Abstract
Microsatellite instability (MSI) is caused by DNA mismatch repair deficiency and is an important prognostic and predictive biomarker in colorectal cancer but relatively few studies have exploited mouse models in the study of its clinical utility. Furthermore, most previous studies have looked at MSI in the small intestine rather than the colon of mismatch repair deficient Msh2-knockout (KO) mice. Here we compared Msh2-KO, p53-KO, and wild type (WT) mice that were treated with the carcinogen azoxymethane (AOM) and the nonsteroidal anti-inflammatory drug sulindac or received no treatment. The induced tumors and normal tissue specimens from the colon were analysed with a panel of five mononucleotide repeat markers. MSI was detected throughout the normal colon in untreated Msh2-KO mice and this involved contraction of the repeat sequences compared to WT. The markers with longer mononucleotide repeats (37–59) were the most sensitive for MSI while the markers with shorter repeats (24) showed only minor change. AOM exposure caused further contraction of the Bat37 and Bat59 repeats in the distal colon of Msh2-KO mice which was reversed by sulindac. Thus AOM-induced carcinogenesis is associated with increased instability of mononucleotide repeats in the colon of Msh2-KO mice but not in WT or p53-KO mice. Chemoprevention of these tumors by sulindac treatment reversed or prevented the increased MSI.
1. Introduction
Microsatellite instability (MSI) in cancer occurs as a result of frameshift mutations in tandem repeat sequences known as microsatellites. These replication errors are caused by polymerase slippage and are normally corrected by the DNA mismatch repair (MMR) proteins; however impairment of this pathway through loss of its components prevents this correction and causes the accumulation of MSI throughout the genome in cancer cells, including coding and noncoding sequences [1].
MMR is a highly conserved cellular process that involves several protein complexes [2]. In the eukaryotes, there are two major MutS heterodimers, MSH2/MSH6 (MutSα) and MSH2/MSH3 (MutSβ), which bind to DNA and initiate the repair of the insertion-deletion-loop mismatches. The MutLα complex comprises MLH1/PMS2 and has a coordinator role in MMR. Germline mutations in the MMR genes MLH1, MSH2, MSH6, and PMS2 cause Lynch syndrome, which has a lifetime colorectal cancer risk of up to 80%, and is characterised by MSI in the tumors [3]. MSI is also found in sporadic colon cancers as a result of methylation silencing of MLH1. High-level MSI (MSI-H) is associated with various clinicopathological and molecular features, including proximal location of tumors, BRAF mutation, CpG island methylator phenotype (CIMP), and good prognosis [4–6]. MSI-positive colorectal cancers are more responsive to immunotherapy involving immune checkpoint blockade, possibly due to an increased level of mutation-associated neoantigens [7].
In mice, constitutive deficiency of individual MMR proteins also causes cancer development, but the spontaneous tumors develop in the small intestine rather than the colon. Relatively few studies have looked at MSI in the mouse colon or compared MSI in the normal colon of Msh2-knockout (KO) and wild type (WT) mice, while there are several reports on the small intestine [8, 9]. Methods to chemically induce tumorigenesis have been developed in order to model colorectal tumors in mice. Administration of the carcinogen azoxymethane (AOM) causes multiple tumors in the distal colon and this is prevented or reduced by sulindac, a nonsteroidal anti-inflammatory drug (NSAID) [10, 11]. In our previous study we showed that both Msh2-KO and p53-KO mice have an increased susceptibility to AOM-induced colon carcinogenesis and that sulindac is protective in the distal colon but has an adverse effect in the proximal colon [11]. AOM is known to cause at least low-level MSI in the colon tumors of WT mice but there are a few studies on MSI in AOM-induced tumors in MMR or p53 deficient mice [12]. Therefore, this comparative study aimed to examine MSI in the normal colon and distal tumors in WT, Msh2-KO, and p53-KO mice exposed to AOM and sulindac.
2. Materials and Methods
2.1. Mouse Breeding and Experimentation
The whole body knockout lines Msh2-KO and p53-KO were maintained in a Specific Pathogen Free breeding facility, crossing heterozygotes with wild type C57Bl6J strain. The Msh2-KO mice were initially generated in the 129/OLA strain [13]. Here we analysed Msh2-KO and p53-KO mice from our previous study, all on the C57Bl6J background [11], as well as additional Msh2-KO mice and their WT siblings, which had a mixed strain background. The mice received three injections of AOM (15 mg/kg at 8, 9, and 10 weeks) and feed containing sulindac for 22 weeks (160–320 ppm) and were all culled at 28 weeks of age. Altogether, we analysed 8–13 AOM-treated WT, p53-KO, or Msh2-KO mice and 17 Msh2-KO mice that received both AOM and sulindac. The macroscopic and histopathology analysis of the colon tissue specimens has been previously described in detail. The animal experimentation was approved by the Animal Ethics Committees of the Central Sydney Area Health Service (99/99/MCG/01) and Garvan Institute of Medical Research (02/05; 05/07).
