Abstract
Cancer is a complex disease where cancer stem cells (CSCs) maintain unlimited replicative potential, but evade chemotherapy drugs through cellular quiescence. CSCs are able to give rise to bulk tumor cells that have the capability to override anti-proliferative signals and evade apoptosis. Numerous pathways are dysregulated in tumor cells, where increased levels of pro-oxidant reactive oxygen and nitrogen species (RONS) can lead to localized inflammation to exacerbate all three stages of tumorigenesis: initiation, progression, and metastasis. Modulation of cellular metabolism in tumor cells as well as immune cells in the tumor microenvironment (TME) can impact inflammatory networks. Altering these pathways can potentially serve as a portal for therapy. It is well known that selenium, through selenoproteins, modulates inflammatory pathways in addition to regulating redox homeostasis in cells. Therefore, selenium has the potential to impact the interaction between tumor cells, cancer stem cells, and immune cells. In the sections below, we review the current status of knowledge regarding this interaction, with reference to leukemia stem cells (LSCs), and the importance of selenium-dependent regulation of inflammation as a potential mechanism to affect the TME and tumor cell survival.
Tumor microenvironment- interplay with immune cells and pathways of inflammation
The tumor tissue consists of parenchyma that includes malignant cells and the stromal compartment. This heterogeneous milieu comprises of various cell types that express adhesion molecules, which provide functional activity and structural support to the TME. The TME is also highly vascularized, which allows infiltrating inflammatory cells and a variety of associated tissue-resident cells entrance into the tumor. Most importantly, the infiltrates of inflammatory cells in TME are enriched with different T cell populations, B-cells, and innate immune cells such as macrophages, polymorphonuclear leukocytes (PMNLs; neutrophils) and natural killer cells (NK cells)(Whiteside, 2008). Among these, presence of cytotoxic CD8+ memory T cells (CD8+ CD45RO+) and their supporter CD4+ T helper1 (Th1) cells with signature cytokines such as interleukin -2 (IL-2) and interferon –γ (IFN-γ) in TME correlate with better prognosis (Fridman, Pagès, Sautès-Fridman & Galon, 2012). Other CD4+ cells such as Th2 cells producing IL-4, IL-5, and IL-13 that support B cell responses, macrophages, or CD4+ Th17 T cells that produce IL-17A, IL-17F, IL-21 and IL-22, all of which favor antimicrobial tissue inflammation are generally thought to promote tumor growth (Fridman, Pagès, Sautès-Fridman & Galon, 2012). The immunosuppressive T regulatory cells (Tregs; CD4+CD25hiFOXP3+) exert tumor promoting function through production of IL-10 and transforming growth factor-β (TGF-β) inhibiting recognition and clearance of tumor cells. Furthermore, high numbers of Tregs in TME correlates with poor prognosis in many types of cancer (Bates et al., 2006; Curiel et al., 2004; Hiraoka, Onozato, Kosuge & Hirohashi, 2006). Studies in rodent models have shown that selenium synergizes with fish oil to affect anti-tumor immunity through a decrease in Tregs that is also associated with a decrease in cancer cachexia (Wang et al., 2013). However, in an iodine-induced autoimmune thyroiditis (AIT) model in mice, selenite administration in drinking water (0.3 mg/L) actually increased Tregs with a concomitant decrease in lymphocyte infiltration and anti-thyroglobulin antibody titres leading to decreased AIT (Xue et al., 2010). This clearly suggests divergent effects of selenium supplementation with regard to the regulation of Tregs in immune suppression that adds another layer of complexity to the Yin-Yang functions of these cells in immune regulation.
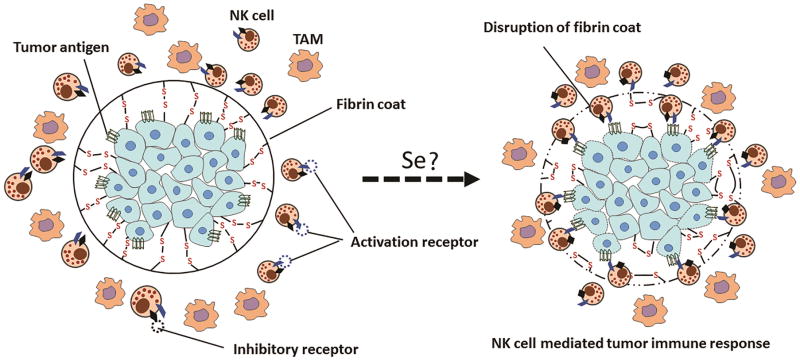
Natural killer cells (NK cells) spontaneously kill cells deemed dangerous to the host. This process is regulated by complex integration of signals from receptors expressed on them including inhibitory receptors (Ly49 in mouse and killer immunoglobulin receptor in humans) that recognize the inhibitory signals and activate receptors that recognize stress-induced ligands on target cells such as antibodies and soluble factors like cytokines (Guillerey, Huntington & Smyth, 2016). In the tumor microenvironment, tumor cells, tumor associated fibroblasts and other tumor –induced aberrant infiltrates including tolerogenic macrophages, dendritic cells and T cells can either secrete immunosuppressive products or interfere with complex receptor array that regulates the activation and anti-tumor activity of NK cells (Vitale, Cantoni, Pietra, Mingari & Moretta, 2014). Additionally, activated platelets can directly inhibit NK cells and platelet cloak formation seems to be critical for inhibition of NK cell mediated killing of circulating tumor cells (Krasnova, Putz, Smyth & Souza-Fonseca-Guimaraes, 2017). Selenium potentiates NK cell activity in two ways (Figure 1). First, it prevents the non-enzymatic formation of parafibrin that surrounds tumor cells rendering the tumor vulnerable to immune surveillance (Lipinski, 2016). Second, selenium activates the NK cell population in TME (Kiremidjian-Schumacher, Roy, Wishe, Cohen & Stotzky, 1996). It is shown that selenite has a property of oxidizing polythiols but not monothiols such as cysteine or glutathione (Frenkel, Falvey & MacVicar, 1991). The oxidant property of selenite is seen only in the presence of polythiols where selenite oxidizes thiol groups to form selenopersulfides at the surface of tumor cells that abolishes proteolytically-resistant fibrin coat formed around the tumor (Lipinski, 2005). These changes provide NK cells an opportunity to recognize tumor cells as foreign and mount an immune response (Lipinski, 2016). Selenite (0.5 μM and higher) induced oxidation of polythiols is implicated in death of prostate cancer cells (Zhong & Oberley, 2001) and hepatoma cells(Shen, Yang & Ong, 1999).
Fig. 1.
Potentiating NK cell function in TME by selenium. Hypoxic environment within the TME can shift the redox equilibrium resulting in an increased interaction between tumor cell membrane and fibrinogen or plasma proteins through disulfide bond formation. As a result, a protective fibrin coat facilitating evasion of immune response in TME is formed. Treatment with selenium could reduce the disulfides within the protective fibrin coat to expose neoantigens on tumor cells to NK cells eventually initiating an immune response against the tumor.
