Abstract
Background and Aims
Investigating the biology of trees that were growing at high latitudes during warmer geological periods is key to understanding the functioning of both past and future forest ecosystems. The aim of this study is to report the first co-occurrence of epicormic shoots and traumatic growth zones in fossil trees from the Triassic of Antarctica and to discuss their biological and environmental implications.
Methods
Permineralized woods bearing scars of epicormic shoots were collected from the Triassic Fremouw Formation in Gordon Valley, Central Transantarctic Mountains, Antarctica in 2010. Samples from different portions of three specimens were prepared using standard thin section and hydrofluoric (HF) acid peel techniques, and anatomical details were studied in transmitted light.
Key Results
The fossil woods represent the outer part of trunks, with at least 40 growth rings that are 0.2–4.8 mm in width. Anatomical comparisons suggest that they represent a new tree taxon for the Triassic of Antarctica. Numerous small epicormic shoots can be seen crossing the wood almost horizontally and are locally branched. Each specimen also contains several occurrences of traumatic growth zones located in the early wood, in the cells produced either at the very start of the growing season or slightly later.
Conclusions
This is the first report of epicormic shoots and traumatic growth zones in the wood of a Triassic tree from Antarctica. Their co-occurrence indicates that these trees from Gordon Valley were subjected to environmental stresses not seen in Triassic trees previously described from this region. This suggests that they had a different biology and/or were growing in a different habitat, which offers a new glimpse into the diversity of high-latitude trees in the Triassic greenhouse climate.
Keywords: Tree, fossil, Triassic, Antarctica, anatomy, epicormics, wood, frost ring, growth, high latitude, Mesozoic, gymnosperm
INTRODUCTION
During the Triassic (251–200 Mya), Antarctica was part of the supercontinent Gondwana and, although it was not centred on the South Pole as it is today, most of it was located at high paleolatitudes (Fig. 1). Thanks to the Triassic greenhouse climate, temperatures were far milder than at comparable latitudes today, and Antarctica was covered by forests with no extant equivalent, growing in a warm temperate climate but under the strongly seasonal light regime characteristic of high latitudes (e.g. Taylor and Taylor, 1990; Escapa et al., 2011; Cantrill and Poole, 2012). Fossil evidence shows that these forests were composed of diverse plants including bryophytes, equisetophytes, lycophytes, ferns, cycads, ginkgos, conifers and several families of pteridosperms, an extinct seed plant group (Escapa et al., 2011; Bomfleur et al., 2011a, b, 2013a, b, 2014a, b). Among the fairly abundant macrofossils from the Triassic of Antarctica, those that are anatomically preserved can provide key information about the biology of these extinct plants and their interactions with their environment. This is especially true in the case of woody plants that can record long-term environmental information in their annual growth rings.
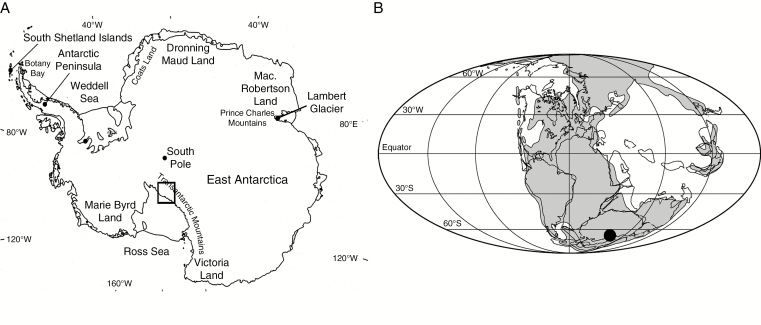
Fig. 1.
(A) Map of present-day Antarctica showing the location of Fremouw Peak and Gordon Valley in the Central Transantarctic Mountains. (B) Paleogeographical reconstruction for the Triassic with the position of the Transantarctic localities indicated.
To date, Triassic trees described in detail from Antarctica belong to two main groups, the conifers and the Corystospermales (=Umkomasiales, pteridosperms). Most of the information on the anatomy and affinities of these trees comes from trunks and young stems with attached leaf bases from the Fremouw Formation at Fremouw Peak, in the Central Transantarctic Mountains (Meyer-Berthaud and Taylor, 1991; Meyer-Berthaud et al., 1993; Bomfleur et al., 2013a; Decombeix et al., 2014). These specimens are preserved in sandstone and represent remains of allochthonous plants transported by a river system (Taylor et al., 1989). A study of tree rings in woods from Fremouw Peak has shown that they are characterized by a very small amount (a few layers of cells) of latewood and have a width of 0.1–6.8 mm (average 1.7 mm; Taylor and Ryberg, 2007). Additional information on Triassic trees in this region of Antarctica comes from an in situ forest discovered in the Fremouw Formation in 1991 at Gordon Valley, about 10 km from Fremouw Peak (Cúneo et al., 2003, and references therein). These trees belong to the corystosperms and formed a forest with a density of 274 trees ha–1 (Cúneo et al., 2003). The trunks studied reached 61 cm in diameter (average 27.7 cm) and their growth rings have the same structure as those found at Fremouw Peak, with only a few cells of latewood. All in all, the analyses of the trees from the Fremouw Formation at both Fremouw Peak and Gordon Valley suggest that they were growing in very favourable conditions. This is consistent with the co-occurrence of frost-sensitive plants, such as cycads (Smoot et al., 1985; Hermsen et al., 2009), and with tree ring analyses from other regions of Antarctica, such as the Allan Hills, in South Victoria Land (Gabites, 1985).
