-induced Ca2+ release (CICR) between L-type Ca2+ channels and ryanodine receptors (RyRs). The FK506-binding protein FKBP12.6 binds to RyR subunits, but its role in stabilizing RyR function has been debated for long. Recent reports of high-resolution RyR structure show that the HD2 domain that binds to the SPRY2 domain of neighbouring subunit in FKBP-bound RyR1 is detached and invisible in FKBP-null RyR2. The present study was to test the consequence of FKBP12.6 absence on the in situ activation of RyR2.
Methods and results
Using whole-cell patch-clamp combined with confocal imaging, we applied a near threshold depolarization to activate a very small fraction of LCCs, which in turn activated RyR Ca2+ sparks stochastically. FKBP12.6-knockout and FK506/rapamycin treatments increased spark frequency and LCC-RyR coupling fidelity without altering LCC open probability. Neither FK506 nor rapamycin further altered LCC-RyR coupling fidelity in FKBP12.6-knockout cells. In loose-seal patch-clamp experiments, the LCC-RyR signalling kinetics, indexed by the delay for a LCC sparklet to trigger a RyR spark, was accelerated after FKBP12.6 knockout and FK506/rapamycin treatments. These results demonstrated that RyRs became more sensitive to Ca2+ triggers without FKBP12.6. Isoproterenol (1 μM) further accelerated the LCC-RyR signalling in FKBP12.6-knockout cells. The synergistic sensitization of RyRs by catecholaminergic signalling and FKBP12.6 dysfunction destabilized the CICR system, leading to chaotic Ca2+ waves and ventricular arrhythmias.
Conclusion:
FKBP12.6 keeps the RyRs from over-sensitization, stabilizes the potentially regenerative CICR system, and thus may suppress the life-threatening arrhythmogenesis.
Keywords: Excitation-contraction coupling, FK506-binding protein, Ryanodine receptor, Calcium signalling
1. Introduction
Ca2+-induced Ca2+ release (CICR) is a fundamental cellular mechanism to generate and amplify intracellular Ca2+ signals.1,2 In healthy heart cells, CICR is operated between L-type Ca2+ channels (LCCs) in the cell membrane/T-tubules and ryanodine receptor (RyR) Ca2+ release channels in the sarcoplasmic reticulum (SR).3,4 The RyR-mediated SR Ca2+ release determines the pace and strength of myocardial contraction. Intuitively, CICR is by nature a positive feedback, and would be expected to operate in an explosive all-or-none manner. However, the existence of solitary RyR Ca2+ release events, Ca2+ sparks,5 suggests that global positive feedback among RyRs is effectively avoided such that the CICR is actually modulated precisely in a graded manner.6 This paradox has been explained by the local control model, in which RyRs are under nanoscopic ‘private’ control by adjacent LCCs within their native Ca2+ release unit (CRU). Although regenerative CICR may still exist within a CRU,7,8 a CRU does not respond to Ca2+ signals propagating from neighbouring CRUs.9–11 This scenario is supported by ultrastructural studies, which show that the RyR-residing junctional SRs meet with cell membrane/T-tubules at a distance (∼15 nm) much shorter than the inter-CRU distance.12 To avoid inter-CRU crosstalk, the RyR sensitivity to Ca2+ triggers must be limited within a certain range.
The 12.6-kd FK506-binding protein (FKBP12.6, also known as calstabin-2) is a cardiac RyR accessory protein13,14 proposed to stabilize RyR Ca2+ release.15,16 However, the role of FKBP12.6 in RyR function has been highly controversial.17 In lipid bilayer experiments, single RyRs from FKBP12.6-knockout mice or treated with rapamycin/FK506 to dissociate FKBP12.6 are found to have increased open probability and partial opening/sub-conductance.18–20 However, single-channel experiments from other labs have shown that the removal of FKBP12.6 from RyRs neither alters channel activity nor prompts sub-conductance.13,21,22 While it has been demonstrated that FKBP12.6 dissociation synergistically enhances the RyR response to protein kinase A (PKA)-mediated phosphorylation,15,23,24 contrary evidence has shown that the PKA-induced RyR regulation does not depend on FKBP12.6.25–27 In intact cardiomyocytes, some reports have shown that FK506 treatment or FKBP12.6 dissociation increases the spontaneous Ca2+ spark frequency and Ca2+ transient amplitude.20,28,29 FKBP12.6 knockout mice develop severe arrhythmia that leads to sudden cardiac death during exercise.24 By contrast, a later report has shown that FKBP12.6 knockout neither promotes spontaneous RyR activity nor causes ventricular arrhythmias under stress conditions.22
Recently, the high-resolution structure of mammalian RyRs has been independently reported by several research groups.30–34 The structure of RyR1-FKBP12 complex shows that that the BSol (HD2) domain binds to the SPRY2 domain of its neighbouring subunit, suggesting that the HD2 domain may play a role in coordinating the allosteric activity among RyR subunits. However, in FKBP-null RyR2, a large portion of HD2 is invisible, indicating that HD2 becomes flexible without FKBPs. Also, FKBP12.6-knockout mice chronically develop cardiac dysfunction due to the activation of a set of signalling cascades.35 These new progresses re-arouse our interest on the role of FKBP12.6 in regulating RyR Ca2+ release in cardiomyocytes. In the present study, we investigated the in situ role of FKBP12.6 in RyR function with a more rigorous experimental design. We compared the in situ behaviour of RyRs in wild-type and FKBP12.6 knockout cardiomyocytes, and provided direct evidence that FKBP12.6 does stabilize the in situ operation of RyRs in intact cardiomyocytes. FKBP12.6 loss-of-function and catecholamine stimulation synergistically over-sensitize the CICR, leading to arrhythmogenic intracellular Ca2+ dynamics and ventricular arrhythmias.
2. Methods
2.1 Preparation of ventricular cardiomyocytes
The investigation conforms with the Guide for Care and Use of Laboratory animals published by the US National Institutes of Health (NIH Publication No.85-23, revised 2011). Animal experiments were approved by the Institutional Animal Care and Use Committee of Peking University. Single ventricular cardiomyocytes were isolated from 3-month-old wild-type and FKBP12.6-knockout male mice,20 as previously described.36 Mice were anesthetized by intraperitoneally injecting with 1g/kg ethyl carbamate. The heart was immediately cut and rinsed in Tyrode solution containing (in mmol/L): 137 NaCl, 5.4 KCl, 1.2 MgCl2, 20 Hepes, 1.2 NaH2PO4·2H2O, 10 glucos, 10 taurine, pH adjusted to 7.4 with NaOH. Then the aorta was cannulated to the Langendorff apparatus and the heart was perfused through the coronary artery retrogradely with Tyrode solution at 37°C to clean the blood in vessels for 5 min. Perfusion flow rate was constant at 3mL/min for mouse hearts. With type II collagenase (200U/ml) and protease type XIV (0.35U/ml) in Tyrode’s solution, the heart was perfused to be digested. When turning soft, the heart was cut into small chunks, which were then dispersed into single cells by shaking at 37°C for 3–5min, 50rpm for 3 times. Single cells were then collected by centrifuging at low speed for 1 min, and re-suspended with Tyrode’s solution containing 4mg/mL bovine serum albumin. Ca2+ concentration was restored step by step from 0.05 to 1mmol/L. Finally, cells were resuspended in extracellular solution containing (in mmol/L): 135 NaCl, 4 KCl, 1 CaCl2, 1 MgCl2, 10 Hepes, 1.2 NaH2PO4·2H2O, 10 glucose, pH adjusted to 7.4 with NaOH.
