Abstract
Aims
Abnormal intracellular Ca2+ cycling contributes to triggered activity and arrhythmias in the heart. We investigated the properties and underlying mechanisms for systolic triggered Ca2+ waves in left atria from normal and failing dog hearts.
Methods and results
Intracellular Ca2+ cycling was studied using confocal microscopy during rapid pacing of atrial myocytes (36 °C) isolated from normal and failing canine hearts (ventricular tachypacing model). In normal atrial myocytes (NAMs), Ca2+ waves developed during rapid pacing at rates ≥ 3.3 Hz and immediately disappeared upon cessation of pacing despite high sarcoplasmic reticulum (SR) load. In heart failure atrial myocytes (HFAMs), triggered Ca2+ waves (TCWs) developed at a higher incidence at slower rates. Because of their timing, TCW development relies upon action potential (AP)-evoked Ca2+ entry. The distribution of Ca2+ wave latencies indicated two populations of waves, with early events representing TCWs and late events representing conventional spontaneous Ca2+ waves. Latency analysis also demonstrated that TCWs arise after junctional Ca2+ release has occurred and spread to non-junctional (cell core) SR. TCWs also occurred in intact dog atrium and in myocytes from humans and pigs. β-adrenergic stimulation increased Ca2+ release and abolished TCWs in NAMs but was ineffective in HFAMs making this a potentially effective adaptive mechanism in normals but potentially arrhythmogenic in HF. Block of Ca-calmodulin kinase II also abolished TCWs, suggesting a role in TCW formation. Pharmacological manoeuvres that increased Ca2+ release suppressed TCWs as did interventions that decreased Ca2+ release but these also severely reduced excitation-contraction coupling.
Conclusion
TCWs develop during the atrial AP and thus could affect AP duration, producing repolarization gradients and creating a substrate for reentry, particularly in HF where they develop at slower rates and a higher incidence. TCWs may represent a mechanism for the initiation of atrial fibrillation particularly in HF.
Keywords: Ca2+ waves, Atrium, Atrial fibrillation, Heart failure
1. Introduction
Much of our understanding of how abnormal Ca2+ cycling contributes to arrhythmogenesis comes from studies in ventricular myocytes where abnormal Ca2+ cycling may promote arrhythmia formation through triggered activity and/or by establishing repolarization gradients leading to reentry.1,2 Abnormal Ca2+ cycling in ventricular myocytes initiates triggered activity by generating delayed afterdepolarizations (DADs)1 which arise when spontaneous Ca2+ release (SCR) from the sarcoplasmic reticulum (SR) occurs during diastole under conditions of cellular Ca2+ overload. Cell depolarization results from subsequent activation of the Na–Ca exchanger (NCX) and its inward current (INCX). Ca2+ cycling has only recently begun to be systemically examined in atrial myocytes from animal models of atrial fibrillation (AF) or human AF.3 Indeed, AF is the most common cardiac arrhythmia, particularly among the elderly and is a complex pathological state often comorbid with structural heart disease such as heart failure (HF). A variety of electrophysiological and structural changes contribute to the formation of AF substrate,3 including abnormal Ca2+ cycling both in the setting of paroxysmal and persistent AF.4,5 Several studies have reported evidence of SCR in atrial myocytes, including the recent demonstration of a close correlation between SCR and DADs in myocytes from patients with AF.6 However, there are significant differences in the characteristics of atrial and ventricular Ca2+ cycling.7 Many differences reflect at least in part the less developed transverse-tubule (TT) system in the atria but the characteristics of SCR are also different between atrial and ventricular myocytes, with some studies describing a unique form of Ca2+-dependent early afterdepolarization (EAD) that occurs in atrial myocytes during the transition from rapid to normal pacing rates.8
Recently, we reported that rapid pacing in ventricular myocytes in intact failing rat hearts results in a unique form of abnormal SR Ca2+ release we termed triggered Ca2+ waves (TCWs)9—‘triggered’ because of their presence during pacing and absence immediately upon a return to rest. In failing ventricular myocytes, TCWs arose in regions with delayed or poor SR Ca2+ release9 largely coinciding with loss of TTs in failing ventricular myocytes. We postulated further that if less extensive TTs are necessary for TCW development, TCWs should develop more in normal atrial myocytes (NAMs) even in larger mammals where the TT system of atrial myocytes is less developed than their ventricular counterparts.10,11 Lower TT density, such as in atrium, should then equate to greater potential for TCW development which should be further enhanced in atrial cells during HF12 because of TT breakdown in HF. TCWs might then develop more readily in atrial myocytes from failing hearts (HFAMs) than in NAMs. The goal of this study was to investigate the characteristics and underlying mechanisms of TCWs in normal atria and in atrial myocytes from failing hearts.
2. Methods
All methods and procedures are described in the Supplementary material online.
3. Results
3.1. TCWs in atrial myocytes from normal and failing canine hearts
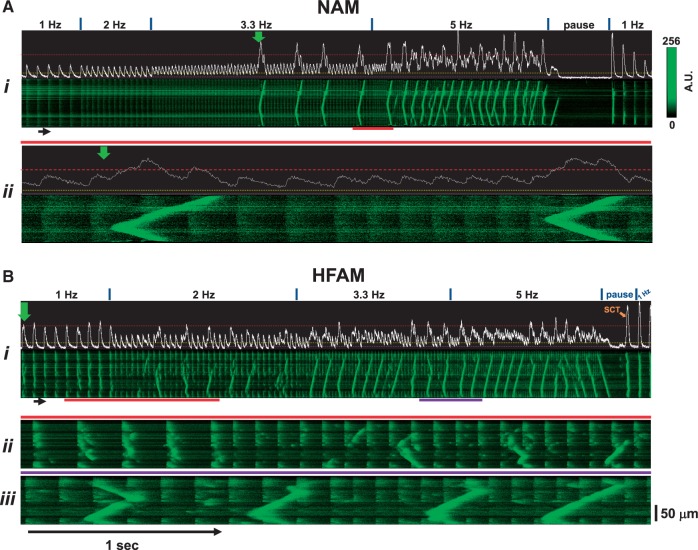
We first investigated whether or not TCWs developed in atrial myocytes when subjected to rapid pacing. Figure 1 shows longitudinal confocal Xt-linescan recordings and (immediately above) the corresponding mean F vs. time (Ft)-profiles of a normal dog left atrial myocyte (NAM, Figure 1A) activated at progressively increasing rates. Ca2+ transients (CaTs) were evoked at 1–2 Hz pacing but as rate increased, a series of large amplitude Ca2+ waves developed (initiated at vertical green arrow) and propagated along the cell. Interestingly, when we repeated this pacing protocol in a HF atrial myocyte (HFAM), these large Ca2+ release events (both propagated and non-propagated) developed at rates as low as 1–2 Hz with much higher frequency during pacing at 3.3–5 Hz (Figure 1B). Note that TCWs were absent immediately upon cessation of rapid pacing, thus distinguishing this form of Ca2+ wave behaviour from typical diastolic spontaneous Ca2+ waves (SCWs) that occur during Ca2+ overload.
Figure 1.
Triggered Ca2+ waves (TCWs) develop during rapid pacing in atrial myocytes from normal dog hearts (NAMs) and from failing dog hearts (HFAMs). (A) Continuous linescan image (Ai) recorded during progressive increases in rate from 1 to 5 Hz followed by a pause and return to basal pacing (1 Hz) with expanded time scale (red bars) shown in Aii. Mean fluorescence (Ft) profile is shown above each image. (B) Same for an HFAM with two expanded regions in Bii (red bars) and in Biii (purple bars). Dashed purple line demarks min, a dashed yellow line demarks ∼15% above that level and a dashed red line demarks 0.5 fractional SR Ca2+ release. Green arrows indicate time of first TCW and orange arrows indicate spontaneous Ca2+ waves (SCWs) or transients (SCTs) during the pause. The fluorescence intensity scale is the same for all figures.