In this study, we describe new fossil trees collected in the Fremouw Formation that show evidence of the formation of epicormic shoots together with traumatic growth zones in their wood. This combination suggests that they were subjected to environmental stresses not noted in previously described Triassic trees from this formation. The biological and environmental implications of this finding are discussed.
MATERIALS AND METHODS
During the 2010–2011 austral field season, silicified wood and peat blocks were collected in the Antarctic locality of Gordon Valley, Central Transantarctic Mountains (Fig. 1). They occurred above the level at which the in situ corystosperm fossil forest was discovered in 1991 (Del Fueyo et al., 1995; Cúneo et al., 2003) but still within the Fremouw Formation. This formation is up to 700 m thick and consists of interbedded sandstones and mudstones. It represents an alluvial plain depositional system within an elongated sedimentary basin (Collinson et al., 1994). The age of the Fremouw Formation is Early to Middle Triassic (Barrett, 1969; Escapa et al., 2011). The specimens collected in 2010–2011 were found in sandstone and represent allochthonous remains, transported by the river system. Among these new fossils, several large pieces of decorticated gymnosperm trunks show conspicuous and numerous small branch scars on their outside and were selected for this study. Three specimens were cut into slices and the polished surfaces etched for 2–3 min in 49 % hydrofluoric (HF) acid to dissolve the silica matrix (Galtier and Philipps, 1999). The remaining organic matter of the cell walls was then transferred onto acetate sheets using acetone. Selected portions of these peels were mounted on slides using Eukitt for microscopic observation. In addition, ten thin sections (Hass and Rowe, 1999) in the tangential and radial planes were made from the wood in order to provide better imaging of fine anatomical details, such as cross-field pitting. Images were taken using a Leica (Leica Microsystems GmbH, Wetzlar, Germany) DC500 digital camera attachment on a Leica MZ16 stereomicroscope and on a Leica DM5000 B compound microscope. Plates were prepared with Photoshop CS5 (Adobe Systems Inc.). Handling of images in Photoshop consisted of cropping, rotation and adjustments of brightness, contrast and white balance. Cell and tissue dimensions were measured using ImageJ version 1.45 (Rasband, 1997–2012). Mean sensitivity was calculated using Fritts’ (1976) formula. The earlywood–latewood boundary was defined as the point where the radial diameter of tracheid lumen becomes smaller than the thickness of the two adjacent cell walls (Mork, 1928; Denne, 1989, formula 2; see also Taylor and Ryberg, 2007).
The specimens, and corresponding peels and slides are housed in the Paleobotany Division of the Natural History Museum and Biodiversity Institute, University of Kansas, Lawrence, under slide accession numbers 30,280–30,293 and specimen numbers 18,291, 18,292 and 18,294.
RESULTS
General aspect
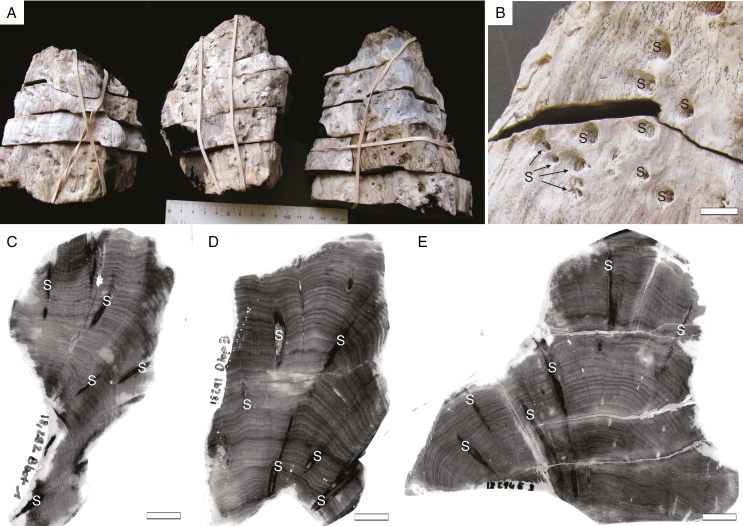
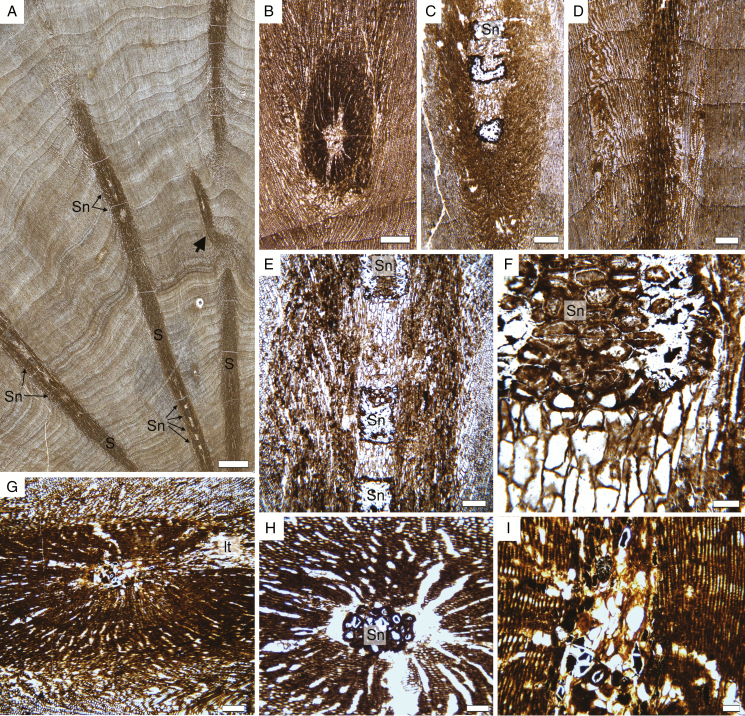
The specimens are respectively 11, 13 and 15 cm in length and 9, 10 and 9 cm in maximum diameter (Fig. 2A). They represent the outer portions of trunks, i.e. the central part (pith and primary xylem) is missing. This suggests that the entire original trunks were at least 18–20 cm in diameter. The outermost tissues (bark) have also been lost and the outer surface of the specimens shows small crowded scars of shoots embedded in the wood (Fig. 2A, B). All three specimens have distinct growth rings of variable width (Fig. 2C, D). In transverse section, the shoots cross the secondary xylem with a horizontal to oblique course and locally branch (Fig. 2C, D).