Selenium also exhibits cancer preventive properties as a regulator of cellular redox via selenoproteins at low concentrations; whereas at higher concentrations selenite is shown to induce apoptosis in malignant cells through oxidative stress (Nilsonne et al., 2006). Further, Se-organic compounds (diselenides) act as glutathione peroxidase (GPx) mimetic drugs in cells with high thiol content such as cells exhibiting a high rate of metabolism and proliferation, all characteristics of tumor (Bartolini et al., 2015b; Frenkel, Falvey & MacVicar, 1991). Furthermore, in malignant cells, toxicity to selenium is reached at considerably lower concentrations compared to non-malignant cells suggesting the cytotoxic effects are specific for tumor cells (Husbeck, Nonn, Peehl & Knox, 2006). While the exact mechanisms are unclear, it is plausible that redox modulation of signalling pathways through changes in cellular oxidant tone combined with changes in redox status of protein thiols, selenium could interfere with the pre-metastatic niche created by platelet mediated fibrin deposition on tumor cells (Bartolini et al., 2015a; Bartolini, et al., 2015b; Quail & Joyce, 2013).
Inflammatory responses play decisive roles at different stages of tumor development, including initiation, promotion, malignant conversion, invasion, and metastasis. Immune cells that infiltrate the tumor engage in an extensive and dynamic crosstalk with cancer cells. The key molecular pathways involved are activation of transcription factors, mainly nuclear factor-κB (NF-κB), signal transducer and activator of transcription 3 (STAT3) and hypoxia-inducible factor-1α (HIF-1α), in tumour cells. These transcription factors coordinate the production of inflammatory mediators, including cytokines and chemokines, as well as the production of cyclooxygenase-2 (COX-2) leading to production of prostaglandins (PGs). These factors recruit and activate various leukocytes, most notably cells of the myelomonocytic lineage. One of the key leukocyte infiltrate include the tumor associated macrophages (TAMs) and related cell types that are involved in carcinogenesis and/or tumour invasion and metastasis (Bunt et al., 2007; Coussens, Tinkle, Hanahan & Werb, 2000; Mantovani, Bottazzi, Colotta, Sozzani & Ruco, 1992). The tumor cells derived factors activate the same key transcription factors in inflammatory cells, stromal cells and tumour cells, resulting in more inflammatory mediators being produced and a cancer-related inflammatory microenvironment is generated. Therefore, targeting of inflammatory mediators (chemokines and TNF-α and IL-1β), key transcription factors involved in inflammation (such as NF-κB and STAT3) or inflammatory cells decreases the incidence and spread of cancer (Mantovani, Allavena, Sica & Balkwill, 2008).
TAMs are a major component of tumor infiltrates that are reprogrammed to be pro-tumorigenic. TAMs often exhibit an alternatively-activated (M2)-like phenotype, which inhibits lymphocyte functions through release of anti-inflammatory cytokines such as IL-10 to negatively impact anti-tumor immune surveillance (Mantovani, Sozzani, Locati, Allavena & Sica, 2002; Martinez, Sica, Mantovani & Locati, 2008). Furthermore, TAMs are major contributors of tumor angiogenesis. The comparative analysis of TAM transcriptomes with available clinical databases show a transcriptional signature predictive of tumor survival (Ojalvo, Whittaker, Condeelis & Pollard, 2010).
Myeloid suppressor cells (MDSCs; CD34+CD33+CD13+CD15−) are a heterogeneous population of bone marrow-derived early progenitors that resemble immature dendritic cells and granulocytes. These cells are characterized by the increased production of extracellular degradative enzymes, cytokines, and RONS (Draghiciu, Lubbers, Nijman & Daemen, 2015). MDSCs promote tumor growth and suppress immune cell functions by blunting leukocyte responses (Ochoa, Zea, Hernandez & Rodriguez, 2007). Given their plasticity and their ability to exhibit variable phenotypes, MDSCs differentiate into Tregs. While there are no reports on the role of selenium in MDSCs per se, it is known that antioxidant vitamin E treatment can significantly reduce the number of MDSCs in a HPV16 E7 expressing TC-1 tumor model (Kang et al., 2014). Like the conventional macrophages, MDSCs also polarize towards M1 or M2 phenotypes. As in the case of macrophages, where selenium skews them towards an M2 phenotype to produce pro-resolution mediators (Figure 2), it remains to be seen if selenium has a similar impact on MDSC polarization to affect the proliferation of tumor cells by locally blunting inflammation to resolve the tumor lesion.
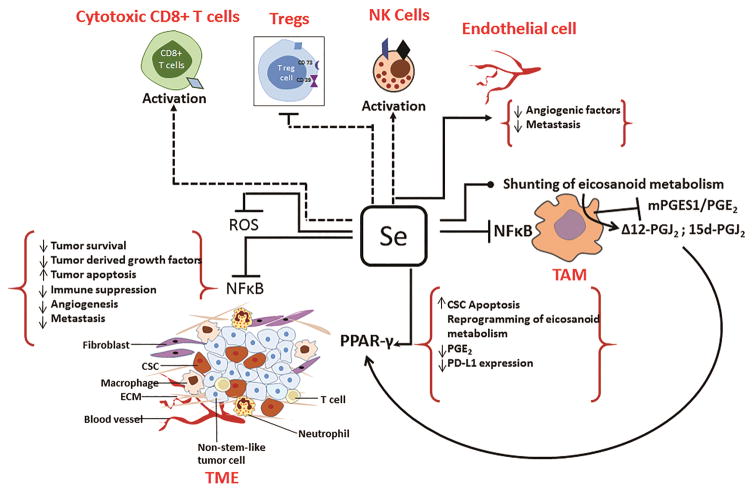
Fig. 2.
Selenium reconditions TME by affecting the pro-tumorigenic phenotype of immune infiltrates within the stromal compartment effecting apoptosis of stem cells and decreased survival of bulk tumor cells. TAMs mediate immune suppression, promote growth, angiogenesis and proliferation of tumor cells through dysregulated activation of NF-κB. Selenium-mediated redox modulation in macrophages inhibits IKKβ leading to decreased activation of NF-κB and its downstream effectors of immune suppression, tumor cell survival, and angiogenesis. Selenium may also sensitize tumor cells to TRAIL-induced apoptosis through NF-κB suppression by increasing the half-life of IKKα in cytosol. The intratumoral angiogenesis is abrogated by selenium through decreased expression of angiopoietin-2, PDGF, and VEGF, which might occur through ROS-mediated DNA damage in endothelial cells. Selenium also enhances the cytotoxic potential of NK cells and CD8+ T cells, while negatively regulating Treg cells, which together promote inhibition of tumor growth. Selenium reprograms arachidonic acid metabolism in macrophages leading to pro resolving CyPGs, while decreasing inflammatory PGs. CyPGs activate PPARγ in CSCs to block quiescence, while decreased PGE2 production by TAMs promotes antitumor effects by decreasing PDL1 expression to affect tumor aggression, angiogenesis, and immune suppression.