2.2. Preparation of Tissue Specimens
Archival tissue samples were selected that would reflect a wide range of treatment conditions, genotypes, and pathological stages. Multiple specimens were analysed for some mice. These included adenocarcinomas and dysplastic tumors from the distal colon and normal tissue from different parts of the colon. Specimens were manually microdissected from formalin-fixed paraffin embedded tissue that had been scored by a specialist Anatomical Pathologist (JED). DNA was isolated using a modified protocol of the Gentra Puregene Tissue Kit (Qiagen).
2.3. Analysis of MSI
For MSI analysis, we chose four mononucleotide repeat markers that were identified by Bacher et al. [14] as sensitive indicators of MSI in the mouse, Bat24, Bat37, Bat59, and Bat64, where the number indicates the length of the poly-A repeat (24–64). We also included the 24-T repeat marker uPAR, which we used previously in the analysis of colitis-associated cancers [15]. PCR amplification was carried out in a multiplex reaction using MyTaq polymerase (Bioline), with primer concentrations ranging from 0.1 to 0.4 μM. The thermal cycling conditions were as follows: initial denaturation at 95°C for 10 minutes; followed by 40 cycles of 95°C for 45 seconds, 55°C for 45 seconds, and 68°C for 1 minute; then a final extension step at 68°C for 10 minutes. PCR fragments were analysed by capillary electrophoresis, ABI3130XL (Life Technologies), and the GeneMapper program (Life Technologies).
2.4. Definition of MSI for Individual Markers
Each marker pattern in the test specimen was compared to the appropriate control tissue as indicated in the results. As some further instability can appear as a result of the polymerase slippage during PCR, the whole pattern of major and minor peaks was compared. MSI-positivity was scored when at least a 1 bp contraction of the repeat was observed, (i.e., deletion, shown as a shift of the highest peak), or if new peaks appeared that were not present in the control tissue. A “minor shift” was recorded, when the tallest peak remained the same but there was otherwise a shift in the overall MSI pattern.
2.5. Statistical Analysis
Graphpad Prism software (GraphPad Inc.) was used for statistical analyses. Unpaired t-test was used to determine the statistical significance of the repeat length variation between genotype and treatment groups.
3. Results
3.1. MSI in the Normal Colon of the Msh2 Knockout Mouse
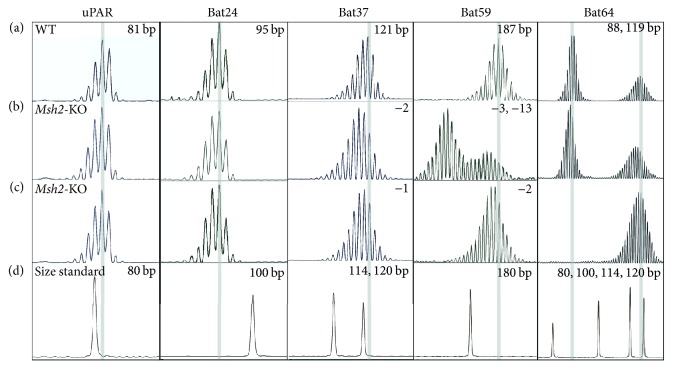
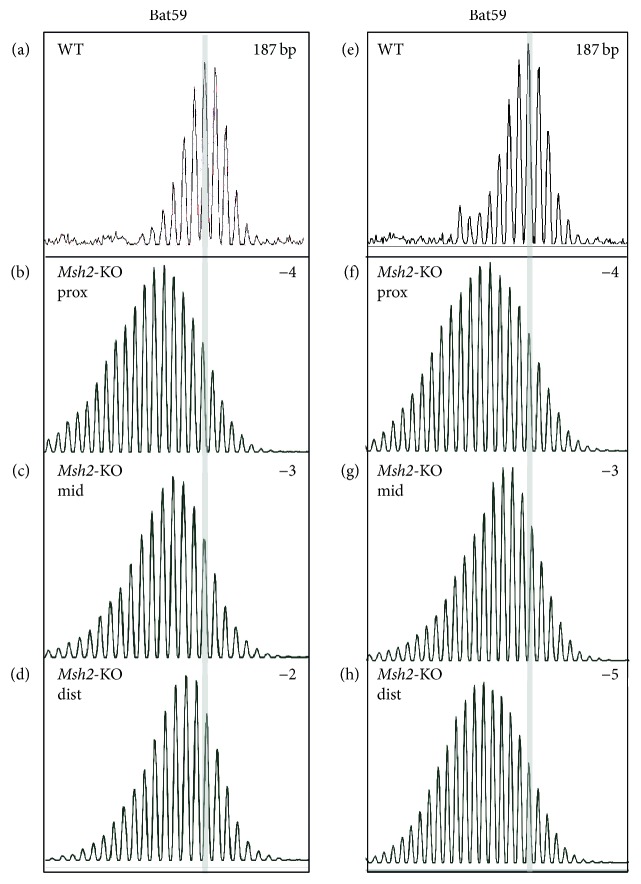