Fig. 2.
Triassic trees with epicormic shoots from Gordon Valley: general aspect. (A) General view of the three specimens. (B) Enlargement showing conspicuous shoot scars (S) within the wood. Scale bar = 1 cm. (C–E) Peels of selected transverse sections in the three specimens showing the distinct growth rings and several shoots (S) crossing the wood. (C) 18,292 Bbot1; (D) 18,291 Dtop3; (E) 18,294 E3. Scale bar = 1 cm.
Ring anatomy
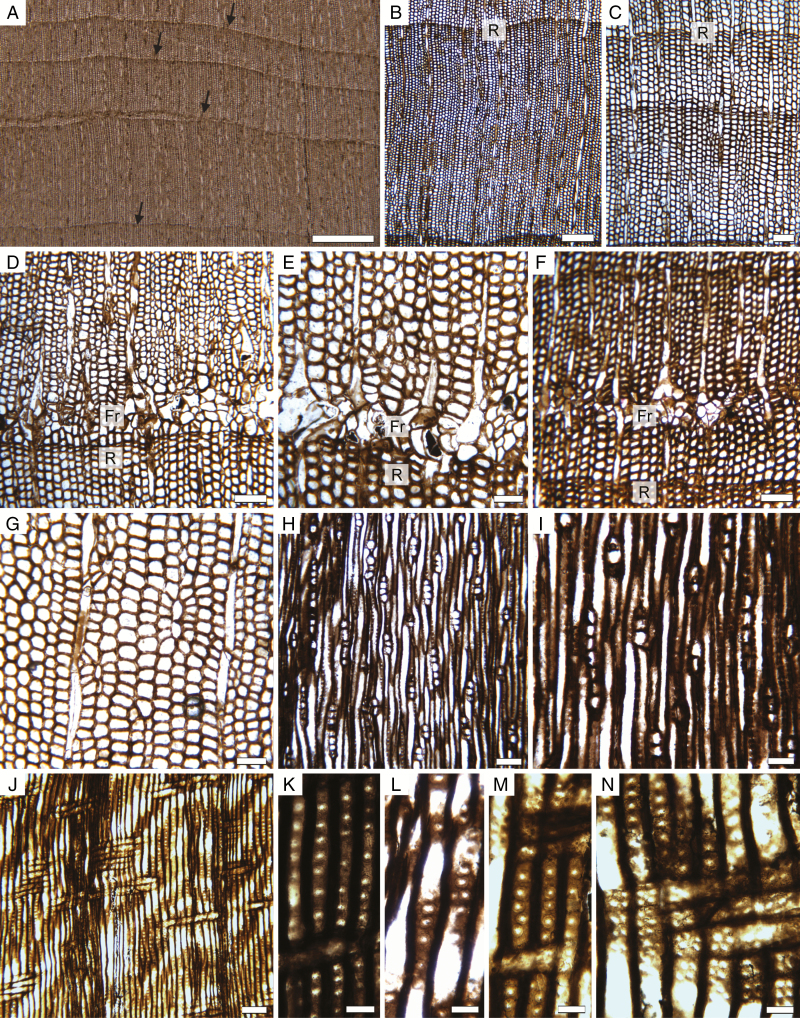
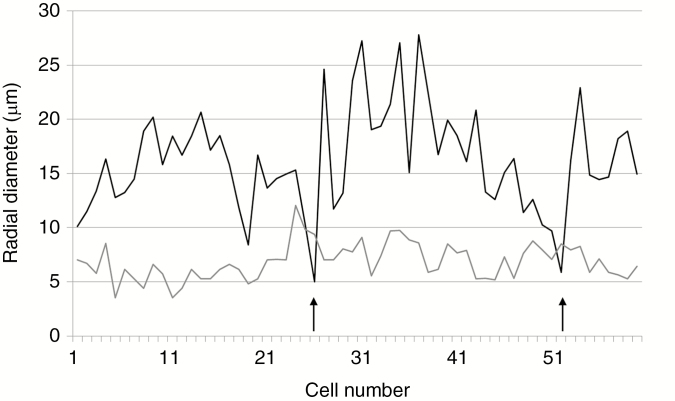
All specimens have ≥40 growth rings (Figs 2C–E, 3A–C and 4). Rings range from 0.2 to 4.8 mm, with an average of 1.2–1.3 mm depending on the specimen (Table 1). One of the largest rings observed had >65 cells radially (Fig. 3B), but average-sized rings typically contain around 20–40 cell layers (Fig. 3C). Mean sensitivity is in the 0.4–0.5 range and the trees are thus all considered as sensitive according to Fritts (1976). The rings typically have a very small amount of latewood, with 1–3 cells characterized by a smaller diameter and thicker cell wall (Fig. 4).
