Abstract
Dietary protein is required for optimal skeletal growth and maturation. Although Recommended Dietary Allowances (RDAs) exist for global dietary protein intake, the level and sources of dietary protein that are optimal for skeletal health over the life continuum have not been established. This is partly due to the difficulty in quantifying the effects of variable levels of a nutrient’s intake over a lifetime as well as the complex nature of the relationships between dietary protein and calcium economy. Areas of current uncertainty include the precise source and amount of dietary protein required for optimal skeletal accretion and maintenance of skeletal mass, as well as the site-specific effects of dietary protein. The cellular and molecular mechanisms that underpin the actions of dietary protein on mineral metabolism and skeletal homeostasis remain unclear. This review attempts to summarize recent data bearing on these questions.
Keywords: Dietary protein, Amino acids, Calcium absorption, Skeletal health, IGF-1
1. Introduction
There is no question that dietary protein is required for skeletal health. Dietary protein is essential for collagen synthesis and the production of non-collagenous matrix proteins in bone (Barbul, 2008). A variety of bone specific extracellular matrix proteins such as osteopontin, bone sialoprotein and fibronectin are also important for the orderly mineralization of the skeleton.
Although recommendations have been made regarding levels of protein intake that are considered adequate for children, adolescents and adults (Food and Nutrition Board IoM, 2002/2005), the level of dietary protein that is optimal for skeletal health is a largely unexplored area. The potential relationship between dietary protein and bone health is particularly relevant to skeletal health in later adult life, at which time bone loss and fracture risks increase. In that context concern has been raised that diets rich in animal protein are deleterious to the adult skeleton (Bushinsky, 2001; Remer, 2000). This is commonly referred to as the acid–ash hypothesis. According to this formulation, the endogenous acid load imposed by the metabolism of sulfur-containing amino acids requires buffering in bone that causes increased bone resorption, which in turn results in the loss of calcium from bone. However, recent data have led to a reconsideration of dietary protein’s actions vis-à-vis the skeleton because of studies suggesting a beneficial effect of protein on mineral metabolism, bone mass and fracture risk (Beasley et al., 2014; Hannan et al., 2000; Kerstetter et al., 2005). This review summarizes recent data bearing on this controversy.
2. The impact of dietary protein on bone accretion during growth
During skeletal growth, it appears that there may be regional differences in the response to a given source of dietary protein. In the setting of a modestly restricted protein intake, 10-week-old mice fed a casein-based diet for 60 days showed a greater increase in femoral bone mineral density (BMD) than mice fed an equivalent soy-based protein diet (Table 1, Rouy et al., 2014). In contrast, spinal BMD was not influenced by either the level or source of dietary protein. Assessment by micro computed tomography revealed significantly greater bone area at the femoral diaphysis, after correcting for changes in weight, in animals on the casein-based, protein restricted diet compared to the soy diet of equal protein level. Indices of bone formation, including osteoid surface and mineral apposition rate, as estimated by micro CT were reduced in the soy fed protein-restricted animals, but not in the animals fed the protein-restricted, casein based diet or a soy-based diet containing a normal amount of protein (20%). Ten-week-old growing rats were fed an energy restricted diet or diet in which both protein and energy were restricted. When casein was supplemented to either one of these diets such that the percent of energy from protein equaled or exceeded that in a normal diet the animals demonstrated a significant protective effect against bone loss (Table 1, Mardon et al., 2009). BMD was lowest in animals that were placed on the energy and protein restricted diets compared to animals whose diets were solely calorically restricted. Conversely, diaphyseal BMD of the femur was not impacted by level of dietary protein when caloric intake was limited, suggesting that during periods of caloric restriction dietary protein may influence trabecular BMD to a greater extent than cortical BMD. As anticipated, restrictions in energy and protein intake prevented increases in body weight in growing animals. However, a shift in the macronutrient composition of the diet to include a greater proportion of calories from casein allowed for some weight gain (albeit not normal) despite deprivation of total energy. Thus, higher casein intakes appear to offset some of the deleterious effects on growth of a calorically restricted diet. In the same study, energy restriction was accompanied by a fall in circulating levels of the anabolic hormone, insulin-like growth factor-1 (IGF-1), which could not be fully compensated by an increase in dietary protein intake even when protein exceeded 20% of total energy. In summary, this latter study suggests that in the setting of limited energy intake and impaired growth rates, dietary casein supplementation may provide site-specific protective effects on bone that are independent of IGF-1.
Table 1.
Impact of animal and vegetable protein sources on skeletal health during periods of growth.
| Reference | Participants/model | Study period/classification | Protein intake | Summary of findings | Favorable effects of animal protein sources | Favorable effects of vegetable protein sources |
|---|---|---|---|---|---|---|
| Animal models | ||||||
| Rouy et al. (2014) | 10-week old Balb/C mice | Sixty days | Four isocaloric diets (pair-fed):
|
Percent change in total and femur BMD by DXA significantly greater with 6% casein compared to 6% soy diet. Greater bone area at femoral diaphysis, after correcting for body weight, with 6% casein compared to 6% soy diet. Indices of bone formation and bone turnover reduced in animals receiving 6% soy vs. 6% casein diet. Circulating IGF-1 levels did not significantly differ between the 6% soy and 6% casein diets. Detrimental skeletal effects of 6% soy diet were not observed with PTH treatment. Twenty percent soy and 6% casein diets induced similar effects on the skeleton. | Yes, under protein restricted conditions. | No, not under protein restricted conditions. |
| Mardon et al. (2009) | 10-week old Wistar male rats | Five months | Six casein based diets:
|
Total and metaphyseal femoral BMD were lowest when energy and protein were concurrently restricted, and significantly higher with increased dietary casein. Increases in casein intake did not completely offset declines in IGF-1 observed with energy restricted diets. | Yes, under energy restricted conditions. | N/A |
| Epidemiological studies | ||||||
| Chevalley et al. (2008) | 232 Healthy prepubertal boys 7.4 ± 0.4 (SD) years | Cross-sectional analysis | Assessed by food frequency questionnaire. | After controlling for physical activity, total protein intake (g/d) from mixed animal sources (approximately 50% from dairy and 50% from meat, fish and eggs) remained a significant predictor of bone mineral content. Protein intake of approximately 2.0 g/kg augmented the positive impact of physical activity on bone mineral content. | Yes | N/A |
| Alexy et al. (2005) | 229 Healthy children and adolescents, Dortmund Nutritional and Anthropometric Longitudinally Designed Study 6–18 years | Four-year prospective longitudinal study | Assessed by weighed and semiquantitative food records. | A significant, positive association between long-term total protein intake (g/d) and one- time pQCT analysis of the forearm (periosteal circumference, cortical area, bone mineral content and polar strength strain index) were observed. Intake of sulfur containing amino acids did not appear to influence the skeletal parameters of interest. | Did not differentiate between sources of animal and vegetable protein. However, long-term intake of sulfur-containing amino acids, which are found at higher concentrations in animal protein sources, did not have a negative impact on bone variables. | |
In children and adolescents up to 18 years of age the current Recommended Dietary Allowance (RDA) for protein ranges from 0.85 to 1.1 g/kg (Food and Nutrition Board IoM, 2002/2005). Recently, it has been suggested that intakes above the current recommendation and in particular certain sources of dietary protein may accelerate bone accretion during growth and thereby increase peak adult bone mass. In healthy, white prepubertal boys, total protein intake from mixed animal sources remained significantly associated with bone mineral content (BMC) after controlling for physical activity (Table 1, Chevalley et al., 2008). In addition, a protein intake of approximately 2.0 g/kg, which is above the current RDA for this age group, augmented the positive impact of physical activity on BMC. Moreover, long-term total protein intake (g/d), assessed by weighed and semiquantitative food records, was found to be a significant positive predictor of bone modeling (as estimated by the rate of cortical apposition) in 229 male and female children (Table 1, Alexy et al., 2005). Dietary sulfur containing amino acids did not appear to attenuate this effect, although the estimated potential renal acid load (PRAL) did.
