Abstract
Aims
Matrix metalloproteinases (MMPs) have been implicated in the development of hypertension in animal models and humans. Mmp2 deletion did not change Ang II-induced blood pressure (BP) rise. However, whether Mmp2 knockout affects angiotensin (Ang) II-induced vascular injury has not been tested. We sought to determine whether Mmp2 knockout will prevent Ang II-induced vascular injury.
Methods and results
A fourteen-day Ang II infusion (1000 ng/kg/min, SC) increased systolic BP, decreased vasodilatory responses to acetylcholine, induced mesenteric artery (MA) hypertrophic remodelling, and enhanced MA stiffness in wild-type (WT) mice. Ang II enhanced aortic media and perivascular reactive oxygen species generation, aortic vascular cell adhesion molecule-1 and monocyte chemotactic protein-1 expression, perivascular monocyte/macrophage and T cell infiltration, and the fraction of spleen activated CD4+CD69+ and CD8+CD69+ T cells, and Ly-6Chi monocytes. Study of intracellular signalling showed that Ang II increased phosphorylation of epidermal growth factor receptor and extracellular-signal-regulated kinase 1/2 in vascular smooth muscle cells isolated from WT mice. All these effects were reduced or prevented by Mmp2 knockout, except for systolic BP elevation. Ang II increased Mmp2 expression in immune cells infiltrating the aorta and perivascular fat. Bone marrow (BM) transplantation experiments revealed that in absence of MMP2 in immune cells, Ang II-induced BP elevation was decreased, and that when MMP2 was deficient in either immune or vascular cells, Ang II-induced endothelial dysfunction was blunted.
Conclusions
Mmp2 knockout impaired Ang II-induced vascular injury but not BP elevation. BM transplantation revealed a role for immune cells in Ang II-induced BP elevation, and for both vascular and immune cell MMP2 in Ang II-induced endothelial dysfunction.
Keywords: MMP2, Blood pressure, Hypertension, Vascular injury, Bone marrow transplantation
1. Introduction
Vascular injury is an early manifestation leading to end-organ damage in hypertension.1 Angiotensin (Ang) II, one of the final mediators of the renin–angiotensin–aldosterone system, plays an important role in the development of hypertension and vascular injury.2 Ang II participates in the pathophysiology of hypertension by increasing blood pressure (BP) through vasoconstriction and its renal actions and by causing small artery injury. The latter is characterized by endothelial dysfunction and vascular remodelling, oxidative stress and inflammation.1,3,4 However, the mechanisms whereby Ang II induces vascular injury are complex, and have not been definitively clarified in their entirety.
Matrix metalloproteinases (MMPs) have been implicated in the pathogenesis of hypertension and its complications.5–8 MMPs are members of a superfamily of zinc-dependent endopeptidases that are involved in extracellular matrix (ECM) remodelling through collagen, elastin, and fibronectin degradation.5,8–10 MMPs also play a role in vascular remodelling by shedding growth factors such as heparin-binding epidermal growth factor (HB-EGF)11 and ECM-bound latent transforming growth factor (TGF) β.12 In addition, MMPs could modulate vascular tone by cleaving big-endothelin-1 and other vasoactive peptides.13 MMPs are expressed in the vascular system in vascular smooth muscle cells (VSMCs) and endothelial cells (ECs).5 A systematic review and meta-analysis showed that plasma levels of MMP2, a gelatinase, are predictive of diastolic left ventricular dysfunction in hypertensive patients.14 MMP2 expression is increased in cardiovascular tissues in hypertensive models such as Ang II-infused mice,15,16 two-kidney, one-clip (2K-1C) rats,17,18 and chronic nitric oxide (NO) synthase inhibition with Nω-nitro-l-arginine methyl ester (l-NAME) in rats.18 Transverse aortic constriction (TAC)-induced pressure overload also causes an elevation in cardiac MMP2 expression.19 Interestingly, inhibition of MMPs with the non-selective MMP inhibitor doxycycline reduced the rise in BP and aortic endothelial dysfunction, hypertrophic remodelling and fibrosis in 2K-1C rats,17 Doxycycline also blunted l-NAME-induced aortic hypertrophic remodelling but not mesenteric artery eutrophic remodelling in rats.18Mmp2 targeted gene deletion prevented pressure overload-induced cardiac hypertrophy, dysfunction and fibrosis.19 A MMP2 selective inhibitor or RNA interference targeting the Mmp2 gene prevented Ang II-induced hypertension but not cardiac hypertrophy and fibrosis.15 In another study, Mmp2 knockout did not affect the development of hypertension but resulted in greater cardiac hypertrophy and fibrosis in Ang II-treated mice.20 However, whether Mmp2 knockout prevents the development of vascular injury in hypertension has never been tested.
We hypothesized that Mmp2-targeted gene deletion could prevent Ang II-induced vascular injury. In order to test this hypothesis, we first determined whether Ang II-induced hypertension and vascular injury were blunted in Mmp2-/- mice. Then we examined if Ang II signalling was reduced by Mmp2 knockout in VSMCs in vitro. Finally, we determined the contribution of MMP2 expressed in vascular and immune cells to Ang II detrimental effects using bone marrow (BM) cell transplantation from wild-type (WT) into irradiated Mmp2-/- mice and vice versa.
2. Methods
An expanded Methods section is available in the Supplementary material online.
2.1 Experimental design
The study was approved by the Animal Care Committee of the Lady Davis Institute for Medical Research and McGill University, and followed recommendations of the Canadian Council of Animal Care. Ten to 12-week-old male C57BL/6J WT (Harlan laboratories, Indianapolis, IN, USA) and Mmp2 knockout (Mmp2-/-) mice (generously provided by Dr Shigeyoshi Itohara21 and reproduced at the Lady Davis Institute for Medical Research) were anaesthetized with 3% isoflurane mixed with O2 at 1 L/min. The depth of anaesthesia was confirmed by the rear foot squeezing. The non-steroidal anti-inflammatory drug carprofen (20 mg/kg) was administered SC to minimize the post-operation pain, and then mice were surgically implanted SC with ALZET osmotic mini pumps (Model 1002, Durect Corporation, Cupertino, CA, USA) infusing Ang II (1000 ng/kg/min) for 14 days, as previously described.22 Control mice underwent sham surgery. A subset of the mice were anaesthetized with isoflurane and surgically instrumented with PA-C10 telemetry transmitters 7–10 days before Ang II treatment and BP was determined from two days before Ang II mini pump or sham surgery until the mice were sacrificed, as previously described.22 At the end of the protocol, mice were weighed and then anaesthetized with isoflurane. The mesenteric arterial vascular bed was dissected, and tissues and tibia harvested in ice-cold phosphate buffered saline. Tissues were weighed and tibia length measured. The spleen was used for monocyte and T cell profiling. Second-order branches of mesenteric arteries (MA) were used for assessment of endothelial function and vessel mechanics by pressurized myography.23 Portions of aorta were embedded in Clear Frozen Section Compound (VWR International, Edmonton, AL, Canada) for determination of reactive oxygen species (ROS) generation with the ROS-sensitive fluorescent dye dihydroethidium, expression of fibronectin, monocyte chemotactic protein-1 (MCP-1) and vascular cell adhesion molecule 1 (VCAM-1) or evaluation of tissue infiltration of monocyte/macrophages and T cells by immunofluorescence, or fixed with 4% paraformaldehyde and embedded in paraffin for quantification of collagen content with Sirius red staining.24 The remaining tissues were frozen in liquid nitrogen and stored at -80 °C until used.
In order to examine the role of MMP2 in Ang II signalling in VSMCs, another set of 8–9-week-old male WT and Mmp2-/- mice were used to isolate the VSMCs from MA and study Ang II-induced epidermal growth factor receptor (EGFR) signalling.
In another subset of WT mice treated or not with Ang II as above, CD45+ immune cells were isolated from thoracic aorta with the surrounding perivascular adipose tissue (PVAT) by fluorescence-activated cell sorting (FACS) and Mmp2 expression was determined by reverse transcription (RT)-quantitative PCR (qPCR).