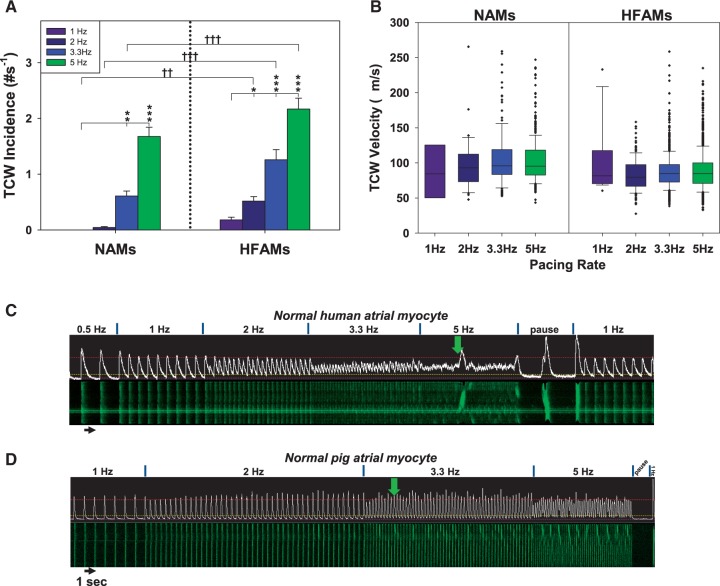
Summarized results from 318 NAMs (eight dogs) and 305 HFAMs (seven dogs) indicate that TCWs typically developed at pacing rates at 3.3–5 Hz in NAMs but as low as 1–2 Hz in HFAMs, then increased in incidence with increased rate (Figure 2A). About 80% of dog NAMs and 90% of dog HFAMs developed TCWs at any cycle length. TCWs exhibited velocities of ∼75–125 µm/s in both NAMs and HFAMs irrespective of pacing rate (Figure 2B) although some propagated at much faster rates, probably reflecting nearly simultaneous activation from multiple initiation sites.
Figure 2.
TCW incidence is greater with increasing rate and in HF while velocity is unchanged. (A) Incidence of TCWs in NAMs (52 myocytes from four atria) and HFAMs (46 HFAMs from four atria). (B) Box-plot summary of rate dependence of TCW propagation velocities. Box boundary closest to zero indicates the 25th percentile, the line in the box marks the median, boundary of the box farthest from zero indicates the 75th percentile while error bars above and below the box indicate the 90th and 10th percentiles, respectively (×’s are outliers). (C) TCWs in a paced human NAM and (D) a pig NAM. *P < 0.05, **P < 0.01, ***P < 0.001 for NAMs or HFAMs within pacing rates, two-way RM ANOVA. †P < 0.05, ††P < 0.01, †††P < 0.001 between NAMs and HFAMs within pacing rates, two-way ANOVA.
We also found TCWs in NAMs from human LA (15/29 myocytes from one normal heart, Figure 2C) and pig hearts (18/30 myocytes from two pigs, Figure 2D). One of the implications of these data is that, while a less extensive TT system may be required for TCW development, it does not insure TCW development, indicating that other mechanisms must also contribute to TCW development.
3.2 Relationship between TCW development and Ca2+ release
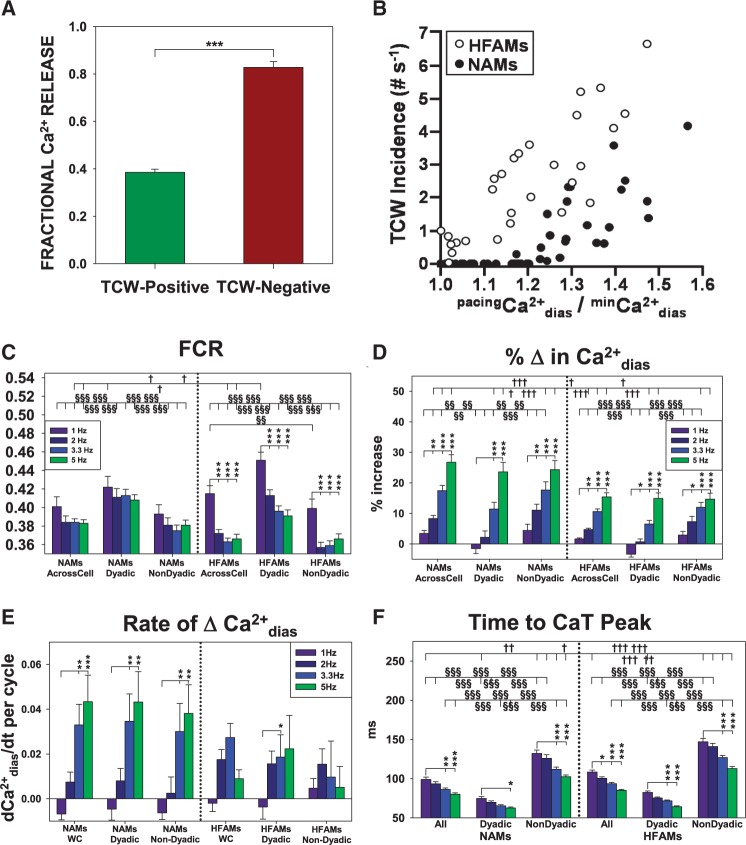
When we analysed the essential properties of TCW formation in NAMs, we found that there were important differences in the Ca2+ cycling properties between TCW-positive and TCW-negative NAMs. First, TCWs developed only when the fractional Ca2+ release (FCR) of the prior peak CaT during basal pacing was ∼0.4–0.5 (Figure 3A). TCW-negative NAMs had a higher FCR. Second, TCWs developed in NAMs when diastolic Ca2+ () at any pacing rate (pacing) rose to a level >∼15% of that at basal pacing at 1 Hz (basal) (Figure 3B). These levels are indicated in all mean Ft profiles above each linescan image (e.g. Figures 1 and 2) where a dashed purple line indicates minimum or basal paced (min), dashed yellow lines indicate 15% above the basal minimum and dashed red lines indicate FCR = 0.5. Note that the human and pig LA myocytes shown in Figure 2C–E obeyed these criteria.
Figure 3.
TCWs are abolished at high fractional Ca2+ release and increase in frequency as diastolic Ca2+ increases in both NAMs and HFAMs. (A) Mean ±SEM fractional Ca2+ release (FCR) normalized to the first post-pacing beat (PP1CaTpeak) at 5 Hz pacing in TCW-positive vs. TCW-negative NAMs (n = 45 from eight atria; ***P < 0.001). (B) Dependence of TCW incidence in NAMs (11 NAMs from three atria) and in HFAMs (seven HFAMs from three atria) on . (C) FCR measured across-cell and in dyadic and non-dyadic regions. (D) Rate dependence of regional and whole cell %↑. (E) Rate of change in (Δ%/cycle) in with increasing pacing rate. (F) Rate dependence of time to the peak of the Ca2+ transients. (C–F) n = 45 NAMs from eight atria; n = 89 HFAMs from seven atria using longitudinal recordings. *P < 0.01, **P < 0.01, ***P < 0.001 between pacing rates within NAMs or HFAMs, 1-way RM ANOVA. §P < 0.05, §§P < 0.01, §§§P < 0.001 whole cell vs. dyadic vs. non-dyadic CaTs of NAMs or HFAMs within pacing rates, two-way ANOVA. †P < 0.05, ††P < 0.01, †††P < 0.001 whole cell vs. dyadic vs. non-dyadic CaTs of NAMs or HFAMs within pacing rates, two-way ANOVA.
