Abstract
Objective
To present a meta-analysis of high-quality case-matched studies comparing laparoscopic (LH) and open hepatectomy (OH) for hepatocellular carcinoma (HCC).
Methods
Studies published up to September 2017 comparing LH and OH for HCC were identified. Selection of high-quality, nonrandomized comparative studies (NRCTs) with case-matched design was based on a validated tool (Methodological Index for Nonrandomized Studies) since no randomized controlled trials (RCTs) were published. Morbidity, mortality, operation time, blood loss, hospital stay, margin distance, recurrence, and survival outcomes were compared. Subgroup analyses were carried out according to the surgical extension (minor or major hepatectomy).
Results
Twenty studies with a total of 830 patients (388 in LH and 442 in OH) were identified. For short-term surgical outcomes, LH showed less morbidity (RR = 0.55; 95% CI, 0.47~0.65; P < 0.01), less mortality (RR = 0.43; 95% CI, 0.18~1.00; P = 0.05), less blood loss (WMD = −93.21 ml, 95% CI, −157.33~−29.09 ml; P < 0.01), shorter hospital stay (WMD = −2.86, 95% CI, −3.63~−2.08; P < 0.01), and comparable operation time (WMD = 9.15 min; 95% CI: −7.61~25.90, P = 0.28). As to oncological outcomes, 5-year overall survival rate was slightly better in LH than OH (HR = 0.66, 95% CI: 0.52~0.84, P < 0.01), whereas the 5-year disease-free survival rate was comparable between two groups (HR = 0.88, 95% CI: 0.74~1.06, P = 0.18).
Conclusion
This meta-analysis has highlighted that LH can be safely performed in selective patients and improves surgical outcomes as compared to OH. Given the limitations of study design, especially the limited cases of major hepatectomy, methodologically high-quality comparative studies are needed for further evaluation.
1. Introduction
Although the incidence of hepatocellular carcinoma (HCC) has decreased, HCC is still the fifth most common malignancy and the third leading cause of cancer-related death worldwide [1]. Since laparoscopic hepatectomy (LH) was first reported in 1996 [2, 3], this treatment has been considered a landmark development in the progress of surgical treatment. However, the majority of HCC patients usually have cirrhosis and hypohepatia. Because of this, hepatectomy increases the risk of developing significant postoperative complications including ascites, hepatic failure, encephalopathy, and portal vein thrombosis [4]. There are some controversial aspects of LH for HCC including complications, postoperative recovery, and long-term survival outcomes.
During the last 6 years, a number of meta-analyses that compare LH with open hepatectomy (OH) for HCC have been published [5–8]. Although randomized controlled trials (RCTs) are the most ideal tools for meta-analysis, no RCTs on this topic have been yet conducted. These meta-analyses included the available nonrandomized comparative studies (NRCTs) to overcome the paucity of RCTs. Therefore unreliable results and little strong evidence had been presented. On the other hand, there was evidence that estimates derived from high-quality NRCTs may be similar to those derived from RCTs [9]. Also, when comparing surgical procedures, pooling of high-quality NRCTs could be as accurate as pooling of RCTs [10]. In addition, several comparative studies on this topic have been published in the last 3 years and none of the published meta-analyses included studies published after 2013. Therefore, we performed an updated meta-analysis evaluating all of the available high-quality published trials to compare LH with OH for HCC.
2. Methods
2.1. Systematic Literature Search
Systematic searches of PubMed, Embase, Cochrane Library, and Web of Science were performed to identify articles published up to September 2017. Searches included the terms “laparoscopic,” “minimally invasive,” “hepatectomy,” “liver resection,” “hepatocellular carcinoma,” and “HCC”. All eligible studies in English were retrieved, and their bibliographies were checked for potential relevant publications.
2.2. Eligibility Criteria and Quality Assessment
In order to reduce bias, our meta-analysis synthesized the existing observational studies while strictly limiting inclusion and exclusion criteria. First of all, papers containing any of the following were excluded: (1) studies that included malignant lesions other than HCC, (2) studies focusing on recurrent HCC, (3) studies that included cases of robotic-assisted hepatectomy. Secondly, only studies designed with case-matched analysis were further evaluated and nonmatched studies were excluded. Then, the methodological quality of the eligible nonrandomized comparative studies (NRCTs) was assessed by the Methodological Index for Nonrandomized Studies (MINORS) [11]. In total, 8 items were evaluated, with a maximum score of 16 points. Studies with 12 or more points were considered of high quality and were included in the meta-analysis. Those with less than 12 points were excluded. Besides, if there was overlap between authors or centers, only the higher-quality or more recent literature was selected.
2.3. Data Extraction and Quality Assessment
Two researchers evaluated all the titles and abstracts. Then they assessed the selected full-text articles for eligibility. This work was then reevaluated and confirmed by a senior researcher. The measured outcomes of all eligible publications can be divided into two categories: ① short-term outcomes (operation time, estimated blood loss, transfusion, length of hospital stay, morbidity, and mortality); ② oncological outcomes (tumor size, margin distance, R0 resection, recurrence, and survival). The postoperative morbidity was cataloged according to the Clavien-Dindo classification. Minor complication refers to Grade I and Grade II complications, and major complication includes Grade III to V complications.
2.4. Subgroup Analysis
Because the different levels of hepatectomy can lead to different outcomes, and major hepatectomy is a technically dependent and time-consuming procedure, subgroup analyses were carried out according to surgical extensions. Included studies were assigned to 3 subgroups: minor hepatectomy, mixed hepatectomy, and major hepatectomy.
2.5. Statistical Analysis
The risk ratio (RR) was utilized to analyze the dichotomous variables, and the weighted mean difference (WMD) was utilized to assess the continuous variables. If the study provided medians and ranges instead of means and standard deviations (SDs), we estimated the means and SDs as described by Hozo et al. [12]. Heterogeneity was evaluated by Cochran's Q statistic and Higgins I2 statistic [13]. If data was not significantly heterogeneous (P > 0.05 or I2 < 50%), the pooled effects were calculated using a fixed model. Otherwise, the pooled effects were calculated using a random model. The hazard ratios (HRs) of a 5-year overall survival rate (OS) and a 5-year disease-free survival rate (DFS) were used with a generic inverse variance meta-analysis. The log HR and its SE were estimated using the method introduced by Tierney et al. [14]. According to the overall morbidity, potential publication bias was determined by carrying out an informal visual inspection of funnel plots. A two-tailed value of P < 0.05 was considered significant. All statistical tests were performed with Review Manager version 5.1 (The Cochrane Collaboration, Oxford, England).
3. Results
3.1. Search Results and Baseline Characteristics
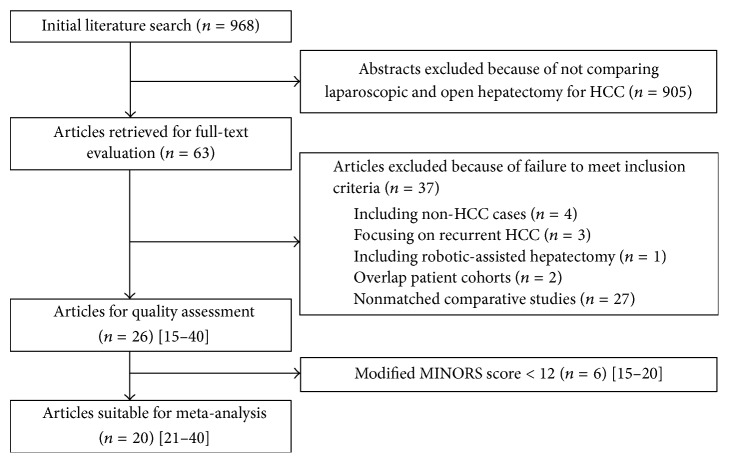
The last search was performed on September 20, 2017. A total of 968 potential published articles were initially identified from the search. Of these, 63 articles were selected based on their titles and abstracts, and a full examination of the texts was performed. Further 37 papers were excluded, after being read thoroughly, due to (1) including non-HCC cases (n = 4), (2) focusing on recurrent HCC (n = 3), (3) robot-assisted hepatectomy (n = 1), (4) overlap patient cohorts (n = 2), or (5) nonmatched comparative studies (n = 27). Then 26 studies were selected for quality assessment, and 6 studies were excluded by a modified MINORS score < 12 [15–20]. Finally, 20 studies were selected for final meta-analysis [21–40]. A flow chart of the search strategies, which contains reasons for excluding studies, is elucidated in Figure 1. The details of the selection process, which included the references of excluded studies and the MINORS assessments of low-quality studies, could be found in Supplementary Materials (available here).
