Abstract
Aims
Rho-associated coiled-coil containing kinase (ROCK)-2 is an important mediator of the actin cytoskeleton. Because changes in the actin cytoskeleton are critical for platelet function, we hypothesized that ROCK2 in platelets will play important role in thrombosis and can be potentially a target for therapeutic intervention in thromboembolic stroke.
Methods and results
We generated platelet-specific ROCK2-deficient mice (ROCK2Plt−/−) from conditional ROCK2fl°x/fl°x and platelet factor (PF)-4-Cre transgenic mice. Platelets from ROCK2Plt−/− mice were less responsive to thrombin stimulation in terms of pseudopodia formation, collagen adhesion, and in the formation of homotypic and heterotypic aggregates. This corresponded to prolonged bleeding time and delayed vascular occlusion following vessel injury. To determine whether these changes in platelet function could affect thrombotic disease, we utilized a clot-embolic model of ischaemic stroke. When pre-formed clots from ROCK2Plt−/− mice were injected into the middle cerebral artery of control mice, cerebral blood flow recovery occurred more rapidly, leading to decreased cerebral injury and neurological deficits, compared to pre-formed clots from control mice. Interestingly, pre-formed clots from control mice produced similar degree of cerebral injury when injected into control or ROCK2Plt−/− mice, suggesting that platelet ROCK2 deficiency affects clot formation but not propagation. Indeed, in a non-thrombotic intra-filament MCA occlusion model of stroke, platelet ROCK2 deletion was not protective. Furthermore, ROCK2Plt−/− mice exhibit similar atherosclerosis severity and vascular remodeling as control mice.
Conclusion
These findings indicate that platelet ROCK2 plays important role in platelet function and thrombosis, but does not contribute to the pathogenesis of atherosclerosis and vascular remodeling.
Keywords: Rho kinase, Platelet, Thrombosis, Stroke, Atherosclerosis
1. Introduction
The activation of platelets is critical for hemostasis,1,2 and is the final common pathway in most acute vascular occlusive diseases.3 Platelet activation is a complex process that involves active rearrangement of the actin cytoskeleton, changes in cell shape, the secretion of granules, and the retraction of clots. However, the signalling pathways that initiate thrombosis are not well understood. Rearrangement of the actin cytoskeleton is mediated, in part, by Rho, and its downstream effector, Rho-associated coiled-coil containing kinases (ROCK).4 Because such rearrangement underlies platelet aggregation,5 vascular contractility,6,7 and leukocyte chemotaxis,8 the Rho/ROCK pathway is likely to play important role in the platelets activation and hemostasis.
ROCK belongs to the serine–threonine kinase family and plays important role in various fundamental cellular processes including contraction, motility, proliferation and migration. There are two isoforms of ROCK: ROCK1 (Rho-kinase β) and ROCK2 (Rho-kinase α). These isoforms share 65% homology in amino acid sequence with 92% homology in their kinase domain. ROCK1 and ROCK2 are ubiquitously expressed in vertebrates with higher expression of ROCK1 in blood, kidney testis, liver and spleen and ROCK2 in heart, brain and vasculature. Homozygous ROCK1 deletion mice show open eyelids and omphalocele, whereas ROCK2 homozygous deletion mice die embryonically because of placental dysfunction.9,10 This indicates that one form of ROCK cannot compensate deletion of another one suggesting that both isoforms mediate different functions in different cell types.
The Rho GTPases, which include RhoA, Rac1, and Cdc42, are critical mediators of platelet function.11–13 In particular, RhoA deficiency in platelets leads to macrothrombocytopenia and defective platelet activation in thrombosis and hemostasis.14 Because ROCKs are not the only downstream targets of RhoA and could be activated by non-RhoA-mediated pathways,4 it is not known what the specific role of ROCKs are in platelets and to what extent they contribute to hemostasis and thrombosis. In addition, it is not known whether platelet ROCKs contribute to vascular remodeling and atherosclerosis. Increased ROCK activity is observed in the context of coronary and cerebral vasospasm,15,16 hypertension,17 vascular inflammation and remodelling,18 arteriosclerosis and atherosclerosis,19,20 Furthermore, increased ROCK activity is observed in humans with acute ischaemic strokes,21 and correlates with cardiovascular risk factors and outcomes.22,23 However, the role of ROCK in platelet function and hemostasis remains largely unknown. Accordingly, with the availability of conditional ROCK2 mice, we investigated the role of ROCK2 in platelet function and thrombosis, and determine whether it is important in the pathogenesis of thromboembolic stroke and other types of vascular diseases.
2. Methods
2.1 Animals
Age-matched male littermates (12–20 weeks, all on C57BL/6 background) were used for experiments. ROCK2fl°x/fl°x mice were generated as previously described.24 Briefly, the ROCK2 locus is on chromosome 12 (EMBL accession: U58513) and contains 33 exons that span over 82 kb. We generated constructs with loxP sites flanking exon 3, which encodes the N-terminal kinase domain of ROCK2. Because many floxed alleles are hypomorphic due to the presence of a residual neomycin cassette, we flanked the neomycin cassette with FRT sites and selectively excised the intervening sequence by crossing these mice to transgenic mice expressing the FLPe recombinase. The resulting ROCK2fl°x/fl°x mice used in this study do not contain the neomycin cassette. To delete ROCK2 in platelets, we bred our conditional ROCK2fl°x/fl°x mice with transgenic mice expressing the Cre recombinase under the control of the platelet factor 4 (PF4) promotor, which is specifically expressed in the megakaryocytic lineage.25 For all surgeries animals were anesthetized initially with 2% isoflurane and after complete anaesthesia isoflurane was reduced to 1.2% and rectal temperature was monitored and maintained at 37 ± 0.5°C. All animals were euthanized with Carbon Dioxide asphyxiation (CO2). A gradual fill method was used with a displacement rate of 10–30% of the chamber volume/minute (3 L/min). CO2 flow will continue for at least 1 min after respiratory arrest. Cervical dislocation, a secondary method of confirming death was always performed on the animals. Animal protocols were in compliance with NIH Guideline for the Care and Use of Laboratory Animals and have been approved by the Standing Committee on Animal Welfare and Protection at Harvard Medical School and the University of Chicago.
2.2 Isolation and characterization of mouse platelets
Blood samples were obtained via cardiac puncture under isoflurane anaesthesia with a syringe containing anticoagulant solution (15.6 mM citric acid anhydrous, 89.4 mM sodium citrate, 18.5 mM sodium phosphate, and 2.56% dextrose, pH 7.35). Whole blood was centrifuged at 450×g for 7 min. Platelet-rich plasma (PRP) was diluted 2.33-fold with platelet wash buffer (10 mM sodium citrate, 150 mM NaCl, 1 mM EDTA, and 1% dextrose, pH 7.4) with prostaglandin E1 (1:10 000). Samples were centrifuged again at 300×g for 4 min to remove any contaminating leukocytes. PRP was further diluted 3-fold with platelet wash buffer and prostaglandin E1. Samples were centrifuged once more, for 11 min at 3500×g. The resulting platelet pellet was resuspended in HEPES buffer (140 mM NaCl, 6 mM KCl, 2.4 mM MgSO4·7H2O, 1.7 mM NaH2PO4, 6 mM Na HEPES, 0.35% BSA, 0.1% Dextrose, pH 7.4). Platelets were used at 2× 108/mL, in aggregation and adhesion experiments.