Nuclear factor kappa β (NF-κB), a redox-sensitive transcription factor and master regulator of inflammatory gene expression, is highly dysregulated in TAMs (Hagemann et al., 2008; Mantovani, Allavena, Sica & Balkwill, 2008; Saccani et al., 2006). Several factors (TNF-α, IL-1, hypoxia, HMGB1, TLR ligands) important for pro-tumor phenotype of TAMs are linked to activation of NF-κB. Interestingly, studies have shown that sodium selenite affects activation of NF-κB by inhibiting IKKβ (Vunta et al., 2007) that is known to be activated in TAMs (Saccani, et al., 2006). Thus, it is plausible that perturbation of NF-κB in TAMs by selenium may result in decreased local immune suppression as well as affecting the expression of cell survival factors (IL-6, TNF-α, COX-2, PGE2) (Mantovani, Allavena, Sica & Balkwill, 2008). Similar effects of selenium were observed in murine macrophages (Vunta, et al., 2007). Selenium also impacted angiogenesis through down regulation of VEGF, IL-8, and COX-2, which affects invasion and metastasis (Mantovani, Allavena, Sica & Balkwill, 2008). COX-2 appears to be one of the key factors regulated by selenium. The bioactive lipid prostaglandin E2 (PGE2) formed from arachidonic acid via COX-2 and microsomal PGE2 synthase-1 (mPGES-1) facilitates both cancer inflammation and immune suppression. In a recent study, the COX-2/mPGES-1/PGE2 pathway was demonstrated to regulate the expression of programmed-death ligand 1 (PD-L1), which is associated with increased tumor aggressiveness and immune suppression in TME (Prima, Kaliberova, Kaliberov, Curiel & Kusmartsev, 2017). Interestingly, selenium through selenoprotein expression, reprograms arachidonic acid metabolism by uncoupling COX-2 from mPEGS-1 during inflammation in macrophages to enhance the production of H-PGDS derived cyclopentenone PGs (CyPGs), Δ12-PGJ2 and 15d-PGJ2 (Gandhi et al., 2011; Vunta, et al., 2007). A consequence of such an “eicosanoid class switching” mechanism is the significant decrease in PGE2 by selenium supplementation. It remains to be seen if selenium decreases tumor aggressiveness through the modulation of expression of PD-L1 by altering the levels of PGE2 in the TME. However, anti-proliferatory CyPGs, Δ12-PGJ2 and 15d-PGJ2 that are produced by BMDMs because of the selenium-dependant eicosanoid class switching were capable of efficiently mediating apoptosis of LSCs in models of chronic myeloid leukemia (CML) suggesting that similar metabolic reprogramming in TAMs could impact the proliferation of tumor cells. However, this needs to be addressed in the future.
Selenium-dependent effects on cancer stem cells in leukemia
A causal inverse relationship between selenium and haematological malignancies exist (Azarm, Fazilati, Azarm & Azarm, 2013; Cai et al., 2016). An early clinical study reported a decrease in total leukocyte counts, immature leukocytes, and spleen size upon oral administration of selenium cystine (diseleno - dialanine) in patients with both CML and AML (Weisberger & Suhrland, 1956). Since then several in vitro studies have demonstrated the anti-leukemic properties of sodium selenite in leukemia cell lines (Jiang Xr, 1992; Liao, Bian, Xie & Peng, 2017; Misra et al., 2016). Sodium selenite supplementation was effective in decreasing circulating leukemia cells in murine Friend erythroleukemia (FV) as well as murine CML models (Gandhi et al., 2014). Lu et al, (Lu et al., 2004) demonstrated that the beneficial effects of selenium was conferred, in part, by improved immune surveillance of leukemia cells by NK cells. In addition, selenite treatment induced cytotoxicity in ex vivo cultures of leukemia cells isolated from peripheral blood of ALL and AML patients (Olm et al., 2009). Intriguingly, no detrimental effects of selenium were observed in cells derived from bone marrow or peripheral blood of normal individuals treated with selenite in this study. The specificity of selenite for LSCs was observed in murine models of CML, which utilized primary hematopoietic stem cells (HSCs) transduced with a retrovirus expressing the fusion oncoprotein BCR-ABL and GFP. Transplantation of these cells leads to disease suggesting that they act as leukemia stem cells (CML-LSCs). Intriguingly, treatment of these cells with selenite (50–500 nM) led to apoptosis that was only restricted to LSCs, while the HSCs expressing GFP were not affected (Finch et al., 2017; Gandhi, et al., 2014; Gandhi, et al., 2011). More importantly, these in-vitro studies corroborated well with in-vivo studies showing similar efficacy towards selenium supplementation on the ablation of LSCs and remission of the disease. While the basis of the selectivity of selenium towards LSCs is unclear, our studies show that selenium induces a number of effects in CML-LSCs that could account, in part, for its efficacy and selectivity. These effects include anti-androgen activity, DNA damage-induced response involving ATM-kinase and its downstream effectors such as p53, caspases, p21, and inhibition of anti-apoptotic pathways via the decrease in Mcl1-dependent control of Bcl2 (Finch, et al., 2017; Gandhi, et al., 2014; Sanmartin, Plano & Palop, 2008). Furthermore, p53 activation could also amplify a positive regulatory feedback loop involving long noncoding RNA (lncRNA) that enhances tumor suppressor activity of p53 (Sánchez et al., 2014).
CML originates in the bone marrow and is primarily due to a chromosomal translocation [t(9:22); Philadelphia chromosome (Ph+)] in progenitor cells resulting in the chimeric fusion oncoprotein, BCR-ABL1, which exhibits dysregulated tyrosine kinase activity (Koretzky, 2007). BCR-ABL drives the development of a two-stage disease with the overproduction of mature myeloid cells in the chronic phase that rapidly progresses to a blast crisis stage, which is an aggressive acute leukemia (Calabretta & Perrotti, 2004). The standard of care for CML patients is tyrosine kinase inhibitor (TKI) therapy. These maintenance drugs inhibit the BCR-ABL tyrosine kinase activity, which limits the over-production of myeloid cells. Although these drugs have been very effective in treating CML, they are often associated with side effects. In addition, patients develop resistance to TKI therapies, which has necessitated the development of newer generation of inhibitors. However, majority of patients will relapse when the TKI therapy is discontinued as these drugs primarily target mature cycling cells (that represent the bulk leukemia cells) and remain ineffective against CML LSCs (Druker et al., 2006; Druker et al., 2001; Gorre et al., 2001). Moreover, because TKIs do not target LSCs, there is a significant gap in our ability to specifically target LSCs to treat CML (Bhatia et al., 2003; Copland et al., 2006; Graham et al., 2002; Jorgensen, Allan, Jordanides, Mountford & Holyoake, 2007). Much like the HSCs that are maintained in specific niches in the bone marrow, CSCs also maintain quiescence with self-renewal capabilities (Bakker, Qattan, Mutti, Demonacos & Krstic-Demonacos, 2016). LSCs are perhaps the best studied CSCs. They give rise to bulk leukemia cells, which are responsible for the clinical presentation of leukemias and the pathologies associated with the disease. In addition, LSCs also self-renew, which maintains a population of quiescent LSCs (Huntly & Gilliland, 2005; Riether, Schürch & Ochsenbein, 2015; Tabe & Konopleva, 2014; Tannishtha, Morrison, Clarke & Weissman, 2001). Thus novel therapeutics that eradicate LSCs could provide a durable remission from the disease.
Interaction of LSCs with immune cells mediated by CyPGs
LSCs are sensitive to intrinsic and extrinsic factors that include components generated by immune cells within the bone marrow or other tissue microenvironment (Bakker, Qattan, Mutti, Demonacos & Krstic-Demonacos, 2016; Konopleva & Jordan, 2011; Tabe & Konopleva, 2014). As introduced in section 1, the extrinsic pathway of LSC control involves monocytes and macrophages that can be reprogrammed with selenium to promote eicosanoid class switching to produce bioactive CyPGs, which are endowed with anti-proliferative activity (Finch, et al., 2017; Gandhi, et al., 2014; Gandhi, et al., 2011). Utilizing conditioned media from bone marrow-derived macrophages (BMDMs), studies have shown that expression of the selenoproteome is critical for eicosanoid class switching to produce CyPGs (Gandhi, et al., 2014; Vunta, et al., 2007).