Figure 1.
Flow chart of literature search strategies.
3.2. Study Characteristics
A total of 830 patients were included in the analysis with 388 undergoing LH (46.8%) and 442 undergoing OH (53.2%). The characteristics of these included studies are summarized in Table 1. Studies were well matched in terms of age, gender, ASA classification, body mass index (BMI), tumor size, and surgical extension. Eight studies reported only minor hepatectomy, and three studies focused on major hepatectomy, whereas the remaining nine studies included both minor and major hepatectomy. The majority of studies graded morbidity according to the Clavien-Dindo classification, with the study by Lee et al. being the only exception [24]. The assessments of the NRCTs are illustrated in Table 2. Each trial received more than 12 points (the maximum possible score is 16) and was considered to be of the highest quality (see Supplementary Materials).
Table 1.
Basic information of the included literature.
| Author | Region | Year | Study period | Sample size | Matching method | Cirrhosis (%) | Surgical extension | Conversion (%) | Clavien-Dindo | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| LH | OH | LH | OH | ||||||||
| Belli et al. | Italy | 2007 | 2000–2004 | 23 | 23 | CCM | 100 | 100 | Minor | 4.3 | Yes |
| Tranchart et al. | France | 2010 | 1999–2008 | 42 | 42 | CCM | 73.8 | 81 | Mixed | 4.8 | Yes |
| Kim et al. | Korea | 2011 | 2005–2009 | 26 | 29 | CCM | 92.3 | 86.2 | Mixed | E | Yes |
| Truant et al. | France | 2011 | 2002–2009 | 36 | 53 | CCM | 100 | 100 | Minor | 19.4 | Yes |
| Lee et al. | Hong Kong | 2011 | 2004–2010 | 33 | 50 | CCM | 84.8 | 64 | Minor | 18.2 | NA |
| Ahn et al. | Korea | 2014 | 2005–2013 | 51 | 51 | PSM | 68.6 | 66.8 | Mixed | 9.8 | Yes |
| Kim et al. | Korea | 2014 | 2000–2012 | 29 | 29 | PSM | 62.1 | 65.5 | Minor | E | Yes |
| Memeo et al. | France | 2014 | 1990–2009 | 45 | 45 | CCM | 100 | 100 | Minor | NA | Yes |
| Lau et al. | USA | 2015 | 2008–2014 | 26 | 26 | CCM | 80.8 | 73.1 | Mixed | 35 | Yes |
| Lee et al. | Canada | 2015 | 2006–2013 | 43 | 86 | CCM | NA | NA | Mixed | 14 | Yes |
| Luo et al. | China | 2015 | 2008–2015 | 53 | 53 | CCM | 100 | 100 | Minor | E | Yes |
| Takahara et al. | Japan | 2015 | 2000–2010 | 387 | 387 | PSM | 61.7 | 59.6 | Mixed | 6.5 | Yes |
| Han et al. | Korea | 2015 | 2004–2013 | 88 | 88 | PSM | 62.5 | 59.1 | Mixed | 9.1 | Yes |
| Yoon et al. | Korea | 2015 | 2007–2011 | 58 | 174 | PSM | NA | NA | Mixed | 0 | Yes |
| Cheung et al. | Hong Kong | 2016 | 2002–2015 | 110 | 330 | PSM | 100 | 100 | Mixed | 5.5 | Yes |
| Sposito et al. | Italy | 2016 | 2006–2013 | 43 | 43 | PSM | 100 | 100 | Minor | 4.7 | Yes |
| Jiang et al. | China | 2016 | 2008–2013 | 59 | 59 | PSM | 100 | 100 | Minor | 5.1 | Yes |
| Komatsu et al. | France | 2016 | 2000–2014 | 38 | 38 | CCM | NA | NA | Major | 31.6 | Yes |
| Yoon et al. | Korea | 2017 | 2008–2015 | 33 | 33 | PSM | 100 | 100 | Major | NA | Yes |
| Xu et al. | China | 2017 | 2015–2017 | 32 | 32 | PSM | 100 | 100 | Major | NA | Yes |
CCM: case by case matching; PSM: propensity score matching; E: conversion cases were excluded from the studies; NA: not available.
Table 2.
Modified MINORS score of all eligible nonrandomized comparative studies.
| Author | ① | ② | ③ | ④ | ⑤ | ⑥ | ⑦ | ⑧ | Score |
|---|---|---|---|---|---|---|---|---|---|
| Belli et al. | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 1 | 13 |
| Tranchart et al. | 2 | 2 | 1 | 1 | 2 | 1 | 2 | 2 | 13 |
| Kim et al. | 2 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 12 |
| Truant et al. | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 1 | 12 |
| Lee et al. | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 1 | 12 |
| Ahn et al. | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 2 | 14 |
| Kim et al. | 2 | 1 | 1 | 2 | 2 | 2 | 1 | 1 | 12 |
| Memeo et al. | 2 | 1 | 1 | 1 | 2 | 1 | 2 | 2 | 12 |
| Lau et al. | 2 | 1 | 2 | 1 | 2 | 2 | 2 | 1 | 13 |
| Lee et al. | 2 | 1 | 1 | 1 | 2 | 2 | 1 | 2 | 12 |
| Luo et al. | 2 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 13 |
| Takahara et al. | 2 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 13 |
| Han et al. | 2 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 13 |
| Yoon et al. | 2 | 1 | 1 | 1 | 2 | 2 | 1 | 2 | 12 |
| Cheung et al. | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 2 | 14 |
| Sposito et al. | 2 | 2 | 1 | 1 | 2 | 2 | 2 | 2 | 14 |
| Jiang et al. | 2 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 13 |
| Komatsu et al. | 2 | 1 | 1 | 1 | 2 | 2 | 2 | 1 | 12 |
| Yoon et al. | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 14 |
| Xu et al. | 2 | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 14 |
① Consecutive patients, ② prospective data collection, ③ reported endpoints, ④ unbiased outcome evaluation, ⑤ appropriate controls, ⑥ contemporary groups, ⑦ groups equivalent, ⑧ sample size.
3.3. Meta-Analysis of Short-Term Outcomes
3.3.1. Operation Time
Operative time was reported in all studies [21–40]. Statistically significant between-study heterogeneity was identified in all subgroups (P < 0.01, I2 = 87.2%). There was no significant difference between the groups' operation times (Table 3). However, in the subgroup of major hepatectomy, the overall effect size of the mean operation time was significantly longer in LH than that in OH (WMD = 77.93 min, 95% CI: 40.45~115.41, P < 0.01).
Table 3.
