Abstract
Background
Many factors may lead to sperm DNA damage. However, it is little known that the correlations of sperm DNA damage with obesity-associated markers, and reproductive hormones and lipids levels in serum and seminal plasma.
Methods
In our prospective study, a total of 1 010 subfertile men, aged from 18 to 50 years old, were enrolled from August 2012 through June 2015. Their obesity-associated markers, semen parameters, sperm acrosomal enzyme activity, seminal plasma biochemical markers, and reproductive hormones and lipids levels in serum and seminal plasma were detected. Sperm DNA fragmentation index (DFI) was determined by sperm chromatin structure assay. The correlations between DFI and each of the above-mentioned variables were analyzed.
Results
Spearman correlation analysis showed that sperm DFI was positively related to age and abstinence time (P<0.001). Sperm DFI was also positively related to semen volume and percent of abnormal sperm head (P<0.001), while negatively related to sperm concentration, progressive motility (PR), sperm motility, total normal-progressively motile sperm count (TNPMS), percent of normal sperm morphology (NSM), percent of intact acrosome and acrosomal enzyme activity (P<0.001). Sperm DFI was positively related to seminal plasma zinc level (P<0.001) but unrelated to seminal plasma total α-glucotase, γ-glutamyl transpeptidase (GGT) and fructose levels. There was no any correlation between sperm DFI and obesity-associated markers such as body mass index (BMI), waist-to-hip ratio (WHR), waist circumference (WC) and waist-to-height ratio (WHtR), and serum lipids levels, but there was positive correlation between sperm DFI and seminal plasma triglyceride (TG) and total cholesterol (TC) levels (P<0.001). Sperm DFI was positively related to serum luteinizing hormone (LH) and follicle stimulating hormone (FSH) levels and seminal plasma FSH and estradiol (E2) levels (P<0.001), but unrelated to serum and seminal plasma testosterone (T) levels. The multivariate regression analysis for the variables which were significantly correlated with sperm DFI in Spearman correlation analysis showed that age, semen volume, sperm concentration, progressive motility, TNPMS and intact acrosome were independently correlated with sperm DFI.
Conclusions
There are many potential factors associated with sperm DFI, including age, abstinence time, spermatogenesis and maturation, seminal plasma lipids and reproductive hormones levels. However, the potential effects of seminal plasma lipids and reproductive hormones on sperm DNA damage need still to be demonstrated by the studies with scientific design and a large size of samples.
Electronic supplementary material
The online version of this article (10.1186/s12958-018-0345-y) contains supplementary material, which is available to authorized users.
Keywords: Sperm DNA fragmentation index, Male infertility, Obesity-associated marker, Lipids, Reproductive hormone
Background
Through searches of “sperm” and “DNA fragmentation index” in PubMed from 2002 to August 2015, we retrieved over 200 literatures associated with sperm DNA fragmentation index (DFI). Comprehensive analyses of these literatures indicated that since the detection of sperm DNA damage was performed in 2002, it had been applied in some clinical andrology laboratories. The detection of sperm DNA damage, as an important supplement to semen routine examination strategies, may predict the outcomes of natural conception and in vitro fertilization, monitor the damage of sperm DNA induced by environmental pollutants and medical interventions, and evaluate the sperm DNA damage related to male reproductive system diseases and their treatments [1]. The factors related to sperm DNA damage included age, environmental pollutants such as organophosphorus and organochloride pesticides, plasticizer, heavy metals such as lead, carcinogens such as polycyclic aromatic hydrocarbons (c-PAHs) and zearalenone (ZEA), male reproductive system diseases or systemic diseases such as varicocele, infection, tumor, spermatogenesis and maturation dysfunction, spinal cord injury and endocrine disorders, seasons and temperature, lifestyle, abstinence time, semen refrigeration, semen handling in vitro, and certain medications [2]. However, controversial results remain.
Obesity and subfertility have become major global public health concerns. Obesity was reportedly associated with lower fertility. Our previously published data showed that, although obesity-associated markers such as body mass index (BMI), waist circumference (WC), waist-to-hip ratio (WHR) and waist-to-height ratio (WHtR) could not predict semen quality [3], the metabolism abnormality of lipids in male reproductive system may affect male fertility [4]. It raises the question of whether sperm DNA damage, as an important factor influencing sperm quality, be associated with obesity and metabolism abnormality of lipids. No data currently available address this question [5]. In this study, we comprehensively analyzed the correlations between sperm DFI and age, abstinence time, obesity-associated markers such as BMI, WC, WHR and WHtR, semen parameters such as semen volume, sperm concentration, total sperm count (TSC), sperm motility, progressive motility (PR), percent of normal sperm morphology (NSM), percent of abnormal sperm head, percent of intact acrosome, acrosomal enzyme activity and total normal-progressively motile sperm count (TNPMS), serum lipids such as total cholesterol (TC), triglyceride (TG), low density lipoprotein cholesterol (LDL-C) and high density lipoprotein cholesterol (HDL-C) levels, serum reproductive hormones such as follicle stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), total testosterone (TT) and sex hormone binding globulin (SHBG) levels, seminal plasma biochemical markers such as total α-glucotase, γ-glutamyl transpeptidase (GGT), zinc and fructose, seminal plasma lipids such as TG, TC, LDL-C and HDL-C, and seminal plasma reproductive hormones such as FSH, E2 and testosterone in 1 010 subfertile Chinese men (The raw data for these variables were shown in Additional file 1).