When we measured SR Ca2+ load following basal (1 Hz) and rapid (3.3 Hz) pacing, we found that that the SR Ca2+ content was greater in HFAMs than in NAMs (see Supplementary material online, Figure S1A and B) which has been reported previously.13 However, there was an important difference in FCR with rate in NAMs and HFAMs; during basal pacing, FCR is the same in NAMs and HFAMs but during rapid pacing FCR is significantly reduced in HFAMs (see Supplementary material online, Figure S1C). The result is that SR load is higher while FCR is lower during rapid pacing than at basal pacing in HFAMs, which could contribute to a higher incidence of TCWs in HFAMs.
We also found that the magnitude of the first post-pacing CaT peak (PP1CaTpeak) in NAMs was very large, so large in fact as to be indistinguishable from the amplitude of release evoked by 10 mM caffeine (caffCaTpeak) (see Supplementary material online, Figure S1D), indicating that the first beat following a pause was sufficient to release total SR Ca2+ load. The fact that there was no difference between caffCaTpeak and PP1CaTpeak also allowed us to normalize Ca2+ release magnitude during pacing to total SR load even in the absence of a caffeine pulse (see Supplementary material online, Figure S1E). The same was true in HFAMs (data not shown). Note that our use of PP1CaTpeak for purposes of normalization for fractional release is also based on the fact that a 3–5 second pause was sufficient for full recovery of Ca2+ release following rapid pacing (see Supplementary material online, Figure S1F).
One key difference between NAMs and HFAMs is that HFAMs often exhibited Ca2+ waves during pacing at or near basal rates (Figures 1, 2A, and 3B). This higher vulnerability to TCWs is in all likelihood largely due to elevated resting Ca2+ level () in HFAMs which has been measured and reported previously in this HF model.13 Hence, during pacing at or near basal rates in most HFAMs is already >15% above the NAM min and is in fact nearly doubled compared to NAMs.13
To determine if regional differences in Ca2+ cycling properties in atrial myocytes might play a role in TCW formation, we measured diastolic Ca2+ and active release behaviour using longitudinal recordings. When we examined the rate dependence of FCR in the different cell regions in NAMs, there was an overall (Across Cell) negative CaT-frequency relationship that was equivalent when comparing dyadic (≤3 µm from the sarcolemma) and non-dyadic regions (>3 µm from sarcolemma, Figure 3C). Furthermore, there was a pronounced rate-dependent decrease in FCR in NAMs that was exaggerated in HFAMs. Moreover, Ca2+ release was reduced more with rate in non-dyadic (central) regions than at dyadic (sarcolemmal) regions of the cell, a difference that was also greater in HFAMs. The fact that FCR decreases with rate, especially in HFAMs, is likely to play a critical part in the development of TCWs. Note that this result differs from the observations of Schotten et al.14 who showed a positive force-frequency relationship in human atrial trabeculae from sinus rhythm patients. However, this relationship became negative at rates faster than 2 hz which is consistent with our observation that triggered waves develop at rates where Ca2+ release is reduced.
increased with rate in both NAMs and HFAMs (Figure 3D) with a greater increase in NAMs than HFAMs, although there were no regional differences (dyadic and nondyadic) in both NAMs or HFAMs. In addition, there was less absolute increase in in HFAMs than in NAMs, reflecting the fact that the starting was higher to begin with in HFAMs.13 This result also explains why there is no requirement for a 15% increase in in HFAMs for induction of TCWs, which can occur even during basal pacing (1 Hz) and causing a leftward shift in the relationship between and TCW incidence for HFAMs (Figure 3B). In addition, the rate of increase in was greater in NAMs than in HFAMs and showed no regional differences but it is important to note that Ca2+ levels are nearly double in HFAMs to start with (Figure 3E). Finally, the time to peak of the CaT is greater in the non-dyadic regions compared to junctional Ca2+ release unit (jCRU)-derived release in both NAMs and HFAMs (Figure 3F), an observation that confirms the delay in central activation compared to the periphery, although no major differences were observed between NAMs and HFAMs.
3.3 Ca2+ sensitivity and density of ryanodine receptors in atrial and ventricular myocytes
Another possible contributor to TCW mechanisms could be altered Ca2+ sensitivity or density of ryanodine receptors (RyRs) in NAMs and HFAMs which could affect Ca2+-induced Ca2+ release (CICR) and activation of Ca2+ release only at high levels such as during rapid pacing or in HF. Because normal ventricular myocytes are not susceptible to TCW formation while NAMs are, we compared the Ca2+ dependence of 3 H-ryanodine binding in normal human atrial and ventricular tissues in order to consider if TCW vulnerability might be a result of a fundamental difference in RyR properties. According to the normalized binding curves, there was no difference in Ca2+-sensitivity of binding between the two tissues (see Supplementary material online, Figure S2A). However, the total binding curves indicated about 50% of maximal binding suggesting that RyR density in atrium is about half that in ventricle (see Supplementary material online, Figure S2B), an observation that was confirmed using Western blot techniques (see Supplementary material online, Figure S2C). Thus, there is unlikely to be a role of altered Ca2+ sensitivity of RyRs in the generation of TCWs.
3.4 Differences between TCWs and SCWs
TCW behaviour indicates a reliance on AP-evoked CaTs which is the basis for referring to them as ‘triggered’ by Ca2+ entry through L-type Ca2+ channels (LTCCs) during the previous CaT. This property is exemplified in Video S1 (see Supplementary material online) which shows that CaTs are activated normally from the periphery and spread to the cell core at 1 Hz pacing (early in the video). During rapid pacing (3.3 Hz), however, centripetal propagation from the periphery then induces TCWs at numerous, almost random, initiation sites throughout the cell core. Notice that a series of small CaTs with low release is followed by beats which initiate TCWs, demonstrating that low release in response to increasing rate is required for subsequent formation of TCWs.