Overall outcomes of the meta-analysis.
| Outcomes | Studies No. | Sample size | Heterogeneity (P, I2) |
Model | Overall effect size | 95% CI of overall effect | P | |
|---|---|---|---|---|---|---|---|---|
| LH | OH | |||||||
| Operation time (min) | 20 | 1255 | 1671 | <0.01, 87% | R | WMD = 9.15 | −7.61~25.90 | 0.28 |
| Minor hepatectomy | 8 | 321 | 355 | <0.01, 78% | R | WMD = 12.04 | −5.31~29.39 | 0.17 |
| Mixed hepatectomy | 9 | 831 | 1213 | <0.01, 83% | R | WMD = −14.28 | −40.76~12.21 | 0.29 |
| Major hepatectomy | 3 | 103 | 103 | 0.05, 67% | R | WMD = 77.93 | 40.45~115.41 | <0.01 |
| Blood loss (mL) | 17 | 1128 | 1425 | <0.01, 92% | R | WMD = −93.21 | −157.33~−29.09 | <0.01 |
| Minor hepatectomy | 7 | 278 | 312 | 0.39, 5% | R | WMD = −76.21 | −98.41~−54.01 | <0.01 |
| Mixed hepatectomy | 7 | 747 | 1010 | 0.05, 52% | R | WMD = −212.94 | −294.57~−131.31 | <0.01 |
| Major hepatectomy | 3 | 103 | 103 | 0.88, 0% | R | WMD = 3.75 | −60.16~67.65 | 0.88 |
| Transfusion | 14 | 979 | 1352 | 0.90, 0% | F | RR = 0.73 | 0.55~0.96 | 0.03 |
| Minor hepatectomy | 4 | 121 | 155 | 0.49, 0% | F | RR = 0.53 | 0.19~1.45 | 0.22 |
| Mixed hepatectomy | 8 | 788 | 1127 | 0.86, 0% | F | RR = 0.75 | 0.55~1.01 | 0.06 |
| Major hepatectomy | 2 | 70 | 70 | 0.28, 15% | F | RR = 0.75 | 0.17~3.25 | 0.70 |
| Hospital stay (days) | 20 | 1255 | 1671 | <0.01, 80% | R | WMD = −2.86 | −3.63~−2.08 | <0.01 |
| Minor hepatectomy | 8 | 321 | 355 | <0.01, 76% | R | WMD = −2.93 | −4.23~−1.63 | <0.01 |
| Mixed hepatectomy | 9 | 831 | 1213 | 0.01, 58% | R | WMD = −2.85 | −3.95~−1.76 | <0.01 |
| Major hepatectomy | 3 | 103 | 103 | 0.15, 47% | R | WMD = −2.76 | −4.60~−0.92 | <0.01 |
| Morbidity | 20 | 1255 | 1671 | 0.28, 14% | F | RR = 0.55 | 0.47~0.65 | <0.01 |
| Minor hepatectomy | 8 | 321 | 355 | 0.29, 17% | F | RR = 0.53 | 0.41~0.69 | <0.01 |
| Mixed hepatectomy | 9 | 831 | 1213 | 0.26, 21% | F | RR = 0.57 | 0.46~0.72 | <0.01 |
| Major hepatectomy | 3 | 103 | 103 | 0.21, 37% | F | RR = 0.55 | 0.36~0.83 | <0.01 |
| Severe complications | 18 | 1196 | 1476 | 0.88, 0% | F | RR = 0.51 | 0.39~0.68 | <0.01 |
| Minor hepatectomy | 7 | 288 | 305 | 0.96, 0% | F | RR = 0.48 | 0.24~0.96 | 0.04 |
| Mixed hepatectomy | 8 | 805 | 1068 | 0.44, 0% | F | RR = 0.54 | 0.38~0.76 | <0.01 |
| Major hepatectomy | 3 | 103 | 103 | 0.35, 6% | F | RR = 0.42 | 0.18~1.00 | 0.05 |
| Mortality | 9 | 789 | 1026 | 0.88, 0 | F | RR = 0.43 | 0.18~1.00 | 0.05 |
| Minor hepatectomy | 3 | 104 | 121 | 0.34, 7 | F | RR = 0.39 | 0.09~1.68 | 0.21 |
| Mixed hepatectomy | 5 | 653 | 873 | 0.84, 0 | F | RR = 0.46 | 0.15~1.43 | 0.18 |
| Major hepatectomy | 1 | 32 | 32 | Not applicable | F | RR = 0.33 | 0.01~7.89 | 0.50 |
| Tumor size (cm) | 19 | 1229 | 1645 | <0.01, 57% | R | WMD = −0.19 | −0.41~0.03 | 0.09 |
| Minor hepatectomy | 8 | 321 | 355 | 0.76, 0% | R | WMD = −0.07 | −0.26~0.12 | 0.48 |
| Mixed hepatectomy | 8 | 805 | 1187 | 0.50, 0% | R | WMD = −0.09 | −0.25~0.07 | 0.28 |
| Major hepatectomy | 3 | 103 | 103 | <0.01, 92% | R | WMD = −1.77 | −4.06~0.53 | 0.13 |
| Margin distance (cm) | 11 | 501 | 694 | 0.14, 47% | R | WMD = 2.61 | 1.06~4.17 | <0.01 |
| Minor hepatectomy | 5 | 186 | 220 | 0.06, 56% | R | WMD = 2.16 | 0.15~4.17 | 0.03 |
| Mixed hepatectomy | 5 | 282 | 441 | 0.15, 41% | R | WMD = 3.20 | 0.41~5.99 | 0.02 |
| Major hepatectomy | 1 | 33 | 33 | Not applicable | R | WMD = 5.90 | −2.69~14.49 | 0.18 |
| R0 resection | 14 | 1010 | 1409 | 0.70, 0% | F | RR = 1.01 | 0.99~1.02 | 0.37 |
| Minor hepatectomy | 6 | 240 | 257 | 0.80, 0% | F | RR = 0.98 | 0.95~1.01 | 0.23 |
| Mixed hepatectomy | 7 | 738 | 1120 | 0.47, 0% | F | RR = 1.01 | 1.00~1.03 | 0.13 |
| Major hepatectomy | 1 | 32 | 32 | Not applicable | F | RR = 1.03 | 0.93~1.15 | 0.56 |
WMD: weighted mean difference; RR: risk ratio; F: fixed; R: random.
3.3.2. Intraoperative Blood Loss
Blood loss was available from 17 studies [21, 22, 24–34, 36, 37, 39, 40]. Statistically significant between-study heterogeneity was identified in all subgroups (P < 0.01, I2 = 88.1%). The pooled results showed that LH was associated with less blood loss than OH (Table 3). However, in the subgroup of major hepatectomy, there was no significant difference between groups (WMD = 3.75 ml, 95% CI: −60.16~67.65, P = 0.88).
3.3.3. Blood Transfusion
Fourteen studies recorded perioperative blood transfusion [21–27, 29, 30, 33–35, 37, 39]. There was no evidence of heterogeneity between subgroups (P = 0.81, I2 = 0%). Although none of the subgroups reached a significant difference, the overall pooled data indicated that transfusion rates were lower in LH (RR = 0.73, 95% CI: 0.55~0.96, P = 0.03) (Table 3).
3.3.4. Duration of Hospital Stay
The length of hospital stays was pooled for all studies [21–40]. Although statistically significant between-study heterogeneity was identified in each subgroup, there was no evidence of heterogeneity between subgroups (P = 0.99, I2 = 0%). Hospital stays in LH group were shorter than those in OH group (WMD = −2.86 d, 95% CI: −3.63~−2.08, P < 0.01) (Table 3).