Methods
Study population
Subfertile men, aged from 18 to 50 years and whose partners had not conceived within 12 months after stopping use of contraception, who attended infertility outpatient clinic at Nanjing Jinling Hospital between August 2012 and June 2015 were included in this prospective study. This study was approved by the Human Subject Committees of Nanjing Jinling Hospital (Approved number: 2012NZKY-012), and informed consent was obtained from all participants. All participants were asked to complete a questionnaire to provide information on occupation, medical and reproductive history and lifestyle factors including intake of alcohol and smoking history. Then, all participants underwent physical examination, and obesity-associated markers were measured, semen samples were collected, and fasting venous blood were drawn during 8:00 am and 10:00 am. Stringent exclusion criteria were employed to exclude regular alcohol drinkers, heavy smokers, and men with chronic diseases, urogenital infections, varicocele, azoospermia and other diseases which might lead to dysspermia, and incomplete data. One thousand and ten (1 010) men were eligible for the inclusion criteria and enrolled in this study.
Measurement of obesity-associated markers
Height and weight were measured with the participants standing without shoes and heavy outer garments. WC was measured at the level midway between the lower rib margin and the iliac crest with participants in standing position without heavy outer garments and with empty pockets, breathing out gently. Hip circumference was recorded as the maximum circumference over the buttocks. BMI was calculated as weight divided by height squared (kg/m2). WHR was calculated as the ratio of WC over the hip circumference. WHtR was calculated as the ratio of WC over height.
With regard to the current Chinese men criteria [6], a BMI under 18.5 kg/m2 was considered underweight; BMI between 18.5 and 23.99 kg/m2 as normal weight; BMI between 24 and 27.99 kg/m2 as overweight; and BMI ≥ 28 kg/m2 as obesity. Generalized obesity and abdominal obesity were defined using WHO Asia Pacific guidelines with WC cutoff as ≥ 90 cm [7], WHR cutoff as ≥ 0.9 [8], and WHtR cutoff as ≥0.5 [9].
Analysis of semen parameters
Semen specimens were collected by masturbation after a period of 2–7 days of sexual abstinence and were kept to liquefy at 37 °C for 30 min. After liquefaction, semen volume was measured by weighing the sample, sperm concentration, total motility and PR were analyzed using a computer-aided sperm analysis (CASA) system (CFT-9201; Jiangsu Rich Life Science Instrument Co., Ltd., Nanjing, China) [10], and sperm morphology was evaluated using Diff-Quik staining, and NSM, percent of abnormal sperm head, percent of intact acrosome and TNPMS (semen volume × sperm concentration × PR × NSM) were calculated. Here TNPMS represents the spermatozoa with motility and normal morphology, and it is a determinative factor for male fertility. For each specimen, at least 200 spermatozoa were counted and analyzed in each replicate. If the difference between the two replicates was acceptable (within 95% confidence interval), the average results of sperm concentration, total motility, PR and NSM were reported. If the difference was too high, two new aliquots from the semen sample were repeatedly assessed [11]. Based on a colorimetric method, the determination of acrosomal enzyme activity was performed strictly according to the manufacturer’s instruction. The kit was purchased from Nanjing Xindi Biological Pharmaceutical Engineering Co., Ltd. (Nanjing, China).
Detection of sperm DFI
Sperm DFI was assessed by the sperm chromatin structure assay (SCSA). The SCSA kit was purchased from CellPro Biotech Co., Ltd. (Ningbo, China). The determination of sperm DFI was performed strictly according to the manufacturer’s instruction. In brief, semen samples were treated for 30 s with 400 μl of a solution of 0.1 % Triton X-100, 0.15 mol/L NaCl, and 0.08 mol/L HCl, pH 1.2. After 30 s, 1.2 ml of staining buffer (6 μg/ml acridine orange [AO], 37 mmol/L citric acid, 126 mmol/L Na2HPO4, 1 mmol/L disodium EDTA, 0.15 mol/L NaCl, pH 6.0) was admixed to the test tube. The sample was placed into the FACS Calibur flow cytometer (Becton Dickinson, San Jose, CA) with the sample flowing to establish optimal sheath/sample flow, and then at exactly 3 min AO staining measurements were taken. A minimum of 5 000 cells from two aliquots of each sample were acquired and analyzed by FACS scan interfaced with a data analysis software (DFIView 2010 Alpha11.15, CellPro Biotech, Ningbo, China). After completion of the sample analysis, the cytogram (red vs green fluorescence) and histogram (total cells vs DFI) plots as well as DFI readings were generated. A mean of the two sperm DFI values was reported. The variability of the replicate DFI measures was less than 5%.
Determination of seminal plasma biochemical markers
After liquefaction, routine analysis of each semen sample was performed, and the remaining semen samples were centrifuged at 12 000 g for 5 min. The upper layer seminal plasma was collected for the determination of biochemical markers. Commercially available kits for the determinations of seminal plasma GGT, total α-glucotase, zinc and fructose were purchased from Nanjing Xindi Biological Pharmaceutical Engineering Co., Ltd. (Nanjing, China). The determinations were carried out using the Olympus AU400 automatic biochemistry analyzer (Olympus Optical Co. Ltd., Japan), and performed strictly according to the manufacturer’s instruction [12–15].
Determinations of serum lipids and reproductive hormones
Fasting venous blood samples were centrifuged at 3 000 g for 5 min to isolate serum for the detections of lipids and reproductive hormones levels. Commercially available kits for the determinations of TG, TC, LDL and HDL were purchased from Shanghai Zhicheng Biotechnology Co., Ltd., China. Calibration and quality control products were purchased from Randox Laboratories Ltd., Northern Ireland, United Kingdom. The determinations of serum lipids were carried out using the Olympus AU400 automatic biochemistry analyzer (Olympus Optical Co. Ltd., Japan). The sample with higher lipid level exceeding the linear range of the kit was diluted with normal saline and the diluted volume was calculated.
