Abstract
Aims
Hypoxic pulmonary vasoconstriction (HPV) redistributes blood flow from poorly ventilated to better aerated areas in the lung, thereby optimizing ventilation-perfusion ratio (V/Q). Pulmonary artery smooth muscle cell (PASMC) contraction in response to hypoxia is triggered by Ca2+ influx via transient receptor potential canonical 6 (TRPC6) cation channels that have translocated to caveolae in the plasma membrane. Since phosphatase and tensin homolog (PTEN) was suggested to regulate TRPC6 in endothelial cells, we aimed to define its role in the hypoxic response of PASMCs and as a putative mediator of HPV.
Methods and results
In isolated perfused mouse lungs, smooth muscle specific PTEN deficiency attenuated pulmonary vasoconstriction in response to hypoxia but not to angiotensin II (Ang II). Analogously, siRNA-mediated knock down of PTEN in human PASMC inhibited the hypoxia-induced increase in cytosolic Ca2+ concentration ([Ca2+]i). Co-immunoprecipitation and proximity ligation assays revealed increased interaction of PTEN with TRPC6 in human PASMC and murine lungs in response to hypoxia. In hypoxic PASMC, both PTEN and TRPC6 translocated to caveolae, and this response was blocked by pharmacological inhibition of Rho-associated protein kinase (ROCK) which in parallel prevented PTEN-TRPC6 interaction, hypoxia-induced [Ca2+]i increase, and HPV in PASMC and murine lungs, respectively.
Conclusion
Our data indicate a novel interplay between ROCK and [Ca2+]i signalling in HPV via PTEN, in that ROCK mediates interaction of PTEN and TRPC6 which then conjointly translocate to caveolae allowing for Ca2+ influx into and subsequent contraction of PASMC.
Keywords: Hypoxia, Phosphatase and tensin homolog (PTEN), Transient receptor potential canonical 6 (TRPC6), Pulmonary artery smooth muscle cells (PASMC), Rho kinase (ROCK)
1. Introduction
Hypoxic pulmonary vasoconstriction (HPV) is a physiological response to alveolar hypoxia which redistributes pulmonary blood flow from poorly aerated lung regions to better ventilated lung segments by an active process of local vasoconstriction. Impaired HPV, as seen in a variety of lung diseases including pulmonary hypertension (PH), pneumonia, or sepsis, results in submaximal oxygenation of arterial blood and limits oxygen supply to systemic organs.1–3 Global hypoxia, as seen at high altitude or during chronic hypoxic lung diseases such as chronic obstructive pulmonary disease, sleep apnea, or lung fibrosis may, on the other hand, cause generalized and sustained pulmonary vasoconstriction leading to vascular remodelling, right ventricular hypertrophy, and ultimately cor pulmonale.4 Although various regulatory pathways involved in HPV have been identified, considerable gaps in our knowledge and understanding remain, and a unifying concept of the underlying signalling pathways has not yet emerged. Thus, HPV continues to be an area of intense biomedical research with important clinical and therapeutic relevance.5,6
Increases in the intracellular Ca2+ concentration ([Ca2+]i) act as second messenger signal triggering pulmonary artery smooth muscle cell (PASMC) contraction in response to hypoxia.7–10 In PASMC, hypoxia causes recruitment of transient receptor potential canonical 6 (TRPC6) to caveolae11 where it is considered to initiate the PASMC [Ca2+]i response.12 However, the mechanisms regulating TRPC6 activation and its recruitment to, or trafficking within, the plasma membrane in hypoxia are poorly understood. In endothelial cells (ECs), phosphatase and tensin homolog (PTEN), a lipid and protein phosphatase, has been shown to serve as a scaffold for TRPC6, enabling cell surface expression of the channel and subsequent Ca2+ entry. Notably, this effect of PTEN is independent of its phosphatase activity, but mediated through direct interaction of PTEN with TRPC6 via its PDZ-binding domain, a common protein-protein binding domain (PDZ is an acronym of three proteins).13
In addition to Ca2+ signalling via TRPC6, HPV requires activation of Rho kinase (ROCK).14,15 which is considered to act predominantly through its Ca2+ sensitizing effects. Consistent with this notion, we recently showed that EC-derived sphingosine-1-phosphate (S1P) mediates HPV in a ROCK-dependent manner, and triggers the translocation of TRPC6 to caveolae and its activation in PASMC.16
Importantly, PTEN activity has previously been reported to be regulated by RhoA and its effector ROCK in pre-osteoblasts, as siRNA-based silencing of RhoA or inhibition of ROCK by Y27632 both decrease PTEN activity. This effect has been proposed to be dependent on direct interaction of PTEN with ROCK as shown by co-immunoprecipitation.17 Rho furthermore regulates PTEN’s localization in chemotaxing neutrophils via phosphorylation and interaction of the two proteins.18 Activation of the Rho-ROCK-PTEN pathway has also been shown to mediate EC permeability changes in response to S1P.19
Based on the reported roles and regulation of PTEN in other cell types, we hypothesized that PTEN may play a critical role in HPV, and provide a key missing link between Rho/ROCK signalling and TRPC6-mediated Ca2+ influx into PASMC. Here we show that PTEN and TRPC6 interact in PASMC in response to hypoxia or S1P in a ROCK dependent manner, and that this interaction is required for their translocation to caveolae and the subsequent increase in PASMC [Ca2+]i that result in smooth muscle contraction and HPV.
2. Methods
This is a short version of the protocols used, further details are provided in the Supplementary Material.
2.1 Animals
Male C57/Bl6 mice (25–30 g) were obtained from Charles River (Canada); mice with a conditional deletion in PTEN in smooth muscle cells (SMCs) were generated by crossing PTENfl°x/fl°x (from Dr Tak W. Mak)20 with tet-O-Cre and SMA-rtTA strains (kindly provided by Dr Dean Sheppard; UCSF)21 to yield tet-O-Cretg/−;SMA-rtTAtg/−; PTENfl/fl (SMC-specific knock out mice) and tet-O-Cretg/−;SMA-rtTAtg/−;PTENWT/WT (control). All animals received care in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publication 8th edition, 2011). Experiments were approved by the Animal Care and Use Committee of St Michael’s Hospital. PTEN knockout mice and the control group received doxycycline in food (2 g/kg, TD.140011, Envigo, Madison, WI) for 1 week prior to experiments to induce gene knock out.
2.2 Isolated perfused mouse lung
Isolated perfused mouse lung (IPL) were prepared from male C57Bl6, tet-O-Cretg/−;SMA-rtTAtg/−:PTENfl/fl, and tet-O-Cretg/−; SMA-rtTAtg/−;PTENWT/WT mice as previously described.22 In brief, mice were anaesthetized with 250 mg/kg avertin via IP injection, depth of anaesthesia was confirmed by toe pinch. Following anaesthesia and tracheotomy the heart was catheterized and pulmonary artery pressure (PAP) was recorded continuously. Lungs were only included in the study if perfusion pressure was stable and below 20 cm H2O during the initial 10 min of baseline perfusion and if lungs showed no macroscopic signs of hemorrhage, atelectasis, or edema.