2.2 Patch clamp
The patch clamp was made at room temperature (∼25°C) using an EPC7 amplifier. Cells were bathed in extracellular solution containing (in mmol/L) 135 NaCl, 4 KCl, 1 CaCl2, 1 MgCl2, 10 Hepes, 1.2 NaH2PO4·2H2O, 10 glucose, pH adjusted to 7.4 with NaOH. For whole-cell patch clamp of ICa,L recording, 15 µM tetrodotoxin and 4 mM 4-aminopyridine were added in the extracellular solution. A glass pipette with a resistance (Rp) of 2-3 MΩ was filled with (in mmol/L) 110 CsCl, 6 MgCl2, 5 Na2ATP, 10 Hepes, 15 TEA·Cl, 0.2 Fluo-4 pentapotassium, pH adjusted to 7.2 with CsOH. In loose-seal experiments, cells were loaded with fluo-4-AM and the pipette electrode with Rp of 4-6 MΩ was filled with (in mmol/L) 120 TEA·Cl, 10 Hepes, 0.01 TTX, 20 CaCl2 and 0.01 FPL64176, pH adjusted to 7.4 with TEA·OH, and was gently pressed onto the cell surface to form a low-resistance seal (Rs = 20–30 MΩ). The patch membrane voltage (VP) was determined based on the resting potential (RP) and command voltage (Vcom) by VP = RP−Vcom(Rs−Rp)/Rs.
2.3 Confocal Ca2+ imaging
Ca2+ imaging was recorded as previously described.37 In whole-cell patch clamp experiments, the Ca2+ indicator Fluo-4 pentapotassium salt (200μmol/L) was already included in the pipette solution. In loose-patch and Ca2+ wave experiments, cells were loaded with 10μmol/L fluo-4-AM (Invitrogen) in 37°C for 5 min in extracellular solution. After the incubation, cells were washed with extracellular solution. Ca2+ imaging was recorded with a Zeiss LSM-510 inverted confocal microscope (Carl Zeiss) with 488 nm laser excitation and a 40X 1.3 N.A. oil-immersion objective. All images were acquired along the long axis of cells in line-scan mode at a sampling rate of 0.768 ms/line for sparklet-spark experiments and 15.36 ms/line for other experiments. Local [Ca2+] was determined by the formula [Ca2+] = kd·R/(kd/C0 + 1−R), where R is the relative fluo-4 fluorescence normalized by the resting level, kd = 1.1 μM is the apparent dissociation constant of fluo-4, and C0 = 100nM is the resting Ca2+ concentration.
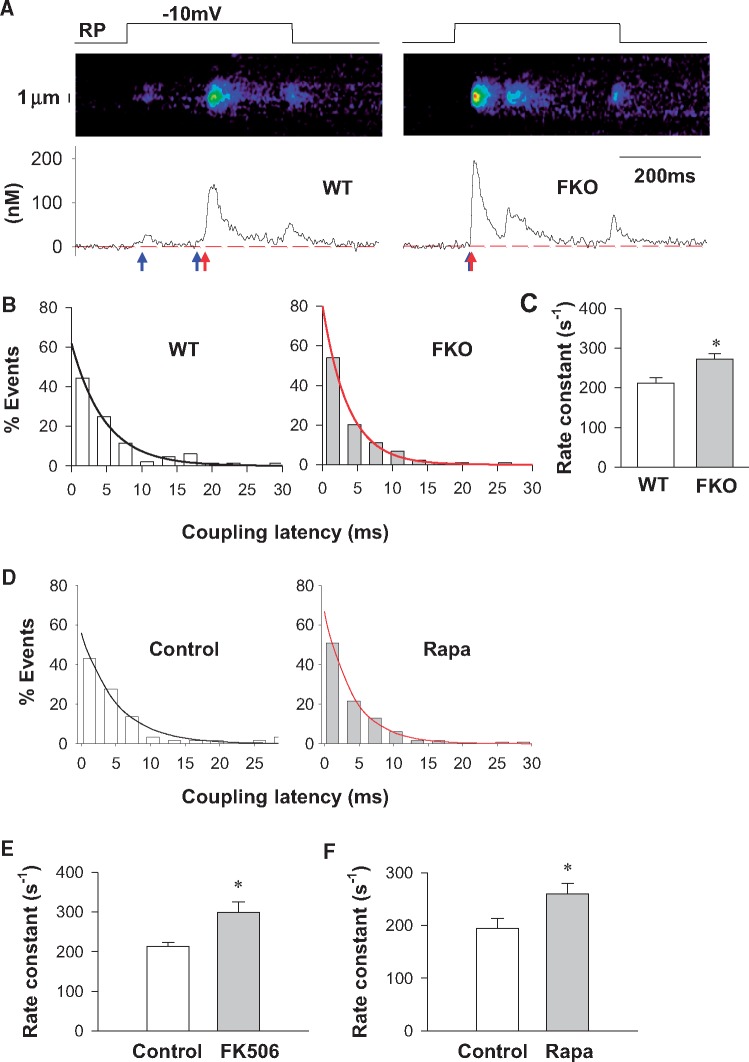
We measured the sparklet-spark latency only for the first spark triggered during a depolarization. The latency was determined by tracing back from the upstroke of a spark to the first datum point that fell below the SD of baseline. Given that the LCC-RyR coupling is a stochastic process, a significant portion of the latency data, represented by the first bar of the latency histogram, were too short for a sparklet to be visually identified (for example, see the right panel of Figure 3A). However, this does not influence the reliability of the latency histogram, which follows an exponential distribution. The apparent rate constant (k) for RyRs to respond to LCC Ca2+ sparklets was determined by fitting the distribution with the formula N = N0e−kt, where N is the number of observations and N0 is the N when t = 0.
Figure 3.
FKBP12.6 dysfunction accelerates the kinetics of LCC-RyR coupling. (A) Representative recordings from WT and FKO groups showing that depolarization to − 10 mV from resting potential (RP) (upper panels) triggered Ca2+ sparklets and Ca2+ sparks (middle panels for images and lower panels for time profiles) in a probabilistic manner. The blue and red arrows indicate the beginning of Ca2+ sparklets and Ca2+ sparks, respectively. (B) Distributions (bars) and exponential fittings (curves) of the coupling latency in WT and FKO groups. (C) Rate constants of LCC-RyR coupling in WT and FKO. (D) Distributions (bars) and exponential fittings (curves) of the coupling latency in WT under control and rapamycin-treated conditions. (E) Rate constants of LCC-RyR coupling in WT under control and FK506-treated conditions. (F) Rate constants of LCC-RyR coupling in WT under control and rapamycin-treated conditions. Data from ≥86 recordings in cells from ≥15 mice in each group. *P < 0.05 vs. WT or control.
2.4. Electrocardiography
Mice were lightly anesthetized with isoflurane (1%) and placed supine on a heated pad of 37°C. Needle ECG electrodes were placed under the skin to record from a Lead I configuration. After a 20-min baseline period, epinephrine (2 mg/kg) was injected intraperitoneally. Arrhythmias were defined as either non-sustained ventricular tachycardia (VT) (e.g. a series of 4–10 consecutive repetitive ventricular ectopic beats) or sustained VT (e.g. a run of 11 or more consecutive repetitive ventricular ectopic beats).
2.5 Data analysis and statistics
All data are expressed as mean ± SE unless otherwise indicated. The linear mixed effects model (by R program and lme4) or χ2 test, where appropriate, were applied for unpaired samples to determine statistical differences. Bonferroni correction was applied when more than two groups were compared to the same control. Curve fitting was performed using Sigmaplot software (Systat Software, Inc). Fitted data were compared using the u test. P < 0.05 was considered statistically significant.