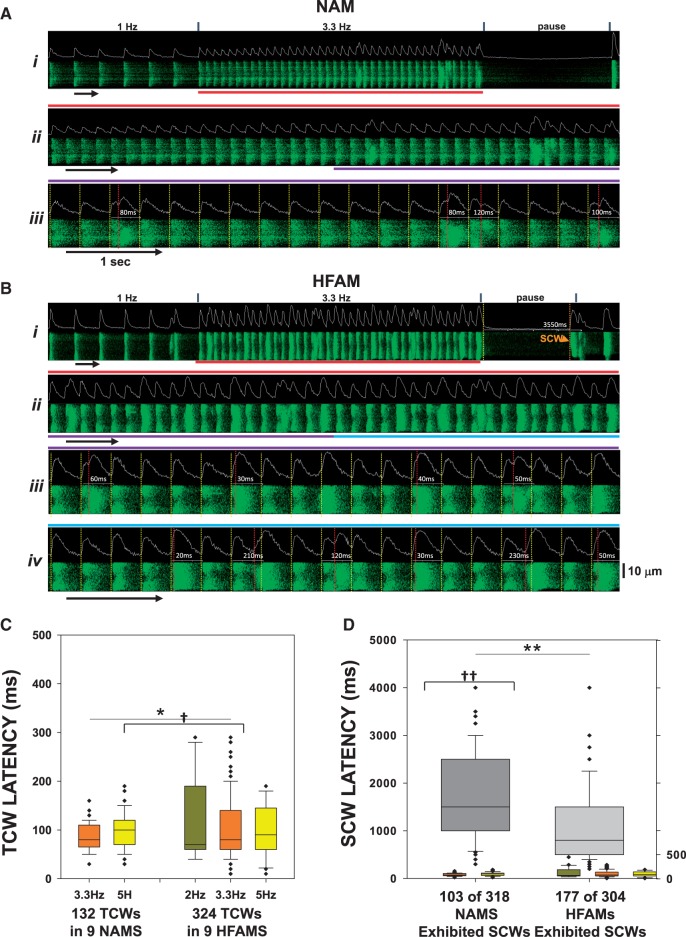
When we measured TCW and SCW latencies in both NAMs and HFAMs with respect to the previous CaT initiation time, we found two distinct populations of Ca2+ waves. Figure 4A and B shows transverse linescans from a NAM (Figure 4A) and an HFAM (Figure 4B) in which jCRU-mediated CaT initiations are denoted by yellow vertical lines. TCW and SCW (during rest) initiations are denoted by red and orange vertical lines, respectively, with each corresponding measured latency shown within the interval. Another example of this analysis in a NAM is shown in Figure S3A and B (see Supplementary material online) at a higher resolution so that precise latencies and the approach to the measurements are apparent. Summary data for latencies of all TCW- and SCW-positive recordings (Figure 4C) show that TCWs in both NAMs and HFAMs were initiated at times less than the halfway point of each respective pacing cycle, the majority of which did not begin earlier than ∼60 ms after the jCRU-mediated CaT initiation. Since atrial AP durations at 3.3 and 5 Hz are approximately 175 and 140 ms, respectively,15 virtually all TCWs occurred during the AP. This important property of TCWs indicates that they originate at non-junctional CRUs (njCRUs) because TCW activation does not coincide with activation of jCRUs and accompanying CaTs. TCWs are in fact a result of activation of internal njCRUs with a delay after jCRU activation which results from normal centripetal spread of CICR into the cell core. As indicated by the SCW latency summary (Figure 4D), SCWs are rarely initiated at latencies <1000 ms in NAMs or <500 ms in HFAMs after pacing, nearly 10 times that of TCWs (Figure 4D). These results demonstrate several of the critical differences between triggered and spontaneous waves and support the idea that TCWs are manifestations of increased activation of infrequently activated core-region njCRUs during rapid pacing and are not just another form of SCWs. However, it is possible that a small subset of overlapping TCW latencies (Figure 4D) could indeed occur because of SCR but it would be difficult to identify such events definitively.
Figure 4.
Latencies for TCWs are greater than for SCWs. (A i) Transverse recording of a paced NAM. (Ii–iv) Time-expansion of recording in (i) indicated by red, purple and blue bars. Vertical yellow lines indicate junctional CaT initiations and vertical red lines indicate the initiation time for each TCW with respect to prior CaT initiation within that cycle. (B) Latency measurements in an HFAM. (C) Box-plot summary of TCW initiation latency in NAMs (eight atria) and HFAMs (seven atria). Two-way ANOVA was used for comparisons between means. (D) Box-plot summary of SCWs in NAMs and HFAMs with TCW data from C included for comparison. See legend in Figure 2 for explanation of box plot details. †P < 0.05, ††P < 0.01 using unpaired t-test was used for comparisons between means.
3.5 Relationship between TCWs and T-tubules
Based on our previous work demonstrating TCWs in failing rat ventricular myocytes with severely disrupted TTs during HF is associated with TT depletion,10 we postulated that HFAMs would be more prone to develop TCWs than NAMs because of a low density of TTs. When we measured TTs in NAMs and HFAMs, we found that 89% of NAMs had TTs whereas only 29% of HFAMs had any TTs whatsoever (48/54 myocytes and 16/56, N = 4 dogs each, P < 0.01). This is an important observation since most NAMs demonstrated TCWs with an even higher incidence of TCWs in HFAMs despite an even lower incidence of TTs. However, in order to investigate further the possibility that TTs might in fact serve as the initiation site for TCWs, we also measured the site of initiation of TCWs in relation to the closest TTs. Overall, we found that 16 myocytes had TTs in a total of 35 myocytes from three normal dog hearts. Figure S4A (see Supplementary material online) shows an example of a TCW (white arrow) that was initiated 10 µm from the closest TT (blue arrow). In those myocytes with TTs, the mean distance between the site of initiation and the closest TT was 13.1 ± 1.70 µm. In the other 19 myocytes without TTs (an example of which is shown in Figure S4B, see Supplementary material online), each had at least one initiation site. These results shows that the presence of TTs is not critical to TCW initiation.
3.6 Effects of increasing CICR on TCWs
Given the requirement for reduced Ca2+ release for TCW development, we considered the possibility that manoeuvres that either severely alter FCR or prohibit the rise in should effectively suppress TCWs. To test this idea, we subjected NAMs and HFAMs to a number of pharmacological agents known to significantly affect SR Ca2+ release and/or SR Ca2+ reuptake.
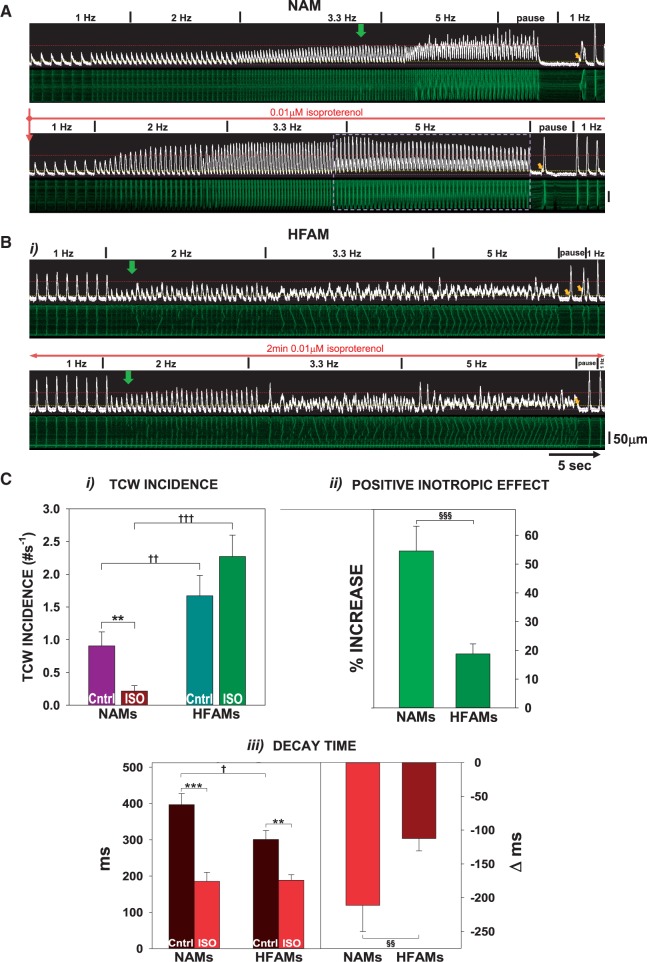
Figure 5 shows the effects of isoproterenol (ISO) which is known to increase both Ca2+ release and reuptake into the SR. ISO nearly abolished TCWs in NAMs (Figure 5A and C) but not in HFAMs (Figure 5B and C). In NAMs, the positive inotropic effect increased FCR well above 0.4 [Figure 5C(i-ii), from 0.036 ± 0.08 to 0.61 ± 0.09 at 5 Hz with ISO, P < 0.01] and produced a potent lusitropic effect [Figure 5C(iii)]. In fact, CaT alternans developed when the TCWs were abolished [white dashed box in Figure 5A(ii)]. In contrast, ISO had only a small positive inotropic effect in HFAMs [Figures 5B and 5C(ii)] with little lusitropy [Figure 5C(iii)]. These results demonstrate the blunted responsiveness of β-adrenergic stimulation in HF16,17 which leads to its inability to suppress TCWs in HFAMs.
