Abstract
Introduction:
This systematic review intended to compare the biocompatibility and sealing ability of mineral trioxide aggregate (MTA) and biodentine as root-end filling material.
Materials and Methods:
A computerized literature search was performed on March 1, 2016, in MEDLINE, PubMed, and COCHRANE LIBRARY for data published from January 2011 to March 2016. Quality assessment of the selected studies was performed according to the PRISMA guidelines, 2009.
Results:
A total of 12 in vitro studies were included in this review. Of these, four studies compared the biocompatibility and eight compared the sealing ability. With regard to biocompatibility, two articles showed biodentine to be better and two showed comparable results, while in the case of sealing ability, one article showed MTA to be better, six articles showed biodentine to be better, and the rest one article showed the comparable result.
Conclusion:
It may be concluded that good sealing ability of biodentine along with its favorable biological properties show that materials can be used competently in clinical practice as a retrograde filling material. However, long-term assessment in clinical situations is necessary for further inferences.
Keywords: Biocompatibility, biodentine, in vitro studies, mineral trioxide aggregate, root-end filling, sealing ability
INTRODUCTION
The varying concepts in surgical techniques along with newer inventions in the equipment and materials made endodontic surgery an expected treatment option in cases that have not responded to initial endodontic therapy or when nonsurgical root canal therapy may not be successful.[1,2,3,4]
Four critical steps are involved in the eradication of the persistent endodontic pathogens which are as follows: (1) removal of the pathological tissues from the periapical area by means of surgery, (2) apical 3 mm of the root resection, (3) root canal preparation apically, and (4) retrograde filling of the root canal.[1,5]
The definitive success of the root-end surgery relies on the regeneration of a functional periodontal attachment apparatus, including cementum overlying the resected root-end surface, periodontal ligament (PDL), and alveolar bone. To achieve this goal, it has been recommended to place a root-end filling material that not only prevents egress of any remaining bacteria or their by-products but also allows for the development of a normal periodontium across its surface.[6,7] Ideally, the root-end filling material should be impermeable to moisture, antibacterial, noncorrosive, nontoxic, nonresorbable, easy to manipulate, radiopaque, cost-effective, easily adaptable, and adhesive to dentin. It should promote the regeneration of the periodontal apparatus and should be biocompatible.[8,9] A wide range of materials have been used for root-end fillings in endodontic surgery – amalgam, glass ionomer cement, zinc oxide eugenol–based materials (Super-Ethoxy Benzoic Acid, intermediate restorative material, Rickert), mineral trioxide aggregate (MTA), zinc phosphate cements, calcium hydroxide cements, and sealer based on epoxy resins (AH plus) – of which MTA is the most preferred one (gold standard).[8,10,11,12]
MTA was discovered by Torabinejad at the Loma Linda University, CA, USA, in 1993. This material contains tricalcium silicate, tricalcium aluminate, tricalcium oxides, silicate oxide, and other material oxides forming a hydrophilic powder which sets in the presence of water. Hydration of the powder results in a colloidal gel which solidifies to a hard structure. It has a long setting time (2 h 45 min) and hence the material must be protected until it is fully set. The pH of MTA rises from 10.2 after mixing to 12.5 after 3 h, remaining unaffected afterward. Similarly, the compressive strength of MTA increases with time, from 40 MPa after 24 h to 67.3 MPa after 21 days.[13] Its use has always remained a challenge despite its excellent physical and biological properties due to its technique sensitivity, prolonged setting time, poor mechanical properties, and high cost. Hence to overcome all these drawbacks, new experimental calcium silicate-based bioactive restorative cement has been discovered in 2011 under the name of biodentine.[14,15,16,17,18,19,20]
The main constituent of the powder is a tricalcium silicate, with the addition to the powder of calcium carbonate and zirconium oxide. The liquid is a solution of calcium chloride with a water-reducing agent. Benefits of this material are chemicomechanical bonding with the tooth and composite, high compressive strength, and flexural strength. Biodentine exhibited a compressive strength of 170 MPa at 24 h that increased substantially to 304 MPa after the material was placed for 21 days in moisture (close to that reported for human dentine).[21,22,23,24,25]
Since apical seal and biocompatibility are among the most important properties of the root-end filling materials, various methods are employed to evaluate it. Current methods to evaluate the efficacy of apical seal and degree of adaptation are dye penetration, radioisotopes, bacterial penetration, scanning electron and confocal laser scanning microscopy, electrochemical means, and fluid filtration technique.[26] Various biocompatibility testing parameters comprise initial tests, secondary tests, and usage studies. The preliminary evaluation should comprise basic in vitro methods of assessing the biological properties. The resultant assessments should be performed in vivo in laboratory animals and can comprise implantation experiments. The usage studies are performed in primates or human beings.[27]
Therefore, in the present review, the aim is to systematically evaluate the apical sealing ability and biocompatibility of the two materials, namely MTA (gold standard) and the newly introduced biodentine as a retrograde obturation material.
