Abstract
Background and Aims
Soil waterlogging adversely impacts most plants. Melilotus siculus is a waterlogging-tolerant annual forage legume, but data were lacking for the effects of root-zone hypoxia on nodulated plants reliant on N2 fixation. The aim was to compare the waterlogging tolerance and physiology of M. siculus reliant on N2 fixation or with access to NO3−.
Methods
A factorial experiment imposed treatments of water level (drained or waterlogged), rhizobia (nil or inoculated) and mineral N supply (nil or 11 mm NO3−) for 21 d on plants in pots of vermiculite in a glasshouse. Nodulation, shoot and root growth and tissue N were determined. Porosity (gas volume per unit tissue volume) and respiration rates of root tissues and nodules, and O2 microelectrode profiling across nodules, were measured in a second experiment.
Key Results
Plants inoculated with the appropriate rhizobia, Ensifer (syn. Sinorhizobium) medicae, formed nodules. Nodulated plants grew as well as plants fed NO3−, both in drained and waterlogged conditions. The growth and total N content of nodulated plants (without any NO3− supplied) indicated N2 fixation. Respiration rates (mass basis) were highest in nodules and root tips and lowest in basal root tissues. Secondary aerenchyma (phellem) formed along basal root parts and a thin layer of this porous tissue also covered nodules, which together enhanced gas-phase diffusion of O2 to the nodules; O2 was below detection within the infected zone of the nodule interior.
Conclusions
Melilotus siculus reliant on N2 fixation grew well both in drained and waterlogged conditions, and had similar tissue N concentrations. In waterlogged conditions the relatively high respiration rates of nodules must rely on O2 movement via the aerenchymatous phellem in hypocotyl, roots and the outer tissue layers of nodules.
Keywords: Aerenchyma, messina, nitrogen fixation, oxygen microelectrode profiling, pasture legume, phellem, root nodules, respiration, soil flooding, tissue porosity, wetland legume plant
INTRODUCTION
Waterlogging, or soil flooding, typically results in severe hypoxia or anoxia in soils and roots (Armstrong, 1979). Soil flooding has been estimated to affect >1700 Mha of land worldwide every year (Voesenek and Sasidharan, 2013). This abiotic stress is a challenge for agriculture in many parts of the world and especially in areas with high rainfall, poor soil drainage or both (Bailey-Serres et al., 2012), with the exception of rice production (Kirk et al., 2014). Roots require O2 for aerobic respiration and lack thereof will cause a shift in sugar catabolism to low ATP-yielding fermentation and result in an ‘energy crisis’ that impedes root growth and functioning for water and nutrient uptake (Gibbs and Greenway, 2003; Colmer and Greenway, 2011; Shabala et al., 2014). An important trait for roots in waterlogged soils is effective internal aeration via development of aerenchyma to provide a pathway of low resistance for O2 diffusion along roots (Armstrong, 1979; Colmer, 2003). Plant species with well-developed root aerenchyma to facilitate gas-phase diffusion of O2 from above-ground parts can thrive in waterlogged soils, whereas species with little aerenchyma typically suffer severe growth reductions and can eventually die during waterlogging (Justin and Armstrong, 1987; Colmer and Voesenek, 2009).
Legumes possess the ability to form a symbiosis with rhizobia (N2-fixing bacteria), which involves the formation of nodules within which rhizobia fix atmospheric N2 (Sprent, 2007). The poor performance of some forage legumes under flooded conditions has been attributed mainly to a reduction in the supply of O2 to root nodules (Shiferaw et al., 1992; Arrese‐Igor et al., 1993; Pugh et al., 1995). Relatively large amounts of O2 are required by nodules to support the respiration needed to provide ATP and reductant to enable the energetically demanding process of N2 fixation catalysed by nitrogenase (Schulze, 2004). Nevertheless, since nitrogenase is sensitive to O2, within the infected zone of nodules the O2 partial pressure is maintained at low levels owing to a diffusion barrier and leghaemoglobin, which binds O2 (Minchin et al., 2008). The additional requirement for assimilates and O2 by nodulated N2-fixing root systems of legumes further emphasizes the importance of internal aeration to supply O2 to below-ground parts of legumes during soil flooding.
Various forage legume species are used in different climates and farming systems of the world (Phelan et al., 2015), but only a few species appear to be suitable for flood-prone areas (Striker and Colmer, 2017). Forage legumes differ in tolerance to waterlogging; examples of sensitive species are Medicago sativa, Trifolium pratense and Trifolium repens, whereas examples of tolerant species are Trifolium michelianum and Melilotus siculus (Striker and Colmer, 2017). The high tolerance of M. siculus to root-zone hypoxia, as well as to salinity, when compared with some other annual pasture legume species, has been emphasized (Rogers et al., 2011; Teakle et al., 2012). Internal aeration of roots of M. siculus benefits from the development of aerenchymatous phellem, a type of secondary aerenchyma, which develops along the hypocotyl and the older parts of roots (Teakle et al., 2011; Verboven et al., 2012; Striker et al., 2015) and phellem has also been found to develop in several species of aquatic legumes in the genera Aeschynomene, Discolobium and Neptunia (James et al., 1992, 2001; Loureiro et al., 1994, 1995). All previous controlled-environment studies of the waterlogging tolerance of M. siculus (e.g. those cited above) have used plants supplied with mineral nitrogen (N) and lacking nodules (reviewed by Striker and Colmer, 2017). The goal of growing a forage legume is to produce high-quality herbage for grazing livestock, especially in areas with low or no N fertilizer inputs (Phelan et al., 2015), so it is of importance to evaluate waterlogging tolerance of forage legumes when reliant on their symbiosis with N2-fixing bacteria and of high interest to compare the physiology of nodulated N2-fixing plants with that of non-nodulated mineral N-fed plants.
The objective of the present study was to evaluate the waterlogging tolerance and physiology of nodulated M. siculus when grown reliant on N2 fixation, in comparison also with plants supplied with mineral N. The first hypothesis tested was that M. siculus is able to form nodules when inoculated with the appropriate rhizobia, and that these nodules are capable of fixing N2 to provide the tissue N required for growth and development in waterlogged conditions. The second hypothesis tested was that phellem-type secondary aerenchyma would develop under waterlogged conditions and provide an internal path for gas-phase diffusion for nodules to receive O2. Finally, we also quantified the gas-filled porosity and O2 consumption rates for different root tissues and nodules.
MATERIALS AND METHODS
Plant culture
Melilotus siculus (syn. Melilotus messanensis, accession SA 36983) was sourced from the Temperate Pasture Genetic Resource Centre (now Australian Pastures Genebank) at the South Australian Research and Development Institute (SARDI). Seeds were scarified by rubbing with fine sandpaper, washed in 0.04 % NaHClO solution and rinsed thoroughly in deionized (DI) water. Seeds were imbibed in aerated 0.5 mm CaSO4 solution in the dark for 3 h and then transferred to plastic mesh floating on aerated 10 %-strength nutrient solution (N-free, full composition described below), still in darkness, for 3 d. The mesh with seedlings was then transferred to aerated 25 %-strength N-free nutrient solution and exposed to light. Nine days after germination, seedlings were transplanted into pots (150 mm high, 80 mm diameter) containing washed, coarse vermiculite (one seedling per pot). Half of the pots received a pinch of rhizobia inoculant, which was peat containing Ensifer (syn. Sinorhizobium) medicae strain SRDI554 (Bonython et al., 2011) kindly provided by R. A. Ballard (SARDI), placed near the root base of these transplanted seedlings. All pots were watered with a 100 %-strength N-free nutrient solution and placed into tubs containing this same solution, which came to a height of 50 mm above the base of the pots; inoculated pots were in tubs separate from non-inoculated pots. The N-free nutrient solution at full concentration (i.e. 100 %-strength) contained macronutrients (mm: 0.50 KH2PO4, 1.5 K2SO4, 4.0 CaSO4 and 1.0 MgSO4) and micronutrients (µM: 37.5 FeNa3EDTA, 23.0 H3BO3, 4.5 MnCl2, 4.0 ZnSO4, 1.5 CuSO4 and 0.050 MoO3). Solution pH was buffered with 2.5 mm MES (2-(N-morpholino)ethanesulphonic acid) and adjusted with KOH to pH 6.3. The nutrient solution was renewed weekly and the level in each tub was maintained by topping up with DI water as required to replace evaporative losses. The N-free nutrient solution was continued for all seedlings during this phase, as seedlings were still relatively small and it takes time for nodules to form and to become functional on the inoculated seedlings. The experiment was conducted in a temperature-controlled (20/15 °C day/night), naturally lit phytotron (glasshouse) located in Perth, Western Australia (September–November).
