Abstract
Background
Due to the heterogeneity of depressive symptoms—which can include depressed mood, anhedonia, negative cognitive biases, and altered activity levels—researchers often use a combination of depression rating scales to assess symptoms. This study sought to identify unidimensional constructs measured across rating scales for depression and to evaluate these constructs across clinical trials of a rapid-acting antidepressant (ketamine).
Methods
Exploratory factor analysis (EFA) was conducted on baseline ratings from the Beck Depression Inventory (BDI), the Hamilton Depression Rating Scale (HAM-D), the Montgomery-Asberg Depression Rating Scale (MADRS), and the Snaith-Hamilton Pleasure Rating Scale (SHAPS). Inpatients with major depressive disorder (n=76) or bipolar depression (n=43) were participating in clinical ketamine trials. The trajectories of the resulting unidimensional scores were evaluated in 41 subjects with bipolar depression who participated in clinical ketamine trials.
Results
The best solution, which exhibited excellent fit to the data, comprised eight factors: Depressed Mood, Tension, Negative Cognition, Impaired Sleep, Suicidal Thoughts, Reduced Appetite, Anhedonia, and Amotivation. Various response patterns were observed across the clinical trial data, both in treatment effect (ketamine versus placebo) and in degree of placebo response, suggesting that use of these unidimensional constructs may reveal patterns not observed with traditional scoring of individual instruments.
Limitations
Limitations include: 1) small sample (and related inability to confirm measurement invariance); 2) absence of an independent sample for confirmation of factor structure; and 3) the treatment-resistant nature of the population, which may limit generalizability.
Conclusions
The empirical identification of unidimensional constructs creates more refined scores that may elucidate the connection between specific symptoms and underlying pathophysiology.
Keywords: Depression, Ketamine, Clinical Trials, Factor Analysis, Psychometrics
Introduction
Under DSM-5 criteria, an estimated 227 combinations of symptoms will lead to a diagnosis of a depressive episode. As a result, a wide range of individuals who meet criteria for depression may overlap on only a limited number of symptoms (Ostergaard et al., 2011; Zimmerman et al., 2015). Indeed, the heterogeneity inherent in the diagnosis of major depressive disorder (MDD) has been a consistent obstacle for identifying viable depression-specific biomarkers that could signal the presence of the disorder as well as predict and track treatment response (Leuchter et al., 2010; Zarate et al., 2013).
Isolating specific clusters of the depressive syndrome with a particular biological signature may be an important step towards advancing translational research into depression and, concomitantly, developing novel therapeutics. However, the depression rating scales commonly used in clinical trials survey a variety of symptoms that reflect DSM criteria, which limits research in several key ways. For instance, such rating scales are useful in dichotomizing individuals into depressed vs. non-depressed samples, but provide little insight into specific symptom clusters that would lead to more homogeneous subgroups, as advocated by efforts such as the NIMH RDoC (Woody and Gibb, 2015). In this context, using unidimensional depressive symptom constructs could reduce variability in the data and increase the precision of attempts to connect specific symptoms with pathophysiology. However, it can be difficult to translate the multifaceted construct of depression across modalities—that is, from depressed patients to healthy control samples or to preclinical models. For example, a cross-method translational approach might first involve isolating a particular symptom construct (e.g, anhedonia or approach motivation) into specific neural circuits in patient samples, followed by an experimental paradigm to induce anhedonic symptoms in non-depressed healthy control participants, and finally into preclinical models of anhedonia in animal studies (Treadway and Zald, 2011). In a similarly translational fashion, findings from preclinical models of anhedonia could have implications for both healthy control and patient samples. However, this approach may be unnecessarily complicated by use of diffuse constructs like ‘depression’. Moreover, depression symptom domains may not have uniform response to treatment. For example, some symptom clusters may be particularly vulnerable to the placebo effect, some may exhibit differential response latency, and others still may not respond to a given intervention. These properties may have unexpected effects on the efficiency and precision of clinical trials, and it is possible—even likely—that researchers are unnecessarily handicapped by redundant use of multidimensional outcome measures.
This analysis sought to identify the unidimensional constructs measured by commonly used rating scales of depression and anhedonia, including the Beck Depression Inventory (BDI), the Hamilton Depression Rating Scale (HAM-D), the Montgomery-Asberg Depression Rating Scale (MADRS), and the Snaith-Hamilton Pleasure Rating Scale (SHAPS). Identifying such unidimensional constructs could then inform the identification of neurobiologically distinct subtypes (also known as biotypes) of depression. In particular, the inclusion of anhedonia and cognitive symptom-specific measures of depression across both clinician-administered and self-report assessments would allow the comprehensive examination of a range of experiences associated with depression. As an initial demonstration of these unidimensional constructs, and in order to assess whether the identified constructs have neurobiological relevance, we examined how these symptoms change in response to a rapid-acting pharmacologic intervention (the glutamatergic modulator ketamine) compared with traditional measures of depression. The literature on depressive biotypes is growing rapidly—in part related to imaging connectivity analyses (Drysdale et al., 2017; Williams, 2017) and the ongoing search for central or peripheral biomarkers (Lamers et al., 2013)—and we believe that careful parcellation of depressive symptoms and behaviors is critical to ensuring that these biotypes have clinical relevance and significance.
Methods
Participants
One hundred nineteen currently depressed patients (61 male, 58 female; aged 21–66, mean age=45.28 years, SD=12.45) were recruited from inpatient studies conducted at the National Institute of Mental Health, National Institutes of Health (NIMH-NIH), Bethesda, MD, USA. The patient sample comprised 76 subjects diagnosed with major depressive disorder (MDD) and 43 diagnosed with bipolar depression (either I or II); the presence of psychotic features was an exclusion criterion for both diagnoses. All patients participated in trials on the same research unit and were assessed and treated by the same clinical and research staff. All trials examined the use of ketamine as a rapid-acting antidepressant; results have been previously published (Diazgranados et al., 2010b; Ibrahim et al., 2012; Zarate et al., 2012; Zarate et al., 2006).