In order to elucidate the contribution of vascular tissue and BM-derived cell MMP2 to Ang II-induced hypertension and vascular injury, irradiation-BM transplantation experiments were performed using 8–10-week-old male WT and Mmp2-/- mice as BM donor and recipient. Ten million BM cells isolated from WT or Mmp2-/- mice were transplanted into γ-irradiated WT (WT → WT or Mmp2-/- → WT) or Mmp2-/- (WT → Mmp2-/- or Mmp2-/- → Mmp2-/-) mice as previously described.25 Four weeks after transplantation, mice were instrumented or not with PA-C10 telemetry transmitters for BP determination, and 7–10 days later BP was determined for two consecutive days before and during the 14-day period of treatment with or without Ang II and studied as above. In addition, blood was collected by cardiac puncture on EDTA. One hundred microlitre of blood was used for confirmation of BM reconstitution and the remaining blood was used for determination of plasma MMP2 and pro-MMP9. BP was also determined in an additional group of BM-transplanted mice infused with l-norepinephrine (4.17 µg/kg/min) using ALZET osmotic pumps for 14 days as previously described.26
2.2 Data analysis
Results are presented as means ± SEM. Data were compared with two-way analysis of variance (ANOVA) or two-way ANOVA for repeated measures, with all ANOVA tests followed by a Student–Newman–Keuls posthoc test, or with an unpaired t-test, as appropriate. P < 0.05 was considered statistically significant.
3. Results
3.1 BP, body and organ weights
Ang II infusion for 2 weeks caused a similar elevation in systolic BP in WT and Mmp2-/- mice (Figure 1A). Body weight, and heart, lung and kidney weight/tibia length were similar, whereas liver and spleen weight/tibia length were, respectively, smaller and greater in Mmp2-/- compared with WT mice (Table 1). Ang II induced an increase in the heart weight/tibia length to a lesser extent in Mmp2-/- mice than WT mice. Ang II also caused an increase in spleen weight/tibia length in WT, but not in Mmp2-/- mice.
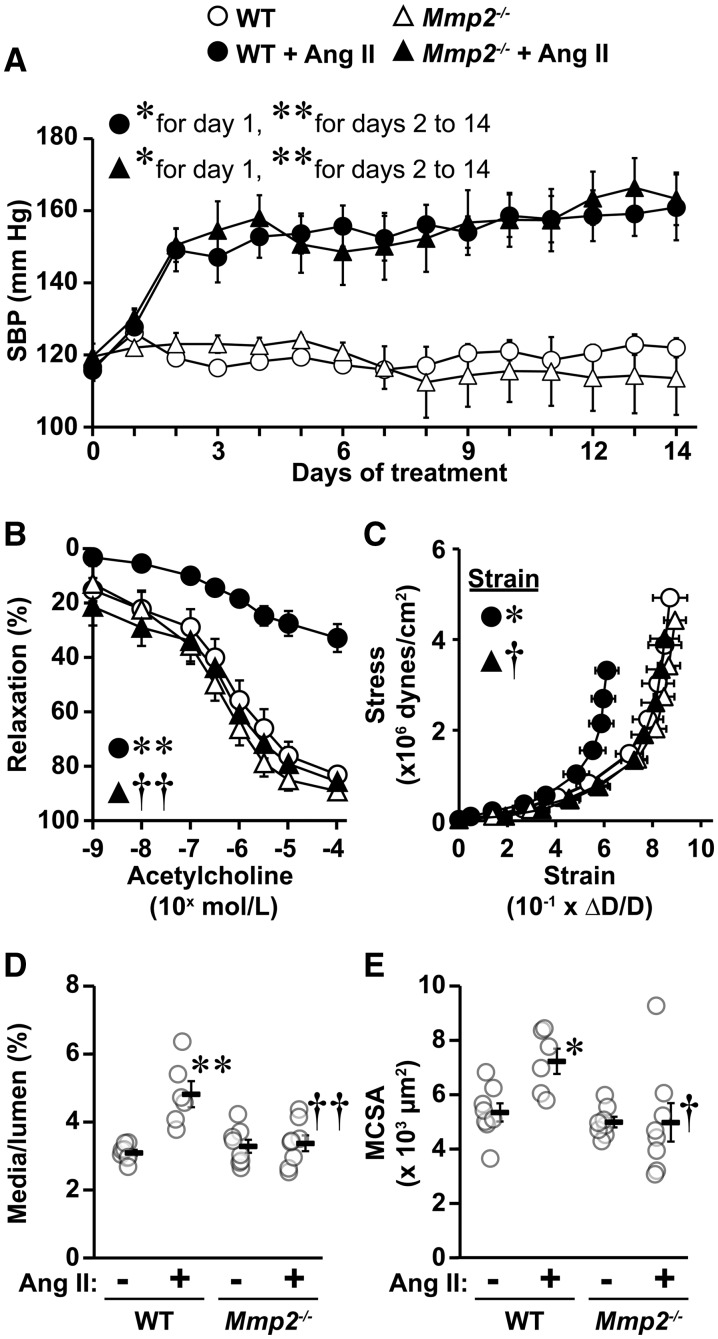
Figure 1.
Mmp2 gene deletion prevented angiotensin (Ang) II-induced endothelial dysfunction and vascular remodelling but not hypertension. Mean 24-h systolic blood pressure (SBP, A) by telemetry and vasodilator responses to acetylcholine (B), vascular stiffness (C), media/lumen (D), and media cross-sectional area (MCSA, E) of small mesenteric arteries using pressurized myography were determined in wild-type (WT) and Mmp2 knockout (Mmp2-/-) mice infused or not with Ang II for 14 days. Media/lumen and MCSA were determined at an intraluminal pressure of 45 mm Hg. Stiffness was determined by comparing values at 140 mm Hg. Values are means ± SEM. Number of samples per group for A: control groups = 3 and Ang II-treated groups = 5. For B: WT = 7, WT + Ang II = 6 and Mmp2-/- and Mmp2-/- + Ang II = 8. For C–E: WT + Ang II = 6 and other groups = 8. Data were analysed using two-way ANOVA for repeated measures in A and B and two-way ANOVA in C–E, with all ANOVA followed by a Student–Newman–Keuls post hoc test. Only the SBP of WT + Ang II and Mmp2-/- +Ang II were statistically analysed in A using respective days 0 as untreated controls. The SBP of WT and Mmp2-/- control groups is presented for reference, and is similar to day 0 of Ang II-treated WT and Mmp2-/- mice. The strain at 140 mm Hg was analysed in C. *P < 0.05 and **P < 0.001 vs. their respective controls and †P < 0.05 and ††P < 0.001 vs. WT + Ang II.
Table 1.
Body and tissue weights
| WT | WT + Ang II | Mmp2-/- | Mmp2-/- + Ang II | |
|---|---|---|---|---|
| BW, g | 27.6 ± 0.6 | 25.4 ± 0.5 | 27.1 ± 0.9 | 25.5 ± 0.8 |
| HW/TL, mg/mm | 7.5 ± 0.1 | 9.4 ± 0.4** | 6.9 ± 0.3 | 8.4 ± 0.3*† |
| LuW/TL, mg/mm | 10.2 ± 0.6 | 10.6 ± 0.5 | 10.8 ± 0.4 | 9.9 ± 0.9 |
| KW/TL, mg/mm | 10.9 ± 0.16 | 10.0 ± 0.3 | 11.0 ± 0.4 | 9.6 ± 0.4 |
| LiW/TL, mg/mm | 91.8 ± 1.6 | 88.8 ± 4.4 | 77.6 ± 4.4* | 81.4 ± 5.5 |
| SW/TL, mg/mm | 3.7 ± 0.2 | 4.5 ± 0.4* | 4.6 ± 0.1* | 4.9 ± 0.2 |
Body weight (BW), tibia length (TL) and heart (HW), lung (LuW), kidney (KW), liver (LiW), and spleen (SW) weight were measured in wild-type (WT) and Mmp2 knockout (Mmp2-/-) mice infused or not with Ang II for 14 days. Values are mean ± SEM. Number of samples per group: Mmp2-/-+Ang II = 8 and other groups = 9. Data were analyzed using two-way ANOVA followed by a Student-Newman-Keuls post hoc test.
P < 0.05 vs. their respective WT or Mmp2-/- controls.
P < 0.001 vs. their respective WT or Mmp2-/- controls.
P < 0.05 vs. other Ang II-infused group.
