Abstract
Aim:
The study evaluated the antibacterial effects of dental restorations such as nano-hybrid composite (Empress), a glass ionomer cement (GIC Gold label Type 9) and silver amalgam against Streptococcus mutans.
Materials and Methods:
A modified bacterial suspension within the material assay was used to study the antibacterial effects. A volume of 20 μl of bacterial suspensions were placed in a narrow conical cavity within the materials. They were incubated for 0, 3, 6, 12, 24, and 48 h at 37°C. After the incubation period, the number of viable cells in the suspension was evaluated. In liquid culture assay, growth inhibition was measured at A600 nm up to 6 h in bacterial suspensions treated with the eluates of the restorative materials.
Statistical Analysis:
Kruskal–Wallis test and Mann–Whitney's test were performed to determine the significant differences between the control and restorative materials for given incubation periods at 5% level of significance (P < 0.05)
Results and Conclusion:
After 6 h of incubation, all restorative materials showed an inhibitory effect when compared to the controls. Silver amalgam showed the highest inhibition, followed by GIC Type 9 and composite. Silver amalgam showed marked inhibition after 2 h in comparison to the other material groups. These techniques employed to study the antibacterial effects showed that the silver amalgam had the pronounced inhibitory effect followed by GIC and composite. Further research on these aspects is necessary to determine whether the material can prevent secondary caries formation.
Keywords: Antibacterial effect, composite, glass ionomer cement, secondary caries, silver amalgam
INTRODUCTION
Esthetic restorations are high on demand in the present market due to its effective restoration of the very own tooth color. Also to prevent the side effects of amalgams, many other newer restorative materials are being used. Among them are nano composites and reinforced glass ionomer Cements. Any of these restorative materials may develop gap formation along the line of restoration and can cause microleakage due to varied stress factors. These gaps can inhabit microorganisms to colonize, thereby promoting secondary caries formation.[1] Secondary caries is termed as one of the major factors in failure of dental restorations.[2] Hence, study on inhibitory functions of the material against bacterial growth and its bonding capability are of paramount importance to be considered as the best restorative materials in preventing secondary caries formation.[3] In attempting to study these effects, various researchers have used different material to decrease the caries formation after restorations.[4] Various in vitro studies have shown that GIC had potential antimicrobial activity[5,6,7,8] and also reduced formation of plaque by Streptococcus mutans strains.[9,1] The conventional restorative material silver amalgam has the highest inhibitory effect against bacterial growth. However, it is been only recommended for temporary fillings due to potential side effects.[10] Another most commonly used restorative group is composites, less known for its antibacterial properties.[11] A highly esthetic nanohybrid filling composite restorative material containing ytterbium trifluoride are recommended for its unique radiopaque properties. Its unique components (F, Ca) are known to favor remineralization and prevent demineralization.[12] It is been assumed that the ions released by these materials can inhibit the bacterial growth thus preventing the secondary caries formation. The study thus aims (i) to evaluate the antibacterial effect of impress direct composite, GIC type 9 and silver amalgam against S. mutans by performing modified bacterial suspension within material assay and liquid culture growth assay and (ii) to determine the growth inhibitory activity of these dental restorative materials.
MATERIALS AND METHODS
Bacterial strain culture
S. mutans (MTCC) strain was used throughout the study. The bacterial strain were initially cultured on Mutan-Sanguis agar and incubated at 37°C in a 5% CO2 incubator. Todd Hewitt Broth were used for liquid cultures supplemented with 0.5% yeast extract (THY) for further studies. The concentration of bacterial suspensions was determined using spectrophotometer, by measuring its absorbance at 600 nm based on its colony-forming units (CFUs)/ml at various optical densities.
Dental restorative materials
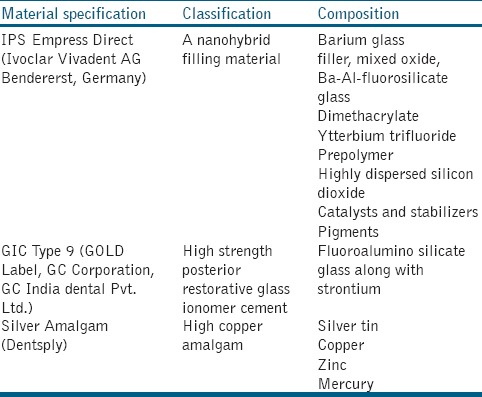
The restorative materials used in the study were obtained commercially. Total of three materials were used, a nano hybrid composite (IPS Empress Direct, Vivadent), (GIC, gold Label Type 9, GC), and silver amalgam (Dentsply). The characteristics of these restorative materials are given in Table 1.
