Abstract
Purpose:
The purpose of this study is to compare the physical, mechanical, and biocompatibility properties of a new dual-cure white mineral trioxide aggregate (D-W-MTA) and a commercial W-MTA.
Materials and Methods:
Diametral tensile strength (DTS), water sorption (WSp), and water solubility (WSl) tests were performed. Cytotoxicity was observed in primary culture of human pulp fibroblasts (HPFs) and mouse 3T3/NIH fibroblast lineage. Specimens of both materials were embedded in 1 mL of Dulbecco's modified essential medium for 24 h. Cells were incubated for 24 h with the eluates. Cytotoxicity was evaluated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay and genotoxicity by micronucleus (MN) formation. Data were analyzed by ANOVA and Kruskal–Wallis tests considering P < 0.05.
Results:
D-MTA and W-MTA not showed cytotoxic effect on the two cell lines. However, D-MTA stimulated HPF growth. The MN count was similar to that of the control group for D-MTA and W-MTA. D-MTA presented lower DTS and WSl. Nevertheless, WSp was similar in the two groups.
Conclusion:
The results suggest that D-MTA is a promising material for pulp capping. Thus, in vivo tests should be performed to evaluate the performance of this material.
Keywords: Mineral trioxide aggregate, pulp capping and pulpectomy agents, surfactant, tensile strength, toxicity
INTRODUCTION
Conservative treatments are indicated for reversible injuries to maintain the vitality and function of dental pulp. Undifferentiated mesenchymal cells present in the dental pulp respond to injury by proliferating, migrating, and differentiating into odontoblasts, leading to the formation of tertiary dentin.[1] Direct pulp capping is an alternative treatment for mechanically exposed pulp tissue and its success depends mainly on the type of injury, presence of bacteria, quality of pulp tissue, patient's age, capping material, and tooth integrity.[1]
For many years, calcium hydroxide was the most appropriate material for direct or indirect pulp capping. It is widely known to have a strong antibacterial activity.[2] Moreover, it has been demonstrated that it is capable of dissolving bioactive molecules from dentin matrix that promote the regulation of odontoblast activity.[3] Dentin matrix molecules are also involved in the recruitment of undifferentiated mesenchymal cells to replace the lost odontoblasts.[3] However, undesirable properties such as high solubility, low mechanical resistance, and lack of bond to dental structures are strongly associated with calcium hydroxide.[2] Furthermore, calcium hydroxide often produces poorly organized tertiary dentin with multiple tunnel defects.[2]
Thus, currently, mineral trioxide aggregate (MTA) has been used for pulp capping due to desirable properties such as high biocompatibility, low solubility, production of better quality tertiary dentin, and encouraging clinical results.[4] Despite its favorable qualities, some of its characteristics often make it difficult to use MTA. Its limitations are mainly related to the difficulty of handling and long setting time.[5] However, these limitations have not prevented a better clinical performance of MTA as direct pulp-capping agent when compared with CaOH2 in vivo.[6]
In an attempt to overcome these limitations, experimental resin-based MTA formulations have been proposed.[7] The incorporation of monomers into pulp-capping materials is not recent but remains controversial due to the potential cell cytotoxicity and allergic reactions to the monomers and polymerization products.[8]
On the other hand, in the last years, some researches have demonstrated the potential use of surfactant dimethacrylates, such as the higher ethoxylated ethoxylated bisphenol-A dimethacrylate (Bis-EMAs), as alternative to the usual monomers in the development and reformulation of methacrylate-based dental materials.[7,8,9] Bis-EMA has been used as analogous to the bisphenol A diglycidyl dimethacrylate, in which the hydroxyls of their structure were removed, providing less viscosity. Bis-EMA may exhibit long chains of ethylene oxide, which in addition to conferring greater flexibility to the monomer, increases the polarity of the hydrophilic site of the molecule. Consequently, these methacrylate monomers demonstrate an amphiphilic behavior since they also have two polar regions corresponding to the two polyoxyethylene chains. Recent studies have demonstrated that Bis-EMA molecules may act as solubility enhancers in dental adhesive blends, increasing the polymerization kinetics and degree conversion.[7,8,9] Thus, these characteristics may hypothetically favor a possible contact of the material with the dental pulp.