Co-incubation of macrophage-conditioned media with LSCs activated pathways of apoptosis, which were inhibited when the macrophages were treated with NSAIDs prior to addition of selenite (Gandhi, et al., 2014). These CyPGs downregulated the anti-apoptotic factor, NF-κB (Gandhi, et al., 2011; Vunta, et al., 2007). In fact, osteoblasts that are part of osteoblastic niche of bone marrow play an important role in quiescence of LSCs. Selenium (in the form of methylseleninic acid) modified the osteoblast inflammatory stress response to bone metastatic breast cancer cells by downregulating the activation of NF-κB leading to decreased expression of COX-2 and INOS and pro-inflammatory mediators such as IL-6, MCP-1 (Chen et al., 2009). This observation demonstrates the efficacy of selenium to modify extrinsic factors of tumor microenvironment and the importance of the microenvironment where macrophage-derived factors, including CyPGs, possibly act as key mediators of LSC apoptosis. While the mechanism of action of CyPGs in LSC apoptosis continues to be investigated, studies suggest that CyPGs exacerbate the production of ROS through a mechanism involving NADPH oxidase (Nox1), which in turn leads to the activation of ATM kinase-p53-dependent downstream pathways of apoptosis (Gandhi, et al., 2014; Hegde et al., 2011). While the mechanism of activation of Nox1 by CyPGs is intriguing, it appears that certain membrane–bound receptors for CyPGs could be involved in the cross-talk, which needs to be substantiated further.
On the other hand, constitutive activation of the NF-κB pathway allows LSCs to evade mechanisms of apoptosis (Bosman, Schuringa & Vellenga, 2016; Konopleva & Jordan, 2011; Zhou, Ching & Chng, 2015). As part of the intrinsic pathway, selenium is shown to suppress activation of NF-κB in murine model of esophageal carcinogenesis (Yang, Jia, Chen, Yang & Li, 2012), human prostate cancer cells (Christensen, Nartey, Hada, Legg & Barzee, 2007), during hepatocarcinogenesis (Alwahaibi, Mohamed & Alhamadani, 2010) and in colorectal cancer (Méplan & Hesketh, 2012; Ravasco et al., 2010). While it is known that LSCs can also endogenously produce CyPGs in response to supplementation with selenium, albeit at much lower concentration than macrophages, these CyPGs can diffuse into cells to bind to intracellular receptors such as PPARγ. Interestingly, PPARγ agonists target CML LSCs in vitro by decreasing transcription of STAT-5a, which is required for LSC self-renewal (Prost et al., 2015). Furthermore, PPARγ agonists can potentiate the action of TKIs in CML (Glodkowska-Mrowka et al., 2016). In a more recent ACTIM phase 2 clinical trial, PPARγ agonist pioglitazone in combination with imatinib (first generation TKI) showed positive outcome in treatment of CML. However, pioglitazone is associated with side effects that limits it use (Gavrilova et al., 2003; Nesto et al., 2003). Along these lines, a recent study demonstrated the activation PPARγ-dependent signalling in LSCs by selenium led to decreased expression of STAT-5a and its two targets, CITED2 and HIF-2α, which are both known to maintain LSC quiescence (Finch, et al., 2017). Treatment of CML-mice or isolated LSCs with selenium in the presence GW9662, a PPARγ antagonist, blocked the ability of selenium to down-regulate the expression of Stat-5a and its targets leading to leukemia. These studies suggest that changes in downstream effectors of PPARγ, which includes metabolic enzymes, could impart some selectivity to ablation of LSCs by selenium.
Targeting LSC metabolism with selenium
Generally, cancer cells prefer aerobic glycolysis to shunt glucose towards cytoplasmic lactate instead of pyruvate-derived acetyl-CoA that would enter the tricarboxylic acid (TCA) cycle coupled to oxidative phosphorylation (OXPHOS). This unusual capability of tumor cells to reprogram glucose metabolism is an emerging avenue for cancer therapeutics that was originally demonstrated by Otto Warburg (Warburg, 1956). Cancer cells prefer the energetically unfavorable glycolysis that produces 18 times less ATP per mole of glucose than OXPHOS. However, this process is more efficient in generating precursors for nucleotide and lipid biosynthesis for rapid cell division and is several orders of magnitude faster, which aids in metabolic coupling within the tumor niche leading to enhanced proliferation (Martinez-Outschoorn, Peiris-Pagés, Pestell, Sotgia & Lisanti, 2016). Moreover, acquired oncogenic mutations alter the metabolic network, where many of tumor-promoting genes are intricately associated with metabolic regulation. It is also evident that reprogramming of energy metabolism and evading immune destruction is a hallmark of cancer cells that navigate through the TME (Hanahan & Weinberg, 2011; Hsu & Sabatini, 2008). The size of the tumor niche population in which CSCs exist determines the availability of nutrients and oxygen. The regulation of nutrient availability, in turn, affects the metabolic phenotype of CSCs, which is poorly understood in the context of tumorigenesis. It is established that not all malignant cells are metabolically and functionally equivalent, which has been demonstrated in leukemia, breast cancer and various other cancers (Sancho, Barneda & Heeschen, 2016). This functional disparity is often attributed to genetic and epigenetic heterogeneity within multiple sub-clonal populations, which contribute to various metabolic adaptations specific to each tumor type (Sancho, Barneda & Heeschen, 2016). Consequently, CSCs exhibit diverse metabolic phenotypes. Based on the existing literature, though CSCs are broadly classified to be primarily glycolytic, they are also known to selectively rely on OXPHOS to maintain their stemness. CSCs selectively adopt to nutrient deprivation and tumor niche metabolic phenotype. Mechanistically, the glycolytic program is also associated with stemness by keeping high antioxidative capacity and increased pentose phosphate pathway (Sancho, Barneda & Heeschen, 2016). High aerobic glycolytic rates and glutaminolysis promote fatty acid oxidation for ATP and reducing equivalents such as NADPH. Notably leukemic and pancreatic CSCs utilize fatty acid oxidation regulated by PPARδ pathway (Ito et al., 2012; Samudio et al., 2010; Sancho, Barneda & Heeschen, 2016). In these cells, NADPH generation was key for self-renewal, maintaining stemness as well as the CSC phenotype. To this end, selenoproteins within cells could play a critical role in the modulation of glucose and fatty acid metabolism.
It is well established that deficiency of GPX1 and other selenoproteins can increase the expression of p53, a tumor suppressor gene and a master controller of cellular metabolism (Cheng, Zheng, Quimby, Roneker & Lei, 2003). Activation of p53 induces the expression of genes associated with DNA repair, apoptosis, cell cycle and senescence, and more importantly, glucose and fatty acid metabolism (Bensaad et al., 2006). Similarly, nucleolar oxidoreductase selenoprotein H (SelenoH) overexpression in neuronal cells protected against UV-induced DNA damage by suppressing p53 and caspase-mediated apoptosis (Mendelev, Witherspoon & Li, 2009). Recent studies by Cox et al. demonstrated that SelenoH deficiency exacerbated oxidative stress-dependent activation of p53, where inflammation was a key factor, leading to gastrointestinal tumorigenesis in a zebrafish model (Cox et al., 2016). Given that selenium supplementation can downregulate inflammation, it remains to be seen if SelenoH regulates expression of inflammatory genes such as Tnf-α and IL-1β (Figure 3).
