Abstract
Extracellular vesicles (EVs)—particularly exosomes and microvesicles (MVs)—are attracting considerable interest in the cardiovascular field as the wide range of their functions is recognized. These capabilities include transporting regulatory molecules including different RNA species, lipids, and proteins through the extracellular space including blood and delivering these cargos to recipient cells to modify cellular activity. EVs powerfully stimulate angiogenesis, and can protect the heart against myocardial infarction. They also appear to mediate some of the paracrine effects of cells, and have therefore been proposed as a potential alternative to cell-based regenerative therapies. Moreover, EVs of different sources may be useful biomarkers of cardiovascular disease identities. However, the methods used for the detection and isolation of EVs have several limitations and vary widely between studies, leading to uncertainties regarding the exact population of EVs studied and how to interpret the data. The number of publications in the exosome and MV field has been increasing exponentially in recent years and, therefore, in this ESC Working Group Position Paper, the overall objective is to provide a set of recommendations for the analysis and translational application of EVs focussing on the diagnosis and therapy of the ischaemic heart. This should help to ensure that the data from emerging studies are robust and repeatable, and optimize the pathway towards the diagnostic and therapeutic use of EVs in clinical studies for patient benefit.
Keywords: Exosomes, Microvesicles, Extracellular vesicles, Ischaemia, Reperfusion, Cardioprotection, Heart failure, Remote conditioning, Preconditioning, Postconditioning, Co-morbidities, Regenerative medicine
1. Introduction
1.1 Cellular secretion for communication; extracellular vesicles
Cells in multicellular organisms must communicate efficiently with each other in order to propagate signals and co-ordinate function. In addition to distinct chemical signals (paracrine and endocrine) and direct cell-cell contact, a growing body of evidence shows that cells communicate via a variety of small, membrane-enclosed vesicles, collectively termed ‘extracellular vesicles’ (EVs). EVs ranging from ∼40 nm to several microns in size (Figure 1), are released into all extracellular fluids including blood. They transport a cell-specific cargo of proteins, lipids, metabolites, and nucleic acids that can affect target cells. This process occurs during normal cellular physiology as well as during stress and disease. In this rapidly evolving area of research, with its associated technical challenges, it is vital to establish standardized techniques for their isolation and criteria for their identification. EVs are released by all cardiac, endothelial and inflammatory cell types, suggesting they have an important role in the cardiovascular system, including the ischaemic heart.1–6
Figure 1.
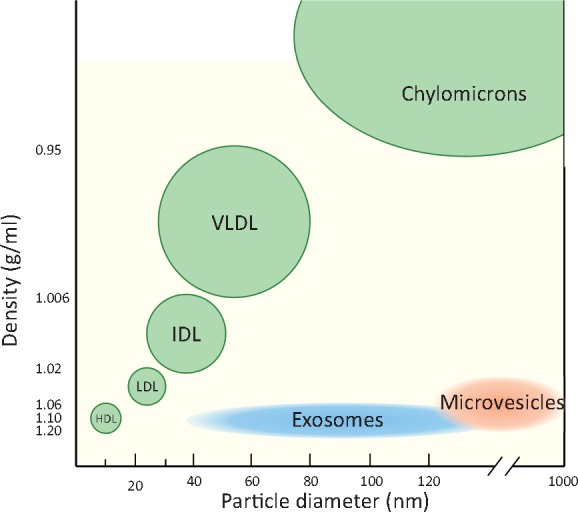
Exosomes and MVs overlap in size with VLDL and chylomicrons, and in density with HDL/LDL particles. Exosome density is typically 1.06–1.20 g/mL. MV density is not well defined but they have been found between ∼1.03–1.08 g/mL.10–12
Therefore, the overall objective of this position paper is to provide a set of recommendations for the isolation, characterization, analysis, and translational application of EVs focussing mainly on the ischaemic heart, i.e. acute myocardial infarction and post-ischaemic heart failure. Coronary atherosclerosis is not discussed in this position paper in detail (see recent reviews and position papers of the European Society of Cardiology that covers this topic extensively7–9).
2. Isolation and characterization of EVs
2.1 Definition of EVs
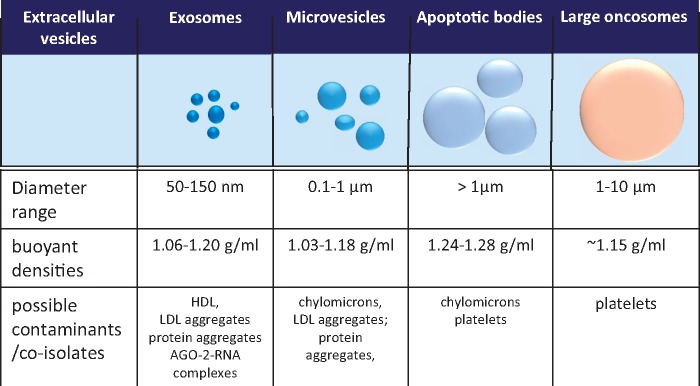
Eukaryotic EVs include (i) exosomes released by exocytosis of multivesicular bodies (usually 50–150 nm), (ii) Microvesicles (MVs, also called microparticles or ectosomes), vesicles around 0.1–1 μm in diameter shed from the plasma membrane,10–12 and (iii) apoptotic vesicles released by blebbing of apoptotic cells some of which are > 1 μm (apoptotic bodies),13,14 and (iv) large oncosomes released by migratory tumour cells (> 1 µm)15,16 (Figure 2). However, since current isolation protocols only result in relative enrichment of vesicle subpopulations rather than their complete purification,17 and that no specific markers are available for the subpopulations, it is preferable to refer to purified vesicles as ‘EVs’ and accurately report the purification method used and characteristics present. An operational definition of small EVs (sEVs) is appropriate for the exosome-enriched population pelleting at high speeds.17
Figure 2.
The major classes of EV. Typical size and density of EV classes and some of the contaminants that may be co-isolated, depending on biofluid.
2.2 Methods for the isolation and characterization of EVs
Several methods of isolating EVs have been developed (Figure 3). The optimal EV isolation procedure will depend on the source biofluid, the EV subpopulation of interest, and their intended end-use, whether that is to be diagnostic biomarker studies, mechanistic studies, or for in vivo administration. However, no EV isolation method yet exists that can be considered as a gold standard, since residual proteins and/or lipoproteins remains problematic.18 Complete removal of lipoproteins (present in both blood and tissue culture serum) remains challenging due to overlapping size and/or densities between EVs and different lipoprotein particles (Figures 1 and 2).12,19,20 Moreover, low density lipoprotein (LDL) and exosomes may associate, rendering their complete separation from blood samples impossible using any technique.12 This, however, might be used in isolating a subset of EVs co-precipitated with LDL or high density lipoprotein (HDL) particles.21,22
Figure 3.
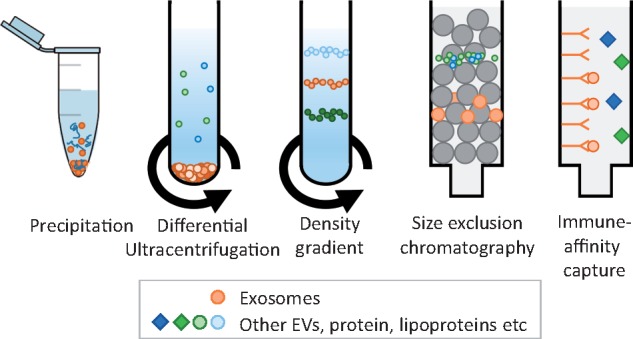
Standard techniques used for isolating exosomes from other EVs, protein, and lipoproteins present in blood and cell-culture medium.
In the simplest technique based on precipitation (e.g. ExoQuickTM), the biofluid sample is mixed and incubated with a hydrophilic polymer prior to low speed centrifugation. The polymers attract water molecules away from the solvation layer around the EVs causing their precipitation. Several manufacturers now market products based on this technique. However, they result in high levels of contaminants including serum proteins and lipoproteins as well as residual matrix that can affect EV biological functions.23,24
Differential centrifugation has long been regarded as the gold standard technique.19 In the most commonly used protocol, cells are removed by centrifugation at 300 x g for 10 min; the supernatant is then cleared of apoptotic bodies by centrifugation at 2000 x g for 10 min followed by 10 000 x g for 30 min to preferentially pellet MVs; exosome-enriched sEVs are then purified from the supernatant by ultracentrifugation at 100 000 x g for 70 min.19 However, optimal parameters are highly dependent on the type of centrifuge rotor used.25 For further purification of EVs from co-pelleted protein complexes26 and lipoproteins, a density gradient is recommended (sucrose or preferably iodixanol).19 Because of the number of steps involved and the length of the procedure, it is clearly unsuited for the analysis of large numbers of samples. More recently, concerns have been raised that ultracentrifugation can cause damage, fusion, and/or aggregation of vesicles.27
There is increasing enthusiasm for the use of size-exclusion chromatography.28 Suitable matrices include Sepharose 2B, Sepharose CL-4B, or Sephacryl S-400.29 The resolution of size separation is dependent on column length. This technique is effective at separating EVs from proteins and some lipoproteins, but samples usually become considerably diluted and efficient separation of lipoproteins remain challenging.12,29,30
Other techniques are less well established. Filtration (0.2 μm–0.8 μm) aids in removal of larger vesicles, but under high pressure it is possible that it could cause the fragmentation of larger EVs into smaller vesicles. Immuno-affinity can be an effective means to isolate specific EV populations, using columns or magnetic beads for example, but for functional follow-up studies this is still challenging. Flow cytometry-based analysis of individual EVs is a valuable tool for EV characterization and quantification, although it remains challenging due to size and sensitivity limitations.31 Progress has been made in developing these technologies for specific EV sorting, but some pre-requisite steps need to be taken.32 Furthermore, this approach requires certainty as to the specificity of epitopes expressed by the desired population of EVs–which may not be the case for exosomes.33 Other isolation techniques such as microfluidics are under development but have not yet been rigorously tested. For pre-clinical or clinical studies of the ischaemic heart, measurement of EVs can be performed from in vivo blood, lymphatic or pericardial fluid samples, ex vivo heart perfusate samples, and tissue culture media samples that may require different isolation techniques.
2.2.1 Isolation from blood
Pre-analytical procedures can have a large impact on blood EV measurements. For example, since clotting may increase the number of EVs in blood by 10-fold,34 it is usually preferable to use plasma. On the other hand, serum may be useful when overall yield of platelet MVs is more important than accurate quantification of particle number. A crucial concern is the minimization of platelet activation and EV release. Standardized procedures to minimize platelet activation during plasma isolation should be followed.35,36 Fasting before blood sampling can help to minimize chylomicron contamination.12 Blood should be collected in citrated or acid-citrate-dextrose anticoagulant tubes,23,35,37 such as vacutainers, and the first tube of blood should be discarded.23,35 It is recommended to dilute blood plasma or serum at least 2x in Ca2+-free phosphate buffered saline (PBS) prior to centrifugation in order to reduce the viscosity.19 However, if annexin V binding will be assessed (which requires Ca2+), PBS should be avoided in order to prevent formation of calcium-phosphate micro-precipitates. The plasma or serum should be centrifuged within 2 h, and agitation avoided.35,38 After centrifugation at 2500 x g for 15 min at room temperature without application of the centrifuge brake, plasma can be carefully collected, and re-centrifuged under identical conditions. This platelet-free-plasma may be snap frozen and stored at –80 °C prior to analysis.
Even when using the same protocol, inter-laboratory variability in plasma EV counts can vary by an order of magnitude.35 Given these problems of irreproducibility, The International Society on Thrombosis, and Haemostasis has advised that further refinements are required before flow cytometric enumeration of platelet MV numbers is ready for clinical use.35
2.2.2 Isolation from pericardial fluid
Pericardial fluid contains EVs that may provide useful biomarker information about cardiac health.39,40 As yet there is no consensus as to the ideal method for isolation of EVs from pericardial fluid.
