Abstract
Nosocomial sequence types of Staphylococcus epidermidis dominate in prosthetic joint infections. We examined caspase-1 activation in human neutrophils after incubation with Staphylococcus epidermidis isolated from prosthetic joint infections and normal skin flora. Active caspase-1 was lower after incubation with isolates from prosthetic joint infections than after incubation with commensal isolates. Both host and isolate dependent differences in active caspase-1 were noted. Our results indicate that there might be a host-dependent incapacity to elicit a strong caspase-1 response towards certain strains of S. epidermidis. Further experiments with a larger number of individuals are warranted.
Keywords: Staphylococcus epidermidis; prosthetic joint infections, host-pathogen interaction; caspase-1; neutrophils
Introduction
Prosthetic joint infection (PJI) after arthroplasty surgery is an unwelcome complication, debilitating for the patient and associated with great costs for the healthcare system 1. Staphylococcus epidermidis is a common causative microorganism of PJIs 2, but also a ubiquitous skin commensal beneficial to the host 3. The majority of PJIs are thought to result from contamination of the implant during surgery or immediately post-operatively 2. In the presence of foreign material, the inoculum needed to establish an S. epidermidis infection is believed to be small (104 colony-forming units) 4. Many implants and/or joints (39-54%) are contaminated during prosthetic joint surgery 5, primarily by coagulase-negative staphylococci 5. However, the incidence of PJIs is low: approximately 1-2% 2. PJIs caused by S. epidermidis are predominantly due to healthcare-associated multidrug-resistant sequence types (STs), such as ST2 and ST215, and not by STs commonly found in the normal skin flora 6. The reason for this predominance of healthcare-associated strains is still unknown.
In an animal model of arthroplasty surgery, higher bacterial burden and markedly less neutrophilic response were demonstrated in IL-1β-deficient mice compared to TLR2-deficient mice or wild-type mice 7. IL-1β is produced as an inactive precursor protein, pro-IL-1β, that is cleaved by proteases to its active form. Cleavage of pro-IL-1β in neutrophils is accomplished both by active caspase-1, formed after autoproteolysis of pro-caspase-1 following oligomerization of inflammasome proteins, and by, for example, serine proteases. In human whole blood, treatment with caspase-1 inhibitor (YVAD) ex vivo reduced the production of IL-1β after stimulation with S. epidermidis in a dose-dependent manner 8, suggesting that caspase-1 is important for IL-1β production after stimulation with S. epidermidis. Experiments with knock-out mice have demonstrated that caspase-1 is essential for neutrophil secretion of IL-1β after stimulation with inflammasome activators 9, and that reduced production of active caspase-1 and IL-1β is associated with higher bacterial burden in a mouse model of corneal infection 10.
An immune evasion mechanism involving capase-1 dependent IL-1β production has been proposed as a possible explanation for the predominance of certain S. pneumoniae serotypes in invasive disease 11. In line with this, a previous study by our group demonstrated less caspase-1 activity in human neutrophils stimulated with Propionibacterium acnes isolates from orthopedic implant infections, compared to neutrophils stimulated with P. acnes isolates from normal skin flora 12. Our hypothesis is that a similar difference between clinical and commensal strains of S. epidermidis might explain the predominance of ST2 in PJIs. The aim of this study was thus to compare levels of active caspase-1 elicited in human neutrophils after stimulation with S. epidermidis isolates obtained from PJIs and S. epidermidis isolates from normal skin flora.
Methods
Human whole blood from healthy controls
Peripheral whole blood was obtained from three healthy controls (denoted A, B, and C). The neutrophil concentration was determined by automated hematology analyzer (X-E-5000; Sysmex Corporation, Kobe, Japan) after washing, as previously described 12. Experiments were performed in blocks of five commensal isolates, five PJI isolates, four positive controls, and one negative control, respectively. For each block, caspase-1 was determined in neutrophils from one individual/day, after 0.5h and 2h of incubation.
