Abstract
In this review, we focus on the emerging role of microRNAs, non-coding RNAs that regulate gene expression and signaling pathways, in dysfunctional adipose tissue. We highlight current paradigms of microRNAs involved in adipose differentiation and function in depots such as white, brown, and beige adipose tissues and potential implications of microRNA dysregulation in human disease such as obesity, inflammation, microvasculature dysfunction, and related cardiovascular diseases. We highlight accumulating studies indicating that adipocyte-derived microRNAs may not only serve as biomarkers of cardiometabolic disease, but also may directly regulate gene expression of other tissues. Finally, we discuss the future prospects, challenges, and emerging strategies for microRNA delivery and targeting for therapeutic applications in cardiovascular disease states associated with adipocyte dysfunction.
Keywords: MicroRNA, Adipocytes, Brown adipose tissue, White adipose tissue
1. Introduction
Type 2 diabetes (T2D) is a worldwide problem that is observed both in developed and developing countries. Hallmark events in T2D are elevated blood glucose that stems from impaired responsiveness to insulin and insufficient insulin production.1 Insulin is a hormone that is produced by the pancreas, plays a fundamental and evolutionary conserved role in maintaining energy homeostasis.2,3 In response to food intake, there is a rapid increase in circulating insulin levels where it exerts its effect on three main target tissues: fat, liver, and skeletal muscle.4 Under homeostatic conditions, cellular signaling by insulin is initiated by binding to insulin receptors that help modulate pathways controlling lipid uptake, lipolysis, and lipogenesis.3,5,6
Insulin resistance, a major risk factor for both type 2 diabetes (T2D) and ischaemic cardiovascular disease, is defined as decreased responsiveness to insulin stimulated glucose transport and metabolism in adipocytes and skeletal muscle, reduced expression of insulin signaling components in skeletal muscle as well as impaired suppression of hepatic glucose output.7–13 Insulin resistance is a key component in the development of T2D and obesity, which in turn significantly contributes to the development of cardiovascular disease and diabetic-associated macrovascular complications such coronary artery disease (CAD), peripheral vascular disease (PVD), and stroke, and microvascular complications such as retinopathy, neuropathy, and nephropathy.14 Diabetes can also contribute to cardiomyopathy resulting in diabetes-associated changes in the structure and function of the myocardium.
Adipose tissue plays a central role in health and disease. Under periods of excess energy intake, its primary function is to store energy in the form of triglycerides (TGs) and release energy during starvation or fasting.15 In addition to being an energy reservoir, adipose tissue is also an endocrine organ relaying hormonal signals that regulate the hypothalamic-pituitary-gonadal axis.16 Distinct roles have emerged for white, brown, and beige (brite) adipose tissue depots in regulating susceptibility to obesity, insulin resistance, and maladaptive metabolism. In addition to the major adipocyte cell subtypes, other cellular constituents such as endothelial cells, fibroblasts, stem cells, neurons, and immune cells may contribute to adipose dysfunction and obesity.17 For example, there is a complex relationship between increased energy intake, increased insulin levels followed by activation of biochemical pathways by insulin that regulate expansion of white adipose tissue (WAT) through adipocyte hypertrophy, or adipogenesis.18,19 Deletion of insulin receptors from WAT protects mice from obesity pointing out the key role insulin plays in adipocyte differentiation or adipocyte hyperthrohy. However, the mechanisms controlling these pathways are not fully understood.20,21
Brown adipose tissue (BAT) has garnered significant attention in recent years in regulating human energy expenditure and metabolism. It is a thermogenic tissue that is important, especially in small mammals, to regulate their body temperature and keep the core body temperature constant under cold ambient temperatures. This is due to its high content of mitochondria, a property that is unique from WAT.22
MiRNAs, evolutionarily conserved small non-coding RNAs of ∼22 nucleotides in length, are involved in a range of developmental and pathophysiological processes in metazoans and collectively target over one-half of protein-coding transcripts.23,24 MicroRNAs are important mediators in adipose function, T2D, obesity, and cardiovascular pathologies.25–29 Accumulating studies provide novel molecular and cellular insights into their impact on pathophysiological pathways in adipocyte differentiation, function, and insulin resistance and obesity.
MicroRNAs have garnered considerable attention not only for their ability to regulate adipogenesis and adipose function, but also for their extracellular presence, such as in circulating blood or urine, raising their potential use as biomarkers for diagnosis or prognosis. In this review, we highlight emerging roles for miRNAs and their target genes in regulating adipocyte function both in a cell intrinsic and extrinsic manner with a special emphasis on links to cardiovascular risk and disease states. Finally, we discuss opportunities and challenges of relevant miRNA delivery strategies that may impact adipocyte function, T2D, obesity, and cardiovascular disease.
2. MiRNA biogenesis and function
MiRNAs are transcribed by RNA polymerase II in the nucleus to form primary-miRNAs (pri-miRNAs) with a stem-loop structure that harbors the mature miRNA sequences.30,31 Next, the nuclear ribonuclease (RNase)-III enzyme, Drosha, cleaves pri-miRNA into precursor-miRNAs (pre-miRNAs), hairpin-shaped RNAs of ∼70 base pairs (bp) harboring an imperfect stem-loop structure.32 Drosha then forms a protein complex with the essential cofactor DGCR8 (also known as Pasha; a protein containing two double-stranded (ds)RNA-binding domains) which directs the cleavage of the stem-loop of the hairpin RNA structure of pri-miRNA.33,34 This complex, also known as microprocessor, plays an important role in the post-transcriptional cross-regulation between Drosha and DGCR8.35–37 Pre-miRNAs are then exported by a Ran-guanosine triphosphate (GTP)-dependent nucleo/cytoplasmic transporter named exportin 5 (Exp5) into the cytoplasm where the maturation of miRNA takes place.38,39 In the cytoplasm, the pre-miRNA is cleaved by a RNase-III enzyme, Dicer, into a small dsRNA duplex that contains both the mature miRNA strand and its complementary strand.40–43
After cleavage by Dicer, the resulting 21-24 nt miRNA duplex unwinds to release one of the strands for entering the RNA-induced silencing complex (RISC) and binding to Argonaute which facilitates its function.44,45 Both strands are capable of being loaded onto Argonaute and repressing target mRNAs.31,46 Typically, the more thermodynamically stable strand of the two becomes the mature miRNA strand that is loaded into the RISC, whereas the other strand of this short-lived duplex disappears45 Subsequently, the miRNA directs the RISC to the 3’ UTR of the target mRNA to negatively regulate mRNA expression by promoting mRNA cleavage47,48 and/or translational repression.49,50 In animals, the 7 nt ‘seed region’, mapping to positions 2 - 8 at the 5’ end, of miRNA51,52 serves an essential role for target recognition. Indeed, the seed region is highly conserved across species.50,53 Upon a perfectly matched complementary sequence between the miRNA and its target, the target mRNA is cleaved.54–56 However, miRNA partial sequence complementarity may also exert target gene translational repression.57–60
3. MicroRNAs in adipose tissue
Adipose tissue is known to have two major roles in the body: a storage depot for triglyceride for times of caloric restriction and an endocrine organ that regulates whole body homeostasis.61,62 However, excess adipose tissue, especially in the abdomen area increases the risk for a number of conditions including type 2 diabetes63,64 hypertriglyceridemia,65 low grade chronic inflammation,66,67 hypertension,68,69 and coronary artery disease (CAD).69 While adipocytes account for the majority of fat pad volume, the non-adipocyte cell subsets, such as endothelial cells, fibroblasts, immune cells, among others, predominate by overall cell number.70
Accumulating studies indicate that miRNAs play an integral role in adipose tissue formation and function. Initial findings demonstrated that inhibition of Drosha and Dicer in human mesenchymal stem cells inhibited differentiation into adipocytes, and inhibition of Drosha in 3T3-L1 cells inhibited adipogenesis.71,72 Interestingly, adipocyte-specific deletion of Dicer in mice resulted in severe depletion of white adipose tissue with reduced adipogenic-associated transcripts. Although Dicer deletion was not required for brown fat lipogenesis, the expression of genes involved in thermoregulation were reduced.73 Adipose-specific ablation of Dgcr8 in mice resulted in enlarged but pale interscapular brown adipose tissue (BAT), decreased expression of signature brown fat genes, and cold intolerance.74 Collectively, these studies of regulators of miRNA biogenesis suggest an important role for miRNAs in adipose tissue homeostasis.
3.1 White adipose tissue (WAT)
There are a growing number of miRNAs that are expressed and regulated in WAT in response to obesity and T2D (Figure 1). WAT is distributed throughout the body and is a vascularized organ. Adipocytes are the main constituents of adipose tissue by volume and play an important role in insulin resistance and T2D. Important contributors to WAT dysfunction include adipocytes and their cross-talk between the microvasculature in adipose tissue (also known as perivascular adipose tissue (PVAT))75 and macrophages that release cytokines such as TNF-α, an effect promoting low grade chronic inflammation.76 Indeed, inflammation in WAT can be quantified histologically by staining for the number of macrophages surrounding adipocytes in what has been termed as ‘crown-like structures’.77
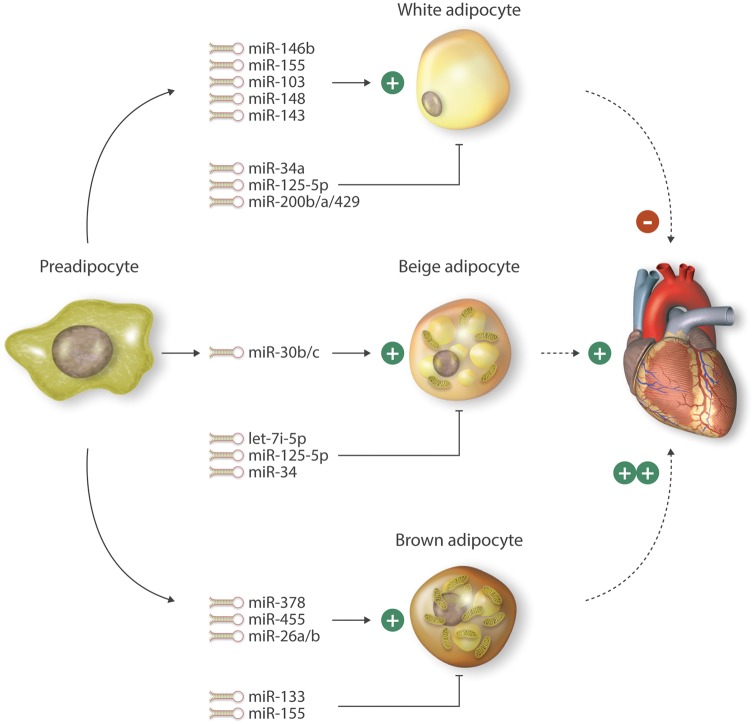
Figure 1.
