Abstract
Aims
Tissue transglutaminase (tTG) is induced in injured and remodelling tissues, and modulates cellular phenotype, while contributing to matrix cross-linking. Our study tested the hypothesis that tTG may be expressed in the pressure-overloaded myocardium, and may regulate cardiac function, myocardial fibrosis and chamber remodelling.
Methods and results
In order to test the hypothesis, wild-type and tTG null mice were subjected to pressure overload induced through transverse aortic constriction. Moreover, we used isolated cardiac fibroblasts and macrophages to dissect the mechanisms of tTG-mediated actions. tTG expression was upregulated in the pressure-overloaded mouse heart and was localized in cardiomyocytes, interstitial cells, and in the extracellular matrix. In contrast, expression of transglutaminases 1, 3, 4, 5, 6, 7 and FXIII was not induced in the remodelling myocardium. In vitro, transforming growth factor (TGF)-β1 stimulated tTG synthesis in cardiac fibroblasts and in macrophages through distinct signalling pathways. tTG null mice had increased mortality and enhanced ventricular dilation following pressure overload, but were protected from diastolic dysfunction. tTG loss was associated with a hypercellular cardiac interstitium, reduced collagen cross-linking, and with accentuated matrix metalloproteinase (MMP)2 activity in the pressure-overloaded myocardium. In vitro, tTG did not modulate TGF-β-mediated responses in cardiac fibroblasts; however, tTG loss was associated with accentuated proliferative activity. Moreover, when bound to the matrix, recombinant tTG induced synthesis of tissue inhibitor of metalloproteinases (TIMP)-1 through transamidase-independent actions.
Conclusions
Following pressure overload, endogenous tTG mediates matrix cross-linking, while protecting the remodelling myocardium from dilation by exerting matrix-preserving actions.
Keywords: Transglutaminase, Cardiac fibrosis, Collagen cross-linking, Fibroblast, Matrix metalloproteinase
1. Introduction
The extracellular matrix plays a critical role in regulating cardiac geometry and function, not only by providing structural support and by determining the mechanical properties of the ventricle, but also by modulating cellular phenotype and behaviour.1–4 In the normal heart, the cardiac matrix shields interstitial cells from mechanical stress preventing their activation; matrix components may also transduce important homeostatic signals in cardiomyocytes, promoting their survival and regulating their function. In the pressure-overloaded myocardium, the cardiac extracellular matrix undergoes profound changes that critically regulate phenotype and function of both cardiomyocytes and interstitial cells. As the ventricle remodels, newly-synthesized matricellular macromolecules are incorporated into the matrix, serving as molecular bridges that transduce, or modulate growth factor- and cytokine-mediated signals, conferring plasticity to the cardiac interstitium.5–7 Because all myocardial cells are enmeshed in the matrix network, matricellular actions drive the dynamic cellular changes that occur in the remodelling pressure-overloaded heart.
Tissue transglutaminase (tTG, transglutaminase 2/TG2), a member of the transglutaminase family, is a ubiquitously expressed 80 kDa protein that exerts a wide range of effects on the extracellular matrix and on cellular elements.8,9 tTG may be secreted into the extracellular space, where it may influence cell behaviour through both enzymatic and non-enzymatic actions. As a typical transglutaminase, tTG catalyses protein deamidation, transamidation and cross-linking;10 some of its enzymatic substrates have been identified in extracellular compartments, on the cell surface, and in the matrix.8,11 tTG-mediated cross-linking of matrix proteins such as fibrinogen, fibronectin, collagen and laminin–nidogen basement membrane complexes12,13 may contribute to the generation of a mechanically stable, stiff and protease-resistant matrix network. More recently, non-enzymatic actions of tTG have been documented, including adapter/scaffolding functions that may directly regulate cell adhesion, migration and differentiation.8,9,14 Because activation of tTG in the extracellular environment appears to be transient due to its oxidation,15 the non-enzymatic functions of the molecule may be particularly important in vivo. tTG is a stress-inducible gene in most mammalian tissues.16 In failing hearts, tTG is one of the most upregulated proteins, exhibiting marked induction in experimental models of cardiac volume and pressure overload.17,18 Although transgenic cardiac-specific overexpression of tTG in mice causes mild hypertrophy and diffuse interstitial fibrosis,19 the role of endogenous tTG in cardiac remodelling has not been studied. We hypothesized that tTG induction in the pressure-overloaded myocardium may play an important role in fibrosis, dysfunction and geometric remodelling of the ventricle. We demonstrate for the first time that tTG exerts potent matrix-preserving effects on the pressure-overloaded myocardium, promoting fibrosis, matrix cross-linking, and diastolic dysfunction, while protecting from dilative remodelling and systolic dysfunction. Our in vivo and in vitro findings suggest that in addition to its actions on matrix cross-linking, tTG inhibits fibroblast proliferation and promotes a matrix-preserving phenotype in cardiac fibroblasts. When bound to the extracellular matrix, tTG stimulates TIMP1 synthesis through non-enzymatic effects, acting as a matricellular protein.
2. Methods (detailed description of the methodology is provided in the online supplement)
2.1 Animal protocols
Animal experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Baylor College of Medicine and Albert Einstein College of Medicine institutional review committees. Male and female, 2- to 4-month-old wild-type (WT) and tTG knockout (KO)20 mice in a C57BL/6J background were genotyped using established protocols. Animals were anesthetized with inhaled isoflurane (4% for induction, 2% for maintenance). Aortic banding was achieved by creating a constriction between the right innominate and left carotid arteries, as previously described.21 The degree of pressure overload was assessed by measuring right-to-left carotid artery flow velocity ratio after constricting the transverse aorta. For analgesia, buprenorphine (0.05–0.2 mg/kg s.c.) was administered at the time of surgery and q12h thereafter for 2 days. Intraoperatively, heart rate, respiratory rate and electrocardiogram were monitored and the depth of anaesthesia was assessed using the toe pinch method. At the end of the experiment, euthanasia was performed using 2% inhaled isoflurane followed by cervical dislocation. Early euthanasia was performed following criteria indicating suffering of the animal: weight loss > 20%, vocalization, dehiscent wound, hypothermia, signs of heart failure (cyanosis, dyspnoea, and tachypnea), lack of movement, hunched back, lack of food or water ingestion.
2.2 Echocardiography
Echocardiography was performed prior to instrumentation and before the end of each experiment using the Vevo 2100 system (VisualSonics, Toronto ON).
2.3 Pressure:volume loop analysis
Left ventricular pressure–volume analysis was performed using progressive isovolumic Langendorff retrograde perfusion of isolated murine hearts, as previously described.22,23
2.4 Immunohistochemistry, dual fluorescence and quantitative histology
Formalin-fixed, paraffin-embedded tissue samples were used for immunohistochemical staining. Collagen was labelled using picrosirius red. Quantitative assessment of myofibroblast and macrophage density was performed by counting the number of cells/myocardial area. Cardiomyocytes were outlined using wheat germ agglutinin (WGA) histochemistry.
2.5 Assessment of apoptosis using TUNEL staining and WGA lectin fluorescence
Apoptotic cardiomyocytes and interstitial cells were identified using fluorescent In situ Cell Death Detection Kit (Roche) and WGA staining.
2.6 RNA extraction and qPCR
RNA extraction and quantitative PCR were performed using established protocols.
2.7 Hydroxyproline biochemical assay
To assess cross-linking of collagen in pressure overloaded hearts we adopted established methods.24
2.8 Zymography
MMP activity in the pressure overloaded myocardium was examined by gelatin zymography.23
2.9 Flow cytometry
Suspensions of interstitial cells were prepared from WT and tTG KO hearts after 7 days of TAC. Cell suspensions were analysed with a Cell Lab Quanta SC flow cytometer (Beckman Coulter).
2.10 Cardiac fibroblast isolation and stimulation
Cardiac fibroblasts were isolated from WT, Smad3 KO and tTG KO animals as previously described.25
2.11 Western blotting
Protein was isolated from stimulated cells and western blotting was performed using standard protocols.
2.12 Studies on cardiac fibroblasts populating collagen pads
Cardiac fibroblasts isolated from WT and tTG KO animals were cultured to passage 2 and serum-starved overnight (16 h), then cultured in collagen pads as previously described.26,27 After incubation, the pads were used for RNA extraction and subsequent analyses. For experiments examining the effects of tTG on fibroblast phenotype, enzymatically active recombinant mouse tTG, or inactive tTG with a point mutation in the catalytic site (Zedira GmbH) was incorporated into collagen pads at a concentration of 50 µg/mL.