3. The effect of dietary protein on adult skeletal health
The effect of dietary protein on the mature skeleton after epiphyseal closure and particularly on the aging skeleton remains an area of considerable controversy. For nearly 90 years, we have known that dietary protein affects calcium metabolism (Sherman, 1920). It has long been known that increasing dietary protein increases urinary calcium excretion (Kerstetter et al., 2003). Balanced studies conducted in the 1970s and 1980s suggested that dietary protein did not change intestinal calcium absorption despite the hypercalcuria (Allen et al., 1979; Anand and Linkswiler, 1974; Hegsted and Linkswiler, 1981; Kim and Linkswiler, 1979), leading to the conclusion that increasing dietary protein resulted in a net negative calcium balance. Consistent with this, earlier epidemiologic studies suggested that increasing dietary protein was associated with an increased risk for fracture in middle-aged and elderly women and men (Table 2, Abelow et al., 1992; Feskanich et al., 1996; Frassetto et al., 2000; Meyer et al., 1997). However, more recent short-term studies using dual-stable calcium isotopes have demonstrated that increasing dietary protein improves intestinal calcium absorption and that the increment in urinary calcium can be quantitatively explained by the increase in intestinal calcium absorption (Kerstetter et al., 1998, 2005). Furthermore, data from the Framingham Osteoporosis Study showed that higher protein intakes in both men and women was associated with slower rates, not higher rates, of bone loss at the femoral neck and spine (Table 2, Hannan et al., 2000). Two recent epidemiological studies also reported a positive relationship between dietary protein and skeletal health, with higher protein intakes being associated with greater total and hip BMD and total BMC, and lower rates of forearm fracture (Table 2) (Beasley et al., 2014; Kim et al., 2013). Potential mechanisms by which dietary protein affects calcium metabolism and skeletal homeostasis will be reviewed later.
Table 2.
Impact of animal and vegetable protein sources on skeletal health in adulthood.
| Reference | Participants/model | Study period/classification | Protein intake | Summary of findings | Favorable effects of animal protein source | Favorable effects of vegetable protein source |
|---|---|---|---|---|---|---|
| Epidemiological studies | ||||||
| Abelow et al. (1992) | 16 countries women >50 years | Retrospective analysis | Per capita food consumption data estimated from Food Balance Sheets. | Strong positive association between rates of hip fracture and animal protein intake. | No, potentially harmful. | N/A |
| Feskanich et al. (1996) | 85,900 women Nurses’ Health Study 35–59 years | Twelve-year prospective study | Assessed by semiquantitative food frequency questionnaire. | Higher intakes of total protein, animal protein and red meat were positively associated with risk of forearm fracture. No associations were observed for vegetable protein intake and forearm fracture risk, nor were there any observed associations for protein intake and hip fracture risk. | No, potentially harmful. | No, neutral relationship. |
| Meyer et al. (1997) | 19,752 women and 20,035 men National Health Screening Service of Norway 35–49 years | Eleven-year prospective study | Assessed by semiquantitative food frequency questionnaire. Emphasis placed on food items representing important fat sources. Usual intake of foods common in the Norwegian diet was also captured. | Intake of nondairy animal protein was not related to hip fracture. Combination of high nondairy animal protein intakes and low calcium intakes increased risk of hip fracture in women. Total protein intake was not associated with hip fracture risk. | No, potentially harmful in low calcium environment. | N/A |
| Frassetto et al. (2000) | 33 countries women ≥50 years | Retrospective analysis | Per capita food consumption data estimated from Food Balance Sheets. | Incidences of hip fractures were positively associated with total protein and animal protein intakes. Vegetable protein intake was negatively associated with hip fracture incidence. | No, potentially harmful. | Yes |
| Hannan et al. (2000) | 224 men and 392 women Framingham Osteoporosis Study 68–91 years | Four-year prospective study | Assessed by semiquantitative food frequency questionnaire. | Lower total protein and animal protein intake was associated with greater bone loss at the femoral neck and spine. No observed relationship between vegetable protein sources and bone loss. | Yes | No, neutral relationship. |
| Kim et al. (2013) | 6,149 men and women NHANES ≥50 years | Cross-sectional study | Assessed by 24-hr recall. | Higher protein intakes were associated with higher total bone mineral content, appendicular bone mineral content and total BMD. | N/A, did not differentiate between animal and vegetable sources. However, there was an overall positive effect of total protein intake on bone mass. | |
| Beasley et al. (2014) | 144,580 women Women’s Health Initiative 50–79 years | Six-year prospective study | Assessed by food frequency questionnaire. | Higher protein intakes, when expressed as a percentage of total energy intake, were associated with a lower risk of forearm fracture. Greater protein intakes were also positively associated with total and hip BMD at 3 years. The association between protein intake and total body BMD persisted at the 6-year follow-up. No associations were observed for dietary protein and hip or spinal fractures. | N/A, did not differentiate between animal and vegetable sources. However, there was an overall positive effect of total protein intake on bone mass. | |
3.1. Dietary protein-induced changes in calcium absorption efficiency
For every 40 g increment in dietary protein, urinary calcium increases by approximately 50 mg (Kerstetter et al., 2003). It was generally assumed that the dietary protein-induced increases in urinary calcium results from the release of skeletal buffer and calcium in response to the metabolic load imposed by sulfur-containing amino acids (Barzel and Massey, 1998; Bushinsky, 2001; Bushinsky and Frick, 2000; Remer, 2000). However, as just noted, studies using dual stable calcium isotopes have found that in the short term, dietary protein profoundly affects intestinal calcium absorption (Fig. 1). In particular, Kerstetter et al. demonstrated that as compared to a high protein diet (2.1 g protein/kg/d), restricting dietary protein to 0.7 g protein/kg/d results in hypocalciuria caused by a reduction in fractional intestinal calcium absorption (Kerstetter et al., 1998). Additionally, increasing dietary protein from 1.0 to 2.1 g protein/kg/d resulted in an increase in urinary calcium that is not accompanied by evidence for increased bone resorption. The incremental change in urinary calcium could be nearly quantitatively explained by the improvement in intestinal calcium absorption that accompanied the increase in dietary protein (Kerstetter et al., 2005). In this experiment, natural foodstuffs were used and the dietary contents of calcium, sodium and phosphorus were controlled so that they were the same or very similar on both the 1.0 g/kg and 2.1 g/kg protein diets. Dietary protein was increased using both animal and vegetable protein sources (Fig. 1).