3.3.5. Morbidity
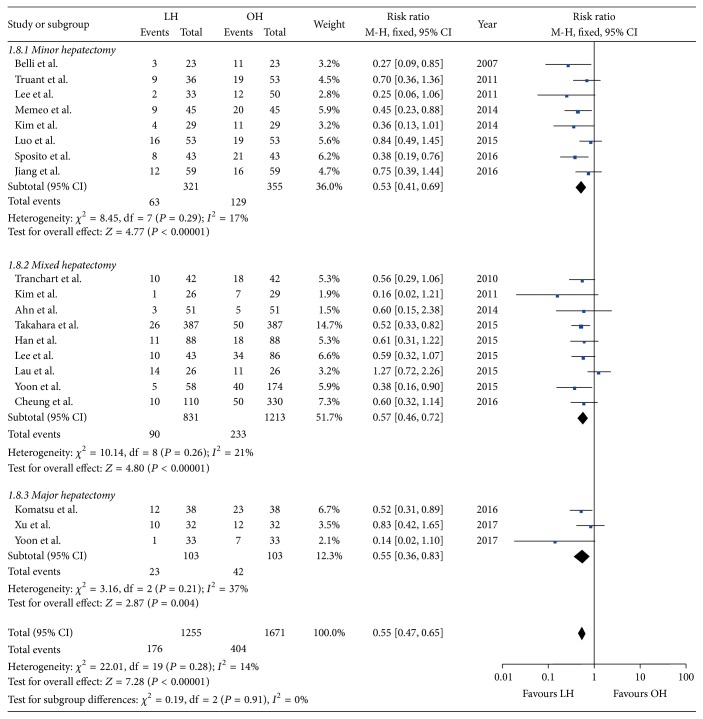
All studies reported their overall complication rates [21–40]. Because there was no statistical evidence of heterogeneity, the effect sizes of all subgroups were synthesized to generate the overall effect size (P = 0.91, I2 = 0%) (Table 3) (Figure 2). The postoperative morbidity rates were 14.0% (176/1255) in LH and 24.2% (404/1671) in OH. In addition the pooled data showed that LH significantly reduced postoperative complications (RR = 0.55; 95% CI, 0.47~0.65; P < 0.01) (Table 3). Moreover, each subgroup also revealed reduced overall morbidity in the LH group (Table 3).
Figure 2.
Forest plot of overall morbidity.
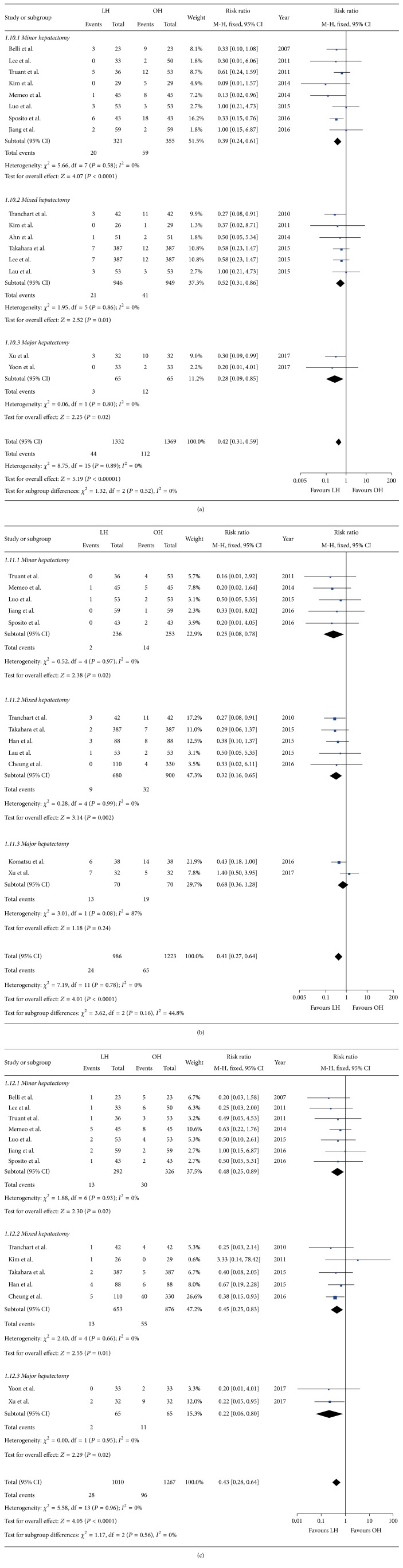
Eighteen studies recorded severe complications [21, 22, 25–40]. Similar to overall morbidity, the results showed that patients in the LH group suffered less severe complications (Table 3). We identified specified complications of ascites, liver failure, and the respiratory system. The results implied that postoperative ascites in patients, regardless of whether they underwent minor or major hepatectomy, was less in LH than in OH (RR = 0.42; 95% CI, 0.31~0.59; P < 0.01) (Figure 3(a)). Studies that recorded postoperative liver failure reported a lower incidence of liver failure in LH than in OH with one exception by Xu et al. [39]. The overall pooled data revealed that patients in the LH group were less likely to suffer liver failure than those in the OH group (RR = 0.41; 95% CI, 0.27~0.64; P < 0.01) (Figure 3(b)). LH was also associated with a significant reduction in respiratory complications regardless of different surgical extension (RR = 0.43, 95% CI: 0.28~0.64, P < 0.01) (Figure 3(c)).
Figure 3.
Forest plot of specific complications: (a) ascites, (b) liver failure, (c) respiratory complications.
3.3.6. Mortality
Nine studies recorded cases of postoperative death [21, 22, 25, 28–30, 33, 35, 39]. There was no evidence of heterogeneity between subgroups (P = 0.97, I2 = 0%). These studies showed very low incidences of mortality. However, the overall pooled data indicated a more reduced postoperative mortality in LH than that in OH (RR = 0.43; 95% CI, 0.18~1.00; P = 0.05) (Table 3).
3.4. Meta-Analysis of Oncological Outcomes
3.4.1. Tumor Size
Only one study did not report tumor size [30]. There was trifling heterogeneity between subgroups, mainly due to the major hepatectomy subgroup (P = 0.35, I2 = 4.2%) (Table 3). Meta-analysis showed that the tumor size of OH was longer than that of LH with a marginal difference (WMD = −0.19 cm; 95% CI: −0.41~−0.03, P = 0.09), which was mainly due to smaller tumors in LH than those in OH in the major hepatectomy subgroup (Table 3).
3.4.2. Margin Distance
Only 11 studies mentioned the distance of the tumor margin [22, 24–29, 31, 34, 38, 40]. Although statistical significant between-study heterogeneity was identified in each subgroup, there was no evidence of heterogeneity between subgroups (P = 0.63, I2 = 0%). On pooling the results, the margin distance was longer in the LH group than that in the OH group (WMD = 2.61 cm; 95% CI: 1.06~4.17, P < 0.01) (Table 3).
3.4.3. R0 Resection
The R0 resection was reported in 14 studies [21, 23, 24, 27, 29–36, 38, 39]. There was no obvious heterogeneity (P = 0.18, I2 = 41.8%). The pooled estimate for margin distance indicated comparative outcomes between groups (RR = 1.01, 95% CI: 0.99~1.02, P = 0.37) (Table 3).
3.4.4. Overall Survival Rate and Disease-Free Survival Rate
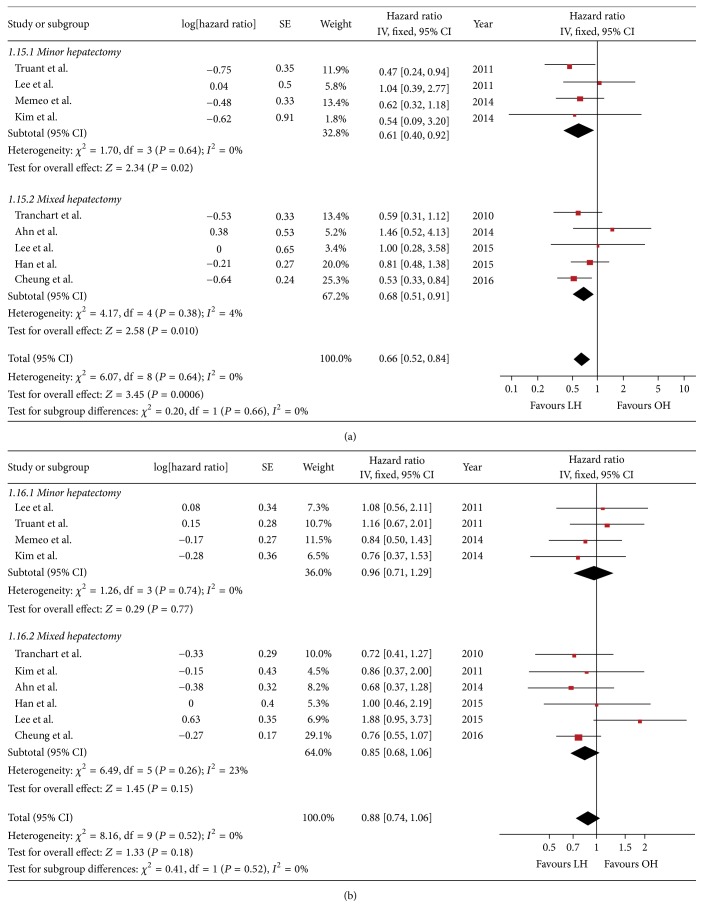
Summary of follow-up time, recurrence, and long-term survival rates is listed in Table 4. Nineteen studies reported the detailed long-term outcomes. Among them, the data for 5-year OS rates can be extracted from nine studies and the data for 5-year DFS rates can be extracted from ten studies. The follow-up periods in six studies were less than five years. The survival data of three studies cannot be extracted due to a technical problem with figures. Unfortunately, none of the three major hepatectomy studies can be included in our survival analysis [37, 39, 40]. In all, the pooled 5-year OS rate was slightly better in LH than in OH (HR = 0.66, 95% CI: 0.52~0.84, P < 0.01) (Figure 4(a)). The 5-year DFS rate was comparable between groups (HR = 0.88, 95% CI: 0.74~1.06, P = 0.18) (Figure 4(b)).