MATERIALS AND METHODS
Literature search
A computerized literature search was performed on March 1, 2016, in MEDLINE, PubMed, and COCHRANE LIBRARY for data published from January 2011 to March 2016 using the following MeSH terms in various combinations: root-end filling materials, retrograde obturation, MTA and biodentine, direct comparison, biocompatibility, AND sealing ability. Related articles that appeared in various search engines were evaluated, and their reference lists were manually checked.
Inclusion and exclusion criteria
The full texts of the studies were obtained and independently reviewed by two reviewers to ascertain whether the studies met the inclusion criteria. The inclusion criteria were as follows: (1) MTA and biodentine as root-end filling materials, (2) direct comparison of MTA and biodentine, (3) comparison in terms of biocompatibility and sealing ability, (4) gray MTA should be used in the studies, and (5) in vitro studies and articles in English language. The exclusion criteria included the following: (1) MTA and biodentine compared in other aspects such as pulp capping agent, repair material, apexogenesis, and sealer; (2) various articles – review articles, clinical studies, ex vivo studies, and case reports; (3) white MTA used in the study; (4) MTA and biodentine compared in terms of properties other than sealing ability and biocompatibility; (5) articles in language other than English; (6) inaccessible articles; and (7) studies that are funded.
Reference lists from identified articles were scanned to find out other potentially relevant articles by two observers. Any disagreement between the authors was resolved through discussion.
Data extraction and quality assessment
Studies that fulfilled the inclusion criteria were processed for data extraction. The spotlight of this review was the MTA and biodentine used as root-end filling material in terms of biocompatibility and sealing ability. The appraisal step was performed in a standardized manner by using quality assessment checklists (PRISMA guidelines, 2009) that included items such as study design and analysis and recognized the deficiencies that might arise from bias. This step was performed by two independent reviewers for enhanced reliability of the results.
RESULTS
Figure 1 summarizes the search strategy process. The search in MEDLINE database using evident web-based search engine covered all articles published in dental journals in English from January 2011 to March 2016, i.e., a total of 334 articles, of which 52 articles were eligible for inclusion on the basis of their titles and abstracts after writing the following “MeSH terms” such as gray MTA AND biodentine AND direct comparison AND biocompatibility AND sealing ability. The other 282 articles were rejected as they were found to be irrelevant to the topic or they did not compare MTA and biodentine directly in the same article.
Figure 1.