3. Results
3.1 FKBP12.6-knockout increases LCC-RyR coupling fidelity
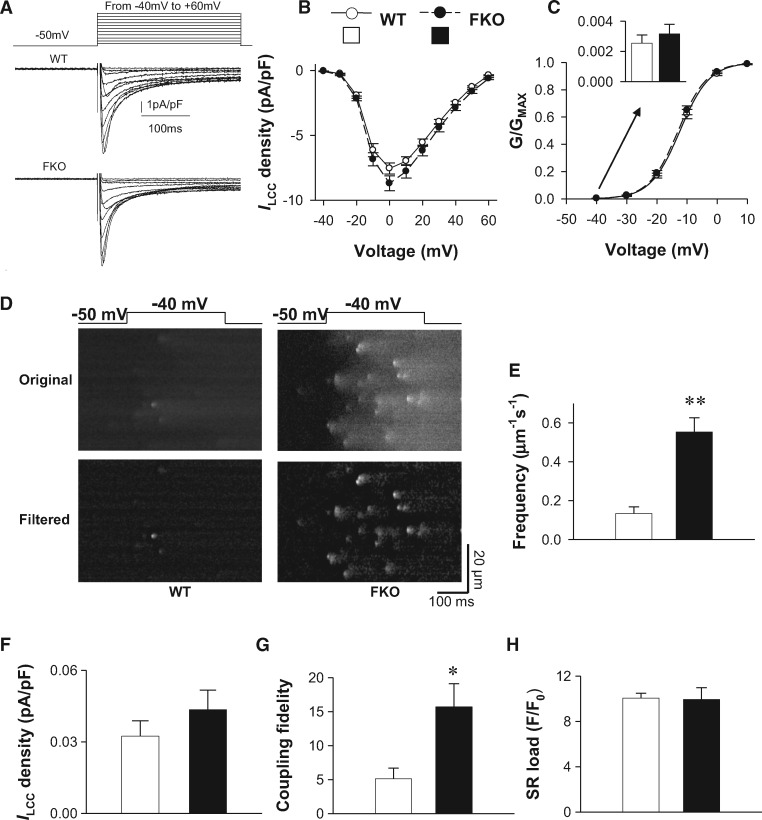
In order to test the effect of FKBP12.6 on the in situ RyR response to LCC Ca2+ influx, we sought to trigger discrete Ca2+ sparks by minimal LCC activation in ventricular cardiomyocytes from wild-type (WT) and FKBP12.6 knockout (FKO) mice. In cardiomyocytes loaded with the Ca2+ indicator fluo-4, we recorded LCC Ca2+ current (ILCC) using the whole-cell patch clamp technique (Figure 1A). Neither the ILCC density (Figure 1B) nor its voltage dependence (Figure 1C) differed between WT and FKO. By Boltzmann fitting of the activation curves, we determined that the probability of LCC activation at −40 mV in both FKO and WT groups was only around 0.003 (Figure 1C). The low probability of LCC openings at the near-threshold depolarization allowed us to compare the frequency of solitary RyR Ca2+ sparks triggered probabilistically by LCCs (Figure 1D). We found that the frequency of Ca2+ sparks triggered by the depolarization from −50 to −40 mV was significantly higher in FKO than in WT (Figure 1E). Due to the low probability of LCC openings, the majority of Ca2+ spark should be activated by a single LCC, confirming that the Ca2+ influx through a single LCC is capable of triggering a Ca2+ spark.38 Because the ILCC at −40 mV (I-40), calculated based on Boltzmann fitting, was comparable between FKO and WT groups (Figure 1F), the higher frequency of Ca2+ sparks in FKO reflected increased RyR response to LCCs. We therefore derived the LCC-RyR coupling fidelity by calculating the Fspark per unit I-40 (Fspark/I-40, Figure 1G), and found that the coupling fidelity was significantly higher in FKO than in WT.
Figure 1.
FKBP12.6 knockout increases LCC-RyR coupling probability by near-threshold depolarization. (A) Currents were triggered from -50 mV to different depolarizations (-40 mV to +60 mV) in WT and FKO cardiomyocytes. Red curves were currents triggered at to -40 mV. (B) Whole cell ILCC density in WT and FKO cardiomyocytes. (C) The activation curves derived from the ILCC-V curves in (B). The G/GMAX at -40 mM (insert) was determined by fitting the data with Boltzmann equation G/GMAX+1/[1+exp(a-bV)], where a and b are fitting constants. (D) Representative recordings showing that a 300 ms depolarization from −50 to − 40mV (upper panels) under the whole-cell patch clamp condition activates Ca2+ sparks in a probabilistic manner (middle panels). In order to identify individual sparks clearly, the original images were high-pass filtered by subtracting a smoothed image (kernel size 10 μm×50 ms) (lower panels). (E) The frequency of Ca2+ sparks at − 40 mV in WT and FKO cardiomyocytes. Data from ≥23 cells from ≥6 mice in each group. (F) The ILCC density at −40mV was determined by ILCC =G(V−VR), where G is the LCC conductance, V is the membrane potential and VR is the reverse potential. (G) The frequency of Ca2+ sparks per unit ILCC (coupling fidelity = Fspark/ILCC). The unit for Fspark/ILCC is μm − 1·s − 1·pA − 1·pF. (H) SR Ca2+ load measured as the amplitude of Ca2+ transients evoked by 20 mM caffeine under the whole-cell patch clamp. Data were from ≥7 cells from 3 mice in each group. *P <0.05 and **P <0.01 vs. WT.
To exclude any possible influence of SR Ca2+ load on the above results, we measured the Ca2+ transient induced by rapidly applying 20 mM caffeine, which opens RyRs simultaneously and allows stored Ca2+ in the SR to diffuse into the cytosol. The caffeine-induced Ca2+ transient amplitude was the same in WT and FKO cells (Figure 1H), indicating that the SR load was not altered in FKO cells. It has been reported that the gain of excitation–contraction coupling is increased by early reverse Na+/Ca2+ exchange (NCX) activated during depolarization.39 To avoid possible involvement of NCX, we applied minimal depolarization (from −50 to −40mV) when the Na+ channels were blocked by tetrodotoxin. Therefore, under our experimental conditions, the increased coupling fidelity in the FKO group was attributable to increased responsivity of RyRs to ILCC.
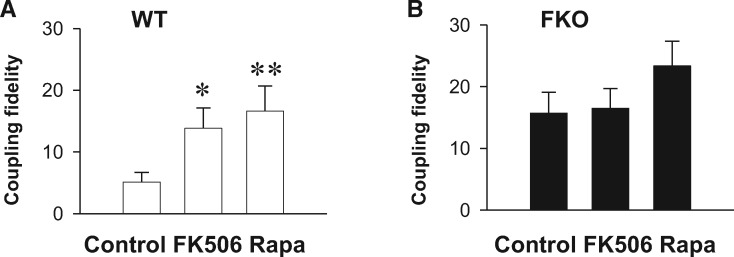
3.2 FK506 and rapamycin increases LCC-RyR coupling fidelity in WT but not FKO cells
In heart cells, both FKBP12 and FKBP12.6 bind to RyRs but their binding affinity differs.13,40,41 In order to check whether FKBP12 has a compensatory effect to FKBP12.6 knockout, we performed pharmacological experiments with 30 μmol/L FK506 and 10 μmol/L rapamycin, both are known to dissociate FKBPs from RyRs.18,19 Agreeing with previous reports,19,28,42 neither FK506 nor rapamycin changed the ILCC (see Supplementary material online, Figure S1). We found that both FK506- and rapamycin-treatments increased the LCC-RyR coupling fidelity (Fspark/ILCC) of WT cardiomyocytes to the same extent as that caused by FKO (comparing Figure 2A with Figure 1G). In contract, neither treatment could further change the Fspark/ILCC in FKO cells (Figure 2B), suggesting that FKBP12 did not exert a compensatory effect. The fact that FKBP12.6 knockout and FK506-/rapamycin-treatment have the same effect on Fspark/ILCC indicated that the knockout-/treatment-induced increase in RyR responsivity was fully attributable to FKBP12.6 dissociation.
Figure 2.
FK506 and rapamycin increases LCC-RyR coupling probability in WT but not FKBP12.6 knockout cells. (A) The effect of FK506 and rapamycin treatment on coupling fidelity in WT. (B) The effect of FK506 and rapamycin treatment on coupling fidelity in FKO. Data from ≥23 cells from ≥6 mice in each group. *P <0.05 and **P <0.01 vs. Control.