2.2.3 Isolation from conditioned media of cultured cells
For the isolation of vesicles produced by cells in tissue culture the important considerations are quite different. The main potential source of contamination is typically from foetal calf serum (FCS) added to the culture medium.41 FCS contains large number of vesicles including exosomes as well as lipoproteins. Exosomes can be largely removed by pre-treating FCS by 18 h ultracentrifugation at 100 000 × g,41 and removal is enhanced by diluting FCS five-fold in culture medium to reduce viscosity.23 Several companies market FCS which has been processed to remove exosomes, though the method used is not specified. However, some caution should be taken for FBS-associated RNA which might be co-isolated with cell-culture derived extracellular RNA (exRNA), thereby interfering with the downstream RNA analysis.42 Alternatively, pre-defined serum or serum-free conditions can be used, and indeed is essential if preparing EVs for clinical use.43 However, cells may undergo apoptosis or autophagy and release apoptotic bodies after extended periods in the absence of serum. Conditioned medium is usually collected after 24–48 h culture. Although sequential filtration offers the advantage of using large volumes of culture media,44 its effect on biological activity of the isolated EVs has not been well characterized. HPLC has been successfully used to purify exosomes.45
2.2.4 Isolation from isolated heart perfusate
EVs can be isolated from hearts perfused with buffer such as those mounted on a Langendorff apparatus.46 Pre-concentration of the perfusate by ultrafiltration may be necessary for a sufficient yield, but subsequently any of the techniques described above may be used. It is important to be aware that exosome-sized, calcium-phosphate nanoparticles form spontaneously in Ca2+-containing bicarbonate buffer, which can interfere with some analyses such as nanoparticle tracking analysis.47
2.2.5 Storage of EVs
EVs appear to be relatively stable when stored at –80 °C or less,48 but repeated freeze-thaw cycles should be avoided and cryo-preservatives such as glycerol and DMSO should not be added as they may lyse EVs.48 Trehalose has recently been proposed to preserve exosomes.49
2.2.6 Visualization and quantitation of EVs
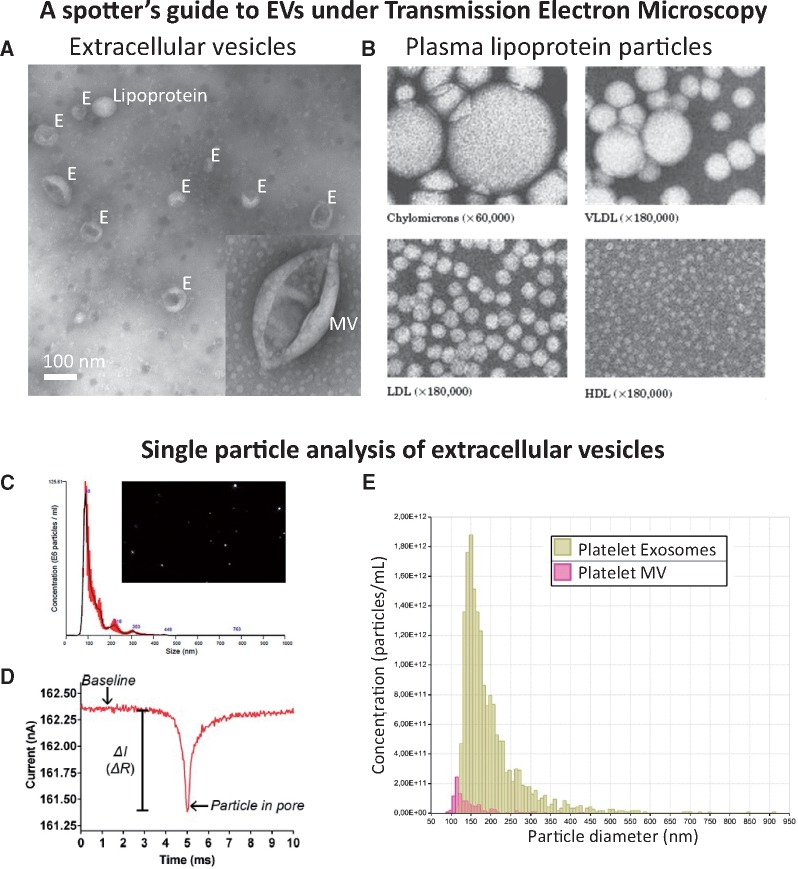
In most studies, it is informative to quantify EVs using either nanoparticle tracking analysis, tuneable resistive pulse sensing, or dynamic light scattering (Figure 4). Importantly, these methods usually do not discriminate EVs from non-vesicular events such as LDL particles.12 The ratio of particle number or lipid content to protein content of the preparations can give an indication of relative EV purity, as compared to protein concentration only.50,51 Exosome morphology should be confirmed by transmission electron microscopy,19 cryo-electron microscopy,52 or atomic force microscopy.53 In addition, presented images should contain multiple EVs per field (Figure 4A). Using flow cytometry, the lower size-limit of detection is steadily decreasing, but it remains technically challenging to obtain comprehensive analyses of MVs let alone exosome-sized particles.32
Figure 4.
Standard methods of characterizing EVs. (A) Transmission electron microscopy (TEM) of negative-stained EVs reveals the ‘cup-shaped’ appearance of exosomes (E) and MVs once they have been dried for TEM (they are spherical in solution). (B) The spherical appearance of lipoprotein particles by TEM is quite distinct (image courtesy of Robert L. Hamilton and the Arteriosclerosis Specialized Center of Research, University of California, San Francisco). (C) Nanoparticle tracking analysis (NTA) provides a size distribution of particles based on calculating their size by their random Brownian motion. (D, E) Tuneable resistance pulse sensing (TRP) determines size distribution by the change in resistance as the particle crosses a small pore in a membrane (which is selected according to the size range examined).
2.2.7 Characterization of EVs
Further characterization of EVs should include the detection of specific markers. It is generally recommended to demonstrate presence of tetraspanin proteins such as CD9, CD81, CD63, and the intra-vesicular protein Alix, which are involved in exosome biogenesis, in addition to other typical marker proteins such as HSP70, flotillin-1, or major histocompatibility complex (MHC) class I and class II. However, recent studies indicate some of these are not as specific for exosomes as thought.17,54 Co-enrichment of CD63, CD9, and CD81 tetraspanins and endosome markers such as syntenin-1 and TSG101 may be seen as indicative of exosome presence.17 MV surface marker expression is a useful index of cell-type of origin, and can be quantified by flow cytometry in order to analyse sub-populations. In addition, MVs may be characterized as ‘calcium-dependent annexin V binding’ or ‘calcium-independent lactadherin (MFGE8) binding’. Gating strategies for flow cytometry involve the use of fluorescent reference beads of known sizes. Preferred beads are the silica beads because their refractive index is close to the one of biological particles.55 Of note, not all MVs are detectable this way. To quantitatively detect exosome proteins, a nano-plasmonic exosome sensor was developed that comprises arrays of periodic nanoholes patterned in a metal film. The arrays are functionalized with affinity ligands for different exosomal protein markers and offers highly sensitive and label-free exosome analyses by continuous and real-time monitoring of molecular binding.56
In order to demonstrate EV functionality it may be useful to demonstrate their interaction with, fusion, or uptake into recipient cells. To this end, EVs can be fluorescently labelled with lipophilic dyes, or by transfection of parent cells with GFP-tagged proteins packaged in EVs. Control experiments using ‘dye-only’ samples prepared in parallel the same way as EVs are essential to confirm the involvement of EVs57 and that free dye has been removed, which can otherwise form micelles. Density gradient ultracentrifugation is a frequently used approach to remove this non-EV-associated dye. Co-incubation with receptor antagonists or uptake inhibitors will provide insights in mechanisms of uptake although these compounds are notorious for their toxicity and limited specificity.58
2.2.8 Analysis of nucleic acid, protein, and lipid contents of EVs
Proteomics can provide a more comprehensive description of EV protein content, but the isolation protocol must be carefully optimized in order to remove plasma protein contamination in blood EV samples.59,60 Similar concerns arise with transcriptomic analyses of miRNA, mRNA, or DNA contents, particularly with regard to potentially contaminating HDL particles and argonaute 2-RNA complexes, which are known to transport miRNA.61 Care must be taken to avoid haemolysis which can affect circulating miRNA levels.62
EV proteomics has been studies in large clinical cohorts,63 but undoubtedly the greatest interest is currently focused at the RNA content of EVs as potential biomarkers.63EV lipidomics is an interesting but under-explored area.64 Nucleic acid, protein and lipid entries from EV studies are available in EV databases (http://www.exocarta.org/, http://student4.postech.ac.kr/evpedia2_xe/xe/ and http://microvesicles.org/ (10 November 2017, date last accessed)). However, because of the diversity of EV isolation protocols used, these database entries should be treated with caution.
2.2.9 Technical control experiments
RNase or protease may be applied to remove extra-vesicular nucleic acids and proteins and confirm intra-vesicular localization. Detergent control during flow cytometry or sizing performed by tuneable resistive pulse sensing or dynamic light scattering enables distinguishing EVs from protein aggregates (the latter being more resistant to detergents lysis than EVs).26,65,66 Of note, small and large EVs have different detergent sensitivities67 and lipoproteins also show a limited sensitivity to detergent lysis.12
2.3 Recommendations for isolation and purification of EVs
At present, there is no universally agreed protocol for isolation of pure populations of EVs or subpopulations of EVs. Even their precise nomenclature is in flux, and will presumably remain so until clear surface protein signatures of individual EV subtypes will be established. Given the rapid developments in this area it is important to remain cognizant of experimental limitations and caveats in order to avoid overstating conclusions. At present, it is challenging to isolate EVs from tissue homogenates. The likely contamination of EV preparations with proteins and lipoproteins renders many results suspect unless crucial control experiments are performed. Differential detergent lysis is a simple, inexpensive EV control for flow cytometry.26,65 Furthermore, to avoid swarm detection at high particle concentrations, analysis of serial dilution of samples is recommended during flow cytometry.32 A characterization of EV proteins will provide a basis on purity without excluding a potential biological role. Table 1 provides a check-list of criteria for the successful isolation and characterization of EVs, as well as more specific criteria for exosomes.
Table 1.
Recommendations for the isolation and characterization of EVs [adapted from Ref. 18]
| General recommendations for EVs |
|
| Specific recommendations for exosomes |
|
| Specific recommendations for MVs |
|
Some markers such as CD63 may not be completely specific for exosomes.17
3. Mechanism of EV actions in the heart
EVs appear to be released from all major cell types found in the heart. For example, exosomes have been shown to be released by primary adult cardiomyocytes,68,69 primary cardiac endothelial cells,70 primary cardiac fibroblasts,71 and vascular smooth muscle cells.72 Most MVs present in the plasma of healthy individuals are derived from platelets and erythrocytes, but plasma MVs also originate from leucocytes, endothelial cells, monocytes neutrophils, and lymphocytes.73,74 Most mechanistic experiments to date use EVs isolated from cultured cells, since isolation of cell-type specific EVs in vivo is not currently feasible. Recently, a clear overview was provided on the role of EVs in coronary artery disease.7 This section will discuss the most relevant examples of the function of EVs released from cells of the heart tissue and their postulated mechanisms of action.
3.1 Cardiomyocyte-derived EVs
Cardiomyocytes are potentially an important source of diagnostic EVs particularly in situations of stress such as myocardial ischaemia and failure.1 However, few studies have examined exosomes from adult as opposed to neonatal cardiomyocytes, and even here it is unclear to what extent this relates to the situation in vivo. In vitro, hypoxia and re-oxygenation leads to the release of heat shock proteins (HSPs) HSP70 and HSP90, as well as HSP60, a ‘danger signal’, via exosomes in primary adult rat cardiomyocytes.68 Cardiomyocytes also release EVs containing tumour necrosis factor (TNF)-α,75 potentially participating in the propagation of an inflammatory response. Glucose deprivation induced the loading of neonatal rat cardiomyocyte exosomes with functional glucose transporters and glycolytic enzymes. When internalized by endothelial cells, these exosomes increased glucose uptake, glycolytic activity, and pyruvate production in recipient cells.76 The transfer of exosomes from glucose-deprived H9C2 cardiac myoblasts to endothelial cells also induced changes in transcriptional activity of pro-angiogenic genes.77 Hyperglycaemia altered cardiomyocyte-derived exosomes in a model of diabetes-associated cardiomyopathy,78 as well as from the hypoxic myocardium which can activate in vitro cultured endothelial cells.79,80 Finally, external stretch caused cardiomyocytes to release exosomes enriched in functional angiotensin II type-1 receptors (AT1Rs).69 Administration of AT1R-enriched exosomes restored responsiveness to angiotensin II in the vessels of AT1R-KO mice. Together, these data indicates that cardiomyocytes respond to environmental changes by releasing specific EVs that specifically modulate neighbour-cell function.
3.2 Cardiac progenitor cells-derived EVs
A variety of cells with the capacity to proliferate and differentiate into cardiac cells have been termed cardiac progenitor cells (CPCs). Exosomes released from these cell types appear to recapitulate the cardioprotective and regenerative benefits of their parent cells.81,82 Hypoxia stimulated exosome release from CPCs and upregulated their expression of pro-angiogenic genes, anti-fibrotic genes, and a cluster of miRs, and increased their ability to improved cardiac function after IR injury in rats.83,84 Vrijsen et al. reported that human CPCs release exosomes into their environment, and that exosomes from CPCs are able to stimulate the migration of endothelial cells in an in vitro scratch wound assay.85 They also showed that CPC-exosomes contain matrix metalloproteinases (MMPs) and the extracellular matrix metalloproteinase inducer (EMPRINN), which mediate their angiogenic potential.86
3.3 Endothelial cell-derived EVs
Endothelial cells are an important source of EVs. Vascular endothelial cells secrete exosomes and MVs that exchange biological messages with other cell types of the heart.4,7 There is extensive literature demonstrating the effects of endothelial MV in promoting angiogenesis (reviewed in Ref. 87). Endothelial exosomes have also been shown to stimulate angiogenesis via a mechanism that is believed to involve transfer of miR-146a.88 Hypoxia alters mRNA and protein composition of exosomes released by cultured endothelial cells.89 TNF-α-treated endothelial cells release exosomes expressing increased levels of intercellular adhesion protein (ICAM)-1.89 These findings exemplify the potential function of endothelial cell-derived exosomes and MV that may also make them useful as biomarkers of cardiac stress and disease.4,9
3.4 Vascular progenitor cell-derived EVs
While their exact differentiation potential has been debated, it is evident that CD34+ cells from bone marrow secrete exosomes that possess angiogenic characteristics, enhance tube formation of endothelial cells, and increase neovascularization in vivo.90 Further analysis revealed enrichment of several pro-angiogenic miRs (miR-126 and 130a) in CD34+ cell-derived exosomes. MVs derived from human vascular progenitor cells carry several markers similar to receptors expressed on their membranes.91 In various disease models, these MVs were shown to enhance expression of angiogenic miRs (miR-126 and 296), and promote neovascularization of pancreatic islets92 and ischaemic hind limb.93
3.5 EVs from fibroblasts, smooth muscle cells, and mesenchymal stromal cells
Ischaemia, pressure, and volume overload induce hypertrophic cellular responses mediated by cross-talk among fibroblast cardiomyocytes, endothelial cells, and inflammatory cells via EVs. In response to angiotensin II, cardiac fibroblasts secreted exosomes that stimulated angiotensin II production and its receptor expression in cardiomyocytes, and stimulated myocyte hypertrophy.94 Exosomes released from cardiac fibroblasts contained high levels of miR-21-3p/miR-21, which induced cardiomyocyte hypertrophy.94 Smooth muscle cells also release exosomes and are implicated in vessel calcification and atherosclerosis.72,95 Mesenchymal stromal/stem cells (MSCs) are resident in almost all tissues, including the heart, and play a major role in tissue repair and regeneration.96 Characterization of the MSC exosome proteome has revealed many cytokines, growth factors, inflammatory molecules, components of the extracellular matrix, and proteases. Analysis of protein content revealed >400 different proteins.97 Many signalling molecules related to MSC self-renewal, differentiation, and signalling pathways were found to be enriched in MSC exosomes, potentially affecting a diverse range of cellular processes, including cell cycle, proliferation, cell adhesion, cell migration, and cell morphogenesis. Similarly, miRNAs shuttle within MSC exosomes but mostly in precursor form, driving downstream signalling pathways. Additionally, MSC exosomes possess immunologic properties, including secretion of anti-inflammatory cytokines, such as interleukin-10, tumour growth factor (TGF)-β, and promote inhibition of lymphocyte proliferation.98
3.6 Immune cell-derived EVs
Immune system cells, such as B cells and dendritic cells, mediate MHC-dependent immune responses upon EV secretion.99,100 For this purpose, vesicles express particular adhesion molecules for specific targeting of recipient cells.101 Other immune cells release MVs with immune functions, for example, NK-derived exosomes enclose perforin and granzyme B and mediate anti-tumour activities either in vitro or in vivo.102 Furthermore, peptides expressed in exosomes released by mast cells are presented by DCs and induce specific immune responses in vivo.103 It has also been reported that macrophages release IL-1β on inflammasome activation, suggesting that these MVs play a role in pro-inflammatory activity and innate immune response.104
3.7 Platelet-derived EVs
Platelets release both exosomes and MVs,105 and release is strikingly enhanced by many stimuli, including physicochemical stresses and apoptosis.106 Platelet-derived exosomes are able to regulate the coagulation response,105 and mediate platelet atherogenic interactions with endothelial cells and monocytes.107,108 Platelet MV stimulate angiogenesis and intramyocardial injection improved post-ischaemic revascularization.109 Platelet exosomal cargoes include diverse cytokines, chemokines, growth factors, coagulation factors, lipoproteins, and other lipids, as well as several types of RNA.105,106,110,111 Platelet exosomal membrane proteins also reflect their platelet source, including the constitutively expressed glycoprotein GPIb, as well as GPVI, αIIbβ3, CD40 ligand, and P-selectin from activated platelets.105,106
It has been suggested that platelet activation in some vascular diseases will elevate loading of cyto-adhesive, thrombogenic, and inflammatory factors into platelet exosomal cargo to promote their delivery to endothelial cells and macrophages at sites of vascular lesions. Augmented delivery of platelet exosomal atherogenic cargo to lesional endothelial cells and macrophages, may consequently accelerate development of vascular plaques, clots, and atherosclerosis.107,108,112
3.8 Technical control experiments for mechanistic studies
Given that current methods of purification are imperfect, the inclusion of appropriate control experiments is crucial (Table 1). First and foremost, any biological effects observed using purified EVs should be absent in ‘sham’ control samples depleted of EVs. Furthermore, when using EVs isolated from tissue culture medium, inhibition of exosome release may be used to confirm EV involvement in a process (e.g. Rab27a and Rab27b silencing).113 Current chemical inhibitors inhibiting sphingomyelinase (e.g. GW4869) are not specific to exosome release.57
3.9 Recommendations for mechanistic studies
EVs and exosomes appear to mediate a regulated process of intercellular communication, which could be capitalized on in order to obtain information more easily and less invasively than from in vivo experiments. However, for studies of exosomal communication, advanced cell models are needed that can compare the use of single cell types with multiple cell types in a 2D or 3D structure. Once identified, exosomal signalling molecules could potentially be used as biomarkers that reflect the cellular state during cardiovascular disease.
