Abstract
With obesity being a leading cause of preventable death, it is vital to understand how best to identify individuals with greater risk of metabolic disease, especially those with high visceral adipose tissue (VAT). This study aimed to determine whether three commonly used waist circumference (WC) measurement sites could provide accurate estimations of VAT, as determined by magnetic resonance imaging (MRI), which is a gold standard for measuring VAT, in postmenopausal women with obesity. VAT volume was measured by MRI of the total abdomen in 97 women aged 57.7 ± 0.4 years (mean ± SEM), mean body mass index 34.5 ± 0.2 kg/m2. WC was measured at the midpoint between the lowest rib and the iliac crest (WCmid), the narrowest point of the torso (WCnarrow), and at the level of the umbilicus (WCumbilicus). WC differed significantly according to measurement site, with WCnarrow (102.1 ± 0.7 cm) < WCmid (108.3 ± 0.7 cm) < WCumbilicus (115.7 ± 0.8 cm) (p < 0.001). WCmid, WCnarrow and WCumbilicus were all significantly correlated with VAT, as measured by MRI (r = 0.581, 0.563 and 0.390, respectively; p < 0.001 for all), but the relationships between WCmid or WCnarrow and VAT determined by MRI were stronger than for WCumbilicus. Measurement of either WCmid or WCnarrow provides valid estimates of VAT in postmenopausal women with obesity, with WCnarrow being favoured in light of its greater ease and speed of measurement in this population.
Keywords: waist circumference, magnetic resonance imaging, visceral adipose tissue, obesity
1. Introduction
Obesity, which can be defined as an excess of body fat, is an ever-growing public health crisis related to the development of many disturbances, including type 2 diabetes and cardiovascular disease [1,2]. Body fat is distributed in two main compartments with different metabolic characteristics: subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT). However, the metabolic risks associated with obesity, such as impaired glucose and lipid metabolism, diabetes, insulin resistance [3,4,5], hypertension [6] and metabolic syndrome [7], have been attributed to increases in VAT [8].
An accurate measure of VAT requires the use of gold-standard imaging techniques, such as computed tomography (CT) or magnetic resonance imaging (MRI) [9]. However, while MRI, avoids the radiation exposure encountered with CT, both CT and MRI are relatively inaccessible, expensive, and image analysis is labour intensive [10]. Dual-energy X-ray absorptiometry (DXA) is an alternative method for estimating VAT [11] that has been shown to correlate strongly with VAT measured using CT [12,13,14,15,16] and MRI [17,18]. DXA delivers a minimal radiation dose and is less costly and time-consuming than CT or MRI for measuring VAT [19]. However, DXA too requires expensive equipment and trained technicians [19].
A simple index of VAT is waist circumference (WC), which provides a recognised estimate of abdominal adiposity and metabolic risk [20]. There are different WC measurement sites that are routinely used [21]. For example, a literature review identified 14 different descriptions of the site for WC measurements [22]. However, there is no consensus regarding the optimal WC measurement site [23]. The World Health Organisation recommends measuring WC at the midpoint between the bony landmarks of the lowest rib and the top of the iliac crest (WCmid) [24]. The Anthropometric Standardization Reference Manual (developed in 1985 by experts attending the Anthropometric Standardization Conference) recommends measuring WC at the narrowest point of the torso (WCnarrow) [25]. In contrast, some research studies have measured WC at the level of the umbilicus (WCumbilicus) [26,27], or have failed to describe the WC measurement site used at all [28,29]. A systematic review looking at 120 studies of WC measurement sites and morbidity or mortality found that most WC measurements were performed at WCmid (30%), WCnarrow (27%) or WCumbilicus (29%) [26]. Further, in the few studies that have investigated the relationship between VAT (using the gold standard techniques of CT and MRI) and WC, they did so only in zero [30], one [31,32,33] or two [34] of the three common WC measurement sites.
To identify those with greater levels of VAT and therefore, greater risk of metabolic disease, especially in higher risk populations, such as older adults or those with obesity, we aimed to determine which of the three common measures of WC (WCmid, WCnarrow and WCumbilicus) is the better predictor of VAT, as determined by the gold standard method of MRI, specifically in postmenopausal women with obesity.
2. Materials and Methods
2.1. Ethics Statement and Participants
The study was approved by the Sydney Local Health District Ethics Committee (Royal Prince Alfred Hospital Zone) and registered with the Australia and New Zealand Clinical Trials registry (number 12612000651886). All participants provided informed, written consent prior to participation.
Ninety-seven women, aged 57.7 ± 0.4 years (mean ± SEM), with a mean body mass index (BMI) of 34.4 ± 0.3 kg/m2 participated in this study. Participants were at least five years postmenopausal at the time of recruitment, and were predominately Caucasian (n = 92). Participants were recruited as part of a broader study: the randomised controlled TEMPO Diet Trial (Type of Energy Manipulation for Promoting optimum metabolic health and body composition in Obesity). All participants were required to be weight stable (±2 kg) for ≥6 months, and sedentary (defined as <3 h of structured physical activity per week). Exclusion criteria were not being ambulatory, or having osteoporosis, hyperthyroidism or hypothyroidism, diabetes mellitus, any loose metal in the body (e.g., pacemaker or bullet) that is contraindicated for MRI for safety reasons, or which may result in artefacts in medical imaging, tobacco use, alcohol or drug dependency.
