Abstract
Aim: Visualization of Staphylococcus aureus biofilm using histochemical staining and combined histochemistry (HC) and immunohistochemistry (IHC).
Methods: The ability of S. aureus S54F9 to form biofilm was tested in vitro. Hereafter, infected bone tissue was collected from two different porcine models of osteomyelitis inoculated with S. aureus strain S54F9. The infection time was five and fifteen days, respectively. Twenty-five different histochemical staining protocols were tested in order to find the stains that could identify extracellular biofilm matrix. Protocols with an optimal visualization of biofilm extracellular matrix were combined with an immunohistochemical protocol based on a specific antibody against S. aureus. The combined protocols were applied to the tissue from the porcine models and to infected bone tissue from a child suffering from chronic staphylococcal osteomyelitis for more than a year.
Results: S. aureus S54F9 showed an ability to form biofilm in vitro. Visualization of biofilm, i.e. bacterial cells and extracellular matrix in different colours, was seen when the immunohistochemical protocol was combined with Alcian Blue pH3, Luna and Methyl-pyronin green. The bacterial cells were red to light brown and the extracellular matrix either light blue, blue or orange depending on the histochemical stain. In the porcine models and the human case 10 and 90 percent, respectively, of the bacterial aggregates in a 100x magnification field displayed both the extracellular matrix and the bacterial cells simultaneously in two different colours.
Conclusions: A combination of HC and IHC can be used to diagnose and characterise biofilm infections on a routine basis.
Keywords: Biofilm, Staphylococcus aureus, bone infection, histology, light microscopy
Introduction
Bacterial biofilms are microbial derived communities, characterized by aggregates of bacterial cells that are attached to a substratum/interface or to each other and embedded in a matrix of extracellular polymeric substances 1-3. Furthermore, the bacteria exhibit an altered phenotype in regard to growth, gene expression and protein production 2,3 and show increased tolerance towards antibiotics and the host immune system 3. In most in-vitro biofilms, the bacteria account for less than 10 % of the dry mass, whereas the extracellular matrix can account for more than 90 % 4. The extracellular matrix of a biofilm is composed of molecules produced both by the bacteria and the host and includes polysaccharides, structural proteins, enzymes, nucleic acids and lipids 4. All the different molecules have specific roles in favour of the survival and growth of the embedded bacteria (Table 1) 4. Despite extensive research focusing on bacterial biofilms, a comprehensive analysis of which histochemical stains that can identify the components of the extracellular biofilm matrix is lacking. Therefore, pathologists are often not aware of the presence of biofilm formation when examining slides for diagnosing bacterial infection in routine diagnostic laboratories.
Table 1.
Function of major components of extracellular matrix (ECM) in bacterial biofilms, modified from 4.
| Component | Function |
|---|---|
| Polysaccharides and proteins | -Adhesion between biofilm components and surfaces -Aggregation and immobilization of bacterial cells -Polysaccharide-enzyme binding causing accumulation, retention and stabilization of enzymes -Cohesion of biofilms ensuring mechanical stability, architecture and cell communication -Enzymatic activity (just proteins) -Electron donor or acceptor, permitting redox activity within the biofilm matrix (just proteins) -Nutrient source for utilization by biofilm cells -Protective barrier conferring host defenses against infection and tolerance to antimicrobials -Retention of water providing a hydrated microenvironment -Promoting nutrient accumulation and detoxification of xenobiotics -Promoting ion exchange, mineral formation, detoxification of toxic metal ions and polysaccharide gel formation |
| Nucleic acids | -Adhesion between biofilm components and surfaces -Aggregation and immobilization of bacterial cells -Cohesion of biofilms ensuring mechanical stability, architecture and cell communication -Exchange of genetic information between biofilm cells -Nutrient source for utilization by biofilm cells |
| Lipids | -Adhesion between biofilm components and surfaces (amphiphilic molecules) -Nutrient source for utilization by biofilm cells |
Biofilm formation facilitates bacterial tolerance to elimination by antimicrobial agents, host phagocytic clearance and host oxygen radicals and proteases 5. Therefore, biofilms are difficult to eradicate and present in many chronic diseases like cystic fibrosis, chronic wounds and orthopaedic infections 3. In orthopaedics, biofilm has been identified inside necrotic bone tissue and on the surface of orthopaedic implants 4. Staphylococcus aureus is one of the most common infectious agents in orthopaedics 5. Recently, the porcine S. aureus strain S54F9 spa type t1333, originating from a porcine lung abscess 6, was characterized by whole-genome sequencing 7. The strain has been used in various reproducible and discriminative porcine models of haematogenous osteomyelitis 8, implant-associated osteomyelitis 9, endocarditis 10 and sepsis 11. Due to the infectious potential of S. aureus S54F9, its in-vitro production of biofilm was tested in the present study. Furthermore, histochemical stains, alone or combined with immunohistochemistry (IHC), were applied to tissue from experimental S. aureus S54F9 bone infections in pigs 8,9 and from a clinical S. aureus bone infection 12 in order to demonstrate S. aureus biofilm using light microscopy.