3.2 MMP2 is required for Ang II-induced endothelial dysfunction and vascular remodelling of small MA
Functional and mechanical properties of small MA were comparable in untreated WT and Mmp2-/- mice (Figure 1B–E and see Supplementary material online, Figure S1). Moreover, contractile responses to norepinephrine were unaffected by Ang II (see Supplementary material online, Figure S1A). Ang II caused impairment of the vasodilatory response to acetylcholine, as indicated by a 50% decrease in the maximal response, in WT but not in Mmp2-/- mice (Figure 1B). NO was the major mediator of the vasodilator response, since acetylcholine-induced relaxation was abrogated in all groups in the presence of the NO synthase inhibitor l-NAME (see Supplementary material online, Figure 1B). The impaired endothelial vasodilatory response was not due to a VSMC defect since endothelium-independent relaxation responses to the NO donor sodium nitroprusside were similar in all groups (see Supplementary material online, Figure 1C). In WT mice, Ang II also increased small artery stiffness as indicated by a leftward shift in the stress/strain curve (Figure 1C), and caused hypertrophic remodelling demonstrated by a ≥1.4-fold increase in the media/lumen and cross-sectional area of mesenteric resistance arteries (Figure 1D and E). Mmp2 deletion prevented Ang II-induced endothelium-dependent relaxation response dysfunction, increase in small artery stiffness and hypertrophic remodelling.
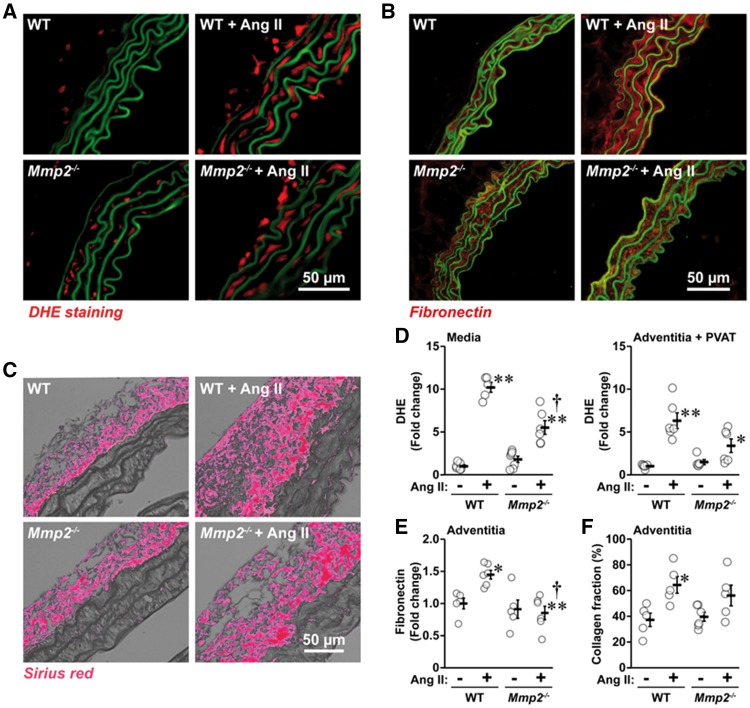
3.3 MMP2 is required for Ang II-induced vascular ROS generation and ECM remodelling
Ang II increased ROS generation 10-fold in the aortic media and six-fold in the adventitia and PVAT in WT mice (Figure 2A and D). ROS generation was reduced in Mmp2-/- mice. Ang II-treated WT mice presented a 1.5-fold increase in the expression of aortic media fibronectin (Figure 2B and E) and a 1.7-fold increase in adventitial collagen fraction (Figure 2C and F) compared with untreated WT mice, changes not found in Mmp2-/- mice.
Figure 2.
Mmp2 gene deletion reduced Ang II-induced reactive oxygen species (ROS) generation and extracellular matrix remodelling. ROS generation by dihydroethidium (DHE) staining in the aortic media and adventitia and perivascular adipose tissue (PVAT) (A and D), media fibronectin expression by immunofluorescence (B and E) and adventitial collagen content by Sirius red staining (C and F) were determined in the same groups as in Figure 1. Representative images of DHE staining (A), fibronectin (B) immunofluorescence images and RGB thresholded images of Sirius red staining (C) of aortic sections are shown. Green fluorescence in A and B represents elastin autofluorescence. Values are means ± SEM. Number of samples per group for media in D: Mmp2-/- = 7 and other groups = 6. For Adventitia + PVAT in D: Mmp2-/- = 5 and other groups = 6. For E: controls = 5 and Ang II-treated groups = 6. For F: Mmp2-/-+Ang II = 6 and other groups = 5. Data were analysed using two-way ANOVA followed by a Student–Newman–Keuls post hoc test. *P < 0.05 and **P < 0.001 vs. their respective controls and †P < 0.001 vs. WT + Ang II.
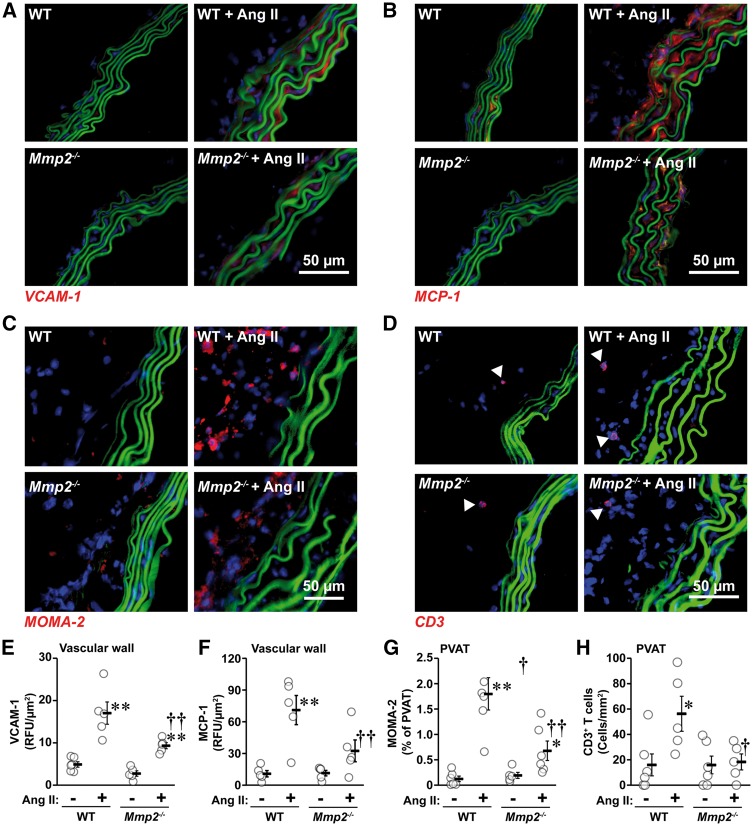
3.4 MMP2 is required for Ang II-induced vascular inflammation and immune response
Ang II increased aortic VCAM-1 and MCP-1 expression four- and seven-fold, respectively, in WT mice, increases that were reduced in Mmp2-/- mice (Figure 3A, B and E, F). Ang II caused an elevation in monocyte/macrophage (15-fold) and CD3+ T cell (four-fold) infiltration in PVAT of WT mice, effect that was markedly reduced in Mmp2-/- mice (Figure 3C, D and G, H).
Figure 3.
Mmp2 gene deletion reduced Ang II-induced inflammation. Aortic VCAM-1 (A and E) and MCP-1 (B and F) expression and MOMA-2+ monocyte/macrophage (C and G) and CD3+ T cell (D and H) infiltration were determined by immunofluorescence in the same groups as in Figure 1. Representative VCAM-1 (A, in red), MCP-1 (B, in red), MOMA-2+ monocyte/macrophages (C, in red), and CD3+ T cell (D, in red) immunofluorescence images of aortic sections are shown. Elastin autofluorescence and nuclear stain DAPI are shown in green and blue, respectively. Arrow heads indicate CD3+ T cells in D. Values are means ± SEM. Number of samples per group for E: WT = 6 and other groups = 5. For F: all groups = 5. For G: Mmp2-/- = 5 and other groups = 6. For H: WT and Mmp2-/- = 5 and Ang II-treated groups = 6. Data were analysed using two-way ANOVA followed by a Student–Newman–Keuls post hoc test. *P < 0.05 and **P < 0.001 vs. their respective untreated controls and †P < 0.05 and vs. ††P < 0.01 WT + Ang II.