Mechanistic experiments should include ‘sham’ EV-depleted control groups. A dose-response curve should be performed to relate the mechanism of action to the EV concentration. Because of the hundreds of different bioactive molecular species in any EV preparation it is often difficult to ascribe function to a specific EV component. At minimum, an association between a protein/miRNA and EVs in support of any function ascribed to them, e.g. using immuno-EM, or co-purification on a density gradient should be performed. It is important to consider the likelihood that a single miRNA or EV component mediates all of its effects, as opposed to there being a network effect of multiple miRNAs, and other mediators.83
4. EVs as biomarkers for the ischaemic heart
Given their easily-accessible presence in bodily extracellular fluids, EVs have been investigated as potential biomarkers for many diseases, and their presence in blood and pericardial or lymphatic fluid lends promise to their use in ischaemic heart disease. Proteins, lipids, coding and non-coding RNAs, and even DNA specific to their cells of origin are incorporated into EVs and released into bio-fluids. They have therefore been described as a form of ‘liquid biopsy’ of the cell of origin, and its pathophysiological status, and are attractive sources of biomarkers for clinical diagnostic applications in ischaemic heart disease.114 Their power derives from the enrichment of potential protein markers which otherwise constitute only a very small proportion (less than 0.01%) of the total proteome of body fluids.115 Their sequestration within membranes might also protect the cargo from degradation. Similarly, many miRNAs detectable in serum and saliva are concentrated in exosomes,116 where they are protected against RNases.117 The RNA content of EVs, especially the miRNA, has provoked great interest as diagnostic biomarkers in exosomal RNA research.118 Technical issues related to analysis of EV RNA and proteins contents are described in Section 2.2.8.
Several potential applications for novel biomarkers can be identified. Blood-borne biomarkers of persistent myocardial ischaemia or vascular injury without concomitant cell death are lacking. Subclinical or silent myocardial ischaemia without infarction, different variants of angina and especially microvascular angina86 would benefit from additional non-invasive diagnostic options in addition to ECG.119 Furthermore, in population studies, biomarkers of persistent low grade myocardial ischaemia without cell death would help to determine the prevalence of such conditions. Similarly, diagnosis of acute coronary syndrome (ACS) need to be improved, especially at early time points after coronary occlusion,120 for microvascular angina and in the case of non-ST segment elevation ACS.121 Given the wide range of cardiac cell types that are capable of releasing EVs upon exposure towards stress, it is clear that circulating EVs offer great potential for the identification of biomarkers to aid diagnosis. Indeed, circulating MV signature (characterized by e.g. CD66b+/CD62E+/CD142+, or CD235a+) in coronary and/or peripheral has been shown to reflect the formation of coronary thrombotic occlusions in STEMI-patients.122,123 Moreover, EVs of lymphatic origin have been recently shown in mice as promising biomarkers for lymphatic dysfunction or inflammatory disease progression in atherosclerosis.124
4.1 EV number as biomarker
The number of EVs including exosomes seem to be associated with the presence of cardiac ischaemia and correlates with the severity of cardiac injury. The number of procoagulant EVs, particularly MVs, is elevated in the peripheral blood of patients with ACS and chronic ischaemic heart disease.3,5 The quantity of endothelium-derived MVs even allowed discrimination between patients with stable angina, first time-, and recurring myocardial infarction.125 Both systemic and intracoronary endothelial and platelet MV correlated with the degree of thrombosis and ischaemic area at risk.126,127 MV protein levels are associated with increased risk for future vascular events and mortality in patients with clinically manifest vascular disease.128 In atherosclerosis, increases in EVs of various cell origins have been demonstrated, predicting cardiovascular morbidity and mortality.129 However, since reports on both pro- and anti-atherosclerotic effects of different EV populations have been published (see for review: Ref.130), their usefulness as diagnostics needs further exploration. Given the caveats mentioned previously with regards the accuracy of current methods of EV quantification, it is important to establish rigorous guidelines for consistency, and to determine the accuracy and precision (coefficient of variation) of the methods used.
4.2 EV content as a source of biomarkers
Since EV content is altered by pathology, diagnostics based on the analysis of exosomal content of body fluids may provide further benefit for diagnosing ischaemic heart diseases. The concentration of certain miRNAs in the blood, such as miR-208a, miR-133a, and miR-499 is elevated after ACS.80,131 Circulating miRNA have therefore been proposed as diagnostic biomarkers in cardiovascular diseases.118,119,132–134 Some of these may be transported by EVs, especially under pathological conditions80,116,135,136 or after coronary-artery by-pass graft surgery.137 MVs containing miR-126 and miR-199a, but not freely circulating miRNA expression, predict the occurrence of CV events in patients with stable coronary artery disease.138 Assessing miRNAs carried specifically by exosomes can even improve diagnostic sensitivity and precision: exosomal miR-208a content correlated well with cTn-I levels after CABG surgery, while the whole-blood miR-208a levels did not.137 Similar results were recently reported in ACS patients, where sensitivity of exosomal miR-208a measurement was superior to that of the serum, which even showed a certain degree of prognostic value regarding 1-year survival rate.139 Exosomal miR-192, miR-194, and miR-34a have been identified as prognostic biomarkers predicting the development of heart failure and pathological remodelling after myocardial infarction.140
The diagnostic value of EV protein content has been little studied. De Hoog et al. found that the proteome of ExoQuickTM-precipitated EVs from ACS patients differed from those from non-ACS patients: elevated vesicular levels of polygenic immunoglobulin receptor, cystatin C, and complement factor C5a was associated with ACS diagnosis.141 Zhang et al. showed that protein levels in EV sub-fractions are associated with heart failure in dyspnea cohort.22
4.3 The effect of co-morbidities on EVs
It is well known that cardiovascular risk factors and co-morbidities (especially aging, gender, and metabolic diseases such as hyperlipidaemia and diabetes), and their medications alter the phenotype of the normal and pathological myocardium including its response to ischaemia/reperfusion and cardioprotective or regenerative therapies (see for extensive reviews).142–145 The presence of these confounding factors may interfere with diagnostic opportunities by means of MVs, however, very little is known in this field. Some recent reviews on the diagnosis of diabetes and dyslipidaemia concluded that since EVs may be involved in these pathologies, analysis of EVs might reveal additional biomarkers.146–148 For example, the quantity of MVs/microparticles is elevated in the blood plasma of patients with metabolic syndrome,149,150 and elevated levels of certain miRNAs derived from ExoQuick-precipitated EVs have been shown to be associated with metabolic syndrome or diabetes,151 but the practical relevance of these reports is yet to be assessed. Since the cardiac transcriptome including miRNA expression profile has been shown to be dramatically changed in the rat myocardium by hyperlipidemia,152 analysis of transcriptome including non-coding RNA content of EVs may provide specific diagnostic markers of the heart affected by this and other co-morbidities.153 In a study on over 1000 patients, EV-associated cystatin C content was found to be significantly elevated (a marginal, 9% difference), in metabolic syndrome patients with cardiovascular disease, and a proteomics study on EVs derived from adipocytes of obese rats revealed 200 proteins with differential expression.154 Moreover, it has been shown that at similar plasma cholesterol levels, patients on statin treatment had a significant lower number of circulating MVs carrying markers of activated cells.155 These studies suggest that EVs have the potential to evolve into useful biomarkers of various metabolic diseases in the future, however, a substantial amount of research has to be done before their clinical use might be realized.
4.4 Recommendations for study of EVs as biomarkers
Since miRNAs are transferred by either protein complexes, HDL, or EVs,134 and since protein contamination of isolated EVs is difficult to control, adequate isolation techniques are of the utmost importance if exosomal miRNAs or proteins are to be assessed as biomarkers. Other recommendations relevant to plasma miRNA biomarker studies in general apply equally to EV miRNA studies.156 It should be noted that although detectable amounts of relatively clean EVs can be isolated, e.g. with size exclusion chromatography,29 methods for the isolation of EVs need to be improved to allow clinical utilization of EV-based diagnostics.
Current technical limitations for EV isolation do not allow definitive guidelines for the use of EVs as biomarkers. Several factors that are lacking include: the lack of standardized pre-analytical and isolation procedures; lack of gold standard for processing, characterization and purity; an unknown influence of comorbidities, co-medication and other confounding factors; lack of disease specificity; lack of tissue-specific markers (Table 2). As with any biomarker study, pilot observations from small cohorts should be validated in larger patient datasets, and the reproducibility of isolation procedures, including markers of EVs and normal range levels in the healthy population should be reported. Moreover, evidence should be provided of the additional value of EVs over current gold-standard biomarkers.
Table 2.
Current technical limitations for clinical translation of EV biomarker
| (1) Lack of standardized pre-analytical and isolation procedures. |
| (2) Currently no gold standard. |
| (3) Need to establish methods of purifying specific subpopulations of EVs originating from the heart, vasculature, or blood cells, no golden standard for processing, characterization, and purity. |
| (4) Unknown influence of confounding factors of EV quality, including disease specificity and presence of comorbidities and their medications. |
| (5) Small yields of EV subpopulations obtained for content analysis: transcriptomics, lipidomics, and proteomics. |
| (6) Validation of novel biomarkers in large patient cohorts, including normal range levels in the healthy population. |
| (7) Determine additional value of EV markers over current golden standard clinical biomarkers, or its use as a combinatory marker. |
5. Therapeutic potential of EVs for the ischaemic heart
EVs purified from defined cell types have been suggested as novel therapeutic options for various cardiac diseases including ischaemic heart disease, myocardial infarction, and heart failure, as well as for pathogen vaccination, immune-modulatory and regenerative therapies and drug delivery. However, the first clinical steps have been made using exosomes as an anti-tumour therapy: an effective way to destroy non-small cell lung cancer (NSCLC) is to activate tumour-specific cytotoxic T cells (CTL), and for this autologous dendritic cell (DC)-derived exosomes (DEX) loaded with tumour antigens have been tested not only in phase I157 but also in a phase II Trial (ClinicalTrials.gov Identifier: NCT01159288). Although this did not induce detectable effector T cell responses, a positive effect on natural killer (NK) cells could be observed in some patients.158 This first exosome Phase I trial highlighted the feasibility of large scale exosomes production and the safety of exosome administration.159 Several other Phase I studies have been initiated, exploring the use of EVs/exosomes as a therapy, including the effect of MSC-derived exosomes on β-cell mass in Type 1 diabetes mellitus (T1DM) Patients (ClinicalTrials.gov Identifier: NCT02138331).
Little is known about whether EVs derived from animals might be feasible for human therapy. However, EV fractions derived from human umbilical cord MSCs and administered to different healthy and diseased animal species (including rats with myocardial infarction) were found to be well tolerated.160 After further study, these results demonstrating a lack of adverse immunological reactions may open the possibility of producing therapeutic EVs from non-human sources. To develop the application of exosome-based therapeutics, a clear understanding of exosome pharmacokinetics in vivo is of utmost importance. Currently, only limited information is available, but unmodified EVs derived from tumours are rapidly cleared in liver and spleen by the reticuloendothelial system and cells of the innate immune system.161 Interestingly, there may be concerns about intravenous injection of high concentrations of EVs since the authors observed rapid asphyxiation of mice when injecting over 400 µg.161 To date, most in vivo bio-distribution studies have used small lipophilic fluorescent dyes to label EVs. Although useful for first impressions, the reliability of these analyses might be impaired by the free dye released from EVs.162 Bioluminescence imaging of a fusion protein consisting of Gaussia luciferase and lactadherin has been used to demonstrate that EVs quickly disappear from the blood circulation and are mainly distributed to the liver after intravenous injection into mice, with a half-life of approximately 2–4 min.163 It is likely that surface molecules such as phospholipids and proteins are important for determining the pharmacokinetics and cellular uptake of EVs.164 A further question relates to the ideal route of EV administration. In this regard, a recent study demonstrated that MSC-derived EVs were effective in pigs after intramyocardial but not intracoronary delivery post reperfusion.165
5.1 Progenitor and stem cell-derived EVs
Stem cell therapy has been investigated as a potential approach to prevent cardiac damage and stimulate cardiac repair in ischaemic heart disease.143 Recent developments in regenerative medicine that explore the use of cell-free approaches, hence using the isolated paracrine effectors from cells, focus on EVs that are release by cultured cells.2,81 The use of these vesicles provide an alternative strategy for the paracrine effects of cell transplantation and activation of the repair mechanisms of the resident myocardium that captures the complexity of the signalling required to stimulate these regenerative processes.