Table 1.
Characteristics of the investigated restorative materials
Determination of viable counts of Streptococcus mutans using bacterial suspension within material assay
Material cavity preparation
The unmolded restorative materials were transferred into sterile 0.5 ml Eppendorf tubes. A 13 mm narrow conical cavity is prepared in the center of the material by impressing the tip of the Eppendorf tube. The impression of the cavity is performed before its setting time and it holds 20 μl of the bacterial suspension. When using impress direct, two cavifils (0.5 g) of the material were transferred to the Eppendorf tubes and impressed using the tip of the tube. The composites were then light-cured (Heliolux DLX, Vivadent) for 40 s and maintained in a sterile condition. For GIC Type 9, the cement was manipulated with agate spatula on a paper pad and impressed using Eppendorf tube. The silver amalgam was triturated using an amalgamator[13] and filled into the Eppendorf tubes, impressed and allowed to set. All the materials were prepared in a sterile condition to prevent contamination.
Culture within cavity
The subculture of S. mutans that has reached 16 h were diluted with THY liquid medium (50 fold) and continued to incubate in the standard culture conditions. The absorbance of the liquid culture is determined frequently at A600 nm and when the growth has reached the log phase that corresponds to 2 × 107 CFUs/ml, the incubation process is terminated. The bacterial suspensions were centrifuged at 2000 g for 5 min and sediments were dissolved in a mixture of artificial saliva and 0.9% NaCl suspension at equal volumes. The above prepared bacterial suspensions were pipetted to the conical test cavity. Bacterial suspensions in Eppendorf tubes without restorative material served as growth control. The tubes were then incubated at 37°C in a humid chamber for 0, 3, 6, 9, 12, 24, and 48 h, each in separate cavity tubes. Post to the incubation period, the bacterial suspensions were removed from the test cavities and serially diluted with sterile NaCl. At t = 0 h, suspensions were immediately taken out of the test cavities without incubation. Aliquots from the diluted suspensions were plated on to the Mutans-sanguis agar solid media. The plates were incubated for 24 h and the number of colonies formed was counted. From the obtained result, the numbers of CFUs per milliliter were calculated (CFUI). After the initial removal of suspension, the test material cavities were filled with 20 μl of 0.9% NaCl and vortexed for 30 s to obtain those bacteria that had been attached to the walls of the cavities. As mentioned above, the suspensions were serially diluted, plated on to the solid media and CFUII was determined. The total numbers of CFUs per milliliter (CFUI + CFUII) were calculated. The experiments for each time interval were performed in triplicates.
Determination of effects of restorative materials on liquid growth cultures
The liquid growth culture inhibition assays were performed according to Friedl et al. method.[11] The restorative materials were transformed into disks of 7 mm in diameter with 1 mm thickness. The discs were immersed in 20 ml 0.9% NaCl solution for 48 h at 37°C. 10 ml of these eluate solutions were inoculated with precultured S. mutans strain in 20 ml THY liquid medium containing 106 CFUs/ml. The liquid culture growth were incubated at 37°C and the growth pattern were observed from lag phase to stationary phase by measuring A600 for every 45 min. Liquid culture with 0.9% NaCl without material eluates served as control. Culture with eluates solution and without bacterial inoculation served as negative control. Each experiment was carried out in a series of triplicates.
Statistical analysis
Statistical analysis were performed using statistic Software SPSS 16.0 (SPSS Ltd, Westlands Centre, Quarry Bay, Hong Kong) Descriptive analysis was performed and the values measured were denoted as median values. Nonparametric tests were conducted and for each test material Kruskal–Wallis test was followed to determine the significant differences between the control and restorative materials for given incubation periods. After confirming the level of significance (P < 0.05), Mann–Whitney's test was performed to study pair-wise comparison between the groups.
RESULTS
The number of colonies formed after incubating the bacterial culture in artificial saliva and saline solution had shown an initial increase up to 3 h and drastically decreased in later experimental periods. The total number of CFUs in silver amalgam and GIC showed complete inhibition after 24 h and impress direct composite showed no growth at 48 h. The effect of dental material on S. mutans has studied by bacterial suspension within material assay up to 48 h are represented in Figure 1. The control group maintained steady growth throughout the experimental period. The effect of restorative materials on S. mutans was analyzed statistically at each experimental period using Kruskal–Wallis test. At all the different test intervals, a significant decrease in CFUs was observed in cavities that included restorative material in comparison to the control group. The inhibitory effect of silver amalgam was the highest followed by GIC and composite with a significant difference of P < 0.05.