Participants were initially screened and evaluated for eligibility for research participation, which included an initial MADRS score ≥ 20 or a HAM-D score ≥ 18 across all trials. Once at the NIH, diagnosis was established using the Structured Clinical Interview for Axis I Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV (First, 2002)) and corroborated by a team of clinicians using all available information. All subjects were in good physical health as determined by medical history, physical examination, and laboratory tests. Exclusion criteria included pregnancy, nursing, or illicit comorbid substance abuse in the previous three months. Written informed consent was obtained from all participants, and the NIH combined Neuroscience Institute Review Board approved the study.
Across all trials, the co-occurrence of Axis I anxiety disorders was permitted if it was not the primary focus of treatment within the past 12-month period. At hospital admission, all subjects were currently experiencing a major depressive episode lasting at least four weeks. Once admitted and where necessary to comply with individual protocols, subjects were tapered off of their existing medications and underwent a two-week drug-free period (five weeks for fluoxetine, three weeks for aripiprazole) before study baseline. All patients diagnosed with bipolar depression were maintained on a therapeutic dose of either lithium (serum lithium, 0.6–1.2 mEq l−1) or valproic acid (50–125 μg ml−1) for four weeks without exhibiting an antidepressant response to the prescribed medication. No other psychotropic medication or psychotherapy was permitted during the drug-free period prior to study baseline or throughout the study. All subjects, with one exception, were considered treatment-resistant, defined as having failed to respond at least one adequate treatment trial, as determined by the Antidepressant Treatment History Form (Sackeim, 2001).
Design
Details regarding study designs can be found elsewhere (Diazgranados et al., 2010b; Ibrahim et al., 2012; Zarate et al., 2012; Zarate et al., 2006). Briefly, patients were administered psychiatric scales in the morning, approximately one hour before beginning their first infusion (regardless of whether the study was open-label or placebo-controlled). This pre-infusion baseline was a time where patients had been medication-free for at least two weeks, with the exception of those patients with bipolar depression who were maintained on lithium or valproate. Ketamine was administered intravenously at 0.5 mg/kg; in the placebo-controlled studies, saline infusions were used as the control condition. The psychiatric rating scales were re-administered to patients at 40, 80, 120, and 230 minutes post-infusion and at Days 1, 2, and 3.
From the larger patient group of 119 depressed participants, longitudinal data from 41 subjects with bipolar depression were used to assess the unidimensional scores in clinical trials. Most of the bipolar depression patients (n=33) had participated in one of two randomized, placebo-controlled, crossover trials of ketamine (an initial trial and a replication) (Diazgranados et al., 2010b; Zarate et al., 2012). The remaining eight participants were drawn from ongoing biomarker studies. These studies were specifically selected for use in this preliminary analysis due to the uniformity of diagnosis, use of all relevant measures, and similarity of research design; it should be noted that for the eight participants drawn from our ongoing biomarker studies, identical methods were used regarding recruitment procedures, inclusion/exclusion criteria, and study protocols. Patient demographics and treatment response did not differ across sources (see Supplementary Table 1).
Measures
The BDI (Beck et al., 1961) is a 21-item self-reported measure of depression severity. Items are framed as aspects of depressive symptomology such as “Sadness”. Answers are measured on a 0–3 scale, with higher scores indicating increased severity of depressive symptoms (e.g., “I do not feel sad” to “I am so sad or unhappy I can’t stand it”). The BDI has high internal reliability (Beck et al., 1961) and concurrent validity with the HAM-D (Beck et al., 1974).
The HAM-D (Hamilton, 1960) is a 17-item clinician-administered scale that assesses severity of depression. As with the BDI, a range of depressive symptomology is assessed; answers range from 0–4, and higher scores indicate increased severity of depressive symptoms (e.g., for the item “Insomnia: Early in the Night”, answers range from “No difficulty” to “Complains of nightly difficulty falling asleep”).
The MADRS (Montgomery and Asberg, 1979) is a 10-item clinician-administered scale. Ratings of depressive symptomology are made on a scale of 0–6, with higher scores indicating increased severity of depressive symptoms (e.g., for the item “Reduced Appetite”, answers range from “Normal or increased appetite” to “Needs persuasion to eat at all”).
The SHAPS (Snaith et al., 1995) is a 14-item self-reported measure of anhedonia. Items assess anhedonia on a 1–4 scale ranging from “strongly agree” to “strongly disagree”. An example of an item from the SHAPS is “I would find pleasure in my hobbies and pastimes”. Higher scores indicate increased levels of anhedonia.
Statistical Analysis
An exploratory factor analysis (EFA) using Mplus version 7.4 (Muthen and Muthen, 2012) was used to explore baseline data from the 119 participants. An EFA was selected rather than a principal components analysis because the former is the most appropriate method for identifying unidimensional constructs, whereas the latter is most appropriate for data reduction.
First, data were prepared by collapsing response categories with fewer than 10 respondents (e.g., if a BDI item score of 2 was endorsed by only seven participants, those participants were pooled with those who received a score of 1). Second, items with insufficient variability were deleted (e.g., items where fewer than 10 individuals received a non-zero score, which eliminated two items from the HAM-D). Third, given the substantial overlap in some items from different scales, the correlation matrix of all items was examined for values greater than .90, and one item was removed (e.g., the MADRS reduced appetite item was retained, but the HAM-D reduced appetite item was excluded; this occurred for two additional HAM-D items). Finally, an EFA with promax (oblique) rotation was performed on the polychoric correlation matrix, with solutions of 1 through 10 factors. Model fit was evaluated using standard interpretation (Hu and Bentler, 1999) of common fit statistics: the root mean square error of approximation (RMSEA; values <.05 considered good), the comparative fit index (CFI; values >.95 considered good), and the Tucker-Lewis fit index (TLI, values >.95 considered good). The chi-square statistic for improvement in model fit was also evaluated.
Using the best model from the EFA stage, unidimensional constructs (i.e., equally weighted item totals) were created. First, items were selected onto factors where the loading was statistically significant and greater than .30; if an item loaded significantly onto more than one factor, the factor with the strongest loading was selected. Second, items from the different measures were put onto the same scale by dividing the score by the “points possible” on the item (i.e., a score of 1 on a 0-to-3 scale was transformed to 0.33). Finally, to facilitate comparison across unidimensional scores, the scaled item scores were summed and divided by the number of items to generate an item-mean score for each unidimensional construct.