Example of microRNAs involved in the differentiation of white, beige, and brown adipocytes. These microRNAs are involved in regulating adipocyte differentiation through effects on their targets that control expression of metabolic genes, inflammation, or insulin resistance. Activation of brown and/or beige adipocytes may contribute to favorable effects on energy expenditure, insulin resistance, and potentially cardiovascular remodeling. Solid lines with arrows followed by (+) indicate positive regulation of the indicated adipocyte differentiation pathway. Dashed lines followed by (+) or ( ++) indicate that the adipocyte differentiation pathway may be favorably implicated in regulating cardiovascular disease.
Since there is complex relationship between the many different cell types involved in the adipose tissue and their involvement in adipogenesis and obesity, a recent study focused on identifying a miRNA signature profile following 6 weeks of endurance training in obese, human male subjects. Interestingly enough, there were no significant changes in the miRNA expression profiles in adipocytes isolated pre- or post-training from abdominal subcutaneous (ABD) and gluteofemoral (GF) adipose tissue, raising the possibility that endurance exercise does not dynamically change adipose tissue miRNA expression compared to other pathophysiological stimuli.78
The association between adipose tissue and the cardiovascular disease (CVD) is a complex one. Obesity-related hyperlipidemia is well-studied where obesity-induced elevation of triglycerides and cholesterol leads to atherosclerosis.79,80 However, there is also a strong correlation between obesity, inflammation, and CVD. Although adipose tissue is not considered an immune organ, it is an endocrine organ and many pro-inflammatory genes such as TNF-α, IL-6, IL1-β PAI-1, angiotensinogen, C-reactive protein or adipokines are highly expressed in dysfunctional adipose tissue, resulting in both local and systemic inflammation that is characterized by infiltration of macrophages and T cells to the vessel wall and thereby increasing the risk for CVD such as atherosclerosis.81–86 Another important factor predisposing patients to CVD is the location of the adipose tissue. Obese human subjects with excess visceral adipose tissue (VAT), or abdominal obesity, are at higher risk for T2D and CVD components than those whose fat is located predominantly in the lower body, subcutaneously.63,64
3.1.1 Adipocyte
The role of miRNAs in adipogenesis is well established and have been shown to act as either pro- and anti-adipogenesis regulators. Examples of pro-adipogenic microRNAs include miR-143, miR-103, miR-146b, miR-148, and miR-33b. Overexpression of miR-143 or miR-103 in preadipocytes accelerated adipogenesis. This process was associated with increased expression of transcription factors such as PPARγ2, cell cycle regulators such as G0/G1 switch 2 (G0S2), FABP4, GLUT4, and adiponectin.87 Interestingly, several studies demonstrate an inverse correlation between miRNAs that are implicated in adipogenesis and obesity.87–89 Consistent with this notion, in contrast to their pro-adipogenic role, expression of miR-143 and miR-103 were significantly decreased in adipocytes of obese mice.87 MiR-146b expression is increased in HFD-induced obese mice and in ob/ob and db/db mice, whereas the expression of its target gene SIRT1 was decreased in WAT. MiR-146b induced adipogenesis by inhibiting SIRT1-dependent acetylation of the transcription factor FOXO1. Indeed, in vivo neutralization of miR-146a expression increased SIRT1 expression and ameliorated diet-induced obesity.90 Expression of another miRNA, miR-148, increased in both obese human subjects and mice fed a HFD. Furthermore miR-148 targeted an inhibitor of adipogenesis, Wnt1, thereby acting as a pro-adipogenic miRNA regulating the differentiation of human adipose-derived mesenchymal stem cells (hMSCs-Ad). Interestingly, the promoter region of miR-148a contains a functional cAMP-response element-binding protein (CREB), which was required for miR-148a expression in hMSCs-Ad.91 Finally, the intronic miRNA miR-33b and its host gene SREBP-1 are highly induced upon preadipocyte differentiation. Inhibition of miR-33b suppressed preadipocyte differentiation and lipid droplet accumulation, whereas its overexpression promoted differentiation.92 These effects were mediated in part by targeting of HMGA2 and cyclin-dependent kinase 6 (CDK6), an important regulator of cell cycle progression, specifically the G1-S transition. These findings have translational relevance as mutations in miR-33 binding sites of the 3'-UTR of HMG2A cause liposarcomes in humans.92 Taken together, these examples highlight that miRNAs may exhibit pro-adipogenic activity by regulating divergent transcriptional and epigenetic targets known to be involved in adipogenesis.
In contrast to the above miRNAs, miR34a, miR-125-5p and miR-200b/a/429 cluster are examples of miRNAs that inhibit adipogenesis. MiR-34a is an excellent example of an anti-adipogenic miRNA in which its expression is increased in serum of patients with T2D.93 There is a positive correlation with increased miR‐34a expression in human subcutaneous WAT (scWAT) and increased miR-34a expression during adipocyte differentiation in vitro.94 Compared to controls, miR-34a KO mice gained more weight at baseline and in response to HFD. In addition, miR‐34a KO epididymal white adipose tissue (eWAT) had a smaller adipocyte area that increased with HFD accompanied with increased expression of metabolic genes such as CD36, HMGCR, LXRα, PGC1α, and FASN. The eWAT from miR-34a KO mice also showed increased inflammation reflected by higher accumulation of F4/80 positive macrophages.95 The expression of another miRNA, miR-125b-5p, increased during human adipocyte differentiation. Overexpression of miR125b-5p reduced adipogenesis; however, the target gene(s) involved in this process remain unclear.96 Adipocyte-specific knockout of the miR-200b/a/429 cluster in mice resulted in HFD-induced weight gain, decreased glucose tolerance and insulin sensitivity, and impaired lipolysis compared to the WT control, effects mediated in part through targeting the EPS8 and GLIS2 genes.97
The role of miRNA, miR-155 in adipogenesis is somewhat unclear. In human obese subjects and in 3T3-L1 cells, increased miR-155 expression correlated to body mass index (BMI) and TNF-α expression, respectively. Moreover in-vitro studies performed in 3T3-L1 cells linked induced expression of miR-155 to activation of the NF-κB signaling pathway. The anti-adipogenic and anti-lipogenic function ascribed to miR-155 may be mediated through direct targeting of PPAR-γ.98 However, in another study, miR-155 was found to function as a pro-adipogenic miRNA. Female miR-155 knock out (KO) mice were protected from high fat diet (HFD)-induced weight gain, obesity, and insulin resistance through decreased WAT accumulation, adipose size, inflammation, and increased energy expenditure.99 While it is unclear how to reconcile the systemic KO studies in mice with the in vitro studies performed using human adipocytes, it is likely that miR-155 may regulate non-adipocyte cell types that may indirectly influence adipocyte function, or miR-155 may have a species or gender-specific role.99 MiR-155 also plays an important role in the regulation of lipid metabolism in liver. Interestingly, deficiency of miR-155 may lead to hepatic steatosis in mice through targeting Nr1h3 (LXRα).100 Collectively, miRNAs can either positively or negatively regulate adipogenesis and thereby having an impact on glucose tolerance, insulin sensitivity, or obesity.
3.1.2 The microvasculature
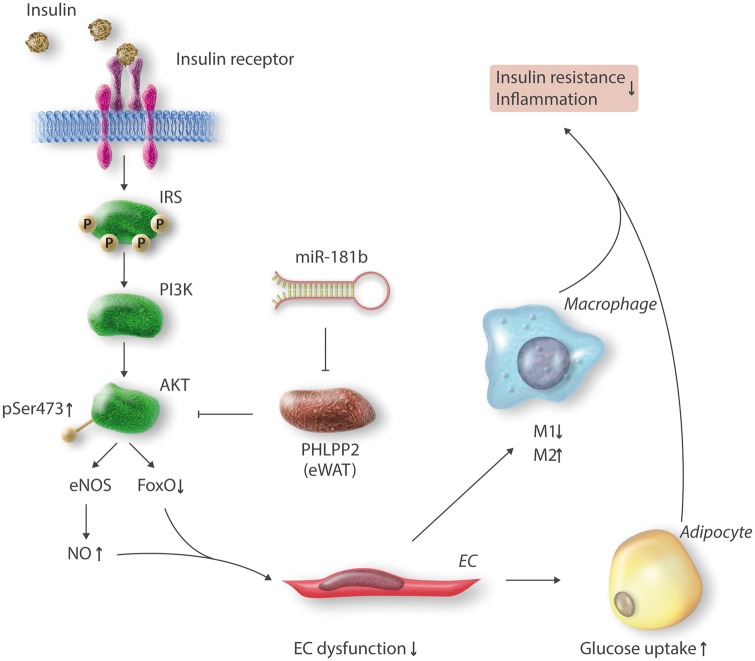
There is a functional and anatomical relationship between adipose tissue and the microvasculature. The crosstalk between the two organs is essential in controlling vascular homeostasis. However, the mechanism regulating this relationship is poorly understood. Perivascular adipose tissue (PVAT) generates vasorelaxants or vasoconstriction factors under physiological conditions or inflammation, respectively, and thereby regulates vessel contractility. Under inflammatory conditions, PVAT can also augment vascular dysfunction by secreting pro-inflammatory adipokines such as adipocyte fatty acid binding protein or TNF-α, hormones, and reactive oxygen species (ROS), which are all known to contribute to endothelial activation in both the macro- and microvasculature leading to vascular wall inflammation and dysfunction.75,101 A study by our group identified that in response to HFD-induced insulin resistance, the anti-inflammatory microRNA, miR-181b,102,103 is significantly reduced in adipose tissue endothelial cells (ECs), but not adipocytes, from eWAT after 1 week. In the light of this finding, we delivered via tail-vein injection liposomally encapsulated miR-181b to HFD-induced insulin resistant mice. Compared to controls, these miR-181b injected mice exhibited markedly improved glucose homeostasis and insulin sensitivity.102 From a mechanistic perspective, miR-181b overexpression in adipose ECs enhanced insulin-mediated Akt phosphorylation at Ser473, and reduced endothelial dysfunction, an effect that shifted macrophage polarization toward an M2 anti-inflammatory phenotype in eWAT. Importantly, these effects were associated with induction of endothelial nitric oxide synthase (eNOS), nitric oxide activity, and FoxO1 phosphorylation specifically in eWAT, but not in liver or skeletal muscle. In contrast, overexpression of miR-181b in peripheral blood mononuclear cells (PBMCs) had no effect on macrophage activation, proliferation, or recruitment to visceral fat. Similarly, adipocytes overexpressing miR-181b had no intrinsic effect on glucose uptake. Using complementary bioinformatics, gene profiling studies, and siRNA-mediated knockdown approaches, pleckstrin homology domain leucine-rich repeat protein (PHLPP2), a phosphatase known to dephosphorylate Akt at Ser473, was revealed as a bona-fide target of miR-181b and PHLPP2 inhibition recapitulated the effects of miR-181b. Interestingly, PHLPP2 is highly expressed in eWAT, whereas it is barely detectable in liver and skeletal muscle, which may help explain the miR-181b tissue-specific effect in eWAT. Taken together, these findings solidified the role of 181b in the maintenance of homeostasis in the microvasculature of visceral fat to control EC inflammation and insulin resistance (Figure 2).