We analysed MSI in Msh2-KO and p53-KO mice that did not receive any specific treatment and compared the pattern of instability to their WT siblings. The Msh2 or p53 deficient mice are susceptible to carcinogenesis in various organs but do not usually develop spontaneous colon tumors [13, 16]. MSI was only observed in the Msh2-KO mice where a subset of the markers detected consistent instability in the normal colon (Figure 1). This involved contraction of the repeats compared to WT, but there was variation in the length of the deletion for each marker. Bat37 revealed deletions of 1-2 bp while Bat59 showed deletions of 2–5 nucleotides in 8 of the 9 mice (Figure 2) and additional new peaks in one mouse (~13 nucleotides shorter). The differences between the WT and Msh2-KO mice were statistically significant, P < 0.001. The shorter repeats were not as sensitive indicators of MSI as the longer repeats. The Msh2-KO specimens commonly showed a minor shift in the overall MSI pattern for the uPAR and Bat24 markers. Therefore, in subsequent analyses, we focused on Bat37 and Bat59. One WT mouse showed two alleles for Bat59, but all other mice appeared monomorphic. Bat64 was found to be polymorphic in the Msh2-KO and p53-KO lines (Figure 1). Altogether, we identified four alleles in the group of mice studied here, 80 bp for the 129/OLA strain and 88, 103, or 119 bp for C57Bl6J. This indicates that there is genetic variation for this repeat even within C57Bl6J.
Figure 1.
MSI in the normal colon of the Msh2-KO mouse. (a) Reference pattern of mononucleotide repeat markers uPAR, Bat24, Bat37, Bat59, and Bat64 in a WT mouse. Bat64 shows two alleles. The position of the highest peak in the WT mouse is highlighted with the grey bar and the size is indicated in the top right corner; (b, c) contraction of the repeats in two representative Msh2-KO mice compared to WT. (d) The position and size of the peaks for the size standard ROX.
Figure 2.
The length of the Bat59 mononucleotide repeat varies slightly within individual Msh2-KO mice. (a, e) Bat59 in the colon of two WT mice; (b–d); (f–h) Bat59 in the proximal, mid, and distal colon of two Msh2-KO mice. The position of the highest peak in the WT mouse is highlighted with the grey bar; 187 bp refers to the size of the PCR fragment in the WT and the number in the top right corner indicates the number of nucleotides by which the repeat is shortened in each Msh2-KO mouse.
3.2. MSI in Mice Exposed to AOM and Sulindac
Injection of the carcinogen AOM causes the development of multiple tumors in the mouse distal colon. Our previous study showed that these tumors feature low- to high-grade dysplasia (LGD/HGD) or adenocarcinoma. The combined frequency of HGD and adenocarcinoma was 35% in WT, 45% in Msh2-KO, and 63% in p53-KO mice [11]. Msh2-KO and p53-KO groups developed significantly more and larger tumors per mouse than the WT group [11].