Therefore, the aim of this study was to compare an experimental dual-cure MTA-based cement (D-MTA) with a commercial white MTA (W-MTA) with respect to its cytotoxic and genotoxic effects on primary human pulp cells and a mouse fibroblast lineage. In addition, the diametral tensile strength (DTS) and sorption/solubility in water of the different cements were compared.
MATERIALS AND METHODS
This project was approved by the Institutional Research Ethics Committee and was carried out in accordance with the principles of the Declaration of Helsinki.
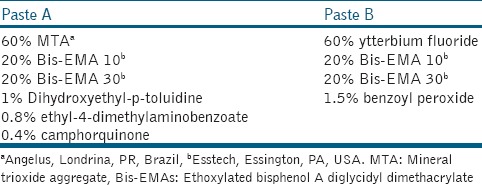
Preparation of test materials
Samples of commercial W-MTA (Angelus, Londrina, Brazil) were prepared with a powder/liquid ratio of 3:1, on a glass slab for 1 min, according to the manufacturer's instructions. The experimental D-MTA-based cement (D-MTA) was developed in two different pastes (base and catalyst). The ingredients of both D-MTA pastes are described in Table 1. To make the base paste (paste A), a binary light-curing system consisting of 0.4 wt% camphorquinone and 0.8 wt% ethyl-4-dimethylaminobenzoate was dissolved in the mixture of 60 wt% MTA, 20 wt% Bis-EMA 10, 20 wt% Bis-EMA 30, and 1 wt% dihydroxyethyl-p-toluidine. To make the catalyst paste (paste B), an oxidizing agent (1.5 wt% benzoyl peroxide) was added to the mixture of 60 wt% ytterbium fluoride, 20 wt% Bis-EMA 10, and 20 wt% Bis-EMA 30. The reagents were used as received, without further purification. An analytical balance (AG 200, Gehaka Electric and Electronic Industry and Commerce, São Paulo, Brazil) was used to weigh the components used to formulate the two different pastes. All components were mixed manually and subsequently homogenized for 15 min in an ultrasonic tank (100 CBU/1 LDG, Flat, São Paulo, Brazil). After this, they were stored in hermetically closed flasks isolated from light and moisture for 24 h to eliminate bubbles. Then, the same amount of base and catalyst of D-MTA pastes was mixed on a glass slab for 1 min. Then, cylindrical specimens were obtained using a metal matrix and a polyester strip (5.5 mm inner diameter × 1 mm thick). D-MTA was light activated for 40 s on each side with a light-emitting diode light source (Radii, SDI, Australia). The irradiance was measured with a digital power meter (Ophir Optronics, Danvers, MA, USA) and was approximately 1400 mW/cm2. Samples were stored in plastic tubes at 37°C in a humidified chamber for 24 h to guarantee the complete reaction. The completion of reaction was tested with a metal instrument against the surface.
Table 1.
Chemical composition of the dual-cure mineral trioxide aggregate in percentage of mass
Obtaining the eluate
Dulbecco's modified essential medium (DMEM, Gibco Invitrogen, Grand Island, NY, USA) without serum was used as elution medium. Eluates were prepared by placing MTA disks into sterile vials with 1 mL of DMEM supplemented with 1% penicillin/streptomycin (Gibco Invitrogen). The ratio of the surface area of discs to the volume of culture medium was 0.95 cm2/mL as recommended by the International Standard Organization 10993-5.[10] Specimens were maintained at 37°C for 24 h. After this, the specimens were removed, and the eluate was kept at a temperature of −80°C.
Human pulp fibroblast culture
Human pulp fibroblasts (HPFs) were cultured from third molars with open apices. Pulp tissues were removed, chopped with a surgical knife, and put into 25 cm2 cell culture plates containing DMEM supplemented with 10% fetal bovine serum (FBS, Gibco Invitrogen) and 1% penicillin/streptomycin. The cells were maintained at 37° C in a humidified atmosphere consisting of 5% CO2. Cells were grown from tissue fragments, collected by trypsinization (0.2% trypsin and 0.02% ethylenediaminetetraacetic acid; Gibco) and subcultured. The medium was changed every 4 days, and the cells between the fourth and eighth passages were used.
