Abstract
Exposure to drug-associated cues can induce drug craving and relapse in abstinent addicts. Cue-induced craving that progressively intensifies (“incubates”) during withdrawal from cocaine has been observed in both rats and humans. Building on recent evidence that aberrant protein translation underlies incubation-related adaptations in the NAc, we used male rats to test the hypothesis that translation is dysregulated during cocaine withdrawal and/or when rats express incubated cocaine craving. We found that intra-NAc infusion of anisomycin, a general protein translation inhibitor, or rapamycin, an inhibitor of mammalian target of rapamycin, reduced the expression of incubated cocaine craving, consistent with previous results showing that inhibition of translation in slices normalized the adaptations that maintain incubation. We then examined signaling pathways involved in protein translation using NAc synaptoneurosomes prepared after >47 d of withdrawal from cocaine or saline self-administration, or after withdrawal plus a cue-induced seeking test. The most robust changes were observed following seeking tests. Most notably, we found that eukaryotic elongation factor 2 (eEF2) and eukaryotic initiation factor 2α (eIF2α) are dephosphorylated when cocaine rats undergo a cue-induced seeking test; both effects are consistent with increased translation during the test. Blocking eIF2α dephosphorylation and thereby restoring its inhibitory influence on translation, via intra-NAc injection of Sal003 just before the test, substantially reduced cocaine seeking. These results are consistent with dysregulation of protein translation in the NAc during cocaine withdrawal, enabling cocaine cues to elicit an aberrant increase in translation that is required for the expression of incubated cocaine craving.
SIGNIFICANCE STATEMENT Cue-induced cocaine craving progressively intensifies (incubates) during withdrawal in both humans and rats. This may contribute to persistent vulnerability to relapse. We previously demonstrated a role for protein translation in synaptic adaptations in the NAc closely linked to incubation. Here, we tested the hypothesis that translation is dysregulated during cocaine withdrawal, and this contributes to incubated craving. Analysis of signaling pathways regulating translation suggested that translation is enhanced when “incubated” rats undergo a cue-induced seeking test. Furthermore, intra-NAc infusions of drugs that inhibit protein translation through different mechanisms reduced expression of incubated cue-induced cocaine seeking. These results demonstrate that the expression of incubation depends on an acute increase in translation that may result from dysregulation of several pathways.
Keywords: cocaine, eIF2α, incubation, mTOR, NAc, protein translation
Introduction
Relapse to cocaine use is often trigged by exposure to drug-associated cues that provoke craving. Cue-induced cocaine craving that progressively intensifies (incubates) over the first month of forced abstinence and then plateaus for additional months has been observed in rodents that undergo specific regimens of cocaine self-administration (Lu et al., 2004). Incubation of cue-induced cocaine craving has also been observed in human cocaine users (Parvaz et al., 2016). This phenomenon is significant because it may contribute to continued vulnerability to relapse long after drug use has been discontinued (Li et al., 2016).
Incubation of cocaine craving involves neuroadaptations in the circuitry underlying reward and motivation (Wolf, 2016). The NAc, which plays a major role in this circuitry, is comprised mainly of medium spiny neurons (MSNs) that receive inputs from cortical and limbic regions and send projections to motor circuitry to mediate motivated behaviors, including drug seeking (Sesack and Grace, 2010). It is now well established that incubation is associated with a delayed increase in high-conductance Ca2+-permeable AMPA receptors (CP-AMPARs) at excitatory synapses onto MSNs in both core and shell subregions of the NAc (Wolf, 2016). Once this occurs, CP-AMPAR activation is required for the expression of incubated craving (Conrad et al., 2008; Lee et al., 2013; Loweth et al., 2014; Ma et al., 2014; Pascoli et al., 2014). Based on these findings and evidence that CP-AMPARs enhance the baseline responsiveness of NAc MSNs to glutamate transmission (Purgianto et al., 2013), we propose that CP-AMPAR accumulation strengthens glutamate synapses onto MSNs, enabling cue-induced glutamate release to produce an augmented synaptic response, which in turns leads to intensified cocaine seeking (Wolf, 2016).
Recently, we showed that maintenance of elevated CP-AMPAR levels in the NAc core is sensitive to inhibitors of translation. Thus, in brain slices prepared from rats that had undergone incubation, pharmacological disruption of protein translation for 1 h reduced CP-AMPARs to saline control levels and also normalized other incubation-related neuroadaptations (Scheyer et al., 2014). These findings suggest that protein translation may be dysregulated during incubation. Here we tested this hypothesis by comparing signaling pathways regulating protein translation in the NAc core of rats following prolonged withdrawal from saline versus cocaine self-administration. A second goal was to determine whether protein translation was further altered when rats express incubated craving during a cue-induced seeking test.
We examined a number of pathways and factors implicated in protein translation and its contribution to synaptic plasticity, including the following: the mammalian target of rapamycin (mTOR) pathway, which is involved in dendritic protein translation (Costa-Mattioli et al., 2009; Liu-Yesucevitz et al., 2011); extracellular signal-regulated protein kinase (ERK), which also regulates dendritic translation (Kindler and Kreienkamp, 2012); eukaryotic elongation factor 2 (eEF2), which is necessary for ribosomal translocation during polypeptide elongation (Taha et al., 2013); and eukaryotic initiation factor 2α (eIF2α), which is required for initiation (Trinh and Klann, 2013). While trends toward changes in the translation machinery were observed in the NAc core after cocaine withdrawal, the most robust changes were observed following a cue-induced seeking test. Interestingly, evidence for increased translation during the test was observed in both saline and cocaine rats, suggesting that increased translation accompanies responding for a salient cue. However, cocaine rats also showed additional and specific alterations consistent with increased translation, including dephosphorylation of both eEF2 and eIF2α. By introducing specific inhibitors into the NAc core before a seeking test, we demonstrated that expression of incubated cocaine craving depends on mTOR activity and on acute dephosphorylation of eIF2α during the test. This dephosphorylation relieves inhibitory control on translation initiation (Trinh and Klann, 2013) and is implicated in cocaine-induced LTP in the VTA (Huang et al., 2016; Placzek et al., 2016a). Thus, combined with our prior work, two levels of protein translation dysregulation are revealed: ongoing changes during withdrawal that maintain synaptic adaptations necessary for incubation (Scheyer et al., 2014) and acute changes occurring during the expression of incubation.
Materials and Methods
Subjects and surgeries for in vivo experiments
Adult male Sprague Dawley rats (250–275 g on arrival; Envigo) were maintained on a reverse 12 h light/dark cycle with food and water available ad libitum. After ∼7 d to acclimate, intravenous jugular catheters were implanted and rats were singly housed. During recovery and training, catheters were flushed every 24 h with 0.9% sterile saline (w/v) and the antibiotic cefazolin (15 mg, i.v.; Webster Veterinary Supply). After 5–7 d of recovery, rats underwent 10 d of self-administration training. Some animals received intracranial surgery to implant guide cannula aimed at the NAc core (anteroposterior: 1.4; mediolateral: 2.4; dorsoventral: −6.3) 32–37 d after completion of training (Experiments 1, 3, 6, 7) or at the same time as jugular catheter implantation (Experiment 2). All experimental procedures were approved by the Rosalind Franklin University Institutional Animal Care and Use Committee in accordance with the United States Public Health Service Guide for the Care and Use of Laboratory Animals.
Drugs
Cocaine HCl was obtained from the National Institute on Drug Abuse and dissolved in 0.9% saline. For intracranial injections, ACSF contained the following: 145 mm NaCl, 2.7 mm KCl, 1.0 mm MgCl2, 1.2 mm CaCl2, 2.0 mm NaH2PO4, and 2.0 mm Na2HPO4. The protein translation inhibitor anisomycin (Sigma-Aldrich) was dissolved in HCl + ACSF, brought to a pH of 7.2 with NaOH (final anisomycin concentration 125 μg/μl). The mTOR inhibitor rapamycin (GenDepot) was dissolved in 100% DMSO as a stock solution and used at a final concentration of 5.47 mm in 8% DMSO/2% β-cyclodextrin in PBS. This is a lower concentration of DMSO than was used in prior studies involving intracranial rapamycin administration (Cota et al., 2006; Parsons et al., 2006; James et al., 2014). Sal003 (Tocris Bioscience), an inhibitor of eIF2α phosphatases, was dissolved in 1% DMSO as a stock solution and used for intracranial injections at a final concentration of 20 μm in 0.1% DMSO in saline. For the cell culture experiments described below, Sal003 was used at a final concentration of 40 μm.
Extended-access self-administration
Rats were randomly assigned to self-administer cocaine (0.5 mg/kg/infusion in a 100 μl/kg volume over 3 s) or saline (32 μl/infusion) for 6 h/d × 10 d on a fixed ratio 1 schedule as previously described (Conrad et al., 2008; McCutcheon et al., 2011a, b; Loweth et al., 2014; Scheyer et al., 2014; Christian et al., 2017a). Briefly, operant chambers (MED Associates) located in sound-attenuating cabinets were equipped with two nose-poke holes. Responding in the active hole resulted in an intravenous infusion of cocaine or saline delivered by a pump that was paired with a 20 s cue light and a 20 s time-out period. Responding in the inactive hole had no consequence.
Withdrawal and cue-induced seeking tests
After completion of training, rats were returned to home cages to undergo withdrawal and were handled several times per week. Rats used for baseline withdrawal measurements were killed on WD47-52 without further testing. Others were returned to the operant chamber on WD1-3 or WD47-52 for a 30 min test to measure cue-induced seeking. Tests were performed under extinction conditions (i.e., responding in the active hole delivered the light cue previously paired with infusions but no infusion was dispensed). The number of responses in the previously active hole was the operational measure of cocaine seeking or craving. For studies in which rats received intracranial injections before testing, vehicle or drug was microinfused 1 h (anisomycin, rapamycin, Sal003) or 15 min (Sal003) before placement into the operant chamber. The amount of drug delivered (anisomycin: 62.5 μg/0.5 μl/hemisphere; rapamycin: 5 μg/1 μl/hemisphere; Sal003: 20 μm/0.5 μl/hemisphere) was based on previous studies. For example, this amount of anisomycin infused into the medial PFC blocked cocaine memory reconsolidation (Sorg et al., 2015). This amount of rapamycin administered into the NAc attenuated cocaine reinstatement and reduced mTOR complex 1 (mTORC1) activity and GluA1 levels (James et al., 2014). This amount of Sal003 infused into the BLA disrupted reconsolidation of morphine- or cocaine-induced conditioned place preference (CPP), cue-induced heroin seeking and dephosphorylation of eIF2α (Jian et al., 2014). After testing, animals were returned to their home cages. When a crossover design was used, vehicle and drug infusions were counterbalanced such that rats received 2 seeking tests, 2–5 d apart (see Experimental design and statistical analysis).
Synaptoneurosome preparation
After rats were decapitated, the NAc was dissected from 2 mm coronal slices prepared with a brain matrix (ASI Instruments). The NAc dissection is intended to emphasize the core, where our characterizations of incubation-related changes in synaptic transmission have been focused (e.g., Conrad et al., 2008; McCutcheon et al., 2011a,b; Loweth et al., 2014) but also includes some shell (see diagrams in McCutcheon et al., 2011a; Werner et al., 2015). Incubation is accompanied by similar CP-AMPAR plasticity in both subregions (McCutcheon et al., 2011a; Wolf, 2016). Immediately following dissection, synaptoneurosomes were prepared as previously described (Most et al., 2015; Workman et al., 2015) with slight modifications. Briefly, NAc punches were homogenized in 500 μl of homogenization buffer [HB; 20 mm HEPES, 0.5 mm EGTA, 1× Proteasome Inhibitor Cocktail Set 1 (Millipore)]. Homogenates were passed through a 100-μm-pore filter and then through a 5-μm-pore filter (Millipore; both filters were prewashed with HB). After homogenates were passed through each filter, filters were washed with 50 μl of HB, and the washes were added to homogenates to maximize yield. Homogenates were then centrifuged at 14,000 × g for 20 min at 4°C. The pellet, which contains the synaptoneurosomes, was frozen on dry ice, stored at −80°C, and ultimately lysed in lysis buffer [0.605 g Tris-HCl, 0.25 g sodium deoxycholate, 0.876 g NaCl, 1 μg/ml PMSF, 5 ml of 20% SDS, and 1× Protease Inhibitor Cocktail Set 1 (Millipore) in 100 ml dH2O] for immunoblotting.
Immunoblotting
NAc synaptoneurosomes prepared from individual rats (10 μg protein/lane) were mixed 1:1 with 2× sample treatment buffer (161–0737, Bio-Rad) and analyzed by SDS-PAGE and immunoblotting (Ferrario et al., 2010). We used α-tubulin as a loading control, as in the original characterization of this synaptoneurosome protocol (Workman et al., 2015). To maximize our ability to quantify multiple antigens, we cut blots so that one blot could be used to probe for multiple antigens in different molecular weight ranges and used primary antibodies raised in different species to reprobe blots (we stripped when this was not possible). When analyzing total and phosphorylated proteins, we probed with the phospho-antibody first, stripped, and then probed with the pan antibody. To quantify target protein levels, SNAP ID 2.0 Protein Detection System (Millipore) was used according to the manufacturer's user guide with minor modifications. Briefly, each hydrated membrane was placed into a prewetted blot holder and then into the blot frame. Blocking buffer [30 ml; 0.5% dry milk, 1% goat serum in double distilled (dd)H2O] was passed through the membrane by vacuum. Next, primary antibody (10 ml) was applied and allowed to incubate for 30 min (tilted every 10 min to ensure even distribution). The vacuum was then applied until the frame was empty and the membrane was then washed (30 ml; 0.36× TBS, 0.1% Tween 20 in ddH2O) 4 times. Secondary antibody (10 ml) was then added to the membrane and incubated for 12 min (tilted at 6 min to ensure even distribution). The vacuum was then applied until the frame was empty, and the membrane was then washed 4 times.