Experimental design
The study consisted of two experiments. In experiment 1, eight different treatments were imposed on plants at 23 d after seed imbibition as a 2 × 2 × 2 factorial experiment (with three replicates; n = 3): with or without rhizobia inoculant (applied 9 d after seed imbibition) × drained or waterlogged (at 23 d) × 0 or 11 mm N as NO3− (at 23 d). Pots assigned to the waterlogged treatment were closed at the bottom with a rubber bung and filled up with deoxygenated (i.e. N2-flushed) 0.1 % (w:v) agar 100 %-strength nutrient solution (composition above or with NO3− added, see below). This agar method simulates the decrease in dissolved O2 and accumulation of plant-produced ethylene that occur in waterlogged soils (Wiengweera et al., 1997). Drained pots were placed in tubs with nutrient solution, which came to a height of 35 mm above the base of the pots; the total volume of nutrient solution applied to the number of drained pots in each tub gave a mean volume per pot equal to the volume applied to each waterlogged pot. The pots in the drained treatment were watered at the top with 50 mL of nutrient solution from the tub each day. The plants in the 0 N treatment continued to receive the N-free 100 %-strength nutrient solution, whereas the plants in the 11 mm N treatment received a nutrient solution as above but with the 1.5 mm K2SO4 replaced by 3.0 mm KNO3 and the 4.0 mm CaSO4 replaced by 4.0 mm Ca(NO3)2. Thus, all plants received equal amounts of all cations, but those plants receiving NO3− were exposed to a lower (but adequate) level (1.0 mm) of SO42−. Nutrient solutions in all treatments were renewed weekly and topped up with DI water as required to replace any evaporative losses.
An initial harvest (n = 3) was taken at the commencement of treatments, and then plants were harvested after 21 d of treatments (plants were then 44 d old). Plants were rinsed in deionized water and separated into shoots, roots and nodules. The number of nodules was counted. The fractions were dried at 70 °C and dry mass was recorded. Relative growth rate (RGR) was calculated as (ln final mass – ln initial mass)/growth period in days. Shoots, roots and nodules were finely ground and sub-samples of 2–3 mg were analysed for total N by gas chromatography after combustion (Carlo Erba Elemental Analyzer, Model EA1108, Rodano, Italy). Three extra plants had been grown for each treatment, and those inoculated with rhizobia, without mineral N and waterlogged, were used for measurements of nodule tissue O2 profiles and nodule gas-phase connectivity with the roots (described below).
In experiment 2, respiration and porosity of roots and nodules were assessed for plants in two flooding regimes (drained or waterlogged) × two N levels (0 or 11 mm NO3−) imposed on inoculated (i.e. nodulated) plants at 28 d after seed imbibition (with three replicates; n = 3). Growth conditions, nutrient solutions and other techniques were as in experiment 1. The plants were sampled and measurements of respiration and porosity were taken at 21 d of treatments (plants were then 49 d old).
Tissue O2 consumption rates (respiration)
Rates of O2 consumption by various root tissues and nodules were measured using a micro-respiration system (MicroResp, Unisense A/S, Aarhus, Denmark). Four different tissues were measured (n = 3): basal zone of adventitious roots with secondary aerenchyma (i.e. with phellem; 1-cm segments taken 3 cm from the shoot–root junction); mature root zone without phellem (1-cm segment 5–6 cm behind the root tip); root tips (1 cm); and root nodules. Fresh mass was recorded and the samples placed onto a mesh platform in 4-mL glass chambers that contained a small, glass-coated magnetic stir bar and a glass stopper lid with a capillary hole (MR Ch-4000, Unisense A/S). Measurements were taken in humid air as each chamber contained 20 µL of DI water in the base. An O2 microelectrode (OX-MR, Unisense A/S) was inserted through the capillary hole of the glass stopper so that the tip of the electrode was within the chamber and in air above the sample. The gap between the electrode shaft and the hole in the lid was sealed with one or two drops of glycerol. Four chambers were run simultaneously at 20 °C in darkness. We monitored O2 continuously with electrode signals recorded by two, two-channel pA meters (PA2000, Unisense A/S) logged every 10 s (ADC16, Pico Technology, St Neots, UK). Measurements were taken after the system had taken a few minutes to stabilize and O2 consumption rates were constant, and rates were expressed on a tissue fresh mass basis.
Tissue porosity
Tissue porosity (gas volume per unit tissue volume) of the same four types of tissues as measured for O2 consumption rates (listed above) was determined using the pycnometer method (Jensen et al., 1969). A 25-mL pycnometer (Isolab GmbH, Wertheim, Germany) was filled with DI water and weighed. Plant tissue samples (n = 3) were rinsed with water and gently blotted with tissue paper and weighed. Each sample was then inserted into a water-filled pycnometer and this was weighed. The tissue sample was subsequently retrieved and ground with a mortar and pestle, and the resulting homogenate was transferred to the pycnometer, which was then topped up with DI water and again weighed. The temperature of the water was maintained constant during all measurements (20 °C). Tissue porosity was calculated using the formula of Jensen et al. (1969).
Nodule O2 profiles and nodule gas-phase connectivity with the root aerenchyma
The gas-phase connectivity of nodules to the roots, whereby atmospheric O2 could diffuse internally via aerenchyma along the root and into nodules, was investigated using O2 microelectrodes to measure nodule tissue O2 when roots and nodules were in an O2-free medium and so reliant on internal O2 movement. A set of additional plants grown alongside experiment 1, inoculated with rhizobia, without mineral N and waterlogged, were used for these experiments. Clark-type O2 microelectrodes with a guard cathode and tip diameter of 25 µm (OX-25, Unisense A/S) were positioned using a motorized micromanipulator and motor controller (Unisense A/S), with the electrode connected to a pA meter (Unisense Multimeter, Unisense A/S). The electrode tip was advanced in steps of 20 µm and the signal was logged with SensortracePRO (Unisense A/S) to obtain radial O2 profiles towards and into the nodule. The radial O2 profiles were measured for nodules of intact plants with the roots submerged in an O2-free 100 %-strength nutrient solution with 0.1 % (w/v) agar in a Petri dish and with the shoot in air, all at 20 °C (n = 2). Nodules of M. siculus are indeterminate and the microelectrode was inserted into the middle of one side, with the expectation that with advancement of the tip into the nodule it would eventually enter into the infected zone III (Soupène et al., 1995; James et al., 1998; Sprent et al., 2017). The gas-phase connectivity of the nodule with the root aerenchyma was also assessed by measuring O2 at the surface of the nodule and then within the nodule (outer porous tissue and further into the nodule infected zone) to a maximum depth of ~400 μm, when the root system was in the O2-free medium but with the shoot excised and the stump of the main root exposed to air, then high-purity N2 gas, and again air (n = 2).
Statistical analyses
Data were analysed by analysis of variance (ANOVA) using type III sum of squares with the software Statgraphics XVI Centurion ver. 16.1.11 (StatPoint Inc.). All data were tested for homogeneity of variance by Bartlett’s test. If necessary, logarithmic or square-root transformations were performed to ensure homogeneity of variance, but for clarity all data are presented as untransformed values.