2.13 Macrophage isolation and stimulation
CD11b+ macrophages were isolated from the mouse spleen using immunomagnetic sorting.
2.14 Statistical analysis
For comparisons between more than two groups, parametric or non-parametric one-way ANOVA was used followed by t-test corrected for multiple comparisons. Comparisons between two groups were performed using unpaired t-test, or the Mann–Whitney test. Mortality was compared using the log rank test. Data were expressed as mean ± SEM. Statistical significance was set at 0.05.
3. Results
3.1 tTG is upregulated in the pressure-overloaded myocardium and is deposited in the extracellular matrix
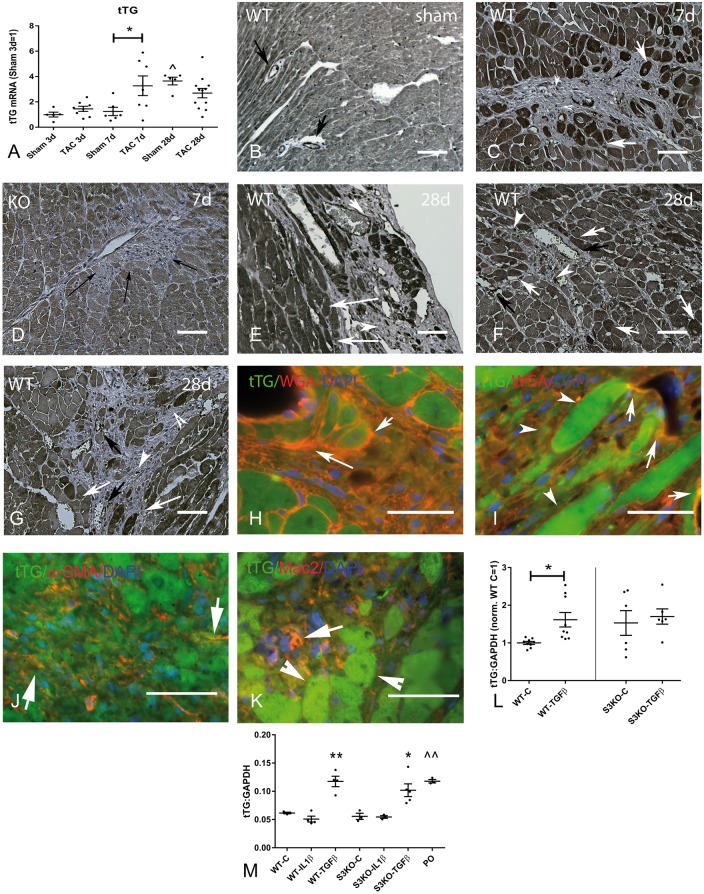
qPCR analysis showed that tTG expression is markedly upregulated in the pressure-overloaded myocardium after 7 days of transverse aortic constriction (TAC) (Figure 1A). In contrast, myocardial expression of other transglutaminases (TG1, TG3, TG4, TG5, TG6, TG7 and factor XIIIA) was not upregulated following pressure overload (see Supplementary material online, Figure S1). We confirmed the specificity of the anti-tTG antibody using tissues from WT and tTG KO mice (see Supplementary material online, Figure S2). Immunohistochemical staining demonstrated that after 7–28 days of TAC, tTG was expressed in cardiomyocytes and interstitial cells in the remodelling myocardium, and was deposited in the fibrotic extracellular matrix (Figure 1B–I). Sections from tTG KO mice were used as negative controls, showing minimal tTG immunofluorescence (Figure 1D). Dual immunofluorescence localized intracellular tTG in cardiomyocytes (Figure 1H and I), α-SMA+ myofibroblasts (Figure 1J) and Mac2+ macrophages (Figure 1K).
Figure 1.
tTG regulation and localization in the pressure-overloaded myocardium. (A) qPCR analysis demonstrated marked tTG mRNA upregulation in the pressure-overloaded myocardium after 7 days of TAC (*P < 0.05 vs. corresponding sham; ^P < 0.05 vs. 3-day sham; n = 5–12/group). (B–G) Immunohistochemical staining demonstrated tTG localization in cardiomyocytes, interstitial matrix, and interstitial cells in the pressure-overloaded heart after 7–28 days of TAC. The specificity of the anti-tTG antibody was validated using tissues from WT and tTG KO animals (see Supplementary material online, Figure S2). In sham WT myocardium, tTG was expressed in cardiomyocytes and vascular cells (arrows). In the pressure-overloaded myocardium, tTG was highly expressed in a subset of cardiomyocytes (white arrows), was localized in interstitial cells (arrowheads), and was deposited in the perivascular extracellular matrix (black arrows) after 7–28 days of TAC. Sections from pressure-overloaded tTG KO (D) hearts showed minimal immunoreactivity (despite evidence of extensive fibrotic remodelling—arrows) and served as negative controls. Significant heterogeneity in cardiomyocyte tTG expression was noted in the pressure overloaded myocardium with some cells (white arrows, F) exhibiting very high levels of immunoreactivity. (H and I) Dual immunofluorescence for tTG and WGA localized tTG (green) in cardiomyocytes (arrowheads) and in the WGA-positive interstitial matrix (arrows—orange). (J) tTG immunoreactivity was also noted in a subset of infiltrating spindle-shaped myofibroblasts (arrows—orange), identified with α-SMA staining (red). (K) A subset of interstitial macrophages (orange—arrows), stained with Mac2 (red) also expressed tTG (green). Please note that cardiomyocytes adjacent to areas of fibrosis are intensely positive for tTG (arrowheads). (L) TGF-β stimulation upregulated tTG in cardiac fibroblasts through Smad-dependent signalling. In cardiac fibroblasts harvested from WT hearts, TGF-β1 stimulation (25 ng/ml for 4 h) induced tTG mRNA upregulation (*P < 0.05 vs. control, n = 6–9). In contrast, no significant tTG upregulation was noted in TGF-β1-stimulated Smad3−/ − (S3KO) fibroblasts (P = NS, n = 6–9). (M) In isolated splenic macrophages, TGF-β1, but not IL-1β induced tTG in both WT and Smad3 KO cells. Macrophages harvested from the pressure-overloaded (PO) myocardium after 7 days of TAC had higher tTG expression levels than control splenic macrophages (*P < 0.01, **P < 0.01 vs. corresponding controls; ^^P < 0.01 vs. WT C, n = 9–15/group). Scale bar = 40 μm.
3.2 Transforming growth factor (TGF)-β induces tTG synthesis in cardiac fibroblasts in a Smad3-dependent manner
TGF-β1 is induced and activated in the remodelling pressure-overloaded myocardium and may exert potent fibrogenic actions, inducing myofibroblast transdifferentiation and expression of matrix proteins.28 Because cardiac myofibroblasts were identified as a major cellular source of tTG in the remodelling myocardium, we examined whether TGF-β stimulation upregulates tTG synthesis in isolated cardiac fibroblasts. TGF-β1 (25 ng/mL) induced a 2.0-fold increase in tTG mRNA expression by cardiac fibroblasts after 4 h of stimulation (Figure 1L). The effects of TGF-β were abrogated in Smad3 null cardiac fibroblasts, suggesting that TGF-β-induced tTG upregulation is Smad-dependent (Figure 1L). Although tTG was the TG showing the highest level of expression in cardiac fibroblasts, TG4 was also expressed. Expression levels of TG1, TG3, TG5, TG6, TG7 and FXIIIA were very low and were not upregulated upon stimulation with TGF-β1 (see Supplementary material online, Figure S3A–H). Dual staining combining pan-cadherin fluorescence to label the cytoplasmic membrane and tTG staining also showed tTG expression in cardiac fibroblasts and documented release into the extracellular matrix upon stimulation. Stimulation with serum, or TGF-β1 increased the intensity of tTG immunofluorescence and induced release of immunoreactive tTG into the surrounding matrix (see Supplementary material online, Figure S4). Cells from tTG KO hearts were used as a negative control and showed negligible immunofluorescence (see Supplementary material online, Figure S4).
3.3 TGF-β1 induces tTG upregulation in mouse macrophages through a Smad-independent pathway
Because tTG was localized in a subset of macrophages in the pressure-overloaded heart (Figure 1K), we examined whether cytokine stimulation upregulates tTG expression in mouse macrophages. TGF-β1, but not IL-1β stimulation induced tTG synthesis is mouse splenic macrophages harvested from WT or Smad3 KO animals (Figure 1M), suggesting that TGF-β1-induced tTG upregulation is mediated through a Smad3-independent pathway. Macrophages harvested from the pressure overloaded heart after 7 days of TAC showed significantly higher levels of tTG expression in comparison to mouse splenic macrophages (Figure 1M). tTG was the highest expressed TG in splenic macrophages (see Supplementary material online, Figure S3I). Macrophages also expressed lower levels of TG4 and FXIIIA; other TGs were not detected (see Supplementary material online, Figure S3J and K). TGF-β1 and IL-1β stimulation did not significantly modulate macrophage TG4 and FXIII expression (see Supplementary material online, Figure S3J and K).