Mouse fibroblast 3T3/NIH culture
The cell lineage was obtained from our stock and maintained in DMEM supplemented with 10% FBS, 1% penicillin and streptomycin, and incubated at 37°C in a humidified atmosphere of 5% CO2 in air until subconfluency.
Cytotoxicity assay (3-[4,5-dimethylthiazol-2-yl] -2,5-diphenyltetrazolium bromide assay)
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to assess cell metabolic function by mitochondrial dehydrogenase activity. 3T3/NIH fibroblasts (2 × 104/well) were maintained in DMEM in 96-well plates for 24 h and pulp fibroblasts (2 ×104/well) for 48 h due to differences in metabolism. Cytotoxicity produced by different MTAs was assessed by 24-h cell exposure to 200 μL of the eluates or DMEM (control group). Eluate was removed and the cells washed with phosphate-buffered saline (PBS). Immediately, 180 μL of DMEM with 20 μL of MTT solution (Gibco, Grand Island, 5 mg of MTT/mL PBS) was added to each well. Plates were maintained at 37°C in darkness for 5 h, and 200 μL/well of dimethyl sulfoxide (Gibco) was added. The absorption at 540 nm was determined spectrophotometrically. All experiments were performed independently at least three times.
Genotoxicity assay (micronucleus test)
A total of 4 × 104/well 3T3/NIH fibroblasts were grown on glass slides in 24-well plates. Cells were submitted to 400 μl of the MTA eluates or DMEM (control). After 24 h, cells were fixed in 3:1 methanol/acetic acid for 30 min. Cells were dried, lysed in 1N hydrochloric acid for 40 min, and stained with Schiff's reagent for 2 h. Slides were rinsed in water, immersed in Fast Green for 10 s, and washed in ethanol. Slides were assessed by light microscopy at ×400 magnification. Two thousand cells per group were analyzed. Micronuclei were identified as DNA-containing structures inside the cytoplasm, separated from the main nucleus, with total area smaller than 1/3 of the main nucleus.
Diametral tensile strength
Ten cylindrical specimens for each group were obtained using a metal matrix and a polyester strip (4 mm diameter × 2 mm thick). The specimens were stored in distilled water at 37°C for 24 h. DTS test was performed in a universal testing machine (DL500; EMIC, PR, Brazil) at a crosshead speed of 0.5 mm/min. Specimens were positioned vertically and subjected to compressive loading until failure. DTS means were calculated in MPa.
Water sorption and solubility
The thickness and diameter of 10 specimens of each group were measured with a digital caliper, and the volume (V in mm3) calculated. Samples were stored in a desiccator at 42°C and repeatedly weighed in regular 24 h intervals, using an analytical digital balance (AG200, Gehaka, São Paulo, SP, Brazil) until a constant mass (m1) was obtained. The specimens were individually placed in sealed plastic vials, immersed in 1 mL of distilled water, and stored at 37°C for 7 days. Afterwards, specimens were blotted with absorbent paper and air-dried for 15 s. Weighing procedures were repeated, during which m2 was recorded. The specimens were placed in a desiccator at 42°C and reweighed until a constant mass (m3) was obtained. Water sorption (Wsp) and Water solubility (Wsl) in mg/mm3 were calculated as follows:
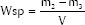
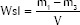
Statistical analysis
Cytotoxicity and genotoxicity results were analyzed by one-way ANOVA and Kruskal–Wallis tests, respectively. DTS and WSp and solubility results were analyzed by one-way ANOVA and Tukey tests using SigmaStat 3.5 software (Systat Inc., Richmond, CA, US). Statistical analysis was performed at a 5% level of significance.