RESULTS
Growth, biomass allocation and tissue N concentrations and organ N contents
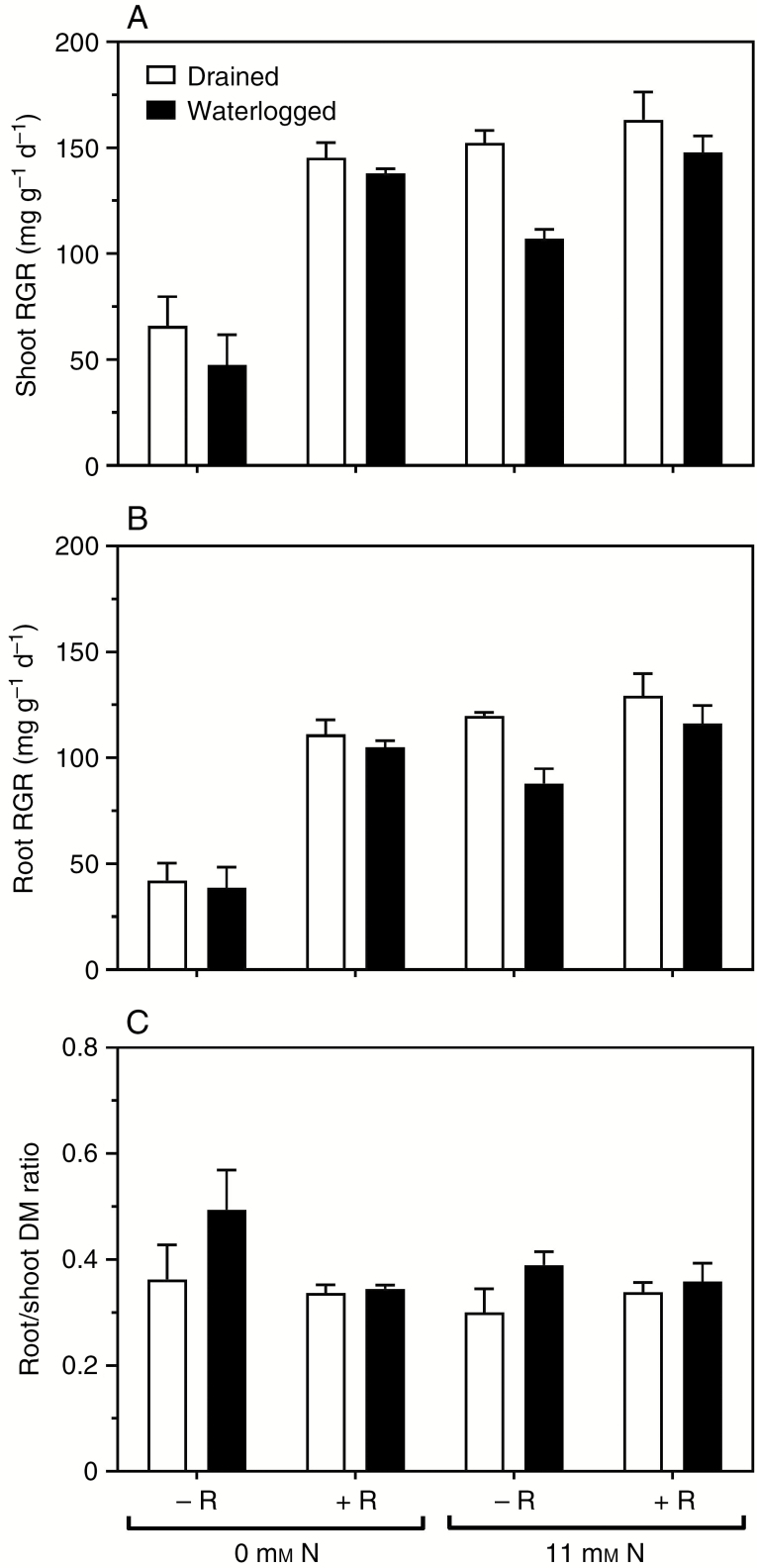
All three treatment factors of rhizobia, waterlogging and mineral N supply had effects on shoot and root growth (Fig. 1A, B and Table 1). Plants inoculated with rhizobia all formed root nodules (considered in the next section), whereas those without rhizobia did not. Plants with rhizobia or fed with mineral N, or both, had substantially greater growth than those without the capacity to fix N2 and not supplied with mineral N, for both drained and waterlogged conditions (Fig. 1A, B). There was a significant interaction between rhizobia and mineral N supply, as the effect of adding rhizobia was higher for plants that were not fed mineral N (Fig. 1A, B). The root-to-shoot ratio was in the range 0.30–0.49 with no significant effect of treatment (Fig. 1C and Table 1).
Fig. 1.
Effects of mineral nitrogen (N) supply (0 or 11 mm NO3−), rhizobia (without or with rhizobia inoculant added, -R or +R) and drained or waterlogged conditions on Melilotus siculus (A) shoot relative growth rate (RGR), (B) root RGR and (C) root/shoot dry mass (DM) ratio. Treatments were imposed 23 d after seed imbibition, for 21 d (plants were 44 d old when harvested) (20/15 °C day/night). Plants were grown in vermiculite and provided with a nutrient solution. Values are means ± s.e. (n = 3; one plant from an individual pot was partitioned to provide one replicate of the various tissues measured). For statistics see Table 1.
Table 1.
Summary of three-way ANOVA (sum of squares [SS] given as percentage of total SS) showing the effects of two mineral nitrogen (N) concentrations (0 or 11 mm NO3−), rhizobia (with or without) and two flooding regimes (drained or waterlogged) on growth, biomass allocation and tissue N concentrations of Melilotus siculus. Treatments were imposed 23 d after imbibition, for 21 d (plants were 44 d old when harvested)
| Parameter | Figure where data are shown | Main effects | Two-way interactions | Three-way interaction | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | Rhizobia | Flooding | N × rhizobia | N × flooding | Rhizobia × flooding | N × rhizobia × flooding | Residual | ||
| Shoot RGR | 1A | 26.1*** | 42.4*** | 6.5** | 12.1*** | 1.1ns | 1.4ns | 0.3ns | 10.1 |
| Root RGR | 1B | 32.2*** | 39.3*** | 3.9* | 12.5*** | 1.7ns | 0.3ns | 0.6ns | 9.5 |
| Root dry mass/shoot dry mass | 1C | 5.5ns | 6.8ns | 15.0ns | 7.9ns | 0.2ns | 8.8ns | 0.7ns | 55.0 |
| Shoot N concentration | 2A | 18.6*** | 50.1*** | 1.1ns | 5.8* | 0.7ns | 9.2** | 1.4ns | 13.1 |
| Shoot N contenta | 2B | 25.0*** | 42.7*** | 7.4* | 0.2ns | 3.4ns | 0.2ns | 0.6ns | 20.5 |
| Root N concentration | 2C | 33.4*** | 43.4*** | 4.8** | 2.3ns | 0.5ns | 6.7** | 0.3ns | 8.6 |
| Root N contenta | 2D | 39.6*** | 27.5*** | 8.4* | 0.1ns | 7.3* | 0.1ns | 0.1ns | 16.9 |
d.f. = 1 for all factors and interactions except residual, where d.f. = 16.
*P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.
aTissue N concentration × tissue dry mass.