The item-mean unidimensional scores over the course of the crossover trial were analysed using a linear mixed model with restricted maximum likelihood estimation and a compound symmetry covariance structure (selected based on fit indices, calculated separately for drug condition), a random subject effect, and fixed effects of time, drug, and their interaction. The period-specific baseline value was entered as a covariate. Effect sizes with 95% confidence intervals were calculated using the least-square mean estimates of difference (and 95% CI) between ketamine and placebo conditions. These analyses combined multiple trials and were purely exploratory. For that reason, no adjustments for multiple comparisons were made.
Results
Patient demographics and baseline scores on psychiatric scales are reported in Table 1. Patients were moderately to severely depressed, as indicated by an average MADRS score of 34 and an average BDI score of 28.
Table 1.
Participant Demographics
|
|
All Participants n=119 (MDD and BD)
|
Longitudinal Data n=41(Bipolar Depression only) | ||
|---|---|---|---|---|
|
|
||||
| Mean | SD | Mean | SD | |
| Age | 45.28 | 12.45 | 46.38 | 10.73 |
|
|
|
|
|
|
| Age of onset of first depressive episode | 18.82 | 10.05 | 18.03 | 7.10 |
|
|
|
|
|
|
| Length of current episode (months) | 54.43 | 94.44 | 17.87 | 20.68 |
|
|
|
|
|
|
| BMI | 29.56 | 6.53 | 29.96 | 5.81 |
|
|
|
|
|
|
| Baseline BDI | 27.97 | 7.97 | 29.32 | 7.65 |
|
|
|
|
|
|
| Baseline HAM-D | 21.40 | 4.18 | 21.98 | 4.22 |
|
|
|
|
|
|
| Baseline MADRS | 33.51 | 4.80 | 33.90 | 5.14 |
|
|
|
|
|
|
| Baseline SHAPS | 37.95 | 6.72 | 36.90 | 7.75 |
|
|
|
|
|
|
| n | % | n | % | |
|
|
|
|
|
|
| Female | 58 | 49 | 23 | 44 |
|
|
|
|
|
|
| Caucasian | 106 | 89 | 34 | 85 |
|
|
|
|
|
|
| Bipolar Depression | 43 | 36 | 41 | 100 |
|
|
|
|
|
|
| History of suicide attempt | 47 | 40 | 18 | 46 |
|
|
|
|
|
|
Abbreviations: BDI: Beck Depression Inventory; HAM-D: Hamilton Rating Scale for Depression; MADRS: Montgomery-Åsberg Depression Rating Scale; SHAPS: Snaith-Hamilton Pleasure Scale; BMI: Body Mass Index, MDD: major depressive disorder.
Note: All patients whose longitudinal data were used had bipolar depression and were maintained on therapeutic doses of lithium or valproic acid during the study. These patients were selected for a sample longitudinal analysis due to the consistency of scale administration and the similarity of their study designs. One person was missing BMI, and two people were missing data on history of suicide attempt. Longitudinal data were drawn from three identically designed studies (n=18, n=15, and n=8). Participant demographics did not differ by study source (see Supplementary Table 1).
Results of the EFA indicated that an eight-factor solution was the best fit to the data (Table 2). The fit of the model to the data was good; the RMSEA was .025 (95% CI: .008–.036), the CFI was 0.97, and the TLI was 0.95. Factor correlations were negligible to moderate and non-significant in most cases. We labelled the subsequent unidimensional constructs as:
Table 2.
Results of exploratory factor analysis (n=119)
| Number of factors | Model parameters | Χ2 | RMSEA (.06) | RMSEA 90% CI | CFI (.95) | TLI (.95) | SRMR (.08) | Χ2 (p) for fit improvement (<.05) |
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
| 1 | 57 | 2420.38 | 0.07 | (0.064 – 0.075) | 0.65 | 0.64 | 0.15 | n/a |
|
|
|
|
|
|
|
|
|
|
| 2 | 113 | 1923.23 | 0.05 | (0.043 – 0.056) | 0.83 | 0.81 | 0.12 | <.0001 |
|
|
|
|
|
|
|
|
|
|
| 3 | 168 | 1765.78 | 0.05 | (0.037 – 0.051) | 0.87 | 0.85 | 0.10 | <.0001 |
|
|
|
|
|
|
|
|
|
|
| 4 | 222 | 1618.75 | 0.04 | (0.030 – 0.046) | 0.90 | 0.89 | 0.09 | <.0001 |
|
|
|
|
|
|
|
|
|
|
| 5 | 275 | 1511.97 | 0.04 | (0.025 – 0.043) | 0.92 | 0.91 | 0.09 | <.0001 |
|
|
|
|
|
|
|
|
|
|
| 6 | 327 | 1413.98 | 0.03 | (0.019 – 0.040) | 0.94 | 0.93 | 0.08 | <.0001 |
|
|
|
|
|
|
|
|
|
|
| 7 | 378 | 1338.81 | 0.03 | (0.016 – 0.038) | 0.95 | 0.94 | 0.07 | 0.0035 |
|
|
|
|
|
|
|
|
|
|
| 8 | 428 | 1257.14 | 0.03 | (0.008 – 0.036) | 0.97 | 0.95 | 0.07 | 0.0003 |
|
|
|
|
|
|
|
|
|
|
| 9 | 477 | 1187.82 | 0.02 | (0 – 0.034) | 0.97 | 0.96 | 0.06 | 0.0175 |
|
|
|
|
|
|
|
|
|
|
| 10 | 525 | 1133.41 | 0.02 | (0 – 0.034) | 0.98 | 0.96 | 0.06 | 0.1069 |
|
|
|
|
|
|
|
|
|
|
Note: RMSEA: root mean square error of approximation; CFI: comparative fit index; TLI: Tucker-Lewis fit index; SRMR: standardized root mean square residual. The cut-off for each fit statistic, or the value to which a given fit statistic should be close to in order to signal “good” fit (Hu and Bentler, 1999), is listed in each column header; these are rules of thumb. Chi-square is scaled to reflect the use of the mean- and variance-adjusted weighted least squares estimator.