2.2. Measurements
2.2.1. Anthropometry
Body weight (Tanita BWB-800 digital scale, Wedderburn Pty. Ltd., Sydney, Australia) and standing height (Harpenden Stadiometer, Holtain Ltd., Crymych, UK) were both measured twice to the nearest 0.1 kg and 0.1 cm, respectively. If the difference between the measurements was >0.5 kg for body weight or >0.5 cm for standing height, a third measurement was taken. The average of the two measurements (or the average of the two closest measurements if a third measurement was taken) was recorded as the result. Participants were dressed in leggings and a sports bra and were not wearing shoes during measurement.
2.2.2. MRI
Abdominal fat volumes (i.e., VAT) were measured by MRI, with participants lying in a supine position in a hospital gown, having removed any metal accessories. Axial T1-weighted fast field echo images were acquired with a 3.0T MRI scanner (the Discovery MR750 3.0T model from GE Healthcare, Milwaukee, WI, USA), from diaphragm to pelvis (repetition time = 3.8 ms, echo time = 2.1 ms, flip angle = 12°), with a slice thickness of 10 mm and an inter-slice gap (i.e., the distance between the surfaces of adjacent slices) of 10 mm. Images were acquired during suspended end-expiration, with a breath-hold duration of approximately 15–18 s per acquisition. Following the scan, all image slices from the base of the lungs to the pelvic floor were segmented manually. VAT was quantified using the Region Growing mode of the analysis software (SliceOMatic Version 5.0 rev-6b, Tomovision Inc., Montreal, QC, Canada), with thresholds adjusted manually, as required. The software automatically calculated the surface area of each slice by multiplying the number of pixels tagged by the surface area of one pixel. The inter-slice volume (i.e., the volume of the inter-slice gap) was extrapolated using a cone formula that considered the surface area of the superior and inferior surfaces of the inter-slice gap, as well as the thickness of the inter-slice gap. The total volume of each of the inter-slice gaps was then added to the total volume of each of the slices (surface area × slice thickness) to calculate the total abdominal volumes of VAT. All analyses were conducted by the same researcher (ALWT).
2.2.3. WC
WC was measured to the nearest 0.1 cm, directly on skin, using a narrow, flexible and inelastic steel tape (Lufkin W606PM, Apex Tool Group, North Carolina, USA). Participants were dressed in leggings and a sports bra. WC was measured at the three most commonly used sites worldwide [26]; WCmid, WCnarrow and WCumbilicus. Participants were asked to breathe normally and to stand with their weight evenly distributed and their arms crossed over their shoulders during measurements. At each measurement site, two measurements were taken, and if the difference between the measurements was >1 cm, a third measurement was taken. The average of each of the two measurements at each site (or the average of the two closest measurements if a third measurement was taken) was recorded as the result. Measurements were taken by the same two researchers (SM and HAF) throughout the study. The inter-observer coefficient of variation (CV) in a subset of five participants was 1.44% for WCmid, 0.66% for WCnarrow, and 0.69% for WCumbilicus. The intra-observer CV for the two measurements on all 97 participants was 0.27% for WCmid, 0.32% for WCnarrow and 0.23% for WCumbilicus.
2.3. Statistical Analysis
All data are presented as means ± standard error of the mean (SEM), unless otherwise stated. A Shapiro–Wilk test of normality demonstrated the normal distribution of all data used in this study. Comparisons between the three different WC measurement sites were performed using one-way repeated measures ANOVA plus a post hoc test for multiple comparisons. Pearson correlations were used to assess the relationships between VAT volume and different WC measurement sites, or BMI. A Fisher’s z-transformation was used to determine if the correlation coefficients between each of the relationships were significantly different from each other. Statistical significance was accepted as p < 0.05. Statistical analyses were performed using SPSS Version 24 (IBM SPSS Statistics, IBM Corporation, New York, NY, USA).
3. Results
Descriptive statistics for the participants in this study are presented in Table 1.
Table 1.
Clinical and anthropometric characteristics of participants.
| Age (years) | 57.7 ± 0.4 (45–65) |
| Weight (kg) | 91.1 ± 0.9 (76.6–116.4) |
| BMI (kg/m2) | 34.5 ± 0.2 (29.6–40.1) |
| Waist circumferences (cm) | |
| WCmid | 108.3 ± 0.7 (93.8–125.8) |
| WCnarrow | 102.1 ± 0.7 (85.3–120.8) |
| WCumbilicus | 115.7 ± 0.8 (97.2–139.3) |
| VAT (cm3) | 5,430 ± 200 (1510–10,840) |
Data are presented as means ± SEM (range) of 97 postmenopausal women. BMI, body mass index; WCmid, waist circumference measured at the midpoint; WCnarrow, WC measured at the narrowest point; WCumbilicus, WC measured at the umbilicus; VAT, visceral adipose tissue volume (measured by magnetic resonance imaging).