Figure 5.
β-adrenergic stimulation suppresses TCWs in NAMs but has little effect in HFAMs. (A) Example of a NAM before and during exposure to isoproterenol (ISO). Dashed box indicates presence of CaT alternans. (B) Example of ISO effects in an HFAM. (C i) Summary of ISO action on TCW incidence in NAMs vs. HFAMs at 3.3–5 Hz; (ii) F/F0 and change in F/F0 with ISO (1 Hz); (iii) CaT 90–10% decay time and change in decay time with ISO (1 Hz). n = 16 NAMs from six atria; n = 43 HFAMs from nine atria. **P < 0.01; ***P < 0.001 between ISO and Control. †P < 0.05, ††P < 0.01, †††P < 0.001; §§P < 0.01, §§§P < 0.001 between NAMs and HFAMs using two-way ANOVA or unpaired and paired t-tests.
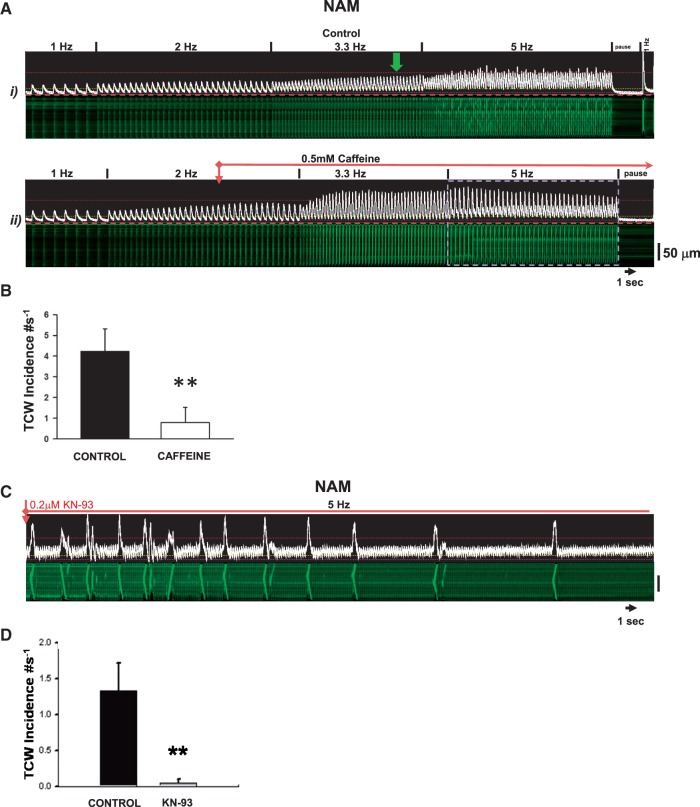
To further test the notion that increasing FCR should suppress TCWs, we used low concentrations of caffeine (0.5 mM) to increase RyR sensitivity to Ca2+. Figure 6A shows an example where 0.5 mM caffeine was applied during constant 5 Hz pacing in a NAM after TCWs had developed which immediately increased FCR and abolished TCWs. The summary of caffeine effects in all cells is shown in Figure 6B and indicates TCW suppression when SR Ca2+ release is increased via sensitizing RyRs. In addition, low caffeine caused no change in diastolic [Ca2+] (−1.14 ± 1.54%, NS) and fractional release increased from 69 ± 2.6% to 93 ± 3.1% of total load (P < 0.001, n = 28 NAMs in four atria).
Figure 6.
Low concentrations of caffeine and KN-93 suppress TCWs. (A) Example of a NAM during pacing at 5 Hz before and during exposure to caffeine. (B) Summary of caffeine effects (n = 14 NAMs from five normal atria). (C) Effects of KN-93 on TCWs during constant pacing at 5 Hz with summary shown in D (n = 16 NAMs from six atria). **P < 0.01 (paired t-test).
3.7 Inhibition of CaMKII and TCWs
One of the possible outcomes of increased during pacing is the activation of Ca-calmodulin kinase II (CaMKII) which increases LTCC current and RyR open probability. Furthermore, there is evidence that cardiac CaMKII-δc activity is increased in HF18 and AF19 which could contribute to the higher incidence of TCWs in HFAMs compared to NAMs. Additionally, CaMKII-mediated phosphorylation of cardiac Na+ channels promotes the late Na+ current, INa, L both in normal and failing heart.20–22Figure 6B(i) shows an example of frequent TCWs during 5 Hz pacing in a NAM. After 1 min exposure to 0.2 μM of the CaMKII antagonist KN-93, there was a decrease in TCW incidence. There was a significant effect on [Ca2+]dias (−19 ± 2.7%, P < 0.001, n = 27 myocytes in five normal dogs) as well as on fractional release (−14 ± 1.9%, P < 0.001). Exposure to the inactive analog KN-92 had no effect on TCWs (data not shown, n = 9 NAMs in four normal dogs) in which both [Ca2+]dias and fractional release were decreased (−15 ± 4.8%, P < 0.01; −10 ± 3.7%, P < 0.03). The summarized data [Figure 6B(ii)] confirm these results and suggest that CaMKII plays an important role in TCW formation in the atrium. Note that there was no significant difference in the changes in either fractional release or [Ca2+]dias between KN-93 and Kn-92, suggesting that the ability of the active inhibitor is likely to suppress triggered waves based on cellular mechanisms unrelated to these modest effects on Ca2+ cycling that are more related to CaMKII inhibition directly.
3.8 Effects of decreasing CICR on TCWs
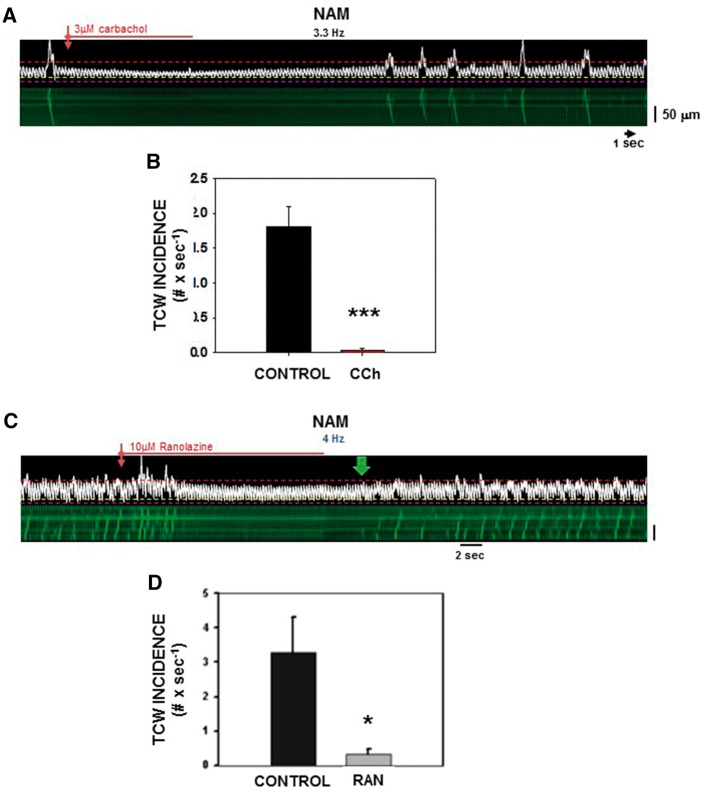
In contrast to the efficacy of TCW suppression by those agents to increase Ca2+ release, we would also expect that negative inotropic agents might also suppress TCWs by reducing overall Ca2+ influx, thus reducing the amount of Ca2+ available for release during rapid pacing. Activation of muscarinic receptors in AMs markedly reduces CICR and thus could suppress TCW development. Figure 7A shows a NAM exhibiting TCWs during 3.3 Hz pacing which were abolished during exposure to carbachol (CCh, 3 µM) and which also reduced overall Ca2+ release (CaT magnitude decreased from 1.41 ± 0.09 to 1.07 ± 0.07, P < 0.001) and (−12.3 ± 0.13%; P < 0.001; n = 24 myocytes from five normal atria). The summary of TCW suppression for combined data from NAMs and HFAMs is shown in Figure 7B.