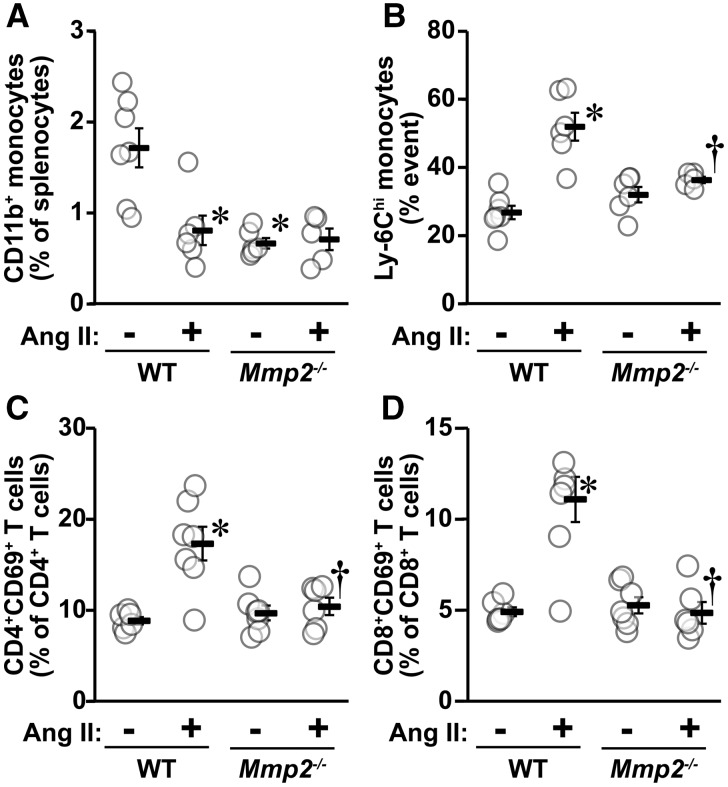
MMP2 is required for activation of immune cells at a systemic level. This was demonstrated by the finding that Ang II caused a 50% decrease in the fraction of CD11b+ monocytes and a two-fold increase in activated Ly-6Chi monocytes in the spleen of WT mice, but not in Mmp2-/- mice (Figure 4A and B). It should be noted that the fraction of CD11b+ monocytes was lower in Mmp2-/- than in WT mice and not affected by Ang II treatment. The fraction of pan (CD3+) T cells, T helper (CD3+CD4+) cells, cytotoxic (CD3+CD8+) T cells, and T regulatory cells (Treg, CD4+CD25+FOXP3+) in the spleen of WT mice was unaffected by Ang II treatment (see Supplementary material online, Figure S2). The fraction of pan T cells was lower in the spleen of Mmp2-/- mice, and Ang II increased this fraction to a comparable level to that observed in WT mice. The fraction of T helper cells, cytotoxic T cells, and Treg was unaltered by deficiency of MMP2 or by Ang II treatment. Ang II increased the fraction of activated T helper (CD4+CD69+) and cytotoxic (CD8+CD69+) T cells ≥two-fold in the spleen of WT mice, but not in Mmp2-/- mice (Figure 4C and D).
Figure 4.
Mmp2 gene deletion blunted Ang II-induced immune responses. Spleen CD11b+ monocytes (A), activated Ly-6Chi monocytes (B), CD4+CD69+ T cells (C), and CD8+CD69+ T cells (D) were determined by flow cytometry in the same groups as in Figure 1. Values are means ± SEM. Number of samples per group for A and B: WT = 7, WT + Ang II and Mmp2-/- = 6 and Mmp2-/- + Ang II = 5. For C and D: WT and Mmp2-/- + Ang II = 6 and other groups = 7. Data were analysed using two-way ANOVA followed by a Student–Newman–Keuls post hoc test. *P < 0.001 vs. their respective controls and †P < 0.01 and ††P < 0.001 vs. WT + Ang II.
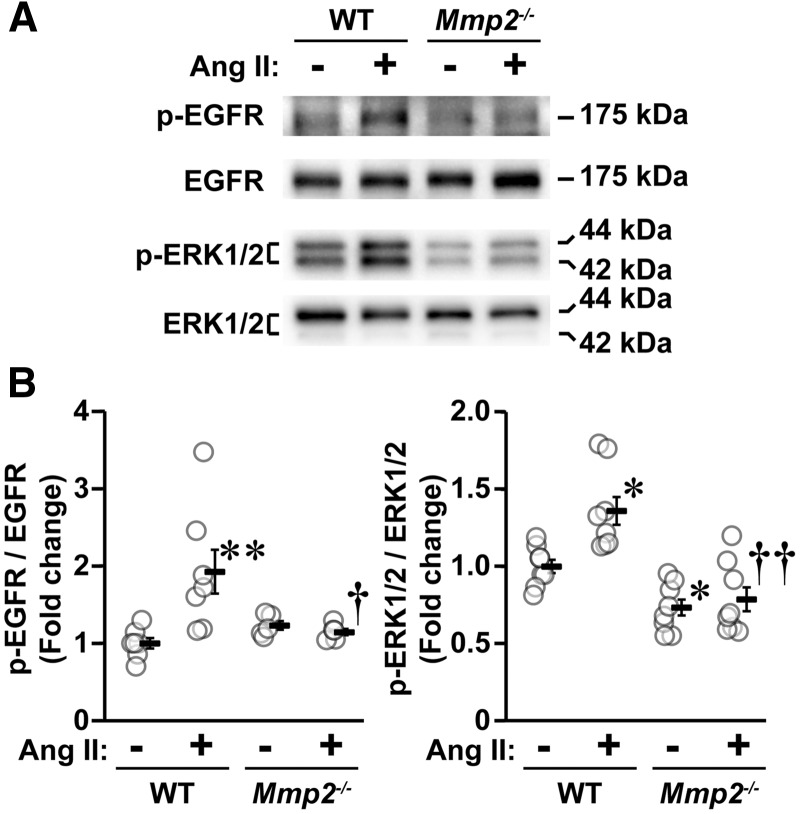
3.5 MMP2 is required for Ang II-EGFR-dependent signalling in VSMCs
The function of MMPs is not limited to turnover and degradation of ECM, but also involves shedding of HB-EGF with subsequent activation of EGFR and downstream mitogen-activated protein kinases (MAPKs), which could be involved in Ang II-induced vascular injury.27 Since MMP2 is highly expressed in VSMCs, we investigated whether Mmp2 deficiency affects Ang II signalling in VSMCs isolated from MA in order to demonstrate the mechanism whereby MMP2 deficiency results in the effects demonstrated in this study. Ang II-induced phosphorylation of EGFR and the downstream extracellular-signal-regulated kinases 1/2 (ERK1/2), MAPKs, were therefore examined in VSMCs. Ang II increased phosphorylation of EGFR two-fold and ERK1/2 1.4-fold in VSMCs isolated from MA of WT (Figure 5). These effects were absent in VSMCs of Mmp2-/- mouse, indicating that MMP2 acts by mediating MAPK activation via EGFR phosphorylation, presumably through contributing to the shedding of HB-EGF.11
Figure 5.
Mmp2 gene deletion blunted Ang II-induced signalling in vascular smooth muscle cells (VSMCs). The level of phosphorylation of epidermal growth factor receptor (EGFR) and of p44/42 mitogen-activated protein kinase (extracellular-signal-regulated kinase 1/2, ERK1/2) in VSMCs from small mesenteric arteries cultured in presence or absence of Ang II for 5 min were determined by western blot. Representative western blots of phosphorylated (p) and total EGFR and ERK1/2 (A) and corresponding dot plots (B) are represented. Values are means ± SEM, number of sample per group for p-EGFR/EGFR: WT = 6, Mmp2-/- = 7 and other groups = 5, and for p-ERK1/2/ERK1/2: eight per group. Data were analysed using two-way ANOVA followed by a Student–Newman–Keuls post hoc test. *P < 0.01 and **P < 0.001 and vs. their respective controls and †P < 0.01 and vs. ††P < 0.01 WT + Ang II.
3.6 Ang II induced Mmp2 expression in infiltrating immune cells
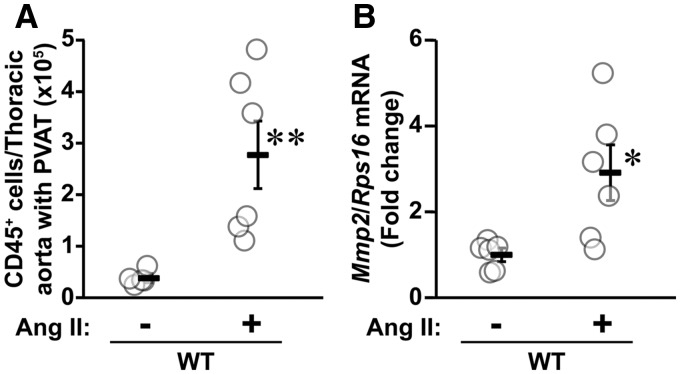
Since MMP2 is expressed in peripheral blood monocytes, resident macrophages and T lymphocytes,9,28,29 we investigated whether Ang II treatment increased the expression of Mmp2 in immune cells infiltrating the aorta/PVAT in WT mice. Ang II increased seven-fold the infiltration of pan (CD45+) immune cells in the aorta/PVAT (Figure 6A), and caused a three-fold rise in Mmp2 expression in these cells (Figure 6B).