3.4 tTG loss does not significantly affect baseline cardiac morphology and function
Although both WT and tTG KO mice were normoglycemic, tTG KOs had lower fasting blood glucose levels (see Supplementary material online, Figure S5A). Systolic and diastolic blood pressure was comparable between groups (see Supplementary material online, Figure S5B). tTG absence had no effects on baseline cardiac morphology and did not affect cardiac geometry and systolic function. Mitral inflow Doppler and tissue Doppler imaging showed no significant effects of tTG loss on baseline diastolic function (see Supplementary material online, Figure S5).
3.5 tTG null mice exhibit increased mortality following pressure overload
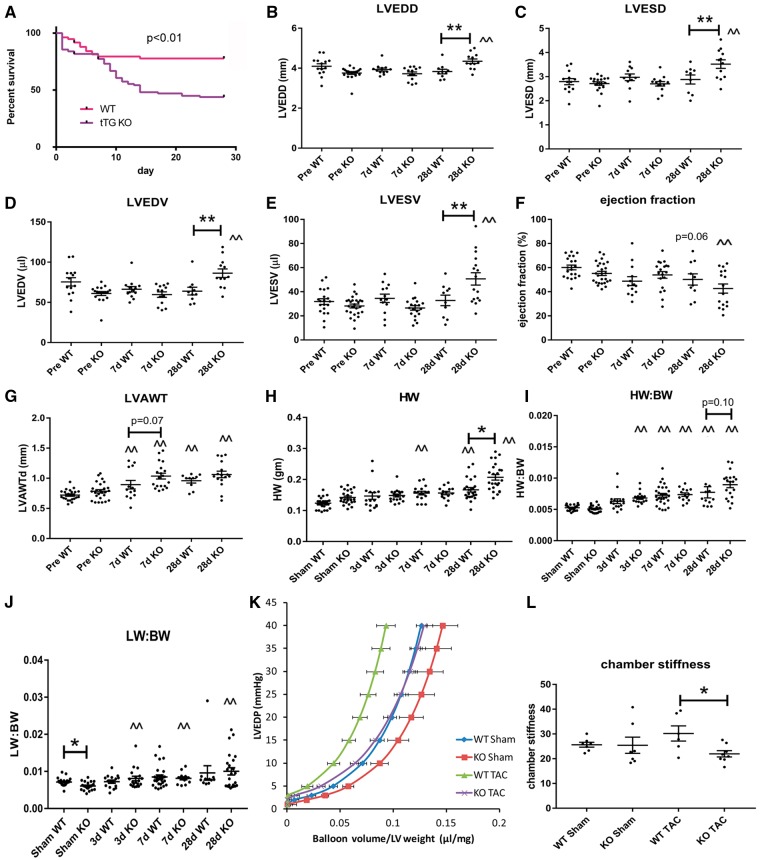
tTG−/− (n = 129) and WT mice (n = 128) underwent TAC protocols. Carotid flow ratio after TAC was comparable between groups (WT, 7.14 + 0.23; KO, 7.16 + 0.3; P = NS) indicating comparable pressure load. Sham WT and KO mice had comparable carotid flow ratios before and after the procedure (WT sham: pre, 0.96 + 0.05 and post, 1.05 + 0.30; KO sham: pre, 1.2 + 0.26 and post, 1.08 + 0.04, P = NS). When compared with WT animals, tTG KO mice had significantly increased mortality following pressure overload (P < 0.01, Figure 2A). There were no deaths in sham animals (WT, n = 31; KO, n = 39).
Figure 2.
tTG loss increases mortality and dilative ventricular remodelling, but protects from the development of diastolic dysfunction following pressure overload. (A) When compared with WT animals, tTG KO mice had increased mortality following pressure overload (P < 0.01; WT, n = 129; tTG KO, n = 128, log-rank test). (B–E) tTG KO mice had increased dilative remodelling after 28 days of TAC, exhibiting significantly higher LVEDD (B), LVESD (C), LVEDV (D) and LVESV (E) in comparison to WT animals (**P < 0.01 vs. corresponding WT, n = 13–18/group, one-way ANOVA followed by Sidak’s test). (F) tTG KO mice had a trend towards reduced ejection fraction after 28 days of TAC. (G) Both WT and tTG KO mice exhibited cardiac hypertrophy following pressure overload (^P < 0.05, ^^P < 0.01 vs. corresponding baseline values, Kruskal–Wallis test). tTG KO mice had trends towards increased anterior wall thickness after 7–28 days of TAC. (H and I) Both heart weight and heart weight:body weight ratio was increased in both WT and tTG KO animals following pressure overload (**P < 0.01 vs. corresponding sham, Kruskal–Wallis test). KO mice had significantly increased heart weight and a trend towards an increase in HW:BW after 28 days of TAC. (J) Lung weight:body weight ratio was also significantly increased in both WT and tTG KO mice after 7–28 days of TAC indicating the development of heart failure (*P < 0.05, **P < 0.01 vs. sham, n = 12–28/group, Kruskal–Wallis test). However, no significant differences in lung weight were noted between WT and tTG KO groups. (K and L) tTG loss attenuated diastolic dysfunction in the pressure overloaded myocardium. (K) Pressure:Volume analysis in isolated perfused hearts showed that tTG KO mice had a rightward shift of the curve. (L) Following TAC, chamber stiffness was significantly higher in WT animals when compared with tTG KOs (*P < 0.05, n = 6–8/group, ANOVA followed by Sidak’s test).
3.6 tTG absence is associated with accentuated dilative remodelling of the pressure-overloaded heart
After 28 days of pressure overload, tTG null mice exhibited marked ventricular dilation, evidenced by markedly higher LVEDD, LVESD, LVEDV and LVESV in comparison to WT animals (Figure 2B–E). Accentuated dilative remodelling in pressure-overloaded tTG KO mice was not associated with a significant deterioration in systolic function (Figure 2F). Both WT and tTG KO mice exhibited marked hypertrophic changes following pressure overload, showing increases in LVAWTd, in heart weight, and in the heart weight: body weight ratio after 7 and 28 days of TAC (Figure 2G–I). tTG null mice showed a trend towards increased hypertrophic remodelling after 7 and 28 days of TAC (Figure 2G–I). Lung weight:body weight ratio after TAC was comparable between groups (2J). Pressure:volume loop analysis in pressure-overloaded hearts using a Langendorff system showed that after 28 days of TAC, tTG KO hearts were more compliant than corresponding WT hearts, suggested by significantly lower chamber stiffness (Figure 2K and L). Thus, tTG loss enhanced chamber dilation and systolic dysfunction, but increased ventricular compliance and attenuated diastolic dysfunction following pressure overload.
3.7 tTG loss does not affect cardiomyocyte apoptosis following pressure overload
Next, we examined whether accentuated dilation in pressure-overloaded tTG null hearts was due to increased cardiomyocyte apoptosis. We identified rare apoptotic cells in the pressure-overloaded myocardium. Dual fluorescence combining WGA histochemistry to outline cardiomyocytes and TUNEL showed that the majority of apoptotic cells were interstitial cells. After 3, 7 and 28 days of TAC, tTG null and WT mice had comparable numbers of apoptotic cardiomyocytes and non-cardiomyocytes (see Supplementary material online, Figure S6).
3.8 Effects of tTG loss on collagen deposition in the pressure-overloaded myocardium
Alterations in composition of the interstitial matrix critically regulate geometric remodelling of the ventricle. Chamber dilation is often associated with accentuated matrix degradation, whereas deposition of cross-linked collagen in the cardiac interstitium causes diastolic dysfunction. Accordingly, we examined whether the geometric and functional changes in pressure-overloaded tTG null hearts are associated with alterations in collagen deposition and remodelling.