Antibodies against the following targets were used in wash buffer: p-4E-BP1 (Thr37/46; 1:175, Cell Signaling Technology), p-4E-BP1 (Ser65; 1:175, Cell Signaling Technology), 4E-BP1 (1:200, Cell Signaling Technology), 4E-BP2 (1:600, Cell Signaling Technology), p-AKT (Thr308; 1:750, Cell Signaling Technology), AKT (1:750, Cell Signaling Technology), ATF4 (1:100, Cell Signaling Technology), p-eEF2 (Thr56; 1:200, Cell Signaling Technology), eEF2 (1:150, Cell Signaling Technology), p-eIF2α (Ser51; 1:150, Cell Signaling Technology), eIF2α (1:333, Cell Signaling Technology), p-eIF4e (Ser209; 1:150, Cell Signaling Technology), eIF4E (1:333, Cell Signaling Technology), p-ERK1/2 (Thr202/Tyr204; 1:700, Cell Signaling Technology), ERK1/2 (1:700; Cell Signaling Technology), p-fragile X mental retardation protein (FMRP) (Ser499; 1:200, Abcam), FMRP (1:175, Cell Signaling Technology), FXR1P (1:500, Cell Signaling Technology), FXR2P (1:500, Millipore), p-mTOR (Ser2448; 1:200, Cell Signaling Technology), p-mTOR (Ser2481; 1:200, Cell Signaling Technology), mTOR (1:200, Cell Signaling Technology), OPHN1 (1:333, Cell Signaling Technology), p-p70s6k (Thr389; 1:500, Cell Signaling Technology), p70s6k (1:500, Cell Signaling Technology), p-s6 (Ser235/236; 1:200, Cell Signaling Technology), s6 (1:200, Cell Signaling Technology), and α-tubulin (1:10,000, Cell Signaling Technology). Primary antibodies were detected with HRP-conjugated anti-rabbit or anti-mouse IgG (1:3000, Invitrogen), except for the α-tubulin secondary, which was detected at 1:10,000. Proteins were visualized by ECL (GE Healthcare) and quantified with TotalLab (Life Sciences Analysis Essentials).
Postnatal NAc/PFC cocultures
Pregnant Sprague Dawley rats (Harlan), obtained at 18–20 d of gestation, were housed individually in breeding cages. Postnatal day 1 (P1) offspring were decapitated and used to obtain NAc neurons. PFC neurons were obtained from P1 offspring of homozygous enhanced cyan fluorescent protein (ECFP)-expressing mice [strain: B6.129(ICR)-Tg(ACTB-ECFP)1Nagy/J; The Jackson Laboratory]. The homozygous ECFP transgenic mouse strain was maintained by mating ECFP male and female mice in-house. As described previously (Sun et al., 2008; Sun and Wolf, 2009; Werner et al., 2017), the medial PFC of ECFP-expressing P1 mice was dissociated with papain (20–25 U/ml; Worthington Biochemical) at 37°C for 30 min. PFC cells were then plated at a density of 30,000 cells/well onto coverslips coated with poly-d-lysine (100 μg/ml; Sigma-Aldrich). One to three days later, the NAc from P1 rats was dissociated with papain as described above and plated at a density of 30,000 cells/well with the PFC cells. Cocultures were grown in Neurobasal medium (Invitrogen) supplemented with 2 mm GlutaMAX, 0.5% Gentamicin, and 2% B27 (Invitrogen). Half of the media was replaced every 4 d. Two to three weeks later, cocultures were used for experiments in which fluorescent noncanonical amino acid tagging (FUNCAT) was used to visualize newly translated proteins in the processes of MSNs, as detailed under Experiment 5 in Experimental design and statistical analysis.
Experimental design and statistical analysis
Data collection and analyses for all experiments were performed blind to experimental conditions. Sample sizes were determined based on our previous studies (Conrad et al., 2008; Sun et al., 2008; Ferrario et al., 2011; McCutcheon et al., 2011a,b; Purgianto et al., 2013; Loweth et al., 2014; Werner et al., 2015, 2017), as well as other related reports (James et al., 2014; Jian et al., 2014; Sorg et al., 2015).
Experiment 1: Intra-NAc core injection of anisomycin attenuates incubated cue-induced cocaine seeking.
Rats underwent jugular catheter implantation and self-administration as described above. On WD32-37, rats underwent intracranial surgery to implant guide cannula aimed at the NAc core. After recovery (∼8–10 d later; WD40-47), rats were returned to the operant chamber for a cue-induced seeking test. One hour before the seeking test, the protein translation inhibitor anisomycin or vehicle was bilaterally injected into the NAc core of 15 rats. Five days later, using a crossover design, vehicle or anisomycin was injected bilaterally into the NAc core of the same rats 1 h before a second seeking test. The data from the extinction tests for cue-induced cocaine seeking were analyzed using paired parametric t tests.
Experiment 2: Intra-NAc core injection of anisomycin does not affect cue-induced cocaine seeking during early withdrawal.
Rats underwent double surgeries for jugular catheter implantation and intracranial implantation of guide cannula aimed at the NAc core and then began self-administration training as described above. On WD1, rats were returned to the operant chamber for a cue-induced seeking test. One hour before the seeking test, anisomycin or vehicle was bilaterally injected into the NAc core of 18 rats. Two days later (WD3), using a crossover design, vehicle or anisomycin was injected bilaterally into the NAc core of the same rats 1 h before a second seeking test. The data from the extinction tests for cue-induced cocaine seeking were analyzed using paired parametric t tests.
Experiment 3: Intra-NAc core injection of rapamycin attenuates incubated cue-induced cocaine seeking.
Rats underwent jugular catheter implantation and self-administration as described above. On WD32-37, rats underwent intracranial surgery to implant guide cannula aimed at the NAc core. Approximately 8–10 d later, rats were returned to the operant chamber for a cue-induced seeking test on WD40-47. One hour before the seeking test, the mTOR inhibitor rapamycin or vehicle was bilaterally injected into the NAc core of 8 rats. Five days later, using a crossover design, vehicle or rapamycin was injected bilaterally into the NAc core of the same rats 1 h before a second seeking test. The data from the extinction tests for cue-induced cocaine seeking were analyzed using paired parametric t tests.
Experiment 4: Biochemical studies of signaling pathways regulating protein translation after prolonged withdrawal or a cue-induced seeking test after prolonged withdrawal.
Following self-administration, rats were killed on WD47-52 without any seeking test (n = 10 saline rats, n = 10 cocaine rats) or 30 min after a 30 min cue-induced seeking test on WD47-52 (n = 8 saline/test rats, n = 10 cocaine/test rats). The 30 min time point was selected based on other studies of biochemical changes associated with expression of incubation (e.g., Lu et al., 2005), including our own studies of protein degradation after the same regimens (Werner et al., 2015). Synaptoneurosomes were prepared from the NAc and used for immunoblotting studies. The data from the extinction tests for cue-induced seeking were analyzed using unpaired parametric t tests. Results of immunoblotting studies were analyzed using two-way ANOVA with treatment (saline or cocaine) and test (no test or test) as factors, followed by Fisher's least significant difference (LSD) post hoc comparisons.
Experiment 5: Blocking eIF2α dephosphorylation reduces de novo protein synthesis in NAc MSNs in vitro.
To confirm that Sal0003, a selective inhibitor of eIF2α phosphatases, reduces translation in MSNs, we quantified newly translated proteins in the processes of NAc MSNs cocultured with PFC cells (see Postnatal NAc/prefrontal cortex (PFC) cocultures, above). The experiment was performed using NAc/PFC cocultures, rather than cultures containing NAc neurons only, because PFC neurons restore excitatory synapses onto the NAc neurons (Sun et al., 2008; Sun and Wolf, 2009; Werner et al., 2017). Newly translated proteins were detected using a modification of FUNCAT (Wolf and Stefanik, 2016) that was adapted from earlier studies (Dieterich et al., 2010; tom Dieck et al., 2012; Hinz et al., 2013). On the day of the experiment, supplemented Neurobasal media (see above) was replaced with supplemented methionine-free DMEM (Invitrogen) for 30 min. Methionine (Met) starvation enhances tagging by the noncanonical amino acid l-azidohomoalanine (AHA, Invitrogen) that incorporates at Met codons. After Met starvation, cells were incubated with DMEM containing AHA (1 mm, pH 7.4) (AHA group), DMEM containing AHA + 40 μm Sal003 (AHA + Sal003 group), or DMEM supplemented with 1 mm Met (Met group; negative control used to define background signal). After this incubation, cells were washed, fixed in 4% PFA for 15 min, and stored in PBS. Click chemistry was then used to visualize AHA labeling. Briefly, cells were permeabilized with 0.25% Triton X-100, washed with 3% BSA, and the click chemistry reaction was performed by incubating the coverslips upside-down on droplets of PBS, pH 7.8, containing a Cy-5-conjugated copper-free click chemistry reagent, dibenzocyclooctyne (DBCO, 20 nm, Click Chemistry Tools) for 30 min. Coverslips were then mounted on slides using ProLong Gold Antifade mountant (Thermo Fisher Scientific). Fluorescence microscopy and MetaMorph software were used to quantify AHA labeling in processes of NAc MSNs. NAc MSNs were distinguished from other neuronal cell-types based on morphology and from ECFP-expressing PFC cells based on fluorescence (Sun et al., 2008; Werner et al., 2017) and selected under phase contrast to avoid experimenter bias. Fluorescence (DBCO-AHA) was measured in a fixed 15 μm length of a process at least one soma diameter away from the soma. The background threshold was set based on an average of background fluorescence in unstained areas in processes of cells from the Met group. The area of fluorescence was compared in processes of NAc MSNs from the AHA group (n = 20 cells) and the AHA + Sal0003 group (n = 27 cells). Data were analyzed using unpaired parametric t tests.
Experiment 6: Blocking eIF2α dephosphorylation in NAc core 1 h before a seeking test attenuates incubated cue-induced cocaine seeking.
Rats underwent jugular catheter implantation and self-administration as described above. On WD32-37, rats underwent intracranial surgery to implant guide cannula aimed at the NAc core. On WD40-47, Sal003 or vehicle was injected into the NAc core of 10 rats 1 h before a cue-induced seeking test to time-match the previous microinjection experiments (Experiments 1–3). Five days later, using a crossover design, vehicle or Sal003 was injected bilaterally into the NAc core of the same rats 1 h before a second seeking test. The data from the extinction tests for cue-induced cocaine seeking were analyzed using paired parametric t tests.
Experiment 7: Blocking eIF2α dephosphorylation in NAc core 15 min before a seeking test attenuates incubated cue-induced cocaine seeking.
Rats were treated exactly as described in Experiment 6, except that Sal003 (n = 8 rats) or vehicle (n = 8 rats) was injected into the NAc core 15 min before a cue-induced seeking test. In Experiment 6, we injected Saal003 1 h before the test to match the timing of other studies showing that 1 h pretreatment with translation inhibitors normalized CP-AMPAR levels in brain slices (Scheyer et al., 2014) and decreased the expression of incubated craving (Experiments 1 and 3). However, because Experiment 7 was designed to specifically test the role of eIF2α in behavioral responding during a seeking test, we designed this experiment to match the timing of previous studies that injected the same concentration of Sal003 before a behavioral test and demonstrated its effectiveness at reversing behavior and preventing eIF2α dephosphorylation 30 min later (Costa-Mattioli et al., 2007; Jian et al., 2014; Huang et al., 2016). Furthermore, because reversal of CP-AMPAR elevation by translation inhibitors occurred after 1 h pretreatment of slices with the inhibitors (Scheyer et al., 2014), we thought that a shorter pretreatment would be more likely to affect cocaine seeking by opposing test-dependent effects as opposed to reversing neuroadaptations present during cocaine withdrawal. Each rat received only one infusion. Data from the extinction tests for cue-induced cocaine seeking were analyzed using unpaired parametric t tests.
Results
For all experiments, rats were trained to nose-poke to receive intravenous cocaine or saline infusions (paired with a light cue) using an extended-access regimen (6 h/d × 10 d) and then underwent withdrawal/forced abstinence in home cages. Training data are provided in Figure 1 for rats from Experiments 1–3, in Figure 2 for rats in Experiment 4, and in Figure 4 for rats in Experiments 6 and 7. For subsequent tissue harvesting/synaptoneurosome preparation or cue-induced seeking tests, we focused on withdrawal periods ranging from 47 to 52 d because incubation has plateaued by this time (Grimm et al., 2001) and CP-AMPAR levels in the NAc core are maximally and stably elevated (Conrad et al., 2008; Wolf and Tseng, 2012).