2.3 Transmission electron microscopy
After treatment with 0.05 U/mL mouse thrombin (Enzyme Research Laboratories) for 10 min in 1× HBSS with calcium and magnesium (or resting) for 10 min, platelets were fixed with 2.0% paraformaldehyde and 2.5% glutaraldehyde in 0.1 M Sorensen’s phosphate buffer (pH 7.4). Samples were washed and post-fixed with 1.0% osmium tetroxide in 0.1 M PBS (pH 7.4). Samples were then dehydrated in serial dilutions of ethanol, and embedded in Epon 812. Samples were sectioned, and uranyl acetate and lead citrates were applied to provide contrast. TEM was performed using a Philips CM10 electron microscope (Eindhoven, Nederland) at 32 000× magnification. To quantify structural changes in platelets we combine blood from eight mice in each sample and measured platelets size of 20 cells and number of pseudopodia from 40 cells per group. Each experiment was independently repeated 5 times.
2.4 Confocal microscopy
Washed platelets at rest and activated with 0.5 U/mL thrombin were stained with Phalloidin-FITC (Sigma). Images were obtained using a Nikon TE-2000E2 Inverted Microscope with a modified Yokogawa CSU10 Spinning Disk Confocal Scan Head. MetaMorph Software was used for acquisition and analysis.
2.5 Complete blood count
Blood was collected from mice by retro-orbital plexus bleeding into EDTA after isoflurane anaesthesia. Peripheral blood platelet counts were performed using an ADVIA 120/2120 automated haematology analyzer (Bayer Health-Care).
2.6 Flow cytometry
For the tests of actin assembly, resting and activated platelets were fixed and permeabilized, and then incubated with 2 μM TRITC-labelled phalloidin (Sigma-Aldrich, St. Louis, MO). For the measurement of levels of P-selectin and active αIIbβ3, resting or thrombin activated platelets were incubated with a FITC-labelled anti-P-selectin antibody (BD Bioscience, San Jose, CA) or Oregon Green 488-labelled fibrinogen (Thermo-Fisher Scientific, Waltham, MA) for 30 min at room temperature. Fluorescence was quantified using a FACSCalibur flow cytometer with CellQuest software (BD Biosciences, San Jose, CA). For each sample, a total of 20 000 events were analysed.
2.7 Platelet aggregation
Aggregation of washed platelets was measured using a PAP-4 aggregometer (Bio/Data Corporation, Horsham, PA). Platelets in HEPES buffer with 1 mM CaCl2, 2 mM MgCl2, and 3 mM fibrinogen (Enzyme Research Laboratories, South Bend, IN) were either left untreated (Resting) or were treated with 25 μM mouse TRAP (Anaspec, San Jose, CA) for 10 min, at 37 °C, while being stirred at 1200 rpm (see Supplementary material online).
2.8 Platelet adhesion
Calcein-AM labelled platelets were either left untreated (Resting) or were treated with 25 μM mouse TRAP for 10 min. Samples were then flow circulated over collagen-coated (100 mg/mL; Chrono-Log) coverslips for 20 min. Images were taken using an Olympus IX70 Inverted Microscope at 20×, and analysed using Universal Imaging MetaMorph Software. Analysis was done using Image J software v1.33 (NIH, Bethesda, MD).
2.9 Bleeding-time assay
PF4 and ROCK2Plt−/− mice were anesthetized with isoflurane and 5–6 mm of the tail tip was cut off and tails were immersed in 0.9% isotonic saline at 37 °C. The amount of time required for bleeding to stop (defined as no blood flow for 1 min) was measured.
2.10 Ferric chloride-induced thrombotic vascular occlusion
After mice were anesthetized with isoflurane, a midline incision was made in the neck to expose the left common carotid artery. Thrombosis was induced by applying two pieces of filter paper (1×2 mm) saturated with 5% FeCl3. Blood flow through the carotid artery was monitored continuously from time 0 (before the FeCl3 paper was applied) for up to 60 min. Blood flow was measured using a miniature Doppler flow probe (Perimed Inc. OH) connected to a laser flow module (Perimed Inc. OH). The data were recorded and analysed using the PowerLab 16/30 data acquisition unit (Transonic Systems) and Chart software (AD Instruments). The end points of the experiment are: when blood flow has ceased for >30 s, or if complete occlusion is not seen after 60 min.
2.11 Non-thrombotic model of focal cerebral ischaemia
A transient intraluminal filament model of MCAO was performed on PF4 and ROCK2Plt−/− mice for 1 h as previously described.26 MCA was occluded by inserting a commercially available 8-0 nylon filament (Doccol Corp., Redlands, CA) into the internal carotid artery and advancing it to the origin of the MCA and leaving it there for 1 h. The tissue was reperfused for 24 h after MCAO. Relative cerebral blood flow returned to >95% of baseline values after withdrawal of the filament, indicating that reperfusion was almost complete, without residual occlusion. Male PF4 and ROCK2Plt−/− mice (20–25 g) were used.
2.12 Thromboembolic model of focal cerebral ischaemia
An embolic occlusion method involving whole blood clots was used to induce focal cerebral ischaemia, as previously described.27 One day before induction of ischaemia, arterial blood from donor mice (i.e. PF4 and ROCK2Plt−/− mice) was drawn into PE-50 tubing, stored at room temperature for 2 h, and then kept at 4°C for 22 h. Coagulated blood was sectioned into 12.5 mm segments, washed with saline, transferred to a modified PE-50 catheter with a diameter of 0.2 mm, and injected into the internal carotid artery. Successful occlusion of the middle cerebral artery was verified by laser Doppler flowmetry (Perimed Inc. OH) for 30 min. This procedure allows for homologous or heterologous injection of blood clots, i.e. platelet clots from PF4 mice to PF4 mice (control) and from ROCK2Plt−/− mice to PF4 mice.
2.13 Neurological deficit score
The neurological score was measured using an expended six-point scale scoring test (0: normal; 1: mild circling behaviour with or without inconsistent rotation; 2: mild but consistent circling; 3: consistent strong and immediate circling, and holding of a rotation position for >1–2 s; 4: severe rotation progressing into barrelling, and loss of righting reflex; 5: comatose or moribund). Animals with a score of 5 were excluded from the experiment, as this status is correlated with severe deficits and high mortality.
2.14 Infarct and residual clot size
About 24 h after reperfusion animals were sacrificed as previously described. Brains were quickly removed, captured under dissecting microscope to measure residual clot size and sliced into 2-mm-thick sections to measure infarct size after triphenyltetrazolium chloride staining (TTC). Images were analysed by using the NIH Image J program, v1.33 (NIH, Bethesda, MD).
2.15 Carotid artery ligation model
The left common carotid artery was dissected and ligated near the carotid bifurcation. PF4 and ROCK2Plt−/− mice were sacrificed at 15 days after ligation of the carotid artery. The common carotid arteries were collected and the segments were embedded in paraffin and analysed by haematoxylin-and-eosin staining for morphometry.
2.16 Atherosclerosis model
For bone marrow transplantation (BMT), 8-week-old male LDL receptor knockout mice (LDLr−/−) mice were subjected to 1000 rad of total body irradiation followed by reconstitution with 2 × 106 bone marrow cells from PF4-Cre or ROCK2Plt−/− mice via retro-orbital injection. Animals were allowed to recover for 4 weeks after BMT on normal chow diet. Blood counts including differential counts were not different between pre- and post-bone marrow transplantation. Thereafter received an atherogenic diet containing 15.8% (wt/wt) fat and 1.25% cholesterol (Harlan Teklad, Madison, WI) for 16 weeks.