Figure 1.
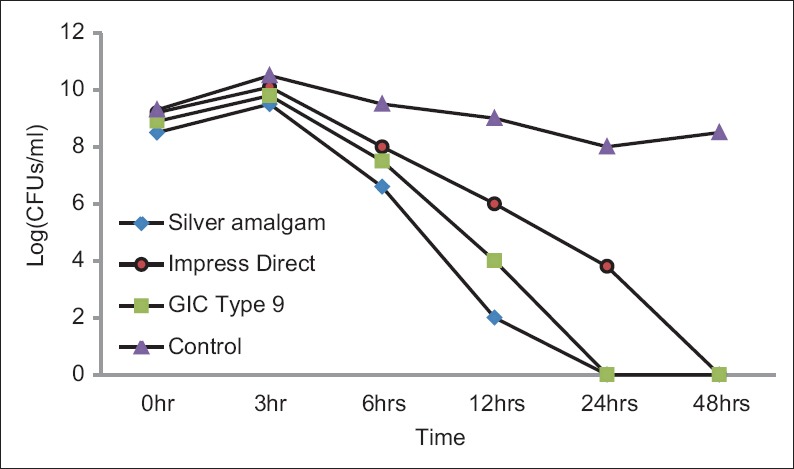
Log (median colony-forming units/ml) showing the effect of dental material on Streptococcus mutans as studied by bacterial suspension within material assay up to 48 h
In the second assay, bacterial growth in the control cultures showed the log phase at 1.5 h and continued up to about 6 h. The results are depicted in Figure 2. The inhibitory effects of eluates of dental restorative materials showed statistically significant difference in comparison to the control group. Silver amalgam had pronounced-inhibitory effects followed by GIC and composite.
Figure 2.
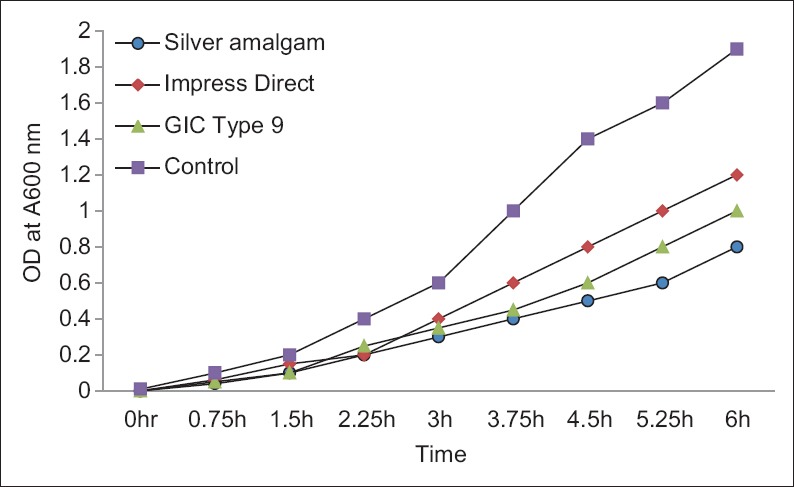
Medians of A600 at different time interval up to 6 h showing the effect of dental materials on the growth of Streptococcus mutans as studied by liquid culture assay
DISCUSSION
Different in vitro methods have been used in the past to study antimicrobial activity and in this study novel assay[5,14] has been used to analyze viable bacterial counts after incubation of a small amount of bacterial suspension within the materials (BSWM model). The limited volume of bacterial suspension surrounded by restorative material was retained and it was formulated in a manner to restrict dilution of components released from the materials. The results attained by the BSWM assay were reconfirmed by determination of growth inhibition in liquid cultures. The advantageous factor of this assay is that bacterial growth can be observed continuously by measuring absorption at 600 nm throughout at the study intervals. In comparison to both the studies BSWM assay is highly recommended for prolonged studies, because the dental materials are in continuous contact with the culture which enables better clinical setup and provides accuracy than any other assays.