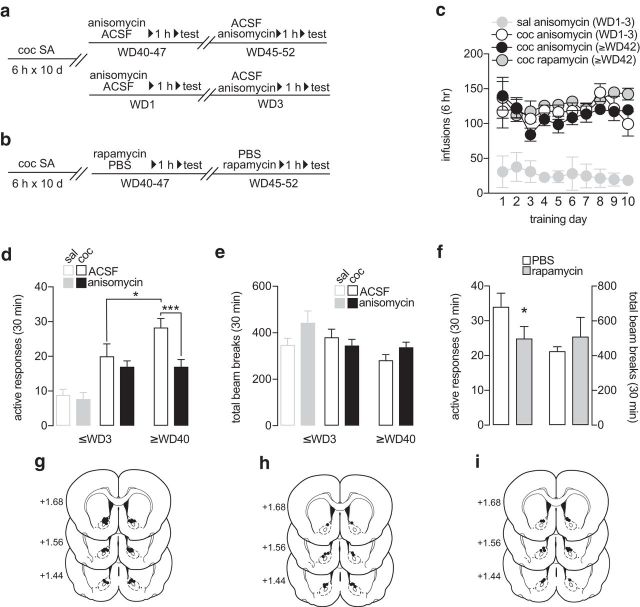
Figure 1.
Incubated cocaine seeking is attenuated by blocking protein translation in the NAc. a, b, Timelines for Experiments 1–3. c, Self-administration training data for rats destined for Experiment 1 (n = 15), Experiment 2 (n = 9), or Experiment 3 (n = 16). Nose-pokes in the active hole resulted in an intravenous infusion of saline or cocaine (0.5 mg/kg) paired with a light cue. d–i, Anisomycin or rapamycin was infused into the NAc core 1 h before a cue-induced seeking test on WD1-3 or ≥WD40. During the test, nose-pokes in the active hole (termed “active responses” in y axis labels of panels d and f) delivered the cue previously associated with cocaine but no cocaine infusion. A crossover design was used so that each rat received vehicle or treatment and then treatment or vehicle 2 d (WD1 and WD3 tests) or 5 d (WD40–47 and WD45-52 tests) later. Anisomycin attenuated cue-induced cocaine seeking in late withdrawal but not early withdrawal (d) without significantly altering locomotor activity (e). Rapamycin also attenuated incubated cocaine seeking but did not alter locomotion (f). Infusion sites for the early withdrawal anisomycin experiment (Experiment 1) (g), late withdrawal anisomycin experiment (Experiment 2) (h), and rapamycin experiment (Experiment 3) (i). Data are mean ± SEM. *p < 0.05, ***p < 0.001.
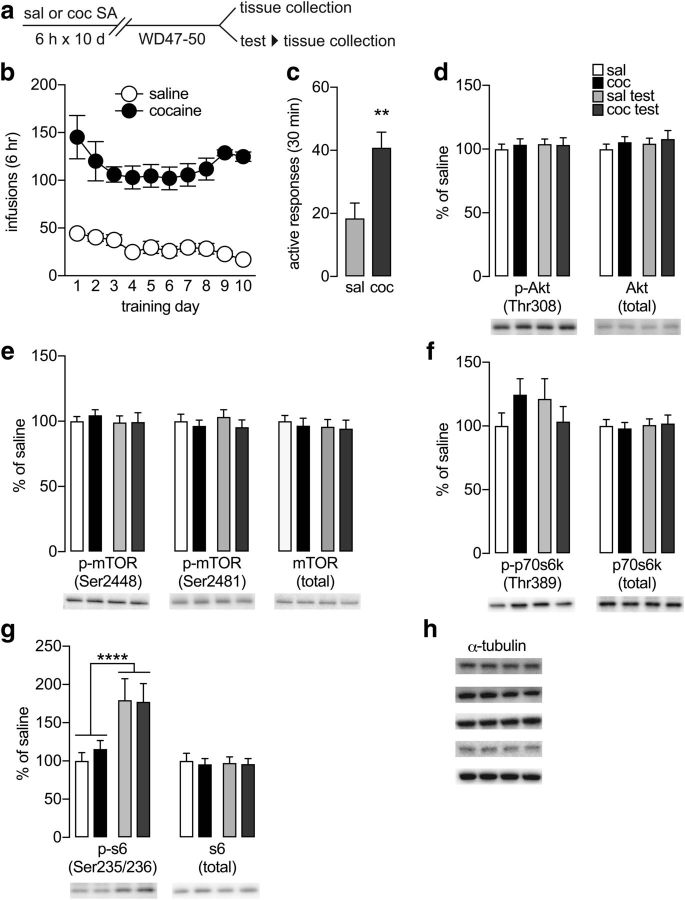
Figure 2.
Examination of the mTOR pathway in NAc synaptoneurosomes prepared after prolonged withdrawal from extended-access cocaine self-administration or after a cue-induced seeking test. a, Timeline for Experiment 4. All rats underwent self-administration training (18 saline rats, 20 cocaine rats). Half were killed for NAc synaptoneurosome preparation on WD47-52 (n = 10 saline, n = 10 cocaine), and the other half underwent cue-induced seeking tests on WD47-52 and were killed 30 min later (n = 8 saline/test, n = 10 cocaine/test). b, Self-administration training. Nose-pokes in the active hole resulted in an intravenous infusion of saline or cocaine (0.5 mg/kg) paired with a light cue. c, Seeking tests on >WD47. Shown are nose-pokes in the previously active hole (active responses; a measure of cocaine seeking) during a 30 min test performed under extinction conditions (nose-poke delivers cue but no infusion). **p = 0.008 (two-tailed t test). d–h, Immunoblotting results in NAc synaptoneurosomes from saline, cocaine, saline/test, and cocaine/test groups (n = 6–9 rats/group): (d) phosphorylated (p)-Akt (Thr308) and total Akt, (e) p-mTOR (Ser2448), p-mTOR (Ser2481) and total mTOR levels, (f) p-p70s6k (Thr389) and total p70s6k, (g) p-s6 (Ser235/236) and total s6 levels, and (h) α-tubulin (loading control). For α-tubulin, we show representative images from immunoblots used to generate data for this figure and all other biochemical data, with each row indicating a lane from each of the four experimental groups (for description of how a single gel was used for multiple analyses, see Materials and Methods). Data are mean ± SEM. ****p < 0.0001.
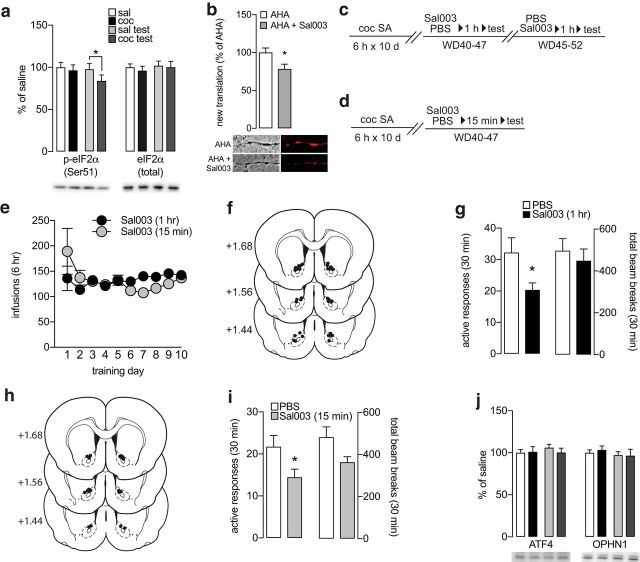
Figure 4.
eIF2α is dephosphorylated in the NAc of cocaine rats during a cue-induced seeking test, and blocking eIF2α dephosphorylation in the NAc core reduces cue-induced cocaine seeking. a, Phosphorylated eIF2α and total eIF2α levels in NAc synaptoneurosomes from saline, cocaine, saline/test, and cocaine/test groups. b, Validation of Sal003 efficacy in NAc MSNs using incorporation of the noncanonical amino acid AHA as a measure of new protein translation. Cocultured NAc and PFC neurons were incubated with AHA (1 mm) or AHA + Sal003 (40 μm; inhibitor of eIF2α phosphatases) for 2 h and then tagged using click chemistry with 20 nm DBCO-Cy5 for visualization of AHA. Shown are representative images of Cy5 signal in NAc MSN processes and a bar graph depicting significant inhibition of AHA incorporation by Sal003. *p < 0.05. (c, d) Time-lines for Experiments 6 and 7, respectively. For Experiment 6, vehicle or Sal003 was infused into the NAc core of 10 rats 1 h before a cue-induced seeking test. A crossover design was used so that a total of 10 measurements were made under each condition. For Experiment 7, vehicle (n = 8) or the Sal003 (n = 8) was infused into the NAc core 15 min before a cue-induced seeking test. Each rat received only one infusion. e, Self-administration training data for Experiments 6 and 7. (f, g), Infusion sites and cue-induced seeking data for Experiment 6 (rats infused with Sal003 1 h prior to the test). Left bars in panel g show active hole responses and right bars show locomotor activity during the test. (h, i) Infusion sites and cue-induced seeking data for Experiment 7 (rats infused with Sal003 15 min prior to the test). Left bars in panel i show active hole responses and right bars show locomotor activity during the test. j, Readouts of eIF2α-dependent translation, ATF4 and OPHN1, remain unchanged following a cue-induced seeking test. Biochemical data were obtained from rats in Experiment 4 (n = 7–10 rats/group). Timeline and training data for these rats are shown in Figure 2. Data are mean ± SEM. *p < 0.05.
Inhibitors of translation reduce incubated cocaine craving
We showed previously that ongoing protein translation is necessary to maintain the elevation in CP-AMPAR levels that occurs in the NAc after incubation of craving. Thus, in acutely prepared slices from “incubated rats,” bathing slices for 1 h with translation inhibitors (anisomycin, cycloheximide, or rapamycin) before recording was found to return CP-AMPAR levels in NAc synapses to saline control levels and also normalize other incubation-related plasticity (Scheyer et al., 2014). Because the expression of incubated craving depends on elevated CP-AMPAR levels (Conrad et al., 2008; Loweth et al., 2014), these results suggest that in vivo inhibition of protein translation in the NAc should reduce incubated cocaine craving.
We tested this hypothesis in Experiments 1–3 by examining the effect of intra-NAc core injection of anisomycin on cue-induced cocaine craving in early withdrawal (WD1–3), before craving has incubated, and in late withdrawal (WD40–52), when incubation has occurred (Fig. 1a; Fig. 1d, middle and right open bars; unpaired one-tailed t test: ≤WD3 cocaine/ACSF vs ≥WD40 cocaine/ACSF: t(22) = 1.889, p = 0.036). We also tested the effect of intra-NAc rapamycin on incubated cocaine craving (Fig. 1b). Rats underwent self-administration (Fig. 1c) and then, on selected withdrawal days (above), were returned to the operant chamber for a cue-induced seeking test. One hour before the seeking test, we injected anisomycin or rapamycin into the NAc core to match the timing of slice recordings in Scheyer et al. (2014). Intra-NAc injection of anisomycin did not affect cocaine or saline seeking in early withdrawal but reduced incubated cocaine seeking after prolonged withdrawal (Fig. 1d; two-tailed t test: ≤WD3 saline/anisomycin vs saline/ACSF p = 0.712; ≤WD3 cocaine/anisomycin vs cocaine/ACSF p = 0.525; ≥WD40 cocaine/anisomycin vs cocaine/ACSF: t(14) = 4.298, p = 0.0007). In addition, rapamycin also reduced incubated cocaine seeking (Fig. 1f, left bars; two-tailed t test: ≥WD40 rapamycin vs PBS: t(8) = 2.698, p = 0.027). Neither of these translation inhibitors significantly influenced locomotor activity (Fig. 1e; Fig. 1f, bars on right; two-tailed t test: ≤WD3 saline/anisomycin vs saline/ACSF p = 0.185; ≤WD3 cocaine/anisomycin vs cocaine/ACSF p = 0.462; ≥WD40 cocaine/anisomycin vs cocaine/ACSF p = 0.202; ≥WD40 rapamycin vs PBS p = 0.489). These results support the hypothesis that ongoing protein translation is required to maintain neuroadaptations that enable the expression of incubated cocaine craving. Along with our electrophysiological results (Scheyer et al., 2014), they raise the possibility that protein translation is dysregulated in the NAc of “incubated rats” compared with saline controls. In particular, dysregulation of dendritic protein translation is implicated, given that mTOR is implicated in dendritic synthesis of synaptic proteins (Costa-Mattioli et al., 2009; Liu-Yesucevitz et al., 2011) and its inhibitor rapamycin was effective in both electrophysiological experiments (Scheyer et al., 2014) and behavioral experiments (Fig. 1f); the speed of the effect in both instances is also consistent with dendritic translation.