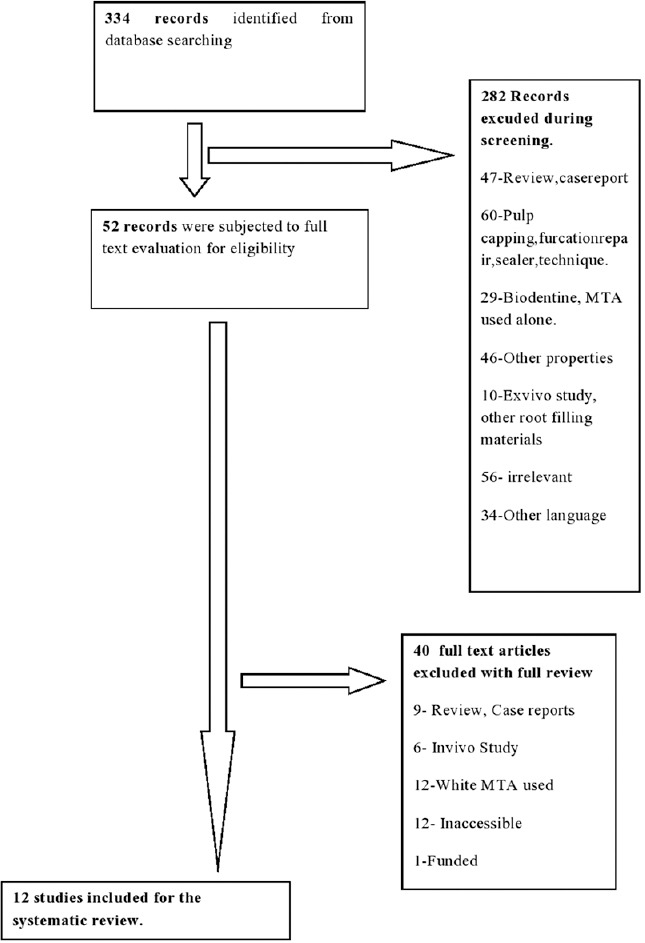
Search strategy flowchart
The combined search through the electronic databases and a manual search resulted in a total of 52 articles/studies, which were subjected to a suitability test in accordance with the criteria for considering studies for this review. Forty articles were excluded as they did not fulfill the inclusion criteria set for this review. Eventually, 12 articles submitted to full-text evaluation satisfied the inclusion criteria and were ultimately selected for the review.
In terms of biocompatibility, 18 articles were selected, of which four met the inclusion criteria and the remaining 14 articles were excluded. Out of the four articles, two articles showed that MTA and biodentine both showed comparable biocompatibility and exhibited no statistically significant difference,[28,29] whereas the remaining two articles showed that biodentine was better than MTA in terms of biocompatibility.[30,31]
A total of 16 articles were selected in terms of sealing ability, of which eight articles met the inclusion criteria and the rest eight were excluded. Out of the eight articles, six articles showed that the biodentine has better sealing ability than MTA,[26,32,33,34,35,36] one article showed the comparable sealing ability,[37] and one article proved MTA to be better than biodentine.[38]
DISCUSSION
Hindlekar and Raghavendra[32] stated that the endodontic treatment failure may be possibly because of the variations in the apical root anatomy such as ramifications, apical delta, and anastomoses. They may contribute to failure as they are difficult to clean. More than 75% of the teeth have canal variations in the apical 3 mm. Hence, the resection of the apical third will include most lateral and accessory canals and eliminate most of the residual microbes and irritants. The primary goal of surgical treatment is to prevent the recontamination of the periapical area by any irritants remaining within the root canal and help in the regeneration of periodontium, a marker for the success of the procedure.
Various materials have been used as root-end filling materials. The quality and stability of any dental material are a key component for the survival of a restoration in clinical conditions; the marginal adaptation and the intimate contact at the interface with the surrounding tissues are determinative features.
Research focusing on issues relevant to the materials to be used as retrograde filling aimed to provide evidence to support clinical decisions. In recent decades, much discussion on the materials to be used has gained attention; however, no consensus has been reached. A systematic review has several purposes when the related studies had conflicting results or small sample sizes, for instance to increase power and precision and to answer questions not posed by the individual studies. This review aimed to compare the outcomes of MTA and biodentine used as retrograde filling materials.
This review identified 12 studies that compared MTA and biodentine directly in terms of various properties of materials such as biocompatibility and sealing ability. Unlike orthograde root canal filling materials, root-end filling materials are positioned in direct contact with vital periapical tissues. The tissue response of these materials, therefore, becomes essential and may control the result of surgical endodontic treatment.