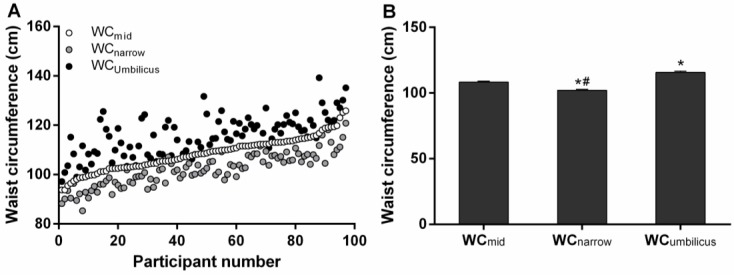
The three different WC measurement sites produced significantly different results, with WCnarrow < WCmid < WCumbilicus (Table 1, Figure 1). The greatest differences were observed between WCnarrow and WCumbilicus (13.5 ± 0.6 cm, p < 0.05), then between WCmid and WCumbilicus (7.1 ± 0.6 cm, p < 0.05), with the smallest difference being between WCnarrow and WCmid (6.3 ± 0.3 cm, p < 0.05).
Figure 1.
Waist circumference (WC) measurement at different sites. WC at the midpoint (WCmid), narrowest point (WCnarrow) and umbilicus (WCumbilicus) for each individual participant (A); and as means ± SEM (B), in postmenopausal women with obesity (n = 97). * p < 0.05 versus WCmid, # p < 0.05 versus WCumbilicus.
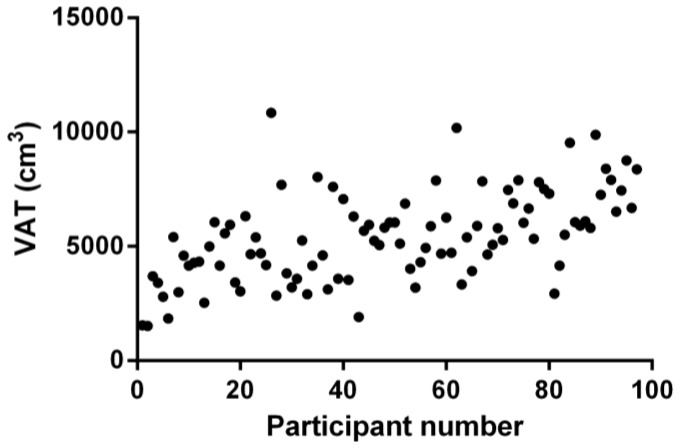
Despite the narrow BMI range of participants in this study (Table 1), in keeping with the selection criteria, there was significant variation in WC (Table 1, Figure 1) and VAT volumes (Table 1, Figure 2) across the cohort. These wide ranges of WC and VAT values are helpful for the current study, in order to demonstrate the relative utility of different WC measurement indexes.
Figure 2.
Visceral adipose tissue volume (VAT), as determined by magnetic resonance imaging for each of the postmenopausal women with obesity in this study (n = 97).
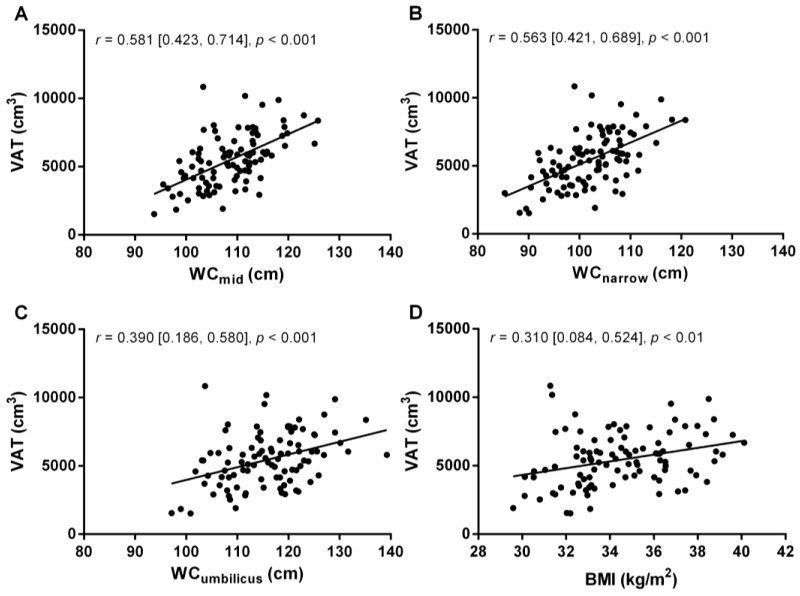
The three different waist circumference measures, and BMI, were significantly correlated with VAT, as determined by MRI (Figure 3). WCmid and WCnarrow were both moderate correlates of VAT, while WCumbilicus was a weak, and BMI a very weak, correlate of VAT (as shown by the correlation coefficients (r) and 95% confidence intervals in Figure 3).