Therefore, in Experiment 4, we assessed signaling pathways regulating translation using synaptoneurosomes, a subcellular fraction enriched for the postsynaptic density (Hollingsworth et al., 1985; Quinlan et al., 1999) that has previously been used in studies of dendritic translation (Hollingsworth et al., 1985; Quinlan et al., 1999; Aoto et al., 2008; Workman et al., 2015). We prepared synaptoneurosomes from the NAc of rats that self-administered saline or cocaine (Fig. 2a,b). Some of these rats were killed on WD47-52 (without any seeking test), hereafter termed saline and cocaine groups (n = 10 and n = 10, respectively). The other rats were subjected to a cue-induced seeking test on WD47-52 (Fig. 2c; two-tailed t test: saline vs cocaine: t(15) = 3.09, p = 0.008) and killed 30 min later (hereafter termed saline/test and cocaine/test groups, n = 8 and n = 10, respectively) (for justification of 30 min time point, see Materials and Methods). Synaptoneurosome fractions from the NAc core (see Materials and Methods) of saline, cocaine, saline/test, and cocaine/test groups were used to evaluate multiple signaling pathways, with results grouped below according to their relationship to mTOR, initiation of translation, ERK1/2, or RNA binding proteins. By comparing biochemical findings in the saline and cocaine groups, we can determine whether regulation of translation is altered during cocaine withdrawal. In addition, these groups provide a baseline from which to assess changes in the protein translational machinery resulting from cue-induced seeking tests. We confirmed purity of the synaptoneurosome fraction by immunoblotting experiments. Relative to homogenates, synaptoneurosomes contained nearly undetectable levels of histone proteins, indicating minimal contamination by cell bodies and enrichment of postsynaptic density proteins (data not shown).
Incubation of craving and mTOR-related signaling
To determine whether incubation is accompanied by adaptations in mTOR signaling, we began by examining Akt, which is upstream of mTOR (Ruggero and Sonenberg, 2005) and implicated in cocaine-induced CPP (Shi et al., 2014), but found no changes in p-Akt (Thr308; Fig. 2d, left; Fisher PLSD test: saline vs cocaine p = 0.824; saline vs saline/test p = 0.613; cocaine vs cocaine/test p = 0.928; saline/test vs cocaine/test p = 0.836) or total Akt (Fig. 2d, right; Fisher PLSD test: saline vs cocaine p = 0.559; saline vs saline/test p = 0.618; cocaine vs cocaine/test p = 0.522; saline/test vs cocaine/test p = 0.538). Next, we examined mTOR itself, which has multiple phosphorylation sites with unique functions (Foster and Fingar, 2010) and has been widely linked with actions of drugs of abuse, including cocaine (Dayas et al., 2012; Neasta et al., 2014). We examined mTOR phosphorylation on Ser2448, which is regulated by Akt (Navé et al., 1999; but see Holz et al., 2005) and p70s6 kinase (p70s6k) (Chiang and Abraham, 2005; Holz et al., 2005), and Ser2481, an autophosphorylation site that reflects intrinsic catalytic activity (Peterson et al., 2000; Soliman et al., 2010; Dalle Pezze et al., 2012). We found no group differences in mTOR phosphorylation at Ser2448 (Fig. 2e, left; Fisher PLSD test: saline vs cocaine p = 0.746; saline vs saline/test p = 0.751; cocaine vs cocaine/test p = 0.476; saline/test vs cocaine/test p = 0.990) or Ser2481 (Fig. 2e, middle; Fisher PLSD test: saline vs cocaine p = 0.276; saline vs saline/test p = 0.732; cocaine vs cocaine/test p = 0.946; saline/test vs cocaine/test p = 0.157). There was also no change in total mTOR levels (Fig. 2e, right; Fisher PLSD test: saline vs cocaine p = 0.360; saline vs saline/test p = 0.442; cocaine vs cocaine/test p = 0.844; saline/test vs cocaine/test p = 0.783).
In many instances, exposure to drugs of abuse has revealed transient activation of mTOR (Neasta et al., 2014). Therefore, despite our failure to detect mTOR activation, we went on to assess its downstream targets, which may show longer-lasting adaptations. mTOR promotes translation of a subset of mRNAs at the initiation stage through phosphorylation of two targets, p70s6k and eukaryotic initiation factor 4E-binding proteins (4E-BPs) (Holz et al., 2005; Sonenberg and Hinnebusch, 2009). Phosphorylation of p70s6k (Thr389), mediated exclusively by mTOR, provides a read-out of mTOR activity, although they do not always correspond (James et al., 2014). p70S6k then phosphorylates ribosomal protein s6 (s6), a component of the 40S subunit of the ribosome (Chung et al., 1992). Changes in p70s6k have been reported following intraperitoneal cocaine injections (Sutton and Caron, 2015; but see Bailey et al., 2012), CPP (Yu et al., 2013; Shi et al., 2014), and cue-induced reinstatement (Wang et al., 2010; Gao et al., 2017). Here, we found a modest trend toward increased p-p70s6k (Thr389) levels in the cocaine group compared with saline controls (Fig. 2f, left; Fisher PLSD test: p = 0.170) but no differences between other groups (Fisher PLSD test: saline vs saline/test p = 0.266; cocaine vs cocaine/test p = 0.200; saline/test vs cocaine/test p = 0.304) and no change in total p70s6k protein (Fig. 2f, right; Fisher PLSD test: saline vs cocaine p = 0.486; saline vs saline/test p = 0.948; cocaine vs cocaine/test p = 0.474; saline/test vs cocaine/test p = 0.934). We also evaluated s6, which directly increases translation initiation when phosphorylated (Dufner and Thomas, 1999). While no group differences in total s6 were observed (Fig. 2g, right; Fisher PLSD test: saline vs cocaine p = 0.533; saline vs saline/test p = 0.765; cocaine vs cocaine/test p = 0.909; saline/test vs cocaine/test p = 0.839), we found that the seeking test increased phosphorylation of s6 (Ser235/236) independent of whether rats had previously self-administered saline or cocaine (Fig. 2g, left; two-way ANOVA: main effect of test: F(1,33) = 24.39, p = 0.00002). Previously, we found that saline rats subjected to a cue-induced seeking test prefer the nose-poke hole that delivers the light cue (Werner et al., 2015). Thus, both cocaine and saline rats are responding to a salient cue during the test, and the present results suggest that such a response is associated with phosphorylation of s6.
Next, we looked at two alternative, downstream, translation-related targets of mTOR: eukaryotic elongation factor 2 (eEF2) and 4E-BPs. eEF2 catalyzes ribosomal translocation during polypeptide elongation, and phosphorylation of eEF2 at Thr56 inhibits its activity and decreases translation (Taha et al., 2013). We found a decrease in p-eEF2 in the cocaine/test group compared with the saline/test group and the cocaine group (Fig. 3a, left; two-way ANOVA: interaction of test and treatment: F(1,28) = 3.335, p = 0.079; Fisher PLSD test: saline vs cocaine p = 0.819; saline vs saline/test p = 0.896; cocaine vs cocaine/test p = 0.020, saline/test vs cocaine/test p = 0.030), suggesting increased translation in the cocaine/test group. No group differences in total eEF2 were found (Fig. 3a, right; Fisher PLSD test: saline vs cocaine p = 0.631; saline vs saline/test p = 0.739; cocaine vs cocaine/test p = 0.595; saline/test vs cocaine/test p = 0.759). It is interesting that the cocaine group shows a trending increase in p-p70s6k (Fig. 2f, left) and the cocaine/test group shows decreased phosphorylation of p-eEF2 (Fig. 3a, left). Increased p-p70s6k phosphorylation is associated with reduced phosphorylation of eEF2 kinase at Ser366 (Redpath et al., 1996; Wang et al., 2001). As phosphorylation of eEF2 kinase inhibits its activity (Herbert and Proud, 2006), this would lead to an increase in p-eEF2. Thus, one interpretation of these results is that the mTOR pathway is primed during cocaine withdrawal such that it is better able to dephosphorylate eEF2 and enhance translation during a seeking test. Altered NMDAR transmission, leading to altered regulation of eEF2 dephosphorylation, is another possibility (see Discussion).
Figure 3.
eEF2, 4E-BP1, and 4E-BP2 in NAc synaptoneurosomes prepared after prolonged withdrawal (WD47-52) from extended-access cocaine self-administration or after a cue-induced seeking test. (a) p-eEF2 and total eEF2, (b) p-4E-BP1 (Thr37/46) and total 4E-BP1, (c) total 4E-BP2 levels, and (d) electrophoretic mobility of 4E-BP2 in saline, cocaine, saline/test and cocaine/test groups (top arrowhead, “b form”; bottom arrowhead, “a form”; see Results section for details). Data were obtained from rats in Experiment 4 (n = 7–9 rats/group). Timeline and training data are shown in Figure 2. Data are mean ± SEM. *p < 0.05.
4E-BPs repress translation by sequestering eukaryotic initiation factor 4E (eIF4E), a critical component of the eukaryotic initiation factor 4E (eIF4E) complex (Sonenberg and Hinnebusch, 2009). Phosphorylation of 4E-BP1 promotes its dissociation from eIF4E, which in turn promotes translation. Deamidation serves the same role for 4E-BP2, which can be detected as reduced electrophoretic mobility (i.e., a shift in intensity between the “a form” [17 kDa] and “b form” [19.5 kDa]; Bidinosti et al., 2010; Ayuso et al., 2015). A previous study reported increases in both p-4E-BP1 and p-eIF4E following repeated cocaine injections (Sutton and Caron, 2015). Here, we observed a trending decrease in total 4E-BP1 in cocaine/test compared to cocaine rats (Fig. 3b, right; Fisher PLSD test: saline vs cocaine p = 0.353; saline vs saline/test p = 0.419; cocaine vs cocaine/test p = 0.072; saline/test vs cocaine/test p = 0.103) but no group differences in p-4E-BP1 (Thr37/46; Fig. 3b, left; Fisher PLSD test: saline vs cocaine p = 0.341; saline vs saline/test p = 0.765; cocaine vs. cocaine/test p = 0.441, saline/test vs cocaine/test p = 0.886), total 4E-BP2 (Fig. 3c; Fisher PLSD test: saline vs cocaine p = 0.862; saline vs saline/test p = 0.900; cocaine vs cocaine/test p = 0.586; saline/test vs cocaine/test p = 0.625) or electrophoretic mobility of 4E-BP2 (Fig. 3d).
Together, these results suggest that mTOR signaling is perturbed during incubation, but the specific pathway affected is not clear-cut. The cocaine group showed a trending increase in phosphorylation/activation of p70s6k compared with the saline group (Fig. 2f, left), but this corresponded only partially to observed changes in phosphorylation of its substrate s6 following a seeking test because increased p-s6 was observed in both cocaine/test and saline/test groups (Fig. 2g, left). One possibility is the p-s6 increase observed in the cocaine/test group occurred through mTOR signaling, as suggested by the trending increase in p70s6k phosphorylation in the cocaine group, whereas the increase in p-s6 observed in the saline/test group may occur via another kinase (Meyuhas, 2015).
Incubation and initiation of translation
Protein translation can be broadly broken down into two phases: initiation and elongation. While the elongation step can be regulated by mTOR via eEF2 (see above), initiation is the rate-limiting step and is controlled, in part, by eIF2α. Phosphorylation of eIF2α suppresses general translation (Trinh and Klann, 2013; but see below). Using synaptoneurosomes from Experiment 4, we found no change in total protein levels of eIF2α in the NAc among our four experimental groups (Fig. 4a, right; Fisher PLSD test: saline vs cocaine p = 0.332; saline vs saline/test p = 0.919; cocaine vs cocaine/test p = 0.446; saline/test vs cocaine/test p = 0.749). However, there was a significant decrease in p-eIF2α in the cocaine/test group compared with the saline/test group (Fig. 4a, left; two-way ANOVA: main effect of treatment: F(1,28) = 4.722, p = 0.038; Fisher PLSD test: saline vs cocaine p = 0.352; saline vs saline/test p = 0.641; cocaine vs cocaine/test p = 0.096; saline/test vs cocaine/test p = 0.046), reminiscent of effects reported in VTA after cocaine exposure (Huang et al., 2016; Placzek et al., 2016a) (see Discussion). This suggests that an increase in protein translation occurs when cocaine rats undergo a cue-induced seeking test.
Having shown that protein translation is required for expression of incubation (Fig. 1), we hypothesized that this reflects a requirement for the observed dephosphorylation of eIF2α. We tested this in two ways. First, in Experiment 5, we determined the effects of Sal003, a selective inhibitor of eIF2α phosphatases (Costa-Mattioli et al., 2007), on levels of newly translated proteins in processes of NAc MSNs. To do so, we used NAc/PFC cocultures and an adapted a FUNCAT approach (Wolf and Stefanik, 2016), which allows for the visualization of newly translated proteins using fluorescent labeling. We have shown previously that newly translated proteins detected in processes of MSNs in this manner are located in proximity to ribosomes and synapses (Wolf and Stefanik, 2016). Here, we found that treatment with Sal003 significantly reduced labeling in NAc MSN processes compared with a vehicle control group (Fig. 4b; two-tailed t test: AHA vs Sal003: t(45) = 2.367, p = 0.022), confirming that Sal003 inhibits translation in NAc MSNs.