Bacterial strains
Ten commensal isolates of S. epidermidis (various STs), isolated from nares and wrists of healthy volunteers, and ten S. epidermidis isolates (ST2) from hip and knee PJIs were used in this study. The isolates were characterized in a previous study 6, and the origin of the isolates, sequence types, prevalence of virulence factors, multidrug resistance, and blocks are presented in table 1. Isolates were subcultured on blood agar plates overnight, after which two to five colonies were added to trypticase soy broth (BBL Trypticase Soy Broth, Beckton, Dickinson and Co., Sparks, MD, USA) and incubated for 24h. On the day of the experiment, 50 μL of the bacterial suspension was added to 950 μL PBS and the bacterial concentration of the suspension was then calculated using a Bürker chamber.
Table 1.
Isolate ID, type, origin, ST (sequence types), prevalence of virulence genes (icaADB, aap), biofilm production (phenotypic), SCCmec types, and multidrug resistance of S. epidermidis isolates (n=20).
| Isolate ID | Type | Origin | ST | icaADB | aap | Biofilm | SCCmec type | Multidrug resistant | Block |
|---|---|---|---|---|---|---|---|---|---|
| H2 | commensal | wrist | 190 | n/a | + | + | NT | no | 1 |
| H3 | commensal | wrist | 291 | n/a | + | - | NT | no | 1 |
| H4 | commensal | wrist | 283 | + | + | + | NT | no | 1 |
| N4 | commensal | nostril | 2 | + | + | + | NT | no | 1 |
| N11 | commensal | nostril | 38 | n/a | + | + | IV | no | 1 |
| H6 | commensal | wrist | 315 | n/a | + | - | NT | no | 2 |
| N7 | commensal | nostril | 307 | + | + | + | NT | no | 2 |
| N12 | commensal | nostril | 190 | n/a | n/a | - | NT | no | 2 |
| N14 | commensal | nostril | 278 | + | n/a | - | NT | no | 2 |
| N17 | commensal | nostril | 297 | + | + | + | NT | no | 2 |
| 115 | PJI | THR | 2 | + | + | + | NT | yes | 1 |
| 117 | PJI | THR | 2 | + | + | + | Ivc | yes | 1 |
| 118 | PJI | THR | 2 | + | + | + | Ivc | yes | 1 |
| 126 | PJI | THR | 2 | + | + | - | III | yes | 1 |
| 128 | PJI | THR | 2 | + | + | + | III? | no | 1 |
| 108 | PJI | THR | 2 | + | + | - | NT | yes | 2 |
| 122 | PJI | TKR | 2 | + | + | + | Ivc | yes | 2 |
| 139 | PJI | THR | 2 | + | + | + | NT | yes | 2 |
| 152 | PJI | THR | 2 | + | + | + | III? | yes | 2 |
| 157 | PJI | THR | 2 | + | + | + | III | yes | 2 |
Block refers to isolates included in the same experiment. THR = total hip replacement, TKR = total knee replacement.
Detection of active caspase-1 in neutrophils
Active caspase-1 in neutrophils was quantified by flow cytometry using FAM-FLICA® Caspase-1 assay kit (ImmunoChemistry Technologies, Bloomington, MN, USA) in parallel with leukocyte labeling, as previously described 12. Human washed whole blood (270 µl) was incubated with 20 μL of bacterial suspension and 10 μL of x30 FLICA for 30min and 2h, respectively. The concentrations of the bacterial suspensions were corrected to MOI of 1:2. Unstimulated cells and four positive controls (ATP, LPS + ATP, nigericin, and LPS + nigericin) were included in all experiments. LPS was added from the start, and ATP and nigericin were added 30 min before end of incubation. Flow cytometry was performed with an EPICS® ALTRA (Beckman Coulter, Fullerton, CA, USA) equipped with an Argon laser (488nm) and EXPO 32 software. FLICA fluorescence was measured with a 525 ± 30 nm band pass filter. For each sample, the fluorescence intensity was determined for 50,000 events. The flow cytometry data were analyzed with Kaluza Analysis software (Beckman Coulter). Leukocytes were distinguished as previously described 12. Neutrophils were gated manually (population identified via high SS, presence of CD45, and lack of CD14). Median fluorescence intensity (MFI) was determined for each sample, and the data were then analyzed by comparing median MFI values in neutrophils incubated with isolates from PJIs, and neutrophils incubated with isolates from normal skin flora. The mean discrepancy in MFI for unstimulated controls between experimental days (the intra-individual variability) was 8.0% (SD ±4.6%).