Figure 2.
MiR-181b improves endothelial dysfunction, glucose homeostasis, and insulin sensitivity in white adipose tissue by binding to the 3’-UTR of the phosphatase PHLPP2 and reducing its expression. Consequently, decreased PHLPP2 improves insulin signaling with enhanced AKT and eNOS expression in eWAT. Abbreviations: IRS, insulin receptor substrate; PI3K, phosphoinositide 3-kinase; AKT, also known as protein kinase B; PHLPP2, pleckstrin homology domain leucine-rich repeat protein 2; pSer473, phosphorylation of serine residue 473 of AKT; eNOS, endothelial nitric oxide synthase; NO, nitric oxide; FoxO, Forkhead box O; eWAT, epididymal white adipose tissue; EC, endothelial cells; M1, pro-inflammatory macrophages; M2, anti-inflammatory macrophages.
A unique fat depot that has recently garnered attention is epicardial adipose tissue (EAT) that surrounds the heart and epicardial vessels; however, its role is not fully understood. Under physiological conditions, EAT is thought to be cardioprotective and is composed of a mixed lineage of fat cells that is close to a beige phenotype.104 However, under pathological conditions, its thickness is associated with insulin resistance,105 and is a risk factor for CAD, atrial fibrillation, and heart disease, suggesting it may play a role in inflammation.106–108 Through a whole genome microarray analysis of patients with CAD, miR-103-3p was shown to be overexpressed in EAT. Furthermore, miR-103-3p targeted the 3’UTR of the pro-inflammatory chemokine CCL13 suggesting that it may regulate EAT inflammation and may be important as a biomarker for CAD.109 Finally, microarray profiling of EAT from hyperglycemic pigs revealed increased expression levels of miR-193a-3p and miR-675-5p, whereas the expression of miR-144-3p was reduced.110 The functional roles for these miRNAs in EAT will require further study.
3.1.3 Macrophage
Studies to date indicate that monocytes are recruited to peripheral tissues such as pancreas, liver, and adipose tissue to become resident macrophages where they may contribute to local inflammation and development of insulin resistance.111 During the pathogenesis of insulin resistance, macrophage populations can switch from an anti-inflammatory (M2) population to an inflammatory (M1) population, a process termed macrophage polarization.112 This event results in the release of proinflammatory cytokines and chemotactic factors that exacerbate the local inflammatory environment, thereby increasing the risk for atherosclerosis and CVD.113
There are a number of miRNAs that regulate monocyte/macrophage activation, differentiation, or recruitment. For example, oxidized low density lipoprotein (oxLDL) increased the expression of miR-155 and promoted macrophage-derived foam cell formation. Interestingly, inhibition of miR-155 decreased lipid-loading in macrophages and reduced atherosclerotic plaques in ApoE-/- mice.114 However, when ApoE-/-/miR-155-/- mice were placed on a HFD, they surprisingly developed obesity, adipocyte hypertrophy, and fatty liver but did not develop glucose intolerance or insulin resistance.115 Overexpression of a different miRNA, miR-144-3p, increased the secretion of inflammatory cytokines such as IL-6, IL-1β, and TNF-α to promote THP-1 macrophage differentiation into foam cells through targeting the ATP-binding cassette transporter A1 (ABCA1). Conversely, inhibition of miR-144-3p in ApoE-/- mice increased macrophage accumulation in atherosclerotic lesions suggesting that miR-144-3p accelerated the progression of atherosclerosis.114 Furthermore, miR-144 was shown to regulate cholesterol metabolism via suppressing ABCA1 expression.116 Moreover, in response to activation of the bile acid receptor farnesoid X receptor (FXR), miR-144 was found to regulate ABCA1 and plasma HDL-cholesterol.117 A role of this miRNA in diet-induced obesity and insulin resistance will require further investigation. Activation of Liver X receptors (LXRs) in macrophages promotes cholesterol efflux118,119 and protects against the development of insulin resistance and atherosclerosis.120 Overexpression of miR-206 in Tamm Horsfall Protein 1(THP-1) cells increased LXRα expression and enhanced cholesterol efflux. Furthermore, LXRα activation reduced miR-206 expression, indicating the presence of a feedback loop between miR-206 and LXRα in regulating cholesterol efflux, insulin resistance, vascular inflammation, and atherosclerosis.121
3.2 Brown adipose tissue (BAT)
Brown adipose tissue (BAT) is one of the major fat depots. It has a unique function of converting stored chemical energy into heat. The uncoupling protein-1 (UCP-1), a BAT-specific protein located within the mitochondria, plays an important role in this process. UCP-1 ‘uncouples’ fuel oxidation from ATP synthesis to generate heat without muscle energy.17 Brown adipocytes are located in the interscapular and perirenal regions of rodents and are highly innervated, vascularized and made up of cells with multiocular lipid droplets.22,122 Although initially BAT was thought only to be active in rodents and human infants, studies employing PET/CT scans where healthy subjects were exposed to ambient room temperature or 18 °C confirmed the presence of metabolically active BAT in adult humans.123 Its activity is significantly decreased in obesity in humans by mechanisms that are not fully understood yet.
Interestingly, although miRNAs are known to regulate brown adipocyte differentiation such as miR-133,124 miR-378,125 miR-155,126 miR-455,127 miR-26,128 and miR-34,129 activation of BAT can also regulate miRNA expression profiles.124
BAT activation may also play an important role in reducing CVD events through its ability to increase energy expenditure and regulate glucose and lipid metabolism.130 However, in APOE and LDL KO mice, cold activation of BAT increased lipolysis that resulted in an increase of LDL cholesterol levels thereby potentially increasing their risk of cardiovascular events in severe hypercholesterolemic states.131
3.2.1 MiRNA regulation of brown fat adipocyte differentiation
Because of BAT’s unique ability to increase energy expenditure rather than store it in the form of WAT, a major interest in recent years has been how to expand BAT. Several miRNAs have been implicated in differentiating adipocytes into brown fat. For example (Figure 1), transgenic overexpression of miR-378, a miRNA that is enriched in BAT and induced with cold temperature exposure, promoted BAT adipogenesis and increased BAT, but not WAT. MiR-378 directly targeted PDE1b, a phosphodiesterase that catalyses the turnover of cAMP and cGMP.125 Overexpression of members of the miR-26 family, miR-26a and miR-26b, in adipose-derived stem cells (hMADS cells) upregulated the BAT marker UCP-1 both at the mRNA and protein level, an effect that may be mediated by targeting ADAM metallopeptidase domain 17 (ADAM17) thereby allowing increased UCP-1 expression in hMADS cells.128 MiR-455, induced by cold temperature exposure and bone morphogenetic protein (BMP7), regulates BAT differentiation by targeting the hypoxia-inducible factor 1 α inhibitor (HIFan), a hydroxylase that modifies AMP‐activated kinase α1 subunit (AMPKα1) by hydroxylation and AMPK, thereby promoting thermogenesis. Furthermore, adipose-specific overexpression of miR-455 induced UCP-1 expression and brown adipogenesis.127 MiR-155 is a miRNA with a dual role of brown adipocyte differentiation and browning of WAT. MiR-155 KO mice have increased UCP-1 and PGC-1α expression and an activated thermogenic program in WAT thereby allowing for the increased ability to adapt to cold exposure by targeting CCAAT/enhancer-binding protein β (C/EBPβ).126 Finally, miR-133 is a negative regulator of BAT adipogenesis through direct targeting of PRDM16, inhibiting its expression and reducing brown adipogenesis in white adipocyte progenitor cells.124,132 Collectively, these studies suggest that microRNAs can effectively regulate brown fat differentiation, providing potential targets for modulating energy expenditure.
3.2.2 MiRNA regulation of skeletal muscle progenitor brown fat metabolism
A brown fat enriched miRNA cluster, miR-193b-365 located on chromosome 6 as a ∼5 kb gene, is significantly increased during brown fat adipogenesis. Furthermore, in obese mice, expression levels of miR-193 and miR-365 are significantly reduced in BAT. Blocking of miR-193a/b and miR-365 reduced lipid accumulation in brown adipocytes. Furthermore, miR-193 directly targets RUNX1T1. Interestingly, due to the common development origin of brown adipocytes and skeletal muscle, the authors hypothesized that blockage of the brown adipocyte lineage by miR-193 or miR-365 may switch brown fat preadipocyte fate to the muscle lineage. Indeed, expression of miR193 and miR-365 in C2C12 skeletal muscle myoblasts induced their differentiation into brown adipocytes. Mechanistically, this is partially controlled by the induction of miR-193 and miR-365 by PRDM16 through peroxisome proliferator-activated receptor alpha (PPARα).133 Activation of PPARα, a member of the nuclear hormone receptor family, was previously shown to prevent inflammation in WAT.134 Similarly, miR-133, a miR that is decreased in mice in response to cold exposure, regulates the lineage commitment between myogenic and brown adipocytes by targeting the 3’UTR of PRDM16. In diet-induced obese mice, inhibition of miR-133 during muscle regeneration increased uncoupled respiration, glucose uptake, thermogenesis in locally-treated muscle, energy expenditure, glucose tolerance, and induced metabolically active brown adipocytes in skeletal muscle.132 Inhibition of miR-328, a regulator of BAT differentiation, blocked preadipocyte commitment, whereas miR-328 overexpression promoted BAT differentiation and impaired muscle progenitor commitment through inhibition of the β-secretase BACE1. In vivo BACE1 inhibition delayed diet induced obesity and improved glucose tolerance and insulin sensitivity.135 Collectively, these miRNAs highlight the dynamic relationship between brown fat preadipocytes and skeletal muscle progenitors.
