Abstract
Background
Dilated cardiomoypathies (DCM) are a heterogeneous group of inherited and acquired diseases characterized by decreased contractility and enlargement of cardiac chambers and a major cause of morbidity and mortality. Mice with Glu54Lys mutation in α-tropomyosin (Tm54) demonstrate typical DCM phenotype with reduced myofilament Ca2+ sensitivity. We tested the hypothesis that early sensitization of the myofilaments to Ca2+ in DCM can prevent the DCM phenotype.
Methods and results
To sensitize Tm54 myofilaments, we used a genetic approach and crossbred Tm54 mice with mice expressing slow skeletal troponin I (ssTnI) that sensitizes myofilaments to Ca2+. Four groups of mice were used: non-transgenic (NTG), Tm54, ssTnI and Tm54/ssTnI (DTG). Systolic function was significantly reduced in the Tm54 mice compared to NTG, but restored in DTG mice. Tm54 mice also showed increased diastolic LV dimensions and HW/BW ratios, when compared to NTG, which were improved in the DTG group. β-myosin heavy chain expression was increased in the Tm54 animals compared to NTG and was partially restored in DTG group. Analysis by 2D-DIGE indicated a significant decrease in two phosphorylated spots of cardiac troponin I (cTnI) in the DTG animals compared to NTG and Tm54. Analysis by 2D-DIGE also indicated no significant changes in troponin T, regulatory light chain, myosin binding protein C and tropomyosin phosphorylation.
Conclusion
Our data indicate that decreased myofilament Ca2+ sensitivity is an essential element in the pathophysiology of thin filament linked DCM. Sensitization of myofilaments to Ca2+ in the early stage of DCM may be a useful therapeutic strategy in thin filament linked DCM.
Keywords: DCM, Myofilament Ca2+ sensitivity, New therapy
1. Introduction
Dilated cardiomyopathy (DCM) is a cause of significant morbidity and mortality and the main indication for cardiac transplant in patients with refractory heart failure. Despite significant research efforts, DCM-related mortality remains high, approaching 50% at 5 years in symptomatic patients. DCM is characterized by chamber dilation, systolic dysfunction and is often associated with arrhythmia and sudden cardiac death (SCD) (for review see Ref. 1). The etiology of DCM varies, but currently it is estimated that about 30–50% of DCM cases have genetic causes mainly with autosomal dominant inheritance and usually associated with mutations in cytoskeletal and sarcomeric proteins.2,3 Identification of mutations that cause hypertrophic cardiomyopathy (HCM), restrictive cardiomyopathy (RCM) or DCM have guided both in vitro and in vivo studies to better understand how the specific mutation alters molecular, cellular and whole heart function leading to development of cardiomyopathy.4 However, there are relatively few animal models that recapitulate human DCM and studies testing specific treatments are limited.5,6 Moreover, the few reports involving therapies for treatment of DCM mice have significant limitations.6–9
At the level of the cardiac sarcomere, most of the mutations in thin filament proteins that are linked to DCM show decreased myofilament sensitivity to Ca2+5,6,10–14 with only few exceptions.15–18 Increasing sarcomere activity and Ca2+ sensitivity have been demonstrated to have beneficial effects in acute treatment of acquired HF in humans.19–21 Nevertheless, little is known as to whether early interventions that promote the myofilament response to Ca2+ would be beneficial, but also long-lasting in DCM in which a potential decrease in the myofilament response to Ca2+ can be predicted in children based on family history and genetic screening. Since the primary defect of DCM in most cases is associated with decreased myofilament sensitivity to Ca2+, the most straightforward therapy would be to sensitize the myofilaments to Ca2+, bringing the sensitivity close to normal physiological levels.
There are several good targets within myofilaments for altering Ca2+ sensitivity, including the troponin subunits TnI and TnC, as well as the thick filament protein myosin.22,23 Our demonstration that expression of the neonatal (slow skeletal) isoform of TnI (ssTnI) in the adult mouse induces a number of beneficial effects including an increase in myofilament responsiveness to Ca2+24–28 provides the basis for a genetic approach to restoring sarcomere function in DCM. In the current studies, we therefore employed a transgenic mouse model documented to mimic DCM29 that expresses mutated tropomyosin at position 54 (TmGlu54Lys; Tm54).5 There are at least 50 sarcomeric mutations that are linked to DCM from which 12 have been identified in Tm (TPM1).30 As proof of principle that shifting the myofilament sensitivity close to the normal level is therapeutic in DCM, we crossed Tm54 mice with TG mice that express ssTnI within the myocardium. Our data show a long-lasting, protective effect of myofilament Ca2+ sensitization and indicate that myofilament sensitization to Ca2+ may be a useful preventative therapeutic strategy in sarcomere-linked DCM associated with decreased sensitivity.
2. Methods
For more detailed methods see Supplementary material online.
2.1 Generation of new transgenic (TG) mice
New TG mouse line was generated by crossbreeding existing lines of mice, TG mice with mutated tropomyosin (Tm) at position 54 (TmGlu54Lys)5 and TG mice expressing skeletal isoform of troponin I (ssTnI).25 All mice used in this work were in the same mixed genetic background. Four groups of mice were used for experiments: (1) NTG (non-transgenic), which expresses wild-type (WT) Tm and cardiac TnI; (2) ssTnI, which expresses WT Tm and ssTnI; (3) Tm54, which expresses Tm54 and cardiac TnI; (4) Tm54/ssTnI (DTG), which expresses Tm54 and ssTnI.
All animal procedures were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Review Board of the University of Illinois at Chicago.
2.2 pCa-Force relationship in skinned fibre preparation
Measurements of pCa-force relations were performed as previously described.31
2.3 Echocardiography
Echocardiography was performed using a Vevo 770 High-Resolution In Vivo Imaging System, RMVTM 707B scan head with a centre frequency of 30 MHz (VisualSonics, Toronto, ON, Canada), and Analytic Software as previously described.32,33 Echocardiographic studies were performed in each animal at 5 months of age.
2.4 In situ hemodynamic
In situ pressure-volume measurements were performed as previously described.34 A tracheotomy was performed, the right common carotid artery was then isolated and the artery cannulated with an ultra-miniature P-V catheter (1.4F PVR1045, Millar Instruments, Houston, TX, USA). LV pressure (LVP) and volume were continuously monitored and digitally recorded on Chart software (v.5.5, AD Instruments). To record P-V loops in different loading conditions, an abdominal access was obtained to allow transitory vena cava occlusion right below the diaphragm.
2.5 Western blots
The Western blots were performed as previously described33,35 with slight modifications. For immuno-detection, the membranes were probed with specific primary anti-rabbit antibodies from Cell Signaling, MA, USA: (1) Phospho (T202, Y204 sites of phosphorylation)-p44/42 MAPK (mitogen-activated protein kinase 1) Erk1/2 (extracellular signal-regulated kinase 1 and 2) 1:1000; (2) p44/42 MAPK (Erk1/2) 1:2500; (3) Phospho (Ser21/9) -GSK-3α/β (Glycogen synthase kinase 3 alpha/beta) 1:1000; (4) GSK-3α/β, 1:2000; (5) Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) 1:50,000; (6) Phospho (S473)-Akt (protein-kinase-B)1:2000; (7) Akt 1:2000. Membranes were also probed with antibodies from Abcam Cambridge, MA, USA: (1) Phospho (S105)-GATA4 (GATA binding factor-4) 1:1000; (2) GATA4 1:1000; and anti-mouse Troponin I from Fitzgerald Acton, MA, USA.