RESULTS
Cytotoxicity assay
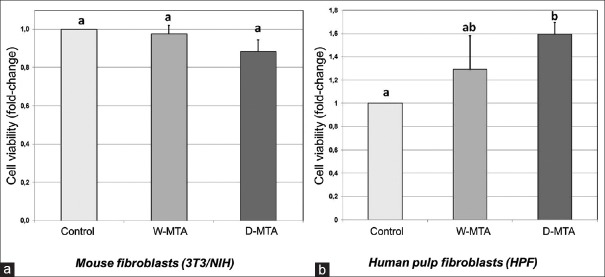
In 3T3/NIH mouse fibroblasts, both MTA cements and the control group showed the same level of cytotoxicity (P = 0.723) [Figure 1a]. In primary human dental pulp fibroblasts, no difference in viability was observed between the two MTA cements. However, D-MTA stimulated HPFs proliferation when compared with the control group (P = 0.021) [Figure 1b].
Figure 1.
(a) Mouse fibroblast 3T3/NIH cytotoxicity after 24 h stimulation with white mineral trioxide aggregate or dual-cure mineral trioxide aggregate eluates. Control group was incubated with Dulbecco's modified essential medium supplemented with fetal bovine serum and antibiotics. No statistical difference was found between groups. (b) Human pulp fibroblast viability after stimulation with eluates (24 h) from white mineral trioxide aggregate or dual-cure mineral trioxide aggregate. Control group was incubated with Dulbecco's modified essential medium supplemented with fetal bovine serum and antibiotics. Mean and standard deviation was based on at least three independent experiments. Results were shown as mean ± standard deviation (shown by bar). Different letters indicate statistical difference (P < 0.05)
Genotoxicity assay
Micronucleus (MN) formation was statistically similar when the two MTA cements were compared with the control group. However, D-MTA produced a higher count of micronuclei in comparison with W-MTA [Figure 2].
Figure 2.
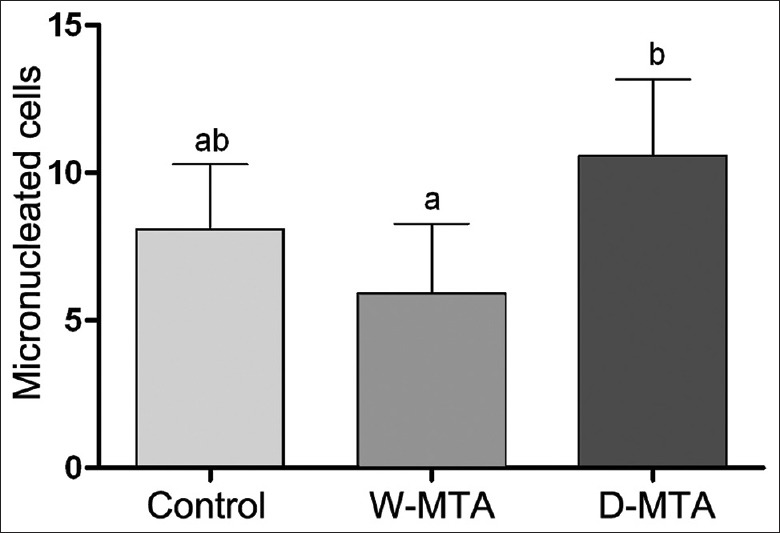
Micronucleus count in 2000 mouse fibroblast 3T3/NIH. Mean and standard deviation was based on at least three independent experiments. Different letters indicate statistical difference (P < 0.05)
Diametral tensile strength
D-MTA showed a low DTS (4.81 ± 1.15 MPa) when compared with W-MTA (7.60 ± 1.99 MPa) (P < 0.001).
Water sorption and solubility
WSp values were equal between W-MTA (11.3 ± 3.0 mg/mm3) and D-MTA (12.2 ± 0.5 mg/mm3) samples (P = 0.4559). However, D-MTA (2.9 ± 0.2 mg/mm3) was less soluble than W-MTA (5.8 ± 1.8 mg/mm3) (P = 0.0002).