2.3 Cell culture
PASMCs, culture media kits (SmGM-2 BulletKit, CC-3182) and subculture supplies including HEPES buffered saline solution (CC-5024), trypsin/EDTA solution (CC-5012) and trypsin neutralizing solution (CC-5002) were purchased from Lonza (Clonetics PASMC, Lonza, Basel, Switzerland). Cells were cultured according to manufacturer’s instructions and used within the first six passages of growth from three different batches.
2.4 Protein extraction
Cells were grown to confluence and treated as indicated in text, then lysed following standard protocols. Protein concentration was measured by BCA assay (Thermo Fischer Scientific, Waltham, MA) and samples were stored at −80 °C.
2.5 Hypoxia exposure in vitro
Experiments for hypoxia exposure of PASMC were performed in a custom-built hypoxia chamber. After 5 min of hypoxia cells were lysed following standard protocols and placed on ice immediately.
2.6 Western blotting
The following antibodies were used following standard western blotting procedures: TRPC6 1:1000—Alomone Lab, Jerusalem, Israel: ACC-01723; PTEN 1:1000—Cell Signaling, Boston, MA: 9552S24; GAPDH 1:1000—Santa Cruz, Dallas, TX: sc-25778; Caveolin-1 1:500—BD Biosciences, San Jose, CA: 610407.
2.7 Immunoprecipitation
One millilitre PASMC lysate was rotated with 20 µL Protein A/G beads (GE Healthcare, Mississauga, ON) for 30 min at 4 °C for pre-clearance. After centrifugation at 6000 rpm and 4 °C for 1 min the supernatant was collected, 1:100 anti-PTEN (Cell Signaling, Boston, MA: 9552S) was added to 750 µg total protein sample, and samples were rotated overnight at 4 °C. The next day, 25 µL of Protein A/G beads (GE Healthcare, Mississauga, ON) was added and samples were kept rotating for 1 h. Beads were washed thrice with lysis buffer and boiled with 30 µL Laemmli buffer (BioRad, Canada).
2.8 Ca2+ imaging in PASMC
Changes in PASMC [Ca2+]i were measured as previously described.16 PASMCs cultured on coverslips were loaded with HBSS containing 5 µmol/L fura-2-acetoxymethyl ester (fura-2AM) (Life Technologies, Carlsbad, CA) dissolved in Pluronic F-127 (20% solution in DMSO) (Life Technologies, Carlsbad, CA), then mounted in a heated chamber (Warner Industries; Saint-Laurent, QC, RC-21B + PH-2) at 37 °C. Fura-2 fluorescence was excited by monochromatic illumination (Polychrome V; TillPhotonics, Victor, NY) at λ = 340 and 380 nm and collected at an emission wavelength of 510 nm via a custom-built upright fluorescence microscope equipped with appropriate dichroic and emission filters and a digital camera. After background correction, the 340/380 ratio was calculated using TillVision 4.0 software (Till Photonics, Germany).
2.9 siRNA transfection
PASMC were seeded on 6-well plates. At 70% confluency cells were transfected with siRNA (On Target PTEN siRNA and non-targeting control; Dharmacon, Ottawa, ON) using Effectene Transfection Reagent from Qiagen (Toronto, ON) according to manufacturer’s instructions.
2.10 Immunofluorescence microscopy
Following exposure to normoxia or hypoxia, cells were fixed with paraformaldehyde and standard immunofluorescence staining protocols were performed.
2.11 Proximity ligation assay
Protein–protein interaction was assessed by proximity ligation assay (PLA) (DuoLink assay; Sigma Aldrich, Oakville, ON). Samples were prepared as described for immunofluorescence microscopy. After incubation with two primary antibodies from different species and directed against two putatively interacting proteins, two species-specific secondary antibodies directed against the different primary antibodies with complementary oligonucleotide sequences at their ends (PLA probes) were added. PLA probes in close proximity (<40 nm) were joined by enzymatic ligation, and the resulting signal was amplified with rolling cycle amplification and visualized using fluorescently labelled complementary oligonucleotides by confocal laser spinning disk microscopy. Raw, single plain images were quantified in ImageJ (National Institute of Health, USA).
2.12 Isolation of caveolar fractions by sucrose density gradient ultracentrifugation
Isolation of caveolar fractions from PASMC and probing for caveolar abundance of proteins of interest was performed as recently reported using sucrose gradient centrifugation.16
2.13 Isolation of PASMC from murine lungs
Under deep anaesthesia, the murine heart and lungs were carefully removed. The trachea, the vena cava and the left lungs were pinned down. The pulmonary artery (PA) was microsurgically separated from the adjacent vein and bronchi, and small pieces of the isolated artery were placed in a T25 flask with droplets of SMC medium containing an additional 15% FBS. These were cultured for 2 weeks before use.
2.14 Statistical analysis
Statistical analyses were performed with GraphPad Prism using Mann-Whitney U test for two independent groups, or One-way Anova for more than two groups. For analysis of multiple cells per isolation (Figures 2C,D and 5B) a hierarchical linear model (with random effect, build nested terms) was used with SPSS Statistics. Data are shown as mean ± SEM, differences were considered significant (*) at P < 0.05.
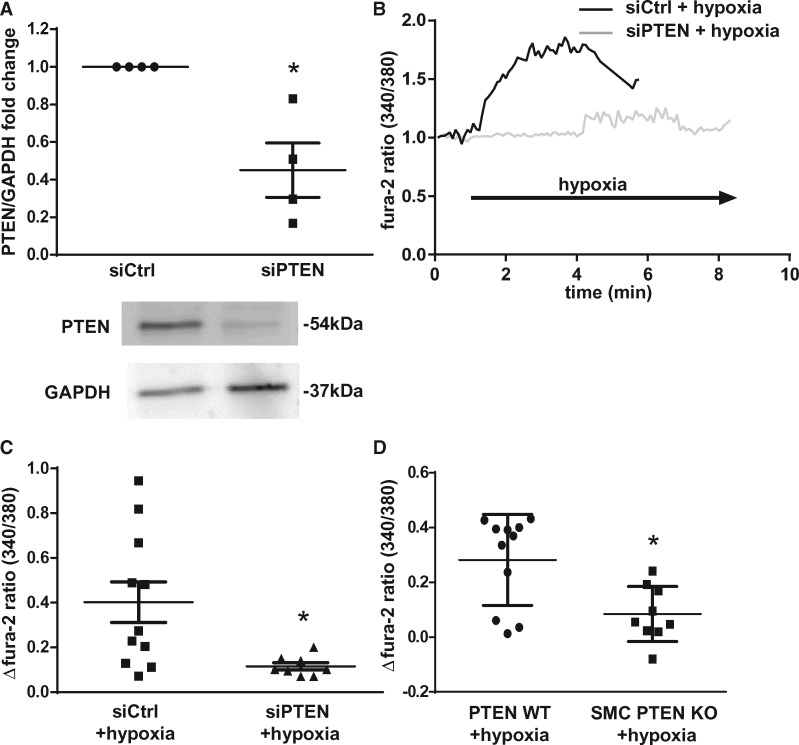
Figure 2.