Fig. 1.
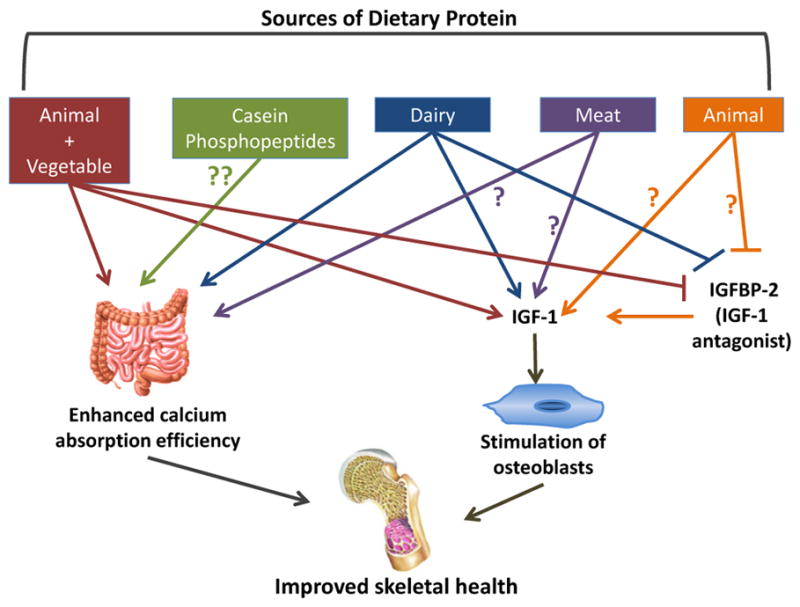
Potential mechanisms by which animal and vegetable sources of dietary protein promote skeletal health. There are likely source-specific effects of dietary protein on skeletal metabolism, the intestine and the IGF-1/IGFBP axis. A high, mixed protein diet (animal + vegetable sources) enhances intestinal calcium absorption and increases circulating levels of IGF-1. Dietary casein, a component of milk, may improve intestinal calcium absorption efficiency. Diary increases the intestinal absorption of calcium and circulating levels of IGF-1, and suppresses IGFBP-2. Meat protein may impact calcium absorption efficiency and increase circulating levels of IGF-1. Animal protein suppresses IGFBP-2, an IGF-1 antagonist, and increases circulating levels of IGF-1. Higher IGF-1 levels stimulate osteoblasts, leading to increases in bone formation. Dietary protein-induced increases in calcium absorption efficiency and circulating levels of IGF-1 can positively impact skeletal health. Abbreviations used: IGF-1: insulin-like growth factor-1; IGFBP-2: insulin-like growth factor-1 binding protein-2.
There is some evidence to suggest that the effect of dietary protein on intestinal calcium absorption differs by protein source (Fig. 1; Heaney et al., 2000; Kerstetter et al., 2006). In one study in a small cohort of adult women calcium absorption tended to be lower when soy protein was substituted for meat protein (Kerstetter et al., 2006). A second study in 16 young and middle-aged males compared the bioavailability of calcium from a fortified soy protein beverage to that from cow’s milk (Heaney et al., 2000). The investigators observed a 25% reduction in calcium absorption from the soy beverage as compared to milk. These differences may be due to the phytic acid contained in soy, which is known to bind calcium. The aforementioned studies solely evaluated acute changes in intestinal calcium absorption efficiency and thus, it is currently unknown if source-specific effects persist over time.
The components of dietary protein responsible for the previously observed increases in calcium absorption are unknown, but several candidates have been identified. In particular, casein phosphopeptides (CPPS), which are a component of milk as well as a digestion product of casein, have been hypothesized to increase calcium absorption (Fig. 1). Calcium binding to CPPS maintains calcium solubility and increases the bioavailability of calcium in the small intestine (Lee et al., 1980). However, data in animal models have provided inconsistent results. The effect of CPPS on calcium absorption was assessed in normal and rachitic chicks using inverted gut sacs and ligated duodenal loop methodology (Mykkanen and Wasserman, 1980). In this study, calcium absorption was increased to a similar extent by CPPs in the duodenum and ileum in both chick models. Phosphate, which may be released during the absorption of CPPS, had no effect on calcium absorption. These data suggest that the stimulatory effect of CPPS on calcium absorption may be independent of vitamin D and phosphate. In contrast, in adult female rats as well as ovariectomized rats CPPS had no independent effect on calcium absorption (Brommage and Jost, 1991; Tsuchita et al., 1993).
The effect of CPPs on calcium metabolism has also been examined in humans. A randomized, double-blind crossover study in postmenopausal women examined the effect of a one-time dose of CPP-supplemented milk on urinary calcium (Narva et al., 2003). CPP-supplemented milk did not increase urinary calcium compared to control milk. Although this study had several limitations, a more recent crossover intervention trial evaluating the effect of a onetime CPP bolus on intestinal calcium handling using dual stable calcium isotopes also found no increase in calcium absorption with CPPs compared to control (Teucher et al., 2006). Both of these studies were single dose interventions and it may be that a more prolonged exposure is required to see the effect of CPPs on calcium absorption.
There are obviously other components of dietary protein that could be responsible for the previously observed increases in calcium absorption (Kerstetter et al., 1998, 2005). Two studies conducted over 50 years ago using rat models reported a dibasic amino acid effect on calcium metabolism (Fig. 1; Comar et al., 1956; Wasserman et al., 1957). Enteral administration of L-lysine followed by L-arginine resulted in the greatest accumulation of 45Ca in bone compared to the other 16 amino acids studied (Comar et al., 1956). In subsequent work in vitamin D deficient rats separate administration of either L-lysine or vitamin D increased calcium accretion to bone (Wasserman et al., 1957). Combining the two interventions resulted in an additive effect. The authors hypothesized that L-lysine and vitamin D may be acting via different cellular pathways to increase intestinal calcium absorption. We recently undertook a crossover feeding study in 14 young Asian and Caucasian women and assessed the effect of amino acid supplementation on intestinal calcium absorption (Bihuniak et al., 2014). Each participant ingested a low-protein diet (0.7 g protein/kg) supplemented with: (1) L-tryptophan, L-phenylalanine, and L-histidine, (2) L-arginine and L-lysine, and (3) methylcellulose capsules (control) and calcium absorption was assessed after 6 days using dual stable calcium isotopes. Consistent with the work by Wasserman and colleagues (Comar et al., 1956; Wasserman et al., 1957), we observed an increase in urinary calcium and improvements in intestinal calcium absorption with L-arginine and L-lysine that were not observed with L-tryptophan, L-phenylalanine, and L-histidine. It is important to note that this effect was observed with amino acids that have no sulfur content, thus arguing against the notion that an imposed metabolic acid load contributes to the effect of dietary protein on urinary calcium. Whether dibasic amino acids have a sustained effect on intestinal calcium transport requires further investigation.