3.3 beige (brite) adipose tissue
Adipose tissue is a complex endocrine organ. Islands of thermogenic adipocytes, termed ‘brite’ (brown-in-white) or beige are a subtype of adipose depot that has recently attracted attention. These adipocytes emerge within WAT with positive expression of UCP-1 and the capability to burn fat and carbohydrates. Beige adipocytes emerge as a physiological response to cold temperature exposure136 by chronic PPARγ activation followed by the activation of the classical brown adipose tissue transcriptional regulator, PRDM16, in subcutaneous WAT (scWAT)137 or by stimulation with β3-adrenoceptor agonists such as CL 316,243 hydrate.138–140 The precise origin of brite adipocytes is still under debate as they are thought to either transdifferentiate from white adipocytes in scWAT136,141 or arise from de-novo differentiation from precursor adipocytes.142 Brite adipocytes are considered to be potential therapeutic targets in the settings of energy expenditure, exercise, and metabolism as they are considered as an ‘inducible’ brown fat subtype.
3.3.1 MiRNA regulation of beige (brite) fat differentiation
In response to a range of stimuli, global miRNA expression profiling of adipocytes has identified a number of miRNAs that are regulated in the browning of WAT (Figure 1). For example, let-7i-5p, a member of the one of the first described let-7 miRNA family was identified from gene profiling of hMSCs-Ad cells that were differentiated from white adipocytes to a brite phenotype using the PPARγ agonist rosiglitazone. Expression of let-7i-5p was reduced in brite adipocytes that negatively correlated with UCP1 expression in mouse and human cells and tissues. Furthermore, let-7i-5p regulated brite adipocyte function in vitro through the specific inhibition of UCP1 expression, which in turn impaired mitochondrial oxygen consumption. Overexpression of let-7i-5p in vivo through injection into scWAT impaired the formation and function of brite adipocytes through partial inhibition of β3-adrenergic activation of the browning process.143 Activation of β3-adrenergic receptors by epinephrine, norepinephrine, or specific agonists typically results in the Gs-dependent activation of adenylate cyclase, increased intracellular cAMP, and stimulation of protein kinase A (PKA) and several downstream kinases including p38 MAPK.144,145 Activation of p38 MAPK induces a transcriptional program including PGC-1a, ATF-2, and UCP-1 leading to brown fat activation and thermogenesis.146 MiR-125-5p is another example of a miRNA whose expression is reduced during brite adipocyte formation in hMSCs-Ad. In response to β3-adrenergic receptor stimulation in vivo, miR-125b-5p expression was reduced in scWAT as well as in interscapular BAT. Overexpression of miR-125-5p inhibited brite adipocyte formation in WAT as evidenced by decreased expression of brite adipocyte markers such as UCP1, CPT1M, CIDEA, and a defect in the formation of multilocular lipid droplet-containing adipocytes representative of activated brite/brown adipocytes. In contrast, loss-of-function studies in mouse scWAT promoted activated brite adipocyte formation.147 MiR-30 family members, specifically miR-30b and miR-30c, regulated not only BAT function and energy homeostasis, but they are also important regulators of brite adipocyte cell formation. Expression of miR-30b and miR-30c increased in response to physiological stimulation such as cold temperature exposure and with chemical activators of β3-adrenergic signaling pathway in primary adipocytes through direct targeting of the 3’-UTR of nuclear co-repressor RIP140. Furthermore, overexpression of miR-30b and -30c significantly induced UCP1 and CIDEA expression and increased mitochondrial activity in white adipocytes from the stromal vascular fraction (SVF) derived from scWAT indicating that miR’s-30b and -30c could induce brite adipocytes in scWAT.148 MiR-34a, a miRNA with increased expression in obesity, is another example of a miRNA with dual role in both brown and brite adipocyte formation in-vivo. Lentiviral-mediated inhibition of miR-34a in mice with diet-induced obesity reduced adiposity, improved serum profile, and increased oxidative function in adipose tissue. Reduced miR-34a expression increased expression of the beige fat-specific marker CD137 and UCP1 in WAT including visceral fat and promoted additional browning in brown fat. Mechanistically, miR-34a directly targets fibroblast growth factor receptor 1 (FGFR1). Furthermore, in-vivo inhibition of miR-34 increased adipocyte SIRT1 levels and deacetylation of PGC-1α, which has an important role in the browning of WAT.129 Collectively, a number of miRNAs that contribute to browning also participate in beige adipocyte differentiation. It will be of interest to elucidate their adipocyte-specific roles in cardiovascular pathophysiological states such as heart failure, atherosclerosis, exercise, and a range of vascular disease states.
4. Adipose-associated circulating microRNAs
Emerging studies indicate that adipocytes can release miRNAs in exosomes. Indeed, adipose-derived circulating miRNAs may regulate gene expression in other tissues highlighting their ‘endocrine-like’ systemic effects.149 Another study highlighted that exosomes isolated from both human and mouse serum may harbor miRNAs such as miR-92a. Interestingly, there was an inverse correlation of miR-92a expression and BAT activity in healthy individuals as measured by PET/CT scans.150 The ability to detect circulating miRNAs and their correlation with adipose tissue activity holds promise that they may serve as metabolic biomarkers. Plasma samples collected from a European cohort of children who were healthy, overweight, or obese were screened for differentially expressed circulating miRNAs. Of the 372 miRNAs that were screened for, miR-31-5p, miR-2355-5p, and miR-206 were differentially expressed and correlated with obesity.151 MiRNA profiling of 85 lean vs. 40 obese children revealed increased concentrations in plasma of miR-486-5p, miR-486-3p, miR-142-3p, miR-130b, and miR-423-5p—all of which associated with factors such as body mass index (BMI), percent fat mass, waist and regional fat distribution, insulin resistance, high-molecular-weight adiponectin, C-reactive protein, and circulating lipids. Plasma concentrations of some of these circulating miRNAs changed significantly during the 3-year follow-up in children who showed an increase or decrease in their weight. These results suggest that the circulating miRNAs are promising candidates as potential biomarkers in the detection of metabolic syndrome even in childhood.152 Additional studies will be required to determine whether adipose-derived circulating miRNAs will have a major impact in cardiovascular pathologies. However, these findings raise the possibility that similar paradigms may exist for miRNAs to be derived from other cells (e.g. endothelial cells, platelets, leucocytes) or tissues (e.g. liver, skeletal muscle) that are also prominently involved in homeostatic control of cardiometabolism.
5. Future directions and conclusion
Accumulating studies demonstrate that microRNAs serve as critical players of gene regulation including the pathways involved in the regulation of adipocyte differentiation and function. They regulate not only white adipose differentiation, but also beige (brite) and brown adipose differentiation and brown fat expansion. Consequently, miRNA-based therapeutics targeting these adipocyte depots may have the ability to reverse adipose tissue dysfunction, insulin resistance, diabetes, obesity, and a range of diabetes-associated cardiovascular complications including accelerated coronary and peripheral atherosclerosis, cardiac lipotoxicity, and microvascular disease in tissues.
There are a number of challenges for miRNA delivery to human tissue. For example, naked miRNAs are readily degraded by endogenous circulating or tissue RNAses. Consequently, chemical modifications have been employed to protect miRNAs and improve durability. Current paradigms for local or systemic delivery utilize a range of chemically modified anti-miRs (to inhibit miRNA) or miRNA mimics (to overexpress miRNA). Several strategies have been used to chemically modify anti-miR oligonucleotides to enhance target stability, affinity, or tissue uptake.153–155 For example, locked nucleic acids exhibit high binding efficiencies and improved stability with the addition of a methylene link between the 2’-oxygen and the 4’-carbon resulting in a locked position and reducing the flexibility of the ribose ring.156 There are examples of other modifications including ribose 2’-OH group modifications, such as 2’4’-constrained 2’O’-ethyl and 2’methoxyethyl, and phosphorothioate modification. However, it has recently been reported that phosphorothioate modifications may facilitate nucleotide-based drugs to bind and activate platelets eliciting thrombus formation in response to carotid injury, pulmonary thromboembolism, and mesenteric artery injury in mice.157 Future studies in human subjects will require careful attention to potential toxicities.
In contrast to their double-stranded counterparts (miRNA mimics), single-stranded anti-miR oligonucleotides can be formulated in saline or phosphate buffered saline for subcutaneous or intravenous delivery and do not require lipid- or nanoparticle-based delivery systems. Following systemic delivery, these anti-miRs rapidly leave the plasma and are taken up by multiple tissues, most notably liver, spleen, kidney, adipose tissue, and bone marrow.158 Upon cellular uptake, the anti-miR generates a high-affinity, stable bond with the miRNA reducing the availability of the endogenous miRNA for binding to the 3’-UTR of the mRNA target(s).
Preclinical studies in nonhuman primates using naked anti-miR oligonucleotides have shown promise for targeting miRNAs expressed highly in the liver such as miR-122 or miR-33.159 Cholesterol analogs have been added to anti-miRs to facilitate cellular uptake, particularly in the liver, and this enhances their incorporation into HDL and LDL.160 Alternative approaches for miRNA inhibition include the use of competitive miRNA inhibitors, such as miRNA sponges or decoy transcripts that contain miRNA binding sites complementary to the seed sequence of the miRNA of interest.161 Several miRNA inhibitors have already advanced into human clinical trials. For example, a human phase 2 study of miR-122 inhibitors (Miravirsen) demonstrated dose-dependent antiviral activity when given as a 4-week monotherapy.162
In disease states where there is a deficiency of a certain miRNA, the delivery of double-stranded therapeutic miRNA molecules in vivo has many of the same challenges as siRNAs. Therefore, drug delivery vehicles such as liposomes, polymeric micelles, and nanoparticle-based carriers are being developed to deliver these oligonucleotides into the cells and tissues. A few miRNA ‘mimics’ are being evaluated in clinical trials. For example, miR-29 mimics are being evaluated in phase 1 studies for safety and tolerability in the context of dermal remodeling (e.g. to prevent fibrous scar tissue formation); NCT02603224.
Another major challenges associated with miRNA mimics delivery is the difficulty to target miRNA to a specific cell type to bypass uptake by other tissues, and the potential requirement of multiple doses to achieve sustained levels for continued target repression. Viral vectors including short hairpin RNAs that can be locally delivered and processed in the target cell to the mature miRNA, are attractive candidates for such a task and this approach has been used in multiple preclinical studies.163,164 However, viral delivery systems will require careful examination for clinical use. Cell-type specific ligands, peptides, and nanoparticles directed at white adipocytes, for example, are likely to provide novel delivery platforms enabling sustained miRNA expression or knockdown for targeted delivery and minimal toxicity to WAT. Collectively, adipocyte-specific miRNA targeting may facilitate beneficial metabolic effects on energy expenditure, insulin resistance, and subsequently cardiovascular remodeling, for example, via induction of browning markers while minimizing any potential non-adipocyte toxicity.
