Abstract
The vascular smooth muscle cell (SMC) is one of the most plastic cells in the body. Understanding how non-coding RNAs (ncRNAs) regulate SMC cell-fate decision making in the vasculature has significantly enhanced our understanding of disease development, and opened up exciting new avenues for potential therapeutic applications. Recent studies on SMC physiology have in addition challenged our traditional view on their role and contribution to vascular disease, mainly in the setting of atherosclerosis as well as aneurysm disease, and restenosis after angioplasties. The impact of SMC behaviour on vascular disease is now recognized to be context dependent; SMC proliferation and migration can be harmful or beneficial, whereas their apoptosis, senescence, and switching into a more macrophage-like phenotype can promote inflammation and disease progression. This is in particular true for atherosclerosis-related diseases, where proliferation of SMCs was believed to promote lesion formation, but may also prevent plaque rupture by stabilizing the fibrous cap. Based on newer findings of genetic lineage tracing studies, it was revealed that SMC phenotypic switching can result in less-differentiated forms that lack classical SMC markers while exhibiting functions more related to macrophage-like cells. This switching can directly promote atherogenesis. The aim of this current review is to summarize and discuss how ncRNAs (mainly microRNAs and long ncRNAs) are involved in SMC plasticity, and how they directly affect vascular disease development and progression. Finally, we want to critically assess where potential future therapies could be useful to influence the burden of vascular diseases.
Keywords: Non-coding RNAs, MicroRNAs, Long non-coding RNAs, Smooth muscle cells, Vascular disease, Atherosclerosis, Aortic aneurysms, Restenosis
1. Non-coding RNAs
New technologies such as next-generation deep sequencing have revealed that the vast majority of our genome is transcribed into RNAs. Interestingly, <2% of the human genome codes for proteins,1 leaving many RNAs without coding potential. These RNAs are referred to as non-coding RNAs (ncRNAs). Historically DNA being transcribed into ncRNA was considered ‘junk DNA’. This view has shifted quite dramatically with increasing evidence that suggests a crucial mediating role for ncRNA on the molecular level. Furthermore, the amount of ncRNAs in an organism correlates with its complexity, which lets scientists assume a key influence of ncRNAs on the development and organization of higher developed animals.2
NcRNAs are divided into two subclasses according to a relatively broad size threshold. NcRNAs longer than 200 nucleotides (nt) are termed long non-coding RNAs (lncRNAs), while shorter ncRNAs (<200 nt) are called small or short ncRNAs. Small ncRNAs can range from very few to 200 nt, while lncRNAs have a size up to several kilobases.3 MicroRNAs (miRNAs) with a size of ∼20 nt have certainly received most of the attention over the last two decades, in particular for their role in tempering gene expression. Several clinical trials have been initiated that utilize miRNA inhibitors in various diseases, mainly targeting kidney and liver pathologies, but also cancer.4 Currently no miRNA-based trial in the cardiovascular field has been initiated, but several candidates are being pre-clinically explored.5
MiRNAs are defined as single-stranded, endogenously expressed ncRNAs that regulate gene expression on the post-transcriptional level. Genes encoding for miRNAs are located throughout the genome, with a large proportion being transcribed within clusters.2 miRNA biogenesis is comprised of a multistep process that involves transcription, nuclear processing, export into the cytoplasm, and maturation before reaching the stage of a functional molecule.6
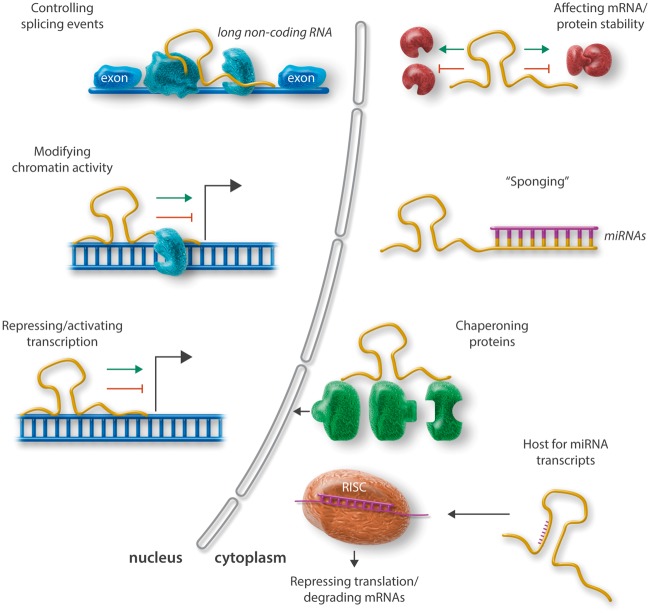
Unlike miRNAs, the mechanisms of action for lncRNAs thus far are only poorly understood. Importantly, lncRNAs can regulate gene expression at multiple levels in the cell, either in the nucleus or the cytoplasm, through complex molecular mechanisms (summarized in Figure 1). Furthermore, lncRNAs are unlike miRNAs less well-conserved across different species when it comes to the nucleotide sequence, even though lncRNA transcripts can be found in almost all living cells.7 As one can imagine, this substantially limits experimental in vitro and in vivo models for investigation of lncRNA functionality, even though a difference in nucleotide composition can still lead to the same 3D geometrical pattern and genomic or epigenetic function.8,9 LncRNAs appear as crucial epigenetic regulators of almost every cellular process, and expression of these molecules is distinctly mediated under physiological and pathological conditions, including cardiovascular disease (CVD). Intriguingly, the vast majority of disease-associated single nucleotide polymorphisms (SNPs) derived from recent genome wide association studies appear in non-coding regions of the human genome.9–11
Figure 1.
Schematic overview of prominent mechanisms of action for ncRNAs (miRNAs and lncRNAs). lncRNAs can act within the nucleus, where they exert regulation on the transcriptional level, as well as in the cytoplasm (mainly affecting mRNA/miRNA and protein stability). Within the nucleus lncRNAs have been shown to be important regulators for splicing events, to modify chromatin activity, and to repress or activate transcription of genes. Within the cytoplasm, lncRNAs mediate mRNA and protein stability and thus translational activity; they can act as sponges for miRNAs, meaning that they capture them before they get uploaded into the RISC where they suppress target gene expression. Furthermore, lncRNAs can serve as chaperons (scaffolds) for proteins (e.g. transcription factors) trying to enter the nucleus. Mature, single-stranded miRNAs can originate from lncRNA transcripts and act upon loading into the RISC via repressing translation or induction of mRNA degradation.
Based on the large number of existing lncRNAs, it becomes evident that only a minority have already been described and investigated thoroughly. However, the ones that have been functionally characterized have proven to regulate gene expression at the transcriptional and post-transcriptional level through structural and sequence-specific manners.12,13 One important feature regarding their true classification as a non-coding transcript is obviously the exclusion of their potential to code for protein. This becomes increasingly evident, as it has indeed been recently reported that some previously considered lncRNAs actually do code for (micro)-peptides.14 Whether these peptides are really functional and have any relevance for the molecular signalling control of lncRNAs remains to be determined.
In addition to these classic linear RNA transcripts, it appears that there is also a back-splicing variant of expression which gives rise to circular RNAs (circRNAs). CircRNAs lack a 5′ cap and 3′ tail, and they are processed as covalently closed continuous loops.15 Interestingly, circRNAs are stably conserved across species and appear relatively tissue-specific compared with other ncRNAs.16 Due to their stability in circulation and tissue specificity, circRNAs might in particular become interesting as future biomarkers in CVD.17,18
2. SMCs and vascular disease development
As this current article is part of a series of reviews for a Spotlight Issue on ‘Novel concepts for the role of smooth muscle cells (SMCs) in vascular disease’, the physiology and contribution to pathology surrounding SMCs in the vasculature will only be briefly discussed. Under physiological—somewhat un-diseased conditions in healthy arteries—SMCs express a range of SMC markers, including SMC-α actin (ACTA2), smoothelin, SMC myosin heavy chain (MYH11), and transgelin (TGLN), among others. SMCs in culture—as well as during disease development and progression—reduce expression of these markers, and at least in vitro become prone for mechanisms that involve proliferation, migration, calcification, and production of various extracellular matrix (ECM) proteins and cytokines.19 In the following paragraphs, several SMC-related mechanisms, their contribution to vascular disease, and the regulatory role specific ncRNAs play in this context, will be discussed (summarized in Tables 1and2).
Table 1.
miRNAs in SMC fate and functionality
| miRNA (cluster) | Regulation | Target(s) | Role and function in SMC dynamics | References |
|---|---|---|---|---|
| miR-1/-133a | ↑ | KLF4, SRF, CCND, RUNX2 | Differentiation, migration, proliferation | 33,42–45,84 |
| miR-21 | ↑ | PTEN, PDCD4, BCL2 | Proliferation, apoptosis | 86–90 |
| miR-23b/-24/-27b | ↑↓ | TLP3, CHI3L1, FOXO4, | Proliferation, differentiation, cytokine production & release | 34–35,91,92 |
| miR-26a | ↓ | SMAD1, SMAD4 | Differentiation | 93 |
| miR-29a/b/c | ↑↓ | COL1A1, COL3A1, COL5A1, ELN, MMP2, MMP9, PTEN | ECM production, proliferation | 34,116–120 |
| miR-34a | ↑↓ | SIRT1, NOTCH1 | Proliferation, migration | 97 |
| miR-130a | ↑ | MEOX1 | Proliferation, migration | 99 |
| miR-138 | ↑↓ | SIRT1 | Proliferation, migration | 98 |
| miR-143/-145 | ↑↓ | KLF4/5, MYOCD, ELK1, SRF | Differentiation, proliferation | 23,48–56 |
| miR-146a | ↑ | KLF4/5 | Differentiation, proliferation | 57 |
| miR-155 | ↑ | SMAD2, BCL6, CTLA4 | Differentiation, inflammation | 58–60 |
| miR-195 | ↓ | ELN, MMP2 | ECM production, remodelling | 118 |
| miR-204 | ↓ | PTPN11 | Proliferation | 100 |
| miR-205 | ↓ | SMAD1, RUNX2 | Proliferation, calcification | 95 |
| miR-206 | ↓ | ARF6, SLC8A1 | Differentiation | 94 |
| miR-210 | ↓ | APC | Apoptosis | 125 |
| miR-221/-222 | ↑ | CDKN1B, CDKN1C | Proliferation, migration, and anti-apoptotic effects | 101,102 |
| miR-424/-322 | ↓ | CCND1, CALU, STIM1 | Proliferation | 103 |
| miR-663 | ↓ | JUNB | Proliferation | 104 |
miRNAs and their regulation (up- or down) under vascular disease relevant conditions and stimuli. Further depicted in the table are the direct downstream targets of specific miRNAs, their main effect in SMC dynamics, and the respective references. Abbreviations of target genes are explained in the running text.