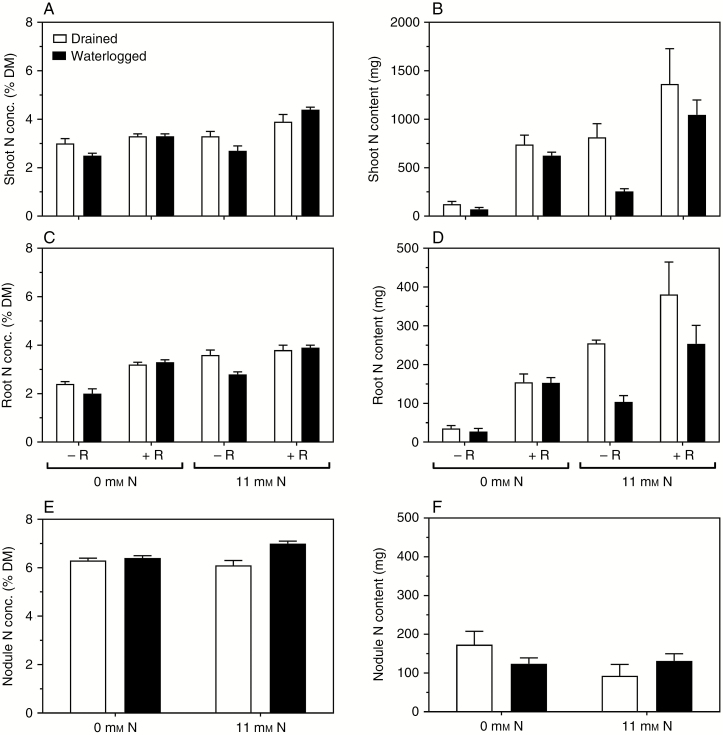
Shoot tissue N concentrations were in the range 2.5–4.4 % (w/w, dry mass), and there were main effects of rhizobia and mineral N treatment with tissue concentrations being higher in plants with rhizobia and mineral N supplied compared with the three other treatments (Fig. 2A and Table 1). An interaction between rhizobia and waterlogging was evident as the waterlogged plants had lower shoot tissue N concentrations when there were no rhizobia, but addition of rhizobia removed this difference between drained and waterlogged plants. Likewise, there was an interaction between rhizobia and mineral N treatment as the shoot N concentration was increased by provision of mineral N, but more so in plants with rhizobia than in those without rhizobia.
Fig. 2.
Effects of mineral nitrogen (N) supply (0 or 11 mm NO3−), rhizobia (without or with rhizobia inoculant added, -R or +R) and drained or waterlogged conditions on Melilotus siculus (A) shoot N concentration, (B) shoot N content, (C) root N concentration, (D) root N content, (E) nodule N concentration and (F) nodule N content. DM, dry mass. Only plants inoculated with rhizobia formed nodules. Treatments were imposed 23 d after seed imbibition, for 21 d (plants were 44 d old when harvested) (20/15 °C day/night). Plants were grown in vermiculite and provided with a nutrient solution. Values are means ± s.e. (n = 3; one plant from an individual pot was partitioned to provide one replicate of the various tissues measured). For statistics see Tables 1 and 2.
For roots, the tissue N concentrations were in the range 2.0–3.9 % (w/w, dry mass) and there were main effects of rhizobia, waterlogging and mineral N treatment, with the lowest root N concentration in waterlogged plants without rhizobia and without mineral N (Fig. 2C and Table 1). There was an interaction between rhizobia and waterlogging; the waterlogged plants had lower root N concentration than drained plants when without rhizobia, but the drained and waterlogged plants did not differ for root N concentration when rhizobia were present (Fig. 2C). Since only plants with rhizobia formed nodules, a two-way ANOVA was performed to analyse the effects of waterlogging and mineral N treatment on nodule tissue N concentration (Fig. 2E and Table 2). The nodule tissue N concentrations were in the range 6.1–7.0 % (w/w, dry mass); these values were higher than those for the shoots and roots. There was an effect of waterlogging and interaction between waterlogging and mineral N treatment on nodule N concentration; nodulated, waterlogged plants with mineral N had the highest tissue N concentration in the nodules.
Table 2.
Summary of two-way ANOVA (sum of squares [SS] given as percentage of total SS) showing the effects of two mineral nitrogen (N) concentrations (0 or 11 mm NO3−) and two flooding regimes (drained or waterlogged) on nodule characteristics of Melilotus siculus. Treatments were imposed 23 d after imbibition, for 21 d (plants were 44 d old when harvested)
| Parameter | Figure where data are shown | Main effects | Two-way interaction | ||
|---|---|---|---|---|---|
| N | Flooding | N × flooding | Residual | ||
| Nodule N concentration | 2E | 8.4ns | 46.4** | 24.7* | 20.5 |
| Nodule N contenta | 2F | 15.7ns | 0.3ns | 22.7ns | 61.3 |
| Total number of nodules | 3A | 6.8ns | 24.5ns | 1.5ns | 67.3 |
| Number of nodules on main root system | 3B | 12.5ns | 34.8* | 9.3ns | 43.5 |
| Number of nodules on adventitious roots | 3C | <0.01ns | 1.0ns | 4.4ns | 94.6 |
| Nodulation (nodules formed during treatment) | 3D | 6.8ns | 24.5ns | 1.5ns | 67.3 |
| Individual nodule dry mass | 3E | 43.0* | 4.8ns | 5.0ns | 47.1 |
| Nodule dry mass/root dry mass | 3F | 71.4*** | 0.6ns | 20.4** | 7.6 |
d.f. = 1 for all factors and interactions except residual, where d.f. = 8.
*P < 0.05; **P < 0.01; ***P < 0.001; ns, not significant.
aTissue N concentration × tissue dry mass.
Both shoot and root N contents (tissue concentration × tissue mass) were affected by N treatment, waterlogging and rhizobia (Table 1). The differences amongst treatments in the N contents of shoots and roots reflected that plants in some treatments had less growth (Fig. 2B, D). Especially the N starved plants without rhizobia and without mineral N had low rates of growth (Fig. 1A, B) and low N contents of shoots and roots (Fig. 2B, D). Waterlogged plants fed with mineral N but lacking rhizobia had the second lowest shoot and root N contents, whereas drained plants with rhizobia and supplied with mineral N had the highest shoot and root N contents. For the nodulated plants, the N content of the nodules did not show any significant effect of treatments (Fig. 2F and Table 2).
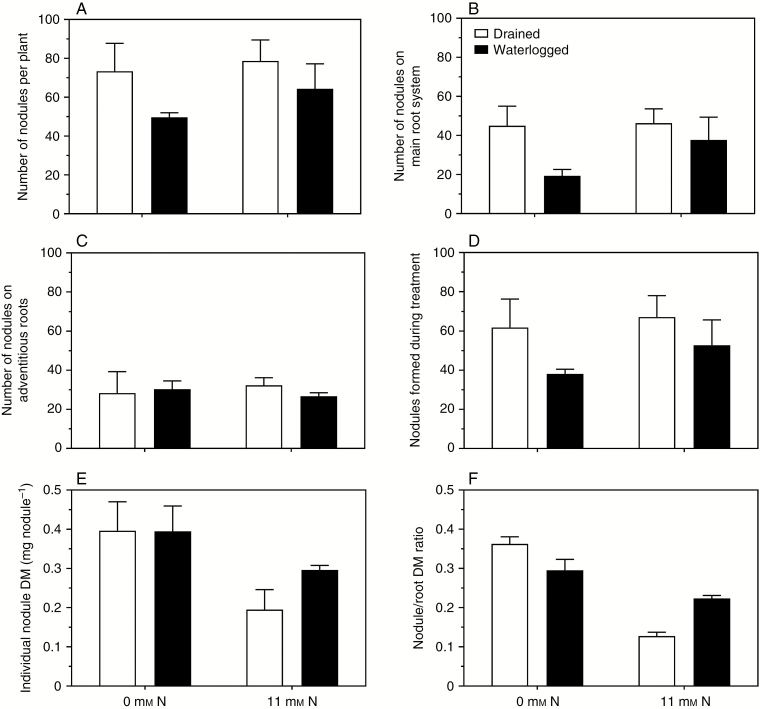
Nodulation
For the plants inoculated with rhizobia, the total number of nodules per plant was between 50 and 79 and there was neither an effect of waterlogging nor of mineral N treatment (Fig. 3A and Table 2). There was, however, a significant effect of waterlogging on the number of nodules on the main root system, which decreased owing to waterlogging (Fig. 3B and Table 2). Thus, the waterlogged plants had a larger proportion of their nodules on adventitious roots, although the absolute numbers on the adventitious roots did not differ significantly from that of the drained plants (Fig. 3C). In all treatments, the majority of the nodules recorded at the final sampling had developed during the treatments (compare panel D with panel A in Fig. 3). Although there appeared to be a tendency towards fewer nodules being developed by plants in the waterlogged treatment, this was not significant at the 5 % probability level (Fig. 3D); there was no significant difference in the numbers of nodules formed during the treatments (Table 2). Interestingly, plants fed with mineral N produced as many nodules as plants not supplied with mineral N, but the nodules were smaller in plants supplied with mineral N (Fig. 3E and Table 2). Thus, the nodule-to-root dry mass ratio was greater in plants that were without the added mineral N (Fig. 3F and Table 2).