6. Conclusion
In summary, accumulating studies highlight the importance of miRNAs in adipocyte dysfunction and their impact on a range of cardiovascular disease states including diabetes, obesity, and atherosclerosis. Harnessing protective miRNAs associated with browning of WAT may provide a novel strategy to improve energy expenditure, insulin resistance, body fat mass, and cardiometabolic health. Conversely, targeting maladaptive miRNAs in adipocytes may also figure prominently to ameliorate a range of cardiovascular disease states. To this end, a number of miRNAs have been identified in white, beige, and brown adipose tissue depots that can positively or negatively regulate adipose cell differentiation and function. In addition, the function of adipocytes may be regulated by microRNA-mediated effects from neighboring cells, such as microvascular endothelial cells or leucocyte subsets, providing alternative non-adipocyte targeted strategies for regulating cardiometabolic disease. Finally, emerging studies highlight that circulating adipocyte-derived miRNAs may not only serve as potential biomarkers of metabolism, but also may directly impact gene expression and potentially function in remote tissues. Future studies will be informative to ascertain the relative importance of adipocyte-derived miRNAs in health and cardiovascular disease states.
Disclosure Statement: The authors have nothing to disclose.
Conflict of interest: none declared.
Funding
This work was supported by the National Institutes of Health (HL115141, HL117994, HL134849, and GM115605 to M.W.F.), the Arthur K. Watson Charitable Trust (to M.W.F.), the Ralph & Marian Falk Medical Research Trust (to M.W.F.), American Diabetes Association grant (#1-16-JDF-046 to B.I.) and Watkins Discovery Award (to B.I.).
References
- 1. Nolan CJ, Ruderman NB, Kahn SE, Pedersen O, Prentki M.. Insulin resistance as a physiological defense against metabolic stress: implications for the management of subsets of type 2 diabetes. Diabetes 2015;64:673–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fontana L, Partridge L, Longo VD.. Extending healthy life span–from yeast to humans. Science 2010;328:321–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Templeman NM, Skovso S, Page MM, Lim GE, Johnson JD.. A causal role for hyperinsulinemia in obesity. J Endocrinol 2017;232:R173–R183. [DOI] [PubMed] [Google Scholar]
- 4. Zierath JR, Krook A, Wallberg-Henriksson H.. Insulin action and insulin resistance in human skeletal muscle. Diabetologia 2000;43:821–835. [DOI] [PubMed] [Google Scholar]
- 5. Rieser P. Tryptophan residues may be at the insulin receptor site in muscle. Life Sci 1967;6:1269–1275. [DOI] [PubMed] [Google Scholar]
- 6. Freychet P, Roth J, Neville DM.. Insulin receptors in the liver: specific binding of [(125)I]insulin to the plasma membrane and its relation to insulin bioactivity. Proc Natl Acad Sci USA 1971;68:1833–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am 2004;88:787–835. [DOI] [PubMed] [Google Scholar]
- 8. Zenni GC, McLane MP, Law WR, Raymond RM.. Hepatic insulin resistance during chronic hyperdynamic sepsis. Circ Shock 1992;37:198–208. [PubMed] [Google Scholar]
- 9. Reynisdottir S, Ellerfeldt K, Wahrenberg H, Lithell H, Arner P.. Multiple lipolysis defects in the insulin resistance (metabolic) syndrome. J Clin Invest 1994;93:2590–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iwatsuka H, Shino A.. Studies on diabetogenic action of obesity in mice congenital insulin resistance of KK mice. Endocrinol Jpn 1970;17:535–540. [DOI] [PubMed] [Google Scholar]
- 11. Duong M, Petit JM, Piroth L, Grappin M, Buisson M, Chavanet P, Hillon P, Portier H.. Association between insulin resistance and hepatitis C virus chronic infection in HIV-hepatitis C virus-coinfected patients undergoing antiretroviral therapy. J Acquir Immune Defic Syndr 2001;27:245–250. [DOI] [PubMed] [Google Scholar]
- 12. Mlinar B, Marc J, Janež A, Pfeifer M.. Molecular mechanisms of insulin resistance and associated diseases. Clin Chim Acta 2007;375:20–35. [DOI] [PubMed] [Google Scholar]
- 13. Tewari N, Awad S, Macdonald IA, Lobo DN.. Obesity-related insulin resistance: implications for the surgical patient. Int J Obes 2015;39:1575–1588. [DOI] [PubMed] [Google Scholar]
- 14. Kahn BB, Flier JS.. Obesity and insulin resistance. J Clin Invest 2000;106:473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sethi JK, Vidal-Puig AJ.. Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res 2007;48:1253–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Michalakis K, Mintziori G, Kaprara A, Tarlatzis BC, Goulis DG.. The complex interaction between obesity, metabolic syndrome and reproductive axis: a narrative review. Metabolism 2013;62:457–478. [DOI] [PubMed] [Google Scholar]
- 17. Rosen ED, Spiegelman BM.. What we talk about when we talk about fat. Cell 2014;156:20–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lim GE, Albrecht T, Piske M, Sarai K, Lee JT, Ramshaw HS, Sinha S, Guthridge MA, Acker-Palmer A, Lopez AF, Clee SM, Nislow C, Johnson JD.. 14-3-3zeta coordinates adipogenesis of visceral fat. Nat Comms 2015;6:7671.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Berry R, Jeffery E, Rodeheffer MS.. Weighing in on adipocyte precursors. Cell Metab 2014;19:8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Softic S, Boucher J, Solheim MH, Fujisaka S, Haering MF, Homan EP, Winnay J, Perez-Atayde AR, Kahn CR.. Lipodystrophy due to adipose tissue-specific insulin receptor knockout results in progressive NAFLD. Diabetes 2016;65:2187–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boucher J, Mori MA, Lee KY, Smyth G, Liew CW, Macotela Y, Rourk M, Bluher M, Russell SJ, Kahn CR.. Impaired thermogenesis and adipose tissue development in mice with fat-specific disruption of insulin and IGF-1 signalling. Nat Comms 2012;3:902.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Enerback S. Human brown adipose tissue. Cell Metab 2010;11:248–252. [DOI] [PubMed] [Google Scholar]
- 23. Li XH, Gong QM, Ling Y, Huang C, Yu DM, Gu LL, Liao XW, Zhang DH, Hu XQ, Han Y, Kong XF, Zhang XX.. Inherent lipid metabolic dysfunction in glycogen storage disease IIIa. Biochem Biophys Res Commun 2014;455:90–97. [DOI] [PubMed] [Google Scholar]
- 24. Ambros V. microRNAs: tiny regulators with great potential. Cell 2001;107:823–826. [DOI] [PubMed] [Google Scholar]
- 25. Icli B, Nabzdyk CS, Lujan-Hernandez J, Cahill M, Auster ME, Wara AK, Sun X, Ozdemir D, Giatsidis G, Orgill DP, Feinberg MW.. Regulation of impaired angiogenesis in diabetic dermal wound healing by microRNA-26a. J Mol Cell Cardiol 2016;91:151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Icli B, Wara AK, Moslehi J, Sun X, Plovie E, Cahill M, Marchini JF, Schissler A, Padera RF, Shi J, Cheng HW, Raghuram S, Arany Z, Liao R, Croce K, MacRae C, Feinberg MW.. MicroRNA-26a regulates pathological and physiological angiogenesis by targeting BMP/SMAD1 signaling. Circ Res 2013;113:1231–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sun X, He S, Wara AK, Icli B, Shvartz E, Tesmenitsky Y, Belkin N, Li D, Blackwell TS, Sukhova GK, Croce K, Feinberg MW.. Systemic delivery of microRNA-181b inhibits nuclear factor-kappaB activation, vascular inflammation, and atherosclerosis in apolipoprotein E-deficient mice. Circ Res 2014;114:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lin J, He S, Sun X, Franck G, Deng Y, Yang D, Haemmig S, Wara AK, Icli B, Li D, Feinberg MW.. MicroRNA-181b inhibits thrombin-mediated endothelial activation and arterial thrombosis by targeting caspase recruitment domain family member 10. Faseb J 2016;30:3216–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang Y, Sun X, Icli B, Feinberg MW.. Emerging roles for microRNAs in diabetic microvascular disease–novel targets therapy. Endocr Rev 2017;2017:1–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee Y, Jeon K, Lee JT, Kim S, Kim VN.. MicroRNA maturation: stepwise processing and subcellular localization. EMBO J 2002;21:4663–4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol 2005;6:376–385. [DOI] [PubMed] [Google Scholar]
- 32. Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Radmark O, Kim S, Kim VN.. The nuclear RNase III Drosha initiates microRNA processing. Nature 2003;425:415–419. [DOI] [PubMed] [Google Scholar]
- 33. Gregory RI, Yan K-P, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, Shiekhattar R.. The microprocessor complex mediates the genesis of microRNAs. Nature 2004;432:235–240. [DOI] [PubMed] [Google Scholar]
- 34. Denli AM, Tops BBJ, Plasterk RHA, Ketting RF, Hannon GJ.. Processing of primary microRNAs by the Microprocessor complex. Nature 2004;432:231–235. [DOI] [PubMed] [Google Scholar]
- 35. Han J, Pedersen JS, Kwon SC, Belair CD, Kim Y-K, Yeom K-H, Yang W-Y, Haussler D, Blelloch R, Kim VN.. Posttranscriptional crossregulation between Drosha and DGCR8. Cell 2009;136:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yeom K-H, Lee Y, Han J, Suh MR, Kim VN.. Characterization of DGCR8/Pasha, the essential cofactor for Drosha in primary miRNA processing. Nucleic Acids Res 2006;34:4622–4629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kadener S, Rodriguez J, Abruzzi KC, Khodor YL, Sugino K, Marr MT, Nelson S, Rosbash M.. Genome-wide identification of targets of the drosha–pasha/DGCR8 complex. RNA 2009;15:537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lund E, Güttinger S, Calado A, Dahlberg JE, Kutay U.. Nuclear export of microRNA precursors. Science 2004;303:95–98. [DOI] [PubMed] [Google Scholar]