Commercially available kits for the determinations of FSH, LH, TT, E2 and SHBG were purchased from Beckman Coulter, Inc., USA. Serum TT, LH, FSH, E2 and SHBG levels were determined by chemiluminescence assay using an automated Unicel Dxi 800 Access Immunoassay System (Beckman Coulter, Inc., USA). The assay sensitivities were 0.35 nmol/L for TT, 0.2 IU/L for LH, 0.2 IU/L for FSH, 73 pmol/L for E2 and 0.33 nmol/L for SHBG. The intra-assay coefficients of variation (CV) for LH, FSH, TT, E2 and SHBG were all less than 5%, and the inter-assay CVs were all less than 8%.
Determinations of seminal plasma lipids and reproductive hormones
After liquefaction, the routine analysis of each semen sample was performed, and the remaining was centrifuged at 12 000 g for 5 min. The upper layer seminal plasma was collected for the determinations of lipids and reproductive hormones [4]. Commercially available kits for the determinations of TG, TC, LDL and HDL were purchased from Shanghai Zhicheng Biotechnology Co., Ltd., China. Calibration and quality control products were purchased from Randox Laboratories Ltd., Northern Ireland, United Kingdom. Determination of lipids in seminal plasma was carried out using the Olympus AU400 automatic biochemistry analyzer (Olympus Optical Co. Ltd., Japan). For lower LH level in seminal plasma, we didn’t obtain its information. So, we only determined seminal plasma FSH, TT and E2 levels. Commercially available kits for the determinations of FSH, TT and E2 were purchased from Beckman Coulter, Inc., USA. Seminal plasma FSH, TT and E2 levels were determined by chemiluminescence assay using an automated Unicel Dxi 800 Access Immunoassay System (Beckman Coulter, Inc., USA). The sample with higher or lower level exceeding the linear range of the kit was diluted with normal saline or added sample size.
Statistical analysis
All data analyses were conducted using SPSS 11.0 software (SPSS Inc., Chicago, IL, USA). First, nonparametric tests (one-sample Kolmogorov–Smirnov test) were used to determine whether analyzed parameters were normally distributed. If the parameter was consistent with normal distribution, correlations between sperm DFI and age, obesity-associated markers, semen parameters, seminal plasma biochemical markers, and serum and seminal plasma lipids and reproductive hormones levels were examined by Pearson test. If the parameter was consistent with skewed distribution, correlations were examined by Spearman’s rho test. The variables which were significantly correlated with sperm DFI in Spearman’s rho test were further assessed by the multivariate logistic regression analysis. The differences among different groups and between two groups with different number of samples were assessed by one-way ANOVA test and independent-samples t test, respectively. P-value < 0.05 was considered statistically significant.
Results
The results of obesity-associated markers and sperm DFI were obtained from all of 1 010 subfertile men. The numbers of men with intact and effective semen parameters (32 men with 100% of spermatia or 100% of teratospermia were excluded), seminal plasma biochemical markers, serum lipids, serum reproductive hormones, seminal plasma lipids and seminal plasma reproductive hormones were 978, 959, 974, 954, 887 and 396, respectively. Table 1 showed the mean, standard deviation and range of all these variables. The representative figure of sperm DFI was shown in Fig. 1.
Table 1.
The mean, standard deviation and range of the investigated parameters in 1 010 subfertile men
| Variables | Number | Mean (SD) | Range |
|---|---|---|---|
| Age (years) | 1010 | 28.89 (4.60) | 18-50 |
| DFI (%) | 1010 | 18.61 (12.46) | 1.61-85.78 |
| BMI (kg/m2) | 1010 | 23.98 (3.08) | 17.36-41.03 |
| WC (cm) | 1010 | 82.14 (9.27) | 60-131 |
| WHR | 1010 | 0.86 (0.06) | 0.70-1.13 |
| WHtR | 1010 | 0.47 (0.05) | 0.34-0.74 |
| Abstinence time (day) | 1010 | 4.28 (1.72) | 1-20 |
| Semen volume (ml) | 978 | 3.79 (1.42) | 1.1-12 |
| Sperm concentration (106/ml) | 978 | 60.78 (51.97) | 0.67-340.44 |
| Total sperm count (106/ejaculate) | 978 | 220.95 (199.10) | 1.35-1364.86 |