S. mutans bacterial strain was used for the whole experiment based on its etiology in the caries development.[15] In this study, the silver amalgam showed the best antibacterial potential. In bacterial suspension within material assay, all the three materials yielded significant reductions in CFUs at all the time intervals compared with the growth control. The inhibitory effect of silver amalgam was highly pronounced than that of the GIC and composite. Whereas composite impress direct showed less inhibition comparable to that of the GICs. The strongest inhibition by silver amalgam is due to the presence of silver particles that has antibacterial properties.[16] The growth inhibition of bacterial culture by of GIC is due to the presence of fluoride[14,17] a strontium-based fluoride that releases for internal remineralization within tooth structure as well as to increase surface strength over time. Very few studies have shown a correlation between antibacterial properties of GICs containing flouride.[7,18,19] In addition, GIC has low pH,[20] that creates an unfavorable environment for the growth of bacteria.
The composites containing fluoride exhibit antibacterial properties[21] and also prevent demineralization of enamel thus forming a protective mechanism against caries.[22] The composite used in the study IPS empress direct contains fluoride in the form of ytterbium-trifluoride which increases the radiopacity of the material. However, fluoride release from these composites is comparatively low compared to that of GICs.[23,24] This could be one of the reasons for composites to have less inhibitory effects in both assays used in this study. Due to favorable pH and ions released, it may contribute to the reduction of bacterial counts. In this study, a statistically significant reduction in CFUs confirms the growth-inhibitory effects of the materials. The results clearly denote that all the restorative materials showed the bacterial growth inhibition in both the assays. Although controversies arise on the usage of silver amalgam, the traditional material had the pronounced inhibition than other materials. The presence of silver particles has promoted the best antibacterial properties for the material. Alternatively, even if it inhibits the bacterial growth, it creates marginal gaps within the vicinity of the teeth which results in colonizations.[25] Whereas GIC and composite do not create gaps in restoration to the most extent, and also it has clinically significant secondary caries prevention action. Hence, these materials are safe to use in restorations with minimal side effects comparable to that of silver amalgam.
CONCLUSION
This study has investigated the potential action of material against the most common caries forming bacteria S. mutans. Hence, further studies need to be conducted using other microorganisms to prove antibacterial properties against the oral microflora. The studies can promote and develop best restorative materials with antibacterial properties. Later to all these experiments, clinical trials need to be performed in vivo to check for the efficacy of dental restorations.
Financial support and sponsorship
The Project was funded by Nitte University Project funds.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Svanberg M, Krasse B, Ornerfeldt HO. Mutans streptococci in interproximal plaque from amalgam and glass ionomer restorations. Caries Res. 1990;24:133–6. doi: 10.1159/000261255. [DOI] [PubMed] [Google Scholar]
- 2.Friedl KH, Hiller KA, Schmalz G. Placement and replacement of composite restorations in Germany. Oper Dent. 1995;20:34–8. [PubMed] [Google Scholar]
- 3.Tobias RS. Antibacterial properties of dental restorative materials: A review. Int Endod J. 1988;21:155–60. doi: 10.1111/j.1365-2591.1988.tb00969.x. [DOI] [PubMed] [Google Scholar]
- 4.Weerheijm KL, Kreulen CM, de Soet JJ, Groen HJ, van Amerongen WE. Bacterial counts in carious dentine under restorations: 2-year in vivo effects. Caries Res. 1999;33:130–4. doi: 10.1159/000016506. [DOI] [PubMed] [Google Scholar]
- 5.Benderli Y, Ulukapi H, Balkanli O, Külekçi G. In vitro plaque formation on some dental filling materials. J Oral Rehabil. 1997;24:80–3. doi: 10.1046/j.1365-2842.1997.00425.x. [DOI] [PubMed] [Google Scholar]