Ethical considerations
Approval from ethics committee was not applicable by the Swedish Act concerning the Ethical Review of Research Involving Humans (2003:460). The blood samples were anonymized. Limited clinical data from the original microbiology request form were provided with the subcultured bacterial isolates from prosthetic joint infections, but identification of patients was not possible, and no human tissue material was stored.
Statistical analyses
The Shapiro-Wilk test was used to test data sets for normality. Non-parametric data are presented as median and interquartile range. A two-tailed Mann-Whitney U-test was used to test for differences between median MFI values between isolate groups (IBM SPSS Statistics for Macintosh, version 23.0). A p-value ≤ 0.05 was considered significant.
Results
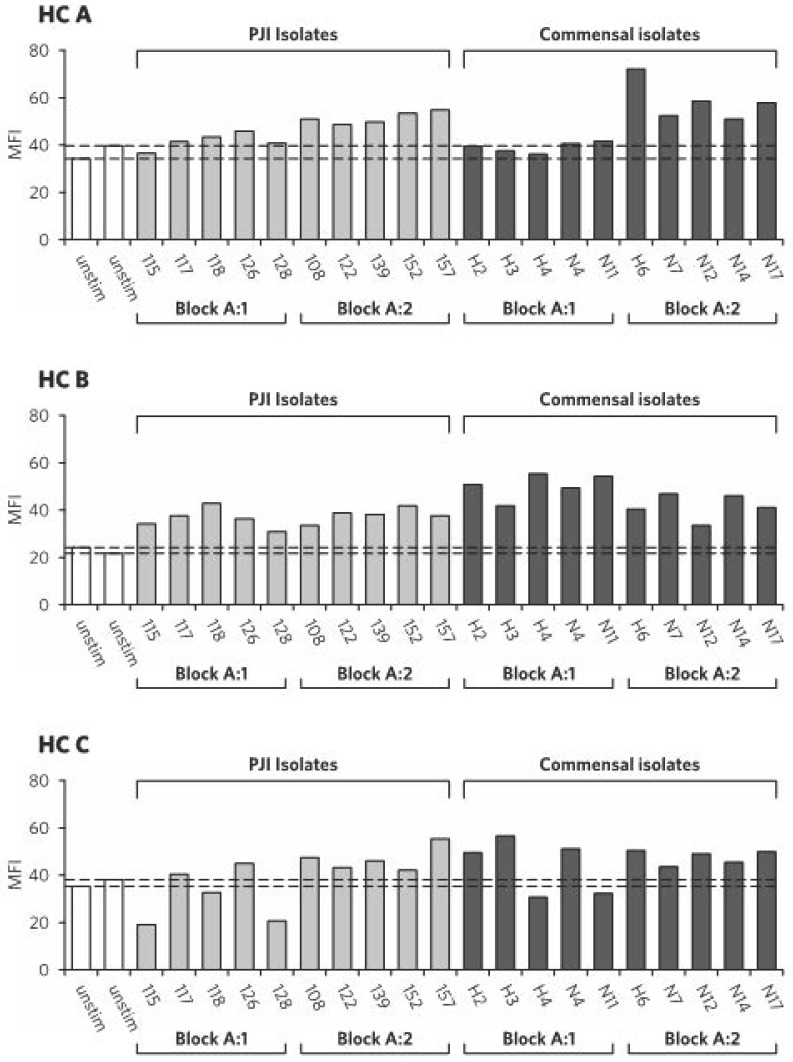
The production of active caspase-1 in human neutrophils was compared under resting conditions and after incubation with S. epidermidis isolates from normal skin flora (n=10) and PJIs (n=10). Under resting conditions, the expression of active caspase-1 in neutrophils was similar for two of the control individuals (A and C), but lower for individual B (demonstrated after 2h in Figure 1, unstimulated neutrophils, white bars).
