This editorial refers to ‘Decreased thromboembolic stroke but not atherosclerosis or vascular remodeling in mice with ROCK2-deficient platelets’ by N. Sladojevic et al., pp. 1307–1317.
Platelets are best known as the cellular mediator of haemostasis and thrombosis. They also possess immune and inflammatory functions that contribute to many vascular pathologies, including atherogenesis, vascular remodelling, valvar calcification, and atherothrombosis.1,2 Platelet activation is a complex process that requires intracellular signal transduction initiated and then amplified by surface receptors for platelet agonists and involves active rearrangement of the actin cytoskeleton. Dynamic rearrangement of the actin cytoskeleton underlie the production of functional platelets by megakaryocytes as well as the secretion of platelet granules, platelet shape change, spreading, and aggregation events necessary for proper haemostasis.3 Among the cytoskeletal regulatory proteins, members of the Rho family of GTP binding proteins, RhoA, Rac1, and Cdc42 have emerged as critical regulators of platelet function.4 These small GTPases have overlapping redundancies in signalling processes, but also exhibit distinct features. Thus, RhoA governs platelet contractility and thrombus stability,3 Rac1 drives lamellipodia formation and Cdc42 may play predominant roles in filopodia formation and granule secretion.4 Megakaryocyte-specific RhoA deficiency results in thrombocytopenia and defective platelet activation.5 The most studied downstream effectors of RhoA are the two isoforms of Rho-associated coiled-coil containing kinase (ROCK), ROCK1, and ROCK2, members of the AGC family of serine/threonine kinases. Genetic deletion studies have shown that ROCK1 and ROCK2 mediate diverse functions in different cell types.6,7 Extending these studies, Sladojevic et al.8 provide evidence that ROCK2 plays a pivotal role in platelet activation and thrombosis, but does not mediate platelets’ contribution to atherosclerotic lesion and vascular remodelling.
Sladojevic et al.8 developed mice with megakaryocyte-specific deletion of ROCK2 (ROCK2Plt−/−). ROCK2 deficiency leads to increased size of resting platelets and impairs cytoskeletal rearrangement that occurs during platelet activation. Loss of platelet ROCK2 leads decreased αIIbβ3 integrin binding to fibrinogen and reduced expression of CD62P (P-selectin) without affecting surface expression of major glycoproteins. These findings would indicate a role for ROCK2 in the initial activation of αIIbβ3 and triggering the release of CD62P from α-granules, rather than maintenance of receptor activation on activated platelets. Consequently, ROCK2-deficient platelets form considerably lesser amounts of heterotypic (platelets and leucocytes) and homotypic aggregates than wild-type ones. Using the ferric chloride-induced carotid artery injury model, the authors show that platelet-specific loss of ROCK2 decreases thrombus formation in situ. Likewise, in a model of acute thrombo-embolic stroke evoked by injection of pre-formed blood clots from wild-type or ROCK2Plt−/− mice into the middle cerebral artery, ROCK2-deficient thrombotic clots are less stable and consequently, cause less cerebral injury and neurological deficits.
ROCK2Plt−/− mice displays slight macrothrombocytopenia, forming about 80% of the numbers of platelets relative to wild-type mice. Although the underlying mechanism is unknown, these observations would suggest a limited contribution of ROCK2 signalling to platelet generation as opposed to the dual requirement for Rac1 and Cdc42 for proper platelet production.5 The modest ∼20% reduction of platelet count in ROCK2Plt−/− mice is unlikely by itself to affect primary haemostasis,5 indicating that the antithrombotic action of ROCK2 deficiency can be attributed to inhibition of platelet function. These findings underscore the importance of platelet ROCK2 in thrombosis and thrombo-embolic stroke and are consistent with increased ROCK activity detected in experimental models of vascular inflammation,9 and patients with acute ischemic strokes.10 An obvious limitation of the study of Sladojevic et al. is that it provides no information on how platelet ROCK2 deficiency leads to protection against thrombo-embolic stroke. Furthermore, the authors’ observations do not exclude a role for ROCK in the vessel wall, which may be activated by mediators released from platelets. Clinical studies have shown that antiplatelet therapy is associated with systemic anti-inflammatory actions in patients with atherothrombotic disease.11 One perspective is to investigate whether genetic deletion or pharmacological blockade of ROCK2 could also activate anti-inflammatory mechanisms.