Figure 7.
CCh and ranolazine suppress TCWs. (A) Effects of CCh on TCWs in a NAM before, during and after washout during constant pacing at 3.3 Hz. (B) Summary of effects of CCh (n = 21 NAMs from five normal atria). ***P < 0.001 (paired t-test). (C) Actions of ranolazine on TCWs. Typical linescan recording of a NAM before and during a brief exposure to 10 µM ranolazine. (D) Summary of TCW incidence before and after ranolazine (n = 6 NAMs from three normal atria). *P < 0.05 (paired t-test).
Recent studies have also highlighted the importance of the late sodium current (INa, L) in cardiac Ca2+ cycling, most likely through increased Na+ influx especially in disease leading to increased intracellular Ca2+ via reverse-mode NCX.22 Block of INa, L by ranolazine abolished TCWs in a reversible manner. The linescan image in Figure 7C shows that ranolazine (10 µM) eliminated TCWs in a NAM paced at 4 Hz. Washout of ranolazine allowed full recovery of TCWs. Figure 7B summarizes the results of ranolazine action on TCW incidence and demonstrates that inhibition of INa, L and resulting reduction of Na+ influx reduce CICR and block TCWs, In addition, ranolazine also reduced overall Ca2+ release (CaT magnitude decreased from 1.41 ± 0.13 to 1.20 ± 0.06, P < 0.05) and (−13.5 ± 4.8%; P < 0.01; n = 6 NAMs from three atria). It is important to acknowledge, however, that these results are also consistent with a reported action of ranolazine to reduce RyR open probability,23 which would also decrease FCR and block TCWs. Either way, ranolazine is effective in preventing TCWs, most likely through its ability to reduce the amount of Ca2+ available for release and .
4. Discussion
4.1 Mechanisms of TCWs and the role of CaMKII
One of the major differences between atrial and ventricular excitation–contraction (E–C) coupling is based on a key ultrastructural difference, particularly a much less well developed T-tubule system in atrium. As a consequence, trigger Ca2+ enters via LTCCs along the atrial sarcolemma, activating junctional RyRs. Ca2+ release at the cell centre occurs in a wave-like fashion with junctional SR Ca2+ activating non-junctional RyRs progressively deeper in the cell core (known as centripetal propagation) which are too far from the cell surface to be activated directly by trigger Ca2+. Aside from these major ultrastructural and activation mechanisms, few molecular differences have been identified in E–C coupling between atrium and ventricle, including identical RyR Ca2+ sensitivities as demonstrated in this study.
It was therefore surprising that Ca2+ cycling properties were so different in the two tissues during rapid pacing. Unlike ventricular myocytes from dog and other large animals, there was a pronounced negative CaT-frequency relationship in NAMs and HFAMs. The result is not only a marked decrease in FCR but also increased diastolic Ca2+ during rapid pacing. The latter is true in ventricle but only at high rates and, in fact, dog and human ventricular myocytes usually show a positive force-frequency relationship over this range of rates. The fact that FCR is reduced at high rates in NAMs yet TCW frequency increases also suggests that reduced release in the setting of high SR load is also likely to be an essential precondition for TCW formation, allowing a low trigger from the cell periphery at jCRUs to initiate centripetal activation of njCRUs that are poised for massive release under high load conditions. Rapid pacing increases producing more Ca2+-induced inactivation of LTCCs at the jCRUs so that only small CaTs are produced throughout the cell. This low release during rapid pacing occurs when CaTs do not propagate well into the myocyte core regions probably because of a reduced number of RyRs and low density of TTs. Occasionally, however, trigger Ca2+ penetrates deep enough into the cell to activate njCRUs in the cell core, possibly assisted by increased activating CaMKII which promotes release by RyRs from the highly loaded SR, effects consistent with our observations that CaMKII inhibition suppresses TCW development. njCRUs in the cell core are then activated and, because the local SR load is extremely high due to the ongoing low level of release during CaTs at high rates, those njCRUs are poised for a large release which is then propagated to neighbouring highly loaded njCRUs and a TCW is born. One implication of these processes underlying TCWs is that TCWs should always exhibit some discernable latency before activation with respect to the preceding jCRU-mediated CaT initiation during the same excitation cycle which was exactly what we observed. Conversely, a random distribution of TCW initiations across excitation cycles would indicate that TCWs are a form of SCWs, behaviour that was not observed. The fact that there are multiple initiation sites in nearly all cells suggests that there is a great deal of heterogeneity in the balance between depth of penetration of trigger Ca2+ and in local SR load, probably reflecting the fact that the Ca2+ reuptake machinery in atrial myocytes is very active, providing a buffer for low amplitude centripetal propagation of release among njRYR clusters some of which have had recent releases while others have not. Furthermore, CaMKII may contribute to TCW generation at least in part by phosphorylation of the cardiac Na+ channel which increases INa, L24 since exposure to ranolazine blocked TCWs. Thus INa, L may contribute to TCW generation by increasing Na+ influx which increases intracellular Ca2+ via NCX, contributing to the higher SR load necessary for TCW generation and possibly further activating CaMKII.
In HF, RyR2 and SERCA2a expression levels are decreased in this experimental model while CaMKIIδc and phospho-CaMKIIδc are upregulated.13 The lower RyR expression could contribute to reduced SR release during CaTs at higher rates, increasing both SR load and the likelihood of TCW formation when trigger Ca2+ manages to activate internal RyRs. The high level of CaMKII activation not only regulates RyR activity in HF but also insures a high activity of SERCA2a and corresponding SR load in HF.
Our results also demonstrate an additional feature of TCWs related to SR release; interventions that either dramatically increase or decrease release are effective in eliminating TCWs. Increasing release (ISO, caffeine) reduces SR accumulation and promotes the efficacy of Ca2+ trigger whereas reducing Ca2+ load (ranolazine, CCh) reduces the likelihood of triggered release, possibly by reducing Ca2+ sensitivity of RyRs. In addition, any agents that reduce (ranolazine, CCh) will in turn reduce CaMKII activation, thus reducing LTCC current, Ca2+ influx, SR Ca2+ load and release, all of which will contribute to suppression of TCWs.
It is also very interesting that triggered waves do not interfere with underlying Ca2+ transients raising the possibility of multiple pools of Ca2+ with different release properties in dog atrium. Although there is no experimental support for such a notion at this time, it is important to bear in mind that IP3 receptors play an extremely important role in atrial E–C coupling that has no direct parallel in ventricle. The role and behaviour of IP3 in triggered waves and, for that matter, normal Ca2+ cycling in atrium are not well understood and could play a role in the apparent independence of Ca2+ waves and transients in dog atrium.