Table 4.
Summary of recurrence and long-term survival.
| Author | Group | Follow-up | R | Survival (time: month; rate: %) |
|---|---|---|---|---|
| Tranchart et al. | LH | 29.7 | 10 | 1, 3, 5 y-DFS: 81.6, 60.9, 45.6; 1, 3, 5 y-OS: 93.1, 74.4, 59.5. |
| OH | 24.6 | 12 | 1, 3, 5 y-DFS: 70.2, 54.3, 37.2; 1, 3, 5 y-OS: 81.8, 73, 47.4. | |
| Kim et al. | LH | 21.8 | 7 | MDFS: 13.4; 1 y-DFS: 84.6. |
| OH | 24.8 | 10 | MDFS: 14.6; 1 y-DFS: 82.8. | |
| Truant et al. | LH | 35.7 | 16 | 5 y-DFS: 35.5; 5 y-OS: 70. |
| OH | 23 | 5 y-DFS: 33.6; 5 y-OS: 46. | ||
| Lee et al. | LH | 35.4 | 15 | 1, 3, 5 y-DFS: 78.8, 51, 45.3; 1, 3, 5 y-OS: 86.9, 81.8, 76.0. |
| OH | 28.5 | 19 | 1, 3, 5 y-DFS: 69.2, 55.9, 55.9; 1, 3, 5 y-OS: 98, 80.6, 76.1. | |
| Ahn et al. | LH | 38.6 | 12 | 5 y-DFS: 67.8; 5 y-OS: 80.1. |
| OH | 52.3 | 21 | 5 y-DFS: 54.8; 5 y-OS: 85.7. | |
| Kim et al. | LH | 47.9 | 11 | MDFS: 15.4; MOS: 47.9; 1, 3, 5 y-DFS: 81.1, 61.7, 54.0; 1, 3, 5 y-OS: 100, 100, 92.2. |
| OH | 59.5 | 16 | MDFS: 32.6; MOS: 59.5; 1, 3, 5 y-DFS: 78.6, 60.9, 40.1; 1, 3, 5 y-OS: 96.5, 92.2, 87.7. | |
| Memeo et al. | LH | NR | 25 | 1, 5, 10 y-DFS: 80, 19, 0; 1, 5, 10 y-OS: 88, 59, 12. |
| OH | NR | 28 | 1, 5, 10 y-DFS: 60, 23, 9; 1, 5, 10 y-OS: 63, 44, 22. | |
| Lee et al. | LH | 22.7 | NR | 1, 3, 5 y-DFS: 60.5, 53.5, 53.5; 1, 3, 5 y-OS: 95.3, 89.7, 89.7. |
| OH | 44.4 | NR | 1, 3, 5 y-DFS: 81.5, 66.7, 58.6; 1, 3, 5 y-OS: 93.9, 89.5, 87.3. | |
| Luo et al. | LH | 35 | 20 | MDFS: 21. |
| OH | 37 | 24 | MDFS: 18. | |
| Takahara et al. | LH | 46.7 | NR | 1, 3, 5 y-DFS: 83.7, 58.3, 40.7; 1, 3, 5 y-OS: 95.8, 86.2, 76.8. |
| OH | 51.7 | NR | 1, 3, 5 y-DFS: 79.6, 50.4, 39.3; 1, 3, 5 y-OS: 95.8, 84.0, 70.9. | |
| Han et al. | LH | 44.0 | 43 | 1, 3, 5 y-DFS: 69.7, 52.0, 44.2; 1, 3, 5 y-OS: 91.6, 87.5, 76.4. |
| OH | 48.7 | 46 | 1, 3, 5 y-DFS: 74.7, 49.5, 41.2; 1, 3, 5 y-OS: 93.1, 87.8, 73.2. | |
| Yoon et al. | LH | NR | 16 | 1, 2, 3, 4 y-DFS: 82.0, 63.0, 56.0, 56.0; 1, 2, 3, 4 y-OS: 95.0, 92.0, 86.0, 86.0. |
| OH | NR | 31 | 1, 2, 3, 4 y-DFS: 88.0, 79.0, 62.0, 62.0; 1, 2, 3, 4 y-OS: 98.0, 93.0, 84.0, 68.0. | |
| Cheung et al. | LH | 34.6 | 36 | MDFS: 66.4; MOS: 136; 1, 3, 5 y-DFS: 87.7, 65.8, 52.2; 1, 3, 5 y-OS: 98.9, 89.8, 83.7. |
| OH | 46.6 | 160 | MDFS: 52.4; MOS: 120; 1, 3, 5 y-DFS: 75.2, 56.3, 47.9; 1, 3, 5 y-OS: 94, 79.3, and 67.4. | |
| Sposito et al. | LH | 39.3 | NR | MDFS: 25.5; MOS: 48.8; 3, 5 y-DFS: 41, 25; 3, 5 y-OS: 75, 38. |
| OH | 44.5 | NR | MDFS: 31.7; MOS: 57.8; 3, 5 y-DFS: 44, 11; 3, 5 y-OS: 79, 46. | |
| Jiang et al. | LH | NR | 26 | MDFS: 17; 5 y-DFS: 44. |
| OH | NR | 30 | MDFS: 15; 5 y-DFS: 40. | |
| Komatsu et al. | LH | 24.7 | NR | 3 y-DFS: 50.3; 3 y-OS: 73.4. |
| OH | NR | 3 y-DFS: 29.7; 3 y-OS: 69.2. | ||
| Yoon et al. | LH | NR | NR | 2 y-DFS: 85.1; 2 y-OS: 100. |
| OH | NR | NR | 2 y-DFS: 83.9; 2 y-OS: 88.8. | |
| Xu et al. | LH | 13.8 | NR | 1, 2 y-DFS: 95.5, 72.9; 1, 2 y-OS: 100, 85.7. |
| OH | NR | 1, 2 y-DFS: 93.5, 81.5; 1, 2 y-OS: 96.3, 86.7. |
Follow-up was shown as median month; R: recurrence; DFS: disease-free survival rate; OS: overall survival rate; MDFS: median disease-free survival time; MOS: median overall survival time; y: year; NR: not reported.
Figure 4.
Forest plot of survival rate: (a) 5-year OS, (b) 5-year DFS.
3.4.5. Publication Bias
The study by Lau et al. was outside the funnel [30], and the remaining representative plots were distributed symmetrically. We believed such publication bias was acceptable in the studies (Figure 5).
Figure 5.