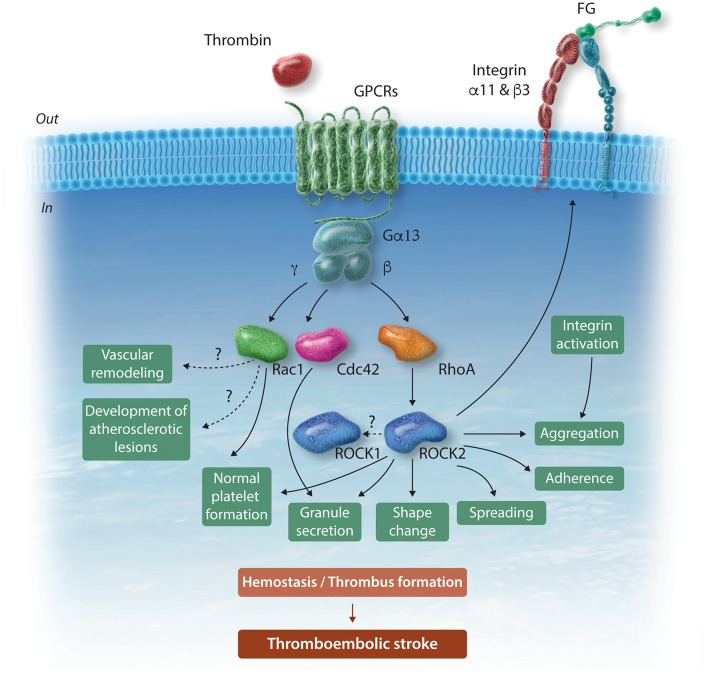
The RhoA-ROCK signalling pathway has extensively been studied.4 Like ROCK2, ROCK1 also triggers phosphorylation of myosin-binding subunit of myosin light chain phosphatase (MYPT1) that drives actin remodelling events. It is unclear why ROCK1 does not compensate for loss of ROCK2. Interestingly, ROCK2-deficient platelets also exhibit slightly reduced ROCK1 expression. Whether ROCK2 regulates ROCK1 expression and whether ROCK1 is downstream of ROCK2 remain speculative. The currently available ROCK inhibitors, such as fasudil or Y27632 lack selectivity towards the two isoforms and could also inhibit other serine/threonine kinases, thereby limiting their usefulness in addressing this issue. Figure 1 summarizes the contribution of platelet ROCK2 to normal haemostasis and thrombosis and the possible mechanisms of action.
Figure 1.
Schematic representation of ROCK2 signalling in platelets, underlying haemostasis, thrombus formation and thromboembolic stroke. Thrombin and other platelet agonists through GPCRs induce RhoA-mediated activation of ROCK2. ROCK2 drives separate steps of platelet activation, leading to thrombus formation and ultimately to thromboembolic stroke. ROCK2 also regulates platelet formation by megakaryocytes albeit to a considerably lesser degree than Rac1 or Cdc42. For the sake of simplicity, RhoA (and possibly ROCK2)-driven platelet contraction and clot retraction are not shown. Intriguingly, platelet-selective deletion of ROCK2 does not affect vascular remodelling and atherogenesis. Cdc42, cell division control protein 42 homolog (Rho-type GTPase); FG, fibrinogen; GPCRs, G-protein-coupled receptors; Gα13, β, γ, G-protein subunits coupling to GPCRs; Rac1, Ras-related C3 botulinum toxin substrate 1 (Rho-type GTPase); RhoA, Ras homolog gene family member A; ROCK, Rho-associated coiled-coil containing kinase.
An unexpected observation in this study was the failure of ROCK2-deficient platelets to attenuate neointima formation following carotid artery ligation or development of atherosclerotic lesions in Ldlr−/− mice fed with high-fat diet for 16 weeks. Earlier studies have reported reduced vascular remodelling after balloon-injury12 and early atherosclerotic lesion formation following ROCK inhibition in mice.13 ROCK activity in peripheral blood leucocytes has been proposed as a biomarker of cardiovascular events in patients.14 A plausible hypothesis posits that Rho-ROCK-independent signalling pathways mediate platelet activation and ROCK signalling in the cells of the vessel wall or leucocytes rather than in platelets contributes to these pathologies. Indeed, ROCK2 inhibits reverse cholesterol transport in macrophages,15 thereby promoting foam cell formation. Clearly, further studies are warranted to address the cell-specific and context-dependent impact of ROCK signalling under these pathological conditions.
Recent progress reveals more complex signalling and amplification networks in platelet activation than those previously established. The discovery of new circuits challenges some of the long-standing concepts of platelet signalling. The study of Sladojevic et al.8 identifies ROCK2 as an important signalling molecule that could eventually be targeted for limiting thrombotic events. Although Sladojevic et al. do not report on the signalling pathways that underlie platelets’ contribution to vascular remodelling or atherogenesis, their findings raise the intriguing possibility of therapeutic blockade of these pathways with minimal interference with haemostasis in patients on long-term antiplatelet therapy. Considering the complexity of Rho family GTPase signalling, it remains a future challenge to investigate the benefits of therapeutic targeting of ROCK2 (and perhaps ROCK1) in patients with thrombo-embolic diseases.