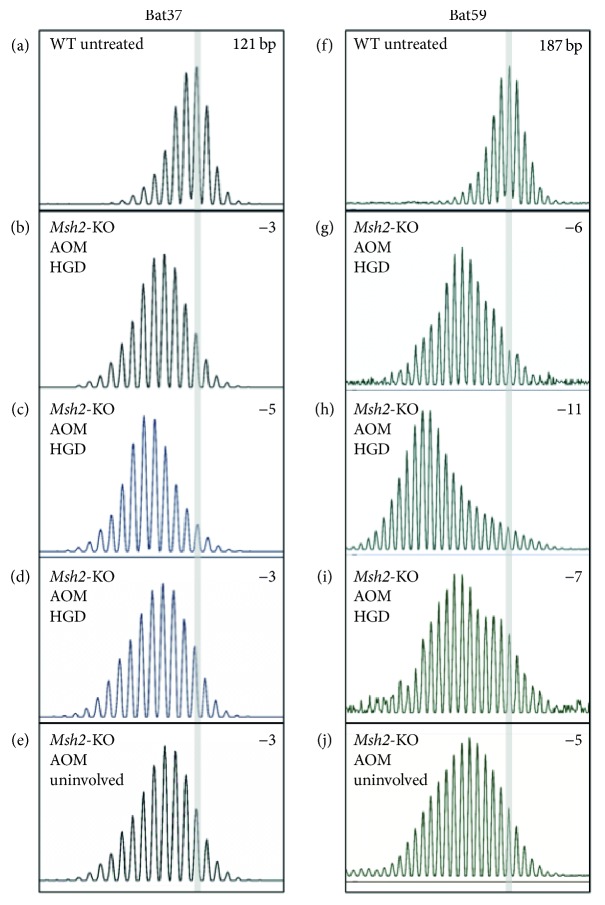
Here we first analysed MSI in a subset of the AOM-treated mice that developed either HGD or adenocarcinoma. The Msh2-KO mice showed MSI, which appeared to involve additional contraction of the mononucleotide repeats. The length of the deletions was variable, 3–10 bp for Bat59 and 2–5 bp for Bat37, compared to WT (Figures 3 and 4). This additional contraction of the two repeats was detected in both the tumors and uninvolved tissue from the distal colon of AOM-treated mice and was not observed in the proximal colon.
Figure 3.

AOM exposure causes a further contraction of the Bat37 and Bat59 repeats in the distal colon of Msh2-KO mice which is reversed by sulindac (sul) treatment. The error bars indicate SEM. p53-KO (grey), WT (black), Msh2-KO, AOM (red), and Msh2-KO, AOM + sulindac (blue).
Figure 4.
AOM-treated Msh2-KO mice display further contraction of the mononucleotide repeats in the distal colon. (a, f) Bat37 and Bat59 in the colon of a WT mouse; (b–d); (g–i) AOM-induced tumors from a Msh2-KO mouse; (e, j) uninvolved colon tissue from an AOM-treated Msh2-KO mouse. The position of the highest peak in the WT mouse is highlighted with the grey bar; 121 and 187 bp refer to the size of the PCR fragment in the WT and the number in the top right corner indicates the number of nucleotides by which the repeat is shortened in the Msh2-KO mouse.
Administration of dietary sulindac to AOM-treated mice can prevent the tumors from developing in the distal colon. In our previous experiment this treatment completely prevented neoplastic distal tumors in WT and lowered the frequency of HGD/adenocarcinoma to 3% in Msh2-KO and 7% in p53-KO mice [11]. Here we analysed the effect of this double treatment on MSI. Sulindac reversed the additional contraction of Bat37 and Bat59 in the distal colon of AOM-treated Msh2-KO mice. This was evident in both the few remaining tumors and in the uninvolved surrounding tissue of the distal colon. There was no change in the uninvolved tissue of the proximal colon in mice exposed to both AOM and sulindac compared to AOM alone (Figure 3).
4. Discussion
This study used a panel of proven mononucleotide repeat markers to analyse MSI in the mouse colon. MSI was not observed in the chemically induced colon cancers in most WT and p53-KO mice. Thus, p53 deficiency promotes AOM-induced colon carcinogenesis [11], but this does not commonly involve instability of mononucleotide repeats. It is possible that these tumors still harbor a defect in the MMR process that repairs dinucleotide or tetranucleotide repeats, which were not analysed here. Low-level MSI of dinucleotide repeats was previously demonstrated in AOM-induced colon tumors in the A/J WT strain [12].
In contrast, MSI was commonly observed in the normal colon of Msh2-KO mice that do not develop colon cancer spontaneously. This instability was found to a varying degree with each marker. Msh2 deficiency clearly causes contraction of the mononucleotide repeats Bat37 and Bat59 in the normal mouse colon, but the deletions were not as extensive in the shorter repeats (uPAR and Bat24). Here we also demonstrate that further contraction of the Bat37 and Bat59 repeats occurs in the distal colon of AOM-treated Msh2-KO mice. This effect was not seen in the proximal colon, which is consistent with the selectivity of AOM to the distal colon.