- 39. Zeng Y, Cullen BR.. Structural requirements for pre-microRNA binding and nuclear export by Exportin 5. Nucleic Acids Res 2004;32:4776–4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hutvagner G, McLachlan J, Pasquinelli AE, Balint E, Tuschl T, Zamore PD.. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science 2001;293:834–838. [DOI] [PubMed] [Google Scholar]
- 41. Grishok A, Pasquinelli AE, Conte D, Li N, Parrish S, Ha I, Baillie DL, Fire A, Ruvkun G, Mello CC.. Genes and mechanisms related to RNA interference regulate expression of the small temporal RNAs that control C. elegans developmental timing. Cell 2001;106:23–34. [DOI] [PubMed] [Google Scholar]
- 42. Ketting RF, Fischer SE, Bernstein E, Sijen T, Hannon GJ, Plasterk RH.. Dicer functions in RNA interference and in synthesis of small RNA involved in developmental timing in C. elegans. Genes Dev 2001;15:2654–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang H, Kolb FA, Jaskiewicz L, Westhof E, Filipowicz W.. Single processing center models for human Dicer and bacterial RNase III. Cell 2004;118:57–68. [DOI] [PubMed] [Google Scholar]
- 44. Carthew RW, Sontheimer EJ.. Origins and mechanisms of miRNAs and siRNAs. Cell 2009;136:642–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Finnegan EF, Pasquinelli AE.. MicroRNA biogenesis: regulating the regulators. Crit Rev Biochem Mol Biol 2013;48:51–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rawal S, Manning P, Katare R.. Cardiovascular microRNAs: as modulators and diagnostic biomarkers of diabetic heart disease. Cardiovasc Diabetol 2014;13:44. doi: 10.1186/1475-2840-1113-1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zeng Y, Wagner EJ, Cullen BR.. Both natural and designed microRNAs can inhibit the expression of cognate mRNAs when expressed in human cells. Mol Cell 2002;9:1327–1333. [DOI] [PubMed] [Google Scholar]
- 48. Zeng Y, Yi R, Cullen BR.. MicroRNAs and small interfering RNAs can inhibit mRNA expression by similar mechanisms. Proc Natl Acad Sci USA 2003;100:9779–9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM.. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature 2005;433:769–773. [DOI] [PubMed] [Google Scholar]
- 50. Bartel DP. MicroRNA target recognition and regulatory functions. Cell 2009;136:215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Brennecke J, Stark A, Russell RB, Cohen SM.. Principles of microRNA–target recognition. PLoS Biol 2005;3:e85.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lewis BP, Burge CB, Bartel DP.. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 2005;120:15–20. [DOI] [PubMed] [Google Scholar]
- 53. Wheeler BM, Heimberg AM, Moy VN, Sperling EA, Holstein TW, Heber S, Peterson KJ.. The deep evolution of metazoan microRNAs. Evol Dev 2009;11:50–68. [DOI] [PubMed] [Google Scholar]
- 54. Meister G, Tuschl T.. Mechanisms of gene silencing by double-stranded RNA. Nature 2004;431:343–349. [DOI] [PubMed] [Google Scholar]
- 55. Martinez J, Patkaniowska A, Urlaub H, Lührmann R, Tuschl T.. Single-stranded antisense siRNAs guide target RNA cleavage in RNAi. Cell 2002;110:563–574. [DOI] [PubMed] [Google Scholar]
- 56. Yekta S, Shih I-h, Bartel DP.. MicroRNA-directed cleavage of HOXB8 mRNA. Science 2004;304:594–596. [DOI] [PubMed] [Google Scholar]
- 57. Wakiyama M, Takimoto K, Ohara O, Yokoyama S.. Let-7 microRNA-mediated mRNA deadenylation and translational repression in a mammalian cell-free system. Genes Dev 2007;21:1857–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rehwinkel J, Behm-Ansmant I, Gatfield D, Izaurralde E.. A crucial role for GW182 and the DCP1:DCP2 decapping complex in miRNA-mediated gene silencing. RNA 2005;11:1640–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Behm-Ansmant I, Rehwinkel J, Doerks T, Stark A, Bork P, Izaurralde E.. mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Genes Dev 2006;20:1885–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Wu L, Fan J, Belasco JG.. MicroRNAs direct rapid deadenylation of mRNA. Proc Natl Acad Sci USA 2006;103:4034–4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rosen ED, Spiegelman BM.. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 2006;444:847–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rajala MW, Scherer PE.. Minireview: The adipocyte–at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology 2003;144:3765–3773. [DOI] [PubMed] [Google Scholar]
- 63. Hall AM, Kou K, Chen Z, Pietka TA, Kumar M, Korenblat KM, Lee K, Ahn K, Fabbrini E, Klein S, Goodwin B, Finck BN.. Evidence for regulated monoacylglycerol acyltransferase expression and activity in human liver. J Lipid Res 2012;53:990–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hamdy O, Porramatikul S, Al-Ozairi E.. Metabolic obesity: the paradox between visceral and subcutaneous fat. CDR 2006;2:367–373. [DOI] [PubMed] [Google Scholar]
- 65. Garg A. Regional Adiposity and Insulin Resistance. J Clin Endocrinol Metab 2004;89:4206–4210. [DOI] [PubMed] [Google Scholar]
- 66. Patel P, Abate N.. Body fat distribution and insulin resistance. Nutrients 2013;5:2019–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006;444:860–867. [DOI] [PubMed] [Google Scholar]
- 68. Faria AN, Ribeiro Filho FF, Gouveia Ferreira SR, Zanella MT.. Impact of visceral fat on blood pressure and insulin sensitivity in hypertensive obese women. Obes Res 2002;10:1203–1206. [DOI] [PubMed] [Google Scholar]
- 69. Chandra A, Neeland IJ, Berry JD, Ayers CR, Rohatgi A, Das SR, Khera A, McGuire DK, de Lemos JA, Turer AT.. The relationship of body mass and fat distribution with incident hypertension: observations from the Dallas Heart Study. J Am Coll Cardiol 2014;64:997–1002. [DOI] [PubMed] [Google Scholar]
- 70. Kanneganti TD, Dixit VD.. Immunological complications of obesity. Nat Immunol 2012;13:707–712. [DOI] [PubMed] [Google Scholar]
- 71. Oskowitz AZ, Lu J, Penfornis P, Ylostalo J, McBride J, Flemington EK, Prockop DJ, Pochampally R.. Human multipotent stromal cells from bone marrow and microRNA: regulation of differentiation and leukemia inhibitory factor expression. Proc Natl Acad Sci USA 2008;105:18372–18377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang Q, Li YC, Wang J, Kong J, Qi Y, Quigg RJ, Li X.. miR-17-92 cluster accelerates adipocyte differentiation by negatively regulating tumor-suppressor Rb2/p130. Proc Natl Acad Sci USA 2008;105:2889–2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mudhasani R, Puri V, Hoover K, Czech MP, Imbalzano AN, Jones SN.. Dicer is required for the formation of white but not brown adipose tissue. J Cell Physiol 2011;226:1399–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kim HJ, Cho H, Alexander R, Patterson HC, Gu M, Lo KA, Xu D, Goh VJ, Nguyen LN, Chai X, Huang CX, Kovalik JP, Ghosh S, Trajkovski M, Silver DL, Lodish H, Sun L.. MicroRNAs are required for the feature maintenance and differentiation of brown adipocytes. Diabetes 2014;63:4045–4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gu P, Xu A.. Interplay between adipose tissue and blood vessels in obesity and vascular dysfunction. Rev Endocr Metab Disord 2013;14:49–58. [DOI] [PubMed] [Google Scholar]
- 76. Heilbronn LK, Campbell LV.. Adipose tissue macrophages, low grade inflammation and insulin resistance in human obesity. CPD 2008;14:1225–1230. [DOI] [PubMed] [Google Scholar]
- 77. Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS.. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 2005;46:2347–2355. [DOI] [PubMed] [Google Scholar]
- 78. Tsiloulis T, Pike J, Powell D, Rossello FJ, Canny BJ, Meex RC, Watt MJ.. Impact of endurance exercise training on adipocyte microRNA expression in overweight men. Faseb J 2017;31:161–171. [DOI] [PubMed] [Google Scholar]
- 79. Ross R, Harker L.. Hyperlipidemia and atherosclerosis. Science 1976;193:1094–1100. [DOI] [PubMed] [Google Scholar]
- 80. Steinberg D. Thematic review series: the pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy, part V: the discovery of the statins and the end of the controversy. J Lipid Res 2006;47:1339–1351. [DOI] [PubMed] [Google Scholar]
- 81. Berg AH, Scherer PE.. Adipose tissue, inflammation, and cardiovascular disease. Circ Res 2005;96:939–949. [DOI] [PubMed] [Google Scholar]
- 82. Paul A, Ko KW, Li L, Yechoor V, McCrory MA, Szalai AJ, Chan L.. C-reactive protein accelerates the progression of atherosclerosis in apolipoprotein E-deficient mice. Circulation 2004;109:647–655. [DOI] [PubMed] [Google Scholar]
- 83. Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation 2003;107:363–369. [DOI] [PubMed] [Google Scholar]
- 84. Shimomura I, Funahasm T, Takahashi M, Maeda K, Kotani K, Nakamura T, Yamashita S, Miura M, Fukuda Y, Takemura K, Tokunaga K, Matsuzawa Y.. Enhanced expression of PAI-1 in visceral fat: possible contributor to vascular disease in obesity. Nat Med 1996;2:800–803. [DOI] [PubMed] [Google Scholar]
- 85. Kern PA, Ranganathan S, Li C, Wood L, Ranganathan G.. Adipose tissue tumor necrosis factor and interleukin-6 expression in human obesity and insulin resistance. Am J Physiol Endocrinol Metab 2001;280:E745–E751. [DOI] [PubMed] [Google Scholar]
- 86. Rocha VZ, Libby P.. Obesity, inflammation, and atherosclerosis. Nat Rev Cardiol 2009;6:399–409. [DOI] [PubMed] [Google Scholar]
- 87. Xie H, Lim B, Lodish HF.. MicroRNAs induced during adipogenesis that accelerate fat cell development are downregulated in obesity. Diabetes 2009;58:1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Moraes RC, Blondet A, Birkenkamp-Demtroeder K, Tirard J, Orntoft TF, Gertler A, Durand P, Naville D, Bégeot M.. Study of the alteration of gene expression in adipose tissue of diet-induced obese mice by microarray and reverse transcription-polymerase chain reaction analyses. Endocrinology 2003;144:4773–4782. [DOI] [PubMed] [Google Scholar]
- 89. Soukas A, Cohen P, Socci ND, Friedman JM.. Leptin-specific patterns of gene expression in white adipose tissue. Genes Dev 2000;14:963–980. [PMC free article] [PubMed] [Google Scholar]