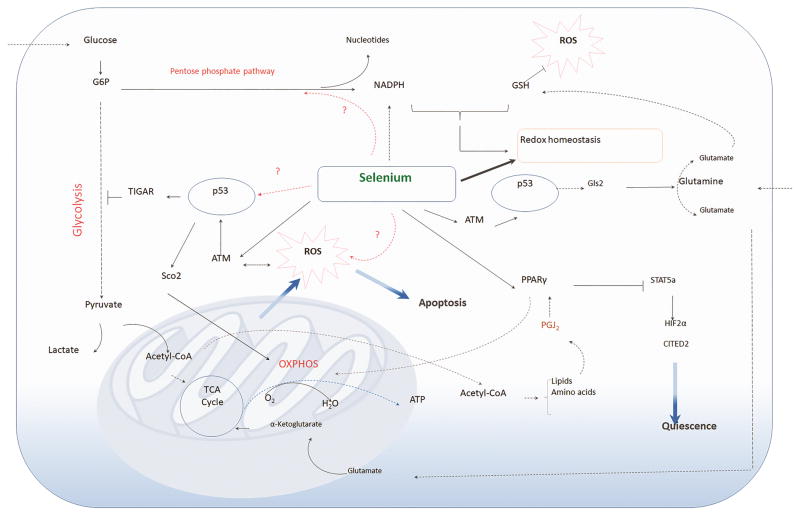
Fig. 3.
Significance of selenium in cancer stem cell metabolism. CSCs generally display glycolytic metabolic phenotype selectively coupled with TCA cycle and OXPHOS to generate energy and cellular components (amino acids and lipids) to support their stemness. Metabolically-active CSCs generate ROS, which is compensated by production of antioxidants, including GSH, NADPH derived from the pentose phosphate pathway. Selenoproteins through their redox gatekeeper functions can effectively modulate redox homeostasis. Selenium treatment of CSCs activate ATM kinase, which in turn activates p53 leading to metabolic reprogramming. p53 targets pathways via TIGAR-mediated glycolytic arrest, Sco2-mediated potentiation of OXPHOS, and Gls2-mediated regulation of glutamine metabolism to ultimately exacerbate ROS production to activate pathways of apoptosis. Selenium-mediated eicosanoid class switching leads to the production of PGJ2 as an adaptive response for protection against ROS, which is mediated through PPARγ that transcriptionally represses STAT5a to effectively inhibit downstream target genes, HIF2α and CITED2, known for maintenance of CSC quiescence, as in CML LSCs.
While the studies mentioned above suggest an inverse causal relationship between selenium status and p53 activation, these mechanisms may operate differently when the tumor has already been initiated, as in the case of expression of the oncoprotein BCR-ABL in HSCs in the murine CML model. Comparison of HSCs (which represent normal stem cells) and LSCs prior to treatment with selenium showed HSCs to have higher ROS compared to LSCs (Gandhi, et al., 2014). Contrary to the prevailing idea with other cell types is the ability of LSCs to tightly regulate ROS and p53 expression, which could enable the LSCs to evade autophagy and/or apoptosis. However, treatment of both these cell types, HSCs and LSCs, with supraphysiological levels of selenium (as selenite; 250–500 nM) selectively increased ROS by several orders of magnitude only in LSCs. This increase was also accompanied by increased ATM kinase-p53-caspase 3-dependent pathway of apoptosis (Gandhi, et al., 2014). Intriguingly, such an increase in ROS was accompanied by increased expression of many antioxidant genes, including Gpx4 that were unable to compensate and protect the LSCs from ROS. The ability of selenium supplementation at levels where most of the selenoproteins are saturated to increase ROS selectively in one cell type (LSCs) suggests a cross-talk between selenium and cysteine thiols in other proteins, particularly phosphatases, in the form of selenopersulfides, which modulates their activities leading to increased cellular oxidative stress. Of interest to us is the ability of p53 to activate pathways of metabolism in the LSCs that may also contribute to increased cellular oxidative stress. For instance, Gandhi et al (2014) demonstrated that selenite treatment increased Sco2 (synthesis of cytochrome oxidase-2) and Rm2b (P53r2; ribonucleotide-diphosphate reductase subunit RM2B), which are likely to increase the ability to LSCs to favor OXPHOS that further contributes to increase in ROS. In addition, TP53 inducible glycolysis and apoptosis regulator (TIGAR), a fructose bisphosphatase that negatively regulates glycolysis was increased in selenite-treated LSCs, which corroborates data from other cancers where TIGAR is overexpressed (Ko et al., 2016; Wanka, Steinbach & Rieger, 2012). TIGAR overexpression is implicated in inhibition of glycolysis and protective mechanisms against ROS in glioma cells (Wanka, Steinbach & Rieger, 2012).
Metabolic adaptations that govern quiescence in CSCs and processes involving self-renewal, tumor initiation, and chemo-resistance call for thorough studies involving flux of key metabolites through specific pathways in conjunction with the use of genetic methods to elucidate the underpinnings of the resiliency of these cells. A better understanding of these pathways and how they are controlled by altered oncogenes and tumor suppressors needs to be integrated with studies of the alterations in cellular redox tone of cells and how these changes impact metabolomic shifts that ultimately dictate cell fate decisions. It is here that the redox gatekeeper functions of selenoproteins are likely to play a pivotal role leading to mechanistic insights involving metabolomic changes within CSCs in collaboration with immune cells in the tumor niche.
Summary
In conclusion, this review highlights various mechanisms by which selenium and selenoproteins affect various stages of tumorigenesis that involves a cross talk with the immune component. In particular, based on the recent work in murine models of CML, the remarkable ability of selenite supplementation to target LSCs for apoptosis is dependent on the enhanced production of CyPGs that drastically decreases the disease burden. The ablation of LSCs in murine models highlight a new area within selenium biology where selenium-mediated changes in metabolic pathways within LSCs as well as immune cells that comprise the microenvironment in the bone marrow and/or spleen underlie the protective effects of selenium supplementation. Even though the underlying mechanism of anti-leukemic activity of selenium is still not completely understood, the ability of selenium to affect quiescence of CML-LSCs is mediated through the enhanced production of CyPGs that are not only generated within the LSCs, but also by immune cells (macrophages) via reprogramming of cellular metabolism is a promising observation. However, one of the key questions that needs to be addressed is the identity of selenoprotein(s) that is pivotal for the eicosanoid-class switching, which is currently being pursued. Regardless, these findings suggest new options for the development of new treatments for CML, where dietary selenium supplementation could be substituted for synthetic PPARγ agonists.
Acknowledgments
This work was supported, in part, by grants from the National Institutes of Health R01CA162665, R01 DK077152, Office of Dietary Supplements (ODS-NIH), USDA-NIFA Hatch project numbers 4605 (K.S.P) and 4736 (R.F.P). We thank current and former members of the Prabhu laboratory for their invaluable contributions.