| Progressive motility (%) | 978 | 32.29 (12.93) | 0.91-81.60 |
| Sperm motility (%) | 978 | 45.37 (19.22) | 1.51-98.89 |
| Normal sperm morphology (%) | 978 | 4.52 (2.01) | 0.41-17.56 |
| TNPMS (106/ejaculate) | 978 | 3.81 (5.00) | 0.01-45.20 |
| Abnormal head (%) | 978 | 67.84 (7.86) | 11.10-95.15 |
| Intact acrosome (%) | 978 | 57.00 (10.61) | 8.18-83.11 |
| Acrosomal enzyme activity (IU/106 sperm) | 978 | 48.46 (15.69) | 15.13-127.65 |
| Seminal plasma total α-glucotase (U/ml) | 959 | 416.93 (191.90) | 48.66-1019.84 |
| Seminal plasma GGT (U/ml) | 959 | 2436.08 (836.43) | 17.62-4477.56 |
| Seminal plasma fructose (mmol/L) | 959 | 14.24 (7.18) | 3.48-53.83 |
| Seminal plasma zinc (mmol/L) | 959 | 2.62 (1.16) | 0.14-7.62 |
| Serum TG (mmol/L) | 974 | 1.65 (1.48) | 0.05-23.34 |
| Serum TC (mmol/L) | 974 | 4.39 (0.93) | 0.54-12.19 |
| Serum LDL (mmol/L) | 974 | 2.59 (0.73) | 0.02-5.77 |
| Serum HDL (mmol/L) | 974 | 1.22 (0.26) | 0.67-3.04 |
| Serum LH (IU/L) | 954 | 4.25 (2.13) | 0.98-25.45 |
| Serum FSH (IU/L) | 954 | 4.84 (2.55) | 1.14-26.43 |
| Serum testosterone (nmol/L) | 954 | 13.14 (4.06) | 1.80-30.41 |
| Serum estradiol (pmol/L) | 954 | 108.74 (48.63) | 3.0-331.0 |
| Serum SHBG (nmol/L) | 954 | 26.02 (10.92) | 5.7-70.7 |
| Seminal plasma TG (mmol/L) | 887 | 0.14 (0.11) | 0.01-0.90 |
| Seminal plasma TC (mmol/L) | 887 | 0.85 (0.51) | 0.01-3.11 |
| Seminal plasma LDL (mmol/L) | 887 | 0.66 (0.34) | 0.01-2.70 |
| Seminal plasma HDL (mmol/L) | 887 | 0.32 (0.21) | 0.01-1.64 |
| Seminal plasma FSH (IU/L) | 396 | 0.29 (0.26) | 0.10-2.13 |
| Seminal plasma testosterone (nmol/L) | 396 | 4.44 (2.15) | 1.18-14.57 |
| Seminal plasma estradiol (pmol/L) | 396 | 276.17 (90.61) | 62.06-505.73 |
TNPMS Total normal-progressively motile spermatozoa count, SHBG sex hormone-binding globulin
Fig. 1.
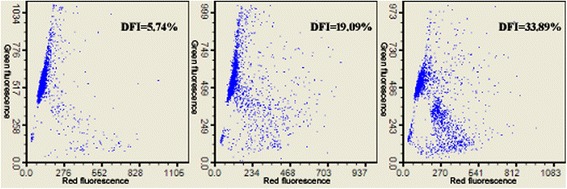
Sperm DFI detected by flow cytometry. Sperm DFI was assessed by the sperm chromatin structure assay (SCSA). In brief, semen samples were treated for 30 s with 400 μl of a solution of 0.1 % Triton X-100, 0.15 mol/L NaCl, and 0.08 mol/L HCl, pH 1.2. After 30 s, 1.2 ml of staining buffer (6 μg/ml acridine orange [AO], 37 mmol/L citric acid, 126 mmol/L Na2HPO4, 1 mmol/L disodium EDTA, 0.15 mol/L NaCl, pH 6.0) was admixed to the test tube. The sample was placed into the FACS Calibur flow cytometer with the sample flowing to establish optimal sheath/sample flow, and then at exactly 3 min AO staining measurements were taken. A minimum of 5 000 cells from two aliquots of each sample were acquired and analyzed by FACS scan interfaced with a data analysis software. After completion of the sample analysis, the cytogram (red vs green fluorescence) and DFI readings were generated.
Next, we analyzed the correlations between sperm DFI and age, obesity-associated markers, semen parameters, seminal plasma biochemical markers, serum lipids and reproductive hormones, and seminal plasma lipids and reproductive hormones in subfertile men. All these variables were consistent with skewed distribution, so the correlations between these variables were analyzed by Spearman’s rho test, and the obtained results were shown in Table 2. The results showed that Sperm DFI was positively related to age and abstinence duration (P<0.001). Sperm DFI was also positively related to semen volume and percentage of abnormal sperm head (P<0.001), while negatively related to sperm concentration, progressive motility, sperm motility, percentage of normal sperm morphology, TNPMS, percentage of intact acrosome sperm and acrosomal enzyme activity (P<0.001). Sperm DFI was positively related to seminal plasma zinc but unrelated to seminal plasma total α-glucotase, GGT and fructose levels. There was no any significant correlation between sperm DFI and obesity-associated markers such as BMI, WC, WHR and WHtR, and serum lipids levels. However, sperm DFI was positively related to seminal plasma TG and TC levels. Sperm DFI was also positively related to serum LH and FSH levels and seminal plasma FSH and estradiol levels, but unrelated to serum and seminal plasma testosterone levels.
Table 2.