- 90. Ahn J, Lee H, Jung CH, Jeon TI, Ha TY.. MicroRNA-146b promotes adipogenesis by suppressing the SIRT1-FOXO1 cascade. EMBO Mol Med 2013;5:1602–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Shi C, Zhang M, Tong M, Yang L, Pang L, Chen L, Xu G, Chi X, Hong Q, Ni Y, Ji C, Guo X.. miR-148a is Associated with Obesity and Modulates Adipocyte Differentiation of Mesenchymal Stem Cells through Wnt Signaling. Sci Rep 2015;5:9930.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Price NL, Holtrup B, Kwei SL, Wabitsch M, Rodeheffer M, Bianchini L, Suarez Y, Fernandez-Hernando C.. SREBP-1c/microRNA 33b genomic loci control adipocyte differentiation. Mol Cell Biol 2016;36:1180–1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kong L, Zhu J, Han W, Jiang X, Xu M, Zhao Y, Dong Q, Pang Z, Guan Q, Gao L, Zhao J, Zhao L.. Significance of serum microRNAs in pre-diabetes and newly diagnosed type 2 diabetes: a clinical study. Acta Diabetol 2011;48:61–69. [DOI] [PubMed] [Google Scholar]
- 94. Ortega FJ, Moreno-Navarrete JM, Pardo G, Sabater M, Hummel M, Ferrer A, Rodriguez-Hermosa JI, Ruiz B, Ricart W, Peral B, Fernández-Real JM, Wang Y.. MiRNA expression profile of human subcutaneous adipose and during adipocyte differentiation. PLoS ONE 2010;5:e9022.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lavery CA, Kurowska-Stolarska M, Holmes WM, Donnelly I, Caslake M, Collier A, Baker AH, Miller AM.. miR-34a(-/-) mice are susceptible to diet-induced obesity. Obesity (Silver Spring) 2016;24:1741–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Rockstroh D, Loffler D, Kiess W, Landgraf K, Korner A.. Regulation of human adipogenesis by miR125b-5p. Adipocyte 2016;5:283–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Tao C, Ren H, Xu P, Cheng J, Huang S, Zhou R, Mu Y, Yang S, Qi D, Wang Y, Li K.. Adipocyte miR-200b/a/429 ablation in mice leads to high-fat-diet-induced obesity. Oncotarget 2016;7:67796–67807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Karkeni E, Astier J, Tourniaire F, El Abed M, Romier B, Gouranton E, Wan L, Borel P, Salles J, Walrand S, Ye J, Landrier JF.. Obesity-associated inflammation induces microRNA-155 expression in adipocytes and adipose tissue: outcome on adipocyte function. J Clin Endocrinol Metab 2016;101:1615–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Gaudet AD, Fonken LK, Gushchina LV, Aubrecht TG, Maurya SK, Periasamy M, Nelson RJ, Popovich PG.. miR-155 deletion in female mice prevents diet-induced obesity. Sci Rep 2016;6:22862.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Miller AM, Gilchrist DS, Nijjar J, Araldi E, Ramirez CM, Lavery CA, Fernández-Hernando C, McInnes IB, Kurowska-Stolarska M, Federici M.. MiR-155 has a protective role in the development of non-alcoholic hepatosteatosis in mice. PLoS One 2013;8:e72324.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Even SE, Dulak-Lis MG, Touyz RM, Nguyen Dinh Cat A.. Crosstalk between adipose tissue and blood vessels in cardiometabolic syndrome: implication of steroid hormone receptors (MR/GR). Horm Mol Biol Clin Investig 2014;19:89–101. [DOI] [PubMed] [Google Scholar]
- 102. Sun X, Lin J, Zhang Y, Kang S, Belkin N, Wara AK, Icli B, Hamburg NM, Li D, Feinberg MW.. MicroRNA-181b improves glucose homeostasis and insulin sensitivity by regulating endothelial function in white adipose tissue. Circ Res 2016;118:810–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sun X, Icli B, Wara AK, Belkin N, He S, Kobzik L, Hunninghake GM, Vera MP, Registry M, Blackwell TS, Baron RM, Feinberg MW.. MicroRNA-181b regulates NF-kappaB-mediated vascular inflammation. J Clin Invest 2012;122:1973–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sacks HS, Fain JN, Bahouth SW, Ojha S, Frontini A, Budge H, Cinti S, Symonds ME.. Adult epicardial fat exhibits beige features. J Clin Endocrinol Metab 2013;98:E1448–E1455. [DOI] [PubMed] [Google Scholar]
- 105. Pierdomenico SD, Pierdomenico AM, Cuccurullo F, Iacobellis G.. Meta-analysis of the relation of echocardiographic epicardial adipose tissue thickness and the metabolic syndrome. Am J Cardiol 2013;111:73–78. [DOI] [PubMed] [Google Scholar]
- 106. Mazurek T, Kiliszek M, Kobylecka M, Skubisz-Głuchowska J, Kochman J, Filipiak K, Królicki L, Opolski G.. Relation of proinflammatory activity of epicardial adipose tissue to the occurrence of atrial fibrillation. Am J Cardiol 2014;113:1505–1508. [DOI] [PubMed] [Google Scholar]
- 107. Mazurek T, Zhang L, Zalewski A, Mannion JD, Diehl JT, Arafat H, Sarov-Blat L, O'brien S, Keiper EA, Johnson AG, Martin J, Goldstein BJ, Shi Y.. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003;108:2460–2466. [DOI] [PubMed] [Google Scholar]
- 108. Venteclef N, Guglielmi V, Balse E, Gaborit B, Cotillard A, Atassi F, Amour J, Leprince P, Dutour A, Clement K, Hatem SN.. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur Heart J 2013;36:795–805. [DOI] [PubMed] [Google Scholar]
- 109. Vacca M, Di Eusanio M, Cariello M, Graziano G, D'amore S, Petridis FD, D'orazio A, Salvatore L, Tamburro A, Folesani G, Rutigliano D, Pellegrini F, Sabba C, Palasciano G, Di Bartolomeo R, Moschetta A.. Integrative miRNA and whole-genome analyses of epicardial adipose tissue in patients with coronary atherosclerosis. Cardiovasc Res 2016;109:228–239. [DOI] [PubMed] [Google Scholar]
- 110. Ocłoń E, Latacz A, Zubel–Łojek J, Pierzchała–Koziec K.. Hyperglycemia-induced changes in miRNA expression patterns in epicardial adipose tissue of piglets. J Endocrinol 2016;229:259–266. [DOI] [PubMed] [Google Scholar]
- 111. Kraakman MJ, Murphy AJ, Jandeleit-Dahm K, Kammoun HL.. Macrophage polarization in obesity and type 2 diabetes: weighing down our understanding of macrophage function? Front Immunol 2014;5:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Chang R, Ying W, Bazer F, Zhou B.. MicroRNAs control macrophage formation and activation: the inflammatory link between obesity and cardiovascular diseases. Cells 2014;3:702–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Stöhr R, Federici M.. Insulin resistance and atherosclerosis: convergence between metabolic pathways and inflammatory nodes. Biochem J 2013;454:1–11. [DOI] [PubMed] [Google Scholar]
- 114. Tian F-J, An L-N, Wang G-K, Zhu J-Q, Li Q, Zhang Y-Y, Zeng A, Zou J, Zhu R-F, Han X-S, Shen N, Yang H-T, Zhao X-X, Huang S, Qin Y-W, Jing Q.. Elevated microRNA-155 promotes foam cell formation by targeting HBP1 in atherogenesis. Cardiovasc Res 2014;103:100–110. [DOI] [PubMed] [Google Scholar]
- 115. Virtue A, Johnson C, Lopez-Pastrana J, Shao Y, Fu H, Li X, Li YF, Yin Y, Mai J, Rizzo V, Tordoff M, Bagi Z, Shan H, Jiang X, Wang H, Yang XF.. MicroRNA-155 deficiency leads to decreased atherosclerosis, increased white adipose tissue obesity, and non-alcoholic fatty liver disease: A NOVEL MOUSE MODEL OF OBESITY PARADOX. J Biol Chem 2017;292:1267–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ramírez CM, Rotllan N, Vlassov AV, Dávalos A, Li M, Goedeke L, Aranda JF, Cirera-Salinas D, Araldi E, Salerno A, Wanschel A, Zavadil J, Castrillo A, Kim J, Suárez Y, Fernández-Hernando C.. Control of cholesterol metabolism and plasma high-density lipoprotein levels by microRNA-144. Circ Res 2013;112:1592–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. de Aguiar Vallim TQ, Tarling EJ, Kim T, Civelek M, Baldan A, Esau C, Edwards PA.. MicroRNA-144 regulates hepatic ATP binding cassette transporter A1 and plasma high-density lipoprotein after activation of the nuclear receptor farnesoid X receptor. Circ Res 2013;112:1602–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Chawla A, Boisvert WA, Lee C-H, Laffitte BA, Barak Y, Joseph SB, Liao D, Nagy L, Edwards PA, Curtiss LK, Evans RM, Tontonoz P.. A PPARγ-LXR-ABCA1 pathway in macrophages is involved in cholesterol efflux and atherogenesis. Mol Cell 2001;7:161–171. [DOI] [PubMed] [Google Scholar]
- 119. Laffitte BA, Repa JJ, Joseph SB, Wilpitz DC, Kast HR, Mangelsdorf DJ, Tontonoz P.. LXRs control lipid-inducible expression of the apolipoprotein E gene in macrophages and adipocytes. Proc Natl Acad Sci USA 2001;98:507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Song C, Hiipakka RA, Liao S.. Auto-oxidized cholesterol sulfates are antagonistic ligands of liver X receptors: implications for the development and treatment of atherosclerosis. Steroids 2001;66:473–479. [DOI] [PubMed] [Google Scholar]
- 121. Vinod M, Chennamsetty I, Colin S, Belloy L, De Paoli F, Schaider H, Graier WF, Frank S, Kratky D, Staels B, Chinetti-Gbaguidi G, Kostner GM.. miR-206 controls LXRalpha expression and promotes LXR-mediated cholesterol efflux in macrophages. Biochim Biophys Acta 2014;1841:827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Bartness TJ, Vaughan CH, Song CK.. Sympathetic and sensory innervation of brown adipose tissue. Int J Obes Relat Metab Disord 2010;34:S36–S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Betz MJ, Enerback S.. Human brown adipose tissue: what we have learned so far. Diabetes 2015;64:2352–2360. [DOI] [PubMed] [Google Scholar]
- 124. Trajkovski M, Ahmed K, Esau CC, Stoffel M.. MyomiR-133 regulates brown fat differentiation through Prdm16. Nat Cell Biol 2012;14:1330–1335. [DOI] [PubMed] [Google Scholar]