Depressed Mood, Tension, Negative Cognition, Impaired Sleep, Suicidal Thoughts, Reduced Appetite, Anhedonia, and Amotivation; all were of variable size (range three to 12 items, see Table 3). The summed item-mean scores, which were used in subsequent analyses, were strongly related to the factor scores (Pearson correlations ranging from r=.84 to r=.98, all p<.0001) (see Supplementary Table 3 and Supplementary Figure 1).
Table 3.
Eight-factor solution (n=119)
| Source | Item | Loading | Source | Item | Loading |
|---|---|---|---|---|---|
|
|
|
|
|
|
|
| Depressed Mood | Impaired Sleep | ||||
|
|
|
|
|
||
| MADRS | Reported Sadness | 0.79 | MADRS | Reduced Sleep | 0.72 |
|
|
|
|
|
|
|
| HAM-D | Depressed Mood | 0.84 | HAM-D | Early Insomnia | 0.70 |
|
|
|
|
|
|
|
| MADRS | Concentration Difficulties | 0.37 | HAM-D | Middle Insomnia | 0.62 |
|
|
|
|
|
|
|
| MADRS | Inability to Feel | 0.61 | HAM-D | Late Insomnia | 0.58 |
|
|
|
|
|
|
|
| HAM-D | Inability to Work | 0.54 | BDI | Insomnia | 0.65 |
|
|
|
|
|
|
|
| MADRS | Apparent Sadness | 0.77 | Reduced Appetite | ||
|
|
|
|
|
|
|
| HAM-D | Psychomotor Retardation | 0.55 | MADRS | Reduced Appetite | 0.92 |
|
|
|
|
|
|
|
| BDI | Sadness | 0.51 | HAM-D | Weight Loss | 0.49 |
|
|
|
|
|
|
|
| Tension | BDI | Reduced Appetite | 0.76 | ||
|
|
|
|
|
|
|
| HAM-D | Psychological Anxiety | 0.90 | SHAPS | Reduced Enjoyment Eating | 0.53 |
|
|
|
|
|
|
|
| MADRS | Inner Tension | 0.86 | Anhedonia | ||
|
|
|
|
|
|
|
| HAM-D | Hypochondriasis | 0.32 | SHAPS | Reduced Enjoyment TV/Radio | 0.60 |
|
|
|
|
|
|
|
| BDI | Fatigue | 0.30 | SHAPS | Reduced Enjoyment w/Family | 0.43 |
|
|
|
|
|
|
|
| Negative Cognition | SHAPS | Reduced Enjoyment Hobbies | 0.41 | ||
|
|
|
|
|
||
| MADRS | Pessimistic Thoughts | 0.39 | SHAPS | Reduced Enjoyment Smells | 0.60 |
|
|
|
|
|
|
|
| HAM-D | Guilt | 0.61 | SHAPS | Reduced Enjoyment Others’ Happiness | 0.72 |
|
|
|
|
|
|
|
| BDI | Thoughts of Failure | 0.49 | SHAPS | Reduced Enjoyment Appearance | 0.80 |
|
|
|
|
|
|
|
| BDI | Guilt | 0.71 | SHAPS | Reduced Enjoyment Reading | 0.58 |
|
|
|
|
|
|
|
| BDI | Feelings of Punishment | 0.48 | SHAPS | Reduced Enjoyment Tea/Coffee | 0.66 |
|
|
|
|
|
|
|
| BDI | Disappointment in Self | 0.53 | SHAPS | Reduced Enjoyment Small Pleasures | 0.75 |
|
|
|
|
|
|
|
| BDI | Self-Criticism | 0.74 | SHAPS | Reduced Enjoyment Landscape | 0.62 |
|
|
|
|
|
|
|
| BDI | Increased Crying | 0.59 | SHAPS | Reduced Enjoyment Helping Others | 0.57 |
|
|
|
|
|
|
|
| BDI | Worthlessness | 0.70 | SHAPS | Reduced Enjoyment Receiving Praise | 0.86 |
|
|
|
|
|
|
|
| BDI | Reduced Sexual Interest | Amotivation | |||
|
|
|
|
|
|
|
| Suicidal Thoughts | BDI | Dissatisfaction with Life | 0.57 | ||
|
|
|
|
|
||
| MADRS | Suicidal Thoughts | 0.70 | BDI | Loss of Interest in People | 0.33 |
|
|
|
|
|
|
|
| BDI | Hopelessness | 0.67 | BDI | Indecisiveness | 0.34 |
|
|
|
|
|
|
|
| BDI | Suicidal Thoughts | 0.78 | BDI | Inability to Work | 0.72 |
BDI: Beck Depression Inventory; HAM-D: Hamilton Rating Scale for Depression; MADRS: Montgomery-Åsberg Depression Rating Scale; SHAPS: Snaith-Hamilton Pleasure Scale; BMI: Body Mass Index
Note: Items were selected onto subscales when the standardized loading was statistically significant (p<.05) and greater than .30; if an item loaded significantly onto more than one factor, the factor with the strongest loading was selected. Eight items (three from the BDI, three from the HAM-D, one from the MADRS, and one from SHAPS) were excluded, leaving 51 items across the eight subscales. The full loading table is shown in Supplementary Table 2.