3.2. Dietary protein-induced alterations in IGF-1 in relation to longitudinal bone growth and bone density
Dietary protein is known to affect the IGF-1/GH axis (Fig. 1). Dietary protein can affect circulating levels of IGF binding proteins (IGFBPs), which in turn can alter the bioavailability of IGF-1. In children and adolescents, IGF-1 regulates skeletal growth (Yakar et al., 2002), and part of its mechanism of action involves stimulation of mature osteoblasts (Giustina et al., 2008). Thus, protein-associated increases in IGF-1 levels in early life (Hoppe et al., 2004) may in part explain how dietary protein exerts its effects on bone during periods of growth.
Two studies have reported changes in the IGF-1/IGFBP axis in children with dietary protein manipulation. Hoppe et al. reported an increase in IGF-1 and the IGF-1:IGFBP-3 ratio with the addition of skim milk to the usual diet of young boys, the latter change suggesting an increase in unbound, biologically active IGF-1 (Fig. 1; Hoppe et al., 2004). However, this effect was not observed with increased meat intake. The varying amino acid and mineral profiles of these two different protein sources may explain this differential response. If dietary protein has a salutary effect on the GH/IGF-1 axis then it might be predicted that protein restriction could have the opposite effect. Consistent with that, Smith et al. reported increases in serum levels of IGFBP-2 in children after a 6-day protein restricted diet (Smith et al., 1995). IGFBP-2 is thought to be an IGF-1 antagonist (Fisher et al., 2005). Thus, overexpression of IGFBP-2 in mice (Eckstein et al., 2002) and chicks (Fisher et al., 2005) impaired longitudinal bone growth. Therefore, higher protein intakes may positively impact bone growth by altering the expression pattern of circulating IGFBPs to favor an increase in free IGF-1. However, it is currently unclear if the effect of dietary protein on the IGF-1/IGFBPs axis during periods of growth is source-specific.
Several studies have examined the influence of protein source on the IGF-1/IGFBPs axis in adolescent males and females. A cross-sectional analysis of circulating IGF-1/IGFBP levels and nutrient intakes in adolescent girls found that total dietary protein was positively associated with IGF-1 concentrations (Kerver et al., 2010). Recent findings from the Dortmund Nutritional and Anthropometric Longitudinally Designed Study demonstrate a potential long-term effect of dietary animal protein intake on IGF-1 levels (Joslowski et al., 2013). Dietary intake was assessed in 130 male and female subjects between the ages of 6 months and 2 years (early life) and in 213 male and female individuals between the ages of 9 and 15 years (adolescence) using 3-day weighed dietary records. Measurements of IGF-1, IGFBP-3 and IGFBP-2 were obtained in these same individuals during young adulthood (ages 18–36), and the association of these measures to dietary protein intake earlier in their lives was evaluated. The analysis was restricted to individuals for whom data were available from at least 2 diet records during either early life or adolescence. Higher intakes of animal protein (meat + dairy products) during puberty were associated with higher circulating levels of IGF-1 and IGFBP-3 and lower levels of IGFBP-2 in young adulthood for females, but not for males (Fig. 1). When type of animal protein was examined, habitual meat intake during puberty was positively associated with circulating levels of IGF-1 in females during young adulthood. This association was not observed for male participants. However, greater animal protein intake during early life was associated with a reduction in circulating levels of IGF-1 in young adulthood for male participants. There was no association of animal protein intake during early life with circulating levels of IGF-1 in young adulthood for female participants. There was also no observed association between plant protein intake during early life and IGF-1 levels during young adulthood for either sex. A major limitation of this study was the use of 3-day weighed dietary records to assess long-term usual intake. It is highly likely that the dietary intake of the study cohort changed over the years making the 3-day diet record obtained during childhood of uncertain value in predicting dietary habits during adolescents. While in the aggregate these studies in children and adolescents establish an effect of dietary protein the IGF-1/IGFBP axis, the duration of this effect, the influence of sex as well as source of dietary protein remain uncertain and require further investigation.
In middle aged adults, dietary protein appears to be a modifiable determinant of circulating IGF-1 levels. This does not appear to be the case in later adult life. Epidemiological studies support this concept in so far as that below age 66 there is a positive association between dietary protein and serum IGF-1 (Crowe et al., 2009; Levine et al., 2014). A cross-sectional analysis of 2253 NHANES III participants revealed a significantly higher mean serum IGF-1 level in adults ages 50–65 with protein intakes ≥20% of total calories compared to individuals whose dietary macronutrient composition included <10% protein (Levine et al., 2014). However, this difference was not observed in adults over the age of 65. It could be that a greater level of dietary protein is required to induce a rise in IGF-1 levels later in life. It is important to note that dietary protein was expressed as a percent of energy and was not corrected for body weight. Caloric consumption tends to decline with age (Morley, 1997), and thus in a reduced energy environment a protein intake of 20% of total calories may not be sufficient to stimulate a rise in IGF-1 levels. Moreover, the source of dietary protein in the two age groups studied was not discussed leaving open the possibility that age-dependent differences in types of dietary protein could, in part, underlie these discrepant findings. The source of dietary protein could also modify its effect on serum IGF-1 levels. For example, in a European cohort of 4731 men and women with an average age of 57, diets containing a higher percentage of calories from protein were positively associated with IGF-1 and negatively associated with IGFBP-2 (Fig. 1, Crowe et al., 2009). Dietary protein intakes were categorized as being from mixed sources, animal products (meat, dairy, fish, shell fish, eggs and egg products) or plant sources. When the different sources of animal proteins were analyzed separately, there was no association of animal protein intake with IGF-1/IGFBPs when dairy products were excluded from the analysis suggesting a key role for dairy products in mediating the effects of animal protein on IGF-1. In addition higher plant based protein intakes were not associated with higher circulating levels of IGF-1/IGFBPs. The differing amino acid compositions between the various protein foods may partially explain the source-specific association with IGF-1 levels. Findings from a short-term amino acid supplementation trial in older adults suggest that different groups of amino acids induce opposing effects on IGF-1 (Fig. 2; Dawson-Hughes et al., 2007). Supplementation with the branched chain amino acids, isoleucine and leucine, caused a reduction in serum levels of this growth factor, while the aromatic amino acids, phenylalanine and histidine, increased IGF-1 levels. Milk contains greater amounts of phenylalanine and lower quantities of isoleucine compared to meat products such as beef and chicken (Sosulski and Imafidon, 1990). The amino acid profile of milk may therefore be more conducive to supporting a rise in circulating IGF-1 levels.