In Experiments 6 and 7, we tested the hypothesis that dephosphorylation of eIF2a leading to increased protein translation contributes to incubated cocaine seeking by performing bilateral injections of Sal003 into the NAc core of WD40-47 cocaine animals before a cue-induced seeking test (Fig. 4c,d). In Experiment 6, we injected Sal0003 into the NAc 1 h before the seeking test. This timing was selected based the ability of anisomycin or rapamycin to reverse CP-AMPAR elevation when added to slices 1 h before patch-clamp recordings (Scheyer et al., 2014) and inhibit expression of incubation when injected into the NAc 1 h before a seeking test (Fig. 1). With this timing, intra-NAc injections of Sal003 (Fig. 4f) significantly reduced cue-induced cocaine craving (Fig. 4g, left bars; two-tailed t test: PBS vs Sal003: t(18) = 2.345, p = 0.031) but did not affect locomotor activity (Fig. 4g, right bars; two-tailed t test: PBS vs Sal003 p = 0.305). One possible explanation for Sal003's ability to reduce seeking is that it is acting similarly to anisomycin and rapamycin, that is, reversing adaptations occurring during withdrawal that are required for incubated seeking (Scheyer et al., 2014).
However, given that eIF2α dephosphorylation in cocaine rats is observed only after a test (cocaine/test group), not after withdrawal only (cocaine group; Fig. 4a, left), it seemed more likely that Sal003 is acting to oppose test-dependent dephosphorylation of eIF2α. To evaluate this latter possibility, we repeated this experiment in a different group of rats, but, this time, injected Sal003 only 15 min before the seeking test (Experiment 7). As detailed in Experimental design, this timing enabled us to more directly target biochemical changes triggered by the test. Again, we found a significant reduction in cue induced seeking (Fig. 4i, left bars; two-tailed t test: PBS vs Sal003: t(17) = 2.16, p = 0.045). There was also a nearly significant reduction in locomotion during the seeking test (Fig. 4i, right; two-tailed t test: PBS vs Sal003 p = 0.054). However, there was no significant correlation between the reduction in locomotion and seeking for individual animals (data not shown). Together, the results presented so far suggest that there may be persistent alterations in translation during withdrawal as well as increased translation depending on eIF2α dephosphorylation during the seeking test.
While phosphorylation of eIF2α generally reduces the rate of translational efficiency by reducing the availability of the ternary complex, this can have the paradoxical effect of increasing translational efficiency of a subset of mRNAs that contain multiple upstream open reading frames (uORFs), such as the transcriptional modulator activating transcription factor 4 (Vattem and Wek, 2004; Trinh and Klann, 2013; ATF4) and Rho-GTPase-activating protein oligophrenin-1 (OPHN1) (Di Prisco et al., 2014). This might predict that dephosphorylation of eIF2α in the cocaine/test group would be accompanied by increased ATF4 and/or OPHN1 protein levels, both of which have previously been shown to be involved in cocaine-mediated plasticity and behavior (Jian et al., 2014; Huang et al., 2016; Liao et al., 2016). However, using NAc synaptoneurosomes from Experiment 4 rats, we found no alterations in ATF4 (Fig. 4j, left; Fisher PLSD test: saline vs cocaine p = 0.834, saline vs saline/test p = 0.427, cocaine vs cocaine/test p = 0.917, saline/test vs cocaine/test p = 0.303) or OPHN1 (Fig. 4j, right; Fisher PLSD test: saline vs cocaine p = 0.868, saline vs saline/test p = 0.571, cocaine vs cocaine/test p = 0.303, saline/test vs cocaine/test p = 0.767) either during withdrawal or 30 min following a seeking test. These results suggest that the behavioral consequences of eIF2α dephosphorylation during the seeking test are associated with general reduction of translation rather than increased translation of ATF4, OPHN1, or other mRNAs with uORFs. Combined with data showing that inhibition of eIF2α dephosphorylation with Sal003 reduces incubated cocaine seeking, we interpret our findings to indicate that the seeking test elicits an increase in protein translation that is required for the expression of incubated seeking.
ERK signaling and the incubation of cocaine craving
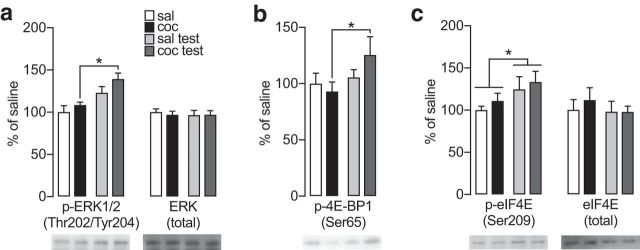
ERK signaling has been widely implicated in the regulation of translation and in cocaine action, including the incubation of cocaine craving (see Introduction and Discussion). Using NAc synaptoneurosomes from Experiment 4, we found a significant increase in phosphorylation of ERK1/2 (Thr202/Tyr204) in the cocaine/test group compared with the cocaine group and a trend toward an increase in the cocaine/test group versus the saline/test group (Fig. 5a, left; two-way ANOVA: main effect of test: F(1,32) = 8.36, p = 0.007; Fisher PLSD test: saline vs cocaine p = 0.484; saline vs saline/test p = 0.162; cocaine vs cocaine/test p = 0.011; saline/test vs cocaine/test p = 0.100). No group differences in total ERK1/2 were found (Fig. 5a, right; Fisher PLSD test: saline vs cocaine p = 0.230; saline vs saline/test p = 0.368; cocaine vs cocaine/test p = 0.878; saline/test vs cocaine/test p = 0.926). ERK1/2 phosphorylates 4E-BP1 at Ser65 (Herbert et al., 2002), which leads to dissociation of p-4E-BP1 from cap-binding factor eIF4E (Gingras et al., 2001). Paralleling our p-ERK1/2 results in the cocaine/test group, we found a significant increase in p-4E-BP1 (Ser65) in cocaine/test group compared with the cocaine group (Fig. 5b; Fisher PLSD test: saline vs cocaine p = 0.513; saline vs saline/test p = 0.810; cocaine vs cocaine/test: t(29) = 2.142, p = 0.041; saline/test vs cocaine/test p = 0.282). When p-4E-BP1 dissociates from eIF4E, eIF4E can be phosphorylated at Ser209 and translation is enhanced (Browne and Proud, 2002; Scheper and Proud, 2002; Senthil et al., 2002). Interestingly, we found a test-dependent increase in p-eIF4E (Ser209; Fig. 5c, left; two-way ANOVA: main effect of test: F(1,26) = 5.02, p = 0.034) but no change in total eIF4E (Fig. 5c, right; Fisher PLSD test: saline vs cocaine p = 0.559; saline vs saline/test p = 0.867; cocaine vs cocaine/test: t(29) = 2.142, p = 0.411; saline/test vs cocaine/test p = 0.953). For the cocaine/test group, we suggest that this is a consequence of the significantly increased p-4E-BP1 observed in this group (Fig. 5b), which in turn may be related to ERK activation (Fig. 5a, left). Therefore, phosphorylation of eIF4E (Fig. 5c, left) may be regulated through distinct mechanisms in the saline/test and cocaine/test groups (see Discussion), reflecting complex cross talk between signaling pathways regulating translation (Wang and Proud, 2007).
Figure 5.
ERK, 4E-BP1, and eIF4E in NAc synaptoneurosomes prepared after prolonged withdrawal from extended-access cocaine self-administration or after a cue-induced seeking test. (a) p-ERK1/2 (Thr202/Tyr204) and total ERK, (b) phosphorylated 4-E-BP1 (Ser65), and (c) phosphorylated eIF4E (Ser209) and total eIF4E in saline, cocaine, saline/test, and cocaine/test groups. Data were obtained from rats in Experiment 4 (n = 8–10 rats/group). Timeline and training data are shown in Figure 2. Data are mean ± SEM. *p < 0.05.
RNA binding proteins and incubation
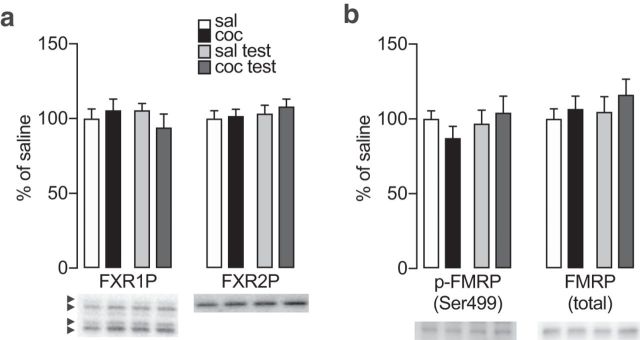
Whereas GluA2-containing AMPARs normally mediate most excitatory synaptic transmission in the NAc, a critical adaptation for incubation of cocaine craving is an increase in homomeric GluA1 CP-AMPARs (Conrad et al., 2008). Therefore, using synaptoneurosomes from Experiment 4, we examined two RNA binding proteins that can specifically regulate translation of AMPAR subunits: fragile X related protein 1 (FXR1P), which has 4 isoforms a-d (Cook et al., 2011) and represses translation of GluA2 (Cook et al., 2014), and fragile X related protein 2 (FXR2P), which enhances translation of GluA1 (Guo et al., 2015) and GluA2 (Cook et al., 2014). However, we did not find any group differences in FXR1P (Fig. 6a, left; Fisher PLSD test: saline vs cocaine p = 0.727, saline vs saline/test p = 0.673, cocaine vs cocaine/test p = 0.243, saline/test vs cocaine/test p = 0.233) or FXR2P (Fig. 6a, right; Fisher PLSD test: saline vs cocaine p = 0.907; saline vs saline/test p = 0.738; cocaine vs cocaine/test p = 0.194; saline/test vs cocaine/test p = 0.399).
Figure 6.
RNA binding proteins FXR1P, FXR2P, and FMRP in NAc synaptoneurosomes prepared after prolonged withdrawal from extended-access cocaine self-administration or after a cue-induced seeking test. a, FXR1P [arrowheads indicate isoforms a (70 kDa), b (72 kDa), c (78 kDa), d (80 kDa)] and FXR2P. b, p-FMRP (Ser499) and total FMRP. Data were obtained from rats in Experiment 4 (n = 8–10 rats/group). Timeline and training data are shown in Figure 2. Data are mean ± SEM.
We also examined FMRP, which regulates the translation of proteins critical to synaptic function (Bassell and Warren, 2008; Sidorov et al., 2013) and is implicated in certain responses to noncontingent cocaine (Fish et al., 2013; Kumar et al., 2013; Smith et al., 2014). Although FMRP does not bind GluA1 mRNA (Zalfa et al., 2007; Darnell et al., 2011; Guo et al., 2015) or GluA2 mRNA (Darnell et al., 2011; Cook et al., 2014), its deficiency can affect AMPAR levels and function (Nakamoto et al., 2007; Hu et al., 2008; Lim et al., 2014; Guo et al., 2015). We also examined FMRP phosphorylation at Ser499 because dephosphorylation of this site is associated with enhanced local translation (Ceman et al., 2003) and is regulated by p70s6k (Narayanan et al., 2008; Bernard et al., 2013; but see Bartley et al., 2014). We did not observe significant group differences in total FMRP protein levels (Fig. 6b, right; Fisher PLSD test: saline vs cocaine p = 0.720; saline vs saline/test p = 0.799; cocaine vs cocaine/test p = 0.388; saline/test vs cocaine/test p = 0.355) or in p-FMRP (Fig. 6b, left; Fisher PLSD test: saline vs cocaine p = 0.181; saline vs saline/test p = 0.735; cocaine vs cocaine/test p = 0.103; saline/test vs cocaine/test p = 0.567), although there was a trend toward increased p-FMRP in the cocaine/test group versus the cocaine group that could suggest enhanced translation during the seeking test.
Discussion
Complex pathways regulate protein translation (Fig. 7). By infusing inhibitors of translation into the NAc core, we found that translation is required for the expression of incubated cocaine seeking (>WD40) but not for cocaine seeking before incubation (WD1/3). Consistent with these behavioral results, immunoblotting results indicated increased translation in NAc tissue from rats that expressed incubated craving, although we cannot definitively link these biochemical changes specifically to incubated craving because we did not conduct parallel biochemical studies in early withdrawal. Nonetheless, our results point to an important role for specific translation pathways in the expression of incubation.
Figure 7.