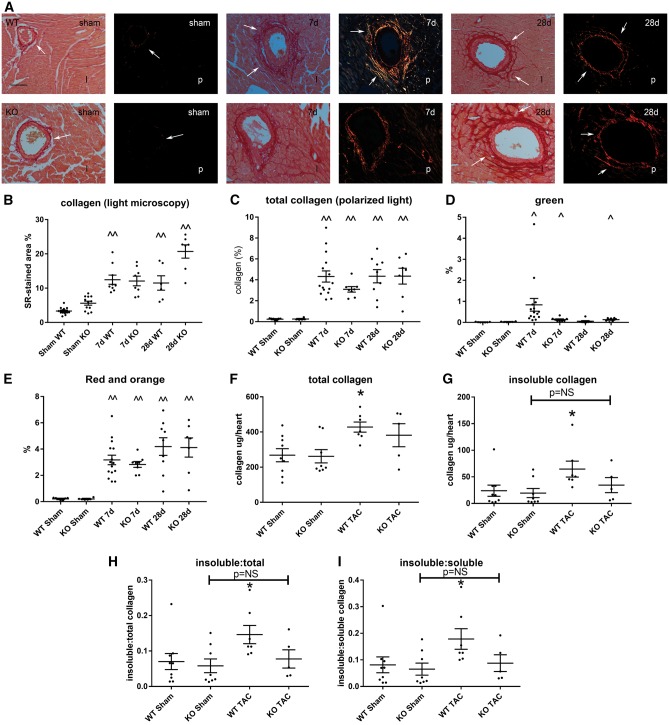
We used light microscopy, polarized microscopy and biochemical analysis to assess collagen deposition and structure in the remodelling myocardium (Figure 3A–I). Quantitative analysis of total collagen staining showed no significant effects of tTG loss on the collagen-stained area after 7–28 days of TAC (Figure 3B and C). Assessment of green and orange-red collagen fibres (reflecting thinner and thicker collagen fibres, respectively) using polarized light showed no significant differences between WT and tTG KO mice (Figure 3D and E). Biochemical analysis showed that in WT mice, pressure overloaded hearts had markedly higher levels of total collagen, insoluble collagen, and an increased ratio of insoluble:soluble and insoluble:total collagen (Figure 3F–I). In contrast, in pressure-overloaded tTG KO hearts, no statistically significant increase in collagen and insoluble collagen levels was noted in comparison to sham hearts.
Figure 3.
Effects of tTG loss on collagen deposition in the pressure-overloaded myocardium. (A) Light microscopy (l) of sirius red-stained sections and polarized light microscopy (p) were used to study the distribution and birefringence of collagen fibres in sham and pressure-overloaded myocardium (arrows). (B and C) Quantitative analysis showed that in both WT and tTG KO animals, pressure overloaded hearts had significantly increased collagen-stained area (^P < 0.05, ^^P < 0.01 vs. corresponding sham, Kruskal–Wallis test, n = 6–14/group) after 7–28 days of TAC. No significant differences were noted in total collagen content (B and C) and in the amount of thinner green (D) and thicker orange-red (E) collagen fibres between WT and tTG KO groups. (F–I) A hydroxyproline biochemical assay showed that after 28 days of TAC, WT animals exhibited a significant increase in total myocardial collagen content (F), insoluble collagen (G), insoluble:total collagen (H) and insoluble:soluble collagen (I) when compared with sham mice (*P < 0.05 vs. sham, n = 6–9/group, Kruskal–Wallis test). In comparison to corresponding sham animals, tTG KO mice had no significant increase in total collagen content after 28 days of pressure overload and no significant increase in the fraction of insoluble collagen. The findings suggest that tTG loss may impair collagen cross-linking in the pressure-overloaded myocardium.
3.9 tTG absence is associated with increased MMP2 activity and accentuated expression of cross-linking enzymes in the pressure-overloaded myocardium
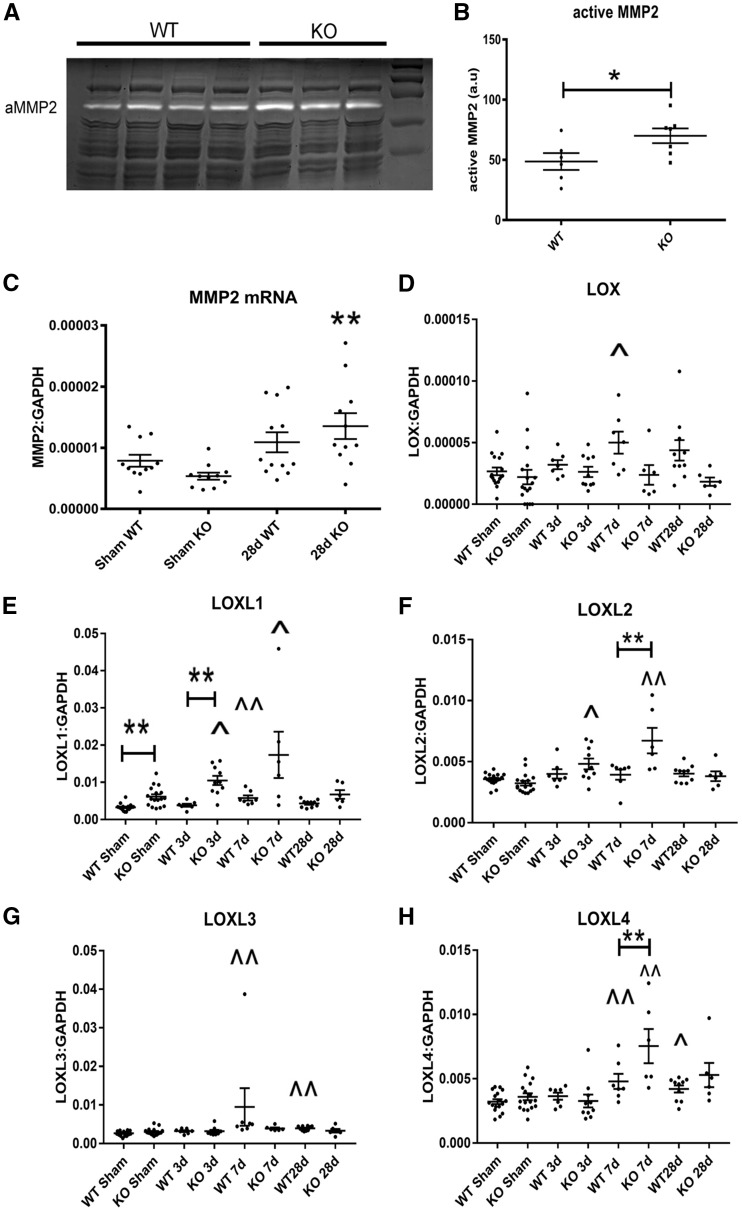
Zymography demonstrated that tTG null hearts had increased levels of active MMP2 after 28 days of TAC, when compared with WT hearts (Figure 4A and B). Accentuated MMP activity in tTG null hearts was not due to significantly higher MMP2 mRNA levels (Figure 4C). tTG loss was associated with a significant accentuation in expression of the matrix cross-linking enzymes lysyl oxidase-like (LOXL)1, LOXL2 and LOXL4. (**P < 0.01 vs. corresponding WT). In contrast, LOX and LOXL3 expression was comparable between WT and tTG KO animals (Figure 4D–H).
Figure 4.
tTG loss increases myocardial MMP2 activity and is associated with accentuated upregulation of LOXL1, LOXL2 and LOXL4 in the pressure-overloaded myocardium. (A and B) Zymography demonstrated that after 28 days of pressure overload tTG KO animals had increased myocardial MMP2 activity when compared with WT animals (*P < 0.05 vs. WT, n = 6–7/group, unpaired t-test). (C) Increased MMP2 activity in tTG null hearts was not due to increased MMP2 transcription. After 28 days of TAC, MMP2 mRNA levels were comparable between WT and tTG KO hearts (**P < 0.01 vs. corresponding sham, Kruskal–Wallis test, n = 6–12/group). (D–H) tTG absence had significant effects on expression of matrix cross-linking genes in the pressure overloaded myocardium (^P < 0.05, ^^P < 0.01 vs. corresponding shams). In comparison to corresponding WT animals, tTG KO mice had significantly increased LOXL1, LOXL2 and LOXL4 mRNA levels after 3–28 days of TAC (*P < 0.05, **P < 0.01, n = 6–17/group, parametric ANOVA followed by Sidak’s test or Kruskal–Wallis test).