Fig. 2.
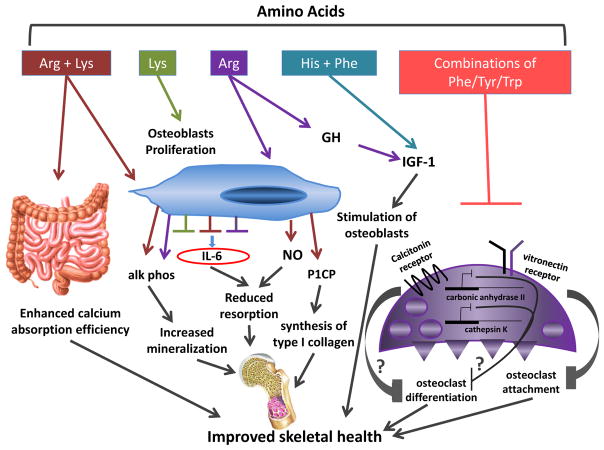
Potential mechanisms of action of amino acids in skeletal metabolism. The effect of dietary protein on bone is likely due, at least in part, to direct and indirect effects of a variety of amino acids on the intestine, osteoblasts, osteoclasts and the GH/IGF-1 axis. When provided in combination, Arg and Lys enhance intestinal calcium absorption in vivo and increase secretion of alk phos, P1CP, and NO, and decrease production of IL-6 from osteoblasts in vitro. Increases in alk phos, P1CP and NO, and a reduction in IL-6 levels may result in increases in bone collagen synthesis and bone formation and reduced bone resorption. Additionally, in vitro, Lys increases osteoblast proliferation and reduces secretion of IL-6 from osteoblasts. Arg also decreases the production of IL-6 and is a potent stimulus of GH secretion, which in turn results in a rise in circulating levels of IGF-1, a known anabolic stimulus for osteoblasts. Together, His and Phe increase levels of circulating IGF-1 in vivo. Different combinations of Phe, Tyr and Trp reduce the expression of the vitronectin receptor in osteoclasts in vitro, potentially decreasing osteoclast precursor attachment. Further, combinations of Phe, Tyr and Trp reduce the expression of the calcitonin receptor, carbonic anhydrase II and cathepsin K in osteoclasts in vitro, which may reflect a suppression of osteoclast differentiation. Amino acid-induced increases in calcium absorption efficiency, osteoblast proliferation and bone mineralization, synthesis of type I collagen and circulating levels of IGF-1 along with reduced bone resorption, osteoclast attachment and suppressed osteoclast differentiation would result in a net increase in bone mass. Abbreviations used: Alk phos: alkaline phosphatase; Arg: arginine; GH: growth hormone; His: histidine; IGF-1: insulin-like growth factor-1; IL-6: interleukin 6; Lys: lysine; NO: nitric oxide; P1CP: carboxy-terminal propeptide of type 1 procollagen; Phe: phenylaline; Tyr: tyrosine; Trp: tryptophan.
Research in growth-hormone deficient models has firmly established arginine as a potent stimulus of growth hormone secretion (Fig. 2; Ghigo et al., 2001; Stanley, 2012), suggesting an indirect mechanism for amino acid-induced increases in IGF-1. It is unclear exactly how arginine stimulates growth hormone release. There is some evidence to suggest that arginine enhances the action of growth hormone releasing hormone, thereby increasing the secretion of growth hormone from the anterior pituitary (Ghigo et al., 2001). These data would suggest that dietary supplementation with arginine should increase circulating levels of IGF-1. However, the data from supplementation trials in healthy adults are mixed (Alvares et al., 2014; Colombani et al., 1999; da Silva et al., 2014; Fayh et al., 2007). Colombani et al. supplemented fourteen long-distance runners with 15 grams of arginine aspartate for 14 days prior to a marathon run and reported an increase in plasma growth hormone levels with arginine supplementation compared to a carbohydrate placebo (Colombani et al., 1999). Conversely, Alvares et al. found no difference in circulating levels of GH or IGF-1 in trained runners who received L-arginine hydrochloride supplementation (6 grams/d, n = 8) versus a cornstarch placebo (6 grams/d, n = 7) for 4 weeks (Alvares et al., 2014). Differences in study duration as well as supplement type and amount may have contributed to the discrepant findings in these two studies. Tightly controlled, dose-dependent trials are needed. Furthermore, typical servings of arginine-rich foods contain milligram amounts of this amino acid (Sosulski and Imafidon, 1990), not gram quantities as were used in these two studies. Therefore, the rise in circulating IGF-1 levels observed with higher protein intakes is unlikely to be due solely to the effects of dietary arginine. Rather more likely is the possibility that multiple amino acids contribute to this effect.
Whether higher circulating levels of IGF-1 are beneficial to overall health in adults is less clear. The relationship between IGF-1 and different types of cancer has gained attention over the past 15 years. For adults between the ages of 50 and 65 who consume ≥20% of total calories from protein, higher concentrations of IGF-1 have been associated with a greater risk of mortality from cancer (Levine et al., 2014). IGF-1 levels and tumor size were also significantly greater in adult mice receiving a high protein diet compared to those on a low protein diet (Levine et al., 2014). Tumor size was suppressed in GHR/IGF-1-deficient mice, further suggesting a role for IGF-1 in cancer progression. However, higher IGF-1 levels in adults over the age of 65 appear to have a neutral impact on cancer mortality risk (Levine et al., 2014). Although the effects of IGF-1 on oncogenesis and cancer progression are unclear, it is very clear that IGF-1 has tropic effects on muscle (Gaffney-Stomberg et al., 2009). Since sarcopenia is a major problem in aging individuals identifying the unique cellular pathways by which IGF-1 augments myogenesis is an area of current investigative interest.