- 6.Boeckh C, Schumacher E, Podbielski A, Haller B. Antibacterial activity of restorative dental biomaterials in vitro . Caries Res. 2002;36:101–7. doi: 10.1159/000057867. [DOI] [PubMed] [Google Scholar]
- 7.Friedl KH, Schmalz G, Hiller KA, Shams M. Resin-modified glass ionomer cements: Fluoride release and influence on Streptococcus mutans growth. Eur J Oral Sci. 1997;105:81–5. doi: 10.1111/j.1600-0722.1997.tb00184.x. [DOI] [PubMed] [Google Scholar]
- 8.Yap AU, Khor E, Foo SH. Fluoride release and antibacterial properties of new-generation tooth-colored restoratives. Oper Dent. 1999;24:297–305. [PubMed] [Google Scholar]
- 9.Herrera M, Castillo A, Bravo M, Liébana J, Carrión P. Antibacterial activity of resin adhesives, glass ionomer and resin-modified glass ionomer cements and a compomer in contact with dentin caries samples. Oper Dent. 2000;25:265–9. [PubMed] [Google Scholar]
- 10.Sivakumar JS, Kumar BNS, and Shyamala PV. Role of provisional restorations in endodontic therapy. J Pharm Bioallied Sci. 2013;5(Suppl 1):S120–S124. doi: 10.4103/0975-7406.113311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beyth N, Domb AJ, Weiss E. An in vitro quantitative antibacterial analysis of amalgam and composite resins. J Dent. 2007;35:201–206. doi: 10.1016/j.jdent.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Collares FM, Ogliari FA, Lima GS, Fontanella VR, Piva E, Samuel SM, et al. Ytterbium trifluoride as a radiopaque agent for dental cements. Int Endod J. 2010;43:792–7. doi: 10.1111/j.1365-2591.2010.01746.x. [DOI] [PubMed] [Google Scholar]
- 13.Osborne JW, Ferguson GW, Sorensen SE, Gale EN. Compressive strength of amalgam triturated by a high-speed amalgamator and by an ultrahigh-speed mixer. J Prosthet Dent. 1968;19:598–604. doi: 10.1016/0022-3913(68)90261-8. [DOI] [PubMed] [Google Scholar]
- 14.Meryon SD, Johnson SG. The modified model cavity method for assessing antibacterial properties of dental restorative materials. J Dent Res. 1989;68:835–9. doi: 10.1177/00220345890680051701. [DOI] [PubMed] [Google Scholar]
- 15.Forssten SD, Björklund M, and Ouwehand AC. Streptococcus mutans, Caries and Simulation Models. Nutrients. 2010;2(3):290–298. doi: 10.3390/nu2030290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Losasso C, Belluco S, Cibin V, Zavagnin P, Micetic I, Gallocchio F, et al. Antibacterial activity of silver nanoparticles: sensitivity of different Salmonella serovars. Front Microbiol. 2014;5:227. doi: 10.3389/fmicb.2014.00227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forss H, Jokinen J, Spets-Happonen S, Seppä L, Luoma H. Fluoride and mutans streptococci in plaque grown on glass ionomer and composite. Caries Res. 1991;25:454–8. doi: 10.1159/000261410. [DOI] [PubMed] [Google Scholar]
- 18.Loyola-Rodriguez JP, Garcia-Godoy F, Lindquist R. Growth inhibition of glass ionomer cements on mutans streptococci. Pediatr Dent. 1994;16:346–9. [PubMed] [Google Scholar]
- 19.Palenik CJ, Behnen MJ, Setcos JC, Miller CH. Inhibition of microbial adherence and growth by various glass ionomers in vitro. Dent Mater. 1992;8:16–20. doi: 10.1016/0109-5641(92)90047-g. [DOI] [PubMed] [Google Scholar]
- 20.Karantakis P, Helvatjoglou-Antoniades M, Theodoridou-Pahini S, Papadogiannis Y. Fluoride release from three glass ionomers, a compomer, and a composite resin in water, artificial saliva, and lactic acid. Oper Dent. 2000;25:20–5. [PubMed] [Google Scholar]
- 21.Coogan MM, Creaven PJ. Antibacterial properties of eight dental cements. Int Endod J. 1993;26:355–61. doi: 10.1111/j.1365-2591.1993.tb00769.x. [DOI] [PubMed] [Google Scholar]
- 22.DeSchepper EJ, White RR, von der Lehr W. Antibacterial effects of glass ionomers. Am J Dent. 1989;2:51–6. [PubMed] [Google Scholar]
- 23.Cosma C, Prejmerean M, Moldovan M, Brie A, Colceriu L. Vezsenyi Fluoride release from dental resin composites. Materials Science and Engineering. 2005;25:231–6. [Google Scholar]
- 24.Dionysopoulos D. The effect of fluoride-releasing restorative materials on inhibition of secondary caries formation. Research review Fluoride. 2014;47:258–65. [Google Scholar]
- 25.Torresyap V, Moshaverinia A, Chee WW. Biofilms in restorative dentistry: A clinical report. Journal of prosthetic Dentistry. 2005;25:231–6. doi: 10.1016/j.prosdent.2015.01.003. [DOI] [PubMed] [Google Scholar]