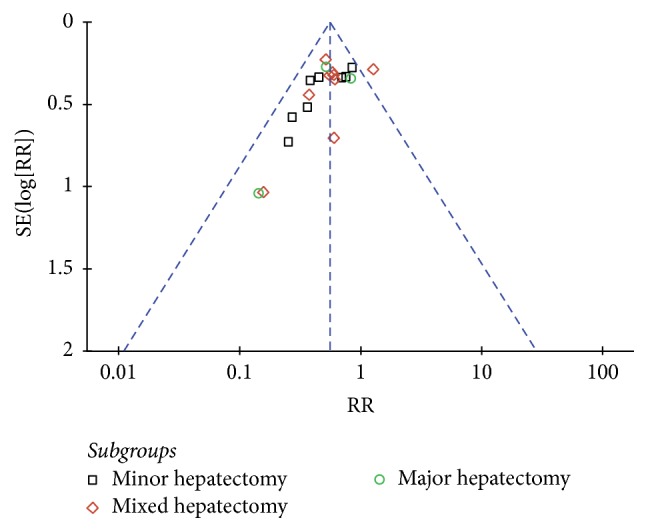
Funnel plot of the overall postoperative morbidity.
4. Discussion
This meta-analysis selected and summarized high-quality literature that compared the short- and long-term outcomes of LH and OH for the treatment of HCC. All of the studies had case-matched design and were of high quality according to the modified MINORS scale. For short-term surgical outcomes, LH exhibited advantages in terms of blood loss, hospital stay, overall postoperative morbidity, and mortality, whereas no statistically significant differences were identified regarding operation time. As for oncological outcomes, R0 and survival rates of LH were also not inferior to OH.
To date, there have been several meta-analyses comparing LH to OH for HCC [5–8]. The results have demonstrated that LH is comparable to OH regarding the operation time and postoperative mortality and is associated with less blood loss, as well as a shorter hospital stay (Table 5). Previous meta-analyses included all available research [5, 6, 8] but had some limitations. Pooling of low-quality studies could undermine the strength of results, whereas selectively pooling high-quality NRCTs could strengthen the power of results [10]. Patients' characteristics and surgical extension have a major impact on the surgical outcomes of hepatectomy. Previous meta-analyses pooled studies, which did not balance the combined factors of tumor size, location, the severity of cirrhosis, and other underlying liver diseases between LH and OH. These factors would have influenced the decision of surgeons and patients and further influenced the major factors of both short- and long-term outcomes. In addition, previous meta-analyses studied LH confined to minor resection. With the accumulation of surgical techniques, major resection of LH has become more commonly performed, but various efficacy and safety concerns for the procedure are warranted. Furthermore, since the publication of previous meta-analyses, several notable clinical observational studies have become available and some of them are from China, where HCC has the highest prevalence in the world [8, 19]. Therefore, our comprehensive meta-analysis will contribute to a more systematic and objective evaluation of the safety and HCC treatment of LH.
Table 5.
Previous meta-analyses comparing LH to OH for HCC.
| Variables | Zhou | Li | Xiong | Yin |
|---|---|---|---|---|
| Year | 2011 | 2012 | 2012 | 2013 |
| Included studies | 10 | 10 | 9 | 15 |
| Total LH numbers | 213 | 244 | 234 | 485 |
| Surgical extension | Minor resection | Minor resection | Minor resection | Minor resection |
| Operation time | NS | NS | NS | NS |
| Blood loss | Favor LH | Favor LH | Favor LH | Favor LH |
| Overall morbidity | Favor LH | Favor LH | N/A | Favor LH |
| Severe complications | N/A | N/A | N/A | N/A |
| ascites | N/A | N/A | Favor LH | N/A |
| Liver failure | NS | N/A | Favor LH | N/A |
| Respiratory complications | NS | N/A | NS | N/A |
| Mortality | NS | N/A | NS | N/A |
| Hospital stay | Favor LH | Favor LH | Favor LH | Favor LH |
| Tumor size | N/A | N/A | N/A | N/A |
| Margin distance | NS | NS | N/A | NS |
| R0 resection | N/A | NS | NS | NS |
| Survival | N/A | N/A | N/A | NS |
NS: not significant, N/A: not available.
Several previous studies have demonstrated that LH can be feasible and beneficial for minor resections or nonanatomical resections of peripheral HCC. This is in accordance with our study that showed minor resection of LH with similar operation time and less blood loss than OH. However, minor hepatectomy is insufficient for large lesions or those located in posterosuperior liver segments to ensure an adequate resection margin and eliminate intrahepatic recurrence. Major hepatectomy is more frequently performed with a curative intent for multifocal or large size HCC or those with a high propensity to invade the portal vein branches [41–43]. Laparoscopic major hepatectomy is, because of the same steps and principles used in laparotomy, technically demanding. Mobilization of a heavy as well as fragile organ, excisions of bulky parenchyma, and major vascular dissection with its associated risk of major vessel injury are all considered risky under laparoscopy. As expected, the present study revealed longer operation times in laparoscopic major hepatectomy. Furthermore, unlike minor resections, the blood loss of laparoscopic major hepatectomy was not superior to its open counterpart.
Patients with HCC and concurrent cirrhosis tend to have higher incidences of postoperative complications and of greater severity. Therefore, the decreased complications in the LH group should be our most striking finding. In detail, postoperative ascites and liver failure tend to decrease in LH. Postoperative decompensation after hepatectomy occurs more frequently in patients with liver cirrhosis or portal hypertension, even for limited resections. The minimization of surgical incision and the subsequent preservation of abdominal wall circulation and lymphatic flow can explain fewer ascites and liver failure in LH. Moreover, a small incision limits the evacuation of ascites through the wall and decreases the risk of infection, thus facilitating wound healing. Laparoscopic surgery also decreases the manipulation of abdominal organs and exposure of bowels, which will also contribute to reduced ascites. Since refractory ascites and progressive liver insufficiency are major causes of severe postoperative morbidities, reduced severe postoperative morbidities and mortality could be expected. Major surgery was often thought to be unsuitable for those with severely impaired pulmonary function due to a higher risk of postoperative respiratory complications. Hepatectomy involving multiple systems, especially the water and electrolyte balance, is a major risk factor for medical complications. It was observed from the reviewed studies that respiratory complications were the most common medical complications, mainly pulmonary infection, followed by cardiovascular complications. Improved preservation of liver functions in LH maintains enough albumin synthesis and decreases the pleural effusion. The pain caused by large incisions, as well as the use of tension sutures and abdominal bandages after laparotomy, can make it difficult for patients to cough. Earlier postoperative ambulation in the laparoscopic group also helped to reduce respiratory complications and promote the postoperative recovery of gastrointestinal function. In accordance with other laparoscopic surgeries, LH achieved enhanced postoperative recovery. The postoperative hospitalization of LH decreases by more than two days. This can be explained by the milder surgical trauma of LH and subsequent faster bowel recovery. Less postoperative morbidities also contribute to shorter length of hospitalization.
The oncologic results of LH for HCC remain a matter of debate. Adequate surgical margins independently improve the long-term oncological outcomes. Our analysis showed that LH could achieve enough surgical margins (more than 2 cm) as OH. The 5-year OS and DFS also showed that LH was comparable to OH. However, the results warrant prudent interpretation because of the discrepancies among the pooled studies, such as tumor size, tumor number, and status of the vascular invasion. Other biases lie in other factors including preoperative TACE and postoperative adjuvant therapies. Unfortunately, none of the three major hepatectomy studies can be included in our survival analysis. Thus, well-designed RCTs, that balance all potential factors, preferably containing major resection are needed to confirm our results.
In the process of our research and manuscript review, two similar articles by Sotiropoulos et al. were published [44, 45], which also had limitations. Examples include pooling the low-quality studies together, failing to evaluate extension on surgical outcomes, and one paper only investigating studies conducted in Europe [45]. Besides, since these studies were published, several clinical observational studies have become available. Therefore, our comprehensive meta-analysis will contribute to a more systematic and objective evaluation of this subject.