3.10 Pressure-overloaded tTG null hearts have a higher number of non-cardiomyocytes than WT hearts, exhibiting increased macrophage and myofibroblast density
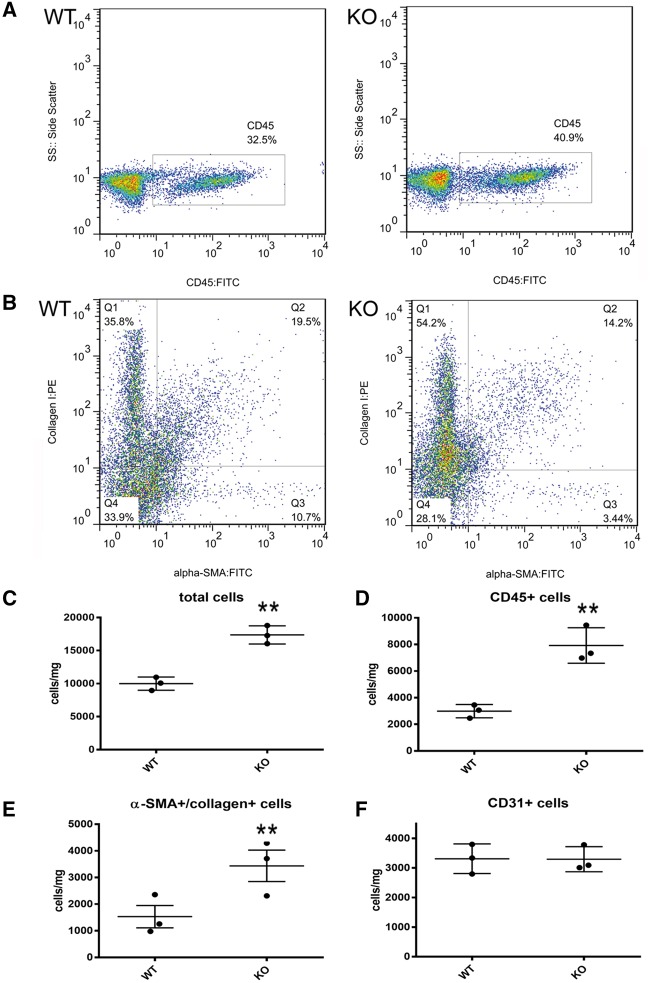
Hematoxylin/eosin staining demonstrated that tTG KO hearts had a highly cellular interstitium after 7–28 days of pressure overload (see Supplementary material online, Figure S7). We compared the cellular composition of the interstitium between pressure-overloaded WT and tTG null hearts using two independent techniques: flow cytometry and immunohistochemistry. Flow cytometric analysis of non-cardiomyocytes harvested from pressure-overloaded hearts (Figure 5A–F) demonstrated that after 7 days of TAC, tTG KO mice had higher cellular content (Figure 5C), increased number of CD45+ hematopoietic cells (Figure 5D), more α-smooth muscle actin (SMA)+/collagen I+ myofibroblasts (Figure 5E), but comparable numbers of CD31+ endothelial cells (Figure 5F). Flow cytometric findings were supported by immunohistochemical studies: tTG KO hearts had higher numbers of Mac2+ macrophages and α-SMA+ myofibroblasts after 7 and 28 days of TAC (Figure 6). Taken together, the quantitative analysis of collagen content (Figure 3) and the flow cytometric, histological and immunohistochemical study of the cellular infiltrate (Figures 5 and 6; see Supplementary material online, Figure S7) supported the notion that tTG loss is associated with a hypercellular interstitium that contains lower amounts of collagen, findings consistent with a matrix-degrading environment.
Figure 5.
tTG loss is associated with increased infiltration of the remodelling myocardium with non-cardiomyocytes. (A and B) Representative flow cytometry identifying CD45+ hematopoietic cells (A) and α-SMA/collagen-expressing myofibroblasts (B) in the remodelling myocardium after 7 days of TAC. (C–F) Quantitative analysis of flow cytometry data showed that tTG KO mice had increased numbers of interstitial cells (C), CD45+ hematopoietic cells (D), α-SMA+/collagen+ myofibroblasts (E), but comparable numbers of CD31+ endothelial cells (F) after 7 days of pressure overload (**P < 0.01 vs. WT, n = 3/group, unpaired t-test).
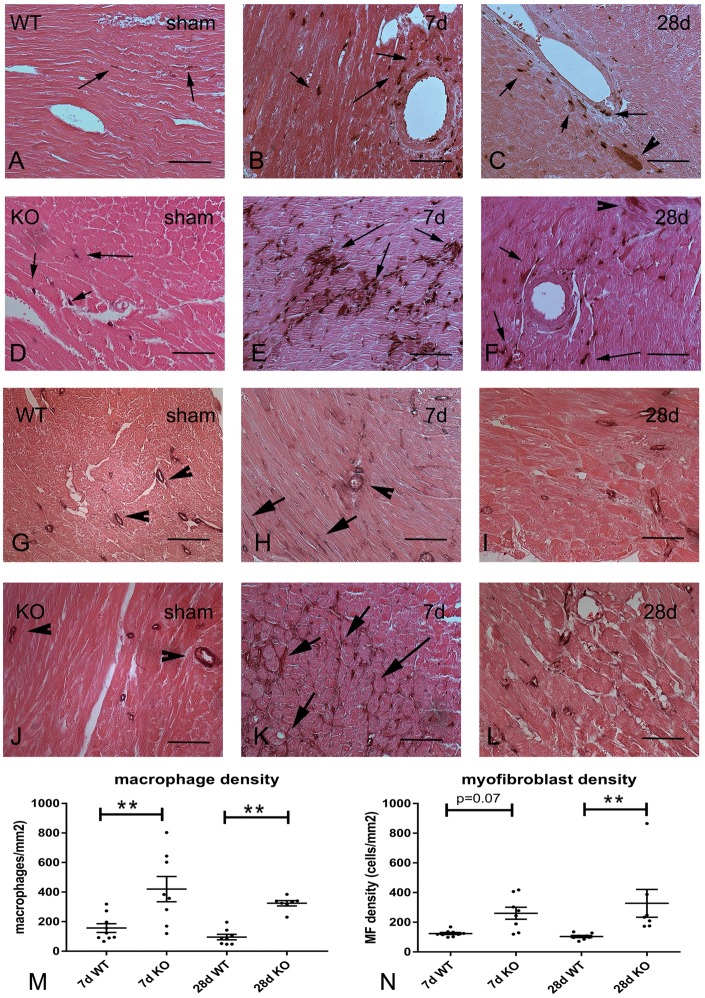
Figure 6.
Immunohistochemical staining demonstrates that tTG KO animals have increased macrophage density and enhanced myofibroblast infiltration after 7–28 days of TAC. (A–F) Mac2 immunohistochemistry (black) was used to identify macrophages in the remodelling myocardium of WT (A–C) and tTG KO animals (D–F) (arrows). WT (A) and tTG KO (D) sham hearts exhibited small populations of Mac2+ macrophages. Intense infiltration with macrophages was noted in perivascular and interstitial areas after 7–28 days of TAC. tTG KO mice (E and F) exhibited more intense infiltration than WT animals (B and C). It should be noted that in the pressure-overloaded myocardium, a subset of cardiomyocytes exhibits Mac2 immunoreactivity41 (arrowhead). For quantitative analysis, only cells with interstitial localization are identified as macrophages (arrows). (G–L) Immunohistochemical staining for α-SMA was used to identify myofibroblasts in the pressure overloaded myocardium of WT (G–I) and tTG KO mice (J–L) as immunoreactive cells located outside the vascular media. In sham WT (G) and tTG KO (J) animals α-SMA immunoreactivity was exclusively localized in vascular smooth muscle cells (arrowheads). After 7–28 days of TAC, both WT (H and I) and tTG KO (K and L) mice exhibited infiltration with α-SMA+ interstitial myofibroblasts (arrows). Myofibroblast infiltration was more intense in tTG KO animals (K and L). Quantitative analysis showed that macrophage density (M) and myofibroblast density (N) was markedly higher in tTG KO animals after 7–28 days of pressure overload (**P < 0.01 vs. corresponding WT, n = 7–9/group, Kruskal–Wallis test) (Scale bar = 50 μm; counterstained with eosin).
3.11 Accentuated MMP activity in tTG null hearts is not due to changes in macrophage or fibroblast MMP synthesis
Recently, tTG has been proposed as a specific marker for M2 macrophages that is conserved between species;29 however, its role in modulation of macrophage phenotype remains unknown. In order to examine whether the effects of tTG on cardiac remodelling are mediated through alterations in macrophage phenotype we harvested myocardial CD11b+ myeloid cells from WT and tTG KO mice undergoing TAC protocols. CD11b+ macrophages isolated from tTG null animals exhibited lower levels of MMP2 and MMP9 expression and comparable MMP3 and TIMP1 expression (see Supplementary material online, Figure S8). Moreover, fibroblasts isolated from tTG KO hearts also expressed lower MMP2 mRNA levels than WT cells (see Supplementary material online, Figure S9). The findings suggested that the increased MMP2 activity observed in pressure-overloaded tTG null hearts was not due to modulation of macrophage or fibroblast MMP2 mRNA synthesis.