Figure 6.
Angiotensin (Ang) II caused an increase in Mmp2 mRNA expression in aorta/perivascular adipose tissue (PVAT) infiltrating pan (CD45+) immune cells. The infiltrating CD45+ immune cells isolated from aorta/perivascular adipose tissue (PVAT) by fluorescence-activated cell sorting and the mRNA expression of Mmp2 and ribosomal protein S16 (Rps16) assessed by reverse transcription-quantitative PCR were determined in wild-type (WT) mice infused or not with Ang II for 14 days. Values are means ± SEM, number of sample per group: WT = 5 and WT + Ang II = 6. Data were analysed using an unpaired T test. *P < 0.05 and **P < 0.001 and vs. WT controls.
3.7 Relative role of immune and vascular cell MMP2 in development of Ang II-induced hypertension and endothelial dysfunction
Since MMP2 expressed in immune cells has been implicated in atherosclerotic plaque rupture28,29 and in Ang II-induced hypertension and vascular injury,3,4 we investigated using BM cell transplantation whether MMP2 expressed in vascular tissue, immune cells, or both, contributed to Ang II-induced BP elevation, endothelial dysfunction and vascular remodelling. WT and Mmp2-/- mice were irradiated and transplanted with BM cells from WT or Mmp2-/- mice, and vice versa. Successful BM cell reconstitution was demonstrated by quantitative PCR of Mmp2 gene (WT donor marker) and neomycin (neo) resistance gene (Mmp2 KO donor marker) in whole blood (see Supplementary material online, Figure S3).
To further characterize the results of BM transplantation mice, plasma MMP2 was determined. MMP2 was detected in the plasma of WT mice transplanted with WT or Mmp2-/- BM cells (WT → WT and Mmp2-/- → WT), and barely or not at all in Mmp2-/- mice transplanted with WT or Mmp2-/- BM cells (WT → Mmp2-/-and Mmp2-/- → Mmp2-/-; see Supplementary material online, Figure S4A and B). Ang II infusion caused a 3.6-fold elevation in plasma MMP2 in WT → WT mice. Mmp2-/- → WT mice presented 2.3-fold more plasma MMP2 than WT → WT mice, which was not further altered by Ang II infusion. In order to determine whether lack of MMP2 could be compensated by another MMP, plasma pro-MMP9 was measured. Plasma pro-MMP9 levels were similar in WT → WT, Mmp2-/- → WT and WT → Mmp2-/- mice, and were unaffected by Ang II infusion (see Supplementary material online, Figure S4C and D). However, Mmp2-/- → Mmp2-/- mice presented 36% lower plasma pro-MMP9 compared with WT → Mmp2-/- mice, which upon Ang II infusion increased to a level similar to that observed in the other groups.
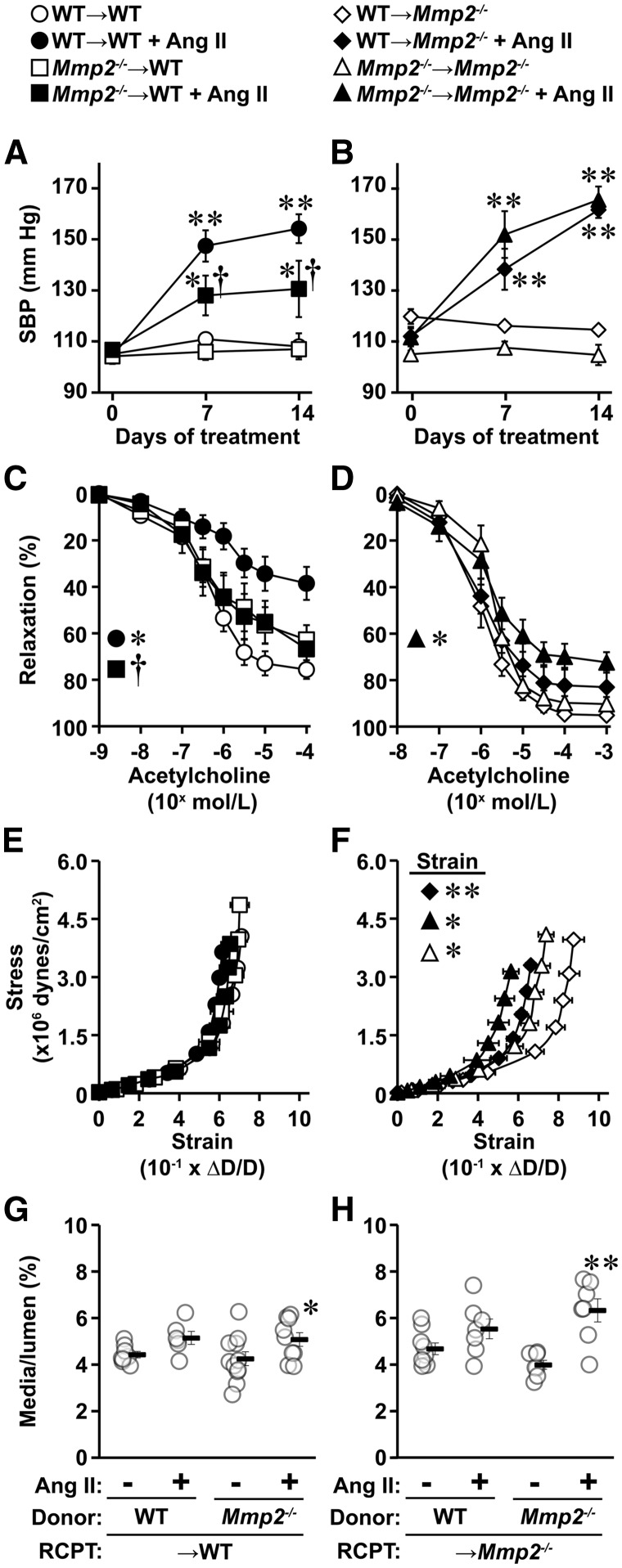
Fourteen days of Ang II caused a 46 and 61 mm Hg mean rise in systolic BP in WT → WT and Mmp2-/- → Mmp2-/- mice, respectively (Figure 7A and B). The systolic BP was 24 mm Hg lower in Mmp2-/- → WT mice than WT → WT mice, and similar in WT → Mmp2-/- compared with Mmp2-/- → Mmp2-/- mice. After Ang II treatment, the MA vasodilatory response to acetylcholine was reduced by ∼50% in WT → WT and to a lesser extent (20%) in Mmp2-/- → Mmp2-/-, but was not blunted in either in Mmp2-/- → WT or WT → Mmp2-/- mice (Figure 7C and D). Small MA stiffness of WT → WT and Mmp2-/- → WT mice was unaffected by Ang II treatment, whereas it was increased in both WT → Mmp2-/- and Mmp2-/- → Mmp2-/- mice, as indicated by a leftward shift in the stress/strain curves (Figure 7E and F). It should be noted that control Mmp2-/- → Mmp2-/- mice presented stiffer MA than control WT → Mmp2-/- mice (Figures 7F). Finally, Ang II caused vascular remodelling in small MA in Mmp2-/- → WT and Mmp2-/- → Mmp2-/- mice, demonstrated by a 1.2- and 1.6-fold increase in media/lumen, respectively, but not in the other groups (Figure 7G and H). Media cross-sectional area was smaller in Mmp2-/- → Mmp2-/- compared with WT → Mmp2-/- mice, and increased 1.6-fold by Ang II infusion in Mmp2-/- → Mmp2-/-, and was unaffected in the other groups (see Supplementary material online, Figure S5).
Figure 7.