3.3 FKBP12.6 absence accelerates RyR response to a single LCC
The whole-cell detection of Ca2+ sparks usually involves out-of-focus events, and may also induce global feedback of CICR, particularly when the RyRs are sensitized. In order to further quantify the role of FKBP12.6 on LCC-RyR signalling, we visualized Ca2+ sparklets from LCCs and triggered Ca2+ sparks from RyRs by confocal imaging by loose-seal patch clamp.37,38 When the patched membrane was depolarized from resting potential (RP) to −10 mV, line-scan imaging focused at the pipette tip detected that Ca2+ sparklets from LCCs (blue arrows in Figure 3A) activated Ca2+ sparks from RyRs (red arrows in Figure 3A) in a stochastic manner. For those Ca2+ sparklets that successfully triggered Ca2+ sparks, the latency from the onset of an LCC sparklet to the takeoff of a triggered RyR spark varied, exhibiting exponential distributions in both WT and FKO groups (Figure 3B). The apparent rate constant (k) for RyRs to respond to LCC sparklets was then determined by fitting the distributions with the formula
where N is the number of observations and N0 is the N when t = 0. The fitting showed that the k was 29% higher in FKO cells than in WT cells (Figure 3C), indicating that the LCC-RyR signalling was accelerated after FKBP12.6 knockout.
In order to exclude any chronic compensatory effect of FKBP12.6 knockout, we further measured the LCC-RyR coupling latency with FK506 or rapamycin treatment in WT cardiomyocytes to acutely dissociate FKBP12.6 from RyR. Similarly with FKO cells, both FK506 and rapamycin accelerated LCC-RyR coupling rate (Figure 3D–F).
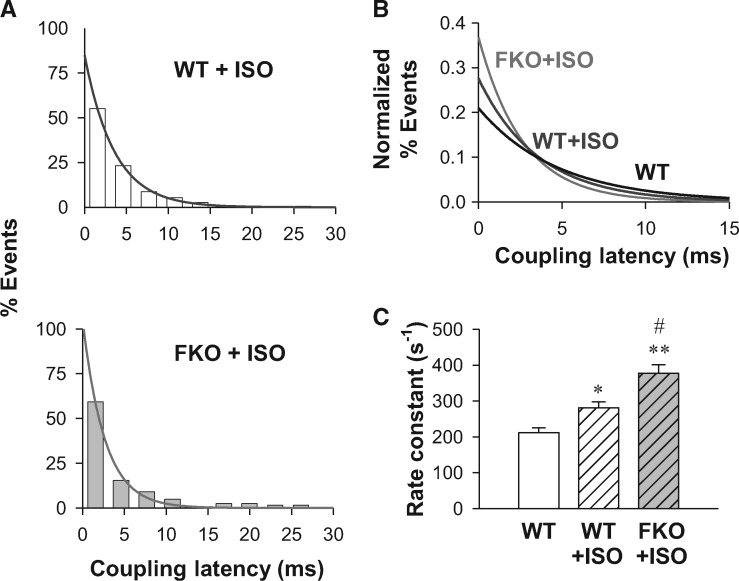
3.4 β-adrenergic stimulation further accelerates RyR response to a single LCC in FKO cells
Next, we sought to probe whether the absence of FKBP12.6 influences the in situ RyR response to the stimulation of β-adrenergic receptors (βARs). In the whole-cell experiment, βAR stimulation will increase the LCC open probability, making it unreliable to quantify RyR response by near-threshold coupling fidelity (Fspark/ILCC). By the loose-patch activation of Ca2+ sparks, we measured the coupling latency from LCC sparklets to RyR sparks when βARs was activated by 1 μmol/L isoproterenol (ISO, Figure 4A). Normalized exponential fitting of the LCC-RyR latency distribution (Figure 4B) showed that the LCC-RyR coupling latency was shortened after ISO treatment and further shortened in the ISO-treated FKO group. Compared with the WT group, the k of RyR response was accelerated by 78% in the ISO-treated FKO group (Figure 4C). Because ISO and FKO alone increased the k by 33 and 29%, respectively, the 78% increase of k indicated that FKBP12.6 knockout and βAR stimulation additively sensitize the RyR response to Ca2+ triggers.
Figure 4.
FKBP12.6 knockout and β-adrenergic stimulation additively sensitize LCC-RyR coupling. (A) Distributions (bars) and exponential fittings (curves) of the coupling latency in WT (upper), FKO (lower) cardiomyocytes bathed in 1 µmol/L ISO. (B) Comparison of normalized curves fitting in WT (black), WT + ISO (blue) and FKO + ISO (purple) groups. (C) Rate constants of LCC-RyR coupling in WT, WT + ISO and FKO + ISO groups. Data from ≥104 recordings in cells from ≥15 mice. *P <0.05 and **P <0.01 vs. WT; #P <0.05 vs. WT + ISO.
3.5 Destabilization of CICR due to the absence of FKBP12.6
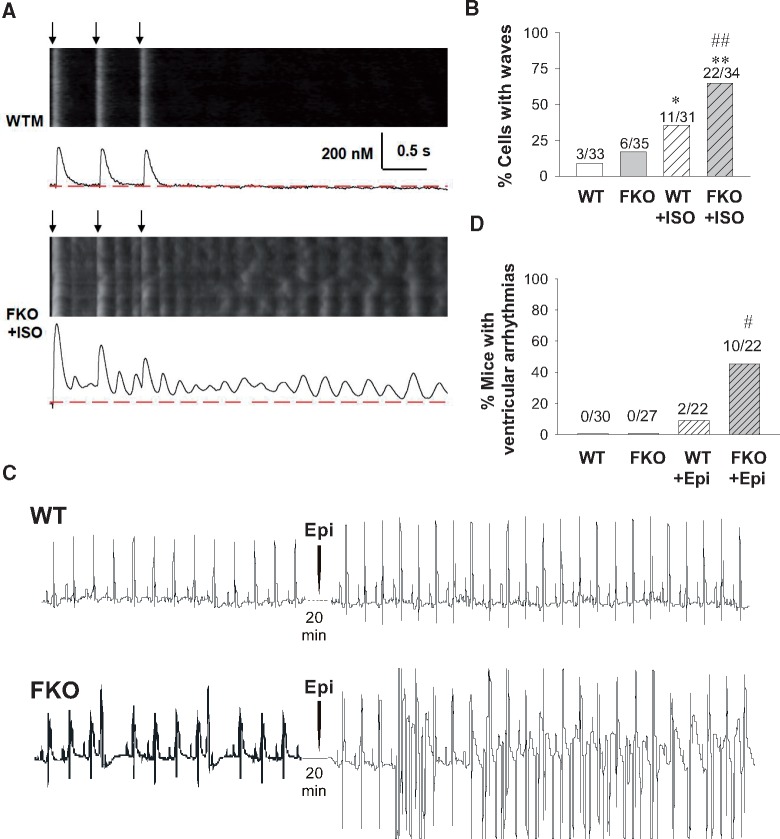
We then tested the stability of the CICR system at the whole-cell level by analysing intracellular Ca2+ activity after 30 pulses of field stimulation at 2 Hz in mouse cells. To avoid the possible toxic effects of laser scanning, we only imaged the cells during and after the last three beats. WT cells without ISO treatment usually displayed synchronized Ca2+ transients with clean diastolic background between successive stimulations and thereafter (Figure 5A, upper). The treatment with 1 μM ISO moderately increased the chance for WT cells to develop Ca2+ waves after field stimulation (Figure 5B white and striped white bars). Although FKO cells without ISO treatment also tended to keep regular Ca2+ transients, most FKO cells treated with ISO fell into chaotic Ca2+ waves (Figure 5A, lower and Figure 5B grey and striped grey bars). This result suggested that the CICR system loses stability under β-adrenergic stimulation in the absence of FKBP12.6.
Figure 5.