Non-parametric (Spearman) correlation coefficients (r) for relationships between sperm DFI and other parameters
| Variables | Number | DFI | |
|---|---|---|---|
| r | P value | ||
| Age | 1010 | 0.115 | <0.001 |
| BMI | 1010 | -0.051 | 0.108 |
| WC | 1010 | -0.065 | 0.050 |
| WHR | 1010 | -0.009 | 0.785 |
| WHtR | 1010 | -0.057 | 0.068 |
| Abstinence time | 1010 | 0.171 | <0.001 |
| Semen volume | 978 | 0.145 | <0.001 |
| Sperm concentration | 978 | -0.115 | <0.001 |
| Total sperm count | 978 | -0.054 | 0.091 |
| Progressive motility | 978 | -0.474 | <0.001 |
| Sperm motility | 978 | -0.487 | <0.001 |
| Normal sperm morphology | 978 | -0.222 | <0.001 |
| TNPMS | 978 | -0.288 | <0.001 |
| Abnormal head | 978 | 0.184 | <0.001 |
| Intact acrosome | 978 | -0.191 | <0.001 |
| Acrosomal enzyme activity | 978 | -0.307 | <0.001 |
| Seminal plasma total α-glucotase | 959 | 0.089 | 0.057 |
| Seminal plasma GGT | 959 | 0.080 | 0.063 |
| Seminal plasma fructose | 959 | 0.002 | 0.957 |
| Seminal plasma zinc | 959 | 0.137 | <0.001 |
| Serum TG | 974 | 0.026 | 0.411 |
| Serum TC | 974 | -0.007 | -0.820 |
| Serum LDL | 974 | -0.005 | -0.865 |
| Serum HDL | 974 | 0.008 | 0.814 |
| Serum LH | 954 | 0.134 | <0.001 |
| Serum FSH | 954 | 0.113 | <0.001 |
| Serum estradiol | 954 | -0.039 | 0.234 |
| Serum testosterone | 954 | -0.042 | 0.194 |
| Serum SHBG | 954 | 0.038 | 0.239 |
| Seminal plasma TG | 887 | 0.136 | <0.001 |
| Seminal plasma TC | 887 | 0.131 | <0.001 |
| Seminal plasma LDL | 887 | 0.013 | 0.695 |
| Seminal plasma HDL | 887 | -0.064 | 0.058 |
| Seminal plasma FSH | 396 | 0.184 | <0.001 |
| Seminal plasma estradiol | 396 | 0.099 | 0.048* |
| Seminal plasma testosterone | 396 | -0.039 | 0.433 |
TNPMS Total normal-progressively motile spermatozoa count, SHBG sex hormone-binding globulin. *: P<0.05. Sperm DFI was positively related to age and abstinence time. Sperm DFI was also positively related to semen volume and the percentage of abnormal sperm head, while negatively related to sperm concentration, progressive motility, sperm motility, the percentage of normal sperm morphology, TNPMS, the percentage of intact acrosome sperm and acrosomal enzyme activity. Sperm DFI was positively related to seminal plasma zinc but unrelated to seminal plasma α-glucotase, GGT and fructose levels. There was no any significant correlation between sperm DFI and obesity-associated markers such as BMI, WC, WHR and WHtR, and serum lipids levels. However, sperm DFI was positively related to seminal plasma TG and TC levels. Sperm DFI was also positively related to serum LH and FSH levels and seminal plasma FSH and estradiol levels, but unrelated to serum and seminal plasma testosterone levels
Although Spearman correlation analysis found that many variables were correlated with sperm DFI, some of them may be confounders. Therefore, we further performed the multivariate regression analysis to assess the variables which were significantly correlated with sperm DFI in Spearman’s rho test. It was found that age, semen volume, sperm concentration, progressive motility, TNPMS and intact acrosome were independently correlated with sperm DFI (Table 3).
Table 3.
The multivariate regression analysis of the variables correlated with sperm DFI in Spearman correlation (n=298)
| Variables | Odds Ratio | 95% CI | P value |
|---|---|---|---|
| Age | 0.865 | 0.785-0.954 | 0.004 |
| Abstinence time | 0.926 | 0.719-1.193 | 0.551 |
| Semen volume | 0.548 | 0.390-0.771 | 0.001 |
| Sperm concentration | 0.978 | 0.962-0.994 | 0.008 |
| Progressive motility | 1.173 | 1.041-1.323 | 0.009 |
| Sperm motility | 0.985 | 0.907-1.069 | 0.713 |
| Normal sperm morphology | 0.716 | 0.487-1.051 | 0.088 |
| TNPMS | 1.307 | 1.012-1.687 | 0.040 |
| Abnormal head | 0.996 | 0.947-1.048 | 0.876 |
| Intact acrosome | 1.082 | 1.006-1.163 | 0.033 |
| Acrosomal enzyme activity | 0.996 | 0.975-1.019 | 0.750 |
| Seminal plasma zinc | 1.229 | 0.705-2.142 | 0.466 |
| Serum LH | 0.976 | 0.768-1.239 | 0.839 |
| Serum FSH | 1.086 | 0.888-1.328 | 0.422 |
| Seminal plasma TG | 1.677 | 0.046-60.819 | 0.778 |
| Seminal plasma TC | 0.807 | 0.224-2.913 | 0.744 |
| Seminal plasma FSH | 0.455 | 0.075-2.752 | 0.391 |
| Seminal plasma estradiol | 0.987 | 0.970-1.003 | 0.111 |
TNPMS Total normal-progressively motile spermatozoa count. Age, semen volume, sperm concentration, progressive motility, TNPMS and intact acrosome were independently correlated with sperm DFI
In addition, for further verifying the correlations of sperm DFI with obesity-associated markers, we set different groups according to the criteria of obesity, and compared sperm DFI based on the dichotomized analyses for BMI, WC, WHR and WHtR (Table 4). We found that there was no any significant difference in sperm DFI among different groups, demonstrating again that sperm DFI was not correlated with obesity-assocaited markers.
Table 4.