Saxena et al.[39] stated that the deposition of cementum on the cut root face is considered a required response and a requirement for the restoration of a functional periodontal attachment. Cementum deposition takes place from the circumference of the root end and proceeds centrally toward the resected root canal. The cementum induces a biological seal in addition to the physical seal of the root-end filling, thereby creating a double seal.
According to Mori et al.,[40] the biocompatibility of dental materials is essential for avoiding considerable inflammatory reactions and for allowing repair. A biocompatible material should present low toxicity without promoting an inflammatory reaction, which should be nonsignificant or mild when present. The material can be considered biocompatible if the inflammatory reaction is reduced to nonsignificant levels in a sensible amount of time, such as 14 days.
Two of four studies proposed that MTA and biodentine are comparable. A study by Nunez et al.[28] stated that both MTA and biodentine are analogous in terms of biocompatibility with potential to provide positive environment for the cell, showing cell proliferation and osteogenic capability. Further, the cytotoxic effects of biodentine and MTA on human pulp cell cultures were tested, stating an absence of toxicity for biodentine compared with MTA. It was also observed that MTA and biodentine do not affect the specific function of target cells. Both the materials allowed for cellular viability and the proliferation of cells over 72 h. Further, it was quoted that cells in contact with biodentine and MTA showed similar cell viability. Furthermore, the mRNA expression of interleukin (IL)-1α and IL-6 in contact with biodentine was similar to cells in contact with MTA.
The second study by Khedmat et al.[29] stated that monocyte viability significantly improved with time for both MTA and biodentine, and this may be due to a decrease in leached cytotoxic substances from the materials with time, thereby decreasing their cytotoxic effects on cells.
While rest of the two studies by Jung et al. and Ceci et al. concluded biodentine to be better than MTA, Jung et al.[30] proposed that the quantity of PDL cells was much higher in biodentine, thereby promoting repair and better biocompatibility. The possible reason is that the biodentine is chiefly composed of tri- and dicalcium silicate which enhances the bioactivity of those materials on osteoblast and osteoclast-like cells which may lead to the release of silicon from the cement. Furthermore, biodentine showed considerably higher levels of calcium and silicon ion release than MTA. Hence, it may be speculated that biodentine shows better biocompatibility. The second study by Ceci et al.[31] has reported biodentine to have more number of viable cells. No possible explanation was given regarding the same.
Out of the eight studies, six studies showed that the biodentine has better sealing ability than MTA. A study by Hindlekar et al.[32] stated that the tricalcium oxide in the cement reacts with the tissue fluid and stimulates dentine regeneration by inducing odontoblast differentiation from pulp progenitor cells. Further, Malhotra and Hegde[26] proposed that the smaller size of Biodentine particles aids in enhanced adaptation at the cavity surface and filling interface. The decreased pore volume and porosity of biodentine as compared to MTA resulted in better sealing ability. The modified composition of the Biodentine powder such as the absence of calcium aluminate, calcium sulfate and presence of calcium chloride in liquid has improved its physical properties mainly handling and the sealing ability. The faster setting of Biodentine would have prevented the prolonged leakage thereby reducing the bacterial contamination. In Biodentine the formation of biomineralization-tag (apatite forming ability in the presence of phosphate solution) have improved the sealing ability of Biodentine compared to MTA. The hydration products of calcium silicate cements provide a highly alkaline environment which causes degradation of collagen present in interfacial dentine. Later in another study, Han and Okiji[34] stated the phenomenon of uptake of calcium and silicate in conjugation with phosphate-buffered saline in the case of calcium silicate-based cements. A solid-liquid interface forms on the mineral particles, and ion dissociation takes place immediately. Ca2+ ions are rapidly migrated into the mixing solution and portlandite (Ca[OH]2) forms. After this, the OH ion attacks the silicates in an alkaline environment and a calcium silicate hydrate (CSH) phase forms on mineral particles. CSH is a porous, fine-grained/fibrous, and disorganized hydrated silicate gel layer containing silanol groups (Si-OH) and negative surface charges that may serve as nucleation sites for apatite formation. This CSH contains an excess of calcium hydroxide formed by OH− causing a marked rise in the pH (11–12) and a rise in the calcium ion concentration in the surrounding environment.