PASMC [Ca2+]i increase in response to hypoxia requires PTEN. (A) Representative immunoblot and quantitative data from four independent isolations show effective knockdown of PTEN by PTEN-specific siRNA (siPTEN) as compared with scrambled siRNA (siCtrl). Each experiment has been normalized to its corresponding control group from the same gel. (B) Representative tracings of the 340/380 nm fura-2 fluorescence ratio (normalized to baseline) in PASMC show a reduced [Ca2+]i response to hypoxia (1% O2) in PASMC transfected with siPTEN as compared with siCtrl. Group data show hypoxia-induced [Ca2+]i increase in siPTEN and siCtrl PASMC (C) or murine PASMC isolated from SMC PTEN KO or WT mice (D) (data from three to five independent experiments, respectively).Group data are means ± SEM, *P < 0.05 vs. siCtrl (A,C) or WT (D).
Figure 5.
ROCK inhibition prevents [Ca2+]i signalling and HPV response. (A) Representative tracings of the 340/380 nm fura-2 fluorescence ratio (normalized to baseline) in PASMC show reduced cytosolic [Ca2+]i response to hypoxia (1% O2) in PASMC in the presence of Y27632 (5 µMol/L). (B) Group data show effect of Y27632 on the hypoxia-induced [Ca2+]i increase (data from 3 independent experiments each). (C) Group data show inhibition of the PAP increase (ΔPAP) by Y27632 in IPL 3 min after start of hypoxia (data from four independent experiments each). Group data are means ± SEM, *P < 0.05 vs. hypoxia (B) or control (Ctrl; C).
3. Results
3.1 PTEN is required for intact HPV
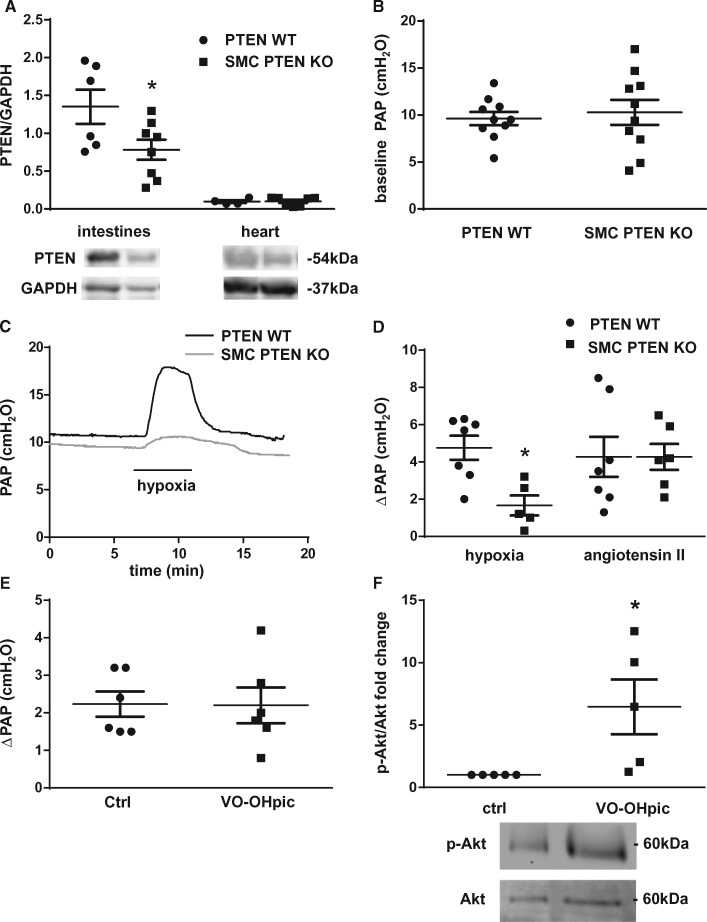
To test for a potential role of PTEN in HPV we generated SMC-specific PTEN knockout mice (SMC PTEN KO). Inducible deletion of PTEN from SMCs in mice was confirmed by western blot analysis of an SMC rich organ (intestine) and an organ containing minimal amounts of SMC (heart). PTEN levels were significantly reduced in the intestines of KO mice as compared with wild type (WT) controls while no significant difference in PTEN expression was detectable in the heart (Figure 1A). Baseline perfusion pressures in isolated lungs did not differ between WT and SMC PTEN KO mice (Figure 1B). We next tested the vasoconstriction response to hypoxia and Ang II in the IPL model by measuring the PAP change in response to a switch from normoxic (21% O2) to hypoxic (1% O2) ventilation or bolus infusion of Ang II, respectively. Although WT lungs showed the characteristic pressure response to hypoxia, SMC PTEN KO lungs had a largely reduced HPV (Figures 1C and D). PTEN deficiency in SMCs did, however, not prevent vasoconstriction in response to Ang II (Figure 1D). Inhibition of PTEN’s phosphatase activity by the vanadate compound VO-OHpic did also not attenuate HPV (Figure 1E), suggesting that the role of PTEN in HPV is independent of its phosphatase activity. VO-OHpic increased Akt phosphorylation (Figure 1F), demonstrating that the applied dose was effective in inhibiting PTEN25–27 in the isolated lung preparation.
Figure 1.
HPV requires SMC PTEN. (A) Representative immunoblots and quantitative data (n = 4–8) show that SMC PTEN KO reduced PTEN expression in SMC-rich intestines, but not in the heart. (B) Quantitative data shows that baseline pressures are similar in IPL of KO animals compared with WT controls (n = 10 each). (C) Representative tracings of PAP in IPL experiments show attenuated vasoconstriction in response to hypoxia (1% O2) in lungs of SMC PTEN KO mice compared with WT. (D) Group data show attenuated PAP increase (ΔPAP) 5 min after start of hypoxia but not in response to Ang II (1 µg bolus for 5 min) in SMC PTEN KO mice; (n = 5–6, respectively) compared with WT mice (n = 7 both). (E) VO-OHpic (10 µMol/L) did not affect the PAP response to hypoxia in isolated lungs of C57Bl/6 mice (n = 6 each). (F) Lungs were collected and snap-frozen after 30 min of perfusion in the presence or absence of VO-OHpic (10 µMol/L). Representative western blot of lung lysates and quantitative data show levels of p-Akt normalized to total Akt in the presence or absence of VO-OHpic (10 µMol/L) (n = 5). Group data are means ± SEM, *P < 0.05 vs. WT (A, D) or control (Ctrl; F).
3.2 PTEN knockdown attenuates hypoxia-induced PASMC contraction and [Ca2+]i increase
Ca2+ entry into PASMCs is a known prerequisite for HPV. Hence, we next tested whether loss of PTEN in PASMC may attenuate PASMC [Ca2+]i signalling in response to hypoxia. PASMCs were transfected with PTEN-specific or control siRNA (siPTEN or siCtrl, respectively), and effective silencing of PTEN by ∼50% was confirmed by Western Blot (Figure 2A). PASMC treated with scrambled siRNA showed the characteristic [Ca2+]i increase in response to hypoxia; yet, silencing PTEN with siPTEN significantly reduced this response (Figure 2B and C). A similar effect was observed in murine PASMC in that cells isolated from SMC PTEN KO mice had a reduced [Ca2+]i response to hypoxia compared with cells from WT animals (Figure 2D).