Materials and methods
In vitro biofilm formation
The ability of S. aureus S54F9 to form biofilm was tested in a microtiter biofilm assay system as described by O'toole 13. Both S. aureus S54F9 and S. aureus RN4220 (a reference strain) 14 were diluted to a final cell density of OD 0.005 in TSB (Sigma, USA) with 1% glucose. Of this solution, 150 µl was applied to each well of a 96-well microtiter plate (Thermo Fisher, USA). Plates were incubated for 24 hours at 37˚C. After incubation the content was gently removed by inverting the plate. The wells were gently rinsed by adding 200 µl saline (0.9%) to each well. In order to fixate biomass, 180 µl methanol (Sigma, USA) was added to each well for 15 min. For staining, 180 µl Crystal Violet solution (CV) (Sigma, USA) was applied to the wells for 15 min. Two wells were left unstained for microscopy. The CV solution was removed by inverting the plate. Residue CV was removed by gently rinsing each well with 200 µl saline (0.9%). The plate was air-dried at room temperature. Bound CV was expelled by incubating the plate in 180 µl 96.5% (V/V) EtOH for 15 min. The intensity of the CV staining was evaluated by OD measuring at 594 nm on a VictorX4 plate reader (Perkin Elmer, USA). A t-test was used to show differences in OD measurements between the two S. aureus strains. For confocal microscopy, biofilm in the two CV unstained microtiter wells was stained with 0.3mM Syto9 (Molecular Probes, USA) and incubated for 15 min. Microscopy was performed using an inverted Zeiss LSM 880 confocal microscope running the program Zen 2.1 (Ziess, Germany). Images were obtained with a 488 nm laser with an emission peak at 498-501 nm by using a 10x/0.3 and a 63x/1.4 objective. 3D projections of these images were produced using the program Imaris (Bitplane, Switzerland).
Sampling of porcine bone tissue
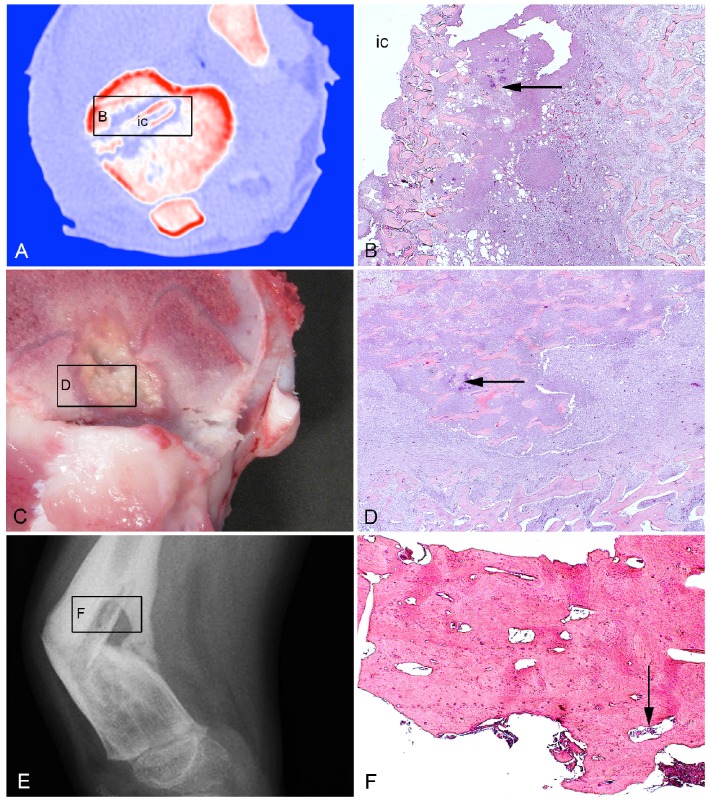
Two blocks of formalin-fixed, decalcified and paraffin embedded porcine infected bone tissue were used 9. The first block contained tissue from a female pig inoculated with 104 CFU of S. aureus S54F9 locally in the right tibia followed by insertion of a small steel implant (2x15 mm). The pig was euthanized following five days of inoculation 9. The tissue block included the implant cavity and the surrounding infected metaphyseal (trabecular) bone tissue (Figures 1A + 1B). The second block originated from a female pig inoculated with 104 CFU/kg BW of S. aureus S54F9 into the right femoral artery 8. This pig was euthanized following 15 days of inoculation 8. The tissue block was sampled from a bone lesion in the distal metaphysis of the right femur (Figures 1C + 1D).
Figure 1.
Bone tissue used in the present study. A: Porcine model of implant-associated osteomyelitis. CT scan after five days of inoculation demonstrating the tibial implant cavity (ic) used for injection of S. aureus and insertion of a small steel implant. B: Histology of picture A demonstrating bacteria (arrow) in the peri-implant tissue adjacent to the implant cavity (ic). HE. C: Porcine model of haematogenous osteomyelitis euthanized fifteen days after inoculation. A lesion is shown in the right femoral metaphysis. D: Histology of picture C demonstrating bacteria (arrow) centrally in the lesion. HE. E: X-ray of a child with haematogenous osteomyelitis and pathological fracture in the right femur. F: Histology of picture E demonstrating bacteria (arrow) in a cortical sequester. HE.
Sampling of human bone tissue
One block of formalin fixed, decalcified and paraffin embedded infected bone tissue from a 5 year-old boy with chronic, haematogenous osteomyelitis in the right femur, lasting for more than one year, was used 12. The block had been sampled during therapeutic surgery and contained a large cortical sequester (Figures 1E + 1F). Microbiological testing demonstrated S. aureus as the infecting organism 12.
Histochemistry (HC)
Tissue sections of 4-5 μm from each of the two cases of porcine osteomyelitis and from the human case of osteomyelitis were stained with haematoxylin and eosin (HE). The HE stained sections of the lesions were used to describe the pathomorphology with regards to bone destruction, inflammatory cells and localization of bacteria. Furthermore, 25 consecutive sections of 4-5 μm were cut from both blocks of porcine tissue and stained with the HC stains listed in Table 2. For all stains, the colour of bacterial clusters and the ability to visualize an extracellular biofilm matrix were recorded. Furthermore, the level of contrast between the colour of the bacterial clusters and surrounding tissue (background) was scored: poor contrast (+), fair contrast (++), good contrast (+++).