Mouse hearts embedded in optimal cutting temperature compound were sectioned in a cryostat until all three leaflets within the aortic valve were visible. From this point, 10 mm sections were taken for the next 300 mm of the valve region, and each section was collected on a Superfrost slide (Fisher Scientific, Tustin, CA). The lesions were visualized by staining with Oil Red O, followed by counterstaining with haematoxylin. A total of five sections taken every 40 mm were used to quantitate lesion areas. For each sample, the remaining aorta was dissected out, cut longitudinally from the aortic root to the iliac bifurcations, pinned onto a black surface, stained with Oil Red O and photographed. The total and atherosclerotic areas of each sample were measured using the NIH Image J program, v1.33 (NIH, Bethesda, MD).
2.17 Data analysis
Results are shown as the mean ± SEM from at least three individual experiments per group unless indicated otherwise. Statistical analyses comparing PF4, ROCK2fl°x/fl°x, and ROCK2Plt−/− animals were performed using either the Student’s t-test, two-way ANOVA followed by Scheffé or Tukey test, or by Nested ANOVA using RStudio software (Boston, MA), with P < 0.05 considered statistically significant.
3. Results
3.1 Characterization of platelet-specific ROCK2 deficiency
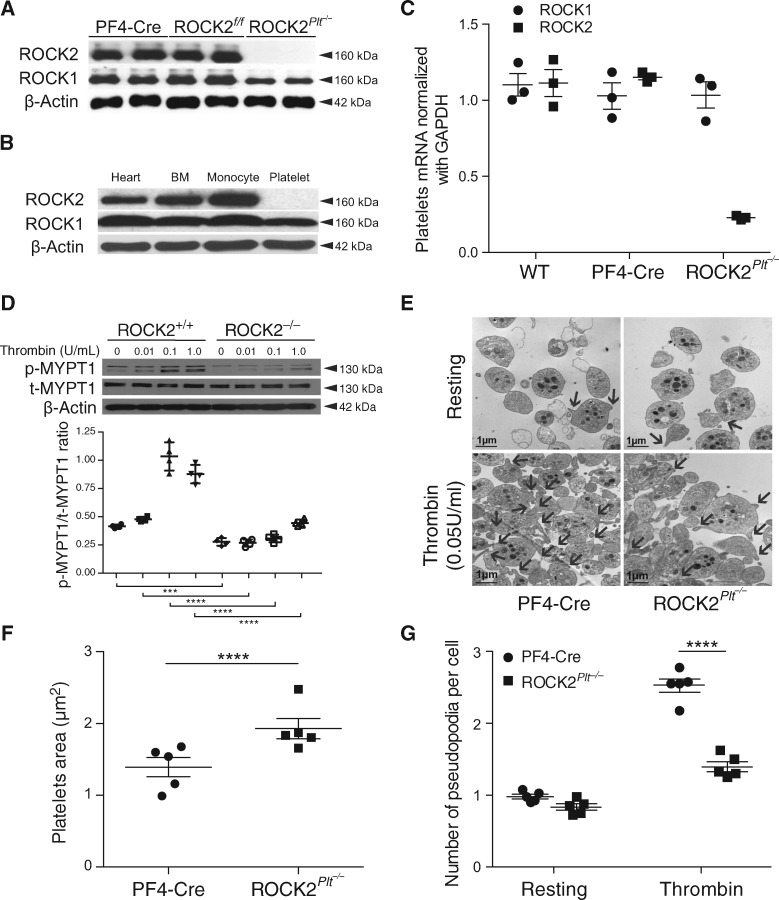
Comparative qPCR and western-blot analysis of ROCK2 expression in wild-type and ROCK2Plt−/− mice platelets have shown only ROCK2 specific deletion in platelets of ROCK2Plt−/− mice (n = 5, 3, Figure 1A and C) but not in the heart, monocytes, or other bone marrow-derived cells (n = 5, Figure 1B). Interestingly, in platelets with ROCK2 deficiency, ROCK1 levels were also slightly decreased, suggesting that ROCK2 may be an important regulator of ROCK1 protein levels in platelets. As expected, thrombin-induced ROCK activity [ratio between phosphorylated and total amount of myosin phosphatase target subunit 1 (p-MYPT1/t-MYPT1)] was reduced in ROCK2-deficient platelets (n = 5, Figure 1D). These findings confirm specific deletion of ROCK2 in the platelets of ROCK2Plt−/− mice.
Figure 1.
Deletion of ROCK2 and decreased ROCK activity in platelets from mice with ROCK2 deficiency. (A) Expression of ROCK1 and ROCK2 in platelets from PF4-Cre, ROCK2fl°x/fl°x, ROCK2Plt−/− mice (n = 5). (B) ROCK expression in heart, bone marrow (BM), monocyte, and platelet of ROCK2Plt−/− mice (n = 5), (C) ROCK1 and ROCK2 mRNA expression in platelets normalized with GAPDH (n = 3), (D) ROCK activity as determined as a relative blot density ratio of Thr853 phosphorylation of myosin phosphatase target 1 (p-MYPT1) to total myosin phosphatase target 1 (t-MYPT1). β-actin expression served as loading control (n = 5). (E) Representative transmission electron microscopy images of resting and thrombin-stimulated platelets from control (PF4-Cre) and ROCK2Plt−/− mice (Scale bar, 1 μm. n = 5) used to quantified (F) platelets area (n = 100) and (G) number of pseudopodia per cell (n = 40). *P<0.05, ***P<0.001, ****P<0.00001. All data are expressed as mean±SEM.
3.2 ROCK2 deficiency in platelets causes cytoskeletal rearrangements in the resting and activated states and leads to macrothromobocytopenia
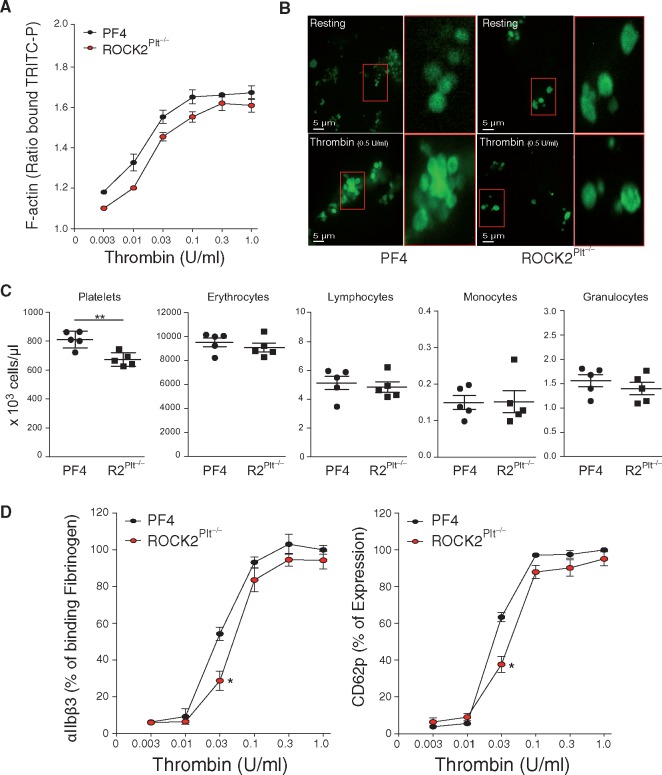
Whether ROCK2 deficiency leads to structural changes in platelets was assessed qualitatively by transmission electron microscopy (TEM) on isolated resting and thrombin-stimulated platelets from PF4-Cre (control) and ROCK2Plt−/− mice (Figure 1E). Platelet-specific ROCK2 deficiency leads to increased size of resting platelets compared to PF4-Cre mice (F1,194 = 20.89, P≤0.0001, Figure 1F). Stimulation of platelets from the PF4-Cre mice led to increased formation of pseudopodia, which was reduced in platelets from ROCK2Plt−/− mice (F1,793 = 301.967, P ≤ 0.0001, Figure 1G), after determining the overall model with stimulation and between isolated cells to be significantly different (F1,789 = 109.4, P ≤ 0.0001) as reported by the Nested ANOVA. The quantitative reduction of pseudopodia formation in ROCK2Plt−/− mice suggests that cytoskeletal rearrangements are impaired in ROCK2-deficient platelets. To determine the integrity of the actin cytoskeleton in the platelets, flow cytometry was used to quantitate the amount of TRITC-phalloidin bound within permeabilized cells. There was a tendency for platelets from ROCK2Plt−/− mice to bind slightly less phalloidin than their control counterparts (PF4-Cre mice) at lower concentrations of thrombin (n = 5, Figure 2A). However, overall differences in platelet TRITC-phalloidin binding between those two strains were not significant.