3.3 Hypoxia and S1P induce PTEN interaction with TRPC6 through ROCK
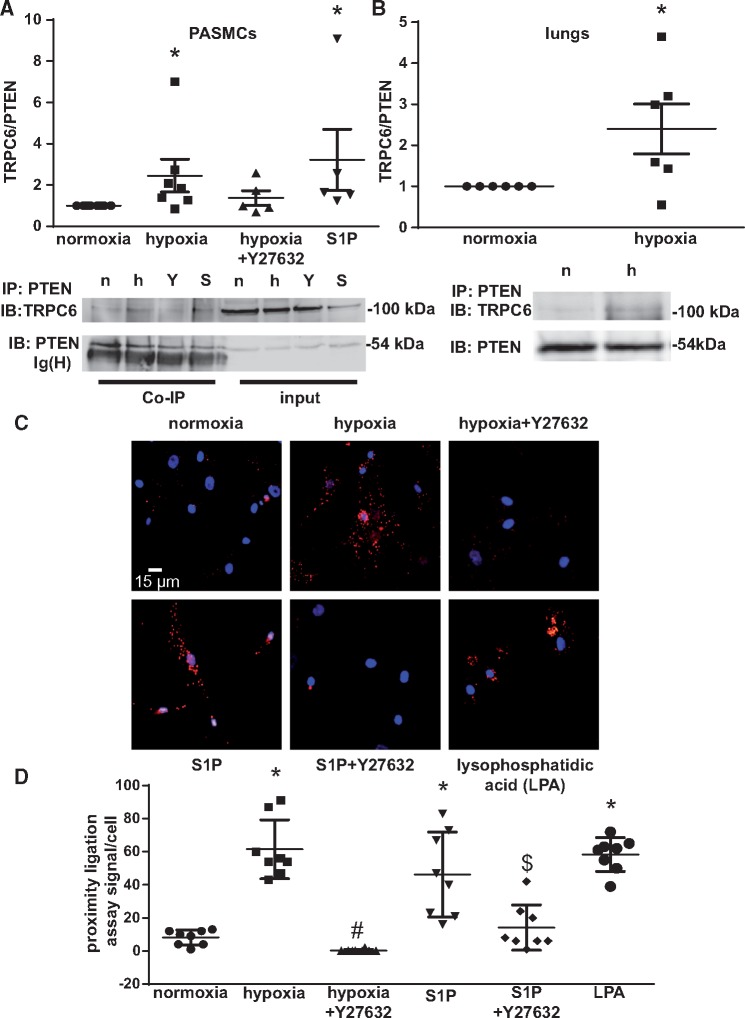
In ECs PTEN has been shown to interact with TRPC6, a cation channel that plays a key role in HPV. To probe for a similar interaction of PTEN with TRPC6 in PASMCs in response to either hypoxia or S1P, a central mediator of HPV, we immunoprecipitated PTEN from PASMC following either 5 min of hypoxia (in the presence or absence of the ROCK inhibitor Y27632) or normoxia, or S1P treatment (Figure 3A), and blotted the immunoprecipitates for both PTEN and TRPC6. Co-immunoprecipitation showed increased interaction of PTEN with TRPC6 in PASMC stimulated with either hypoxia or S1P as compared to normoxic controls. As TRPC6 and PTEN are both regulated by ROCK,17,19,28 we probed for the effects of the ROCK inhibitor Y27632 on the interaction of TRPC6 with PTEN. Y27632 blunted the hypoxia-induced interaction, indicating a regulatory role of ROCK that will be followed up further below. A similar increase in TRPC6/PTEN interaction was detected in whole lung lysates (Figure 3B) of IPLs exposed to hypoxia for 3 min as compared with normoxic controls. Increased interaction of PTEN with TRPC6 in PASMC in response to hypoxia or S1P was also confirmed by PLA (Figure 3C and D) where red puncta indicate interaction of the 2 proteins while nuclei were stained with DAPI (blue). Y27632 again blocked TRPC6/PTEN interaction in response to hypoxia or S1P, while lysophosphatidic acid (LPA), a known ROCK activator, recapitulated the effects of S1P and hypoxia, thus corroborating a regulatory role of ROCK in the interaction of PTEN with TRPC6.
Figure 3.
Hypoxia increases the interaction of PTEN with TRPC6 in a ROCK-dependent manner. (A) PASMCs were exposed to either normoxia (n), hypoxia (h; 1% O2), or hypoxia in the presence of Y27632 (Y; 5µMol/L), or to S1P (S; 10 µMol/L) for 5 min. Representative immunoblots show TRPC6 and PTEN expression in PASMC for whole cell lysate (input) and after immunoprecipitation for PTEN (Co-IP). Group data from n = 5–7 independent replicates showing TRPC6-over-PTEN ratio normalized to the normoxic control group from the same gel, demonstrate increased interaction of PTEN with TRPC6 in response to hypoxia and S1P, respectively. (B) Isolated lung were ventilated with normoxic or hypoxic (1% O2) gas for 3 min, and tissue was snap-frozen and lysed. Representative immunoblots show TRPC6 and PTEN expression after immunoprecipitation for PTEN; group data from n = 6 replicates show quantification of TRPC6-over-PTEN ratio normalized to the normoxic control group from the same gel. (C) Representative images show PLA for the interaction between PTEN and TRPC6 in PASMC following exposure to either normoxia, hypoxia, or S1P(10 µMol/L) for 5 min, in the presence or absence of Y27632 (5 µMol/L), or LPA (3 µMol/L) for 15 min. Red puncta indicate sites of interaction between PTEN and TRPC6, nuclei are counterstained in blue with DAPI. (D) Group data show quantification of the PLA from eight cells from three independent experiments each. Group data are means ± SEM, *P < 0.05 vs. normoxia, #P < 0.05 vs. hypoxia and $P < 0.05 vs. S1P.
3.4 PTEN mediates TRPC6 recruitment to caveolae in a ROCK-dependent manner
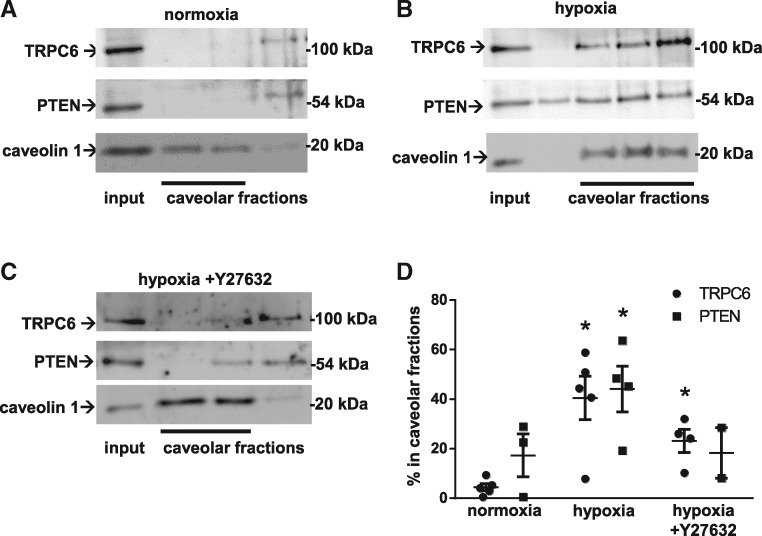
Caveolae are specialized membrane microdomains enriched in cholesterol, sphingolipids, and proteins that serve as signalling hubs in the plasma membrane. Caveolae play a critical role in Ca2+ influx via TRPC6 in pulmonary microvascular ECs29 and PASMC,11 in that they regulate the abundance of functional TRPC6 at the plasma membrane. To test whether PTEN and/or ROCK may play a role in TRPC6 translocation to caveolae, we prepared caveolar fractions by sucrose gradient centrifugation of PASMC lysates. When compared with normoxic controls (Figure 4A, B, and D), we found both PTEN and TRPC6 translocated to caveolae. As TRPC6/PTEN interaction had been inhibitable by Y27632, we next tested whether the trafficking of the two proteins to caveolae in response to hypoxia similarly required ROCK. Pretreatment of PASMC with Y27632 reduced the abundance of TRPC6 and PTEN in caveolae, indicating that the translocation of both proteins occurred in a ROCK-dependent manner (Figure 4C and D).