Comparison of sperm DFI based on the dichotomized analyses for BMI, WHR, WC and WHtR
| Variables | Number | DFI (%) | |
|---|---|---|---|
| BMI (kg/m2) | <18.5 | 20 | 17.60 ± 10.61 |
| 18.5-23.99 | 507 | 18.91 ± 12.43 | |
| 24-27.99 | 389 | 18.63 ± 12.77 | |
| ≥28 | 94 | 17.17 ± 11.76 | |
| F | 0.556 | ||
| P | 0.644 | ||
| WC (cm) | <90 | 807 | 18.76 ± 12.50 |
| ≥90 | 203 | 18.04 ± 12.33 | |
| t | 0.548 | ||
| P | 0.459 | ||
| WHR | <0.9 | 747 | 18.75 ± 12.54 |
| ≥0.9 | 263 | 18.24 ± 12.26 | |
| t | 0.321 | ||
| P | 0.571 | ||
| WHtR | <0.5 | 686 | 19.00 ± 12.71 |
| ≥0.5 | 324 | 17.80 ± 11.91 | |
| t | 2.037 | ||
| P | 0.154 | ||
Discussion
The determination of sperm DNA damage, as an important supplement to semen routine determination strategies, has been applied in some clinical andrology laboratories worldwide. What factors may lead to sperm DNA damage remains one of the major concerns. There were increasingly accumulated evidence for the correlation between obesity and male subfertility. It was reported that obesity was closely related to male subfertility [16]. Obesity and related abnormal lipids metabolism and the change of reproductive hormones might lead to the decrease of sperm quality [3, 4]. However, it has little reports whether obesity and related abnormal lipids metabolism and reproductive hormones may lead to the increase of sperm DNA damage. Based on these, we prospectively investigated the correlations between sperm DFI and obesity-associated markers, serum and seminal plasma lipids and reproductive hormones, as well as, age, abstinence time and semen parameters.
First, as expected, our results showed that sperm DFI was positively related to age and abstinence time in subfertile men, which was consistent with other reports [17–19]. Moreover, it was reported that sperm DFI in men with age ≤ 35 years was significantly lower than that in men with age between 36-39 years and above 40 years, and no significant difference in the latter two [17, 18], indicating that the proportion of sperm with DNA damage increased with increasing age, and that the best child-bearing age in males would be before 35 years old. In addition, in order to guarantee sperm quality, the abstinence time in males should not too long.
Next, we investigated the correlations between sperm DFI and semen parameters and seminal plasma biochemical markers. Similar to others’ results [20–22], we found that sperm DFI was positively related to semen volume and percent of abnormal head sperm (P<0.001), while negatively related to sperm concentration, PR, sperm motility, TNPMS, percent of normal sperm morphology, percent of intact acrosome and acrosomal enzyme activity (P<0.001). It was reported that sperm DFI was independent of and could predict male fertility better than routine semen parameters [23]. Our data showed that sperm DFI was almost significantly related to all parameters reflecting semen quality, especially TNPMS, indicating that sperm DNA damage might be key factor leading to the decrease of semen quality, that is, abnormal semen parameters.
We found that sperm DFI was positively related to seminal plasma zinc level (P<0.001), but unrelated to seminal plasma total α-glucosidase, GGT and fructose levels. Seminal plasma total α-glucosidase, GGT and fructose levels may be used to evaluate the secretory function of epididymis, prostate and seminal vesicle, indicating that the auxiliary gonad might be less effect on sperm DNA damage, and that sperm DNA damage might be existed before sperm transferred into epididymis. It was reported that zinc played an important role not only in normal testis development but also in spermatogenesis and the maintenance of sperm motility [24, 25]. However, the excess of zinc in seminal plasma may accumulate in sperm, produce adverse effect on sperm DNA quality [26], and reduce sperm motility and survival [25]. It is indicated that the excess of seminal plasma zinc might lead to sperm DNA damage, and that the supplement of zinc in clinic should be appropriate.
Further investigations on the relationship between obesity-associated markers and sperm DFI showed that there was no any correlation between sperm DFI and BMI, WC, WHR and WHtR. Moreover, there was no any correlation between sperm DFI and serum lipids levels. This was consistent with our early investigation results, that is, obesity-associated markers could not predict semen quality in subfertile men [3], and there was no any correlation between serum lipids levels and semen parameters [4]. Furthermore, recently, Bandel et al. [5] found that there was no any correlation between BMI and sperm DNA integrity, which was similar to our results. However, we found that sperm DFI was positively related to seminal plasma TG and TC levels. As observed in our early study [4], unlike lipids levels in serum, TG, TC, LDL and HDL levels in seminal plasma were all negatively related to some of semen parameters. Likewise, this study demonstrated that the increase of seminal plasma TG and TC levels might lead to increased sperm DFI, indicating that the elevated lipids levels in seminal plasma might have adverse effect on sperm quality. However, the detail mechanism needs to be further elucidated.
As obesity could lead to the change of reproductive hormones, we also investigated the correlations between sperm DFI and seminal plasma reproductive hormones levels. The results showed that sperm DFI was positively related to not only serum LH and FSH levels but also seminal plasma FSH and E2 levels (P<0.001), but unrelated to serum and seminal plasma testosterone. Our early investigations had demonstrated that serum LH and FSH levels were negatively correlated with sperm concentration and percent of normal sperm morphology [3]. Moreover, it was reported that the change of serum LH and FSH levels had adverse effect on male fertility [27], and that serum FSH level was the risk factor of sperm DFI [28], which demonstrated that serum FSH and LH levels might have adverse effect on sperm maturation, and might interfere sperm DNA integrity. In addition, we also detected the reproductive hormones levels in seminal plasma, and found that sperm DFI was also positively related to seminal plasma FSH and E2 levels, indicating that the higher FSH and E2 levels in local genital tract likewise had adverse effect on sperm DNA integrity. All these demonstrated that reproductive hormones levels might influence on the integrity of sperm chromatin and then on sperm fertilizing ability [29].