Fig. 3.
Effects of mineral nitrogen (N) supply (0 or 11 mm NO3−) and drained or waterlogged conditions on Melilotus siculus inoculated with rhizobia. (A) Number of nodules per plant, (B) number of nodules on main root system, (C) number of nodules on adventitious roots, (D) nodules formed during treatments, (E) individual nodule dry mass (DM) and (F) nodule/root DM ratio. Treatments were imposed 23 d after seed imbibition, for 21 d (plants were 44 d old when harvested) (20/15 °C day/night). Plants were grown in vermiculite and provided with a nutrient solution. Values are means ± s.e. (n = 3; one plant from an individual pot was partitioned to provide one replicate of the various tissues measured). For statistics see Table 2.
Tissue respiration and porosity
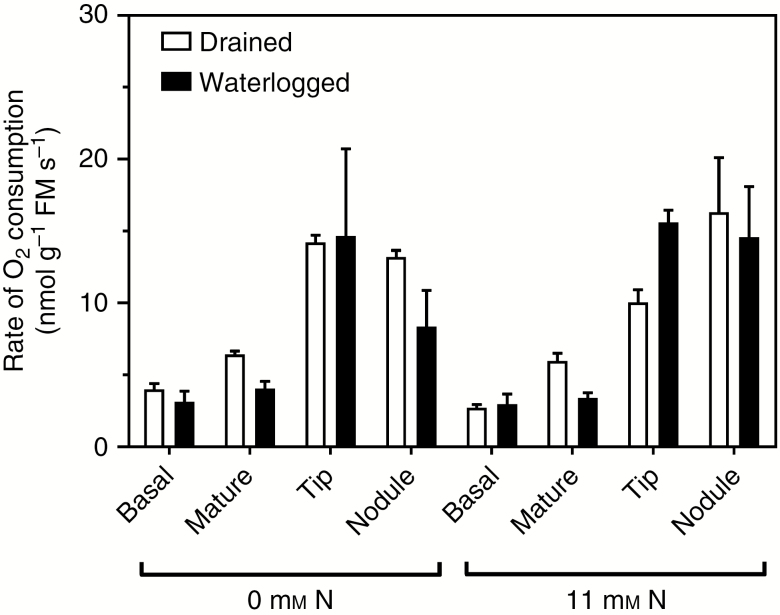
Tissue respiration rate (fresh mass basis) differed markedly amongst tissue types (measured in humid air; Fig. 4 and Table 3). Respiration rates were highest in root tips and nodules and lowest in the basal zones of adventitious roots, and rates were also relatively low in the mature (expanded) segments of adventitious roots sampled from between the tips and the base (Fig. 4). The rates are expressed on a fresh mass basis (Fig. 4); if these were expressed on a volume basis, the rates of the basal zones of adventitious roots with secondary aerenchyma (phellem) and thus high gas-filled porosity (see next paragraph) would be even lower in comparison with the tissues containing less gas-filled space. In contrast with the basal tissues, the mature (expanded) root segments measured contained primary aerenchyma, but had not yet formed phellem. Respiration rate was not affected by mineral N supply, and prior waterlogging had only a small influence on the rate of respiration (Fig. 4 and Table 3). It is important to note that the intact nodules of M. siculus contained 17–19 kPa O2 in the outer porous tissues when roots were in an O2-free medium and reliant on internal O2 transport (see next section), so that the nodules from the waterlogged treatment did not experience any sudden exposure to substantially higher O2 for the measurements of respiration; nodules can be sensitive to sudden changes in O2 (e.g. soybean nodules; Minchin et al., 1986).
Fig. 4.
Respiration measured as rates of O2 consumption by four different tissues from adventitious roots: basal zone of root with secondary aerenchyma (1-cm segment taken 3 cm below root–shoot junction); mature zone of root (5–6 cm behind the root tip); root tips (1 cm); and root nodules of Melilotus siculus as affected by mineral nitrogen (N) supply (0 or 11 mm NO3−) and drained or waterlogged conditions. Treatments were imposed 28 d after seed imbibition, for 21 d (plants were 49 d old when harvested) (20/15 °C day/night). Measurements were conducted in darkness at 20 °C in sealed vials with humid air and rates were calculated based on fresh mass (FM). Plants were grown in vermiculite and provided with a nutrient solution. Values are means ± s.e. (n = 3; one plant from an individual pot was partitioned to provide one replicate of the various tissues measured). For statistics see Table 3.
Table 3.
Summary of three-way ANOVA (sum of squares [SS] given as percentage of total SS) showing the effects of fraction (i.e. type) of tissue from adventitious roots (basal zone of root with secondary aerenchyma, mature zone of root, root tip or nodule), two mineral nitrogen (N) concentrations (0 or 11 mm NO3−) and two flooding regimes (drained or waterlogged) on respiration and porosity of Melilotus siculus. Treatments were imposed 28 d after imbibition, for 21 d (plants were 49 d old when measured)
| Parameter | Figure where data appear | Main effects | Two-way interaction | Three-way interaction | |||||
|---|---|---|---|---|---|---|---|---|---|
| Tissue | N | Flooding | Tissue × N | Tissue × flooding | N × flooding | Flooding × N × tissue | Residual | ||
| Respiration | 4 | 73.9*** | <0.01ns | 2.3* | 2.5ns | 2.8ns | 1.3ns | 0.9ns | 16.3 |
| Porosity | 5 | 64.2*** | 0.1ns | 9.0*** | 0.6ns | 10.0*** | <0.01ns | 1.2ns | 14.8 |
d.f. = 3 for all factors and interactions except N, flooding and N × flooding, where d.f. = 1; d.f. = 32 for residuals.
*P < 0.05; ***P < 0.001; ns, not significant.
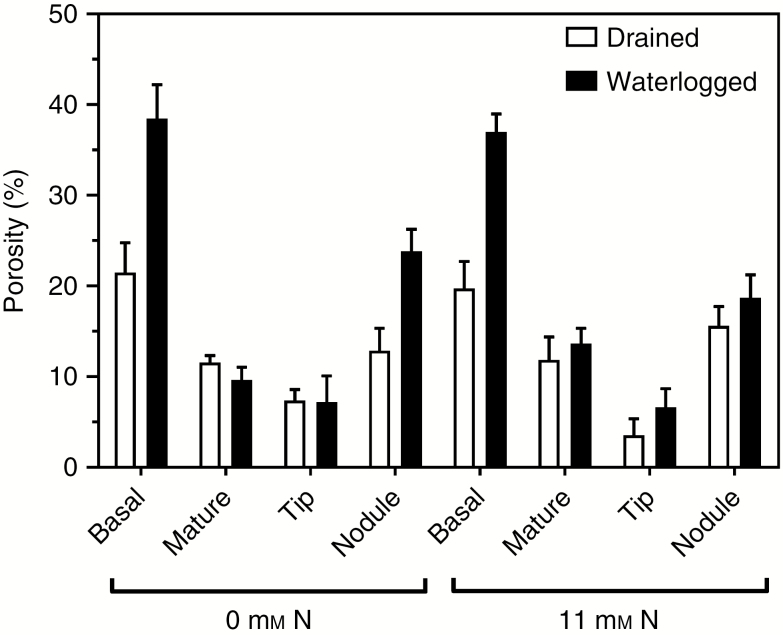
Gas-filled porosity differed amongst the various tissue types, being least in the root tips, moderately higher in the mature (expanded) root zone behind the tips, and substantially higher in the basal root zones with secondary aerenchyma (phellem) and for nodules that were also observed to have formed phellem (see also the next section) (Fig. 5 and Table 3). There was a significant interaction between waterlogging and tissue type since the differences in tissue porosity between drained and waterlogged plants were more pronounced for the basal zones of roots with secondary aerenchyma, than for the other root tissues.
Fig. 5.