References
- Alwahaibi N, Mohamed J, Alhamadani A. Supplementation of selenium reduces chemical hepatocarcinogenesis in male Sprague-Dawley rats. Journal of Trace Elements in Medicine and Biology. 2010;24:119–123. doi: 10.1016/j.jtemb.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Azarm T, Fazilati M, Azarm H, Azarm A. Serum selenium levels in chronic lymphocytic leukemia. Advanced biomedical research. 2013;2:44–44. doi: 10.4103/2277-9175.114177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker E, Qattan M, Mutti L, Demonacos C, Krstic-Demonacos M. The role of microenvironment and immunity in drug response in leukemia. Biochimica et Biophysica Acta. 2016;1863:414–426. doi: 10.1016/j.bbamcr.2015.08.003. [DOI] [PubMed] [Google Scholar]
- Bartolini D, Commodi J, Piroddi M, Incipini L, Sancineto L, Santi C, Galli F. Glutathione S-transferase pi expression regulates the Nrf2-dependent response to hormetic diselenides. Free Radical Biology & Medicine. 2015a;88:466–480. doi: 10.1016/j.freeradbiomed.2015.06.039. [DOI] [PubMed] [Google Scholar]
- Bartolini D, Piroddi M, Tidei C, Giovagnoli S, Pietrella D, Manevich Y, Tew KD, Giustarini D, Rossi R, Townsend DM, Santi C, Galli F. Reaction kinetics and targeting to cellular glutathione S-transferase of the glutathione peroxidase mimetic PhSeZnCl and its D,L-polylactide microparticle formulation. Free Radical Biology & Medicine. 2015b;78:56–65. doi: 10.1016/j.freeradbiomed.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. Journal of Clinical Oncology. 2006;24:5373–5380. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- Bensaad K, Tsuruta A, Selak MA, Vidal MNC, Nakano K, Bartrons R, Gottlieb E, Vousden KH. TIGAR, a p53-Inducible Regulator of Glycolysis and Apoptosis. Cell. 2006;126:107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- Bhatia R, Holtz M, Niu N, Gray R, Snyder DS, Sawyers CL, Arber Da, Slovak ML, Forman SJ. Persistence of malignant hematopoietic progenitors in chronic myelogenous leukemia patients in complete cytogenetic remission following imatinib mesylate treatment. Blood. 2003;101:4701–4707. doi: 10.1182/blood-2002-09-2780. [DOI] [PubMed] [Google Scholar]
- Bosman MC, Schuringa JJ, Vellenga E. Constitutive NF-kappaB activation in AML: Causes and treatment strategies. Critical Reviews in Oncology/Hematology. 2016;98:35–44. doi: 10.1016/j.critrevonc.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Research. 2007;67:10019–10026. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Wang C, Yu W, Fan W, Wang S, Shen N, Wu P, Li X, Wang F. Selenium Exposure and Cancer Risk: an Updated Meta-analysis and Meta-regression. Scientific reports. 2016;6:19213–19213. doi: 10.1038/srep19213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabretta B, Perrotti D. Review in translational hematology The biology of CML blast crisis. Blood. 2004;103:4010–4022. doi: 10.1182/blood-2003-12-4111. [DOI] [PubMed] [Google Scholar]
- Chen YC, Sosnoski DM, Gandhi UH, Novinger LJ, Prabhu KS, Mastro AM. Selenium modifies the osteoblast inflammatory stress response to bone metastatic breast cancer. Carcinogenesis. 2009;30:1941–1948. doi: 10.1093/carcin/bgp227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng WH, Zheng X, Quimby FR, Roneker CA, Lei XG. Low levels of glutathione peroxidase 1 activity in selenium-deficient mouse liver affect c-Jun N-terminal kinase activation and p53 phosphorylation on Ser-15 in pro-oxidant-induced aponecrosis. The Biochemical journal. 2003;370:927–934. doi: 10.1042/BJ20021870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen MJ, Nartey ET, Hada AL, Legg RL, Barzee BR. High selenium reduces NF-kappaB-regulated gene expression in uninduced human prostate cancer cells. Nutrition and cancer. 2007;58:197–204. doi: 10.1080/01635580701328701. [DOI] [PubMed] [Google Scholar]
- Copland M, Hamilton A, Elrick LJ, Baird JW, Allan EK, Jordanides N, Barow M, Mountford JC, Holyoake TL. Dasatinib (BMS-354825) targets an earlier progenitor population than imatinib in primary CML but does not eliminate the quiescent fraction. Blood. 2006;107:4532–4539. doi: 10.1182/blood-2005-07-2947. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Tinkle CL, Hanahan D, Werb Z. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox AG, Tsomides A, Kim AJ, Saunders D, Hwang KL, Evason KJ, Heidel J, Brown KK, Yuan M, Lien EC, Lee BC, Nissim S, Dickinson B, Chhangawala S, Chang CJ, Asara JM, Houvras Y, Gladyshev VN, Goessling W. Selenoprotein H is an essential regulator of redox homeostasis that cooperates with p53 in development and tumorigenesis. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E5562–E5571. doi: 10.1073/pnas.1600204113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature medicine. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- Draghiciu O, Lubbers J, Nijman HW, Daemen T. Myeloid derived suppressor cells-An overview of combat strategies to increase immunotherapy efficacy. Oncoimmunology. 2015;4:e954829. doi: 10.4161/21624011.2014.954829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, Deininger MWN, Silver RT, Goldman JM, Stone RM, Cervantes F, Hochhaus A, Powell BL, Gabrilove JL, Rousselot P, Reiffers J, Cornelissen JJ, Hughes T, Agis H, Fischer T, Verhoef G, Shepherd J, Saglio G, Gratwohl A, Nielsen JL, Radich JP, Simonsson B, Taylor K, Baccarani M, So C, Letvak L, Larson Ra. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. The New England journal of medicine. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- Druker BJ, Sawyers CL, Kantarjian H, Resta DJ, Reese SF, Ford JM, Capdeville R, Talpaz M. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. The New England Journal of Medicine. 2001;344:1038–1042. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- Finch ER, Tukaramrao DB, Goodfield LL, Quickel MD, Paulson RF, Prabhu KS. Activation of PPARγ by endogenous prostaglandin J 2 mediates the antileukemic effect of selenium in murine leukemia. Blood. 2017;129:1802–1810. doi: 10.1182/blood-2016-08-736405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel GD, Falvey D, MacVicar C. Products of the reaction of selenite with intracellular sulfhydryl compounds. Biological Trace Element Research. 1991;30:9–18. doi: 10.1007/BF02990338. [DOI] [PubMed] [Google Scholar]
- Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nature Reviews Cancer. 2012;12:298–306. doi: 10.1038/nrc3245. [DOI] [PubMed] [Google Scholar]
- Gandhi UH, Kaushal N, Hegde S, Finch ER, Kudva AK, Kennett MJ, Jordan CT, Paulson RF, Prabhu KS. Selenium suppresses leukemia through the action of endogenous eicosanoids. Cancer Research. 2014;74:3890–3901. doi: 10.1158/0008-5472.CAN-13-3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi UH, Kaushal N, Ravindra KC, Hegde S, Nelson SM, Narayan V, Vunta H, Paulson RF, Prabhu KS. Selenoprotein-dependent up-regulation of hematopoietic prostaglandin D2 synthase in macrophages is mediated through the activation of peroxisome proliferator-activated receptor (PPAR) gamma. Journal of Biological Chemistry. 2011;286:27471–27482. doi: 10.1074/jbc.M111.260547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilova O, Haluzik M, Matsusue K, Cutson JJ, Johnson L, Dietz KR, Nicol CJ, Vinson C, Gonzalez FJ, Reitman ML. Liver Peroxisome Proliferator-activated Receptor ?? Contributes to Hepatic Steatosis, Triglyceride Clearance, and Regulation of Body Fat Mass. Journal of Biological Chemistry. 2003;278:34268–34276. doi: 10.1074/jbc.M300043200. [DOI] [PubMed] [Google Scholar]