Figure 3.
Visceral adipose tissue volume (VAT), measured by magnetic resonance imaging, versus waist circumference measured at the midpoint (WCmid, A); narrowest point (WCnarrow, B) and umbilicus (WCumbilicus, C); as well as body mass index (BMI, D), in postmenopausal women with obesity (n = 97). Data on each panel represent correlation coefficients (r) as well as their 95% confidence intervals [lower limit, upper limit], and corresponding p values.
There was no significant difference between the correlations of VAT–WCmid and VAT–WCnarrow (z = 0.18, p = 0.86). The difference in correlations between VAT–WCmid and VAT–WCumbilicus approached, but did not reach statistical significance (z = 1.73, p = 0.08), as did the correlations between VAT–WCnarrow and VAT–WCumbilicus (z = 1.55, p = 0.12). In contrast, there was a significant difference between the correlations of VAT–BMI and VAT–WCmid (z = 2.35, p = 0.02) and VAT–BMI and VAT–WCnarrow (z = 2.17, p = 0.03), but there was no significant difference in correlations between VAT–BMI and VAT–WCumbilicus (z = 0.63, p = 0.53). In addition, when comparing each of the three different WC measurement sites against one another, there was a strong correlation between WCmid and WCnarrow (r = 0.91), which was significantly stronger than either of the correlations between WCmid and WCumbilicus (r = 0.715), or WCnarrow and WCumbilicus (r = 0.702). This data, combined with the correlations between VAT and different WC measurement sites, shows that the most useful anthropometric indices of VAT volume are WCmid or WCnarrow.
4. Discussion
Our study investigated the correlation between VAT, as quantified by the gold standard method of MRI, and all three of the most common WC measurement sites [26], namely WCmid, WCnarrow and WCumbilicus. Our results indicate that WCmid and WCnarrow are the most appropriate WC measurement sites for the estimation of VAT by MRI in postmenopausal women with obesity. Additionally, there was no significant difference in the correlations between WCmid and WCnarrow with respect to their correlations with VAT, indicating that both are very similar measurement sites, while WCnarrow is easier and faster to measure than WCmid, as explained below.
While previous studies have investigated the relationship between VAT (determined by MRI or CT) and WC, they only investigated zero [30], one [31,32,33] or two [34] of the three common WC measurement sites. In the studies measuring only one WC site, they found WCmid [31], WCnarrow [32,33], or WC measured at the halfway point between the L4 and L5 vertebrae [30], were all associated with VAT. The study that looked at two of the three common WC measurement sites (WCnarrow and WCumbilicus)—in an overweight to mildly obese population (average BMI 30.2 kg/m2, range 25–35 kg/m2)—found that VAT area measured by CT correlated slightly more strongly with WCnarrow (r = 0.63) than with WCumbilicus (r = 0.57) [34]. The current study extends knowledge from this existent literature by directly comparing all three of the common WC measurement sites in the same study, thereby allowing for selection of the most appropriate WC measurement site(s) in postmenopausal women with obesity (WCmid or WCnarrow).
When considering the practicality of measuring WCmid or WCnarrow, WCnarrow is advantageous. This is because when measuring WCmid, the observer needs to identify and mark two anatomical landmarks (the bottom of the lowest rib and the top of the iliac crest), and then mark the midpoint between these two landmarks before WC can be measured. In contrast, when measuring WCnarrow, the observer can estimate the narrowest point of the torso by simply viewing the torso from the back prior to measuring WC. As such, measuring WCnarrow is less time consuming than WCmid. While some studies emphasise the need for bony landmarks to guide WC measurement [23], this can be problematic. The identification of both the rib and the iliac crest for the measurement of WCmid can be difficult in people with increased adiposity. To identify these two landmarks, the observer may need to palpate the abdomen thoroughly, which can be intrusive and embarrassing for the subject [35]. In addition, at higher levels of adiposity, these landmarks might not be able to be located precisely, which would make the measurement imprecise, because inaccurate localization of the border of either the rib or the iliac crest can have a significant effect on the WC measured, thus leaving more room for error [22]. In summary, WCnarrow is not only a good estimate of VAT in postmenopausal women with obesity, but is also quicker and easier to measure than WCmid and offers the advantage of avoiding the less practical procedure and potential imprecision of landmark identification. However, it should be noted that these results are valid for an environment where highly trained researchers take the measures, which may not apply to clinical practice.