Figure 1.
Active caspase-1 in neutrophils after 2h of stimulation, per healthy control (HC A-C) and isolate number. White bars = unstimulated neutrophils, light grey bars = neutrophils stimulated with S. epidermidis isolates from prosthetic joint infections, dark grey bars = neutrophils stimulated with S. epidermidis isolates from normal skin flora. Dotted lines show values for unstimulated controls. MFI = median fluorescence intensity.
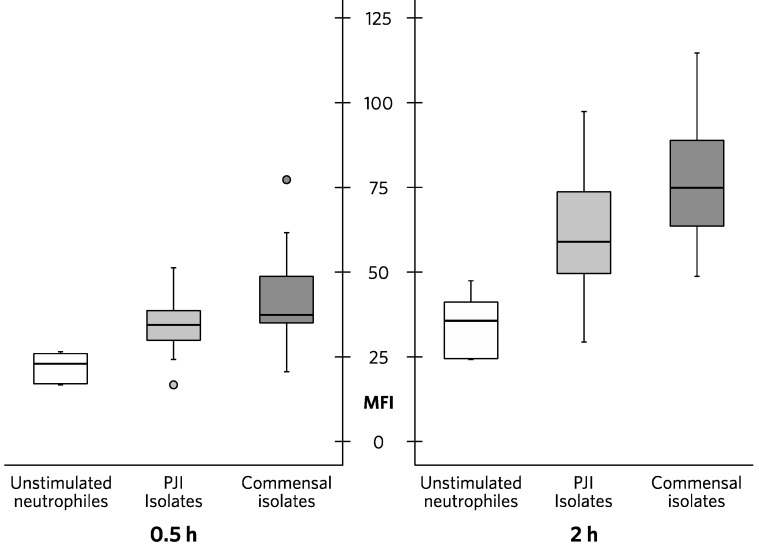
After incubation with S. epidermidis isolates, the expression of active caspase-1 in neutrophils was increased compared to unstimulated cells. Median MFI after 0.5h was 37.3 (35.1-47.5) for neutrophils incubated with commensal isolates and 34.5 (30.2-38.5) for neutrophils incubated with PJI isolates (p=0.029) (Figure 2). After 2h of incubation, median MFI was 74.9 (64.5-87.8) for neutrophils incubated with commensal isolates and 59.1 (50.5-73.3) for neutrophils incubated with PJI isolates (p=0.003) (Figure 2.). Active caspase-1 in neutrophils after 2h of stimulation is presented by healthy control and isolate in Figure 1.
Figure 2.
Active caspase-1 in neutrophils from three healthy controls after 0.5h and 2h of stimulation. White bars = unstimulated neutrophils, light grey bars = neutrophils stimulated with S. epidermidis isolates from prosthetic joint infections (PJIs), dark grey bars = neutrophils stimulated with S. epidermidis isolates from normal skin flora (commensal isolates).
Discussion
In this study, we determined active caspase-1 in human neutrophils after incubation with S. epidermidis isolates from PJIs and normal skin flora, in order to investigate if there was a difference in host immune response towards isolates of different origin that could contribute to the predominance of nosocomial S. epidermidis genotypes in PJIs. S. epidermidis isolates from PJIs as well as from normal skin flora induced caspase-1 activity in neutrophils, but commensal bacteria generally produced a stronger response than PJI isolates.
Two PJI isolates, numbers 115 and 128, consistently produced low levels of caspase-1 activity (Fig 1B), indicating isolate-specific differences in virulence responsible for affecting host inflammatory response. Neutrophils from one individual (C) demonstrated even lower caspase-1 activity after incubation with these isolates compared to the unstimulated setting. It remains to be clarified whether such a low level of active caspase-1 can influence bacterial clearance by inducing insufficient levels of IL-1β or other caspase-1 mediated inflammatory responses; the potential underlying mechanism for this phenomenon of host-pathogen interaction is also still unclear.