Absence of MMP2 in immune cells decreased Ang II-induced rise in SBP, and lack of MMP2 in immune or vascular cells blunted Ang II-induced endothelial dysfunction. Bone marrow from wild-type (WT) and Mmp2-/- donor mice was transplanted into γ-irradiated WT (A, C, E and G, WT → WT and Mmp2-/- → WT) and Mmp2-/- (B, D, F and H, WT → Mmp2-/- and Mmp2-/- → Mmp2-/-) recipient (RCPT) mice. One month later, mice were infused or not with Ang II for 14 days. Mean 24-h SBP was determined by telemetry (A and B). Vasodilator responses to acetylcholine (C and D), vascular stiffness (E and F), and remodelling (G and H) of small mesenteric arteries were determined by pressurized myography at the end of the infusion period. Media/lumen was determined with an intraluminal pressure of 45 mm Hg. Values are means ± SEM. Number of samples per group for A: WT → WT and Mmp2-/- → WT + Ang II = 6 and other groups = 7. For B: WT → Mmp2-/- + Ang II = 6 and other groups = 5. For C: WT → WT = 8, WT → WT + Ang II = 7, Mmp2-/- → WT = 9, and Mmp2-/- → WT + Ang II = 10. For D: WT → Mmp2-/- = 8, Mmp2-/- → Mmp2-/- = 7, and other groups = 6. For E and G: WT → WT = 8, WT → WT +Ang II = 6, Mmp2-/- → WT = 11, and Mmp2-/- → WT +Ang II = 9. For F and H: WT → Mmp2-/- = 9 and other groups = 7. Data were analysed using two-way ANOVA for repeated measures in A–D and two-way ANOVA in E–H, with all ANOVA followed by a Student–Newman–Keuls post hoc test. SBP at days 7 and 14 (A and B) and vasodilator responses to acetylcholine 10−6 to 10−4 mol/L (C and D) were statically analysed. The strain at 140 mm Hg was analysed in F. *P < 0.05 and **P < 0.001 vs. their respective controls, and †P < 0.05 and ††P < 0.001 vs. other Ang II-treated group.
ROS generation was similar in the aortic media of all BM transplanted mice (see Supplementary material online, Figure S6A–D). However, ROS generation was increased in adventitia and PVAT upon Ang II infusion in WT → WT, WT → Mmp2-/- and Mmp2-/- → Mmp2-/- mice but not in Mmp2-/- → WT mice (see Supplementary material online, Figure S6A, B, and E, F). Control Mmp2-/- → Mmp2-/- mice presented lower ROS generation than control WT → Mmp2-/- mice (see Supplementary material online, Figure S6B and F).
In order to determine whether the alteration in Ang II-induced BP rise in absence of vascular or immune MMP2 was selective to this model, an alternative hypertensive agent was tested. Norepinephrine infusion caused similar SBP rise in all groups (see Supplementary material online, Figure S7).
4. Discussion
The present study showed that Mmp2 deletion prevents Ang II-induced endothelial dysfunction, vascular remodelling, oxidative stress, and inflammation in mice. These vascular protective effects could be mediated at least in part through the inhibition of the role that MMP2 plays via shedding of HB-EGF11 leading to EGFR/ERK1/2 signalling in VSMCs. Ang II increased Mmp2 expression in aorta/PVAT infiltrating immune cells. As well, BM cell transplantation experiments revealed that Mmp2 deficiency in immune cells reduced BP rise, and lack of Mmp2 in both vascular and immune cells blunted endothelial dysfunction in Ang II infused mice.
In this study, endothelial dysfunction, vascular stiffness and remodelling of small MA, oxidative stress and inflammation caused by Ang II were blunted by Mmp2 deletion, whereas BP elevation was not. This is the first study that demonstrates a role of MMP2 in the development of vascular injury independent of BP elevation. The role of MMPs in hypertension has been examined previously using doxycycline, a broad-spectrum MMP inhibitor. Bouvet et al. showed that doxycycline reduced BP elevation and aortic remodelling but not mesenteric artery remodelling in rats subjected to chronic NO synthase inhibition with l-NAME.18 In 2K-1C hypertension, Castro et al. observed that doxycycline reduced BP and prevented development of aortic endothelial dysfunction and remodelling.17 The blunting of BP elevation in the above studies could be due to inhibition of multiple MMPs and also to off-target effects of the non-selective MMP inhibitors. Odenbach et al. showed that specific pharmacologic inhibition of MMP2 using the lipid analogue MMP2 inhibitor I, and RNA interference to knock down Mmp2, prevented Ang II-induced hypertension but not cardiac hypertrophy and fibrosis.15 More recently, the same group showed that the extent of BP elevation was unaffected but cardiac hypertrophy and pericoronary artery fibrosis were exaggerated in Ang II-treated Mmp2-/- compared with WT mice.20 This latter study is in agreement with our current study with respect to BP. However, in our study cardiac hypertrophy was unaffected by absence of MMP2.
MMP2 is expressed in different tissues including vascular cells. It is highly expressed in VSMCs as demonstrated by in silico human expression profile (http://ds.biogps.org/?dataset=GSE1133&gene=4313, accessed on 7 May 2017, date last accessed). Furthermore, Ang II increased expression of Mmp2 in vitro in VSMCs and in vivo in mouse aorta.16,30–33 Therefore, it is possible that prevention of Ang II-induced vascular injury by Mmp2 knockout could be due, at least in part, to the lack of MMP2 in VSMCs. MMPs including MMP2 have been involved in activation or shedding of growth factors and peptides such as latent TGFβ,34 big endothelin-1,13 and HB-EGF.27 The mitogenic effects of the G protein-coupled receptors, including Ang type 1 receptor (AGTR1), are mediated through activation of EGFR and subsequent activation of phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) and MAPKs including ERK1/2 and p38 MAPK.11,27 Indeed, our study of cultured mesenteric artery VSMCs demonstrated that Ang II signalling via EGFR and ERK1/2 was blunted in absence of MMP2. Also, Schreier et al. showed that postnatal VSMC Egfr knockout blunted acute vasoconstriction induced by Ang II.35 Accordingly, vascular protective effects of Mmp2 knockout could be mediated at least in part by inhibition of Ang II signalling in VSMCs. A similar protective mechanism may be occurring in other cell types such as immune cells. Indeed, monocyte/macrophages have been shown to express AGTR1,36Mmp2,28,29 and EGFR.37,38
We and others have shown that the innate and adaptive immune systems play an important role in the development of hypertension and vascular injury.3,4 PVAT infiltrating immune cells could cause endothelial dysfunction through ROS generation. MMP2 expression is induced in monocytes via interaction with ECM components such as fibronectin, while it is constitutively expressed in differentiated macrophages.28 MMP2 is expressed to a higher level in CD4+ T helper (Th)1 effector cells than in Th2 effector or naive Th0 cells. Furthermore, Th1 effector cells can stimulate MMP2 expression in macrophages.29 In this study, we demonstrated for the first time that Ang II increased Mmp2 expression in aorta/PVAT infiltrating immune cells, which could contribute to Ang II-induced vascular injury. This possibility was addressed using BM cell transplantation from WT or Mmp2-/- mice into WT or Mmp2-/- mice.
BM cell transplantation experiments demonstrated a role for immune cell MMP2 in the development of hypertension. Ang II-induced BP rise was reduced in WT recipient mice that received Mmp2-/- BM cells, and was delayed in Mmp2-/- recipient mice that received WT or Mmp2-/- BM cells. This finding might be unique to the Ang II-infused model since it was not observed using BM-transplanted mice infused with norepinephrine as an alternative hypertensive agent. It is unclear why BP did not reach the same levels after Ang II infusion in WT and Mmp2-/- recipient mice. However, this difference was not observed with norepinephrine infusion.
A role for both immune and vascular cell MMP2 was also demonstrated for mesenteric artery endothelial function. The most severe endothelial dysfunction caused by Ang II was observed in mice having MMP2 in both immune and vascular cells, and deletion of Mmp2 in either immune cells, vascular cells or both blunted or prevented Ang II-induced endothelial dysfunction. Ang II-induced endothelial dysfunction is mediated at least in part by increased production of oxidative stress by immune cells.22,39 Deletion of MMP2 in immune cells accompanied by persistence of vascular MMP2 was associated with blunted Ang II-induced perivascular ROS production. However, a dissociation between endothelial function and perivascular ROS generation was observed in Mmp2-/- recipient mice receiving either WT or Mmp2-/- BM cells.
The Ang II-induced increase in vascular stiffness was found only in Mmp2-/- recipient mice receiving WT or Mmp2-/- BM cells, whereas Ang II-induced vascular remodelling was observed only in WT and Mmp2-/- recipient mice receiving Mmp2-/- BM cells. The slightly greater increase in BP observed in Mmp2-/- recipient mice receiving WT or Mmp2-/- BM cells compared with WT mice receiving either BM cells could have resulted in the observed vascular stiffening. Differences in vascular wall composition (perhaps due to the irradiation necessary for BM transplantation) could explain the vascular stiffening and remodelling results. As a consequence, BM cell transplantation experiments did not totally reproduce the experiments performed originally with WT and Mmp2-/- mice infused with Ang II. However, they allowed teasing out differential effects of MMP2 expressed in either immune cells or in vascular tissue. The limitations of BM cell transplantation experiments could only be resolved by using an inducible conditional gene knockout mouse. Unfortunately, floxed Mmp2 mice are unavailable.