Figure 2.
ROCK2 deficiency leads to decreased platelet function. (A) Amount of F-actin as determined by ratio of binding to TRICT-P in response to thrombin in platelets from control (PF4-Cre) and ROCK2Plt−/− mice. (B) Representative confocal microscopic images of phalloidin-FITC in resting and thrombin-stimulated platelets from PF4-Cre and ROCK2Plt−/− mice. Scale bar, 5 μm. (C) Blood cell count in PF4-Cre and ROCK2Plt−/− mice (n = 5). (D) Percent expression of αIIbβ3 and CD62p in response to thrombin in platelets from control (PF4-Cre) and ROCK2Plt−/− mice. n = 5, *P < 0.05, **P < 0.01. All data are expressed as mean ± SEM.
This observation prompted us to perform confocal imaging of both resting and thrombin-stimulated platelets. In both sets of mice, platelets in the resting state exhibited uniform actin staining (n = 5, Figure 2B, upper panels). Upon activation with 0.5 U/mL thrombin, the platelets from control mice formed aggregates and the actin staining changed to a ring pattern, indicative of actin accumulation at the periphery (n = 5, Figure 2B, lower left panel). In contrast, the ROCK2-deficient platelets formed less aggregates and did not exhibit this ring staining, consistent with ROCK2 contributing to cytoskeletal rearrangements that occur during platelet activation (n = 5, Figure 2B, lower right panel).
Platelet-specific ROCK2 deficiency leads to macrothrombocytopenia with the number of platelets reduced by 17.1% compared to PF4-Cre mice and the production of other bone marrow-derived cells, including erythrocytes, lymphocytes, monocytes, and granulocytes, did not differ significantly (n = 5, P < 0.01, Figure 2C).
3.3 Platelet ROCK2 deficiency impairs the initiation, but not maintenance, of expression of glycoproteins and CD62p on the platelet surface
Flow cytometry was used to measure baseline expression of major glycoproteins in platelets from PF4-Cre and ROCK2Plt−/− mice (Table 1). Data do not show differences in glycoprotein levels between PF4-Cre and ROCK2Plt−/− mice. We tested the possibility that even if ROCK2 does not affect the expression of these surface receptors, they may still affect their activation and function. Using flow cytometry to assess the activity of integrin αIIbβ3 in platelets from mice treated with increasing concentrations of thrombin, we found that αIIbβ3 binding to fibrinogen in ROCK2Plt−/− platelets was lower than that in PF4-Cre counterparts, but only at a low concentration of thrombin, i.e. 0.03 U/mL (n = 5, P < 0.05, Figure 2D, left panel).
Table 1.
Expression of major surface glycoproteins on PF4 and R2Plt−/− platelets
| Parameter | PF4 (n=4–6) | R2Plt−/−(n=4–6) | P-value |
|---|---|---|---|
| GPIbα (CD42b) | 47.1 ± 3 | 43.3 ± 2 | 0.16 |
| GP1bβ (CD42c) | 286.3 ± 9 | 282.4 ± 9 | 0.54 |
| GPIX (CD42a) | 75.6 ± 6 | 62.2 ± 4.7 | 0.31 |
| GPV (CD42d) | 55 ± 2 | 53 ± 1 | 0.17 |
| GPIIIa (CD61, integrin β3) | 531 ± 20 | 517 ± 35 | 0.70 |
| GPVI | 14 | 14.1 | 0.51 |
Data are expressed as mean fluorescence intensity bound to the surface of PF4 and R2Plt−/− platelets and represent mean ± SEM.
To test the level of platelet activation, we stimulated platelets from ROCK2Plt−/− and PF4-Cre mice with increasing concentrations of thrombin and then analysed them by flow cytometry for the percentage of CD62P expression. At 0.03 U/mL thrombin, CD62P was expressed at lower levels in the ROCK2Plt−/− vs. PF4 platelets (n = 5, P < 0.05, Figure 2D, right panel).
3.4 ROCK2 deficiency leads to reduced aggregation and adhesion of platelets
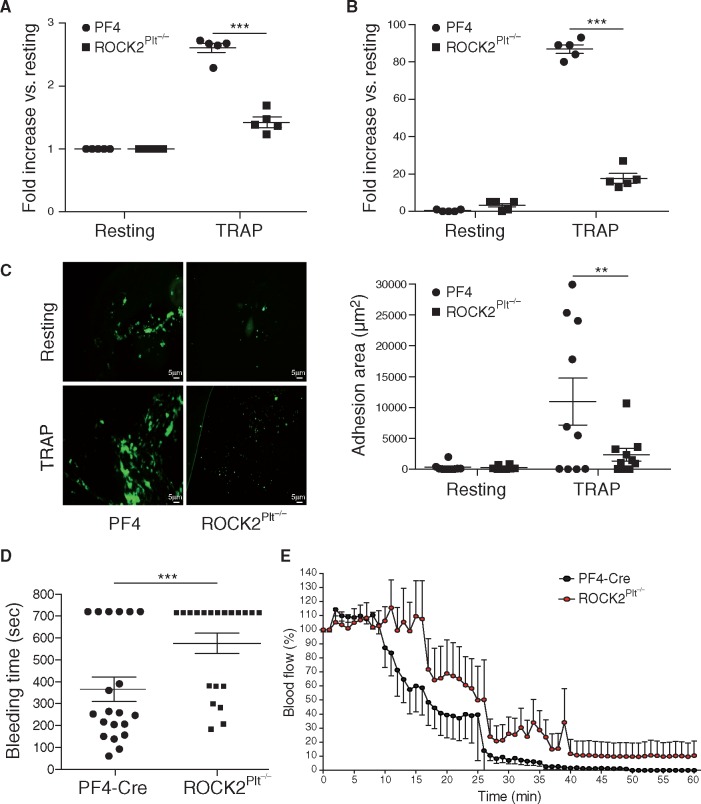
To assess heterotypic (platelets and leukocytes) and homotypic aggregation (between platelets) thrombocytes rich fraction with contaminated leukocytes and fraction with only platelets, isolated from PF4-Cre and ROCK2Plt−/− mice were treated with thrombin-receptor activation peptide (TRAP). Compared to PF4-Cre mice, there was a reduction in heterotypic (1.4 ± 0.07 vs. 2.6 ± 0.07-fold increase from resting, n= 5, P < 0.001, Figure 3A) and homotypic (17.6 ± 2.4 vs. 87 ± 2.2-fold increase from resting, n= 5, P < 0.001, Figure 3B) aggregation of platelets from ROCK2Plt−/− mice in response to TRAP. Furthermore, to assess adhesion to collagen, washed platelets from PF4-Cre and ROCK2Plt−/− mice were either left untreated (resting) or were treated with TRAP, and then flow circulated over collagen-coated coverslips. Under resting conditions, very few platelets adhered to collagen. In the presence of TRAP, fewer adherent platelets as determined by adhesion area were observed with platelets from ROCK2Plt−/− mice compared to that of PF4-Cre mice (2339 ± 1011 vs. 10968 ± 3468 mm2, n = 3 P < 0.01) (Figure 3C). Notably, those platelets that did adhere to collagen (mainly among the PF4-Cre samples) also did not form large aggregates.