This study showed that although WCumbilicus was significantly correlated with VAT, as had also been shown in a previous study [34], it further showed that WCumbilicus was of limited value in estimating VAT in postmenopausal women with obesity, when compared to WCmid or WCnarrow. This may be related to the relatively low position of this the umbilicus site in the abdomen. A study in overweight or obese participants found that the correlation between VAT area, measured by single-slice MRI, and VAT volume, measured by multi-slice MRI, was strongest higher in the abdomen compared to lower [36]. These findings suggest that WC measured in the upper abdomen may correlate more closely with VAT than WC measured in the lower abdomen. This can potentially explain the differences in the correlations between VAT and different measurement sites, and supports our findings where WCmid and WCnarrow—which are higher in the abdomen than WCumbilicus—are more strongly correlated with VAT than WCumbilicus. In addition, although measuring WCumbilicus is the least time-consuming of all three methods, a limitation in the use of WCumbilicus is the variability in its position, as the umbilicus is a soft tissue landmark [37]. A study looking at umbilical position and BMI in males and females found that the median position of the umbilicus was 0.88 cm, 1.20 cm, and 3.50 cm below the middle of the torso in people who had a BMI in the normal, overweight, or obese range, respectively [38]. While this change in the position of the umbilicus could indicate important VAT changes, the panniculus (the so called ‘apron of fat’ upon which the umbilicus sits) consists of subcutaneous fat, and therefore a drop in umbilical position is more likely to indicate subcutaneous fat accumulation than any change in VAT [39]. Therefore, one might expect WCumbilicus to be a poorer correlate of VAT, especially in people with obesity, due to its varying position on the torso.
In this study we also looked at the correlation between VAT (as determined by MRI) and BMI in our 97 participants, and found that the correlation was very weak—significantly weaker than the relationship between WCmid or WCnarrow and VAT. This finding underscores the strength and specificity of the correlation between WCmid or WCnarrow and VAT. This finding is in contrast to large-scale population-based studies, including ~3,000–4,500 people, where BMI was significantly correlated with VAT, as measured by CT [4,40]. This highlights that although BMI may be an appropriate estimator of metabolic risk in large-scale population studies, it is not appropriate for evaluation of metabolic risk in smaller studies or in individuals [41].
Another finding from this study is that the site of WC measurement significantly influences the magnitude of the measurement, with WCnarrow < WCmid < WCumbilicus in our population of postmenopausal women with obesity. This finding is consistent with previous investigations suggesting that WC measurements made using different protocols are not all comparable [22,34,42]. For instance, in a study of WC measurements taken at four sites in 57 women, there were significant differences between all measurement sites (WCnarrow < WC immediately below the lowest rib < WCmid < WC immediately above the iliac crest; p < 0.05 for all comparisons) [22]. The current study advances on existing findings by showing—within the same study—that even the three commonly used WC measurement sites are significantly different from each other, even in a relatively homogenous population of postmenopausal women with obesity. This calls into question the practice of not reporting the specific site at which WC was measured, as in some publications [28,29].
This study has several limitations and strengths. Selection criteria for the present study were narrow, and while this limits the ability to generalise the results to other population groups, it also strengthens the findings by making them specific to a high-risk population of postmenopausal women with obesity—a population where WC measurement site differences were relatively unknown. Another limitation of our study is that the WC measures were not investigated under conditions of weight change. In addition, a potential source of measurement error for all WC sites is incorrectly positioning the tape measure on the subject’s body, however, this was minimised in the current study with the use of only two researchers assessing WC. In addition, the heavy-duty tape measure used in our study was flexible, inelastic, and firm, making it easy to place around the trunk region of the body in the same plane. Another strength of our study was that data variability was reduced by the consistent analysis of MRI scans by one researcher for the whole study.
In conclusion, measurement of WCmid or WCnarrow are accurate, practical and cost-effective means of estimating VAT in postmenopausal women with obesity, with WCnarrow offering several advantages for ease, speed and precision of measurement over WCmid.
Acknowledgments
This work was supported by the National Health and Medical Research Council (NHMRC) of Australia via an Early Career Research Fellowship to R.V.S. (1072771) and S.E.K. (1122190), as well as a Project Grant (1026005) to A.S., N.M.B. and I.D.C., and a Senior Research Fellowship (1042555) to A.S. F.Q.dL. was supported by the CAPES Foundation, Ministry of Education of Brazil, via a postgraduate scholarship. The Australian Government’s Department of Education & Training also supported this research via an Australian Postgraduate Award to A.A.G., and an International Postgraduate Research Scholarship to H.A.F. S.M.G. acknowledges the support of the Parker-Hughes Bequest and Frecker Family Trust.
Author Contributions
R.V.S. and A.L.W.-T. analyzed and interpreted data and wrote the manuscript. A.A.G., C.H., S.M., H.A.F., M.S.H.H. performed experiments and drafted manuscript. S.E.K. analyzed and interpreted data and drafted manuscript. N.A.J. and S.M.G. contributed to the design of experiments, interpreted data and drafted manuscript. F.Q.d.L., T.P.M., I.D.C. and N.M.B. interpreted data and drafted manuscript. A.S. conceived the study, designed experiments, interpreted data, and wrote the manuscript.