There are similarities between these results and our previous findings showing that Ang II-induced BP rise and endothelial dysfunction were reduced or blunted in osteopetrotic mice that have a mutation in the colony-stimulating factor gene (Csf1) and an associated decrease in functional monocyte/macrophages,40 as well as in mice adoptively transferred with T regulatory cells,22 in which effector T cells and innate immune cells such as dendritic cells, monocytes, and macrophages are suppressed.3,4 In this study, Mmp2 deficiency in immune cells prevented Ang II-induced BP rise and endothelial dysfunction. MMP2 could play a role in innate or adaptive immune responses in the pathophysiology of hypertension and vascular injury. This could be mediated through a local MMP2 action, since immune cells contribute very little to circulating MMP2. It has been shown that interaction of monocytes with matrix leads to up-regulation of several MMPs including MMP2.28 Interestingly, we found that Ang II enhanced MMP2 expression in infiltrating immune cells. MMP2 could contribute to immune cell activation via cytokines such as interleukin-1β.9 We also observed that the lack of Mmp2 in vascular tissue did not affect Ang II-induced BP rise but blunted endothelial dysfunction. It is unclear why the lack of vascular MMP2 did not impede Ang II pressor responses. However, vascular MMP2 might be important for Ang II-induced vascular injury. Expression of MMP2 in vascular cells could play a role in the attraction and recruitment of monocytes and T cells, as has been suggested in atherosclerosis.28 This could occur via Ang II-dependent MMP2-mediated EGFR signalling by vascular cells or infiltrating monocyte/macrophages expressing AGTR1, MMP2, and EGFR.22,41 This could also rely on MMP2 secretion by vascular or immune cells causing degradation of ECM or activation of nearby immune cells.
5. Limitations
The present study has focused on determining the role of MMP2 in Ang II-induced hypertension and vascular injury. MMP2 is part of the MMP family that includes 24 members.9 MMPs are regulated by tissue inhibitors of metalloproteinases (TIMPs) that inhibit their activity by binding to their catalytic site. There are four TIMPs (TIMP-1, -2, -3 and -4). TIMP2 acts as inhibitor or activator of MMPs. TIMP-2 is required with MMP14 for the activation of pro-MMP2. The present study has not addressed the role of the complex interactional network of MMPs/TIMPS in hypertension and vascular injury, for which additional studies will be required.
6. Conclusions and perspectives
This study demonstrated using Mmp2 null mice that MMP2 mediates effects of Ang II leading to endothelial dysfunction, vascular remodelling, oxidative stress, and inflammation. These effects are mediated at least in part through the role that MMP2 plays via shedding of HB-EGF that stimulate EGFR/ERK1/2 signalling in VSMCs, or through enhanced Mmp2 expression in infiltrating immune cells. BM transplantation experimental results revealed a role for immune cell MMP2 in Ang II-induced BP elevation and for both vascular and immune cell MMP2 in Ang II-induced endothelial dysfunction. Understanding the mechanism of action of MMP2 in immune and vascular cells may reveal new therapeutic targets for the treatment of hypertension and vascular disease.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Supplementary Material
Acknowledgements
We are grateful to Adriana Cristina Ene, Guillem Colell Dinarès, and Véronique Michaud for excellent technical support.
Conflict of interest: none declared.
Funding
This work was supported by Canadian Institutes of Health Research (CIHR) grants 82790, 102606, and 123465, a CIHR First Pilot Foundation Grant 143348, a Canada Research Chair (CRC) on Hypertension and Vascular Research by the CRC Government of Canada/CIHR Program, and by the Canada Fund for Innovation (CFI), all to E.L.S., by a CIHR grant 142479, a CRC in Cardiovascular Physiology and a CFI to S.L., and by fellowships to M.O.R.M. (Canadian Vascular Network and Lady Davis Institute/TD Bank studentship), T.B. (Société québécoise d’hypertension artérielle [SQHA] and Richard and Edith Strauss Postdoctoral Fellowship), N.I.K. (SQHA and ‘Fonds de recherché du Québec en Santé’), J.C.F.A. (Science without Borders (CsF) of the National Council for Scientific and Technological Development (CNPq) of Brazil), S.O. (CIHR Canada Graduate Scholarship-Master’s scholarship), and B.D.-G. (Division of Experimental Medicine, McGill University).
References
- 1. Schiffrin EL. Vascular remodeling in hypertension: mechanisms and treatment. Hypertension 2012;59:367–374. [DOI] [PubMed] [Google Scholar]
- 2. Paradis P, Schiffrin EL, Renin–angiotensin–aldosterone system and pathobiology of hypertension. In DeMello WC, Frohlich ED (eds). Renin Angiotensin System and Cardiovascular Disease. New York, N.Y: Humana Press, 2009. pp. 35–58. [Google Scholar]
- 3. Idris-Khodja N, Mian MO, Paradis P, Schiffrin EL.. Dual opposing roles of adaptive immunity in hypertension. Eur Heart J 2014;35:1238–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mian MO, Paradis P, Schiffrin EL.. Innate immunity in hypertension. Curr Hypertens Rep 2014;16:413.. [DOI] [PubMed] [Google Scholar]
- 5. Fontana V, Silva PS, Gerlach RF, Tanus-Santos JE.. Circulating matrix metalloproteinases and their inhibitors in hypertension. Clin Chim Acta 2012;413:656–662. [DOI] [PubMed] [Google Scholar]
- 6. Marchesi C, Paradis P, Schiffrin EL.. Role of the renin–angiotensin system in vascular inflammation. Trends Pharmacol Sci 2008;29:367–374. [DOI] [PubMed] [Google Scholar]
- 7. Wang M, Kim SH, Monticone RE, Lakatta EG.. Matrix metalloproteinases promote arterial remodeling in aging, hypertension, and atherosclerosis. Hypertension 2015;65:698–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Berry E, Bosonea AM, Wang X, Fernandez-Patron C.. Insights into the activity, differential expression, mutual regulation, and functions of matrix metalloproteinases and a disintegrin and metalloproteinases in hypertension and cardiac disease. J Vasc Res 2013;50:52–68. [DOI] [PubMed] [Google Scholar]
- 9. Parks WC, Wilson CL, Lopez-Boado YS.. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol 2004;4:617–629. [DOI] [PubMed] [Google Scholar]
- 10. Zavadzkas JA, Stroud RE, Bouges S, Mukherjee R, Jones JR, Patel RK, McDermott PJ, Spinale FG.. Targeted overexpression of tissue inhibitor of matrix metalloproteinase-4 modifies post-myocardial infarction remodeling in mice. Circ Res 2014;114:1435–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eguchi S, Dempsey PJ, Frank GD, Motley ED, Inagami T.. Activation of MAPKs by angiotensin II in vascular smooth muscle cells. Metalloprotease-dependent EGF receptor activation is required for activation of ERK and p38 MAPK but not for JNK. J Biol Chem 2001;276:7957–7962. [DOI] [PubMed] [Google Scholar]
- 12. Annes JP, Munger JS, Rifkin DB.. Making sense of latent TGFbeta activation. J Cell Sci 2003;116:217–224. [DOI] [PubMed] [Google Scholar]
- 13. Fernandez-Patron C, Radomski MW, Davidge ST.. Vascular matrix metalloproteinase-2 cleaves big endothelin-1 yielding a novel vasoconstrictor. Circ Res 1999;85:906–911. [DOI] [PubMed] [Google Scholar]
- 14. Marchesi C, Dentali F, Nicolini E, Maresca AM, Tayebjee MH, Franz M, Guasti L, Venco A, Schiffrin EL, Lip GY, Grandi AM.. Plasma levels of matrix metalloproteinases and their inhibitors in hypertension: a systematic review and meta-analysis. J Hypertens 2012;30:3–16. [DOI] [PubMed] [Google Scholar]