- Glodkowska-Mrowka E, Manda-Handzlik A, Stelmaszczyk-Emmel A, Seferynska I, Stoklosa T, Przybylski J, Mrowka P. PPARγ ligands increase antileukemic activity of second- and third-generation tyrosine kinase inhibitors in chronic myeloid leukemia cells. Blood Cancer Journal. 2016;6:e377. doi: 10.1038/bcj.2015.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, Sawyers CL. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- Graham SM, Jorgensen HG, Allan E, Pearson C, Alcorn MJ, Richmond L, Holyoake TL. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99:319–325. doi: 10.1182/blood.v99.1.319. [DOI] [PubMed] [Google Scholar]
- Guillerey C, Huntington ND, Smyth MJ. Targeting natural killer cells in cancer immunotherapy. Nature Immunology. 2016;17:1025–1036. doi: 10.1038/ni.3518. [DOI] [PubMed] [Google Scholar]
- Hagemann T, Lawrence T, McNeish I, Charles Ka, Kulbe H, Thompson RG, Robinson SC, Balkwill FR. “Re-educating” tumor-associated macrophages by targeting NF-kappaB. The Journal of experimental medicine. 2008;205:1261–1268. doi: 10.1084/jem.20080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hegde S, Kaushal N, Ravindra KC, Chiaro C, Hafer KT, Gandhi UH, Thompson JT, van den Heuvel JP, Kennett MJ, Hankey P, Paulson RF, Prabhu KS. Δ12-prostaglandin J3, an omega-3 fatty acid-derived metabolite, selectively ablates leukemia stem cells in mice. Blood. 2011;118:6909–6919. doi: 10.1182/blood-2010-11-317750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraoka N, Onozato K, Kosuge T, Hirohashi S. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clinical Cancer Research. 2006;12:5423–5434. doi: 10.1158/1078-0432.CCR-06-0369. [DOI] [PubMed] [Google Scholar]
- Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134:703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Huntly BJP, Gilliland DG. Leukaemia stem cells and the evolution of cancer-stem-cell research. Nature reviews Cancer. 2005;5:311–321. doi: 10.1038/nrc1592. [DOI] [PubMed] [Google Scholar]
- Husbeck B, Nonn L, Peehl DM, Knox SJ. Tumor-selective killing by selenite in patient-matched pairs of normal and malignant prostate cells. Prostate. 2006;66:218–225. doi: 10.1002/pros.20337. [DOI] [PubMed] [Google Scholar]
- Ito K, Carracedo A, Weiss D, Arai F, Ala U, Avigan DE, Schafer ZT, Evans RM, Suda T, Lee CH, Pandolfi PP. A PML-PPAR-delta pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nature Medicine. 2012;18:1350–1358. doi: 10.1038/nm.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Xr MMGLHXNAC. The anti-leukaemic effects and the mechanism of sodium selenite. Leukocyte research. 1992;16:347–352. doi: 10.1016/0145-2126(92)90136-u. [DOI] [PubMed] [Google Scholar]
- Jorgensen HG, Allan EK, Jordanides NE, Mountford JC, Holyoake TL. Nilotinib exerts equipotent antiproliferative effects to imatinib and does not induce apoptosis in CD34 + CML cells Brief report Nilotinib exerts equipotent antiproliferative effects to imatinib and does not induce apoptosis in CD34 + CML cells. Blood. 2007;109:4016–4019. doi: 10.1182/blood-2006-11-057521. [DOI] [PubMed] [Google Scholar]
- Kang TH, Knoff J, Yeh WH, Yang B, Wang C, Kim YS, Kim TW, Wu TC, Hung CF. Treatment of tumors with vitamin e suppresses myeloid derived suppressor cells and enhances CD8+ T cell-mediated antitumor effects. PLoS ONE. 2014;9:1–10. doi: 10.1371/journal.pone.0103562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiremidjian-Schumacher L, Roy M, Wishe HI, Cohen MW, Stotzky G. Supplementation with selenium augments the functions of natural killer and lymphokine-activated killer cells. Biological Trace Element Research. 1996;52:227–239. doi: 10.1007/BF02789164. [DOI] [PubMed] [Google Scholar]
- Ko YH, Domingo-Vidal M, Roche M, Lin Z, Whitaker-Menezes D, Seifert E, Capparelli C, Tuluc M, Birbe RC, Tassone P, Curry JM, Navarro-Sabate A, Manzano A, Bartrons R, Caro J, Martinez-Outschoorn U. TP53-inducible Glycolysis and Apoptosis Regulator (TIGAR) Metabolically Reprograms Carcinoma and Stromal Cells in Breast Cancer. Journal of Biological Chemistry. 2016;291:26291–26303. doi: 10.1074/jbc.M116.740209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopleva MY, Jordan CT. Leukemia stem cells and microenvironment: biology and therapeutic targeting. Journal of Clinical Oncology. 2011;29:591–599. doi: 10.1200/JCO.2010.31.0904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koretzky Ga. Review series introduction The legacy of the Philadelphia chromosome. The Journal of clinical investigation. 2007;117:2030–2032. doi: 10.1172/JCI33032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasnova Y, Putz EM, Smyth MJ, Souza-Fonseca-Guimaraes F. Bench to bedside: NK cells and control of metastasis. Clinical Immunology. 2017;177:50–59. doi: 10.1016/j.clim.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Liao K, Bian Z, Xie D, Peng Q. A Selenium-Modified Ginseng Polysaccharide Promotes the Apoptosis in Human Promyelocytic Leukemia (HL-60) Cells via a Mitochondrial-Mediated Pathway. Biological Trace Element Research. 2017;177:64–71. doi: 10.1007/s12011-016-0879-9. [DOI] [PubMed] [Google Scholar]
- Lipinski B. Rationale for the treatment of cancer with sodium selenite. Medical Hypotheses. 2005;64:806–810. doi: 10.1016/j.mehy.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Lipinski B. Sodium Selenite as an Anticancer Agent. Anti-Cancer Agents in Medicinal Chemistry. 2016;16:1–4. doi: 10.2174/1871520616666160607011024. [DOI] [PubMed] [Google Scholar]
- Lu DY, Chi J, Lin LP, Huang M, Xu B, Ding J. Effect of anti-cancer drugs on the binding of 125I-Fibrinogen to two leukaemia cell lines in vitro. Journal of International Medical Research. 2004;32:488–91. doi: 10.1177/147323000403200505. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Bottazzi B, Colotta F, Sozzani S, Ruco L. The origin and function of tumor-associated macrophages. Immunology Today. 1992;13:265–270. doi: 10.1016/0167-5699(92)90008-U. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends in Immunology. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- Martinez-Outschoorn UE, Peiris-Pagés M, Pestell RG, Sotgia F, Lisanti MP. Cancer metabolism: a therapeutic perspective. Nature reviews. Clinical oncology. 2016:1–21. doi: 10.1038/nrclinonc.2016.60. [DOI] [PubMed] [Google Scholar]
- Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Frontiers in Bioscience. 2008;13:453–461. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- Mendelev N, Witherspoon S, Li PA. Overexpression of human selenoprotein H in neuronal cells ameliorates ultraviolet irradiation-induced damage by modulating cell signaling pathways. Experimental Neurology. 2009;220:328–334. doi: 10.1016/j.expneurol.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méplan C, Hesketh J. The influence of selenium and selenoprotein gene variants on colorectal cancer risk. Mutagenesis. 2012;27:177–186. doi: 10.1093/mutage/ger058. [DOI] [PubMed] [Google Scholar]