Signaling pathways related to protein translation mechanisms. Schematic of major signaling pathways regulating protein translation, based on recent reviews (Ruggero and Sonenberg, 2005; Tai and Schuman, 2008; Costa-Mattioli et al., 2009; Liu-Yesucevitz et al., 2011; Kindler and Kreienkamp, 2012; Trinh and Klann, 2013). The directionality of phosphorylation-induced changes are as follows: eIF2α, inhibits; Akt, activates; Erk1/2, activates; 4E-BP1, activates; mTOR, activates; p70s6k, activates; s6, activates; FMRP, inhibits; eEF2, inhibits; eIF4E, activates.
mTOR pathway
mTOR signaling is implicated in animal models of cocaine addiction (Neasta et al., 2014), including sensitization (Wu et al., 2011; Bailey et al., 2012), CPP (Bailey et al., 2012; Yu et al., 2013; Shi et al., 2014; Liu et al., 2017), and measures of motivation or relapse following cocaine self-administration (Wang et al., 2010; James et al., 2014; Gao et al., 2017). In addition, mTOR regulates GluA1 expression (Slipczuk et al., 2009; Neasta et al., 2010; James et al., 2014, 2016) through local translation (Schratt et al., 2004; Beckley et al., 2016). Because rats analyzed after incubation (>WD40) have elevated GluA1 protein and homomeric GluA1 CP-AMPARs (Conrad et al., 2008; Ferrario et al., 2011), and mTOR inhibition normalizes CP-AMPAR levels in NAc neurons from such rats (Scheyer et al., 2014), we expected to observe mTOR activation in the NAc of analogous “incubated rats” (cocaine group) in the present study. Surprisingly, we found no evidence of upstream Akt activation, no changes in mTOR levels or phosphorylation, and only a trend toward activation of mTOR's downstream effector p70s6k (Fig. 2g). If the latter trend is indicative of low-level mTOR activation during withdrawal, this could explain the ability of rapamycin to normalize CP-AMPAR levels in NAc MSN of “incubated rats” (Scheyer et al., 2014). Regardless, our findings suggest that GluA1 upregulation in such rats is mechanistically distinct from other instances in which increased GluA1 translation is associated with robust Akt and mTOR activation (e.g., Beckley et al., 2016). Failure to observe mTOR activation in our cocaine group may reflect the fact that tissue was collected >47 d after the last cocaine exposure, which contrasts with the design of cocaine/mTOR studies cited above.
In contrast, we obtained evidence of mTOR activation in both cocaine/test and saline/test groups, most strikingly a robust increase in p-s6 (Ser235/236) (Fig. 2g), suggesting a role for mTOR in responding to salient cues regardless of preexposure condition (see Werner et al. (2015) for evidence that cues are also salient for saline rats). However, we then demonstrated a specific requirement for mTOR signaling in expression of incubated cocaine seeking. Thus, intra-NAc core injection of anisomycin, a general translation inhibitor, or rapamycin, an mTOR-specific inhibitor, 1 h before a seeking test significantly reduced expression of incubation following prolonged withdrawal (>WD40) (Fig. 1d,f). Because we previously showed that 1 h pretreatment of brain slices with the same inhibitors normalizes CP-AMPAR levels in NAc MSNs from “incubated rats” (Scheyer et al., 2014) and that expression of incubation depends on elevated CP-AMPARs (Conrad et al., 2008; Loweth et al., 2014), translation inhibitors may reduce cocaine seeking by normalizing CP-AMPAR levels. Alternatively, they may be preventing an acute increase in translation during the seeking test (based on Fig. 2g and data on ERK, eIF2α, and eEF2 discussed below). Finally, we cannot rule out other potential mechanisms, including translation-independent effects associated with anisomycin (Canal et al., 2007).
ERK
ERK signaling is implicated in regulation of translation (Kindler and Kreienkamp, 2012) and actions of drugs of abuse (Lu et al., 2006; Girault et al., 2007). Phosphorylation/activation of ERK in the central nucleus of the amygdala, but not basolateral amygdala or prefrontal cortex, is critical for expression of incubated cocaine craving (Lu et al., 2005; Koya et al., 2009), but NAc has not been examined. We began by comparing saline and cocaine groups (WD47-52) and found no differences in ERK levels or phosphorylation (Fig. 5a). We previously reported a very modest increase in pERK2/total ERK2 in a PSD fraction prepared from analogous cocaine rats (Ferrario et al., 2011).
After a seeking test, ERK phosphorylation was significantly increased in cocaine/test rats versus cocaine rats (Fig. 5a). The cocaine/test group also showed increased phosphorylation of the ERK substrate 4E-BP1 (Ser65; Fig. 5b) and its downstream target eIF4E (Ser209; Fig. 5c), indicative of increased translation (Browne and Proud, 2002; Scheper and Proud, 2002; Senthil et al., 2002). Our results in saline/test rats also suggest increases in phosphorylation of eIF4E (Fig. 5c); however, unlike cocaine/test rats, the saline/test group showed no evidence of increased 4E-BP1 phosphorylation (Fig. 5b). Together, these results may suggest that pathways downstream of ERK are differentially regulated in cocaine/test versus saline/test groups. This is consistent with evidence for unique regulation of translation in the cocaine/test group through other effectors, namely, eEF2 (Fig. 3a) and eIF2α (Fig. 4a). However, our results suggesting activation of ERK pathways in saline/test rats are reminiscent of an earlier study in which increased ERK phosphorylation was found in NAc core during the expression of incubated context-induced cocaine seeking but also in response to sucrose seeking (Edwards et al., 2011). Thus, ERK may have a general role in responding to cues, as discussed above for s6. Another study found increased ERK phosphorylation in NAc core after extinction of cocaine self-administration, both before and after cue presentation leading to reinstatement (Gao et al., 2017).
eEF2 and eIF2α
Dephosphorylation of eEF2 or eIF2α enhances protein translation by regulating elongation and initiation, respectively (Taha et al., 2013; Trinh and Klann, 2013). Here, we found a decrease in p-eEF2 (Thr56) in the cocaine/test group compared with cocaine and saline/test groups (Fig. 3a). This could be the result of mTOR- and/or ERK-dependent signaling. mTOR dephosphorylates eEF2 through a signaling cascade involving p70s6k, whereas ERK dephosphorylates eEF2 via p90RSK1 and these pathways signal independently of one another (Wang et al., 2001). In addition, NMDAR transmission suppresses local translation and NMDAR antagonists enhance translation, including translation of GluA1, via bidirectional control of eEF2 (Sutton et al., 2007; Autry et al., 2011; Kavalali and Monteggia, 2015). It is possible that NMDAR plasticity during incubation (Christian et al., 2017b) lessens the ability of NMDAR transmission to maintain eEF2 in a phosphorylated/inactivated state, setting the stage for its dephosphorylation/activation during a cocaine seeking test.
We also found decreased p-eIF2α (Ser51) in the cocaine/test group compared with the saline/test group (and a trend between cocaine/test and cocaine groups; Fig. 4a). To investigate its functional significance, we blocked dephosphorylation of eIF2α by injecting the phosphatase inhibitor Sal003 into the NAc core before a seeking test and found that this attenuated incubated cocaine craving (Fig. 4g,i). These results suggest that eIF2α dephosphorylation is required for expression of incubation, presumably by enabling increased translation during the test. Previous work has shown that eIF2α dephosphorylation/activation in the VTA increased susceptibility to cocaine-induced synaptic potentiation and CPP (Huang et al., 2016) and mediated cocaine-induced LTP (Placzek et al., 2016a). Furthermore, dephosphorylation of eIF2α in VTA potentiated nicotine-dependent excitatory synaptic transmission in mice, and a single nucleotide polymorphism in the Eif2s1 gene (encoding eIF2α) modulates mesolimbic neuronal reward responses in human smokers (Placzek et al., 2016b). Combined with our results, these findings suggest increased protein translation resulting from eIF2α dephosphorylation is critical for responding to drugs and drug cues.
Conclusion
Here we conducted the first examination of the regulation of protein translation during the incubation of cocaine craving. We showed that protein translation, mTOR-dependent signaling, and eIF2α dephosphorylation are required for expression of incubated cocaine seeking (WD>40), using intra-NAc core injections of anisomycin, rapamycin, and Sal003, respectively. In contrast, inhibiting translation did not affect cocaine seeking before incubation (WD1/3). We then conducted biochemical studies of NAc tissue after prolonged withdrawal from self-administration or 30 min after a cue-induced seeking test (all assessments on WD47-52). Analysis of signaling pathways (Fig. 7) supported an increase in protein translation in the NAc core when cocaine rats express incubated cocaine seeking. Interestingly, responding for a cue may also increase translation in saline rats, albeit through different mechanisms. We found no significant changes in levels of RNA binding proteins FMRP, FX1RP, and FX2RP, or in FMRP phosphorylation.
It will be important to explore the changes in synaptic transmission that are upstream of the alterations in translation-related signaling cascades described here. To this end, we are studying the ability of ionotropic and metabotropic glutamate receptors to modulate translation in the NAc of drug-naive and “incubated rats” (Stefanik et al., 2018) and in cultured NAc neurons (Wolf and Stefanik, 2016). Another challenge is to identify specific proteins whose translation is regulated during incubation of craving. The present results lay the foundation for such work. Given that protein translation is a target for pharmacotherapies in other brain disorders (e.g., Bhakar et al., 2012; Kavalali and Monteggia, 2015), a better understanding of the regulation of translation after cocaine exposure may identify novel treatment strategies.
Footnotes
This work was supported by United States Public Health Service Grant DA015835 to M.E.W. and individual National Research Service Awards F31 DA036950 to C.T.W. and F32 DA040414 to M.T.S. We thank Dr. Dana Most and Dr. R. Adron Harris for generously providing their protocol for synaptoneurosome preparation.
The authors declare no competing financial interests.
References
- Aoto J, Nam CI, Poon MM, Ting P, Chen L (2008) Synaptic signaling by all-trans retinoic acid in homeostatic synaptic plasticity. Neuron 60:308–320. 10.1016/j.neuron.2008.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM (2011) NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 475:91–95. 10.1038/nature10130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayuso MI, Martinez-Alonso E, Salvador N, Bonova P, Regidor I, Alcázar A (2015) Dissociation of eIF4E-binding protein 2 (4E-BP2) from eIF4E independent of Thr37/Thr46 phosphorylation in the ischemic stress response. PLoS One 10:e0121958. 10.1371/journal.pone.0121958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J, Ma D, Szumlinski KK (2012) Rapamycin attenuates the expression of cocaine-induced place preference and behavioral sensitization. Addict Biol 17:248–258. 10.1111/j.1369-1600.2010.00311.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley CM, O'Keefe RA, Bordey A (2014) FMRP S499 is phosphorylated independent of mTORC1–S6K1 activity. PLoS One 9:e96956. 10.1371/journal.pone.0096956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassell GJ, Warren ST (2008) Fragile X syndrome: loss of local mRNA regulation alters synaptic development and function. Neuron 60:201–214. 10.1016/j.neuron.2008.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckley JT, Laguesse S, Phamluong K, Morisot N, Wegner SA, Ron D (2016) The first alcohol drink triggers mTORC1-dependent synaptic plasticity in nucleus accumbens dopamine D1 receptor neurons. J Neurosci 36:701–713. 10.1523/JNEUROSCI.2254-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard PB, Castano AM, O'Leary H, Simpson K, Browning MD, Benke TA (2013) Phosphorylation of FMRP and alterations of FMRP complex underlie enhanced mLTD in adult rats triggered by early life seizures. Neurobiol Dis 59:1–17. 10.1016/j.nbd.2013.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakar AL, Dölen G, Bear MF (2012) The pathophysiology of fragile X (and what it teaches us about synapses). Annu Rev Neurosci 35:417–443. 10.1146/annurev-neuro-060909-153138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidinosti M, Ran I, Sanchez-Carbente MR, Martineau Y, Gingras AC, Gkogkas C, Raught B, Bramham CR, Sossin WS, Costa-Mattioli M, DesGroseillers L, Lacaille JC, Sonenberg N (2010) Postnatal deamidation of 4E-BP2 in brain enhances its association with raptor and alters kinetics of excitatory synaptic transmission. Mol Cell 37:797–808. 10.1016/j.molcel.2010.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne GJ, Proud CG (2002) Regulation of peptide-chain elongation in mammalian cells. Eur J Biochem 269:5360–5368. 10.1046/j.1432-1033.2002.03290.x [DOI] [PubMed] [Google Scholar]
- Canal CE, Chang Q, Gold PE (2007) Amnesia produced by altered release of neurotransmitters after intraamygdala injections of a protein synthesis inhibitor. Proc Natl Acad Sci U S A 104:12500–12505. 10.1073/pnas.0705195104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceman S, O'Donnell WT, Reed M, Patton S, Pohl J, Warren ST (2003) Phosphorylation influences the translation state of FMRP-associated polyribosomes. Hum Mol Genet 12:3295–3305. 10.1093/hmg/ddg350 [DOI] [PubMed] [Google Scholar]