Further, when exposed to the phosphate-containing fluid, a sequence of reaction takes place between calcium from cement and phosphate from the solution, namely the absorption of calcium and phosphate ions on the silanol groups (Si-OH) of the silica-rich CSH surface and the precipitation of calcium phosphates and apatite. The calcium phosphate apatite deposits form a layer of spherulites filling the superficial porosities. However, the mechanochemical bonding is the same for both MTA and biodentine. Various studies stated that there were wider calcium- and silicate-rich dentine areas and larger incorporation depths and hence the lesser penetration of dyes in case of biodentine. Furthermore, Radeva et al., Han and Okiji, Kokate and Pawar, and Chalas et al.[33,34,35,36] stated that the thickness of the calcium- and silicate-rich layers has enlarged over time and was larger in the case of biodentine than MTA after 30 and 90 days, concluding that the dentine element uptake was greater for biodentine than for MTA.
A study by Soundappan et al.[38] proved MTA to be better than biodentine, which stated that MTA has better marginal adaptation due to the absence of a gap between MTA and dentin and the possible expansion of the cement on the setting.
Only one study by Bolhari et al.[37] showed the comparable sealing ability of MTA and biodentine, which stated that both the materials had a similar composition with calcium silicate as their main constituent.
CONCLUSION
The evidence-based data regarding the sealing ability and biocompatibility of MTA and biodentine are lacking. Hence, according to the results of this review, it may be concluded that good sealing ability of biodentine associated with its favorable biological properties indicate that the material can be used efficiently in clinical practice as a retrograde filling material. The fast setting time is the major advantage of biodentine in comparison to MTA. However, long-standing evaluation in clinical situations is required for further inferences.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ravichandra PV, Vemisetty H, Deepthi K, Reddy JP, Ram Kiran D, Nagendra Krishna MJ, et al. Comparative evaluation of marginal adaptation of biodentine and other commonly used root end filling materials – An in vitro study. J Clin Diagn Res. 2014;8:243–5. doi: 10.7860/JCDR/2014/7834.4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papancheva TB, Panov V, Peev S, Papanchev G. Root End Filling Materials-Review. Scripta Scientifica Medicinae Dentalis. 2015;1:9–15. [Google Scholar]
- 3.Parmar J, Choksi D, Idnani B. Root end filling materials: Review literature. Heal Talk (A Journal of clinical dentistry) 2014;6:32–4. [Google Scholar]
- 4.Bhavana V, Chaitanya KP, Gandi P, Patil J, Dola B, Reddy RB, et al. Evaluation of antibacterial and antifungal activity of new calcium-based cement (Biodentine) compared to MTA and glass ionomer cement. J Conserv Dent. 2015;18:44–6. doi: 10.4103/0972-0707.148892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mandava P, Bolla N, Thumu J, Vemuri S, Chukka S. Microleakage evaluation around retrograde filling materials prepared using conventional and ultrasonic techniques. J Clin Diagn Res. 2015;9:ZC43–6. doi: 10.7860/JCDR/2015/11071.5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Küçükkaya S, Görduysus MÖ, Zeybek ND, Müftüoǧlu SF. In vitro cytotoxicity of calcium silicate-based endodontic cement as root-end filling materials. Scientifica (Cairo) 2016. 2016 doi: 10.1155/2016/9203932. 9203932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khan S, Fareed MA, Kaleem M, Din SU, Iqbal K. An updated review of MTA Part 2: Biological properties, clinical applications and alternative materials. J Pak Dent Assoc. 2015;24:2–10. [Google Scholar]