Table 2.
lncRNAs in SMC fate and functionality
| lncRNA | Regulation | Related targets and factors | Role and function in SMC dynamics | References |
|---|---|---|---|---|
| ANRIL | ↓↑ | CDKN2A, CDKN2B, DAB2IP, LRP1, LRPR, CNTN3 | Proliferation | 10,109–112 |
| RNCR3 | ↑ | KLF2, miR-185 | Proliferation | 108 |
| H19 | ↑ | miR-675, IGF2, let7, p53, NOTCH1, miR-106a, MYC, miR-19b, SOX6 | Differentiation and proliferation | 64–76 |
| lnc-362 | ↑ | miR-221/222 | Host-gene for miRNAs, proliferation | 105 |
| SENCR | ↑ | FLI1 | Migration and proliferation | 77,78 |
| Lnc-GAS5 | ↓ | ANXA2 | Differentiation, proliferation, and migration | 106,107 |
| Lnc-MEG3 | – | p53, MMP2, IFN-γ | Proliferation, migration, and apoptosis | 137,138 |
| MYOSLID | – | MYOCD, MRTF-A, TGF-β (SMAD) | Differentiation and proliferation | 79 |
| HIF1α-AS1 | ↑ | HIF1A, BRG1 | Apoptosis and proliferation | 45,135,136 |
| HAS-AS1 | ↑ | HAS2 | ECM remodelling and deposition | 122 |
| lincRNA-p21 | ↑ | p53, p300 | Apoptosis | 133,134 |
lncRNAs and their regulation (up- or down; if applicable) under vascular disease relevant conditions and stimuli. Further depicted in the table are the associated up- and downstream targets, their main effect in SMC dynamics, and the respective references. Abbreviations of lncRNAs and associated mediators/genes are explained in the running text.
3. SMC de- and trans-differentiation
Several ncRNAs have been shown to control the varying mechanisms, which govern SMC plasticity and fate (Figure 2). A strong indicator for the importance of miRNAs in this context, are studies being performed in mice with a genetic deletion of Dicer.20–22 The enzyme Dicer plays a central role in miRNA biogenesis, as it has the ability to cleave pre-miRNA (as well as other double-stranded RNAs) into short double-stranded RNA fragments (of about 18–25 base pairs), facilitating the activation of the RNA-induced silencing complex (RISC), in which mature miRNAs act upon their target mRNAs.6 The consequences of deficiency in SMCs are impaired vascular development and embryonic lethality.23,24 Recent data from Zahedi et al.25 revealed that conditional deletion of Dicer in SMCs of Apoe−/− mice reduces their proliferation rate. The SMC-specific deletion mediated a network of anti-proliferative miRNAs and targets in wire-injured mouse carotid arteries. Albinsson et al.23,26 further revealed that Dicer-deficient mice displayed significant blood pressure reductions with limited SMC contractile functionality. Interestingly, the same researchers were able to show that miR-145 can to some extent rescue the contractile phenotype of Dicer-deficient SMCs.
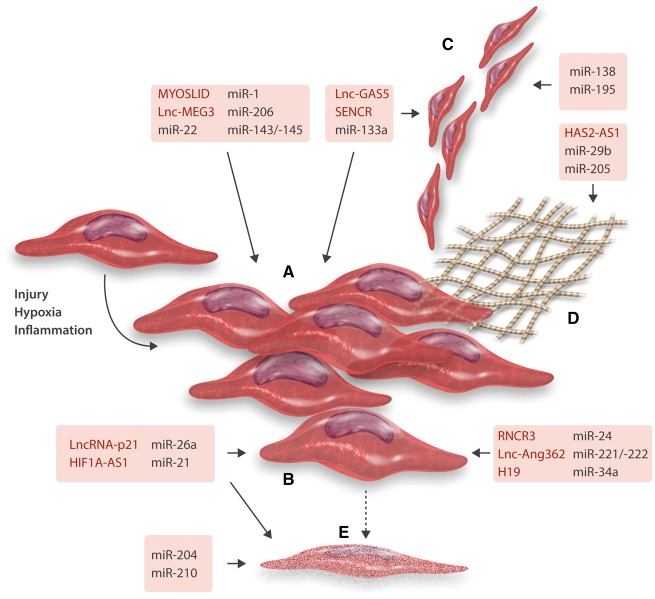
Figure 2.
Schematic overview of miRNAs and lncRNAs involved in SMC fate decisions. Contractile SMCs upon different stimuli (injury, hypoxia, inflammation, etc.) undergo phenotypic switching (A) to a more synthetic, proliferative cellular subtype. Proliferation (B), migration (C), ECM production (D), and cell death (apoptosis) (E) of SMCs has been shown to be regulated by several miRNAs as well as more recently lncRNAs. For details on specific mechanisms of action and targets of all displayed ncRNAs, please refer to the main text.
Phenotypic switching of SMCs has long been considered as an important contributing factor to vascular disease development. Recent studies have discovered that SMCs that undergo phenotypic switching resemble a more macrophage-like phenotype, which is mainly based on the markers being expressed on their surface.27 The stem cell and differentiation mediator Kruppel-like factor 4 (KLF4) has been shown previously to be required for phenotypic switching of cultured SMCs in response to platelet-derived growth factor BB (PDGF-BB),28,29 oxidized phospholipids,30,31 and interleukin (IL)-1β,32 while repressing SMC marker genes to limit the activation of myocardin (MYOCD)-responsive genes.29,33 One of the first studies investigating the role of miRNAs in SMC differentiation led to the discovery of the miR-1/miR-133a family in this context. In particular miR-1, which becomes activated through MYOCD, destabilizes the cytoskeleton in contractile state-bound SMCs.34 miR-24 and miR-29a are additional miRNAs that get induced through MYOCD. Both have been investigated for their regulatory role in migrative and proliferative processes in SMCs through facilitating the expression of PDGFRβ levels.35 miR-24 has further been shown to mediate Tribbles-like protein 3 upon PDGF-BB stimulation, which through SMURF1 and reduced SMAD1 enhances SMC differentiation.36,37
Regarding the specific role of KLF4 in SMCs in vivo, it could be shown that this transcription factor is strongly associated with an augmented phenotypic transitioning in response to carotid ligation injury.38 More recent studies from the Owens laboratory have shown that conditional SMC-specific genetic deletion of KLF4 does not necessarily prevent SMC phenotypic switching, but substantially limits the size of atherosclerotic lesions in parallel with increased fibrous cap and plaque stability.27 Interestingly, overall SMC numbers were not affected by KLF4 deletion, but a substantial reduction in SMC-derived macrophage-like and mesenchymal stem cell-like cells was discovered, indicating a crucial and novel role for KLF4 regulating SMC-macrophage transition. The gene expression of SMC-derived macrophages was importantly different from classical macrophages, dendritic cells, or monocytes.39 The SMC-derived macrophages showed a reduction in phagocytic capacity when being compared with activated peritoneal macrophages. This study challenges our more classical view on the role of SMCs, being considered as purely protective in fibrous caps of advanced atherosclerotic lesions.40,41 Certainly SMC function might vary quite drastically depending on the stimulus and outcome of their phenotypic switching and transformation.
The aforementioned miR-1 directly targets KLF4, guiding the differentiation process of embryonic stem cells into SMC progenitors,42 while miR-1 depleted mice display abnormal SMC differentiation.43 Two human miR-1 genes exist (miR-1-1 and miR-1-2), and both are co-transcribed with the respective miR-133a genes (miR-1-2 and miR-133a-1 on chromosome 18; miR-1-1 and miR-133a-2 on chromosome 20).44 miR-1-1 and miR-1-2 become stimulated by MYOCD and myocyte enhancer factor-2 in cardiac and skeletal muscle precursor cells.45 Mice with genetic deletions of miR-133a display abnormal SMC gene expression in the heart and limited cardiomyocyte proliferation during development, which the authors at least partially attribute to increased expression of its direct targets serum response factor (SRF) and cyclin D2 (CCND2).46 An additional study was able to indicate a role for miR-133a in SMC-driven arterial calcification processes.47 In this study, miR-133a overexpression limited the trans-differentiation of SMCs into osteoblast-like cells, whereas inhibition of miR-133a was able to promote a pro-osteogenic response by inducing the expression of its direct target RUNX2.
The miR-143/-145 cluster originates from a bi-cistronic transcript sharing a common promoter,48 harbouring various binding sites for transcription factors known to be involved in SMC differentiation.49–51 Both miRNAs are augmented in SMC progenitors in vascular development,52 and influence SMC dynamics by directly targeting KLF4 and KLF5, which then induces MYOCD.49,53 In most studies, induction of miR-145 limits neointima formation upon experimental vascular injury.48,53 Contrary results were reported for cultured SMCs originating from clinical tissue specimen and experimental animal models of pulmonary arterial hypertension (PAH). Here, miR-145 appeared initially up-regulated, and Caruso et al.54 revealed that vector-based repression of miR-145 halted SMC differentiation and switching towards a pro-proliferative phenotype.
Two interesting studies have assessed the intercellular communication and potential differentiation of SMCs and endothelial cells (ECs) being triggered through miR-143/-145. Climent et al.55 discovered that SMCs can at least partially control EC functionality via sending miR-143/-145 through tunnelling nanotubes, enhancing the pro-angiogenic capabilities of ECs in a transforming growth factor-β (TGF-β) dependent manner. Hergenreider et al.56 have demonstrated the exchange of miRNAs (as well as other RNAs) from ECs to SMCs, discovering a key role for travelling miR-143-/-145 in SMC differentiation and vascular functionality. In this study, laminar shear stress was able to KLF2-dependently increase expression, exosomal release, and intercellular transfer of miR-143/145, exerting atheroprotective effects.