Figure 4.
Hypoxia causes ROCK-dependent translocation of TRPC6 and PTEN to caveolae. Caveolar fractions were isolated from PASMC (input: whole cell lysate) by sucrose gradient centrifugation, and identified by the presence of the marker protein caveolin-1. Representative immunoblots show (A) absence of PTEN and TRPC6 from caveolae of normoxic PASMC, but (B) recruitment to caveolar fractions within 15 min of hypoxia (1% O2), that was (C) blocked by Y27632 (5 µMol/L). (D) Group data from n = 3–5 independent experiments each show caveolar recruitment of TRPC6 and PTEN, expressed as fraction of protein detected in caveolar fractions. Group data are means ± SEM, *P < 0.05 vs. normoxia.
3.5 ROCK mediates PASMC [Ca2+]i response to hypoxia and HPV
Analogous to the effects of PTEN knock-down, inhibition of ROCK also inhibited the hypoxia induced [Ca2+]i increase in PASMC as measured by fura-2 ratiometric imaging (Figure 5A and B). Finally, ROCK inhibition attenuated HPV in IPLs (Figure 5C), consolidating the functional relevance of ROCK for the outlined signalling pathway.
4. Discussion
In this study, we identify a previously unrecognized regulatory role for PTEN in HPV. This role relates to its function in PASMC, as SMC-specific knockout of PTEN attenuated the characteristic pulmonary vasoconstrictive response to hypoxia, but not to the systemic vasoconstrictor Ang II. This role of PTEN in PASMC is independent of its phosphatase activity, as it was not blocked by the PTEN inhibitor VO-OHpic at effective pharmacological concentrations. Instead, PTEN may act as a scaffold for the polymodal cation channel TRPC6 which mediates PASMC Ca2+ entry in response to hypoxia,12 as (i) hypoxia triggered the interaction of PTEN with TRPC6 in both intact lungs and isolated PASMC, and (ii) PTEN knock-down blocked the hypoxia-induced [Ca2+]i increase in PASMC. Both PTEN and TRPC6 translocated to caveolae in PASMC in response to hypoxia in a ROCK-dependent manner. ROCK inhibition in turn blocked PTEN-TRPC6 interaction, inhibited the PASMC [Ca2+]i response to hypoxia, and attenuated HPV. Taken together, these findings identify a new signalling pathway for HPV in that hypoxia, and/or the hypoxia-generated mediator S1P, stimulate a ROCK-dependent interaction between PTEN and TRPC6 in PASMC which is required for the effective recruitment of TRPC6 to caveolae and the subsequent influx of Ca2+ that ultimately triggers PASMC contraction (Figure 6).
Figure 6.
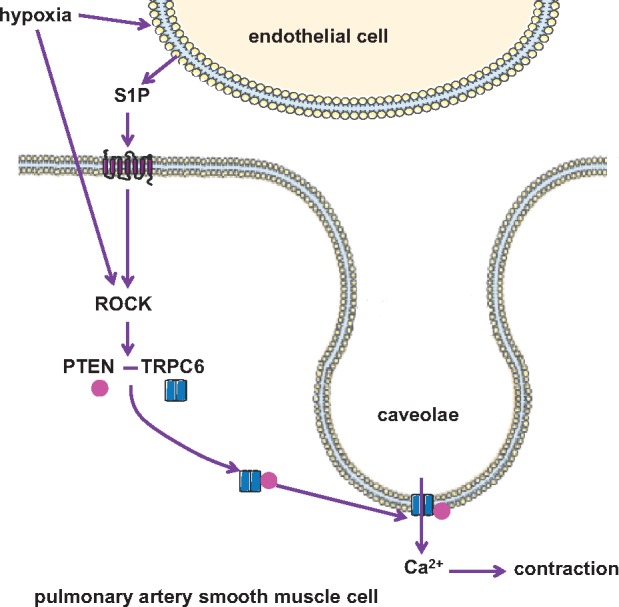
Schematic outline of the proposed role of PTEN in HPV: Hypoxia, either directly and/or via formation of S1P, activates ROCKin PASMC, which mediates the interaction between PTEN and TRPC6 and their translocation to caveolae, where TRPC6 facilitates Ca2+ entry and subsequent PASMC contraction.
Ca2+ influx is the essential second messenger that triggers actin-myosin interaction and thus, the characteristic contraction of PASMCs in response to hypoxia. TRPC6 has been identified as a cation channel that is critical for both hypoxia-induced Ca2+ entry into PASMC and subsequent HPV in intact lungs.12,30,31 Although TRPC6 by itself may not be the only cation channel mediating hypoxia-induced Ca2+ influx in PASMC, which has also been shown to involve voltage-gated Ca2+ channels,32,33 TRPC6 activation has emerged as a key initiating event. Specifically, (i) TRPC616, yet not L-type channel inhibition34,35 results in an almost complete loss of the PASMC Ca2+ response to hypoxia and (ii) TRPC6-mediated cation entry serves as initial trigger for activation of voltage-gated Ca2+ channels via membrane depolarization.29 Following stimulation by hypoxia,11 TRPC6 translocates to caveolae, specialized membrane microdomains that act as signalling hubs for outside-in-signalling and play key roles in regulating the abundance of ion channels at the plasma membrane and resulting ion fluxes, including those of Ca2+.11,16 However, the exact mechanisms underlying the hypoxia-induced recruitment of TRPC6 to caveolae remain unclear.
Recently, our group identified a critical role for endothelial-derived S1P as putative intercellular mediator during HPV, downstream of neutral sphingomyelinase activation.16 In this work, we showed that S1P signalling is required for the translocation of TRPC6 to caveolae, and its activation to trigger Ca2+ influx into PASMC. We further demonstrated that S1P receptor-2 mediated activation of PLC is required for HPV, presumably acting via DAG synthesis and subsequent TRPC6 activation36 that synergized with a parallel activation of the Rho/ROCK signalling pathway to elicit pulmonary vasoconstriction.16 Notably, although the sphingolipid-mediated recruitment and activation of TRP channels in response to hypoxia is present in PASMC per se,16 the HPV response in the intact lung requires additional input from the endothelium as a conducer of the hypoxic signal from the alveolar capillaries to the feeding arteries.