3.3. Amino acid regulation of bone cell metabolism
A more direct mechanism by which dietary protein could affect skeletal homeostasis is via the actions of protein-derived polypeptides and amino acids on bone cells. Relatively little has been reported on the effects of diet-derived polypeptides in bone cells. There are more data available from studies exploring the effects of amino acids on osteoblasts, osteoclasts, and bone marrow stromal cells. Torricelli et al. cultured human osteoblasts derived from healthy (Torricelli et al., 2002) and osteopenic (Torricelli et al., 2003) patients and treated them with arginine, lysine or a combination of the two essential amino acids for 7 days. Osteoblast proliferation and alkaline phosphatase production were increased by exposure to one or more of the amino acid conditions in both cell types (Fig. 2, Torricelli et al., 2002, 2003). In osteoblasts derived from normal individuals, the three amino acid treatments reduced levels of the resorptive cytokine, interleukin-6 (IL-6) (Kwan Tat et al., 2004), and co-treatment with arginine and lysine were synergistic for this effect (Fig. 2; Torricelli et al., 2002). Individual amino acid treatments were unable to elicit a significant decline in secreted IL-6 levels from osteopenic bone-derived osteoblasts; however, a synergistic effect of the arginine/lysine combination was still observed (Torricelli et al., 2003). When compared to control, treatment with arginine resulted in a significant increase in secreted procollagen I C-terminal propeptide, a marker of type I collagen synthesis, by osteoblasts derived from healthy (Torricelli et al., 2002), but not osteopenic bone (Torricelli et al., 2003) (Fig. 2). Exposure to lysine, but not arginine, reduced levels of transforming growth factor-β1 (TGF-β1) (Torricelli et al., 2002, 2003), a modulator of bone remodeling (Bonewald, 1999). When provided in combination, arginine and lysine induced a rise in nitric oxide (NO) levels from healthy bone-derived osteoblasts (Fig. 2, Torricelli et al., 2002), which suggests a potential indirect anti-resorptive effect of the amino acid co-treatment (Wimalawansa, 2010). This effect was not observed in osteopenic bone-derived osteoblasts (Torricelli et al., 2003). Although these data demonstrate a direct effect of amino acids on isolated human osteoblasts in vitro and further demonstrate differences in cells isolated from normal bone as compared to osteopenic bone they do not provide an easily integrated view of the overall impact of dibasic amino acids on osteoblast function. There are some data to suggest these in vitro findings have relevance in vivo. As noted earlier, studies from Wasserman and colleagues reported an effect of lysine and arginine on calcium absorption in rats (Comar et al., 1956; Wasserman et al., 1957). We also recently demonstrated a trend toward higher calcium absorption in young women when a low-protein diet was supplemented with arginine and lysine (Bihuniak et al., 2014). The data from Torricelli et al. (2002, 2003) underscore the importance of a comprehensive analysis of the effects of amino acids on bone cells both individually as well as in functionally relevant combinations. They provide initial insight into potential regulatory actions of amino acids on bone and highlight differences in the responses of bone cells in normal and osteopenic individuals. Furthermore, it appears that there may be a multitude of effects of specific amino acids on the bone microenviroment as evidenced by both independent and synergistic actions of arginine and lysine (Fig. 2).
A recent study by Refaey et al. examined the effect of combinations of aromatic amino acids, phenylalanine, tyrosine, and tryptophan, on markers of osteoclast differentiation, structure and resorptive activity in vitro (Refaey et al., 2014). Gene expression of the vitronectin receptor, calcitonin receptor, carbonic anhydrase II and cathepsin K were significantly reduced by treatment with a variety of aromatic amino acid combinations, suggesting amino acid-induced suppression of osteoclast differentiation, and/or function (Fig. 2). These findings are consistent with recent data from the same group reporting that the addition of aromatic amino acids to an 8% protein diet ameliorated bone loss in ovariectomized mice (Ding et al., 2014).
4. Conclusion
The effects of dietary protein on calcium economy are complex and likely involve multiple cellular pathways. It is currently unknown if the potential positive impact of dietary protein on calcium metabolism is sustained over time and further it appears that this effect may be protein source-specific. Results from both in vivo and in vitro studies suggest that components of dietary protein, specifically amino acids, may act as direct regulators of skeletal and calcium economy. Future studies should direct their attention to the skeletal effects of protein at different stages in the lifespan, to improve our ability to optimize the salutary effect of this vital nutrient.
References
- Abelow BJ, Holford TR, Insogna KL. Cross-cultural association between dietary animal protein and hip fracture: a hypothesis. Calcif Tissue Int. 1992;50:14–18. doi: 10.1007/BF00297291. [DOI] [PubMed] [Google Scholar]
- Alexy U, Remer T, Manz F, Neu CM, Schoenau E. Long-term protein intake and dietary potential renal acid load are associated with bone modeling and remodeling at the proximal radius in healthy children. Am J Clin Nutr. 2005;82:1107–1114. doi: 10.1093/ajcn/82.5.1107. [DOI] [PubMed] [Google Scholar]
- Allen LH, Oddoye EA, Margen S. Protein-induced hypercalciuria: a longer term study. Am J Clin Nutr. 1979;32:741–749. doi: 10.1093/ajcn/32.4.741. [DOI] [PubMed] [Google Scholar]
- Alvares TS, Conte-Junior CA, Silva JT, Paschoalin VM. L-arginine does not improve biochemical and hormonal response in trained runners after 4 weeks of supplementation. Nutr Res. 2014;34:31–39. doi: 10.1016/j.nutres.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Anand CR, Linkswiler HM. Effect of protein intake on calcium balance of young men given 500 mg calcium daily. J Nutr. 1974;104:695–700. doi: 10.1093/jn/104.6.695. [DOI] [PubMed] [Google Scholar]
- Barbul A. Proline precursors to sustain Mammalian collagen synthesis. J Nutr. 2008;138:2021S–2024S. doi: 10.1093/jn/138.10.2021S. [DOI] [PubMed] [Google Scholar]
- Barzel US, Massey LK. Excess dietary protein can adversely affect bone. J Nutr. 1998;128:1051–1053. doi: 10.1093/jn/128.6.1051. [DOI] [PubMed] [Google Scholar]