Figure 3.
Decreased aggregation and adhesion of ROCK2 deficient platelets with prolonged bleeding time and vascular occlusion. (A) Heterotypic and (B) homotypic aggregation in resting and in response to TRAP in platelets from PF4-Cre and ROCK2Plt−/− mice (n = 5). (C) Confocal microscopy images and quantification of adhesion area (μm2) in resting and TRAP-activated platelets from PF4-Cre and ROCK2Plt−/−mice (n = 3), (D) Bleeding times of PF4-Cre and ROCK2Plt−/− mice after tail snipping in saline at 37 °C (n = 20), (E) relative changes in common carotid artery blood flow after application of 5% FeCl3 in PF4-Cre and ROCK2Plt−/−mice. Blood flow was monitored continuously from time 0 for up to 60 min (n = 8). *P < 0.05, **P < 0.01, ***P < 0.001. All data are expressed as mean±SEM.
3.5 ROCK2 deficiency in platelets leads to defective hemostasis and vascular thrombosis
To determine whether ROCK2 deficiency could lead to defective hemostasis, we measured tail bleeding times in control and ROCK2Plt−/−mice. The amount of time needed for tail bleeding to stop was substantially longer in ROCK2Plt−/− mice than in PF4-Cre mice (575 ± 46 vs. 365 ± 56 s, n = 20, P < 0.01, Figure 3D). To test whether platelet ROCK2 plays an important role in vascular thrombosis, we assess vascular occlusion using a ferric chloride-induced carotid artery injury model. Compared to PF4-Cre mice where thrombotic occlusion occurred in <30 min, thrombotic vascular occlusion occurred at ∼40 min in ROCK2Plt−/− mice. Indeed, in contrast to PF4-Cre mice, ROCK2Plt−/− mice approached but never reached total occlusion in terms of blood flow cessation (n = 8, P< 0.05; Figure 3E).
3.6 Platelet ROCK2 deficiency is protective in thromboembolic stroke
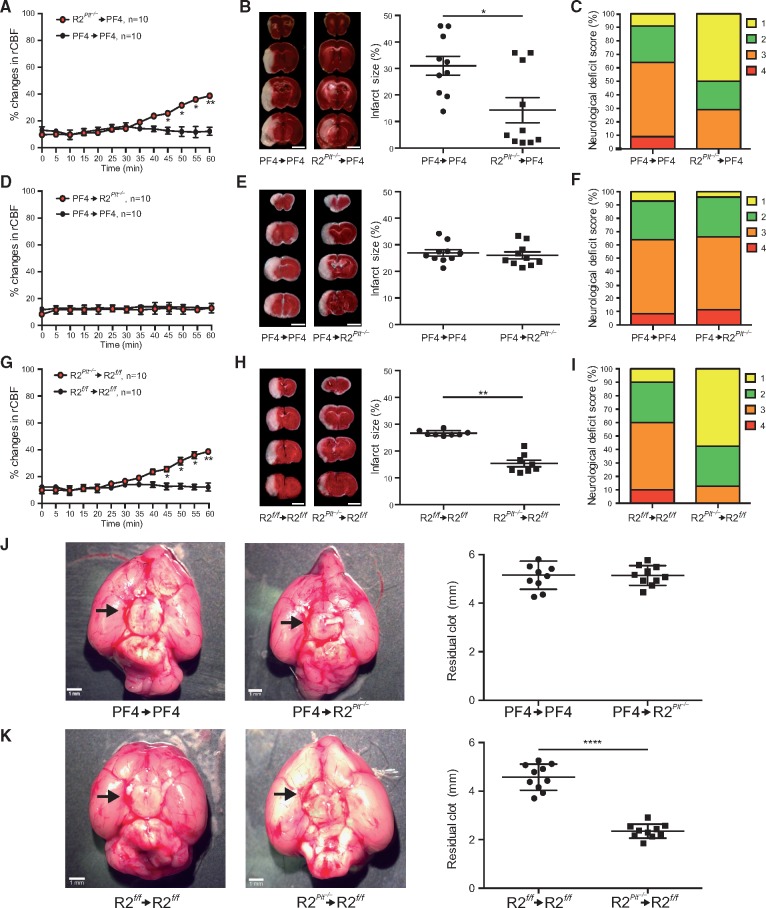
Because ROCK2 deficiency in platelets leads to decreased thrombosis in situ, we next determined whether platelet ROCK2 could play a pathogenic role in acute thromboembolic stroke. When pre-formed blood clots from PF4-Cre (control) or ROCK2Plt−/− mice were injected into the middle cerebral artery (MCA) of PF4-Cre mice, blood flow recovery as measured by increases in relative cerebral blood flow (rCBF) in the MCA territory occurred by 45 min after embolic occlusion in PF4-Cre mice receiving clots from ROCK2Plt−/−, but not PF4-Cre mice (Figure 4A). This correlated with reduction in cerebral infarct volume (31 ± 3 vs. 14 ± 4%, n = 10, P < 0.05; Figure 4B) and improvement in neurological deficit score (1.80 ± 0.24 vs. 2.60 ± 0.24, n = 10, P < 0.05; Figure 5C) in ROCK2Plt−/−→PF4-Cre mice compared to that of PF4-Cre→PF4-Cre mice. To confirm that the neuroprotective effects were due to platelets from donor mice, we repeated the same experiment using clots from control PF4-Cre mice and injecting them into recipient ROCK2Plt−/− and PF4-Cre mice. There were no differences in rCBF, cerebral infarct size (27 ± 1 vs. 26 ± 1%), and neurological deficit score (3.6 ± 0.25 vs. 3.2 ± 0.26) between PF4-Cre→PF4-Cre mice and PF4-Cre→ROCK2Plt−/− mice (P > 0.05 for all measurements, n = 10) (Figure 4D–F) with no difference in residual clot size (5 ± 0.2 vs. 5 ± 0.1 mm, n = 10, P > 0.05, Figure 4J). Similar to ROCK2Plt−/−→PF4-Cre mice, ROCK2Plt−/−→ROCK2fl°x/fl°x mice also exhibited faster recovery of rCBF than their ROCK2fl°x/fl°x→ROCK2fl°x/fl°x counterparts (Figure 4G), lower cerebral infarct volume (26 ± 1 vs. 15 ± 1%, n = 10, P < 0.01; Figure 4H), improved neurological deficit score (1.6 ± 0.14 vs. 3.1 ± 0.32, n = 10, P < 0.05; Figure 4I) and smaller residual clot size (4.5 ± 0.1 vs. 2.3 ± 0.1 mm, n = 10, P < 0.0001, Figure 4K).
Figure 4.