- 8.Torabinejad M, Watson TF, Pitt Ford TR. Sealing ability of a mineral trioxide aggregate when used as a root end filling material. J Endod. 1993;19:591–5. doi: 10.1016/S0099-2399(06)80271-2. [DOI] [PubMed] [Google Scholar]
- 9.Singh S, Podar R, Dadu S, Kulkarni G, Purba R. Solubility of a new calcium silicate-based root-end filling material. J Conserv Dent. 2015;18:149–53. doi: 10.4103/0972-0707.153053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makkar S, Vashisht R, Kalsi A, Gupta P. The effect of altered pH on push out bond strength of biodentin, GIC, MTA and theracal. Serbian Dent J. 2015;62:7–13. [Google Scholar]
- 11.Grech L, Mallia B, Camilleri J. Characterization of set intermediate restorative material, biodentine, bioaggregate and a protype calcium silicate cement for use as root end filling materials. Int Endod J. 2013;46:632–41. doi: 10.1111/iej.12039. [DOI] [PubMed] [Google Scholar]
- 12.Lee JH, Shon WJ, Lee WC, Baek SH. The effect of several root end filling materials on MG63 osteoblast like cells. J Korean Acad Conserv Dent. 2010;35:222–8. [Google Scholar]
- 13.Meshack RA, Velkrishna K, Chakravarthy P, Nerali J. Overview of root end filling materials. Int J Clin Dent Sci. 2012;3:63–9. [Google Scholar]
- 14.Pawar AM, Kokate S, Shah R. Management of a large periapical lesion using biodentine as retrograde restoration with eighteen month evident follow up. J Conserv Dent. 2013;16:573–5. doi: 10.4103/0972-0707.120934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Attik GN, Villat C, Hallay F, Pradelle-Plasse N, Bonnet H, Moreau K, et al. In vitro biocompatibility of a dentine substitute cement on human MG63 osteoblasts cells: Biodentine ™ versus MTA ®. Int Endod J. 2014;47:1133–41. doi: 10.1111/iej.12261. [DOI] [PubMed] [Google Scholar]
- 16.Maaita AM, Qualtrough AJ, Watts DC. The effects of smear layer on the push out bond strength of root canal calcium silicate cements. Dent Mater J. 2013;29:797–803. doi: 10.1016/j.dental.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 17.Butt N, Talwar S, Chaudhry S, Nawal RR, Yadav S, Bali A, et al. Comparison of physical and mechanical properties of mineral trioxide aggregate and biodentine. Indian J Dent Res. 2014;25:692–7. doi: 10.4103/0970-9290.152163. [DOI] [PubMed] [Google Scholar]
- 18.Prati C, Gandolfi MG. Calcium silicate bioactive cements: Biological perspectives and clinical applications. Dent Mater. 2015;31:351–70. doi: 10.1016/j.dental.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Setbon HM, Devaux J, Iserentant A, Leloup G, Leprince JG. Influence of composition on setting kinetics of new injectable and/or fast setting tricalcium silicate cements. Dent Mater. 2014;30:1291–303. doi: 10.1016/j.dental.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Kaup M, Dammann CH, Schäfer E, Dammaschke T. Shear bond strength of Biodentine, ProRoot MTA, glass ionomer cement and composite resin on human dentine ex vivo. Head Face Med. 2015;11:14. doi: 10.1186/s13005-015-0071-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singh H, Kaur M, Markan S, Kapoor P. Biodentine: A promising dentin substitute. J Interdiscipl Med Dent Sci. 2014;2:1–5. [Google Scholar]
- 22.Bachoo IK, Seymour D, Brunton P. A biocompatible and bioactive replacement for dentine: Is this a reality?. The properties and uses of a novel calcium-based cements. Br Dent J. 2013;214:E5. doi: 10.1038/sj.bdj.2013.57. [DOI] [PubMed] [Google Scholar]
- 23.Zeid S, Alothmani O, Yousef MK. Biodentine and MTA: An analysis of solubility, pH changes and leaching elements. Life Sci J. 2015;12:18–23. [Google Scholar]
- 24.Gandolfi MG, Siboni F, Polimeni A, Bossu M, Riccitiello F, Rengo S, et al. In-vitro screening of the apatite forming ability, biointeractivity and physical properties of a tri calcium silicate material for endodontics and restorative dentistry. Dent J. 2013;1:41–60. [Google Scholar]