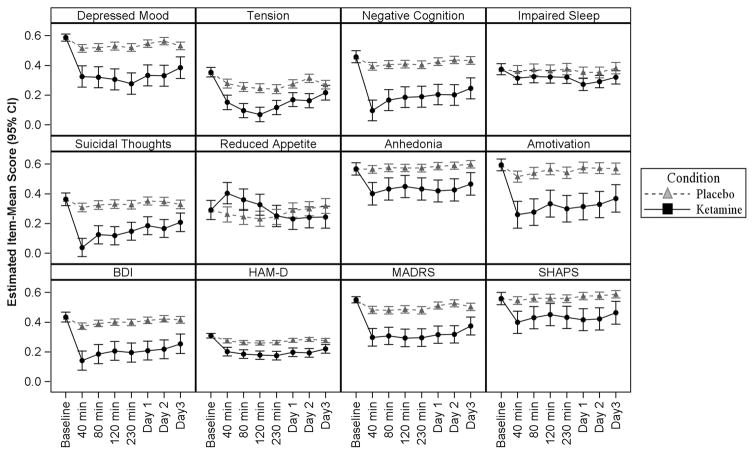
Using the longitudinal data (n=41), we then evaluated the effect of ketamine on both the unidimensional scores and the conventional instrument scores originally used in the trials (BDI, MADRS, HAM-D, and SHAPS) (Table 4). Trajectories of change are shown in Figure 1. With the exceptions of Tension, Impaired Sleep, and Reduced Appetite, a significant effect (p<.05) of ketamine relative to placebo at all post-baseline timepoints was observed on both the unidimensional scores and the conventional rating instrument scores. The effect of ketamine on Tension was significant at all timepoints except Day 3. The effect of ketamine on Impaired Sleep was not significant until 230 minutes. The effect of drug on Reduced Appetite reversed midway through the study, such that the ketamine condition was significantly worse at timepoints 40, 80, and 120 minutes, but not different from placebo at 230 minutes, Day 1, and Day 2. Taken together, these results suggest that unique symptom clusters associated with treatment response to ketamine may be more readily identified using statistically identified unidimensional constructs than conventional rating instruments.
Table 4.
Type III tests of fixed effects (n=41)
|
|
Drug F (p)a | Minutes F (p)b | Drug*Minutes F (p)b |
|---|---|---|---|
| Constructs | |||
|
|
|
|
|
| Depressed Mood | 43.40 (<.0001) | 3.30 (.004) | 2.00 (.07) |
|
|
|
|
|
| Tension | 41.22 (<.0001) | 9.02 (<.0001) | 3.31 (.004) |
|
|
|
|
|
| Negative Cognition | 48.90 (<.0001) | 9.8 (<.0001) | 3.08 (.006) |
|
|
|
|
|
| Impaired Sleep | 13.19 (.0009) | 1.45 (.194) | 0.30 (.94) |
|
|
|
|
|
| Suicidal Thoughts | 47.96 (<.0001) | 9.42 (<.0001) | 4.32 (.0003) |
|
|
|
|
|
| Reduced Appetite | 0.59 (.44) | 2.78 (.01) | 7.33 (<.0001) |
|
|
|
|
|
| Anhedonia | 18.07 (.0001) | 1.99 (.07) | 0.71 (.64) |
|
|
|
|
|
| Amotivation | 38.50 (<.0001) | 3.83 (.001) | 0.55 (.77) |
|
|
|
|
|
| Conventional Scores | |||
|
|
|
|
|
| BDI Total | 44.19 (<.0001) | 8.10 (<.0001) | 1.38 (.22) |
|
|
|
|
|
| HAM-D Total | 36.09 (<.0001) | 4.68 (.0001) | 1.22 (.30) |
|
|
|
|
|
| MADRS Total | 47.37 (<.0001) | 3.90 (.0009) | 1.62 (.14) |
|
|
|
|
|
| SHAPS Total | 15.41 (.0003) | 2.32 (.03) | 0.77 (.60) |
|
|
|
|
|
Numerator DF was 1, denominator DF ranged from 31.3 to 57.2.
Numerator DF was 6, denominator DF ranged from 290 to 384.
Note: BDI: Beck Depression Inventory; HAM-D: Hamilton Rating Scale for Depression; MADRS: Montgomery-Asberg Depression Rating Scale; SHAPS: Snaith Hamilton Pleasure Scale; DF: degrees of freedom. Results of a mixed model with compound symmetry covariance structure for repeated measures, random subject effect, and Satterthwaite’s approximation for degrees of freedom. Period-specific baseline was entered as a covariate.
Figure 1. Results of mixed models in Bipolar Depression analysis (n=41).
Item-mean scores reflect the average proportion of points endorsed to points available across items on the subscale. For all constructs except Tension, Impaired Sleep, and Reduced Appetite, the difference between ketamine and placebo was statistically significant (p<.05) at all post-baseline assessments. The effect of drug on Tension was significant except at Day 3. The effect of drug on Impaired Sleep was not significant until 230 minutes. The effect of drug on Reduced Appetite was significant (in favour of placebo) until 230, after which it was not significant.
BDI: Beck Depression Inventory; HAM-D: Hamilton Rating Scale for Depression; MADRS: Montgomery-Asberg Depression Rating Scale; SHAPS: Snaith Hamilton Pleasure Scale.
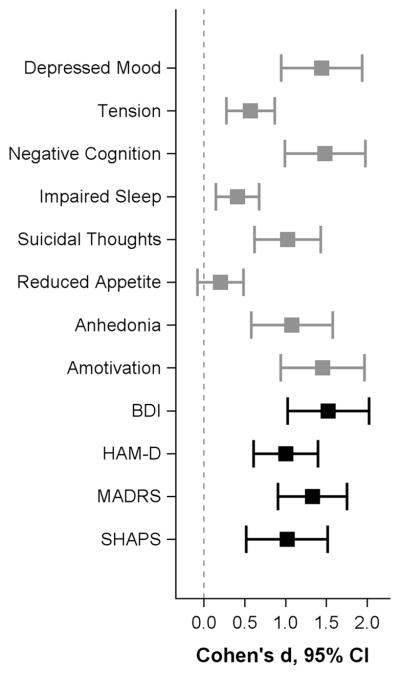
Although the significant effect of drug was apparent across both unidimensional and conventional scores, the magnitude of the effect varied widely. Using Day 1 for the purposes of illustration (Figure 2), we noted that effect sizes for ketamine versus placebo ranged from very small and non-significant (Reduced Appetite: d=0.10, 95% CI: −0.04–0.24) to large (Amotivation: d=0.73, 95% CI: 0.47–0.98). Less variability was observed in the conventional rating instrument scores, which all exceeded 0.50.