Porosity (% gas-filled volume per unit tissue volume) of four different tissues from adventitious roots: basal zone of root with secondary aerenchyma (1-cm segment taken 3 cm below root–shoot junction); mature zone of root (5–6 cm behind the root tip); root tips (1 cm); and root nodules of Melilotus siculus as affected by mineral nitrogen (N) supply (0 or 11 mm NO3−) and drained or waterlogged conditions. Treatments were imposed 28 d after seed imbibition, for 21 d (plants were 49 d old when harvested) (20/15 °C day/night). Plants were grown in vermiculite and provided with a nutrient solution. Values are means ± s.e. (n = 3; one plant from an individual pot was partitioned to provide one replicate of the various tissues measured). For statistics see Table 3.
Nodule O2 profiles and nodule gas-phase connectivity with the root aerenchyma
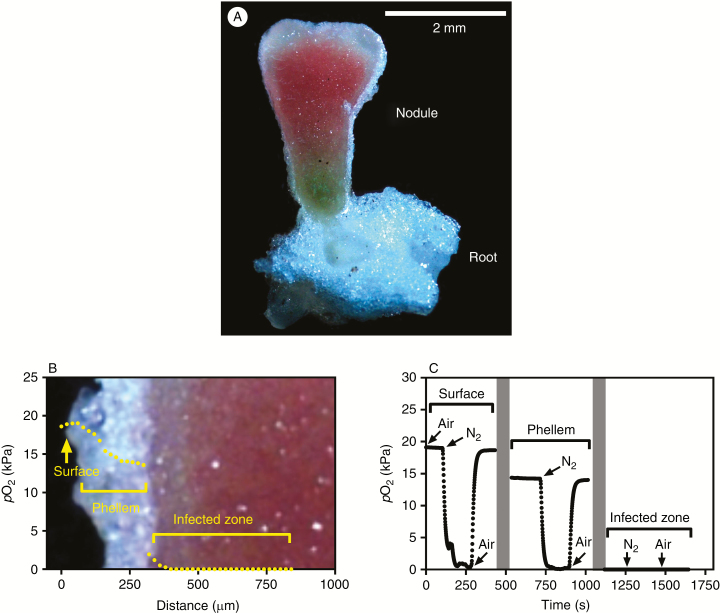
Cross-sections of nodules attached to roots were examined under a microscope (Zeiss Stemi 2000-C, Thornwood, NY, USA). We chose to observe nodules from the plants without added mineral N, as N acquisition by these plants depended on N2 fixation by the nodules. The nodules were located along the basal parts of roots that had formed secondary aerenchyma (phellem) and a thin layer of this porous (high gas-filled volume) tissue had also developed around the nodules (Fig. 6A), which hereafter we refer to as nodule phellem. We tested (1) whether the aerenchyma (phellem) was functional by O2 microelectrode profiling across the nodules when the roots were in an O2-free medium and reliant on internal O2 diffusion along the aerenchyma, and (2) followed by further tests using manipulation of the O2 available to the basal end of the root and the influence of this on pO2 in the various tissues of the nodule.
Fig. 6.
(A) Cross-section of a nodule and root of Melilotus siculus inoculated with rhizobia and grown without mineral N. (B) Profile of O2 partial pressure (pO2) in a nodule attached to a root in an O2-free medium with the shoot in air. (C) Tissue pO2 at three positions (at the surface, 200 µm into the phellem and then an additional 400 µm to place the tip within the infected zone) within a nodule attached to a root with the shoot excised and the stub of the main root (with phellem) initially exposed to air, then high-purity N2, and returned to air. The nodule was ~6 cm from the exposed stub of the main root and the O2 microelectrode was inserted into the side of the nodule. Grey bars indicate that the O2 microelectrode was moved to a new position. Plants were grown in vermiculite under waterlogged conditions (20/15 °C day/night) with rhizobia but without mineral N in the nutrient solution. Treatments were imposed 23 d after seed imbibition, and the plants were measured after 21 d of treatments. The root system was in an O2-free medium, so that nodule O2 supply was dependent on internal movement via the aerenchymatous phellem of the root and of the attached nodule.
Radial O2 profiles of nodules (measured commencing on the side of the nodule) on intact plants with the roots submerged in an O2-free medium and shoots in air showed that pO2 at the surface of the nodule was ~18 kPa, which is only a few kilopascals below the level in air of 20.6 kPa. This demonstrates a low-resistance pathway for O2 (and other gases) to diffuse along the phellem tissue, as well as the potential for high rates of radial O2 loss from the nodules. Tissue pO2 declined to 13 kPa across the phellem tissue around the nodule, and then pO2 declined sharply across what would be the ‘diffusion barrier’ and was below detection (<0.02 kPa) within the infected zone in the interior of the nodule (Fig. 6B).
We tested the connectivity of the nodule phellem to that of the root by measuring O2 at three positions across a nodule attached to a root system in an O2-free medium but with the shoot excised so that either air or high-purity N2 could be supplied at the stub of the main root (Fig. 6C). When air was switched to N2 at the root stub, the pO2 at the nodule surface rapidly declined from ~19 kPa to near 0 kPa, and it increased again to ~19 kPa when air was re-supplied. The same pattern was demonstrated when the microelectrode tip was 200 µm inside the nodule phellem, albeit with a lower initial pO2 of ~14 kPa. By contrast, when the microelectrode tip was 400 µm inside the infected zone of the nodule, pO2 was below detection regardless of whether the cut end of the root stub was exposed to air or to N2. This dynamic response of pO2 in the nodule outer tissues to O2 supply at the base of the root again demonstrates a low-resistance internal pathway for diffusion of O2 (and other gases) along the roots and into the nodules.
DISCUSSION
Melilotus siculus is a waterlogging-tolerant forage legume (Rogers et al., 2008, 2011; Teakle et al., 2012; Striker et al., 2015) but previous controlled-environment studies used plants grown with NO3−, so data were lacking for this species when reliant on N2 fixation (Striker and Colmer, 2017). Nodulated M. siculus grown in vermiculite without a supply of mineral N did not differ in RGR between drained or waterlogged conditions, and these N2-fixing plants grew almost equally well as plants supplied with 11 mm NO3− (Fig. 1A, B). The whole-shoot N concentrations were 3.3–4.4 % (w/w, dry mass) in the M. siculus plants with the greatest shoot RGRs (138–163 mg g−1 d−1) across a few of the treatments, whereas plants with 2.5–3.0 % N in shoots grew significantly less (compare Figs 1A and 2A). Critical shoot tissue N concentrations are not available for M. siculus, but a benchmark for comparison is the 3.2–3.6 % N regarded as marginal deficiency in shoots of Trifolium repens (Reuter and Robinson, 1997). Moreover, M. siculus developed nodules during the waterlogging treatment (Fig. 3D), although the main root system possessed fewer nodules than in the drained controls (Fig. 3B). In addition, M. siculus maintained its root-to-shoot ratio when waterlogged (Fig. 1C), which is a characteristic of waterlogging-tolerant forage species, whereas this ratio declines in sensitive species (Striker and Colmer, 2017). Thus, the present study demonstrated that nodulated M. siculus tolerated 3 weeks of waterlogging in vermiculite and the physiology of these plants reliant on N2 fixation is the main focus of our discussion.
The impaired growth of some nodulated forage legumes under waterlogged conditions has been attributed mainly to a reduction in the supply of O2 to the roots and nodules (Shiferaw et al., 1992; Arrese‐Igor et al., 1993; Pugh et al., 1995). The altered root growth, morphology and metabolism caused by O2-deficient/anoxic waterlogged soil could each disturb the formation and/or functioning of the plant–rhizobia symbiosis, with adverse consequences for N nutrition of forage legumes (Striker and Colmer, 2017). Functional nodules have been demonstrated to form under waterlogged conditions on Lotus pedunculatus, a waterlogging-tolerant forage species (James and Sprent, 1999). Interestingly, field observations of L. pedunculatus described that most of the nodules were present on adventitious roots growing just above the soil surface (Allan et al., 2000). Melilotus siculus also formed nodules during the waterlogging treatment (Fig. 3D), but had a larger proportion of its nodules on adventitious roots when waterlogged compared with plants in drained conditions (calculated from Fig. 3B, C). Although nodules are present, these may not always be functional in N2 fixation. When grown in deoxygenated water, Lotus corniculatus formed fewer nodules and these senesced prematurely, possibly due to the limited access to O2, whereas the more tolerant L. pedunculatus maintained nodulation, which might be associated with internal O2 supply via an extensive network of aerenchymatous tissue that covered stems, roots and nodules (James and Crawford, 1998). Comparison of the nodulated M. siculus without applied mineral N between waterlogged and drained conditions showed that these plants have similar growth and tissue N concentrations (and total N contents) and in both cases were substantially greater than those of the non-nodulated plants (Figs 1A, B and 2A–D), which together demonstrates that the nodules of M. siculus were functional in N2 fixation under waterlogged conditions, being similar to that in drained conditions.