- 25.Kaup M, Schäfer E, Dammaschke T. An in vitro study of different material properties of Biodentine compared to ProRoot MTA. Head Face Med. 2015;11:16. doi: 10.1186/s13005-015-0074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malhotra S, Hegde M. Analysis of marginal seal of ProRoot MTA, MTA angleus, biodentine and glass ionomer cement as root end filling materials: An in vitro study. J Oral Res Rev. 2015;7:44–9. [Google Scholar]
- 27.Sousa CJ, Loyola AM, Versiani MA, Biffi JC, Oliveira RP, Pascon EA, et al. A comparative histological evaluation of the biocompatibility of materials used in apical surgery. Int Endod J. 2004;37:738–48. doi: 10.1111/j.1365-2591.2004.00861.x. [DOI] [PubMed] [Google Scholar]
- 28.Nunez CM, Bosomworth HJ, Field C, Whitworth JM, Valentine RA. Biodentine and MTA induce similar cellular responses in a fibroblast cell line. J Endod. 2014;40:406–11. doi: 10.1016/j.joen.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 29.Khedmat S, Dehghan S, Hadjati J, Masoumi F, Nekoofar MH, Dummer PM, et al. In vitro cytotoxicity of four calcium silicate-based endodontic cements on human monocytes, a colorimetric MTT assay. Restor Dent Endod. 2014;39:149–54. doi: 10.5395/rde.2014.39.3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jung S, Mielert J, Kleinheinz J, Dammaschke T. Human oral cells' response to different endodontic restorative materials: An in vitro study. Head Face Med. 2014;10:55. doi: 10.1186/s13005-014-0055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ceci M, Beltrami R, Chiesa M, Colombo M, Poggio C. Biological and chemical-physical properties of root-end filling materials: A comparative study. J Conserv Dent. 2015;18:94–9. doi: 10.4103/0972-0707.153058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hindlekar A, Raghavendra SS. Comparative evaluation of sealing ability of three root end filling materials – An in-vitro study. Int J Dent Clin. 2014;6:4–7. [Google Scholar]
- 33.Radeva E, Uzunov T, Kosturkov D. Microleakage associated with retrograde filling after root end resection-in vitro study. J IMAB. 2014;20:578–83. [Google Scholar]
- 34.Han L, Okiji T. Uptake of calcium and silicon released from calcium silicate-based endodontic materials into root canal dentine. Int Endod J. 2011;44:1081–7. doi: 10.1111/j.1365-2591.2011.01924.x. [DOI] [PubMed] [Google Scholar]
- 35.Kokate S, Pawar A. An in-vitro comparative sterreomicroscopic evaluation of marginal seal between MTA, glass ionomer cement and biodentine as root end filling materials using 1% methylene blue as tracer. Endodontology. 2012;24:36–42. [Google Scholar]
- 36.Chalas R, Mielko E, Wrobel JZ, Nowak J. A chemical activity evaluation of two dental calcium silicate based materials. Curr Issues Pharm Med Sci. 2015;28:89–91. [Google Scholar]
- 37.Bolhari B, Ashofteh Yazdi K, Sharifi F, Pirmoazen S. Comparative scanning electron microscopic study of the marginal adaptation of four root-end filling materials in presence and absence of blood. J Dent (Tehran) 2015;12:226–34. [PMC free article] [PubMed] [Google Scholar]
- 38.Soundappan S, Sundaramurthy JL, Raghu S, Natanasabapathy V. Biodentine versus mineral trioxide aggregate versus intermediate restorative material for retrograde root end filling: An in vitro study. J Dent (Tehran) 2014;11:143–9. [PMC free article] [PubMed] [Google Scholar]
- 39.Saxena P, Gupta SK, Newaskar V. Biocompatibility of root-end filling materials: Recent update. Restor Dent Endod. 2013;38:119–27. doi: 10.5395/rde.2013.38.3.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mori GG, Teixeira LM, de Oliveira DL, Jacomini LM, da Silva SR. Biocompatibility evaluation of biodentine in subcutaneous tissue of rats. J Endod. 2014;40:1485–8. doi: 10.1016/j.joen.2014.02.027. [DOI] [PubMed] [Google Scholar]