Figure 2. Effect sizes (ketamine versus placebo) at Day 1 in the Bipolar Depression analysis (n=41).
Effect sizes were calculated using least square mean estimates at Day 1 from the mixed model.
BDI: Beck Depression Inventory; HAM-D: Hamilton Rating Scale for Depression; MADRS: Montgomery-Asberg Depression Rating Scale; SHAPS: Snaith Hamilton Pleasure Scale.
Degree of improvement on placebo (i.e., change from baseline during the placebo condition) also varied. For the purposes of modelling placebo response, baseline scores were moved from covariate to dependent variable so later timepoints could be directly compared to baseline, and the models were re-run. Three of the conventional rating instrument scores exhibited significant placebo response (i.e., change from baseline) at 40 minutes: BDI (d=0.38, 95% CI: 0.24–0.52), HAM-D (d=0.28, 95% CI: 0.15–0.42), and MADRS (d=0.34, 95% CI: 0.20–0.47). In contrast, five of the eight unidimensional scores exhibited significant placebo response at 40 minutes: Depressed Mood (d=0.34, 95%CI 0.21–0.48), Tension (d=0.27, 95% CI: 0.14–0.41), Negative Cognition (d=0.32, 95% CI: 0.19–0.46), Suicidal Thoughts (d=0.22, 95% CI: 0.08–0.35), and Amotivation (d=0.30, 95% CI: 0.16–0.43). With the exceptions of Amotivation and Suicidal Thoughts, these placebo effects remained significant (though attenuated) at Day 1.
Discussion
This EFA of the MADRS, HAM-D, BDI, and SHAPS was conducted with ratings obtained from subjects with treatment–resistant MDD and bipolar depression in an effort to identify and reduce heterogeneity within depression rating scales. The scales were found to measure several unidimensional constructs, which we identified as: Depressed Mood, Tension, Negative Cognition, Impaired Sleep, Suicidal Thoughts, Reduced Appetite, Anhedonia, and Amotivation. The Anhedonia construct comprised items from the SHAPS, whereas the Negative Cognition construct was primarily composed of items from the BDI, highlighting the importance of using a range of clinical and self-report measures of both depressive and anhedonic symptoms to obtain a more comprehensive picture of depressive symptomatology.
As a first demonstration of the use of these constructs, we reanalyzed existing clinical trial data using the unidimensional scores as outcomes. The effect of ketamine varied from small to moderate across the unidimensional scores, demonstrating that the depression symptom domains did not respond uniformly to ketamine administration. In contrast, the effect sizes across total scores from the conventional measures (MADRS, HAM-D, BDI, and SHAPS) were uniformly moderate. Furthermore, variable patterns of placebo response were observed; five of the eight unidimensional scores were significantly improved at 40 minutes from baseline, while three of the four conventional measures were improved after placebo. Overall, the results of the EFA indicated that several unidimensional constructs can be measured by widely used depression rating scales. In addition, our preliminary evaluation of clinical trial data in inpatients with treatment-resistant MDD and bipolar depression suggests that using these unidimensional scores may reveal response trajectories that were previously obscured by use of heterogeneous conventional rating instruments.
Our study extends the work of other researchers who identified homogenous constructs that could be measured by depression and anhedonia rating scales by widening the EFA to include constructs across—rather than within—scales. In one respect, the resulting EFA is quite similar to other factor analyses of MDD symptoms (Vrieze et al., 2014), including analyses of the HAM-D6 as a distilled measure of melancholia (Bech et al., 2011; Ostergaard et al., 2014), and other evaluations of the SHAPS as a unidimensional scale with divergent validity from other depression rating measures (Nakonezny et al., 2010; Nakonezny et al., 2015). Similarly, Grunebaum and colleagues, in their separate factor analyses of the HAM-D and BDI, identified constructs that reflect psychic depression, loss of motivated behavior, disturbed thinking, anxiety, and sleep disturbance from the HAM-D, and subjective depression, self-blame, and somatic complaints from the BDI (Grunebaum et al., 2005). These subscale scores have also been linked to distinct neuroimaging findings using PET, as well as response to treatment (Grunebaum et al., 2013; Milak et al., 2005). The present study builds upon this work by combining scales that represent all facets of depressive symptoms, thus enabling us to more holistically model their heterogeneity. Although we excluded redundant items, many items remained that were similar across scales. This allowed us to identify factors represented by few items (for instance, suicidal ideation) that, traditionally, would have been assessed by only one item in each scale, resulting in greater measurement bias.
The variable effects of ketamine observed across unidimensional scores are consistent with previously published findings of ketamine’s rapid and broad antidepressant, anti-anxiolytic, anti-anhedonic, and anti-suicidal effects (Diazgranados et al., 2010a; Feder et al., 2014; Lally et al., 2014). Previous analyses of these data revealed that ketamine had observable effects on both anhedonia and suicidal ideation as well as on neural correlates identified via PET imaging, and that these effects occurred independently from its overall effect on depressive symptoms (Ballard et al., 2014a; Ballard et al., 2014b; Lally et al., 2014; Lally et al., 2015). It is likely that the PET imaging findings were most closely related to the constructs assessed by the Depressed Mood, Anhedonia, and Suicidal Ideation factors. Similarly, a recent meta-analysis on functional connectivity suggested that depressed and anxious patients exhibited a number of depressive and anxious biotypes (Williams, 2017). We suspect that these biotypes may be more easily validated with unidimensional constructs like those proposed here than with conventional, heterogeneous measures. For example, the rumination biotype (Williams, 2017), characterized by default mode hyper-activation, could be linked to the Negative Cognition construct. In turn, the apprehension biotype, which is characterized by hypoactivation in the salience circuit, could be linked to the Tension construct, and the anhedonia biotype, characterized by reward circuit hypoactivation, could be compared to both the Anhedonia and Amotivation constructs. In addition, Drysdale and colleagues developed a series of fMRI-based biotypes derived from frontostriatal and limbic connectivity and found that these differed on measures of anxiety, anhedonia, and psychomotor agitation (Drysdale et al., 2017), providing additional evidence that the constructs identified in the present study might fit into a dimensional approach that could be used to assess the neurobiology of depression, as advocated by efforts such as the NIMH RDoC (Cuthbert, 2014).