Nodules require O2 for the respiration needed to provide the high ATP and reductant demands of N2 fixation (Schulze, 2004). Consistently, nodules of M. siculus had a relatively high respiratory activity of 8.4–16.3 nmol g−1 FM s−1 (where FM is fresh mass), which was similar to the rate in root tips of 10.1–14.7 nmol g−1 FM s−1, whereas the older root parts had lower rates of 2.7–6.4 nmol g−1 FM s−1. For comparison, lateral roots of six species of Trifolium had respiration rates of 2.3–5.1 nmol g−1 FM s−1 (Gibberd et al., 2001) and the stele and phellem tissues of the basal part of the main root of M. siculus had rates of 3.9 and 2.2 nmol g−1 FM s−1, respectively (Verboven et al., 2012). In order to supply sufficient O2 to sustain high respiration rates in nodules of roots in waterlogged soil, internal transport of O2 via aerenchyma is necessary. Aerenchyma provides a pathway enabling gas-phase diffusion of O2, and other gases, along plant organs (Armstrong, 1979). Melilotus siculus has well-developed aerenchyma, both in the primary cortex of roots and notably also phellem, which is a tissue of high gas-filled porosity that is produced by secondary growth. Phellem is present along the hypocotyl and older portions of the roots and this specialized tissue facilitates O2 movement into and along roots of M. siculus, as demonstrated for non-nodulated plants in stagnant nutrient solution (Teakle et al., 2011; Verboven et al., 2012). The present study shows that nodules of M. siculus, like the older portions of the roots, possess an outer layer of phellem (Fig. 6A, B), although more detailed anatomical studies would be needed to confirm the location of the phellogen within the nodules of M. siculus. The presence of phellem has also been reported for nodules and roots of soybean (Shimamura et al., 2003, 2010; Thomas et al., 2005) and various wetland and aquatic legume species (James et al., 1992, 2001; Loureiro et al., 1994, 1995; Stevens et al., 2002).
Since nodules have a high O2 demand, we hypothesized that the porous tissues of the roots and outer parts of nodules would connect to function as aerenchyma. An O2 profile measurement through a nodule of an intact plant with the roots in an O2-free medium showed that pO2 was high in the nodule phellem (13–19 kPa) and then dropped to below detection in the infected zone of the nodule (Fig. 6B). We tested the connectivity of the root aerenchyma with the nodules by positioning an O2 microelectrode within a nodule attached to a root system in an O2-free medium but with the shoot excised and the stump exposed to air and then N2 (Fig. 6C). There was a fast response of the pO2 signal at the nodule surface and in the phellem of the nodule, whereas the infected zone had consistently low pO2; this rapid response demonstrated a gas-phase connectivity (i.e. functional aerenchyma) from the root base (i.e. root–shoot junction) to nodules.
Tjepkema and Yocum (1974) conducted the first O2 microelectrode studies of nodules using soybean, and recorded an abrupt and very substantial decline in pO2 in the inner cortex at a depth of ~250 μm. This pioneering study, and subsequent O2 microelectrode profiling measurements of nodules of other grain and forage legume species (Witty et al., 1987; Soupène et al., 1995) and the wetland legume Sesbania (James et al., 1998), all demonstrate a high resistance to O2 diffusion within the cortex of nodules. Nitrogenase is an O2-sensitive enzyme and O2 concentrations >5 µm (~0.4 kPa) can result in denaturation (Gallon, 1992), so it is essential to maintain low O2 concentrations inside the infected zone of nodules. The low O2 is regulated by a diffusion barrier in the nodule cortex, and the O2 that enters beyond this barrier is bound by leghaemoglobin, which then delivers the O2 to the cells within this tissue for respiration (Minchin et al., 2008).
In summary, the present study is the first controlled-environment experiment to evaluate the waterlogging tolerance of nodulated M. siculus reliant on N2 fixation. Waterlogged plants formed nodules and when reliant on N2 fixation grew well and maintained tissue N concentrations; both plant growth and tissue N concentrations were similar for the waterlogged and the drained N2-fixing plants. The root nodules had a relatively high respiration rate and the O2 demand of nodules was supplied via well-developed secondary aerenchyma (phellem) in hypocotyl, roots and nodules.
SUPPLEMENTARY DATA
Supplementary data are available online at www.aob.oxfordjournals.org and consist of the following. Fig. S1: (A) profile of O2 partial pressure (pO2) in a nodule of Melilotus siculus attached to a root in an O2-free medium with the shoot in air; (B) tissue pO2 at four different positions
ACKNOWLEDGEMENTS
We thank Dr S. Hughes (Australian Pastures Genebank, SARDI) for providing seeds and Dr R. A. Ballard (SARDI) for providing rhizobia. D.K. and O.P. were supported by two grants from the Villum Foundation. G.T. thanks Prof. M. Pinto for encouragement to travel and the Comisión Nacional de Ciencia y Tecnología (CONICYT) Regional/CEAF/R08I1001 for funds to visit the University of Western Australia. The writing of this article was assisted by a UWA Research Collaboration Award to T.D.C. and O.P.