The major limitation of this study was that all included studies are NRCTs and of retrospective design. NRCTs have potential biases that limit an unequivocal conclusion, even though we exclusively included the case-matched studies to minimize the selection biases. Another limitation is the lack of studies on laparoscopic major hepatectomy. The analysis was based on only three pooled studies. Little is known about how these results would hold for a larger sample size, which is particularly important as a fair number of patients with HCC are treated with open major hepatectomy. In addition, data from several studies are extracted using the methods reported by Hozo et al. and Tierney et al., which are not completely accurate and result in bias. Moreover, it is quite possible that surgical teams undertaking research and publishing their results are more experienced and more skillful than others. Publication bias was inevitable since one plot was outside the funnel. The bias would be overcome only with the collection of more reports.
5. Conclusions
This meta-analysis has highlighted that LH can be safely performed in select patients and improves surgical outcomes when compared to OH. The data indicate that laparoscopic minor hepatectomy is acceptable with less blood loss, less postoperative morbidity, shorter hospitalization, and comparable operation times and oncological outcomes. The role of laparoscopic major hepatectomy is promising in terms of decreasing postoperative morbidity and recovery, but the technique also has drawbacks in prolonged operation time. Given the heterogeneity of the patient groups, the limitations of study design, and the small sample size, it is likely that patients have potential to benefit from LH, but further well-designed studies are needed to accurately select them.
Abbreviations
- LH:
Laparoscopic hepatectomy
- OH:
Open hepatectomy
- HCC:
Hepatocellular carcinoma
- NRCT:
Nonrandomized comparative study
- RCT:
Randomized controlled trial
- MINORS:
Methodological Index for Nonrandomized Studies
- RR:
Risk ratio
- WMD:
Weighted mean difference
- SD:
Standard deviation
- OS:
Overall survival rate
- DFS:
Disease-free survival rate
- HR:
Hazard ratio.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Ke Chen designed the study; Yu Pan and Xiao-long Liu collected literature and conducted the analysis of pooled data; Hendi Maher helped to draft the manuscript; Ke Chen and Xue-yong Zheng wrote the manuscript; Xue-yong Zheng proofread and revised the manuscript. All authors have approved the version to be published.
Supplementary Materials
Table of modified MINORS score of studies with score < 12.
References
- 1.Torre L. A., Bray F., Siegel R. L., Ferlay J., Lortet-Tieulent J. Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Azagra J. S., Goergen M., Gilbart E., Jacobs D. Laparoscopic anatomical (hepatic) left lateral segmentectomy - Technical aspects. Surgical Endoscopy. 1996;10(7):758–761. doi: 10.1007/s004649900150. [DOI] [PubMed] [Google Scholar]
- 3.Kaneko H., Takagi S., Shiba T. Laparoscopic partial hepatectomy and left lateral segmentectomy: Technique and results of a clinical series. Surgery. 1996;120(3):468–475. doi: 10.1016/S0039-6060(96)80065-1. [DOI] [PubMed] [Google Scholar]
- 4.Kanazawa A., Tsukamoto T., Shimizu S. Impact of laparoscopic liver resection for hepatocellular carcinoma with F4-liver cirrhosis. Surgical Endoscopy. 2013;27(7):2592–2597. doi: 10.1007/s00464-013-2795-9. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Y.-M., Shao W.-Y., Zhao Y.-F., Xu D.-H., Li B. Meta-analysis of laparoscopic versus open resection for hepatocellular carcinoma. Digestive Diseases and Sciences. 2011;56(7):1937–1943. doi: 10.1007/s10620-011-1572-7. [DOI] [PubMed] [Google Scholar]
- 6.Li N., Wu Y.-R., Wu B., Lu M.-Q. Surgical and oncologic outcomes following laparoscopic versus open liver resection for hepatocellular carcinoma: A meta-analysis. Hepatology Research. 2012;42(1):51–59. doi: 10.1111/j.1872-034X.2011.00890.x. [DOI] [PubMed] [Google Scholar]
- 7.Xiong J.-J., Altaf K., Javed M. A., et al. Meta-analysis of laparoscopic vs open liver resection for hepatocellular carcinoma. World Journal of Gastroenterology. 2012;18(45):6657–6668. doi: 10.3748/wjg.v18.i45.6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin Z., Fan X., Ye H., Yin D., Wang J. Short- and long-term outcomes after laparoscopic and open hepatectomy for hepatocellular carcinoma: a global systematic review and meta-analysis. Annals of Surgical Oncology. 2013;20(4):1203–1215. doi: 10.1245/s10434-012-2705-8. [DOI] [PubMed] [Google Scholar]
- 9.MacLehose R. R., Reeves B. C., Harvey I. M., et al. A systematic review of comparisons of effect sizes derived from randomised and non-randomised studies. Health technology assessment. Vol. 34. Winchester, England: Health Technology Assessment; 2000. [PubMed] [Google Scholar]
- 10.Abraham N. S., Byrne C. J., Young J. M., Solomon M. J. Meta-analysis of well-designed nonrandomized comparative studies of surgical procedures is as good as randomized controlled trials. Journal of Clinical Epidemiology. 2010;63(3):238–245. doi: 10.1016/j.jclinepi.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 11.Slim K., Nini E., Forestier D., Kwiatkowski F., Panis Y., Chipponi J. Methodological index for non-randomized studies (Minors): development and validation of a new instrument. ANZ Journal of Surgery. 2003;73(9):712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 12.Hozo S. P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology. 2005;5, article 13 doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins J. P. T., Thompson S. G., Deeks J. J., Altman D. G. Measuring inconsistency in meta-analyses. British Medical Journal. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tierney J. F., Stewart L. A., Ghersi D., Burdett S., Sydes M. R. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8, article 16 doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laurent A., Cherqui D., Lesurtel M., et al. Laparoscopic liver resection for subcapsular hepatocellular carcinoma complicating chronic liver disease. JAMA Surgery. 2003;138(7):763–769. doi: 10.1001/archsurg.138.7.763. [DOI] [PubMed] [Google Scholar]
- 16.Lai E. C. H., Tang C. N., Ha J. P. Y., Li M. K. W. Laparoscopic liver resection for hepatocellular carcinoma ten-year experience in a single center. JAMA Surgery. 2009;144(2):143–147. doi: 10.1001/archsurg.2008.536. [DOI] [PubMed] [Google Scholar]
- 17.Sarpel U., Hefti M. M., Wisnievsky J. P., Roayaie S., Schwartz M. E., Labow D. M. Outcome for patients treated with laparoscopic versus open resection of hepatocellular carcinoma: Case-matched analysis. Annals of Surgical Oncology. 2009;16(6):1572–1577. doi: 10.1245/s10434-009-0414-8. [DOI] [PubMed] [Google Scholar]
- 18.Aldrighetti L., Guzzetti E., Pulitanò C., et al. Case-matched analysis of totally laparoscopic versus open liver resection for HCC: Short and middle term results. Journal of Surgical Oncology. 2010;102(1):82–86. doi: 10.1002/jso.21541. [DOI] [PubMed] [Google Scholar]