Conflict of interest: none declared.
Funding
This work was supported by grant MOP-97742 from the Canadian Institutes of Health Research.
References
- 1. Davi G, Patrono C.. Platelet activation and atherothrombosis. N Engl J Med 2007;357:2482–2494. [DOI] [PubMed] [Google Scholar]
- 2. von Hundelshausen P, Weber C.. Platelets as immune cells: bridging inflammation and cardiovascular disease. Circ Res 2007;100:27–40. [DOI] [PubMed] [Google Scholar]
- 3. Li Z, Delaney MK, O'Brien KA, Du X.. Signaling during platelet adhesion and activation. Arterioscler Thromb Vasc Biol 2010;30:2341–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aslan JE, McCarty OJT.. Rho GTPases in platelet function. J Thromb Haemost 2013;11:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pleines I, Hagedorn I, Gupta S, May F, Chakarova L, van Hengel J, Offermanns S, Krohne G, Kleinschnitz C, Brakebusch C, Nieswandt B.. Megakaryocyte-specific RhoA deficiency causes macrothrombocytopenia and defective platelet activation in hemostasis and thrombosis. Blood 2012;119:1054–1063. [DOI] [PubMed] [Google Scholar]
- 6. Shimizu Y, Thumkeo D, Keel J, Ishizaki T, Oshima H, Oshima M, Noda Y, Matsumura F, Taketo MM, Narumiya S.. ROCK-I regulates closure of the eyelids and ventral body wall by inducing assembly of actomyosin bundles. J Cell Biol 2005;168:941–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Thumkeo D, Keel J, Ishizaki T, Hirose M, Nonomura K, Oshima H, Oshima M, Taketo MM, Narumiya S.. Targeted disruption of the mouse rho-associated kinase 2 gene results in intrauterine growth retardation and fetal death. Mol Cell Biol 2003;23:5043–5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sladojevic N, Oh GT, Kim H-H, Beaulieu LM, Falet H, Kaminski K, Freedman JE, Liao JK.. Decreased thromboembolic stroke but not atherosclerosis or vascular remodeling in mice with ROCK2-deficient platelets. Cardiovasc Res 2017;113:1307–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kataoka C, Egashira K, Inoue S, Takemoto M, Ni W, Koyanagi M, Kitamoto S, Usui M, Kaibuchi K, Shimokawa H, Takeshita A.. Important role of Rho-kinase in the pathogenesis of cardiovascular inflammation and remodeling induced by long-term blockade of nitric oxide synthesis in rats. Hypertension 2002;39:245–250. [DOI] [PubMed] [Google Scholar]
- 10. Feske SK, Sorond FA, Henderson GV, Seto M, Hitomi A, Kawasaki K, Sasaki Y, Asano T, Liao JK.. Increased leukocyte ROCK activity in patients after acute ischemic stroke. Brain Res 2009;1257:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Steinhubl SR, Badimon JJ, Bhatt DL, Herbert JM, Luscher TF.. Clinical evidence for anti-inflammatory effects of antiplatelet therapy in patients with atherothrombotic diseases. Vasc Med 2007;12:113–122. [DOI] [PubMed] [Google Scholar]
- 12. Sawada N, Itoh H, Ueyama K, Yamashita J, Doi K, Chun TH, Inoue M, Masatsugu K, Saito T, Fukunaga Y, Sakaguchi S, Arai H, Ohno N, Komeda M, Nakao K.. Inhibition of rho-associated kinase results in suppression of neointimal formation of balloon-injured arteries. Circulation 2000;101:2030–2033. [DOI] [PubMed] [Google Scholar]
- 13. Mallat Z, Gojova A, Sauzeau V, Brun V, Silvestre JS, Esposito B, Merval R, Groux H, Loirand G, Tedgui A.. Rho-associated protein kinase contributes to early atherosclerotic lesion formation in mice. Circ Res 2003;93:884–888. [DOI] [PubMed] [Google Scholar]
- 14. Kajikawa M, Noma K, Maruhashi T, Mikami S, Iwamoto Y, Iwamoto A, Matsumoto T, Hidaka T, Kihara Y, Chayama K, Nakashima A, Goto C, Liao JK, Higashi Y.. Rho-associated kinase activity is a predictor of cardiovascular outcomes. Hypertension 2014;63:856–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhou Q, Mei Y, Shoji T, Han X, Kaminski K, Oh GT, Ongusaha PP, Zhang K, Schmitt H, Moser M, Bode C, Liao JK.. Rho-associated coiled-coil-containing kinase 2 deficiency in bone marrow-derived cells leads to increased cholesterol efflux and decreased atherosclerosis. Circulation 2012;126:2236–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]