Fig. 3.
Triassic trees with epicormic shoots from Gordon valley: wood anatomy. (A) Transverse section of wood showing growth rings of various thicknesses. Arrows indicate ring boundaries. 18,292 Cbot1 (slide 30,291). (B) Detail of a wide growth ring (R: ring boundary). 18,292 Cbot1 (slide 30,291). (C) Detail of a smaller ring (R: ring boundary). 18,291 Ctop1 (slide 30,290). (D) Frost ring (Fr) occurring early in growth. 18,291 Ctop1 (slide 30,290). (E) Detail of cell distortion in a frost ring (Fr). 18,294 Btop1 (slide 30,293). (F) Frost ring (Fr) occurring later during the growth season. 18,291 Ctop1 (slide 30,290). (G) Disturbed radial arrangement of tracheid files. 18,294 Btop1 (slide 30,293). (H) General view in tangential longitudinal section showing low uniseriate rays. 18,294 A1 (slide 30,282). (I) Detail of rays in tangential longitudinal section. 18,294 A1 (slide 30,282). (J) Radial longitudinal section showing low parenchymatous rays, pitting on the radial wall of tracheids and a ring boundary. 18,294 A1 (slide 30,282). (K–N) Radial pitting. 18,294 A1 (slide 30,282). (K) Vertically spaced rounded pits. (L) Partly biseriate pitting with opposite pits. (M) Uniseriate crowded pits. (N) Detail of numerous, small crowded cross-field pits. Scale bar in (A) 1 mm, (B) 250 µm, (C), (D), (F), (H), (J) 100 µm, (E), (G), (I) 50 µm, (K), (L), (M), (N) 25 µm.
Fig. 4.
Triassic trees with epicormic shoots from Gordon Valley: variations of tracheid lumen radial diameter (black line) vs. wall thickness (grey line) along a typical growth ring. Arrows indicate the location of latewood (sensu Mork, 1928; Denne 1989, formula 2, i.e. where the radial diameter of the tracheid lumen becomes smaller than the thickness of the two succeeding cell walls).
Table 1.
Growth ring data for the three specimens
| Specimen | 18,291 | 18,292 | 18,294 |
|---|---|---|---|
| Minimum trunk radius | 9 cm | 10 cm | 9 cm |
| Number of rings measured | 44 | 43 | 65 |
| Average ring thickness | 1.3 mm | 1.3 mm | 1.2 mm |
| Minimum and maximum ring thickness | 0.5–2.7 mm | 0.2–4.8 mm | 0.3–3.6 mm |
| Mean sensitivity | 0.42 | 0.49 | 0.48 |
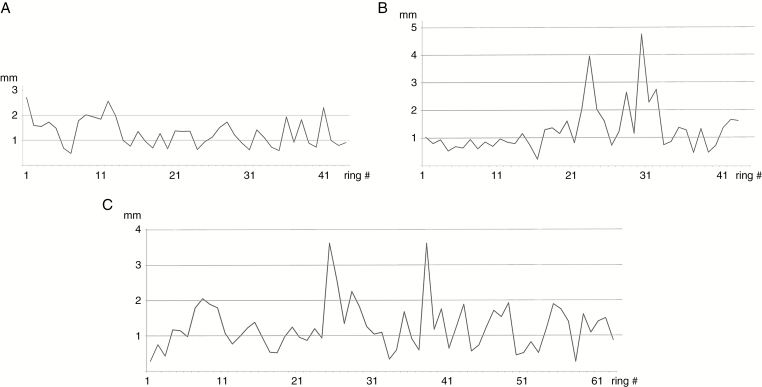
In order to test whether the three specimens could actually belong to different parts of the same trunk, we compared their variations in ring width to look for similar trends in growth rhythms (Fig. 5). Specimens 18,292 and 18,294 show very similar trends, especially in the occurrence of their smallest and largest rings, but it was not possible to correlate them exactly. Specimen 18,291 has a different ring pattern.
Fig. 5.
Triassic trees with epicormic shoots from Gordon Valley: variations of growth ring size (mm) along a transverse section in each specimen. (A) 18,291. (B) 18,292. (C) 18,294.
In addition to normal rings, all the specimens contain one or several so-called ‘frost rings’, characterized by collapsed tracheids with thin walls, and bent rays with enlarged cells (Fig. 3D–F). These traumatic growth zones occur either in the first part of the earlywood (Fig. 3D) or, more rarely, further in the ring (Fig. 3F). Most have a limited lateral extent.
Other variations in cambial growth are indicated by the repeated occurrence of irregular files of tracheids, with periclinal divisions and/or loss of initials (Fig. 3G). These perturbations occur even in zones of the wood that are located away from any epicormic shoots.
Wood anatomy
The secondary xylem is composed of parenchymatous rays and tracheids. Possible axial parenchyma cells have been observed in only two instances. The rays are typically uniseriate, 1–10 cells in height (average = 3; n = 50) (Fig. 3H, I). Partly biseriate rays are scarce (approx. 2 %). In tangential longitudinal section, rays cells are 16–33 µm high (average = 24; n = 25) and 9–27 µm wide (average = 17; n = 25). In radial longitudinal section, they are about 60–130 µm long with vertical to slightly oblique walls (Fig. 3J). Secondary xylem tracheids are 13–37 µm (mean = 23 µm; n = 60) in transverse section (Fig. 3B, C). Their radial walls display variable pitting (Fig. 3J–N). Over 90 % of tracheids have a single row of pits, independent of their diameter. These pits are circular, with a circular to elliptical aperture. They appear either crowded (araucarian) or spaced (abietinean). A few occurrences of tracheids with two rows of radial pits were observed and, in those cases, the pit arrangement is either alternate or opposite. Pits are 10–18 µm in diameter (average = 13 µm; n = 25). Cross-fields are araucarioid. They typically contain 2–5 small (average = 8 µm; n = 25) crowded pits (Fig. 3N), with no variation in shape or number within a given growth ring.