The best studied population of cells for its paracrine effects in the heart are the mesenchymal stromal cells (MSCs). Conditioned medium of these cells reduced infarct size in both murine and porcine animal models,166,167 after which the exosome-containing fraction was demonstrated to be the functional fraction, which decreased oxidative stress and activated the PI3K/Akt pathway in the myocardium.45,168 As indicated above, several subclasses of EVs might be released by cells. A recent study showed that MSCs produce at least three subtypes of EVs, isolated based on their affinities for membrane lipid-binding ligands, and are likely to have a different biogenesis pathway and possibly different functions.169,170
In addition to the cardioprotective effects of EVs, pro-angiogenic effects have been observed,170 not only by MSCs but also by cardiac-derived progenitor cells (CPCs), which are at least partly mediated via extracellular matrix metalloproteinase inducer (EMMPRIN).85,86,171 In the EVs of CPCs, several miRNA clusters are enriched and important for their therapeutic effects.82,83 EVs derived from blood-outgrowth endothelial cells91,172 and especially circulating CD34+ stem cells90 were also shown to exert pro-angiogenic paracrine activity. EVs are now being reported to have anti-inflammatory effects as well, e.g. endothelial cell-derived EVs were found to suppress monocyte activation.173 EVs harvested from Treg cells have been found to exert an immune-suppressive function by the suppression of monocyte activation,174 and EVs from MSCs can suppress T-Lymphocyte proliferation.175,176
Mouse embryonic stem cells secrete exosomes which can also augment neovascularization, myocyte proliferation, and survival after MI.177 Importantly, they were shown to augment the survival, retention, and proliferation of CPCs in ischaemic myocardium by delivery of miR-294.177 Exosomes have also been identified as key mediators of regeneration induced by cardiosphere-derived cells (CDCs), which they achieved partly by the transfer of miR-146a.81 Recently, CDC exosomes isolated by filtration and precipitation were injected into the myocardium of pigs following ischaemia and reperfusion, which reduced infarct size and improved cardiac function 4 weeks later.165
Recent studies described the isolation of EVs from iPSCs, with healing ability in in vivo model of myocardial ischaemia.178 Finally, plasma EVs themselves have been shown to be able to protect the myocardium from ischaemia and reperfusion injury179 and EVs have been shown to be necessary for endogenous cardioprotective mechanisms such as remote ischaemic conditioning.46
5.2 Hybrid nanomedicine to mimic EVs
A further approach to the use of EVs as therapeutic tools is to load them with a range of molecules or pharmacological compounds from siRNA to small molecules or proteins, serving as tool for empowering cell-derived EVs and for targeted-drug delivery.180 For example, liposomes with a phospholipid bilayer can carry a variety of proteins and nucleic acids as well as pharmaceutically active substances to the injured heart.181 Such exosome-mimetic structures can also include specific targeting molecules that enhance their targeting. Currently, liposomal transport of drug molecules are in clinical practice and under clinical trial.182,183 Exosome-mimetic delivery systems offer the advantage of being more controllable and scalable for clinical settings.184,185 Conversely, the advantage of using EVs in targeted drug delivery over the existing synthetic delivery systems (such as liposomes) includes their potentially increased circulation time, bio-distribution, decreased immunogenicity and toxicity, loading of complex cargo, cellular interactions and the different methods for therapeutic cargo loading (both post-loading and pre-loading) and administration.
5.3 Important limitations to overcome in the development of EV therapeutics
The use of EVs provides several advantages over the use of cells for therapeutic application. These include the absence of tumourigenicity, conservation of activity between species, lower immunogenic potential, and theoretically improved tissue-targeting potential. However, before EVs can enter the clinic as medicinal products several important steps have to be taken (Table 3)43:
Table 3.
Current limitations for cardiac therapeutic use of EVs
| (1) Unestablished regulatory aspects. |
| (2) Scalable production and stability of EVs. |
| (3) Purification problem, including potential heterogeneity of EVs and presence of co-purified molecules. |
| (4) Lack for standardized quality control methods for EV production. |
| (5) Determine which proportion of EVs mediates therapeutic effects, including unknown mode of action, including potential retention issues. |
| (6) Unknown pharmacokinetics of EVs as a therapeutic. |
| (7) Unknown safety and toxicity, including immunogenicity. |
| (8) Difficulty for freedom to operate due to regulatory protection issues. |
Therapeutic differences from independent cell preparations have been observed among comparable EV fractions, which might be caused by variability between donors or heterogeneity of EVs harvested.169 Since cellular growth status varies between cell cultures, as it does for embryonic stem cells or MSCs,186 these differences might also be reflected in their secreted EVs.
Upon EV isolation and usage, contamination by co-purified and bound moleculesmight affect functional assays.12
Since isolated EVs might be heterogeneous by nature, only a proportion of produced EVs might be responsible for observedtherapeutic effects.
The EV pharmacokinetics are largely unknown and need detailed follow-up in patients to understand their bio-distributions.
The approach of using non-autologous EVs in the setting of cardiovascular disease should be taken carefully, since EVs can potentially carry pathogen-specific antigens.
Currently, as it is unknown which EV fraction (content, membranes or both) is responsible for the biological activity of EVs, the mechanism of actionshould investigated.
Prior to entering into clinical trials, industrial-scale, highly reproducible methods of isolating GMP-quality EVs, as well as validation procedures are required.
6. Consensus statement on the steps required to take EVs forward to clinical applications as biomarkers or therapeutics
Biomarkers: As indicated in Section 4.4., the current technical limitations for EV isolation do not allow specific recommendations for the use of EVs as biomarkers, though guidelines for biomarkers in general are applicable. In the coming years, standardized pre-analytic and isolation procedures need to be further defined and simplified, including the definition of gold standards for processing, characterization, and identification of EV purity (Table 4). By exploring these areas, the influence of confounding factors, including co-morbidities, co-medication and whether identified EV markers are disease specific will be evaluated. The typically small yields of isolated EVs will hamper a clear full EV characterization, but technical developments that allow, for example, single cell sequencing will lower input needs for these techniques. Most important is the validation of pilot observation from small cohorts in larger dataset of patients, thereby improving the reproducibility of isolation procedures, and to provide evidence of additional value of EVs over current golden standard biomarkers. To strengthen the process of identification of EVs or EV-components as biomarkers, experiments may also be designed to identify the tissue source of the biomarker, combined with the functional validation of the identified molecule (protein and/or RNA) in the target tissues.
Table 4.
Future perspectives: Developments required to take EVs forward to clinical applications as biomarkers or therapeutics
| – Improvement of flow cytometric methods and standardization of analytical procedures. (Until this is achieved, bead-based bulk detection of EVs may provide a feasible flow cytometric detection approach irrespective of the instrument used). |
| – Development of novel high-resolution methodologies for EV isolation and visualizations. |
| – Understand mechanism of inter-cell or inter-organ communication. |
| – Potential source for cardiac tissue and disease specific biomarkers. |
| – Potential packages for cardiac specific therapies. |
| – Potential multi-targeting effects of EVs for the complex mechanisms of ischaemic heart disease. |
| – Potential advantage for EV therapeutic application over cells, including absence of tumourigenicity and cross species efficacy. |
Therapy: The translation of EVs into clinical therapies will require categorization of EV-based therapeutics in compliance with existing regulatory frameworks. Since EVs will probably be considered as biological medicinal products more specifically advanced therapy medicinal products (ATMPs), new rules explicitly regulating EV-based therapies are probably not needed. However, although existing European guidance on biological active substances covers the manufacturing and clinical evaluation of novel EV-based therapeutics, special guidelines addressing EV-based therapeutics may be needed.43 The regulatory classification of most biological medicinal products, including ATMPs depends on a pharmaceutically active substance that does not require a molecular definition, but could mean the cells themselves in case of cellular therapeutics.187 Here, one needs to identify, quantify, and characterize the main effector that is causing the biologic effect and define the mode or mechanism of action.
Similar to human cell-based products,188 preclinical safety testing might be needed as well as a risk-analysis approach for the transition from preclinical to clinical development, thereby taking into account the heterogeneity among independent cell donors and different preparations, as well as the EV heterogeneity in obtained donor cell samples. A test for biological activity should be included unless otherwise justified. However, since many cell products are composed of a heterogeneous mixture of cells, identification of the cellular component(s) responsible for a proposed biological activity will be a major challenge. General clinical requirements and developments have been recently reviewed for cardiac cellular therapeutics.143
Based on the legislation for ATMPs including tissues, cells, a set of minimal criteria for the characterization of such products needs to be considered before use in clinical trials. One needs to define whether a product is (i) of autologous, allogeneic or xenogeneic origin; (ii) extensively or minimally manipulated in vitro; and (iii) immunologically active or neutral, (iv) is the proliferative capacity of cells and tissue-like organization, as well as defining (v) the dynamic interaction between these elements, (vi) the intended use.43,189
In summary, to advance towards first-in-man-clinical trials approval of the technical requirements and quality risk management is needed, donor safety, recipient safety, and release criteria for EV-based therapeutics should be defined and quality control should be in place, including molecular and physical EV characterization, and in vitro potency assays. Moreover, the identified recommendation criteria for the characterization of isolated EV are critical for both their therapeutic use and for biomarker discovery.
Funding
JS is supported by Horizon2020 ERC-2016-COG EVICARE (725229), the Project SMARTCARE-II of the BioMedicalMaterials Institute, co-funded by the ZonMw‐TAS program (#116002016), the Dutch Ministry of Economic Affairs, Agriculture and Innovation and the Netherlands CardioVascular Research Initiative (CVON): the Dutch Heart Foundation, Dutch Federations of University Medical Centers, the Netherlands Organization for Health Research and Development, and the Royal Netherlands Academy of Sciences. SD is supported by Project grant PG/16/85/32471 from the British Heart Foundation. PF and RS were supported by the European Cooperation in Science and Technology (COST EU-ROS BM1203). DH is the chair, PF is the vice chair, and SD and RS are working group leaders of COST Action EU-Cardioprotection (CA16225). PF holds grants from the Hungarian National Research, Development, and Innovation Office (OTKA K 109737, OTKA ANN 107803, NVKP 16-1-2016-0017, and VEKOP-2.3.2-16-2016-00002). EIB is supported by NVKP 16-1-2016-0017, OTKA 20237, OTKA 111958, and VEKOP-2.3.3-15-2017-00016. RK is supported by National Institutes of Health Grants HL091983, HL126186 and HL134608. CP is supported by the Italian Ministry of University, Research and Technology (RBFR124FEN and 2015583WMV grants) and by Programme STAR, financially supported by Compagnia di San Paolo and Federico II University, Naples (Italy). SL holds grant from the South African National Research Foundation. FBE is supported by the German Research Foundation (DFG Research Unit FOR2149). DdK is supported by Queen of Hearts Program Dutch Heart Foundation 2013T084; BMRC CS-IRG, ATTRaCT SPF grant. CMB is supported by Insitut National de la Santé et de la Recherche Medicale, Université Paris Descartes and ANR-16-CE92-0032-02. DH was supported by the British Heart Foundation (FS/10/039/28270), Duke-National University Singapore Medical School, the National Institute for Health Research University College London Hospitals Biomedical Research Centre, Singapore Ministry of Health's National Medical Research Council under its Clinician Scientist-Senior Investigator scheme (NMRC/CSA-SI/0011/2017) and Collaborative Centre Grant scheme (NMRC/CGAug16C006), the Singapore Ministry of Education Academic Research Fund Tier 2 (MOE2016-T2-2-021), and the National Research Foundation (NRF) Singapore (NRF-CRP13-2014-05).
Conflict of interest: PF is the founder and CEO of Pharmahungary Group, a group of R&D companies.