Conflicts of Interest
A.A.G. has received payment for oral presentations from the Pharmacy Guild of Australia and Nestlé Health Science. T.P.M. is on the NovoNordisk Obesity Advisory Board and Health Science Optifast® VLCDTM Advisory Board, has received funds for performing clinical trials from Pfizer, NovoNordisk, SFI, Australia Egg Corporation and has given talks on obesity for NovoNordisk. I.D.C. is president of the World Obesity Federation, has received funds for performing clinical trials from the NHMRC, SFI, the Australia Egg Corporation, NovoNordisk, BMS and Pfizer, and has given talks on obesity for Servier Laboratories, Ache Pharmaceuticals. A.S. is the author of The Don’t Go Hungry Diet (Bantam, Australia and New Zealand, 2007) and Don’t Go Hungry For Life (Bantam, Australia and New Zealand, 2011). She has also received payment from Eli Lilly, the Pharmacy Guild of Australia, Novo Nordisk, the Dietitians Association of Australia, Shoalhaven Family Medical Centres and the Pharmaceutical Society of Australia for presentation at conferences, and has served on the Nestlé Health Science Optifast® VLCDTM Advisory Board since 2016.
References
- 1.Kelly T., Yang W., Chen C.S., Reynolds K., He J. Global burden of obesity in 2005 and projections to 2030. Int. J. Obes. 2008;32:1431–1437. doi: 10.1038/ijo.2008.102. [DOI] [PubMed] [Google Scholar]
- 2.Afshin A., Forouzanfar M.H., Reitsma M.B., Sur P., Estep K., Lee A., Marczak L., Mokdad A.H., Moradi-Lakeh M., Naghavi M., et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017;377:13–27. doi: 10.1056/NEJMoa1614362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ritchie S.A., Connell J.M. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr. Metab. Cardiovasc. Dis. 2007;17:319–326. doi: 10.1016/j.numecd.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Fox C.S., Massaro J.M., Hoffmann U., Pou K.M., Maurovich-Horvat P., Liu C.Y., Vasan R.S., Murabito J.M., Meigs J.B., Cupples L.A., et al. Abdominal visceral and subcutaneous adipose tissue compartments: Association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 5.Goodpaster B.H., Krishnaswami S., Resnick H., Kelley D.E., Haggerty C., Harris T.B., Schwartz A.V., Kritchevsky S., Newman A.B. Association between regional adipose tissue distribution and both type 2 diabetes and impaired glucose tolerance in elderly men and women. Diabetes Care. 2003;26:372–379. doi: 10.2337/diacare.26.2.372. [DOI] [PubMed] [Google Scholar]
- 6.Sironi A.M., Gastaldelli A., Mari A., Ciociaro D., Positano V., Buzzigoli E., Ghione S., Turchi S., Lombardi M., Ferrannini E. Visceral fat in hypertension: Influence on insulin resistance and beta-cell function. Hypertension. 2004;44:127–133. doi: 10.1161/01.HYP.0000137982.10191.0a. [DOI] [PubMed] [Google Scholar]
- 7.Goodpaster B.H., Krishnaswami S., Harris T.B., Katsiaras A., Kritchevsky S.B., Simonsick E.M., Nevitt M., Holvoet P., Newman A.B. Obesity, regional body fat distribution, and the metabolic syndrome in older men and women. Arch. Intern. Med. 2005;165:777–783. doi: 10.1001/archinte.165.7.777. [DOI] [PubMed] [Google Scholar]
- 8.Lee S.W., Son J.Y., Kim J.M., Hwang S.S., Han J.S., Heo N.J. Body fat distribution is more predictive of all-cause mortality than overall adiposity. Diabetes Obes. Metab. 2018;20:141–147. doi: 10.1111/dom.13050. [DOI] [PubMed] [Google Scholar]
- 9.Shuster A., Patlas M., Pinthus J.H., Mourtzakis M. The clinical importance of visceral adiposity: A critical review of methods for visceral adipose tissue analysis. Br. J. Radiol. 2012;85:1–10. doi: 10.1259/bjr/38447238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheung A.S., de Rooy C., Hoermann R., Gianatti E.J., Hamilton E.J., Roff G., Zajac J.D., Grossmann M. Correlation of visceral adipose tissue measured by Lunar Prodigy dual X-ray absorptiometry with MRI and CT in older men. Int. J. Obes. 2016;40:1325–1328. doi: 10.1038/ijo.2016.50. [DOI] [PubMed] [Google Scholar]