Anatomy of the shoots
The shoots crossing the wood are about 1–2.5 mm in diameter. They cross the trunk at an obtuse angle in the vertical plane and are typically seen in oblique to longitudinal section on transverse sections of the trunk (Fig. 6A–D). Some of them give rise to lateral axes (Fig. 6A, arrow; Fig. 6G, lt). They are typically composed of a pith 0.4–0.6 mm in diameter and a small amount of secondary xylem (Fig. 6A–C, E, G). The pith is composed of parenchyma cells and conspicuous sclerotic nests (Fig. 6C, H). The parenchyma cells are polygonal and isodiametric in transverse section and range from 19 to 61 µm in diameter (average = 55 µm; n = 25) (Fig. 6B). In longitudinal section they are conspicuously elongated, with a height of 35–124 µm (average = 77 µm; n = 25) (Fig. 6E, F). A few have dark contents. The sclerotic nests occur at regular intervals as seen in longitudinal section (Fig. 6A, E) and are 0.5–1.1 mm in height and 0.2–05 mm in diameter. As a result of the size and distribution of the sclerotic nests, the pith of the shoots commonly appears to be composed either only of parenchyma (e.g. Fig. 6B) or only of sclerotic cells (e.g. Fig. 6H) when seen in cross-section. Individual sclereids are 39–71 µm (average = 55 µm; n = 25) in diameter. Unlike the parenchyma cells, they tend to be wider than high when seen in longitudinal section, with a height of 22–56 µm (average 36 µm, n = 25) (Fig. 6F). The wood of the shoots contains small tracheids with one row of bordered pits similar to that seen in the wood of the trunks (Fig. 6I). Rays could not be observed in the epicormic shoots.
Fig. 6.
Triassic trees with epicormic shoots from Gordon Valley: shoot anatomy. (A) View of a cross-section of wood showing several epicormic traces (S) with an oblique to horizontal trajectory. The shoot on the right is branching (arrow). Note regularly spaced sclerotic nests in the pith of the shoots (Sn). 18,292 Dbot1 (slide 30,292). (B) Cross-section of a shoot at a level with no sclerotic nest in the pith. 18,294 Btop1. (C) Oblique view of a shoot showing some sclerotic nests in the pith. 18,292 Cbot1 (slide 30,291). (D) Oblique view of a shoot for which only the secondary xylem is visible. Note that there are no conspicuous frost rings associated with the trace. 18,291 Cbot1 (slide 30,291). (E) Detail of a shoot seen in longitudinal section, with an alternation of parenchyma and sclerotic nests in the pith. 18,292 Cbot1 (slide 30,291). (F) Detail of the pith on (E) 18,292 Cbot1 (slide 30,291). (G) Shoot producing a trace to a lateral organ (lt). 18,294 A6 (slide 30,286). (H) Transverse-oblique section of a shoot showing a large sclerotic nest (Sn) in the pith. 18,294 B3 (slide 30,289). (I) Longitudinal section of a shoot showing the pith and secondary xylem with uniseriate pits on the tracheid walls. 18,294 A6 (slide 30,286). Scale bars: (A) 1 cm; (B–D) 500 µm; (E, G) 250 µm; (H) 100 µm; (F) 50 µm.
DISCUSSION
Anatomical comparison with previously described trees from the Fremouw Formation
Until now, two genera of anatomically preserved arborescent gymnosperms have been reported from the Fremouw Formation: the voltzialean conifer Telemachus and the corystosperm Kykloxylon (Meyer-Berthaud and Taylor, 1991; Meyer-Berthaud et al., 1993; Bomfleur et al., 2013a; Decombeix et al., 2014). Even in the absence of their distinctive leaf bases, stems of the two genera can easily be differentiated by details of the primary vascular system and wood anatomy. The new specimens from Gordon Valley can be distinguished from stems and trunks of Telemachus (morphogenus Notophytum) by two conspicuous anatomical characters: (1) the presence of sclerotic nests in their pith vs. no sclerotic cells in the pith of Notophytum; and (2) the presence of small crowded cross-field pits in the wood vs. 1–2, rarely four large pits in the wood of Notophytum (Meyer-Berthaud and Taylor, 1991). On the other hand, these two characters are shared with Kykloxylon, and all other available characters except two are also consistent with Kykloxylon. The first difference is a slightly different type of radial pitting on the secondary xylem tracheids. In Kykloxylon it is typically araucarian, whereas the new Gordon Valley specimens have some occurrences of pits that are more spaced vertically and can be opposite when in two rows (mixed type). A second difference is the lack of the unusual secondary growth that is evident in Kykloxylon specimens from the Fremouw Formation, both at Fremouw Peak and in the Gordon Valley in situ forest (Decombeix et al., 2014), as well as in recently reported specimens from North Victoria Land (Oh et al., 2016). In all these specimens, the secondary xylem of stems 5 years and older contains conspicuous radial and tangential parenchymatous zones that give them a distinctive aspect. A similar situation exists in other Triassic corystosperm woods reported from South America, such as Cuneumxylon (Artabe and Brea, 2003). Current evidence suggests that this unusual cambial activity has a taxonomic value, and its absence in the new specimens suggests that they might represent a new taxon. As nothing is known about the bark anatomy, mode of leaf trace production, or leaf base anatomy in the new trunks, we chose not to assign them to a new taxon until further anatomical data are discovered. At the current stage of our knowledge, they appear consistent with the corystosperms. In this context, it is interesting to note that several morphospecies of Dicroidium, the foliage of the corystosperms, have been reported in the Fremouw Formation, including two in Gordon Valley (Cúneo et al., 2003).