We could not find a previous report in the literature analysing MSI in the normal colon of Msh2-KO mice. The Bat24, Bat37, and Bat59 markers were used by Bacher et al. [14] to show instability in the spontaneous tumors that develop in the small intestine of the Msh2-KO mouse. It remains unexplained why these mice do not develop spontaneous tumors in the colon. However, this is likely to require the generation of further cancer driver mutations. The coding regions of genes usually only harbor short repeat sequences, such as the 10-A and 8-G repeats in TGFBR2 and BAX genes, respectively, that are frequently mutated in MSI-H human tumors [17]. Our data show that the two markers with 24-mononucleotide repeats were less affected by Msh2 deficiency in the colon than the 37–59-mononucleotide repeats. Therefore, it is possible that the coding regions of genes are also less frequently mutated in the colon of these mice.
We also report for the first time that exposure to the NSAID sulindac can reverse the increased instability that was caused by AOM in Msh2-KO mice. This was observed in the specimens obtained from the distal colon of AOM-treated mice and was most prominent for the Bat37 and Bat59 markers. This may indicate an opposite effect on the repeat length, contraction for AOM and expansion for sulindac exposure in vivo. Alternatively sulindac treatment may selectively target cells with MSI. It was previously described that aspirin, another NSAID, can reduce MSI in vivo in the intestinal epithelium of mice with Msh2 deficiency [18]. Aspirin has also been found effective in reducing the risk of colorectal cancer in carriers of Lynch syndrome mutations [19]. In vitro studies with both mouse and human MMR deficient cells indicated that this may be due to NSAID-mediated apoptosis that selectively targets cells with MSI, thus having an overall stabilizing effect on the repeats [20, 21]. However, it should be noted that, in our study, AOM-induced distal colon tumors were also prevented by sulindac in WT mice and on the background of p53 deficiency [11], in the absence of mononucleotide repeat instability. This indicates that there are multiple chemopreventive mechanisms.
In conclusion, instability of mononucleotide repeats is present in the normal colon of Msh2-KO mice that do not develop spontaneous colon cancers. AOM exposure caused further contraction of the repeats in Msh2-KO mice but this was reversed by administration of dietary sulindac in both the AOM-induced tumors and uninvolved tissue of the distal colon. There was no MSI found in the AOM-induced colon tumors in the p53-KO and WT mice.
Acknowledgments
The authors thank Elaine G. Bean for processing the mouse tissue specimens for histopathology. The Msh2 knockout mice were provided by Hein te Riele and the p53 knockout mice by Tyler Jacks. This work was supported by funding from the National Health and Medical Research Council of Australia (NHMRC 1020406), Gastroenterological Society of Australia, Cancer Institute NSW, and Cancer Council NSW (RG17-05). Joseph J. Daniel was supported by the NHMRC Dora Lush Biomedical Scholarship.
Disclosure
The contents of the published material are solely the responsibility of the administering institution, a participating institution, or individual authors and do not reflect the views of the NHMRC. The funders had no role in study design, data collection and analysis, decision to publish, preparation of the manuscript, or the decision to publish the results.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Nicola Currey and Joseph J. Daniel contributed equally.