2.6 Assessment of β-MHC abundance
The expression level of βMHC was assessed as previously described.36
2.7 Assessment of myofilament modifications by 2D-DIGE
2D-DIGE gels were run to determine the post translational modifications of sarcomeric proteins.35
2.8 Statistical analysis
All statistical analysis was performed using GraphPad Prism 6. Data in the manuscript are presented as mean ± SE, n = number of samples. Differences among four groups were analysed by one-way ANOVA followed by post-hoc analysis indicated in figure legends or text. Differences were considered significant when P < 0.05.
3. Results
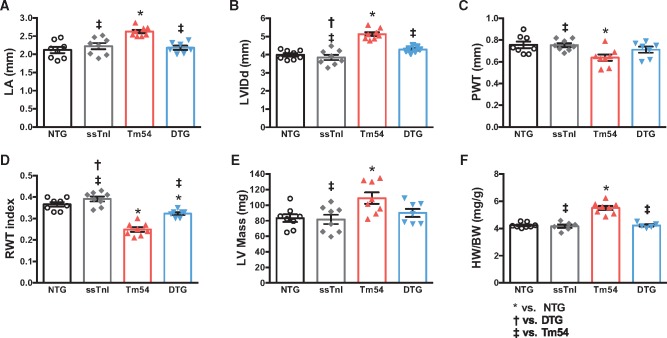
Expression of ssTnI prevents Tm54 hearts from pathological remodeling. High resolution echocardiography and gross pathological evaluation demonstrated that Tm54 hearts developed cardiac chamber dilation that was prevented by expression of ssTnI (Figure 1). Compared to NTG hearts, Tm54 hearts displayed significantly enlarged left atria (LA) (Figure 1A) and severe LV dilation (increased LV internal diastolic dimension (LVIDd) (Figure 1B); eccentric hypertrophy, with lower posterior and relative wall thickness (Figure 1C and D) and higher LV calculated mass (Figure 1E). Heart weight to body weight (HW/BW)) ratio was also increased in Tm54 hearts compared to NTG and ssTnI mice (Figure 1F). Most of these altered parameters were restored to normal values in DTG mice (Tm54 hearts expressing ssTnI).
Figure 1.
DTG mice show improved cardiac morphology compared to Tm54 mice. Cardiac morphology was evaluated by high resolution echocardiography (panels A–E) and whole heart gross morphology (panel F). (A) Left atrium (LA) size, (B) LV internal diastolic dimension (LVIDd), (C) posterior wall thickness (PWT), (D) relative wall thickness (RWT), (E) left ventricular (LV) calculated mass, and (F) heart weight to body weight (HW/BW) ratio. Data are presented as mean ± SE. *Significantly different from NTG, †significantly different from DTG, ‡significantly different from Tm54 based on post-hoc multiple comparison analysis (Newman-Keuls test). n = 7–8 per group.
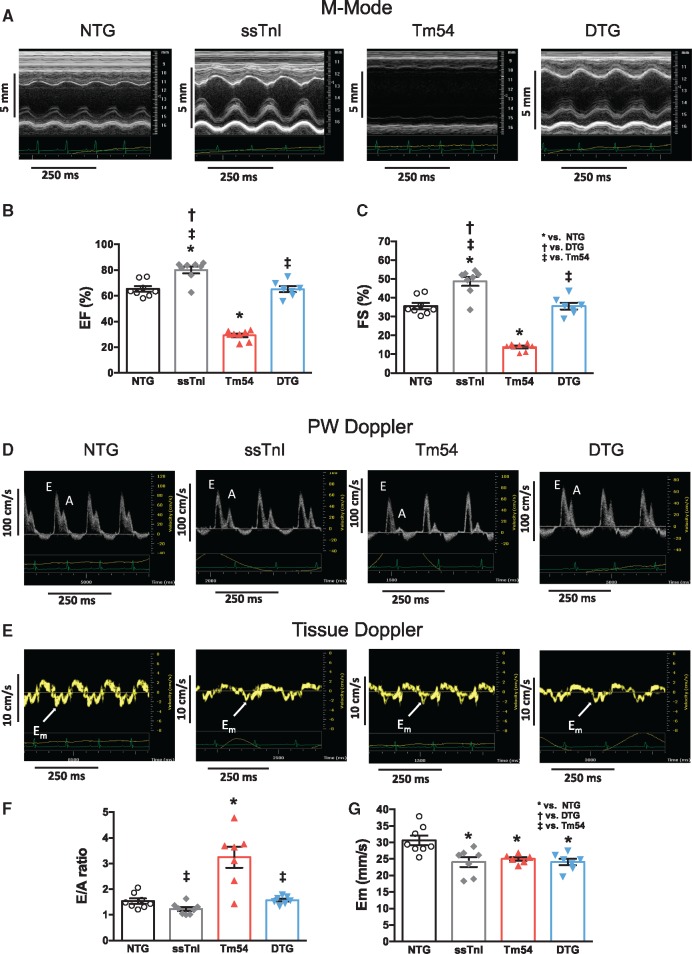
Expression of ssTnI prevents the LV dysfunction observed in Tm54 hearts. Echocardiography and Doppler studies showed that Tm54 mice developed severe systolic and diastolic left ventricular dysfunction (Figure 2 and see Supplementary material online, Table S1). Figure 2 shows the representative M-mode echo images (panel A), pulsed Doppler (panel D) and tissue Doppler (TDI, panel E) in all groups of mice. Figure 2B, C, F, G and see Supplementary material online, Table S1 summarize the evaluation of the systolic and diastolic left ventricular functions in all four groups of mice. Compared to the NTG group Tm54 mice showed reduced ejection fraction (EF; Figure 2B) and fractional shortening (FS; Figure 2C), peak myocardial velocity (Sm) and stroke volume (see Supplementary material online, Table S1). These parameters, with the exception of Sm, were similar to NTG levels in DTG mice. Figure 2F–G and see Supplementary material online, Table S1 summarize the evaluation of diastolic function by pulsed Doppler and TDI studies. Tm54 mice also showed a restrictive LV filling pattern with higher the E/A ratio (Figure 2F), prolonged isovolumic relaxation time (IVRT) (see Supplementary material online, Table S1) and lower peak myocardial velocities in the early phase of diastole (Em) (Figure 2G) and after atrial contraction (Am) (see Supplementary material online, Table S1), when compared to NTG mice. The expression of ssTnI in Tm54 mice partially prevented the development of diastolic dysfunction as it is seen by restoration of the E/A ratio to normal level (Figure 2F), but prolonged IVRT and lower myocardial velocities Em and Am (Figure 2G and see Supplementary material online, Table S1)
Figure 2.