Table 2.
Results of 25 histochemical stains, modified from the cited references, applied to bone tissue from porcine models of haematogenous and implant associated osteomyelitis induced with Staphylococcus aureus S45F9.
| Stain | Staining pattern | Bacteria color | Matrix | Contrast | Protocol Reference |
|---|---|---|---|---|---|
| Connective tissue stains | |||||
| Alkaline congo red | Red: Amyloid (via. H-bonds), elastic fibers, eosinophil granules Blue: Nuclei |
Purple | No | ++ | 15 |
| Crystal violet | Red-purple: Amyloid, mucin, renal hyaline Blue: Background |
Purple | Yes Purple |
++ | 15 |
| Gomori's reticular fibers | Black: Reticular fibers Gray: Nuclei |
Brown/purple | No | + | 15 |
| Luna | Red: Eosinophils, RBC (red blood cells) Blue: Background |
Blue | Yes Blue |
+++ | 16 |
| Martius scarlet blue | Blue: Nuclei, collagen, (old fibrin) Yellow: RBC, (early fibrin) Red: Muscle, fibrin |
Purple | No | + | 15 |
| Masson-trichome | Blue/black: Nuclei Red: Cytoplasm, muscle, RBC Blue: Collagen |
Brown/purple | No | ++ | 15 |
| Picro-sirius red | Red: Collagen type 1-3, keratohyalin granules Polarized light: Collagen 1 - yellow/orange/red, collagen 3 - green |
Orange | Yes Orange |
++ | 17 |
| PTAH | Dark blue: Muscle striations, neuroglia, fibrin, amoebae Blue: Nuclei, cilia, RBC Light blue: Myelin Deep red-brown: Collagen, osteoid, cartilage, elastic fibers Pale pink-brown: Cytoplasm |
Brown/purple | No | + | 16 |
| Van Gieson | Blue/black: Nuclei Red: Collagen |
Brown | No | + | 15 |
| Verhöeff's | Black: Elastic tissue fibers | Purple | No | + | 15 |
| Carbohydrate stains | |||||
| Alcian blue ph1 | Blue: Acid mucins, proteoglycans, hyaluronic acid Red: Nuclei |
Blue | Yes Blue |
+ | 15 |
| Alcian blue ph3 | Blue: Acid mucins, proteoglycans, hyaluronic acid Red: Nuclei |
Blue | Yes Blue |
+++ | 15 |
| PAS | Magenta: Glycogen, neutral/sialomucins, glycoproteins Blue: Nuclei |
Purple | Yes Purple |
++ | 15 |
| Safranin O | Black: Nuclei Grey/green: Cytoplasm Orange/red: Cartilage, mast cells |
Purple | Yes Purple |
+++ | 18 |
| Toluidine blue-acetone | Blue/purple: Mast cell granula Blue: Nuclei and background |
Blue | Yes Blue |
++ | 15 |
| Lipid stains | |||||
| Oil-red O | Red: Fat Blue: Nuclei |
Purple | Yes Purple |
++ | 15 |
| Pigment and mineral stains | |||||
| Perls' Prussian blue | Blue: Ferric iron Red: Nuclei |
Red | No | ++ | 15 |
| Von kossa | Black: Mineralized bone Red: Osteoid |
Brown | No | + | 15 |
| Alizarin red S | Orange-red: Calcium deposits (especially small deposits) | Yellow/brown | No | +/++ | 15 |
| Microorganism stains | |||||
| Giemsa | Dark blue: Protozoa, microorganisms Pink-pale blue: Background Blue: Nuclei |
Blue | Yes Blue |
+++ | 15 |
| Grocott methenamine-silver | Black: Fungi, pneumocystis, melanin, hyphae, yeast form cells of fungi Taupe-dark gray: Mucins, glycogen Pale green: Background |
Black | No | ++ | 15 |
| Levaditis | Black: Spirochaetes, some organisms and fungi Yellow: Background |
Black | No | ++ | 16 |
| Gram | Blue/black: Gram positive bacteria Red: Gram negative bacteria |
Purple/blue | Yes Purple/blue |
+++ | 15 |
| Nucleic acid stains | |||||
| Feulgen nuclear reaction for DNA | Red/purple: DNA Green: Cytoplasm |
Turquoise | No | + | 15 |
| Methyl green-pyronin | Green/blue: DNA Red: RNA |
Red/pink | Yes Red/pink |
+++ | 15 |
Contrast: poor contrast (+), fair contrast (++), good contrast (+++) between bacteria and background.
Immunohistochemistry (IHC) towards S. aureus
Porcine and human tissue sections of 4-5 μm were prepared and processed for indirect in situ identification of S. aureus. Primary S. aureus specific antibodies (ab37644, Abcam, Cambridge, UK, diluted 1:2000 in 5% swine serum) targeting at antigen protein A were used. The specific protocol has recently been described (see also next section) 19. The three smallest and three largest (diameter) IHC positive bacterial aggregates were measured using the software Delta Pix Insight (Smørum, Denmark).