- Chiang GG, Abraham RT (2005) Phosphorylation of mammalian target of rapamycin (mTOR) at ser-2448 is mediated by p70S6 kinase. J Biol Chem 280:25485–25490. 10.1074/jbc.M501707200 [DOI] [PubMed] [Google Scholar]
- Christian DT, Wang X, Chen EL, Sehgal LK, Ghassemlou MN, Miao JJ, Estepanian D, Araghi CH, Stutzmann GE, Wolf ME (2017a) Dynamic alterations of rat nucleus accumbens dendritic spines over 2 months of abstinence from extended-access cocaine self-administration. Neuropsychopharmacology 42:748–756. 10.1038/npp.2016.168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian DT, Tseng KY, Wolf ME (2017b) Extended access cocaine self-administration leads to increased GluN3-containing NMDA receptor function in the rat nucleus accumbens. Washington, DC: Society for Neuroscience. [Google Scholar]
- Chung J, Kuo CJ, Crabtree GR, Blenis J (1992) Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell 69:1227–1236. 10.1016/0092-8674(92)90643-Q [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME (2008) Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature 453:118–121. 10.1038/nature06995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D, Sanchez-Carbente Mdel R, Lachance C, Radzioch D, Tremblay S, Khandjian EW, DesGroseillers L, Murai KK (2011) Fragile X related protein 1 clusters with ribosomes and messenger RNAs at a subset of dendritic spines in the mouse hippocampus. PLoS One 6:e26120. 10.1371/journal.pone.0026120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook D, Nuro E, Jones EV, Altimimi HF, Farmer WT, Gandin V, Hanna E, Zong R, Barbon A, Nelson DL, Topisirovic I, Rochford J, Stellwagen D, Béïque JC, Murai KK (2014) FXR1P limits long-term memory, long-lasting synaptic potentiation, and de novo GluA2 translation. Cell Rep 9:1402–1416. 10.1016/j.celrep.2014.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Mattioli M, Gobert D, Stern E, Gamache K, Colina R, Cuello C, Sossin W, Kaufman R, Pelletier J, Rosenblum K, Krnjević K, Lacaille JC, Nader K, Sonenberg N (2007) eIF2alpha phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell 129:195–206. 10.1016/j.cell.2007.01.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N (2009) Translational control of long-lasting synaptic plasticity and memory. Neuron 61:10–26. 10.1016/j.neuron.2008.10.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ (2006) Hypothalamic mTOR signaling regulates food intake. Science 312:927–930. 10.1126/science.1124147 [DOI] [PubMed] [Google Scholar]
- Dalle Pezze P, Sonntag AG, Thien A, Prentzell MT, Gödel M, Fischer S, Neumann-Haefelin E, Huber TB, Baumeister R, Shanley DP, Thedieck K (2012) A dynamic network model of mTOR signaling reveals TSC-independent mTORC2 regulation. Sci Signal 5:ra25. 10.1126/scisignal.2002469 [DOI] [PubMed] [Google Scholar]
- Darnell JC, Van Driesche SJ, Zhang C, Hung KY, Mele A, Fraser CE, Stone EF, Chen C, Fak JJ, Chi SW, Licatalosi DD, Richter JD, Darnell RB (2011) FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 146:247–261. 10.1016/j.cell.2011.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, Smith DW, Dunkley PR (2012) An emerging role for the mammalian target of rapamycin in “pathological” protein translation: relevance to cocaine addiction. Front Pharmacol 3:13. 10.3389/fphar.2012.00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieterich DC, Hodas JJ, Gouzer G, Shadrin IY, Ngo JT, Triller A, Tirrell DA, Schuman EM (2010) In situ visualization and dynamics of newly synthesized proteins in rat hippocampal neurons. Nat Neurosci 13:897–905. 10.1038/nn.2580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Prisco GV, Huang W, Buffington SA, Hsu CC, Bonnen PE, Placzek AN, Sidrauski C, Krnjević K, Kaufman RJ, Walter P, Costa-Mattioli M (2014) Translational control of mGluR-dependent long-term depression and object-place learning by eIF2alpha. Nat Neurosci 17:1073–1082. 10.1038/nn.3754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufner A, Thomas G (1999) Ribosomal S6 kinase signaling and the control of translation. Exp Cell Res 253:100–109. 10.1006/excr.1999.4683 [DOI] [PubMed] [Google Scholar]
- Edwards S, Bachtell RK, Guzman D, Whisler KN, Self DW (2011) Emergence of context-associated GluR(1) and ERK phosphorylation in the nucleus accumbens core during withdrawal from cocaine self-administration. Addict Biol 16:450–457. 10.1111/j.1369-1600.2010.00296.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Li X, Wang X, Reimers JM, Uejima JL, Wolf ME (2010) The role of glutamate receptor redistribution in locomotor sensitization to cocaine. Neuropsychopharmacology 35:818–833. 10.1038/npp.2009.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Loweth JA, Milovanovic M, Ford KA, Galiñanes GL, Heng LJ, Tseng KY, Wolf ME (2011) Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca(2)(+)-permeable AMPA receptors during the incubation of cocaine craving. Neuropharmacology 61:1141–1151. 10.1016/j.neuropharm.2011.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish EW, Krouse MC, Stringfield SJ, Diberto JF, Robinson JE, Malanga CJ (2013) Changes in sensitivity of reward and motor behavior to dopaminergic, glutamatergic, and cholinergic drugs in a mouse model of fragile X syndrome. PLoS One 8:e77896. 10.1371/journal.pone.0077896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster KG, Fingar DC (2010) Mammalian target of rapamycin (mTOR): conducting the cellular signaling symphony. J Biol Chem 285:14071–14077. 10.1074/jbc.R109.094003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao XJ, Yuan K, Cao L, Yan W, Luo YX, Jian M, Liu JF, Fang Q, Wang JS, Han Y, Shi J, Lu L (2017) AMPK signaling in the nucleus accumbens core mediates cue-induced reinstatement of cocaine seeking. Sci Rep 7:1038. 10.1038/s41598-017-01043-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingras AC, Raught B, Sonenberg N (2001) Regulation of translation initiation by FRAP/mTOR. Genes Dev 15:807–826. 10.1101/gad.887201 [DOI] [PubMed] [Google Scholar]
- Girault JA, Valjent E, Caboche J, Hervé D (2007) ERK2: a logical AND gate critical for drug-induced plasticity? Curr Opin Pharmacol 7:77–85. 10.1016/j.coph.2006.08.012 [DOI] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y (2001) Incubation of cocaine craving after withdrawal. Nature 412:141–142. 10.1038/35084134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Polich ED, Su J, Gao Y, Christopher DM, Allan AM, Wang M, Wang F, Wang G, Zhao X (2015) Fragile X proteins FMRP and FXR2P control synaptic GluA1 expression and neuronal maturation via distinct mechanisms. Cell Rep 11:1651–1666. 10.1016/j.celrep.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert TP, Proud CG (2006) Regulation of translation elongation and cotranslational protein targeting. In: Translational control of gene expression (Sonenberg N, Hershey JW, Mathews MB, eds). Cold Spring Harbor, NY: Cold Spring Harbor Laboratory. [Google Scholar]
- Herbert TP, Tee AR, Proud CG (2002) The extracellular signal-regulated kinase pathway regulates the phosphorylation of 4E-BP1 at multiple sites. J Biol Chem 277:11591–11596. 10.1074/jbc.M110367200 [DOI] [PubMed] [Google Scholar]
- Hinz FI, Dieterich DC, Schuman EM (2013) Teaching old NCATs new tricks: using non-canonical amino acid tagging to study neuronal plasticity. Curr Opin Chem Biol 17:738–746. 10.1016/j.cbpa.2013.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingsworth EB, McNeal ET, Burton JL, Williams RJ, Daly JW, Creveling CR (1985) Biochemical characterization of a filtered synaptoneurosome preparation from guinea pig cerebral cortex: cyclic adenosine 3′:5′-monophosphate-generating systems, receptors, and enzymes. J Neurosci 5:2240–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz MK, Ballif BA, Gygi SP, Blenis J (2005) mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell 123:569–580. 10.1016/j.cell.2005.10.024 [DOI] [PubMed] [Google Scholar]
- Hu H, Qin Y, Bochorishvili G, Zhu Y, van Aelst L, Zhu JJ (2008) Ras signaling mechanisms underlying impaired GluR1-dependent plasticity associated with fragile X syndrome. J Neurosci 28:7847–7862. 10.1523/JNEUROSCI.1496-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W, Placzek AN, Viana Di Prisco G, Khatiwada S, Sidrauski C, Krnjević K, Walter P, Dani JA, Costa-Mattioli M (2016) Translational control by eIF2alpha phosphorylation regulates vulnerability to the synaptic and behavioral effects of cocaine. Elife 5:e12052. 10.7554/eLife.12052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Quinn RK, Ong LK, Levi EM, Charnley JL, Smith DW, Dickson PW, Dayas CV (2014) mTORC1 inhibition in the nucleus accumbens “protects” against the expression of drug seeking and “relapse” and is associated with reductions in GluA1 AMPAR and CaMKIIalpha levels. Neuropsychopharmacology 39:1694–1702. 10.1038/npp.2014.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Quinn RK, Ong LK, Levi EM, Smith DW, Dickson PW, Dayas CV (2016) Rapamycin reduces motivated responding for cocaine and alters GluA1 expression in the ventral but not dorsal striatum. Eur J Pharmacol 784:147–154. 10.1016/j.ejphar.2016.05.013 [DOI] [PubMed] [Google Scholar]
- Jian M, Luo YX, Xue YX, Han Y, Shi HS, Liu JF, Yan W, Wu P, Meng SQ, Deng JH, Shen HW, Shi J, Lu L (2014) eIF2alpha dephosphorylation in basolateral amygdala mediates reconsolidation of drug memory. J Neurosci 34:10010–10021. 10.1523/JNEUROSCI.0934-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavalali ET, Monteggia LM (2015) How does ketamine elicit a rapid antidepressant response? Curr Opin Pharmacol 20:35–39. 10.1016/j.coph.2014.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler S, Kreienkamp HJ (2012) Dendritic mRNA targeting and translation. Adv Exp Med Biol 970:285–305. 10.1007/978-3-7091-0932-8_13 [DOI] [PubMed] [Google Scholar]
- Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y (2009) Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology 56:177–185. 10.1016/j.neuropharm.2008.04.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Kim K, Joseph C, Kourrich S, Yoo SH, Huang HC, Vitaterna MH, de Villena FP, Churchill G, Bonci A, Takahashi JS (2013) C57BL/6N mutation in cytoplasmic FMRP interacting protein 2 regulates cocaine response. Science 342:1508–1512. 10.1126/science.1245503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BR, Ma YY, Huang YH, Wang X, Otaka M, Ishikawa M, Neumann PA, Graziane NM, Brown TE, Suska A, Guo C, Lobo MK, Sesack SR, Wolf ME, Nestler EJ, Shaham Y, Schlüter OM, Dong Y (2013) Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat Neurosci 16:1644–1651. 10.1038/nn.3533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao K, Guo M, Niu F, Yang L, Callen SE, Buch S (2016) Cocaine-mediated induction of microglial activation involves the ER stress-TLR2 axis. J Neuroinflammation 13:33. 10.1186/s12974-016-0501-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Venniro M, Shaham Y (2016) Translational research on incubation of cocaine craving. JAMA Psychiatry 73:1115–1116. 10.1001/jamapsychiatry.2016.2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CS, Hoang ET, Viar KE, Stornetta RL, Scott MM, Zhu JJ (2014) Pharmacological rescue of ras signaling, GluA1-dependent synaptic plasticity, and learning deficits in a fragile X model. Genes Dev 28:273–289. 10.1101/gad.232470.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Li Y, Yu L, Vickstrom CR, Liu QS (2017) VTA mTOR signaling regulates dopamine dynamics, cocaine-induced synaptic alterations, and reward. Neuropsychopharmacology. Advance online publication. Retrieved Oct. 17, 2017. doi: 10.1038/npp.2017.247. 10.1038/npp.2017.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Yesucevitz L, Bassell GJ, Gitler AD, Hart AC, Klann E, Richter JD, Warren ST, Wolozin B (2011) Local RNA translation at the synapse and in disease. J Neurosci 31:16086–16093. 10.1523/JNEUROSCI.4105-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loweth JA, Scheyer AF, Milovanovic M, LaCrosse AL, Flores-Barrera E, Werner CT, Li X, Ford KA, Le T, Olive MF, Szumlinski KK, Tseng KY, Wolf ME (2014) Synaptic depression via mGluR1 positive allosteric modulation suppresses cue-induced cocaine craving. Nat Neurosci 17:73–80. 10.1038/nn.3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y (2004) Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology 47 [Suppl 1]:214–226. [DOI] [PubMed] [Google Scholar]
- Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y (2005) Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci 8:212–219. 10.1038/nn1383 [DOI] [PubMed] [Google Scholar]
- Lu L, Koya E, Zhai H, Hope BT, Shaham Y (2006) Role of ERK in cocaine addiction. Trends Neurosci 29:695–703. 10.1016/j.tins.2006.10.005 [DOI] [PubMed] [Google Scholar]
- Ma YY, Lee BR, Wang X, Guo C, Liu L, Cui R, Lan Y, Balcita-Pedicino JJ, Wolf ME, Sesack SR, Shaham Y, Schlüter OM, Huang YH, Dong Y (2014) Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron 83:1453–1467. 10.1016/j.neuron.2014.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M (2011a) Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J Neurosci 31:5737–5743. 10.1523/JNEUROSCI.0350-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Loweth JA, Ford KA, Marinelli M, Wolf ME, Tseng KY (2011b) Group I mGluR activation reverses cocaine-induced accumulation of calcium-permeable AMPA receptors in nucleus accumbens synapses via a protein kinase C-dependent mechanism. J Neurosci 31:14536–14541. 10.1523/JNEUROSCI.3625-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyuhas O. (2015) Ribosomal protein S6 phosphorylation: four decades of research. Int Rev Cell Mol Biol 320:41–73. 10.1016/bs.ircmb.2015.07.006 [DOI] [PubMed] [Google Scholar]