Epicormic shoots
The most conspicuous feature of the new specimens is the presence of numerous epicormic shoots. Because the centre of the trunks is not preserved, it is impossible to assess the preventitious vs. adventitious nature of these shoots. Their presence does, however, indicate that these trees had the potential to produce new shoots on their trunks in addition to their normal architecture. In extant trees, such shoots are produced in response to various stimuli (Del Tredici et al., 2001; Meier et al., 2012). They can contribute either (1) to the repair of damaged structures (e.g. by fire Burrows et al., 2010); (2) to the adjustment of plant architecture to the available resources (e.g. Hallé et al., 1978; Nicolini et al., 2001, 2003); or, more rarely, (3) to the intrinsic architecture of the tree (e.g. Barthélémy and Caraglio, 2007; Ishii et al., 2007). Because of the co-occurring traumatic growth zones and perturbed wood (see next paragraph), it is more likely that, in the present case, the epicormic shoots were produced in reaction to environmental signal(s) and not as part of the normal architectural development of the trees.
The new specimens from the Triassic of Gordon Valley represent the second example of a fossil gymnosperm from high latitudes able to produce epicormic shoots, the other one being another extinct seed plant, the Permian gymnosperm Glossopteris (Decombeix et al., 2010). In Glossopteris, the epicormic shoots were grouped in clusters that initiated from a few original shoots branching profusely. They might have been produced in reaction to a wound/infection around a dead branch (Decombeix et al., 2010). The only other detailed description of epicormic shoots in a fossil gymnosperm is that of the conifer Woodworthia, from the Permian and Triassic of North America and Brazil (Creber and Collinson, 2006).
Traumatic growth zones in the wood
The traumatic rings in the wood of the specimens differ significantly from the ‘unusual rings’ that have been documented previously in corystosperm woods from Fremouw Peak. Those typically contain groups of small thin-walled tracheids that are up 10–20 cells in radial thickness and occur within a parenchymatous matrix (e.g. Taylor and Ryberg, 2007; Decombeix et al., 2014, plate III, 4). In contrast, the traumatic zones in the wood of the new trees show all the characters of typical ‘frost rings’ as encountered in extant trees, i.e. thin-walled, collapsed tracheids, bent rays, callus tissue and morphologically regenerating tracheids. In contrast to their name, ‘frost rings’ are created not only by unseasonable freezing episodes, but also by other events that similarly cause a loss of pressure in the xylem, either by transpiration pull or by local injury (Schweingruber, 2007, and references therein). Possible causes of ‘frost rings’ include frost, but also lightning strikes, severe drought, defoliation and mechanical injuries. In extant trees, finding their exact cause in retrospect can only be achieved by comparing meteorological data with the intra-annual position of the traumatic growth zone (Schweingruber, 2007), and it is thus not possible to find their exact cause in the new fossil trees. To take into account this uncertainty, the term ‘traumatic growth zones’ is used here instead of the more misleading ‘frost ring’. Damage by fire is a possible cause of traumatic growth zones in the new specimens from Gordon Valley, but no evidence of charring was found in woods from the locality. One possible clue, however, is the frequent disorientation of the radial arrangement of tracheid files in the wood. In extant trees, this type of phenomenon occurs especially in trees that have suffered some kind of crown damage (Schweingruber, 2007, and references therein).
Stressed vs. non-stressed Triassic trees in the Fremouw Formation
This is the first report of the presence of traumatic growth zones in the wood of a tree from the Fremouw Formation and, combined with other perturbations in the wood and the production of epicormic shoots, it suggests that these trees were under some environmental stresses that have not been observed in trees found previously as fossils in the Fremouw Formation. Among the factors that could lead to a different recording of external conditions, two can be excluded, bark thickness and position in the tree. Bark plays a crucial role in the thermal protection of the cambium (e.g. Chapman, 1994). Because bark thickness tends to increase with time in a given tree, traumatic growth zones are mostly represented in the first few centimetres from the pith, when the axis was small and the bark thin (e.g. Arco Molina et al., 2016). Both young and old stems of Kykloxylon and Notophytum are known at Fremouw Peak, and none shows traumatic growth zones. This indicates that even in their theoretically most sensitive stage, they were not subjected to environmental stresses strong enough to cause such structures to form. Along the trunks of extant trees, traumatic growth zones also occur more commonly near the ground than higher up in the stem (Schweingruber, 2007, and references therein). Since the material from Fremouw Peak includes both whole trunks (including their bases) and young branches, however, a difference in position in the tree cannot explain the difference in presence/absence of traumatic growth zones in the present case. Two main hypotheses should thus be considered.
(1) A genetic/taxonomic difference. In extant trees, the threshold at which a traumatic growth zone will be formed is very variable between taxa. Similarly, the ability to produce epicormic shoots varies between taxa. The fact that the wood anatomy of the new specimens does not match entirely with taxa described previously from the Fremouw Formation suggests that they could represent a different species.