Active caspase-1 was particularly high after incubation with some of the S. epidermidis isolates from normal skin flora. However, isolate number H6, for example, which evoked by far the highest caspase-1 activity of individual A, resulted in a more average active caspase-1 response in neutrophils from individual C, suggesting inter-individual variations of host-microbe interaction.
Single nucleotide polymorphism (SNP) in NLRP3 and CARD8 genes (involved in inflammasome-dependent activation of caspase-1) has been associated with elevated levels of IL-1β in healthy blood donors 13 and a number of inflammatory diseases characterized by elevated levels of IL-1β 14. Inter-donor variation in IL-1β production in human monocytes after stimulation with canonical inflammasome activators has been described 15, as have variable expression and function of the P2X7 receptor (which mediates ATP-driven NLRP3 inflammasome-dependent IL-1β secretion) on human neutrophils 16. Polymorphism in the human P2X7 receptor gene has been associated with increased susceptibility to Mycobacteria, and in vitro data imply that the P2X7 receptor is important also in modulating immune response towards other intracellular bacteria, parasites and extracellular bacteria such as Staphylococcus aureus and Escherichia coli 17. However, most work on inflammasome activation by bacteria has been performed with inbred mice and a limited number of strains 18, and hence little is known about inter-individual variation in human innate immune response towards different strains of a specific bacterial species. A study by Fatykhova et al. found serotypic differences in NLRP3 inflammasome-dependent responses to pneumococci in human tissue 11, but not inter-individual differences.
A strength of the present study is the large number of isolates from both PJIs and normal skin flora, as the variable response to different isolates implies that it is difficult to draw conclusions on a species level from results based on experiments with just one or two strains. Unstimulated cells were similar between experimental days, indicating a stable assay, but repeated experiments were not performed with the same isolate and healthy control. A major limitation of this study is the small number of healthy controls; further studies of inflammasome activation by S. epidermidis would benefit from including a larger number of individuals. To clarify if there are functional differences between clinical and commensal strains with regard to induction of downstream inflammatory response, measurements of IL-1β in future experiments would also be important. Neutrophil supernatants from this experimental setting could not be used for IL-1β analyses as the fluorescent inhibitor probe FAM-YVAD-FMK forms a covalent interaction with the active site of the enzyme and thus inhibits caspase-1 activity. Another limitation of the present study is that the addition of FLICA at the beginning of incubation meant that no positive or negative feedback by active caspase-1 could take place. On the other hand, the addition of FLICA at the beginning also meant that there was less risk of inadvertently missing the caspase-1 peak, and so time-sensitivity was less of a problem.
To conclude, lower activation of caspase-1 was demonstrated in human neutrophils after incubation with S. epidermidis isolates from PJIs, compared to S. epidermidis isolates from normal skin flora. Differences in active caspase-1 were found to be host and isolate dependent. Further studies are needed in order to clarify if there are individual patients at increased risk for PJI due to sub-optimal activation of caspase-1 in neutrophils when challenged with S. epidermidis; and if so, whether the increased risk is isolate-specific due to specific virulence determinants.
Acknowledgments
Funding for this study was provided by grants from Örebro County Council Research Committee, Örebro, Sweden and the County Council of Västmanland Research Fund.