FKBP12.6 knockout increases the global Ca2+ release after field stimulation with ISO treatment and the ventricular arrhythmias after catecholaminergic stimulation in mice. (A) Representative confocal images and their time profiles of the last 3 Ca2+ transients of 30 pulses of 2 Hz stimulation in WT (upper) and ISO-treated FKO (lower) cardiomyocytes. Black arrows denote the timing of field stimulations. (B) Percentage of cells displaying Ca2+ waves in mouse cells under 2-Hz stimulation. The number of observations in each group is marked in the panels. *P <0.05 and **P <0.01 vs. WT; ##P <0.01 vs. WT + ISO. (C) Representative echocardiographic recordings from WT (upper) and FKO (lower) mice before and after epinephrine (Epi) treatment. (D) Percentage of mice with ventricular arrhythmias. # P <0.05 vs. WT + Epi.
To further test whether the cellular alterations described above predispose FKBP12.6 knockout mice to cardiac arrhythmias, we performed electrocardiography (ECG) in WT and FKO mice before and after catecholaminergic challenge by intraperitoneal administration of epinephrine (Epi) (Figure 5C). At basal condition, neither WT nor FKO displayed ventricular arrhythmias. Catecholaminergic challenge elicited few arrhythmic events in WT mice, but led to more frequent ventricular arrhythmias in FKO mice (Figure 5D).
4. Discussion
FK506-binding proteins, including FKBP12 and FKBP12.6, are important modulators of RyRs.13,43,44 In heart cells, FKBP12.6 binds RyRs with a much higher affinity than FKBP12.13,40,41,45 In the present study, we investigated the role of FKBP12.6 in modulating RyR Ca2+ release using FKBP12.6-knockout mice and FK506/rapamycin pharmacology. Either of these two experimental systems may have limitations: the acute treatment with FK506/rapamycin may contaminate with FKBP12 effect, while FKBP12.6-knockout strategy may involve chronic compensation. Therefore, we have exerted both experimental systems for complementary. The acute treatment with FK506/rapamycin excluded the chronic compensation and adaptation possibly associated with gene manipulation, but might involve an effect of FKBP12 dissociation. This latter concern was eliminated by the FKBP12.6 knockout results, which were fully attributable to the absence of FKBP12.6. Both lines of evidence demonstrated that FKBP12.6 dissociation (i) increased the CICR sensitivity and activation probability of RyRs, (ii) further sensitized RyRs under β-adrenergic stimulation, and (iii) elevated the risk for heart cells to develop arrhythmogenic Ca2+ activity. Therefore, the presence of FKBP12.6 should play an important role in stabilizing the CICR system in intact heart cells.
In previous studies, one of the problems keeping the role of FKBP12.6 from clarification is that RyRs are intracellular channels inaccessible to direct electrophysiological measurements. Although lipid bilayers have been widely used to study the interaction between FKBP12.6 and RyRs, the experimental settings vary greatly from lab to lab. For example, an experiment supporting FKBP12.6-modulation of RyR was done in the presence of Mg2+ and 50 mmol/L trans(luminal)-side Ca2+,24 whereas counteracting data were collected with symmetrical high concentration of K+ without Mg2+.22 Despite the differences in ionic composition, lipid bilayer experiments cannot reconstruct the native environment of interacting proteins, such as calsequestrin, junctin, and triadin. Therefore, the results in lipid bilayers may not necessarily represent the in situ effect of FKBP12.6.
In intact cardiomyocytes, early reports have shown that spontaneous Ca2+ spark amplitude is either decreased19 or unchanged28 after FK506 treatment. However, studies in FKO mice showed that Ca2+ spark amplitude is either increased22 or unchanged31 in the absence of FKBP12.6. We noticed that, in reports showing increased Ca2+ spark amplitude, spark duration and full-width at half-maximum (FWHM) were also increased.22 Given that the Ca2+ spark frequency was increased by several folds, FKO cells display a lot of overlapping sparks and local wavelets,20,29 which caused overestimation of Ca2+ spark amplitude, duration, and FWHM. In the present study, we did not measure overlapping sparks. Our measurement of spontaneous sparks showed that spark amplitude, time-to-peak, FWHM, and full-duration at half-maximum were comparable between WT and FKO cells (see Supplementary material online, Figure S2). However, when the RyR Ca2+ release was activated by native LCC current under the loose-patch and whole-cell depolarization conditions, we did find that the rate constant and probability of spark activation were significantly increased when FKBP12.6 was absent, presumably due to increased open probability of RyRs. These data indicated that the kinetic change of RyR activation is not reflected in spontaneous Ca2+ sparks. The mixture of in-focus and off-focus Ca2+ release events in spontaneous sparks makes it difficult to quantify RyR properties. Therefore, the difference in experimental design at least partially explains why the role of FKBP12.6 in stabilizing RyR Ca2+ release is not detected in some studies.
In the present study, the triggering of RyR Ca2+ sparks by unitary LCC current made it more feasible than previously to investigate RyR Ca2+-sensitivity in intact cells. Our LCC-RyR coupling experiments provided two lines of evidence for the enhanced Ca2+ sensitivity of RyRs after FKBP12.6 knockout or FK506/rapamycin treatment: (i) an increased probability for LCC openings to activate RyRs, and (ii) an accelerated response of RyRs to single LCC Ca2+ influx. Given that an LCC opening has limited lifetime, a quicker response implies that a RyR has a higher chance of being activated before the LCC closes. Therefore, the accelerated coupling kinetics agreed well with the increased probability of LCC-RyR coupling. Both measurements provided unequivocal evidence that RyRs are sensitized to Ca2+ triggers after FKBP12.6 dissociation. In other words, the sensitivity of RyR activation is under tonic suppression by FKBP12.6 under physiological conditions.
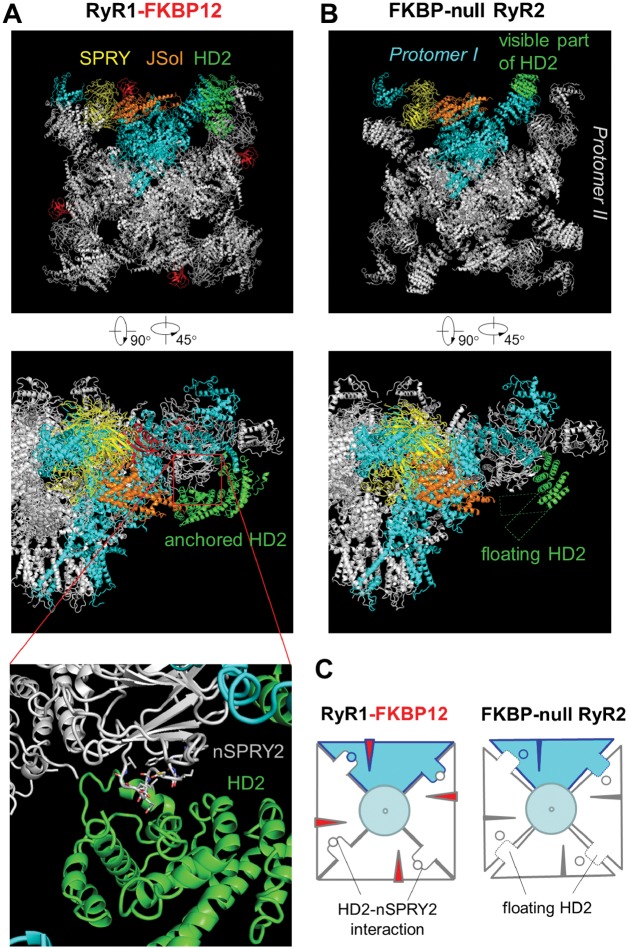
Guo et al.41 reported that only <20% FKBP12.6 binding sites on RyRs are occupied by FKBP12.6 in mouse heart cells. As each RyR has 4 FKBP12.6 binding sites on its 4 subunits, <41% RyRs would bind with one FKBP12.6 molecule, <15% with 2, <2.5% with 3, and only <0.16% RyRs would be fully occupied by FKBP12.6. The robust effect of FKBP12.6 knockout could not be explained if multiple FKBP12.6 binding was required to modify RyR behaviour. Therefore, we infer that single occupation of the FPBP12.6-binding sites should be effective in stabilizing RyR function. Based on the recent reports of RyR structure,31–34 FKBP is inserted into the gap between JSol (handle) domain and SPRY triangle. Structural comparison using the data of RyR1-FKBP12 complex32 and FKBP-null RyR234 suggested that the insertion of FKBP may adjust the positioning of certain domains such that the BSol (HD2) domain can be anchored onto the SPRY2 domain of the neighbouring protomer (nSPRY2) (Figure 6A and B). The putative HD2-nSPRY2 interaction thus provides a possible mechanism for inter-subunit coordination (Figure 6C). In this scenario, a single-subunit occupation of FKBP12.6 would be expected to stabilize 2 adjacent subunits of a RyR, and a 20% occupation of FKBP12.6-binging sites would stabilize ∼59% (41 + 15 + 2.5 + 0.16)% RyRs. This prediction explains the robust effect of FKBP12.6 knockout on LCC-RyR signalling.