- Beasley JM, LaCroix AZ, Larson JC, Huang Y, Neuhouser ML, Tinker LF, et al. Biomarker-calibrated protein intake and bone health in the Women’s Health Initiative clinical trials and observational study. Am J Clin Nutr. 2014;99:934–940. doi: 10.3945/ajcn.113.076786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bihuniak JD, Sullivan RR, Simpson CA, Caseria DM, Huedo-Medina TB, O’Brien KO, et al. Supplementing a low-protein diet with dibasic amino acids increases urinary calcium excretion in young women. J Nutr. 2014;144:282–288. doi: 10.3945/jn.113.185009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonewald LF. Regulation and regulatory activities of transforming growth factor beta. Crit Rev Eukaryot Gene Expr. 1999;9:33–44. [PubMed] [Google Scholar]
- Brommage MJ, Jost R. Influence of casein phosphopeptides and lactulose on intestinal calcium absorption in adult female rats. Lait. 1991;71:173–180. [Google Scholar]
- Bushinsky DA. Acid-base imbalance and the skeleton. Eur J Nutr. 2001;40:238–244. doi: 10.1007/s394-001-8351-5. [DOI] [PubMed] [Google Scholar]
- Bushinsky DA, Frick KK. The effects of acid on bone. Curr Opin Nephrol Hypertens. 2000;9:369–379. doi: 10.1097/00041552-200007000-00008. [DOI] [PubMed] [Google Scholar]
- Chevalley T, Bonjour JP, Ferrari S, Rizzoli R. High-protein intake enhances the positive impact of physical activity on BMC in prepubertal boys. J Bone Miner Res. 2008;23:131–142. doi: 10.1359/jbmr.070907. [DOI] [PubMed] [Google Scholar]
- Colombani PC, Bitzi R, Frey-Rindova P, Frey W, Arnold M, Langhans W, et al. Chronic arginine aspartate supplementation in runners reduces total plasma amino acid level at rest and during a marathon run. Eur J Nutr. 1999;38:263–270. doi: 10.1007/s003940050076. [DOI] [PubMed] [Google Scholar]
- Comar CL, Nold MM, Wasserman RH. The influence of amino acids and other organic compounds on the gastrointestinal absorption of calcium 45 and strontium 89 in the rat. J Nutr. 1956;59:371–383. doi: 10.1093/jn/59.3.371. [DOI] [PubMed] [Google Scholar]
- Crowe FL, Key TJ, Allen NE, Appleby PN, Roddam A, Overvad K, et al. The association between diet and serum concentrations of IGF-I, IGFBP-1, IGFBP-2, and IGFBP-3 in the European Prospective Investigation into Cancer and Nutrition. Cancer Epidemiol Biomarkers Prev. 2009;18:1333–1340. doi: 10.1158/1055-9965.EPI-08-0781. [DOI] [PubMed] [Google Scholar]
- da Silva DV, Conte-Junior CA, Paschoalin VM, da Alvares TS. Hormonal response to L-arginine supplementation in physically active individuals. Food Nutr Res. 2014:58. doi: 10.3402/fnr.v58.22569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson-Hughes B, Harris SS, Rasmussen HM, Dallal GE. Comparative effects of oral aromatic and branched-chain amino acids on urine calcium excretion in humans. Osteoporos Int. 2007;18:955–961. doi: 10.1007/s00198-006-0320-x. [DOI] [PubMed] [Google Scholar]
- Ding KZQ, Bollag W, Xu J, Hill W, Shi X, Refaey M, et al. Aromatic amino acids ameliorate ovariectomy induced bone loss. J Bone Miner Res. 2014;29(Suppl 1) < http://www.asbmr.org/education/AbstractDetail?aid=c409d540-7178-4d18-86df-1d9aba64d773>. [Google Scholar]
- Eckstein F, Pavicic T, Nedbal S, Schmidt C, Wehr U, Rambeck W, et al. Insulin-like growth factor-binding protein-2 (IGFBP-2) overexpression negatively regulates bone size and mass, but not density, in the absence and presence of growth hormone/IGF-I excess in transgenic mice. Anat Embryol (Berl) 2002;206:139–148. doi: 10.1007/s00429-002-0282-5. [DOI] [PubMed] [Google Scholar]
- Fayh AP, Friedman R, Sapata KB, Oliveira AR. Effect of L-arginine supplementation on secretion of human growth hormone and insulin-like growth factor in adults. Arq Bras Endocrinol Metabol. 2007;51:587–592. doi: 10.1590/s0004-27302007000400013. [DOI] [PubMed] [Google Scholar]
- Feskanich D, Willett WC, Stampfer MJ, Colditz GA. Protein consumption and bone fractures in women. Am J Epidemiol. 1996;143:472–479. doi: 10.1093/oxfordjournals.aje.a008767. [DOI] [PubMed] [Google Scholar]
- Fisher MC, Meyer C, Garber G, Dealy CN. Role of IGFBP2, IGF-I and IGF-II in regulating long bone growth. Bone. 2005;37:741–750. doi: 10.1016/j.bone.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Food and Nutrition Board IoM. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. 2002/2005 doi: 10.1016/s0002-8223(02)90346-9. [DOI] [PubMed] [Google Scholar]
- Frassetto LA, Todd KM, Morris RC, Jr, Sebastian A. Worldwide incidence of hip fracture in elderly women: relation to consumption of animal and vegetable foods. J Gerontol A Biol Sci Med Sci. 2000;55:M585–M592. doi: 10.1093/gerona/55.10.m585. [DOI] [PubMed] [Google Scholar]
- Gaffney-Stomberg E, Insogna KL, Rodriguez NR, Kerstetter JE. Increasing dietary protein requirements in elderly people for optimal muscle and bone health. J Am Geriatr Soc. 2009;57:1073–1079. doi: 10.1111/j.1532-5415.2009.02285.x. [DOI] [PubMed] [Google Scholar]
- Ghigo E, Aimaretti G, Arvat E, Camanni F. Growth hormone-releasing hormone combined with arginine or growth hormone secretagogues for the diagnosis of growth hormone deficiency in adults. Endocrine. 2001;15:29–38. doi: 10.1385/ENDO:15:1:029. [DOI] [PubMed] [Google Scholar]
- Giustina A, Mazziotti G, Canalis E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr Rev. 2008;29:535–559. doi: 10.1210/er.2007-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan MT, Tucker KL, Dawson-Hughes B, Cupples LA, Felson DT, Kiel DP. Effect of dietary protein on bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res. 2000;15:2504–2512. doi: 10.1359/jbmr.2000.15.12.2504. [DOI] [PubMed] [Google Scholar]
- Heaney RP, Dowell MS, Rafferty K, Bierman J. Bioavailability of the calcium in fortified soy imitation milk, with some observations on method. Am J Clin Nutr. 2000;71:1166–1169. doi: 10.1093/ajcn/71.5.1166. [DOI] [PubMed] [Google Scholar]
- Hegsted M, Linkswiler HM. Long-term effects of level of protein intake on calcium metabolism in young adult women. J Nutr. 1981;111:244–251. doi: 10.1093/jn/111.2.244. [DOI] [PubMed] [Google Scholar]
- Hoppe C, Molgaard C, Juul A, Michaelsen KF. High intakes of skimmed milk, but not meat, increase serum IGF-I and IGFBP-3 in eight-year-old boys. Eur J Clin Nutr. 2004;58:1211–1216. doi: 10.1038/sj.ejcn.1601948. [DOI] [PubMed] [Google Scholar]
- Hoppe C, Udam TR, Lauritzen L, Molgaard C, Juul A, Michaelsen KF. Animal protein intake, serum insulin-like growth factor I, and growth in healthy 2.5-y-old Danish children. Am J Clin Nutr. 2004;80:447–452. doi: 10.1093/ajcn/80.2.447. [DOI] [PubMed] [Google Scholar]