References
- 1. Sluijter JP, Verhage V, Deddens JC, van den Akker F, Doevendans PA.. Microvesicles and exosomes for intracardiac communication. Cardiovasc Res 2014;102:302–311. 10.1093/cvr/cvu022 [DOI] [PubMed] [Google Scholar]
- 2. Davidson SM, Takov K, Yellon DM.. Exosomes and cardiovascular protection. Cardiovasc Drugs Ther 2017;31:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lawson C, Vicencio JM, Yellon DM, Davidson SM.. Microvesicles and exosomes: new players in metabolic and cardiovascular disease. J Endocrinol 2016;228:R57–R71. [DOI] [PubMed] [Google Scholar]
- 4. Barile L, Moccetti T, Marbán E, Vassalli G.. Roles of exosomes in cardioprotection. Eur Heart J 2017;38:1372–1379. [DOI] [PubMed] [Google Scholar]
- 5. VanWijk MJ, VanBavel E, Sturk A, Nieuwland R.. Microparticles in cardiovascular diseases. Cardiovasc Res 2003;59:277–287. 10.1016/S0008-6363(03)00367-5 [DOI] [PubMed] [Google Scholar]
- 6. Ibrahim A, Marban E.. Exosomes: fundamental biology and roles in cardiovascular physiology. Annu Rev Physiol 2016;78:67–83. 10.1146/annurev-physiol-021115-104929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boulanger CM, Loyer X, Rautou PE, Amabile N.. Extracellular vesicles in coronary artery disease. Nat Rev Cardiol 2017;14:259–272. 10.1038/nrcardio.2017.7 [DOI] [PubMed] [Google Scholar]
- 8. Ridger VC, Boulanger CM, Angelillo-Scherrer A, Badimon L, Blanc-Brude O, Bochaton-Piallat M-L, Boilard E, Buzas EI, Caporali A, Dignat-George F, Evans PC, Lacroix R, Lutgens E, Ketelhuth DFJ, Nieuwland R, Toti F, Tunon J, Weber C, Hoefer IE.. Microvesicles in vascular homeostasis and diseases. Position Paper of the European Society of Cardiology (ESC) Working Group on Atherosclerosis and Vascular Biology. Thromb Haemost 2017;117:1296.. [DOI] [PubMed] [Google Scholar]
- 9. Hoefer IE, Steffens S, Ala-Korpela M, Back M, Badimon L, Bochaton-Piallat ML, Boulanger CM, Caligiuri G, Dimmeler S, Egido J, Evans PC, Guzik T, Kwak BR, Landmesser U, Mayr M, Monaco C, Pasterkamp G, Tunon J, Weber C. Atherosclerosis ESCWG Vascular B. Novel methodologies for biomarker discovery in atherosclerosis. Eur Heart J 2015;36:2635–2642. [DOI] [PubMed] [Google Scholar]
- 10. Ettelaie C, Collier ME, Maraveyas A, Ettelaie R.. Characterization of physical properties of tissue factor-containing microvesicles and a comparison of ultracentrifuge-based recovery procedures. J Extracell Vesicles 2014;3:23592.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu R, Greening DW, Rai A, Ji H, Simpson RJ.. Highly-purified exosomes and shed microvesicles isolated from the human colon cancer cell line LIM1863 by sequential centrifugal ultrafiltration are biochemically and functionally distinct. Methods 2015;87:11–25. 10.1016/j.ymeth.2015.04.008 [DOI] [PubMed] [Google Scholar]
- 12. Sódar BW, Kittel Á, Pálóczi K, Vukman KV, Osteikoetxea X, Szabó-Taylor K, Németh A, Sperlágh B, Baranyai T, Giricz Z, Wiener Z, Turiák L, Drahos L, Pállinger É, Vékey K, Ferdinandy P, Falus A, Buzás EI.. Low-density lipoprotein mimics blood plasma-derived exosomes and microvesicles during isolation and detection. Sci Rep 2016;6:24316.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bautista D, Rodriguez LS, Franco MA, Angel J, Barreto A.. Caco-2 cells infected with rotavirus release extracellular vesicles that express markers of apoptotic bodies and exosomes. Cell Stress Chaperones 2015;20:697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thery C, Boussac M, Veron P, Ricciardi-Castagnoli P, Raposo G, Garin J, Amigorena S.. Proteomic analysis of dendritic cell-derived exosomes: a secreted subcellular compartment distinct from apoptotic vesicles. J Immunol 2001;166:7309–7318. [DOI] [PubMed] [Google Scholar]
- 15. Di Vizio D, Morello M, Dudley AC, Schow PW, Adam RM, Morley S, Mulholland D, Rotinen M, Hager MH, Insabato L, Moses MA, Demichelis F, Lisanti MP, Wu H, Klagsbrun M, Bhowmick NA, Rubin MA, D'souza-Schorey C, Freeman MR.. Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am J Pathol 2012;181:1573–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Minciacchi VR, You S, Spinelli C, Morley S, Zandian M, Aspuria PJ, Cavallini L, Ciardiello C, Reis Sobreiro M, Morello M, Kharmate G, Jang SC, Kim DK, Hosseini-Beheshti E, Tomlinson Guns E, Gleave M, Gho YS, Mathivanan S, Yang W, Freeman MR, Di Vizio D.. Large oncosomes contain distinct protein cargo and represent a separate functional class of tumor-derived extracellular vesicles. Oncotarget 2015;6:11327–11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, Dingli F, Loew D, Tkach M, Théry C.. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci USA 2016;113:E968–E977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lötvall J, Hill AF, Hochberg F, Buzás EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, Sahoo S, Tahara H, Wauben MH, Witwer KW, Théry C.. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles 2014;3:26913.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thery C, Amigorena S, Raposo G, Clayton A.. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr Protoc Cell Biol 2006;Chapter 3 Unit 3:22.. [DOI] [PubMed] [Google Scholar]
- 20. Yuana Y, Levels J, Grootemaat A, Sturk A, Nieuwland R.. Co-isolation of extracellular vesicles and high-density lipoproteins using density gradient ultracentrifugation. J Extracell Vesicles 2014;3:23262.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang J, Hawari FI, Shamburek RD, Adamik B, Kaler M, Islam A, Liao DW, Rouhani FN, Ingham M, Levine SJ.. Circulating TNFR1 exosome-like vesicles partition with the LDL fraction of human plasma. Biochem Biophys Res Commun 2008;366:579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang Y-N, Vernooij F, Ibrahim I, Ooi S, Gijsberts CM, Schoneveld AH, Sen KW, den Ruijter HM, Timmers L, Richards AM, Jong CT, Mazlan I, Wang J-W, Lam CSP, de Kleijn DPV, Lionetti V.. Extracellular vesicle proteins associated with systemic vascular events correlate with heart failure: an observational study in a dyspnoea cohort. PLoS One 2016;11:e0148073.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Witwer KW, Buzás EI, Bemis LT, Bora A, Lässer C, Lötvall J, Nolte-‘T Hoen EN, Piper MG, Sivaraman S, Skog J, Théry C, Wauben MH, Hochberg F.. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles 2013;2:20360.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Paolini L, Zendrini A, Di Noto G, Busatto S, Lottini E, Radeghieri A, Dossi A, Caneschi A, Ricotta D, Bergese P.. Residual matrix from different separation techniques impacts exosome biological activity. Sci Rep 2016;6:23550.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Livshits MA, Khomyakova E, Evtushenko EG, Lazarev VN, Kulemin NA, Semina SE, Generozov EV, Govorun VM.. Isolation of exosomes by differential centrifugation: theoretical analysis of a commonly used protocol. Sci Rep 2015;5:17319.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gyorgy B, Modos K, Pallinger E, Paloczi K, Pasztoi M, Misjak P, Deli MA, Sipos A, Szalai A, Voszka I, Polgar A, Toth K, Csete M, Nagy G, Gay S, Falus A, Kittel A, Buzas EI.. Detection and isolation of cell-derived microparticles are compromised by protein complexes resulting from shared biophysical parameters. Blood 2011;117:e39–e48. [DOI] [PubMed] [Google Scholar]
- 27. Linares R, Tan S, Gounou C, Arraud N, Brisson AR.. High-speed centrifugation induces aggregation of extracellular vesicles. J Extracell Vesicles 2015;4:29509.. 10.3402/jev.v4.29509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boing AN, van der Pol E, Grootemaat AE, Coumans FA, Sturk A, Nieuwland R.. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J Extracell Vesicles 2014;3:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baranyai T, Herczeg K, Onódi Z, Voszka I, Módos K, Marton N, Nagy G, Mäger I, Wood MJ, El Andaloussi S, Pálinkás Z, Kumar V, Nagy P, Kittel Á, Buzás EI, Ferdinandy P, Giricz Z, Rito-Palomares M.. Isolation of exosomes from blood plasma: qualitative and quantitative comparison of ultracentrifugation and size exclusion chromatography methods. PLoS One 2015;10:e0145686.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Welton JL, Webber JP, Botos LA, Jones M, Clayton A.. Ready-made chromatography columns for extracellular vesicle isolation from plasma. J Extracell Vesicles 2015;4:27269.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nolte-'T Hoen EN, van der Vlist EJ, de Boer-Brouwer M, Arkesteijn GJ, Stoorvogel W, Wauben MH.. Dynamics of dendritic cell-derived vesicles: high-resolution flow cytometric analysis of extracellular vesicle quantity and quality. J Leukoc Biol 2013;93:395–402. [DOI] [PubMed] [Google Scholar]
- 32. Groot Kormelink T, Arkesteijn GJ, Nauwelaers FA, van den Engh G, Nolte-'T Hoen EN, Wauben MH.. Prerequisites for the analysis and sorting of extracellular vesicle subpopulations by high-resolution flow cytometry. Cytometry A 2016;89:135–147. [DOI] [PubMed] [Google Scholar]
- 33. Scientific Program 2012 ISEV meeting Wednesday 18th April. J Extracell Vesicles 2012;1:18182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. George JN, Thoi LL, McManus LM, Reimann TA.. Isolation of human platelet membrane microparticles from plasma and serum. Blood 1982;60:834–840. [PubMed] [Google Scholar]
- 35. Lacroix R, Judicone C, Mooberry M, Boucekine M, Key NS, Dignat-George F, The ISSCW. Standardization of pre-analytical variables in plasma microparticle determination: results of the International Society on Thrombosis and Haemostasis SSC Collaborative workshop. J Thromb Haemost 2013;11:1190–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yuana Y, Boing AN, Grootemaat AE, van der Pol E, Hau CM, Cizmar P, Buhr E, Sturk A, Nieuwland R.. Handling and storage of human body fluids for analysis of extracellular vesicles. J Extracell Vesicles 2015;4:29260.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. György B, Pálóczi K, Kovács A, Barabás E, Bekő G, Várnai K, Pállinger É, Szabó-Taylor K, Szabó TG, Kiss AA, Falus A, Buzás EI.. Improved circulating microparticle analysis in acid-citrate dextrose (ACD) anticoagulant tube. Thromb Res 2014;133:285–292. [DOI] [PubMed] [Google Scholar]
- 38. Lacroix R, Judicone C, Poncelet P, Robert S, Arnaud L, Sampol J, Dignat-George F.. Impact of pre-analytical parameters on the measurement of circulating microparticles: towards standardization of protocol. J Thromb Haemost 2012;10:437–446. [DOI] [PubMed] [Google Scholar]
- 39. Beltrami C, Besnier M, Shantikumar S, Shearn AI, Rajakaruna C, Laftah A, Sessa F, Spinetti G, Petretto E, Angelini GD, Emanueli C.. Human pericardial fluid contains exosomes enriched with cardiovascular-expressed microRNAs and promotes therapeutic angiogenesis. Mol Ther 2017;25:679–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Foglio E, Puddighinu G, Fasanaro P, D'arcangelo D, Perrone GA, Mocini D, Campanella C, Coppola L, Logozzi M, Azzarito T, Marzoli F, Fais S, Pieroni L, Marzano V, Germani A, Capogrossi MC, Russo MA, Limana F.. Exosomal clusterin, identified in the pericardial fluid, improves myocardial performance following MI through epicardial activation, enhanced arteriogenesis and reduced apoptosis. Int J Cardiol 2015;197:333–347. [DOI] [PubMed] [Google Scholar]
- 41. Shelke GV, Lasser C, Gho YS, Lotvall J.. Importance of exosome depletion protocols to eliminate functional and RNA-containing extracellular vesicles from fetal bovine serum. J Extracell Vesicles 2014;3:24783.. 10.3402/jev.v3.24783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wei Z, Batagov AO, Carter DR, Krichevsky AM.. Fetal bovine serum RNA interferes with the cell culture derived extracellular RNA. Sci Rep 2016;6:31175.. 10.1038/srep31175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lener T, Gimona M, Aigner L, Börger V, Buzas E, Camussi G, Chaput N, Chatterjee D, Court FA, Del Portillo HA, O'driscoll L, Fais S, Falcon-Perez JM, Felderhoff-Mueser U, Fraile L, Gho YS, Görgens A, Gupta RC, Hendrix A, Hermann DM, Hill AF, Hochberg F, Horn PA, de Kleijn D, Kordelas L, Kramer BW, Krämer-Albers E-M, Laner-Plamberger S, Laitinen S, Leonardi T, Lorenowicz MJ, Lim SK, Lötvall J, Maguire CA, Marcilla A, Nazarenko I, Ochiya T, Patel T, Pedersen S, Pocsfalvi G, Pluchino S, Quesenberry P, Reischl IG, Rivera FJ, Sanzenbacher R, Schallmoser K, Slaper-Cortenbach I, Strunk D, Tonn T, Vader P, van Balkom BWM, Wauben M, Andaloussi SE, Théry C, Rohde E, Giebel B.. Applying extracellular vesicles based therapeutics in clinical trials–an ISEV position paper. J Extracell Vesicles 2015;4:30087.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Heinemann ML, Ilmer M, Silva LP, Hawke DH, Recio A, Vorontsova MA, Alt E, Vykoukal J.. Benchtop isolation and characterization of functional exosomes by sequential filtration. J Chromatogr A 2014;1371:125–135. [DOI] [PubMed] [Google Scholar]
- 45. Lai RC, Arslan F, Lee MM, Sze NS, Choo A, Chen TS, Salto-Tellez M, Timmers L, Lee CN, El Oakley RM, Pasterkamp G, de Kleijn DP, Lim SK.. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res 2010;4:214–222. [DOI] [PubMed] [Google Scholar]