- Most D, Ferguson L, Blednov Y, Mayfield RD, Harris RA (2015) The synaptoneurosome transcriptome: a model for profiling the emolecular effects of alcohol. Pharmacogenomics 15:177–188. 10.1038/tpj.2014.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto M, Nalavadi V, Epstein MP, Narayanan U, Bassell GJ, Warren ST (2007) Fragile X mental retardation protein deficiency leads to excessive mGluR5-dependent internalization of AMPA receptors. Proc Natl Acad Sci U S A 104:15537–15542. 10.1073/pnas.0707484104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan U, Nalavadi V, Nakamoto M, Thomas G, Ceman S, Bassell GJ, Warren ST (2008) S6K1 phosphorylates and regulates fragile X mental retardation protein (FMRP) with the neuronal protein synthesis-dependent mammalian target of rapamycin (mTOR) signaling cascade. J Biol Chem 283:18478–18482. 10.1074/jbc.C800055200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navé BT, Ouwens M, Withers DJ, Alessi DR, Shepherd PR (1999) Mammalian target of rapamycin is a direct target for protein kinase B: identification of a convergence point for opposing effects of insulin and amino-acid deficiency on protein translation. Biochem J 344:427–431. 10.1042/bj3440427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neasta J, Ben Hamida S, Yowell Q, Carnicella S, Ron D (2010) Role for mammalian target of rapamycin complex 1 signaling in neuroadaptations underlying alcohol-related disorders. Proc Natl Acad Sci U S A 107:20093–20098. 10.1073/pnas.1005554107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neasta J, Barak S, Hamida SB, Ron D (2014) mTOR complex 1: a key player in neuroadaptations induced by drugs of abuse. J Neurochem 130:172–184. 10.1111/jnc.12725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons RG, Gafford GM, Helmstetter FJ (2006) Translational control via the mammalian target of rapamycin pathway is critical for the formation and stability of long-term fear memory in amygdala neurons. J Neurosci 26:12977–12983. 10.1523/JNEUROSCI.4209-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvaz MA, Moeller SJ, Goldstein RZ (2016) Incubation of cue-induced craving in adults addicted to cocaine measured by electroencephalography. JAMA Psychiatry 73:1127–1134. 10.1001/jamapsychiatry.2016.2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoli V, Terrier J, Espallergues J, Valjent E, O'Connor EC, Lüscher C (2014) Contrasting forms of cocaine-evoked plasticity control components of relapse. Nature 509:459–464. 10.1038/nature13257 [DOI] [PubMed] [Google Scholar]
- Peterson RT, Beal PA, Comb MJ, Schreiber SL (2000) FKBP12-rapamycin-associated protein (FRAP) autophosphorylates at serine 2481 under translationally repressive conditions. J Biol Chem 275:7416–7423. 10.1074/jbc.275.10.7416 [DOI] [PubMed] [Google Scholar]
- Placzek AN, Prisco GV, Khatiwada S, Sgritta M, Huang W, Krnjević K, Kaufman RJ, Dani JA, Walter P, Costa-Mattioli M (2016a) eIF2alpha-mediated translational control regulates the persistence of cocaine-induced LTP in midbrain dopamine neurons. Elife 5:e17517. 10.7554/eLife.17517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placzek AN, Molfese DL, Khatiwada S, Viana Di Prisco G, Huang W, Sidrauski C, Krnjević K, Amos CL, Ray R, Dani JA, Walter P, Salas R, Costa-Mattioli M (2016b) Translational control of nicotine-evoked synaptic potentiation in mice and neuronal responses in human smokers by eIF2alpha. Elife 5:e12056. 10.7554/eLife.12056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purgianto A, Scheyer AF, Loweth JA, Ford KA, Tseng KY, Wolf ME (2013) Different adaptations in AMPA receptor transmission in the nucleus accumbens after short vs long access cocaine self-administration regimens. Neuropsychopharmacology 38:1789–1797. 10.1038/npp.2013.78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan EM, Olstein DH, Bear MF (1999) Bidirectional, experience-dependent regulation of N-methyl-d-aspartate receptor subunit composition in the rat visual cortex during postnatal development. Proc Natl Acad Sci U S A 96:12876–12880. 10.1073/pnas.96.22.12876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redpath NT, Foulstone EJ, Proud CG (1996) Regulation of translation elongation factor-2 by insulin via a rapamycin-sensitive signalling pathway. EMBO J 15:2291–2297. [PMC free article] [PubMed] [Google Scholar]
- Ruggero D, Sonenberg N (2005) The akt of translational control. Oncogene 24:7426–7434. 10.1038/sj.onc.1209098 [DOI] [PubMed] [Google Scholar]
- Scheper GC, Proud CG (2002) Does phosphorylation of the cap-binding protein eIF4E play a role in translation initiation? Eur J Biochem 269:5350–5359. 10.1046/j.1432-1033.2002.03291.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheyer AF, Wolf ME, Tseng KY (2014) A protein synthesis-dependent mechanism sustains calcium-permeable AMPA receptor transmission in nucleus accumbens synapses during withdrawal from cocaine self-administration. J Neurosci 34:3095–3100. 10.1523/JNEUROSCI.4940-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schratt GM, Nigh EA, Chen WG, Hu L, Greenberg ME (2004) BDNF regulates the translation of a select group of mRNAs by a mammalian target of rapamycin-phosphatidylinositol 3-kinase-dependent pathway during neuronal development. J Neurosci 24:7366–7377. 10.1523/JNEUROSCI.1739-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senthil D, Choudhury GG, Abboud HE, Sonenberg N, Kasinath BS (2002) Regulation of protein synthesis by IGF-I in proximal tubular epithelial cells. Am J Physiol Renal Physiol 283:F1226–F1236. 10.1152/ajprenal.00109.2002 [DOI] [PubMed] [Google Scholar]
- Sesack SR, Grace AA (2010) Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology 35:27–47. 10.1038/npp.2009.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Miller JS, Harper LJ, Poole RL, Gould TJ, Unterwald EM (2014) Reactivation of cocaine reward memory engages the Akt/GSK3/mTOR signaling pathway and can be disrupted by GSK3 inhibition. Psychopharmacology (Berl) 231:3109–3118. 10.1007/s00213-014-3491-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidorov MS, Auerbach BD, Bear MF (2013) Fragile X mental retardation protein and synaptic plasticity. Mol Brain 6:15. 10.1186/1756-6606-6-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slipczuk L, Bekinschtein P, Katche C, Cammarota M, Izquierdo I, Medina JH (2009) BDNF activates mTOR to regulate GluR1 expression required for memory formation. PLoS One 4:e6007. 10.1371/journal.pone.0006007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LN, Jedynak JP, Fontenot MR, Hale CF, Dietz KC, Taniguchi M, Thomas FS, Zirlin BC, Birnbaum SG, Huber KM, Thomas MJ, Cowan CW (2014) Fragile X mental retardation protein regulates synaptic and behavioral plasticity to repeated cocaine administration. Neuron 82:645–658. 10.1016/j.neuron.2014.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman GA, Acosta-Jaquez HA, Dunlop EA, Ekim B, Maj NE, Tee AR, Fingar DC (2010) mTOR ser-2481 autophosphorylation monitors mTORC-specific catalytic activity and clarifies rapamycin mechanism of action. J Biol Chem 285:7866–7879. 10.1074/jbc.M109.096222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenberg N, Hinnebusch AG (2009) Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 136:731–745. 10.1016/j.cell.2009.01.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg BA, Todd RP, Slaker M, Churchill L (2015) Anisomycin in the medial prefrontal cortex reduces reconsolidation of cocaine-associated memories in the rat self-administration model. Neuropharmacology 92:25–33. 10.1016/j.neuropharm.2014.12.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik MT, Milovanovic M, Wolf ME (2018) Examining protein synthesis in the nucleus accumbens after withdrawal from extended-access cocaine self-administration. Washington, DC: Society for Neuroscience, Neuroscience Meeting Planner. [Google Scholar]
- Sun X, Wolf ME (2009) Nucleus accumbens neurons exhibit synaptic scaling that is occluded by repeated dopamine pre-exposure. Eur J Neurosci 30:539–550. 10.1111/j.1460-9568.2009.06852.x [DOI] [PubMed] [Google Scholar]
- Sun X, Milovanovic M, Zhao Y, Wolf ME (2008) Acute and chronic dopamine receptor stimulation modulates AMPA receptor trafficking in nucleus accumbens neurons cocultured with prefrontal cortex neurons. J Neurosci 28:4216–4230. 10.1523/JNEUROSCI.0258-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton LP, Caron MG (2015) Essential role of D1R in the regulation of mTOR complex1 signaling induced by cocaine. Neuropharmacology 99:610–619. 10.1016/j.neuropharm.2015.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton MA, Taylor AM, Ito HT, Pham A, Schuman EM (2007) Postsynaptic decoding of neural activity: eEF2 as a biochemical sensor coupling miniature synaptic transmission to local protein synthesis. Neuron 55:648–661. 10.1016/j.neuron.2007.07.030 [DOI] [PubMed] [Google Scholar]
- Taha E, Gildish I, Gal-Ben-Ari S, Rosenblum K (2013) The role of eEF2 pathway in learning and synaptic plasticity. Neurobiol Learn Mem 105:100–106. 10.1016/j.nlm.2013.04.015 [DOI] [PubMed] [Google Scholar]
- Tai HC, Schuman EM (2008) Ubiquitin, the proteasome and protein degradation in neuronal function and dysfunction. Nat Rev Neurosci 9:826–838. 10.1038/nrn2499 [DOI] [PubMed] [Google Scholar]
- tom Dieck S, Müller A, Nehring A, Hinz FI, Bartnik I, Schuman EM, Dieterich DC (2012) Metabolic labeling with noncanonical amino acids and visualization by chemoselective fluorescent tagging. Curr Protoc Cell Biol 7:11. 10.1002/0471143030.cb0711s56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh MA, Klann E (2013) Translational control by eIF2alpha kinases in long-lasting synaptic plasticity and long-term memory. Neurobiol Learn Mem 105:93–99. 10.1016/j.nlm.2013.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vattem KM, Wek RC (2004) Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci U S A 101:11269–11274. 10.1073/pnas.0400541101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Proud CG (2007) Methods for studying signal-dependent regulation of translation factor activity. Methods Enzymol 431:113–142. 10.1016/S0076-6879(07)31007-0 [DOI] [PubMed] [Google Scholar]
- Wang X, Li W, Williams M, Terada N, Alessi DR, Proud CG (2001) Regulation of elongation factor 2 kinase by p90(RSK1) and p70 S6 kinase. EMBO J 20:4370–4379. 10.1093/emboj/20.16.4370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Luo YX, He YY, Li FQ, Shi HS, Xue LF, Xue YX, Lu L (2010) Nucleus accumbens core mammalian target of rapamycin signaling pathway is critical for cue-induced reinstatement of cocaine seeking in rats. J Neurosci 30:12632–12641. 10.1523/JNEUROSCI.1264-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner CT, Milovanovic M, Christian DT, Loweth JA, Wolf ME (2015) Response of the ubiquitin proteasome system to memory retrieval after extended-access cocaine or saline self-administration. Neuropsychopharmacology 40:3006–3014. 10.1038/npp.2015.156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner CT, Murray CH, Reimers JM, Chauhan NM, Woo KK, Molla HM, Loweth JA, Wolf ME (2017) Trafficking of calcium-permeable and calcium-impermeable AMPA receptors in nucleus accumbens medium spiny neurons co-cultured with prefrontal cortex neurons. Neuropharmacology 116:224–232. 10.1016/j.neuropharm.2016.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. (2016) Synaptic mechanisms underlying persistent cocaine craving. Nat Rev Neurosci 17:351–365. 10.1038/nrn.2016.39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Stefanik MT (2016) Mechanisms regulating protein synthesis in cultured nucleus accumbens neurons. San Diego, CA: Society for Neuroscience, Neuroscience Meeting Planner. [Google Scholar]
- Wolf ME, Tseng KY (2012) Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how, and why? Front Mol Neurosci 5:72. 10.3389/fnmol.2012.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman ER, Haddick PC, Bush K, Dilly GA, Niere F, Zemelman BV, Raab-Graham KF (2015) Rapid antidepressants stimulate the decoupling of GABA(B) receptors from GIRK/Kir3 channels through increased protein stability of 14–3-3eta. Mol Psychiatry 20:298–310. 10.1038/mp.2014.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, McCallum SE, Glick SD, Huang Y (2011) Inhibition of the mammalian target of rapamycin pathway by rapamycin blocks cocaine-induced locomotor sensitization. Neuroscience 172:104–109. 10.1016/j.neuroscience.2010.10.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Zhong P, Liu X, Sun D, Gao HQ, Liu QS (2013) Metabotropic glutamate receptor I (mGluR1) antagonism impairs cocaine-induced conditioned place preference via inhibition of protein synthesis. Neuropsychopharmacology 38:1308–1321. 10.1038/npp.2013.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalfa F, Eleuteri B, Dickson KS, Mercaldo V, De Rubeis S, di Penta A, Tabolacci E, Chiurazzi P, Neri G, Grant SG, Bagni C (2007) A new function for the fragile X mental retardation protein in regulation of PSD-95 mRNA stability. Nat Neurosci 10:578–587. 10.1038/nn1893 [DOI] [PMC free article] [PubMed] [Google Scholar]