DTG mice show improved cardiac function compared to Tm54 mice. Systolic (panels A–C) and diastolic (panels D–G) functions were assessed by high resolution echocardiography. (A) representative M-mode echo images of all four group of mice, (B) Ejection fraction (EF) and (C) fractional shortening (FS) calculated from B-mode. (D) representative pulsed Doppler and (E) tissue Doppler images of four groups of mice, (F) peak velocity of mitral blood inflow in early diastole (E) to peak velocity of mitral blood inflow in late diastole (A) (E/A ratio), (G) peak myocardial velocity in early diastole (Em). Data are presented as mean ± SE. *Significantly different from NTG, †significantly different from DTG, ‡significantly different from Tm54 based on post-hoc multiple comparison analysis (Newman-Keuls test). n = 7–8 per group.
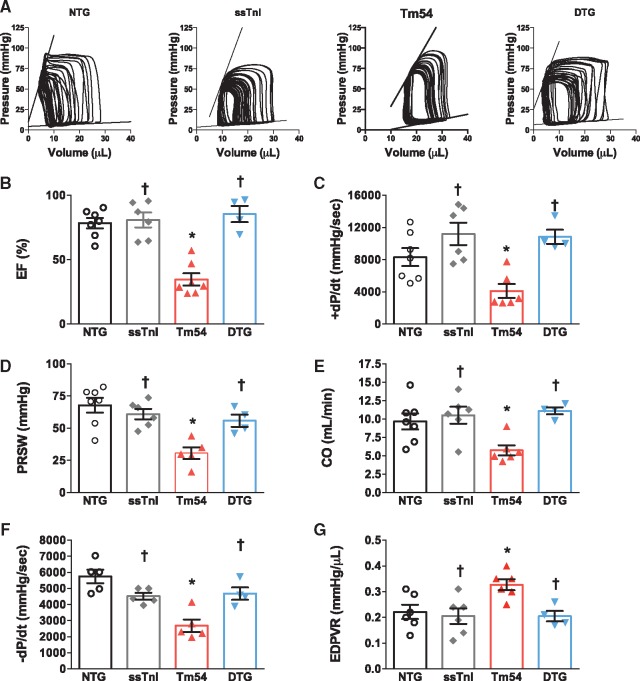
We also used a P-V conductance catheter to perform hemodynamic studies in all four groups of mice (Figure 3). The representative P-V loops are presented in Figure 3A. Compared to NTG mice Tm54 mice showed reduced values for all assessed contractile parameters: ejection fraction (EF) (Figure 3B), rate of pressure development (+dP/dt) (Figure 3C) and preload recruited stroke work (PRSW) (Figure 3D). The depressed contractile parameters and cardiac output in Tm54 hearts were restored in DTG mice (Figure 3B–E). The diastolic function was also impaired in Tm54 mice as assessed by reduced rate of pressure decay (–dP/dt) (Figure 3F) and by the load-independent parameter end diastolic pressure volume relation (EDPVR) (Figure 3G). Overall, cardiac performance was near normal in DTG mice, shown by the normal values of cardiac output (Figure 3E).
Figure 3.
In situ cardiac function was improved in DTG compared to Tm54 mice. (A) Representative P-V loops in NTG, ssTnI, Tm54 and DTG mice, (B) ejection fraction (EF), (C) the maximal rate of contraction (+dP/dt), (D) Preload recruited stoke work (PRSW), (E) the cardiac output (CO), (F) maximal rate of relaxation (-dP/dt), and (G) end diastolic pressure-volume relation (EDPVR). Data are presented as mean ± SE. P < 0.05. *Significantly different from NTG and †significantly different from Tm54 based on post-hoc multiple comparison analysis (Newman-Keuls test). n = 4–8 per group.
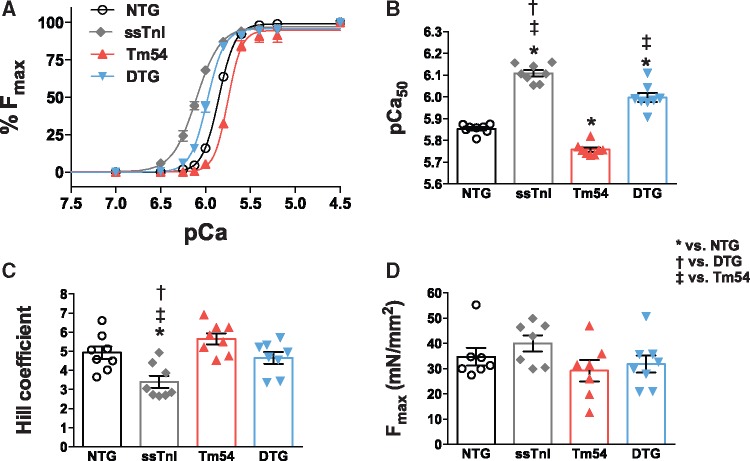
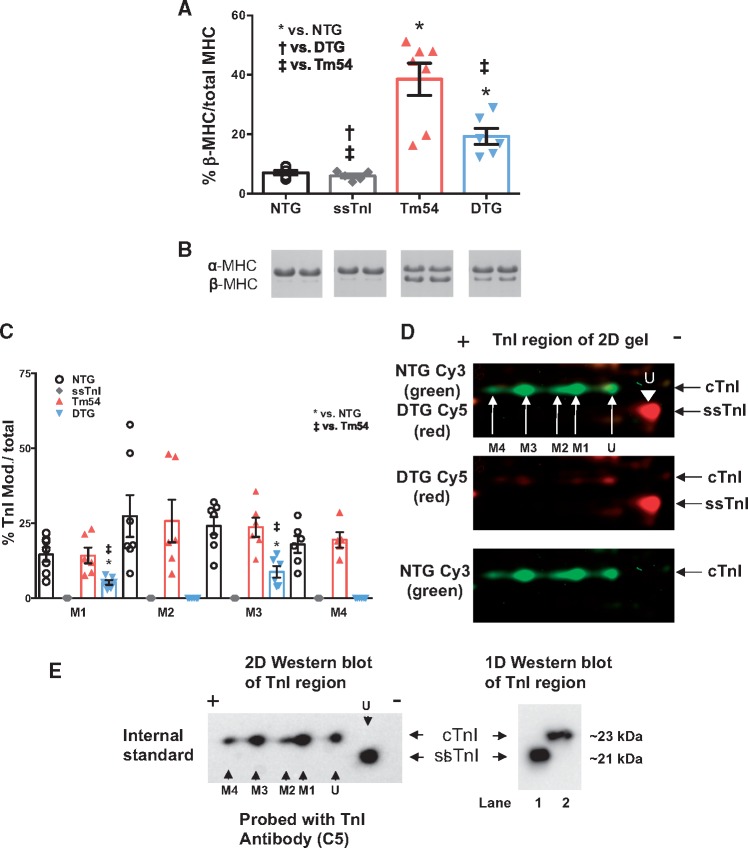
Expression of ssTnI in Tm54 mice increases myofilament Ca2+ sensitivity and partially restores expression of β-MHC. We have previously reported that myofilaments from ssTnI mouse hearts are more sensitive to Ca2+.25 To test the degree to which expressing ssTnI in Tm54 hearts restored myofilament Ca2+ sensitivity and contributed to restoration of cardiac function in DTG mice, we evaluated the myofilament Ca2+ sensitivity in skinned fibres prepared from NTG, ssTnI, Tm54 and DTG mouse hearts (Figure 4). The pCa-force relations showed that myofilaments from Tm54 hearts had lower Ca2+ sensitivity (pCa50 = 5.75 ± 0.004; n = 8) than NTG (pCa50 =5.85 ± 0.002, n = 8), while ssTnI hearts had higher Ca2+ sensitivity (pCa50 = 6.10 ± 0.003, n = 8). The Ca2+ sensitivity of fibres from DTG hearts (pCa50 = 5.98 ± 0.003, n = 8) was significantly higher than Tm54 and NTG fibres (Figure 4A and B). The Hill coefficient, an indicator of cooperativity or steepness of the pCa-force relationship, was lower in ssTnI fibres compared to other groups (Figure 4C). There were no significant differences in the maximal generated tensions between groups (Figure 4D). Since myofilament functional properties depend on expression of myofilament protein isoforms we assessed the level of expression of myosin heavy chain isoforms (Figure 5A and B). Figure 5A and B indicates that expression of β-MHC is significantly increased in Tm54 (38.5 ± 5.4%, n = 6) compared to NTG (7.09 ± 0.74%, n = 6) hearts and partially restored in DTG (19.2 ± 2.7%, n = 6) hearts. The ssTnI hearts expressed a normal level of βMHC (5.98 ± 0.67%, n = 6). Since Tm54 and ssTnI were co-expressed in DTG mice, we assessed the levels of expression of Tm54 in Tm54 and DTG hearts. There was no significant difference in Tm54 expression between hearts from Tm54 and DTG mice. The % replacement of WT Tm in the Tm54 and DTG was 43.2 ± 3.7% (n = 6) and 43.4 ± 5.4% (n = 5), respectively.