Finally, our observations that few HFAMs possessed TTs is important since one possible mechanism for TCW formation involves a role for TTs. The fact that initiation of TCWs occurs far from the nearest TTs in the cell as well as the number of myocytes with TTs decreases so dramatically in HF despite the increase in TCW incidence suggests that it is unlikely that TTs play much of a role in TCW formation although it is possible that, if anything, the absence of TTs rather than their presence might actually promote TCWs in the atrium. However, a recent report25 suggests that RyR hyperphosphorylation along specific areas of the axial elements of the atrial T-tubule system may promote fast and well-coordinated njCRU-based Ca2+ release that occurs along the cell core. This local hyperphosphorylation occurs despite decreases in RyR2 cluster density and protein expression in LA hypertrophy presumably helping to ensure a rapid and homogenous internal Ca2+ release and contraction in disease. Furthermore, CaMKII is at least partly responsible for the elevated RyR phosphorylation along axial tubules, making them particularly sensitive to Ca2+ release. It is possible that these axial tubules, rather than TTs themselves, might increase the sensitivity of njCRUs to trigger Ca2+ influx during rapid pacing as LTCCs are inactivated also increasing the likelihood of TCW formation.25
4.2 Effects of β-adrenergic signalling on TCW generation
Since alterations in autonomic tone (including β-adrenergic signalling) are known to contribute to the genesis of AF,26 we examined TCW behaviour in the presence of β-adrenergic stimulation. Surprisingly, ISO attenuated TCW generation in NAMs, but had little effect in HFAMs. The latter effect is in fact not so paradoxical and could arise as a consequence of the well-known down-regulation of β-adrenergic signalling in HF. Even though β-adrenergic signalling increases Ca2+ influx and SR release and might be expected to promote TCWs, it actually prevents TCW formation probably as the result of increased efficiency of SR Ca2+ re-uptake and release. As such, β-adrenergic signalling may be playing a protective role at high atrial rates by attenuating the substrate for TCW formation. Conversely, the down-regulation of β-adrenergic signalling in HF might then actually be pro-arrhythmic in the setting of high atrial rates during HF.
4.3 Differences between triggered and spontaneous Ca2+ waves
In HF ventricular myocytes, hyperphosphorylated leaky RyRs in combination with a greatly diminished T-tubule system are typically thought to underlie SCR which contributes to triggered arrhythmias.7,27 Yeh et al.13 suggested that increased CaMKII-phosphorylation of phospholamban (but not RyRs) in conjunction with decreased calsequestrin were responsible for increased SR Ca2+ content causing SCR in HFAM. Indeed, we also observed both a higher incidence of SCWs in HFAMs as well as a greater SR Ca2+ load.13 However, as we have proposed here and elsewhere,28 TCWs are distinct from traditional SCWs and must rely on different mechanisms for their generation. Most importantly, TCWs occur only during rapid pacing where activation of LTCCs is ongoing and cease immediately upon cessation of pacing, behaviour that is opposite of that for SCWs. The result of this difference is our observation that there are two distinct populations of Ca2+ release events with little overlap in their timing; early events clustered around 100 ms latencies following AP activation and later events that occur at rest clustering around latencies approximately 10–20-fold greater. Almost all early events occur during the AP which we interpret as being triggered events arising from LTCC activation during depolarization whereas the late population most likely represents conventional SCR events. This is in part due to the fact that, by definition of their timing during diastole, SCWs occur in the absence of LTCC activation. It is possible that there may be some small overlap as indicated in our results but there is generally a clear separation between these two subpopulations of triggered Ca2+ events. It is also important to consider that there appears to be another novel requirement of TCWs for a progressive increase in diastolic in the presence of high SR Ca2+ loads at baseline, both of which appear to be characteristics of NAM which are exaggerated in HFAM but which is not required for SCWs.
Our results also suggest that there are several important properties of Ca2+ cycling in both NAMs and HFAMs that are likely to establish critical conditions necessary for TCW formation; these include a decrease in normalized Ca2+ release (FCR), a high SR load, and pronounced increase in with rate. These effects are exaggerated in HFAMs because of a pre-existing elevation in at rest which is likely to explain why TCW initiation occurs at slower rates and with less increase in , all results strongly supporting the key role that plays in TCW initiation. Furthermore, the fact that FCR decreases more in the cell core than at the periphery is likely to promote regional SR overload, probably explaining why TCWs are generally initiated within the cell rather than at the cell boundary. Finally, it is also important to note that it is unlikely that the rate of increase in is important to TCW formation since there is in fact a slower rate of increase in HFAMs compared to NAMs despite a much higher rate sensitivity to TCWs in HFAMs.
4.4 Clinical implications of atrial TCWs
In this study, we felt it was important to establish the fact that novel Ca2+ cycling behaviour in isolated myocytes has physiological and clinical relevance by demonstrating its presence in intact cardiac tissue. We addressed this by recording cellular Ca2+ cycling in one intact normal dog atrium ex vivo. Upon subjecting the left atrium to a similar pacing protocol used for the isolated myocyte studies (see Supplementary material online, Video S1), we found that TCWs developed in most myocytes during rapid pacing. Video S2 (see Supplementary material online) shows that normal CaTs occur uniformly in all myocytes in the recording field during basal pacing at a cycle length of 700 ms (first four beats). However, after a decrease in cycle length to 360 ms, TCWs develop within a few beats and appear as very bright irregular flashes superimposed upon dim CaTs in almost all myocytes in the recording field. When pacing is paused, both CaTs and TCWs cease immediately followed after many seconds of quiescence by SCWs that occurred in several cells. Thus, (i) TCWs are not an artefact of isolated myocyte studies, (ii) their development and characteristics in intact atria are very similar to those in isolated atrial myocytes, and (iii) the fact that TCWs do occur in intact atria suggests a role in atrial arrhythmogenesis.
In the current study, we identified a novel form of abnormal Ca2+ cycling, TCWs, that occur only during pacing in atrial myocytes in large animals including dog, human, and pig atria. The incidence of triggered waves increased at lower heart rates in HF raising the potential clinical relevance of these events, since we found that they occur even in the physiological range of heart rates in dog and human, often occurring at 120 bpm and even at 60 bpm. The implication is that TCWs might contribute to the initiation of AF in the setting of HF and possibly in normal heart during the transition to paroxysmal AF. It remains to be seen if TCWs contribute to the initiation and/or maintenance of AF once electrical and structural remodelling take place during chronic, sustained AF. Because of their timing, TCWs may prolong the AP establishing conditions for EAD development and/or repolarization gradients at rapid atrial rates. Thus TCWs generated at rapid rates, such as in the setting of AF in HF, may contribute not only to the creation but the perpetuation of AF thereby allowing AF to ‘beget’ more AF. Furthermore, β-adrenergic de-sensitization—as typically seen in HF—may make the atrium more susceptible to TCW-mediated arrhythmias, especially under conditions of enhanced CAMKII signalling. Finally, our finding that TCWs manifest in the intact dog atrium with characteristics nearly identical to those in isolated dog atrial myocytes also suggests that TCWs could contribute to atrial arrhythmogenesis.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by grants from the National Institutes of Health (HL093490 to R.A., HL119095 to J.A.W. and Y.S. and RO1-HL55438, PO1-HL094291, and RO1-108175 to H.H.V.).