In ECs PTEN, a lipid and protein phosphatase protein, serves as a scaffold for TRPC6 after thrombin stimulation, enabling cell surface expression of the channel and subsequent Ca2+ entry. Notably, this effect of PTEN is independent of its phosphatase activity, but mediated through direct interaction of PTEN with TRPC6 via its PDZ-binding domain.13 This is in line with our finding that inhibition of PTEN phosphatase activity did not affect HPV. Instead, we show by two different approaches, namely co-immunoprecipitation in intact lungs and PLA in cultured PASMC that hypoxia as well as S1P trigger the interaction of PTEN with TRPC6. This finding is in line with previous data from ECs demonstrating that cell migration is regulated by S1P through activation of PTEN.37 The relevance of the detected interaction between PTEN and TRPC6 is highlighted by the fact that siRNA-mediated knock-down of PTEN attenuated the characteristic increase in PASMC [Ca2+]i in response to hypoxia, which had been identified to be mediated by TRPC6.12 The importance of PTEN/TRPC6 interaction is furthermore underlined by our finding that SMC PTEN KO using a Cre-lox system attenuated HPV in ex vivo perfused mouse lungs. Loss of PTEN in SMC did; however, not attenuate vasoconstriction in response to Ang II. Although this finding does not preclude differences in the vasoconstriction response to other pharmacological agonists, it suggests that the role of PTEN in vasoconstriction may be specific for the response to hypoxia. Of relevance, SMC-specific PTEN deletion in mice has been demonstrated to result in age-dependent spontaneous PH.38 We and others have shown that HPV is attenuated in mice with chronic hypoxic PH,39,40 which is considered to contribute to systemic hypoxemia in PH patients.41 In this study, however, experiments were conducted at an age of 8–12 weeks, i.e. prior to the onset of spontaneous PH, which was confirmed by the fact that lungs of SMC PTEN KO mice had similar baseline perfusion pressures as those of WT mice. Hence, while other key signalling molecules involved in HPV including TRPC6,12 connexin 40,42 CFTR,16 or TRPV443 are typically also involved in the development of chronic hypoxic PH,16,44–50 the described role of PTEN seems to present a rare case of a signalling pathway with opposing effects on HPV and PH. Importantly, this notion opens up the possibility that the inhibition of HPV (and thus, the hypoxemia caused by the resulting V/Q mismatch) are not exclusively caused by an increased basal tone of the pulmonary resistance vessels which limits their ability to constrict further, but may in part result from activation of signalling pathways with opposing effects on HPV and PH.
Rho/ROCK signalling plays a central signalling role in SMC contraction, largely through its Ca2+ sensitizing role by inhibiting myosin light chain phosphatase.15 Accordingly, Rho/ROCK signalling has been implicated in HPV,14,51 a notion that was confirmed in this study in that HPV was markedly reduced by Y27632. In addition, however, Y27632 has been shown to directly inhibit hypoxia-induced increases in PASMC [Ca2+]i,15 a finding that was again confirmed in this study, and that indicates that Rho/ROCK signalling may, in addition to its Ca2+-sensitizing effects, act upstream of TRPC6-mediated Ca2+ entry in hypoxic PASMC. The latter notion is consistent with previous data from primary podocytes in which Rho-mediated PLCε stimulation was shown to activate TRPC6.28
In line with the newly identified role of PTEN in HPV and its previously reported regulation by ROCK,16,19 we found inhibition of ROCK to prevent the interaction of TRPC6 with PTEN and their translocation to caveolae. The mechanism, however, by which ROCK mediates the interaction and caveolar recruitment of PTEN and TRPC6 in hypoxia remains to be resolved. Notably, dephosphorylated PTEN was shown to recruit to a protein complex to the plasma membrane whereas phosphorylation of the molecule prevented this response.52,53 Hence, PTEN may become indirectly dephosphorylated in a ROCK-dependent manner, as previously shown for neuronal cells54,55 through a pathway that has not yet been identified. Dephosphorylated PTEN may then facilitate, through direct protein–protein interaction, the recruitment of TRPC6 to caveolae. However, as co-immunoprecipitation as well as proximity ligation and caveolar recruitment assays using specific phospho- and non-phospho-PTEN antibodies failed to yield reproducible results (data not shown), presumably due to limited antibody specificity, the exact mechanism by which ROCK mediates PTEN-TRPC6 interaction must remain speculative at the present stage.
In conclusion, we report here a new critical role of PTEN in hypoxia-induced vasoconstriction and Ca2+ signalling in PASMC. In response to hypoxia or S1P, PTEN interacts and translocates with TRPC6 into caveolae in a ROCK-dependent manner, thus highlighting the intricate interdependence between Ca2+ and Rho/ROCK signalling in HPV.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Author contributions
A.K., K.S., and W.M.K., designed the study and wrote the article. T.M. provided key materials. All authors interpreted the results and approved the final version of the article.
Supplementary Material
Acknowledgements
We would like to gratefully thank Dean Sheppard, UCSF, for providing us the tet-O-Cre and SMA-rtTA transgenic mice.
Conflict of interest: none declared.
Funding
This work was supported by a Canadian Institutes of Health Research (CIHR) open operating grant no. 273746 to W.M.K.