Figure 4.
pCa-force relations in skinned fibre bundles indicate significant changes in Ca2+ sensitivity. (A–B) Expression of ssTnI in the presence of Tm54 mutations (DTG) caused an increase in the myofilament Ca2+ (pCa50) sensitivity. (C) Hill coefficient and (D) Maximal tension (Fmax). Data are presented as mean ± SE; P < 0.05. *Significantly different from NTG, †significantly different from DTG, ‡significantly different from Tm54 based on post-hoc multiple comparison analysis (Newman-Keuls test). n = 8 per group.
Figure 5.
Expression of cardiac β-myosin heavy chain (MHC) was significantly reduced in DTG vs. Tm54 via SDS_PAGE analysis and troponin I (TnI) analysis via 2D-DIGE revealed significantly reduced overall phosphorylation of DTG vs. Tm54. Data shown as mean ± SEM; P < 0.05, *Significantly different from NTG, †significantly different from DTG, ‡significantly different from Tm54 based on an 1-way ANOVA with a Newman-Keuls post-hoc test. n = 5–7 per group. (A) % expression of β-MHC in each group of mice and (B) two representative lanes of MHC from each group of mice stained with Coomassie blue. (C) Histogram of the 2D-DIGE TnI quantitation (D) Representative 2D-DIGE region of interest image comparing the NTG vs. DTG (Tm54 + ssTnI) shown in green and red respectively. (E) Representative Western blot analysis of both 1D and 2D blots probed with troponin I C5 antibody recognizing both ssTnI and cTnI. cTnI = cardiac troponin I, ssTnI = slow skeletal troponin I, M = site of modification, U= unmodified spot. The 2D blot confirms spots in the 2D-DIGE are troponin I and ssTnI has only one unmodified spot. Lane 1 represents ssTnI sample and lane 2 represents cTnI sample. The 1D blot confirms ssTnI samples do not contain cTnI. Note the modifications 1-4 are likely phosphorylation based on previous phosphatase data,37 but may also include other modifications.
Expression of ssTnI in Tm54 mice decreases the overall phosphorylation level of TnI in the DTG group. Figure 5C summarizes M1–M4 modification levels of TnI calculated from 2D DIGE gels (Figure 5D). 2D-DIGE gels separate protein based on both molecular weight and isoelectric point and are TnI isoform independent, therefore the horizontal shifts are due to charge changes of the proteins. M1 through M4 spots are distinct charge variants of cTnI, the M indicates modified spot. Therefore M2 is more negatively charged than the M1 spot hence M4 spot is the most negatively charged cTnI spot detected. In previous studies it has been shown that the M1–M4 spots are phosphorylated entities based on phosphatase experiments.37 Mice expressing ssTnI lack modifications M1–M4 due to no detectable phosphorylation in TnI likely due to the missing N-terminal cardiac extension of TnI. Only modifications in M1 and M3 are detected in the DTG group with significantly reduced levels compared to the NTG and Tm54 groups likely due to decreased cardiac TnI expression and significantly lower levels of phosphorylation, thus M2 and M4 spots were below the level of detection (Figure 5E). In the DTG group the % replacement of cTnI with ssTnI was 78 ± 3% n = 6. No significant changes in modifications of TnT (see Supplementary material online, Figure S1), Tm (see Supplementary material online, Figure S2), MLC2 (see Supplementary material online, Figure S3) and MyBP-C (see Supplementary material online, Figure S4) were detected by 2D-DIGE.
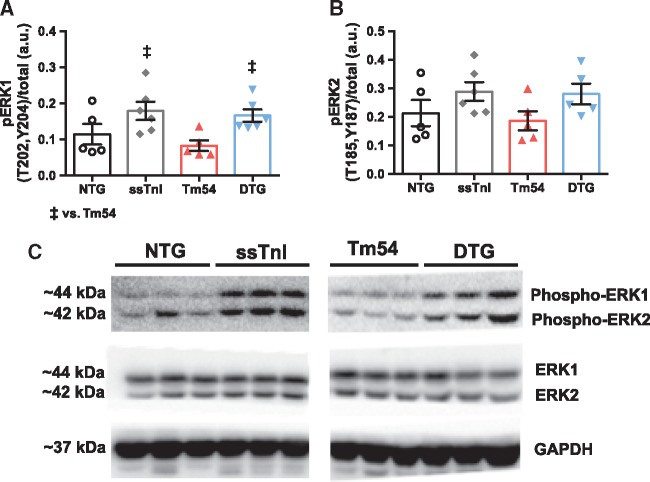
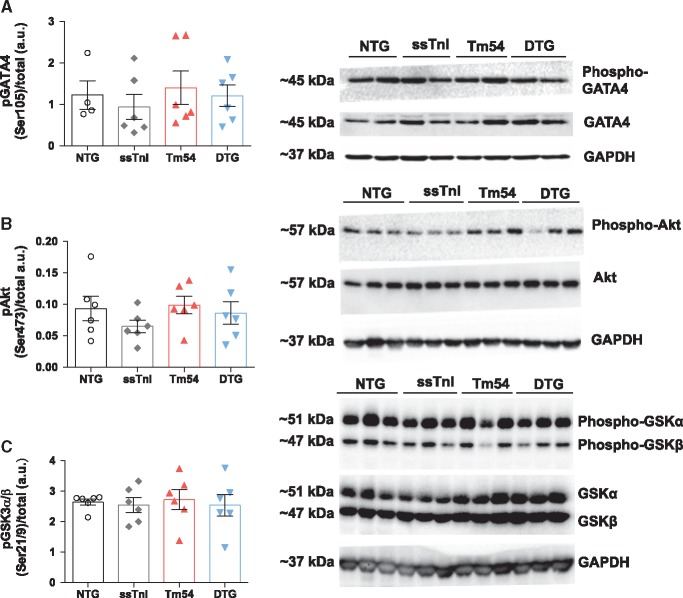
Expression of ssTnI in Tm54 hearts results in increased phosphorylation of ERK1 at T202 and Y204. We have previously shown that activity of ERK1/2 and GATA4 is altered in the HCM mouse model which bears a mutation in Tm at position 180,34 and likely contributes to hypertrophic remodeling. Whether this may also occur in DCM models with eccentric hypertrophy is yet unknown. Therefore, to address this question, and further assess whether signaling may be attenuated in our DTG mice, we assessed both ERK1/2 and GATA4 levels in hearts from all four groups of mice. Figure 6A and C shows that the level of phosphorylation of ERK1 was higher in ssTnI and DTG hearts compared to NTG group. Although ERK2 phosphorylation trended to increase in these two groups as well, no significant changes were found (Figure 6B and C). In addition, there were no observed differences in the phosphorylation of GATA4 (Ser105), Akt (Ser473) or GSK3α/β (Ser21/9) between groups (Figure 7).