Another interesting KLF-4 related mechanism in SMC differentiation is exerted by miR-146a. This miRNA directly targets KLF4, but at the same time has binding sites for both KLF4 and KLF5 in its own promoter region. The two KLF transcription factors have opposing effects on SMC phenotypic switching and proliferation depending on active binding to the miRNA gene.57
Finally, miR-155 has been indicated to revoke SMC differentiation by limiting SM-MHC levels.58,59 Apart from its role in differentiating SMCs, miR-155 is well known in vascular disease-related mechanisms for its repression of B-cell CCL, lymphoma 6 (BCL6) in macrophages.60
Apart from miRNAs, several lncRNAs have been suggested to play a role in SMC differentiation. H19 was one of the first RNAs discovered to act as a transcript without protein-coding ability.61 Initially, H19 was discovered as a paternally imprinted ncRNA, which remains highly expressed throughout embryonic and foetal development, with its expression being shut down in most tissues (including the vasculature) shortly after birth.62 This onco-fetal behaviour pattern and its uni-parental monoallelic expression are considered principal characteristics of imprinted genes, with many of these genes becoming altered in various malignancies.63
The multiple functions of H19 in vascular disease progression in relation to pathological processes involving SMCs are under current investigation by several labs. Of interest to SMC dynamics, H19 is a known regulator of p53,64 which mediates cellular differentiation and apoptosis. Other important functions of H19 include its hosting of the pri-miR-675 gene that suppresses growth and migration,65,66 as a modulator of miRNA expression (including let-7 and miR-106a),67–69 or mediating RNA: protein interactions.70,71 One SMC-specific study revealed that let-7a attenuates migration and proliferation in vitro and in vivo by targeting V-myc avian myelocytomatosis viral oncogene homologue (MYC).72 Additional effects in the cardiovascular system of H19 include increasing proliferation, while blocking apoptosis during late-stage cardiac differentiation via regulation of miR-19b and SOX6.73 H19 inhibition was further detected to limit human umbilical vein endothelial cell (HUVEC) growth and capillary formation,74 while H19-bourne miR-675 was shown to magnify restenosis development by targeting phosphatase and tensin homologue (PTEN) in SMCs.75 Recently, altered DNA methylation in the promoter region of H19 in calcified aortic valve disease was shown to accelerate mineralization by silencing NOTCH1.76
The Miano Lab has recently shown the first lncRNA being predominantly expressed in ECs and SMCs.77 The investigators assessed human aortic SMCs via RNA-sequencing and revealed an antisense RNA overlapping the Friend Leukaemia Integration virus 1 (FLI1) gene, an established regulator of endothelial development.78 They termed the transcript smooth muscle and endothelial cell enriched migration/differentiation-associated lncRNA (SENCR), which has two variants with enhanced expression in arterial ECs and SMCs, as well as other tissues (e.g. lung, skeletal muscle). SENCR depletion led to SMC de-differentiation and induction of migration, mainly being indicated through increased expression of midkine and pleiotrophin. Repression of both pro-migratory genes upon SENCR inhibition halted this effect. Interestingly, SENCR appears to be mainly expressed and active cytoplasmaticly, and thus not regulating FLI1 on the transcriptional level.77 Additional studies looking at the mesodermal and endothelial lineage commitment upon SENCR modulation discovered that its overexpression promotes proliferation, migration, and angiogenesis in HUVECs.78
A novel transcript termed myocardin-induced smooth muscle lncRNA (MYOSLID)79 has been shown to increase SMC phenotypic switching while reducing SMC proliferation rates. MYOSLID gets induced via TGF-β/SMAD pathways, as well as MYOCD and SRF. Inhibition of MYOSLID unsettles actin formation by blocking nuclear translocation of the MYOCD-related transcription factor A (MRTF-A), while limiting the TGF-β1-induced phosphorylation of SMAD2.
4. SMC migration, proliferation, and ECM production
Absolute or relative quantification of SMC migration in human arteries is considered very difficult—if not impossible to perform—due to the lack of solid and specific markers. Thus, in vitro studies are providing us with the only evidence that human SMCs can migrate in response to varying stimuli.39 As a result, the contribution of SMC migration to aneurysm development (or its limitation), as well as restenosis, or the maturation of atherosclerotic plaques in patients appears largely unclear. Also, until now it could not be clarified whether migration occurs independently—or is dependent on SMC proliferation.80 Classically, the presence of a large number of intimal SMCs in aortic aneurysms as well as in fibrous cap formation in advanced atherosclerosis has been taken as evidence that SMC migration from the media plays an important role in vascular disease progression. Unlike humans, rodents have no SMCs in the intima of a healthy artery, which leads to the assumption that neointimal SMCs must have migrated from the intima—or originate from invading myeloid cells from the lumen that underwent trans-differentiation.39,81
Accelerated SMC proliferation rates can be observed in response to vascular injury as well as during early atherogenesis and upon vascular injury.82 On the contrary, SMCs derived from either aged arteries, aneurysmal disease, or advanced atherosclerotic plaques display augmented proliferation rates and extended population doubling times.83,84 The aforementioned miR-1 has been reported as an important regulator to limit SMC proliferation via mediating the expression of PIM1.85 A more prominent and well-studied miRNA in determining SMC fate in aortic aneurysm development as well as after vascular injury and neointima formation is miR-21, which provides mainly pro-proliferative and anti-apoptotic effects in SMCs.86,87 Inhibition of miR-21 diminishes the neointimal response via induction of PTEN expression (Figure 3). In studies investigating the development of in-stent restenosis (ISR), miR-21-depleted mice appeared protected from this complication by limiting the burden of neointima formation in response to vascular injury. The authors of the study associated this to an increase of anti-inflammatory M2 macrophage signalling in conjunction with a compromised proliferative reaction in miR-21−/− SMCs.88 Wang et al.89 were able to show that local delivery using anti-miR-21 coated drug eluting stents limited SMC-driven myointimal hyperplasia, and thus effectively blocked ISR development. One of the early studies on miR-21 by Davis et al.90 was able to discover an opposing effect for this miRNA in SMC differentiation and proliferation. Here, miR-21 down-regulated programmed cell death 4 (PDCD4), which acts as a negative regulator of SMC contractile genes. Interestingly, enhanced signalling of TGF-β and bone morphogenic protein pathways led to increased expression of mature miR-21 through a post-transcriptional regulatory circuit, which triggered the processing of the pri-miR-21 gene into pre-miR-21.
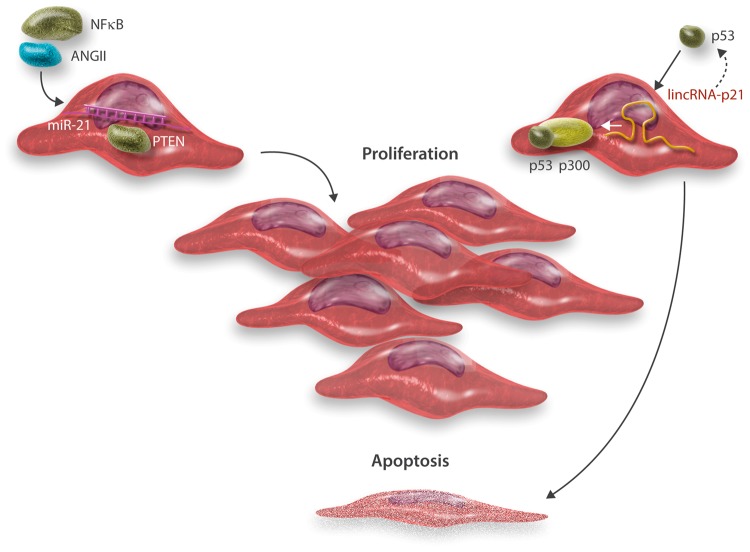
Figure 3.
miR-21 regulates SMC proliferation, while lincRNA-p21 mediates apoptosis. miR-21 has been reported to get induced by different factors involved in vascular disease evolvement and progression, such as nuclear factor kappa-b and ANGII.86 SMC-enriched miR-21 targets and down-regulates expression of PTEN, activating proliferation.86,87 lincRNA-p21 is a transcriptional target of p53, which is enabled to feed back to accelerate p53 activity through binding to other factors (mouse double minute 2 = MDM2, an E3 ubiquitin-protein ligase not shown in the scheme).133 Increased levels of lincRNA-p21 allow p53 to interact with p300, which leads to SMC apoptosis.