Figure 6.
Structural comparison between RyR1-FKBP12 complex and FKBP-null RyR2. The images were generated based on the original structural data from Zalk et al.32 for RyR1 and from Peng et al.34 for RyR2. (A) In RyR1-FKBP12 complex, the JSol (HD2) domain potentially interacts with the SPRY2 domain of the neighbouring protomer (nSPRY2). (B) In FKBP-null RyR2, the HD2 domain is largely invisible, indicating that it may be floating without HD2-nSPRY2 interaction. (C) A hypothetic scheme based on the above information illustrating that the putative HD2-nSPRY2 interaction provides a possible mechanism for FKBPs to coordinate the allosteric activity among protomers. This HD2-nSPRY2 interaction may become destabilized without FKBP.
The role of FKBP12 in modulating RyRs in cardiac cells is another emerging controversy. It is shown that FKBP12 binds RyRs with very low affinity in heart cells,41 and overexpression of FKBP12 reduces the sensitivity of RyRs to Ca2+.46 However, it has been reported that FKBP12 is a high affinity activator of RyR2, sensitizing RyRs to cytosolic Ca2+.47 In the present study, we showed that FK506 did not further influence coupling fidelity in FKBP12.6-null cardiomyocytes, suggesting that FK506-induced FKBP12 dissociation had little effects on RyR sensitivity in intact ventricular cardiomyocytes.
The sympathetic system regulates heart function through ARs. It has been reported that FKBP12.6 knockout mice develop severe arrhythmia that leads to sudden cardiac death during exercise, well mimicking catecholaminergic polymorphic ventricular tachycardia.24,25 However, many experiments do not support the above idea.17,26,27 It has been shown that FKBP12.6 knockout neither promotes RyR activity nor causes ventricular arrhythmias under stress conditions.22 To determine whether and how FKBP12.6 interferes with the β-AR regulation of RyRs, we compared the effect of ISO, a selective β-AR agonist, on the LCC-RyR signalling kinetics in WT and FKO cardiomyocytes. In both groups, ISO treatment accelerated the coupling rate constant. The result that β-AR regulation further accelerated the LCC-RyR signalling kinetics in FKO cells provided in situ evidence at the molecular level that β-AR stimulation is able to sensitize Ca2+-induced RyR activation via an FKBP12.6-independent mechanism. Because FKBP12.6 absence per se sensitizes RyR activation, our results indicated that FKBP12.6-dependent and β-AR-mediated FKBP12.6-independent sensitization mechanisms cooperate in an additive manner in ISO-treated FKO cells.
The effect of FKBP12.6 on RyR2 Ca2+ release has also been investigated previously by overexpressing the FKBP12.6 gene in rabbit16,48 or rat49 cardiac myocytes, and by FKBP12.6-overexpression mouse models50–54. FKBP12.6 overexpression decreases spontaneous Ca2+ spark frequency48,49,51 and SR Ca2+ leak,16 which in turn increases SR Ca2+ load16,48,49 and Ca2+ transient peak.48,49 FKBP12.6 overexpression also increases the degree of synchronicity of SR Ca2+ release.48 Taken together, FKBP12.6 overexpression stabilizes RyR2 and prevents arrhythmogenic SR Ca2+ leak at the cellular level, which is in agreement with our findings by FKO cells. Furthermore, FKBP12.6-overexpressed mouse model shows improved cardiac function after myocardial infarction,50 decreased ventricular tachycardia after catecholaminergic stimulation,51 ameliorated post-thoracic aortic constriction (TAC) survival rates,52 protection against maladaptive left ventricular hypertrophy and reduced ventricular arrhythmias after TAC.53 The results from FKBP12.6 overexpression mouse model and our FKBP12.6 knockout model double confirmed that FKBP12.6 exerts a protective effect on heart function.
CICR is intrinsically a positive feedback loop, and would be expected to be explosive.55 However, the Ca2+ release of RyRs in heart cells is modulated precisely in the forms of both global Ca2+ transients and local Ca2+ sparks. As the key mechanism to avoid the self-excitation of CICR in heart cells, RyRs and LCCs are clustered to form discrete CRUs.12,56,57 Within each CRU, the Ca2+ influx through LCCs travels across a ∼15 nm junctional cleft, and activates RyRs in a stochastic manner.58 Between CRUs, however, the longer distance prevents crosstalk between adjacent CRUs.12 In this scenario, the RyR sensitivity must be limited within a certain dynamic range, or stability margin, such that the RyRs respond promptly to Ca2+ signals from LCCs but not to those from adjacent CRUs. Based on our findings, β-AR stimulation and FKBP12.6 dissociation both sensitized RyRs. When either of these factors act solo, the chance of generating regenerative Ca2+ activity is kept at a low level comparable with that in wild-type/control conditions, suggesting that moderate sensitization by FKBP12.6 dissociation per se was still within the stability margin of the CICR system. However, when both factors concur, the additive sensitization of RyRs causes chaotic Ca2+ waves in most cells, indicating that the sensitization of RyRs have stepped beyond the stability margin of the CICR system. Once inter-CRU CICR is enabled due to over-sensitization of RyRs, the intracellular Ca2+ release runs out-of-control, and Ca2+ waves occur in a regenerative manner. The chaotic Ca2+ waves not only prevent cardiomyocytes from rhythmic contraction and relaxation but also activate Na+/Ca2+ exchange and lead to arrhythmogenic excitation.59 Therefore, preventing RyRs from the wave generation is a prerequisite for the healthy operation of heart cells. The FKBP12.6-mediated suppression of RyR sensitivity is a key mechanism to keep the CICR system from wave generation and cardiac arrhythmia, allowing an indispensable stability margin for dynamic regulation of blood pumping power.
5. Limitations
The constitutive FKBP12.6 knockout mouse model has limitations for this study, since the chronic knockout might bring adaptive or compensatory effect. Recently, it has been reported that aged FKBP12.6 knockout mice (1-year old) develop cardiac dysfunction due to the activation of the AKT/mTOR pathway.35 Although the above activation has not been detected at the age of 3-month-old mice as the same age we used in the present study, we should be cautious that other adaptation might occur. Although we used FK506/rapamycin to acutely dissociate FKBP12.6 from RyRs in order to exclude any adaptive effect that might be brought about by the constitutive knockout of FKBP12.6, there was still limitation, because FK506/rapamycin also dissociates FKBP12. The perfect model for this study is cardiac-specific conditional FKBP12.6 knockout animal. Unfortunately, this model has not been available yet.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Supplementary Material
Acknowledgements
We thank Dr. Xue-Mei Hao for professional technical support and Dr. Iain C. Bruce for editing.
Funding
This study was supported by the State Major Research and Development Program (2016YFA0500401), National Natural Science Foundation of China (31271228, 31630035, 81370203, 81461148026, and 31327901), the Project of Beijing Municipal Science and Technology Commission (Z141100000214006) and the National Institutes of Health, USA (NIH R01 TW007269).
Conflict of interest: None declared.
Footnotes
The first two authors contributed equally.