References
- 1. Wright J, Levy R, Churg A.. Pulmonary hypertension in chronic obstructive pulmonary disease: current theories of pathogenesis and their implications for treatment. Thorax 2005;60:605–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marshall BE, Hanson CW, Frasch F, Marshall C.. Role of hypoxic pulmonary vasoconstriction in pulmonary gas exchange and blood flow distribution. 2. Pathophysiol Intens Care Med 1994;20:379–389. [DOI] [PubMed] [Google Scholar]
- 3. McCormack DG, Paterson NAM.. Loss of hypoxic pulmonary vasoconstriction in chronic pneumonia is not mediated by nitric oxide. Am J Physiol 1993; 265:H1523–H1528. [DOI] [PubMed] [Google Scholar]
- 4. Naeije R, Barbera JA.. Pulmonary hypertension associated with COPD. Crit Care 2001;5:286–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sommer N, Dietrich A, Schermuly RT, Ghofrani HA, Gudermann T, Schulz R, Seeger W, Grimminger F, Weissmann N.. Regulation of hypoxic pulmonary vasoconstriction: basic mechanisms. Eur Respir J 2008;32:1639–1651. [DOI] [PubMed] [Google Scholar]
- 6. Moudgil R, Michelakis ED, Archer SL.. Hypoxic pulmonary vasoconstriction. Essays Biochem 2007;43:61–76. [DOI] [PubMed] [Google Scholar]
- 7. Berridge MJ, Lipp P, Bootman MD.. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol 2000;1:11–21. [DOI] [PubMed] [Google Scholar]
- 8. Berridge MJ, Bootman MD, Lipp P.. Calcium-a life and death signal. Nature 1998; 395:645–648. [DOI] [PubMed] [Google Scholar]
- 9. Jabr RI, Toland H, Gelband CH, Wang XX, Hume JR.. Prominent role of intracellular Ca2+ release in hypoxic vasoconstriction of canine pulmonary artery. Br J Pharmacol 1997;122:21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smith KA, Voiriot G, Tang H, Fraidenburg DR, Song S, Yamamura H, Yamamura A, Guo Q, Wan J, Pohl NM, Tauseef M, Bodmer R, Ocorr K, Thistlethwaite PA, Haddad GG, Powell FL, Makino A, Mehta D, Yuan JX-J.. Notch activation of Ca 2+ signaling in the development of hypoxic pulmonary vasoconstriction and pulmonary hypertension. Am J Respir Cell Mol Biol 2015;53:355–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Keserü B, Barbosa-Sicard E, Popp R, Fisslthaler B, Dietrich A, Gudermann T, Hammock BD, Falck JR, Weissmann N, Busse R, Fleming I.. Epoxyeicosatrienoic acids and the soluble epoxide hydrolase are determinants of pulmonary artery pressure and the acute hypoxic pulmonary vasoconstrictor response. FASEB J 2008; 22:4306–4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weissmann N, Dietrich A, Fuchs B, Kalwa H, Ay M, Dumitrascu R, Olschewski A, Storch U, Mederos y Schnitzler M, Ghofrani HA, Schermuly RT, Pinkenburg O, Seeger W, Grimminger F, Gudermann T.. Classical transient receptor potential channel 6 (TRPC6) is essential for hypoxic pulmonary vasoconstriction and alveolar gas exchange. Proc Natl Acad Sci USA 2006;103:19093–19098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kini V, Chavez A, Mehta D.. A new role for PTEN in regulating transient receptor potential canonical channel 6-mediated Ca2+ entry, endothelial permeability, and angiogenesis. J Biol Chem 2010;285:33082–33091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Robertson TP, Dipp M, Ward JP, Aaronson PI, Evans AM.. Inhibition of sustained hypoxic vasoconstriction by Y-27632 in isolated intrapulmonary arteries and perfused lung of the rat. Br J Pharmacol 2000;131:5–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang J, Weigand L, Foxson J, Shimoda LA, Sylvester JT.. Ca2+ signaling in hypoxic pulmonary vasoconstriction: effects of myosin light chain and Rho kinase antagonists. Am J Physiol Lung Cell Mol Physiol 2007;293:L674–85. [DOI] [PubMed] [Google Scholar]
- 16. Tabeling C, Yu H, Wang L, Ranke H, Goldenberg NM, Zabini D, Noe E, Krauszman A, Gutbier B, Yin J, Schaefer M, Arenz C, Hocke AC, Suttorp N, Proia RL, Witzenrath M, Kuebler WM.. CFTR and sphingolipids mediate hypoxic pulmonary vasoconstriction. Proc Natl Acad Sci USA 2015;112:E1614–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang S, Kim H-M.. The RhoA-ROCK-PTEN pathway as a molecular switch for anchorage dependent cell behavior. Biomaterials 2012;33:2902–2915. [DOI] [PubMed] [Google Scholar]
- 18. Li Z, Dong X, Wang Z, Liu W, Deng N, Ding Y, Tang L, Hla T, Zeng R, Li L, Wu D.. Regulation of PTEN by Rho small GTPases. Nat Cell Biol 2005;7:399–404. [DOI] [PubMed] [Google Scholar]
- 19. Sanchez T, Skoura A, Wu MT, Casserly B, Harrington EO, Hla T.. Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler Thromb Vasc Biol 2007; 27:1312–1318. [DOI] [PubMed] [Google Scholar]
- 20. Kimura T, Suzuki A, Fujita Y, Yomogida K, Lomeli H, Asada N, Ikeuchi M, Nagy A, Mak TW, Nakano T.. Conditional loss of PTEN leads to testicular teratoma and enhances embryonic germ cell production. Development 2003;130:1691–1700. [DOI] [PubMed] [Google Scholar]
- 21. Hogmalm A, Sheppard D, Lappalainen U, Bry K.. beta6 Integrin subunit deficiency alleviates lung injury in a mouse model of bronchopulmonary dysplasia. Am J Respir Cell Mol Biol 2010;43:88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tabeling C, Scheer H, Schönrock SM, Runge F, Gutbier B, Lienau J, Hamelmann E, Opitz B, Suttorp N, Mayer K, Behrens GM, Tschernig T, Witzenrath M.. Nucleotide oligomerization domain 1 ligation suppressed murine allergen-specific T-cell proliferation and airway hyperresponsiveness. Am J Respir Cell Mol Biol 2014;50:903–911. [DOI] [PubMed] [Google Scholar]
- 23. Hassock SR, Zhu MX, Trost C, Flockerzi V, Authi KS.. Expression and role of TRPC proteins in human platelets: Evidence that TRPC6 forms the store-independent calcium entry channel. Blood 2002;100:2801–2811. [DOI] [PubMed] [Google Scholar]
- 24. Shnitsar I, Bashkurov M, Masson GR, Ogunjimi AA, Mosessian S, Cabeza EA, Hirsch CL, Trcka D, Gish G, Jiao J, Wu H, Winklbauer R, Williams RL, Pelletier L, Wrana JL, Barrios-Rodiles M.. PTEN regulates cilia through Dishevelled. Nat Commun Nature Publishing Group; 2015;6:8388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang J, Kontos CD.. PTEN modulates vascular endothelial growth factor-mediated signaling and angiogenic effects. J Biol Chem 2002;277:10760–10766. [DOI] [PubMed] [Google Scholar]
- 26. Kanamori Y, Kigawa J, Itamochi H.. Correlation between loss of PTEN expression and Akt phosphorylation in endometrial carcinoma. Clin Cancer Res 2001;7:892–895. [PubMed] [Google Scholar]
- 27. Nemenoff RA, Simpson PA, Furgeson SB, Kaplan-Albuquerque N, Crossno J, Garl PJ, Cooper J, Weiser-Evans MCM.. Targeted deletion of PTEN in smooth muscle cells results in vascular remodeling and recruitment of progenitor cells through induction of stromal cell-derived factor-1alpha. Circ Res 2008;102:1036–1045. [DOI] [PubMed] [Google Scholar]