Another cluster with implication for SMC dynamics and cell-fate decision making is the miR-23b/-24/-27b family.91 miR-23b limits SMC proliferation and migration, effecting neointimal hyperplasia in an arterial injury model through targeting of FOXO4.92 miR-24 has further been shown to limit aortic aneurysm progression by targeting cytokine release and survival rates in SMCs (and macrophages) by targeting chitinase 3-like 1 (CHI3L1).91
miR-26a has been shown to limit serum starvation-induced differentiation, while enhancing proliferation of SMCs. Leeper et al.93 identified SMAD1 and SMAD4 as direct targets of deregulated miR-26a with direct implications for angiotensin II (ANGII)-induced aortic aneurysms in ApoE−/− mice. Interestingly, another study discovered that miR-206 in HUVECs helps to maintain the contractile phenotype of SMCs by suppressing exosome release of miR-26a-enriched particles via the adenosine diphosphate (ADP)-rybosilation factor 6 (ARF6) and soluble carrier family 8 member A1 (SLC8A1, previously known as NCX1).94 Another miRNA targeting SMAD1 (as well as RUNX2) expression levels is miR-205, which adversely regulates β-glycerophosphate-induced calcification of human SMCs.95
Another prominent miRNA regulating migration and proliferation is miR-34a, which modulates Sirtuin1 (SIRT1),96 as well as NOTCH1 expression levels.97 Induction of miR-34a led to limited neointima formation in murine models of arterial injury. Another miRNA targeting SIRT1 is miR-138, which has been indicated to enhance SMC migration and proliferation in a diabetic model utilizing db/db mice.98 A similar mechanism aiming at the proliferative response in SMCs could be reported for miR-130a, which regulates Mesenchyme Homeobox 1 (MEOX1) in this process.99
Interesting observations on the regulation of miRNAs in SMC proliferation and contribution to vascular disease progression stem from studies investigating PAH. Here, it was discovered that miR-204 was repressed in human PAH disease specimens and murine models. Upon down-regulation of miR-204, Protein tyrosine phosphatase, non-receptor type 11 (PTPN11, also known as SHP2) increases, enhancing STAT signalling, which mediates SMC proliferation and intimal hyperplasia in pulmonary arteries.100
miR-221 is another miRNA that has been shown to stimulate SMC proliferation downstream of PDGF. Induction of this miRNA decreased the expression of c-Kit and p27Kip1, which are both critical mediators of cell proliferation.101 A similar mechanism was observed for miR-221’s cluster member miR-222.102
MiRNA profiling studies using microarray technology led to the discovery of miR-424 and its rat ortholog miR-322 in studies investigating myointimal hyperplasia and SMC responsiveness. Functional in vitro and in vivo studies identified CCND, as well as Ca(2+)-regulating proteins calumenin (CALU) and stromal-interacting molecule 1 (STIM1) as direct targets.103 Adenoviral overexpression of miR-424/-322 limited the pro-proliferative response in an arterial injury model in rats.
Through direct targeting of the oncogene and transcription factor JUNB, miR-663 was identified as a crucial inducer of SMC proliferation.104 Again, utilization of adenoviral overexpression limited SMC proliferation in a mouse model of carotid ligation injury.
Similar to the molecular mechanisms regulating SMC differentiation, several lncRNAs were identified in recent years to contribute to SMC proliferation and migration. One of the first studies using RNA deep sequencing in rat SMCs discovered that ANGII stimulation deregulates several lncRNAs. Interestingly one of them, lncRNA-362 functions as a host for the aforementioned miR-221/-222 cluster. Inhibition of lncRNA-362 limited SMC proliferation rates in response to ANGII treatment.105
Further profiling approaches using microarrays identified several deregulated transcripts in varicose great saphenous veins compared with un-diseased controls. Co-expression analysis revealed their potential importance in metabolic pathways; however, none of the lncRNAs was experimentally or functionally analysed.106 A second study performed in saphenous veins and SMCs originating from these vessels identified lncRNA-GAS5A as a novel mediator in SMC proliferation. RNA pull-down experiments indicated a direct binding for this lncRNA to the RNA-binding protein Annexin A2 (ANXA2). Inhibition of lncRNA-GAS5 was able to limit ANXA2 expression and SMC proliferation, while ANXA2 overexpression increased proliferation rates.107
The retinal lncRNA 3 (lncRNA–RNCR3) has been linked to atherosclerosis-related vascular dysfunction with a potential mechanism relating to SMC dynamics.108 RNCR3 levels are augmented in human and mouse atherosclerotic lesions, in which the lncRNA co-locates with ECs and SMCs. Inhibition of RNCR3 sufficiently enhances pro-inflammatory signalling during atherogenesis and hypercholesterolaemia, while blocking proliferation in SMCs. Cell-fate decisions in SMCs are regulated by RNCR3 through the formation of a feedback loop with KLF2 and miR-185.
The anti-sense RNA in the INK4 locus (ANRIL) is localized in the CVD-associated 9p21.3 region of the human genome.9,109 ANRIL is a crucial regulator of cell cycle genes, which it mediates in cis through polycomb repressive complexes.110 SMCs stemming from humans with a SNP variant in the ANRIL locus exhibit increased proliferation rates.111 Holdt et al.112 reported that the molecular mechanism controlled by ANRIL affects target-genes in trans, which leads to cellular proliferation, adhesion, and concomitantly a reduction in apoptosis. Importantly, the reported trans regulatory mechanisms relied on Alu motifs, which were responsible for identifying the promoters of the respective ANRIL targets.
SMCs synthesize components of the ECM, in which they are embedded. Earlier studies revealed that the ECM suppresses phenotypic switching, keeping SMCs in a contractile state while being less responsive to varying stimuli (e.g. cytokines). On the contrary, ECM breakdown and anti-fibrotic factors (like matrix-metalloproteinases released from macrophages and SMCs) were shown to promote phenotypic switching and to induce a pro-migratory and -proliferative response in the arterial wall as well as the plaque.113,114 Interestingly, no study has been conducted until now that uses knockout of a certain ECM gene exclusively in SMCs to prove that this affects disease progression by de-stabilizing atherosclerotic lesions.115
Regarding miRNA involvement into ECM production and release mechanism, it was shown that miR-29b and miR-195 are targeting several pro-fibrotic components (collagen isoforms) and mediators of inflammation,116–118 and that their inhibition can limit aneurysm progression, as well as stabilize experimental atherosclerotic lesions.119 miR-29b is again part of a cluster, together with miR-29a and miR-29c. Interestingly, miR-29b appears lower expressed in SMCs compared with the other two, underlining differences in miRNA processing between the cluster members in SMC-related cell-fate decisions. In this study by Bretschneider et al.,120 aldosterone was labelled as a distinct regulator being capable of adjusting miR-29b expression levels.
Accumulation of hyaluronan into the matrix of arteries increases wall thickening, and thus contributes to atherogenesis and vascular disease progression.121 The natural antisense transcript (NAT) hyaluronan synthase 2 gene (HAS2)-AS1 is transcribed opposite to the HAS2, and appears generally required to induce the transcription of the protein-coding gene, and its depository and remodelling effects in the vascular matrix.122
5. SMC apoptosis
Cell death of any kind plays a crucial role in vascular disease development.123 Aortic aneurysms continue to expand rapidly if SMC survival is augmented,86 and the importance of apoptosis in advanced atherosclerotic lesions has been indicated in numerous studies.82,83,124,125 In the setting of atherosclerosis, the level of apoptosis seems low in early lesions (Stary126 Grades I–III), but substantially increases as lesions progress. Apoptosis appears predominantly in SMCs, but can also be detected in macrophages.127 However, in general all cellular subtypes of the vasculature can potentially undergo apoptosis. As with other mechanisms driving SMC functionality and fate; interpretation of the apoptosis effect in actual human patients is limited due to the lack of specific markers.
Although apoptosis is seen in all kinds of different vascular diseases, conclusions about the absolute rates of cell death are almost impossible to be estimated, as our understanding of the initiation and duration of the process in vivo is limited.115,128 Chronic SMC apoptosis accelerates vascular disease progression (in particular aneurysm disease as well as atherosclerosis-related pathologies), promotes calcification, and induces features of medial degeneration, like atrophy, elastin fragmentation, as well as enhanced glycosaminoglycan deposition.129 Cystic medial degeneration, which becomes visible for example in Erdheim-Chester disease, Marfan syndrome, and to a lesser extent in normal aging, are good examples of SMC loss being connected with apoptosis.130–132
A recent study from our lab by Eken et al.125 has shown that miR-210 can stabilize fibrous caps of advanced atherosclerotic lesions by blocking SMC apoptosis via inhibition of the tumour suppressor Adenomatous polyposis coli (APC) and canonical Wnt-pathway signalling. p53 was identified as a negative upstream regulator of miR-210.
Another molecular process involving p53-mediated apoptosis signalling in atherosclerotic lesions relates to lincRNA-p21, which was recently identified as a crucial mediator of SMC and macrophage survival.133 lincRNA-p21 is decreased in lesions of ApoE-deficient mice and plaque specimens from individuals suffering from coronary artery disease. Inhibition of lincRNA-p21 enhanced neointimal hyperplasia in a murine model of carotid artery injury. The overall effect of this lncRNA could be linked to p53:p300 interactions, allowing these factors to bind to their promoters and enhancers in apoptotic signalling pathways (Figure 3). In addition, p53 and lincRNA-p21 form a feedback loop, in which p53 regulates the transcriptional activity of the lncRNA.134
The NAT to the transcription factor HIF1α (HIF1α-AS1) has been discovered to be elevated in circulation of patients with aortic aneurysms.135 siRNA-guided inhibition of this NAT in aortic SMCs limited the apoptotic response as indicated by different markers of cell death (e.g. Caspases 3 and 8).45
Another study linking HIF1α-AS1 to thoracic aneurysms was able to identify Brahma-related gene 1 (BRG1) as a potential target. Overexpression of BRG1 in human aortic SMCs could increase apoptosis, while inhibition of HIF1α-AS1 not only reduced BRG1 levels, but also promoted cellular proliferation.136 The direct molecular mechanism behind this regulation remains to be determined.
The lncRNA MEG3 is another recently unravelled transcript with important implications in vascular disease. Boon et al.137 have shown that MEG3 regulates endothelial aging while mediating angiogenesis. For SMC plasticity, MEG3 seems of potential interest, as it has been shown to be under the control of dNK-derived interferon γ (IFN-γ), negatively affecting SMC survival and migration in uterine spiral arteries during vascular transformation.138
6. Conclusion and perspectives
The emerging links between ncRNAs and diseases have opened up a new field of therapeutic and diagnostic opportunities. Many miRNAs have already successfully been shown to serve as biomarkers or therapeutic targets for many different pathologies. There is also evidence that the same holds true for lncRNAs (and maybe circRNAs). It is evident that RNA molecules exhibit many more functions beyond their classic role as templates for protein synthesis. Considering the ability of RNA to form 3D structures and interact with DNA, proteins, and other RNA molecules, non-coding transcripts are assumed to be as versatile as proteins, allowing them to mediate all major cellular processes. The classical view on SMCs and how they are involved in vascular disease development and progression has been challenged in recent times. Many studies in the field have moved SMCs, with their plastic and dynamic features, into the centre of attention. Potential new therapies should be considered for being directed towards manipulating SMC fate decisions, enabling us to limit the burden of SMC apoptosis and transformation into macrophage-like cells with enhanced pro-inflammatory activity. Strategies that modulate the expression of ncRNAs, like miRNAs and lncRNAs, could play an important role in this regard, as they have been shown to be master regulators of SMC plasticity in vascular disease evolvement.