Combined IHC and HC
HC stains demonstrating extracellular biofilm matrix and good contrast to the surrounding tissue (Table 2) were selected to be combined with IHC towards S. aureus. Consecutive tissue sections of 4-5 μm from the porcine and human tissue blocks were mounted on adhesive glass slides (Thermo Scientific, Menzel GmbH & CoKG, Baunschweig, Germany). Following deparaffinization, antigen retrieval procedures were carried out by treatment with a Trypsin solution (Sigma-Aldrich, St. Louis, Missouri, USA) pH 7.8 for 30 min at 37 ºC. This was followed by blocking of endogenous peroxidase by 0.6% H2O2 for 15 min and blocking of unspecific protein binding by Ultra V Block (AH-diagnostics, Aarhus, Denmark) for five min. Tissue sections were incubated with the primary antibodies overnight followed by the application of a primary antibody enhancer, horseradish peroxidase polymer and aminoethylcabazol/3,3'- diaminobenzidine as described by the manufacturer (AH-diagnostics, Aarhus, Denmark). Throughout the immune-staining protocol, tissue sections were washed in Tris-buffered saline pH 7.6. After the IHC steps, the HC protocols with good contrast (Table 2) were applied, and all tissue sections were mounted with glycerol-gelatin and a cover slip. For each of the porcine and human tissue sections stained with the combined IHC and HC protocols, the number of bacterial aggregates with and without a visible biofilm matrix were registered in a 100x magnification field.
Image analysis
Combined IHC and HC stained porcine and human tissue sections were scanned using Zeiss Axio Scan.Z1 at a 10x objective and imported into Visiopharm Integrator System 4.6.857. The Visiopharm software was trained to recognize and stain positive areas of S. aureus (yellow) and extracellular matrix (purple). Pre-processing median filters for red/green/blue bands and the bayessian classification method were applied to improve image quality and recognition ability. On each tissue section, a representative bacterial aggregate demonstrating both bacterial cells and extracellular matrix was outlined. The Visiopharm software was used to identify and calculate the percentage of S. aureus and extracellular matrix positive areas within the outlined bacterial aggregate.
Results
In vitro biofilm formation
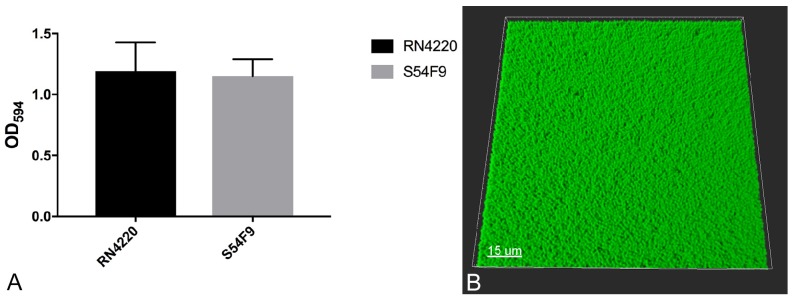
S. aureus S54F9 produced the same amount (P = 0.4693) of attached biomass as the known biofilm producing strain RN4220 (Figure 2A). A dense layer of biomass was visible in the bottom of the microtiter trays wells. At a higher magnification (630x), a compact layer of coccid bacteria comparable to S. aureus was seen (Figure 2B) 13,20.
Figure 2.
Biofilm formation in vitro. A: Attached biomass of reference strain RN4220 and S54F9 in the wells of microtiter trays based on the binding of crystal violet. Mean OD594 with SEM. B: 3D projection of the bottom of a microtiter well stained for bacterial cells with green Syto9. 630 x magnification.
Histochemistry
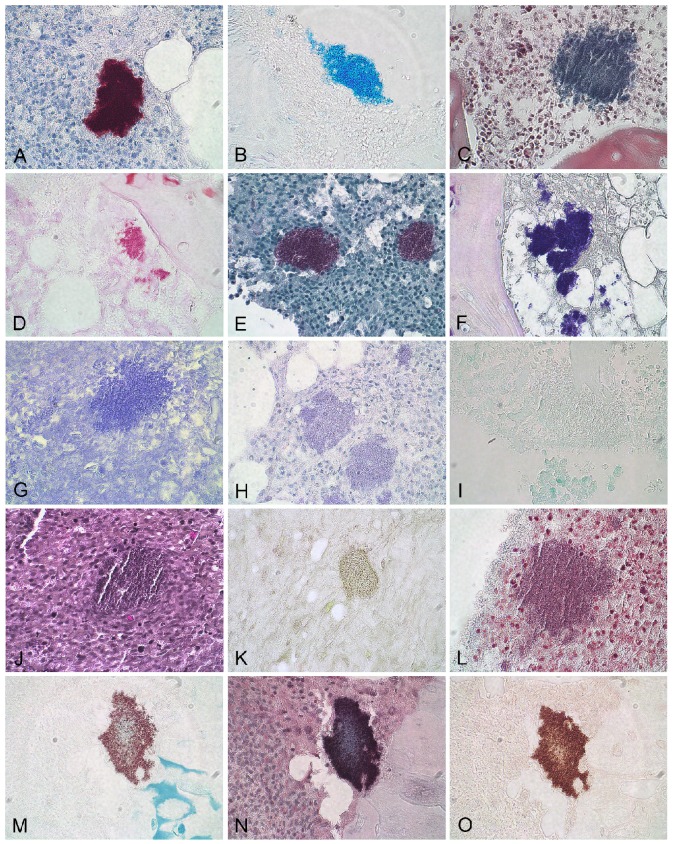
The porcine (5 day) tibial lesion consisted of necrotic trabecular bone debris, intermingled with accumulations of neutrophilic granulocytes and bacterial aggregates (Figure 1B). The porcine (15 day) femoral lesion consisted centrally of sequestered trabecular bone tissue with empty lacuna. The dead bone tissue was surrounded by neutrophilic granulocytes and aggregates of bacteria (Figure 1D). Macrophages and fibroblasts encapsulated the area (Figure 1D). The human tissue consisted of necrotic cortical bone tissue. The lamellar cortical bone structure could be observed in the sequestrum, and aggregates of bacteria and few inflammatory cells were located within Volkmann canals and the Haverisian system (Figure 1F). In the porcine tissues, six HC stains (Table 2) revealed the presence of an extracellular biofilm matrix and good contrast to the surrounding tissue (Figures 3B-F, Table 2).