- 11.Xia Y., Ergun D.L., Wacker W.K., Wang X., Davis C.E., Kaul S. Relationship between dual-energy X-ray absorptiometry volumetric assessment and X-ray computed tomography-derived single-slice measurement of visceral fat. J. Clin. Densitom. 2014;17:78–83. doi: 10.1016/j.jocd.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Kaul S., Rothney M.P., Peters D.M., Wacker W.K., Davis C.E., Shapiro M.D., Ergun D.L. Dual-energy X-ray absorptiometry for quantification of visceral fat. Obesity. 2012;20:1313–1318. doi: 10.1038/oby.2011.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Micklesfield L.K., Goedecke J.H., Punyanitya M., Wilson K.E., Kelly T.L. Dual-energy X-ray performs as well as clinical computed tomography for the measurement of visceral fat. Obesity. 2012;20:1109–1114. doi: 10.1038/oby.2011.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Choi Y.J., Seo Y.K., Lee E.J., Chung Y.S. Quantification of visceral fat using dual-energy X-ray absorptiometry and its reliability according to the amount of visceral fat in Korean adults. J. Clin. Densitom. 2015;18:192–197. doi: 10.1016/j.jocd.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Lin H., Yan H., Rao S., Xia M., Zhou Q., Xu H., Rothney M.P., Xia Y., Wacker W.K., Ergun D.L., et al. Quantification of visceral adipose tissue using lunar dual-energy X-ray absorptiometry in Asian Chinese. Obesity. 2013;21:2112–2117. doi: 10.1002/oby.20325. [DOI] [PubMed] [Google Scholar]
- 16.Svendsen O.L., Hassager C., Bergmann I., Christiansen C. Measurement of abdominal and intra-abdominal fat in postmenopausal women by dual energy X-ray absorptiometry and anthropometry: Comparison with computerized tomography. Int. J. Obes. Relat. Metab. Disord. 1993;17:45–51. [PubMed] [Google Scholar]
- 17.Neeland I.J., Grundy S.M., Li X., Adams-Huet B., Vega G.L. Comparison of visceral fat mass measurement by dual-X-ray absorptiometry and magnetic resonance imaging in a multiethnic cohort: The Dallas Heart Study. Nutr. Diabetes. 2016;6:e221. doi: 10.1038/nutd.2016.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamel E.G., McNeill G., Han T.S., Smith F.W., Avenell A., Davidson L., Tothill P. Measurement of abdominal fat by magnetic resonance imaging, dual-energy X-ray absorptiometry and anthropometry in non-obese men and women. Int. J. Obes. Relat. Metab. Disord. 1999;23:686–692. doi: 10.1038/sj.ijo.0800904. [DOI] [PubMed] [Google Scholar]
- 19.Naboush A., Hamdy O. Measuring visceral and hepatic fat in clinical practice and clinical research. Endocr. Pract. 2013;19:587–589. doi: 10.4158/EP12331.OR. [DOI] [PubMed] [Google Scholar]
- 20.Alberti K.G., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A., Fruchart J.C., James W.P., Loria C.M., Smith S.C., Jr. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 21.Harrington D.M., Staiano A.E., Broyles S.T., Gupta A.K., Katzmarzyk P.T. Waist circumference measurement site does not affect relationships with visceral adiposity and cardiometabolic risk factors in children. Pediatr. Obes. 2013;8:199–206. doi: 10.1111/j.2047-6310.2012.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J., Thornton J.C., Bari S., Williamson B., Gallagher D., Heymsfield S.B., Horlick M., Kotler D., Laferrere B., Mayer L., et al. Comparisons of waist circumferences measured at 4 sites. Am. J. Clin. Nutr. 2003;77:379–384. doi: 10.1093/ajcn/77.2.379. [DOI] [PubMed] [Google Scholar]
- 23.Bosy-Westphal A., Booke C.A., Blocker T., Kossel E., Goele K., Later W., Hitze B., Heller M., Gluer C.C., Muller M.J. Measurement site for waist circumference affects its accuracy as an index of visceral and abdominal subcutaneous fat in a Caucasian population. J. Nutr. 2010;140:954–961. doi: 10.3945/jn.109.118737. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization (WHO) Waist Circumference and Waist-Hip Ratio: Report of a WHO Expert Consultation. WHO; Geneva, Switzerland: 2008. [Google Scholar]
- 25.Lohman T.G., Roche A.F., Martorell R. Anthropometric Standardization Reference Manual. Human Kinetics; Champaign, IL, USA: 1988. [Google Scholar]
- 26.Ross R., Berentzen T., Bradshaw A.J., Janssen I., Kahn H.S., Katzmarzyk P.T., Kuk J.L., Seidell J.C., Snijder M.B., Sorensen T.I., et al. Does the relationship between waist circumference, morbidity and mortality depend on measurement protocol for waist circumference? Obes. Rev. 2008;9:312–325. doi: 10.1111/j.1467-789X.2007.00411.x. [DOI] [PubMed] [Google Scholar]
- 27.Rexrode K.M., Carey V.J., Hennekens C.H., Walters E.E., Colditz G.A., Stampfer M.J., Willett W.C., Manson J.E. Abdominal adiposity and coronary heart disease in women. JAMA. 1998;280:1843–1848. doi: 10.1001/jama.280.21.1843. [DOI] [PubMed] [Google Scholar]