- 46. Giricz Z, Varga ZV, Baranyai T, Sipos P, Paloczi K, Kittel A, Buzas E, Ferdinandy P.. Cardioprotection by remote ischemic preconditioning of the rat heart is mediated by extracellular vesicles. J Mol Cell Cardiol 2014;68:75–78. [DOI] [PubMed] [Google Scholar]
- 47. Termine JD, Posner AS.. Calcium phosphate formation in vitro. I. Factors affecting initial phase separation. Arch Biochem Biophys 1970;140:307–317. [DOI] [PubMed] [Google Scholar]
- 48. Lőrincz ÁM, Timár CI, Marosvári KA, Veres DS, Otrokocsi L, Kittel Á, Ligeti E.. Effect of storage on physical and functional properties of extracellular vesicles derived from neutrophilic granulocytes. J Extracell Vesicles 2014;3:25465.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bosch S, de Beaurepaire L, Allard M, Mosser M, Heichette C, Chretien D, Jegou D, Bach JM.. Trehalose prevents aggregation of exosomes and cryodamage. Sci Rep 2016;6:36162.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Webber J, Clayton A.. How pure are your vesicles? J Extracell Vesicles 2013;2:19861.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Osteikoetxea X, Balogh A, Szabó-Taylor K, Németh A, Szabó TG, Pálóczi K, Sódar B, Kittel Á, György B, Pállinger É, Matkó J, Buzás EI, Caporali A.. Improved characterization of EV preparations based on protein to lipid ratio and lipid properties. PLoS One 2015;10:e0121184.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Arraud N, Linares R, Tan S, Gounou C, Pasquet JM, Mornet S, Brisson AR.. Extracellular vesicles from blood plasma: determination of their morphology, size, phenotype and concentration. J Thromb Haemost 2014;12:614–627. [DOI] [PubMed] [Google Scholar]
- 53. Yuana Y, Oosterkamp TH, Bahatyrova S, Ashcroft B, Garcia Rodriguez P, Bertina RM, Osanto S.. Atomic force microscopy: a novel approach to the detection of nanosized blood microparticles. J Thromb Haemost 2010;8:315–323. 10.1111/j.1538-7836.2009.03654.x [DOI] [PubMed] [Google Scholar]
- 54. Yoshioka Y, Konishi Y, Kosaka N, Katsuda T, Kato T, Ochiya T.. Comparative marker analysis of extracellular vesicles in different human cancer types. J Extracell Vesicles 2013;2:20424.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. van der Pol E, van Gemert MJ, Sturk A, Nieuwland R, van Leeuwen TG.. Single vs. swarm detection of microparticles and exosomes by flow cytometry. J Thromb Haemost 2012;10:919–930. [DOI] [PubMed] [Google Scholar]
- 56. Im H, Shao H, Park YI, Peterson VM, Castro CM, Weissleder R, Lee H.. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nat Biotechnol 2014;32:490–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tkach M, Thery C.. Communication by extracellular vesicles: where we are and where we need to go. Cell 2016;164:1226–1232. 10.1016/j.cell.2016.01.043 [DOI] [PubMed] [Google Scholar]
- 58. Rautou PE, Leroyer AS, Ramkhelawon B, Devue C, Duflaut D, Vion AC, Nalbone G, Castier Y, Leseche G, Lehoux S, Tedgui A, Boulanger CM.. Microparticles from human atherosclerotic plaques promote endothelial ICAM-1-dependent monocyte adhesion and transendothelial migration. Circ Res 2011;108:335–343. [DOI] [PubMed] [Google Scholar]
- 59. Van Deun J, Mestdagh P, Sormunen R, Cocquyt V, Vermaelen K, Vandesompele J, Bracke M, De Wever O, Hendrix A.. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J Extracell Vesicles 2014;3:24858.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pocsfalvi G, Stanly C, Vilasi A, Fiume I, Capasso G, Turiak L, Buzas EI, Vekey K.. Mass spectrometry of extracellular vesicles. Mass Spectrom Rev 2016;35:3–21. [DOI] [PubMed] [Google Scholar]
- 61. Boon RA, Vickers KC.. Intercellular transport of microRNAs. Arterioscler Thromb Vasc Biol 2013;33:186–192. 10.1161/ATVBAHA.112.300139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pritchard CC, Kroh E, Wood B, Arroyo JD, Dougherty KJ, Miyaji MM, Tait JF, Tewari M.. Blood cell origin of circulating microRNAs: a cautionary note for cancer biomarker studies. Cancer Prev Res (Phila) 2012;5:492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bank IE, Timmers L, Gijsberts CM, Zhang YN, Mosterd A, Wang JW, Chan MY, De Hoog V, Lim SK, Sze SK, Lam CS, De Kleijn DP.. The diagnostic and prognostic potential of plasma extracellular vesicles for cardiovascular disease. Expert Rev Mol Diagn 2015;15:1577–1588. 10.1586/14737159.2015.1109450 [DOI] [PubMed] [Google Scholar]
- 64. Kreimer S, Belov AM, Ghiran I, Murthy SK, Frank DA, Ivanov AR.. Mass-spectrometry-based molecular characterization of extracellular vesicles: lipidomics and proteomics. J Proteome Res 2015;14:2367–2384. [DOI] [PubMed] [Google Scholar]
- 65. Gyorgy B, Szabo TG, Turiak L, Wright M, Herczeg P, Ledeczi Z, Kittel A, Polgar A, Toth K, Derfalvi B, Zelenak G, Borocz I, Carr B, Nagy G, Vekey K, Gay S, Falus A, Buzas EI.. Improved flow cytometric assessment reveals distinct microvesicle (cell-derived microparticle) signatures in joint diseases. PLoS One 2012;7:e49726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Amabile N, Renard JM, Caussin C, Boulanger CM.. Circulating immune complexes do not affect microparticle flow cytometry analysis in acute coronary syndrome. Blood 2012;119:2174–2175. author reply 2175–2176. [DOI] [PubMed] [Google Scholar]
- 67. Osteikoetxea X, Sódar B, Németh A, Szabó-Taylor K, Pálóczi K, Vukman KV, Tamási V, Balogh A, Kittel Á, Pállinger É, Buzás EI.. Differential detergent sensitivity of extracellular vesicle subpopulations. Org Biomol Chem 2015;13:9775–9782. [DOI] [PubMed] [Google Scholar]
- 68. Gupta S, Knowlton AA.. HSP60 trafficking in adult cardiac myocytes: role of the exosomal pathway. Am J Physiol Heart Circ Physiol 2007;292:H3052–H3056. [DOI] [PubMed] [Google Scholar]
- 69. Pironti G, Strachan RT, Abraham D, Mon-Wei Yu S, Chen M, Chen W, Hanada K, Mao L, Watson LJ, Rockman HA.. Circulating exosomes induced by cardiac pressure overload contain functional angiotensin II type 1 receptors. Circulation 2015;131:2120–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Song J, Chen X, Wang M, Xing Y, Zheng Z, Hu S.. Cardiac endothelial cell-derived exosomes induce specific regulatory B cells. Sci Rep 2015;4:7583.. 10.1038/srep07583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bang C, Batkai S, Dangwal S, Gupta SK, Foinquinos A, Holzmann A, Just A, Remke J, Zimmer K, Zeug A, Ponimaskin E, Schmiedl A, Yin X, Mayr M, Halder R, Fischer A, Engelhardt S, Wei Y, Schober A, Fiedler J, Thum T.. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J Clin Invest 2014;124:2136–2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kapustin AN, Chatrou ML, Drozdov I, Zheng Y, Davidson SM, Soong D, Furmanik M, Sanchis P, De Rosales RT, Alvarez-Hernandez D, Shroff R, Yin X, Muller K, Skepper JN, Mayr M, Reutelingsperger CP, Chester A, Bertazzo S, Schurgers LJ, Shanahan CM.. Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circ Res 2015;116:1312–1323. [DOI] [PubMed] [Google Scholar]
- 73. Horstman LL, Ahn YS.. Platelet microparticles: a wide-angle perspective. Crit Rev Oncol Hematol 1999;30:111–142. 10.1016/S1040-8428(98)00044-4 [DOI] [PubMed] [Google Scholar]
- 74. Loyer X, Vion AC, Tedgui A, Boulanger CM.. Microvesicles as cell-cell messengers in cardiovascular diseases. Circ Res 2014;114:345–353. 10.1161/CIRCRESAHA.113.300858 [DOI] [PubMed] [Google Scholar]
- 75. Yu X, Deng L, Wang D, Li N, Chen X, Cheng X, Yuan J, Gao X, Liao M, Wang M, Liao Y.. Mechanism of TNF-alpha autocrine effects in hypoxic cardiomyocytes: initiated by hypoxia inducible factor 1alpha, presented by exosomes. J Mol Cell Cardiol 2012;53:848–857. [DOI] [PubMed] [Google Scholar]
- 76. Garcia NA, Moncayo-Arlandi J, Sepulveda P, Diez-Juan A.. Cardiomyocyte exosomes regulate glycolytic flux in endothelium by direct transfer of GLUT transporters and glycolytic enzymes. Cardiovasc Res 2016;109:397–408. [DOI] [PubMed] [Google Scholar]
- 77. Garcia NA, Ontoria-Oviedo I, González-King H, Diez-Juan A, Sepúlveda P, Busson P.. Glucose starvation in cardiomyocytes enhances exosome secretion and promotes angiogenesis in endothelial cells. PLoS One 2015;10:e0138849.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wang X, Huang W, Liu G, Cai W, Millard RW, Wang Y, Chang J, Peng T, Fan GC.. Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. J Mol Cell Cardiol 2014;74:139–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Deddens JC, Vrijsen KR, Girao H, Doevendans PA, Sluijter JP.. Cardiac-released extracellular vesicles can activate endothelial cells. Ann Transl Med 2017;5:64.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Deddens JC, Vrijsen KR, Colijn JM, Oerlemans MI, Metz CHG, van der Vlist EJ, Nolte-’T Hoen ENM, den Ouden K, Jansen Of Lorkeers SJ, van der Spoel TIG, Koudstaal S, Arkesteijn GJ, Wauben MHM, van Laake LW, Doevendans PA, Chamuleau SAJ, Sluijter JPG.. Circulating extracellular vesicles contain miRNAs and are released as early biomarkers for cardiac injury. J Cardiovasc Trans Res 2016;9:291–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ibrahim AG, Cheng K, Marban E.. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Rep 2014;2:606–619. 10.1016/j.stemcr.2014.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Barile L, Lionetti V, Cervio E, Matteucci M, Gherghiceanu M, Popescu LM, Torre T, Siclari F, Moccetti T, Vassalli G.. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc Res 2014;103:530–541. [DOI] [PubMed] [Google Scholar]
- 83. Gray WD, French KM, Ghosh-Choudhary S, Maxwell JT, Brown ME, Platt MO, Searles CD, Davis ME.. Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circ Res 2015;116:255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Agarwal U, George A, Bhutani S, Ghosh-Choudhary S, Maxwell JT, Brown ME, Mehta Y, Platt MO, Liang Y, Sahoo S, Davis ME.. Experimental, systems, and computational approaches to understanding the microRNA-mediated reparative potential of cardiac progenitor cell-derived exosomes from pediatric patients. Circ Res 2017;120:701–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Vrijsen KR, Sluijter JP, Schuchardt MW, van Balkom BW, Noort WA, Chamuleau SA, Doevendans PA.. Cardiomyocyte progenitor cell-derived exosomes stimulate migration of endothelial cells. J Cell Mol Med 2010;14:1064–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Vrijsen KR, Maring JA, Chamuleau SA, Verhage V, Mol EA, Deddens JC, Metz CH, Lodder K, van Eeuwijk EC, van Dommelen SM, Doevendans PA, Smits AM, Goumans MJ, Sluijter JP.. Exosomes from cardiomyocyte progenitor cells and mesenchymal stem cells stimulate angiogenesis via EMMPRIN. Adv Healthcare Mater 2016;5:2555–2565. [DOI] [PubMed] [Google Scholar]
- 87. Dignat-George F, Boulanger CM.. The many faces of endothelial microparticles. Arterioscler Thromb Vasc Biol 2011;31:27–33. 10.1161/ATVBAHA.110.218123 [DOI] [PubMed] [Google Scholar]
- 88. van Balkom BW, de Jong OG, Smits M, Brummelman J, den Ouden K, de Bree PM, van Eijndhoven MA, Pegtel DM, Stoorvogel W, Wurdinger T, Verhaar MC.. Endothelial cells require miR-214 to secrete exosomes that suppress senescence and induce angiogenesis in human and mouse endothelial cells. Blood 2013;121:3997–4006. S3991–3915. [DOI] [PubMed] [Google Scholar]
- 89. de Jong OG, Verhaar MC, Chen Y, Vader P, Gremmels H, Posthuma G, Schiffelers RM, Gucek M, van Balkom BWM.. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J Extracell Vesicles 2012;1:18396.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sahoo S, Klychko E, Thorne T, Misener S, Schultz KM, Millay M, Ito A, Liu T, Kamide C, Agrawal H, Perlman H, Qin G, Kishore R, Losordo DW.. Exosomes from human CD34(+) stem cells mediate their proangiogenic paracrine activity. Circ Res 2011;109:724–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Deregibus MC, Cantaluppi V, Calogero R, Lo Iacono M, Tetta C, Biancone L, Bruno S, Bussolati B, Camussi G.. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood 2007;110:2440–2448. [DOI] [PubMed] [Google Scholar]
- 92. Cantaluppi V, Biancone L, Figliolini F, Beltramo S, Medica D, Deregibus MC, Galimi F, Romagnoli R, Salizzoni M, Tetta C, Segoloni GP, Camussi G.. Microvesicles derived from endothelial progenitor cells enhance neoangiogenesis of human pancreatic islets. Cell Transplant 2012;21:1305–1320. [DOI] [PubMed] [Google Scholar]
- 93. Ranghino A, Cantaluppi V, Grange C, Vitillo L, Fop F, Biancone L, Deregibus MC, Tetta C, Segoloni GP, Camussi G.. Endothelial progenitor cell-derived microvesicles improve neovascularization in a murine model of hindlimb ischemia. Int J Immunopathol Pharmacol 2012;25:75–85. [DOI] [PubMed] [Google Scholar]
- 94. Lyu L, Wang H, Li B, Qin Q, Qi L, Nagarkatti M, Nagarkatti P, Janicki JS, Wang XL, Cui T.. A critical role of cardiac fibroblast-derived exosomes in activating renin angiotensin system in cardiomyocytes. J Mol Cell Cardiol 2015;89:268–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kapustin AN, Shanahan CM.. Emerging roles for vascular smooth muscle cell exosomes in calcification and coagulation. J Physiol (Lond) 2016;594:2905–2914. 10.1113/JP271340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hematti P. Mesenchymal stromal cells and fibroblasts: a case of mistaken identity? Cytotherapy 2012;14:516–521. [DOI] [PubMed] [Google Scholar]