Figure 3.
Immunohistochemical (A), histochemical (B-L) and combined histochemical/immunohistochemical (M-O) stains applied to porcine S. aureus infected bone tissue. A: S. aureus immunohistochemistry, B: Alcian blue pH3, C: Luna, D: Methyl green-pyronin, E: Safranin O, F: Gram, G: Crystal Violet, H: Oil red, I: Feulgen, J: Verhoeff, K: Alzian Red, L: Masson trichome, M: Alcian blue pH 3 + S. aureus immunohistochemistry, N: Luna + S. aureus immunohistochemistry, O: Methyl green-pyronin + S. aureus immunohistochemistry. All pictures are at 60x magnification
Immunohistochemistry towards S. aureus
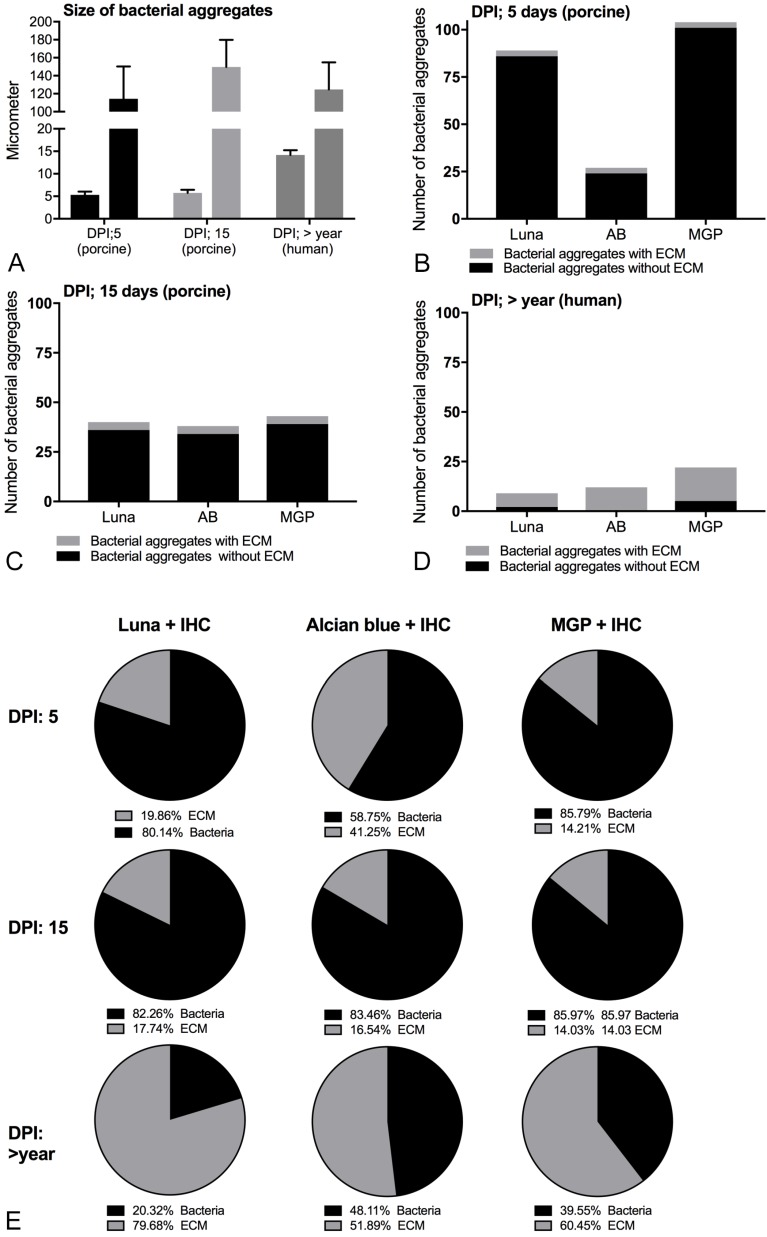
IHC staining towards S. aureus successfully demonstrated the presence of S. aureus in both porcine and human bone tissue (Figure 3A). Positive S. aureus bacteria were stained red. The size of the three smallest and three largest bacterial aggregates are shown in Figure 4A.
Figure 4.
Composition and size of biofilm forming infections in porcine and human bone tissue. A: Size of the three smallest and three largest bacterial aggregates seen with immunohistochemistry towards S. aureus, Mean ± SD. B-D: Number of bacterial aggregates with and without a visible extracellular biofilm matrix at 100x magnification following combined histochemistry with immunohistochemistry. The x-axis shows the histochemical stains. E: Percentage of bacterial cells and extracellular matrix in single representative S. aureus biofilm aggregates. AB: Alcian Blue pH3, MGP: Methyl-pyronin green, DPI: days post infection, ECM: extracellular matrix.