A major strength of this approach is that data obtained from multiple trials conducted by the same clinical researchers on the same clinical inpatient unit were combined, which reduces variability in the administration and interpretation of the original rating instruments. However, the study is also associated with several limitations. These include: 1) the small sample size; 2) the absence of an independent sample for confirmation of the EFA; 3) the fact that the participant sample comprised individuals with treatment-resistant MDD or bipolar depression, which might not translate across the continuum of individuals with mood disorders or healthy individuals; 4) the possibility that instruments such as the Temporal Experience of Pleasure Scale, which makes a clear distinction between anticipatory and consummatory anhedonia (Gard et al., 2006), could ultimately be more useful in future analyses than the SHAPS; and 5) that while these factors somewhat align with biotypes as proposed in the neuroimaging literature, future analyses should explicitly link these factors to functional connectivity data, experimental paradigms, plasma markers, and treatment outcomes. Finally, the small sample size precluded our ability to assess measurement invariance across diagnosis (MDD versus bipolar disorder) or over time. The possibility that the measurement properties of the factor model may differ across disorders or across timepoints is a limitation that must be addressed in future analyses.
Conclusions
As interest in identifying biotypes of subjects with mood disorders develops, so will the need for appropriate and specific clinical assessments. The present study has underscored that factor analysis techniques can identify unidimensional constructs from a range of depressive symptoms. By using these unidimensional scores instead of total measure scores, we may be able to increase the precision with which we clinically describe these new and emerging biotypes, as well as measure response to treatment. Such an approach may be an important step towards parsing the heterogeneity of depressive symptoms.
Supplementary Material
Highlights.
Depression is a heterogeneous disorder with a variety of presenting symptoms
Depression rating scales may capture a number of constructs
This factor analysis studied rating scales often used in depression clinical trials
The identified constructs showed differential responses to ketamine
The constructs may help investigate the neurobiology of depression
Acknowledgments
The authors thank the 7SE research unit and staff for their support. Ioline Henter (NIMH) provided invaluable editorial assistance.
Role of Funding Source
Funding for this work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH) (NCT00088699; ZIA-MH002927; 04-M-0222), by a NARSAD Independent Investigator Award to Dr. Zarate, and by a Brain and Behavior Mood Disorders Research Award to Dr. Zarate. These organizations had no further role in study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Declaration of Interest
Dr. Zarate is listed as a coinventor on a patent for the use of ketamine and its metabolites in major depression and suicidal ideation. Dr. Zarate is listed as a co-inventor on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydro and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain. Dr. Zarate is listed as co-inventor on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders; he has assigned his patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government. All other authors have no conflict of interest to disclose, financial or otherwise.
Contributors
EDB: conceptualized the study; drafted the manuscript; helped interpret the statistical analysis; edited the manuscript for critical intellectual content; approved the final version of the manuscript.
JY: helped conceptualize the study; helped interpret the statistical analysis; revised and edited the manuscript for critical intellectual content; approved the final version of the manuscript.
CAF: helped conceptualize the study; conducted the statistical design; revised and edited the manuscript for critical intellectual content; approved the final version of the manuscript.
MSL: helped conceptualize the study; revised and edited the manuscript for critical intellectual content; approved the final version of the manuscript.
BK: helped conceptualize the study; revised and edited the manuscript for critical intellectual content; approved the final version of the manuscript.
NL: helped conceptualize the study; revised and edited the manuscript for critical intellectual content; approved the final version of the manuscript.
DW: helped conceptualize the study; revised and edited the manuscript for critical intellectual content; approved the final version of the manuscript.
RMV: helped conceptualize the study; revised and edited the manuscript for critical intellectual content; approved the final version of the manuscript.
MJN: helped conceptualize the study; revised and edited the manuscript for critical intellectual content; approved the final version of the manuscript.
LP: helped conceptualize the study, revised and edited the manuscript for critical intellectual content; approved the final version of the manuscript.
CAZ: helped conceptualize the study; provided research supervision; edited the manuscript for critical intellectual content; approved the final version of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ballard ED, Ionescu DF, Vande Voort JL, Niciu MJ, Richards EM, Luckenbaugh DA, Brutsche NE, Ameli R, Furey ML, Zarate CA., Jr Improvement in suicidal ideation after ketamine infusion: Relationship to reductions in depression and anxiety. J Psychiatr Res. 2014a;58:161–166. doi: 10.1016/j.jpsychires.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard ED, Lally N, Nugent AC, Furey ML, Luckenbaugh DA, Zarate CA., Jr Neural correlates of suicidal ideation and its reduction in depression. Int J Neuropsychopharmacol. 2014b:18. doi: 10.1093/ijnp/pyu069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bech P, Fava M, Trivedi MH, Wisniewski SR, Rush AJ. Factor structure and dimensionality of the two depression scales in STAR*D using level 1 datasets. J Affect Disord. 2011;132:396–400. doi: 10.1016/j.jad.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Beck AT, Rial WY, Rickets K. Short form of depression inventory: cross-validation. Psychol Rep. 1974;34:1184–1186. [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN. The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. 2014;13:28–35. doi: 10.1002/wps.20087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Ameli R, Henter ID, Luckenbaugh DA, Machado-Vieira R, Zarate CA. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-aspartate antagonist in patients with treatment resistant major depressive disorder. J Clin Psychiatry. 