- 15. Odenbach J, Wang X, Cooper S, Chow FL, Oka T, Lopaschuk G, Kassiri Z, Fernandez-Patron C.. MMP-2 mediates angiotensin II-induced hypertension under the transcriptional control of MMP-7 and TACE. Hypertension 2011;57:123–130. [DOI] [PubMed] [Google Scholar]
- 16. Wu J, Thabet SR, Kirabo A, Trott DW, Saleh MA, Xiao L, Madhur MS, Chen W, Harrison DG.. Inflammation and mechanical stretch promote aortic stiffening in hypertension through activation of p38 mitogen-activated protein kinase. Circ Res 2014;114:616–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Castro MM, Rizzi E, Figueiredo-Lopes L, Fernandes K, Bendhack LM, Pitol DL, Gerlach RF, Tanus-Santos JE.. Metalloproteinase inhibition ameliorates hypertension and prevents vascular dysfunction and remodeling in renovascular hypertensive rats. Atherosclerosis 2008;198:320–331. [DOI] [PubMed] [Google Scholar]
- 18. Bouvet C, Gilbert LA, Girardot D, deBlois D, Moreau P.. Different involvement of extracellular matrix components in small and large arteries during chronic NO synthase inhibition. Hypertension 2005;45:432–437. [DOI] [PubMed] [Google Scholar]
- 19. Matsusaka H, Ide T, Matsushima S, Ikeuchi M, Kubota T, Sunagawa K, Kinugawa S, Tsutsui H.. Targeted deletion of matrix metalloproteinase 2 ameliorates myocardial remodeling in mice with chronic pressure overload. Hypertension 2006;47:711–717. [DOI] [PubMed] [Google Scholar]
- 20. Wang X, Berry E, Hernandez-Anzaldo S, Takawale A, Kassiri Z, Fernandez-Patron C.. Matrix metalloproteinase-2 mediates a mechanism of metabolic cardioprotection consisting of negative regulation of the sterol regulatory element-binding protein-2/3-hydroxy-3-methylglutaryl-CoA reductase pathway in the heart. Hypertension 2015;65:882–888. [DOI] [PubMed] [Google Scholar]
- 21. Kuzuya M, Nakamura K, Sasaki T, Cheng XW, Itohara S, Iguchi A.. Effect of MMP-2 deficiency on atherosclerotic lesion formation in apoE-deficient mice. Arterioscler Thromb Vasc Biol 2006;26:1120–1125. [DOI] [PubMed] [Google Scholar]
- 22. Barhoumi T, Kasal DA, Li MW, Shbat L, Laurant P, Neves MF, Paradis P, Schiffrin EL.. T regulatory lymphocytes prevent angiotensin II-induced hypertension and vascular injury. Hypertension 2011;57:469–476. [DOI] [PubMed] [Google Scholar]
- 23. Diep QN, El Mabrouk M, Cohn JS, Endemann D, Amiri F, Virdis A, Neves MF, Schiffrin EL.. Structure, endothelial function, cell growth, and inflammation in blood vessels of angiotensin II-infused rats: role of peroxisome proliferator-activated receptor-gamma. Circulation 2002;105:2296–2302. [DOI] [PubMed] [Google Scholar]
- 24. Li MW, Mian MO, Barhoumi T, Rehman A, Mann K, Paradis P, Schiffrin EL.. Endothelin-1 overexpression exacerbates atherosclerosis and induces aortic aneurysms in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol 2013;33:2306–2315. [DOI] [PubMed] [Google Scholar]
- 25. Ebrahimian T, Simon D, Lemarie CA, Simeone S, Heidari M, Mann KK, Wassmann S, Lehoux S.. Absence of four-and-a-half LIM domain protein 2 decreases atherosclerosis in ApoE-/- mice. Arterioscler Thromb Vasc Biol 2015;35:1190–1197. [DOI] [PubMed] [Google Scholar]
- 26. Briet M, Barhoumi T, Mian MO, Coelho SC, Ouerd S, Rautureau Y, Coffman TM, Paradis P, Schiffrin EL.. Aldosterone-induced vascular remodeling and endothelial dysfunction require functional angiotensin type 1a receptors. Hypertension 2016;67:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shah BH, Catt KJ.. Matrix metalloproteinase-dependent EGF receptor activation in hypertension and left ventricular hypertrophy. Trends Endocrinol Metab 2004;15:241–243. [DOI] [PubMed] [Google Scholar]
- 28. Newby AC. Metalloproteinase expression in monocytes and macrophages and its relationship to atherosclerotic plaque instability. Arterioscler Thromb Vasc Biol 2008;28:2108–2114. [DOI] [PubMed] [Google Scholar]
- 29. Oviedo-Orta E, Bermudez-Fajardo A, Karanam S, Benbow U, Newby AC.. Comparison of MMP-2 and MMP-9 secretion from T helper 0, 1 and 2 lymphocytes alone and in coculture with macrophages. Immunology 2008;124:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chowdhury A, Sarkar J, Pramanik PK, Chakraborti T, Chakraborti S.. Cross talk between MMP2-Spm-Cer-S1P and ERK1/2 in proliferation of pulmonary artery smooth muscle cells under angiotensin II stimulation. Arch Biochem Biophys 2016;603:91–101. [DOI] [PubMed] [Google Scholar]
- 31. Luchtefeld M, Grote K, Grothusen C, Bley S, Bandlow N, Selle T, Struber M, Haverich A, Bavendiek U, Drexler H, Schieffer B.. Angiotensin II induces MMP-2 in a p47phox-dependent manner. Biochem Biophys Res Commun 2005;328:183–188. [DOI] [PubMed] [Google Scholar]
- 32. Wang C, Chang Q, Sun X, Qian X, Liu P, Pei H, Guo X, Liu W.. Angiotensin II induces an increase in matrix metalloproteinase 2 expression in aortic smooth muscle cells of ascending thoracic aortic aneurysms through JNK, ERK1/2, and p38 MAPK activation. J Cardiovasc Pharmacol 2015;66:285–293. [DOI] [PubMed] [Google Scholar]
- 33. Wang C, Qian X, Sun X, Chang Q.. Angiotensin II increases matrix metalloproteinase 2 expression in human aortic smooth muscle cells via AT1R and ERK1/2. Exp Biol Med (Maywood) 2015;240:1564–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shen M, Lee J, Basu R, Sakamuri SS, Wang X, Fan D, Kassiri Z.. Divergent roles of matrix metalloproteinase 2 in pathogenesis of thoracic aortic aneurysm. Arterioscler Thromb Vasc Biol 2015;35:888–898. [DOI] [PubMed] [Google Scholar]
- 35. Schreier B, Hunerberg M, Rabe S, Mildenberger S, Bethmann D, Heise C, Sibilia M, Offermanns S, Gekle M.. Consequences of postnatal vascular smooth muscle EGFR deletion on acute angiotensin II action. Clin Sci (Lond) 2016;130:19–33. [DOI] [PubMed] [Google Scholar]
- 36. Nataraj C, Oliverio MI, Mannon RB, Mannon PJ, Audoly LP, Amuchastegui CS, Ruiz P, Smithies O, Coffman TM.. Angiotensin II regulates cellular immune responses through a calcineurin-dependent pathway. J Clin Invest 1999;104:1693–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chan G, Nogalski MT, Yurochko AD.. Activation of EGFR on monocytes is required for human cytomegalovirus entry and mediates cellular motility. Proc Natl Acad Sci U S A 2009;106:22369–22374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dreux AC, Lamb DJ, Modjtahedi H, Ferns GA.. The epidermal growth factor receptors and their family of ligands: their putative role in atherogenesis. Atherosclerosis 2006;186:38–53. [DOI] [PubMed] [Google Scholar]
- 39. Mian MO, Barhoumi T, Briet M, Paradis P, Schiffrin EL.. Deficiency of T-regulatory cells exaggerates angiotensin II-induced microvascular injury by enhancing immune responses. J Hypertens 2016;34:97–108. [DOI] [PubMed] [Google Scholar]
- 40. De Ciuceis C, Amiri F, Brassard P, Endemann DH, Touyz RM, Schiffrin EL.. Reduced vascular remodeling, endothelial dysfunction, and oxidative stress in resistance arteries of angiotensin II-infused macrophage colony-stimulating factor-deficient mice: evidence for a role in inflammation in angiotensin-induced vascular injury. Arterioscler Thromb Vasc Biol 2005;25:2106–2113. [DOI] [PubMed] [Google Scholar]
- 41. Kasal DA, Barhoumi T, Li MW, Yamamoto N, Zdanovich E, Rehman A, Neves MF, Laurant P, Paradis P, Schiffrin EL.. T regulatory lymphocytes prevent aldosterone-induced vascular injury. Hypertension 2012;59:324–330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.