Neuroprotective effects of platelet ROCK2 deficiency in thromboembolic stroke. Pre-formed clots from control (PF4-Cre) or ROCK2Plt−/− mice were injected into the middle cerebral artery of recipient PF4-Cre mice with corresponding (A) percent change in relative cerebral blood flow, (B) coronal brain sections stained with 2,3,5-triphenyltetrazolium chloride (TTC) (left panel, scale bar 5 mm) and cerebral infarct volume (right panel), and (C) neurological deficit score (n = 10). Pre-formed clots from control (PF4-Cre) mice were injected into the middle cerebral artery of recipient PF4-Cre or ROCK2Plt−/− mice with corresponding (D) percent change in relative cerebral blood flow, (E) coronal brain sections stained with (TTC) (left panel) and cerebral infarct volume (right panel), and (F) neurological deficit score (n = 10). Pre-formed clots from control (ROCK2fl°x/fl°x) or ROCK2Plt−/− mice were injected into the middle cerebral artery of recipient ROCK2fl°x/fl°x mice with corresponding (G) percent change in relative cerebral blood flow, (H) coronal brain sections stained with TTC (left panel) and cerebral infarct volume (right panel), and (I) neurological deficit score (n = 10). (J) and (K) Residual clot size after thromboembolic stroke (n = 10). *P < 0.05, **P < 0.01, ****P < 0.0001. All data are expressed as mean±SEM.
Figure 5.
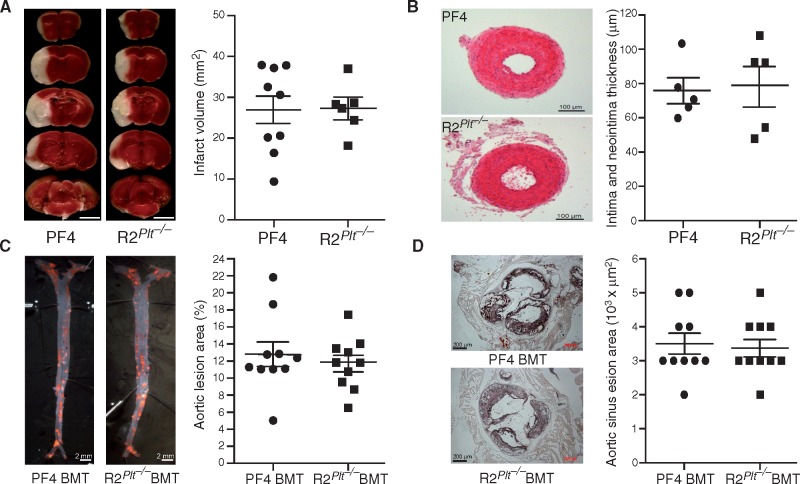
Platelet ROCK2 deficiency does not contribute to non-thrombotic stroke, vascular remodeling, and atherosclerosis. (A) Coronal brain sections stained with 2,3,5-triphenyltetrazolium chloride (TTC) from control (PF4-Cre) and ROCK2Plt−/− mice following middle cerebral artery occlusion (MCAO) (n = 9–6, left panel), and quantification of cerebral infarct volume from TTC-stained brain section (right panel, scale bar 5 mm). (B) H&E staining of common carotid artery 15 days following ligation in PF4-Cre and ROCK2Plt−/− mice (n = 5). (C) Oil Red-O staining and quantification of atherosclerotic lesions of en face aorta and (D) aortic sinus areas cross sections in PF4 BMT and ROCK2Plt−/− BMT mice after 16 weeks of atherogenic diet (n = 10).
3.7 Platelet ROCK2 does not contribute to non-thrombotic stroke, vascular remodeling or atherosclerosis
When the non-thrombotic transient intra-filament MCA occlusion stroke model was used, there were no differences in rCBF (>95% reduction from baseline, data not shown), cerebral infarct volume (27 ± 3.5 vs. 27.3 ± 2.5%, n = 9–6, P > 0.05), or neurological deficit score (data not shown) between PF4-Cre and ROCK2Plt−/− mice (Figure 5A).
To investigate whether platelet ROCK2 could contribute to vascular remodeling we performed a vascular injury model using carotid artery ligation. Morphometric analysis of the common carotid artery after ligation injury did not show any differences in neointima formation between PF4-Cre and ROCK2Plt−/− mice (79 ± 18 vs. 79 ± 13 μm, n = 5, P > 0.05; Figure 5B).
When bone marrows (BM) from PF4-Cre and mice were transplanted into irradiated LDLr−/− mice that were subsequently fed an atherogenic diet, the resultant PF4 BMT and ROCK2Plt−/− BMT mice did not exhibit any differences in the degree of atherosclerosis with respect to en face aortic lesions (13 ± 1 vs. 12 ± 1%, n = 10, P > 0.05; Figure 5C) or aortic sinus lesion areas (468 ± 36 vs. 452 ± 33×103 μm2, n = 10, P > 0.05; Figure 5D).
4. Discussion
We have shown that ROCK2 in platelets is an important mediator of platelet function and thrombosis. Loss of platelet ROCK2 leads to decreased MYPT1 phosphorylation and loss of thrombin-induced pseudopodia formation. Furthermore, ROCK2-deficient platelets exhibited decreased adhesion to collagen and do not readily form homotypic and heterotypic aggregates despite no detectable changes in platelet glycoprotein expression. These findings suggest that platelet function and activation are impaired by the loss of ROCK2 rather than due to changes in cell surface receptor expression. The functional hemostatic consequences of platelet ROCK2 deficiency include increased bleeding time and a longer time period required for thrombotic vascular occlusion following vessel injury. Indeed, thrombotic clots from platelet-specific ROCK2-deficient mice are less stable compared to that of control mice, thereby producing faster cerebral blood flow recovery when injected into the MCA of control mice. Consequently, ROCK2-deficient thrombotic clots cause less cerebral injury and neurological deficits compared to thrombotic clots from control mice. These findings suggest that inhibition of ROCK2 in platelets may have therapeutic benefits in thrombotic vascular diseases such as thrombotic-embolic strokes.
RhoA is a critical for megakaryocytes development and the production of normal, mature platelets. Analysis of RhoA-null megakaryocytes showed that changes in membrane rheology caused the premature release of aberrant, unstable platelets and that this led to macrothrombocytopenia.28 Similar to mice with RhoA-deficient platelets,14 platelet-specific ROCK2 deficiency also leads to macrothrombocytopenia. Reduction in platelets number is actually more pronounced in mice with RhoA-deficient platelets than that of ROCK2-deficient platelets, probably because deficiency of RhoA, which is upstream of ROCK2, affects other downstream RhoA effectors such as ROCK1, mDia, formin, phosphatidylinositol 5 kinase (PIP5K) that are known to contribute to the assembly of the actin ring in megakaryopoiesis. Although the mechanism underlying the macrothrombocytopenia in ROCK2Plt−/− mice is unknown, our data suggest that it may be related to defects in ROCK2-mediated MYPT1 phosphorylation. Our finding that ROCK2-deficient platelets are not forming peripheral actin ring-like structures after thrombin stimulation in contrast to their WT counterparts also indicates a defect in their actin stress fibre assembly and membrane rheology. Indeed, the Rho/ROCK signalling pathway has been implicated in promoting changes in platelet shape in response to thrombin stimulation by mediating MLC2 phosphorylation.14,29 The ROCK inhibitors, HA1077 and Y-27632, inhibits RhoA-dependent MYPT1 phosphorylation,26 leading to decreased actin stress fiber assembly. Interestingly, in platelets with ROCK2 deficiency, ROCK1 levels were also mildly reduced, suggesting that ROCK2 may also be an important regulator of ROCK1 protein levels in platelets.