2010a;71:1605–1611. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diazgranados N, Ibrahim L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji HK, Zarate CA., Jr A randomized add-on trial of an N-methyl-D-aspartate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010b;67:793–802. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drysdale AT, Grosenick L, Downar J, Dunlop K, Mansouri F, Meng Y, Fetcho RN, Zebley B, Oathes DJ, Etkin A, Schatzberg AF, Sudheimer K, Keller J, Mayberg HS, Gunning FM, Alexopoulos GS, Fox MD, Pascual-Leone A, Voss HU, Casey BJ, Dubin MJ, Liston C. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23:28–38. doi: 10.1038/nm.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder A, Parides MK, Murrough JW, Perez AM, Morgan JE, Saxena S, Kirkwood K, aan het Rot M, Lapidus KAB, Wan LB, Iosifescu D, Charney DS. Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder. A randomized clinical trial. JAMA Psychiatry. 2014;71:681–688. doi: 10.1001/jamapsychiatry.2014.62. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Biometrics Research. New York: New York State Psychiatric Institute; 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P) [Google Scholar]
- Gard DE, Gard MG, Kring AM, John OP. Anticipatory and consummatory components of the experience of pleasure: A scale development study. J Res Personal. 2006;40:1086–1102. [Google Scholar]
- Grunebaum MF, Keilp J, Li S, Ellis SP, Burke AK, Oquendo MA, Mann JJ. Symptom components of standard depression scales and past suicidal behavior. J Affect Disord. 2005;87:73–82. doi: 10.1016/j.jad.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Grunebaum MF, Keilp JG, Ellis SP, Sudol K, Bauer N, Burke AK, Oquendo MA, Mann JJ. SSRI versus bupropion effects on symptom clusters in suicidal depression: post hoc analysis of a randomized clinical trial. J Clin Psychiatry. 2013;74:872–879. doi: 10.4088/JCP.12m08000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Lt, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1–55. [Google Scholar]
- Ibrahim L, Diazgranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, Moaddel R, Wainer I, Luckenbaugh DA, Manji HK, Zarate CA., Jr Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology. 2012;37:1526–1533. doi: 10.1038/npp.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lally N, Nugent AC, Luckenbaugh DA, Ameli R, Roiser JP, Zarate CA. Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Transl Psychiatry. 2014;4:e469. doi: 10.1038/tp.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lally N, Nugent AC, Luckenbaugh DA, Niciu MJ, Roiser JP, Zarate CA., Jr Neural correlates of change in major depressive disorder anhedonia following open-label ketamine. J Psychopharmacol. 2015;29:596–607. doi: 10.1177/0269881114568041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers F, Vogelzangs N, Merikangas KR, de Jonge P, Beekman AT, Penninx BW. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol Psychiatry. 2013;18:692–699. doi: 10.1038/mp.2012.144. [DOI] [PubMed] [Google Scholar]
- Leuchter AF, Cook IA, Hamilton SP, Narr KL, Toga A, Hunter AM, Faull K, Whitelegge J, Andrews AM, Loo J, Way B, Nelson SF, Horvath S, Lebowitz BD. Biomarkers to predict antidepressant response. Curr Psychiatry Rep. 2010;12:553–562. doi: 10.1007/s11920-010-0160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milak MS, Parsey RV, Keilp J, Oquendo MA, Malone KM, Mann JJ. Neuroanatomic correlates of psychopathologic components of major depressive disorder. Arch Gen Psychiatry. 2005;62:397–408. doi: 10.1001/archpsyc.62.4.397. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO. Mplus User's Guide. 7. Muthen & Muthen; Los Angeles, CA: 2012. [Google Scholar]
- Nakonezny PA, Carmody TJ, Morris DW, Kurian BT, Trivedi MH. Psychometric evaluation of the Snaith-Hamilton pleasure scale in adult outpatients with major depressive disorder. Int Clin Psychopharmacol. 2010;25:328–333. doi: 10.1097/YIC.0b013e32833eb5ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakonezny PA, Morris DW, Greer TL, Byerly MJ, Carmody TJ, Grannemann BD, Bernstein IH, Trivedi MH. Evaluation of anhedonia with the Snaith-Hamilton Pleasure Scale (SHAPS) in adult outpatients with major depressive disorder. J Psychiatr Res. 2015;65:124–130. doi: 10.1016/j.jpsychires.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostergaard SD, Bech P, Trivedi MH, Wisniewski SR, Rush AJ, Fava M. Brief, unidimensional melancholia rating scales are highly sensitive to the effect of citalopram and may have biological validity: implications for the research domain criteria (RDoC) J Affect Disord. 2014;163:18–24. doi: 10.1016/j.jad.2014.03.049. [DOI] [PubMed] [Google Scholar]
- Ostergaard SD, Jensen SO, Bech P. The heterogeneity of the depressive syndrome: when numbers get serious. Acta Psychiatr Scand. 2011;124:495–496. doi: 10.1111/j.1600-0447.2011.01744.x. [DOI] [PubMed] [Google Scholar]
- Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(Suppl 16):10–17. [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. Br J Psychiatry. 1995;167:99–103. doi: 10.1192/bjp.167.1.99. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci Biobehav Rev. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze E, Demyttenaere K, Bruffaerts R, Hermans D, Pizzagalli DA, Sienaert P, Hompes T, de Boer P, Schmidt M, Claes S. Dimensions in major depressive disorder and their relevance for treatment outcome. J Affect Disord. 2014;155:35–41. doi: 10.1016/j.jad.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM. Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: a theoretical review of the evidence and future directions for clinical translation. Depress Anxiety. 2017;34:9–24. doi: 10.1002/da.22556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woody ML, Gibb BE. Integrating NIMH Research Domain Criteria (RDoC) into Depression Research. Curr Opin Psychol. 2015;4:6–12. doi: 10.1016/j.copsyc.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Brutsche NE, Ibrahim L, Franco-Chaves J, Diazgranados N, Cravchik A, Selter J, Marquardt CA, Liberty V, Luckenbaugh DA. Replication of ketamine's antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71:939–946. doi: 10.1016/j.biopsych.2011.12.010. Epub 2012 Jan 2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Mathews DC, Furey ML. Human biomarkers of rapid antidepressant effects. Biol Psychiatry. 2013;73:1142–1155. doi: 10.1016/j.biopsych.2012.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Ellison W, Young D, Chelminski I, Dalrymple K. How many different ways do patients meet the diagnostic criteria for major depressive disorder? Compr Psychiatry. 2015;56:29–34. doi: 10.1016/j.comppsych.2014.09.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.