Supplementary Material
References
- 1. Ter Keurs HE, Boyden PA.. Calcium and arrhythmogenesis. Physiol Rev 2007;87:457–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aistrup GL, Balke CW, Wasserstrom JA.. Arrhythmia triggers in heart failure: the smoking gun of [Ca2+]i dysregulation. Heart Rhythm 2011;8:1804–1808. [DOI] [PubMed] [Google Scholar]
- 3. Schotten U, Verheule S, Kirchhof P, Goette A.. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev 2011;91:265–325. [DOI] [PubMed] [Google Scholar]
- 4. Voigt N, Heijman J, Wang Q, Chiang DY, Li N, Karck M, Wehrens XHT, Nattel S, Dobrev D.. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation 2014;129:145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Greiser M, Kerfant BG, Williams GS, Voigt N, Harks E, Dibb KM, Giese A, Meszaros J, Verheule S, Ravens U, Allessie MA, Gammie JS, van der Velden J, Lederer WJ, Dobrev D, Schotten U.. Tachycardia-induced silencing of subcellular Ca2+ signaling in atrial myocytes. J Clin Invest 2014;124:4759–4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, Sun Q, Wieland T, Ravens U, Nattel S, Wehrens XHT, Dobrev D.. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+–Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation 2012;125:2059–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Trafford AW, Clarke JD, Richards MA, Eisner DA, Dibb KM.. Calcium signalling microdomains and the t-tubular system in atrial mycoytes: potential roles in cardiac disease and arrhythmias. Cardiovasc Res 2013;98:192–203. [DOI] [PubMed] [Google Scholar]
- 8. Burashnikov A, Antzelevitch C.. Reinduction of atrial fibrillation immediately after termination of the arrhythmia is mediated by late phase 3 early afterdepolarization-induced triggered activity. Circulation 2003;107:2355–2360. [DOI] [PubMed] [Google Scholar]
- 9. Wasserstrom JA, Sharma R, Kapur S, Kelly JE, Kadish AH, Balke CW, Aistrup GL.. Multiple defects in intracellular calcium cycling in whole failing rat heart. Circ Heart Fail 2009;2:223–232. [DOI] [PubMed] [Google Scholar]
- 10. Ferrantini C, Crocini C, Coppini R, Vanzi F, Tesi C, Cerbai E, Poggesi C, Pavone FS, Sacconi L.. The transverse-axial tubular system of cardiomyocytes. Cell Mol Life Sci 2013;70:4695–4710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arora R, Aistrup GL, Supple S, Frank C, Singh J, Tai S, Zhao A, Chicos L, Marszalec W, Guo A, Song LS, Wasserstrom JA.. Regional distribution of T-tubule density in left and right atria in dogs. Heart Rhythm 2017;14:273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dibb KM, Clarke JD, Horn MA, Richards MA, Graham HK, Eisner DA, Trafford AW.. Characterization of an extensive transverse tubular network in sheep atrial myocytes and its depletion in heart failure. Circ Heart Fail 2009;2:482–489. [DOI] [PubMed] [Google Scholar]
- 13. Yeh YH, Wakili R, Qi XY, Chartier D, Boknik P, Kaab S, Ravens U, Coutu P, Dobrev D, Nattel S.. Calcium-handling abnormalities underlying atrial arrhythmogenesis and contractile dysfunction in dogs with congestive heart failure. Circ Arrhythm Electrophysiol 2008;1:93–102. [DOI] [PubMed] [Google Scholar]
- 14. Schotten U, Greiser M, Benke D, Buerkel K, Ehrenteidt B, Stellbrink C, Vazquez-Jimenez JF, Schoendube F, Hanrath P, Allessie M.. Atrial fibrillation-induced atrial contractile dysfunction: a tachycardiomyopathy of a different sort. Cardiovasc Res 2002;53:192–201. [DOI] [PubMed] [Google Scholar]
- 15. Hara M, Shvilkin A, Rosen MR, Danilo P, Boyden PA.. Steady-state and nonsteady-state action potentials in fibrillating canine atrium: abnormal rate adaptation and its possible mechanisms. Cardiovasc Res 1999;42:455–469. [DOI] [PubMed] [Google Scholar]
- 16. Vatner DE, Sato N, Ishikawa Y, Kiuchi K, Shannon RP, Vatner SF.. Beta-adrenoceptor desensitization during the development of canine pacing-induced heart failure. Clin Exp Pharmacol Physiol 1996;23:688–692. [DOI] [PubMed] [Google Scholar]
- 17. Vatner DE, Asai K, Iwase M, Ishikawa Y, Shannon RP, Homcy CJ, Vatner SF.. Beta-adrenergic receptor-G protein-adenylyl cyclase signal transduction in the failing heart. Am J Cardiol 1999;83:80–85. [DOI] [PubMed] [Google Scholar]
- 18. Zhang T, Brown JH.. Role of Ca2+/calmodulin-dependent protein kinase II in cardiac hypertrophy and heart failure. Cardiovasc Res 2004;63:476–486. [DOI] [PubMed] [Google Scholar]
- 19. Dobrev D, Wehrens XHT.. Calmodulin kinase II, sarcoplasmic reticulum Ca2+ leak, and atrial fibrillation. Trends Cardiovasc Med 2010;20:30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wagner S, Dybkova N, Rasenack ECL, Jacobshagen C, Fabritz L, Kirchhof P, Maier SKG, Zhang T, Hasenfuss G, Brown JH, Bers DM, Maier LS.. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest 2006;116:3127–3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maltsev VA, Reznikov V, Undrovinas NA, Sabbah HN, Undrovinas A.. Modulation of late sodium current by Ca2+, calmodulin, and CaMKII in normal and failing dog cardiomyocytes: similarities and differences. Am J Physiol Heart Circ Physiol 2008;294:H1597–H1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zaza A, Rocchetti M.. The late Na+ current: origin and pathophysiological relevance. Cardiovasc Drugs Ther 2013;27:61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Parikh A, Mantravadi R, Kozhevnikov D, Roche MA, Ye Y, Owen LJ, Puglisi JL, Abramson JJ, Salama G.. Ranolazine stabilizes cardiac ryanodine receptors: a novel mechanism for the suppression of early afterdepolarization and torsades de pointes in long QT type 2. Heart Rhythm 2012;9:953–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Aiba T, Hesketh GG, Liu T, Carlisle R, Villa-Abrille MC, O'rourke B, Akar FG, Tomaselli GF.. Na+ channel regulation by Ca2+/calmodulin and Ca2+/calmodulin-dependent protein kinase II in guinea-pig ventricular myocytes. Cardiovasc Res 2010;85:454–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brandenburg S, Kohl T, Williams GS, Gusev K, Wagner E, Rog-Zielinska EA, Hebisch E, Dura M, Didie M, Gotthardt M, Nikolaev VO, Hasenfuss G, Kohl P, Ward CW, Lederer WJ, Lehnart SE.. Axial tubule junctions control rapid calcium signaling in atria. J Clin Invest 2016;126:3999–4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Arora R. Recent insights into the role of the autonomic nervous system in the creation of substrate for atrial fibrillation: implications for therapies targeting the atrial autonomic nervous system. Circ Arrhythm Electrophysiol 2012;5:850–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bers DM. Cardiac sarcoplasmic reticulum calcium leak: basis and roles in cardiac dysfunction. Annu Rev Physiol 2014;76:107–127. [DOI] [PubMed] [Google Scholar]
- 28. Shiferaw Y, Aistrup GL, Wasserstrom JA.. Intracellular Ca2+ waves, afterdepolarizations, and triggered arrhythmias. Cardiovasc Res 2012;95:265–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.