References
- 1.Ionov Y., Peinado M. A., Malkhosyan S., Shibata D., Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993;363(6429):558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- 2.Fishel R. The selection for mismatch repair defects in hereditary nonpolyposis colorectal cancer: Revising the mutator hypothesis. Cancer Research. 2001;61(20):7369–7374. [PubMed] [Google Scholar]
- 3.Sehgal R., Sheahan K., O'Connell P. R., Hanly A. M., Martin S. T., Winter D. C. Lynch Syndrome: An updated review. Gene. 2014;5(3):497–507. doi: 10.3390/genes5030497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weisenberger D. J., Siegmund K. D., Campan M., et al. CpG island methylator phenotype underlies sporadic microsatellite instability and is tightly associated with BRAF mutation in colorectal cancer. Nature Genetics. 2006;38(7):787–793. doi: 10.1038/ng1834. [DOI] [PubMed] [Google Scholar]
- 5.Wright C. M., Dent O. F., Barker M., et al. Prognostic significance of extensive microsatellite instability in sporadic clinicopathological stage C colorectal cancer. British Journal of Surgery. 2000;87(9):1197–1202. doi: 10.1046/j.1365-2168.2000.01508.x. [DOI] [PubMed] [Google Scholar]
- 6.Kohonen-Corish M. R. J., Daniel J. J., Chan C., et al. Low microsatellite instability is associated with poor prognosis in stage C colon cancer. Journal of Clinical Oncology. 2005;23(10):2318–2324. doi: 10.1200/JCO.2005.00.109. [DOI] [PubMed] [Google Scholar]
- 7.Le D. T., Uram J. N., Wang H., et al. PD-1 blockade in tumors with mismatch-repair deficiency. The New England Journal of Medicine. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kucherlapati M. H., Lee K., Nguyen A. A., et al. An Msh2 conditional knockout mouse for studying intestinal cancer and testing anticancer agents. Gastroenterology. 2010;138(3):993–1002. doi: 10.1053/j.gastro.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee K., Tosti E., Edelmann W. Mouse models of DNA mismatch repair in cancer research. DNA Repair. 2016;38:140–146. doi: 10.1016/j.dnarep.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cruz-Correa M., Hylind L. M., Romans K. E., Booker S. V., Giardiello F. M. Long-term treatment with sulindac in familial adenomatous polyposis: A prospective cohort study. Gastroenterology. 2002;122(3):641–645. doi: 10.1053/gast.2002.31890. [DOI] [PubMed] [Google Scholar]
- 11.Mladenova D., Daniel J. J., Dahlstrom J. E., et al. The NSAID sulindac is chemopreventive in the mouse distal colon but carcinogenic in the proximal colon. Gut. 2011;60(3):350–360. doi: 10.1136/gut.2010.208314. [DOI] [PubMed] [Google Scholar]
- 12.Guda K., Upender M. B., Belinsky G., et al. Carcinogen-induced colon tumors in mice are chromosomally stable and are characterized by low-level microsatellite instability. Oncogene. 2004;23(21):3813–3821. doi: 10.1038/sj.onc.1207489. [DOI] [PubMed] [Google Scholar]
- 13.De Wind N., Dekker M., Van Rossum A., Van Der Valk M., Te Riele H. Mouse models for hereditary nonpolyposis colorectal cancer. Cancer Research. 1998;58(2):248–255. [PubMed] [Google Scholar]
- 14.Bacher J. W., Abdel Megid W. M., Kent-First M. G., Halberg R. B. Use of mononucleotide repeat markers for detection of microsatellite instability in mouse tumors. Molecular Carcinogenesis. 2005;44(4):285–292. doi: 10.1002/mc.20146. [DOI] [PubMed] [Google Scholar]
- 15.Kohonen-Corish M. R. J., Daniel J. J., Riele H. T., Buffinton G. D., Dahlstrom J. E. Susceptibility of Msh2-deficient mice to inflammation-associated colorectal tumors. Cancer Research. 2002;62(7):2092–2097. [PubMed] [Google Scholar]
- 16.Jacks T., Remington L., Williams B. O., et al. Tumor spectrum analysis in p53-mutant mice. Current Biology. 1994;4(1):1–7. doi: 10.1016/S0960-9822(00)00002-6. [DOI] [PubMed] [Google Scholar]
- 17.Shima K., Morikawa T., Yamauchi M., et al. TGFBR2 and BAX mononucleotide tract mutations, microsatellite instability, and prognosis in 1072 colorectal cancers. PLoS ONE. 2011;6(9) doi: 10.1371/journal.pone.0025062.e25062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mcilhatton M. A., Tyler J., Kerepesi L. A., et al. Aspirin and low-dose nitric oxide-donating aspirin increase life span in a Lynch syndrome mouse model. Cancer Prevention Research. 2011;4(5):684–693. doi: 10.1158/1940-6207.CAPR-10-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burn J., Gerdes A., Macrae F., et al. Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. The Lancet. 2011;378(9809):2081–2087. doi: 10.1016/S0140-6736(11)61049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McIlhatton M. A., Tyler J., Burkholder S., et al. Nitric oxide-donating aspirin derivatives suppress microsatellite instability in mismatch repair-deficient and hereditary nonpolyposis colorectal cancer cells. Cancer Research. 2007;67(22):10966–10975. doi: 10.1158/0008-5472.CAN-07-2562. [DOI] [PubMed] [Google Scholar]
- 21.Rüschoff J., Wallinger S., Dietmaier W., et al. Aspirin suppresses the mutator phenotype associated with hereditary nonpolyposis colorectal cancer by genetic selection. Proceedings of the National Acadamy of Sciences of the United States of America. 1998;95(19):11301–11306. doi: 10.1073/pnas.95.19.11301. [DOI] [PMC free article] [PubMed] [Google Scholar]