Combined immunohistochemistry and histochemistry
Of the six HC stains with good contrast and visible biofilm matrix, Alcian blue pH 3, Luna and Methyl green-pyronin (Table 2) were found to be optimal for combination with IHC. The remaining three good HC stains, i.e. Safranin O, Giemsa and Gram (Table 2) resulted in purple or blue bacterial aggregates which combined with IHC resulted in a brown indefinite mass. Alcian blue pH3, Luna or Methyl green-pyronin combined with IHC stained the bacterial cells red to light brown and the extracellular matrix light blue, blue or orange, respectively (Figures 3M-O). Extracellular matrix and bacteria were simultaneously seen in 3-10 % and 80-100 % of the bacterial aggregates in the porcine and human bone tissue, respectively (Figures 4B-D). For the single representative bacterial aggregates used to determine the bacterial cell/extracellular matrix ratio, a higher ratio of extracellular matrix than bacterial cells was seen in the human tissue compared to the two porcine models (Figure 4E and Figure 5).
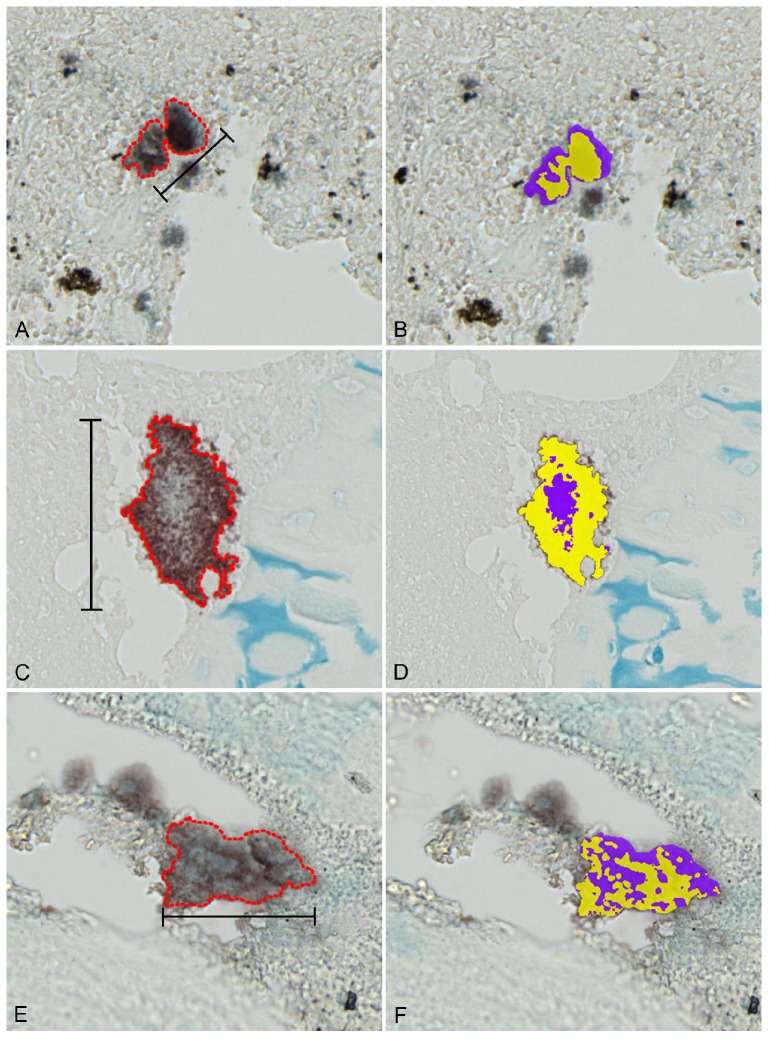
Figure 5.
Percentage of bacteria and extracellular matrix in S. aureus biofilm infections. Left column: combined immunohistochemistry (towards S. aureus) and Alcian blue pH3 staining of representative bacterial biofilms (red outline) displaying both bacteria (red/brown) and extracellular matrix(blue). Right column demonstrates calculations of % bacterial cells (yellow) and extracellular matrix (purple). A+B: Bone tissue from a porcine model of S. aureus implant-associated osteomyelitis infected for five days. Bar = 40 μm. C+D: Bone tissue from a porcine model of S. aureus haematogenous osteomyelitis infected for fifteen days. Bar = 78 μm. E+F: Bone tissue from a patient suffering from S. aureus haematogenous osteomyelitis for more than one year. Bar = 72 μm.
Discussion
Biofilm is typically found in chronic infections, which means that the infection persists despite adequate antibiotic therapy and host immune defence mechanisms 21. Therefore, increased focus has been on diagnosing and treating bacterial biofilm infections 21. In the present study, S. aureus S54F9 showed the ability to form biofilm in vitro. The histochemically stained bacterial aggregates seen in the porcine osteomyelitis models (inoculated with S54F9), therefore, likely represent in vivo biofilm produced by S. aureus S54F9. Likewise, the HC and IHC double coloured bacterial clusters seen in the human tissue also represent biofilm produced by S. aureus.
Recently, ESCMID (European Society for Clinical Microbiology and Infectious Diseases) published guidelines for diagnosing biofilm infections 21. According to these guidelines, biofilm detection requires that tissue microscopy shows evidence of an infective process, such as the presence of leucocytes, and that the microorganisms present form aggregates embedded in a self-produced matrix distinct from the surrounding tissue 21. These requirements were achieved with the present combination of HC and IHC staining's for light microscopy. Light microscopy is one of the most common methods by which bacteria are demonstrated in tissue 22. Bacteria can be visualized in routine HE stains, although a disadvantage of HE staining is that haematoxylin stains all nucleic acids, which may make it difficult to distinguish microbial cells from host cells 22. To specifically detect bacteria with HC stains, the Gram stain can be used 15. HC stains are commonly used to demonstrate different molecules in tissue sections based on chemical properties. Therefore, it is reasonable to suggest that the biofilm extracellular matrix can be visualized by different HC stains due to its content of carbohydrates, proteins, lipids and nucleic acids (Table 2). A limited number of studies demonstrating bacterial biofilm by HC and light microscopy exists 23-25. In these studies, PAS, Giemsa, Gram or HE stains have been applied to demonstrate bacterial biofilms 23-25. Also more advanced microscopic techniques have been used to visualize biofilm, e.g. confocal laser scanning microscopy, fluorescence microscopy, scanning electron microscopy, and transmissions electron microscopy 26. A combination of confocal microscopy and reverse transcriptase-polymerase chain reaction has been described as a powerful tool for detection of biofilms in operative tissue specimens 27. Confocal laser scanning microscopy and electron microscopy are, however, specialized and time consuming techniques based on equipment not generally available in diagnostic laboratories 28.