- Joslowski G, Remer T, Assmann KE, Krupp D, Cheng G, Garnett SP, et al. Animal protein intakes during early life and adolescence differ in their relation to the growth hormone-insulin-like-growth-factor axis in young adulthood. J Nutr. 2013;143:1147–1154. doi: 10.3945/jn.113.175877. [DOI] [PubMed] [Google Scholar]
- Kerstetter JE, O’Brien KO, Insogna KL. Dietary protein affects intestinal calcium absorption. Am J Clin Nutr. 1998;68:859–865. doi: 10.1093/ajcn/68.4.859. [DOI] [PubMed] [Google Scholar]
- Kerstetter JE, O’Brien KO, Insogna KL. Low protein intake: the impact on calcium and bone homeostasis in humans. J Nutr. 2003;133:855S–861S. doi: 10.1093/jn/133.3.855S. [DOI] [PubMed] [Google Scholar]
- Kerstetter JE, O’Brien KO, Caseria DM, Wall DE, Insogna KL. The impact of dietary protein on calcium absorption and kinetic measures of bone turnover in women. J Clin Endocrinol Metab. 2005;90:26–31. doi: 10.1210/jc.2004-0179. [DOI] [PubMed] [Google Scholar]
- Kerstetter JE, Wall DE, O’Brien KO, Caseria DM, Insogna KL. Meat and soy protein affect calcium homeostasis in healthy women. J Nutr. 2006;136:1890–1895. doi: 10.1093/jn/136.7.1890. [DOI] [PubMed] [Google Scholar]
- Kerver JM, Gardiner JC, Dorgan JF, Rosen CJ, Velie EM. Dietary predictors of the insulin-like growth factor system in adolescent females: results from the Dietary Intervention Study in Children (DISC) Am J Clin Nutr. 2010;91:643–650. doi: 10.3945/ajcn.2009.28205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JE, Zanovec M, Fulgoni VL, Campbell WW. Effect of dietary protein on bone status in US adults aged 50 years and older; NHANES 1999–2004. FASEB J. 2013:27. [Google Scholar]
- Kim Y, Linkswiler HM. Effect of level of protein intake on calcium metabolism and on parathyroid and renal function in the adult human male. J Nutr. 1979;109:1399–1404. doi: 10.1093/jn/109.8.1399. [DOI] [PubMed] [Google Scholar]
- Kwan Tat S, Padrines M, Theoleyre S, Heymann D, Fortun Y. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004;15:49–60. doi: 10.1016/j.cytogfr.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Lee YS, Noguchi T, Naito H. Phosphopeptides and soluble calcium in the small intestine of rats given a casein diet. Br J Nutr. 1980;43:457–467. doi: 10.1079/bjn19800113. [DOI] [PubMed] [Google Scholar]
- Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, Madia F, et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014;19:407–417. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardon J, Trzeciakiewicz A, Habauzit V, Davicco MJ, Lebecque P, Mercier S, et al. Dietary protein supplementation increases peak bone mass acquisition in energy-restricted growing rats. Pediatr Res. 2009;66:513–518. doi: 10.1203/PDR.0b013e3181b9b4bb. [DOI] [PubMed] [Google Scholar]
- Meyer HE, Pedersen JI, Loken EB, Tverdal A. Dietary factors and the incidence of hip fracture in middle-aged Norwegians. A prospective study Am J Epidemiol. 1997;145:117–123. doi: 10.1093/oxfordjournals.aje.a009082. [DOI] [PubMed] [Google Scholar]
- Morley JE. Anorexia of aging: physiologic and pathologic. Am J Clin Nutr. 1997;66:760–773. doi: 10.1093/ajcn/66.4.760. [DOI] [PubMed] [Google Scholar]
- Mykkanen HM, Wasserman RH. Enhanced absorption of calcium by casein phosphopeptides in rachitic and normal chicks. J Nutr. 1980;110:2141–2148. doi: 10.1093/jn/110.11.2141. [DOI] [PubMed] [Google Scholar]
- Narva M, Karkkainen M, Poussa T, Lamberg-Allardt C, Korpela R. Caseinphosphopeptides in milk and fermented milk do not affect calcium metabolism acutely in postmenopausal women. J Am Coll Nutr. 2003;22:88–93. doi: 10.1080/07315724.2003.10719280. [DOI] [PubMed] [Google Scholar]
- Refaey ME, Zhong Q, Ding KH, Shi XM, Xu J, Bollag WB, et al. Impact of dietary aromatic amino acids on osteoclastic activity. Calcif Tissue Int. 2014;95:174–182. doi: 10.1007/s00223-014-9878-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remer T. Influence of diet on acid-base balance. Semin Dial. 2000;13:221–226. doi: 10.1046/j.1525-139x.2000.00062.x. [DOI] [PubMed] [Google Scholar]
- Rouy E, Vico L, Laroche N, Benoit V, Rousseau B, Blachier F, et al. Protein quality affects bone status during moderate protein restriction in growing mice. Bone. 2014;59:7–13. doi: 10.1016/j.bone.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Sherman HC. Calcium requirement of maintenance in man. J Biol Chem. 1920;44:21–27. doi: 10.1073/pnas.6.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WJ, Underwood LE, Clemmons DR. Effects of caloric or protein restriction on insulin-like growth factor-I (IGF-I) and IGF-binding proteins in children and adults. J Clin Endocrinol Metab. 1995;80:443–449. doi: 10.1210/jcem.80.2.7531712. [DOI] [PubMed] [Google Scholar]
- Sosulski FW, Imafidon GI. Amino acid composition and nitrogen-to-protein conversion factors for animal and plant foods. J Agric Food Chem. 1990;38:1351–1356. [Google Scholar]
- Stanley T. Diagnosis of growth hormone deficiency in childhood. Curr Opin Endocrinol Diabetes Obes. 2012;19:47–52. doi: 10.1097/MED.0b013e32834ec952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teucher B, Majsak-Newman G, Dainty JR, McDonagh D, FitzGerald RJ, Fairweather-Tait SJ. Calcium absorption is not increased by caseinophosphopeptides. Am J Clin Nutr. 2006;84:162–166. doi: 10.1093/ajcn/84.1.162. [DOI] [PubMed] [Google Scholar]
- Torricelli P, Fini M, Giavaresi G, Giardino R, Gnudi S, Nicolini A, et al. L-arginine and L-lysine stimulation on cultured human osteoblasts. Biomed Pharmacother. 2002;56:492–497. doi: 10.1016/s0753-3322(02)00287-1. [DOI] [PubMed] [Google Scholar]
- Torricelli P, Fini M, Giavaresi G, Giardino R. Human osteopenic bone-derived osteoblasts: essential amino acids treatment effects. Artif Cells Blood Substit Immobil Biotechnol. 2003;31:35–46. doi: 10.1081/bio-120018002. [DOI] [PubMed] [Google Scholar]
- Tsuchita H, Sekiguchi I, Kuwata T, Igarashi C, Ezawa I. The effect of casein phosphopeptides on calcium utilization in young ovariectomized rats. Z Ernahrungswiss. 1993;32:121–130. doi: 10.1007/BF01614755. [DOI] [PubMed] [Google Scholar]
- Wasserman RH, Comar CL, Schooley JC, Lengemann FW. Interrelated effects of L-lysine and other dietary factors on the gastrointestinal absorption of calcium 45 in the rat and chick. J Nutr. 1957;62:367–376. doi: 10.1093/jn/62.3.367. [DOI] [PubMed] [Google Scholar]
- Wimalawansa SJ. Nitric oxide and bone. Ann N Y Acad Sci. 2010;1192:391–403. doi: 10.1111/j.1749-6632.2009.05230.x. [DOI] [PubMed] [Google Scholar]
- Yakar S, Rosen CJ, Beamer WG, Ackert-Bicknell CL, Wu Y, Liu JL, et al. Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest. 2002;110:771–781. doi: 10.1172/JCI15463. [DOI] [PMC free article] [PubMed] [Google Scholar]