3.12 tTG loss does not affect TGF-β-mediated activation of Smad2/3 signalling
tTG has been implicated in TGF-β activation.30,31 Accordingly, we asked whether tTG loss affects TGF-β-induced Smad2/3 activation. Western blotting experiments demonstrated that WT and tTG KO cardiac fibroblasts had comparable activation of Smad2 signalling (evidenced through a marked increase in Smad2 phosphorylation), upon stimulation with TGF-β1 for 5–30 min. Moreover, in fibroblasts cultured in collagen pads, tTG KO cells and WT cells had comparable p-Smad2 immunoreactivity after 24 h of stimulation with TGF-β1 (see Supplementary material online, Figure S10).
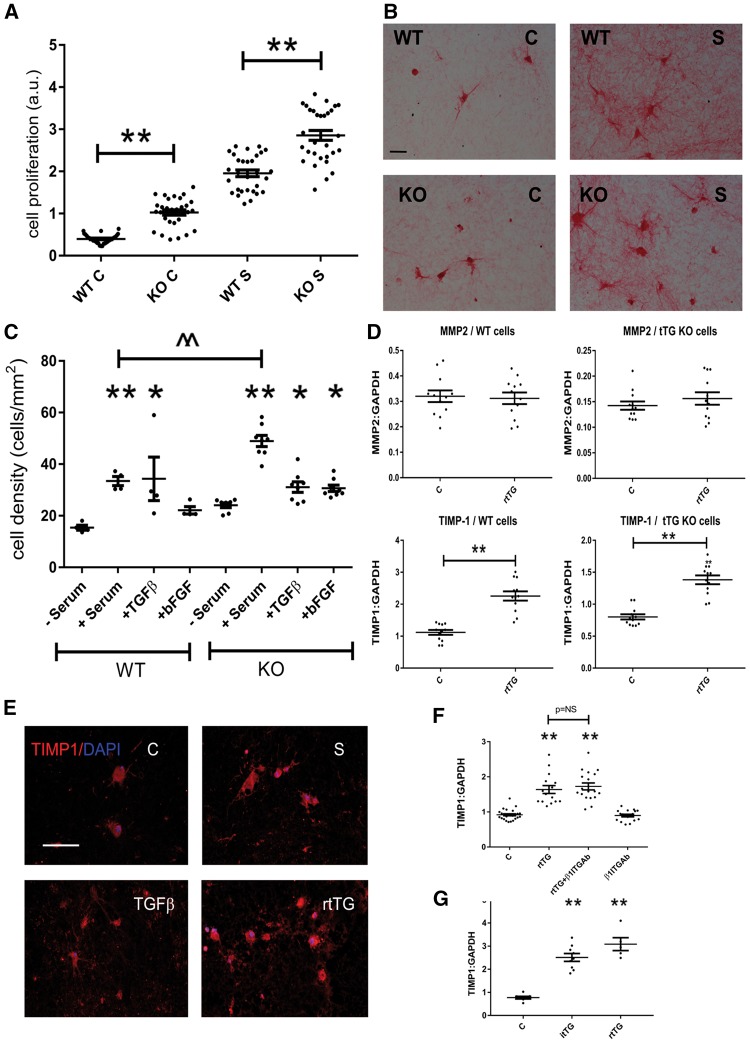
3.13 tTG loss is associated with increased fibroblast proliferative activity
Using an in vitro proliferation assay, we found that tTG KO fibroblasts had markedly increased proliferative activity upon stimulation with serum (Figure 7A). Experiments in fibroblasts cultured in collagen pads (Figure 7B) provided further support to the role of intracellular tTG in inhibition of proliferative activity. Collagen pads populated with tTG KO cells had higher fibroblast density upon stimulation with serum (Figure 7C).
Figure 7.
Actions of matrix-bound tTG on cardiac fibroblasts. (A–C) Loss of intracellular tTG increases fibroblast proliferative activity. Using a BrdU proliferation assay, we found that tTG KO cardiac fibroblasts had significantly higher proliferative activity than WT cells at baseline and after stimulation with serum (S) (**P < 0.01 vs. corresponding WT, n = 30, one-way ANOVA followed by Sidak’s test). (B and C) The effects of tTG in suppression of fibroblast proliferation were supported by experiments in fibroblasts populating collagen pads. Fibroblasts were identified using sirius red staining (B, scale bar = 50 μm). Incubation with serum, TGF-β, or bFGF increased cell density in pads populated with WT or tTG KO cells (*P < 0.05, **P < 0.01 vs. corresponding control, Kruskal–Wallis test). Upon serum stimulation, pads populated with tTG KO cells had markedly higher density than pads stimulated with WT cells (^^P < 0.01, n = 5, Kruskal–Wallis test). (D) Extracellular matrix-bound rtTG induces TIMP-1 synthesis by cardiac fibroblasts through both enzymatic and non-enzymatic actions. To examine the effects of extracellular rtTG on cardiac fibroblasts, we performed 24-h tTG stimulation experiments in fibroblasts populating collagen pads. rtTG did not affect MMP2 synthesis in WT or tTG KO cells. rtTG markedly induced TIMP-1 mRNA expression in both WT and tTG KO cardiac fibroblasts (^P < 0.05, ^^P < 0.01 vs. control, n = 10–12/group, unpaired t-test). (E) Immunofluorescent staining showed that serum, TGF-β1, or rtTG stimulation accentuated TIMP1 immunoreactivity in fibroblasts populating collagen pads. (F) Incubation with an antibody to β1 integrin (β1ITGAb) demonstrated that the effects of rtTG were independent of β1 integrin signalling. (G) Stimulation with inactive tTG with a point mutation in the catalytic site (itTG) also induced TIMP1 in cardiac fibroblasts, suggesting effects independent of transamidase activity (**P < 0.01 vs. control, one-way ANOVA followed by Sidak’s test).
3.14 Matrix-bound tTG enhances TIMP-1 expression by cardiac fibroblasts through a non-enzymatic pathway
In order to test the hypothesis that extracellular tTG may modulate fibroblast phenotype, we examined the effects of matrix-bound recombinant tTG (rtTG) on gene expression by cardiac fibroblasts populating collagen pads. When incorporated into the matrix, tTG induced TIMP1 mRNA expression in both WT and tTG KO cardiac fibroblasts (Figure 7D), suggesting the role of extracellular tTG actions in modulating fibroblast profile. Matrix-bound tTG did not significantly modulate MMP2 mRNA expression in WT, or in tTG KO fibroblasts (Figure 7D). Immunofluorescent staining confirmed that rtTG increases TIMP1 immunoreactivity in fibroblasts populating collagen pads (Figure 7E). Antibody neutralization experiments demonstrated that the effects of rtTG were independent of β1 integrin activation (Figure 7F). Inactive Cys277Ser-mutant recombinant tTG (itTG) with a point mutation in the catalytic centre also increased fibroblast TIMP-1 synthesis, documenting that this effect is transamidase-independent (Figure 7G).
4. Discussion
We document for the first time a critical role for endogenous tTG in cardiac remodelling. We demonstrate that tTG is markedly upregulated in the pressure-overloaded myocardium. tTG loss has profound effects on the ventricular response to a pressure load, attenuating diastolic dysfunction, while increasing mortality and accentuating chamber dilation. The effects of tTG absence are not due to actions on cardiomyocyte survival, but reflect activation of a proliferative phenotype in cardiac fibroblasts and modulation of matrix metabolism resulting in overactive matrix-degrading pathways.
Most cell types are capable of producing tTG; vascular cells, fibroblasts and M2 macrophages have been identified as important sources of tTG in vitro and in vivo.29,32,33 Neonatal rat cardiomyocytes constitutively express tTG; its expression is upregulated upon stimulation with endothelin-1,34 or reactive oxygen species.35 Published evidence suggests that tTG is markedly upregulated in experimental rat models of heart failure due to pressure or volume overload;17,18 its expression is associated with transition to heart failure. Our experiments show persistent tTG upregulation in the remodelling mouse myocardium after 7–28 days of pressure overload and suggest that tTG is localized in cardiomyocytes, interstitial cells and in the remodelling extracellular matrix (Figure 1).