Although the present combinations of HC and IHC protocols were able to demonstrate biofilm formation by S. aureus, a negative result can not exclude the presence of biofilm. Furthermore, one should be aware of the fact that extracellular biofilm matrix components differ amongst different bacteria and bacterial strains. In a clinical situation, a combined staining technique can not be applied before the results of microbiology are available in order to select adequate antibodies for IHC. Therefore, the introduction of combined HC and IHC staining, as demonstrated in this study, is not useful in facilitating fast histopathological diagnosis of infections. However, it is useful for facilitating the demonstration of bacterial biofilm formation, which may impact the selected treatment protocol. Histologically, diagnosis of bone infection should not rely only on bacterial identification, but also on the patho-morphology present.
Biofilm extracellular matrix components include polysaccharides, lipids, proteins and extracellular DNA (Table 1). The light blue colour of the biofilm matrix seen with the Alcian Blue pH3 stain is due to a chemical reaction with polysaccharides. The blue and orange colours of biofilm matrix seen with Luna and Methyl-green pyronin, respectively, are due to haematoxylin and methyl-green which both stain nucleic acids. Extracellular DNA has been identified as a major structural component in the biofilm matrix of S. aureus, whereas it is only a minor component of biofilm formed by S. epidermidis 29. In a review concerning biofilm formation in vivo, it was recently shown that tissue biofilm aggregates in chronic infections of humans, including orthopaedic infections, despite anatomical location and type of bacteria involved have an upper size limit of approximately 200 μm 30. None of the measured bacterial aggregates in the present study exceeded this limit. According to Bjarnsholt et al. 30 and Parsek et al. 31 the human osteomyelitis case of the present study fulfils all criteria for a biofilm infection. This includes tissue adherent pathogenic bacteria found in clusters with a diameter within the range of ~5-200 µm 30, infection localized to a particular anatomic site and persistent infection despite exposure to antibiotics 30.
Conclusion
The porcine S. aureus strain S54F9 has the ability to form biofilm both in vitro and in vivo and is, therefore, a reliable agent to be used in animal models of human chronic infectious diseases caused by S. aureus.
A combination of HC and IHC can be used to diagnose and characterise biofilm infections. Furthermore, a list of how biofilm is stained by histochemical stains available in most routine diagnostic laboratories is provided.
Acknowledgments
We would like to thank Betina Andersen and Elizabeth Petersen for excellent laboratory assistance and the Core Facility for Integrated Microscopy at The University of Copenhagen for assistance with scanning of tissue sections.
Funding
This study was financed by grant no. 4005-00035B from the Danish Research Council and the European Union's Horizon 2020 research and innovation program under NOMORFILM project grant agreement No 634588 and grant no. R173-2014-584 and R105-A9791 from the Lundbeck Foundation to TB.
Ethical Approval
The porcine tissue blocks were collected from studies 8,9 approved by the Danish Animal Experimental Act (Licence no. 2013/15-2934-00946 and 2008/561-37). The human tissue block was collected from a study 12 approved by the Danish National Committee on Biomedical Research Ethics (Protocol no. H-2-2011-102).
Abbreviations
- IHC
immunohistochemistry
- HC
histochemistry, CFU: colony-forming unit, OD: optical density
- CV
Crystal Violet solution
- HE
haematoxylin and eosin.