- Misra S, Selvam AK, Wallenberg M, Ambati A, Matolcsy A, Magalhaes I, Lauter G, Bjornstedt M. Selenite promotes all-trans retinoic acid-induced maturation of acute promyelocytic leukemia cells. Oncotarget. 2016;7:74686–74700. doi: 10.18632/oncotarget.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesto RW, Bell D, Bonow RO, Fonseca V, Grundy SM, Horton ES, Le Winter M, Porte D, Semenkovich CF, Smith S, Young LH, Kahn R. Thiazolidinedione Use, Fluid Retention, and Congestive Heart Failure: A Consensus Statement from the American Heart Association and American Diabetes Association. Circulation. 2003;108:2941–2948. doi: 10.1161/01.CIR.0000103683.99399.7E. [DOI] [PubMed] [Google Scholar]
- Ochoa AC, Zea AH, Hernandez C, Rodriguez PC. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clinical Cancer Research. 2007;13:721–726. doi: 10.1158/1078-0432.CCR-06-2197. [DOI] [PubMed] [Google Scholar]
- Ojalvo LS, Whittaker CA, Condeelis JS, Pollard JW. Gene expression analysis of macrophages that facilitate tumor invasion supports a role for Wnt-signaling in mediating their activity in primary mammary tumors. Journal of Immunology. 2010;184:702–712. doi: 10.4049/jimmunol.0902360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olm E, Jonsson-Videsater K, Ribera-Cortada I, Fernandes AP, Eriksson LC, Lehmann S, Rundlof AK, Paul C, Bjornstedt M. Selenite is a potent cytotoxic agent for human primary AML cells. Cancer Letters. 2009;282:116–123. doi: 10.1016/j.canlet.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Prima V, Kaliberova LN, Kaliberov S, Curiel DT, Kusmartsev S. COX2/mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells. Proceedings of the National Academy of Sciences of the United States of America. 2017;114:1117–1122. doi: 10.1073/pnas.1612920114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prost S, Relouzat F, Spentchian M, Ouzegdouh Y, Saliba J, Massonnet G, Beressi JP, Verhoeyen E, Raggueneau V, Maneglier B, Castaigne S, Chomienne C, Chrétien S, Rousselot P, Leboulch P. Erosion of the chronic myeloid leukaemia stem cell pool by PPARγ agonists. Nature. 2015;525:380–383. doi: 10.1038/nature15248. [DOI] [PubMed] [Google Scholar]
- Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nature Medicine. 2013;19:1423–1437. doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravasco P, Aranha MM, Borralho PM, Moreira da Silva IB, Correia L, Fernandes A, Rodrigues CMP, Camilo M. Colorectal cancer: Can nutrients modulate NF-κB and apoptosis? Clinical Nutrition. 2010;29:42–46. doi: 10.1016/j.clnu.2009.06.006. [DOI] [PubMed] [Google Scholar]
- Riether C, Schürch CM, Ochsenbein AF. Regulation of hematopoietic and leukemic stem cells by the immune system. Cell death and differentiation. 2015;22:187–98. doi: 10.1038/cdd.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saccani A, Schioppa T, Porta C, Biswas SK, Nebuloni M, Vago L, Bottazzi B, Colombo MP, Mantovani A, Sica A. p50 nuclear factor-κB overexpression in tumor-associated macrophages inhibits M1 inflammatory responses and antitumor resistance. Cancer Research. 2006;66:11432–11440. doi: 10.1158/0008-5472.CAN-06-1867. [DOI] [PubMed] [Google Scholar]
- Samudio I, Harmancey R, Fiegl M, Kantarjian H, Konopleva M, Korchin B, Kaluarachchi K, Bornmann W, Duvvuri S, Taegtmeyer H, Andreeff M. Pharmacologic inhibition of fatty acid oxidation sensitizes human leukemia cells to apoptosis induction. Journal of Clinical Investigation. 2010;120:142–156. doi: 10.1172/JCI38942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez Y, Segura V, Marín-Béjar O, Athie A, Marchese FP, González J, Bujanda L, Guo S, Matheu A, Huarte M. Genome-wide analysis of the human p53 transcriptional network unveils a lncRNA tumour suppressor signature. Nature communications. 2014;5:5812–5812. doi: 10.1038/ncomms6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho P, Barneda D, Heeschen C. Hallmarks of cancer stem cell metabolism. British Journal of Cancer. 2016;114:1305–1312. doi: 10.1038/bjc.2016.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanmartin C, Plano D, Palop JA. Selenium compounds and apoptotic modulation: a new perspective in cancer therapy. Mini-Reviews in Medicinal Chemistry. 2008;8:1020–31. doi: 10.2174/138955708785740625. [DOI] [PubMed] [Google Scholar]
- Shen HM, Yang CF, Ong CN. Sodium selenite-induced oxidative stress and apoptosis in human hepatoma HepG2 cells. International Journal of Cancer. 1999;81:820–828. doi: 10.1002/(sici)1097-0215(19990531)81:5<820::aid-ijc25>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Tabe Y, Konopleva M. Advances in understanding the leukaemia microenvironment. British Journal of Haematology. 2014;164:767–778. doi: 10.1111/bjh.12725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tannishtha R, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- Vitale M, Cantoni C, Pietra G, Mingari MC, Moretta L. Effect of tumor cells and tumor microenvironment on NK-cell function. European Journal of Immunology. 2014;44:1582–92. doi: 10.1002/eji.201344272. [DOI] [PubMed] [Google Scholar]
- Vunta H, Davis F, Palempalli UD, Bhat D, Arner RJ, Thompson JT, Peterson DG, Reddy CC, Prabhu KS. The anti-inflammatory effects of selenium are mediated through 15-deoxy-Delta12,14-prostaglandin J2 in macrophages. The Journal of biological chemistry. 2007;282:17964–17973. doi: 10.1074/jbc.M703075200. [DOI] [PubMed] [Google Scholar]
- Wang H, Chan YL, Li TL, Bauer BA, Hsia S, Wang CH, Huang JS, Wang HM, Yeh KY, Huang TH, Wu GJ, Wu CJ. Reduction of Splenic Immunosuppressive Cells and Enhancement of Anti-Tumor Immunity by Synergy of Fish Oil and Selenium Yeast. PLoS ONE. 2013;8:1–11. doi: 10.1371/journal.pone.0052912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanka C, Steinbach JP, Rieger J. Tp53-induced glycolysis and apoptosis regulator (TIGAR) protects glioma cells from starvation-induced cell death by up-regulating respiration and improving cellular redox homeostasis. Journal of Biological Chemistry. 2012;287:33436–33446. doi: 10.1074/jbc.M112.384578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O. On Respiratory Impairment in Cancer Cells. Science. 1956;124:269–70. [PubMed] [Google Scholar]
- Weisberger AS, Suhrland LG. Studies on analogues of L-cysteine and L-cystine. III. The effect of selenium cystine on leukemia. Blood. 1956;11:19–30. [PubMed] [Google Scholar]
- Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27:5904–5912. doi: 10.1038/onc.2008.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue H, Wang W, Li Y, Shan Z, Li Y, Teng X, Gao Y, Fan C, Teng W. Selenium upregulates CD4(+)CD25(+) regulatory T cells in iodine-induced autoimmune thyroiditis model of NOD.H-2(h4) mice. Endocrine Journal. 2010;57:595–601. doi: 10.1507/endocrj.k10e-063. [DOI] [PubMed] [Google Scholar]
- Yang H, Jia X, Chen X, Yang CS, Li N. Time-selective chemoprevention of vitamin E and selenium on esophageal carcinogenesis in rats: the possible role of nuclear factor kappaB signaling pathway. International journal of cancer. 2012;131:1517–1527. doi: 10.1002/ijc.27423. [DOI] [PubMed] [Google Scholar]
- Zhong W, Oberley TD. Redox-mediated effects of selenium on apoptosis and cell cycle in the LNCaP human prostate cancer cell line. Cancer research. 2001;61:7071–7078. [PubMed] [Google Scholar]
- Zhou J, Ching YQ, Chng WJ. Aberrant nuclear factor-kappa B activity in acute myeloid leukemia: from molecular pathogenesis to therapeutic target. Oncotarget. 2015;6:5490–500. doi: 10.18632/oncotarget.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]