Acknowledgements
We apologize to all researchers whose work could not be cited due to space limitations.
Conflict of interest: none declared.
Funding
Research on ncRNAs in vascular diseases in Munich and Stockholm are supported by the European Research Council (ERC Starting Grant NORVAS), a German Center for Cardiovascular Research (DZHK) junior research group (JRG_LM_MRI), the Heisenberg Programme of the German Research Council (DFG, MA4688/2-1), the Ragnar Söderberg Foundation (M55/14), the Swedish Research Council/Vetenkapsrådet (2015-03140), and the Swedish Heart-Lungfoundation (20140186, 20160732). Research in Nick Leeper’s laboratory at Stanford University is supported by the National Institutes of Health via R01 HL12337002.
References
- 1. Consortium EP. An integrated encyclopedia of DNA elements in the human genome. Nature 2012;489:57–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beermann J, Piccoli MT, Viereck J, Thum T.. Non-coding RNAs in development and disease: background, mechanisms, and therapeutic approaches. Physiol Rev 2016;96:1297–1325. [DOI] [PubMed] [Google Scholar]
- 3. Rinn JL, Chang HY.. Genome regulation by long noncoding RNAs. Annu Rev Biochem 2012;81:145–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rupaimoole R, Slack FJ.. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov 2017;16:203–222. [DOI] [PubMed] [Google Scholar]
- 5. Maegdefessel L. The emerging role of microRNAs in cardiovascular disease. J Intern Med 2014;276:633–644. [DOI] [PubMed] [Google Scholar]
- 6. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 2004;116:281–297. [DOI] [PubMed] [Google Scholar]
- 7. Guttman M, Rinn JL.. Modular regulatory principles of large non-coding RNAs. Nature 2012;482:339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pang KC, Frith MC, Mattick JS.. Rapid evolution of noncoding RNAs: lack of conservation does not mean lack of function. Trends Genet 2006;22:1–5. [DOI] [PubMed] [Google Scholar]
- 9. Boon RA, Jae N, Holdt L, Dimmeler S.. Long noncoding RNAs: from clinical genetics to therapeutic targets? J Am Coll Cardiol 2016;67:1214–1226. [DOI] [PubMed] [Google Scholar]
- 10. Wapinski O, Chang HY.. Long noncoding RNAs and human disease. Trends Cell Biol 2011;21:354–361. [DOI] [PubMed] [Google Scholar]
- 11. Coll-Bonfill N, de la Cruz-Thea B, Pisano MV, Musri MM.. Noncoding RNAs in smooth muscle cell homeostasis: implications in phenotypic switch and vascular disorders. Pflugers Arch 2016;468:1071–1087. [DOI] [PubMed] [Google Scholar]
- 12. Mercer TR, Dinger ME, Mattick JS.. Long non-coding RNAs: insights into functions. Nat Rev Genet 2009;10:155–159. [DOI] [PubMed] [Google Scholar]
- 13. Wilusz JE, Sunwoo H, Spector DL.. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev 2009;23:1494–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Anderson DM, Anderson KM, Chang CL, Makarewich CA, Nelson BR, McAnally JR, Kasaragod P, Shelton JM, Liou J, Bassel-Duby R, Olson EN.. A micropeptide encoded by a putative long noncoding RNA regulates muscle performance. Cell 2015;160:595–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Greene J, Baird AM, Brady L, Lim M, Gray SG, McDermott R, Finn SP.. Circular RNAs: biogenesis, function and role in human diseases. Front Mol Biosci 2017;4:38.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, Loewer A, Ziebold U, Landthaler M, Kocks C, Le Noble F, Rajewsky N.. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013;495:333–338. [DOI] [PubMed] [Google Scholar]
- 17. Chen LL. The biogenesis and emerging roles of circular RNAs. Nat Rev Mol Cell Biol 2016;17:205–211. [DOI] [PubMed] [Google Scholar]
- 18. Devaux Y, Creemers EE, Boon RA, Werfel S, Thum T, Engelhardt S, Dimmeler S, Squire I; Cardiolinc Network. Circular RNAs in heart failure. Eur J Heart Fail 2017;19:701–709. [DOI] [PubMed] [Google Scholar]
- 19. Alexander MR, Owens GK.. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol 2012;74:13–40. [DOI] [PubMed] [Google Scholar]
- 20. Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, Hannon GJ.. Dicer is essential for mouse development. Nat Genet 2003;35:215–217. [DOI] [PubMed] [Google Scholar]
- 21. Yang WJ, Yang DD, Na S, Sandusky GE, Zhang Q, Zhao G.. Dicer is required for embryonic angiogenesis during mouse development. J Biol Chem 2005;280:9330–9335. [DOI] [PubMed] [Google Scholar]
- 22. Jaskiewicz L, Filipowicz W.. Role of Dicer in posttranscriptional RNA silencing. Curr Top Microbiol Immunol 2008;320:77–97. [DOI] [PubMed] [Google Scholar]
- 23. Albinsson S, Suarez Y, Skoura A, Offermanns S, Miano JM, Sessa WC.. MicroRNAs are necessary for vascular smooth muscle growth, differentiation, and function. Arterioscler Thromb Vasc Biol 2010;30:1118–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pan Y, Balazs L, Tigyi G, Yue J.. Conditional deletion of Dicer in vascular smooth muscle cells leads to the developmental delay and embryonic mortality. Biochem Biophys Res Commun 2011;408:369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zahedi F, Nazari-Jahantigh M, Zhou Z, Subramanian P, Wei Y, Grommes J, Offermanns S, Steffens S, Weber C, Schober A.. Dicer generates a regulatory microRNA network in smooth muscle cells that limits neointima formation during vascular repair. Cell Mol Life Sci 2017;74:359–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Albinsson S, Skoura A, Yu J, DiLorenzo A, Fernández-Hernando C, Offermanns S, Miano JM, Sessa WC, Ruhrberg C.. Smooth muscle miRNAs are critical for post-natal regulation of blood pressure and vascular function. PLoS One 2011;6:e18869.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shankman LS, Gomez D, Cherepanova OA, Salmon M, Alencar GF, Haskins RM, Swiatlowska P, Newman AA, Greene ES, Straub AC, Isakson B, Randolph GJ, Owens GK.. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med 2015;21:628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Deaton RA, Gan Q, Owens GK.. Sp1-dependent activation of KLF4 is required for PDGF-BB-induced phenotypic modulation of smooth muscle. Am J Physiol Heart Circ Physiol 2009;296:H1027–H1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yoshida T, Gan Q, Shang Y, Owens GK.. Platelet-derived growth factor-BB represses smooth muscle cell marker genes via changes in binding of MKL factors and histone deacetylases to their promoters. Am J Physiol Cell Physiol 2007;292:C886–C895. [DOI] [PubMed] [Google Scholar]
- 30. Pidkovka NA, Cherepanova OA, Yoshida T, Alexander MR, Deaton RA, Thomas JA, Leitinger N, Owens GK.. Oxidized phospholipids induce phenotypic switching of vascular smooth muscle cells in vivo and in vitro. Circ Res 2007;101:792–801. [DOI] [PubMed] [Google Scholar]
- 31. Yoshida T, Gan Q, Owens GK.. Kruppel-like factor 4, Elk-1, and histone deacetylases cooperatively suppress smooth muscle cell differentiation markers in response to oxidized phospholipids. Am J Physiol Cell Physiol 2008;295:C1175–C1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alexander MR, Murgai M, Moehle CW, Owens GK.. Interleukin-1beta modulates smooth muscle cell phenotype to a distinct inflammatory state relative to PDGF-DD via NF-kappaB-dependent mechanisms. Physiol Genomics 2012;44:417–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Salmon M, Gomez D, Greene E, Shankman L, Owens GK.. Cooperative binding of KLF4, pELK-1, and HDAC2 to a G/C repressor element in the SM22alpha promoter mediates transcriptional silencing during SMC phenotypic switching in vivo. Circ Res 2012;11:685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jiang Y, Yin H, Zheng XL.. MicroRNA-1 inhibits myocardin-induced contractility of human vascular smooth muscle cells. J Cell Physiol 2010;225:506–511. [DOI] [PubMed] [Google Scholar]
- 35. Talasila A, Yu H, Ackers-Johnson M, Bot M, van Berkel T, Bennett MR, Bot I, Sinha S.. Myocardin regulates vascular response to injury through miR-24/-29a and platelet-derived growth factor receptor-beta. Arterioscler Thromb Vasc Biol 2013;33:2355–2365. [DOI] [PubMed] [Google Scholar]
- 36. Chan MC, Hilyard AC, Wu C, Davis BN, Hill NS, Lal A, Lieberman J, Lagna G, Hata A.. Molecular basis for antagonism between PDGF and the TGFbeta family of signalling pathways by control of miR-24 expression. Embo J 2010;29:559–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chan MC, Nguyen PH, Davis BN, Ohoka N, Hayashi H, Du K, Lagna G, Hata A.. A novel regulatory mechanism of the bone morphogenetic protein (BMP) signaling pathway involving the carboxyl-terminal tail domain of BMP type II receptor. Mol Cell Biol 2007;27:5776–5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yoshida T, Kaestner KH, Owens GK.. Conditional deletion of Kruppel-like factor 4 delays downregulation of smooth muscle cell differentiation markers but accelerates neointimal formation following vascular injury. Circ Res 2008;102:1548–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bennett MR, Sinha S, Owens GK.. Vascular Smooth Muscle Cells in Atherosclerosis. Circ Res 2016;118:692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Libby P, Ridker PM, Hansson GK.. Progress and challenges in translating the biology of atherosclerosis. Nature 2011;473:317–325. [DOI] [PubMed] [Google Scholar]