Figure 6.
Phosphorylation of ERK1 was significantly increased in the DTG vs. Tm54 and pERK2 was increased in DTG vs. Tm54 but not significantly. (A) phosphorylation of ERK1 and (B) phosphorylation of ERK2. Data are presented as mean ± SE. Phosphorylated protein bands were normalized to total protein. No significant differences in total protein expression were observed. ‡significantly different from Tm54 based on post-hoc multiple comparison analysis (Newman-Keuls test). n = 5-6 per group. (C) Representative western blots for ERK1/2.
Figure 7.
Phosphorylation of GATA4, Akt and GSK3α/β was not significantly altered. (A) phosphorylation of GATA4 at Ser105, (B) phosphorylation of Akt at Ser473, and (C) phosphorylation of GSK3α/β at Ser21/9. Data are presented as mean ± SE n = 4–6 per group. Phosphorylated protein bands were normalized to total protein. No significant differences in total protein expression or phosphorylation levels were observed.
4. Discussion
A major novel finding from experiments reported here is the demonstration that an early intervention restoring myofilament Ca2+ sensitivity in familial DCM is able to prevent progression of the disorder. Although our approach involved an intervention with pleiotropic effects, a major aspect of our use of expression of ssTnI in the DCM54 model is an increase in myofilament Ca2+ sensitivity. Thus, our data provide proof of principle for the development of therapies more specifically affecting sarcomeres than traditional therapies in current use. These therapies comprise a combination of angiotensin converting enzyme (ACE) inhibitors, angiotensin II (AngII) receptor blockers, β-blockers, aldosterone antagonists and diuretics.4 Despite a need for new specific treatments for DCM, there are few in vivo studies in mouse models with DCM and these studies are limited to one model of DCM with a deletion mutation ΔK210 in TnT.6,7,8,9,38 Moreover, some of the studies tested the traditional therapies such as β- and angiotensin II receptor (ARB) blockers.7,9 Zhang et al.7 reported that treatment of TnT ΔK210 DCM mice with β1-selective β-blocker metoprolol was able to prolong survival, reduce myocardial remodeling and cardiac dysfunction compared to vehicle treated DCM mice, but treated DCM mice were still morphologically and functionally different to a significant degree from age-matched wild type (WT) mice. These beneficial effects were not seen in mice treated with non-specific β-blocker carvedilol and hydrophilic β1-selective β-blocker atenolol. In another study using ΔK210 TnT mouse model Odagiri et al.9 have shown that candesartan, an ARB blocker also had a beneficial effect on improving survival rate, cardiac histology and preventing some electrical remodeling, but most of the hemodynamic parameters remained maladaptive when compared with WT age matched mice. As a potential new treatment it was recently reported that ghrelin, a growth-hormone releasing peptide has the beneficial effects in the ΔK210 TnT mouse model, however there was only partial restoration of morphological and functional parameters in the treated DCM mice.38
Since most of the mutations that are linked to DCMs show decreased sarcomere activation resulting in systolic dysfunction and HF, interventions that increase myofilament sensitivity to Ca2+ should be studied in different DCM mouse models. We and others have previously shown that in HCM and RCM mouse models that display increased myofilament sensitivity to Ca2+ bringing sensitivity closer to normal levels has beneficial effects.33,34,39,40 Here, we provided support for the concept that early manipulation in myofilament Ca2+ sensitivity in a DCM model is also beneficial and can significantly prevent both morphological (Figure 1) and functional (Figure 2 and 3) changes. At 5 months of age DTG mice do not show any dilation and most systolic and diastolic parameters are not different from NTG mice. These beneficial changes are associated with increased myofilament sensitivity to Ca2+ slightly above the NTG level (Figure 4) at the same level of Tm54 expression. Importantly, this slight over sensitization did not result in any occurrence of arrhythmias during the echocardiographic assessments or episodes of SCD in DTG mice that we observed up to 5 months which was the time of final non-survival experiment. It has been previously reported that increased myofilament Ca2+ sensitivity causes susceptibility to cardiac arrhythmia in mice.41,42 Although over sensitization of myofilament to Ca2+ can be dangerous and should be taken into account with regard to the development of new therapies and establishing the proper therapeutic window of new drugs, in normal physiological conditions myofilaments sensitivity changes and they operate in a specific zone of Ca2+ sensitivity.
This increased myofilament Ca2+ sensitivity observed in DTG mice can be explained mainly by expression of ssTnI instead of cTnI (Figure 5) and was not associated with changes in phosphorylation of TnT, Tm, myosin binding protein C, or myosin light chain 2 (see Supplementary material online, Figures S1–4). Moreover, we found no significant modifications in cTnI between NTG and Tm54 groups. As predicted, overall phosphorylation of TnI was reduced in DTG mice (Figure 5C and E), inasmuch as they mainly express ssTnI, which cannot be phosphorylated by PKA.24,25 In addition we found that Tm54 mice express β-MHC and its level was significantly reduced in DTG mice (Figure 5A and B). Expression of β-MHC was also reported in other mouse models of DCM ΔK210 TnT.7 Although, the DTG mice still express higher relative amounts of β-myosin in the myosin isoform population compared to NTG group, they show improved relaxation. There are several potential mechanisms that may contribute to this observation. Overall, DTG hearts show reduced remodeling compared to Tm54 hearts that may in part explain our finding that expression of ssTnI enhanced myocardial relaxation in the DTG model despite increased sensitivity to Ca2+. Moreover, the DTG mice most likely maintain better responsiveness to β-adrenergic stimulation. The better responsiveness to β-adrenergic stimulation would allow increasing the rate of relaxation through phosphorylation of other cellular proteins such as phosholamban. It is also possible that the Ca2+ fluxes are altered in Tm54 mice and maintained close to normal in DTG mice.