LITERATURE CITED
- Allan CE, Wheeler CT, Handley LL, Murphy KJ. 2000. Adaptations of Lotus pedunculatus Cav. for nitrogen fixation in a riverine wetland plant community. Botanical Journal of Scotland 52: 149–158. [Google Scholar]
- Armstrong W. 1979. Aeration in higher plants. Advances in Botanical Research 7: 225–332. [Google Scholar]
- Arrese-Igor C, Royuela M, Lorenzo C, Felipe MR, Aparicio-Tejo PM. 1993. Effect of low rhizosphere oxygen on growth, nitrogen fixation and nodule morphology in lucerne. Physiologia Plantarum 89: 55–63. [Google Scholar]
- Bailey-Serres J, Lee SC, Brinton E. 2012. Waterproofing crops: effective flooding survival strategies. Plant Physiology 160: 1698–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonython AL, Ballard RA, Charman N, Nichols PGH, Craig AD. 2011. New strains of rhizobia that nodulate regenerating messina (Melilotus siculus) plants in saline soils. Crop and Pasture Science 62: 427–436. [Google Scholar]
- Colmer TD. 2003. Long-distance transport of gases in plants: a perspective on internal aeration and radial oxygen loss from roots. Plant, Cell & Environment 26: 17–36. [Google Scholar]
- Colmer TD, Greenway H. 2011. Ion transport in seminal and adventitious roots of cereals during O2 deficiency. Journal of Experimental Botany 62: 39–57. [DOI] [PubMed] [Google Scholar]
- Colmer TD, Voesenek LACJ. 2009. Flooding tolerance: suites of plant traits in variable environments. Functional Plant Biology 36: 665–681. [DOI] [PubMed] [Google Scholar]
- Gallon JR. 1992. Reconciling the incompatible: N2 fixation and O2. New Phytologist 122: 571–609. [Google Scholar]
- Gibberd MR, Gray JD, Cocks PS, Colmer TD. 2001. Waterlogging tolerance among a diverse range of Trifolium accessions is related to root porosity, lateral root formation and ‘aerotropic rooting’. Annals of Botany 88: 579–589. [Google Scholar]
- Gibbs J, Greenway H. 2003. Mechanisms of anoxia tolerance in plants. I. Growth, survival and anaerobic catabolism. Functional Plant Biology 30: 1–47. [DOI] [PubMed] [Google Scholar]
- James EK, Crawford RMM. 1998. Effect of oxygen availability on nitrogen fixation by two Lotus species under flooded conditions. Journal of Experimental Botany 49: 599–609. [Google Scholar]
- James EK, Sprent JI. 1999. Development of N2-fixing nodules on the wetland legume Lotus uliginosus exposed to conditions of flooding. New Phytologist 142: 219–231. [Google Scholar]
- James EK, Sprent JI, Sutherland JM, McInroy SG, Minchin FR. 1992. The structure of nitrogen fixing root nodules on the aquatic mimosoid legume Neptunia plena. Annals of Botany 69: 173–180. [Google Scholar]
- James EK, Minchin FR, Oxborough K et al. 1998. Photosynthetic oxygen evolution within Sesbania rostrata stem nodules. Plant Journal 13: 29–38. [Google Scholar]
- James EK, Loureiro MdF, Pott A et al. 2001. Flooding-tolerant legume symbioses from the Brazilian Pantanal. New Phytologist 150: 723–738. [Google Scholar]
- Jensen CR, Luxmoore RJ, Vangundy SD, Stolzy LH. 1969. Root air measurements by a pycnometer method. Agronomy Journal 61: 474–475. [Google Scholar]
- Justin SHFW, Armstrong W. 1987. The anatomical characteristics of roots and plant response to soil flooding. New Phytologist 106: 465–495. [Google Scholar]
- Kirk GJD, Greenway H, Atwell BJ, Ismail AM, Colmer TD. 2014. Adaptation of rice to flooded soils. In: Lüttge U, Beyschlag W, Cushman J, eds. Progress in botany, Vol. 75 Berlin: Springer, 215–253. [Google Scholar]
- Loureiro MF, Faria SMd, James EK, Pott A, Franco AA. 1994. Nitrogen-fixing stem nodules of the legume, Discolobium pulchellum Benth. New Phytologist 128: 283–295. [DOI] [PubMed] [Google Scholar]
- Loureiro MF, James EK, Sprent JI, Franco AA. 1995. Stem and root nodules on the tropical wetland legume Aeschynomene fluminensis. New Phytologist 130: 531–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minchin FR, Sheehy JE, Witty JF. 1986. Further errors in the acetylene reduction assay: effects of plant disturbance. Journal of Experimental Botany 37: 1581–1591.
- Minchin FR, James EK, Becana M. 2008. Oxygen diffusion, production of reactive oxygen and nitrogen species, and antioxidants in legume nodules. In: Dilworth MJ, James EK, Sprent JI, Newton WE, eds. Nitrogen-fixing leguminous symbioses. Dordrecht: Springer, 321–362. [Google Scholar]
- Phelan P, Moloney AP, McGeough EJ et al. 2015. Forage legumes for grazing and conserving in ruminant production systems. Critical Reviews in Plant Sciences 34: 281–326. [Google Scholar]
- Pugh R, Witty JF, Mytton LR, Minchin FR. 1995. The effect of waterlogging on nitrogen fixation and nodule morphology in soil-grown white clover (Trifolium repens L.). Journal of Experimental Botany 46: 285–290. [Google Scholar]
- Reuter D, Robinson JB. 1997. Plant analysis: an interpretation manual. Collingwood: CSIRO Publishing. [Google Scholar]
- Rogers ME, Colmer TD, Frost K et al. 2008. Diversity in the genus Melilotus for tolerance to salinity and waterlogging. Plant and Soil 304: 89–101. [Google Scholar]
- Rogers ME, Colmer TD, Nichols PGH et al. 2011. Salinity and waterlogging tolerance amongst accessions of messina (Melilotus siculus). Crop and Pasture Science 62: 225–235. [Google Scholar]
- Schulze J. 2004. How are nitrogen fixation rates regulated in legumes?Journal of Plant Nutrition and Soil Science 167: 125–137. [Google Scholar]
- Shabala S, Shabala L, Barcelo J, Poschenrieder C. 2014. Membrane transporters mediating root signalling and adaptive responses to oxygen deprivation and soil flooding. Plant, Cell & Environment 37: 2216–2233. [DOI] [PubMed] [Google Scholar]
- Shiferaw W, Shelton HM, So HB. 1992. Tolerance of some subtropical pasture legumes to waterlogging. Tropical Grasslands 26: 187–195. [Google Scholar]
- Shimamura S, Mochizuki T, Nada Y, Fukuyama M. 2003. Formation and function of secondary aerenchyma in hypocotyl, roots and nodules of soybean (Glycine max) under flooded conditions. Plant and Soil 251: 351–359. [Google Scholar]
- Shimamura S, Yamamoto R, Nakamura T, Shimada S, Komatsu S. 2010. Stem hypertrophic lenticels and secondary aerenchyma enable oxygen transport to roots of soybean in flooded soil. Annals of Botany 106: 277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soupène E, Foussard M, Boistard P, Truchet G, Batut J. 1995. Oxygen as a key developmental regulator of Rhizobium meliloti N2-fixation gene expression within the alfalfa root nodule. Proceedings of the National Academy of Sciences of the USA 92: 3759–3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprent JI. 2007. Evolving ideas of legume evolution and diversity: a taxonomic perspective on the occurrence of nodulation. New Phytologist 174: 11–25. [DOI] [PubMed] [Google Scholar]
- Sprent JI, Ardley J, James EK. 2017. Biogeography of nodulated legumes and their nitrogen-fixing symbionts. New Phytologist 215: 40–56. [DOI] [PubMed] [Google Scholar]
- Stevens KJ, Peterson RL, Reader RJ. 2002. The aerenchymatous phellem of Lythrum salicaria (L.): a pathway for gas transport and its role in flood tolerance. Annals of Botany 89: 621–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striker GG, Colmer TD. 2017. Flooding tolerance of forage legumes. Journal of Experimental Botany 68: 1851–1872. [DOI] [PubMed] [Google Scholar]
- Striker GG, Teakle NL, Colmer TD, Barrett-Lennard EG. 2015. Growth responses of Melilotus siculus accessions to combined salinity and root-zone hypoxia are correlated with differences in tissue ion concentrations and not differences in root aeration. Environmental and Experimental Botany 109: 89–98. [Google Scholar]
- Teakle NL, Armstrong J, Barrett-Lennard EG, Colmer TD. 2011. Aerenchymatous phellem in hypocotyl and roots enables O2 transport in Melilotus siculus. New Phytologist 190: 340–350. [DOI] [PubMed] [Google Scholar]
- Teakle NL, Bowman S, Barrett-Lennard EG, Real D, Colmer TD. 2012. Comparisons of annual pasture legumes in growth, ion regulation and root porosity demonstrate that Melilotus siculus has exceptional tolerance to combinations of salinity and waterlogging. Environmental and Experimental Botany 77: 175–184. [Google Scholar]
- Thomas AL, Guerreiro SMC, Sodek L. 2005. Aerenchyma formation and recovery from hypoxia of the flooded root system of nodulated soybean. Annals of Botany 96: 1191–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjepkema JD, Yocum CS. 1974. Measurement of oxygen partial pressure within soybean nodules by oxygen microelectrodes. Planta 119: 351–360. [DOI] [PubMed] [Google Scholar]
- Verboven P, Pedersen O, Herremans E et al. 2012. Root aeration via aerenchymatous phellem: three-dimensional micro-imaging and radial O2 profiles in Melilotus siculus. New Phytologist 193: 420–431. [DOI] [PubMed] [Google Scholar]
- Voesenek LACJ, Sasidharan R. 2013. Ethylene – and oxygen signalling – drive plant survival during flooding. Plant Biology 15: 426–435. [DOI] [PubMed] [Google Scholar]
- Wiengweera A, Greenway H, Thomson CJ. 1997. The use of agar nutrient solution to simulate lack of convection in waterlogged soils. Annals of Botany 80: 115–123. [Google Scholar]
- Witty JF, Skøt L, Revsbech NP. 1987. Direct evidence for changes in the resistance of legume root nodules to O2 diffusion. Journal of Experimental Botany 38: 1129–1140. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.