References
- 1. Berridge MJ, Dupont G.. Spatial and temporal signalling by calcium. Curr Opin Cell Biol 1994;6:267–274. [DOI] [PubMed] [Google Scholar]
- 2. Bers DM. Cardiac excitation-contraction coupling. Nature 2002;415:198–205. [DOI] [PubMed] [Google Scholar]
- 3. Fabiato A, Fabiato F.. Contractions induced by a calcium-triggered release of calcium from the sarcoplasmic reticulum of single skinned cardiac cells. J Physiol 1975;249:469–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lopez-Lopez JR, Shacklock PS, Balke CW, Wier WG.. Local calcium transients triggered by single L-type calcium channel currents in cardiac cells. Science 1995;268:1042–1045. [DOI] [PubMed] [Google Scholar]
- 5. Cheng H, Lederer WJ, Cannell MB.. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science 1993;262:740–744. [DOI] [PubMed] [Google Scholar]
- 6. Cheng H, Lederer WJ.. Calcium sparks. Physiol Rev 2008;88:1491–1545. [DOI] [PubMed] [Google Scholar]
- 7. Guo T, Gillespie D, Fill M.. Ryanodine receptor current amplitude controls Ca2+ sparks in cardiac muscle. Circ Res 2012;111:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao YT, Valdivia HH.. Ca2+ nanosparks: shining light on the dyadic cleft but missing the intensity of its signal. Circ Res 2014;114:396–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stern MD. Theory of excitation-contraction coupling in cardiac muscle. Biophys J 1992;63:497–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wier WG, Egan TM, Lopez-Lopez JR, Balke CW.. Local control of excitation-contraction coupling in rat heart cells. J Physiol 1994;474:463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Santana LF, Cheng H, Gomez AM, Cannell MB, Lederer WJ.. Relation between the sarcolemmal Ca2+ current and Ca2+ sparks and local control theories for cardiac excitation-contraction coupling. Circ Res 1996;78:166–171. [DOI] [PubMed] [Google Scholar]
- 12. Franzini-Armstrong C, Protasi F, Ramesh V.. Shape, size, and distribution of Ca(2+) release units and couplons in skeletal and cardiac muscles. Biophys J 1999;77:1528–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Timerman AP, Onoue H, Xin HB, Barg S, Copello J, Wiederrecht G, Fleischer S.. Selective binding of FKBP12.6 by the cardiac ryanodine receptor. J Biol Chem 1996;271:20385–20391. [DOI] [PubMed] [Google Scholar]
- 14. Lam E, Martin MM, Timerman AP, Sabers C, Fleischer S, Lukas T, Abraham RT, O'Keefe SJ, O'Neill EA, Wiederrecht GJ.. A novel FK506 binding protein can mediate the immunosuppressive effects of FK506 and is associated with the cardiac ryanodine receptor. J Biol Chem 1995;270:26511–26522. [DOI] [PubMed] [Google Scholar]
- 15. Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR.. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 2000;101:365–376. [DOI] [PubMed] [Google Scholar]
- 16. Prestle J, Janssen PM, Janssen AP, Zeitz O, Lehnart SE, Bruce L, Smith GL, Hasenfuss G.. Overexpression of FK506-binding protein FKBP12.6 in cardiomyocytes reduces ryanodine receptor-mediated Ca(2+) leak from the sarcoplasmic reticulum and increases contractility. Circ Res 2001;88:188–194. [DOI] [PubMed] [Google Scholar]
- 17. Houser SR. Role of RyR2 phosphorylation in heart failure and arrhythmias: protein kinase A-mediated hyperphosphorylation of the ryanodine receptor at serine 2808 does not alter cardiac contractility or cause heart failure and arrhythmias. Circ Res 2014;114:1320–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaftan E, Marks AR, Ehrlich BE.. Effects of rapamycin on ryanodine receptor/Ca(2+)-release channels from cardiac muscle. Circ Res 1996;78:990–997. [DOI] [PubMed] [Google Scholar]
- 19. Xiao RP, Valdivia HH, Bogdanov K, Valdivia C, Lakatta EG, Cheng H.. The immunophilin FK506-binding protein modulates Ca2+ release channel closure in rat heart. J Physiol 1997;500(Pt 2):343–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Xin HB, Senbonmatsu T, Cheng DS, Wang YX, Copello JA, Ji GJ, Collier ML, Deng KY, Jeyakumar LH, Magnuson MA, Inagami T, Kotlikoff MI, Fleischer S.. Oestrogen protects FKBP12.6 null mice from cardiac hypertrophy. Nature 2002;416:334–338. [DOI] [PubMed] [Google Scholar]
- 21. Barg S, Copello JA, Fleischer S.. Different interactions of cardiac and skeletal muscle ryanodine receptors with FK-506 binding protein isoforms. Am J Physiol 1997;272:C1726–C1733. [DOI] [PubMed] [Google Scholar]
- 22. Xiao J, Tian X, Jones PP, Bolstad J, Kong H, Wang R, Zhang L, Duff HJ, Gillis AM, Fleischer S, Kotlikoff M, Copello JA, Chen SR.. Removal of FKBP12.6 does not alter the conductance and activation of the cardiac ryanodine receptor or the susceptibility to stress-induced ventricular arrhythmias. J Biol Chem 2007;282:34828–34838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Doi M, Yano M, Kobayashi S, Kohno M, Tokuhisa T, Okuda S, Suetsugu M, Hisamatsu Y, Ohkusa T, Matsuzaki M. Propranolol prevents the development of heart failure by restoring FKBP12.6-mediated stabilization of ryanodine receptor. Circulation 2002;105:1374–1379. [DOI] [PubMed] [Google Scholar]
- 24. Wehrens XH, Lehnart SE, Huang F, Vest JA, Reiken SR, Mohler PJ, Sun J, Guatimosim S, Song LS, Rosemblit N, D'Armiento JM, Napolitano C, Memmi M, Priori SG, Lederer WJ, Marks AR.. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell 2003;113:829–840. [DOI] [PubMed] [Google Scholar]
- 25. Zhao YT, Valdivia CR, Gurrola GB, Hernández JJ, Valdivia HH.. Arrhythmogenic mechanisms in ryanodine receptor channelopathies. Sci China Life Sci 2015;58:54–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stange M, Xu L, Balshaw D, Yamaguchi N, Meissner G.. Characterization of recombinant skeletal muscle (Ser-2843) and cardiac muscle (Ser-2809) ryanodine receptor phosphorylation mutants. J Biol Chem 2003;278:51693–51702. [DOI] [PubMed] [Google Scholar]
- 27. Xiao B, Sutherland C, Walsh MP, Chen SR.. Protein kinase A phosphorylation at serine-2808 of the cardiac Ca2+-release channel (ryanodine receptor) does not dissociate 12.6-kDa FK506-binding protein (FKBP12.6). Circ Res 2004;94:487–495. [DOI] [PubMed] [Google Scholar]
- 28. McCall E, Li L, Satoh H, Shannon TR, Blatter LA, Bers DM.. Effects of FK-506 on contraction and Ca2+ transients in rat cardiac myocytes. Circ Res 1996;79:1110–1121. [DOI] [PubMed] [Google Scholar]
- 29. Zhang X, Tallini YN, Chen Z, Gan L, Wei B, Doran R, Miao L, Xin HB, Kotlikoff MI, Ji G.. Dissociation of FKBP12.6 from ryanodine receptor type 2 is regulated by cyclic ADP-ribose but not beta-adrenergic stimulation in mouse cardiomyocytes. Cardiovasc Res 2009;84:253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang LG. Near-atomic resolution structure of the largest known Ca2+ channel: ryanodine receptor. Sci China Life Sci 2015;58:221–222. [DOI] [PubMed] [Google Scholar]
- 31. Efremov RG, Leitner A, Aebersold R, Raunser S.. Architecture and conformational switch mechanism of the ryanodine receptor. Nature 2015;517:39–43. [DOI] [PubMed] [Google Scholar]