(2) Different site conditions. Because the range of growth ring width and the sensitivity of the new specimens are similar to those of other trees from Fremouw Peak and the in situ forest at Gordon Valley, we hypothesize that their general growth conditions were roughly comparable. However, small differences in spatial or temporal occurrence of these trees might explain the existence of different environmental constraints. In situ trees from Gordon Valley grew in a channel, but both the specimens from Fremouw Peak and the new specimens in this study represent allochthonous trees that were transported away from their growth site by a river system. It is thus possible that the new trees originated from a habitat that was drier/colder than that of the Fremouw Peak trees, either further away from the river bank or at a higher altitude. Other possibilities are that the forest they originated from had a different stand organization that induced a different microclimate or that these trees were subjected to attacks by pathogens or insects. It is also possible that these trees grew during a slightly colder and/or dryer climatic episode. Finally, an isolated event such as a storm might have caused damage to the crown of these trees and resulted in the production of the shoots and traumatic growth zones.
Conclusion
The presence in the specimens of both epicormic shoots and traumatic growth zones, combined with the observation of some disorientation of the wood structure in some parts of the trunks, suggest that these trees were growing under more stressful conditions than previously described trees from the Fremouw Formation. This can be explained by a different biology of the trees and/or different local environmental conditions at their growth site. It suggests an additional diversity of plants and/or environments in Antarctica during the Triassic and provides an example of tree response to stress in a high-latitude ecosystem of the past.
ACKNOWLEDGEMENTS
We thank Dorothée Letellier (Montpellier) for preparing the thin sections, Eric Nicolini and Yves Caraglio (Montpellier) for discussion on epicormic shoots in extant trees, and the other members of the 2010–2011 G-496 team, Ignacio Escapa, Erik Gulbranson, Patricia Ryberg, Andrew Schwendemann and Brian Staite, for their help in the field. This work was partially supported by the US National Science Foundation [award 1443546].
LITTERATURE CITED
- Arco Molina JG, Hadad MA, Patón Domínguez D, Roig FA. 2016. Tree age and bark thickness as traits linked to frost ring probability on Araucaria araucana trees in northern Patagonia. Dendrochronologia 37: 116–125. [Google Scholar]
- Artabe A, Brea M. 2003. A new approach to Corystospermales based on Triassic permineralized stems from Argentina. Alcheringa 27: 209–229. [Google Scholar]
- Barret PJ. 1969. Stratigraphy and petrology of the mainly fluviatile Permian and Triassic Beacon rocks, Beardmore Glacier, Antarctica: Institute of Polar Studies Report, 34. [Google Scholar]
- Barthélémy D, Caraglio Y. 2007. Plant architecture: a dynamic, multilevel and comprehensive approach to plant form, structure and ontogeny. Annals of Botany 99: 375–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomfleur B, Krings M, Taylor EL, Taylor TN. 2011a. Macrofossil evidence for pleuromeialean lycophytes from the Triassic of Antarctica. Acta Palaeontologica Polonica 56: 195–203. [Google Scholar]
- Bomfleur B, Taylor EL, Taylor TN, Serbet R, Krings M, Kerp H. 2011b. Systematics and paleoecology of a new peltaspermalean seed fern from the Triassic polar vegetation of Gondwana. International Journal of Plant Sciences 172: 807–835. [Google Scholar]
- Bomfleur B, Decombeix A-L, Escapa IH, Schwendemann AB, Axsmith B. 2013a. Whole-plant concept and environment reconstruction of a Telemachus conifer (Voltziales) from the Triassic of Antarctica. International Journal of Plant Sciences 174: 425–444. [Google Scholar]
- Bomfleur B, Escapa IH, Taylor EL, Taylor TN. 2013b. A reappraisal of Neocalamites and Schizoneura (fossil Equisetales) based on material from the Triassic of East Antarctica. Alcheringa 37: 1–17. [Google Scholar]
- Bomfleur B, Decombeix A-L, Schwendemann AB et al. . 2014a. Habit and ecology of the Petriellales, an unusual group of seed plants from the Triassic of Gondwana. International Journal of Plant Sciences 175: 1062–1075 [Google Scholar]
- Bomfleur B, Klymiuk AA, Taylor EL, Taylor TN, Gulbranson EL, Isbell JL. 2014b. Diverse bryophyte mesofossils from the Triassic of Antarctica. Lethaia 47: 120–132. [Google Scholar]
- Burrows GE. 2002. Epicormic strand structure in Angophora, Eucalyptus and Lophostemon (Myrtaceae): implications for fire resistance and recovery. New Phytologist 153: 111–131. [Google Scholar]
- Cantrill DJ, Poole I. 2012. The vegetation of Antarctica through geological time. Cambridge: Cambridge University Press. [Google Scholar]
- Chapman JL. 1994. Distinguishing internal developmental characteristics from external palaeoenvironmental effects in fossil wood. Review of Palaeobotany and Palynology 81: 19–32. [Google Scholar]