- 41. Ho-Tin-Noe B, Vo S, Bayles R, Ferriere S, Ladjal H, Toumi S, Deschildre C, Ollivier V, Michel JB.. Cholesterol crystallization in human atherosclerosis is triggered in smooth muscle cells during the transition from fatty streak to fibroatheroma. J Pathol 2017;241:671–682. [DOI] [PubMed] [Google Scholar]
- 42. Xie C, Huang H, Sun X, Guo Y, Hamblin M, Ritchie RP, Garcia-Barrio MT, Zhang J, Chen YE.. MicroRNA-1 regulates smooth muscle cell differentiation by repressing Kruppel-like factor 4. Stem Cells Dev 2011;20:205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Heidersbach A, Saxby C, Carver-Moore K, Huang Y, Ang YS, de Jong PJ, Ivey KN, Srivastava D.. microRNA-1 regulates sarcomere formation and suppresses smooth muscle gene expression in the mammalian heart. Elife 2013;2:e01323.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rao PK, Kumar RM, Farkhondeh M, Baskerville S, Lodish HF.. Myogenic factors that regulate expression of muscle-specific microRNAs. Proc Natl Acad Sci U S A 2006;103:8721–8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhao Y, Samal E, Srivastava D.. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature 2005;436:214–220. [DOI] [PubMed] [Google Scholar]
- 46. Liu N, Bezprozvannaya S, Williams AH, Qi X, Richardson JA, Bassel-Duby R, Olson EN.. microRNA-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart. Genes Dev 2008;22:3242–3254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liao XB, Zhang ZY, Yuan K, Liu Y, Feng X, Cui RR, Hu YR, Yuan ZS, Gu L, Li SJ, Mao DA, Lu Q, Zhou XM, de Jesus Perez VA, Yuan LQ.. MiR-133a modulates osteogenic differentiation of vascular smooth muscle cells. Endocrinology 2013;154:3344–3352. [DOI] [PubMed] [Google Scholar]
- 48. Elia L, Quintavalle M, Zhang J, Contu R, Cossu L, Latronico MV, Peterson KL, Indolfi C, Catalucci D, Chen J, Courtneidge SA, Condorelli G.. The knockout of miR-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: correlates with human disease. Cell Death Differ 2009;16:1590–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, Lee TH, Miano JM, Ivey KN, Srivastava D.. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 2009;460:705–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Long X, Miano JM.. Transforming growth factor-beta1 (TGF-beta1) utilizes distinct pathways for the transcriptional activation of microRNA 143/145 in human coronary artery smooth muscle cells. J Biol Chem 2011;286:30119–30129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Boucher JM, Peterson SM, Urs S, Zhang C, Liaw L.. The miR-143/145 cluster is a novel transcriptional target of Jagged-1/Notch signaling in vascular smooth muscle cells. J Biol Chem 2011;286:28312–28321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Boettger T, Beetz N, Kostin S, Schneider J, Kruger M, Hein L, Braun T.. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Invest 2009;119:2634–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cheng Y, Liu X, Yang J, Lin Y, Xu DZ, Lu Q, Deitch EA, Huo Y, Delphin ES, Zhang C.. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res 2009;105:158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Caruso P, Dempsie Y, Stevens HC, McDonald RA, Long L, Lu R, White K, Mair KM, McClure JD, Southwood M, Upton P, Xin M, van Rooij E, Olson EN, Morrell NW, MacLean MR, Baker AH.. A role for miR-145 in pulmonary arterial hypertension: evidence from mouse models and patient samples. Circ Res 2012;111:290–300. [DOI] [PubMed] [Google Scholar]
- 55. Climent M, Quintavalle M, Miragoli M, Chen J, Condorelli G, Elia L.. TGFbeta triggers miR-143/145 transfer from smooth muscle cells to endothelial cells, thereby modulating vessel stabilization. Circ Res 2015;116:1753–1764. [DOI] [PubMed] [Google Scholar]
- 56. Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, Braun T, Urbich C, Boon RA, Dimmeler S.. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol 2012;14:249–256. [DOI] [PubMed] [Google Scholar]
- 57. Sun SG, Zheng B, Han M, Fang XM, Li HX, Miao SB, Su M, Han Y, Shi HJ, Wen JK.. miR-146a and Kruppel-like factor 4 form a feedback loop to participate in vascular smooth muscle cell proliferation. EMBO Rep 2011;12:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Danielson LS, Menendez S, Attolini CS, Guijarro MV, Bisogna M, Wei J, Socci ND, Levine DA, Michor F, Hernando E.. A differentiation-based microRNA signature identifies leiomyosarcoma as a mesenchymal stem cell-related malignancy. Am J Pathol 2010;177:908–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zheng L, Xu CC, Chen WD, Shen WL, Ruan CC, Zhu LM, Zhu DL, Gao PJ.. MicroRNA-155 regulates angiotensin II type 1 receptor expression and phenotypic differentiation in vascular adventitial fibroblasts. Biochem Biophys Res Commun 2010;400:483–488. [DOI] [PubMed] [Google Scholar]
- 60. Nazari-Jahantigh M, Wei Y, Noels H, Akhtar S, Zhou Z, Koenen RR, Heyll K, Gremse F, Kiessling F, Grommes J, Weber C, Schober A.. MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. J Clin Invest 2012;122:4190–4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Brannan CI, Dees EC, Ingram RS, Tilghman SM.. The product of the H19 gene may function as an RNA. Mol Cell Biol 1990;10:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pachnis V, Belayew A, Tilghman SM.. Locus unlinked to alpha-fetoprotein under the control of the murine raf and Rif genes. Proc Natl Acad Sci U S A 1984;81:5523–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Matouk IJ, Halle D, Raveh E, Gilon M, Sorin V, Hochberg A.. The role of the oncofetal H19 lncRNA in tumor metastasis: orchestrating the EMT-MET decision. Oncotarget 2016;7:3748–3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Raveh E, Matouk IJ, Gilon M, Hochberg A.. The H19 Long non-coding RNA in cancer initiation, progression and metastasis - a proposed unifying theory. Mol Cancer 2015;14:184.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Keniry A, Oxley D, Monnier P, Kyba M, Dandolo L, Smits G, Reik W.. The H19 lincRNA is a developmental reservoir of miR-675 that suppresses growth and Igf1r. Nat Cell Biol 2012;14:659–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Vennin C, Spruyt N, Dahmani F, Julien S, Bertucci F, Finetti P, Chassat T, Bourette RP, Le Bourhis X, Adriaenssens E.. H19 non coding RNA-derived miR-675 enhances tumorigenesis and metastasis of breast cancer cells by downregulating c-Cbl and Cbl-b. Oncotarget 2015;6:29209–29223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kallen AN, Zhou XB, Xu J, Qiao C, Ma J, Yan L, Lu L, Liu C, Yi JS, Zhang H, Min W, Bennett AM, Gregory RI, Ding Y, Huang Y.. The imprinted H19 lncRNA antagonizes let-7 microRNAs. Mol Cell 2013;52:101–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Imig J, Brunschweiger A, Brummer A, Guennewig B, Mittal N, Kishore S, Tsikrika P, Gerber AP, Zavolan M, Hall J.. miR-CLIP capture of a miRNA targetome uncovers a lincRNA H19-miR-106a interaction. Nat Chem Biol 2014;11:107–114. [DOI] [PubMed] [Google Scholar]
- 69. Liang WC, Fu WM, Wong CW, Wang Y, Wang WM, Hu GX, Zhang L, Xiao LJ, Wan DC, Zhang JF, Waye MM.. The lncRNA H19 promotes epithelial to mesenchymal transition by functioning as miRNA sponges in colorectal cancer. Oncotarget 2015;6:22513–22525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zou T, Jaladanki SK, Liu L, Xiao L, Chung HK, Wang JY, Xu Y, Gorospe M, Wang JY.. H19 Long Noncoding RNA Regulates Intestinal Epithelial Barrier Function via MicroRNA 675 by Interacting with RNA-Binding Protein HuR. Mol Cell Biol 2016;36:1332–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Giovarelli M, Bucci G, Ramos A, Bordo D, Wilusz CJ, Chen CY, Puppo M, Briata P, Gherzi R.. H19 long noncoding RNA controls the mRNA decay promoting function of KSRP. Proc Natl Acad Sci U S A 2014;111:E5023–E5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Cao H, Hu X, Zhang Q, Wang J, Li J, Liu B, Shao Y, Li X, Zhang J, Xin S.. Upregulation of let-7a inhibits vascular smooth muscle cell proliferation in vitro and in vein graft intimal hyperplasia in rats. J Surg Res 2014;192:223–233. [DOI] [PubMed] [Google Scholar]
- 73. Han Y, Xu H, Cheng J, Zhang Y, Gao C, Fan T, Peng B, Li B, Liu L, Cheng Z.. Downregulation of long non-coding RNA H19 promotes P19CL6 cells proliferation and inhibits apoptosis during late-stage cardiac differentiation via miR-19b-modulated Sox6. Cell Biosci 2016;6:58.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Voellenkle C, Garcia-Manteiga JM, Pedrotti S, Perfetti A, De Toma I, Da Silva D, Maimone B, Greco S, Fasanaro P, Creo P, Zaccagnini G, Gaetano C, Martelli F.. Implication of Long noncoding RNAs in the endothelial cell response to hypoxia revealed by RNA-sequencing. Sci Rep 2016;6:24141.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lv J, Wang L, Zhang J, Lin R, Wang L, Sun W, Wu H, Xin S.. Long noncoding RNA H19-derived miR-675 aggravates restenosis by targeting PTEN. Biochem Biophys Res Commun 2017; [DOI] [PubMed] [Google Scholar]