- 28.Zhu S., Wang Z., Heshka S., Heo M., Faith M.S., Heymsfield S.B. Waist circumference and obesity-associated risk factors among whites in the third National Health and Nutrition Examination Survey: Clinical action thresholds. Am. J. Clin. Nutr. 2002;76:743–749. doi: 10.1093/ajcn/76.4.743. [DOI] [PubMed] [Google Scholar]
- 29.Thomas G.N., Ho S.Y., Lam K.S., Janus E.D., Hedley A.J., Lam T.H. Impact of obesity and body fat distribution on cardiovascular risk factors in Hong Kong Chinese. Obes. Res. 2004;12:1805–1813. doi: 10.1038/oby.2004.224. [DOI] [PubMed] [Google Scholar]
- 30.Jia W.P., Lu J.X., Xiang K.S., Bao Y.Q., Lu H.J., Chen L. Prediction of abdominal visceral obesity from body mass index, waist circumference and waist-hip ratio in Chinese adults: Receiver operating characteristic curves analysis. Biomed. Environ. Sci. 2003;16:206–211. [PubMed] [Google Scholar]
- 31.Onat A., Avci G.S., Barlan M.M., Uyarel H., Uzunlar B., Sansoy V. Measures of abdominal obesity assessed for visceral adiposity and relation to coronary risk. Int. J. Obes. Relat. Metab. Disord. 2004;28:1018–1025. doi: 10.1038/sj.ijo.0802695. [DOI] [PubMed] [Google Scholar]
- 32.Hill J.O., Sidney S., Lewis C.E., Tolan K., Scherzinger A.L., Stamm E.R. Racial differences in amounts of visceral adipose tissue in young adults: The CARDIA (Coronary Artery Risk Development in Young Adults) study. Am. J. Clin. Nutr. 1999;69:381–387. doi: 10.1093/ajcn/69.3.381. [DOI] [PubMed] [Google Scholar]
- 33.Valsamakis G., Chetty R., Anwar A., Banerjee A.K., Barnett A., Kumar S. Association of simple anthropometric measures of obesity with visceral fat and the metabolic syndrome in male Caucasian and Indo-Asian subjects. Diabet. Med. 2004;21:1339–1345. doi: 10.1111/j.1464-5491.2004.01361.x. [DOI] [PubMed] [Google Scholar]
- 34.Willis L.H., Slentz C.A., Houmard J.A., Johnson J.L., Duscha B.D., Aiken L.B., Kraus W.E. Minimal versus umbilical waist circumference measures as indicators of cardiovascular disease risk. Obesity. 2007;15:753–759. doi: 10.1038/oby.2007.612. [DOI] [PubMed] [Google Scholar]
- 35.Rudolf M.C., Walker J., Cole T.J. What is the best way to measure waist circumference? Int. J. Pediatr. Obes. 2007;2:58–61. doi: 10.1080/17477160601095177. [DOI] [PubMed] [Google Scholar]
- 36.Shen W., Chen J., Gantz M., Velasquez G., Punyanitya M., Heymsfield S.B. A single MRI slice does not accurately predict visceral and subcutaneous adipose tissue changes during weight loss. Obesity. 2012;20:2458–2463. doi: 10.1038/oby.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson S.T., Kuk J.L., Mackenzie K.A., Huang T.T., Rosychuk R.J., Ball G.D. Metabolic risk varies according to waist circumference measurement site in overweight boys and girls. J. Pediatr. 2010;156:247–252. doi: 10.1016/j.jpeds.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 38.Ambardar S., Cabot J., Cekic V., Baxter K., Arnell T.D., Forde K.A., Nihalani A., Whelan R.L. Abdominal wall dimensions and umbilical position vary widely with BMI and should be taken into account when choosing port locations. Surg. Endosc. 2009;23:1995–2000. doi: 10.1007/s00464-008-9965-1. [DOI] [PubMed] [Google Scholar]
- 39.Sabin M.A., Wong N., Campbell P., Lee K.J., McCallum Z., Werther G.A. Where should we measure waist circumference in clinically overweight and obese youth? J. Paediatr. Child Health. 2014;50:519–524. doi: 10.1111/jpc.12626. [DOI] [PubMed] [Google Scholar]
- 40.Nazare J.A., Smith J., Borel A.L., Aschner P., Barter P., Van Gaal L., Tan C.E., Wittchen H.U., Matsuzawa Y., Kadowaki T., et al. Usefulness of measuring both body mass index and waist circumference for the estimation of visceral adiposity and related cardiometabolic risk profile (from the INSPIRE ME IAA study) Am. J. Cardiol. 2015;115:307–315. doi: 10.1016/j.amjcard.2014.10.039. [DOI] [PubMed] [Google Scholar]
- 41.Keys A., Fidanza F., Karvonen M.J., Kimura N., Taylor H.L. Indices of relative weight and obesity. Int. J. Epidemiol. 2014;43:655–665. doi: 10.1093/ije/dyu058. [DOI] [PubMed] [Google Scholar]
- 42.Agarwal S.K., Misra A., Aggarwal P., Bardia A., Goel R., Vikram N.K., Wasir J.S., Hussain N., Ramachandran K., Pandey R.M. Waist circumference measurement by site, posture, respiratory phase, and meal time: Implications for methodology. Obesity. 2009;17:1056–1061. doi: 10.1038/oby.2008.635. [DOI] [PubMed] [Google Scholar]