Although Spearman correlation analysis found that many variables were correlated with sperm DFI, the multivariate regression analysis for the variables which were significantly correlated with sperm DFI in Spearman’s rho test showed that only age, semen volume, sperm concentration, progressive motility, TNPMS and intact acrosome were independently correlated with sperm DFI. Due to the less semen volume for each sample, not all the parameters of each sample investigated in the study were detected. A total of 298 samples were analyzed all the parameters. Perhaps it was the lower size of samples that led to the difference in the results between Spearman correlation analysis and multivariate regression analysis. Therefore, whether sperm DFI is correlated with abstinence time, sperm motility, normal sperm morphology, abnormal head, acrosomal enzyme activity, serum LH and FSH, and seminal plasma zinc, TG, TC, FSH and estradiol should be further demonstrated by a large size of samples.
In the future, the mechanisms that independent correlation factors lead to sperm DNA damage should be clarified, while the potential correlation factors should be further demonstrated. The key goal to investigate sperm DFI-related factors is to prevent sperm DNA damage during spermatogenesis and sperm maturation. Therefore, the intervention experiments to some correlation factors may verify their effects on sperm DNA damage.
Conclusions
In conclusion, there are many potential factors associated with sperm DFI, including age, abstinence time, spermatogenesis and maturation, seminal plasma lipids and reproductive hormones levels. However, the potential effects of seminal plasma lipids and reproductive hormones on sperm DNA damage need still to be demonstrated by the studies with scientific design and a large size of samples.
Additional file
Raw data for DFI and its related variables. (XLS 388 kb)
Acknowledgements
None.
Funding
This study was supported by State Key Development Program for Basic Research of China (2013CB945200).
Availability of data and materials
The data and materials are available from the corresponding author on reasonable requests.
Abbreviations
- DFI
sperm DNA fragmentation index
- SCSA
sperm chromatin structure assay
- AO
acridine orange
- TSC
total sperm count
- PR
progressive motility
- TNPMS
total normal-progressively motile sperm count
- NSM
percent of normal sperm morphology
- CASA
computer-aided sperm analysis
- BMI
body mass index
- WHR
waist-to-hip ratio
- WC
waist circumference
- WHtR
waist-to-height ratio
- TG
triglyceride
- TC
total cholesterol
- LDL-C
low density lipoprotein cholesterol
- HDL-C
high density lipoprotein cholesterol
- GGT
γ-glutamyl transpeptidase
- SHBG
sex hormone binding globulin
- FSH
follicle stimulating hormone
- E2
estradiol
- T
testosterone
- TT
total testosterone
- c-PAHs
polycyclic aromatic hydrocarbons
- ZEA
zearalenone
Authors’ contributions
J-CL and JJ had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. BY contributed to the study concept and design. All authors contributed to the acquisition, analysis, and interpretation of data. J-CL and JJ provided the statistical analysis. J-CL and BY performed the drafting of the manuscript. The funding was obtained by BY. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Human Subject Committees of Nanjing Jinling Hospital (Approved number: 2012NZKY-012), and informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12958-018-0345-y) contains supplementary material, which is available to authorized users.
Contributor Information
Jin-Chun Lu, Email: lujc@androl.cn.
Jun Jing, Email: jingjun36@163.com.
Li Chen, Email: 872276095@qq.com.
Yi-Feng Ge, Email: 957129249@qq.com.
Rui-Xiang Feng, Email: fengrx1975@163.com.
Yuan-Jiao Liang, Email: yuanjiao1965@126.com.
Bing Yao, Phone: +86-25-8086-0174, Email: 2424572228@qq.com.
References
- 1.Lu JC. Clinical application values and existed issues in the determination of sperm DNA damage. Chin Med J. 2015;95:2989–2993. [Google Scholar]
- 2.Lu JC. Related factors of sperm DNA damage: Advances in studies. Zhonghua Nan Ke Xue. 2015;21:675–680. [PubMed] [Google Scholar]
- 3.Lu JC, Jing J, Dai JY, Zhao AZ, Yao Q, Fan K, et al. Body mass index, waist-to-hip ratio, waist circumference and waist-to-height ratio cannot predict male semen quality: A report of 1231 subfertile Chinese men. Andrologia. 2015;47:1047–1054. doi: 10.1111/and.12376. [DOI] [PubMed] [Google Scholar]
- 4.Lu JC, Jing J, Yao Q, Fan K, Wang GH, Feng RX, et al. Relationship between lipids levels of serum and seminal plasma and semen parameters in 631 Chinese subfertile men. PLoS One. 2016;11:e0146304. doi: 10.1371/journal.pone.0146304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bandel I, Bungum M, Richtoff J, Malm J, Axelsson J, Pedersen HS, et al. No association between body mass index and sperm DNA integrity. Hum Reprod. 2015;30:1704–1713. doi: 10.1093/humrep/dev111. [DOI] [PubMed] [Google Scholar]
- 6.Cai L, Liu A, Zhang Y, Wang P. Waist-to-height ratio and cardiovascular risk factors among Chinese adults in Beijing. PLoS One. 2013;8:e69298. doi: 10.1371/journal.pone.0069298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia WP, Lu JX, Xiang KS, Bao YQ, Lu HJ, Chen L. Prediction of abdominal visceral obesity from body mass index, waist circumference and waist-hip ratio in Chinese adults: receiver operating characteristic curves analysis. Biomed Environ Sci. 2003;16:206–211. [PubMed] [Google Scholar]