Oxidative stress, growth factors, and cytokines are activated in the remodelling heart,28 and are capable of inducing tTG transcription in various cell types.8 TGF-β, a prominent mediator in cardiac remodelling and fibrosis36 has been shown to exert cell-specific actions on tTG expression, increasing its transcription in fibroblasts and many other cell types, but downregulating tTG synthesis in epithelial cells.8 Our experiments demonstrate that TGF-β1 induces tTG upregulation in isolated cardiac fibroblasts through activation of Smad3 signalling (Figure 1L). In macrophages, TGF-β1, but not the pro-inflammatory cytokine IL-1β stimulated tTG synthesis; however the effects of TGF-β were independent of Smad3 (Figure 1M).
In order to explore the role of tTG in cardiac remodelling, we studied the effects of tTG gene disruption on the pressure-overloaded myocardium. Baseline blood pressure, left ventricular geometry, systolic and diastolic function were not affected by tTG loss. However, following pressure overload tTG null animals exhibited significantly increased mortality when compared with WT controls, associated with accentuated chamber dilation (Figure 2B–E). Both WT and tTG KO animals had marked increases in heart and lung weight following pressure overload, suggesting comparable severity of heart failure, despite increased dilative remodelling of tTG null hearts. This may reflect the attenuated diastolic dysfunction observed in tTG KO animals (Figure 2K and L); the adverse effects of tTG loss on cardiac geometry may be counterbalanced by beneficial actions that reduce chamber stiffness.
The effects of tTG on function and remodelling of the pressure-overloaded heart may reflect a wide range of actions on many cell types and effects on the cardiac extracellular matrix. Because tTG has been implicated in the regulation of cellular apoptosis,37,38 we examined whether worse remodelling in tTG KO hearts is due to loss of pro-survival signalling and subsequent apoptotic death of cardiomyocytes. TUNEL staining demonstrated that apoptotic cardiomyocytes were rare in the pressure-overloaded heart, and their number was not affected by the absence of tTG (see Supplementary material online, Figure S6). However, despite comparable loss of cardiomyocytes, tTG KO mice exhibited significant alterations in the cardiac interstitium following pressure overload. We used three different methods to examine the effects of tTG loss on collagen deposition in the remodelling myocardium. Both light microscopy and quantitative analysis of birefringence with polarized light microscopy showed no significant differences in collagen deposition between WT and tTG KO animals (Figure 3). Using a biochemical assay we found that pressure overload induced a significant increase in total and insoluble collagen in the remodelling myocardium. In contrast to WT animals, tTG null mice did not exhibit an increase in insoluble collagen content following pressure overload (Figure 3G–H), presumably reflecting the loss of the matrix cross-linking effects of tTG. Moreover, zymography showed that tTG absence significantly enhanced MMP2 activation in the remodelling myocardium (Figure 4A and B). Both flow cytometry and immunohistochemistry demonstrated that tTG KO mice had markedly increased numbers of interstitial myofibroblasts in the remodelling myocardium (Figures 5 and 6). tTG absence also increased expression of LOXL1, LOXL2 and LOXL4 in the pressure-overloaded myocardium. Recently published evidence suggested that LOXL2 stimulates cardiac fibroblasts promoting myofibroblast transdifferentiation and migratory activity.39 Accentuated expression of lysyl oxidases in the absence of tTG may be responsible, at least in part, for the observed expansion of the myofibroblast population in the interstitium of pressure-overloaded tTG KO hearts.
Increased cellularity, impaired matrix cross-linking and enhanced MMP activity may explain the geometric and functional consequences of tTG loss on the remodelling heart. The abundance of interstitial cells, increased matrix-degrading activity, and reduced deposition of cross-linked collagen may on one hand improve compliance and attenuate diastolic dysfunction, while promoting dilative ventricular remodelling.
What is the cell biological basis for the effects of tTG on the remodelling myocardium? Effects of tTG on fibroblast phenotype and function have been previously reported. tTG may affect fibroblast function by modulating growth factor-stimulated signalling cascades. Experiments in human fibroblasts have demonstrated that cell surface tTG facilitates platelet-derived growth factor signalling.31 Moreover, a role for tTG in activation and induction of the TGF-β response has been suggested.30,40 Our in vitro experiments demonstrated that tTG does not play a significant role in regulation of TGF-β responses in cardiac fibroblasts. WT and tTG KO cells had comparable Smad2 phosphorylation upon stimulation with TGF-β1 (see Supplementary material online, Figure S10). However, tTG null cells exhibited increased proliferative activity at baseline, and upon stimulation with serum (Figure 7). Accentuated proliferation may explain the abundance of interstitial fibroblasts in pressure overloaded tTG KO hearts.
In order to examine the effects of tTG in modulating behaviour of fibroblasts in their matrix environment, we used an in vitro model of cardiac fibroblasts cultured in collagen pads enriched with tTG. We found that matrix-bound recombinant tTG exerts potent matrix-preserving actions on cardiac fibroblasts. When incorporated into the collagenous matrix, tTG induced TIMP-1 synthesis in both WT and tTG KO cells (Figure 7). The effect of tTG on TIMP1 expression by fibroblasts is not due to enzymatic actions; mutant tTG that lacks transglutaminase activity due to a point mutation in the active catalytic centre had similar effects on cardiac fibroblasts populating collagen pads (Figure 7G).
Our observations illustrate the critical role of matrix metabolism in cardiac remodelling. In the pressure-overloaded heart, activation of matrix-preserving signals results in fibrosis and diastolic dysfunction. From a theoretical perspective, inhibition of selected matrix-preserving mechanisms (such as tTG) seems an attractive therapeutic approach to reduce fibrosis and to protect from diastolic heart failure. However, this approach has significant risks. Matrix-preserving signals may exert protective actions by providing much needed mechanical support to the pressure-overloaded ventricle. Thus, overzealous inhibition of pathways involved in preservation of the matrix (such as the complete loss of tTG in mutant mice) may perturb the matrix balance leading to chamber dilation and systolic dysfunction.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
Supported by grants from the National Institutes of Health (R01 HL76246 and R01 HL85440 to N.G.F.), the Department of Defense (PR151134 and PR151029 to N.G.F.) and the American Heart Association Founders’ affiliate (to A.V.S.).
Supplementary Material
References
- 1. Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiol Rev 2012;92:635–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Skrbic B, Engebretsen KV, Strand ME, Lunde IG, Herum KM, Marstein HS, Sjaastad I, Lunde PK, Carlson CR, Christensen G, Bjornstad JL, Tonnessen T.. Lack of collagen VIII reduces fibrosis and promotes early mortality and cardiac dilatation in pressure overload in mice. Cardiovasc Res 2015;106:32–42. [DOI] [PubMed] [Google Scholar]
- 3. Gonzalez-Santamaria J, Villalba M, Busnadiego O, Lopez-Olaneta MM, Sandoval P, Snabel J, Lopez-Cabrera M, Erler JT, Hanemaaijer R, Lara-Pezzi E, Rodriguez-Pascual F.. Matrix cross-linking lysyl oxidases are induced in response to myocardial infarction and promote cardiac dysfunction. Cardiovasc Res 2016;109:67–78. [DOI] [PubMed] [Google Scholar]
- 4. Takawale A, Sakamuri SS, Kassiri Z.. Extracellular matrix communication and turnover in cardiac physiology and pathology. Compr Physiol 2015;5:687–719. [DOI] [PubMed] [Google Scholar]
- 5. Goldsmith EC, Bradshaw AD, Spinale FG.. Cellular mechanisms of tissue fibrosis. 2. Contributory pathways leading to myocardial fibrosis: moving beyond collagen expression. Am J Physiol Cell Physiol 2013;304:C393–C402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kong P, Christia P, Frangogiannis NG.. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci 2014;71:549–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schellings MW, Pinto YM, Heymans S.. Matricellular proteins in the heart: possible role during stress and remodeling. Cardiovasc Res 2004;64:24–31. [DOI] [PubMed] [Google Scholar]