Our findings that the baseline expression of major glycoproteins in platelets from PF4-Cre and ROCK2Plt−/− mice are similar to the finding of previous studies that do not show differences in glycoprotein levels between PF4-Cre and RhoAPlt−/− mice.14 This indicates that the loss of ROCK2 does not affect the expression of these surface receptors, but rather that the loss of ROCK2 in platelets affects their activation and function. Interestingly, the binding of fibrinogen to αIIbβ3, a glycoprotein that undergoes structural changes in order to bind to fibrinogen,30 was reduced in ROCK2Plt−/− platelets, but only at a low concentration of thrombin stimulation. These findings suggest that ROCK2 is necessary for the initial activation of αIIbβ3, but perhaps, not for maintenance of receptor activation. Higher concentrations of thrombin may induce granule release of other factors that further activate αIIbβ3.30 Indeed, the release of granules from platelets during their activation requires cytoskeletal rearrangements.31 The α-granules contain CD62P, and upon platelet activation, this protein is expressed on the cell surface. Lower expression of this protein on platelets in response to thrombin stimulation was observed in ROCK2Plt−/− mice, suggesting that the secretion of α-granules at low thrombin concentration is dependent on ROCK2. Thus, it appears that ROCK2 is necessary for the initial release of granules, but not for maintaining their release at the higher concentration of thrombin.
ROCK2 deficiency in platelets alters cytoskeletal reorganization, pseudopodia formation, αIIbβ3 activation, and granule secretion. All of these processes are important for platelet aggregation and adhesion. Because collagen signals through protein kinase C (PKC) rather than through the Rho/ROCK pathway, to active αIIbβ3 and re-arrange the actin cytoskeleton,14 there was no difference in aggregation between platelets from PF4 and ROCK2Plt−/− mice in response to collagen. These findings underscore the importance and specificity of ROCK2 and its dependency on the agonist used to activate platelets.
Compared to PF4-Cre mice, there was a reduction in heterotypic and homotypic aggregation of platelets in response to TRAP from ROCK2Plt−/− mice, indicating the importance of ROCK2 in homotypic and heterotypic aggregation. Indeed, under both resting and TRAP-stimulated conditions, very few ROCK2-deficient platelets adhered to collagen. Of the platelets that did adhere to collagen (mainly from PF4-Cre samples), they did not form large aggregates. These findings indicate that platelet ROCK2 is important in mediating platelet adhesion and contributes to their formation of large aggregates and thrombus formation. The reduction of platelet function in ROCK2Plt−/− mice and prolonged bleeding time suggest that platelet ROCK2 is important for clot stabilization during hemostasis. Although the platelet count in ROCK2Plt−/− mice was reduced by 17.1%, this was not sufficient to affect hemostasis.14 Thus, the observed alter hemostasis is probably due to a reduction in platelet function rather than number. Although bleeding time in vivo could also be affected by changes in endothelial function, the in vivo platelet-specific model used in this study assesses the impact of platelet ROCK2 on vascular hemostasis.
Interestingly, the thromboembolic stroke model rather than the intra-filament MCAO model showed stroke protection with platelet ROCK2 deficiency, suggesting that ROCK2 may be more important in thrombus formation than thrombus propagation. However, previous studies with megakaryocyte-specific RhoA deficiency has shown neuroprotective effects after intra-filament MCAO model,14 suggesting that there may be other downstream signalling effects of RhoA rather than ROCK2 in platelets that contributes to neuroprotection in the MCAO model. Finally, little is known about the precise mechanism by which platelet ROCK2 deficiency leads to thromboembolic stroke protection. Thus, further studies are needed to determine whether PIP5K/PIP2 generation, thrombin receptor expression, and various G-protein signaling could contribute to thromboembolic stroke protection in ROCK2Plt−/− mice.
Because platelets are thought to play an important role in mediating vascular remodeling and atherosclerosis,32,33 we investigated whether platelet ROCK2 could contribute to vascular remodeling. We performed a vascular injury model using carotid artery ligation. This flow-cessation model leads to increased vascular inflammation and neointimal proliferation.8 Surprisingly, morphometric analysis of the common carotid artery after ligation injury did not show any differences in neointima formation between PF4-Cre and ROCK2Plt−/− mice, suggesting that platelet ROCK2 does not contribute to neointima proliferation and vascular remodeling after injury. These findings are consistent with our previous study showing that bone marrow transplantation from ROCK2-deficient mice did not affect neointima formation following carotid artery ligation.8 These findings suggest that platelet ROCK2 does not contribute to neointima proliferation and vascular remodeling after injury. Furthermore, in contrast to the role of ROCK2 in macrophages, which mediates atherogenesis, in part, by inhibiting reverse cholesterol transport and cholesterol efflux,34 ROCK2-deficient platelets do not play an important role in the development of atherosclerosis.
In summary, we found that ROCK2 is an important mediator of platelet activation. This occurs, in part, through the phosphorylation of MYPT1, which may be a final common pathway for platelet activation. It is not known whether ROCK1, which also phosphorylates MYPT1, could also play a similar role in platelet activation and thrombosis. Further studies are needed to address this issue. Nevertheless, this study provides evidence regarding the importance of platelet ROCK2 in hemostasis and the pathogenesis of thromboembolic stroke. It remains to be determining whether inhibition of platelet ROCK2 will have therapeutic benefits in patients with thromboemblic strokes.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: J.K.L. is a consultant for Asahi-Kasei Pharmaceuticals, Inc. All of the other authors have no conflict of interest.
Funding
This study was supported by grants from the National Institutes of Health (NS070001, A1078894, HL126743, HL059561, HL104145, T32-HL0722), the National Research Foundation of Korea (No. 2012R1A3A2026454, G.T.O.), and the Foundation for Polish Science (K.K.), the American Heart Association (SDG Award, H.-H.-K.), the American Society of Hematology (H.F.), and the Brigham Research Institute (Falet).