- 19.Hu B.-S., Chen K., Tan H.-M., Ding X.-M., Tan J.-W. Comparison of laparoscopic vs open liver lobectomy (segmentectomy) for hepatocellular carcinoma. World Journal of Gastroenterology. 2011;17(42):4725–4728. doi: 10.3748/wjg.v17.i42.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meguro M., Mizuguchi T., Kawamoto M., et al. Clinical comparison of laparoscopic and open liver resection after propensity matching selection. Surgery. 2015;158(3):573–587. doi: 10.1016/j.surg.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 21.Belli G., Fantini C., D'Agostino A., et al. Laparoscopic versus open liver resection for hepatocellular carcinoma in patients with histologically proven cirrhosis: Short- and middle-term results. Surgical Endoscopy. 2007;21(11):2004–2011. doi: 10.1007/s00464-007-9503-6. [DOI] [PubMed] [Google Scholar]
- 22.Tranchart H., Di Giuro G., Lainas P., et al. Laparoscopic resection for hepatocellular carcinoma: A matched-pair comparative study. Surgical Endoscopy. 2010;24(5):1170–1176. doi: 10.1007/s00464-009-0745-3. [DOI] [PubMed] [Google Scholar]
- 23.Kim H. H., Park E. K., Seoung J. S., et al. Liver resection for hepatocellular carcinoma: Case-matched analysis of laparoscopic versus open resection. Journal of the Korean Surgical Society. 2011;80(6):412–419. doi: 10.4174/jkss.2011.80.6.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee K. F., Chong C. N., Wong J., Cheung Y. S., Wong J., Lai P. Long-term results: Of laparoscopic hepatectomy versus open hepatectomy for hepatocellular carcinoma: A case-matched analysis. World Journal of Surgery. 2011;35(10):2268–2274. doi: 10.1007/s00268-011-1212-6. [DOI] [PubMed] [Google Scholar]
- 25.Truant S., Bouras A. F., Hebbar M., et al. Laparoscopic resection vs. open liver resection for peripheral hepatocellular carcinoma in patients with chronic liver disease: A case-matched study. Surgical Endoscopy. 2011;25(11):3668–3677. doi: 10.1007/s00464-011-1775-1. [DOI] [PubMed] [Google Scholar]
- 26.Ahn K. S., Kang K. J., Kim Y. H., Kim T.-S., Lim T. J. A propensity score-matched case-control comparative study of laparoscopic and open liver resection for hepatocellular carcinoma. Journal of Laparoendoscopic & Advanced Surgical Techniques. 2014;24(12):872–877. doi: 10.1089/lap.2014.0273. [DOI] [PubMed] [Google Scholar]
- 27.Kim H., Suh K.-S., Lee K.-W., et al. Long-term outcome of laparoscopic versus open liver resection for hepatocellular carcinoma: A case-controlled study with propensity score matching. Surgical Endoscopy. 2014;28(3):950–960. doi: 10.1007/s00464-013-3254-3. [DOI] [PubMed] [Google Scholar]
- 28.Memeo R., De'Angelis N., Compagnon P., et al. Laparoscopic vs. open liver resection for hepatocellular carcinoma of cirrhotic liver: A case-control study. World Journal of Surgery. 2014;38(11):2919–2926. doi: 10.1007/s00268-014-2659-z. [DOI] [PubMed] [Google Scholar]
- 29.Han H.-S., Shehta A., Ahn S., Yoon Y.-S., Cho J. Y., Choi Y. Laparoscopic versus open liver resection for hepatocellular carcinoma: Case-matched study with propensity score matching. Journal of Hepatology. 2015;63(3, article no. 5641):643–650. doi: 10.1016/j.jhep.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Lau B., Franken C., Lee D., Putchakayla K., DiFronzo L. A. Short-term outcomes of laparoscopic versus open formal anatomical hepatectomy: A case matched control study. The American Surgeon. 2015;81(10):1097–1100. doi: 10.1177/000313481508101037. [DOI] [PubMed] [Google Scholar]
- 31.Lee J. J., Conneely J. B., Smoot R. L. Laparoscopic versus open liver resection for hepatocellular carcinoma at a North-American Centre: a 2-to-1 matched pair analysis. HPB: The Official Journal of the International Hepato Pancreato Biliary Association. 2015;17(4):304–310. doi: 10.1111/hpb.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luo L., Zou H., Yao Y., Huang X. Laparoscopic versus open hepatectomy forhepatocellular carcinoma: Short- and long-term outcomes comparison. International Journal of Clinical and Experimental Medicine. 2015;8(10):18772–18778. [PMC free article] [PubMed] [Google Scholar]
- 33.Takahara T., Wakabayashi G., Beppu T., et al. Long-term and perioperative outcomes of laparoscopic versus open liver resection for hepatocellular carcinoma with propensity score matching: A multi-institutional Japanese study. Journal of Hepato-Biliary-Pancreatic Sciences. 2015;22(10):721–727. doi: 10.1002/jhbp.276. [DOI] [PubMed] [Google Scholar]
- 34.Yoon S.-Y., Kim K.-H., Jung D.-H., Yu A., Lee S.-G. Oncological and surgical results of laparoscopic versus open liver resection for HCC less than 5 cm: case-matched analysis. Surgical Endoscopy. 2015;29(9):2628–2634. doi: 10.1007/s00464-014-3980-1. [DOI] [PubMed] [Google Scholar]
- 35.Cheung T. T., Dai W. C., Tsang S. H. Y., et al. Pure laparoscopic hepatectomy versus open hepatectomy for hepatocellular carcinoma in 110 patients with liver cirrhosis: A propensity analysis at a single center. Annals of Surgery. 2016;264(4):612–620. doi: 10.1097/SLA.0000000000001848. [DOI] [PubMed] [Google Scholar]
- 36.Jiang X., Liu L., Zhang Q., et al. Laparoscopic versus open hepatectomy for hepatocellular carcinoma: long-term outcomes. Journal of BUON : official journal of the Balkan Union of Oncology. 2016;21(1):135–141. [PubMed] [Google Scholar]
- 37.Komatsu S., Brustia R., Goumard C., Perdigao F., Soubrane O., Scatton O. Laparoscopic versus open major hepatectomy for hepatocellular carcinoma: a matched pair analysis. Surgical Endoscopy. 2016;30(5):1965–1974. doi: 10.1007/s00464-015-4422-4. [DOI] [PubMed] [Google Scholar]
- 38.Sposito C., Battiston C., Facciorusso A., et al. Propensity score analysis of outcomes following laparoscopic or open liver resection for hepatocellular carcinoma. British Journal of Surgery. 2016;103(7):871–880. doi: 10.1002/bjs.10137. [DOI] [PubMed] [Google Scholar]
- 39.Xu H.-W., Liu F., Li H.-Y., Wei Y.-G., Li B. Outcomes following laparoscopic versus open major hepatectomy for hepatocellular carcinoma in patients with cirrhosis: a propensity score-matched analysis. Surgical Endoscopy. 2017:1–8. doi: 10.1007/s00464-017-5727-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoon Y.-I., Kim K.-H., Kang S.-H., et al. Pure Laparoscopic Versus Open Right Hepatectomy for Hepatocellular Carcinoma in Patients with Cirrhosis. Annals of Surgery. 2017;265(5):856–863. doi: 10.1097/SLA.0000000000002072. [DOI] [PubMed] [Google Scholar]
- 41.Dahiya D., Wu T.-J., Lee C.-F., Chan K.-M., Lee W.-C., Chen M.-F. Minor versus major hepatic resection for small hepatocellular carcinoma (HCC) in cirrhotic patients: A 20-year experience. Surgery. 2010;147(5):676–685. doi: 10.1016/j.surg.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 42.Hasegawa K., Kokudo N., Imamura H., et al. Prognostic impact of anatomic resection for hepatocellular carcinoma. Annals of Surgery. 2005;242(2):252–259. doi: 10.1097/01.sla.0000171307.37401.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lang B. H.-H., Poon R. T.-P., Fan S.-T., Wong J. Perioperative and long-term outcome of major hepatic resection for small solitary hepatocellular carcinoma in patients with cirrhosis. JAMA Surgery. 2003;138(11):1207–1213. doi: 10.1001/archsurg.138.11.1207. [DOI] [PubMed] [Google Scholar]
- 44.Sotiropoulos G. C., Prodromidou A., Kostakis I. D., Machairas N. Meta-analysis of laparoscopic vs open liver resection for hepatocellular carcinoma. Updates in Surgery. 2017;69(3):291–311. doi: 10.1007/s13304-017-0421-4. [DOI] [PubMed] [Google Scholar]
- 45.Sotiropoulos G. C., Prodromidou A., Machairas N. Machairas N: Meta-analysis of laparoscopic vs open liver resection for hepatocellular carcinoma: The European experience. Journal of BUON : Official Journal of the Balkan Union of Oncology. 2017;22(5):1160–1171. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table of modified MINORS score of studies with score < 12.