- 97. Lai RC, Tan SS, Teh BJ, Sze SK, Arslan F, de Kleijn DP, Choo A, Lim SK.. Proteolytic potential of the MSC exosome proteome: implications for an exosome-mediated delivery of therapeutic proteasome. Int J Proteomics 2012;2012:971907.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zhang B, Yin Y, Lai RC, Tan SS, Choo AB, Lim SK.. Mesenchymal stem cells secrete immunologically active exosomes. Stem Cells Dev 2014;23:1233–1244. 10.1089/scd.2013.0479 [DOI] [PubMed] [Google Scholar]
- 99. Muntasell A, Berger AC, Roche PA.. T cell-induced secretion of MHC class II-peptide complexes on B cell exosomes. Embo J 2007;26:4263–4272. 10.1038/sj.emboj.7601842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Quah BJ, O'neill HC.. The immunogenicity of dendritic cell-derived exosomes. Blood Cells Mol Dis 2005;35:94–110. 10.1016/j.bcmd.2005.05.002 [DOI] [PubMed] [Google Scholar]
- 101. Simpson RJ, Lim JW, Moritz RL, Mathivanan S.. Exosomes: proteomic insights and diagnostic potential. Expert Rev Proteomics 2009;6:267–283. 10.1586/epr.09.17 [DOI] [PubMed] [Google Scholar]
- 102. Lugini L, Cecchetti S, Huber V, Luciani F, Macchia G, Spadaro F, Paris L, Abalsamo L, Colone M, Molinari A, Podo F, Rivoltini L, Ramoni C, Fais S.. Immune surveillance properties of human NK cell-derived exosomes. J Immunol 2012;189:2833–2842. [DOI] [PubMed] [Google Scholar]
- 103. Skokos D, Botros HG, Demeure C, Morin J, Peronet R, Birkenmeier G, Boudaly S, Mecheri S.. Mast cell-derived exosomes induce phenotypic and functional maturation of dendritic cells and elicit specific immune responses in vivo. J Immunol 2003;170:3037–3045. [DOI] [PubMed] [Google Scholar]
- 104. Qu Y, Franchi L, Nunez G, Dubyak GR.. Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol 2007;179:1913–1925. 10.4049/jimmunol.179.3.1913 [DOI] [PubMed] [Google Scholar]
- 105. Heijnen HF, Schiel AE, Fijnheer R, Geuze HJ, Sixma JJ.. Activated platelets release two types of membrane vesicles: microvesicles by surface shedding and exosomes derived from exocytosis of multivesicular bodies and alpha-granules. Blood 1999;94:3791–3799. [PubMed] [Google Scholar]
- 106. Burnouf T, Goubran HA, Chou ML, Devos D, Radosevic M.. Platelet microparticles: detection and assessment of their paradoxical functional roles in disease and regenerative medicine. Blood Rev 2014;28:155–166. [DOI] [PubMed] [Google Scholar]
- 107. Goetzl EJ, Goetzl L, Karliner JS, Tang N, Pulliam L.. Human plasma platelet-derived exosomes: effects of aspirin. Faseb J 2016;30:2058–2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Badimon L, Suades R, Fuentes E, Palomo I, Padro T.. Role of platelet-derived microvesicles as crosstalk mediators in atherothrombosis and future pharmacology targets: a link between inflammation, atherosclerosis, and thrombosis. Front Pharmacol 2016;7:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Brill A, Dashevsky O, Rivo J, Gozal Y, Varon D.. Platelet-derived microparticles induce angiogenesis and stimulate post-ischemic revascularization. Cardiovasc Res 2005;67:30–38. 10.1016/j.cardiores.2005.04.007 [DOI] [PubMed] [Google Scholar]
- 110. Aatonen M, Gronholm M, Siljander PR.. Platelet-derived microvesicles: multitalented participants in intercellular communication. Semin Thromb Hemost 2012;38:102–113. 10.1055/s-0031-1300956 [DOI] [PubMed] [Google Scholar]
- 111. Aatonen MT, Ohman T, Nyman TA, Laitinen S, Gronholm M, Siljander PR.. Isolation and characterization of platelet-derived extracellular vesicles. J Extracell Vesicles 2014;3:24692.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Hulsmans M, Holvoet P.. MicroRNA-containing microvesicles regulating inflammation in association with atherosclerotic disease. Cardiovasc Res 2013;100:7–18. 10.1093/cvr/cvt161 [DOI] [PubMed] [Google Scholar]
- 113. Ostrowski M, Carmo NB, Krumeich S, Fanget I, Raposo G, Savina A, Moita CF, Schauer K, Hume AN, Freitas RP, Goud B, Benaroch P, Hacohen N, Fukuda M, Desnos C, Seabra MC, Darchen F, Amigorena S, Moita LF, Thery C.. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol 2010;12:19–30. sup pp 11–13. [DOI] [PubMed] [Google Scholar]
- 114. Lin J, Li J, Huang B, Liu J, Chen X, Chen XM, Xu YM, Huang LF, Wang XZ.. Exosomes: novel biomarkers for clinical diagnosis. Sci World J 2015;2015:657086.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Properzi F, Logozzi M, Fais S.. Exosomes: the future of biomarkers in medicine. Biomark Med 2013;7:769–778. 10.2217/bmm.13.63 [DOI] [PubMed] [Google Scholar]
- 116. Gallo A, Tandon M, Alevizos I, Illei GG.. The majority of microRNAs detectable in serum and saliva is concentrated in exosomes. PLoS One 2012;7:e30679.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M.. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA 2008;105:10513–10518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Navickas R, Gal D, Laucevičius A, Taparauskaitė A, Zdanytė M, Holvoet P.. Identifying circulating microRNAs as biomarkers of cardiovascular disease: a systematic review. Cardiovasc Res 2016;111:322–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Deddens JC, Colijn JM, Oerlemans MI, Pasterkamp G, Chamuleau SA, Doevendans PA, Sluijter JP.. Circulating microRNAs as novel biomarkers for the early diagnosis of acute coronary syndrome. J Cardiovasc Transl Res 2013;6:884–898. [DOI] [PubMed] [Google Scholar]
- 120. Oerlemans MI, Mosterd A, Dekker MS, de Vrey EA, van Mil A, Pasterkamp G, Doevendans PA, Hoes AW, Sluijter JP.. Early assessment of acute coronary syndromes in the emergency department: the potential diagnostic value of circulating microRNAs. EMBO Mol Med 2012;4:1176–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Sara JD, Widmer RJ, Matsuzawa Y, Lennon RJ, Lerman LO, Lerman A.. Prevalence of coronary microvascular dysfunction among patients with chest pain and nonobstructive coronary artery disease. JACC Cardiovasc Interv 2015;8:1445–1453. [DOI] [PubMed] [Google Scholar]
- 122. Suades R, Padró T, Vilahur G, Martin-Yuste V, Sabaté M, Sans-Roselló J, Sionis A, Badimon L.. Growing thrombi release increased levels of CD235a(+) microparticles and decreased levels of activated platelet-derived microparticles. Validation in ST-elevation myocardial infarction patients. J Thromb Haemost 2015;13:1776–1786. [DOI] [PubMed] [Google Scholar]
- 123. Suades R, Padró T, Crespo J, Ramaiola I, Martin-Yuste V, Sabaté M, Sans-Roselló J, Sionis A, Badimon L.. Circulating microparticle signature in coronary and peripheral blood of ST elevation myocardial infarction patients in relation to pain-to-PCI elapsed time. Int J Cardiol 2016;202:378–387. [DOI] [PubMed] [Google Scholar]
- 124. Milasan A, Tessandier N, Tan S, Brisson A, Boilard E, Martel C.. Extracellular vesicles are present in mouse lymph and their level differs in atherosclerosis. J Extracell Vesicles 2016;5:31427.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Bernal-Mizrachi L, Jy W, Jimenez JJ, Pastor J, Mauro LM, Horstman LL, de Marchena E, Ahn YS.. High levels of circulating endothelial microparticles in patients with acute coronary syndromes. Am Heart J 2003;145:962–970. [DOI] [PubMed] [Google Scholar]
- 126. Porto I, Biasucci LM, De Maria GL, Leone AM, Niccoli G, Burzotta F, Trani C, Tritarelli A, Vergallo R, Liuzzo G, Crea F.. Intracoronary microparticles and microvascular obstruction in patients with ST elevation myocardial infarction undergoing primary percutaneous intervention. Eur Heart J 2012;33:2928–2938. [DOI] [PubMed] [Google Scholar]
- 127. Jung C, Sorensson P, Saleh N, Arheden H, Ryden L, Pernow J.. Circulating endothelial and platelet derived microparticles reflect the size of myocardium at risk in patients with ST-elevation myocardial infarction. Atherosclerosis 2012;221:226–231. [DOI] [PubMed] [Google Scholar]
- 128. Kanhai DA, Visseren FL, van der Graaf Y, Schoneveld AH, Catanzariti LM, Timmers L, Kappelle LJ, Uiterwaal CS, Lim SK, Sze SK, Pasterkamp G, de Kleijn DP.. Microvesicle protein levels are associated with increased risk for future vascular events and mortality in patients with clinically manifest vascular disease. Int J Cardiol 2013;168:2358–2363. [DOI] [PubMed] [Google Scholar]
- 129. Yin M, Loyer X, Boulanger CM.. Extracellular vesicles as new pharmacological targets to treat atherosclerosis. Eur J Pharmacol 2015;763:90–103. 10.1016/j.ejphar.2015.06.047 [DOI] [PubMed] [Google Scholar]
- 130. Huber HJ, Holvoet P.. Exosomes: emerging roles in communication between blood cells and vascular tissues during atherosclerosis. Curr Opin Lipidol 2015;26:412–419. 10.1097/MOL.0000000000000214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kuwabara Y, Ono K, Horie T, Nishi H, Nagao K, Kinoshita M, Watanabe S, Baba O, Kojima Y, Shizuta S, Imai M, Tamura T, Kita T, Kimura T.. Increased microRNA-1 and microRNA-133a levels in serum of patients with cardiovascular disease indicate myocardial damage. Circ Cardiovasc Genet 2011;4:446–454. [DOI] [PubMed] [Google Scholar]
- 132. Karakas M, Schulte C, Appelbaum S, Ojeda F, Lackner KJ, Munzel T, Schnabel RB, Blankenberg S, Zeller T.. Circulating microRNAs strongly predict cardiovascular death in patients with coronary artery disease-results from the large AtheroGene study. Eur Heart J 2017;38:516–523. [DOI] [PubMed] [Google Scholar]
- 133. Goren Y, Kushnir M, Zafrir B, Tabak S, Lewis BS, Amir O.. Serum levels of microRNAs in patients with heart failure. Eur J Heart Fail 2012;14:147–154. [DOI] [PubMed] [Google Scholar]
- 134. Creemers EE, Tijsen AJ, Pinto YM.. Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ Res 2012;110:483–495. [DOI] [PubMed] [Google Scholar]
- 135. Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M.. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA 2011;108:5003–5008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Vegter EL, van der Meer P, de Windt LJ, Pinto YM, Voors AA.. MicroRNAs in heart failure: from biomarker to target for therapy. Eur J Heart Fail 2016;18:457–468. 10.1002/ejhf.495 [DOI] [PubMed] [Google Scholar]
- 137. Emanueli C, Shearn AIU, Laftah A, Fiorentino F, Reeves BC, Beltrami C, Mumford A, Clayton A, Gurney M, Shantikumar S, Angelini GD, Qin G.. Coronary artery-bypass-graft surgery increases the plasma concentration of exosomes carrying a cargo of cardiac microRNAs: an example of exosome trafficking out of the human heart with potential for cardiac biomarker discovery. PLoS One 2016;11:e0154274.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Jansen F, Yang X, Proebsting S, Hoelscher M, Przybilla D, Baumann K, Schmitz T, Dolf A, Endl E, Franklin BS, Sinning JM, Vasa-Nicotera M, Nickenig G, Werner N.. MicroRNA expression in circulating microvesicles predicts cardiovascular events in patients with coronary artery disease. J Am Heart Assoc 2014;3:e001249.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Bi S, Wang C, Jin Y, Lv Z, Xing X, Lu Q.. Correlation between serum exosome derived miR-208a and acute coronary syndrome. Int J Clin Exp Med 2015;8:4275–4280. [PMC free article] [PubMed] [Google Scholar]
- 140. Matsumoto S, Sakata Y, Suna S, Nakatani D, Usami M, Hara M, Kitamura T, Hamasaki T, Nanto S, Kawahara Y, Komuro I.. Circulating p53-responsive microRNAs are predictive indicators of heart failure after acute myocardial infarction. Circ Res 2013;113:322–326. [DOI] [PubMed] [Google Scholar]
- 141. de Hoog VC, Timmers L, Schoneveld AH, Wang JW, van de Weg SM, Sze SK, van Keulen JK, Hoes AW, den Ruijter HM, de Kleijn DP, Mosterd A.. Serum extracellular vesicle protein levels are associated with acute coronary syndrome. Eur Heart J Acute Cardiovascular Care 2013;2:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Ferdinandy P, Hausenloy DJ, Heusch G, Baxter GF, Schulz R.. Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev 2014;66:1142–1174. [DOI] [PubMed] [Google Scholar]
- 143. Madonna R, Van Laake LW, Davidson SM, Engel FB, Hausenloy DJ, Lecour S, Leor J, Perrino C, Schulz R, Ytrehus K, Landmesser U, Mummery CL, Janssens S, Willerson J, Eschenhagen T, Ferdinandy P, Sluijter JP.. Position paper of the European Society of Cardiology Working Group Cellular Biology of the Heart: cell-based therapies for myocardial repair and regeneration in ischemic heart disease and heart failure. Eur Heart J 2016;37:1789–1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Varga ZV, Giricz Z, Bencsik P, Madonna R, Gyongyosi M, Schulz R, Mayr M, Thum T, Puskas LG, Ferdinandy P.. Functional genomics of cardioprotection by ischemic conditioning and the influence of comorbid conditions: implications in target identification. CDT 2015;16:904–911. [DOI] [PubMed] [Google Scholar]
- 145. Hausenloy DJ, Garcia-Dorado D, Botker HE, Davidson SM, Downey J, Engel FB, Jennings R, Lecour S, Leor J, Madonna R, Ovize M, Perrino C, Prunier F, Schulz R, Sluijter JPG, Van Laake LW, Vinten-Johansen J, Yellon DM, Ytrehus K, Heusch G, Ferdinandy P.. Novel targets and future strategies for acute cardioprotection: position paper of the European Society of Cardiology Working Group on Cellular Biology of the Heart. Cardiovasc Res 2017;113:564–585. [DOI] [PubMed] [Google Scholar]
- 146. O'Neill S, Bohl M, Gregersen S, Hermansen K, O'driscoll L.. Blood-based biomarkers for metabolic syndrome. Trends Endocrinol Metab 2016;27:363–374. [DOI] [PubMed] [Google Scholar]