- 76. Hadji F, Boulanger M-C, Guay S-P, Gaudreault N, Amellah S, Mkannez G, Bouchareb R, Marchand JT, Nsaibia MJ, Guauque-Olarte S, Pibarot P, Bouchard L, Bossé Y, Mathieu P.. Altered DNA methylation of long noncoding RNA H19 in calcific aortic valve disease promotes mineralization by silencing NOTCH1. Circulation 2016;134:1848–1862. [DOI] [PubMed] [Google Scholar]
- 77. Bell RD, Long X, Lin M, Bergmann JH, Nanda V, Cowan SL, Zhou Q, Han Y, Spector DL, Zheng D, Miano JM.. Identification and initial functional characterization of a human vascular cell-enriched long noncoding RNA. Arterioscler Thromb Vasc Biol 2014;34:1249–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Boulberdaa M, Scott E, Ballantyne M, Garcia R, Descamps B, Angelini GD, Brittan M, Hunter A, McBride M, McClure J, Miano JM, Emanueli C, Mills NL, Mountford JC, Baker AH.. A role for the long noncoding RNA SENCR in commitment and function of endothelial cells. Mol Ther 2016;24:978–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zhao J, Zhang W, Lin M, Wu W, Jiang P, Tou E, Xue M, Richards A, Jourd’heuil D, Asif A, Zheng D, Singer HA, Miano JM, Long X.. Long X. MYOSLID is a novel serum response factor-dependent long noncoding RNA that amplifies the vascular smooth muscle differentiation program. Arterioscler Thromb Vasc Biol 2016;36:2088–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Maegdefessel L, Rayner KJ, Leeper NJ.. MicroRNA regulation of vascular smooth muscle function and phenotype: early career committee contribution. Arterioscler Thromb Vasc Biol 2015;35:2–6. [DOI] [PubMed] [Google Scholar]
- 81. Gomez D, Owens GK.. Reconciling smooth muscle cell oligoclonality and proliferative capacity in experimental atherosclerosis. Circ Res 2016;119:1262–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lutgens E, de Muinck ED, Kitslaar PJ, Tordoir JH, Wellens HJ, Daemen MJ.. Biphasic pattern of cell turnover characterizes the progression from fatty streaks to ruptured human atherosclerotic plaques. Cardiovasc Res 1999;41:473–479. [DOI] [PubMed] [Google Scholar]
- 83. Bennett MR, Evan GI, Schwartz SM.. Apoptosis of human vascular smooth muscle cells derived from normal vessels and coronary atherosclerotic plaques. J Clin Invest 1995;95:2266–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. O'Brien ER, Alpers CE, Stewart DK, Ferguson M, Tran N, Gordon D, Benditt EP, Hinohara T, Simpson JB, Schwartz SM.. Proliferation in primary and restenotic coronary atherectomy tissue. Implications for antiproliferative therapy. Circ Res 1993;73:223–231. [DOI] [PubMed] [Google Scholar]
- 85. Chen J, Yin H, Jiang Y, Radhakrishnan SK, Huang ZP, Li J, Shi Z, Kilsdonk EP, Gui Y, Wang DZ, Zheng XL.. Induction of microRNA-1 by myocardin in smooth muscle cells inhibits cell proliferation. Arterioscler Thromb Vasc Biol 2011;31:368–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Maegdefessel L, Azuma J, Toh R, Deng A, Merk DR, Raiesdana A, Leeper NJ, Raaz U, Schoelmerich AM, McConnell MV, Dalman RL, Spin JM, Tsao PS.. MicroRNA-21 blocks abdominal aortic aneurysm development and nicotine-augmented expansion. Sci Transl Med 2012;4:122ra122.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ji R, Cheng Y, Yue J, Yang J, Liu X, Chen H, Dean DB, Zhang C.. MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ Res 2007;100:1579–1588. [DOI] [PubMed] [Google Scholar]
- 88. McDonald RA, Halliday CA, Miller AM, Diver LA, Dakin RS, Montgomery J, McBride MW, Kennedy S, McClure JD, Robertson KE, Douglas G, Channon KM, Oldroyd KG, Baker AH.. Reducing in-stent restenosis: therapeutic manipulation of miRNA in vascular remodeling and inflammation. J Am Coll Cardiol 2015;65:2314–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wang D, Deuse T, Stubbendorff M, Chernogubova E, Erben RG, Eken SM, Jin H, Li Y, Busch A, Heeger CH, Behnisch B, Reichenspurner H, Robbins RC, Spin JM, Tsao PS, Schrepfer S, Maegdefessel L.. Local microRNA modulation using a novel anti-miR-21-eluting stent effectively prevents experimental in-stent restenosis. Arterioscler Thromb Vasc Biol 2015;35:1945–1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Davis BN, Hilyard AC, Lagna G, Hata A.. SMAD proteins control DROSHA-mediated microRNA maturation. Nature 2008;454:56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Maegdefessel L, Spin JM, Raaz U, Eken SM, Toh R, Azuma J, Adam M, Nagakami F, Heymann HM, Chernugobova E, Jin H, Roy J, Hultgren R, Caidahl K, Schrepfer S, Hamsten A, Eriksson P, McConnell MV, Dalman RL, Tsao PS.. miR-24 limits aortic vascular inflammation and murine abdominal aneurysm development. Nat Commun 2014;5:5214.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Iaconetti C, De Rosa S, Polimeni A, Sorrentino S, Gareri C, Carino A, Sabatino J, Colangelo M, Curcio A, Indolfi C.. Down-regulation of miR-23b induces phenotypic switching of vascular smooth muscle cells in vitro and in vivo. Cardiovasc Res 2015;107:522–533. [DOI] [PubMed] [Google Scholar]
- 93. Leeper NJ, Raiesdana A, Kojima Y, Chun HJ, Azuma J, Maegdefessel L, Kundu RK, Quertermous T, Tsao PS, Spin JM.. MicroRNA-26a is a novel regulator of vascular smooth muscle cell function. J Cell Physiol 2011;226:1035–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lin X, He Y, Hou X, Zhang Z, Wang R, Wu Q, Ro S.. Endothelial cells can regulate smooth muscle cells in contractile phenotype through the miR-206/ARF6&NCX1/exosome axis. PLoS One 2016;11:e0152959.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Qiao W, Chen L, Zhang M.. MicroRNA-205 regulates the calcification and osteoblastic differentiation of vascular smooth muscle cells. Cell Physiol Biochem 2014;33:1945–1953. [DOI] [PubMed] [Google Scholar]
- 96. Yu X, Zhang L, Wen G, Zhao H, Luong LA, Chen Q, Huang Y, Zhu J, Ye S, Xu Q, Wang W, Xiao Q.. Upregulated sirtuin 1 by miRNA-34a is required for smooth muscle cell differentiation from pluripotent stem cells. Cell Death Differ 2015;22:1170–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chen Q, Yang F, Guo M, Wen G, Zhang C, Luong Le A, Zhu J, Xiao Q, Zhang L.. miRNA-34a reduces neointima formation through inhibiting smooth muscle cell proliferation and migration. J Mol Cell Cardiol 2015;89:75–86. [DOI] [PubMed] [Google Scholar]
- 98. Xu J, Li L, Yun HF, Han YS.. MiR-138 promotes smooth muscle cells proliferation and migration in db/db mice through down-regulation of SIRT1. Biochem Biophys Res Commun 2015;463:1159–1164. [DOI] [PubMed] [Google Scholar]
- 99. Wu WH, Hu CP, Chen XP, Zhang WF, Li XW, Xiong XM, Li YJ.. MicroRNA-130a mediates proliferation of vascular smooth muscle cells in hypertension. Am J Hypertens 2011;24:1087–1093. [DOI] [PubMed] [Google Scholar]
- 100. Courboulin A, Paulin R, Giguere NJ, Saksouk N, Perreault T, Meloche J, Paquet ER, Biardel S, Provencher S, Cote J, Simard MJ, Bonnet S.. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med 2011;208:535–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Davis BN, Hilyard AC, Nguyen PH, Lagna G, Hata A.. Induction of microRNA-221 by platelet-derived growth factor signaling is critical for modulation of vascular smooth muscle phenotype. J Biol Chem 2009;284:3728–3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Liu X, Cheng Y, Zhang S, Lin Y, Yang J, Zhang C.. A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res 2009;104:476–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Merlet E, Atassi F, Motiani RK, Mougenot N, Jacquet A, Nadaud S, Capiod T, Trebak M, Lompre AM, Marchand A.. miR-424/322 regulates vascular smooth muscle cell phenotype and neointimal formation in the rat. Cardiovasc Res 2013;98:458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Li P, Zhu N, Yi B, Wang N, Chen M, You X, Zhao X, Solomides CC, Qin Y, Sun J.. MicroRNA-663 regulates human vascular smooth muscle cell phenotypic switch and vascular neointimal formation. Circ Res 2013;113:1117–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Leung A, Trac C, Jin W, Lanting L, Akbany A, Sætrom P, Schones DE, Natarajan R.. Novel long noncoding RNAs are regulated by angiotensin II in vascular smooth muscle cells. Circ Res 2013;113:266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Li X, Jiang XY, Ge J, Wang J, Chen GJ, Xu L, Xie DY, Yuan TY, Zhang DS, Zhang H, Chen YH.. Aberrantly expressed lncRNAs in primary varicose great saphenous veins. PLoS One 2014;9:e86156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Li L, Li X, The E, Wang L-J, Yuan T-Y, Wang S-Y, Feng J, Wang J, Liu Y, Wu Y-H, Ma X-E, Ge J, Cui Y-Y, Jiang X-Y, Wu Q.. Low expression of lncRNA-GAS5 is implicated in human primary varicose great saphenous veins. PLoS One 2015;10:e0120550.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Yao J, Wang X-Q, Li Y-J, Shan K, Yang H, Wang Y-N-Z, Yao M-D, Liu C, Li X-M, Shen Y, Liu J-Y, Cheng H, Yuan J, Zhang Y-Y, Jiang Q, Yan B.. Role of long non-coding RNA-RNCR3 in atherosclerosis-related vascular dysfunction. Cell Death Dis 2016;8:e2248.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Pasmant E, Sabbagh A, Vidaud M, Bieche I.. ANRIL, a long, noncoding RNA, is an unexpected major hotspot in GWAS. Faseb J 2011;25:444–448. [DOI] [PubMed] [Google Scholar]