It has been previously suggested that mutations that cause DCM abolish the relationship between TnI phosphorylation and myofilament Ca2+ sensitivity and that re-coupling by pharmacological drugs may be of potential therapeutic significance for treating of cardiomyopathies.16,43 Our data as well as others6 suggest that this uncoupling between TnI phosphorylation and myofilament Ca2+ sensitivity may be present in some, but not all DCM cases. It should be noted that in the work reported by Memo etal.16 a reconstituted system with human heart muscle TPM1 E54K demonstrated a Ca2+ response similar to controls. Although this finding may indicate a difference between the human and mouse mutant Tm, this conclusion is made difficult by the use of an unloaded system lacking a full complement of sarcomeric proteins, especially titin and myosin binding protein C. In other words, the different findings reported here may reflect the significance of investigating full native preparations rather than reconstituted preparations. Clearly more work is necessary with the human samples to sort this out.
The increased Ca2+ sensitivity in Tm54 myofilaments controlled by ssTnI resulted not only in improved heart morphology and function, but also increased pERK1 and a trend toward increased pERK2 compared to Tm54 mice. The role of ERK1/2 in regulating the balance between concentric vs. eccentric hypertrophy has been reported using mice either lacking ERK1/2 or expressing activated Mek1.44 The authors showed that activation of the ERK1/2 pathway induced concentric hypertrophy, whereas inhibition of ERK1/2 pathway resulted in eccentric hypertrophy as it is seen in pressure vs. volume overload hypertrophy. We and others have shown that HCM is associated with increased ERK1/2 activity and can be reversed in rescued models.33,34,45,46
Although, there are some studies in treating the ΔK210 TnT mouse model with the Ca2+ sensitizers, pimobendan, they are limited. Du et al.6 reported that treatment of 4-week and 20-week old DCM ΔK210 TnT mice for 4 weeks with pimobendan, improved cardiac function and prolonged survival, but the authors presented only a limited set of data and these data were mainly for treatment of 4-week old mice. Moreover, pimobendan is also an inhibitor of phoshodieterase III (PDE3), which raises the concern of increased risk of arrhythmia.47 Recently, Du et al.8 reported that propyl gallate, a phenolic antioxidant in the same DCM TnT mouse model was able to increase survival, reduce pathological remodeling and improve some hemodynamic parameters, but most of the parameters were still different from the WT mice demonstrating persistent maladaptation.
Although our data provide proof of principle regarding the effectiveness of early intervention in preventing the progression of the disorders in DCM, there are limitations to our interpretations of the mechanistic basis of our findings. The effects of ssTnI expression may be better considered to be pleiotropic rather than specific with regard to enhanced Ca2+ sensitivity. Expression of ssTnI in the adult heart has been demonstrated to have effects other than increasing myofilament response to Ca2+. These include effects to reduce length dependence of activation24 as well as induction of metabolic remodeling.27 Moreover, ssTnI lacks both PKA and PKC phosphorylation sites that may modulate contraction in DCM. The extent to which these other effects act in synergy with Ca2+ sensitization is not clear from our study. It is apparent that treatment with a specific sarcomere activator such Omecamtiv Mecarbil, without PDE III inhibition, may provide more definitive evidence that specific effects on myofilament response to Ca2+ are able to prevent DCM progression. Preliminary studies with skinned fibres48 and human inducible pluripotent stem cells49 indicate that Omecamtiv may in fact be effective. Even so it remains to be determined whether long term Ca2+ sensitization induces remodeling similar to that seen with long term ssTnI expression.
In summary, we have demonstrated that early sensitization of myofilaments to Ca2+ should be considered as a new and promising therapeutic intervention for DCM caused by mutations in thin filament proteins that show decreased myofilament Ca2+ sensitivity. Modification of TnI signaling in the myofilaments is an obvious and important target. It has been reported that a small-molecule activator of fast skeletal troponin is able to sensitize myofilaments and offers hope of a therapy for disorders of skeletal muscle.50 It will be important to test thin filament activators in other DCM models, especially those linked to mutations in the thick filaments and titin. Moreover, there are no specific early interventions in children from families with familial cardiomyopathies,51 although early identification of HCM and DCM mutations are possible and more common.52 Our data point to the importance of taking advantage of early childhood diagnosis and to start prevention therapy before the disease develops. A first pilot randomized trial to modify the HCM diseases with diltiazem by treating carriers without LVH has just been published suggesting that pre-clinical administration of diltiazem is safe and may improve LV remodeling53 supporting our conclusion for early treatment of DCM patients before the development of the phenotype.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This research was supported by NIH research grants PO1 HL-62426 (Project 1(RJS and BMW), Core B (BMW) and Core C (CMW), RO1 HL-64035 (RJS and BMW), RO1 HL-128468 (RJS and BMW), RO1 HL-81680 (DFW). RDG and JNS were supported by T32 HL-07692.
Supplementary Material
References
- 1. Luk A, Ahn E, Soor GS, Butany J.. Dilated cardiomyopathy: a review. J Clin Pathol 2009;62:219–225. [DOI] [PubMed] [Google Scholar]
- 2. Burkett EL, Hershberger RE.. Clinical and genetic issues in familial dilated cardiomyopathy. J Am Coll Cardiol 2005;45:969–981. [DOI] [PubMed] [Google Scholar]
- 3. Towbin JA. Inherited cardiomyopathies. Circ J 2014;78:2347–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB, American Heart A, Council on Clinical Cardiology HF, Transplantation C, Quality of C, Outcomes R, Functional G, Translational Biology Interdisciplinary Working G, Council on E, Prevention. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 2006;113:1807–1816. [DOI] [PubMed] [Google Scholar]
- 5. Rajan S, Ahmed RP, Jagatheesan G, Petrashevskaya N, Boivin GP, Urboniene D, Arteaga GM, Wolska BM, Solaro RJ, Liggett SB, Wieczorek DF.. Dilated cardiomyopathy mutant tropomyosin mice develop cardiac dysfunction with significantly decreased fractional shortening and myofilament calcium sensitivity. Circ Res 2007;101:205–214. [DOI] [PubMed] [Google Scholar]