DISCUSSION
Several studies have evaluated not only the physical and chemical properties of MTA-based cements but also the biological responses as well.[6,11] Nevertheless, the problems related to the setting time and manipulation persist.[5] Therefore, the proposal of a dual cure MTA-based cement may be a good strategy to improve the manipulation and properties of these cements. However, this new material should have at least the same biological performance when compared with the traditional MTA. From this aspect, cytotoxicity and genotoxicity tests have been considered a good tool for the initial screening of dental materials as regards possible toxic effects.[12] These tests allow a careful control of the physic-chemical and physiological environment, reduce animal experimentation, and are economical, controllable, and reproducible.[12] In the present study, experimental (D-MTA) and commercial (W-MTA) MTA cements were tested using both mouse fibroblasts of the 3T3/NIH-immortalized cell line [Figure 1a] and a primary culture of HPFs [Figure 1b]. Cell lines are used in many studies because they have been well characterized and are reproducible.[12] On the other hand, primary culture cells from candidate target tissues are correlated more closely with the system under examination.[13] Therefore, to provide a better level of evidence, both cell types were used in the present study.[14] The cytotoxicity of both MTA cements and control group showed no statistically significant difference when evaluated in mouse fibroblast 3T3/NIH culture [Figure 1a]. In the primary fibroblast pulp cell culture, D-MTA was capable of stimulating cell proliferation when compared with control group while the W-MTA had a similar rate of proliferation compared with D-MTA and control group [Figure 1b].
The biological responses of the MTA-based cements also were investigated by the MN test. The induction of MN is considered an effective biomarker to provide information on the cytogenetic damage to the tissues and process associated with the induction of DNA damage.[15] MN is DNA masses with the appearance of small nuclei found in the cytoplasm of cells, which are capable of dividing themselves, representing a variety of segregational DNA defects.[16] In this study, the results demonstrated that D-MTA showed an increase in the frequency of MN after cell exposure when compared with W-MTA [Figure 2]. Nevertheless, this does not seem to be alarming because the D-MTA had an MN count similar to that of the control group. Although there are no studies that have evaluated the formation of MN in cells exposed to MTA, the results presented here are consistent with those of Lauren et al.[17] These authors evaluated the formation of MN in lymphocytes after exposure to tricalcium silicate (the main constituent of MTA)-based cement, indicating that the material was unable to increase the MN count.[17]
The MTA cements were developed for use in humid environments. Blood and saliva are the common fluids that may come into contact with MTA cements. However, the humidity significantly increases the setting time of these materials[18] and negatively influences the chemical and physical properties.[19] Therefore, the study and development of D-MTA-based cements is an interesting initiative to reduce problems mainly related to the handling of contemporary commercial MTA cements.[5] Even after being mixed with water, it is difficult to put MTA into position. Moreover, the physical properties of MTA may be influenced by several factors, such as powder/liquid ratio, manipulation, condensation pressure, and handling time.[20] MTA manipulation with excess water increases the porosity, solubility, and calcium release,[21] which are capable of inducing the production of inflammatory mediators. Although further studies are required, the use of an organic matrix consisting essentially of surfactant monomer (Bis-EMA) does not appear to have deleterious effects on cells in cytotoxicity and genotoxicity tests. Whereas, it is known that most of the methacrylates are highly cytotoxic.[15] However, the Bis-EMA showed fewer cytotoxic results in HPFs and 3T3/NIH cells when compared with Bis-GMA and other methacrylate monomers.[15] Bis-EMA has a long chain composed of oxyethylene groups that allows up to 100% polymerization.[9]