- 28. Kalwa H, Storch U, Demleitner J, Fiedler S, Mayer T, Kannler M, Fahlbusch M, Barth H, Smrcka A, Hildebrandt F, Gudermann T, Dietrich A.. Phospholipase C epsilon (PLCε) induced TRPC6 activation: a common but redundant mechanism in primary podocytes. J Cell Physiol 2015;230:1389–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Samapati R, Yang Y, Yin J, Stoerger C, Arenz C, Dietrich A, Gudermann T, Adam D, Wu S, Freichel M, Flockerzi V, Uhlig S, Kuebler WM.. Lung endothelial Ca2+ and permeability response to platelet-activating factor is mediated by acid sphingomyelinase and transient receptor potential classical 6. Am J Respir Crit Care Med 2012; 185:160–170. [DOI] [PubMed] [Google Scholar]
- 30. Urban N, Wang L, Kwiek S, Rademann J, Kuebler WM, Schaefer M.. Identification and validation of Larixyl acetate as a potent TRPC6 inhibitor. Mol Pharmacol 2016; 89:197–213. [DOI] [PubMed] [Google Scholar]
- 31. Veit F, Pak O, Brandes RP, Weissmann N.. Hypoxia-dependent reactive oxygen species signaling in the pulmonary circulation: focus on ion channels. Antioxid Redox Signal 2015;22:537–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gudermann T, Mederos y Schnitzler M, Dietrich A.. Receptor-Operated Cation Entry–More Than Esoteric Terminology? Sci Signal 2004;2004:pe35–pe35. [DOI] [PubMed] [Google Scholar]
- 33. Soboloff J, Spassova M, Xu W, He LP, Cuesta N, Gill DL.. Role of endogenous TRPC6 channels in Ca2+ signal generation in A7r5 smooth muscle cells. J Biol Chem 2005;280:39786–39794. [DOI] [PubMed] [Google Scholar]
- 34. Wang J, Shimoda LA, Weigand L, Wang W, Sun D, Sylvester JT, Acute JTS.. Acute hypoxia increases intracellular [Ca 2+] in pulmonary arterial smooth muscle by enhancing capacitative Ca2+ entry. Am J Physiol 2005;21224:1059–1069. [DOI] [PubMed] [Google Scholar]
- 35. Salvaterra CG, Goldman WF.. Acute hypoxia increases cytosolic calcium in cultured pulmonary arterial myocytes. Am J Physiol 1993;264:L323–L328. [DOI] [PubMed] [Google Scholar]
- 36. Fuchs B, Rupp M, Ghofrani HA, Schermuly RT, Seeger W, Grimminger F, Gudermann T, Dietrich A, Weissmann N.. Diacylglycerol regulates acute hypoxic pulmonary vasoconstriction via TRPC6. Respir Res BioMed Central Ltd; 2011;12:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sanchez T, Thangada S, Wu M-T, Kontos CD, Wu D, Wu H, Hla T.. PTEN as an effector in the signaling of antimigratory G protein-coupled receptor. Proc Natl Acad Sci USA 2005;102:4312–4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Horita H, Furgeson SB, Ostriker A, Olszewski KA, Sullivan T, Villegas LR, Levine M, Parr JE, Cool CD, Nemenoff RA, Weiser-Evans MCM.. Selective inactivation of PTEN in smooth muscle cells synergizes with hypoxia to induce severe pulmonary hypertension. J Am Heart Assoc 2013;2:e000188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bertero T, Cottrill K, Krauszman A, Lu Y, Annis S, Hale A, Bhat B, Waxman AB, Chau BN, Kuebler WM, Chan SY.. The MicroRNA-130/301 family controls vasoconstriction in pulmonary hypertension. J Biol Chem 2015;290:2069–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Shimoda LA, Sham JSK, Sylvester JT.. Altered pulmonary vasoreactivity in the chronically hypoxic lung. Physiol Res 2000;49:549–560. [PubMed] [Google Scholar]
- 41. Michelakis ED, Thébaud B, Weir EK, Archer SL.. Hypoxic pulmonary vasoconstriction: redox regulation of O2-sensitive K+ channels by a mitochondrial O2-sensor in resistance artery smooth muscle cells. J Mol Cell Cardiol 2004;37:1119–1136. [DOI] [PubMed] [Google Scholar]
- 42. Wang L, Yin J, Nickles HT, Ranke H, Tabuchi A, Hoffmann J, Tabeling C, Barbosa-sicard E, Chanson M, Kwak BR, Shin H, Wu S, Isakson BE, Witzenrath M, Wit C De, Fleming I, Kuppe H, Kuebler WM.. Hypoxic pulmonary vasoconstriction requires connexin 40 – mediated endothelial signal conduction. J Clin Invest 2012;122:4218–4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goldenberg NM, Wang L, Ranke H, Liedtke W, Tabuchi A, Kuebler WM.. TRPV4, Is required for hypoxic pulmonary vasoconstriction. Anesthesiology 2015;122:1–11. [DOI] [PubMed] [Google Scholar]
- 44. Zhang M-F, Liu X-R, Yang N, Lin M-J.. TRPC6 mediates the enhancements of pulmonary arterial tone and intracellular Ca2+ concentration of pulmonary arterial smooth muscle cells in pulmonary hypertension rats. Sheng Li Xue Bao 2010;62:55–62. [PubMed] [Google Scholar]
- 45. Xia Y, Yang X-R, Fu Z, Paudel O, Abramowitz J, Birnbaumer L, Sham JSK.. Classical transient receptor potential 1 and 6 contribute to hypoxic pulmonary hypertension through differential regulation of pulmonary vascular functions. Hypertension 2014;63:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Earley S, Leblanc N.. Serotonin receptors take the TRiPV4 highway in chronic hypoxic pulmonary hypertension. Focus on ‘TRPV4 channel contributes to serotonin-induced pulmonary vasoconstriction and the enhanced vascular reactivity in chronic hypoxic pulmonary hypertension’. Am J Physiol Cell Physiol 2013; 305:C690–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yang X-R, Lin AHY, Hughes JM, Flavahan NA, Cao Y-N, Liedtke W, Sham JSK.. Upregulation of osmo-mechanosensitive TRPV4 channel facilitates chronic hypoxia-induced myogenic tone and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2012;302:L555–L5–68.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Xia Y, Fu Z, Hu J, Huang C, Paudel O, Cai S, Liedtke W, Sham JS.. TRPV4 channel contributes to serotonin-induced pulmonary vasoconstriction and the enhanced vascular reactivity in chronic hypoxic pulmonary hypertension. Am J Physiol Cell Physiol 2013;305:C704–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Billaud M, Dahan D, Marthan R, Savineau J-P, Guibert C.. Role of the gap junctions in the contractile response to agonists in pulmonary artery from two rat models of pulmonary hypertension. Respir Res 2011;12:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Koval M, Billaud M, Straub AC, Johnstone SR, Zarbock A, Duling BR, Isakson BE.. Spontaneous lung dysfunction and fibrosis in mice lacking connexin 40 and endothelial cell connexin 43. Am J Pathol 2011;178:2536–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wang Z, Jin N, Ganguli S, Swartz DR, Li L, Rhoades RA.. Rho-kinase activation is involved in hypoxia-induced pulmonary vasoconstriction. Am J Respir Cell Mol Biol 2001;25:628–635. [DOI] [PubMed] [Google Scholar]
- 52. Das S, Dixon JE, Cho W.. Membrane-binding and activation mechanism of PTEN. Proc Natl Acad Sci USA 2003;100:7491–7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vazquez F, Grossman SR, Takahashi Y, Rokas M V, Nakamura N, Sellers WR.. Phosphorylation of the PTEN tail acts as an inhibitory switch by preventing its recruitment into a protein complex. J Biol Chem 2001;276:48627–48630. [DOI] [PubMed] [Google Scholar]
- 54. Wu J, Li J, Hu H, Liu P, Fang Y, Wu D.. Rho-kinase inhibitor, fasudil, prevents neuronal apoptosis via the Akt activation and PTEN inactivation in the ischemic penumbra of rat brain. Cell Mol Neurobiol 2012;32:1187–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Takata M, Tanaka H, Kimura M, Nagahara Y, Tanaka K, Kawasaki K, Seto M, Tsuruma K, Shimazawa M, Hara H.. Fasudil, a rho kinase inhibitor, limits motor neuron loss in experimental models of amyotrophic lateral sclerosis. Br J Pharmacol 2013; 170:341–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.