- 110. Popov N, Gil J.. Epigenetic regulation of the INK4b-ARF-INK4a locus: in sickness and in health. Epigenetics 2010;5:685–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Motterle A, Pu X, Wood H, Xiao Q, Gor S, Ng FL, Chan K, Cross F, Shohreh B, Poston RN, Tucker AT, Caulfield MJ, Ye S.. Functional analyses of coronary artery disease associated variation on chromosome 9p21 in vascular smooth muscle cells. Hum Mol Genet 2012;21:4021–4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Holdt LM, Hoffmann S, Sass K, Langenberger D, Scholz M, Krohn K, Finstermeier K, Stahringer A, Wilfert W, Beutner F, Gielen S, Schuler G, Gäbel G, Bergert H, Bechmann I, Stadler PF, Thiery J, Teupser D, McCarthy MI.. Alu elements in ANRIL non-coding RNA at chromosome 9p21 modulate atherogenic cell functions through trans-regulation of gene networks. PLoS Genet 2013;9:e1003588.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Koyama H, Raines EW, Bornfeldt KE, Roberts JM, Ross R.. Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of Cdk2 inhibitors. Cell 1996;87:1069–1078. [DOI] [PubMed] [Google Scholar]
- 114. Li DY, Brooke B, Davis EC, Mecham RP, Sorensen LK, Boak BB, Eichwald E, Keating MT.. Elastin is an essential determinant of arterial morphogenesis. Nature 1998;393:276–280. [DOI] [PubMed] [Google Scholar]
- 115. Bennett MR. Life and death in the atherosclerotic plaque. Curr Opin Lipidol 2010;21:422–426. [DOI] [PubMed] [Google Scholar]
- 116. Boon RA, Seeger T, Heydt S, Fischer A, Hergenreider E, Horrevoets AJ, Vinciguerra M, Rosenthal N, Sciacca S, Pilato M, van Heijningen P, Essers J, Brandes RP, Zeiher AM, Dimmeler S.. MicroRNA-29 in aortic dilation: implications for aneurysm formation. Circ Res 2011;109:1115–1119. [DOI] [PubMed] [Google Scholar]
- 117. Maegdefessel L, Azuma J, Toh R, Merk DR, Deng A, Chin JT, Raaz U, Schoelmerich AM, Raiesdana A, Leeper NJ, McConnell MV, Dalman RL, Spin JM, Tsao PS.. Inhibition of microRNA-29b reduces murine abdominal aortic aneurysm development. J Clin Invest 2012;122:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Zampetaki A, Attia R, Mayr U, Gomes RS, Phinikaridou A, Yin X, Langley SR, Willeit P, Lu R, Fanshawe B, Fava M, Barallobre-Barreiro J, Molenaar C, So PW, Abbas A, Jahangiri M, Waltham M, Botnar R, Smith A, Mayr M.. Role of miR-195 in aortic aneurysmal disease. Circ Res 2014;115:857–866. [DOI] [PubMed] [Google Scholar]
- 119. Ulrich V, Rotllan N, Araldi E, Luciano A, Skroblin P, Abonnenc M, Perrotta P, Yin X, Bauer A, Leslie KL, Zhang P, Aryal B, Montgomery RL, Thum T, Martin K, Suarez Y, Mayr M, Fernandez-Hernando C, Sessa WC.. Chronic miR-29 antagonism promotes favorable plaque remodeling in atherosclerotic mice. EMBO Mol Med 2016;8:643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Bretschneider M, Busch B, Mueller D, Nolze A, Schreier B, Gekle M, Grossmann C.. Activated mineralocorticoid receptor regulates micro-RNA-29b in vascular smooth muscle cells. Faseb J 2016;30:1610–1622. [DOI] [PubMed] [Google Scholar]
- 121. Viola M, Karousou E, D’angelo ML, Moretto P, Caon I, Luca G, Passi A, Vigetti D.. Extracellular matrix in atherosclerosis: hyaluronan and proteoglycans insights. Curr Med Chem 2016;23:2958–2971. [DOI] [PubMed] [Google Scholar]
- 122. Vigetti D, Deleonibus S, Moretto P, Bowen T, Fischer JW, Grandoch M, Oberhuber A, Love DC, Hanover JA, Cinquetti R, Karousou E, Viola M, D’angelo ML, Hascall VC, De Luca G, Passi A.. Natural antisense transcript for hyaluronan synthase 2 (HAS2-AS1) induces transcription of HAS2 via protein O-GlcNAcylation. J Biol Chem 2014;289:28816–28826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Rayner KJ. Cell death in the vessel wall: the good, the bad, the ugly. Arterioscler Thromb Vasc Biol 2017;37:e75–e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Patel VA, Zhang QJ, Siddle K, Soos MA, Goddard M, Weissberg PL, Bennett MR.. Defect in insulin-like growth factor-1 survival mechanism in atherosclerotic plaque-derived vascular smooth muscle cells is mediated by reduced surface binding and signaling. Circ Res 2001;88:895–902. [DOI] [PubMed] [Google Scholar]
- 125. Eken SM, Jin H, Chernogubova E, Li Y, Simon N, Sun C, Korzunowicz G, Busch A, Backlund A, Osterholm C, Razuvaev A, Renne T, Eckstein HH, Pelisek J, Eriksson P, Gonzalez Diez M, Perisic Matic L, Schellinger IN, Raaz U, Leeper NJ, Hansson GK, Paulsson-Berne G, Hedin U, Maegdefessel L.. MicroRNA-210 enhances fibrous cap stability in advanced atherosclerotic lesions. Circ Res 2017;120:633–644. [DOI] [PubMed] [Google Scholar]
- 126. Stary HC, Chandler AB, Dinsmore RE, Fuster V, Glagov S, Insull W Jr., Rosenfeld ME, Schwartz CJ, Wagner WD, Wissler RW.. A definition of advanced types of atherosclerotic lesions and a histological classification of atherosclerosis. A report from the Committee on Vascular Lesions of the Council on Arteriosclerosis, American Heart Association. Circulation 1995;92:1355–1374. [DOI] [PubMed] [Google Scholar]
- 127. Karunakaran D, Geoffrion M, Wei L, Gan W, Richards L, Shangari P, DeKemp EM, Beanlands RA, Perisic L, Maegdefessel L, Hedin U, Sad S, Guo L, Kolodgie FD, Virmani R, Ruddy T, Rayner KJ.. Targeting macrophage necroptosis for therapeutic and diagnostic interventions in atherosclerosis. Sci Adv 2016;2:e1600224.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Kockx MM. Apoptosis in the atherosclerotic plaque: quantitative and qualitative aspects. Arterioscler Thromb Vasc Biol 1998;18:1519–1522. [DOI] [PubMed] [Google Scholar]
- 129. Clarke MC, Littlewood TD, Figg N, Maguire JJ, Davenport AP, Goddard M, Bennett MR.. Chronic apoptosis of vascular smooth muscle cells accelerates atherosclerosis and promotes calcification and medial degeneration. Circ Res 2008;102:1529–1538. [DOI] [PubMed] [Google Scholar]
- 130. Ihling C, Szombathy T, Nampoothiri K, Haendeler J, Beyersdorf F, Uhl M, Zeiher AM, Schaefer HE.. Cystic medial degeneration of the aorta is associated with p53 accumulation, Bax upregulation, apoptotic cell death, and cell proliferation. Heart 1999;82:286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Merk DR, Chin JT, Dake BA, Maegdefessel L, Miller MO, Kimura N, Tsao PS, Iosef C, Berry GJ, Mohr FW, Spin JM, Alvira CM, Robbins RC, Fischbein MP.. miR-29b participates in early aneurysm development in Marfan syndrome. Circ Res 2012;110:312–324. [DOI] [PubMed] [Google Scholar]
- 132. Emrich FC, Okamura H, Dalal AR, Penov K, Merk DR, Raaz U, Hennigs JK, Chin JT, Miller MO, Pedroza AJ, Craig JK, Koyano TK, Blankenberg FG, Connolly AJ, Mohr FW, Alvira CM, Rabinovitch M, Fischbein MP.. Enhanced caspase activity contributes to aortic wall remodeling and early aneurysm development in a murine model of Marfan syndrome. Arterioscler Thromb Vasc Biol 2015;35:146–154. [DOI] [PubMed] [Google Scholar]
- 133. Wu G, Cai J, Han Y, Chen J, Huang ZP, Chen C, Cai Y, Huang H, Yang Y, Liu Y, Xu Z, He D, Zhang X, Hu X, Pinello L, Zhong D, He F, Yuan GC, Wang DZ, Zeng C.. LincRNA-p21 regulates neointima formation, vascular smooth muscle cell proliferation, apoptosis, and atherosclerosis by enhancing p53 activity. Circulation 2014;130:1452–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Huarte M, Guttman M, Feldser D, Garber M, Koziol MJ, Kenzelmann-Broz D, Khalil AM, Zuk O, Amit I, Rabani M, Attardi LD, Regev A, Lander ES, Jacks T, Rinn JL.. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell 2010;142:409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Zhao Y, Feng G, Wang Y, Yue Y, Zhao W.. Regulation of apoptosis by long non-coding RNA HIF1A-AS1 in VSMCs: implications for TAA pathogenesis. Int J Clin Exp Pathol 2014;7:7643–7652. [PMC free article] [PubMed] [Google Scholar]
- 136. Wang S, Zhang X, Yuan Y, Tan M, Zhang L, Xue X, Yan Y, Han L, Xu Z.. BRG1 expression is increased in thoracic aortic aneurysms and regulates proliferation and apoptosis of vascular smooth muscle cells through the long non-coding RNA HIF1A-AS1 in vitro. Eur J Cardiothorac Surg 2015;47:439–446. [DOI] [PubMed] [Google Scholar]
- 137. Boon RA, Hofmann P, Michalik KM, Lozano-Vidal N, Berghäuser D, Fischer A, Knau A, Jaé N, Schürmann C, Dimmeler S.. Long noncoding RNA Meg3 controls endothelial cell aging and function: implications for regenerative angiogenesis. J Am Coll Cardiol 2016;68:2589–2591. [DOI] [PubMed] [Google Scholar]
- 138. Liu W, Liu X, Luo M, Liu X, Luo Q, Tao H, Wu D, Lu S, Jin J, Zhao Y, Zou L.. dNK derived IFN-gamma mediates VSMC migration and apoptosis via the induction of LncRNA MEG3: A role in uterovascular transformation. Placenta 2017;50:32–39. [DOI] [PubMed] [Google Scholar]