- 6. Du CK, Morimoto S, Nishii K, Minakami R, Ohta M, Tadano N, Lu QW, Wang YY, Zhan DY, Mochizuki M, Kita S, Miwa Y, Takahashi-Yanaga F, Iwamoto T, Ohtsuki I, Sasaguri T.. Knock-in mouse model of dilated cardiomyopathy caused by troponin mutation. Circ Res 2007;101:185–194. [DOI] [PubMed] [Google Scholar]
- 7. Zhan DY, Morimoto S, Du CK, Wang YY, Lu QW, Tanaka A, Ide T, Miwa Y, Takahashi-Yanaga F, Sasaguri T.. Therapeutic effect of β-adrenoceptor blockers using a mouse model of dilated cardiomyopathy with a troponin mutation. Cardiovasc Res 2009;84:64–71. [DOI] [PubMed] [Google Scholar]
- 8. Du CK, Zhan DY, Morimoto S.. In vivo effects of propyl gallate, a novel Ca2+ sensitizer, in a mouse model of dilated cardiomyopathy caused by cardiac troponin T mutation. Life Sci 2014;109:15–19. [DOI] [PubMed] [Google Scholar]
- 9. Odagiri F, Inoue H, Sugihara M, Suzuki T, Murayama T, Shioya T, Konishi M, Nakazato Y, Daida H, Sakurai T, Morimoto S, Kurebayashi N.. Effects of candesartan on electrical remodeling in the hearts of inherited dilated cardiomyopathy model mice. PLoS One 2014;9:e101838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morimoto S, Lu QW, Harada K, Takahashi-Yanaga F, Minakami R, Ohta M, Sasaguri T, Ohtsuki I.. Ca2+-desensitizing effect of a deletion mutation Delta K210 in cardiac troponin T that causes familial dilated cardiomyopathy. Proc Natl Acad Sci USA 2002;99:913–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hershberger RE, Pinto JR, Parks SB, Kushner JD, Li D, Ludwigsen S, Cowan J, Morales A, Parvatiyar MS, Potter JD.. Clinical and functional characterization of TNNT2 mutations identified in patients with dilated cardiomyopathy. Circ Cardiovasc Genet 2009;2:306–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lakdawala NK, Dellefave L, Redwood CS, Sparks E, Cirino AL, Depalma S, Colan SD, Funke B, Zimmerman RS, Robinson P, Watkins H, Seidman CE, Seidman JG, McNally EM, Ho CY.. Familial dilated cardiomyopathy caused by an alpha-tropomyosin mutation: the distinctive natural history of sarcomeric dilated cardiomyopathy. J Am Coll Cardiol 2010;55:320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mirza M, Marston S, Willott R, Ashley C, Mogensen J, McKenna W, Robinson P, Redwood C, Watkins H.. Dilated cardiomyopathy mutations in three thin filament regulatory proteins result in a common functional phenotype. J Biol Chem 2005; 280:28498–28506. [DOI] [PubMed] [Google Scholar]
- 14. Willott RH, Gomes AV, Chang AN, Parvatiyar MS, Pinto JR, Potter JD.. Mutations in Troponin that cause HCM, DCM AND RCM: what can we learn about thin filament function? J Mol Cell Cardiol 2010;48:882–892. [DOI] [PubMed] [Google Scholar]
- 15. Robinson P, Griffiths PJ, Watkins H, Redwood CS.. Dilated and hypertrophic cardiomyopathy mutations in troponin and alpha-tropomyosin have opposing effects on the calcium affinity of cardiac thin filaments. Circ Res 2007;101:1266–1273. [DOI] [PubMed] [Google Scholar]
- 16. Memo M, Leung MC, Ward DG, dos Remedios C, Morimoto S, Zhang L, Ravenscroft G, McNamara E, Nowak KJ, Marston SB, Messer AE.. Familial dilated cardiomyopathy mutations uncouple troponin I phosphorylation from changes in myofibrillar Ca2+ sensitivity. Cardiovasc Res 2013;99:65–73. [DOI] [PubMed] [Google Scholar]
- 17. Preston LC, Lipscomb S, Robinson P, Mogensen J, McKenna WJ, Watkins H, Ashley CC, Redwood CS.. Functional effects of the DCM mutant Gly159Asp troponin C in skinned muscle fibres. Pflugers Arch 2007;453:771–776. [DOI] [PubMed] [Google Scholar]
- 18. Marston SB. Why is there a limit to the changes in myofilament Ca2+-sensitivity associated with myopathy causing mutations? Front Physiol 2016;7:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kota B, Prasad AS, Economides C, Singh BN.. Levosimendan and calcium sensitization of the contractile proteins in cardiac muscle: impact on heart failure. J Cardiovasc Pharmacol Ther 2008;13:269–278. [DOI] [PubMed] [Google Scholar]
- 20. Parissis JT, Rafouli-Stergiou P, Paraskevaidis I, Mebazaa A.. Levosimendan: from basic science to clinical practice. Heart Fail Rev 2009;14:265–275. [DOI] [PubMed] [Google Scholar]
- 21. Pollesello P, Papp Z.. The cardioprotective effects of levosimendan: preclinical and clinical evidence. J Cardiovasc Pharmacol 2007;50:257–263. [DOI] [PubMed] [Google Scholar]
- 22. Kass DA, Solaro RJ.. Mechanisms and use of calcium-sensitizing agents in the failing heart. Circulation 2006;113:305–315. [DOI] [PubMed] [Google Scholar]
- 23. Feest ER, Steven Korte F, Tu A-Y, Dai J, Razumova MV, Murry CE, Regnier M.. Thin filament incorporation of an engineered cardiac troponin C variant (L48Q) enhances contractility in intact cardiomyocytes from healthy and infarcted hearts. J Mol Cell Cardiol 2014;72:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Konhilas JP, Irving TC, Wolska BM, Jweied EE, Martin AF, Solaro RJ, de Tombe PP.. Troponin I in the murine myocardium: influence on length-dependent activation and interfilament spacing. J Physiol 2003;547:951–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fentzke RC, Buck SH, Patel JR, Lin H, Wolska BM, Stojanovic MO, Martin AF, Solaro RJ, Moss RL, Leiden JM.. Impaired cardiomyocyte relaxation and diastolic function in transgenic mice expressing slow skeletal troponin I in the heart. J Physiol 1999;517 (Pt 1):143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wolska BM, Vijayan K, Arteaga GM, Konhilas JP, Phillips RM, Kim R, Naya T, Leiden JM, Martin AF, de Tombe PP, Solaro RJ.. Expression of slow skeletal troponin I in adult transgenic mouse heart muscle reduces the force decline observed during acidic conditions. J Physiol 2001;536:863–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pound KM, Arteaga GM, Fasano M, Wilder T, Fischer SK, Warren CM, Wende AR, Farjah M, Abel ED, Solaro RJ, Lewandowski ED.. Expression of slow skeletal TnI in adult mouse hearts confers metabolic protection to ischemia. J Mol Cell Cardiol 2011;51:236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Layland J, Cave AC, Warren C, Grieve DJ, Sparks E, Kentish JC, Solaro RJ, Shah AM.. Protection against endotoxemia-induced contractile dysfunction in mice with cardiac-specific expression of slow skeletal troponin I. FASEB J 2005;19:1137–1139. [DOI] [PubMed] [Google Scholar]
- 29. Olson TM, Kishimoto NY, Whitby FG, Michels VV.. Mutations that alter the surface charge of alpha-tropomyosin are associated with dilated cardiomyopathy. JMolCell Cardiol 2001;33:723–732. [DOI] [PubMed] [Google Scholar]
- 30. Redwood C, Robinson P.. Alpha-tropomyosin mutations in inherited cardiomyopathies. J Muscle Res Cell Motil 2013;34:285–294. [DOI] [PubMed] [Google Scholar]
- 31. Evans CC, Pena JR, Phillips RM, Muthuchamy M, Wieczorek DF, Solaro RJ, Wolska BM.. Altered hemodynamics in transgenic mice harboring mutant tropomyosin linked to hypertrophic cardiomyopathy. Am J Physiol Heart Circ Physiol 2000; 279:H2414–H2423. [DOI] [PubMed] [Google Scholar]