Gandolfi et al.[18] developed a light-polymerized MTA composed of 2-hydroxyethyl methacrylate (HEMA) and triethylene glycol dimethacrylate (TEGDMA) that also showed good biological responses. However, the researchers reacted with surprise since HEMA and TEGDMA are widely reported to be highly cytotoxic methacrylate monomers. One possible explanation for this result is the insolubility of dimethacrylates due to the formation of crosslinks. On the other hand, a HEMA/TEGDMA-based resin is more susceptible to hydrolytic degradation since it is formed by small and hydrophilic monomers when compared with Bis-EMA.[22] In the MTA-based cements, the immediate solubility is an important property since the effect of the material is closely related to ion release, mainly Ca2+.[23] In the present study, the results of sorption and solubility showed that the both MTA-based cements had a similar sorption behavior while D-MTA showed less solubility than W-MTA. The lower solubility of the D-MTA can be explained by the presence of Bis-EMA. This monomer has long oxyethylene chain extenders and two hydrophobic methacrylate monomers, dimethacrylates that can form densely cross-linked polymers reducing the susceptibility to hydrolysis in aqueous solutions.[24] The results also showed that D-MTA had a lower DTS when compared with W-MTA. It was expected that the addition of Bis-EMA would increase the mechanical properties of D-MTA due to the monomer molecular structure. Bis-EMA has aromatic phenyl groups that should provide stiffness characteristics to the material. However, a hypothesis that can be suggested to explain these results is that there was not a good interaction between MTA particles and the resin matrix. Nevertheless, other studies have demonstrated values around 4 MPa for DTS of MTA cements and result in agreement with those of the present study.[12]
CONCLUSION
Considering the potential and advantages of a dual MTA-based cement and based on the results, it could be concluded that D-MTA presented a similar performance when compared with W-MTA. The incorporation of Bis-EMA into the W-MTA formulation maintained the biocompatibility of the material as well as the physical and mechanical data. Thus, D-MTA might have a promising application in dentistry. Nevertheless, further studies are necessary to determine the in vivo results.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Tziafas D. The future role of a molecular approach to pulp-dentinal regeneration. Caries Res. 2004;38:314–20. doi: 10.1159/000077771. [DOI] [PubMed] [Google Scholar]
- 2.Mohammadi Z, Dummer PM. Properties and applications of calcium hydroxide in endodontics and dental traumatology. Int Endod J. 2011;44:697–730. doi: 10.1111/j.1365-2591.2011.01886.x. [DOI] [PubMed] [Google Scholar]
- 3.Smith AJ, Scheven BA, Takahashi Y, Ferracane JL, Shelton RM, Cooper PR, et al. Dentine as a bioactive extracellular matrix. Arch Oral Biol. 2012;57:109–21. doi: 10.1016/j.archoralbio.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Parirokh M, Torabinejad M. Mineral trioxide aggregate: A comprehensive literature review – Part III: Clinical applications, drawbacks, and mechanism of action. J Endod. 2010;36:400–13. doi: 10.1016/j.joen.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 5.Chiang TY, Ding SJ. Comparative physicochemical and biocompatible properties of radiopaque dicalcium silicate cement and mineral trioxide aggregate. J Endod. 2010;36:1683–7. doi: 10.1016/j.joen.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 6.Hilton TJ, Ferracane JL, Mancl L. Northwest Practice-based Research Collaborative in Evidence-based Dentistry (NWP). Comparison of caOH with MTA for direct pulp capping: A PBRN randomized clinical trial. J Dent Res. 2013;92:16S–22S. doi: 10.1177/0022034513484336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Formosa LM, Mallia B, Camilleri J. Push-out bond strength of MTA with antiwashout gel or resins. Int Endod J. 2014;47:454–62. doi: 10.1111/iej.12169. [DOI] [PubMed] [Google Scholar]
- 8.Dammaschke T, Stratmann U, Fischer RJ, Sagheri D, Schäfer E. Proliferation of rat molar pulp cells after direct pulp capping with dentine adhesive and calcium hydroxide. Clin Oral Investig. 2011;15:577–87. doi: 10.1007/s00784-010-0409-7. [DOI] [PubMed] [Google Scholar]