- 8.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1. Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 9.Raman R, Rani PK, Gnanamoorthy P, Sudhir RR, Kumaramanikavel G, Sharma T. Association of obesity with diabetic retinopathy: Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetics Study (SN-DREAMS Report no. 8) Acta Diabetol. 2010;47:209–215. doi: 10.1007/s00592-009-0113-8. [DOI] [PubMed] [Google Scholar]
- 10.Lu JC, Huang YF, Lü NQ. Computer-aided sperm analysis: Past, present and future. Andrologia. 2014;46:329–338. doi: 10.1111/and.12093. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization . Laboratory manual for the examination and processing of human semen. 5. Geneva: World Health Organization; 2010. [Google Scholar]
- 12.Lu JC, Lu KG, Zhang HY, Feng RX. Establishment and evaluation of automatic method for seminal plasma γ-L-glutamyl transpeptidase detection. Zhonghua Nan Ke Xue. 2013;19:1077–1081. [PubMed] [Google Scholar]
- 13.Zhang HY, Lu JC, Lu KG, Feng RX. Establishment and evaluation of an automatic method for seminal plasma α-glucosidase detection. Zhonghua Nan Ke Xue. 2014;20:886–889. [PubMed] [Google Scholar]
- 14.Lu JC, Lu KG, Zhang HY, Feng RX. Establishment and evaluation of automatic assay on level of seminal plasma zinc. Chin J Androl. 2015;29:31–35. [Google Scholar]
- 15.Lu JC, Lu KG, Zhang HY, Feng RX. Evaluation of rate method for detection of seminal plasma fructose. Chin J Clin Lab Sci. 2015;33:6–8. [Google Scholar]
- 16.Cabler S, Agarwal A, Flint M, du Plessis SS. Obesity: Modern man’s fertility nemesis. Asian J Androl. 2010;12:480–489. doi: 10.1038/aja.2010.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bojar I, Witczak M, Wdowiak A. Biological and environmental conditionings for a sperm DNA fragmentation. Ann Agric Environ Med. 2013;20:865–868. [PubMed] [Google Scholar]
- 18.Das M, Al-Hathal N, San-Gabriel M, Phillips S, Kadoch IJ, Bissonnette F, et al. High prevalence of isolated sperm DNA damage in infertile men with advanced paternal age. J Assist Reprod Genet. 2013;30:843–848. doi: 10.1007/s10815-013-0015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Håkonsen LB, Spano M, Bonde JP, Olsen J, Thulstrup AM, Ernst E, et al. Exposures that may affect sperm DNA integrity: Two decades of follow-up in a pregnancy cohort. Reprod Toxicol. 2012;33:316–321. doi: 10.1016/j.reprotox.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 20.Alkhayal A, San Gabriel M, Zeidan K, Alrabeeah K, Noel D, McGraw R, et al. Sperm DNA and chromatin integrity in semen samples used for intrauterine insemination. J Assist Reprod Genet. 2013;30:1519–1524. doi: 10.1007/s10815-013-0101-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venkatesh S, Singh A, Shamsi MB, Thilagavathi J, Kumar R, Mitra DK, et al. Clinical significance of sperm DNA damage threshold value in the assessment of male infertility. Reprod Sci. 2011;18:1005–1013. doi: 10.1177/1933719111401662. [DOI] [PubMed] [Google Scholar]
- 22.Chi HJ, Chung DY, Choi SY, Kim JH, Kim GY, Lee JS, et al. Integrity of human sperm DNA assessed by the neutral comet assay and its relationship to semen parameters and clinical outcomes for the IVF-ET program. Clin Exp Reprod Med. 2011;38:10–17. doi: 10.5653/cerm.2011.38.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xue X, Wang WS, Shi JZ, Zhang SL, Zhao WQ, Shi WH, et al. Efficacy of swim-up versus density gradient centrifugation in improving sperm deformity rate and DNA fragmentation index in semen samples from teratozoospermic patients. J Assist Reprod Genet. 2014;31:1161–1166. doi: 10.1007/s10815-014-0287-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Atig F, Raffa M, Habib BA, Kerkeni A, Saad A, Ajina M. Impact of seminal trace element and glutathione levels on semen quality of Tunisian infertile men. BMC Urol. 2012;12:6. doi: 10.1186/1471-2490-12-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan MS, Zaman S, Sajjad M, Shoaib M, Gilani G. Assessment of the level of traceelement zinc in seminal plasma of males and evaluation of its role in male infertility. Int J Appl Basic Med Res. 2011;l:93–96. doi: 10.4103/2229-516X.91152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.García-Contreras A, De Loera Y, García-Artiga C, Palomo A, Guevara JA, Herrera-Haro J, et al. Elevated dietary intake of Zn-methionate is associated with increased sperm DNA fragmentation in the boar. Reprod Toxicol. 2011;3l:570–573. doi: 10.1016/j.reprotox.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 27.Miranda-Contreras L, Gómez-Pérez R, Rojas G, Cruz I, Berrueta L, Salmen S, et al. Occupational exposure to organophosphate and carbamate pesticides affects sperm chromatin integrity and reproductive hormone levels among Venezuelan farm workers. J Occup Health. 2013;55:195–203. doi: 10.1539/joh.12-0144-FS. [DOI] [PubMed] [Google Scholar]
- 28.Smit M, Romijn JC, Wildhagen MF, Weber RF, Dohle GR. Sperm chromatin structure is associated with the quality of spermatogenesis in infertile patients. Fertil Steril. 2010;94:1748–1752. doi: 10.1016/j.fertnstert.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 29.Richthoff J, Spano M, Giwercman YL, Frohm B, Jepson K, Malm J, et al. The impact of testicular and accessory sex gland function on sperm chromatin integrity as assessed by the sperm chromatin structure assay (SCSA) Hum Reprod. 2002;17:3162–3169. doi: 10.1093/humrep/17.12.3162. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Raw data for DFI and its related variables. (XLS 388 kb)
Data Availability Statement
The data and materials are available from the corresponding author on reasonable requests.