References
- 1.Costerton JW, Geesey GG, Cheng K-J. How bacteria stick. Sci Am. 1978;238:86–95. doi: 10.1038/scientificamerican0178-86. [DOI] [PubMed] [Google Scholar]
- 2.Donlan RM, Costerton WJ. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bjarnsholt T. The role of bacterial biofilms in chronic infections. APMIS. 2013;121:1–51. doi: 10.1111/apm.12099. [DOI] [PubMed] [Google Scholar]
- 4.Flemming H-C, Wingender J. The biofilm matrix. Nature Rev Microbiol. 2010;8:623–633. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 5.Brady RA, Leid JG, Calhoun JH. et al. Osteomyelitis and the role of biofilms in chronic infection. FEMS Immunol Med Microbiol. 2008;52:13–22. doi: 10.1111/j.1574-695X.2007.00357.x. [DOI] [PubMed] [Google Scholar]
- 6.Hasman H, Moodley A, Guardabassi L. et al. spa type distribution in Staphylococcus aureus originating from pigs, cattle and poultry. Vet Microbiol. 2010;141:326–331. doi: 10.1016/j.vetmic.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 7.Aalbæk B, Jensen LK, Jensen HE. et al. Whole-genome sequence of Staphylococcus aureus S54F9 isolated from a chronic disseminated porcine lung abscess and used in human infection models. Genome Announc. 2015;3:1–2. doi: 10.1128/genomeA.01207-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johansen LK, Koch J, Frees D. et al. Pathology and biofilm formation in a porcine model of staphylococcal osteomyelitis. J Comp Path. 2012;147:343–353. doi: 10.1016/j.jcpa.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 9.Jensen LK, Koch J, Dich-Jorgensen K, Novel porcine model of implant-associated osteomyelitis: A comprehensive analysis of local, regional and systemic response. J Orthop Res; 2017. doi: 10.1002/jor.23505: 1-11. [DOI] [PubMed] [Google Scholar]
- 10.Christiansen JG, Jensen HE, Johansen LK. et al. Porcine models of non-bacterial thrombotic endocarditis (NBTE) and infective endocarditis (IE) caused by Staphylococcus aureus: A preliminary study. J Heart Valve Dis. 2013;22:368–376. [PubMed] [Google Scholar]
- 11.Sørensen KE, Skovgaard K, Heegaard PMH. et al. The impact of Staphylococcus aureus concentration on the development of pulmonary lesions and cytokine expression after intravenous inoculation of pigs. Vet Pathol. 2012;49:950–962. doi: 10.1177/0300985812439726. [DOI] [PubMed] [Google Scholar]
- 12.Johansen LK, Koch J, Kirketerp-Møller K. et al. Therapy of haematogenous osteomyelitis - a comparative study in a porcine model and Angolan children. In vivo. 2013;27:305–312. [PubMed] [Google Scholar]
- 13.O'Toole GA. Microtiter dish biofilm formation assay. J Vis Exp; 2011. doi:10.3791/2437: 1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malone CL, Boles BR, Lauderdale KJ. et al. Flourescent reporters for Staphylococcus aureus. J Microbiol Methods. 2009;77:251–260. doi: 10.1016/j.mimet.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bancroft J.D. & Gamble, M. Theory and practice of histological techniques. New York, USA: Churchill Livingstone Elsevier; 2008. [Google Scholar]
- 16.Luna LG. Manual of histologic and methods of the armed forces institute of pathology. New York, USA: McGraw-Hill; 1960. [Google Scholar]
- 17.Pearse SGE. Histochemistry theoretical and applied. New York, USA: Churchhill Livingstone; 1985. [Google Scholar]
- 18.Lille RD, Fullmer HM. Histopathologic technic and practical histochemistry. New York, USA: McGraw-Hill; 1965. [Google Scholar]
- 19.Johansen LK, Frees D, Aalbæk B. et al. A porcine model of acute, haematogenous localized osteomyelitis due to Staphylococcus aureus: a pathomorphological study. APMIS. 2011;119:111–118. doi: 10.1111/j.1600-0463.2010.02700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yarwood JM, Bartels DJ, Volper EM. et al. Quorum sensing in Staphylococcus aureus biofilms. J Bacteriol. 2004;186:1838–1850. doi: 10.1128/JB.186.6.1838-1850.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Høiby N, Bjarnsholt T, Moser C. et al. ESCMID guideline for the diagnosis and treatment of biofilm infections 2014. Clin Microbiol Infect. 2015;21:S1–S25. doi: 10.1016/j.cmi.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 22.Rumbaugh KP, Carty NL. In vivo models of biofilm infection. In: Bjarnsholt T, editor. Biofilm infections, 1st ed. New York: Springer-Verlag; 2011. pp. 267–290. [Google Scholar]
- 23.Winther B, Gross BC, Hendley JO. et al. Location of bacterial biofilm in the mucus overlaying the adenoid by light microscopy. Arch Otolaryngol Head Neck Surg. 2009;135:1239–1245. doi: 10.1001/archoto.2009.186. [DOI] [PubMed] [Google Scholar]
- 24.Hochstim CJ, Choi JY, Lowe D. et al. Biofilm detection with hematoxylin-eosin staining. Arch Otolaryngol Head Neck Surg. 2010;136:453–456. doi: 10.1001/archoto.2010.62. [DOI] [PubMed] [Google Scholar]
- 25.Tóth L, Csomor P, Sziklai I. et al. Biofilm detection in chronic rhinosinusitis by combined application of hematoxylin-eosin and gram staining. Eur Arch Otorhinolaryngol. 2011;268:1455–1462. doi: 10.1007/s00405-011-1623-x. [DOI] [PubMed] [Google Scholar]
- 26.Harrison-Balestra C, Cazzangia AL, Davis SC. et al. A wound-isolated Pseudomonas aeruginosa grows a biofilm in vitro within 10 hours and is visualized by light microscopy. Dermatol Surg. 2003;29:631–635. doi: 10.1046/j.1524-4725.2003.29146.x. [DOI] [PubMed] [Google Scholar]
- 27.Stoodley P, Nistico L, Johnson S. et al. Direct demonstration of viable Staphylococcus aureus biofilms in an infected total joint arthroplasty. J Bone Joint Surg Am. 2008;90:1751–1758. doi: 10.2106/JBJS.G.00838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Izano EA, Amarante MA, Kher WB. et al. Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. Appl Environ Microbiol. 2008;74:470–476. doi: 10.1128/AEM.02073-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ziran BH. Osteomyelitis. J Trauma. 2007;62:59–60. doi: 10.1097/TA.0b013e318065abbd. [DOI] [PubMed] [Google Scholar]
- 30.Bjarnsholt T, Alhede M, Alhede M. et al. The in vivo biofilm. Trends Microbiol. 2013;21:466–474. doi: 10.1016/j.tim.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Parsek MR, Singh PK. Bacterial biofilms: an emerging link to disease pathogenesis. Annu Rev Microbiol. 2003;57:677–701. doi: 10.1146/annurev.micro.57.030502.090720. [DOI] [PubMed] [Google Scholar]