- 8. Nurminskaya MV, Belkin AM.. Cellular functions of tissue transglutaminase. Int Rev Cell Mol Biol 2012;294:1–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang Z, Griffin M.. TG2, a novel extracellular protein with multiple functions. Amino Acids 2012;42:939–949. [DOI] [PubMed] [Google Scholar]
- 10. Iismaa SE, Mearns BM, Lorand L, Graham RM.. Transglutaminases and disease: lessons from genetically engineered mouse models and inherited disorders. Physiol Rev 2009;89:991–1023. [DOI] [PubMed] [Google Scholar]
- 11. Facchiano A, Facchiano F.. Transglutaminases and their substrates in biology and human diseases: 50 years of growing. Amino Acids 2009;36:599–614. [DOI] [PubMed] [Google Scholar]
- 12. Barsigian C, Fellin FM, Jain A, Martinez J.. Dissociation of fibrinogen and fibronectin binding from transglutaminase-mediated cross-linking at the hepatocyte surface. J Biol Chem 1988;263:14015–14022. [PubMed] [Google Scholar]
- 13. Aeschlimann D, Paulsson M.. Cross-linking of laminin-nidogen complexes by tissue transglutaminase. A novel mechanism for basement membrane stabilization. J Biol Chem 1991;266:15308–15317. [PubMed] [Google Scholar]
- 14. Belkin AM. Extracellular TG2: emerging functions and regulation. FEBS J 2011; 278:4704–4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stamnaes J, Pinkas DM, Fleckenstein B, Khosla C, Sollid LM.. Redox regulation of transglutaminase 2 activity. J Biol Chem 2010;285:25402–25409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oh K, Park HB, Byoun OJ, Shin DM, Jeong EM, Kim YW, Kim YS, Melino G, Kim IG, Lee DS.. Epithelial transglutaminase 2 is needed for T cell interleukin-17 production and subsequent pulmonary inflammation and fibrosis in bleomycin-treated mice. J Exp Med 2011;208:1707–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iwai N, Shimoike H, Kinoshita M.. Genes up-regulated in hypertrophied ventricle. Biochem Biophys Res Commun 1995;209:527–534. [DOI] [PubMed] [Google Scholar]
- 18. Petrak J, Pospisilova J, Sedinova M, Jedelsky P, Lorkova L, Vit O, Kolar M, Strnad H, Benes J, Sedmera D, Cervenka L, Melenovsky V.. Proteomic and transcriptomic analysis of heart failure due to volume overload in a rat aorto-caval fistula model provides support for new potential therapeutic targets – monoamine oxidase A and transglutaminase 2. Proteome Sci 2011;9:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Small K, Feng JF, Lorenz J, Donnelly ET, Yu A, Im MJ, Dorn GW, 2nd Liggett SB.. Cardiac specific overexpression of transglutaminase II (G(h)) results in a unique hypertrophy phenotype independent of phospholipase C activation. J Biol Chem 1999;274:21291–21296. [DOI] [PubMed] [Google Scholar]
- 20. De Laurenzi V, Melino G.. Gene disruption of tissue transglutaminase. Mol Cell Biol 2001;21:148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xia Y, Dobaczewski M, Gonzalez-Quesada C, Chen W, Biernacka A, Li N, Lee DW, Frangogiannis NG.. Endogenous thrombospondin 1 protects the pressure-overloaded myocardium by modulating fibroblast phenotype and matrix metabolism. Hypertension 2011;58:902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hilfiker-Kleiner D, Hilfiker A, Fuchs M, Kaminski K, Schaefer A, Schieffer B, Hillmer A, Schmiedl A, Ding Z, Podewski E, Podewski E, Poli V, Schneider MD, Schulz R, Park JK, Wollert KC, Drexler H.. Signal transducer and activator of transcription 3 is required for myocardial capillary growth, control of interstitial matrix deposition, and heart protection from ischemic injury. Circ Res 2004;95:187–195. [DOI] [PubMed] [Google Scholar]
- 23. Biernacka A, Cavalera M, Wang J, Russo I, Shinde A, Kong P, Gonzalez-Quesada C, Rai V, Dobaczewski M, Lee DW, Wang XF, Frangogiannis NG.. Smad3 signaling promotes fibrosis while preserving cardiac and aortic geometry in obese diabetic mice. Circ Heart Fail 2015;8:788–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mukherjee D, Sen S.. Collagen phenotypes during development and regression of myocardial hypertrophy in spontaneously hypertensive rats. Circ Res 1990; 67:1474–1480. [DOI] [PubMed] [Google Scholar]
- 25. Dobaczewski M, Bujak M, Li N, Gonzalez-Quesada C, Mendoza LH, Wang XF, Frangogiannis NG.. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res 2010;107:418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Saxena A, Bujak M, Frunza O, Dobaczewski M, Gonzalez-Quesada C, Lu B, Gerard C, Frangogiannis NG.. CXCR3-independent actions of the CXC chemokine CXCL10 in the infarcted myocardium and in isolated cardiac fibroblasts are mediated through proteoglycans. Cardiovasc Res 2014;103:217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shinde AV, Humeres C, Frangogiannis NG.. The role of alpha-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim Biophys Acta 2017; 1863:298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xia Y, Lee K, Li N, Corbett D, Mendoza L, Frangogiannis NG.. Characterization of the inflammatory and fibrotic response in a mouse model of cardiac pressure overload. Histochem Cell Biol 2009;131:471–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Martinez FO, Helming L, Milde R, Varin A, Melgert BN, Draijer C, Thomas B, Fabbri M, Crawshaw A, Ho LP, Ten Hacken NH, Cobos Jimenez V, Kootstra NA, Hamann J, Greaves DR, Locati M, Mantovani A, Gordon S.. Genetic programs expressed in resting and IL-4 alternatively activated mouse and human macrophages: similarities and differences. Blood 2013;121:e57–e69. [DOI] [PubMed] [Google Scholar]
- 30. Nunes I, Shapiro RL, Rifkin DB.. Characterization of latent TGF-beta activation by murine peritoneal macrophages. J Immunol 1995;155:1450–1459. [PubMed] [Google Scholar]
- 31. Zemskov EA, Loukinova E, Mikhailenko I, Coleman RA, Strickland DK, Belkin AM.. Regulation of platelet-derived growth factor receptor function by integrin-associated cell surface transglutaminase. J Biol Chem 2009;284:16693–16703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haroon ZA, Hettasch JM, Lai TS, Dewhirst MW, Greenberg CS.. Tissue transglutaminase is expressed, active, and directly involved in rat dermal wound healing and angiogenesis. FASEB J 1999;13:1787–1795. [DOI] [PubMed] [Google Scholar]
- 33. Jung SA, Lee HK, Yoon JS, Kim SJ, Kim CY, Song H, Hwang KC, Lee JB, Lee JH.. Upregulation of TGF-beta-induced tissue transglutaminase expression by PI3K-Akt pathway activation in human subconjunctival fibroblasts. Invest Ophthalmol Vis Sci 2007;48:1952–1958. [DOI] [PubMed] [Google Scholar]
- 34. Li X, Wei XL, Meng LL, Chi MG, Yan JQ, Ma XY, Jia YS, Liang L, Yan HT, Zheng JQ.. Involvement of tissue transglutaminase in endothelin 1-induced hypertrophy in cultured neonatal rat cardiomyocytes. Hypertension 2009;54:839–844. [DOI] [PubMed] [Google Scholar]
- 35. Song H, Kim BK, Chang W, Lim S, Song BW, Cha MJ, Jang Y, Hwang KC.. Tissue transglutaminase 2 promotes apoptosis of rat neonatal cardiomyocytes under oxidative stress. J Recept Signal Transduct Res 2011;31:66–74. [DOI] [PubMed] [Google Scholar]
- 36. Dobaczewski M, Chen W, Frangogiannis NG.. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J Mol Cell Cardiol 2011;51:600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fesus L, Szondy Z.. Transglutaminase 2 in the balance of cell death and survival. FEBS Lett 2005;579:3297–3302. [DOI] [PubMed] [Google Scholar]
- 38. Verderio EA, Telci D, Okoye A, Melino G, Griffin M.. A novel RGD-independent cel adhesion pathway mediated by fibronectin-bound tissue transglutaminase rescues cells from anoikis. J Biol Chem 2003;278:42604–42614. [DOI] [PubMed] [Google Scholar]
- 39. Yang J, Savvatis K, Kang JS, Fan P, Zhong H, Schwartz K, Barry V, Mikels-Vigdal A, Karpinski S, Kornyeyev D, Adamkewicz J, Feng X, Zhou Q, Shang C, Kumar P, Phan D, Kasner M, Lopez B, Diez J, Wright KC, Kovacs RL, Chen PS, Quertermous T, Smith V, Yao L, Tschope C, Chang CP.. Targeting LOXL2 for cardiac interstitial fibrosis and heart failure treatment. Nat Commun 2016;7:13710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Telci D, Collighan RJ, Basaga H, Griffin M.. Increased TG2 expression can result in induction of transforming growth factor beta1, causing increased synthesis and deposition of matrix proteins, which can be regulated by nitric oxide. J Biol Chem 2009;284:29547–29558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Frunza O, Russo I, Saxena A, Shinde AV, Humeres C, Hanif W, Rai V, Su Y, Frangogiannis NG.. Myocardial galectin-3 expression is associated with remodeling of the pressure-overloaded heart and may delay the hypertrophic response without affecting survival, dysfunction, and cardiac fibrosis. Am J Pathol 2016;186: 1114–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.