- 147. Record M, Poirot M, Silvente-Poirot S.. Emerging concepts on the role of exosomes in lipid metabolic diseases. Biochimie 2014;96:67–74. 10.1016/j.biochi.2013.06.016 [DOI] [PubMed] [Google Scholar]
- 148. Sahoo S, Emanueli C.. Exosomes in diabetic cardiomyopathy: the next-generation therapeutic targets? Diabetes 2016;65:2829–2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Agouni A, Ducluzeau P-H, Benameur T, Faure S, Sladkova M, Duluc L, Leftheriotis G, Pechanova O, Delibegovic M, Martinez MC, Andriantsitohaina R, Reitsma PH.. Microparticles from patients with metabolic syndrome induce vascular hypo-reactivity via Fas/Fas-ligand pathway in mice. PLoS One 2011;6:e27809.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Agouni A, Lagrue-Lak-Hal AH, Ducluzeau PH, Mostefai HA, Draunet-Busson C, Leftheriotis G, Heymes C, Martinez MC, Andriantsitohaina R.. Endothelial dysfunction caused by circulating microparticles from patients with metabolic syndrome. Am J Pathol 2008;173:1210–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Karolina DS, Tavintharan S, Armugam A, Sepramaniam S, Pek SL, Wong MT, Lim SC, Sum CF, Jeyaseelan K.. Circulating miRNA profiles in patients with metabolic syndrome. J Clin Endocrinol Metab 2012;97:E2271–E2276. [DOI] [PubMed] [Google Scholar]
- 152. Varga ZV, Kupai K, Szűcs G, Gáspár R, Pálóczi J, Faragó N, Zvara Á, Puskás LG, Rázga Z, Tiszlavicz L, Bencsik P, Görbe A, Csonka C, Ferdinandy P, Csont T.. MicroRNA-25-dependent up-regulation of NADPH oxidase 4 (NOX4) mediates hypercholesterolemia-induced oxidative/nitrative stress and subsequent dysfunction in the heart. J Mol Cell Cardiol 2013;62:111–121. [DOI] [PubMed] [Google Scholar]
- 153. Perrino C, Barabasi AL, Condorelli G, Davidson SM, De Windt L, Dimmeler S, Engel FB, Hausenloy DJ, Hill JA, Van Laake LW, Lecour S, Leor J, Madonna R, Mayr M, Prunier F, Sluijter JPG, Schulz R, Thum T, Ytrehus K, Ferdinandy P.. Epigenomic and transcriptomic approaches in the post-genomic era: path to novel targets for diagnosis and therapy of the ischaemic heart? Position Paper of the European Society of Cardiology Working Group on Cellular Biology of the Heart. Cardiovasc Res 2017;113:725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Lee JE, Moon PG, Lee IK, Baek MC.. Proteomic analysis of extracellular vesicles released by adipocytes of otsuka long-evans Tokushima fatty (OLETF) rats. Protein J 2015;34:220–235. 10.1007/s10930-015-9616-z [DOI] [PubMed] [Google Scholar]
- 155. Suades R, Padro T, Alonso R, Mata P, Badimon L.. Lipid-lowering therapy with statins reduces microparticle shedding from endothelium, platelets and inflammatory cells. Thromb Haemost 2013;110:366–377. 10.1160/TH13-03-0238 [DOI] [PubMed] [Google Scholar]
- 156. Sunderland N, Skroblin P, Barwari T, Huntley RP, Lu R, Joshi A, Lovering RC, Mayr M.. MicroRNA biomarkers and platelet reactivity: the clot thickens. Circ Res 2017;120:418–435. [DOI] [PubMed] [Google Scholar]
- 157. Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, Valente N, Shreeniwas R, Sutton MA, Delcayre A, Hsu DH, Le Pecq JB, Lyerly HK.. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med 2005;3:9.. 10.1186/1479-5876-3-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Besse B, Charrier M, Lapierre V, Dansin E, Lantz O, Planchard D, Le Chevalier T, Livartoski A, Barlesi F, Laplanche A, Ploix S, Vimond N, Peguillet I, Thery C, Lacroix L, Zoernig I, Dhodapkar K, Dhodapkar M, Viaud S, Soria JC, Reiners KS, Pogge von Strandmann E, Vely F, Rusakiewicz S, Eggermont A, Pitt JM, Zitvogel L, Chaput N.. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology 2016;5:e1071008.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Escudier B, Dorval T, Chaput N, Andre F, Caby MP, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S, Boccaccio C, Bonnerot C, Dhellin O, Movassagh M, Piperno S, Robert C, Serra V, Valente N, Le Pecq JB, Spatz A, Lantz O, Tursz T, Angevin E, Zitvogel L.. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J Transl Med 2005;3:10.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Sun L, Xu R, Sun X, Duan Y, Han Y, Zhao Y, Qian H, Zhu W, Xu W.. Safety evaluation of exosomes derived from human umbilical cord mesenchymal stromal cell. Cytotherapy 2016;18:413–422. [DOI] [PubMed] [Google Scholar]
- 161. Smyth T, Kullberg M, Malik N, Smith-Jones P, Graner MW, Anchordoquy TJ.. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J Control Release 2015;199:145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Kotmakçı M, Bozok Çetintaş V.. Extracellular vesicles as natural nanosized delivery systems for small-molecule drugs and genetic material: steps towards the future nanomedicines. J Pharm Pharm Sci 2015;18:396–413. [DOI] [PubMed] [Google Scholar]
- 163. Takahashi Y, Nishikawa M, Shinotsuka H, Matsui Y, Ohara S, Imai T, Takakura Y.. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J Biotechnol 2013;165:77–84. [DOI] [PubMed] [Google Scholar]
- 164. Morishita M, Takahashi Y, Nishikawa M, Takakura Y.. Pharmacokinetics of exosomes-an important factor for elucidating the biological roles of exosomes and for the development of exosome-based therapeutics. J Pharm Sci 2017;106:2265–2269. [DOI] [PubMed] [Google Scholar]
- 165. Gallet R, Dawkins J, Valle J, Simsolo E, de Couto G, Middleton R, Tseliou E, Luthringer D, Kreke M, Smith RR, Marban L, Ghaleh B, Marban E.. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J 2017;38:201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Gnecchi M, He H, Liang OD, Melo LG, Morello F, Mu H, Noiseux N, Zhang L, Pratt RE, Ingwall JS, Dzau VJ.. Paracrine action accounts for marked protection of ischemic heart by Akt-modified mesenchymal stem cells. Nat Med 2005;11:367–368. [DOI] [PubMed] [Google Scholar]
- 167. Timmers L, Lim SK, Arslan F, Armstrong JS, Hoefer IE, Doevendans PA, Piek JJ, El Oakley RM, Choo A, Lee CN, Pasterkamp G, de Kleijn DP.. Reduction of myocardial infarct size by human mesenchymal stem cell conditioned medium. Stem Cell Res 2008;1:129–137. [DOI] [PubMed] [Google Scholar]
- 168. Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor EN, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, de Kleijn DP.. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res 2013;10:301–312. [DOI] [PubMed] [Google Scholar]
- 169. Lai RC, Tan SS, Yeo RW, Choo AB, Reiner AT, Su Y, Shen Y, Fu Z, Alexander L, Sze SK, Lim SK.. MSC secretes at least 3 EV types each with a unique permutation of membrane lipid, protein and RNA. J Extracell Vesicles 2016;5:29828.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Bian S, Zhang L, Duan L, Wang X, Min Y, Yu H.. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J Mol Med 2014;92:387–397. 10.1007/s00109-013-1110-5 [DOI] [PubMed] [Google Scholar]
- 171. Chen L, Wang Y, Pan Y, Zhang L, Shen C, Qin G, Ashraf M, Weintraub N, Ma G, Tang Y.. Cardiac progenitor-derived Exosomes protect ischemic myocardium from acute ischemia/reperfusion injury. Biochem Biophys Res Commun 2013;431:566–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Burger D, Vinas JL, Akbari S, Dehak H, Knoll W, Gutsol A, Carter A, Touyz RM, Allan DS, Burns KD.. Human endothelial colony-forming cells protect against acute kidney injury: role of exosomes. Am J Pathol 2015;185:2309–2323. [DOI] [PubMed] [Google Scholar]
- 173. Njock MS, Cheng HS, Dang LT, Nazari-Jahantigh M, Lau AC, Boudreau E, Roufaiel M, Cybulsky MI, Schober A, Fish JE.. Endothelial cells suppress monocyte activation through secretion of extracellular vesicles containing antiinflammatory microRNAs. Blood 2015;125:3202–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Chatila TA, Williams CB.. Regulatory T cells: exosomes deliver tolerance. Immunity 2014;41:3–5. 10.1016/j.immuni.2014.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Budoni M, Fierabracci A, Luciano R, Petrini S, Di Ciommo V, Muraca M.. The immunosuppressive effect of mesenchymal stromal cells on B lymphocytes is mediated by membrane vesicles. Cell Transplant 2013;22:369–379. [DOI] [PubMed] [Google Scholar]
- 176. Fierabracci A, Del Fattore A, Luciano R, Muraca M, Teti A, Muraca M.. Recent advances in mesenchymal stem cell immunomodulation: the role of microvesicles. Cell Transplant 2015;24:133–149. 10.3727/096368913X675728 [DOI] [PubMed] [Google Scholar]
- 177. Khan M, Nickoloff E, Abramova T, Johnson J, Verma SK, Krishnamurthy P, Mackie AR, Vaughan E, Garikipati VN, Benedict C, Ramirez V, Lambers E, Ito A, Gao E, Misener S, Luongo T, Elrod J, Qin G, Houser SR, Koch WJ, Kishore R.. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ Res 2015;117:52–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Wang Y, Zhang L, Li Y, Chen L, Wang X, Guo W, Zhang X, Qin G, He SH, Zimmerman A, Liu Y, Kim IM, Weintraub NL, Tang Y.. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int J Cardiol 2015;192:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Vicencio JM, Yellon DM, Sivaraman V, Das D, Boi-Doku C, Arjun S, Zheng Y, Riquelme JA, Kearney J, Sharma V, Multhoff G, Hall AR, Davidson SM.. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J Am Coll Cardiol 2015;65:1525–1536. [DOI] [PubMed] [Google Scholar]
- 180. Kanasty R, Dorkin JR, Vegas A, Anderson D.. Delivery materials for siRNA therapeutics. Nat Mater 2013;12:967–977. 10.1038/nmat3765 [DOI] [PubMed] [Google Scholar]
- 181. Harel-Adar T, Ben Mordechai T, Amsalem Y, Feinberg MS, Leor J, Cohen S.. Modulation of cardiac macrophages by phosphatidylserine-presenting liposomes improves infarct repair. Proc Natl Acad Sci USA 2011;108:1827–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Airoldi M, Amadori D, Barni S, Cinieri S, De Placido S, Di Leo A, Gennari A, Iacobelli S, Ionta MT, Lorusso V, Lotrionte M, Marchetti P, Mattioli R, Minotti G, Pronzato P, Rosti G, Tondini CA, Veronesi A.. Clinical activity and cardiac tolerability of non-pegylated liposomal doxorubicin in breast cancer: a synthetic review. Tumori 2011;97:690–692. [DOI] [PubMed] [Google Scholar]
- 183. Gomez-Cabrero A, Wrasidlo W, Reisfeld RA, Najbauer J.. IMD-0354 targets breast cancer stem cells: a novel approach for an adjuvant to chemotherapy to prevent multidrug resistance in a murine model. PLoS One 2013;8:e73607.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Aryani A, Denecke B.. Exosomes as a nanodelivery system: a key to the future of neuromedicine? Mol Neurobiol 2016;53:818–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Kooijmans SA, Vader P, van Dommelen SM, van Solinge WW, Schiffelers RM.. Exosome mimetics: a novel class of drug delivery systems. Int J Nanomed 2012;7:1525–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Lavoie JR, Rosu-Myles M.. Uncovering the secretes of mesenchymal stem cells. Biochimie 2013;95:2212–2221. 10.1016/j.biochi.2013.06.017 [DOI] [PubMed] [Google Scholar]
- 187. Cheung MM, Kharbanda RK, Konstantinov IE, Shimizu M, Frndova H, Li J, Holtby HM, Cox PN, Smallhorn JF, Van Arsdell GS, Redington AN.. Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol 2006;47:2277–2282. 10.1016/j.jacc.2006.01.066 [DOI] [PubMed] [Google Scholar]
- 188. Candilio L, Malik A, Ariti C, Barnard M, Di Salvo C, Lawrence D, Hayward M, Yap J, Roberts N, Sheikh A, Kolvekar S, Hausenloy DJ, Yellon DM.. Effect of remote ischaemic preconditioning on clinical outcomes in patients undergoing cardiac bypass surgery: a randomised controlled clinical trial. Heart 2015;101:185–192. [DOI] [PubMed] [Google Scholar]
- 189. Gedik N, Thielmann M, Kottenberg E, Peters J, Jakob H, Heusch G, Kleinbongard P.. No evidence for activated autophagy in left ventricular myocardium at early reperfusion with protection by remote ischemic preconditioning in patients undergoing coronary artery bypass grafting. PLoS One 2014;9:e96567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Consortium E-T, Van Deun J, Mestdagh P, Agostinis P, Akay O, Anand S, Anckaert J, Martinez ZA, Baetens T, Beghein E, Bertier L, Berx G, Boere J, Boukouris S, Bremer M, Buschmann D, Byrd JB, Casert C, Cheng L, Cmoch A, Daveloose D, De Smedt E, Demirsoy S, Depoorter V, Dhondt B, Driedonks TA, Dudek A, Elsharawy A, Floris I, Foers AD, Gartner K, Garg AD, Geeurickx E, Gettemans J, Ghazavi F, Giebel B, Kormelink TG, Hancock G, Helsmoortel H, Hill AF, Hyenne V, Kalra H, Kim D, Kowal J, Kraemer S, Leidinger P, Leonelli C, Liang Y, Lippens L, Liu S, Lo Cicero A, Martin S, Mathivanan S, Mathiyalagan P, Matusek T, Milani G, Monguio-Tortajada M, Mus LM, Muth DC, Nemeth A, Nolte-'T Hoen EN, O'driscoll L, Palmulli R, Pfaffl MW, Primdal-Bengtson B, Romano E, Rousseau Q, Sahoo S, Sampaio N, Samuel M, Scicluna B, Soen B, Steels A, Swinnen JV, Takatalo M, Thaminy S, Thery C, Tulkens J, Van Audenhove I, van der Grein S, Van Goethem A, van Herwijnen MJ, Van Niel G, Van Roy N, Van Vliet AR, Vandamme N, Vanhauwaert S, Vergauwen G, Verweij F, Wallaert A, Wauben M, Witwer KW, Zonneveld MI, De Wever O, Vandesompele J, Hendrix A.. EV-TRACK: transparent reporting and centralizing knowledge in extracellular vesicle research. Nat Meth 2017;14:228–232. [DOI] [PubMed] [Google Scholar]