- 9.Zanchi CH, Münchow EA, Ogliari FA, de Carvalho RV, Chersoni S, Prati C, et al. Effects of long-term water storage on the microtensile bond strength of five experimental self-etching adhesives based on surfactants rather than HEMA. Clin Oral Investig. 2013;17:833–9. doi: 10.1007/s00784-012-0791-4. [DOI] [PubMed] [Google Scholar]
- 10.International Standard Organization. Biological Evaluation of Medical Devices. Part 5. Geneva, Switzerland: International Organization for Standardization; 2009. [Google Scholar]
- 11.Gomes-Filho JE, de Moraes Costa MM, Cintra LT, Duarte PC, Takamiya AS, Lodi CS, et al. Evaluation of rat alveolar bone response to angelus MTA or experimental light-cured mineral trioxide aggregate using fluorochromes. J Endod. 2011;37:250–4. doi: 10.1016/j.joen.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 12.Freshney I. Application of cell cultures to toxicology. Cell Biol Toxicol. 2001;17:213–30. doi: 10.1023/a:1012572930721. [DOI] [PubMed] [Google Scholar]
- 13.Geurtsen W, Lehmann F, Spahl W, Leyhausen G. Cytotoxicity of 35 dental resin composite monomers/additives in permanent 3T3 and three human primary fibroblast cultures. J Biomed Mater Res. 1998;41:474–80. doi: 10.1002/(sici)1097-4636(19980905)41:3<474::aid-jbm18>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 14.Thonemann B, Schmalz G, Hiller KA, Schweikl H. Responses of L929 mouse fibroblasts, primary and immortalized bovine dental papilla-derived cell lines to dental resin components. Dent Mater. 2002;18:318–23. doi: 10.1016/s0109-5641(01)00056-2. [DOI] [PubMed] [Google Scholar]
- 15.Fernández MR, Carvalho RV, Ogliari FA, Beira FA, Etges A, Bueno M, et al. Cytotoxicity and genotoxicity of sodium percarbonate: A comparison with bleaching agents commonly used in discoloured pulpless teeth. Int Endod J. 2010;43:102–8. doi: 10.1111/j.1365-2591.2009.01648.x. [DOI] [PubMed] [Google Scholar]
- 16.de Almeida TM, Leitão RC, Andrade JD, Beçak W, Carrilho FJ, Sonohara S, et al. Detection of micronuclei formation and nuclear anomalies in regenerative nodules of human cirrhotic livers and relationship to hepatocellular carcinoma. Cancer Genet Cytogenet. 2004;150:16–21. doi: 10.1016/j.cancergencyto.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Laurent P, Camps J, De Méo M, Déjou J, About I. Induction of specific cell responses to a ca(3)SiO(5)-based posterior restorative material. Dent Mater. 2008;24:1486–94. doi: 10.1016/j.dental.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 18.Gandolfi MG, Iacono F, Agee K, Siboni F, Tay F, Pashley DH, et al. Setting time and expansion in different soaking media of experimental accelerated calcium-silicate cements and proRoot MTA. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:e39–45. doi: 10.1016/j.tripleo.2009.07.039. [DOI] [PubMed] [Google Scholar]
- 19.Budig CG, Eleazer PD. In vitro comparison of the setting of dry ProRoot MTA by moisture absorbed through the root. J Endod. 2008;34:712–4. doi: 10.1016/j.joen.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 20.Gandolfi MG, Taddei P, Siboni F, Modena E, Ciapetti G, Prati C, et al. Development of the foremost light-curable calcium-silicate MTA cement as root-end in oral surgery. Chemical-physical properties, bioactivity and biological behavior. Dent Mater. 2011;27:e134–57. doi: 10.1016/j.dental.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Parirokh M, Torabinejad M. Mineral trioxide aggregate: A comprehensive literature review – Part I: Chemical, physical, and antibacterial properties. J Endod. 2010;36:16–27. doi: 10.1016/j.joen.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Park J, Eslick J, Ye Q, Misra A, Spencer P. The influence of chemical structure on the properties in methacrylate-based dentin adhesives. Dent Mater. 2011;27:1086–93. doi: 10.1016/j.dental.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borges RP, Sousa-Neto MD, Versiani MA, Rached-Júnior FA, De-Deus G, Miranda CE, et al. Changes in the surface of four calcium silicate-containing endodontic materials and an epoxy resin-based sealer after a solubility test. Int Endod J. 2012;45:419–28. doi: 10.1111/j.1365-2591.2011.01992.x. [DOI] [PubMed] [Google Scholar]
- 24.Ferracane JL. Hygroscopic and hydrolytic effects in dental polymer networks. Dent Mater. 2006;22:211–22. doi: 10.1016/j.dental.2005.05.005. [DOI] [PubMed] [Google Scholar]