- Collinson JW, Isbell JL, Elliot DH, Miller MF, Miller JMG. 1994. Permian–Triassic Transantarctic Basin. In: Veevers JJ, Powell CMcA, eds. Permian–Triassic Pangean Basins and Foldbelts along the Panthalassan Margin of Gondwanaland. Geological Society of America Memoir; 184: 173–222. [Google Scholar]
- Creber GT, Collinson ME. 2006. Epicormic shoot traces in the secondary xylem of the Triassic and Permian fossil conifer species Woodworthia arizonica – short communication. IAWA Journal 27: 237–241. [Google Scholar]
- Cúneo NR, Taylor EL, Taylor TN, Krings M. 2003. In situ fossil forest from the upper Fremouw Formation (Triassic) of Antarctica: paleoenvironmental setting and paleoclimate analysis. Palaeogeography, Palaeoclimatology, Palaeoecology 197: 239–261. [Google Scholar]
- Decombeix A-L, Taylor EL, Taylor TN. 2010. Epicormic shoots in a Permian gymnosperm from Antarctica. International Journal of Plant Sciences 171: 772–782 [Google Scholar]
- Decombeix A-L, Bomfleur B, Taylor EL, Taylor TN. 2014. New insights into the anatomy, development, and affinities of corystosperm trees from the Triassic of Antarctica. Review of Palaeobotany and Palynology 203: 22–34. [Google Scholar]
- Del Fueyo GM, Taylor EL, Taylor TN, Cúneo NR. 1995. Triassic wood from the Gordon Valley, central Transantarctic Mountains, Antarctica. IAWA Journal 16: 111–126. [Google Scholar]
- Del Tredici P. 2001. Sprouting in temperate trees: a morphological and ecological review. Botanical Review 67: 121–140. [Google Scholar]
- Denne M. 1989. Definition of latewood according to Mork (1928). IAWA Bulletin 10: 59–62. [Google Scholar]
- Escapa IH, Taylor EL, Cúneo R et al. . 2011. Triassic floras of Antarctica: plant diversity and distribution in high paleolatitude communities. PALAIOS 26: 522–544. [Google Scholar]
- Fritts HC. 1976. Tree rings and climate. London: Academic Press. [Google Scholar]
- Gabites HI. 1985. Triassic Paleoecology of the Lashly Formation, Transantarctic Mts., Antarctica. Unpublished MSc thesis, Victoria University of Wellington, Wellington, New Zealand. [Google Scholar]
- Galtier J, Phillips TL. 1999. The acetate peel technique. In: Jones TP, Rowe NP, eds. Fossil plants and spores: modern techniques. London: The Geological Society, 67–70. [Google Scholar]
- Halle F, Oldeman RAA, Thomlinson PB. 1978. Tropical trees and forests—an architectural analysis. Berlin: Springer. [Google Scholar]
- Hass H, Rowe NP. 1999. Thin sections and wafering. In: Jones TP, Rowe NP, eds. Fossil plants and spores: modern techniques. London: The Geological Society, 76–81. [Google Scholar]
- Hermsen EJ, Taylor EL, Taylor TN. 2009. Morphology and ecology of the Antarcticycas plant. Review of Palaeobotany and Palynology 153: 108–123. [Google Scholar]
- Ishii HT, Ford ED, Kennedy MC. 2007. Physiological and ecological implications of adaptive reiteration as a mechanism for crown maintenance and longevity. Tree Physiology 27: 455–462. [DOI] [PubMed] [Google Scholar]
- Meier AR, Saunders MR, Michler CH. 2012. Epicormic buds in trees: a review of bud establishment, development and dormancy release. Tree Physiology 32: 565–584. [DOI] [PubMed] [Google Scholar]
- Meyer-Berthaud B, Taylor TN. 1991. A probable conifer with podocarpacean affinities from the Triassic of Antarctica. Review of Palaeobotany and Palynology 67: 179–198. [Google Scholar]
- Meyer-Berthaud B, Taylor TN, Taylor EL. 1993. Petrified stems bearing Dicroidium leaves from the Triassic of Antarctica. Palaeontology 36: 337356. [Google Scholar]
- Mork E. 1928. Die Qualität des Fichtenholzes unter besonderer Rücksichtnahme auf Schleif- und Papierholz. Der Papier-Fabrikant 26: 741–747. [Google Scholar]
- Nicolini E, Chanson B, Bonne F. 2001. Stem growth and epicormic branch formation in understorey beech trees (Fagus sylvatica L.). Annals of Botany 87: 737–750. [Google Scholar]
- Nicolini E, Caraglio Y, Pelissier R, Leroy C, Roggy J-C. 2003. Epicormic branches: a growth indicator for the tropical forest tree Dicorynia guianensis Amshoff (Caesalpiniaceae). Annals of Botany 92: 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh C, Park T-YS, Woo J et al. . 2016. Triassic Kykloxylon wood (Umkomasiaceae, Gymnospermopsida) from Skinner Ridge, northern Victoria Land, East Antarctica. Review of Palaeobotany and Palynology 233: 104–114 [Google Scholar]
- Rasband WS. 1997–2012. ImageJ. Bethesda, MD: US National Institutes of Health; http://imagej.nih.gov/ij/ [Google Scholar]
- Schweingruber FH. 2007. Wood structure and environment. Berlin: Springer. [Google Scholar]
- Smoot EL, Taylor TN, Delevoryas T. 1985. Structurally preserved fossil plants from Antarctica. I. Antarcticycas, gen. nov., a Triassic cycad stem from the Beardmore Glacier area. American Journal of Botany 72: 1410–1423. [Google Scholar]
- Taylor TN, Taylor EL, eds 1990. Antarctic paleobiology: its role in the reconstruction of Gondwana. New York: Springer. [Google Scholar]
- Taylor EL, Ryberg PE. 2007. Tree growth at polar latitudes based on fossil tree ring analysis. Palaeogeography, Palaeoclimatology, Palaeoecology 255: 246–264. [Google Scholar]
- Taylor EL, Taylor TN, Collinson JW. 1989. Depositional setting and paleobotany of Permian and Triassic permineralized peat from the central Transantarctic Mountains, Antarctica. International Journal of Coal Geology 12: 657–679. [Google Scholar]