- 125. Pan D, Mao C, Quattrochi B, Friedline RH, Zhu LJ, Jung DY, Kim JK, Lewis B, Wang YX.. MicroRNA-378 controls classical brown fat expansion to counteract obesity. Nat Comms 2014;5:4725.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Chen Y, Siegel F, Kipschull S, Haas B, Frohlich H, Meister G, Pfeifer A.. miR-155 regulates differentiation of brown and beige adipocytes via a bistable circuit. Nat Comms 2013;4:1769.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Zhang H, Guan M, Townsend KL, Huang TL, An D, Yan X, Xue R, Schulz TJ, Winnay J, Mori M, Hirshman MF, Kristiansen K, Tsang JS, White AP, Cypess AM, Goodyear LJ, Tseng YH.. MicroRNA-455 regulates brown adipogenesis via a novel HIF1an-AMPK-PGC1alpha signaling network. EMBO Rep 2015;16:1378–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Karbiener M, Pisani DF, Frontini A, Oberreiter LM, Lang E, Vegiopoulos A, Mossenbock K, Bernhardt GA, Mayr T, Hildner F, Grillari J, Ailhaud G, Herzig S, Cinti S, Amri EZ, Scheideler M.. MicroRNA-26 family is required for human adipogenesis and drives characteristics of brown adipocytes. Stem Cells 2014;32:1578–1590. [DOI] [PubMed] [Google Scholar]
- 129. Fu T, Seok S, Choi S, Huang Z, Suino-Powell K, Xu HE, Kemper B, Kemper JK.. MicroRNA 34a inhibits beige and brown fat formation in obesity in part by suppressing adipocyte fibroblast growth factor 21 signaling and SIRT1 function. Mol Cell Biol 2014;34:4130–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Thoonen R, Hindle AG, Scherrer-Crosbie M.. Brown adipose tissue: The heat is on the heart. Am J Physiol Heart Circ Physiol 2016;310:H1592–H1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Dong M, Yang X, Lim S, Cao Z, Honek J, Lu H, Zhang C, Seki T, Hosaka K, Wahlberg E, Yang J, Zhang L, Lanne T, Sun B, Li X, Liu Y, Zhang Y, Cao Y.. Cold exposure promotes atherosclerotic plaque growth and instability via UCP1-dependent lipolysis. Cell Metab 2013;18:118–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Liu W, Bi P, Shan T, Yang X, Yin H, Wang Y-X, Liu N, Rudnicki MA, Kuang S, Kajimura S.. miR-133a regulates adipocyte browning in vivo. PLoS Genet 2013;9:e1003626.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Sun L, Xie H, Mori MA, Alexander R, Yuan B, Hattangadi SM, Liu Q, Kahn CR, Lodish HF.. Mir193b-365 is essential for brown fat differentiation. Nat Cell Biol 2011;13:958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Tsuchida A, Yamauchi T, Takekawa S, Hada Y, Ito Y, Maki T, Kadowaki T.. Peroxisome proliferator-activated receptor (PPAR)alpha activation increases adiponectin receptors and reduces obesity-related inflammation in adipose tissue: comparison of activation of PPARalpha, PPARgamma, and their combination. Diabetes 2005;54:3358–3370. [DOI] [PubMed] [Google Scholar]
- 135. Oliverio M, Schmidt E, Mauer J, Baitzel C, Hansmeier N, Khani S, Konieczka S, Pradas-Juni M, Brodesser S, Van T-M, Bartsch D, Brönneke HS, Heine M, Hilpert H, Tarcitano E, Garinis GA, Frommolt P, Heeren J, Mori MA, Brüning JC, Kornfeld J-W.. Dicer1-miR-328-Bace1 signalling controls brown adipose tissue differentiation and function. Nat Cell Biol 2016;18:328–336. [DOI] [PubMed] [Google Scholar]
- 136. Rosenwald M, Perdikari A, Rulicke T, Wolfrum C.. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol 2013;15:659–667. [DOI] [PubMed] [Google Scholar]
- 137. Ohno H, Shinoda K, Spiegelman BM, Kajimura S.. PPARgamma agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab 2012;15:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, Spiegelman BM.. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev 2012;26:271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Rosell M, Kaforou M, Frontini A, Okolo A, Chan YW, Nikolopoulou E, Millership S, Fenech ME, MacIntyre D, Turner JO, Moore JD, Blackburn E, Gullick WJ, Cinti S, Montana G, Parker MG, Christian M.. Brown and white adipose tissues: intrinsic differences in gene expression and response to cold exposure in mice. Am J Physiol Endocrinol Metab 2014;306:E945–E964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Mossenbock K, Vegiopoulos A, Rose AJ, Sijmonsma TP, Herzig S, Schafmeier T.. Browning of white adipose tissue uncouples glucose uptake from insulin signaling. PLoS ONE 2014;9:e110428.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Lee YH, Petkova AP, Mottillo EP, Granneman JG.. In vivo identification of bipotential adipocyte progenitors recruited by beta3-adrenoceptor activation and high-fat feeding. Cell Metab 2012;15:480–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Wang QA, Tao C, Gupta RK, Scherer PE.. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 2013;19:1338–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Giroud M, Karbiener M, Pisani DF, Ghandour RA, Beranger GE, Niemi T, Taittonen M, Nuutila P, Virtanen KA, Langin D, Scheideler M, Amri EZ.. Let-7i-5p represses brite adipocyte function in mice and humans. Sci Rep 2016;6:28613.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Guan XM, Amend A, Strader CD.. Determination of structural domains for G protein coupling and ligand binding in beta 3-adrenergic receptor. Mol Pharmacol 1995;48:492–498. [PubMed] [Google Scholar]
- 145. Lindquist JM, Fredriksson JM, Rehnmark S, Cannon B, Nedergaard J.. Beta 3- and alpha1-adrenergic Erk1/2 activation is Src- but not Gi-mediated in Brown adipocytes. J Biol Chem 2000;275:22670–22677. [DOI] [PubMed] [Google Scholar]
- 146. Collins S. beta-adrenoceptor signaling networks in adipocytes for recruiting stored fat and energy expenditure. Front Endocrinol (Lausanne) 2011;2:102.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Giroud M, Pisani DF, Karbiener M, Barquissau V, Ghandour RA, Tews D, Fischer-Posovszky P, Chambard JC, Knippschild U, Niemi T, Taittonen M, Nuutila P, Wabitsch M, Herzig S, Virtanen KA, Langin D, Scheideler M, Amri EZ.. miR-125b affects mitochondrial biogenesis and impairs brite adipocyte formation and function. Mol Metab 2016;5:615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Hu F, Wang M, Xiao T, Yin B, He L, Meng W, Dong M, Liu F.. miR-30 promotes thermogenesis and the development of beige fat by targeting RIP140. Diabetes 2015;64:2056–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Thomou T, Mori MA, Dreyfuss JM, Konishi M, Sakaguchi M, Wolfrum C, Rao TN, Winnay JN, Garcia-Martin R, Grinspoon SK, Gorden P, Kahn CR.. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 2017;542:450–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Chen Y, Buyel JJ, Hanssen MJ, Siegel F, Pan R, Naumann J, Schell M, van der Lans A, Schlein C, Froehlich H, Heeren J, Virtanen KA, van Marken Lichtenbelt W, Pfeifer A.. Exosomal microRNA miR-92a concentration in serum reflects human brown fat activity. Nat Comms 2016;7:11420.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Iacomino G, Russo P, Stillitano I, Lauria F, Marena P, Ahrens W, De Luca P, Siani A.. Circulating microRNAs are deregulated in overweight/obese children: preliminary results of the I.Family study. Genes Nutr 2016;11:7.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Prats-Puig A, Ortega FJ, Mercader JM, Moreno-Navarrete JM, Moreno M, Bonet N, Ricart W, López-Bermejo A, Fernández-Real JM.. Changes in circulating microRNAs are associated with childhood obesity. J Clin Endocrinol Metab 2013;98:E1655–16E1660. [DOI] [PubMed] [Google Scholar]
- 153. Broderick JA, Zamore PD.. MicroRNA therapeutics. Gene Ther 2011;18:1104–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Feinberg MW, Moore KJ.. MicroRNA regulation of atherosclerosis. Circ Res 2016;118:703–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Andreou I, Sun X, Stone PH, Edelman ER, Feinberg MW.. miRNAs in atherosclerotic plaque initiation, progression, and rupture. Trends Mol Med 2015;21:307–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Grunweller A, Hartmann RK.. Locked nucleic acid oligonucleotides: the next generation of antisense agents? BioDrugs 2007;21:235–243. [DOI] [PubMed] [Google Scholar]
- 157. Flierl U, Nero TL, Lim B, Arthur JF, Yao Y, Jung SM, Gitz E, Pollitt AY, Zaldivia MT, Jandrot-Perrus M, Schafer A, Nieswandt B, Andrews RK, Parker MW, Gardiner EE, Peter K.. Phosphorothioate backbone modifications of nucleotide-based drugs are potent platelet activators. J Exp Med 2015;212:129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Stenvang J, Petri A, Lindow M, Obad S, Kauppinen S.. Inhibition of microRNA function by antimiR oligonucleotides. Silence 2012;3:1–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Rayner KJ, Esau CC, Hussain FN, McDaniel AL, Marshall SM, van Gils JM, Ray TD, Sheedy FJ, Goedeke L, Liu X, Khatsenko OG, Kaimal V, Lees CJ, Fernandez-Hernando C, Fisher EA, Temel RE, Moore KJ.. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature 2011;478:404–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Semple SC, Akinc A, Chen J, Sandhu AP, Mui BL, Cho CK, Sah DW, Stebbing D, Crosley EJ, Yaworski E, Hafez IM, Dorkin JR, Qin J, Lam K, Rajeev KG, Wong KF, Jeffs LB, Nechev L, Eisenhardt ML, Jayaraman M, Kazem M, Maier MA, Srinivasulu M, Weinstein MJ, Chen Q, Alvarez R, Barros SA, De S, Klimuk SK, Borland T, Kosovrasti V, Cantley WL, Tam YK, Manoharan M, Ciufolini MA, Tracy MA, de Fougerolles A, MacLachlan I, Cullis PR, Madden TD, Hope MJ.. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol 2010;28:172–176. [DOI] [PubMed] [Google Scholar]
- 161. Ebert MS, Neilson JR, Sharp PA.. MicroRNA sponges: competitive inhibitors of small RNAs in mammalian cells. Nat Meth 2007;4:721–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Janssen HL, Kauppinen S, Hodges MR.. HCV infection and miravirsen. N Engl J Med 2013;369:878.. [DOI] [PubMed] [Google Scholar]
- 163. Rayner KJ, Suarez Y, Davalos A, Parathath S, Fitzgerald ML, Tamehiro N, Fisher EA, Moore KJ, Fernandez-Hernando C.. MiR-33 contributes to the regulation of cholesterol homeostasis. Science 2010;328:1570–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Gentner B, Schira G, Giustacchini A, Amendola M, Brown BD, Ponzoni M, Naldini L.. Stable knockdown of microRNA in vivo by lentiviral vectors. Nat Methods 2009;6:63–66. [DOI] [PubMed] [Google Scholar]