- 32. Rajan S, Jagatheesan G, Karam CN, Alves ML, Bodi I, Schwartz A, Bulcao CF, D'Souza KM, Akhter SA, Boivin GP, Dube DK, Petrashevskaya N, Herr AB, Hullin R, Liggett SB, Wolska BM, Solaro RJ, Wieczorek DF.. Molecular and functional characterization of a novel cardiac-specific human tropomyosin isoform. Circulation 2010; 121:410–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gaffin RD, Pena JR, Alves MS, Dias FA, Chowdhury SA, Heinrich LS, Goldspink PH, Kranias EG, Wieczorek DF, Wolska BM.. Long-term rescue of a familial hypertrophic cardiomyopathy caused by a mutation in the thin filament protein, tropomyosin, via modulation of a calcium cycling protein. J Mol Cell Cardiol 2011;51:812–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alves ML, Dias FA, Gaffin RD, Simon JN, Montminy EM, Biesiadecki BJ, Hinken AC, Warren CM, Utter MS, Davis RT III, Sadayappan S, Robbins J, Wieczorek DF, Solaro RJ, Wolska BM.. Desensitization of myofilaments to Ca2+ as a therapeutic target for hypertrophic cardiomyopathy with mutations in thin filament proteins. Circ Cardiovasc Genet 2014;7:132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Warren CM, Arteaga GM, Rajan S, Ahmed RP, Wieczorek DF, Solaro RJ.. Use of 2-D DIGE analysis reveals altered phosphorylation in a tropomyosin mutant (Glu54Lys) linked to dilated cardiomyopathy. Proteomics 2008;8:100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Warren CM, Greaser ML.. Method for cardiac myosin heavy chain separation by sodium dodecyl sulfate gel electrophoresis. Anal Biochem 2003;320:149–151. [DOI] [PubMed] [Google Scholar]
- 37. Kirk JA, MacGowan GA, Evans C, Smith SH, Warren CM, Mamidi R, Chandra M, Stewart AF, Solaro RJ, Shroff SG.. Left ventricular and myocardial function in mice expressing constitutively pseudophosphorylated cardiac troponin I. Circ Res 2009;105:1232–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Du C-K, Zhan D-Y, Morimoto S, Akiyama T, Schwenke DO, Hosoda H, Kangawa K, Shirai M.. Survival benefit of ghrelin in the heart failure due to dilated cardiomyopathy. Pharmacol Res Perspect 2014;2:e00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jagatheesan G, Rajan S, Petrashevskaya N, Schwartz A, Boivin G, Arteaga GM, Solaro RJ, Liggett SB, Wieczorek DF.. Rescue of tropomyosin-induced familial hypertrophic cardiomyopathy mice by transgenesis. Am J Physiol Heart Circ Physiol 2007; 293:H949–H958. [DOI] [PubMed] [Google Scholar]
- 40. Li Y, Charles PY, Nan C, Pinto JR, Wang Y, Liang J, Wu G, Tian J, Feng HZ, Potter JD, Jin JP, Huang X.. Correcting diastolic dysfunction by Ca2+ desensitizing troponin in a transgenic mouse model of restrictive cardiomyopathy. J Mol Cell Cardiol 2010;49:402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Baudenbacher F, Schober T, Pinto JR, Sidorov VY, Hilliard F, Solaro RJ, Potter JD, Knollmann BC.. Myofilament Ca2+ sensitization causes susceptibility to cardiac arrhythmia in mice. J Clin Invest 2008;118:3893–3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Schober T, Huke S, Venkataraman R, Gryshchenko O, Kryshtal D, Hwang HS, Baudenbacher FJ, Knollmann BC.. Myofilament Ca2+ sensitization increases cytosolic Ca2+ binding affinity, alters intracellular Ca2+ homeostasis, and causes pause-dependent ca-triggered arrhythmia. Circ Res 2012;111:170–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Papadaki M, Vikhorev PG, Marston SB, Messer AE.. Uncoupling of myofilament Ca2+ sensitivity from troponin I phosphorylation by mutations can be reversed by epigallocatechin-3-gallate. Cardiovasc Res 2015;108:99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kehat I, Davis J, Tiburcy M, Accornero F, Saba-El-Leil MK, Maillet M, York AJ, Lorenz JN, Zimmermann WH, Meloche S, Molkentin JD.. Extracellular signal-regulated kinases 1 and 2 regulate the balance between eccentric and concentric cardiac growth. Circ Res 2011;108:176–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Senthil V, Chen SN, Tsybouleva N, Halder T, Nagueh SF, Willerson JT, Roberts R, Marian AJ.. Prevention of cardiac hypertrophy by atorvastatin in a transgenic rabbit model of human hypertrophic cardiomyopathy. Circ Res 2005;97:285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Patel R, Nagueh SF, Tsybouleva N, Abdellatif M, Lutucuta S, Kopelen HA, Quinones MA, Zoghbi WA, Entman ML, Roberts R, Marian AJ.. Simvastatin induces regression of cardiac hypertrophy and fibrosis and improves cardiac function in a transgenic rabbit model of human hypertrophic cardiomyopathy. Circulation 2001;104:317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lubsen J, Just H, Hjalmarsson AC, La Framboise D, Remme WJ, Heinrich-Nols J, Dumont JM, Seed P.. Effect of pimobendan on exercise capacity in patients with heart failure: main results from the Pimobendan in Congestive Heart Failure (PICO) trial. Heart 1996;76:223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Utter MS, Ryba DM, Li BH, Wolska BM, Solaro RJ.. Omecamtiv mecarbil, a cardiac myosin activator, increases Ca2+ sensitivity in myofilaments with a dilated cardiomyopathy mutant tropomyosin E54K. J Cardiovasc Pharmacol 2015; 66:347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Broughton KM, Li J, Sarmah E, Warren CM, Lin YH, Henze MP, Sanchez-Freire V, Solaro RJ, Russell B.. A myosin activator improves actin assembly and sarcomere function of human-induced pluripotent stem cell-derived cardiomyocytes with a troponin T point mutation. Am J Physiol Heart Circ Physiol 2016; 311:H107–H117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Shefner J, Cedarbaum JM, Cudkowicz ME, Maragakis N, Lee J, Jones D, Watson ML, Mahoney K, Chen M, Saikali K, Mao J, Russell AJ, Hansen RL, Malik F, Wolff AA Neals/Cytokinetics Study T Atassi N, Bedlack R, Boylan K, Bradshaw D, Goslin K, Heiman Patterson T, Jackson C, Kasarskis EJ, Katz J, Levine T, Maragakis NJ, Pestronk A, Simmons Z.. Safety, tolerability and pharmacodynamics of a skeletal muscle activator in amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2012;13:430–438. [DOI] [PubMed] [Google Scholar]
- 51. Colan SD. Treatment of hypertrophic cardiomyopathy in childhood. Prog Pediatr Cardiol 2011;31:13–19. [Google Scholar]
- 52. Ackerman MJ, Marcou CA, Tester DJ.. Personalized medicine: genetic diagnosis for inherited cardiomyopathies/channelopathies. Revista Espanola de Cardiologia 2013; 66:298–307. [DOI] [PubMed] [Google Scholar]
- 53. Ho CY, Lakdawala NK, Cirino AL, Lipshultz SE, Sparks E, Abbasi SA, Kwong RY, Antman EM, Semsarian C, Gonzalez A, Lopez B, Diez J, Orav EJ, Colan SD, Seidman CE.. Diltiazem treatment for pre-clinical hypertrophic cardiomyopathy sarcomere mutation carriers: a pilot randomized trial to modify disease expression. JACC Heart Fail 2015;3:180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.