References
- 1.Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic Burden of Periprosthetic Joint Infection in the United States. The Journal of arthroplasty. 2012;27:61–5.e1. doi: 10.1016/j.arth.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 2.Tande AJ, Patel R. Prosthetic Joint Infection. Clinical Microbiology Reviews. 2014;27:302–45. doi: 10.1128/CMR.00111-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallo RL, Nakatsuji T. Microbial symbiosis with the innate immune defense system of the skin. J Invest Dermatol. 2011;131:1974–80. doi: 10.1038/jid.2011.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Widmer AF, Frei R, Rajacic Z, Zimmerli W. Correlation between In Vivo and In Vitro Efficacy of Antimicrobial Agents against Foreign Body Infections. Journal of Infectious Diseases. 1990;162:96–102. doi: 10.1093/infdis/162.1.96. [DOI] [PubMed] [Google Scholar]
- 5.Jonsson EÖ, Johannesdottir H, Robertsson O, Mogensen B. Bacterial contamination of the wound during primary total hip and knee replacement. Acta orthopaedica. 2014;85:159–64. doi: 10.3109/17453674.2014.899848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hellmark B, Söderquist B, Unemo M, Nilsdotter-Augustinsson Å. Comparison of Staphylococcus epidermidis isolated from prosthetic joint infections and commensal isolates in regard to antibiotic susceptibility, agr type, biofilm production, and epidemiology. International Journal of Medical Microbiology. 2013;303:32–9. doi: 10.1016/j.ijmm.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Bernthal NM, Pribaz JR, Stavrakis AI, Billi F, Cho JS, Ramos RI. et al. Protective role of IL-1beta against post-arthroplasty Staphylococcus aureus infection. J Orthop Res. 2011;29:1621–6. doi: 10.1002/jor.21414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stuyt RJ, Kim SH, Reznikov LL, Fantuzzi G, Novick D, Rubinstein M. et al. Regulation of Staphylococcus epidermidis-induced IFN-gamma in whole human blood: the role of endogenous IL-18, IL-12, IL-1, and TNF. Cytokine. 2003;21:65–73. doi: 10.1016/s1043-4666(02)00501-x. [DOI] [PubMed] [Google Scholar]
- 9.Mankan AK, Dau T, Jenne D, Hornung V. The NLRP3/ASC/Caspase-1 axis regulates IL-1beta processing in neutrophils. Eur J Immunol. 2012;42:710–5. doi: 10.1002/eji.201141921. [DOI] [PubMed] [Google Scholar]
- 10.Karmakar M, Katsnelson M, Malak HA, Greene NG, Howell SJ, Hise AG. et al. Neutrophil IL-1beta processing induced by pneumolysin is mediated by the NLRP3/ASC inflammasome and caspase-1 activation and is dependent on K+ efflux. Journal of immunology. 2015;194:1763–75. doi: 10.4049/jimmunol.1401624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fatykhova D, Rabes A, Machnik C, Guruprasad K, Pache F, Berg J. et al. Serotype 1 and 8 Pneumococci Evade Sensing by Inflammasomes in Human Lung Tissue. PloS one. 2015;10:e0137108. doi: 10.1371/journal.pone.0137108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahdo B, Särndahl E, Elgh F, Söderquist B. Propionibacterium acnes activates caspase-1 in human neutrophils. APMIS: acta pathologica, microbiologica, et immunologica Scandinavica. 2013;121:652–63. doi: 10.1111/apm.12035. [DOI] [PubMed] [Google Scholar]
- 13.Sahdo B, Fransén K, Asfaw Idosa B, Eriksson P, Söderquist B, Kelly A. et al. Cytokine profile in a cohort of healthy blood donors carrying polymorphisms in genes encoding the NLRP3 inflammasome. PloS one. 2013;8:e75457. doi: 10.1371/journal.pone.0075457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paramel GV, Sirsjo A, Fransen K. Role of genetic alterations in the NLRP3 and CARD8 genes in health and disease. Mediators Inflamm. 2015;2015:846782. doi: 10.1155/2015/846782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gattorno M, Tassi S, Carta S, Delfino L, Ferlito F, Pelagatti MA. et al. Pattern of interleukin-1beta secretion in response to lipopolysaccharide and ATP before and after interleukin-1 blockade in patients with CIAS1 mutations. Arthritis Rheum. 2007;56:3138–48. doi: 10.1002/art.22842. [DOI] [PubMed] [Google Scholar]
- 16.Karmakar M, Katsnelson MA, Dubyak GR, Pearlman E. Neutrophil P2X7 receptors mediate NLRP3 inflammasome-dependent IL-1beta secretion in response to ATP. Nat Commun. 2016;7:10555. doi: 10.1038/ncomms10555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller CM, Boulter NR, Fuller SJ, Zakrzewski AM, Lees MP, Saunders BM. et al. The role of the P2X(7) receptor in infectious diseases. PLoS Pathog. 2011;7:e1002212. doi: 10.1371/journal.ppat.1002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev. 2017;277:61–75. doi: 10.1111/imr.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]