- 32. Zalk R, Clarke OB, des Georges A, Grassucci RA, Reiken S, Mancia F, Hendrickson WA, Frank J, Marks AR.. Structure of a mammalian ryanodine receptor. Nature 2015;517:44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yan Z, Bai XC, Yan C, Wu J, Li Z, Xie T, Peng W, Yin CC, Li X, Scheres SH, Shi Y, Yan N.. Structure of the rabbit ryanodine receptor RyR1 at near-atomic resolution. Nature 2015;517:50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peng W, Shen H, Wu J, Guo W, Pan X, Wang R, Chen SR, Yan N.. Structural basis for the gating mechanism of the type 2 ryanodine receptor RyR2. Science 2016;354:aah5324-1-10. [DOI] [PubMed] [Google Scholar]
- 35. Yuan Q, Chen Z, Santulli G, Gu L, Yang ZG, Yuan ZQ, Zhao YT, Xin HB, Deng KY, Wang SQ, Ji G.. Functional role of Calstabin2 in age-related cardiac alterations. Sci Rep 2014;4:7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xu M, Zhou P, Xu SM, Liu Y, Feng X, Bai SH, Bai Y, Hao XM, Han Q, Zhang Y, Wang SQ.. Intermolecular failure of L-type Ca2+ channel and ryanodine receptor signaling in hypertrophy. PLoS Biol 2007;5:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhou P, Zhao YT, Guo YB, Xu SM, Bai SH, Lakatta EG, Cheng H, Hao XM, Wang SQ.. Beta-adrenergic signaling accelerates and synchronizes cardiac ryanodine receptor response to a single L-type Ca2+ channel. Proc Natl Acad Sci U S A 2009;106:18028–18033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang SQ, Song LS, Lakatta EG, Cheng H.. Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature 2001;410:592–596. [DOI] [PubMed] [Google Scholar]
- 39. Torres NS, Larbig R, Rock A, Goldhaber JI, Bridge JH.. Na+ currents are required for efficient 0excitation-contraction coupling in rabbit ventricular myocytes: a possible contribution of neuronal Na+ channels. J Physiol 2010;588:4249–4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xin HB, Rogers K, Qi Y, Kanematsu T, Fleischer S.. Three amino acid residues determine selective binding of FK506-binding protein 12.6 to the cardiac ryanodine receptor. J Biol Chem 1999;274:15315–15319. [DOI] [PubMed] [Google Scholar]
- 41. Guo T, Cornea RL, Huke S, Camors E, Yang Y, Picht E, Fruen BR, Bers DM.. Kinetics of FKBP12.6 binding to ryanodine receptors in permeabilized cardiac myocytes and effects on Ca sparks. Circ Res 2010;106:1743–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Su Z, Sugishita K, Li F, Ritter M, Barry WH.. Effects of FK506 on [Ca2+]i differ in mouse and rabbit ventricular myocytes. J Pharmacol Exp Ther 2003;304:334–341. [DOI] [PubMed] [Google Scholar]
- 43. Liu J, Farmer JD Jr, Lane WS, Friedman J, Weissman I, Schreiber SL.. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 1991;66:807–815. [DOI] [PubMed] [Google Scholar]
- 44. Samso M, Shen X, Allen PD.. Structural characterization of the RyR1-FKBP12 interaction. J Mol Biol 2006;356:917–927. [DOI] [PubMed] [Google Scholar]
- 45. Oda T, Yang Y, Uchinoumi H, Thomas DD, Chen-Izu Y, Kato T, Yamamoto T, Yano M, Cornea RL, Bers DM.. Oxidation of ryanodine receptor (RyR) and calmodulin enhance Ca release and pathologically alter, RyR structure and calmodulin affinity. J Mol Cell Cardiol 2015;85:240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Seidler T, Loughrey CM, Zibrova D, Kettlewell S, Teucher N, Kogler H, Hasenfuss G, Smith GL.. Overexpression of FK-506 binding protein 12.0 modulates excitation contraction coupling in adult rabbit ventricular cardiomyocytes. Circ Res 2007;101:1020–1029. [DOI] [PubMed] [Google Scholar]
- 47. Galfre E, Pitt SJ, Venturi E, Sitsapesan M, Zaccai NR, Tsaneva-Atanasova K, O'Neill S, Sitsapesan R.. FKBP12 activates the cardiac ryanodine receptor Ca2+-release channel and is antagonised by FKBP12.6. PLoS One 2012;7:e31956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Loughrey CM, Seidler T, Miller SL, Prestle J, MacEachern KE, Reynolds DF, Hasenfuss G, Smith GL.. Over-expression of FK506-binding protein FKBP12.6 alters excitation-contraction coupling in adult rabbit cardiomyocytes. J Physiol 2004;556:919–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gomez AM, Schuster I, Fauconnier J, Prestle J, Hasenfuss G, Richard S.. FKBP12.6 overexpression decreases Ca2+ spark amplitude but enhances [Ca2+]i transient in rat cardiac myocytes. Am J Physiol Heart Circ Physiol 2004;287:H1987–H1993. [DOI] [PubMed] [Google Scholar]
- 50. Huang F, Shan J, Reiken S, Wehrens XH, Marks AR.. Analysis of calstabin2 (FKBP12.6)-ryanodine receptor interactions: rescue of heart failure by calstabin2 in mice. Proc Natl Acad Sci U S A 2006;103:3456–3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gellen B, Fernandez-Velasco M, Briec F, Vinet L, LeQuang K, Rouet-Benzineb P, Benitah JP, Pezet M, Palais G, Pellegrin N, Zhang A, Perrier R, Escoubet B, Marniquet X, Richard S, Jaisser F, Gomez AM, Charpentier F, Mercadier JJ.. Conditional FKBP12.6 overexpression in mouse cardiac myocytes prevents triggered ventricular tachycardia through specific alterations in excitation-contraction coupling. Circulation 2008;117:1778–1786. [DOI] [PubMed] [Google Scholar]
- 52. Previlon M, Pezet M, Semprez F, Mercadier JJ, Rouet-Benzineb P.. FKBP12.6 mice display temporal gender differences in cardiac Ca(2+)-signalling phenotype upon chronic pressure overload. Can J Physiol Pharmacol 2011;89:769–782. [DOI] [PubMed] [Google Scholar]
- 53. Vinet L, Pezet M, Bito V, Briec F, Biesmans L, Rouet-Benzineb P, Gellen B, Previlon M, Chimenti S, Vilaine JP, Charpentier F, Sipido KR, Mercadier JJ.. Cardiac FKBP12.6 overexpression protects against triggered ventricular tachycardia in pressure overloaded mouse hearts. Basic Res Cardiol 2012;107:246. [DOI] [PubMed] [Google Scholar]
- 54. Bito V, Biesmans L, Gellen B, Antoons G, Macquaide N, Rouet-Benzineb P, Pezet M, Mercadier JJ, Sipido KR.. FKBP12.6 overexpression does not protect against remodelling after myocardial infarction. Exp Physiol 2013;98:134–148. [DOI] [PubMed] [Google Scholar]
- 55. Rios E, Stern MD.. Calcium in close quarters: microdomain feedback in excitation-contraction coupling and other cell biological phenomena. Annu Rev Biophys Biomol Struct 1997;26:47–82. [DOI] [PubMed] [Google Scholar]
- 56. Soeller C, Crossman D, Gilbert R, Cannell MB.. Analysis of ryanodine receptor clusters in rat and human cardiac myocytes. Proc Natl Acad Sci U S A 2007;104:14958–14963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Baddeley D, Jayasinghe ID, Lam L, Rossberger S, Cannell MB, Soeller C.. Optical single-channel resolution imaging of the ryanodine receptor distribution in rat cardiac myocytes. Proc Natl Acad Sci U S A 2009;106: 22275–22280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Inoue M, Bridge JH.. Ca2+ sparks in rabbit ventricular myocytes evoked by action potentials: involvement of clusters of L-type Ca2+ channels. Circ Res 2003;92:532–538. [DOI] [PubMed] [Google Scholar]
- 59. Boyden PA, Barbhaiya C, Lee T, ter Keurs HE.. Nonuniform Ca2+ transients in arrhythmogenic Purkinje cells that survive in the infarcted canine heart. Cardiovasc Res 2003;57:681–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.