Supplementary Material
References
- 1. del Zoppo GJ. The role of platelets in ischemic stroke. Neurology 1998;51:S9–S14. [DOI] [PubMed] [Google Scholar]
- 2. Stoll G, Kleinschnitz C, Nieswandt B.. Molecular mechanisms of thrombus formation in ischemic stroke: novel insights and targets for treatment. Blood 2008;112:3555–3562. [DOI] [PubMed] [Google Scholar]
- 3. Lo EH, Dalkara T, Moskowitz MA.. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci 2003;4:399–415. [DOI] [PubMed] [Google Scholar]
- 4. Riento K, Ridley AJ.. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol 2003;4:446–456. [DOI] [PubMed] [Google Scholar]
- 5. Maekawa M, Ishizaki T, Boku S, Watanabe N, Fujita A, Iwamatsu A, Obinata T, Ohashi K, Mizuno K, Narumiya S.. Signaling from Rho to the actin cytoskeleton through protein kinases ROCK and LIM-kinase. Science 1999;285:895–898. [DOI] [PubMed] [Google Scholar]
- 6. Carroll RC, Butler RG, Morris PA, Gerrard JM.. Separable assembly of platelet pseudopodal and contractile cytoskeletons. Cell 1982;30:385–393. [DOI] [PubMed] [Google Scholar]
- 7. Wang LL, Bryan J.. Isolation of calcium-dependent platelet proteins that interact with actin. Cell 1981;25:637–649. [DOI] [PubMed] [Google Scholar]
- 8. Noma K, Rikitake Y, Oyama N, Yan G, Alcaide P, Liu PY, Wang H, Ahl D, Sawada N, Okamoto R, Hiroi Y, Shimizu K, Luscinskas FW, Sun J, Liao JK.. ROCK1 mediates leukocyte recruitment and neointima formation following vascular injury. J Clin Invest 2008;118:1632–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shimizu Y, Thumkeo D, Keel J, Ishizaki T, Oshima H, Oshima M, Noda Y, Matsumura F, Taketo MM, Narumiya S.. ROCK-I regulates closure of the eyelids and ventral body wall by inducing assembly of actomyosin bundles. J Cell Biol 2005;168:941–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thumkeo D, Keel J, Ishizaki T, Hirose M, Nonomura K, Oshima H, Oshima M, Taketo MM, Narumiya S.. Targeted disruption of the mouse rho-associated kinase 2 gene results in intrauterine growth retardation and fetal death. Mol Cell Biol 2003;23:5043–5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aslan JE, McCarty OJ.. Rho GTPases in platelet function. J Thromb Haemost 2013;11:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McCarty OJ, Larson MK, Auger JM, Kalia N, Atkinson BT, Pearce AC, Ruf S, Henderson RB, Tybulewicz VL, Machesky LM, Watson SP.. Rac1 is essential for platelet lamellipodia formation and aggregate stability under flow. J Biol Chem 2005;280:39474–39484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pleines I, Eckly A, Elvers M, Hagedorn I, Eliautou S, Bender M, Wu X, Lanza F, Gachet C, Brakebusch C, Nieswandt B.. Multiple alterations of platelet functions dominated by increased secretion in mice lacking Cdc42 in platelets. Blood 2010;115:3364–3373. [DOI] [PubMed] [Google Scholar]
- 14. Pleines I, Hagedorn I, Gupta S, May F, Chakarova L, van Hengel J, Offermanns S, Krohne G, Kleinschnitz C, Brakebusch C, Nieswandt B.. Megakaryocyte-specific RhoA deficiency causes macrothrombocytopenia and defective platelet activation in hemostasis and thrombosis. Blood 2012;119:1054–1063. [DOI] [PubMed] [Google Scholar]
- 15. Masumoto A, Mohri M, Shimokawa H, Urakami L, Usui M, Takeshita A.. Suppression of coronary artery spasm by the Rho-kinase inhibitor fasudil in patients with vasospastic angina. Circulation 2002;105:1545–1547. [DOI] [PubMed] [Google Scholar]
- 16. Sato M, Tani E, Fujikawa H, Kaibuchi K.. Involvement of Rho-kinase-mediated phosphorylation of myosin light chain in enhancement of cerebral vasospasm. Circ Res 2000;87:195–200. [DOI] [PubMed] [Google Scholar]
- 17. Masumoto A, Hirooka Y, Shimokawa H, Hironaga K, Setoguchi S, Takeshita A.. Possible involvement of Rho-kinase in the pathogenesis of hypertension in humans. Hypertension 2001;38:1307–1310. [DOI] [PubMed] [Google Scholar]
- 18. Kataoka C, Egashira K, Inoue S, Takemoto M, Ni W, Koyanagi M, Kitamoto S, Usui M, Kaibuchi K, Shimokawa H, Takeshita A.. Important role of Rho-kinase in the pathogenesis of cardiovascular inflammation and remodeling induced by long-term blockade of nitric oxide synthesis in rats. Hypertension 2002;39:245–250. [DOI] [PubMed] [Google Scholar]
- 19. Sawada N, Itoh H, Ueyama K, Yamashita J, Doi K, Chun TH, Inoue M, Masatsugu K, Saito T, Fukunaga Y, Sakaguchi S, Arai H, Ohno N, Komeda M, Nakao K.. Inhibition of rho-associated kinase results in suppression of neointimal formation of balloon-injured arteries. Circulation 2000;101:2030–2033. [DOI] [PubMed] [Google Scholar]
- 20. Mallat Z, Gojova A, Sauzeau V, Brun V, Silvestre JS, Esposito B, Merval R, Groux H, Loirand G, Tedgui A.. Rho-associated protein kinase contributes to early atherosclerotic lesion formation in mice. Circ Res 2003;93:884–888. [DOI] [PubMed] [Google Scholar]
- 21. Feske SK, Sorond FA, Henderson GV, Seto M, Hitomi A, Kawasaki K, Sasaki Y, Asano T, Liao JK.. Increased leukocyte ROCK activity in patients after acute ischemic stroke. Brain Res 2009;1257:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kajikawa M, Noma K, Maruhashi T, Mikami S, Iwamoto Y, Iwamoto A, Matsumoto T, Hidaka T, Kihara Y, Chayama K, Nakashima A, Goto C, Liao JK, Higashi Y.. Rho-associated kinase activity is a predictor of cardiovascular outcomes. Hypertension 2014;63:856–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dong M, Jiang X, Liao JK, Yan BP.. Elevated rho-kinase activity as a marker indicating atherosclerosis and inflammation burden in polyvascular disease patients with concomitant coronary and peripheral arterial disease. Clin Cardiol 2013;36:347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Okamoto R, Li Y, Noma K, Hiroi Y, Liu PY, Taniguchi M, Ito M, Liao JK.. FHL2 prevents cardiac hypertrophy in mice with cardiac-specific deletion of ROCK2. FASEB J 2013;27:1439–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tiedt R, Schomber T, Hao-Shen H, Skoda RC.. Pf4-Cre transgenic mice allow the generation of lineage-restricted gene knockouts for studying megakaryocyte and platelet function in vivo. Blood 2007;109:1503–1506. [DOI] [PubMed] [Google Scholar]
- 26. Rikitake Y, Kim HH, Huang Z, Seto M, Yano K, Asano T, Moskowitz MA, Liao JK.. Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection. Stroke 2005;36:2251–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang Z, Chopp M, Zhang RL, Goussev A.. A mouse model of embolic focal cerebral ischemia. J Cereb Blood Flow Metab 1997;17:1081–1088. [DOI] [PubMed] [Google Scholar]
- 28. Suzuki A, Shin JW, Wang Y, Min SH, Poncz M, Choi JK, Discher DE, Carpenter CL, Lian L, Zhao L, Wang Y, Abrams CS.. RhoA is essential for maintaining normal megakaryocyte ploidy and platelet generation. PLoS One 2013;8:e69315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chang Y, Aurade F, Larbret F, Zhang Y, Le Couedic JP, Momeux L, Larghero J, Bertoglio J, Louache F, Cramer E, Vainchenker W, Debili N.. Proplatelet formation is regulated by the Rho/ROCK pathway. Blood 2007;109:4229–4236. [DOI] [PubMed] [Google Scholar]
- 30. Moers A, Nieswandt B, Massberg S, Wettschureck N, Gruner S, Konrad I, Schulte V, Aktas B, Gratacap MP, Simon MI, Gawaz M, Offermanns S.. G13 is an essential mediator of platelet activation in hemostasis and thrombosis. Nat Med 2003;9:1418–1422. [DOI] [PubMed] [Google Scholar]
- 31. Woronowicz K, Dilks JR, Rozenvayn N, Dowal L, Blair PS, Peters CG, Woronowicz L, Flaumenhaft R.. The platelet actin cytoskeleton associates with SNAREs and participates in alpha-granule secretion. Biochemistry 2010;49:4533–4542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Davi G, Patrono C.. Platelet activation and atherothrombosis. N Engl J Med 2007;357:2482–2494. [DOI] [PubMed] [Google Scholar]
- 33. Ross R, Glomset JA.. The pathogenesis of atherosclerosis (first of two parts). N Engl J Med 1976;295:369–377. [DOI] [PubMed] [Google Scholar]
- 34. Zhou Q, Mei Y, Shoji T, Han X, Kaminski K, Oh GT, Ongusaha PP, Zhang K, Schmitt H, Moser M, Bode C, Liao JK.. Rho-associated coiled-coil-containing kinase 2 deficiency in bone marrow-derived cells leads to increased cholesterol efflux and decreased atherosclerosis. Circulation 2012;126:2236–2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.